
Int J App Pharm, Vol 17, Issue 2, 2025, 314-320Original Article
PRODUCTION OF POTENT NEUTRALIZING POLYCLONAL ANTIBODIES AGAINST SEVERE ACUTE RESPIRATORY SYNDROME-CORONAVIRUS-2 (SARS-CoV-2) IN RABBITS, IMMUNIZED WITH RECEPTOR BINDING DOMAIN HEPATITIS B SURFACE ANTIGEN CONJUGATE PROTEIN AND WHOLE INACTIVATED SARS-CoV-2 (MT416726): A COMPARATIVE STUDY
DHAIRYASHEEL YADAV1,2, NANDKUMAR KADAM1,2, S. MOHAN KARUPPAYIL2, MAYUR VIKHARANKAR3, UMESH SHALIGRAM3, ASHWINI K. JADHAV2*
1SERA Biological Pvt Ltd, Shirala, Sangli-415408, Maharashtra India. 2Department of Stem Cell and Regenerative Medicine and Medical Biotechnology, Centre for Interdisciplinary Research, DY Patil Education Society (Deemed to be University), Kolhapur, Kasaba Bawada-416006, Maharashtra, India. 3Serum Institute of India Pvt Ltd, 212/2, Hadapsar, Off Soli Poonawalla Road, Pune-411028 India
*Corresponding author: Ashwini K. Jadhav; *Email: ashujadhav09@gmail.com
Received: 19 Jul 2024, Revised and Accepted: 04 Feb 2025
ABSTRACT
Objective: The current study aims to produce potent neutralizing polyclonal antibodies against novel Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2) by immunization of rabbits.
Methods: Whole inactivated SARS-CoV-2 and purified Receptor Binding Domain-Hepatitis B surface Antigen (RBD-HBsAg) conjugate protein were used as immunogens along with Freud’s incomplete adjuvant for systematic immunization in rabbits by following a protocol approved by the Committee for Control and Supervision of Experiments on Animals (CCSEA) approved Institutional Ethics Committee (IAEC). During the systematic immunization cycle, blood samples were collected periodically after some intervals and checked for in vitro efficacy against SARS-CoV-2 by using Enzyme-Linked Immunosorbent Assay (ELISA) and Plaque Reduction Neutralization Test (PRNT50) methods.
Results: The study revealed that 28, 35, and 42 d are required to generate high-neutralizing hyperimmune polyclonal antibodies in rabbits against immunogens. A combination of Freud’s incomplete adjuvant with whole inactivated SARS-CoV-2 and RBD-HBsAg conjugate protein has shown good response in the generation of potent highly specific polyclonal antibodies. RBD-HBsAg Conjugate protein has shown threefold more immunogenicity and neutralizing efficacy as compared to a whole inactivated SARS-CoV-2.
Conclusion: Rabbits immunized with RBD-HBsAg Conjugate protein immunogen generated high neutralizing and more specific polyclonal antibodies. After extensive preclinical and clinical studies, such purified polyclonal antibodies can be used as alternative therapeutic drugs against SARS-CoV-2 infection.
Keywords: ELISA, Immunization, Rabbit, RBD, Polyclonal antibodies, SARS-CoV-2
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijap.2025v17i2.52098 Journal homepage: https://innovareacademics.in/journals/index.php/ijap
INTRODUCTION
COVID-19 is an infectious disease caused by the novel Severe Acute Respiratory Syndrome-Corona Virus-2 (SARS-CoV-2), originated in Wuhan, China, in December 2019. It rapidly proliferated globally, gaining pandemic status by March 11, 2020. Statistics from October 2022, show that the virus affected over 618 million confirmed cases, and 6.5 million fatalities were reported globally as of October 9th, 2021 [1]. SARS-CoV-2 has single-stranded RNA as the genetic material of size ~30000 nucleotides with only 15 coding genes [2]. Among all the potential targets of SARS-CoV-2, the spike glycoprotein (S) has been well studied due to its critical role in mediating viral entry and in inducing a protective antibody response in infected individuals [3]. The initial stage of viral entry involves the binding of the S protein of SARS-CoV-2 to the ACE2 receptor located on the surface of host cells of vital organs. This binding event induces conformational changes in the S protein, exposing the fusion peptide located within the S2 subunit [4]. Hence, S protein is one of the best target for development of vaccines.
Currently, an appreciative response is seen among people with a vaccine acceptance [5] there are few drugs approved for the treatment of SARS-CoV-2 infection, and many are under different phases of the trial, finding a vaccine for this virus, therefore, remains a high priority [6]. Various areas explored in the search for an ideal vaccine against SARS-CoV-2 include inactivated virus vaccines, recombinant viral vaccines, subunit vaccines, DNA vaccines, and attenuated vaccines. More than 20 vaccines have been approved for human use in different countries for COVID-19 [7]. Some of those are BNT162b2, mRNA-1273, and Sputnik V after two doses had the highest efficacy (>90%) in preventing symptomatic cases in phase III trials [8]. mRNA vaccines, AZD1222, and CoronaVac were effective in preventing symptomatic COVID-19, developed by Pfizer/BioNTech, Moderna, and Oxford University [9]. It is believed that symptoms of SARS-CoV-2 may appear in 2 to 14 d [10]. A strategy for treating SARS-CoV-2 is directly attacking the virus. Blocking a virus’s ability to recognize, attach to, or penetrate host cells will prevent infection altogether. In many cases, the human body naturally produces antibodies against the SARS-CoV-2 virus [11]. In SARS-CoV-2 infection, in humans, both humoral and cellular immune responses are crucial for the clearance of infections. The immune response can be enhanced by Active Immunization or Passive Immunization [10].
Vaccination introduces a dead or weakened version of the pathogen, resulting in the development of vaccine-induced immunity. In either case, if an immune person later comes into contact with that virus, their immune system will instantly recognize it and develop the antibodies required to attack it. Active immunity can last for a very long time, even a lifetime. When a person receives antibodies to a disease rather than creating them through his or her immune system, passive immunity is supplied [10].
The use of monoclonal antibodies is a new outlook in the prevention of infectious diseases [14]. Monoclonal antibodies are utilized to bind to one specific substance in the body. This binding is very versatile and can mimic, block, or cause changes to enact precise mechanisms and provide an effective therapeutic intervention with a very specific treatment for diseases [14]. Many monoclonal antibodies have been recognized to identify the S1 fragment of SARS-CoV-2 and the Receptor Binding Domain (RBD). Subunit S1 is the most important target for SARS-CoV-2 [1], as monoclonal antibodies can block the interaction of RBD and ACE2 receptors. There is a need to do a detailed investigation on the development of novel therapeutics against COVID-19 disease and develop a more precise approach to deal with it [14, 15].
Passive immunotherapy with sera of animal origin has been in use for more than 120 years to treat bacterial and viral infections, envenomation, and drug intoxications [14]. Equine-based purified antibody fragments are safe and effective therapies approved worldwide for the treatment of Snake bites, Rabies, diphtheria, Tetanus, and hepatitis B [15]. The purified antibody fragments are injected into a new patient providing him/her with passive immunity and helping to neutralize circulating viruses till the patient’s immune system generates its antibodies. More recently, antibody-derived therapy was used to treat patients during outbreaks of Ebola [14], West Nile virus [16], H5N1 influenza virus, severe acute respiratory syndrome virus, and Middle East respiratory syndrome coronavirus [17].
In this study, high-neutralizing hyperimmune polyclonal antibodies were produced by immunizing rabbits with whole inactivated SARS-CoV-2 and RBD-HBsAg Conjugate protein and their potency tested by ELISA and PRNT50 assays. The work highlights the therapeutic and diagnostic potential of highly neutralizing hyperimmune polyclonal antibodies and suggests improvements that can be made in the screening of immunogens and adjuvants for broad application of the treatment against SARS-CoV-2 [17].
MATERIALS AND METHODS
Antigen preparation
Whole inactivated SARS-CoV-2 (MT416726)
SARS-CoV-2 (8004/IND/2020/IRSHA PUNE), Accession no. MT416726 was isolated from a throat/nasal swab specimen of COVID-19-positive patients in Vero CCL-81 cells at the Interactive Research School for Health Affairs (IRSHA) [18]. SARS-CoV-2 stock was prepared by inoculating the known titer of the virus in three passages in Vero CCL-81 cells. Virus titrations were performed in Vero CCL-81 cells using tissue culture infectious dose 50% (TCID50)assay. Virus titer (TCID50/ml) was calculated by the Reed-Muench method and found to be 106 TCID50/ml.
The concentration of gamma-inactivated antigen: Gamma-irradiated SARS-CoV-2-infected tissue culture fluid was concentrated using 30 kDa filters (Pall, Germany) and further passed through 0.2 μm filters. aliquoted and stored at −80 °C. Concentrated viral antigen was also aliquoted in 1 and 2 ml volumes in frosted glass bottles and further lyophilized. The lyophilized vials were stored at −20 °C to be used as a source of whole virus antigen.
Receptor binding domain (RBD)-HBsAg conjugate
Innovative VLP-based recombinant protein RBD-HBsAg conjugate developed by the Serum Institute of India[19]was used as an antigen along with Freud’s incomplete adjuvant (FIA).
All the experiments were performed in Biosafety cabinet Class III in sterile conditions. Antigen was handled with extreme care and significant precautions were taken. The antigen dose was prepared as per the standard immunization schedule.
Selection, quarantine, and handling of rabbits
The rabbits were procured from Crystal Biological Solution, Pune, India. The selection, quarantine, and handling of rabbits were done according to the Institutional Animal Ethics Committee (IAEC) approved protocol no. ISB/COVID-19/S/2020/Rev.00 (table 1).
Table 1: Criteria to select rabbits for experiment
| Species/Common name | Oryctolagus/New Zealand white rabbits |
| Age/weight/size | 1.6 – 1.8 Kg |
| Gender | Male/Female |
| Number used | 15 |
| Number of days each animal housed | 120 d |
| Proposed source of animals | Crystal Biological Solution, Pune |
Immunization of rabbits
The whole inactivated SARS-CoV-2 (MT416726) injected in rabbits of Group 1 (table 2) and RBD-HBsAg Conjugate protein injected in rabbits of Group 2 (table 3).
Immunization of group 1 rabbits
Five rabbits were given gradually increasing doses of a combination of killed viral suspension of SARS-CoV-2 (MT416726) antigen at periodic intervals using FIA adjuvants. After completion of the primary immunization of the Rabbits, the blood samples were collected to check antibody response for inducting rabbits into the bleeding program during which the same procedure was followed for periodic immunization of animals under production (table 2).
Table 2: Immunization schedule in rabbits by whole inactivated SARS-CoV-2 (MT416726) injected in group 1
| S. No. | Injection to group 1 | Days | Concentration of virus suspension in TCID50 (PFU/ml) |
| 1 | 1st: Primary | 0 | 1.2 x 105 |
| 2 | 2nd: Booster | 7 | 2.4 x 105 |
| 3 | 3rd: Booster | 14 | 3.0 x 105 |
| 4 | 4th: Booster | 21 | 3.0 x 105 |
| 5 | 5th: Booster | 28 | 3.0 x 105 |
| 6 | 6th: Booster | 35 | 3.0 x 105 |
Immunization of group 2
Five rabbits were given gradually increasing doses of a combination of SARS-CoV-2 (RBD)-HBsAg conjugate protein antigen at periodic intervals using FIA adjuvants. After completion of the primary immunization of the Rabbits, the blood samples were collected to check antibody response for inducting Rabbits into the bleeding program during which the same procedure was followed for periodic immunization of animals under production (table 3).
Table 3: Immunization time intervals for Rabbits immunized by RBD-HBsAg conjugate protein injected in group 2
| S. No. | Injection to group 2 | Days | (RBD)-HBsAg conjugate protein in µl | Concentration in µg/ml |
| 1 | 1st: Primary | 0 | 60 | 20 |
| 2 | 2nd: Booster | 7 | 120 | 40 |
| 3 | 3rd: Booster | 14 | 150 | 50 |
| 4 | 4th: Booster | 21 | 150 | 50 |
| 5 | 5th: Booster | 28 | 150 | 50 |
| 6 | 6th: Booster | 35 | 150 | 50 |
Group 3: This group was not included in the immunization program.
Blood sample collection from immunized rabbits
After clearly outlining the veins, the needle (21-23 gauge) inserted and compressing the ear facilitated bleeding through the mid-ear artery of rabbits. Pre-immune and final blood were collected on the defined days as mentioned in table 4. The collected blood samples were kept in tubes in a slanting position at room temperature for 1 h to allow them to clot. The serum was separated by centrifugation at 5000 rpm for 5 min at 4 °C and transferred into clean and appropriately labelled tubes. Serum samples of non-immunized Group 3 were considered as negative control.
Table 4: Bleeding schedule in rabbits immunized by whole inactivated SARS-CoV-2 (MT416726), RBD-HBsAg Conjugate protein, and non-immunized rabbits
| S. No. | Days | Blood collection | Blood volume | Tests |
| 1 | 0 | Pre-Immune | 1 ml | To use as a Control |
| 2 | 28 | Test Blood 1 (TB1) | 1 ml | Potency by ELISA |
| 3 | 35 | Test Blood 2 (TB2) | 1 ml | Potency by ELISA |
| 4 | 42 | Final Blood | 10 ml | Potency by ELISA, Potency by PRNT50 |
Testing of potency
Indirect ELISA
In vitro efficacy was measured by an indirect enzyme-linked immunosorbent assay (ELISA) [18] using whole purified RBD as a coating antigen in a Tetramethylbenzidine (TMB) system. 96-well polystyrene microtitre ELISA plates (Nunc, Thermo Fisher Scientific, USA) Microwell plates were coated overnight at 4 °C with each purified COVID-19 RBD protein virus at (1:10 diluted, 100 μl/well) in carbonate-bicarbonate buffer (pH 9.6). The wells were washed three times with 0.05% Tween 20 in PBS (PBS-T) and then blocked with 1 % BSA in PBS-T at 37 °C for 1 h. Following three washes with PBS-T to the coated plate, 100 μl of 1:100 diluted Serum samples were added and incubated at 37 °C for 1 h. Following five washes, 100 μl/well of HRP-conjugated rabbit anti-horse IgG (Sigma) diluted 5000-fold in PBS-T for the detection of bound antibodies. Following incubation at 37 °C for 1 h, the plates were washed and the 100μl/well of substrate TMB solution (Sigma) was added to the wells to generate the color. After incubation at room temperature for 30 min, the reaction was stopped by adding 100μl/well of 2 mmol/l H2SO4. The absorbance value at 450 nm (A450) was determined with an ELISA reader (ModeliMark, BioRad,). Antibody titre was defined as the highest dilution at which the A450 ratio (A450 of negative serum) was greater than 2.0.
Virus neutralization assay by PRNT50
PRNT50 assay was carried out as per previously published standard methods with minor modifications [20, 21]. Four-fold serial dilutions of plasma samples were mixed with an equal amount of virus suspension, including 100 pfu in 0.1 ml. After nurturing the combinations at 37 °C for one hour, each virus-diluted serum sample (0.1 ml) was inoculated onto one well of a 24-well tissue culture plate containing a confluent monolayer of Vero CCL-81 cells. After breeding the plate at 37 °C for one hour, an overlay tool (2 % CMC with 2 % FBS in 2 × MEM) was contributed to the cell monolayer, as well as the plate was further incubated at 37 ° C in five percent CO2 for 4-5 d. Plaques were observed. Antibody titres were defined as the highest possible product dilution that resulted in>50 percent (PRNT50) decrease in the variety of plaques.
Statistical analysis
For statistical significance, five rabbits (n=5) in each group were used in the current study. All experiments were carried out in triplicates, the mean values are plotted in a graph, and error bars denote the standard deviation. Significance testing of Indirect ELISA endpoint titres between two groups is analysed, P value arrived is less than 0.05; based on the data we found there is a significant difference between the groups so we rejected the Null hypothesis, and the alternative hypothesis is accepted. Significance testing between two groups is analysed.
RESULTS
Indirect ELISA
As per mentioned immunization protocol, after the completion of 28, 35, and 45 d of immunization, Test blood samples were collected. The indirect ELISA end-point titration method was performed by coating purified RBD proteins in 96 wells plates, Binding of antibodies found in Rabbits immunized with RBD and whole inactivated virus, Serum collected from test bleed samples of Group 1 and Group 2 rabbits showed increasing high binding efficacy after 28, 35, and 42 d. Group 2 rabbits immunized with RBD-HBsAg Conjugate protein serum samples showed threefold more neutralization binding Efficacy than the serum samples of group 1 rabbits immunized with Whole inactivated virus. Serum samples of non-immunized Group 3 were considered as negative control.
Indirect ELISA results of group 1 rabbits immunized with whole inactivated SARS-CoV-2 (MT416726) virus
Neutralization efficacy was estimated by using the indirect ELISA endpoint titration method for 28, 35, and 42 d intervals. Serum samples of rabbits (Group 1) diluted from 1:50 to 1:102400 and added in ELISA plate coated with RBD protein and O. D. was taken at 490 nm. The results revealed that neutralization efficacy increased from samples of 28, 35, and 42 d. The assay is carried out in triplicates to ensure the reproducibility of results. The mean values are plotted in a graph, and error bars denote the standard deviation (fig. 1).
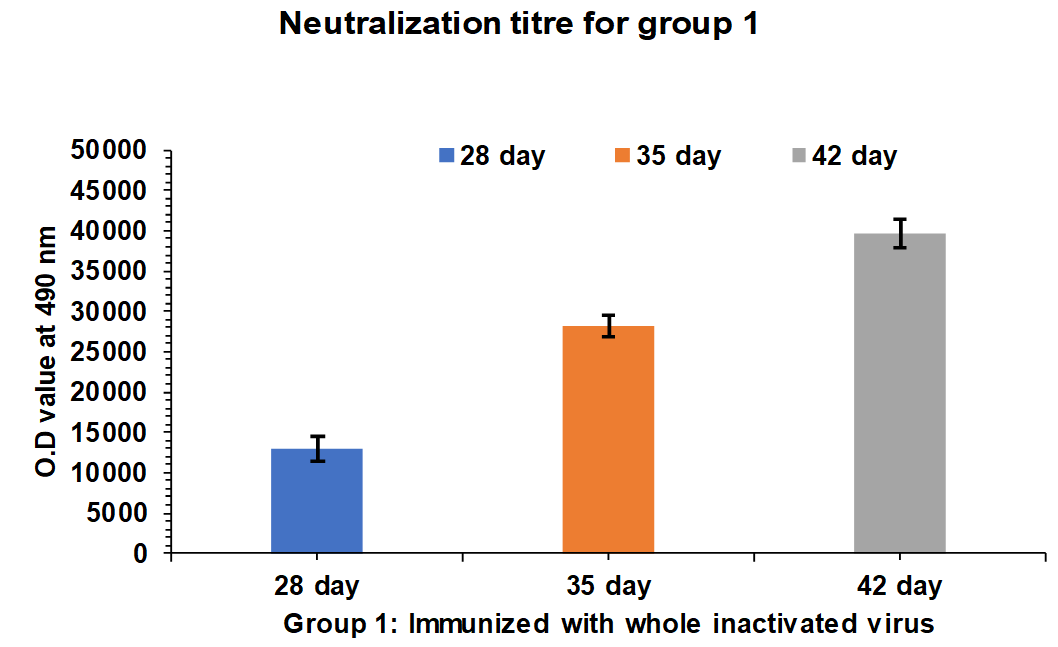
Fig. 1: Neutralization efficacy estimation by Indirect ELISA for 28, 35, and 42-d of intervals for group 1: (G1-R1 to G1-R5) rabbits immunized with whole inactivated SARS-CoV-2 (MT416726). The experiment is carried out in triplicates, the mean values are plotted in a graph, and error bars denotes the standard deviation (SD)
Indirect ELISA results of group 2 rabbits immunized with RBD-HBsAg conjugate protein
Neutralization efficacy was evaluated by Indirect ELISA endpoint titration method for 28, 35, and 42 d samples. Serum samples of rabbits (Group 2) diluted from 1:50 to 1:102400 and added in ELISA plate coated with RBD protein and O. D. were taken at 490 nm. The results revealed that neutralization efficacy increased from samples of 28, 35, and 42 d (fig. 2).
ELISA results of group 1 rabbits antibody against the whole inactivated virus
Serum samples of 28, 35, and 42 d interval from Group 1 Rabbits were pooled equally and three test samples were prepared. The consistency in the neutralization titer was evaluated by the ELISA end-point titration method. ELISA plate coated with RBD protein, and Test sample 1 to Test sample 3 diluted from 1:400 to 1:102400 dilutions added in to plates. O. D. taken at 490 nm and it was revealed that there was no significant difference in titre values of Test sample 1, 2 and 3. The mean values of titre are plotted in a graph, and error bars denote the standard deviation (fig. 3).
ELISA results of group 2 rabbits antibody against the receptor binding domain (RBD)-HBsAg conjugate protein
Serum samples from group 2 rabbits after 28, 35, and 42 d interval were pooled equally and three test samples were prepared. The consistency in the neutralization titer was evaluated by the ELISA end-point titration method. ELISA plate coated with RBD protein, and Test sample 1 to Test sample 3 diluted from 1:400 to 1:102400 dilutions. O. D. taken at 490 nm. The mean values are plotted in a graph, and error bars denote the standard deviation (fig. 4).
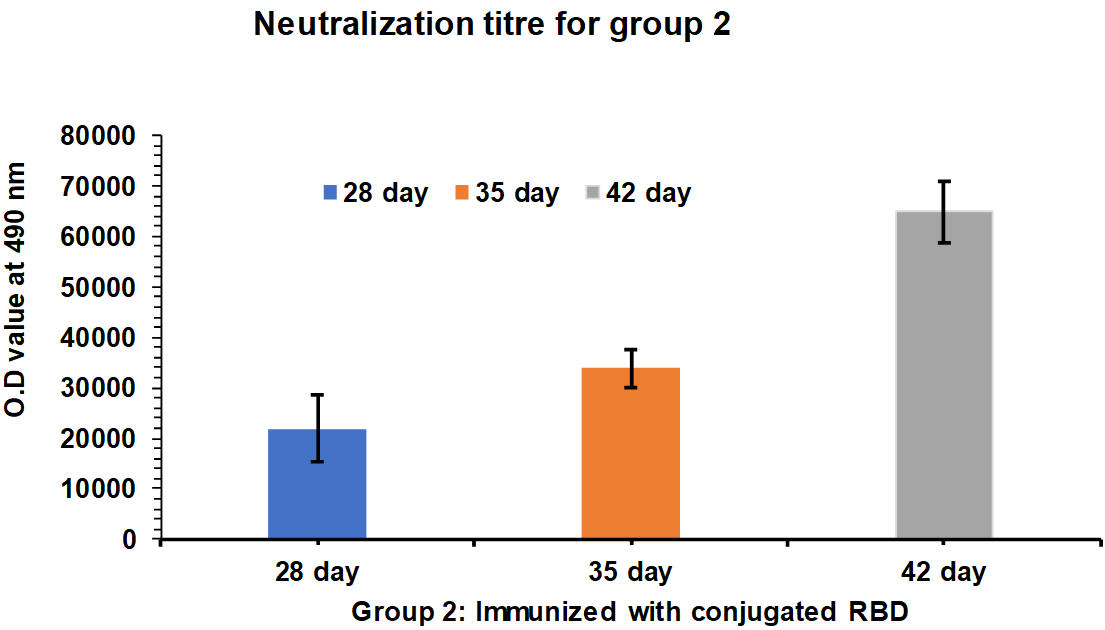
Fig. 2: Neutralization efficacy estimation by Indirect ELISA after 28, 35, and 42 d intervals. Group 2: G2-R1 to G2-R5 rabbits immunized with RBD-HBsAg Conjugate protein, ELISA plate coated with RBD protein, and added serum sample dilutions of 1:400 to 1:102400. and taken O. D. at 490 nm. The experiment is carried out in triplicates, the mean values are plotted in a graph, and error bars denotes the standard deviation.
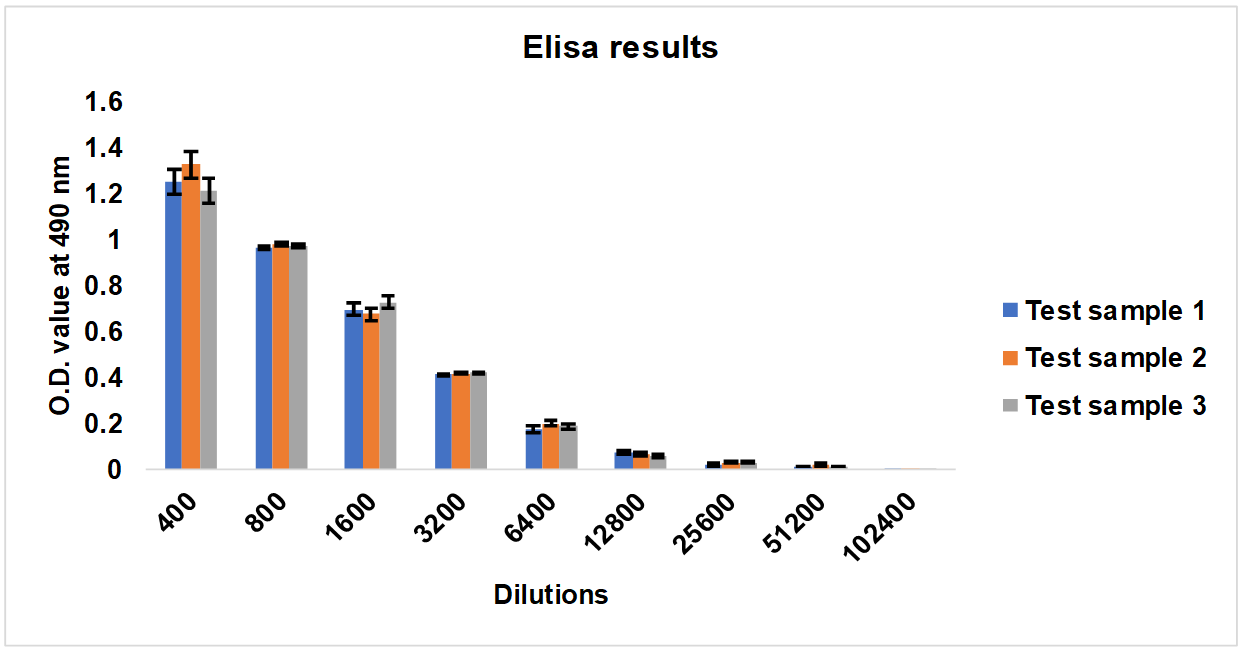
Fig. 3: ELISA results of Test sample 1, Test sample 2, Test sample 3, Serum samples collected from Group 1: G1-R1 to G1-R5 rabbits immunized with whole inactivated SARS-CoV-2 virus. The experiments were carried out in triplicates; the mean values are plotted in a graph, error bars denote the standard deviation (SD)
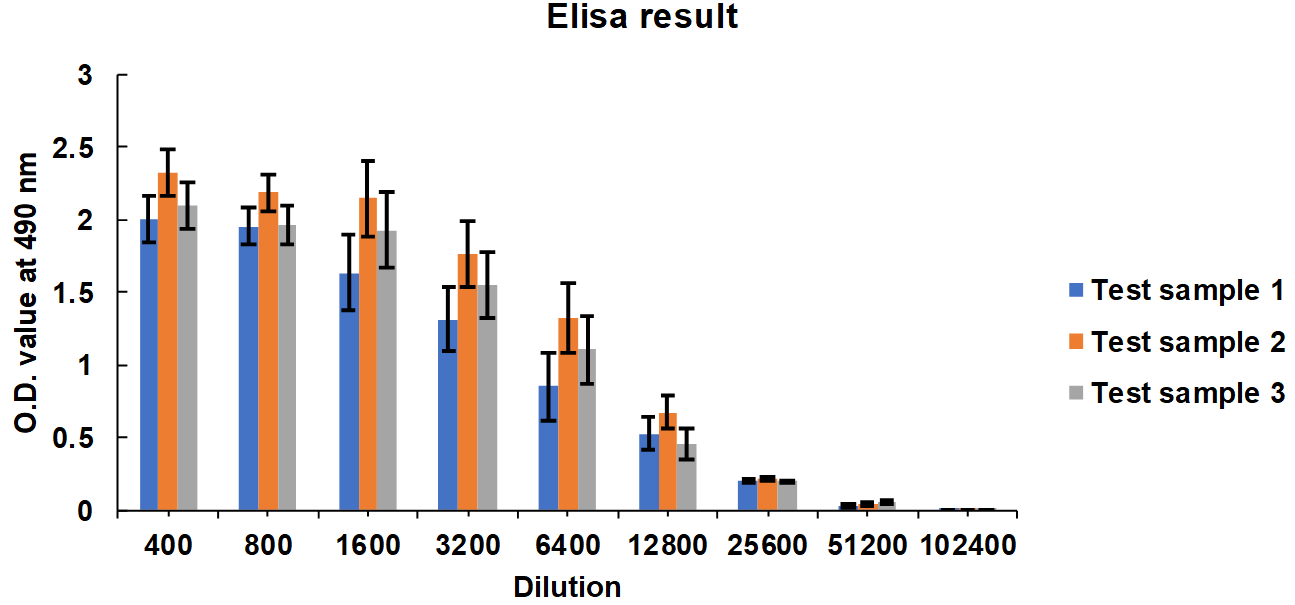
Fig. 4: ELISA results of test sample 1, test sample 2, test sample 3, serum collected from group 2: G2-R1 to G2-R5 rabbits immunized with receptor binding domain (RBD)-HBsAg conjugate protein antigen. The experiment is carried out in triplicates; the mean values are plotted in a graph, error bars denote the standard deviation. Significance testing of Indirect ELISA endpoint titres between two groups is analysed; p-value arrived is less than 0.05; based on the data we found there is a significant difference between the groups so we rejected the Null hypothesis, and the alternative hypothesis is accepted
Virus neutralization assay by PRNT50
Rabbit antibody neutralizing efficacy against antibodies generated by immunizing whole inactivated SARS-CoV-2 and RBD-HBsAg conjugate protein
Serum samples were collected from Group 1 and 2 rabbits after 35 and 42 d intervals and tested for PRNT50 neutralization assay. After 35 d of an interval, the neutralization titre for Group 1 rabbits is found in the range of 2200 to 2350 and after 28, 35, and 42 d the neutralization titre for Group 1 rabbits is found in the range of 4000 to 5300 (fig. 5). In case of Group 2 rabbits After 35 d the neutralization titer for Group 2 rabbits is found in the range of 4100 to 5350 and after 28, 35, and 42 d the neutralization titre for Group 1 rabbits is found in the range of 12700 to 13700.
Group 2 rabbits immunized with RBD-HBsAg Conjugate protein showed three three-fold more immunogenicity and neutralization efficacy than the whole inactivated SARS-CoV-2 (fig. 6). Polyclonal antibodies Generated in rabbits immunized with Receptor Binding Domain (RBD)-HBsAg conjugate has shown three-fold more neutralization efficacy than antibodies generated by whole inactivated SARS-CoV-2. The assay was carried out in triplicates to assure the consistency in results.
Significance testing of PRNT 50 values between two groups were analysed, P value arrived is less than 0.05, based on the data we found. There is a significant difference between the groups so we rejected the Null hypothesis, and the alternative hypothesis is accepted.
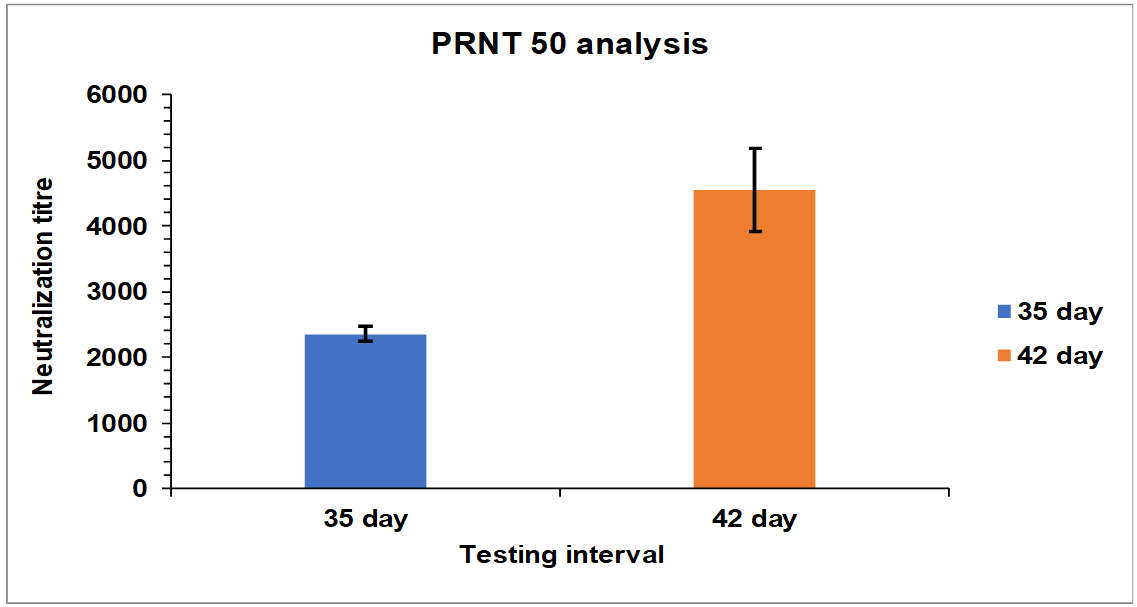
Fig. 5: Neutralization titre estimation by PRNT50 after 35th and 42nd d intervals. Group 1: G1-R1 to-G1 R5 rabbits immunized with whole inactivated SARS-CoV-2. The mean values are plotted in graph; error bars denote the standard deviation
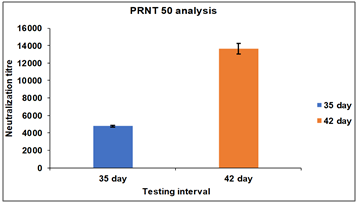
Fig. 6: Neutralization titre estimation by PRNT50 after 35th and 42nd d. Group 2: G2-R1 to G2-R5 rabbits immunized with RBD-HBsAg Conjugate protein, RBD-HBsAg Conjugate protein showed three three-fold more immunogenicity and neutralization efficacy than the Whole inactivated SARS-CoV-2. The mean values are plotted in a graph, and error bars denote the standard deviation.
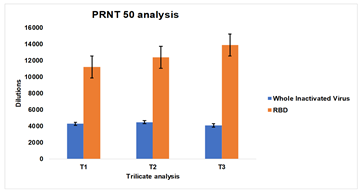
Fig. 7: PRNT50 of serum samples collected from group 1: G1-R1 to G1-R5 rabbits immunized with whole inactivated SARS-CoV-2 virus and Group 2 (G2-R1 to G2-R5) rabbits immunized with receptor binding domain (RBD)-HBsAg conjugate protein. The experiment was carried out in triplicates, the mean values are plotted in a graph, and error bars denote the standard deviation
Rabbit antibody neutralizing efficacy against antibodies generated by immunizing Whole inactivated SARS-CoV-2 and RBD-HBsAg conjugate protein
Serum samples from Group 1 and Group 2 Rabbits serum were pooled equally and three test samples of each group were prepared. The consistency in the neutralization titer was evaluated by the PRNT50 assay (fig. 7).
Group 1 rabbits showed neutralization titre in the range of 4200 to 4500, whereas Group 2 rabbits showed neutralization titre in the range of 11200 to 13872.
DISCUSSION
The COVID-19 pandemic impacted the whole world badly; millions have lost their lives, and those who survived the last phase of infection live with partial or permanent lung damage that affected their quality of life [23]. During the COVID pandemic, global medical services have faced many challenges in the rapid diagnosis of SARS-CoV-2 [24]. The genetic material of SARS-CoV‑2 is highly prone to frequent recombination processes that form new strains with altered virulence. During the last 48 mo, SARS-CoV-2 strains have been mutated several times, out of which some strains are contagious and some strains are lethal and created challenges for our treatments [25].
Scientists from all over the world with the help of research institutes and organizations, have developed different types of vaccines, like Covi-shield, Covaxin, Sputnik, etc. They are manufactured at different places across the globe and distributed through Government vaccination programs that vaccinate more than 60 % population and they showed good protection against SARS-CoV-2 and its variants spread. Monoclonal therapeutic drugs like Regdanvimab, which targets the spike protein receptor-binding domain of SARS-CoV-2 showed good results against native variants [26].
More than 85 % of the RBD antibody epitopes in SARS-CoV-2 exhibited significantly higher binding affinity to the ACE2 receptor than the SARS-CoV-2. This provides a platform to develop anti-SARS-CoV-2 drugs that target the RBD. Hence, there is a need to develop novel fusion inhibitors targeting SARS-CoV-2 [27].
A monoclonal antibody, Tocilizumab (TCZ), acts against the IL-6 Receptor (IL-6R) and has been found effective in SARS-CoV-2 patients. Also, the mAb-based therapy for COVID-19 is effective and useful for the development of mAbs-based therapeutics against emerging SARS-CoV-2 variants [28].
Vaccines will need to be constantly reassessed for their efficacy due to mutations on the pathogens. The purified antibody biotherapeutics are a promising strategy for immediate treatment/prophylaxis or in situations where vaccines are less effective, such as in immunocompromised individuals, young, elderly, and vaccine-hesitant individuals. The purified antibody biotherapeutics can also be rapidly tailored, selected, or mined towards new variants [29].
Purified immunoglobulins obtained from hyper-immune equine sera have been an effective and time-tested approach in various infections such as diphtheria, tetanus, rabies, and bites from snakes, scorpions, arachnids and, more recently SARS-CoV-1, MERS-CoV, Ebola, and avian influenza virus [15].
In this study, the rabbit hyper-immune sera with whole inactivated SARS-CoV-2 and purified RBD-HBsAg Conjugate protein to demonstrated their protective efficacy against SARS-CoV-2 virus using an in vitro live virus neutralization assay. The antiserum was prepared by injecting inactivated whole virus and purified RBD-HBsAg Conjugate protein antigen in rabbits subcutaneously for 35 d. The resulting nAb titers in the plasma of the immunized rabbits displayed high titers against SARS-CoV-2.
In this comparative study, the in vitro virus neutralization efficacy of immunogens has been assessed. Rabbits were immunized with whole inactivated SARS-CoV-2 (MT416726) and purified RBD-HBsAg Conjugate protein with suitable adjuvants. Immunization is done up to 35 d as per immunization protocol; blood samples were taken after the 28th d, 35th d, and 35th d intervals. In vitro efficacy was performed with the ELISA end-point titration method and PRNT50 methods.
After the completion of studies after the 42th d it was observed that Group 1 and group 2 rabbits showed good immune response against the whole inactivated virus, RBD-HBsAg Conjugate protein, respectively. neutralization titers were achieved on the 28th d and gradually increased further till the 42th d. It was found that in ELISA and PRNT50 assay, group 2 rabbits immunized with RBD-HBsAg conjugate protein showed better neutralization titer than Group 1 rabbits which were immunized with whole inactivated virus.
Our results are in agreement with the other studies on animal antisera for SARS-CoV-2, which reported the generation of high nAb in animals against the receptor binding domain of the spike protein of the virus.
Since, polyclonal antibodies are comparatively easy to produce and can be produced in large quantities in small and large animals. In rabbits, with the help of RBD-HBsAg, Conjugate protein as an immunogen which can able to produce highly neutralizing antibodies in large amounts. The result indicates that it can serve as the best therapeutic drug to treat COVID19 patients. Moreover, extensive preclinical and clinical studies needs to be done to confirm the antiviral efficacy of developed polyclonal antibodies. This indigenously developed polyclonal-based antibody therapeutic drug can be used as a cost-effective and efficient alternative to monoclonal antibodies for the treatment of COVID-19.
CONCLUSION
In this study, high-neutralizing hyperimmune polyclonal antibodies were produced by immunizing rabbits with whole inactivated SARS-CoV-2 and RBD-HBsAg conjugate protein. It was found that RBD-HBsAg conjugate protein showed better immunogenicity and neutralization Efficacy compared to the Whole inactivated SARS-CoV-2 virus. RBD-HBsAg conjugate protein has therapeutic and diagnostic potential. It could be used as a potential immunogen to generate highly effective and specific polyclonal antibodies therapeutics that will show good neutralization efficacy against SARS-CoV-2. Results indicate that this indigenously developed polyclonal-based antibody therapeutic drug may be used as a cost-effective and efficient alternative to monoclonal antibodies for the treatment of COVID-19 patients.
ACKNOWLEDGMENT
Authors are thankful to ‘DY Patil Education Society (Deemed to be University), Kolhapur, MS, India’, ‘iSERA Biological Pvt. Ltd., Shirala, Sangli, Maharashtra, India’ and ‘Serum Institute of India Pvt. Ltd. Pune, India’ for their support in current research work.
FUNDING
Nil
AUTHORS CONTRIBUTIONS
Dhairyasheel Yadav: Investigation, Methodology, writing of the original draft; Nandakumar Kadam: Investigation, Methodology, S. Mohan Karuppayil: Conceptualisation, Mayur Vikhrankar: Data curation, Umesh Shaligram: Data curation, Ashwini K. Jadhav:, Data Validation, Supervision.
CONFLICT OF INTERESTS
The authors report no conflict of interest related to the
current research work
REFERENCES
Yang J, Vaghela S, Yarnoff B, DE Boisvilliers S, DI Fusco M, Wiemken TL. Estimated global public health and economic impact of COVID-19 vaccines in the pre-omicron era using real-world empirical data. Expert Rev Vaccines. 2023 Dec 31;22(1):54-65. doi: 10.1080/14760584.2023.2157817, PMID 36527724.
Karia R, Gupta I, Khandait H, Yadav A, Yadav A. COVID-19 and its modes of transmission. SN Compr Clin Med. 2020 Oct;2(10):1798-801. doi: 10.1007/s42399-020-00498-4, PMID 32904860.
Salian VS, Wright JA, Vedell PT, Nair S, LI C, Kandimalla M. COVID-19 transmission current treatment and future therapeutic strategies. Mol Pharm. 2021 Jan 19;18(3):754-71. doi: 10.1021/acs.molpharmaceut.0c00608, PMID 33464914.
Singh MS, Yellaboina S, Ansari MA. A comprehensive review on the multifaceted interactions between host immunity and viral pathogenesis in COVID-19. Int J App Pharm. 2024;16(4):37-45. doi: 10.22159/ijap.2024v16i4.50576.
Ansari MZ, Mehta SK, Priyadarshi K, Mohanty B, Sunder A. The impact of different variables on the three cell lines in cobalamin deficient patients in a tertiary care teaching hospital. Int J Pharm Clin Res. 2024;16(7):905-11.
Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij Rammerstorfer S. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind randomised controlled trial. Lancet. 2020;396(10249):467-78. doi: 10.1016/S0140-6736(20)31604-4, PMID 32702298.
Izda V, Jeffries MA, Sawalha AH. COVID-19: a review of therapeutic strategies and vaccine candidates. Clin Immunol. 2021 Jan 1;222:108634. doi: 10.1016/j.clim.2020.108634, PMID 33217545.
Kaznadzey A, Tutukina M, Bessonova T, Kireeva M, Mazo I. BNT162b2 mRNA-1273 and sputnik V vaccines induce comparable immune responses on a par with severe course of COVID-19. Front Immunol. 2022 Apr 13;13:797918. doi: 10.3389/fimmu.2022.797918, PMID 35493476.
Nicola M, Alsafi Z, Sohrabi C, Kerwan A, Al Jabir A, Iosifidis C. The socio-economic implications of the coronavirus pandemic (COVID-19): a review. Int J Surg. 2020 Jun 1;78:185-93. doi: 10.1016/j.ijsu.2020.04.018, PMID 32305533.
Behnood SA, Shafran R, Bennett SD, Zhang AX, O Mahoney LL, Stephenson TJ. Persistent symptoms following SARS-CoV-2 infection amongst children and young people: a meta-analysis of controlled and uncontrolled studies. J Infect. 2022 Feb 1;84(2):158-70. doi: 10.1016/j.jinf.2021.11.011, PMID 34813820.
Castro Dopico X, Ols S, Lore K, Karlsson Hedestam GB. Immunity to SARS‐CoV‐2 induced by infection or vaccination. J Intern Med. 2022 Jan;291(1):32-50. doi: 10.1111/joim.13372, PMID 34352148.
Jahanshahlu L, Rezaei N. Monoclonal antibody as a potential anti-COVID-19. Biomed Pharmacother. 2020 Sep 1;129:110337. doi: 10.1016/j.biopha.2020.110337, PMID 32534226.
Rosenstein S, Vaisman Mentesh A, Levy L, Kigel A, Dror Y, Wine Y. Production of F (ab) 2 from monoclonal and polyclonal antibodies. Curr Protoc Mol Biol. 2020 Jun;131(1):e119. doi: 10.1002/cpmb.119, PMID 32319727.
Zheng X, Wong G, Zhao Y, Wang H, HE S, BI Y. Treatment with hyperimmune equine immunoglobulin or immunoglobulin fragments completely protects rodents from Ebola virus infection. Sci Rep. 2016 Apr 12;6(1):24179. doi: 10.1038/srep24179, PMID 27067649.
Graham BS, Ambrosino DM. History of passive antibody administration for prevention and treatment of infectious diseases. Curr Opin HIV AIDS. 2015 May 1;10(3):129-34. doi: 10.1097/COH.0000000000000154, PMID 25760933.
Habarugira G, Suen WW, Hobson Peters J, Hall RA, Bielefeldt Ohmann H. West nile virus: an update on pathobiology epidemiology diagnostics control and one health implications. Pathogens. 2020 Jul 19;9(7):589. doi: 10.3390/pathogens9070589, PMID 32707644.
HU T, Liu Y, Zhao M, Zhuang Q, XU L, HE Q. A comparison of COVID-19 sars and mers. Peer J. 2020 Aug 19;8:e9725. doi: 10.7717/peerj.9725, PMID 32879801.
Kulkarni R, Patil HP, Palkar S, Lalwani S, Mishra AC, Arankalle V. Anti SARS-CoV-2 IgG antibody response among Indian COVID-19 patients using β-propiolactone inactivated whole virus-based indirect ELISA. J Virol Methods. 2021 Jan 1;287:113996. doi: 10.1016/j.jviromet.2020.113996, PMID 33126149.
Barbier M, Lee KS, Vikharankar MS, Rajpathak SN, Kadam N, Wong TY. Passive immunization with equine rbd-specific fab protects k18-hace2-mice against alpha or beta variants of SARS-COV-2. Front Immunol. 2022 Aug 15;13:948431. doi: 10.3389/fimmu.2022.948431, PMID 36091051.
Muruato AE, Fontes Garfias CR, Ren P, Garcia Blanco MA, Menachery VD, Xie X. A high throughput neutralizing antibody assay for COVID-19 diagnosis and vaccine evaluation. Nat Commun. 2020 Aug 13;11(1):4059. doi: 10.1038/s41467-020-17892-0, PMID 32792628.
Perera RA, Mok CK, Tsang OT, LV H, KO RL, WU NC. Serological assays for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) March 2020. Euro Surveill. 2020 Apr 23;25(16):2000421. doi: 10.2807/1560-7917.ES.2020.25.16.2000421, PMID 32347204.
Deshpande GR, Sapkal GN, Tilekar BN, Yadav PD, Gurav Y, Gaikwad S. Neutralizing antibody responses to SARS-CoV-2 COVID-19 patients. Ind J Med Res. 2020 Jul 1;152(1):82-7.
Kanne JP, Little BP, Schulte JJ, Haramati A, Haramati LB. Long term lung abnormalities associated with COVID-19 pneumonia. Radiology. 2023;306(2):e221806. doi: 10.1148/radiol.221806, PMID 36040336.
Assefa N, Hassen JY, Admassu D, Brhane M, Deressa M, Marami D. COVID-19 testing experience in a resource-limited setting: the use of existing facilities in public health emergency management. Front Public Health. 2021 Jun 14;9:675553. doi: 10.3389/fpubh.2021.675553, PMID 34195170.
Hebbani AV, Pulakuntla S, Pannuru P, Aramgam S, Badri KR, Reddy VD. COVID-19: comprehensive review on mutations and current vaccines. Arch Microbiol. 2021;204(1):8. doi: 10.1007/s00203-021-02606-x, PMID 34873656.
Takashita E, Kinoshita N, Yamayoshi S, Sakai Tagawa Y, Fujisaki S, Ito M. Efficacy of antibodies and antiviral drugs against COVID-19 omicron variant. N Engl J Med. 2022 Mar 10;386(10):995-8. doi: 10.1056/NEJMc2119407, PMID 35081300.
Omolo CA, Soni N, Fasiku VO, Mackraj I, Govender T. Update on therapeutic approaches and emerging therapies for SARS-CoV-2 virus. Eur J Pharmacol. 2020 Sep 15;883:173348. doi: 10.1016/j.ejphar.2020.173348, PMID 32634438.
Gupta A, Pradhan A, Maurya VK, Kumar S, Theengh A, Puri B. Therapeutic approaches for SARS-CoV-2 infection. Methods. 2021 Nov 1;195:29-43. doi: 10.1016/j.ymeth.2021.04.026, PMID 33962011.
Kumar S, Chandele A, Sharma A. Current status of therapeutic monoclonal antibodies against SARS-CoV-2. PLOS Pathog. 2021 Sep 3;17(9):e1009885. doi: 10.1371/journal.ppat.1009885, PMID 34478455.