
Int J App Pharm, Vol 17, Issue 2, 2025, 78-89Review Article
NANOTECHNOLOGY-DRIVEN THERAPEUTICS: ENHANCING BRAIN DRUG DELIVERY VIA NASAL PATHWAYS
PRABHAT KUMAR1, SHALU VERMA1*, ALKA SINGH2, TARUN PARASHAR3
1Department of Pharmaceutics, Uttaranchal Institute of Pharmaceutical Sciences, Uttaranchal University, Premnagar, Dehradun-248007, India. 2School of Pharmaceutical Sciences, Sardar Bhagwan Singh University, Balawala, Dehradun, Uttarakhand-248007, India. 3School of Pharmacy and Research, Dev Bhoomi Uttarakhand University, Dehradun, Uttarakhand-248007, India
*Corresponding author: Shalu Verma; *Email: vermashalu339@gmail.com
Received: 07 Oct 2024, Revised and Accepted: 06 Feb 2025
ABSTRACT
The use of nanotechnology in drug delivery and targeting has proven to be extremely valuable. The Nose-to-brain route of drug administration acts as a more encouraging alternative to the traditional routes of medications acting on the Central Nervous System (CNS). This approach overcomes the disadvantages of Blood Brain Barrier (BBB), hepatic first-pass metabolism, and systemic circulation. Thus, it is highly suitable for neurodegenerative diseases, brain tumors, and neurological disorders like Alzheimer’s disease, Parkinson's disease, epilepsy, and brain cancer. Anatomy and physiology of the nasal cavity, mechanisms of drug transport to the brain, and different nano-formulations that may enhance the delivery and efficacy of CNS targeted drugs are reviewed here. Nanotechnology has brought new drug delivery systems like nanoparticles, niosomes, liposomes, dendrimers, in-situ gels, nanoemulsions, and nanostructured lipid carriers capable of successfully delivering drugs across the olfactory and trigeminal nerve pathways. It also discusses challenges pertinent to drug delivery across the BBB and the therapeutic application of nose-to-brain delivery, the article also highlighted the nanoformulation development and the ongoing clinical trials along with the marketed formulations related to nose-to-brain delivery.
Keywords: Nasal delivery, Brain, Nanotechnology, Targeted, Challenges
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijap.2025v17i2.52879 Journal homepage: https://innovareacademics.in/journals/index.php/ijap
INTRODUCTION
The two most frequently used methods to administer drugs are orally and intravenously. However, these methods present significant limitations, especially for brain disorders, due to barriers such as the BBB and hepatic first-pass metabolism. Therefore, there is a need to explore alternative drug delivery systems for enhanced therapeutic effects and reduced side effects [1].
This review focuses on nanotechnology-driven formulations for nose-to-brain drug delivery, emphasizing intranasal pathways to bypass the BBB. A systematic literature search was conducted using keywords such as "nanotechnology," "nose-to-brain delivery," "intranasal administration," "CNS disorders," and "nanoformulations." Articles from peer-reviewed journals spanning the past 25 y were selected to cover recent advances and foundational studies. Additional filters included English-language publications and studies focusing on nanoparticle-based drug delivery systems, covering mechanisms, therapeutic applications, and clinical advancements in nose-to-brain formulations.
One such promising route is the intranasal administration of drugs, which provides a direct nose-to-brain pathway through olfactory and trigeminal nerves and reduces exposure to systemic circulation [2]. Knowledge of the anatomy and physiology of the nasal cavity is important for the achievement of fantastic therapeutic efficacy with drug delivery systems. The nasal cavity is divided into three regions: the vestibule, respiratory, and olfactory. Its surface area for the vestibule region is small, and thus, drug absorption in this region is limited [3].
The brain, which receives messages from sense organs and controls most bodily activities, is one of the most intricate and important organs. It regulates the operations of numerous other organs as well as hormone secretion, memory encoding, and both voluntary and involuntary motions [4].
The brain is crucial to the human body and are protected both internally and externally. The skull and its various membrane layers shield it from external harm, while the BBB, the Cerebro Spinal Fluid (CSF), and the CSF-blood barrier provide internal protection. These barriers assist in preserving the brain's equilibrium and protect against endotoxins, harm to the body, infections, and other negative consequences [5].
The nose-to-brain pathways include the respiratory and olfactory regions of the nasal cavity as shown in fig. 1. The septum divides the nasal cavity into two 12 cm long halves containing 13 ml in volume and 150 cm2 in surface area. Each of these two halves contains three sections as follows: the respiratory section, the olfactory section, and finally, the physiological site, the vestibular section, which acts as a physiological site for intranasal transport to the brain. In the respiratory region, goblet cells are mucous-secreting and extremely vital for mucociliary clearance, which aids in the removal of foreign substances. The presence of the trigeminal nerves in the respiratory region can provide a rapid pathway for drug delivery to the brain [6].
The olfactory area also plays an important role in transporting drugs to the brain and CSF. This region is placed in the upper nasal cavity, which may limit the drug’s access to this permeation area [7]. The most important organ in humans for controlling their body functions is the brain [8]. The human brain contains 644 km of blood vessels that supply oxygen, energy, metabolites, and nutrients to brain cells and also remove carbon dioxide and other metabolic wastes from the circulatory system [9]. The endothelial surface area of the brain vasculature, which includes arteries, veins, arterioles, capillaries, and venules, is around 20 sq. meters [10]. The brain is extremely sensitive to toxic substances in circulation and requires an optimum microenvironment that is controlled by three barrier systems to ensure correct neuronal activity. The third barrier is the BBB which is fabricated by the brain micro vessel endothelial cells [11]; the arachnoid barrier is the barrier that borders the CSF in the subarachnoid space from extracellular fluids in the blood vessels of the superficial dura layers, which represents fenestrated capillaries [12], the blood-CSF barrier is at the choroid plexus, which is of spongy, vascularized structure that manufactures CSF and is attached to the wall inside the ventricles of the brain [13].
Some physicochemical parameters can affect the delivery of the nose to the brain. One of the most important aspects of the nose-to-brain delivery system is particle size. The Olfactory Sensory Neuron's diameter ranges from 0.1 to 0.7 µm, restricting the size of the particles to the nanoscale [14]. Additionally, because mucus creates a structure resembling a mesh, smaller particles are less resistant to penetrating the mucous membrane. Since the membranes lining the nasal mucosa are generally negatively charged, positively charged particles are more likely to interact electrostatically with the mucosa. Increased residence time and bio-adhesion to the nasal epithelium will result from this [15]. Because of this property, many research studies have employed positively charged carriers to boost drug bioavailability for nose-to-brain delivery, specifically chitosan and its derivatives. Since hydrophobic carriers form hydrophobic bonds with the hydrophobic domains of mucin, they lengthen the residence period, which increases mucoadhesion. However, because of its hydrophobic interaction with mucin, if the carrier is sufficiently hydrophobic, it will not pass through the mucus and be removed by mucociliary clearance [16]. Thus, striking a delicate balance between lengthening the residency period and allowing mucus penetration would be crucial. Similar to how the charge of the nanoparticle influences mucoadhesion, hydrophobicity may likewise influence the route of nose-to-brain delivery and distribution in the brain.
Intranasal administration represents an easy, non-invasive, and vascular route allowing brain-targeted drug delivery. Intranasal delivery is a convenient and non-invasive way to directly administer drugs to the brain [17]. This delivery mechanism overcomes the BBB, blood-CSF barrier, and hepatic first-pass metabolism, eliminating oral administration of medications [18]. Intranasal delivery systems deliver drugs through the nasal cavity either to general circulation i. e., and systemic effect or to specific targets where other routes cannot do, for example-the brain [19]. Various methods have been explored to bypass the BBB and distribution of drugs to the CNS. Novel approaches to medication delivery to the brain have been made possible by advancements in nanotechnology and nanoscience. Conventional oral delivery for Alzheimer’s disease has some drawbacks, such as low bioavailability, rapid metabolism, limited brain exposure, and even some adverse effects, making it difficult to identify a highly effective therapy [20]. Such as a recently prepared transdermal patch of rivastigmine has improved tolerability, but brain penetration remains limited. Therefore, alternative brain-targeted rivastigmine delivery strategies are urgently needed [21]. Some novel formulations can overcome these problems with the help of nanotechnology. Several nanoformulations are used like in-situ gel, liposomes, nanoparticles, nanoemulsion, Nanostructured Lipid Carrier (NLC), micro-emulsion, niosomal in situ gel, microsphere, niosomes, Solid Lipid Nanoparticles, nasal insert, etc. and we can target this formulation through nose-to-brain delivery. This study aims to provide insights into the function of nanotechnology of nose-to-brain drug delivery. The review mainly focuses on the research work done using various nanoformulations for nose-to-brain drug delivery. In this article, we will discuss the therapeutic application of nose-to-brain delivery, ongoing clinical trials, and marketed products, along with the challenges and future aspects of nanotechnology.
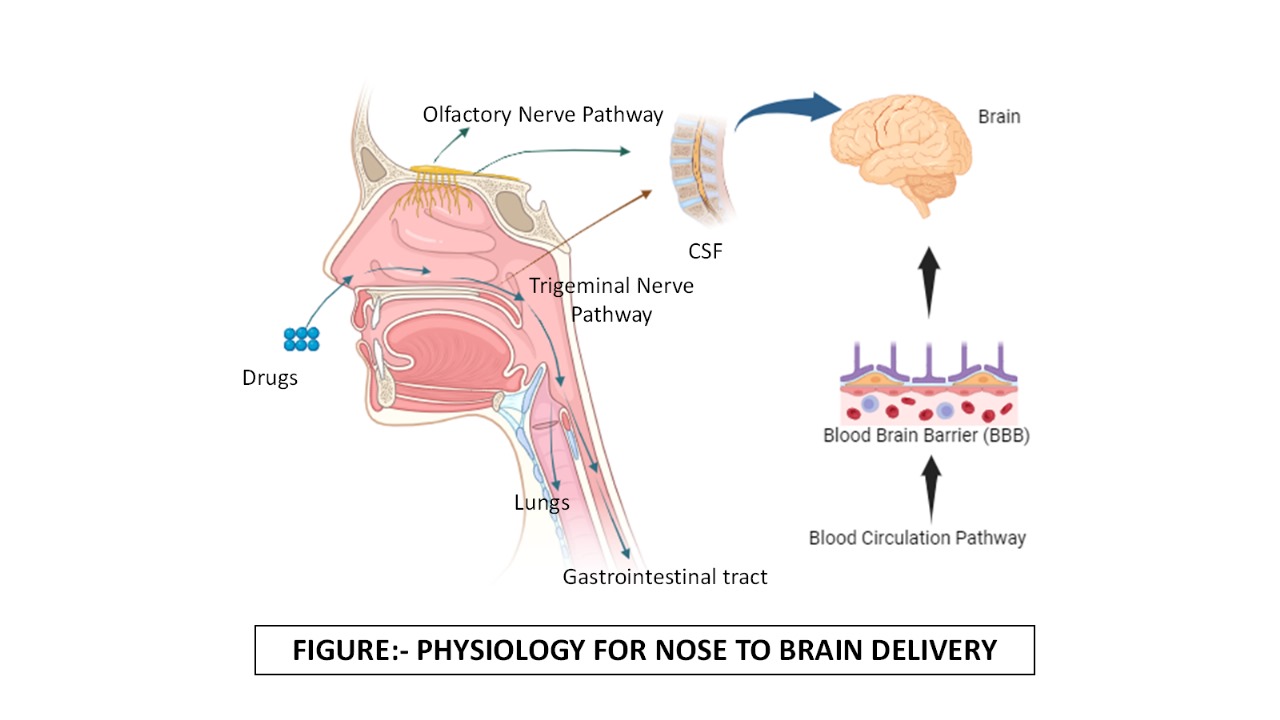
Fig. 1: Physiology for nose to brain delivery
Therapeutic application of nose-to-brain delivery
Alzheimer’s disease
Alzheimer's disease (AD’s) is a chronic and progressive neurodegenerative brain disorder, recognized as the most prevalent cause of dementia globally [22]. Approximately 30 million people around the world suffer from AD’s, and these fig. are expected to triple by 2050[23]. This neurodegeneration results in a constant decline in cognitive, functional, and behavioral processes, causing characteristic disorders like loss of memory, confusion, agitation, and difficulties in executing daily activities [24].
The pathological manifestations of AD’s include the presence of intracellular Neurofibrillary Tangles and extracellular amyloid plaques (amyloid-beta deposits). These are accompanied by neuronal cell loss, which is related to memory impairment [25]. It is a type of multifactorial disorder and its causes are still unclear, but having in mind the important role they play in the cholinergic system changes early neuro as well amyloid plaque production line tangles neurofibrillary [26].
The brain contains billions of neurons, each with an axon and some dendrites. Neurons need communication, metabolism, and self-repair to stay healthy. Β-amyloid plaques and neurofibrillary tangles characterize all three important functions of AD’s. First, neuritic plaques are caused by deposits of the β-amyloid protein fragment building up in the space between nerve cells or neurons. The Amyloid Precursor Protein is the building block of amyloid plaque, which protrudes through the neuron membrane. Enzymes such as βsecretase and α-secretase break down Amyloid Precursor Protein into neurotoxic Aβ42 fragments, which include β-amyloid. These β-amyloid fragments aggregate to form plaques. Neurons from these aggregates grow in AD’s, interfering with neuron function [27].
A variety of nano-formulations have been developed to treat AD’s, in a recent study by Wilson et al., prepared nanoparticles of Sitagliptin using chitosan as a polymer. The particle mean size and zeta potential of the formulation are 188.4±48.1 nm and 20.8 mV, respectively. In the in vitro studies in pH 6.4 phosphate buffer, the range was between 49.55±2.62% w/w and 73.77±2.14% w/w for 24 h. In studies conducted on rats, it was found that sitagliptin-loaded chitosan nanoparticles increased sitagliptin levels in the brain by 5 times more effectively compared to free sitagliptin after intranasal administration [28]. Additionally, in a study by Shehata et al., nanostructured lipid carriers of Astaxanthin (AST) were formulated using a high-pressure homogenization process. The developed Astaxanthin loaded nanostructured lipid carriers (AST-NLCs) have a mean particle size of 141.8±5.02 nm, a polydispersity index (PDI) of 0.247±0.016, a zeta potential of-32.2±7.88 mV, an entrapment efficacy of 94.1±2.46%, and a drug loading of 23.5±1.48%. The nose-to-brain delivery of Astaxanthin loaded nanostructured lipid carriers (AST-NLCs) for AD’s was performed by a rats’ model, which shows anti-cholinesterase, antioxidant, anti-apoptotic, and anti-neuroinflammatory effects through targeting different pathways to treat AD’s. They summarize that the intranasal distribution of Astaxanthin-loaded nanostructured lipid carriers AST-NLCs may be an alternative treatment for AD’s, overcoming its low oral bioavailability [29]. One more study by Kaur et al. developed a nano-emulsion of Memantine for Intranasal delivery to bypass the BBB for the management of AD’s. The developed nano-emulsion has a particle size of 11 nm and a transmittance of 99%. In vitro release studies demonstrated 80% drug release in simulated nasal fluid. In pharmacokinetic studies using a rat model, administration of the radiolabelled formulation through the intra-nasal route showed higher drug absorption in the brain region compared to oral and IV administration [30].
In further studies by, Espinoza et al., developed a nasal microemulsion of Donepezil (DPZ) for intranasal administration. In this respect, the developed microemulsion in these studies had a transparent and homogenous particle size of 58.9±3.2 nm, while the PDI was 0.19±0.04. From morphological studies, it resulted in a spherical shape of droplets with smooth and uniform surface features. The hyperbola kinetic model is used in the in vitro release investigation of medication DPZ microemulsion, which ensures that the drug release studies have a prolonged time, However, the ex-vivo permeation investigations showed that the largest quantity that permeated was around 2000 μg or 80% of the initial medication and that the peak permeation occurred in the first 4 h. They finally conclude that microemulsion can be a novel tool for further research in the field of AD’s [31]. The various nano-formulations for treating Alzheimer's have been discussed in table 1.
Table 1: Nano-formulation for Alzheimer's disease
| Drugs | Formulation type | Methods | Polymer | Key finding | Reference |
| Sitagliptin | Chitosan Nanoparticles |
Ionic gelation method | Chitosan Tripolyphosphate |
Chitosan nanoparticles boosted brain Sitagliptin levels after intranasal administration, but more studies are needed to confirm its effectiveness as a drug delivery system. | [28] |
| Astaxanthin | NLC | The hot high-pressure homogenization method | Poloxamer 188 Glyceryl palmitostearate |
The results we obtained indicate that intranasal administration of Astaxanthin in NLC could serve as an appropriate treatment for AD’s and address its low oral bioavailability. | [32] |
| DPZ | Nano-emulsion | DPZ-loaded nanoemulsion was prepared by incorporating DPZ in oil under stirring at 700 rpm until a drug was dissolved | Polyglyceryl-3 dioleate and oleoyl polyoxyl-6 glycerides. |
The nanotechnology-based drug delivery of DPZ enhanced the pharmacological efficacy. | [33] |
| Memantine | Nano-emulsion | Homogenization and Ultrasonication Method | Propylene glycol. Ethylene glycol |
A nano-emulsion of the drug was prepared based on solubility and transmittance. The formulation's particle size was in the nano range, and it demonstrated cell viability in neuroblastoma cells. | [30] |
| DPZ | DPZ loaded Micro-Emulsion | Developed by Pseudo ternary phase diagrams | Propylene glycol | It minimizes nasal irritation and provides effective delivery while maintaining a low viscosity. | [31] |
| DPZ | Liposomes | Thin layer hydration technique | Polyethylene glycol | The administration of liposomal formulation via the intranasal route significantly increased (P<0.05) the bioavailability of DPZ in plasma and brain. | [34] |
| Intranasal H102 peptide-loaded liposomes | Liposomes | Modified thin film hydration method | Chitosan | The liposomes penetrated Calu-3 cell monolayers, and intranasal administration effectively delivered H102 to the brain, with hippocampal AUC of H102 liposomes about 2.92 times greater than the solution group. | [35] |
| Rivastigmine | Nasal Liposome and Nanoparticle-based Rivastigmine | Lipid layer hydration method for liposome. Nano-precipitation method for Nanoparticle. |
Scopolamine hydrobromide Colchicine Soya lecithin cholesterol |
The results showed that it gave nasally depicted good pharmacokinetic properties, including rapid absorption and increased systemic bioavailability. | [36] |
| Noval Luteolin-loaded Chitosan decorated Nanoparticles | Nanoparticles | Ethanol injection method | Chitosan Cholesterol Phosphatidylserine Streptozotocin |
Chitosomes enable non-invasive intra-nasal delivery with lower doses and increased efficacy compared to the suspension form. | [37] |
| Rivastigmine loaded self-assembled nanostructure | Niosomal in situ gel | Thin film hydration technique | Carbopol-934P HPMC-K4M Chloroform |
The formulation showed enhanced perfusion and is expected to improve bioavailability over conventional drug delivery systems. | [38] |
| Rivastigmine | Microsphere | Lectin was chemically functionalized on microspheres using a two-step carbodiimide activation reaction method. | Chitosan Ethyl-cellulose Polyvinyl alcohol |
The result shows improved memory retention in rats compared to the pure drug solution (p<0.001). | [39] |
| Lacosamide | Niosomes | Thin-film hydration method | Chitosan Cholesterol |
The formulation offers sustained release, better nasal diffusion, and improved stability. | [40] |
| Barberine-laden nanostructured lipid carriers overloaded with Chitosan (BER-CTS-NLCs) | NLC | Hot homogenization and ultrasonication method | Poloxamer 407 Glycerol monostearate Chitosan Soybean lecithin Oleic acid |
The histopathological investigation revealed that BER-CTS-NLCs are acceptable for nasal administration, with higher brain/blood ratios, drug transport, and targeting efficiency than the BER solution. | [41] |
| DPZ | Solid lipid Nano-particles | Solvent emulsification-diffusion technique | Poloxamer 188 Glyceryl mono-stearate o-phosphoric acid Stearic acid Palmitic acid |
The DP-SLN formulation provided sustained release, with imaging confirming drug localization in the rabbit's brain. | [42] |
Parkinson’s disease
The second most common and significant neurodegenerative disease after AD is Parkinson's Disease (PD), affecting millions of people across the globe [43]. Association with PD is due to the loss of dopaminergic neurons within the Substantial nigra pas compacta. The association of the loss with PD is from the deficiency in the dopamine level, as it is a neurodegenerative disorder [44]. This is because dopamine, with less penetration to enter the BBB of the brain, cannot enter into this. Hence, the treatment of PD with the administration of external dopamine is limited [45]. There are various drugs used to treat PD, such as the administration of L-DOPA, which are the direct precursors of dopamine [46].
Various nano-formulations have been developed to treat the PD. A recent study by Saha et al. formulated a poloxamer-stabilized nanosuspension of Rotigotine (RTZ) for intranasal administration. The formulated product demonstrated an average particle size of 73 nanometers and a PDI of 0.286. The lyophilization characteristic of RTZ-nanosuspension showed good stability and was amorphous. In vitro studies confirm that the first 15 min. showed 95% of the drug dissolved from the prepared formulation. They concluded that the amorphous nature of RTZ rapidly enhances the drug's ex-vivo nasal permeation and in vitro nasal dissolution. Therefore, RTZ-nanosuspension can improve RTZ distribution to the brain and could potentially have therapeutic applications [47]. Further studies by Avadh et al., developed a polyelectrolyte complex of levodopa for nasal drug administration. The particle size of the formulation is pH-dependent. In the beginning (pH up to 10), increasing the pH reduces the particle size; after that particle size increases. The drug content was 65.12 mg, and entrapment efficacy was 81.93% to 89.67. They conclude that the nose-to-brain delivery of levodopa medication via the trigeminal neurons and the olfactory route has been studied to enhance brain absorption and prevent levodopa degradation in the peripheral circulation [48].
One more study by Sridhar et al. formulated a nanoparticle of selegiline with enhanced brain delivery in PD. The different formulations were prepared to form nanoparticles due to which the particle size of the nanoparticles ranged from 341-502 nm and the lowest particle size 341.6±56.91 was detected in one batch. The PDI of this batch is 0.317±0.29, which is the lowest of all the batches and the maximum drug entrapment efficacy is 92.20±7.15%. The formulated mixture resulted in spherical nanoparticles with over 90% of the drug loaded. The ex-vivo studies were conducted in rats following intranasal and oral administration. Compared to oral dosing, intranasal therapy resulted in 20-and 12-fold greater amounts of selegiline in the brain and plasma [49]. The various nanoformulations have been discussed for the treatment of Parkinson’s in table 2.
Table 2: Nano-formulation for Parkinson's disease
| Drugs | Formulation type | Method | Polymer | Key finding | Reference |
| Levodopa and Carbidopa | Chitosan-based Poly-electrolyte complex | Ionic gelation method | Chitosan Pectin |
Researchers have investigated delivering levodopa via the olfactory pathway and trigeminal neurons to improve brain uptake and avoid peripheral degradation and carbidopa use. | [48] |
| RTZ | Redispersible Nano-Suspension | Antisolvent precipitation Ultrasonication method | Poloxamer 407 Poloxamer 188 Soluplus |
The nasal ciliotoxicity investigation demonstrated that both RTZ-nanosuspension and drug dispersion were safe for intravenous delivery, whereas RTG-Nanosuspension demonstrated a 20-fold increase in nasal penetration. | [47] |
| Levodopa | Polymeric Nanoparticle | Solvent evaporation technique Ionic gelation method |
Chitosan Sodium tri-polyphosphate Poly (lactic-co-glycolic acid) [PLGA] |
Characterization of the polymeric nanoparticles included particle size distribution, drug content, zeta potential, and morphology. The levodopa-loaded nanoparticles showed an AUC nearly twice as high (p<0.05) as the levodopa solution. | [50] |
| Levodopa | Liposomes | Thin film hydration methodology | Quercetin dihydrate Ascorbic acid Sodium acetate |
This study evaluated the structure-activity relationship of several levodopa-loaded liposomes and preliminarily assessed their potential for intranasal drug delivery. | [51] |
| Selegiline | Nanoparticles | Ionic gelation method | Chitosan Sodium tri-polyphosphate |
Selegiline administered intranasally enhances the amount of medication that reaches the brain by lowering pre-systemic metabolism. It has a higher therapeutic value than oral delivery. | [49] |
| Levodopa | In-situ thermo-sensitive gel | Gelation temperature method | Poloxamer-407 Chitosan Alginate |
The results suggested that in situ gel-forming formulation could be of value for longer retaining of the drug in the nasal cavity and increasing the chance for delivery to the brain. | [52] |
Epilepsy
According to the World Health Organization, epilepsy ranks as the 4th most frequent neurological disorder. It affects more than 50 million individuals globally, primarily in developing nations accounting for over 90% of all cases. Every year, around 5 million people worldwide are diagnosed with epilepsy. It is a condition that disrupts normal nerve cell function in the brain, causing periodic epileptic seizures [53]. To control this condition, multiple strategies have been used, including surgery, medications, and yoga. Medicines continue to play an important role in seizure management [54]. The researchers investigate glutamatergic neurotransmission and gamma-aminobutyric aminergic pathways in the CNS, which regulate excitatory and inhibitory activity [55]. For the treatment of epilepsy natural sources of medicine have several functions, including medicinal, gastronomic, nutritional, and curative purposes. Traditional and alternative plant-based therapies promote optimum health and lower adverse effects [56]. In epilepsy, Pregabalin is used as a first-line drug, which is commonly prescribed for diabetic nerve pain and partial neuropathy [57].
Different nano-formulations have been prepared for the treatment of epilepsy. A recent study by Shah et al. developed Lamotrigine (LMT) loaded poly(lactic-co-glycolic) acid nanoparticles for direct delivery from the nose to brain. The developed formulation exhibited a particle size of 170.0 nm, % an entrapment efficacy is 71%, a PDI of 0.191, and a Zeta potential of-16.60 mV. The biodistribution and gamma scintigraphy tests were conducted in mice, rabbits, and rats. The results revealed that intranasal delivery of LMT-nanoparticles resulted in a greater concentration of LMT in the brain than LMT solution. This method also demonstrated prolonged release, increased bioavailability, and better targeting of the BBB [58]. A further study by Tulbah et al., formulated a novel nasal niosomes loaded with lacosamide (LCA) using the thin film hydration method. The formulation has a vesicle size of 194.3 nm, with entrapment of 58.3%. Surface charge+35.6mV and in vitro release of the formulation is 81.3% during 8 h. The developed formulation has increased residence time on the physical stability, nasal mucosa, and brain bioavailability of LCA in brain tissues. These Niosomal formulations have a delayed release profile compared to solution form. After intranasal administration, it has 2 times increased in bioavailability, nasal diffusion, and brain distribution as compared to other routes of administration [59].
In one more study by, Nerli et al., they formulate niosomes coated with chitosan for nasal delivery of Clonazepam (CLZ). The prepared formulation has a particle size of around 200 nm, PDI less than 0.3, a positive surface charge, a spherical shape, and CLZ encapsulation above 60%. The release of CLZ is 50% after 4 h. in the medium. After encapsulation, it reduces the 1.5 times CLZ toxicity, which is confirmed by the mucoadhesive properties of chitosomes and has 10 times permeation in co with a CLZ solution [60]. The various nanoformulations for the treatment of Epilepsy have been discussed in table 3.
Table 3: Nano-formulation for epilepsy
| Drugs | Formulation type | Method | Polymers | Key finding | Reference |
| CLZ | Chitosan coated Niosomes | Thin layer evaporation method | Chitosan Sorbitan stearate Cholesterol Triton X-100 |
The optimized CLZ-loaded chitosan, with ~200 nm size and PDI<0.3, achieved over 60% encapsulation, 12-week stability, and 50% release in 4 h. They also reduced CLZ toxicity and increased permeability. | [60] |
| LCA | Niosomes | Thin film hydration method | Chitosan Cholesterol |
It shows sustained release, better nasal diffusion, and enhanced stability. Histopathological tests revealed no nasal mucosa toxicity, and it has significantly greater brain distribution than the drug solution. | [59] |
| Phenytoin Sodium loaded NLC’s | NLC’s | Melt emulsification with the ultrasonication method | Poloxamer 188 Cholesterol |
Three sizes of phenytoin sodium-loaded NLCs were developed. NLCs smaller than 50 nm released the drug completely in 15 min, crucial for rapid epilepsy treatment. | [61] |
| LMT | Nano-liposome | Thin film dehydration-rehydration method | Phospholipid 90G Cholesterol |
The LTG nanoliposome demonstrated nanoscale vesicle size, substantial entrapment within the lipid bilayer, and a high release rate, as shown by Plackett-Burman's design and response surface methodology. | [62] |
| LMT | Nasal insert | Ethanol injection method | HPMC K4M Gelatin Potassium dihydrogen phosphate Disodium hydrogen phosphate Glycine |
Based on optimization parameters, nano-plastic nasal inserts can be considered a novel nano-carrier system for targeting the brain with Lamotrigine to enhance its therapeutic effects. | [63] |
| LMT loaded PLGA nano-particles | Nano-particles | Emulsification-solvent evaporation method | Poloxamer-407 Potassium dihydrogen phosphate Poly (lactic-co-glycolic acid) |
The formulation could be used as a once-daily treatment for epilepsy, reducing the need for frequent dosing. Pharmacodynamic studies also confirmed that it quickly addresses involuntary muscle contractions. | [58] |
| Lorazepam (LZM) | Thermo-sensitive Gel | Solvent diffusion and evaporation method | Chitosan β-glycerol phosphate |
In the present study, a thermosensitive gel loaded with LZM-NLCs was investigated for its applicability as a nasal drug delivery system in treating epilepsy | [64] |
| LMT | NLC | Solvent evaporation method | Poloxamer PEG-8 Beeswaxes Glyceryl di-behenate Glycerol disterate |
The drug release followed zero-order kinetics and diffusion according to Fick's law, as described by the Korsmeyer-Peppas model. | [65] |
| Midazolam | Nano-particles | Ionic gelation method | Chitosan Sodium tri-poly-phosphate Glacial acetic acid |
In this study, the nose-to-brain delivery of Midazolam-loaded chitosan Nanoparticles was established with superiority over other formulations andanother route of administration. | [66] |
| Catechin hydrate | Polymeric nanoparticles | Double emulsion-solvent evaporation method | Chitosan Poly-vinyl alcohol Poly (lactic-co-glycolic acid) |
The formulation was optimized using a four-factor, three-level central composite design, and optimized based on particle size, PDI, and zeta potential with high entrapment efficacy. | [67] |
| Clonazepam | Polymeric micelles | Thin film hydration method | Polyethylene glycol Polypropylene glycol |
The produced formulation findings demonstrate the generated polymeric micelles' potential as an effective therapy option for epilepsy in an emergency. | [68] |
| Carbamazepine loaded carboxymethyl chitosan (CBZ-nanoparticles) | Nanoparticles | Etherification method Emulsification method |
Chitosan | This study found that the Intranasal CBZ-nanoparticles administration increased brain-to-plasma exposure (AUC) by 150%, indicating preferential brain delivery. | [69] |
| CBZ | Micro-emulsion | Emulsification method | Oleoyl polyoxylglyce-rides Diethylene glycol monoethyl ether Polyoxyl-40 hydrogenated castor oil Polycarbophil |
The formulation with polycarbophil enhances intranasal delivery of CBZ to the brain, resulting in higher brain concentrations compared to intravenous administration. | [70] |
| LMT | Nano-capsules | Solvent displacement method | Glyceryl mono-oleate Diethylene glycol monoethyl ether, Chitosan hydrochloride Poly-oxyethylene monostearate |
In this study, we confirm that lamotrigine rapidly and significantly releases from nanocapsules in vitro and shows considerable brain tissue penetration in vivo. | [71] |
| CBZ | Mucoadhesive Nano-emulgel | Spontaneous emulsification following the water titration method | Xanthan gum Cholesterol Phosphatidyl-coline Labrasol |
CBZ-loaded mucoadhesive nano-emulgel for intranasal use shows promise as a brain-targeted delivery system through the olfactory mucosa, potentially allowing lower doses and reducing CBZ side effects in epilepsy treatment. | [72] |
| LMT | Hydrogels | Cold production method | Poloxamer-407and188 Carbomer 974P Propylene glycol Polyethylene glycol 400 |
The results show that various formulations exhibited good thermosetting behavior, pH, and lamotrigine release above the minimum effective concentration for treating generalized epilepsy. | [73] |
Ischemic heart stroke
Even in the modern world, ischemic stroke remains one of the main causes of mortality and disability [74], Over 100 million individuals worldwide have been affected by ischemic stroke. Every year, more than 12.2 million fresh cases are reported, causing 6.55 million fatalities [75]. It is the leading cause of disability worldwide and the second most prevalent cause of death [76]. Ischemic stroke occurs when there is a disruption in the blood supply to a specific region of the brain, leading to brain injury and cell death [77], with adverse effects including memory loss, speech impairments, or physical impairments [78]. According to the previously published data, the process of oxidative stress is considered to be the primary contributor to the development of cerebral ischemia and its reperfusion damage. During oxidative stress in the body, the production of free radicles and reactive oxygen species results in the largest amount of oxygen consumption [79]. Some antioxidant drugs, including thioperamide, ropinirole, and curcumin, have been shown to decrease ROS-mediated reactions, protecting neurons from neuronal loss due to reperfusion in animal models with cerebral ischemia [80].
There are different nano-formulations have been prepared for the treatment of Ischemic stroke. A recent study by Yu et al. prepared a formulation of multidrug-loaded liposomes, which are encapsulated by Baicalin (BA) Borneol (BO) and Cholic acid (CA) to avoid ischemic stroke. The prepared formulation of Baicalin, Borneol, and Cholic acid-loaded Liposomes (BBC-LP) has a drug loading was 6.17%and the entrapment efficacy was found to be 42.69%. Formulated liposomes have a low particle size (156.62±2.96 nm), zeta potential (-0.99 mV), and PDI (0.195). Pharmacological studies show that compared to BBC in other forms with BBC-LP has significantly improved neurological deficiency and inhibited neuronal apoptosis more effectively shown in the Middle cerebral artery occlusion rat model and due to the toxicity studies, intranasal delivery of BBC-LP was safe and did not cause discomfort to the nasal mucosa [81].
Further studies by Ahmad et al., in this work, poloxamer chitosan-based Naringenin nanoemulsion was developed for the treatment of cerebral ischemia. The particle size of the developed Naringenin nanoemulsion (NRG-NE) gel formulation was observed to be 98.31±1.17 nm with a PDI of 0.386±0.021. The prepared NRG-NE gel showed a pH and viscosity of about 6.0±0.20 and 2447±24 cp, respectively, with % accuracy ranging from 95.10 – 99.30%. According to the conducted pharmacodynamic studies, the Area under the curve (AUC) of the brain and plasma were observed at 5600.99±144.92 and 995.60±24.59 (ng min/ml g) respectively in rats after intranasal delivery of the prepared formulation. The drug NRG, effectively used for treating cerebral ischemia by decreasing the side effects systematically, emerged as a potent neuroprotective drug against oxidative stress and cellular damage [82]. The various nanoformulations for the treatment of Ischemic heart strokes have been discussed in table 4.
Table 4: Nano-formulation for ischemic heart strokes
| Drugs | Formulation type | Method | Polymers | Key finding | Reference |
| BA-loaded ligand-modified nanoparticles | Nanoparticles | Double emulsification method | PLGA Polyethylene glycol |
PEG-PLGA nanoparticles modified with RVG29 peptide reach the hippocampus via the nasal-to-brain pathway, allowing BA to exert neuroprotective effects after cerebral ischemia. | [83] |
| Melatonin lipidic nano-capsules | Nano-capsules | Phase inversion temperature method | Solutol HS15 Soya bean lecithin |
Melatonin (MEL)-lipidic nanocapsules were evaluated in a cerebral ischemia model using histopathology, pro-inflammatory cytokines, apoptotic markers, and oxidative stress markers. | [84] |
| BO modified transhione (TSA) nanoparticles | Nanoparticles | Double emulsion-solvent evaporation method | Comurine-6 Dimethyl sulfoxide PEG, PLGA |
A novel formulation of BO-TSA-nanoparticles improves brain delivery and enhances TSA's prevention effect on Cerebral ischemia/reperfusion injury (CIRI). | [85] |
| Multidrug-loaded BBC-liposomes | Liposomes | Reverse phase evaporation method | Soybean lecithin Cholesterol Sodium deoxycholate |
In this formulation, pharmacological studies reveal that, compared to the drug alone, the liposome formulation more effectively reduces nerve damage and inhibits neuronal apoptosis. | [81] |
| Naringenin | Nano-emulsion | Ultrasonication method | Poloxamer-407 Chitosan PEG-400 Tri-phenyl-tetrazolium chloride |
The formulation effectively treats cerebral ischemia, reduces systemic side effects, and demonstrates strong neuroprotection against oxidative stress and cellular damage. | [82] |
| Thymoquinone-loaded mucoadhesive nanoemulsion | Nano-emulsion | Titration method | Carbitol Labrasol Tween-20 Formic acid |
The study confirmed that low-dose Thymoquinone-loaded mucoadhesive nanoemulsion was found to improve brain bioavailability and effectively treat cerebral ischemia. | [86] |
Migraine
Globally migraine is one of the most prevalent neurological disorders, which is ranked as 2nd cause of disability among young and middle-aged individuals [87]. About 15% of individuals worldwide suffer from severe neurological conditions [88]. In the United States, approximately 28 million individuals experience migraines, with a higher prevalence of 18.2% among women compared to 6.5% among men [89]. It is characterized by headaches that are paroxysmal and that usually last for four to seventy-two hours. It is divided into two main types: migraine with aura and migraine without aura." and its associated symptoms typically persist for 4-72 h [90]. During the aura phase, 5-hydroxytryptamine concentration in the blood falls, which might result in migraine problems while the symptoms of migraine without aura include unilateral headaches that are worse or prevented from practicing routine activities like walking [91]. Although the pathogenesis of migraine is unknown, a leading theory is that the stimulation of trigeminal nerve fibers by neurogenic inflammation triggers migraine attacks [92].
There are Different nano-formulations have been developed to treat migraine. Recent research by Alastal et al., prepared a nanoparticles of Dihydroergotamine (DHE) using the ionotropic gelation method. The developed formulation has a drug loading percentage is 20%, encapsulation efficiency of 95±13%, and a particle size of 395±59 nm. The In vitro release studies show that DHE-nanoparticles given through intranasal have significantly increased bioavailability (82.5±12.3%) compared to administration solution (53.2±7.7%). The prepared DHE-chitosan nanoparticles may enhance the systemic absorption of the drug, which results in more rapid systemic onset and higher systemic bioavailability compared to the DHE intranasal solution [93].
A further study by Saleh et al. prepared Spanlastics of zolmitriptan to enhance migraine treatment. The optimal formulation was found to have an entrapment efficiency of 45.65% and an average particle size of 117.5 nm. In the ex-vivo permeation studies, it is observed that 70% of the drug gets penetrated within 30 min through the nasal membrane. After 2 h, it is entirely penetrated and has a much larger steady-state flow than normal gel. The formulated drug showed improved permeation across the nasal membrane, confirming the potential efficacy of intranasal delivery to the brain [94].
One more study by Esim et al. developed Eletriptan Hydrobromide (EH) nanoparticles for nose-to-brain delivery. The formulated nanoparticles have a zeta potential value of-17.3±2.11 mV and an average particle size of 201.5±13.6 nm. After conducting studies on cellular uptake and P-glycoprotein efflux, it has been shown that the developed nanoparticles inhibit P-gp-mediated drug efflux. The pharmacokinetics studies show that the administration of EH-nanoparticles improves CNS permeation behavior compared to other formulations. In conclusion for intranasal administration, an approximately 2-fold higher AUC-brain value may indicate a greater amount of drug reaching the brain as compared to other formulations [95]. The various nanoformulations for the treatment of Migraine have been discussed in table 5.
Brain cancer
Cancer is a hereditary disease caused by various factors like Deoxyribose Nucleic Acid mutations, ultraviolet radiation, and certain environmental factors, as well as alterations in cell division [105]. The World Health Organisation reports that cancer caused 10 million deaths in 2020 and affects approximately 4 lakh youngsters annually. By 2040, it's expected that there will be around 29.5 million fresh cases of cancer, and unfortunately, about 16.4 million people may succumb to it [106]. Cancer is characterized by uncontrolled cell growth, lack of cell maturation, cell death, disrupted redox state, stress, metabolic regulation, and the capacity to spread to other parts of the body (metastasis) [107]. Adult patients with Glioblastoma Multiforme (GBM) have one of the most common and malignant primary brain tumors. Even with the use of multimodal treatment, including surgery and chemotherapy, GBM has an occurrence rate of over 90% [108]. There are Different nano-formulations have been prepared for intranasal administration for the treatment of cancer cells. A recent study by Colombo et al. developed a Kaempferol-loaded mucoadhesive nano-emulsion (KPF-MNE). The study aimed to develop nano-emulsions of KPF with or without chitosan to test their efficacy for brain transport and anticancer activity against glioma cells. The prepared KPF-MNE formulation has a globule size of 180.53±4.90 nm, PDI of 0.203±0.022, Zeta potential is+26.09±2.67 mV, and refractive index of 1.359±0.001. The intranasal administration of KPF-MNE in rats' brains significantly increased the amount of drug in the brain leading to cause enhancement of apoptosis and decreased viability of glioma cells, a crucial objective in cancer treatment. In conclusion, the developed formulation significantly decreased C6 glioma cell viability by inducing apoptosis, compared to free KPF and KPF-NE [109].
Further study by Alex et al. developed a nanoparticles of Carboplatin, which is loaded with polycaprolactone (PCL) for intranasal delivery. The formulated carboplatin-PCL-nanoparticles have a zeta potential of-16.3±3.7 mV and a particle size of 311.6±4.7 nm. They exhibit an entrapment efficacy of 27.95±4.21%. The formulation has shown ex-vivo permeation into sheep nasal mucosa and demonstrated a release pattern similar to in vitro experiments. In-situ nasal perfusion studies in Wistar rats revealed that carboplatin-loaded PCL nanoparticles are more effectively absorbed through the nostrils than pure carboplatin solution. Additionally, the generated nanoparticles of Carboplatin exhibits enhanced in vitro anti-tumor efficacy than plain medicines against human glioblastoma cells [110]. The various nanoformulations for treating brain cancer have been discussed in table 6.
Table 5: Nano-formulation for migraine
| Drugs | Formulation type | Method | Polymers | Key finding | Reference |
| Zolmitriptan | SLN | Emulsion solvent evaporation technique | Dimethyl-sulfoxide Tri-methylamine Stearic acid, Cholesterol |
Intranasal delivery of SLN with Zolmitriptan via the olfactory pathway achieves about 90% drug targeting compared to conventional Zolmitriptan tablets. | [96] |
| DHE | Nanoparticles | Modified ionotropic gelation method | Chitosan Trifluoroacetic acid Triethyl-amine |
The formulation of DHE chitosan nanoparticles may increase systemic drug absorption by about 55%, displaying a quick onset of action and higher systemic bioavailability in comparison with DHE solution. | [93] |
| Zolmitriptan | Spanlastics | Ethanol injection method | Span-60 Tween-80 |
The improved formulation resulted in enhanced drug penetration through the nasal membrane, demonstrating the intranasal route's potential for brain delivery. | [94] |
| EH | Nanoparticles | W/O/W emulsion solvent evaporation method | Poly(lactic-co-glycolic acid) Polyvinyl alcohol |
This study explored the antimigraine efficacy of EH polymeric nanoparticles, finding that different pH levels in the water phase affected drug encapsulation efficiency. | [95] |
| Frovatriptan Succinate | Polymeric Nano-particles | Double emulsion method | Poly (lactic-co-glycolic acid), PLGA, Polyvinyl alcohol, Span-80 | Intranasal delivery resulted in much greater drug dispersion in the brain than intravenous administration, suggesting that intranasal delivery is a promising brain-targeting route. | [97] |
| Sumatriptan | NLC’s | Solvent diffusion evaporation method | Cholesterol Triolein Stearic acid |
This intranasal NLC formulation is an effective brain delivery system. A high Direct transport percentage (DTP) of the NLC formulation indicates efficient sumatriptan entry into the brain. | [98] |
| Rizatriptan benzoate | SLN | Solvent diffusion method | Glyceryl monostearate Lecithin Pluronic F127 |
In vivo studies showed that intranasal administration of rizatriptan benzoate consistently resulted in higher brain concentrations than intravenous administration at all time points. | [99] |
| Zolmitriptan | Nanoparticles | Ionic gelation method | Sodium tri-polyphosphate, Chitosan |
The formulation showed maximum entrapment efficacy with a narrow particle size range and provided immediate Zolmitriptan release followed by sustained release. | [100] |
| Sumatriptan containing gliadin nanoparticles. | Nanoparticles | Desolvation method | Pluronic F-68 | The optimized nanoparticles released faster than tablets and nasal sprays, thanks to their small size. Glutaraldehyde increased hardness, F68 improved stability, and gliadin added adhesive properties. | [101] |
| Zolmitriptan | Nano-ethosomes | Ethanol injection method | Carbopol-934 Poloxamer 407 HPMC K100 |
A three-level factorial design optimized the ethosomal formulation, indicating that zolmitriptan-loaded intranasal gel could be a better option for managing recurrent migraines. | [102] |
| Zolmitriptan | Nanoparticles | Double emulsion solvent diffusion technique | Poloxamer-188, Polyvinyl alcohol, Ethyl acetate Poly (lactic-co-glycolic acid) |
The present study successfully applied the quality-by-design approach to develop Zolmitriptan-loaded PLGA nanoparticles achieving maximum brain targeting efficiency. | [103] |
| Propranolol | Thermo-responsive nasal nanogel. | Micro-emulsion ion system | Poloxamer-407 Chitosan Deacetylated chitin |
This research demonstrates that limonene-based microemulsion in situ nanogel effectively manages migraines by bypassing the liver's first-pass metabolism of propranolol. | [104] |
Challenges and Future Prospects for the nose to brain delivery
The need for targeted drug administration may be significantly addressed by intranasal drug delivery. Numerous studies have demonstrated the intranasal route's superiority over other conventional drug delivery techniques for the treatment of CNS disorder with promising applications in bypassing the BBB and directly targeting CNS cells table 7 highlights the ongoing clinical trials for nose-to-brain delivery and table 8 highlights the marketed products for nose-to-brain. Despite these advantages, several limitations still challenge the clinical applications of this method, such as ensuring consistent bioavailability, maintaining stability of formulations, and addressing patient-specific anatomical differences that can affect drug absorption. The BBB remains a significant hurdle in the CNS drug delivery, making current treatments for neurological disorders challenging. Many alternatives have been suggested to get around this issue. Among them, the application of intranasal delivery systems for nanocarriers has demonstrated encouraging outcomes. This is because it circumvents the BBB, allowing for quick access to the brain and getting around the limitations of oral and intravenous delivery methods. However, there is an ongoing need for enhanced formulations that can provide sustained release and minimize systemic side effects without compromising patient safety. Future developments in nose-to-brain drug delivery are expected to focus on personalized medicine, where treatments can be tailored to individual patient needs based on their genetic makeup and disease progression. The nose-to-brain delivery method represents a significant advancement in treating neurological disorders by circumventing the limitations posed by the BBB and first-pass metabolism associated with traditional oral and intravenous routes. Utilizing intranasal delivery alongside nanotechnology formulations, such as lipid carriers and nanoparticles, enhances drug bioavailability and neuroprotection. This method shows improved therapeutic outcomes for conditions like PD, AD, epilepsy, stroke, and brain cancer. Innovations in nanotechnology and nanocarriers will likely improve CNS drugs' targeting efficiency and therapeutic efficacy. The future in nanocarrier design for nose-to-brain drug delivery aims to refine the designs of nanocarriers to enhance treatment safety and efficacy, providing better targeting specificity. Such advanced nanocarriers with varied functionalities, such as controlled release combined with mechanisms that may cross the BBB, will be a critical factor that will improve treatment outcomes in the future. Additionally, combinational therapies that utilize nose-to-brain systems for multifactorial diseases like Alzheimer’s and brain cancer present significant potential, though regulatory and safety considerations for these novel approaches need careful evaluation. By overcoming current challenges such as drug stability, sustained release, and minimizing systemic side effects, nose-to-brain drug delivery could become the preferred method for treating CNS disorders. Despite these encouraging results from preclinical studies, several challenges remain for clinical application, including variability in nasal anatomy, the need for precise dosing, long-term safety concerns, and scalability of nanocarrier production. Furthermore, long-term safety concerns regarding the use of nanocarriers necessitate thorough evaluation to ensure patient safety and minimize potential adverse effects. Continued research is critical for optimizing nose-to-brain delivery systems and ensuring their effectiveness in clinical settings.
Table 6: Nano-formulation for brain cancer
| Drugs | Formulation type | Method | Polymers | Key finding | Reference |
| Temozolomide | Nano lipid-based chitosan Hydrogel | High-pressure homogenization technique | Chitosan, Gelucire 44/14, Transcutol, Labrafil, Labrasol, Poly-oxyethylene sorbitan mono-oleate | We incorporated temozolomide into a chitosan gel to enhance viscosity and extend nasal residence time. Absorption studies revealed higher temozolomide concentrations in the target area with this formulation. | [111] |
| Carboplatin-loaded polycaprolactone nps | Nanoparticles | Double emulsion solvent evaporation technique | Poly-caprolactone Poly-vinyl alcohol |
The optimized formulation showed sustained release with non-Fickian diffusion kinetics. Drug penetration into sheep nasal mucosa followed the in vitro release pattern. | [110] |
| Curcumin-Loaded NLC | NLC’s | Hot pressure homogenization method | Soya lecithin Precirol Capmul-MCM |
We formulated Curcumin-NLC, improving drug entrapment efficiency and drug release properties. DSC and XRD analyses confirmed the drug is in an amorphous state within the NLC particles. | [112] |
| Doxorubicin-loaded PLGA nanoparticles | Nanoparticles | Double emulsion method | PLGA Poly-vinyl alcohol |
Intranasal delivery of Doxorubicin nanoparticles demonstrates the great potential for glioblastoma therapy and its application to other types of brain disease. | [113] |
| KPF-loaded mucoadhesive nanoemulsion | Nanoemulsion | High-pressure homogenization technique | Chitosan Dimethyl sulfoxide Polysorbate-80 |
The mucoadhesive nanoemulsion induced apoptosis and reduced glioma cell viability, showing promise for delivering kaempferol from the nose to the brain as a potential treatment for pre-clinical gliomas. | [109] |
| Temozolomide | In-situ gel | By low energy method | Poloxamer-407 Poloxamer-188 |
The formulation showed sustained release and effective nasal permeation. Gamma scintigraphy confirmed brain accumulation, indicating in situ gel nanoemulsion of TMZ as a promising brain tumor treatment. | [114] |
Table 7: Ongoing clinical trial of the nose to the brain
| Drug | Disease | Phase | Application no. |
| Insulin detemir | AD’s | Phase II completed | NCT01547169 |
| Calcitonin | Acute treatment of migraine headache. | Phase II and III have been completed. | NCT04408794 |
| Oxytocin | Depression premenstrual dysphoric disorder | Completed | NCT02508103 |
| Vasopressin | Schizophrenia | Completed | NCT04190004O |
Insulin and Semaglutide |
Metabolic syndrome and Mild Cognitive Impairment | Phase II enlisting | NCT06072963 |
| Insulin analogue | Hyperglycemia, Stroke | Phase IV completed | NCT048334362 |
| Insulin Aspart | Mild cognitive impairments and AD’s | Phase II completed | NCT02462161 |
| Progesterone | Stroke, Brain injury | Phase IV (unknown status) | NCT04143880 |
| Oxytocin | Dementia | Completed | NCT01002300 |
| Human fibroblast growth factor | PD’s | Phase I, not yet recruiting | NCT05493462 |
| Insulin Aspart | AD’s | Phase I and II completed | NCT00581867 |
| Insulin | PD’s | Phase II completed | NCT02064166 |
| Vasopressin | Austin Spectrum Disorder | Phase II and III enlisting | NCT03204786 |
| Oxytocin (syntocinon) | Psychotic disorders and Schizophrenia | Early phase I completed | NCT02508103 |
| Oxytocin and vasopressin | Social behavior | Completed | NCT04890470 |
| Insulin (Humulin R U-100) | Mild cognitive impairment sand AD’s | Phase II completed | NCT00438568 |
| Oxytocin (syntocinon) | Autism spectrum disorder | Phase II Completed | NCT01908205 |
| Insulin (Humulin R® U-100) | AD’s | Phase II and III completed | NCT01767909 |
| Insulin (Humulin R U-100) Empagliflozin | AD’s | Phase II enlisting | NCT05081219 |
| Insulin | Stroke | Phase II completed | NCT02810392 |
| Foralumab | Multiple sclerosis | Phase I withdrawn | NCT05029609 |
Table 8: Marketed product of nose-to-brain delivery
| Drug | Brand name | Manufacturer | Year |
| Salmon calcitonin | Fortical (200 IU/spray) | Physicians Total Care, Inc. (Tulsa, OK, USA) | 2005 |
| Zolmitriptan | AscoTop/Zoming | AstraZeneca | |
| Desmopressin | DDAVP solution | Ferring Pharmaceuticals Ltd. | 1978 |
| Oxytocin | Syntocinon (40 IU/ml, solution spray) | Novartis | 1960 |
| Nafarelin | Synarel (0.2 mg/spray) | Pfizer Canada Ulc (Monteral. QC, Canada) | 1996 |
| Glucagon | Baqsimi (3 mg powder) | Eli Lillyand co. Ltd. (Basingstoke, UK) | 2019 |
| Buserelin | Suprecur (0.15 mg/spray) | Sanofi Avents | 2001 |
| Desmopressin | Noctiva (spray) | Pharmaceuticals LLC (Dublin, Ireland) | 2017 |
| Salmon calcitonin | Miacalcin (200IU/spray) | Novartis | 1995 |
| Dihydroergotamine | Migranal | Novartis Pharma | |
| Butorphanol tartrate | Stadol NS | Bristol-Myers Squibb | 1979 |
CONCLUSION
The nose-to-brain route of delivery is about a paradigm shift in overcoming critical barriers to neurological diseases, considering that the BBB and first-pass metabolism strongly limit traditional oral and intravenous administration methods. Intranasal delivery combined with nanotechnology-based formulations such as nanostructured lipid carriers, nanoemulsions, nanoparticles, and liposomes has shown considerable promise for improving bioavailability, increasing penetration to brain tissues, and providing neuroprotection. These systems have exhibited improved therapeutic efficacies in neurological disorders like PD’s, AD’s, epilepsy, stroke, and brain cancer, where traditional therapies have been less effective. Nanotechnology-based formulations allow drugs to cross the BBB and provide sustained release, improved stability and reduced systemic side effects. The capability of drugs to directly reach the brain through the olfactory and trigeminal nerve pathways has made the nose-to-brain systems one of the most promising systems to target specific brain regions. Nevertheless, promising results have been demonstrated through animal research with nose-to-brain drug delivery systems. Nose-to-brain uptake and therapeutic outcomes were seen in the pre-clinical studies putting nose to brain delivery system at the center stage of CNS drug development.
ACKNOWLEDGEMENT
The authors conveyed special thanks to Mr. Jitender Joshi, president, and Prof. (Dr.) Dharam Buddhi, Vice-Chancellor of Uttaranchal University, for their research-associated encouragement.
FUNDING
Nil
AUTHORS CONTRIBUTIONS
Shalu Verma: Investigation, Conceptualization, drafting, Supervision. Alka Singh: Review, editing, and visualization. Prabhat Kumar: Writing review and editing. Tarun Parashar: writing and analysis.
CONFLICT OF INTERESTS
Declared none
REFERENCES
Hussain AA. Intranasal drug delivery. Adv Drug Deliv Rev. 1998;29(1-2):39-49. doi: 10.1016/s0169-409x(97)00060-4, PMID 10837579.
Alnasser S. A review on nasal drug delivery system and its contribution in therapeutic management. Asian J Pharm Clin Res. 2019;12(1):40. doi: 10.22159/ajpcr.2019.v12i1.29443.
Illum L. Transport of drugs from the nasal cavity to the central nervous system. Eur J Pharm Sci. 2000;11(1):1-18. doi: 10.1016/s0928-0987(00)00087-7, PMID 10913748.
Alexander A, Agrawal M, Uddin A, Siddique S, Shehata AM, Shaker MA. Recent expansions of novel strategies towards the drug targeting into the brain. Int J Nanomedicine. 2019;14:5895-909. doi: 10.2147/IJN.S210876, PMID 31440051.
Mac Kinnon GE, Mac Kinnon NJ, Pharmacokinetics C, Larry Bauer PA, Larisa Cavallari PH, Francis Lam YW. Pharmacotherapy: A Pathophysiologic Approach; 2008.
Bonferoni MC, Rossi S, Sandri G, Ferrari F, Gavini E, Rassu G. Nanoemulsions for nose to brain drug delivery. Pharmaceutics. 2019;11(2):84. doi: 10.3390/pharmaceutics11020084, PMID 30781585.
Rassu G, Soddu E, Cossu M, Brundu A, Cerri G, Marchetti N. Solid microparticles based on chitosan or methyl-β-cyclodextrin: a first formulative approach to increase the nose to brain transport of deferoxamine mesylate. J Control Release. 2015 Mar 10;201:68-77. doi: 10.1016/j.jconrel.2015.01.025, PMID 25620068.
Zlokovic BV. Neurovascular pathways to neurodegeneration in alzheimers disease and other disorders. Nat Rev Neurosci. 2011;12(12):723-38. doi: 10.1038/nrn3114, PMID 22048062.
Kisler K, Nelson AR, Montagne A, Zlokovic BV. Cerebral blood flow regulation and neurovascular dysfunction in alzheimer disease. Nat Rev Neurosci. 2017;18(7):419-34. doi: 10.1038/nrn.2017.48, PMID 28515434.
Pardridge WM. Blood-brain barrier endogenous transporters as therapeutic targets: a new model for small molecule CNS drug discovery. Expert Opin Ther Targets. 2015;19(8):1059-72. doi: 10.1517/14728222.2015.1042364, PMID 25936389.
Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37(1):13-25. doi: 10.1016/j.nbd.2009.07.030, PMID 19664713.
Saunders NR, Habgood MD, Mollgard K, Dziegielewska KM. The biological significance of brain barrier mechanisms: help or hindrance in drug delivery to the central nervous system? F1000Res. 2016;5:F1000. doi: 10.12688/f1000research.7378.1, PMID 26998242.
Engelhardt B, Sorokin L. The blood-brain and the blood-cerebrospinal fluid barriers: function and dysfunction. Semin Immunopathol. 2009;31(4):497-511. doi: 10.1007/s00281-009-0177-0, PMID 19779720.
Morrison EE, Costanzo RM. Morphology of olfactory epithelium in humans and other vertebrates. Microsc Res Tech. 1992;23(1):49-61. doi: 10.1002/jemt.1070230105, PMID 1392071.
Law SL, Huang KJ, Chou HY. Preparation of desmopressin containing liposomes for intranasal delivery. J Control Release. 2001;70(3):375-82. doi: 10.1016/s0168-3659(00)00369-2, PMID 11182207.
Sosnik A, Das Neves J, Sarmento B. Mucoadhesive polymers in the design of nano drug delivery systems for administration by non parenteral routes: a review. Prog Polym Sci. 2014;39(12):2030-75. doi: 10.1016/j.progpolymsci.2014.07.010.
Mittal D, Ali A, Md S, Baboota S, Sahni JK, Ali J. Insights into direct nose to brain delivery: current status and future perspective. Drug Deliv. 2014;21(2):75-86. doi: 10.3109/10717544.2013.838713, PMID 24102636.
Djupesland PG, Messina JC, Mahmoud RA. The nasal approach to delivering treatment for brain diseases: an anatomic physiologic and delivery technology overview. Ther Deliv. 2014;5(6):709-33. doi: 10.4155/tde.14.41, PMID 25090283.
Serralheiro A, Alves G, Fortuna A, Falcao A. Intranasal administration of carbamazepine to mice: a direct delivery pathway for brain targeting. Eur J Pharm Sci. 2014;60(60):32-9. doi: 10.1016/j.ejps.2014.04.019, PMID 24813112.
Chapman CD, Frey WH, Craft S, Danielyan L, Hallschmid M, Schioth HB. Intranasal treatment of central nervous system dysfunction in humans. Pharm Res. 2013;30(10):2475-84. doi: 10.1007/s11095-012-0915-1, PMID 23135822.
Dhillon S. Rivastigmine transdermal patch: a review of its use in the management of dementia of the alzheimers type. Drugs. 2011;71(9):1209-31. doi: 10.2165/11206380-000000000-00000, PMID 21711064.
Carreiras MC, Mendes E, Perry MJ, Francisco AP, Marco Contelles J. The multifactorial nature of alzheimers disease for developing potential therapeutics. Curr Top Med Chem. 2013;13(15):1745-70. doi: 10.2174/15680266113139990135, PMID 23931435–70.
Onofri E, Mercuri M, Archer T, Rapp Ricciardi M, Ricci S. Legal medical consideration of alzheimers disease patients dysgraphia and cognitive dysfunction: a 6 month follow up. Clin Interv Aging. 2016;11:279-84. doi: 10.2147/CIA.S94750, PMID 27022252.
Mc Khann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of alzheimers disease: report of the nincds-adrda work group under the auspices of department of health and human services task force on alzheimers disease. Neurology. 1984;34(7):939-44. doi: 10.1212/wnl.34.7.939, PMID 6610841.
Hardy J. The amyloid hypothesis for alzheimers disease: a critical reappraisal. J Neurochem. 2009;110(4):1129-34. doi: 10.1111/j.1471-4159.2009.06181.x, PMID 19457065.
D Arrigo JS. Alzheimers disease brain injury and C.N.S. nanotherapy in humans: sonoporation augmenting drug targeting. Med Sci. 2017;5(4):29. doi: 10.3390/medsci5040029.
Trejo Lopez JA, Yachnis AT, Prokop S. Neuropathology of alzheimers disease. Neurotherapeutics. 2022;19(1):173-85. doi: 10.1007/s13311-021-01146-y, PMID 34729690.
Wilson B, Mohamed Alobaid BN, Geetha KM, Jenita JL. Chitosan nanoparticles to enhance nasal absorption and brain targeting of sitagliptin to treat alzheimers disease. J Drug Deliv Sci Technol. 2021 Feb;61:102176. doi: 10.1016/j.jddst.2020.102176.
Shehata MK, Ismail AA, Kamel MA. Nose to brain delivery of astaxanthin loaded nanostructured lipid carriers in rat model of alzheimers disease: preparation in vitro and in vivo evaluation. Int J Nanomedicine. 2023;18:1631-58. doi: 10.2147/IJN.S402447, PMID 37020692.
Kaur A, Nigam K, Srivastava S, Tyagi A, Dang S. Memantine nanoemulsion: a new approach to treat alzheimers disease. J Microencapsul. 2020;37(5):355-65. doi: 10.1080/02652048.2020.1756971, PMID 32293915.
Espinoza LC, Vacacela M, Clares B, Garcia ML, Fabrega MJ, Calpena AC. Development of a nasal donepezil loaded microemulsion for the treatment of alzheimers disease: in vitro and ex vivo characterization. CNS Neurol Disord Drug Targets. 2018;17(1):43-53. doi: 10.2174/1871527317666180104122347, PMID 29299992.
Shehata MK, Ismail AA, Kamel MA. Nose to brain delivery of astaxanthin loaded nanostructured lipid carriers in rat model of alzheimers disease: preparation in vitro and in vivo evaluation. Int J Nanomedicine. 2023;18:1631-58. doi: 10.2147/IJN.S402447, PMID 37020692.
Espinoza LC, Silva Abreu M, Clares B, Rodriguez Lagunas MJ, Halbaut L, Canas MA. Formulation strategies to improve nose to brain delivery of donepezil. Pharmaceutics. 2019;11(2):64. doi: 10.3390/pharmaceutics11020064, PMID 30717264.
Al Asmari AK, Ullah Z, Tariq M, Fatani A. Preparation characterization and in vivo evaluation of intranasally administered liposomal formulation of donepezil. Drug Des Dev Ther. 2016 Jan 12;10:205-15. doi: 10.2147/DDDT.S93937, PMID 26834457.
Zheng X, Shao X, Zhang C, Tan Y, Liu Q, Wan X. Intranasal H102 peptide loaded liposomes for brain delivery to treat alzheimers disease. Pharm Res. 2015;32(12):3837-49. doi: 10.1007/s11095-015-1744-9, PMID 26113236.
Rompicherla SK, Arumugam K, Bojja SL, Kumar N, Rao CM. Pharmacokinetic and pharmacodynamic evaluation of nasal liposome and nanoparticle based rivastigmine formulations in acute and chronic models of alzheimers disease. Naunyn Schmiedebergs Arch Pharmacol. 2021;394(8):1737-55. doi: 10.1007/s00210-021-02096-0, PMID 34086100.
Abbas H, Sayed NS, Youssef NA, ME Gaafar P, Mousa MR, Fayez AM. Novel luteolin loaded chitosan decorated nanoparticles for brain targeting delivery in a sporadic alzheimers disease mouse model: focus on antioxidant anti-inflammatory and amyloidogenic pathways. Pharmaceutics. 2022;14(5):1003. doi: 10.3390/pharmaceutics14051003, PMID 35631589.
Saraswathi TS, Mothilal M. Development of rivastigmine loaded self assembled nanostructures of nonionic surfactants for brain delivery. Int J Appl Pharm. 2021;13(5):205-15. doi: 10.22159/ijap.2021v13i5.42664.
Gao Y, Almalki WH, Afzal O, Panda SK, Kazmi I, Alrobaian M. Systematic development of lectin conjugated microspheres for nose to brain delivery of rivastigmine for the treatment of alzheimers disease. Biomed Pharmacother. 2021;141 Sep:111829. doi: 10.1016/j.biopha.2021.111829, PMID 34147904.
Tulbah AS, Elkomy MH, Zaki RM, Eid HM, Eissa EM, Ali AA. Novel nasal niosomes loaded with lacosamide and coated with chitosan: a possible pathway to target the brain to control partial onset seizures. Int J Pharm X. 2023;6:100206. doi: 10.1016/j.ijpx.2023.100206, PMID 37637477.
Abo El Enin HA, Elkomy MH, Naguib IA, Ahmed MF, Alsaidan OA, Alsalahat I. Lipid nanocarriers overlaid with chitosan for brain delivery of berberine via the nasal route. Pharmaceuticals (Basel). 2022;15(3):281. doi: 10.3390/ph15030281, PMID 35337079.
Yasir M, Sara UV, Chauhan I, Gaur PK, Singh AP, Puri D. Solid lipid nanoparticles for nose to brain delivery of donepezil: formulation optimization by box behnken design in vitro and in vivo evaluation. Artif Cells Nanomed Biotechnol. 2018;46(8):1838-51. doi: 10.1080/21691401.2017.1394872.
Khatri DK, Preeti K, Tonape S, Bhattacharjee S, Patel M, Shah S. Nanotechnological advances for nose to brain delivery of therapeutics to improve the parkinson therapy. Curr Neuropharmacol. 2023;21(3):493-516. doi: 10.2174/1570159X20666220507022701, PMID 35524671.
Dimiou S, Lopes RM, Kubajewska I, Mellor RD, Schlosser CS, Shet MS. Particulate levodopa nose to brain delivery targets dopamine to the brain with no plasma exposure. Int J Pharm. 2022;618:121658. doi: 10.1016/j.ijpharm.2022.121658, PMID 35292396.
Melamed E, Hefti F, Wurtman RJ. Nonaminergic striatal neurons convert exogenous L-dopa to dopamine in parkinsonism. Ann Neurol. 1980;8(6):558-63. doi: 10.1002/ana.410080603, PMID 6260009.
Sharma S, Lohan S, Murthy RS. Formulation and characterization of intranasal mucoadhesive nanoparticulates and thermo reversible gel of levodopa for brain delivery. Drug Dev Ind Pharm. 2014;40(7):869-78. doi: 10.3109/03639045.2013.789051, PMID 23600649.
Saha P, Kathuria H, Pandey MM. Nose to brain delivery of rotigotine redispersible nanosuspension: in vitro and in vivo characterization. J Drug Deliv Sci Technol. 2023;79:104049. doi: 10.1016/j.jddst.2022.104049.
Avhad PS, Gupta RA, Firke SN, Roge AB, Sarje SK, Raut AS. Formulation and evaluation of chitosan based polyelectrolyte complex of levodopa for nasal drug delivery. Int J Health Sci. 2022;S3:1316-27. doi: 10.53730/ijhs.v6nS3.5555.
Sridhar V, Gaud R, Bajaj A, Wairkar S. Pharmacokinetics and pharmacodynamics of intranasally administered selegiline nanoparticles with improved brain delivery in parkinsons disease. Nanomedicine. 2018;14(8):2609-18. doi: 10.1016/j.nano.2018.08.004, PMID 30171904.
Ahmad MZ, Sabri AH, Anjani QK, Dominguez Robles J, Abdul Latip N, Hamid KA. Design and development of levodopa loaded polymeric nanoparticles for intranasal delivery. Pharmaceuticals (Basel). 2022;15(3):370. doi: 10.3390/ph15030370, PMID 35337167.
Allegritti E, Battista S, Maggi MA, Marconi C, Galantini L, Giansanti L. Novel liposomal formulations for protection and delivery of levodopa: structure properties correlation. Int J Pharm. 2023;643:123230. doi: 10.1016/j.ijpharm.2023.123230, PMID 37454830.
Alipour S, Azari H, TIPS AF. In situ thermosensitive gel of levodopa: potential formulation for nose to brain delivery in parkinson disease. Trends Pharm Sci; 2020.
Scharfman HE. The neurobiology of epilepsy. Curr Neurol Neurosci Rep. 2007;7(4):348-54. doi: 10.1007/s11910-007-0053-z, PMID 17618543.
Gupta YK, Malhotra J. Antiepileptic drug therapy in the twenty first century. Indian J Physiol Pharmacol. 2000;44(1):8-23. PMID 10919091.
DE Sarro G, Spagnolo C, Gareri P, Gallelli L, DE Sarro A. Gabapentin potentiates the antiseizure activity of certain anticonvulsants in DBA/2 mice. Eur J Pharmacol. 1998;349(2-3):179-85. doi: 10.1016/s0014-2999(98)00193-9, PMID 9671096.
Goyal SN, Prajapati CP, Gore PR, Patil CR, Mahajan UB, Sharma C. Therapeutic potential and pharmaceutical development of thymoquinone: a multitargeted molecule of natural origin. Front Pharmacol. 2017;8:656. doi: 10.3389/fphar.2017.00656, PMID 28983249.
Pethe A, Hadke A, Agrawal S, Telange D. Intranasal formulation and characterization of chitosan microsphere for improving in vitro mucoadhesion residence time and absorption rate of pregabalin. Int J Appl Pharm. 2023;15(1):156-65. doi: 10.22159/ijap.2023v15i1.46359.
Shah P, Dubey P, Vyas B, Kaul A, Mishra AK, Chopra D. Lamotrigine loaded PLGA nanoparticles intended for direct nose to brain delivery in epilepsy: pharmacokinetic pharmacodynamic and scintigraphy study. Artif Cells Nanomed Biotechnol. 2021;49(1):511-22. doi: 10.1080/21691401.2021.1939709, PMID 34151674.
Tulbah AS, Elkomy MH, Zaki RM, Eid HM, Eissa EM, Ali AA. Novel nasal niosomes loaded with lacosamide and coated with chitosan: a possible pathway to target the brain to control partial onset seizures. Int J Pharm X. 2023;6:100206. doi: 10.1016/j.ijpx.2023.100206, PMID 37637477.
Nerli G, Robla S, Bartalesi M, Luceri C, D Ambrosio M, Csaba N. Chitosan coated niosomes for nose to brain delivery of clonazepam: formulation stability and permeability studies. Carbohydr Polym Technol Appl. 2023 Dec;6:100332. doi: 10.1016/j.carpta.2023.100332.
Nair SC, Vinayan KP, Mangalathillam S. Nose to brain delivery of phenytoin sodium loaded nano lipid carriers: formulation drug release permeation and in vivo pharmacokinetic studies. Pharmaceutics. 2021;13(10):1640. doi: 10.3390/pharmaceutics13101640, PMID 34683933.
Praveen A, Aqil M, Imam SS, Ahad A, Moolakkadath T, Ahmad FJ. Lamotrigine encapsulated intra nasal nanoliposome formulation for epilepsy treatment: formulation design characterization and nasal toxicity study. Colloids Surf B Biointerfaces. 2019;174:553-62. doi: 10.1016/j.colsurfb.2018.11.025, PMID 30502666.
Abdelmonem R, El Enin HA, Abdelkader G, Abdel Hakeem M. Formulation and characterization of lamotrigine nasal insert targeted brain for enhanced epilepsy treatment. Drug Deliv. 2023;30(1):2163321. doi: 10.1080/10717544.2022.2163321, PMID 36579655.
Taymouri S, Minaiyan M, Ebrahimi F, Tavakoli N. In vitro and in vivo evaluation of chitosan based thermosensitive gel containing lorazepam NLCs for the treatment of status epilepticus. IET Nanobiotechnol. 2020;14(2):148-54. doi: 10.1049/iet-nbt.2019.0156, PMID 32433032.
Alam T, Pandit J, Vohora D, Aqil M, Ali A, Sultana Y. Optimization of nanostructured lipid carriers of lamotrigine for brain delivery: in vitro characterization and in vivo efficacy in epilepsy. Expert Opin Drug Deliv. 2015;12(2):181-94. doi: 10.1517/17425247.2014.945416, PMID 25164097.
Shrestha N, Khan S, Neupane YR, Dang S, Md S, Fahmy UA. Tailoring midazolam loaded chitosan nanoparticulate formulation for enhanced brain delivery via intranasal route. Polymers. 2020;12(11):2589. doi: 10.3390/polym12112589, PMID 33158148.
Ahmad N, Ahmad R, Alrasheed RA, Almatar HM, Al Ramadan AS, Amir M. Quantification and evaluations of catechin hydrate polymeric nanoparticles used in brain targeting for the treatment of epilepsy. Pharmaceutics. 2020;12(3):203. doi: 10.3390/pharmaceutics12030203, PMID 32120778.
Nour SA, Abdelmalak NS, Naguib MJ, Rashed HM, Ibrahim AB. Intranasal brain targeted clonazepam polymeric micelles for immediate control of status epilepticus: in vitro optimization ex vivo determination of cytotoxicity in vivo biodistribution and pharmacodynamics studies. Drug Deliv. 2016;23(9):3681-95. doi: 10.1080/10717544.2016.1223216, PMID 27648847.
Liu S, Yang S, HO PC. Intranasal administration of carbamazepine loaded carboxymethyl chitosan nanoparticles for drug delivery to the brain. Asian J Pharm Sci. 2018;13(1):72-81. doi: 10.1016/j.ajps.2017.09.001, PMID 32104380.
Patel RB, Patel MR, Bhatt KK, Patel BG, Gaikwad RV. Microemulsion based drug delivery system for transnasal delivery of carbamazepine: preliminary brain targeting study. Drug Deliv. 2016;23(1):207-13. doi: 10.3109/10717544.2014.908980, PMID 24825492.
Gieszinger P, Stefania Csaba N, Garcia Fuentes M, Prasanna M, Gaspar R, Sztojkov Ivanov A. Preparation and characterization of lamotrigine containing nanocapsules for nasal administration. Eur J Pharm Biopharm. 2020 Aug;153:177-86. doi: 10.1016/j.ejpb.2020.06.003, PMID 32531424.
Samia O, Hanan R, Kamal ET. Carbamazepine mucoadhesive nanoemulgel (MNEG) as brain targeting delivery system via the olfactory mucosa. Drug Deliv. 2012;19(1):58-67. doi: 10.3109/10717544.2011.644349, PMID 22191715.
Melamane S, Walker RB, Khamanga SM. Formulation optimization of smart thermosetting lamotrigine loaded hydrogels using response surface methodology box benhken design and artificial neural networks. Drug Dev Ind Pharm. 2020;46(9):1402-15. doi: 10.1080/03639045.2020.1791163, PMID 32795107.
WU MY, Yiang GT, Liao WT, Tsai AP, Cheng YL, Cheng PW. Current mechanistic concepts in ischemia and reperfusion injury. Cell Physiol Biochem. 2018;46(4):1650-67. doi: 10.1159/000489241, PMID 29694958.
Campbell BC, DE Silva DA, Macleod MR, Coutts SB, Schwamm LH, Davis SM. Ischaemic stroke. Nat Rev Dis Primers. 2019;5(1):70. doi: 10.1038/s41572-019-0118-8, PMID 31601801.
Jurcau A, Simion A. Neuroinflammation in cerebral ischemia and ischemia/reperfusion injuries: from pathophysiology to therapeutic strategies. Int J Mol Sci. 2021;23(1):14. doi: 10.3390/ijms23010014, PMID 35008440.
Gund GM, Jagtap MP, Ingale MV, Patil RY. Stroke: a brain attack. IOSR J Pharm. 2013;3.
Radak D, Katsiki N, Resanovic I, Jovanovic A, Sudar Milovanovic E, Zafirovic S, S, R Isenovic E. Apoptosis and acute brain ischemia in ischemic stroke. Curr Vasc Pharmacol. 2017;15(2):115-22. doi: 10.2174/1570161115666161104095522.
Ahmad N, Ahmad R, Naqvi AA, Alam MA, Ashafaq M, Abdur Rub R. Intranasal delivery of quercetin loaded mucoadhesive nanoemulsion for treatment of cerebral ischaemia. Artif Cells Nanomed Biotechnol. 2018;46(4):717-29. doi: 10.1080/21691401.2017.1337024, PMID 28604104.
Yan H, Zhang X, HU W, MA J, Hou W, Zhang X. Histamine H3 receptors aggravate cerebral ischaemic injury by histamine independent mechanisms. Nat Commun. 2014 Feb 25;5:3334. doi: 10.1038/ncomms4334, PMID 24566390.
YU S, LI D, Shi A, Long Y, Deng J, MA Y. Multidrug loaded liposomes prevent ischemic stroke through intranasal administration. Biomed Pharmacother. 2023;162:114542. doi: 10.1016/j.biopha.2023.114542, PMID 36989725.
Ahmad N, Ahmad R, Ahmad FJ, Ahmad W, Alam MA, Amir M. Poloxamer chitosan based naringenin nanoformulation used in brain targeting for the treatment of cerebral ischemia. Saudi J Biol Sci. 2020;27(1):500-17. doi: 10.1016/j.sjbs.2019.11.008, PMID 31889876.
LI X, LI S, MA C, LI T, Yang L. Preparation of baicalin loaded ligand modified nanoparticles for nose to brain delivery for neuroprotection in cerebral ischemia. Drug Deliv. 2022;29(1):1282-98. doi: 10.1080/10717544.2022.2064564, PMID 35467483.
Bseiso EA, AbdEl Aal SA, Nasr M, Sammour OA, El Gawad NA. Nose to brain delivery of melatonin lipidic nanocapsules as a promising post ischemic neuroprotective therapeutic modality. Drug Deliv. 2022;29(1):2469-80. doi: 10.1080/10717544.2022.2104405, PMID 35892291.
Wang L, XU L, DU J, Zhao X, Liu M, Feng J. Nose to brain delivery of borneol modified tanshinone IIA nanoparticles in prevention of cerebral ischemia/reperfusion injury. Drug Deliv. 2021;28(1):1363-75. doi: 10.1080/10717544.2021.1943058, PMID 34180761.
Ahmad N, Ahmad R, Alam MA, Samim M, Iqbal Z, Ahmad FJ. Quantification and evaluation of thymoquinone loaded mucoadhesive nanoemulsion for treatment of cerebral ischemia. Int J Biol Macromol. 2016;88:320-32. doi: 10.1016/j.ijbiomac.2016.03.019, PMID 26976069.
Hansen JM, Charles A. Differences in treatment response between migraine with aura and migraine without aura: lessons from clinical practice and RCTs. J Headache Pain. 2019;20(1):96. doi: 10.1186/s10194-019-1046-4, PMID 31492106.
Edvinsson L, Haanes KA, Warfvinge K, Krause DN. CGRP as the target of new migraine therapies successful translation from bench to clinic. Nat Rev Neurol. 2018;14(6):338-50. doi: 10.1038/s41582-018-0003-1, PMID 29691490.
Lipton RB, Stewart WF, Diamond S, Diamond ML, Reed M. Prevalence and burden of migraine in the United States: data from the American migraine study II. headache. 2001;41(7):646-57. doi: 10.1046/j.1526-4610.2001.041007646.x, PMID 11554952.
Gargus JJ. Ion channel functional candidate genes in multigenic neuropsychiatric disease. Biol Psychiatry. 2006;60(2):177-85. doi: 10.1016/j.biopsych.2005.12.008, PMID 16497276.
Gupta S, Oosthuizen R, Pulfrey S. Treatment of acute migraine in the emergency department. Can Fam Physician. 2014;60(1):47-9. PMID 24452560.
M A Moskowitz. Basic mechanisms in vascular headache Pub Med. Neurol Clin. 1990 Nov;8(4):801-15.
Alastal A, Kwaik AD, Malkawi AA, Baltzley S, Al Ghananeem AM. Enhancing intranasal delivery and bioavailability of dihydroergotamine utilizing chitosan nanoparticles. J Clin Pharm Ther. 2023;2023:1-8. doi: 10.1155/2023/2284371.
Saleh A, Khalifa M, Shawky S, Bani Ali A, Eassa H. Zolmitriptan intranasal spanlastics for enhanced migraine treatment; formulation parameters optimized via quality by design approach. Sci Pharm. 2021;89(2):2. doi: 10.3390/scipharm89020024.
Esim O, Savaser A, Ozkan CK, Oztuna A, Goksel BA, Ozler M. Nose to brain delivery of eletriptan hydrobromide nanoparticles: preparation in vitro/in vivo evaluation and effect on trigeminal activation. J Drug Deliv Sci Technol. 2020 Oct;59:101919. doi: 10.1016/j.jddst.2020.101919.
Mostafa DA, Khalifa MK, Gad SS. Zolmitriptan brain targeting via intranasal route using solid lipid nanoparticles for migraine therapy: formulation characterization in vitro and in vivo assessment. Int J Appl Pharm. 2020;12(2):86-93. doi: 10.22159/ijap.2020v12i2.36812.
Deepika D, Dewangan HK, Maurya L, Singh S. Intranasal drug delivery of frovatriptan succinate loaded polymeric nanoparticles for brain targeting. J Pharm Sci. 2019;108(2):851-9. doi: 10.1016/j.xphs.2018.07.013, PMID 30053555.
Masjedi M, Azadi A, Heidari R, Mohammadi Samani S. Nose to brain delivery of sumatriptan loaded nanostructured lipid carriers: preparation optimization characterization and pharmacokinetic evaluation. J Pharm Pharmacol. 2020;72(10):1341-51. doi: 10.1111/jphp.13316, PMID 32579251.
Singh A, Ubrane R, Prasad P, Ramteke S. Preparation and characterization of rizatriptan benzoate loaded solid lipid nanoparticles for brain targeting. Mater Today Proc. 2015;2(9):4521-43. doi: 10.1016/j.matpr.2015.10.067.
Mandlik SK, Vidyapeeth B, Adhikari S, Ranpise NS. Formulation and in vitro characterization of chitosan biodegradable nanoparticles of zolmitriptan for migraine treatment pharmacie globale international journal of comprehensive pharmacy formulation and in vitro characterization of chitosan biodegradable nanoparticles of zolmitriptan for migraine treatment 2012.101. Asian J Pharm Clin Res. 2018;11(7):345-7.
Shelke S, Shahi S, Jalalpure S, Dhamecha D. Poloxamer 407-based intranasal thermoreversible gel of zolmitriptan loaded nanoethosomes: formulation optimization evaluation and permeation studies. J Liposome Res. 2016;26(4):313-23. doi: 10.3109/08982104.2015.1132232, PMID 26758957.
Girotra P, Singh SK, Kumar G. Development of zolmitriptan loaded PLGA/poloxamer nanoparticles for migraine using quality by design approach. Int J Biol Macromol. 2016;85:92-101. doi: 10.1016/j.ijbiomac.2015.12.069, PMID 26724690.
Abla KK, Domiati S, El Majzoub R, Mehanna MM. Propranolol loaded limonene based microemulsion thermo responsive mucoadhesive nasal nanogel: design in vitro assessment ex vivo permeation and brain biodistribution. Gels. 2023;9(6):491. doi: 10.3390/gels9060491, PMID 37367161.
The genetics of cancer-NCI; 2024.
DE Martel C, Georges D, Bray F, Ferlay J, Clifford GM. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob Health. 2020;8(2):e180-90. doi: 10.1016/S2214-109X(19)30488-7, PMID 31862245.
Emami Nejad A, Najafgholian S, Rostami A, Sistani A, Shojaeifar S, Esparvarinha M. The role of hypoxia in the tumor microenvironment and development of cancer stem cell: a novel approach to developing treatment. Cancer Cell Int. 2021;21(1):62. doi: 10.1186/s12935-020-01719-5, PMID 33472628.
Morsy AA, Ng WH. Re-do craniotomy for recurrent glioblastoma. CNS Oncol. 2015;4(2):55-7. doi: 10.2217/cns.15.4, PMID 25768328.
Colombo M, Figueiro F, DE Fraga Dias A, Teixeira HF, Battastini AM, Koester LS. Kaempferol loaded mucoadhesive nanoemulsion for intranasal administration reduces glioma growth in vitro. Int J Pharm. 2018;543(1-2):214-23. doi: 10.1016/j.ijpharm.2018.03.055, PMID 29605695.
Alex AT, Joseph A, Shavi G, Rao JV, Udupa N. Development and evaluation of carboplatin loaded PCL nanoparticles for intranasal delivery. Drug Deliv. 2016;23(7):2144-53. doi: 10.3109/10717544.2014.948643, PMID 25544603.
Khan A, Aqil M, Imam SS, Ahad A, Sultana Y, Ali A. Temozolomide loaded nano lipid based chitosan hydrogel for nose to brain delivery: characterization nasal absorption histopathology and cell line study. Int J Biol Macromol. 2018;116:1260-7. doi: 10.1016/j.ijbiomac.2018.05.079, PMID 29775717.
Madane RG, Mahajan HS. Curcumin loaded nanostructured lipid carriers (NLCs) for nasal administration: design characterization and in vivo study. Drug Deliv. 2016;23(4):1326-34. doi: 10.3109/10717544.2014.975382, PMID 25367836.
Chung K, Ullah I, Kim N, Lim J, Shin J, Lee SC. Intranasal delivery of cancer targeting doxorubicin loaded PLGA nanoparticles arrests glioblastoma growth. J Drug Target. 2020;28(6):617-26. doi: 10.1080/1061186X.2019.1706095, PMID 31852284.
Bayanati M, Khosroshahi AG, Alvandi M, Mahboobian MM. Fabrication of a thermosensitive in situ gel nanoemulsion for nose to brain delivery of temozolomide. J Nanomater. 2021;2021:1-11. doi: 10.1155/2021/1546798.