
Int J App Pharm, Vol 17, Issue 1, 2025, 355-364Original Article
DESIGN AND OPTIMIZATION OF ACYCLOVIR LOADED SOLID LIPID NANOPARTICLES: A SUSTAINED RELEASE APPROACH
DEEVAN PAUL A.*, PHUVISAA B. S., KIRUTHIKA S., MOHAMED ARSATH, ABIRAMI D., GOKUL K.
Sri Ramachandra Faculty of Pharmacy, Sri Ramachandra Institute of Research and Education, Chennai–600116, India
*Corresponding author: Deevan Paul A.; *Email: deevanpaul@sriramachandra.edu.in
Received: 24 Jun 2024, Revised and Accepted: 16 Oct 2024
ABSTRACT
Objective: This study aims to develop Acyclovir-Loaded Solid Lipid Nanoparticles (ASLN) prepared through homogenisation and evaluate their efficacy.
Methods: ASLN were formulated using Gelucire 43/01, Polyvinylpyrrolidone (PVP), Tween 80, and Stearic acid in varying ratios through solvent evaporation and homogenisation. Lipids and Acyclovir were melted together and then emulsified using a homogeniser. Particle size distribution was assessed by Dynamic Light Scattering (DLS), and Zeta Potential was measured using electrophoretic mobility. The cumulative drug release profile was analyzed to determine sustained release characteristics. Zero-order kinetic modelling was applied to elucidate the release mechanism, indicating diffusion rate-limited drug release. Comparative studies with marketed Acyclovir formulations were conducted to assess efficacy and performance.
Results: All formulations exhibited satisfactory characteristics: Particle size of 185.6±4.28 nm, Zeta potential of-24.15±5.43 mV, Polydispersity Index of 0.192±3.11, and Drug Entrapment Efficiency of 77.06±4.3%. In vitro release studies of ASLN formulation F12 showed prolonged drug release (90.88% cumulative release by the 8th hour), in sustained drug availability. Comparative studies highlighted the efficacy of ASLN compared to commercial acyclovir products. The kinetic analysis confirmed zero-order kinetics and diffusion rate-limited drug release for all formulations.
Conclusion: In conclusion, the formulation of ASLN using Gelucire 43/01, Polyvinylpyrrolidone (PVP), Tween 80, and Stearic acid produced nanoparticles with favourable characteristics. These included appropriate particle size, zeta potential, and high drug entrapment efficiency. In vitro studies demonstrated sustained drug release, suggesting ASLN as promising carriers for enhancing Acyclovir's therapeutic efficacy.
Keywords: ASLN, Acyclovir, Drug delivery, Zero-order kinetics, Sustained release
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijap.2025v17i1.51877 Journal homepage: https://innovareacademics.in/journals/index.php/ijap
INTRODUCTION
Herpes Simplex Virus (HSV) infections are a significant global health concern, affecting millions of individuals worldwide and necessitating effective antiviral therapies. Among the treatments available, acyclovir has been a cornerstone due to its potent antiviral activity against HSV. However, the clinical efficacy of Acyclovir is hampered by challenges such as low bioavailability and poor aqueous solubility, leading to suboptimal therapeutic outcomes and necessitating frequent dosing regimens [1]. Solid lipid nanoparticles (SLN) have emerged as promising nanocarriers for improving the delivery and efficacy of poorly soluble drugs like SLN. These offer several advantages, including enhanced drug stability, sustained release kinetics, and improved pharmacokinetic profiles. These attributes make SLN an attractive platform for overcoming the limitations of conventional acyclovir formulations. The formulation of Acyclovir-loaded Solid lipid Nanoparticles (ASLN) involves encapsulating acyclovir within a lipid matrix composed of biocompatible materials such as Gelucire 43/01, Polyvinylpyrrolidone (PVP), Tween 80, and Stearic acid [2]. This approach improves drug solubility and bioavailability and enables sustained release of Acyclovir, potentially reducing dosing frequency and enhancing patient compliance. Moreover, SLN can protect acyclovir from enzymatic degradation and systemic clearance, thereby prolonging its therapeutic effect. Previous studies have demonstrated the efficacy of SLN in enhancing the therapeutic outcomes of various drugs by improving their pharmacokinetic and pharmacodynamic profiles. In the following research on antiviral drugs, SLN offer a promising strategy to improve the effectiveness of acyclovir against HSV infections. By optimizing the formulation parameters and characterizing the physicochemical properties of ASLN, this study aims to contribute to the development of advanced drug delivery systems that can address the current challenges in HSV treatment [3-5]. This study shows the formulation and characterisation of ASLN, a novel approach that promises to significantly advance antiviral therapy. While acyclovir has been a cornerstone in treating HSV infections, its effectiveness is often limited by poor drug bioavailability and rapid drug clearance. By incorporating acyclovir into SLN, this research introduces a cutting-edge strategy designed to overcome these limitations. SLNs offer a unique combination of controlled release, enhanced stability, and improved drug permeability, which are not fully exploited in current acyclovir formulations. This study's focus on ASLN represents an innovative shift from traditional delivery methods, aiming to enhance therapeutic efficacy and patient outcomes in HSV treatment. The study has implications for HSV infections and the limitations of current acyclovir treatments.
MATERIALS AND METHODS
Chemicals involved
Acyclovir was obtained as a gift sample from GlaxoSmith (Mumbai), PVP, Dimethyl Sulfoxide (DMSO), and Stearic acid were obtained as a gift sample from Sisco Research Laboratories Pvt. Ltd, Gelucire 43/01 was obtained as a gift sample from Gattefosse Indian Pvt. Ltd, Tween 80, and Oleic acid were obtained as a gift sample from Nice Chemical Pvt. Ltd.
Differential scanning calorimetry (DSC)
Differential scanning calorimetry (DSC) curves of pure Acyclovir, Acyclovir with Gelucire 43/01, and Acyclovir with PVP were generated using a Perkin Elmer thermal analysis instrument. The samples weighed 9.58 mg, 7.700 mg, and 5.150 mg, respectively. The temperature was ramped from 30 °C to 400 °C at a rate of 10 °C/min during the analysis [6].
Fourier transform infrared spectroscopy (FT-IR)
Fourier Transform Infrared Spectroscopy (FT-IR) analysis was conducted to investigate the interactions between Acyclovir and polymers (Gelucire 43/01 and PVP). The FT-IR studies were performed using a PerkinElmer FT-IR spectrometer with a spectral range. The absorption peaks of the drug and excipients were observed within the range [7]. The spectra were recorded and analyzed using FT-IR BRUKER software.
Designing of acyclovir loaded SLN using box-behnken design software
Optimization studies
It is a systematic approach used to plan experiments, control variables, and analyze data to optimize processes or products. The Box-Behnken design is a specific type of DOE where multiple factors are varied simultaneously to observe their effects and interactions on the response variable. This methodology enables efficient experimentation and helps draw robust conclusions about the factors influencing the outcome [8-11].
Procedure for factorial design in DOE
Defining factors and levels
The investigation focused on different concentrations of the drug and polymer, with each factor assigned a name (e.g., Factor A –Tween 80, Factor B – PVP, Factor C – Gelucire 43/01. These factors were considered independent variables. Two levels were specified for each factor, denoted as "low" (-1) and "high" (+1).
Table 1: Formulation of ASL nusing DOE software
| Formulations/ingredients | Acyclovir (mg) | Tween 80(ml) | Pvp (mg) | Gelucire43/01 (mg) | Stearic acid (mg) | Water (ml) |
| F1 | 200 | 0.35 | 20 | 80 | 80 | Q. s |
| F2 | 200 | 0.35 | 22.5 | 70 | 80 | Q. s |
| F3 | 200 | 0.5 | 25 | 70 | 80 | Q. s |
| F4 | 200 | 0.2 | 20 | 70 | 80 | Q. s |
| F5 | 200 | 0.35 | 20 | 60 | 80 | Q. s |
| F6 | 200 | 0.35 | 25 | 80 | 80 | Q. s |
| F7 | 200 | 0.35 | 25 | 60 | 80 | Q. s |
| F8 | 200 | 0.2 | 25 | 70 | 80 | Q. s |
| F9 | 200 | 0.2 | 22.5 | 80 | 80 | Q. s |
| F10 | 200 | 0.5 | 22.5 | 80 | 80 | Q. s |
| F11 | 200 | 0.5 | 22.5 | 60 | 80 | Q. s |
| F12 | 200 | 0.5 | 20 | 70 | 80 | Q. s |
| F13 | 200 | 0.2 | 22.5 | 60 | 80 | Q. s |
Analyzing the design
Built-in analysis tools were utilized for visualizing or calculating effects, including second-order polynomial equations to account for interactions and quadratic terms. The model fit, P-values, ANOVA, and diagnostic graphs for the responses were examined. The model was confirmed by comparing predicted and observed values using the 95% confidence interval and P-value [12].
SLN were prepared using the homogenization and solvent evaporation method. The solid lipid and aqueous phase, consisting of deionized water and Tween 80, were heated to 65 °C and then combined to form a coarse emulsion under magnetic stirring at 600 rpm. For ASLN, 10 mg of Acyclovir was dispersed into the molten lipid phase. A homogenizer operated at 1000 rpm for 1 h was used to emulsify the mixture. Subsequently, the formulation was cooled to room temperature (25 °C) for at least an hour to allow SLN to form and then stored in amber-colored bottles for further analysis.
Characterization studies for ASLN
Characteristic studies of Particle size, Zeta potential, Polydispersity index (PDI) and Drug determined by Malvern Zeta sizer by using the dynamic light scattering approach and a (Malvern Zeta sizer Par analytical) outfitted with Zeta sizer software, particle size and PDI were determined [13]. The measurements were conducted at a constant 90◦degree light scattering angle. Before each measurement, distilled water was diluted in a ratio of 1:100 and utilized as a dispersion solvent. The identical cuvette cells were used for zeta potential and particle size measurements. Since the particles are scattered throughout the aqueous media. A constant temperature of 25 °C was used for all the measurements. For every sample, a total of 13 formulation measurements were made [14]. All data are shown as mean±SD (n = 3), where n is the number of observations.
Drug entrapment efficiency (DEE)
Using the ultrafiltration-centrifugation technique, the concentration of free drug from SLN was found, and the Percentage Entrapment Efficiency (%EE) was computed. Three separate preparations of ASLN samples were centrifuged for ten minutes at 5000 rpm using a fixed 23° angle rotor after being filtered via centrifugal filter devices [15, 16]. The concentration of acyclovir found in the entrapped supernatant was measured at 254 nm using a UV spectrophotometer. The following formula was used to calculate EE:
All data are shown as mean±SD (n = 3), where n is the number of observations.
EE (%) 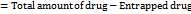 /
/
In vitro dissolution studies using 6.8 pH phosphate buffer
An in vitro drug release profile was obtained using the Franz diffusion cell method to elucidate the effect of the SLN system on the release kinetics of Acyclovir. Gather a magnetic stirrer with beads, a Franz diffusion cell, a burette stand, a syringe, phosphate buffer, and a cellulose sheet. First, soak the cellulose sheet in phosphate buffer for 24 h in a petri dish. Then, fill the diffusion cell-receptor compartment with the buffer and then place the cellophane membrane. Then place the cellophane membrane in both the donor compartment and diffusion cell and tie it with the rubber band [17]. Place the SLN formulation on top of the diffusion cell. Take 2 ml of sample from the donor compartment and replace the buffer with the donor compartment at the time interval of 1-8 h. Add the sample to the test tube and check the absorbance for each sample in UV spectrophotometry at 254 nm.
All data are shown as mean±SD (n = 3), where n is the number of observations.
Stability studies
The stability study of the ASLN formulation was done at 40◦C±2 °C/75% RH ±5%RH. As per ICH Q1A guidelines, the samples for stability analysis must be exposed to an environment of 40 °C±2 °C/75% RH ±5%RH for 90 days. As per the standard protocol, the samples must be analyzed at a 0,30,45 and 90-time point [19-21].
RESULTS AND DISCUSSION
Differential scanning calorimetry (DSC)
The DSC thermogram of pure Acyclovir exhibited a sharp melting peak at 259 °C, indicating its high thermal stability. Formulating acyclovir with Gelucire 43/01, a commonly used solid lipid, slightly reduced the melting peak temperature to 257 °C, suggesting a minimal impact on Acyclovir's thermal stability. Incorporating PVP further decreased the melting peak temperature to 256 °C. These results indicate that Acyclovir maintained its thermal stability during SLN formulation with Gelucire 43/01 and PVP. The observed reduction in melting peak temperature with PVP suggests potential interactions between Acyclovir and the lipid matrix, highlighting PVP role as a stabilizer and solubilizer in nanoparticle formulations. This indicates careful consideration of drug-excipient compatibility in formulation design.
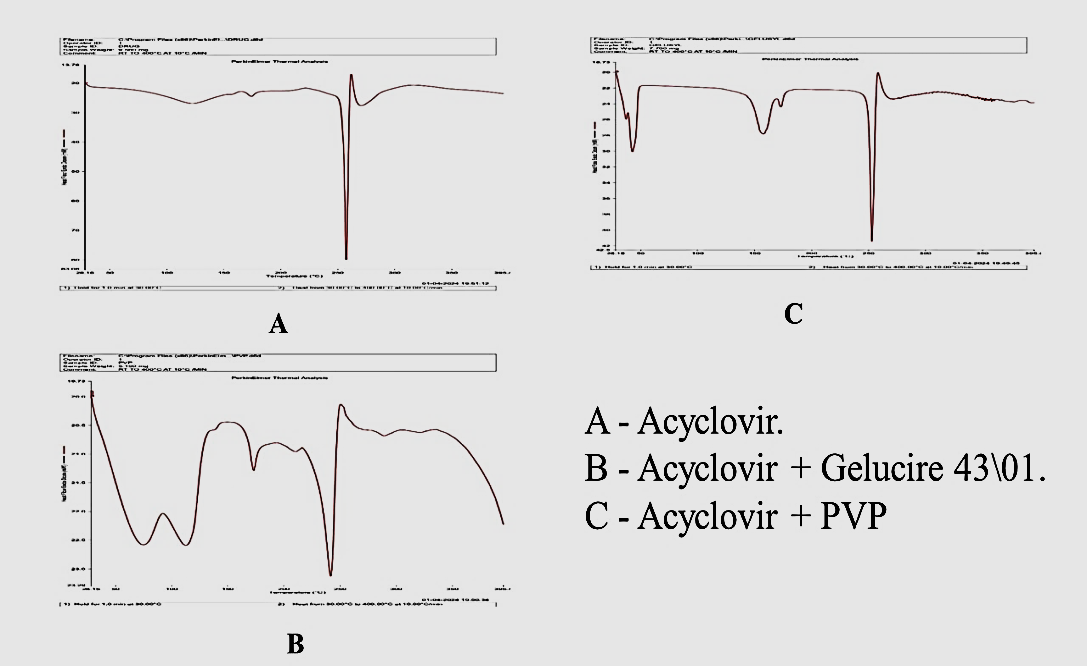
Fig. 1: Endometricpeaks of DSC
Table 2: Melting point analysis of acyclovir and excipients
| Sample | Drug | Drug+Gelucire 43/01 | Drug+PVP |
| Temperature | 259 °C | 257 °C | 256 °C |
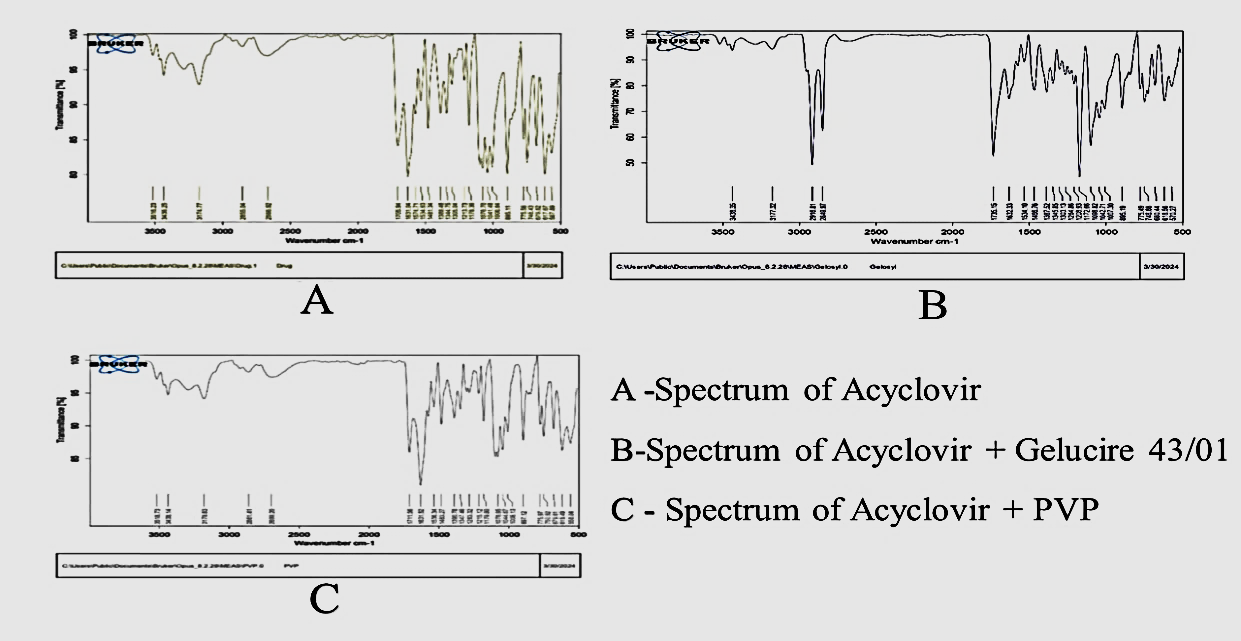
Fig. 2: Spectrum analysis of FT-IR
FTIR spectrum of drug and excipients
The FT-IR spectrum of pure Acyclovir exhibits characteristic peaks at 3436 cm for O-H stretching, 3174 cm for N-H stretching, 1705.94 cm for C=O stretching, 2855 cm for C-H stretching, 1631 cm for R-NH bending, and 1041 cm for C-N stretching. When Acyclovir is formulated with Gelucire 43/01, the FT-IR spectrum shows characteristic peaks at 1632.33 cm for C=O stretching, 2916.81 cm for C-H stretching, and 3438.35 cm for N-H stretching. Formulating Acyclovir with PVP results in characteristic peaks at 1711.50 cm for C=O stretching, 2861 cm for C-H stretching, and 1631 cm for C-N stretching. The distinct differences in peak positions and intensities between the spectra of Acyclovir alone and its formulations with Gelucire 43/01 and PVP suggest that the drug and excipients may not be fully compatible. These differences in FT-IR spectra indicate potential interactions between Acyclovir and the excipients, particularly affecting functional groups involved in stretching and bending vibrations [21]. Therefore, careful consideration of compatibility is crucial in the formulation process.
Table 3: Infrared spectral data of individual drugs and excipients
| Sample | Functional group | Wave number (cm-1) | Vibration |
| Drug | OH | 3436 | O-H Stretching |
| NH | 3174 | N-H Stretching | |
| C=O | 1705.94 | C=O Stretching | |
| CH | 2855 | C-H Stretching | |
| R-NH | 1631 | R-NH Bending | |
| C-N | 1041 | C-N Stretching | |
| Drug+gelucire43/01 | C=O | 1632.33 | C=O Stretching |
| CH | 2916.81 | C-H Stretching | |
| N-H | 3438.35 | N-H Stretching | |
| Drug+PVP | C=O | 1711.50 | C=O Stretching |
| C-H | 2861 | C-H Stretching | |
| C-N | 1631 | C-N Stretching |
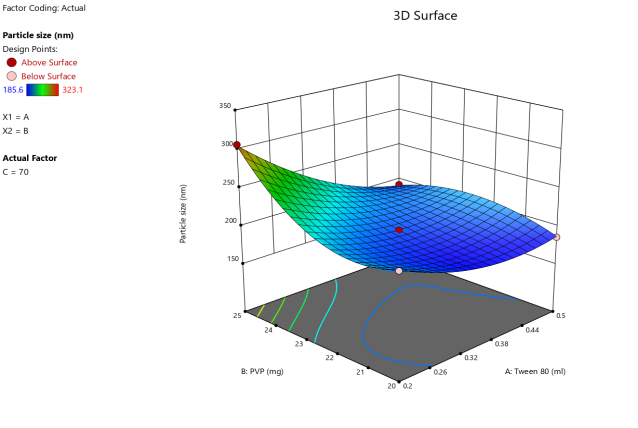
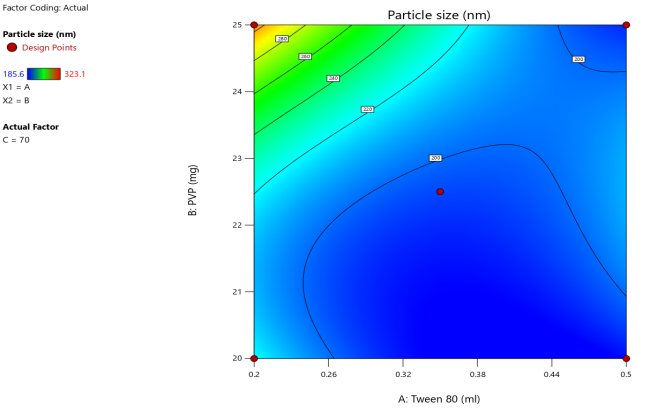
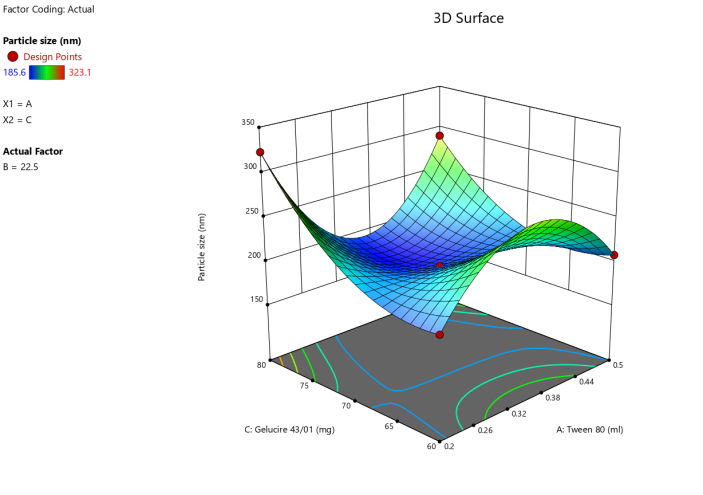
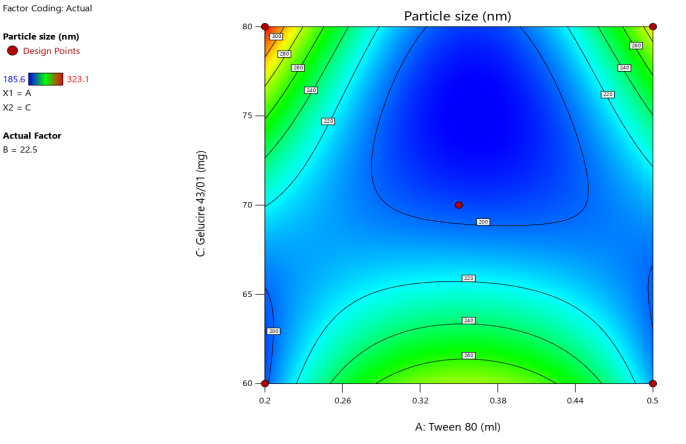
3D surface Contour plot
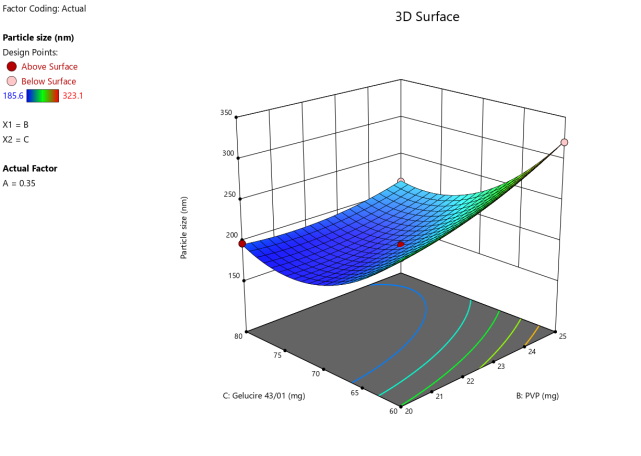
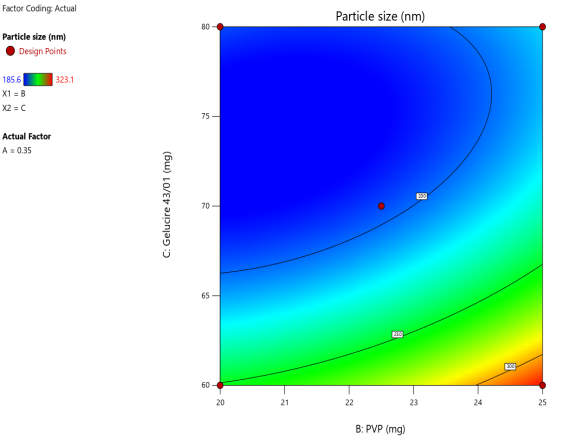
Fig. 3: Anova 3-dimensional graph for particle size
Table 4: Characterization of ASLN by using DOE software
| Factor 1 | Factor 2 | Factor 3 | Response 1 | Response 2 | Response 3 | Response 4 |
| A: Tween 80 | B: PVP | C: Gelucire 43/01 | Particle size | PDI | Zeta potential | Entrapment efficiency |
| ml | mg | mg | nm | mv | % | |
| 0.35 | 20 | 80 | 197.2±6.31 | 0.421±1.45 | -20.23±2.12 | 72.58±1.3 |
| 0.35 | 22.5 | 70 | 195.3±7.45 | 0.395±3.75 | -26.34±3.24 | 73.81±2.5 |
| 0.5 | 25 | 70 | 191.9±8.32 | 0.347±3.23 | -25.93±3.42 | 71.32±3.6 |
| 0.2 | 20 | 70 | 219.3±3.25 | 0.23±2.74 | -30.92±4.23 | 75.43±2.6 |
| 0.35 | 20 | 60 | 252.7±5.43 | 0.362±3.26 | -19.41±2.54 | 76.80±3.1 |
| 0.35 | 25 | 80 | 213.5±3.53 | 0.227±4.12 | -32.47±3.53 | 71.11±1.3 |
| 0.35 | 25 | 60 | 320.6±6.48 | 0.377±2.16 | -36.37±1.34 | 76.42±3.2 |
| 0.2 | 25 | 70 | 306.5±5.23 | 0.358±2.75 | -31.51±2.84 | 73.04±2.7 |
| 0.2 | 22.5 | 80 | 323.1±3.32 | 0.432±3.23 | -34.14±1.45 | 75.78±5.2 |
| 0.5 | 22.5 | 80 | 289.9±4.23 | 0.497±2.65 | -15.72±1.56 | 72.03±2.1 |
| 0.5 | 22.5 | 60 | 208.4±3.65 | 0.208±3.25 | -23.48±2.12 | 76.42±3.2 |
| 0.5 | 20 | 70 | 185.6±4.28 | 0.192±3.11 | -24.15±5.43 | 77.06±4.3 |
| 0.2 | 22.5 | 60 | 197.3±4.71 | 0.232±1.21 | -28.34±3.13 | 73.89±6.4 |
All data are shown as mean±SD (n = 3), where n is the number of observations.
Response 1: Particle size
Final equation in terms of coded factors
Release (Y3) =+195.30-5.63A+22.17B-40.82C-20.22AB-10.98AC-12.98BC+19.64A2+10.89B2+39.64C2+92.75A2C-31.45AB2+0.0000AC2
Response 2: PDI
Final equation in terms of coded factors
Release (Y3) =+0.4020A-0.044B-0.02C-0.02AC-0.05BC-0.06A²-0.05B²+0.11A²B-0.14A²C
The equation in terms of coded factors can be used to make predictions about the response for given levels of each factor. By default, the high levels of the factors are coded as+1 and the low levels are coded as-1. The coded equation is useful for identifying the relative impact of the factors by comparing the factor coefficients [22, 23].
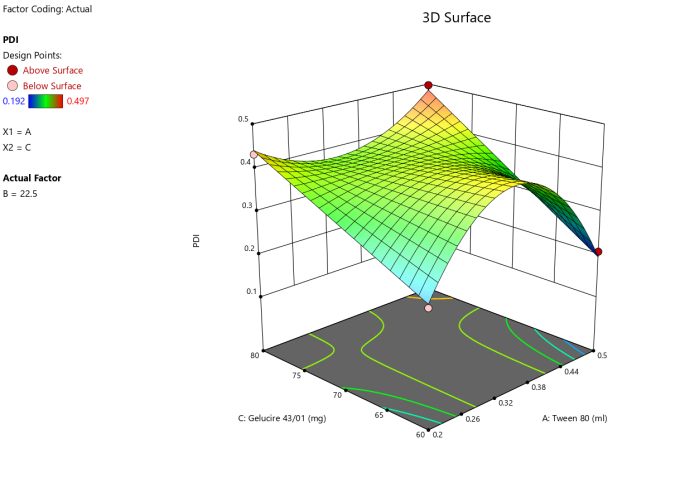
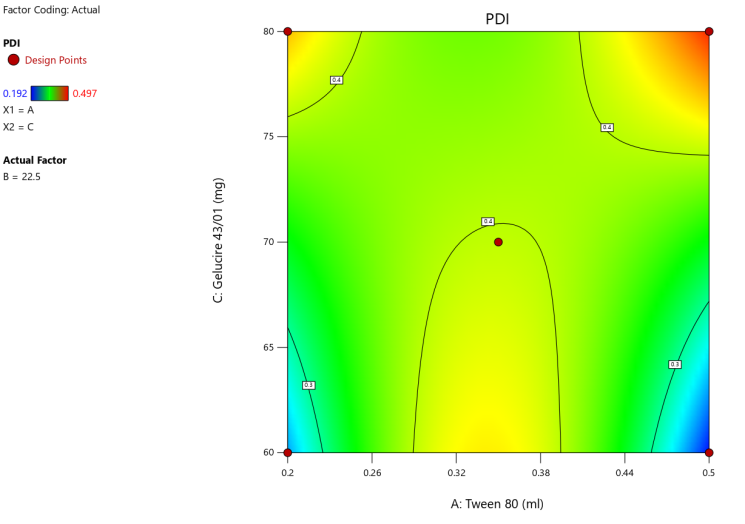
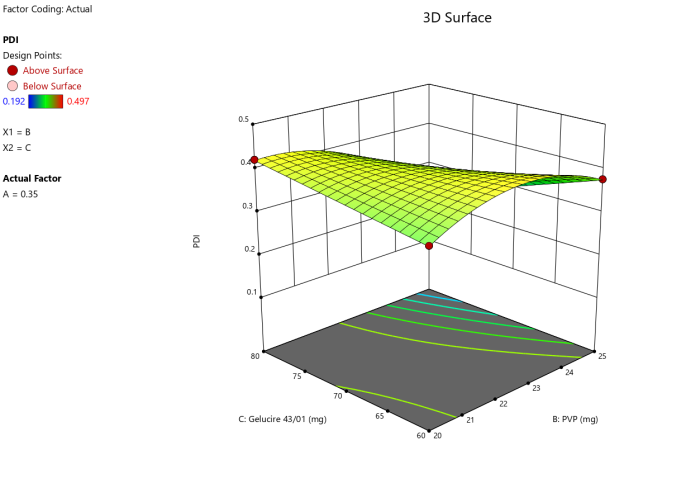
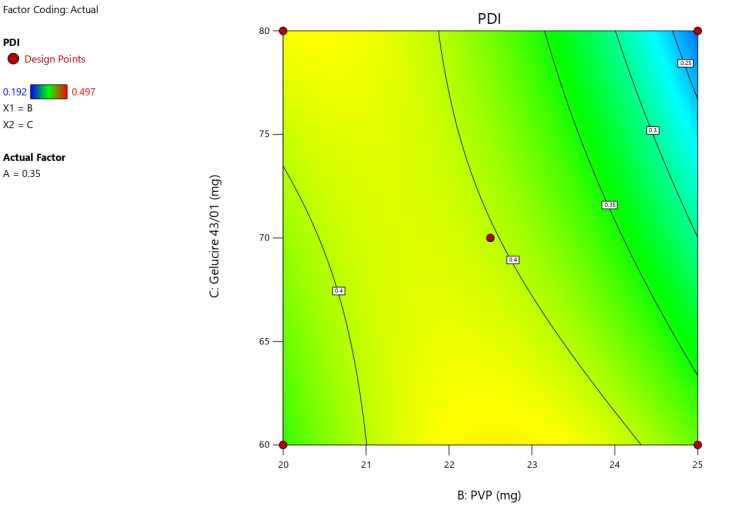
3D surface Contour plot
Fig. 4: Anova 3-dimensional graph for PDI
Response 3: Zeta potential
Final equation in terms of coded factors
Release (Y3) =-26.34+8.33A-3.55B-2.01C+5.20AB+2.43BC-1.67 A²+2.88 B²+5.09 C²-4.04 A²B-9.74 AB²+9.02 B²C
The equation in terms of coded factors can be used to make predictions about the response for given levels of each factor. By default, the high levels of the factors are coded as+1 and the low levels are coded as-1. The coded equation is useful for identifying the relative impact of the factors by comparing the factor coefficients.
Response 4: Entrapment efficiency
Final equation in terms of coded factors
Release (Y3) = 74.28-0.163A+0.25-B1.50C+2.16AB-1.57AC-0.27BC
ASLN underwent comprehensive characterization for Particle size (nm), Polydispersity index (PDI), Zeta potential (-mV), and DEE. Among the formulations tested, F12 was identified as the optimal formulation, with a particle size of 185.6 nm, which enhances permeability characteristics. The PDI value of 0.192 indicates narrow particle size distribution, reflecting the high uniformity and quality of the SLN system.
Zeta potential, a crucial parameter for assessing colloidal dispersion quality, was measured at-24.15 mV. This negative value indicates the presence of a stable colloidal system, where repulsive forces between particles help prevent aggregation. The DEE for ASLN was determined to be 77.06%, indicating that a significant portion of the drug was successfully encapsulated within the nanoparticles [24].
These findings collectively suggest that the F12 formulation of ASLN exhibits favorable characteristics for potential applications in drug delivery systems, highlighting its suitability for further pharmaceutical development and studies.
In vitro dissolution studies using ph6.8 phosphate buffer
All Thirteen formulations were studied in In vitro drug release using phosphate buffer pH 6.8.
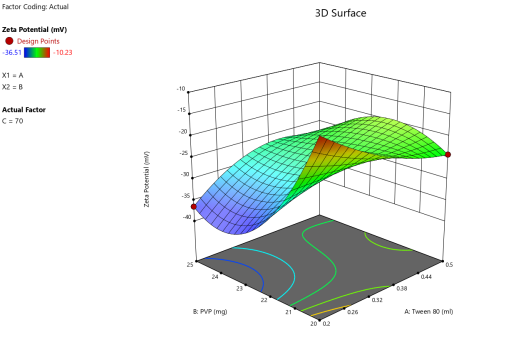
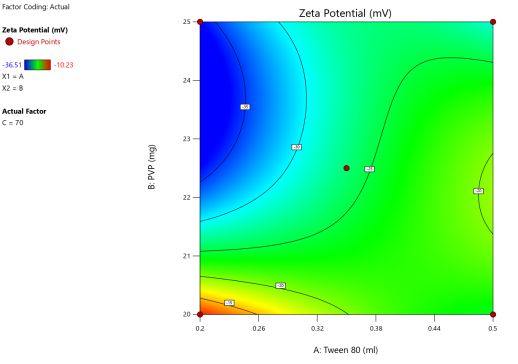
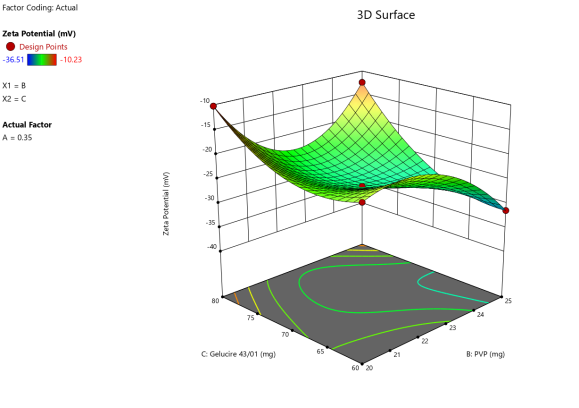
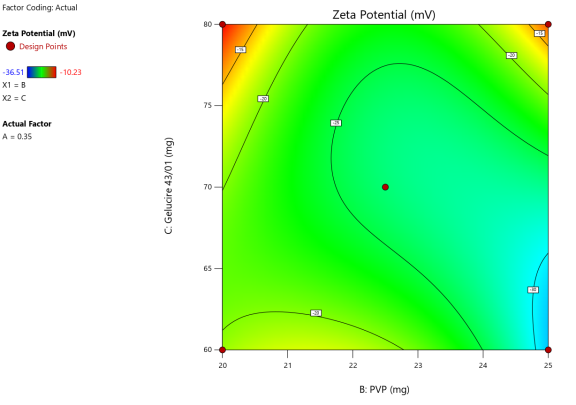
Fig. 5: Anova 3-dimensional graph for zeta potential
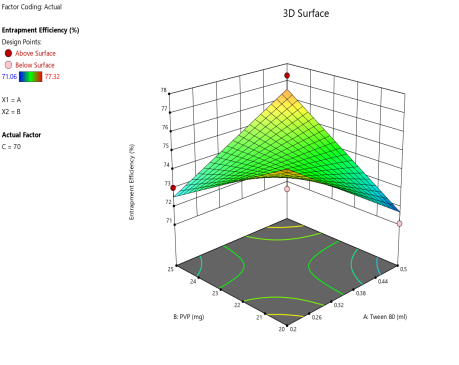
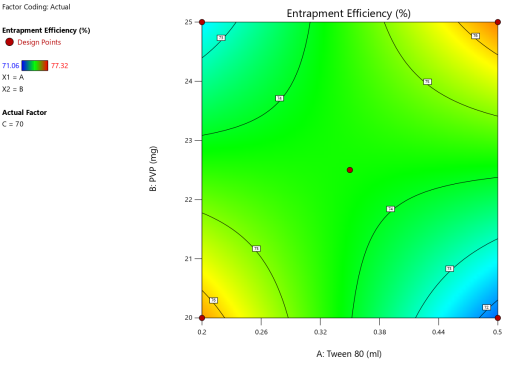
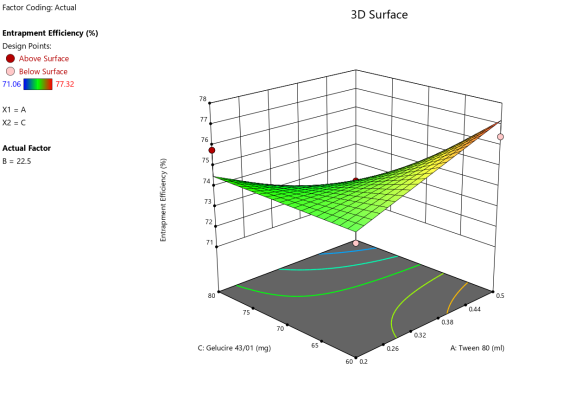
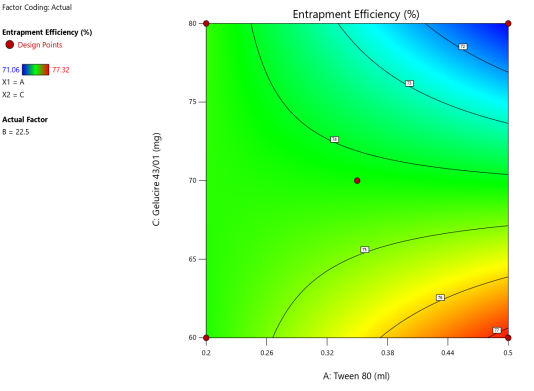
Fig. 6: ANOVA 3-Dimensional graph for entrapment efficiency
Characterization study for ideal formulation
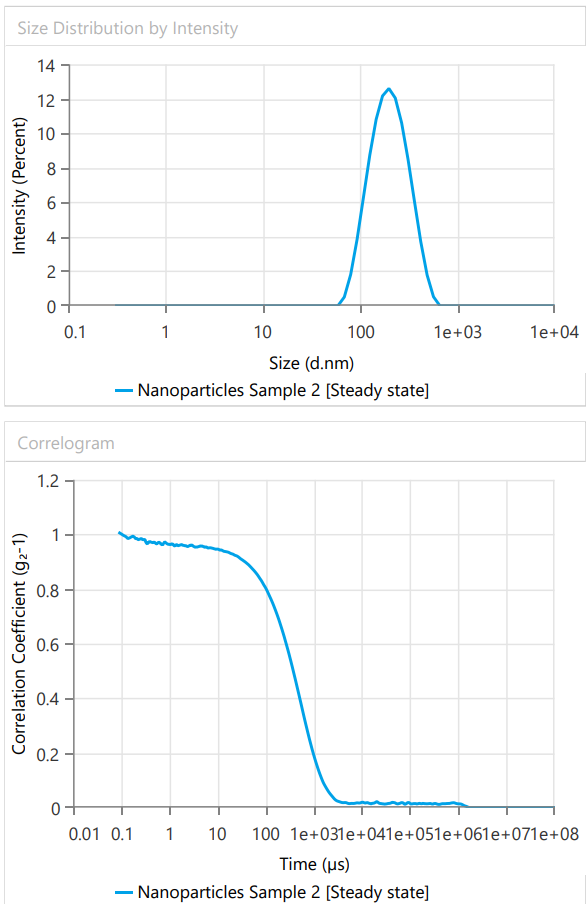

Fig. 7: Response of particle size and polydispersity Index (PDI) for ideal formulation
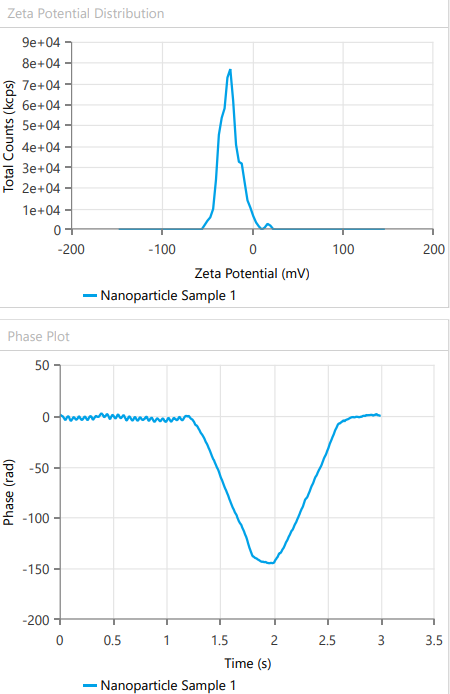

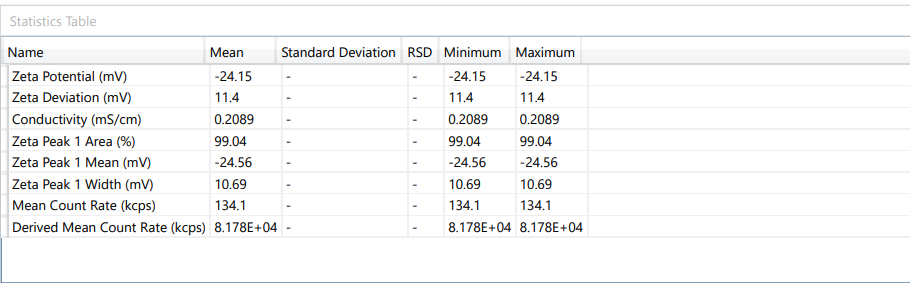
Fig. 8: Response of zeta potential for ideal formulation
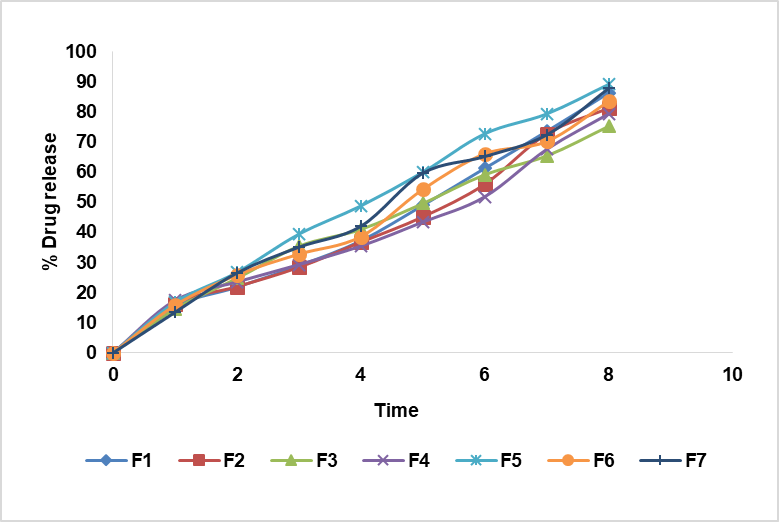
Fig. 9: In vitro drug release curve of nanoparticle from F1-F7. All data showed as mean (n=3); where n is the number of observations
The in vitro drug release of 7 formulated nanoparticles was tabulated. The maximum percentage of drug release at the end of 8 h of F1 to F7 was found to be 89.26±3.21%.
The in vitro drug release of 6 formulated nanoparticles was tabulated. F12 exhibited the highest drug release percentage among the formulations tested, with 90.88±2.21% of the loaded acyclovir released by the 8th h. This sustained release behaviour indicates that the SLN can maintain therapeutic drug levels over an extended period, making it an ideal formulation [25]. The high drug release efficiency of F12 at the 8th h underscores its potential suitability for targeted therapeutic applications, where sustained drug delivery is critical for optimizing therapeutic effects while reducing dosing frequency and potential side effects. This characteristic positions F12 as a promising candidate for further development and potential clinical use in controlled drug delivery systems.
Zero Order, Higuchi, and First Order Kinetics: These models describe the release pattern of the drug over time. Zero-Order Kinetics: The release of the drug is independent of time. Higuchi Kinetics: The release of the drug is proportional to the square root of time, indicating a diffusion-sustained release mechanism. Regression Coefficient Values: These values from the kinetic models indicate how well the experimental data fit each model. Higher regression coefficients signify greater linearity and better fit of the experimental data to the respective kinetic model. Interpretation: A higher regression coefficient implies stronger conformity of the experimental data to the mathematical model, indicating the model's reliability in describing the release kinetics of the drug from the SLN.
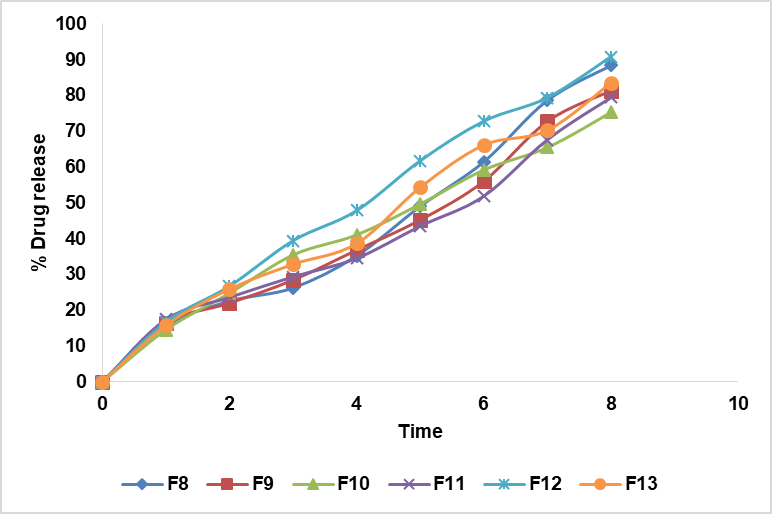
Fig. 10: In vitro drug release curve of nanoparticles F8-F13, all data are presented as mean (n = 3), where n is the number of observations
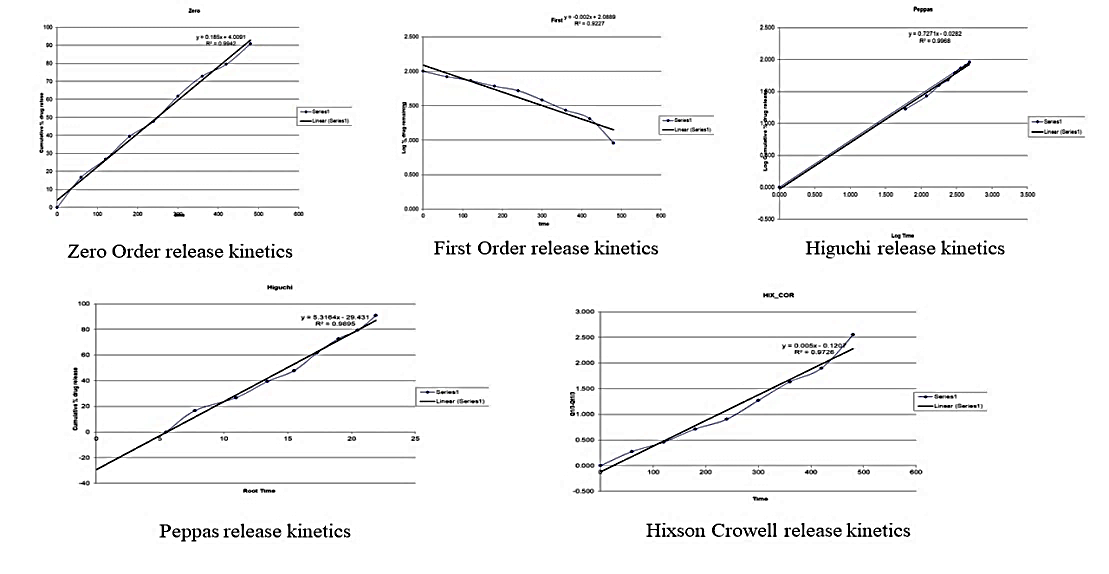
Fig. 11: Graphical representation of release kinetics
Table 8: Accelerated stability studies for the ideal formulation. All data are shown as mean±SD (n = 3), where n is the number of observations
| Days | Particle size (nm) | Polydispersity index (PDI) | Zeta potential (-mV) |
| 0 | 185.6±4.28 | 0.192±3.11 | -24.15±5.43 |
| 15 | 193.2±2.13 | 0.227±2.31 | -16.48±3.12 |
| 30 | 206.8±1.19 | 0.347±1.22 | -10.23±2.53 |
Ideal F12 Solid Lipid Nanoparticle formulated was stored at room temperature at humidity. After the day of manufacture, the dispersions remained stable for up to various study interval periods at 0, 15, and 30 days of stability studies which stored, retaining their original size, PDI, and Zeta potential.
CONCLUSION
This study successfully developed ASL Nanoparticles using gelucire 43/01, PVP, Tween 80, and stearic acid via homogenization. The formulated SLN exhibited desirable characteristics, including a particle size of 185.6 nm, a zeta potential of-24.15±5.43 mV, and a high DEE of 77.06%. In vitro release studies demonstrated sustained drug release profiles, with formulation F12 achieving 90.88±2.21%% cumulative release by the 8th h, indicative of prolonged drug availability. Comparative studies against commercial Acyclovir products underscored the enhanced efficacy and performance of SLN. The kinetic analysis confirmed zero-order kinetics and diffusion rate-limited drug release mechanisms across all formulations. These findings highlight the potential of SLN as effective carriers for improving the therapeutic outcomes of Acyclovir, promising advancements in pharmaceutical formulations for antiviral therapy.
ACKNOWLEDGEMENT
The authors gratefully acknowledge the Principal and Professor of the Department of Pharmacy for using chemicals and labs for our research.
FUNDING
Nil
AUTHORS CONTRIBUTIONS
All authors contributed equally and collaboratively to the conception and design of the study and the collection, analysis, and interpretation of the data. Each author thoroughly reviewed the findings and provided critical insights. Their collective expertise and commitment were essential to completing this work.
CONFLICTS OF INTERESTS
The authors declare no conflict of interest.
REFERENCES
Mahmood S, Kiong KC, Tham CS, Chien TC, Hilles AR, Venugopal JR. Pegylated lipid polymeric nanoparticle encapsulated acyclovir for in vitro controlled release and ex vivo gut sac permeation. AAPS Pharm Sci Tech. 2020;21(7):285. doi: 10.1208/s12249-020-01810-0, PMID 33057878.
Delshadi R, Bahrami A, McClements DJ, Moore MD, Williams L. Development of nanoparticle delivery systems for antiviral agents: a review. J Control Release. 2021 Mar 10;331:30-44. doi: 10.1016/j.jconrel.2021.01.017, PMID 33450319.
Hassan H, Bello RO, Adam SK, Alias E, Meor Mohd Affandi MM, Shamsuddin AF. Acyclovir loaded solid lipid nanoparticles: optimization characterization and evaluation of its pharmacokinetic profile. Nanomaterials (Basel). 2020;10(9):1785. doi: 10.3390/nano10091785, PMID 32916823.
Pandey M, Choudhury H, Abdul Aziz A, Bhattamisra SK, Gorain B, SU JS. Advancement on sustained antiviral ocular drug delivery for herpes simplex virus keratitis: recent update on potential investigation. Pharmaceutics. 2020;13(1):1. doi: 10.3390/pharmaceutics13010001, PMID 33374925.
Kondel R, Shafiq N, Kaur IP, Singh MP, Pandey AK, Ratho RK. Effect of acyclovir solid lipid nanoparticles for the treatment of herpes simplex virus (HSV) infection in an animal model of HSV-1 infection. Pharm Nanotechnol. 2019;7(5):389-403. doi: 10.2174/2211738507666190829161737, PMID 31465287.
Seyfoddin A, Al Kassas R. Development of solid lipid nanoparticles and nanostructured lipid carriers for improving ocular delivery of acyclovir. Drug Dev Ind Pharm. 2013;39(4):508-19. doi: 10.3109/03639045.2012.665460, PMID 22424312.
Kukhanova MK, Korovina AN, Kochetkov SN. Human herpes simplex virus: life cycle and development of inhibitors. Biochemistry (Mosc). 2014;79(13):1635-52. doi: 10.1134/S0006297914130124, PMID 25749169.
El Assal MI. Acyclovir loaded solid lipid nanoparticle-based cream: a novel drug delivery system. Int J Drug Deliv Technol. 2017;7(1):52-62. doi: 10.25258/ijddt.v7i1.8917.
Alsaad A, Hussien A, Ghareeb M. Solid lipid nanoparticles (SLN) as a novel drug delivery system: a theoretical review. Syst Rev Pharm. 2020;11(5):259-73. doi: 10.31838/srp.2020.5.39.
German Cortes J, Vilar Hernandez M, Rafael D, Abasolo I, Andrade F. Solid lipid nanoparticles: multitasking nano-carriers for cancer treatment. Pharmaceutics. 2023;15(3):831. doi: 10.3390/pharmaceutics15030831, PMID 36986692, PMCID PMC10056426.
Beloqui A, Solinis MA, Gascon AR, Del Pozo Rodriguez A, Des Rieux A, Preat V. Mechanism of transport of saquinavir loaded nanostructured lipid carriers across the intestinal barrier. J Control Release. 2013;166(2):115-23. doi: 10.1016/j.jconrel.2012.12.021, PMID 23266764.
Gill S, Lobenberg R, KU T, Azarmi S, Roa W, Prenner EJ. Nanoparticles: characteristics mechanisms of action and toxicity in pulmonary drug delivery a review. J Biomed Nanotechnol. 2007;3(2):107-19. doi: 10.1166/jbn.2007.015.
Jenning V, Thunemann AF, Gohla SH. Characterisation of a novel solid lipid nanoparticle carrier system based on binary mixtures of liquid and solid lipids. Int J Pharm. 2000;199(2):167-77. doi: 10.1016/s0378-5173(00)00378-1, PMID 10802410.
Choi KO, Aditya NP, KO S. Effect of aqueous pH and electrolyte concentration on structure stability and flow behavior of non ionic surfactant based solid lipid nanoparticles. Food Chem. 2014 Mar 15;147:239-44. doi: 10.1016/j.foodchem.2013.09.095, PMID 24206712.
El Gizawy SA, El Maghraby GM, Hedaya AA. Formulation of acyclovir loaded solid lipid nanoparticles: design optimization and in vitro characterization. Pharm Dev Technol. 2019;24(10):1287-98. doi: 10.1080/10837450.2019.1667385, PMID 31507232.
Karpe M, Mali N, Kadam V. Formulation development and evaluation of acyclovir orally disintegrating tablets. J Appl Pharm Sci. 2012 Mar;2(3). doi: 10.7324/JAPS.2012.2317.
Madkhali OA. Perspectives and prospective on solid lipid nanoparticles as drug delivery systems. Molecules. 2022;27(5):1543. doi: 10.3390/molecules27051543, PMID 35268643.
Mahmood A, Ahmad M, Sarfraz RM, Minhas MU, Yaqoob A. Formulation and in vitro evaluation of acyclovir loaded polymeric microparticles: a solubility enhancement study. Acta Pol Pharm. 2016;73(5):1311-24. PMID 29638071.
Nugrahani I, Mussadah MV. Development and validation analysis of acyclovir tablet content determination method using FTIR. Int J Appl Pharm. 2016;8(3):43-7. doi: 10.22159/ijap.2016v8i3.12946.
SG, Chandrakala V, Srinivasan S. Development and evaluation of microsponge gel of an antifungal drug. Int J Curr Pharm Sci. 2023;15(1):30-41. doi: 10.22159/ijcpr.2023v15i1.2069.
Parthiban R, Sathishkumar S, Surendhar S, Ramakrishnan P. Design and evaluation of acyclovir loaded solid lipid nanoparticles for sustained release. Drug Invent Today. 2020;14(1):108.
Bhatnagar A, Bhatnagar E. Evaluation of a combination of gingival physiotherapy flossing and brushing technique through in-office observation. Int J Curr Pharm Sci. 2023;15(3):34-6. doi: 10.22159/ijcpr.2023v15i3.3007.
Rajadhyax A, Shinde U, Desai H, Mane S. Hot melt extrusion in engineering of drug cocrystals: a review. Asian J Pharm Clin Res. 2021;14(8):10-9. doi: 10.22159/ajpcr.2021.v14i8.41857.
Gupta B, Sharma R. Formulation and in vitro characterization of the solid lipid nanoparticles of naftopidil for enhancing oral bioavailability. Asian J Pharm Clin Res. 2023;16(2):77-82. doi: 10.22159/ajpcr.2023.v16i2.46465.
Phalak SD, Bodke V, Yadav R, Pandav S, Ranaware M. A systematic review on nano drug delivery system: solid lipid nanoparticles (SLN). Int J Curr Pharm Sci. 2024;16(1):10-20. doi: 10.22159/ijcpr.2024v16i1.4020.