Int J App Pharm, Vol 17, Issue 1, 2025, 397-409Original Article
ETHOSOMES CARRYING ETODOLAC-EFFECTIVE DRUG CARRIER AS TOPICAL DOSAGE FORM IN THE TREATMENT OF RHEUMATOID ARTHRITIS
ASHWINI A. BACHHAV1,2*, PRASHANT L. PINGALE3, CHANDRASHEKHAR D. UPASANI1
1*Shriman Sureshdada Jain College of Pharmacy, Chandwad-423101, Nashik, Maharashtra, India. 2NDMVPS’s College of Pharmacy, Nashik-422002, Maharashtra, India. 3GES’s Sir Dr. M. S. Gosavi College of Pharmaceutical Education and Research, Nashik-422005, Maharashtra, India
*Corresponding author: Ashwini A. Bachhav; *Email: ashwini27212@gmail.com
Received: 24 Jul 2024, Revised and Accepted: 16 Nov 2024
ABSTRACT
Objective: The priority-based objective of this research was to develop and evaluate the etodolac loaded Ethosomes as topical delivery in Rheumatoid Arthritis autoimmune disease.
Methods: The ethosomes (EE1-EE6) were prepared with varied concentrations (10%-60% v/v) of ethanol by using Rotary evaporator. The prepared Ethosomes evaluated for %EE (Entraptment Efficiency), Zeta potential, SEM (Scanning Electron Microscopy), TEM (Transmission Electron Microscopy), Vesicle size, In vitro release, In vivo study, Irritancy test, stability test etc.
Results: Prepared ethosomes analysed for zeta potential, vesicle size and % entrapment efficiency and ranges are found to be-18.50mV to-64.53mV, 166.66 nm to 848.97 nm and 53.15% to 89.35%, respectively. Furthermore, when these ethosomes were incorporated in Carbopol 940 gels (EEC1-EEC6), it evaluated for appearance, spredability, pH, viscosity study, drug content and In vitro drug release. All ethosomal gels show satisfactory results. After optimization of ethosomes (EE1-EE6) and ethosomal gels (EEC1-EEC6) on previous criteria, optimised formulations EE4 and EEC4 characterised for SEM, TEM, Ex-vivo permeation, Irritancy, In vivo and different analytical evaluation. The values of r2 were found higher for the first-order model and Korsmeyer-Peppas model for all formulations. Irritancy test shows safe use of formulation and in vivo test shows that EEC4 had a significant inhibitory effect on edema, which was developed in the right paw of rat by injecting carrageenan when it compared with plain Carbopol gel and marketed formulation.
Conclusion: The present study has confirmed that the formulated Etodolac Ethosomal gel can be used as best vehicle for topical administration, which may be used for the management of rheumatoid arthritis.
Keywords: Etodolac, Ethosomes, Carbopol 940, Topical, Rheumatoid arthritis
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijap.2025v17i1.52153 Journal homepage: https://innovareacademics.in/journals/index.php/ijap
INTRODUCTION
Rheumatoid arthritis is one of the diseases which is characterized by painful with swollen and stiff joints. The clear etiology for this disease is not known but, some study shows that it occurs when the body’s own immune system attack on its own joints. Complete cure for this disease not known only symptomatic treatment is available to relieve pain and stiffness of joints to improve patient normal daily activity [1, 2]. Pain, fever and tenderness occurs with inflammation due to the release of important chemical in the body known as Prostaglandins [3].
Furtherly to treat pain and inflammation associated with prostaglandin, patients need to use anti-inflammatory drugs like etodolac. Unfortunately, etodolac have many side effects when it is taken by oral route like gastrointestinal disturbances, and it worsens it can cause gastric ulcers and bleeding [4]. With this etodolac also have poor solubility and less bioavailability. Repeated dosing is required because its half-life is 7 h. Thus, to avoid such complications, it is necessary to change the route of administration. There are multiple reasons to prefer Topical route like effectiveness, safety, bypass the first-pass metabolism, easy administration and organ dysfunction due to systemic adverse effects, but with these advantages there is a disadvantage like it delivers in adequate amount of drugs [5]. To avoid this disadvantage, encapsulate the drug in the different vesicles [6, 7]. These delivery systems are capable of carrying drug molecules and show its therapeutic effects at site of action. It never without interfere with the surrounding tissues and can cross hard layer of skin i. e. Stratum corneum [8-10].
Moreover, it not only delivers hydrophilic drugs but also it can deliver lipophilic drugs. The advantage of these vesicles that it has the same properties as skinny as it made from phospholipids, ultimately, it results in improving drug permeability through the skin layers, mostly stratum corneum layer, without barrier which results in better therapeutic activity [11, 12]. But the therapeutic efficacy of drugs across the skin using first generation vesicular system (liposomes and niosomes) is less due to their stiff structure [13]. Thus, researchers are exploring next-generation vesicular system which are flexible in their structure, like transferosomes and ethosomes [14].
Ethosomes are vesicular drug carriers which have lipids and high concentration of ethanol in their structure. Because of high concentration of ethanol helps them to modify the highly compact structure of stratum corneum layer, which results in deep penetration of drugs into the tissue through skin [15, 16]. The high concentration of ethanol results in a net negative charge on the surface of ethosomes, which causes electrostatic repulsion which results in stability of vesicles [17]. Ethanol also responsible for high solubility of lipophilic drugs [18]. Ethosomes are less toxic, irritant and suitable for topical delivery of drugs [19]. Studies have also shown that the incorporation of vesicular carriers into another gel i. e. Carbopol gel can improve their stability and skin permeability [20]. This study, then, aims to formulate ethosomes and incorporate ethosomes vesicles into Carbopol 940 gel to improve skin permeation.
MATERIALS AND METHODS
Materials
Etodolac was procured from Yarrow Chem Ltd, Mumbai. Soya lecithin was procured from Modern chemicals, Nashik. Other chemicals and different solvents, including water were used of analytical grade and purchased from different suppliers.
Formulation of etodolac containing ethosomes
All ethosomes were prepared by using the thin-film hydration technique (Rotary Evaporation). First, oil phase prepared by dissolving etodolac and lipids (Soya Phosphotidyl Choline and cholesterol) in ethanol and aqueous phase was a hydroethanolic solution. Both phases were separately sonicated at 60 °C for 30 min. It became homogenous. Then, this homogenous mixture was added in a round-bottomed flask and a thin lipid film was formed by removal of ethanol using a rotary evaporator (Rotary Vacuum Film Evaporator-Coslab-Model-CRE400) at 40 °C to obtain the thin lipid film after complete evaporation of the organic solvents. The film was hydrated with a phosphate buffer (pH 7.4) by rotation for 1hr. The vesicles were kept for swelling for 1 h. Both previous steps were carried out at room temperature. The resultant vesicles were sonicated for 30 min in a bath sonicator (Digital Ultrasonic Cleaner). The sonicated vesicles were stored at 4 °C [21, 22]. The composition of ethosomal vesicles is shown in the table 1.
Table 1: Formulation code and composition of preparation of Ethosomes
| Formulation | Cholesterol (w/v) | SPC (% w/v) | Ethanol (% V/V) | Water (% V/V) |
| EE1 | 0.5 | 4 | 10 | 90 |
| EE2 | 0.5 | 4 | 20 | 80 |
| EE3 | 0.5 | 4 | 30 | 70 |
| EE4 | 0.5 | 4 | 40 | 60 |
| EE5 | 0.5 | 4 | 50 | 50 |
| EE6 | 0.5 | 4 | 60 | 40 |
SPC: Soya Phosphatidylcholine
Preparation of ethosomal carbopol gel
In the first step, plain Carbopol gel was prepared by incorporating 1 g Carbopol 940 in 100 ml water at 50 °C by continuous stirring. Methyl paraben and propyl paraben were as preservatives. The Ethosomes equivalent to 2% of etodolac was mixed with mentioned Carbopol gel and simultaneously, one plain Carbopol gel also prepared with same concentration (2%). After 2 h swelling, triethanolamine was added to neutralize the prepared Carbopol 940 gel [23].
Fig. 1: Formulation batches of ethosomes
Optimization of prepared ethosomes
Optical microscopy
The prepared ethosomes were observed under light microscope, Metzer Biomedical Model VFM9003. This evaluation used to give an idea about the shape of the ethosomes [24].
Vesicle size, PDI and zeta potential
Vesicle size, PDI and zeta potential of etodolac ethosomes were determined by using Zetasizer (Diya Lab, Mumbai, India), and the values were obtained at 25 °C [25, 26].
Drug loading and entrapment efficiency
Ethosomes were centrifuged at 12000 rpm for 30 min (Remi, Cε, NT 2178GK). The absorbance of supernatant liquid was taken spectrophotometrically after separation from total mixture at 281 nm by using a UV spectrophotometer (UV-2450, Shimadzu, Japan) [27, 28]. The following formulas were used to calculate % EE and % DL.
![]()
![]()
Characterization of prepared ethosomal gel
The prepared ethosomes (EE1-EE6) batches incorporated in Carbopol gel and these gels evaluated for following characteristics. The batches were labelled as EEC-1 to EEC-6, respectively, and the batch was contained only plain Carbopol gel was labelled as EC-0.
Physical appearance, homogeneity, clarity and colour
All the prepared gels (EEC-1 to EEC-6) were evaluated for appearance, homogeneity, colour and clarity [29].
Spredability
Spreadability of gels was measured by using wooden block apparatus, on the wooden base, one glass slide was stuck and another glass slide was attached to the pulley, of which another end has weighed pan. The 2 gm of gel pressed between 2 slides for uniform thickness. The 100 gm of weight was removed, and times (seconds) require by movable slide getting separated from stationary slide were noted [30]. Spreadability was calculated as:
![]()
Where,
L = Length of glass slide (cm),
W = Weight applied (g)
T = Time required for separation of slide (sec)
PH
The pH of the developed gels (EEC1-EEC6 and EC0) was determined in triplicate with pH meter (pH Cal Model). 1 gm gel was dissolved in 100 ml water after 2 h pH was measured [31]. Average pH is reported in table 6.
Rheological study
For viscosity determination a Brookfield viscometer LV DV-II+PRO model was used. T-95 Bar spindle was used and whole study done at room temperature. 50 g of each ethosomes sample was taken in was dipped. The Readings for viscosity were taken at 10, 20, 30, 40, 50 rpm at room temperature. Spindle was rotated for 5 minute for every reading. Evaluations were done in triplicates, and mean±SD viscosities were calculated [32].
Drug content
An accurately 1 g formulated gel was dispersed in a specific volume of phosphate buffer with vigorous shaking. The dispersion was then filtered and drug amount in the filtrate was analysed spectrophotometrically at λmax equal to 281 nm [33]. Equation was used to calculate drug content (%) as follows:
![]()
In vitro drug release study
A soaked cellophane membrane was placed between donor and receptor compartments of the Franz diffusion cell. Ethosomal gel was applied to the cellophane membrane and phosphate buffer was kept in receiver compartment and it stirred continuously with a magnetic bar at 50 rpm. 1 ml sample was taken at 0, 1, 2, 3, 4, 5, 6, and 12 h intervals, volume of receiver compartment replaced with 1 ml of buffer solution, and the sample was analysed by using Ultra-Violetviolet (UV) spectrophotometer at 281 nm. The percentage of drug released was then calculated [34].
Evaluation of optimized formulations
Vesicle morphology
The optimized ethosomes (EE4) formulation was characterized morphologically using SEM and TEM at Cochin, SAIF, India [35-37].
Ex vivo permeation study
For this technique, abdominal skin of albino rats was used. This study was carried out at 37± 2 °C. The Franz diffusion cell was placed on a magnetic stirrer with constant heating equipment. The gels were placed in receptor compartment. Aliquot samples of 1 ml were withdrawn at the regular intervals and replaced with the same volume of fresh buffer. Amount of drug diffused through the membrane was measured by using U. V. spectrophotometer at a wavelength of 281 nm against phosphate buffer (pH 7.4 saline) as the blank. The results were replicated three times as mean±SD [38].
Irritancy test
The skin irritation potential of etodolac-loaded ethosomal gel (EEC-4) was assessed with permission of ethical committee (Biotox/IAEC/03/2024/RP-14) by using rats from species Wistar Albino (200-300 g) sourced from Biotox Laboratory, Naskik, India. The selected rats were acclimatized for one week before the study began. Approximately 4 h prior to the experiment, the dorsal surface of each rat was shaved. Four groups were prepared by dividing rats. Each group contains 3 rats.
Group I-Received plain Carbopol gel (without Etodolac)
Group II-Received Carbopol gel with Etodolac
Group III-Received Ethosomes with Etodolac
Group IV-Received ethosomal gel with Etodolac.
The formulations (100 mg containing 2%) were applied onto a shaved skin area of 1 cm². After application, the rats observed at 24, 48, and 72 h for irritation. The severity of irritation was scored as follows: 0 for no erythema or edema, 1 for slight erythema or edema, 2 for moderate erythema or edema, and 3 for severe erythema or edema [39].
In vivo study (Anti-inflammatory activity)
Anti-inflammatory study of optimised ethosomal gel (EEC4) formulations was evaluated inducing carrageenan into hind paw of rats with permission of ethical committee (Biotox/IAEC/03/2024/RP-14). For this, 24 rats were divided into four groups. Each group had 6 rats. Formulations were topically applied 20 mg/kg of dose to the surface of the left hind paw of the rats and gently massaged before 30 min of intraplanar injection of 100 µl** of carrageenan solution (1% w/v) into the left hind paw. The digital vernier calliper was used to measure the difference in volume between the right and left paws (Baker, DDS Series) at ½, 1, 2, 4, 6, and 8 h after the carrageenan injection. The percentage inhibition of edema was calculated by using the following formula,
![]()
Where, Vt: the paw edema volume of the groups treated with the formulations in rats,
Vc: the mean edema volume of the control group.
In vivo experimental group design
Group 1: Control group, No treatment, (n = 6),
Group 2: Traditional group Etodolac Carbopol gel, (n = 6),
Group 3: Conventional group Marketed etodolac gel (n = 6),
Group 4: Novel group Etodolac loaded ethosomal (n = 6). 40, 41
FTIR study
The FTIR (Fourier Transform Infra-red) of etodolac, Carbopol gel without drug, etodolac Carbopol gel, optimized etodolac ethosomes and optimized etodolac ethosomal Carbopol gel were recorded by FTIR spectroscopy (Thermo Nicolet IS50, SAIF, Cochin, India) [42].
Differential scanning colorimetry study
Etodolac, drug-free Carbopol gel, etodolac Carbopol gel, optimized etodolac ethosomes and optimized etodolac ethosomal Carbopol gel, were analysed by using DSC (Model: DSC 204 F1 PhoenikNetezch, SAIF, Cochin, India) [43].
X-ray diffraction (XRD)
XRD for Etodolac, drug-free Carbopol gel, etodolac Carbopol gel, optimized etodolac ethosomes and optimized etodolac ethosomal Carbopol gel were analysed using X-ray diffractometer (Bruker D8 Advance, SAIF, Cochin, India) [44].
UV analysis
Absorption spectrum of etodolac, drug-free Carbopol gel, etodolac Carbopol gel, optimized etodolac ethosomes and optimized etodolac ethosomal Carbopol gel were analysed in the wavelength range of 200–400 nm to determine its maximum absorption wavelength. A standard stock solution was prepared by measuring 20 mg of formulations and dissolved in 20 ml of methanol [45].
Stability study
The optimised ethosomal formulations were stored at 5 °C±3 °C; 25±2 °C, 60±5%RH; (40±2 °C, 75±5%RH) in stability chamber (Thermolab ISO 000090S) for 6 mo. Then stability of formulations was evaluated by determining % entrapment efficiency at respective temperature [46].
RESULTS AND DISCUSSION
Optimization of prepared transferosomes
Optical microscopy
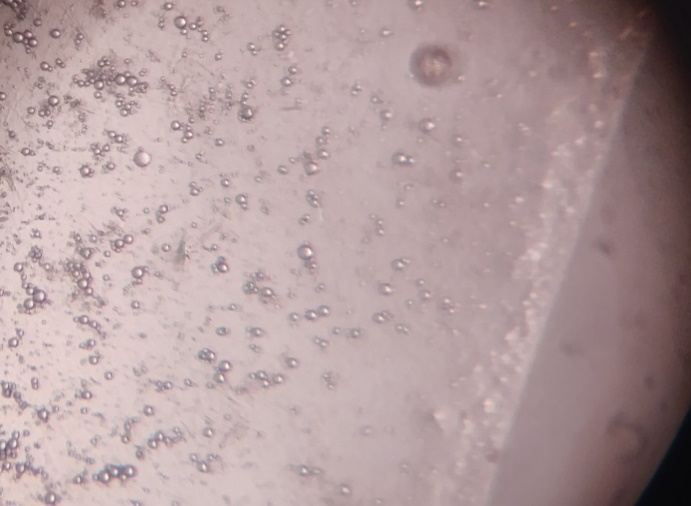
Optical microscopy shows small spherical vesicles of Ethosomes for batches EE1 to EE5 at the magnification power of 45X. But, in Ethosomes EE5, the vesicles quantity is less as compared to other previous four formulations and in EE6 clearly damaged vesicles were observed, it may due to increased concentration of alcohol.
EE1 |
EE2 |
EE3 |
EE4 |
EE5 |
EE6 |
Fig. 2: Optical microscopy of ethosomes (45x)
Vesicle size, PDI and zeta potential
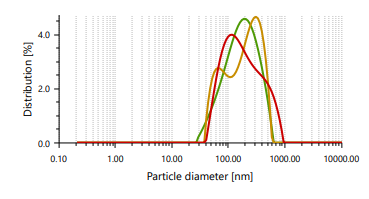
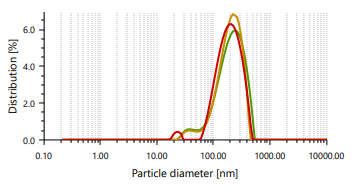
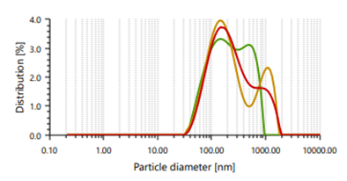
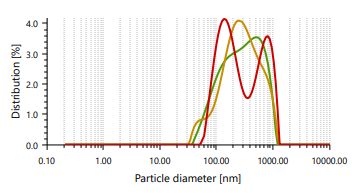
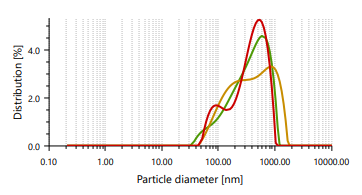
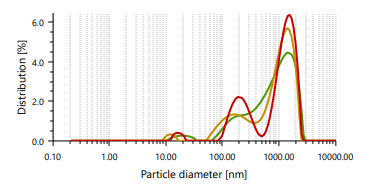
The vesicles sizes ≥600 nm cannot penetrate to deeper into skin layers, but vesicle sizes ≤300 nm can penetrate deeply into the skin [47]. Ethosomes vesicles were found to be in the range of 166.66 nm to 848.97 nm. In case of vesicle size, it was noticed that as the concentration of ethanol increases, the size of the vesicle also increases, which contrast observation than the previous study. PDI found in the range of 0.244 to 0.301 [48]. It indicates that formulated ethosomes have a uniform particle size as it is numerically near to number 0.
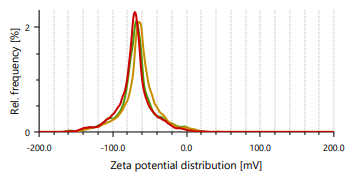
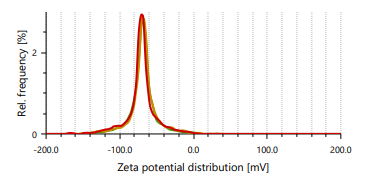
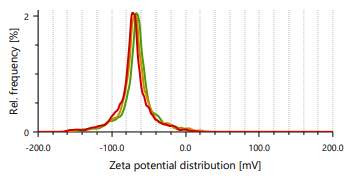
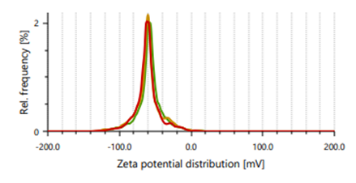
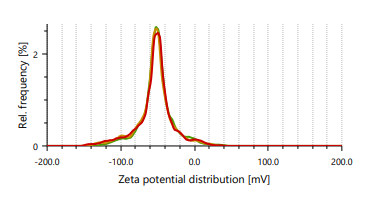
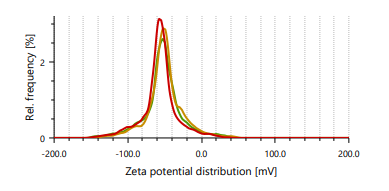
Zeta potential indicates stability of vesicles. A value of zeta potential of ±30 mV charge indicates that the vesicles have repulsion are more which results in stability of formulations. Ethanol is responsible for negative charge to the surface of ethosomes. The repulsive force is not only dependent only on the zeta potential but also on dispersion. Classification for degree of stability and corresponding zeta potential shown in table 2. It shows that a zeta potential of more than ±30 mV provides good stability and obtains excellent stability when the ZP reaches toward ±60 mV [49]. The Vesicle size, PDI, and zeta potential values of optimized formulations are presented in table 3 and fig. 3 and 4.
Table 2: Stability of nanofluids at different zeta potentials
| Zeta potential (mV) | Stability degree |
| 0 | Slight to no stability |
| 15 | Less stability with light settling |
| 30 | Moderate stability |
| 45 | Decent stability with possible settling |
| 60 | Excellent stability with lesser possibility of settling |
Table 3: Vesicle size, PDI, and zeta potential of ethosomes
| Formulation | Particle size (nm) | PDI | Zeta potential (mV) |
| EE1 | 166.66±7.99 | 0.271±0.01 | -34.07±0.74 |
| EE2 | 185.59±04.05 | 0.244±0.01 | -47.77±0.57 |
| EE3 | 249.00±20.70 | 0.251±0.01 | -37.47±4.22 |
| EE4 | 287.17±10.68 | 0.265±0.01 | -64.53±0.39 |
| EE5 | 372.10±09.39 | 0.270±0.01 | -45.73±0.66 |
| EE6 | 848.97±40.42 | 0.301±0.03 | -18.50±0.78 |
Data are expressed as mean±SD (n = 3)
Drug loading and entrapment efficiency
Drug loading (%) indicates the quantity of drug present in net weight of the formulation. Entrapment efficiency (%) indicates the only quantity of drug entrapped in vesicles compared to net weight of the drug added in the formulation. Entrapment efficiency (%) for all formulations ranged between 53.15±0.64 to 89.35±1.19 and Drug loading (%) ranged between 18.96±0.18 to 43.51±0.47. Results are displayed in table 4.
Characterization of prepared ethosomal carbopol gel
Physical appearance and homogeneity, clarity and colour
All ethosomal Carbopol gel (EEC1-EEC6) and plain Carbopol gel without ethosomes (EC0) show smooth, clear and homogeneous visual appearance. All ethosomal formulations appeared opaque and off white in colour while EC0 appearance was transparent and colorless. As the concentration of alcohol was increased, the yellowish coloration also increases (fig. 1). Results are displayed in table 5.
EE1 |
EE2 |
EE1 |
EE2 |
EE3 |
EE4 |
EE3 |
EE4 |
EE5 |
EE6 |
EE5 |
EE6 |
Fig. 3: Particle size distribution and PDI of Fig. 4: Zeta potential of ethosomes ethosomes
Table 4: Entraptment efficiency and drug loading
| Formulation | EE% (±SD) | D.L.%(±SD) |
| EE1 | 74.70±0.90 | 43.51±0.47 |
| EE2 | 77.84±1.33 | 30.92±0.81 |
| EE3 | 80.96±0.66 | 29.40±0.31 |
| EE4 | 89.35±1.19 | 36.22±0.55 |
| EE5 | 56.38±0.47 | 18.96±0.18 |
| EE6 | 53.15±0.64 | 23.20±0.25 |
Data are expressed as mean±SD (n = 3)
Table 5: Physical appearance, texture, homogeneity, clarity, colour, of ethosomal gel and plain carbopol gel
| Formulation | Physical appearance | Texture | Homogeneity | Clarity | Colour |
| EE1 | Opaque | Smooth | Homogenous | Clear | Off white |
| EE2 | Opaque | Smooth | Homogenous | Clear | Off white |
| EE3 | Opaque | Smooth | Homogenous | Clear | Off white |
| EE4 | Opaque | Smooth | Homogenous | Clear | Off white |
| EE5 | Opaque | Smooth | Homogenous | Clear | Off white |
| EE6 | Opaque | Smooth | Homogenous | Clear | Off white |
| EC0 | Transparent | Smooth | Homogenous | Clear | Colourless |
Spreadability
Spreadability evaluation is important to indicate contact time of formulation with the site of action, effortless application to the site and easy and proper extrusion of formulation from container. All ethosomal gel shows good results regarding spreadbility. Plain Carbopol gel shows a spreadability 5.76±013. When ethosomes were added into plain Carbopol gel, spreadability increases. The concentration of ethanol is directly proportional to spreadability, and results are shown in table 6.
PH
pH of the formulated gels were obtained from 6 to 7.05, which is near to the neutral pH and determined pH shown in table 6. Therefore, these gels are suitable for safe and non-irritant topical application as it is compatible with human skin.
Rheological study
The viscosity study indicates that the concentration of ethanol increases in ethosomes decreases the viscosity of plain Carbopol gel. This may be due to ethanol decreases the interactions between the molecules present in Carbopol gel and leading to their thinning effect. Rheological behavior of all formulations shown in fig. 5.
Drug content
The drug content of all formulations obtained in a range of 96.68 to 102.25 %, which confirmed that % drug content was within the limit of United States Pharmacopeia (100±10 %), which indicates uniform distribution of drugs in formulation (except EE2, which have drug content (%) is 87.92). This study confirmed that procedures and protocols followed in current research are suitable for formulation.
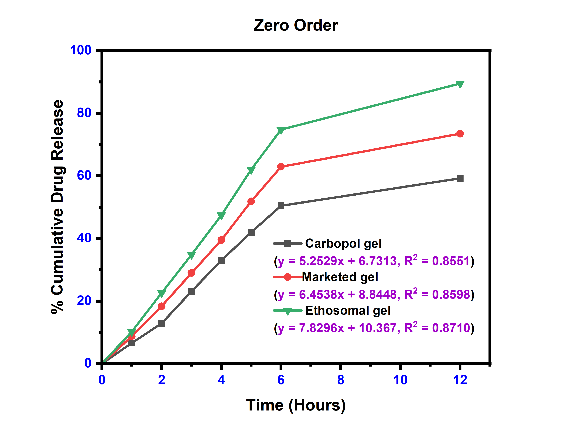
In vitro release study
The results of Cumulative drug release flux. Permeability coefficient and enhancement ratio of Ethosomal Gel were determined and displayed in table 7 and fig. 6. It shows optimized results for EEC4 formulations. All other ethosomal formulations also show better results than plain Carbopol gel (EC0). Fig. 6 gives a clear idea about enhanced diffusion of etodolac drug from ethosomal formulations as compared to plain etodolac Carbopol gel.
Table 6: Spreadability, pH and drug content of ethosomal gel and plain carbopol gel
| Formulation | Spreadability g. cm/sec | pH | Drug content (%) |
| EEC1 | 07.89±0.16 | 6.45±0.09 | 98.06±0.24 |
| EEC2 | 12.88±0.34 | 6.13±0.05 | 87.92±0.17 |
| EEC3 | 14.67±0.18 | 6.78±0.07 | 102.25±0.30 |
| EEC4 | 16.09±0.33 | 6.00±0.05 | 99.67±0.35 |
| EEC5 | 20.41±0.30 | 6.90±0.04 | 98.89±0.53 |
| EEC6 | 24.60±0.38 | 6.22±0.02 | 96.68±0.58 |
| EC0 | 5.76±013 | 7.05±0.02 | 98.43±0.58 |
Data are expressed as mean±SD (n = 3)
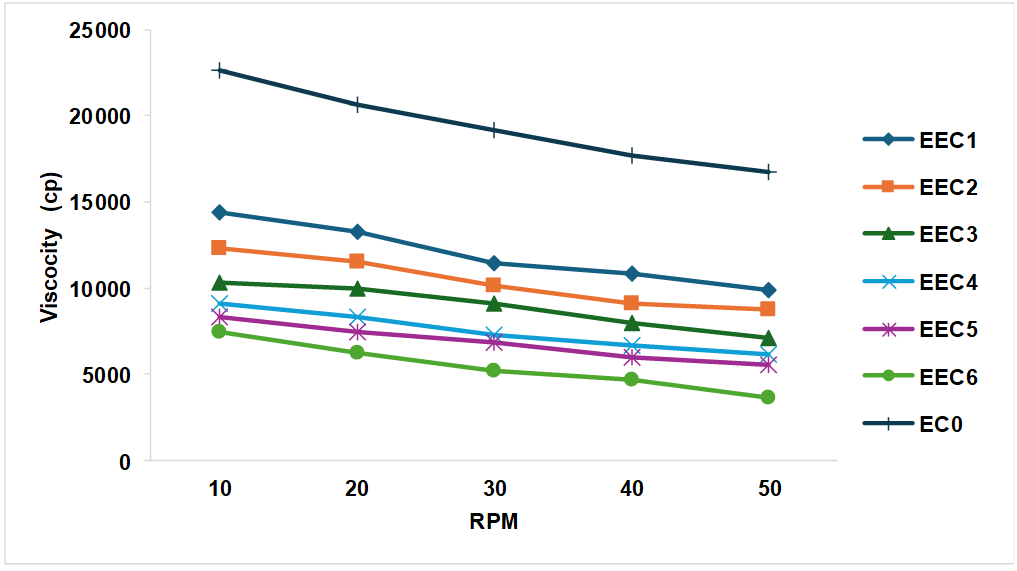
Fig. 5: Rheological study of ethosomal carbopol gel, data is expressed as mean of triplicates.
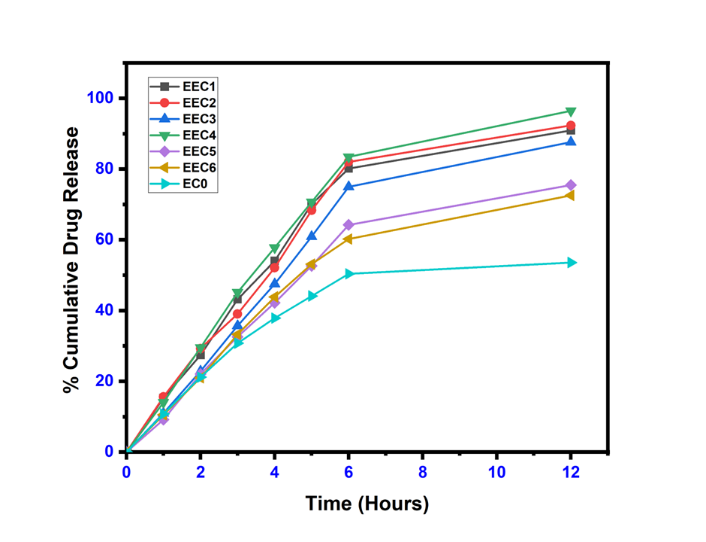
Fig. 6: Cumulative percentage of drug released (%)
Table 7: Cumulative drug release, flux, and permeability coefficient and enhancement ratio of ethosomal gel
| Code | Q12h(µg/cm2) | Flux (µg/cm2/h) | Permeability coefficient X 10-4 (Kp) (cm2/h) | Enhancement ratio (ER) |
| EC0 | 107.12±11.14 | 8.83±0.94 | 4.42±0.47 | 1 |
| EEC1 | 181.83±26.62 | 15.49±2.33 | 7.75±0.11 | 1.75 |
| EEC2 | 184.64±30.76 | 15.76±2.42 | 7.88±0.12 | 1.78 |
| EEC3 | 175.19±18.28 | 15.26±1.7 | 7.63±0.85 | 1.73 |
| EEC4 | 192.95±18.44 | 16.45±1.44 | 8.23±0.72 | 1.86 |
| EEC5 | 150.95±14.02 | 13.01±1.16 | 6.51±0.58 | 1.47 |
| EEC6 | 145.09±12.12 | 12.34±1.06 | 6.17±0.53 | 1.40 |
Data are expressed as mean±SD (n = 3)
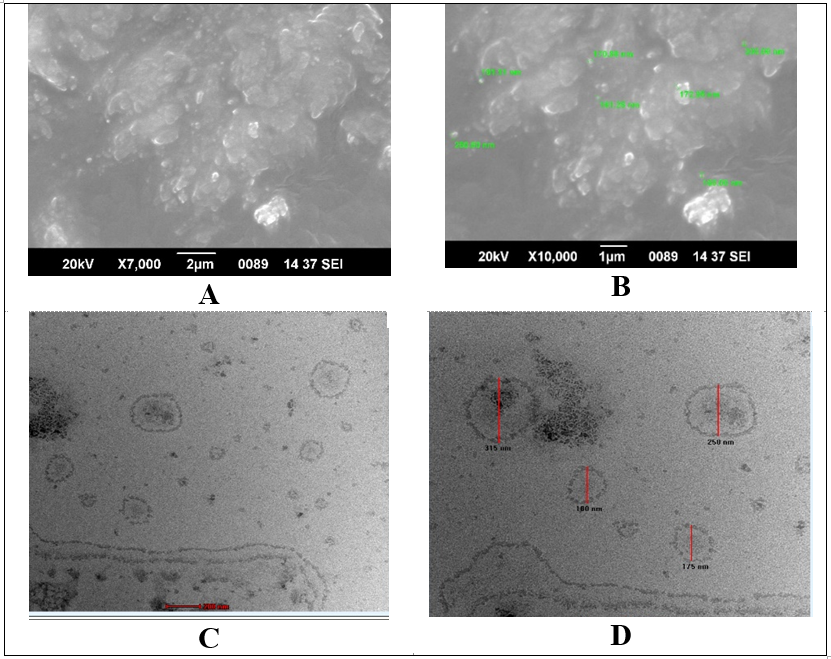
Fig. 7: SEM (A and B) and TEM (C and D) images of optimized ethosomes
Evaluation of optimized formulations
Based on results obtained from above evaluation, formulation EE4 and EEC4 were selected for further evaluation. Specially, results obtained from entrapment evaluation, particle size, zeta potential, In vitro diffusion study etc.
Vesicle morphology
Morphology of Ethosomal vesicles (EE4) was studied by using two techniques namely SEM and TEM. These methods indicated that vesicles were present in the nanometer range. Ethosomes vesicles have loose aggregates and smooth surfaces. TEM images (fig. 7C and 7D) shows almost spherical vesicles with a clear and dark outline. Outline and core of vesicles confirmed vesicular characteristics indicating the integrity of closed structures with drug entrapped in vesicles.
Ex vivo permeation study
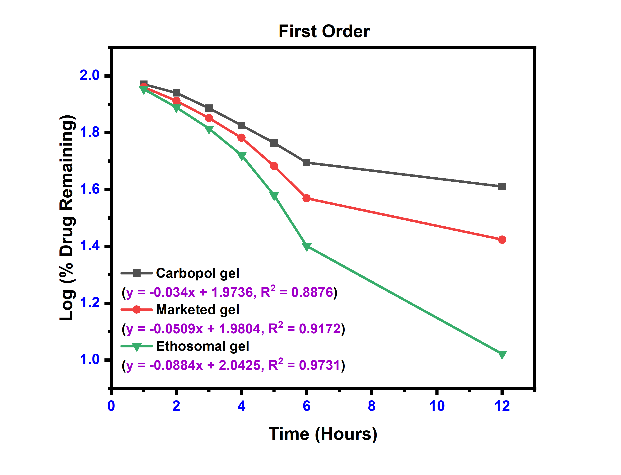
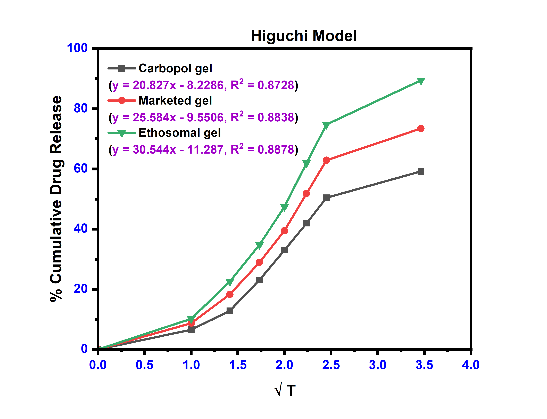
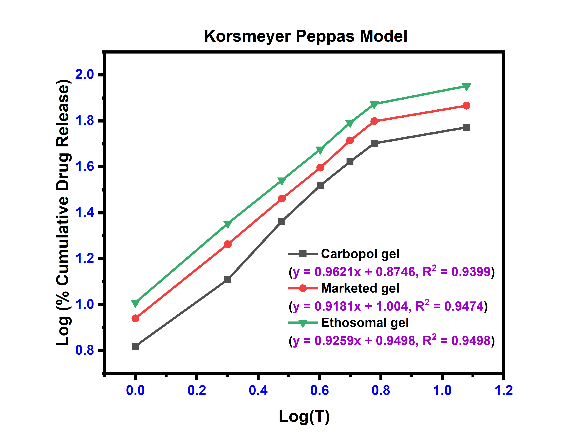
The linear curves and their corresponding equations for kinetics models with correlation coefficient (r2) for etodolac Carbopol gel, Marketed gel and Ethosomal gel using different kinetics are shown in fig. 8 and table 8. The r2 values were found higher for the first-order model and Korsmeyer-Peppas model for all formulations. The release profile of with Korsmeyer-Peppas equation revealed the value of “n” predicts the mechanism of the drug release. If "n" value<0.45 indicates Fickian diffusion, 0.45 to 0.89 denotes non-Fickian transport, n = 0.89 suggests Case II transport, and n>0.89 indicates super Case II transport for a cylindrical system [50]. Super Case-II transport is associated with stresses and transition present in hydrophilic glassy polymers which swell in presence of water or different biological fluids. These three formulations are shown value>0.89, it means a release is super Case II transport [51].
Table 8: Kinetics model data of ethosomal gel formulation
| Formulation | Zero-order | First order | Higuchi’s model | Korsmeyers peppas equation | |
| r2 | r2 | r2 | r2 | n | |
| Plain carbopol gel | 0.8551 | 0.8876 | 0.8728 | 0.9399 | 0.9621 |
| Marketed formulation | 0.8598 | 0.9172 | 0.8838 | 0.9474 | 0.9181 |
| Ethosomal gel (EEC4) | 0.8710 | 0.9731 | 0.8878 | 0.9498 | 0.9259 |
A |
B |
C |
D |
Fig. 8: The release kinetics model fitting curves: (a) zero-order release kinetics; (b) first-order kinetic model; (c) Higuchi model; and (d) Korsmeyerspeppas model
Irritancy test
All gels had no edema, erythema or any irritation on rat skin. These results indicated that topical application of these gels is safe to use and no harmful signs on the skin.
In vivo test
In vivo for anti-inflammatory activity of (EEC4) formulation showed 36.58% reduction significantly (<0.05) of carrageenan-induced paw edema at the end of 8 hr. This result was superior as compared to Plain Etodolac Carbopol gel and Marketed formulation. These formulations show 20.10 % inhibition and 24.92 % inhibition, respectively. Edema produced after administration of carrageenan was observed in fig. 9. % Inhibition of rat edema graphically shown in fig. 10.
FTIR study
FTIR graph of pure Etodolac with all other formulations are summarised in fig. 11. Etodolac and all formulations were show characteristics peaks in the same range because Etodolac drug and carbapol 940 have same functional groups. FTIR spectrum of etodolac revealed a characteristic sharp peak at 3344.07 (-NH stretching), 2971.33 (-OH stretching), 1745.44 (-C=O stretching), 1412.16 (C-H stretching), 1034.08 (C-O-C stretching) and Carbopol 940 show characteristics peaks at 3000-2950 (O-H stretching vibration) intramolecular H-bonded, 1750-1700 (C=O group of acids (C=O stretching vibration) 1450-1400 (C-O stretching vibrations) 1250-1200 (C-O-C stretching vibration) and 850-800 (C-H out of plane bending vibration) [52].
A |
B |
Fig. 9: Picture of left paw of rat A. Before carrageenan injection B. After carrageenan injection
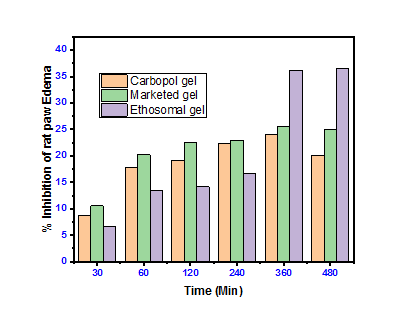
Fig. 10: % Inhibition of rat paw edema, data are expressed as mean
DSC study
The DSC thermogram of etodolac drug showed a sharp endothermic peak at 154.5 °C temperature, which indicates crystalline nature of etodolac pure drug. The thermogram of etodolac transferosomes showed transition temperature 107.1 C, whereas as plain Carbopol gel (without drug) shows at 96.2 °C, etodolac Carbopol gel showed 138.2 °C and etodolac Ethosomal Carbopol gel shows at 120.7 °C. The lower transition temperatures of gels indicate fluidity. Sharpness of peak was very law in fig. 12-D because of double gel system one is ethosomes and another was Carbopol gel. These results suggest that in formulation, the crystalline nature of etodolac was changed from crystalline state to amorphous state [53].
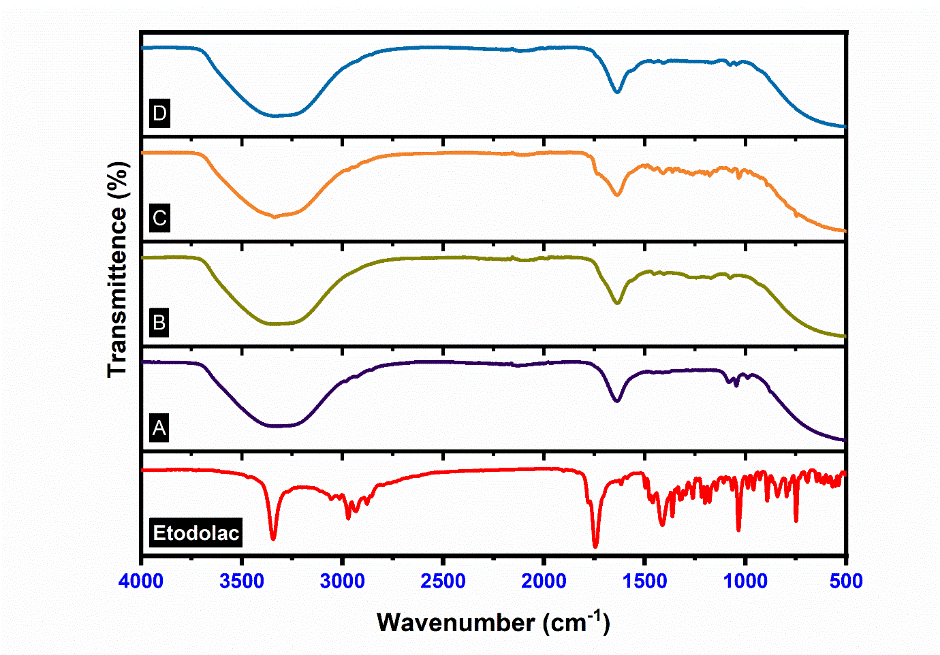
Fig. 11: FTIR spectra of etodolac; A: Etodolac transferosome, B: Plain carbopol gel, C: Etodolac carbopol gel, D: Etodolac ethosomal gel
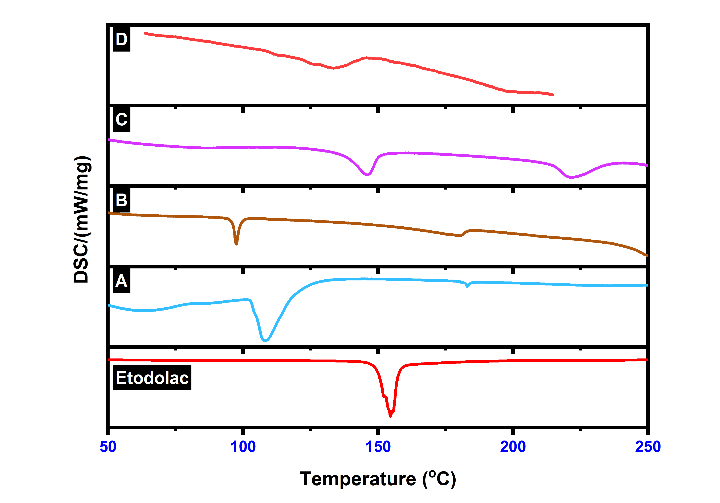
Fig. 12: DSC graph of etodolac; A: Etodolac transferosome, B: Plain carbopol gel, C: Etodolac carbopol gel, D: Etodolac ethosomal gel
XRD analysis
Fig. 14 shows the changes in the XRD peaks of etodolac drug after encapsulation into the ethosomes and plain Carbopol gel. Pure etodolac showed characteristic crystalline peaks, indicating the crystalline nature of the pure drug. Etodolac ethosomes (fig. 13-A) showed low-intensity characteristic peaks as compared to plane pure drug. The change in peak height and intensity confirms that etodolac was soluble in the used lipids. The formation of vesicles shows that the etodolac drug was entrapped in the lipid of vesicles and lost its crystalline nature. The absence of a sharp etodolac peak may be due to the Etodolac-phospholipid complex formation [54].
UV analysis
The Maximum absorption spectra of pure etodolac, plain Carbopol gel, etodolac Carbopol gel, and Etodolac ethosomal gel are shown in fig. 14. All formulations (fig. 14-A, C, D) shows the same spectra because of the presence of the Etodolac drug. Only fig. 14-B shows different spectra may be due to the absence of the drug.
Stability study
Stability study of the optimized ethosomal gel (EEC4) was carried out at different conditions like 5 °C±3 °C, 25 °C±2 °C, 60±5% RH and 40±2 °C, 75±5% RH for 180 d and results are shown in table 9. It indicates that the formulation has been shown different stability behaviour related percentage drug entrapment efficiency, it reduced significantly when this formulation was stored at 40±2 °C, 75±5%RH at more stable at 5 °C±3 °C. These results show that storage condition should maintain for ethosomal formulation to achieve stability.
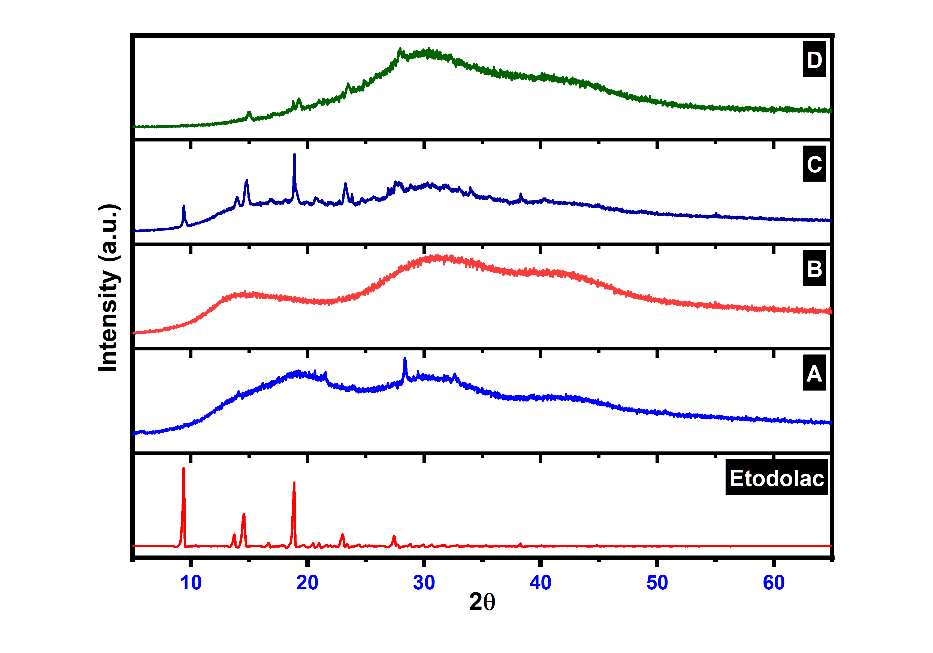
Fig. 13: XRD graph of etodolac; A: Etodolac transferosome, B: Plain carbopol gel, C: Etodolac carbopol gel, D: Etodolac ethosomal gel
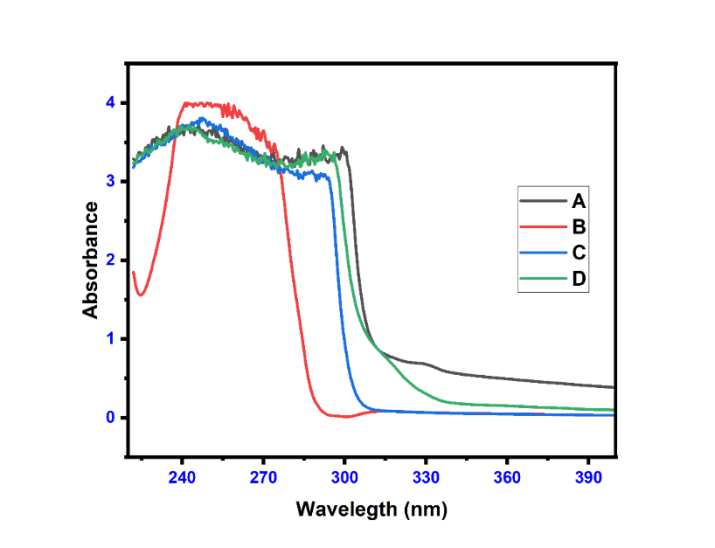
Fig. 14: Maximum absorption spectra of etodolac; A: Etodolac transfer some, B: Plain carbopol gel, C: Etodolac carbopol gel, D: Etodolac ethosomal gel
Table 9: Stability study of optimised transfersomal gel (EEC4)
| Months | 0 | 1 | 3 | 6 |
| Storage | % Entraptment Efficiency | |||
| 5 °C±3 °C | 84.51±0.42 | 81.41±0.60 | 78.60±0.68 | 75.68±0.53 |
| 25±2 °C, 60±5%RH | 84.51±0.42 | 76.96±0.89 | 70.84±1.74 | 66.44±0.71 |
| 40±2 °C, 75±5%RH | 84.51±0.42 | 65.62±0.90 | 66.44±0.71 | 55.96±0.79 |
Data are expressed as mean±SD (n = 3)
CONCLUSION
This research suggests that Ethosomes are an effective drug delivery system through topical route with less side effect. The formulations of ethosomes with different ratios of alcohol (EE1-EE6) were successfully manufactured by thin-film hydration method. The results obtained from vesicle size, zeta potential, polydispersity index (PDI) and entrapment efficiency of etodolac ethosomes were in acceptable ranges. Furthermore, ethosomal formulations were incorporated into carbopol-940 gels. These ethosomal gels were subjected to colour, pH, spreadability, viscosity, homogeneity, and In vitro drug release for different models. The optimized formulation (EE4) was further evaluated for SEM, TEM, Ex vivo, Irritancy, In vivo and different analytical techniques. Finally, concluded that Etodolac Ethosomal gel could become stable at specific condition successful topical dosage form for symptomatic relief in Rheumatoid Arthritis.
FUNDING
Nil
AUTHORS CONTRIBUTIONS
Ashwini A. Bachhav-Conducted the experiments, performed data analysis, and contributed to the interpretation of results. Dr. Prashant L. Pingale-Conceptualized the study, designed the experiments, and wrote the initial draft of the manuscript. Dr. Chadrashekhar D. Upasani- oversaw the statistical analysis, helped with manuscript editing, and supervised the research project.
CONFLICT OF INTERESTS
Declared none
REFERENCES
Madhavi N, Sudhakar B, Reddy KV, Ratna JV. Design by optimization and comparative evaluation of vesicular gels of etodolac for transdermal delivery. Drug Dev Ind Pharm. 2019;45(4):611-28. doi: 10.1080/03639045.2019.1569030, PMID 30712433.
Dhule KD, Nandgude TD. Lipid nanosystem based topical drug delivery for management of rheumatoid arthritis: an overview. Adv Pharm Bull. 2023;13(4):663-77. doi: 10.34172/apb.2023.075, PMID 38022817.
Shilakari Asthana G, Asthana A, Singh D, Sharma PK. Etodolac containing topical niosomal gel: formulation development and evaluation. J Drug Deliv. 2016;2016(1):9324567. doi: 10.1155/2016/9324567, PMID 27478643.
Salah S, Mahmoud AA, Kamel AO. Etodolac transdermal cubosomes for the treatment of rheumatoid arthritis: ex vivo permeation and in vivo pharmacokinetic studies. Drug Deliv. 2017;24(1):846-56. doi: 10.1080/10717544.2017.1326539, PMID 28535740.
Sharma G, Mahajan A, Thakur K, Kaur G, Goni VG, Kumar MV. Exploring the therapeutic potential of sodium deoxycholate-tailored deformable emulsomes of etodolac for effective management of arthritis. Sci Rep. 2023;13(1):21681. doi: 10.1038/s41598-023-46119-7, PMID 38066008.
Gill V, Nanda A. Preparation and characterization of etodolac bearing emulsomes. Int J App Pharm. 2020;12(5):166-72. doi: 10.22159/ijap.2020v12i5.38842.
Moore N, Pollack C, Butkerait P. Adverse drug reactions and drug-drug interactions with over-the-counter NSAIDs. Ther Clin Risk Manag. 2015;11:1061-75. doi: 10.2147/TCRM.S79135, PMID 26203254.
Bachhav AA, Ahire SA. Proniosome: a novel nonionic provesicules as potential drug carrier. Asian J Pharm (AJP). 2016;10(3). doi: 10.22377/ajp.v10i03.757.
David AO, Anton HA. Topical drug delivery formulations. Vol. 1. Marcel Dekker; 1990.
Jeong WY, Kwon M, Choi HE, Kim KS. Recent advances in transdermal drug delivery systems: a review. Biomater Res. 2021;25(1):24. doi: 10.1186/s40824-021-00226-6, PMID 34321111.
Placzek M, Watrobska Swietlikowska D, Stefanowicz Hajduk J, Drechsler M, Ochocka JR, Sznitowska M. Comparison of the in vitro cytotoxicity among phospholipid based parenteral drug delivery systems: emulsions liposomes and aqueous lecithin dispersions (WLDs). Eur J Pharm Sci. 2019;127:92-101. doi: 10.1016/j.ejps.2018.10.018, PMID 30342174.
NG ZY, Wong JY, Panneerselvam J, Madheswaran T, Kumar P, Pillay V. Assessing the potential of liposomes loaded with curcumin as a therapeutic intervention in asthma. Colloids Surf B Biointerfaces. 2018 Dec 1;172:51-9. doi: 10.1016/j.colsurfb.2018.08.027, PMID 30134219.
Mahmood S, Mandal UK, Chatterjee B. Transdermal delivery of raloxifene HCl via ethosomal system: formulation advanced characterizations and pharmacokinetic evaluation. Int J Pharm. 2018;542(1-2):36-46. doi: 10.1016/j.ijpharm.2018.02.044, PMID 29501737.
Mahmood S, Chatterjee B, Mandal UK. Pharmacokinetic evaluation of the synergistic effect of raloxifene loaded transfersomes for transdermal delivery. J Drug Deliv Sci Technol. 2021 Jun;63:1025-45. doi: 10.1016/j.jddst.2021.102545.
Bhandari S. Ethosomes: a novel vesicular innovation to enhance transdermal delivery of drugs. RJPDFT. 2022;14(1):72-8. doi: 10.52711/0975-4377.2022.00012.
Paliwal S, Tilak A, Sharma J, Dave V, Sharma S, Yadav R. Flurbiprofen loaded ethosomes transdermal delivery of anti-inflammatory effect in rat model. Lipids Health Dis. 2019;18(1):133. doi: 10.1186/s12944-019-1064-x, PMID 31170970.
Mistry A, Ravikumar P, Pathare S. Ethosomes: unique elastic vesicular carrier an overview. Int J Pharm Sci Res. 2015;6:4129-36. doi: 10.13040/IJPSR.0975-8232.6(10).4129-36.
Ghanbarzadeh S, Arami S. Enhanced transdermal delivery of diclofenac sodium via conventional liposomes ethosomes and transfersomes. BioMed Res Int. 2013;2013:616810. doi: 10.1155/2013/616810, PMID 23936825.
Fang YP, Tsai YH, WU PC, Huang YB. Comparison of 5‑aminolevulinic acid encapsulated liposome versus ethosome for skin delivery for photodynamic therapy. Int J Pharm. 2008;356(1-2):144-52. doi: 10.1016/j.ijpharm.2008.01.020, PMID 18325699.
Peppas NA, Bures P, Leobandung W, Ichikawa H. Hydrogels in pharmaceutical formulations. Eur J Pharm Biopharm. 2000;50(1):27-46. doi: 10.1016/s0939-6411(00)00090-4, PMID 10840191.
Limsuwan T, Boonme P, Khongkow P, Amnuaikit T. Ethosomes of phenyl ethyl resorcinol as vesicular delivery system for skin lightening applications. Bio Med Res Int. 2017;2017:8310979. doi: 10.1155/2017/8310979, PMID 28804723, PMCID PMC5540262.
Ibrahim TM, Abdallah MH, El Megrab NA, El Nahas HM. Transdermal ethosomal gel nanocarriers; a promising strategy for enhancement of anti-hypertensive effect of carvedilol. J Liposome Res. 2019;29(3):215-28. doi: 10.1080/08982104.2018.1529793, PMID 30272506.
Saifee M, Atre M, Toshniwal R. Formulation and in vitro evaluation of ethosomal gel of repaglinide for transdermal delivery. Int J Pharm Phytopharm Res. 2021;11(4):11-7. doi: 10.51847/lQKgwgUi1l.
Lohani A, Verma A, Hema G, Pathak K. Topical delivery of geranium/calendula essential oil entrapped ethanolic lipid vesicular cream to combat skin aging. Bio Med Res Int. 2021;2021(1):4593759. doi: 10.1155/2021/4593759, PMID 34552986.
Lukhele BS, Bassey K, Witika BA. The utilization of plant material-loaded vesicular drug delivery systems in the management of pulmonary diseases. Curr Issues Mol Biol. 2023;45(12):9985-10017. doi: 10.3390/cimb45120624, PMID 38132470.
Hettiarachchi SD, Kwon YM, Omidi Y, Speth RC. Nanoparticle approaches for the rennin angiotensin system. Heliyon. 2023;9(6):e16951. doi: 10.1016/j.heliyon.2023.e16951, PMID 37484281.
Salatin S, Barar J, Barzegar Jalali M, Adibkia K, Kiafar F, Jelvehgari M. Development of a nanoprecipitation method for the entrapment of a very water soluble drug into Eudragit RL nanoparticles. Res Pharm Sci. 2017;12(1):1-14. doi: 10.4103/1735-5362.199041, PMID 28255308, PMCID PMC5333474.
Negi S, Tandel N, Garg NK, Sharma P, Kumar R, Sharma P. Co-delivery of aceclofenac and methotrexate nanoparticles presents an effective treatment for rheumatoid arthritis. Int J Nanomedicine. 2024;19:2149-77. doi: 10.2147/IJN.S439359, PMID 38482519.
Tiwari A, Bag P, Sarkar M, Chawla V, Chawla PA. Formulation validation and evaluation studies on metaxalone and diclofenac potassium topical gel. Environ Anal Health Toxicol. 2021;36(1):e2021001. doi: 10.5620/eaht.2021001, PMID 33499562, PMCID PMC8207004.
Elena OB, Maria NA, Michael SZ, Natalia BD, Alexander IB. Dermatologic gels spreadability measuring methods comparative study. Int J App Pharm. 2022;14(1):164-8. doi: 10.22159/ijap.2022v14i1.41267.
Kapoor A. Formulation and assessment of stability parameters for acitretin loaded NLC gel. Asian J Pharm (AJP). 2021;15(2). doi: 10.22377/ajp.v15i2.4060.
Magbool FF, Ibrahim Elnima EI, Shayoub ME, Osman Z, Ebied Adam M, Hamedelnie EI. Formulation design development and evaluation of quercun infectoria galls extract oral gels for oral candidiasis. Plant Biotechnol Persa. 2020;2(2):1-13. doi: 10.52547/pbp.2.2.1.
Aldawsari MF, Moglad EH, Alotaibi HF, Alkahtani HM, Khafagy ES. Ophthalmic bimatoprost loaded niosomal in situ gel: preparation optimization and in vivo pharmacodynamics study. Polymers. 2023;15(21):433-6. doi: 10.3390/polym15214336, PMID 37960016.
Rao AA, Arvapalli S, Koteswara Rao GS, Malothu N, Bandaru NR. Design and evaluation of acyclovir niosomes. Res J Pharm Technol. 2021;14(8):4185-8. doi: 10.52711/0974-360X.2021.00724.
Ozdemir S, Celik B, Turkoz Acar E, Duman G, Uner M. Eplerenone nanoemulsions for treatment of hypertension experimental design for optimization of formulations and physical characterization. J Drug Deliv Sci Technol. 2018;45;I:357-66. doi: 10.1016/j.jddst.2018.03.014.
Salem HF, Kharshoum RM, Gamal F A, Abo El Ela FI, Abdellatif KR. Treatment of breast cancer with engineered novel pH-sensitive triaryl-(Z)-olefin niosomes containing hydrogel: an in vitro and in vivo study. J Liposome Res. 2020;30(2):126-35. doi: 10.1080/08982104.2019.1601213, PMID 30935273.
Fouad AG, Ali MR, Naguib DM, Farouk HO, Zanaty MI, El Ela FI. Design optimization and in vivo evaluation of invasome-mediated candesartan for the control of diabetes-associated atherosclerosis. Drug Deliv Transl Res. 2024;14(2):474-90. doi: 10.1007/s13346-023-01412-w, PMID 37605039.
Salem HF, Gamal A, Saeed H, Kamal M, Tulbah AS. Enhancing the bioavailability and efficacy of vismodegib for the control of skin cancer: in vitro and in vivo studies. Pharmaceuticals (Basel). 2022;15(2):126. doi: 10.3390/ph15020126, PMID 35215238.
Adin SN, Gupta I, Rashid MA, Alhamhoom Y, Aqil M, Mujeeb M. Nanotransethosomes for enhanced transdermal delivery of mangiferin against rheumatoid arthritis: formulation characterization in vivo pharmacokinetic and pharmacodynamic evaluation. Drug Deliv. 2023;30(1):2173338. doi: 10.1080/10717544.2023.2173338, PMID 36729134, PMCID PMC9943251.
Karakucuk A, Tort S, Han S, Oktay AN, Celebi N. Etodolac nanosuspension based gel for enhanced dermal delivery: in vitro and in vivo evaluation. J Microencapsul. 2021;38(4):218-32. doi: 10.1080/02652048.2021.1895344, PMID 33752553.
Ozdemir S, Uner B, Karakucuk A, Celik B, Sumer E, Tas C. Nanoemulsions as a promising carrier for topical delivery of etodolac: formulation development and characterization. Pharmaceutics. 2023;15(10):2510. doi: 10.3390/pharmaceutics15102510, PMID 37896270, PMCID PMC10610052.
Sangkana S, Eawsakul K, Ongtanasup T, Boonhok R, Mitsuwan W, Chimplee S. Preparation and evaluation of a niosomal delivery system containing G. mangostana extract and study of its anti-acanthamoeba activity. Nanoscale Adv. 2024;6(5):1467-79. doi: 10.1039/D3NA01016C, PMID 38419876.
Salem HF, Gamal A, Saeed H, Tulbah AS. The impact of improving dermal permeation on the efficacy and targeting of liposome nanoparticles as a potential treatment for breast cancer. Pharmaceutics. 2021;13(10):1633. doi: 10.3390/pharmaceutics13101633, PMID 34683926.
Imam SS, Alshehri S, Altamimi MA, Hussain A, Alyahya KH, Mahdi WA. Formulation and evaluation of luteolin loaded nanovesicles: in vitro physicochemical characterization and viability assessment. ACS Omega. 2022;7(1):1048-56. doi: 10.1021/acsomega.1c05628, PMID 35036768.
Quevedo AC, Guggenheim E, Briffa SM, Adams J, Lofts S, Kwak M. UV vis spectroscopic characterization of nanomaterials in aqueous media. J Vis Exp. 2021;(176):e61764. doi: 10.3791/61764, PMID 34747394.
Keservani RK, Prakash Gautam S. Formulation and evaluation of baclofen liposome vesicles using lecithin. Ars Pharmdoi. 2020;61(3):175-80. doi: 10.30827/ars.v61i3.15279.
Opatha SA, Titapiwatanakun V, Chutoprapat R. Transfersomes: a promising nanoencapsulation technique for transdermal drug delivery. Pharmaceutics. 2020;12(9):855. doi: 10.3390/pharmaceutics12090855, PMID 32916782.
Maurya SD, Dhakar RC, Aggarwal S, Tilak VK, Verma KK, Prajapati SK. Enhancement of transdermal permeation of indinavir sulfate via ethosomevesicles. Afr Jof Pharm Sci Pharm AJPSP. 2021;2:1-33.
Rai VK, Mishra N, Yadav KS, Yadav NP. Nanoemulsion as pharmaceutical carrier for dermal and transdermal drug delivery: formulation development stability issues basic considerations and applications. J Control Release. 2018;270:203-25. doi: 10.1016/j.jconrel.2017.11.049, PMID 29199062.
Khan MI, Yaqoob S, Madni A, Akhtar MF, Sohail MF, Saleem A. Development and in vitro/ex vivo evaluation of lecithin based deformable transfersomes and transfersome-based gels for combined dermal delivery of meloxicam and dexamethasone. Bio Med Res Int. 2022;2022:8170318. doi: 10.1155/2022/8170318, PMID 36483631.
Taymouri S, Hajhashemi V, Tabbakhian M, Torkashvand M. Preparation and evaluation of imatinib loaded transfersomal gel for the treatment of rheumatoid arthritis. Iran J Pharm Res. 2021;20(4):33-46. doi: 10.22037/ijpr.2021.115481.15394, PMID 35194426.
Sharma A, Kaur J, Goyal A. Carbopol 940 Vs carbol 904: a better polymer for hydrogel formulation. Res J Pharm Technol. 2021;14(3):1561-4. doi: 10.5958/0974-360X.2021.00275.4.
Madhavi N, Sudhakar B, Reddy KV, Ratna JV. Design by optimization and comparative evaluation of vesicular gels of etodolac for transdermal delivery. Drug Dev Ind Pharm. 2019;45(4):611-28. doi: 10.1080/03639045.2019.1569030, PMID 30712433.
Zhang S, Gao H, Bao G. Physical principles of nanoparticle cellular endocytosis. ACS Nano. 2015;9(9):8655-71. doi: 10.1021/acsnano.5b03184.