
Int J App Pharm, Vol 17, Issue 3, 2025, 119-126Review Article
MICRONEEDLES: REVOLUTIONIZING TRANSDERMAL DRUG DELIVERY SYSTEMS
DEEPIKA B.*, ANEES BEGUM, NAGA RAJU KANDUKOORI, KATLA VENU MADHAV
Department of Pharmaceutics, St. Pauls College of Pharmacy, Hyderabad, Telangana, India
*Corresponding author: Deepika B.; *Email: deepikab@stpaulscollege.ac.in
Received: 29 Aug 2024, Revised and Accepted: 25 Feb 2025
ABSTRACT
Microneedles (MNDs) have emerged as a transformative technology in transdermal drug delivery, offering a minimally invasive alternative to traditional hypodermic needles. These microscale devices allow the direct delivery of medication into the dermal layers by creating tiny punctures in the skin.
This article looks in-depth at the various types of MNDs, including hollow, hydrogel-forming, coated, dissolvable, and solid MNDs, along with the associated manufacturing techniques. These are used in many medical disciplines to reduce side effects, increase patient compliance, and enhance medication efficacy.
Examples of these specialties include pain treatment, hormone administration, vaccine delivery, and cosmetic surgeries. Despite their promising advantages, problems with patient education, regulatory obstacles, and manufacturing scalability need to be fixed.
This article describes the current status of MNDs technology, discusses potential changes in transdermal medication administration, and suggests future directions for study and development.
Keywords: Microneedles, Transdermal drug delivery, Design, Therapeutics, Skin penetration, Minimally invasive
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijap.2025v17i3.52517 Journal homepage: https://innovareacademics.in/journals/index.php/ijap
INTRODUCTION
Drugs can be administered topically through the skin's dermal layer using Transdermal Drug Delivery Systems (TDDS), which eliminates the need for intrusive needle injections and achieves systemic dispersion. This non-invasive approach has drawn a lot of interest and investigation, making it a well-known substitute for traditional drug delivery methods. To provide the regulated, systemic release of medications in therapeutically appropriate dosages, TDDS uses customized patches that are placed topically and carefully engineered [1]. In this subject, research and study are focused on finding a solution to the main obstacle of medication dispersion across the stratum corneum, the outermost and relatively impermeable layer of skin. Fig. 1 illustrates some of the advantages of TDDS over traditional medication delivery methods.
Conventional drug administration techniques, like injectables and oral medications, have drawbacks in terms of patient compliance, discomfort, and drug degradation [1]. They can cause tolerance, dosage mistakes, and storage issues, and they might not be useful for targeted therapy. Alternative medication delivery techniques that can improve therapeutic efficacy and patient experience are therefore required.
Hypodermic needles are a quick, easy, and economical way to introduce different compounds into the body. They are used in the administration of several biotherapeutics and vaccinations. However, patient self-administration is usually limited by the need for skilled medical staff to utilize and dispose of this approach [2]. Significant obstacles to patient compliance include discomfort, needle anxiety, and worries about the spread of blood-borne pathogens.
Although oral administration can help with some of these problems, it is not recommended for all medications due to complications with the gut and hepatic metabolism during assimilation and breakdown. While other modes of administration have been investigated, none of them can match the adaptability of administration using needles. Some scientists have suggested shrinking needles to the nanoscale as an alternative to totally doing away with them. Without sacrificing the benefits of needle-based medication administration devices, this strategy can increase patient safety and encourage adherence to treatment [3]. Microneedles (MNDs) are devices at the micron scale that need to be compact enough to lessen discomfort and anxiety and big enough to deliver most drugs or formulations with microscopic particles without being overly large and requiring a lot of training. The skin, the suprachoroidal area of the eye, and even cell nuclei can be precisely targeted for drug administration with the help of MNDs. The administration of vaccines and drugs transdermally has been the main focus of MNDs research [4].
With the use of MNDs, which are tiny needles that puncture the skin to form temporary microchannels, medications can more effectively reach the underlying tissues underneath the stratum corneum. With less pain and suffering than traditional methods, this minimally invasive technique combines the efficiency of hypodermic needles with the convenience of use of transdermal patches [5]. Due to its versatility, it is being explored in a number of areas, such as hormone therapy, pain management, vaccine delivery, and cosmetic applications.
The goal of this paper is to present a thorough overview of MNDs technology, including a discussion of the various kinds of MNDs, how they are made, and the range of uses they have in medication delivery. The assessment also looks at the benefits of over traditional drug delivery techniques and the obstacles that need to be removed in order to reach their full potential. This article emphasizes how MNDs are revolutionizing transdermal medication delivery methods by highlighting recent developments and promising future paths [6].
The selections of articles for the present review were searched from Specialized databases (Range of years: 2018-2024) such as Elsevier Pubmed, using the keywords MNDs, Transdermal Drug Delivery, Microneedle Design, Therapeutics, Skin Penetration, Minimally Invasive.
Development and history of medication delivery
A tale of invention and advancement is the development of medicine delivery through microneedle technology. The 1920s are when the earliest ideas for microneedles were first proposed. The primary purposes of the early designs were skin perforation and laboratory use. Nevertheless, at the time, they were not extensively used in the medical industry. MNDs technologies were created throughout the 1950s and 60s, but they were still in the experimental stage7. Following the development of modern MNDs in the late 20th century, namely throughout the 1970s and 1980s, research into MNDs accelerated. Researchers started looking into the potential uses of MNDs for transdermal and medication delivery. Creating a painless, less intrusive way to deliver medicine was the aim [7, 8].
When researchers at the Georgia Institute of Technology and Emory University first proposed the idea of "MNDs arrays" for drug administration in 1998, it was one of the major advances. Several MNDs that could be placed to the skin made up these arrays. Researchers from all over the world achieved major advancements in MNDs technology in the 2000s. They experimented with a range of fabrication designs, materials, and techniques. Because they might distribute medications without causing discomfort and dissolve after usage, biodegradable and dissolvable MNDs attracted attention. Strong validation of the safety and efficacy of microneedle technology came from the FDA's approval of the first MNDs-based product, a glucose-level-measuring device for the interstitial fluid [8].
Three concurrent efforts launched substantial study into the use of for drug delivery in the mid-1990s:
Becton dickinson (BD)
A significant portion of their business was parenteral injection, and the company had a strong interest in improving hypodermic needles for this use [9, 10]. They funded studies at the Universities of Utah and California Berkeley to look into how micro-fabrication technology may be used to make smaller needles with improved pharmacological and therapeutic outcomes [11]. In an effort to improve skin permeability for topical vaccines, Becton Dickinson focused on solid MNDs with blunt tips and short hollow steel needles. In the end, this endeavor produced commercially viable goods such as Soluvia®, an intradermal vaccination injection.
Alza corporation
Beginning with their active involvement in the development of iontophoresis, a method of medication delivery through the skin, in the 1970s, Alza developed a passion with MNDs [12]. They explored the use of strong metallic MNDs in conjunction with iontophoresis to enhance medication delivery. Through the use of this creative strategy, a new company called Zosano Pharma was eventually founded. The technology then advanced to the point of clinical trials, with the goal of supplying medications to treat conditions like osteoporosis [13].
Georgia Institute of Technology: Mark Allen's expertise in microfabrication and Mark Prausnitz's transdermal drug delivery expertise led Georgia Tech's cooperative efforts to publish the first scientific paper on MNDs-based drug administration. Consequently, Georgia Tech has continued to be actively involved in this research.
After the 20th century ended and the 21st century got underway, more businesses and educational establishments started investigating MNDs. Yet, rather than being employed for medication delivery in the early stages of creation, numerous designs and techniques were largely employed for the advancement of microfabrication research. Until recently [8], there was not much academic participation in this research. Even while academic efforts to make microfabricants slowed down in the 2000s, research on drug delivery at universities grew and expanded significantly. Meanwhile, commercial development continued to expand. The condition of the area now is the result of the eventual bridging of the multidisciplinary gap between medication delivery and microfabrication. As of right now, numerous industrial and academic laboratories are home to operational devices [14].
Many applications are now in the phases of human trials after being successfully shown in controlled laboratory environments and animal investigations. A chance to further translate this into clinical applications and integrate MNDs goods into medical practice is presented to the field. Increased study and development into technology was observed in the 2010s. Applications such as hormonal contraception, pain management, vaccine distribution, and the treatment of different medical diseases were among the many that researchers investigated [15].
Cho and associates made an important finding on quills of North American porcupines in 2012 [13]. They discovered that compared to hypodermal needles of the same diameter, the microstructure of these quills made tissue penetration easier. Both real quill testing and finite element models were used in the study. These tests showed that the quills caused less damage than quills without barbs because they could easily pierce pig skin and chicken muscle with little effort. Tests using artificial polyurethane quills confirmed these results.
This novel design has ramifications for the creation of transdermal goods, such MNDs arrays, which require less force to penetrate the skin. The endoparasite Pomphorhynchus laevis, which extends its proboscis to adhere to the intestinal lining of its host, served as an inspiration for a different team of researchers in 2013. As a result, MN arrays with two phases were created. The first phase involved mechanically locking with tissue using swellable tips to boost adhesion strength. The poly (styrene)-block-poly (acrylic acid) structure of these MNs had a non-expanding polystyrene core and a swelling tip. The MNDs with a conical shape showed certain interesting properties, such as strong adhesion, less tissue penetrating force, and shallower insertion depth.
This bioinspired design lowered the danger of infection, minimized injury to soft tissue during adhesion, and improved drug delivery. Zhipeng Chen and his colleagues were inspired in 2018 by the stingers of honeybees, which are renowned for the tiny barbs they deploy to transport venom [14]. They effectively created MNs that resembled honeybee stingers by introducing a unique 3D additive manufacturing technology called Magneto Rheological Drawing Lithography (MRDL). Compared to barbless MNs, these barbed MNs-which were made possible by an external magnetic field—were more difficult to remove but were easier to introduce.
With potential applications in tissue adherence and transdermal medication delivery, the strength ratio between extraction and penetration was increased compared to barbless MNDs. Additionally, the tension at the barbs enhanced adhesive force during removal while reducing insertion force. A micro-adhesive backward-facing barbed MN that demonstrated 18 times stronger tissue adherence than barbless MNs was created in 2020 by Rutgers researchers using micro 3D and 4D printing technologies [15]. The development of a more stable MN appropriate for reliable drug administration was made possible by the combination of various printing processes. Although they provide medication fast, conventional hypodermic needles might not be the best option for long-term use. Although they require reapplication occasionally, MNDs are a less unpleasant option.
In order to overcome these problems, the 4D printed MNDs array was created, which mimics the effectiveness of real barbed needles for improved adhesion. The degree of barb curvature might be adjusted by researchers to maximize adhesion. In the 2020s and later, there have been notable developments in the materials used to create MNDs [17]. Materials that are biocompatible and biodegradable are included, along with advancements in design. Real-time monitoring of indicators, such glucose levels and drug release, is now possible because to the combination of MNDs with sensor technologies. These MNDs have the power to completely change medication delivery and healthcare monitoring. Clinical trials or commercial approval have been granted to a number of medication delivery technologies and devices. These changes suggest that the healthcare sector is coming to terms with this technology [18].
Considering theses are originated in microelectronics, silicon constituted the majority of their construction. Subsequently, a diverse array of materials, such as metal, polymers, glass, and ceramics, have been employed to create MNDs, which are customised in size and form to suit certain uses. A variety of standard microfabrication techniques, including photolithography, silicon patterning, laser ablation, metal electrodeposition, electrolytic polishing, and microstructure molding, are used in the majority of fabrication processes. Four main types of methods can be distinguished, as illustrated in fig. 2.
Types of MNDs
Solid MNDs the main purpose of these is to induce microchannels in the skin, increasing the stratum corneum's permeability. Drugs administered topically can absorb more of these microchannels. It is possible to create solid MNDs out of silicon, metals, and polymers, among other materials [19]. They are applied by first pretreating the skin and then applying a patch or formulation that contains drugs.
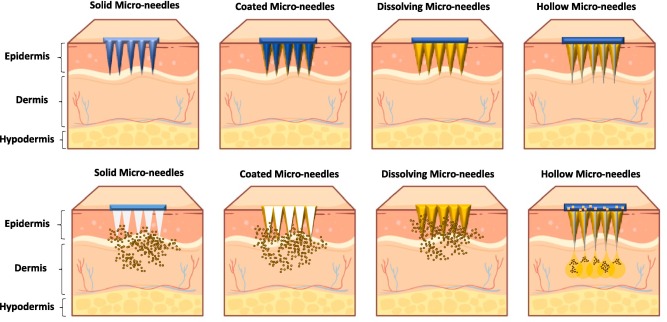
Fig. 2: MNDs according to drug release [18]
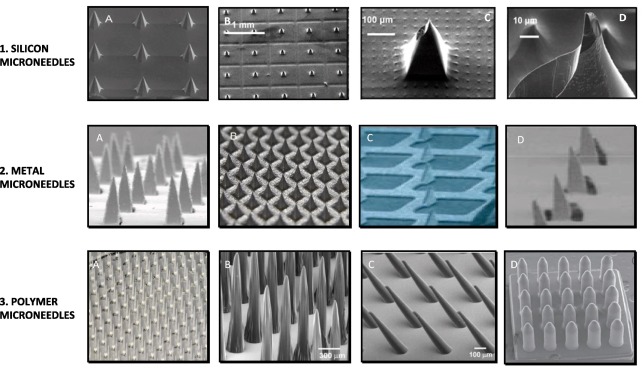
Fig. 3: Various materials are used to create solid MNDs. Three types are available: silicon, metal, and polymer [19]
Coated MNDs
The purpose of coated MNDs is to administer medications that are coated onto the needles' surface. The medication coating dissolves and is released into the dermal layers upon insertion into the skin. This kind of MNDs can be utilized for a variety of medications, such as therapeutic agents and vaccinations, and it provides accurate dosage management. Usually composed of metal or silicon, coated MNDs are applied by a coating process such as spray coating or dip coating [20].
Dissolvable MNDs
The medicine that is enclosed in dissolvable MNDs is released when the needles dissolve when inserted into the skin because they are composed of biocompatible and biodegradable materials [21]. These lower the possibility of sharp waste and do away with the necessity to remove needles. Proteins, polymers, and sugars are among the frequently utilized materials. In particular, dissolvable MNDs can be used to administer biological substances such as vaccines [22].
Hollow MNDs
On a smaller scale, hollow MNDs resemble conventional hypodermic needles. They have a hollow bore that allows liquid medications to be injected straight into the skin. This kind of MNDs can be utilized for bolus or continuous delivery, enabling regulated medication infusion. Usually made of glass, metals, or robust polymers, hollow MNDs are useful for applications like insulin delivery that require for accurate drug delivery [23].
Hydrogel-forming MNDs
MNDs with hydrogel-forming properties are made of materials that can swell and congeal when they absorb interstitial fluid from the skin. Instead of dissolving, these MNDs expand to create a porous matrix that allows medications to slowly seep through [24]. Hydrogel-forming MNDs are helpful for a controlled and prolonged release of medication, which makes them perfect for long-term therapy of chronic illnesses. These are made of cross-linked polymers that are strong mechanically and biocompatible.
Every variety has distinct benefits and is appropriate for a particular use in medication administration. The kind of use depends on the medication, the therapeutic application, and the necessary delivery profile. It is essential to comprehend these variations in order to optimize technology for a range of therapeutic applications [25].
Fabrication methods of MNDs
Using precise engineering processes, MNDs are made into shapes that can effectively penetrate the skin and deliver medicinal substances. Microneedles can be made using a variety of techniques, each with pros and cons. The main techniques for fabrication are as follows:
The process of photolithography
A method frequently employed in the microelectronics sector, photolithography has been modified for the production of MNDs. With this technique, a geometric pattern is transferred from a photo mask to a substrate's light-sensitive chemical photo resist via the use of light. Next, a MNDs mold is formed in the exposed locations. With this process, materials like silicon and other polymers are frequently employed. Photolithography is the perfect method for producing arrays of coated and solid MNDs because it can achieve high precision and consistency in size [26].
Micromolding
Using a mold made of the required MNDs shape, ingredients like sugars, biocompatible polymers, or other dissolvent compounds are poured into the mold. Usually, the procedure entails utilizing methods such as photolithography or laser cutting to create a master template, which is then used to cast the mold [27]. This technique is ideal for producing hydrogel-forming and dissolvable MNDs in large quantities since it is both extremely scalable and economical.
Utilizing laser technology
Using high-precision lasers, laser cutting creates and shapes MNDs out of silicon, metal, and polymers. This process makes it possible to create hollow structures and elaborate shapes. For applications needing great mechanical strength and for precisely sized hollow MNDs, laser cutting is especially helpful [17].
3D printing
Also known as additive manufacturing, 3D printing is a recently developed method for fabricating MNDs that enables quick prototyping and customization. 3D printers can create MNDs layer by layer out of materials like composites and polymers by using digital models. This process produces intricate structures with great design freedom, which makes it perfect for applications involving personalized medicine. MNDs that form hydrogel are soluble and may be made with 3D printing [28, 29].
Electroplating
Electroplating involves depositing a metal layer onto a conductive substrate using an electrochemical process. This method is used to fabricate with high mechanical strength, often from materials such as nickel or gold [40]. The process starts with a patterned substrate, typically created through photolithography, followed by the electroplating of metal onto the pattern. Electroplating is particularly suitable for producing solid and coated MNDs with precise geometries.
Roll-to-roll processing
MNDs may be cheaply and mass-produced in enormous quantities using roll-to-roll processing, a high-throughput manufacturing method. Through the use of a succession of rollers, a flexible substrate is continually printed or cast with MNDs using this technique. For large-scale production, roll-to-roll technology is beneficial and can be used to fabricate coated and dissolvable MNDs [30].
Depending on the intended uses and characteristics of the MNDs, each of these fabrication techniques has a unique set of advantages. Various considerations, including the material to be utilized, the level of precision required, the manufacturing scale, and the particular drug delivery application, impact the choice of fabrication technique [31]. These techniques keep becoming better, which improves MNDs technology's accessibility and performance.
Applications in drug delivery
They have shown immense potential in various applications within drug delivery, leveraging their minimally invasive nature and ability to enhance drug absorption through the skin. Here are some key applications:
Vaccine delivery
MNDs have revolutionized vaccine delivery by enabling efficient and pain-free administration of vaccines. They enhance the immune response by targeting antigen-presenting cells in the skin's dermal layer [32, 33]. MNDs patches for vaccines such as influenza, measles, and COVID-19 have shown promising results in preclinical and clinical studies. These patches simplify logistics, improve patient compliance, and reduce the need for trained healthcare professionals.
Pain management
They offer a localized and controlled release of analgesics, providing effective pain relief with fewer systemic side effects. This is particularly beneficial for chronic pain conditions, where consistent delivery of medication is required. MNDs can deliver nonsteroidal anti-inflammatory drugs (NSAIDs), local anesthetics, and other pain-relief medications directly to the target site [34].
Hormone delivery
Hormone therapies, such as insulin for diabetes or hormones for endocrine disorders, benefit from microneedle technology. MNDs allow for painless and precise delivery of insulin, improving patient adherence to treatment regimens. They also facilitate the controlled release of other hormones, providing a steady therapeutic effect and reducing the frequency of administration [41].
Cosmetic applications
MNDs are increasingly used in cosmetic dermatology for delivering anti-aging agents, vitamins, and other skin treatments. They enhance the penetration of active ingredients, improving their efficacy [35]. MNDs patches can deliver hyaluronic acid, peptides, and other substances that promote skin rejuvenation, reduce wrinkles, and improve skin texture [58].
Transdermal drug delivery
MNDs enhance the transdermal delivery of a wide range of drugs that typically struggle to penetrate the skin. This includes small molecules, peptides, and nucleic acids. Drugs for conditions like hypertension, migraine, and motion sickness can be delivered more effectively through MNDs systems, providing a more consistent therapeutic effect and improving patient compliance [36].
Biologics and macromolecule delivery
Delivering large biologics, such as proteins and monoclonal antibodies, is challenging due to their size and sensitivity. MNDs enable the delivery of these macromolecules directly into the skin, preserving their activity and improving bioavailability. This application is particularly relevant for diseases like rheumatoid arthritis, psoriasis, and certain cancers [37].
Diagnostic applications
MNDs can be used for minimally invasive diagnostic sampling, such as extracting interstitial fluid for glucose monitoring in diabetic patients. This approach offers a pain-free alternative to traditional blood sampling methods and can be integrated into wearable devices for continuous monitoring of biomarkers [39, 55].
Advantages over traditional methods
Minimally invasive
Significantly reduces pain and discomfort compared to conventional needles.
Enhanced patient compliance
Easy self-administration and reduced fear of needles improve adherence to treatment [65].
Improved drug efficacy
Direct delivery to the dermal layer enhances drug absorption and bioavailability.
Reduced side effects
Targeted delivery allows for lower doses, minimizing systemic exposure and adverse effects [62].
Simplified logistics
MNDs patches do not require refrigeration and have a longer shelf life, making them ideal for use in low-resource settings [38, 42].
Challenges and future directions
Despite the promising potential of MNDs in revolutionizing transdermal drug delivery, several challenges need to be addressed to fully realize their capabilities. Here, we discuss key challenges and future directions for research and development in MNDs technology.
Consistency and quality control
Producing MNDs at scale with consistent quality and performance remains a significant challenge. Ensuring uniformity in size, shape, and drug loading across large batches is critical for clinical success [44].
Cost-effectiveness
Developing cost-effective manufacturing processes that can produce MNDs affordably for widespread use, especially in low-resource settings, is essential [43].
Regulatory approval
Safety and efficacy
Comprehensive preclinical and clinical trials are required to demonstrate the safety and efficacy of MNDs-based drug delivery systems. Regulatory agencies need detailed data to approve new MNDs products [42].
Standardization
Establishing industry standards and guidelines for the fabrication, testing, and use of MNDs will facilitate smoother regulatory approval processes [53, 58].
Patient acceptance and education
User training
Educating patients and healthcare providers on the correct use of these systems is vital to ensure proper administration and maximize therapeutic benefits.
Overcoming needle phobia
While these are less intimidating than conventional needles, some patients may still be apprehensive. Effective communication about the minimal pain and benefits of MNDs can help overcome this barrier [48, 59].
Long-term stability
Drug stability
Ensuring the stability of drugs within MNDs formulations during storage and transport is crucial. Factors such as temperature, humidity, and light exposure can affect the efficacy of the encapsulated drugs [46, 47].
MNDs integrity
The physical integrity of MNDs must be maintained over time to ensure they function as intended when applied [48, 52].
Skin reactions
Irritation and Infection: Repeated use of MNDs may cause skin irritation or increase the risk of infection if not properly sanitized. Developing biocompatible materials and ensuring proper hygiene can mitigate these risks [49, 51].
Future directions
Advanced materials
Smart polymers
Research into smart polymers that respond to environmental triggers (e.g. pH, temperature) can lead to MNDs capable of controlled and sustained drug release [61, 62].
Biodegradable and biocompatible materials
Continued development of new materials that are both biocompatible and biodegradable will enhance the safety and effectiveness of MNDs systems [50, 67].
Personalized medicine
Customized MNDs
Utilizing technologies such as 3D printing to create personalized MNDs tailored to individual patient needs, including specific drug dosages and delivery profiles [52, 67].
Point-of-care diagnostics
Integrating MNDs with biosensors for real-time monitoring and diagnosis, enabling personalized treatment regimens based on immediate health data [63, 64].
Integration with digital health
Wearable devices
Combining MNDs patches with wearable health monitoring devices can provide continuous drug delivery and real-time health data tracking, enhancing chronic disease management [56, 57].
Telemedicine
Enabling remote healthcare providers to monitor and adjust treatment protocols based on data from MNDs-based devices [69].
New therapeutic areas
Gene and cell therapy
Exploring the use of MNDs for the delivery of nucleic acids, gene-editing tools, and cellular therapies, potentially revolutionizing treatments for genetic disorders and cancers [68, 70].
Vaccines for emerging diseases
Rapid development and deployment of MNDs-based vaccines for emerging infectious diseases, enhancing global health responses [71, 72].
Long-term clinical studies
Efficacy and safety
Conducting long-term clinical studies to better understand the efficacy, safety, and patient adherence associated with MNDs-based treatments.
Comparative studies
Comparing MNDs systems with traditional delivery methods to quantify benefits and address any lingering concerns about their relative effectiveness [72].
CONCLUSION
MNDs represent a versatile and innovative platform for drug delivery, addressing many limitations associated with traditional administration routes. Their applications in vaccine delivery, pain management, hormone therapy, and more demonstrate their potential to transform healthcare delivery and improve patient outcomes. Addressing the current challenges through advanced research, innovative materials, and comprehensive clinical studies will pave the way for wider adoption of this technology. The future of MNDs lies in their ability to integrate with personalized and digital healthcare solutions, potentially revolutionizing the way we approach treatment and disease management.
ACKNOWLEDGEMENT
The authors would like to thank St. Pauls College of Pharmacy, Hyderabad, Telangana
FUNDING
Nil
AUTHORS CONTRIBUTIONS
Deepika B: Literature review, Data curation, Writing-original draft, review and editing; Naga raju Kandukoori: Literature review, Data curation, Writing-original draft; Venu Madhav: Literature review, Data curation, Writing-original draft; B. Jyothi: Literature review, Data curation, Writing-original draft.
CONFLICTS OF INTERESTS
The authors declare no conflict of interest
REFERENCES
Shahriari MH, Salmani H, Akrami M, Salehi Z. Development of a facile versatile and scalable fabrication approach of solid coated and dissolving microneedle devices for transdermal drug delivery applications. Giant. 2024;18:100284. doi: 10.1016/j.giant.2024.100284.
LV J, Zhao J, LI X, Ling G, Zhang P. Preparation of a novel hyaluronic acid based separable hydrogel microneedle with niacinamide to treat pigment deposition using solvent free solid state crosslinking method. Eur Polym J. 2024;210:113003. doi: 10.1016/j.eurpolymj.2024.113003.
Yang Y, Sheng C, Dong F, Liu S. An integrated wearable differential microneedle array for continuous glucose monitoring in interstitial fluids. Biosens Bioelectron. 2024;256:116280. doi: 10.1016/j.bios.2024.116280, PMID 38603840.
Zhang X, Chen H, Song T, Wang J, Zhao Y. Controllable histotomy based on hierarchical magnetic microneedle array robots. Engineering. 2024;42:166-74. doi: 10.1016/j.eng.2024.05.004.
Zong Q, Wang G, Zhao Z, LI W, Hou MY. Fabrication and characterization of dissolving microneedles for transdermal delivery of hypocrellin A. J Drug Deliv Sci Technol. 2024;95:105594. doi: 10.1016/j.jddst.2024.105594.
Raikar AS, Kalaskar DM, Bhilegaonkar S, Somnache SN, Bodaghi M. Revolutionizing drug delivery by bioinspired 4D transdermal microneedles: advances and future horizons. Eur Polym J. 2024 Apr 24;210:112952. doi: 10.1016/j.eurpolymj.2024.112952.
WU D, WU X, Luan Q, Tang Q, Fan L, Shou X. Dynamic hydrogel integrated microneedle patch with extracellular vesicles encapsulation for wound healing. Chem Eng J. 2024 Aug 1;493:152252. doi: 10.1016/j.cej.2024.152252.
Zhou X, Liu H, YU Z, YU H, Meng D, Zhu L. Direct 3D printing of triple responsive nanocomposite hydrogel microneedles for controllable drug delivery. J Colloid Interface Sci. 2024 Sep 15;670:1-11. doi: 10.1016/j.jcis.2024.05.045, PMID 38749378.
Shahriari MH, Salmani H, Akrami M, Salehi Z. Development of a facile versatile and scalable fabrication approach of solid coated and dissolving microneedle devices for transdermal drug delivery applications. Giant. 2024;18:100284. doi: 10.1016/j.giant.2024.100284.
Yang Y, Sheng C, Dong F, Liu S. An integrated wearable differential microneedle array for continuous glucose monitoring in interstitial fluids. Biosens Bioelectron. 2024;256:116280. doi: 10.1016/j.bios.2024.116280, PMID 38603840.
LV J, Zhao J, LI X, Ling G, Zhang P. Preparation of a novel hyaluronic acid based separable hydrogel microneedle with niacinamide to treat pigment deposition using solvent free solid state crosslinking method. Eur Polym J. 2024 Apr 24;210:113003. doi: 10.1016/j.eurpolymj.2024.113003.
WU D, WU X, Luan Q, Tang Q, Fan L, Shou X. Dynamic hydrogel integrated microneedle patch with extracellular vesicles encapsulation for wound healing. Chem Eng J. 2024 Aug 1;493:152252. doi: 10.1016/j.cej.2024.152252.
Zong Q, Wang G, Zhao Z, LI W, Hou MY. Fabrication and characterization of dissolving microneedles for transdermal delivery of hypocrellin a. J Drug Deliv Sci Technol. 2024 May;95:105594. doi: 10.1016/j.jddst.2024.105594.
Zhou X, Liu H, YU Z, YU H, Meng D, Zhu L. Direct 3D printing of triple-responsive nanocomposite hydrogel microneedles for controllable drug delivery. J Colloid Interface Sci. 2024;670:1-11. doi: 10.1016/j.jcis.2024.05.045, PMID 38749378.
Geng LU, B LI, L Lin, X LI, J Ban. Mechanical strength affecting the penetration in microneedles and PLGA nanoparticle assisted drug delivery importance of preparation and formulation. Biomed Pharmacother. 2024 Apr;173:116339. doi: 10.1016/j.biopha.2024.116339.
Dong CW, Lee CJ, Lee DH, Moon SH, Park WT. Fabrication of barbed microneedle array for bio signal measurement. Sens Actuators A. 2024;367:115040-367. doi: 10.1016/j.sna.2024.115040.
Liang C, Wang R, HE T, Chen D, Zhang G, Yin X. Revolutionizing diabetic wound healing: the power of microneedles. Chin J Plast Reconstr Surg. 2023;5(4):185-94. doi: 10.1016/j.cjprs.2023.12.004.
Biswas AA, Dhondale MR, Singh M, Agrawal AK, Muthudoss P, Mishra B. Development and comparison of machine learning models for in vitro drug permeation prediction from microneedle patch. Eur J Pharm Biopharm. 2024;199:114311. doi: 10.1016/j.ejpb.2024.114311, PMID 38710374.
Kolahi Azar H, Hajian Monfared M, Seraji AA, Nazarnezhad S, Nasiri E, Zeinanloo N. Integration of polysaccharide electrospun nanofibers with microneedle arrays promotes wound regeneration: a review. Int J Biol Macromol. 2024;258(1):128482. doi: 10.1016/j.ijbiomac.2023.128482, PMID 38042326.
Kang J, Kim KY, Kim S, Hong H, Bae BS, Kang SK. A conformable microneedle sensor with photopatternable skin adhesive and gel electrolyte for continuous glucose monitoring. Device. 2023;1(4). doi: 10.1016/j.device.2023.100112.
Ramadon D, Muliawardani F, Nisrina NA, Tri Hamda O, Iswandana R, Wahyuni T. Transdermal delivery of captopril using poly(vinyl pyrrolidone)/poly(vinyl alcohol) based dissolving and hydrogel-forming microneedles: a proof of concept. Eur Polym J. 2024 Mar 25;208:112860. doi: 10.1016/j.eurpolymj.2024.112860.
Ruan S, LI J, Ruan H, Xia Q, Hou X, Wang Z. Microneedle mediated nose to brain drug delivery for improved alzheimers disease treatment. J Control Release. 2024;366:712-31. doi: 10.1016/j.jconrel.2024.01.013, PMID 38219911.
Zhang R, Miao Q, Deng D, WU J, Miao Y, LI Y. Research progress of advanced microneedle drug delivery system and its application in biomedicine. Colloids Surf B Biointerfaces. 2023;226:113302. doi: 10.1016/j.colsurfb.2023.113302, PMID 37086686.
Zhang X, Luo Z, Zhao Y, M LU. Developing hierarchical microneedles for biomedical applications. Engineered Regeneration. 2023 Sep;3(4):316-27. doi: 10.1016/j.engreg.2023.04.004.
Ismail ES, A Mousavi Khaneghah. Advancing food quality assurance: integrating microneedle technology with advanced analytical methods. Nano Today. 2024 Feb;54:102115. doi: 10.1016/j.nantod.2023.102115.
Chen H, Zhou S, Chen J, Zhou J, Fan K, Pan Y. An integrated plant glucose monitoring system based on microneedle enabled electrochemical sensor. Biosens Bioelectron. 2024;248:115964. doi: 10.1016/j.bios.2023.115964, PMID 38160635.
Lammerding LC, Breitkreutz J. Technical evaluation of precisely manufacturing customized microneedle array patches via inkjet drug printing. Int J Pharm. 2023;642:123173. doi: 10.1016/j.ijpharm.2023.123173, PMID 37369288.
Kshirsagar S, Dandekar A, Srivastava RK, Khan J, Muzaffar S, Athar M. Microneedle mediated transdermal delivery of N-acetyl cysteine as a potential antidote for lewisite injury. Int J Pharm. 2023;647:123547. doi: 10.1016/j.ijpharm.2023.123547, PMID 37884214.
Zhang X, Chen H, Song T, Wang J, Zhao Y. Controllable histotomy based on hierarchical magnetic microneedle array robots. Engineering. 2024;42:166-74. doi: 10.1016/j.eng.2024.05.004.
Chen L, Cao P, Zhao P, XU Y, LV G, YU D. Photodynamic controllable microneedle composite with antibacterial antioxidant and angiogenic effects to expedite infected diabetic wound healing. Mater Des. 2024 May;241:112971. doi: 10.1016/j.matdes.2024.112971.
HE M, Jin L, Wang F, Wang X, You Y, HE H. Simple ultrasensitive detection of superoxide anion radical mutations in melanoma mice with SERS microneedles. Spectrochim Acta A Mol Biomol Spectrosc. 2024;316:124292. doi: 10.1016/j.saa.2024.124292, PMID 38669980.
Che QT, Seo JW, Charoensri K, Nguyen MH, Park HJ, Bae H. 4D-printed microneedles from dual sensitive chitosan for non-transdermal drug delivery. Int J Biol Macromol. 2024;261(2):129638. doi: 10.1016/j.ijbiomac.2024.129638, PMID 38266841.
Mulkutkar M, Damani M, Sawarkar S. Polymeric microneedles for the eye: an overview of advances and ocular applications for minimally invasive drug delivery. Eur J Pharm Biopharm. 2024;197:114209. doi: 10.1016/j.ejpb.2024.114209, PMID 38336234.
Sil D, Bhowmik S, Patel P, Balak Das Kurmi. Promising role of microneedles in therapeutic and biomedical applications. J Drug Deliv Sci Technol. 2024 Jan;91:105273. doi: 10.1016/j.jddst.2023.105273.
Babu MR, Vishwas S, Gulati M, Dua K, Singh SK. Harnessing the role of microneedles as sensors: current status and future perspectives. Drug Discov Today. 2024 Jul;29(7):104030. doi: 10.1016/j.drudis.2024.104030.
Chen X, Wang L, YU H, LI C, Feng J, Haq F. Preparation properties and challenges of the microneedles-based insulin delivery system. J Control Release. 2018 Oct 28;288:173-88. doi: 10.1016/j.jconrel.2018.08.042, PMID 30189223.
Chen Z, Ren L, LI J, Yao L, Chen Y, Liu B. Rapid fabrication of microneedles using magnetorheological drawing lithography. Acta Biomater. 2018;283;91-65. doi: 10.1016/j.actbio.2017.10.030.
Maurya A, Nanjappa SH, Honnavar S, Salwa M, Murthy SN. Rapidly dissolving microneedle patches for transdermal iron replenishment therapy. J Pharm Sci. 2018;107(6):1642-7. doi: 10.1016/j.xphs.2018.02.011, PMID 29462631.
Lim DJ, Vines JB, Park H, Lee SH. Microneedles: a versatile strategy for transdermal delivery of biological molecules. Int J Biol Macromol. 2018 Apr 15;110:30-8. doi: 10.1016/j.ijbiomac.2017.12.027, PMID 29223756.
Pere CP, Economidou SN, Lall G, Ziraud C, Boateng JS, Alexander BD. 3D printed microneedles for insulin skin delivery. Int J Pharm. 2018;544(2):425-32. doi: 10.1016/j.ijpharm.2018.03.031, PMID 29555437.
Ronnander P, Simon L, Spilgies H, Koch A. Modelling the in vitro dissolution and release of sumatriptan succinate from polyvinylpyrrolidone based microneedles. Eur J Pharm Sci. 2018;125:54-63. doi: 10.1016/j.ejps.2018.09.010, PMID 30223035.
Ilic T, Savic S, Batinic B, Markovic B, Schmidberger M, Lunter D. Combined use of biocompatible nanoemulsions and solid microneedles to improve transport of a model NSAID across the skin: in vitro and in vivo studies. Eur J Pharm Sci. 2018;125:110-9. doi: 10.1016/j.ejps.2018.09.023, PMID 30287408.
Caudill CL, Perry JL, Tian S, Luft JC, DeSimone JM. Spatially controlled coating of continuous liquid interface production microneedles for transdermal protein delivery. J Control Release. 2018 Aug 28;284:122-32. doi: 10.1016/j.jconrel.2018.05.042, PMID 29894710.
Rzhevskiy AS, Singh TR, Donnelly RF, Anissimov YG. Microneedles as the technique of drug delivery enhancement in diverse organs and tissues. J Control Release. 2018 Jan 28;270:184-202. doi: 10.1016/j.jconrel.2017.11.048, PMID 29203415.
Yan Q, Cheng Z, Liu H, Shan W, Cheng Z, Dai X. Enhancement of Ag85B DNA vaccine immunogenicity against tuberculosis by dissolving microneedles in mice. Vaccine. 2018;36(30):4471-6. doi: 10.1016/j.vaccine.2018.06.025, PMID 29910005.
Ita K. Modulation of transdermal drug delivery with coated microneedles. J Drug Deliv Sci Technol. 2018 Jun;45:203-12. doi: 10.1016/j.jddst.2018.03.021.
Tomono T. A new way to control the internal structure of microneedles: a case of chitosan lactate. Mater Today Chem. 2019;13:79-87. doi: 10.1016/j.mtchem.2019.04.009.
Gholami S, Mohebi MM, Hajizadeh Saffar E, Ghanian MH, Zarkesh I, Baharvand H. Fabrication of microporous inorganic microneedles by centrifugal casting method for transdermal extraction and delivery. Int J Pharm. 2019;558:299-310. doi: 10.1016/j.ijpharm.2018.12.089, PMID 30654056.
Ahmed Saeed AL Japairai K, Mahmood S, Hamed Almurisi S, Reddy Venugopal J, Rebhi Hilles A, Azmana M. Current trends in polymer microneedle for transdermal drug delivery. International Journal of Pharmaceutics. 2020 Sep 25;587:119673. doi: 10.1016/j.ijpharm.2020.119673.
WU M, Zhang Y, Huang H, LI J, Liu H, Guo Z. Assisted 3D printing of microneedle patches for minimally invasive glucose control in diabetes. Mater Sci Eng C Mater Biol Appl. 2020;117:111299. doi: 10.1016/j.msec.2020.111299, PMID 32919660.
Koyani RD. Synthetic polymers for microneedle synthesis: from then to now. J Drug Deliv Sci Technol. 2020 Dec;60:102071. doi: 10.1016/j.jddst.2020.102071.
Azmana M, Mahmood S, Hilles AR, Mandal UK, Saeed Al Japairai KA, Raman S. Transdermal drug delivery system through polymeric microneedle: a recent update. J Drug Deliv Sci Technol. 2020 Dec;60:101877. doi: 10.1016/j.jddst.2020.101877.
Chen L, Ding X, Dong Y, Chen H, Gao F, Cui B. Integration of catalytic hairpin assembly probes into microneedles for detection of microRNA in plants. Sens Actuators B. 2024 Apr 1;404:135277. doi: 10.1016/j.snb.2024.135277.
Khan S, Hasan A, Attar F, Babadaei MM, Zeinabad HA, Salehi M. Diagnostic and drug release systems based on microneedle arrays in breast cancer therapy. J Control Release. 2021 Oct 10;338:341-57. doi: 10.1016/j.jconrel.2021.08.036, PMID 34428480.
Shu W, Heimark H, Bertollo N, Tobin DJ, O Cearbhaill ED, Annaidh AN. Insights into the mechanics of solid conical microneedle array insertion into skin using the finite element method. Acta Biomater. 2021;135:403-13. doi: 10.1016/j.actbio.2021.08.045, PMID 34492370.
Zhang L, Guo R, Wang S, Yang X, Ling G, Zhang P. Fabrication evaluation and applications of dissolving microneedles. International Journal of Pharmaceutics. 2021 Jul 15;604:120749. doi: 10.1016/j.ijpharm.2021.120749.
Radhika C, Gnanavel BK. Buckling analysis of polymer microneedle for transdermal drug delivery. Materials Today: Proceedings. 2021;46:3538-41. doi: 10.1016/j.matpr.2020.12.397.
Sawon MA, Samad MF. Design and optimization of a microneedle with skin insertion analysis for transdermal drug delivery applications. J Drug Deliv Sci Technol. 2021 Jun;63:102477. doi: 10.1016/j.jddst.2021.102477.
DU G, HE P, Zhao J, HE C, Jiang M, Zhang Z. Polymeric microneedle mediated transdermal delivery of melittin for rheumatoid arthritis treatment. J Control Release. 2021;336:537-48. doi: 10.1016/j.jconrel.2021.07.005, PMID 34237400.
Ingrole RS, Azizoglu E, Dul M, Birchall JC, Gill HS, Prausnitz MR. Trends of microneedle technology in the scientific literature patents clinical trials and internet activity. Biomaterials. 2021;267:120491. doi: 10.1016/j.biomaterials.2020.120491, PMID 33217629.
Singh V, Kesharwani P. Recent advances in microneedles based drug delivery device in the diagnosis and treatment of cancer. J Control Release. 2021 Oct 10;338:394-409. doi: 10.1016/j.jconrel.2021.08.054, PMID 34481019.
Glover K, Mishra D, Gade S, Vora LK, WU Y, Paredes AJ. Microneedles for advanced ocular drug delivery. Adv Drug Deliv Rev. 2023 Oct;201:115082. doi: 10.1016/j.addr.2023.115082, PMID 37678648.
Jiang X, Chen P, Niu W, Fang R, Chen H, An Y. Preparation and evaluation of dissolving tofacitinib microneedles for effective management of rheumatoid arthritis. Eur J Pharm Sci. 2023;188:106518. doi: 10.1016/j.ejps.2023.106518, PMID 37419290.
Newell B, Zhan W. Mathematical modelling of microneedle mediated transdermal delivery of drug nanocarriers into skin tissue and circulatory system. J Control Release. 2023 Aug;360:447-67. doi: 10.1016/j.jconrel.2023.07.011, PMID 37429359.
Lin Z, Zheng K, Zhong J, Zheng X. Advances in microneedle based therapy for bone disorders. Biomed Pharmacother. 2023;165:115013. doi: 10.1016/j.biopha.2023.115013, PMID 37531783.
Jose J, Khot KB, Shastry P, Thomas SP, Chopra H, Gopan G. Advances in microneedles based drug delivery system on promoting wound healing. J Drug Deliv Sci Technol. 2023;90:105163. doi: 10.1016/j.jddst.2023.105163.
Ece E, ES I, Inci F. Microneedle technology as a new standpoint in agriculture: treatment and sensing. Materials Today. 2023;68:275-97. doi: 10.1016/j.mattod.2023.07.002.
LI R, Zhang L, Jiang X, LI L, WU S, Yuan X. 3D-printed microneedle arrays for drug delivery. J Control Release. 2022;350:933-48. doi: 10.1016/j.jconrel.2022.08.022, PMID 35977583.
Xiu X, Gao G, Liu Y, MA F. Drug delivery with dissolving microneedles: skin puncture its influencing factors and improvement strategies. J Drug Deliv Sci Technol. 2022;76:103653. doi: 10.1016/j.jddst.2022.103653.
Choi JE, Cha HR, Kim S, Kim JS, Kim MJ, Chung HW. Preparation of particle-attached microneedles using a dry coating process. J Control Release. 2022 Nov;351:1003-16. doi: 10.1016/j.jconrel.2022.10.003, PMID 36216176.
Maru AD, Lahoti SR. Formulation and evaluation of moisturizing cream containing sunflower wax. Int J Pharm Pharm Sci. 2018;10(11):54-9. doi: 10.22159/ijpps.2018v10i11.28645.
Gupta A, Barman S, Roy D. Prescription pattern of antihypertensive medications in patients with hypertension at a Tertiary Care Hospital Assam. Asian J Pharm Clin Res. 2024 Oct;17(10):85-9. doi: 10.22159/ajpcr.2024v17i10.52069.
Bhagyashree N, Veerapaneni K. Correlation between psychological parameters such as depression anxiety and stress and affect score among medical students. Asian J Pharm Clin Res. 2024;17(10)93-4. doi: 10.22159/ajpcr.2024v17i10.52032.