
Int J App Pharm, Vol 17, Issue 2, 2025, 126-133Original Article
PROANTHOCYANIDIN-PLGA NANOPARTICLE INFUSED CALCIUM HYDROXIDE SEALER: ADVANCING MATERIAL PERFORMANCE AND CLINICAL STABILITY IN ENDODONTICS
VANDANA SADANANDA1*, GOWRISH S.2, R. NARAYANA CHARYULU3, MITHRA N. HEGDE4*
1,2,4Department of Conservative Dentistry and Endodontics, Deralakatte, Mangaluru-575018, Karnataka, India. 3NITTE (Deemed to be University), NGSM Institute of Pharmaceutical Sciences, Department of Pharmaceutics, Deralakatte, Mangaluru-575018, Karnataka, India.
*Corresponding author: Vandana Sadananda; *Email: drvandanasadananda@nitte.edu.in
Received: 01 Oct 2024, Revised and Accepted: 21 Dec 2024
ABSTRACT
Objective: The study presents a novel calcium hydroxide-based endodontic sealer infused with proanthocyanidin nanoparticles (PAC-NPs) encapsulated in poly(actic-co-glycolic acid) (PLGA), designed to enhance critical clinical properties and overcome limitations of traditional sealers.
Methods: The sealer's formulation was evaluated for flow, setting time, solubility, dimensional stability, and pH, adhering to ISO 6876:2012 and ANSI/ADA standards 57. The incorporation of PAC-PLGA and calcium hydroxide nanoparticles was analyzed using Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy (ATR-FTIR) to confirm chemical interactions and functional integration.
Results: The sealer exhibited exceptional flow properties (23.78 mm), a balanced setting profile (initial: 65 min; final: 810 min), and low solubility (0.825%), ensuring durability and clinical reliability. Dimensional stability remained within ISO limits (0.417% over 30 days). The sealer maintained a highly alkaline environment (pH 12.3 initially, stabilizing at 10.6 over four weeks), crucial for antimicrobial activity and healing. ATR-FTIR confirmed the stable incorporation of PAC-PLGA NPs, potentially offering antioxidant and collagen-stabilizing benefits.
Conclusion: This novel formulation demonstrates significant improvements in critical clinical parameters, offering a durable and bioactive alternative to traditional calcium hydroxide sealers. While no immediate limitations were observed, further in vivo assessments are recommended to evaluate its long-term sealing efficacy, dentine preservation, and periapical healing outcomes.
Keywords: Calcium hydroxide, Proanthocyanidin nanoparticles, PLGA, Dentine collagen stabilization
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijap.2025v17i2.53094 Journal homepage: https://innovareacademics.in/journals/index.php/ijap
INTRODUCTION
Calcium hydroxide has long been considered a gold standard in endodontic therapy due to its well-established antimicrobial properties and ability to promote hard tissue formation [1]. First introduced in the 1920s, calcium hydroxide revolutionized root canal treatment by facilitating the repair of periapical lesions through its high pH and bactericidal effects [2]. The dissociation of calcium hydroxide into calcium and hydroxyl ions is central to its mechanism of action, creating an alkaline environment that favours mineralization and impedes bacterial survival [3]. However, despite its widespread use, traditional calcium hydroxide-based sealers are associated with several drawbacks, including high solubility, extended setting times, and inadequate mechanical strength [4]. These drawbacks limit the longevity and effectiveness of traditional sealers, particularly due to their susceptibility to dissolution in tissue fluids, leading to a compromised seal over time [1]. Traditional calcium hydroxide-based sealers are widely used for their antimicrobial properties and ability to promote hard tissue formation. However, they suffer from critical limitations, including high solubility, extended setting times, and inadequate mechanical strength, which affect their long-term clinical performance and sealing ability [2]. Addressing these limitations is essential for improving the success of root canal therapies [3]. This increased solubility risks reinfection, undermining the long-term success of root canal treatments [4, 5]. Additionally, the extended setting times inherent to these sealers can complicate clinical procedures, as the delayed setting may lead to premature contamination of the material. Furthermore, the brittle nature of calcium hydroxide contributes to lower compressive strength and poor dimensional stability, making it less durable under the functional stresses of the oral environment [1]. Addressing these limitations requires a material that not only maintains the benefits of calcium hydroxide but also improves mechanical stability and bioactivity [2, 6].
The advent of nanotechnology has presented a promising avenue to address these limitations. Nanoparticles, due to their increased surface area and enhanced reactivity, have the potential to improve the physical and chemical properties of traditional dental materials. However, despite these advancements, challenges remain regarding the long-term clinical stability and bioactivity of nanoparticle-based sealers, necessitating further research to address these gaps. Calcium hydroxide nanoparticles, in particular, have garnered attention for their ability to enhance ion release, improve bioavailability, and reduce setting times [7, 8]. Their smaller particle size allows for better penetration into dentinal tubules, contributing to a tighter seal and improved interaction with the dentine [9, 10].
Beyond mechanical reinforcement, proanthocyanidins (PAC), a class of naturally occurring polyphenolic compounds, have emerged as promising bioactive agents in dental materials. PACs are known for their ability to crosslink collagen, enhance dentine bonding, and increase the mechanical properties of dental tissues [11, 12]. Additionally, PACs exhibit antioxidant properties, which help mitigate the oxidative stress associated with inflammatory responses in periapical tissues, potentially improving healing outcomes [13]. Encapsulating PAC in Poly(lactic-co-glycolic acid) (PLGA) Nanoparticles (NPs) allows for controlled and sustained release, ensuring prolonged bioactivity and enhancing the long-term stability of the sealer [14]. This encapsulation not only protects the PACs from degradation but also facilitates their gradual release, allowing for continuous reinforcement of the dentine-sealer interface.
The development of a calcium hydroxide-based endodontic sealer augmented with PAC nanoparticles represents a significant advancement in dental material science. By combining the bioactivity of calcium hydroxide with the mechanical reinforcement provided by nanoparticles, this formulation aims to overcome the limitations of traditional sealers. The incorporation of PAC-PLGA NPs offers a dual advantage: enhancing the physical properties of the sealer while promoting biocompatibility and potentially improving clinical outcomes through sustained bioactivity [15].
This study focuses on the synthesis and characterization of a novel calcium hydroxide-based endodontic sealer, incorporating PAC NPs encapsulated in PLGA. The physical properties, including flow, setting time, solubility, dimensional stability, and pH, were evaluated according to ISO 6876:2012 and ANSI/ADA standards 57. Attenuated Total Reflectance-Fourier Transform Infrared (ATR-FTIR) spectroscopy was utilized to identify the functional groups in the sealer and confirm the chemical interactions responsible for its enhanced properties. This research aims to provide a comprehensive evaluation of the potential of nanoparticle-enhanced calcium hydroxide-based sealers to address the clinical challenges associated with traditional formulations, ultimately contributing to more effective and durable root canal treatments.
MATERIALS AND METHODS
Synthesis of the calcium hydroxide-based sealer: The sealer was synthesized by preparing two separate components: the base paste and the accelerator paste table 1. The two pastes were mixed in a 1:1 ratio to prepare the final set cement for testing.
Table 1: Composition and role of each ingredient in the base paste and accelerator paste
| Base paste | Accelerator paste |
|
|
Preparation of the base paste
The following components were weighed: calcium hydroxide NPs (40 g), calcium oxide NPs (20 g), silicon dioxide (10 g), PAC-PLGA NPs (10 g), hydrated colophonium (10 g), and phosphoric acid alkyl ester (10 g). The powders were thoroughly mixed in a planetary mixer for 2 h to achieve homogeneity and ensure uniform dispersion of all components.
To break down any agglomerates and further improve dispersion, the base paste mixture was subjected to ultra-sonication for 30 min. This step ensured the fine distribution of nanoparticles throughout the base paste. The base paste was degassed under vacuum for 30 min to remove entrapped air bubbles, which could potentially compromise the mechanical properties of the sealer.
Preparation of accelerator paste
The accelerator paste was prepared by weighing disalicylate (40 g), bismuth hydroxide (20 g), bismuth carbonate (10 g), silicon dioxide (20 g), and phosphoric acid alkyl ester (10 g). The components were mixed using a planetary mixer for 2 h to ensure uniformity. The mixture was subjected to 30 min of ultrasonication to ensure even particle distribution. The accelerator paste was degassed under vacuum for 30 min to eliminate air bubbles and voids.
Final set cement preparation
The base and accelerator pastes were mixed in a 1:1 ratio using a spatula on a glass slab. This ensured a homogenous final set cement for further testing. The final set cement was applied to cylindrical moulds for physical property testing, including flow, setting time, solubility, dimensional stability, and pH analysis.
ATR-FTIR analysis
ATR-FTIR analysis was performed to confirm the presence of key functional groups in the base paste, accelerator paste, and final set cement. Samples of the base paste, accelerator paste, and final set cement were placed on the ATR crystal for direct analysis. FTIR spectra were collected over the wavenumber range of 4000–400 cm⁻¹, with each spectrum obtained by averaging 32 scans at a resolution of 4 cm⁻¹.
Physical and chemical property testing
The physical and chemical properties of the sealer were evaluated following ISO 6876:2012 and ANSI/ADA Specification No. 57 standards.
Setting time
Setting time was determined using the Gilmore needle method. The final set cement was placed into a stainless-steel mould with a 10 mm diameter and 2 mm height. A Gilmore needle with a 2.0 mm diameter flat end was used to assess the initial setting time. The time at which no indentation was observed on the surface of the cement was recorded as the initial setting time. The final setting time was recorded when a 100 g Gilmore needle left no visible indentation on the cement surface. The samples were kept in an incubator at 37 °C with 95% relative humidity during the setting process. Ten measurements were made for the sealer.
Solubility
Ten cylindrical sealer samples were prepared using a 20 mm diameter and 1.5 mm height mould. The samples were allowed to set for three times the recorded setting time. The samples were weighed (W₀) after setting and then placed in distilled water at 37 °C for 24 h. After water immersion, the samples were removed, dried in a desiccator for 48 h, and weighed again (Wf). The difference between the final mass and the initial mass was divided by the initial dry weight of the sample x 100 corresponding to the loss of mass of each specimen expressed as a percentage of solubility. The solubility test was repeated 2 mo later.
Dimensional stability
Dimensional stability was assessed by measuring changes in sample dimensions over time. Cylindrical samples (6 mm diameter, 12 mm height) were immersed in distilled water at 37 °C after setting. Length measurements were taken at 6 h, 24 h, 7 days, and 30 days using a digital calliper to assess dimensional change. As per ISO 6876:2012 requirement sealer should not expand or contract more than 1.0% after setting. The test was implemented ten times for the sealer.
Flow
0.05 ml volume of the sealer was placed on a glass plate (40 mm × 40 mm) and compressed with another glass plate and a mass of 120 g. After 10 min of compression, the diameter of the flattened sealer disc was measured using a digital calliper. Ten tests were done for the sealer.
pH measurement
The pH of both fresh and set samples was measured at different intervals to assess the alkalinity of the sealer: Fresh and set samples were prepared in a 5 mm diameter, 1 mm thick mould and placed in 50 ml of deionized water at 37 °C. pH values were recorded at 3 min, 30 min, 60 min, 12 h, and 24 h for fresh samples. Set samples were measured at 12 h, 24 h, 3 d, 7 d, 2 w, and 4 w. Ten measurements were taken for each time interval.
RESULTS
ATR-FTIR analysis
ATR-FTIR analysis was conducted to identify the functional groups present in the base paste, accelerator paste, and final set cement, including the confirmation of the PAC NPs encapsulated in PLGA. The spectra confirmed the presence of key functional groups associated with the components of the calcium hydroxide-based sealer table 2.
Table 2: Key ATR-FTIR observations in the base paste, accelerator and final set cement
| Component | Peak (cm⁻¹) | Functional group | Significance |
| Base paste | 2950-2850 cm⁻¹ | C-H Stretching | From phosphoric acid alkyl ester, improving adhesion |
| 3200-3600 cm⁻¹ | O-H Stretching | From calcium hydroxide, indicative of high pH | |
| 1750 cm⁻¹ | C=O Stretching | From PLGA encapsulating PAC confirming successful nanoparticle incorporation | |
| 1510-1600 cm⁻¹ | Aromatic C=C Stretching | From PAC, confirming its presence in the sealer matrix | |
| 450-600 cm⁻¹ | Si-O-Si Stretching | From silicon dioxide, contributing to reinforcement | |
| Accelerator paste | 2950-2850 cm⁻¹ | C-H Stretching | From disalicylate, responsible for polymerization |
| 1000-1130 cm⁻¹ | Si-O-Si Stretching | From silicon dioxide, enhancing mechanical properties | |
| Final set cement | 3200-3600 cm⁻¹ | O-H Stretching | From calcium hydroxide, indicating bioactivity |
| 1750 cm⁻¹ | C=O Stretching | Retained from PLGA, confirming the stability of the PAC encapsulation in the final set cement | |
| 1510-1600 cm⁻¹ | Aromatic C=C Stretching | From PAC, confirming that the bioactive nanoparticle remained integrated post-setting | |
| 450-600 cm⁻¹ | Si-O-Si Stretching | Confirming silicon dioxide retention in the final cement |
Basepaste (fig. 1)
3200-3600 cm⁻¹ (O-H Stretching): This broad peak corresponds to O-H stretching vibrations primarily from calcium hydroxide NPs and phenolic groups in PAC. Calcium hydroxide provides the alkaline environment crucial for antibacterial properties, while PAC offers antioxidant and collagen stabilization effects. Hydroxyl groups from calcium hydroxide and phenolic O-H groups from PAC.
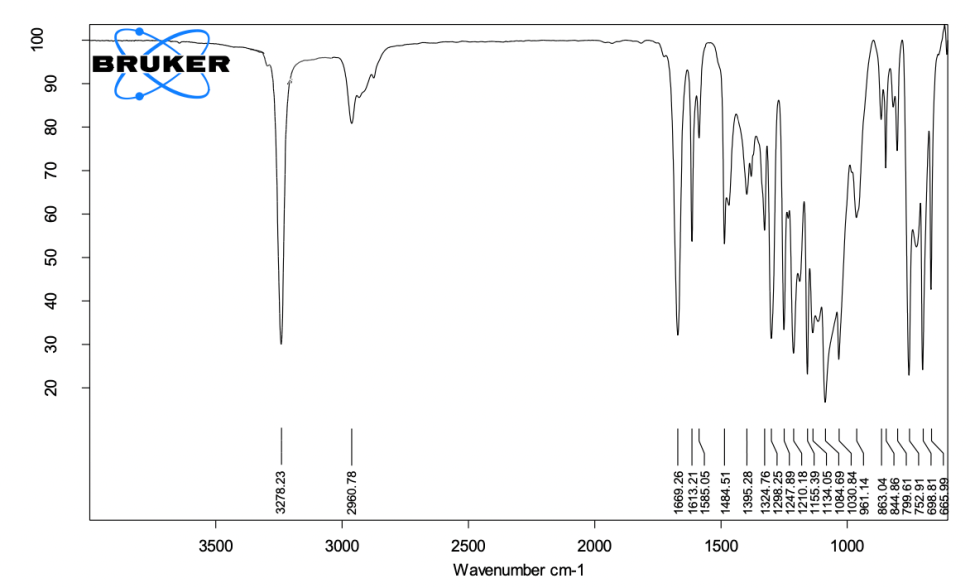
Fig. 1: ATR-FTIR spectra of base paste, the spectrum highlights the key functional groups in the base paste, including O-H stretching (3200–3600 cm⁻¹) from calcium hydroxide nanoparticles and PAC, C=O stretching (1750 cm⁻¹) from PLGA encapsulation, and Si-O stretching (1000–1200 cm⁻¹) from silicon dioxide. Peaks confirm the alkaline environment and the stable incorporation of bioactive nanoparticles
1750 cm⁻¹ (C=O Stretching): This peak is associated with ester carbonyl groups from PLGA (poly(lactic-co-glycolic acid)), which encapsulates the PAC.
This encapsulation ensures the controlled release of the bioactive agent, contributing to prolonged antibacterial and dentine collagen-stabilizing effects. PLGA encapsulates PAC, ensuring its gradual release and sustained bioactivity.
2800-3000 cm⁻¹ (C-H Stretching): The C-H stretching peaks are likely from the organic components in hydrated colophonium, a resinous binder that enhances the mixture's structural stability. C-H bonds from hydrated colophonium, which contributes to the base paste’s mechanical integrity.
1000-1200 cm⁻¹ (Si-O Stretching): Silicon dioxide, included to enhance viscosity and mechanical strength, shows Si-O stretching peaks in this range. Its presence improves the paste’s handling properties and contributes to its final strength. Silicon dioxide filler provides mechanical strength.
700-900 cm⁻¹ (Metal-Oxygen Stretching): Metal-oxygen peaks correspond to calcium oxide and calcium hydroxide nanoparticles. These peaks confirm the essential calcium compounds necessary for the setting process and antibacterial action. Calcium hydroxide and calcium oxide nanoparticles are key for setting reactions and creating an alkaline environment.
Accelerator paste (fig. 2)
3100-3500 cm⁻¹ (N-H Stretching): This peak corresponds to N-H stretching, likely from disalicylate, an essential chelating agent that initiates the setting reaction by interacting with the calcium components of the base paste. Disalicylate helps trigger the cement’s setting and hardening process.
1750 cm⁻¹ (C=O Stretching): This peak reflects the ester functional groups, primarily from the phosphoric acid alkyl ester, which acts as a dispersant and stabilizer. These esters contribute to better mixing and integration of the accelerator paste with the base paste during application. Phosphoric acid alkyl ester stabilises the paste and promotes uniform consistency.
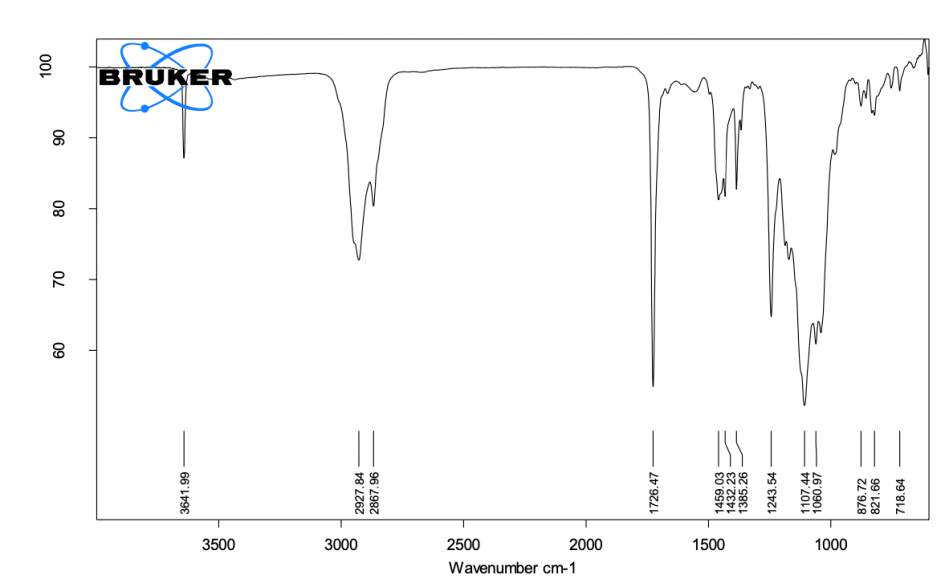
Fig. 2: ATR-FTIR spectra of accelerator paste, the spectrum shows N-H stretching (3100–3500 cm⁻¹) from disalicylate, C=O stretching (1750 cm⁻¹) from phosphoric acid alkyl ester, and Si-O stretching (1000–1200 cm⁻¹) from silicon dioxide. Peaks confirm the functionality of chelating agents and fillers for mechanical stability
1000-1200 cm⁻¹ (Si-O Stretching): Silicon dioxide, used as a viscosity modifier in the accelerator paste, contributes to these peaks. Its presence ensures uniform particle dispersion and enhances the flow properties of the paste. Silicon dioxide provides consistency and viscosity control.
500-700 cm⁻¹ (Bi-O Stretching): The Bi-O stretching peaks correspond to bismuth hydroxide and bismuth carbonate, compounds used to impart radiopacity to the sealer. These peaks confirm that the sealer will be visible in radiographs, which is critical for clinical monitoring. Bismuth hydroxide and bismuth carbonate, are responsible for radiopacity.
Final set cement (fig. 3)
3000-3600 cm⁻¹ (O-H Stretching): This broad peak is associated with O-H stretching, typically from hydroxyl groups. It primarily originates from calcium hydroxide NPS, which contributes to the highly alkaline environment of the sealer, essential for its antibacterial properties. O-H groups from calcium hydroxide NPS and phenolic O-H groups from PAC. These groups contribute to the cement’s biological properties, including antibacterial and antioxidant activity.
1750 cm⁻¹ (C=O Stretching): This peak indicates the presence of carbonyl groups, likely from PLGA used to encapsulate proanthocyanidin nanoparticles. The presence of this peak suggests that the PLGA remains intact in the final set cement, facilitating the controlled release of PAC and its sustained bioactivity. Ester carbonyl groups from PLGA NPS, confirm the encapsulation of PAC, which stabilizes dentine collagen and contributes to the bioactivity of the final cement.
1000-1200 cm⁻¹ (Si-O Stretching): These peaks arise from the silicon dioxide present in both the base and activator pastes. Silicon dioxide is included to enhance the mechanical strength and viscosity of the sealer. In the final set cement, these Si-O peaks confirm the successful integration of the silicon dioxide as a filler, providing structural reinforcement. Si-O bonds from silicon dioxide, contribute to the final cement’s mechanical properties, including improved strength and viscosity.
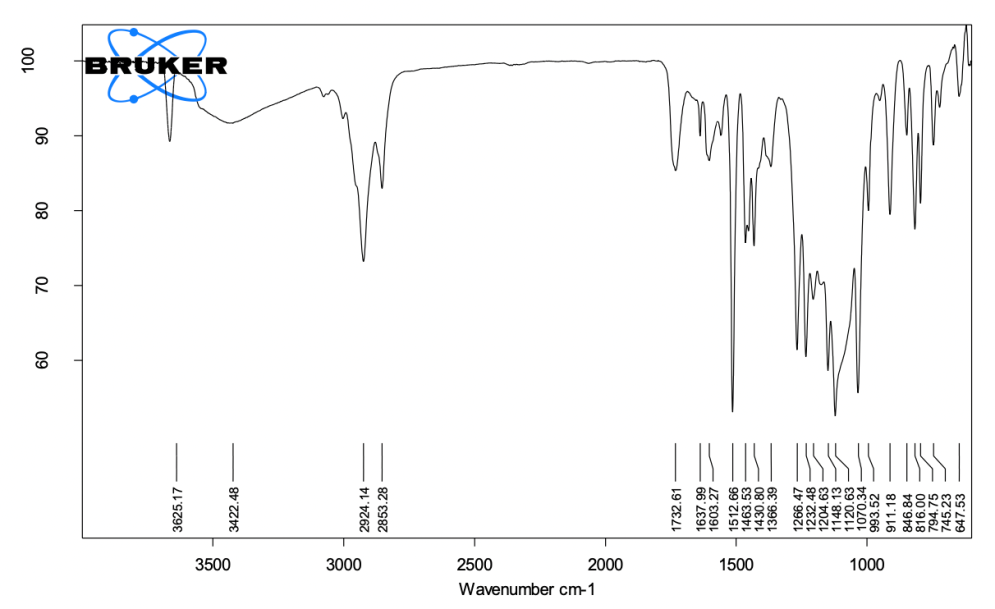
Fig. 3: ATR-FTIR image of the final set cement, the spectrum indicates O-H stretching (3200–3600 cm⁻¹) from calcium hydroxide and PAC, C=O stretching (1750 cm⁻¹) from PLGA encapsulation, and Bi-O stretching (500–700 cm⁻¹) from bismuth compounds, demonstrating radiopacity. Peaks confirm the stability of bioactive components and functional integration
700-900 cm⁻¹ (Metal-Oxygen Stretching): These peaks are associated with metal-oxygen bonds, primarily from calcium oxide and calcium hydroxide nanoparticles. The presence of these peaks indicates that the cement retains its calcium-based components, which are responsible for the alkaline environment and setting reactions of the sealer. Metal-oxygen stretching from calcium hydroxide and calcium oxide is essential for the antibacterial properties and setting mechanism of the cement.
500-700 cm⁻¹ (Bi-O Stretching): These peaks correspond to bismuth compounds, such as bismuth hydroxide and bismuth carbonate, which are included for their radiopacity. The presence of these peaks confirms that the cement has retained its radiopaque properties, crucial for its clinical use in dental applications. Bi-O stretching from bismuth compounds ensures radiopacity, which allows the material to be visible on X-rays.
The ATR-FTIR analysis confirmed the effective integration of both calcium hydroxide and PAC-PLGA NPS within the sealer matrix. Key spectral peaks provide insights into the stability and interaction of these nanoparticles within the sealer. The C=O stretching vibration at 1750 cm⁻¹, associated with the PLGA encapsulation of PAC, demonstrates the encapsulation stability, supporting the controlled and prolonged release of bioactive compounds. This stability is crucial for sustained antioxidant and dentine collagen-stabilizing effects, which enhance the material's clinical performance. Furthermore, the O-H stretching peak between 3200-3600 cm⁻¹, attributed to calcium hydroxide nanoparticles, reflects the sealer’s alkaline nature, a vital factor for its antibacterial efficacy and potential to promote periapical tissue healing. The Si-O-Si stretching peaks observed between 450-600 cm⁻¹, primarily from silicon dioxide, highlight the nanoparticle’s role in reinforcing the sealer matrix, enhancing structural integrity and contributing to the material's dimensional stability as per ISO standards.
Physical and chemical property testing
Setting time
The recorded setting times of 65 min (initial) and 810 min (final) are within the ISO 6876:2012 standards, showcasing an improved setting profile compared to conventional calcium hydroxide sealers. The inclusion of calcium hydroxide nanoparticles plays a critical role here by providing additional nucleation sites that facilitate faster crystallization within the sealer matrix. This optimized setting behaviour minimizes delays in clinical application and reduces risks associated with extended setting times, such as contamination or compromised sealing. Thus, the setting properties of this nanoparticle-infused sealer align well with clinical needs for prompt material hardening and durability table 3.
Table 3: Initial and final setting time of the sealer
| Property | Measured value in min (Mean± SD) | ISO 6876:2012 standard requirement |
| Initial setting time | 65.00±0.3 | ≥ 30 min |
| Final setting time | 810.00±1.5 | ≤ 24 h |
Value represent the mean±standard deviation for initial and final setting time conducted on 10 samples
Solubility
Solubility was calculated by measuring the weight loss of the sealer samples after immersion in distilled water for 24 h. The solubility of the sealer was measured at 0.825%, well within the acceptable limit of 3% according to ISO standards, indicating low solubility and high stability table 4. This low solubility is likely due to the combined effects of silicon dioxide and PAC-PLGA NPS. The hydrophobic nature of PLGA encapsulation minimizes water uptake, thus reducing solubility and enhancing the sealer's durability within the root canal environment. By resisting dissolution, the sealer provides a stable and effective seal, reducing the likelihood of microleakage and promoting successful endodontic outcomes.
Table 4: Percentage of solubility of sealer
| Sample | Initial weight in g (W₀) (mean±SD) |
Final weight in g (Wf) (mean±SD) |
Solubility (%) | ISO requirement (≤ 3%) |
| Calcium hydroxide-based sealer | 0.150±0.02 | 0.1488±0.09 | 0.825% | Compliant |
Solubility was calculated as the percentage weight loss after immersion in distilled water for 24 h at 37 °C. Value represent the mean±standard deviation for 10 replicates.
Dimensional stability
Dimensional stability was assessed by measuring the percentage change in length of the samples after immersion in distilled water for up to 30 d. The dimensional change remained below 1% at all time intervals, in accordance with ISO 6876:2012 standards, indicating excellent dimensional stability table 5. The inclusion of silicon dioxide nanoparticles in the formulation reinforces the material’s structure, while the PAC-PLGA NPs provide an additional stabilizing effect through their encapsulated release system. This dual reinforcement is crucial in ensuring that the sealer does not shrink or expand excessively, which is essential to maintaining a long-lasting seal within the root canal.
Flow
The flow properties of the calcium hydroxide-based sealer were measured by the diameter of the compressed disc formed after 10 min of compression under 120 g of weight. The sealer exhibited a flow of 23.78 mm, exceeding the minimum requirement of 20 mm as per ISO standards, indicating adequate flowability suitable for clinical application table 6.
Table 5: Stability percentage of sealer
| Time interval | Initial length in mm (L₀) (mean±SD) |
Final length in mm (L₁) (mean±SD) |
Dimensional change (%) |
ISO 6876:2012 standard (≤ 1%) |
| 6 h | 12.00± 0.008 | 11.98± 0.003 | 0.167% | Compliant |
| 24 h | 12.00± 0.008 | 11.97± 0.006 | 0.250% | Compliant |
| 7 d | 12.00± 0.008 | 11.95± 0.006 | 0.417% | Compliant |
| 30 d | 12.00± 0.008 | 11.95± 1.008 | 0.417% | Compliant |
Dimensional stability values represent the mean ± standard deviation for 10 replicates, tested over specified time intervals.
Table 6: Flow of sealer
| Property | Measured value in mm (mean±SD) | ISO requirement (≥ 20 mm) |
| Flow | 23.78± 0.008 | Compliant |
Flow measurements represent the mean±standard deviation for 10 replicates
pH measurement
The pH values of both fresh and set samples were recorded at various intervals to evaluate the alkalinity of the calcium hydroxide-based sealer. The results are summarized in the table below table 7. The pH values of the fresh samples remained highly alkaline (pH 12.3 initially) and gradually decreased over time. The set samples maintained alkalinity over 4 w, with a final pH of 10.6. The consistently high pH values, starting at 12.3 in fresh samples and stabilizing at 10.6 over four weeks, reflect the sustained bioactivity provided by the calcium hydroxide NPs. This alkalinity is crucial for antibacterial activity, as well as for creating a favourable environment for periapical tissue healing. The gradual decrease in pH over time indicates a controlled release of hydroxyl ions, aligning with the expected behaviour of calcium hydroxide-based materials. Additionally, the slow, sustained release of PAC from the PLGA NPs offers potential antioxidant benefits, which could further support healing by reducing oxidative stress in periapical tissues. This extended bioactivity aligns with the sealer’s intended role in improving the durability and effectiveness of endodontic treatments.
Table 7: pH of sealer
| Time interval | Fresh sample pH (mean±SD) | Fresh sample pH (mean±SD) |
| 3 min | 12.3±0.005 | - |
| 30 min | 12.2±0.004 | - |
| 60 min | 12.1±0.003 | - |
| 2 h | 12.0±0.003 | - |
| 12 h | 11.8±0.009 | 11.5±0.005 |
| 24 h | 11.7±0.008 | 11.3±0.009 |
| 3 d | - | 11.0±0.009 |
| 7 d | - | 10.8±0.011 |
| 2 w | - | 10.7±0.007 |
| 4 w | - | 10.6±0.008 |
pH values for fresh and set samples are presented as mean±standard deviation for 10 replicates. Measurements were conducted at specified time intervals to evaluate the sustained alkalinity of the sealer.
DISCUSSION
The calcium hydroxide-based endodontic sealer, formulated with PAC NPs encapsulated in PLGA, showed promising improvements in its physical properties. These results suggest that the modified sealer can address the key limitations of traditional calcium hydroxide-based formulations, such as high solubility, prolonged setting times, and poor dimensional stability. Furthermore, the inclusion of PAC NPs, known for their bioactive potential, introduces the possibility of enhanced dentine collagen stabilization, although this was not directly evaluated in this study. The calcium hydroxide NPs enhance the alkaline pH crucial for antibacterial efficacy, contributing to improved healing outcomes. Simultaneously, the controlled release from PAC-PLGA NPs introduces antioxidant properties and dentine collagen stabilization, potentially reducing degradation and ensuring better integration over time. This dual nanoparticle approach enables a sealer formulation that aligns more closely with clinical performance requirements, with increased longevity and reduced solubility. Unlike other nanoparticle-based sealers, such as those incorporating silver or zinc oxide nanoparticles, this formulation leverages the bioactive properties of proanthocyanidin encapsulated in PLGA. While silver nanoparticles primarily offer antimicrobial effects, PAC-PLGA not only provides sustained antibacterial activity but also enhances collagen stabilization and antioxidant properties, addressing a broader spectrum of clinical needs.
Flow is a crucial property that determines the sealer’s ability to penetrate the dentinal tubules and adapt to the complexities of the root canal system. In this study, the calcium hydroxide-based sealer exhibited a flow value of 23.78 mm, surpassing the ISO 6876:2012 minimum requirement of 20 mm. Adequate flow is essential for achieving a complete seal, reducing the risk of voids that could allow for bacterial reinfection [14, 15]. The addition of silicon dioxide nanoparticles, which act as fillers, likely contributed to the increased flow by improving the material’s workability and reducing its viscosity [7, 3]. Hydrated colophonium and phosphoric acid alkyl ester in the base paste further enhanced the material’s rheological properties, making the sealer more manageable during application [10].
The balance between sufficient working time and rapid setting is critical for clinical procedures, where prolonged setting times can increase the risk of contamination and poor sealing. The calcium hydroxide-based sealer demonstrated an initial setting time of 65 min and a final setting time of 810 min, both within the ISO 6876:2012 guidelines. This represents a significant improvement over traditional calcium hydroxide sealers, which are often criticized for their extended setting times [4]. The presence of calcium oxide nanoparticles, which react with water to form additional calcium hydroxide, may have accelerated the setting reaction by providing additional nucleation sites for the crystallization process [8, 17]. This ensures that the sealer achieves optimal mechanical properties in a clinically relevant time frame, minimizing the risk of material displacement before it fully sets. The improved setting profile of the calcium hydroxide-based sealer, with an initial setting time of 65 min and a final setting time of 810 min, highlights the effectiveness of calcium hydroxide nanoparticles in accelerating the setting process. This enhancement is attributed to the nanoparticles’ high surface area, which provides additional nucleation sites, facilitating faster crystallization within the matrix. Faster setting is clinically advantageous as it minimizes the time the material remains vulnerable to contamination, ensuring a more stable seal in the root canal [1, 4]. This optimized setting behaviour is a significant improvement over traditional calcium hydroxide-based sealers, which often have extended setting times that delay treatment completion [1, 18].
The solubility of an endodontic sealer is a critical factor that influences its long-term performance. High solubility can lead to material dissolution, causing voids and reducing the sealer’s ability to maintain an effective seal. In this study, the calcium hydroxide-based sealer demonstrated a solubility of 0.825%, well below the ISO 6876:2012 threshold of 3%. This indicates that the modified sealer possesses excellent resistance to dissolution, which is likely due to the incorporation of silicon dioxide nanoparticles and PLGA encapsulation [6, 17]. The encapsulation of PAC in PLGA provided a hydrophobic barrier that further reduced the material's solubility, enhancing its durability in the aqueous environment of the root canal [12]. Low solubility is essential for maintaining the integrity of the sealer over time, preventing microleakage, and ensuring the long-term success of the endodontic treatment [14, 2]. The observed low solubility (0.825%) indicates a high degree of stability, essential for maintaining the integrity of the sealer over time. Traditional calcium hydroxide sealers are often criticized for high solubility, which can lead to dissolution and jeopardize the treatment's success. The incorporation of PAC-PLGA NPs plays a crucial role in this improvement. The PLGA encapsulation offers a hydrophobic barrier, reducing water uptake and further decreasing solubility. Additionally, silicon dioxide nanoparticles act as structural fillers that improve the packing density and reduce porosity, thus preventing material degradation. Together, these effects contribute to the sealer’s ability to withstand the aqueous environment within the root canal, ultimately reducing microleakage and extending the material’s lifespan [3, 19].
Dimensional stability is another critical property for endodontic sealers. Significant shrinkage or expansion can lead to gaps between the sealer and the root canal walls, increasing the risk of bacterial reinfection. The calcium hydroxide-based sealer exhibited a dimensional change of only 0.417% over 30 d, well within the ISO 6876:2012 limit of ≤1%. This minimal dimensional change suggests that the sealer maintains its volume and integrity after setting, which is essential for long-term performance [5, 9]. The inclusion of silicon dioxide and bismuth hydroxide as reinforcing fillers likely contributed to this stability by enhancing the material’s compressive strength and reducing the risk of shrinkage [10, 8]. The ability of the sealer to retain its dimensions ensures a continuous seal, reducing the likelihood of voids or gaps that could compromise the treatment outcome. The excellent dimensional stability of the sealer, with a minimal dimensional change of 0.417% over 30 d, reflects its resilience against shrinkage or expansion. Dimensional stability is crucial in endodontic applications, as any significant shrinkage or expansion could lead to gaps at the sealer-dentine interface, increasing the risk of bacterial infiltration. The inclusion of silicon dioxide nanoparticles as reinforcing agents provides compressive strength that minimizes the risk of shrinkage, while the sustained release of PAC from the PLGA encapsulation may contribute to internal stabilization by reducing oxidative stress within the sealer matrix [7, 1]. This stability ensures that the sealer maintains a tight seal with dentine, essential for preventing reinfection and ensuring treatment success.
The alkaline pH of calcium hydroxide-based sealers is a key factor in their antimicrobial efficacy and ability to promote periapical healing. In this study, the sealer maintained a highly alkaline pH, starting at 12.3 in fresh samples and gradually decreasing to 10.6 after 4 w. The initial high pH is indicative of the dissociation of calcium hydroxide into calcium and hydroxyl ions, which are known to create an antimicrobial environment and stimulate the formation of hard tissues [18, 13]. Although the pH decreased over time, it remained within an alkaline range which is beneficial for long-term healing and antimicrobial activity. The gradual reduction in pH is typical of calcium hydroxide-based materials and reflects the sustained release of bioactive ions over time, which is essential for promoting tissue repair and preventing reinfection [14, 2].
ATR-FTIR spectroscopy confirmed the presence of key functional groups in the calcium hydroxide-based sealer, including the successful incorporation of PAC NPs encapsulated in PLGA. The characteristic C=O stretching vibration at 1750 cm⁻¹, indicative of the carbonyl group in PLGA, confirmed the stability of the encapsulated nanoparticles throughout the setting process. The presence of aromatic C=C stretching vibrations at 1510-1600 cm⁻¹ confirmed the presence of PACs in the sealer matrix, suggesting that their bioactive properties are retained post-setting. Although dentine collagen stabilization was not directly assessed in this study, PACs have been shown to stabilize collagen fibrils by crosslinking with collagen, reducing enzymatic degradation and enhancing mechanical properties [5, 9]. The slow release of PACs from the PLGA nanoparticles could provide sustained bioactivity, contributing to the long-term stability of the dentine-sealer interface.
While dentine collagen stabilization was not the focus of this study, the release of PACs from PLGA nanoparticles offers a promising avenue for future research. PACs are known to enhance the mechanical properties of the collagen matrix by forming crosslinks that increase resistance to degradation by matrix metalloproteinases (MMPs) [1, 9]. Stabilizing the dentine collagen matrix could reduce the risk of microleakage at the sealer-dentine interface, improving the longevity of the root canal treatment. The sustained release of PACs from the PLGA NPs ensures prolonged bioactivity, which could support the long-term preservation of the dentine structure and contribute to the success of the endodontic treatment [17, 7]. However, the in vivo release kinetics of PAC may vary due to factors such as enzymatic activity, fluid exchange within the root canal system, and interactions with periapical tissues. Future studies should investigate these dynamics to optimize the formulation for sustained clinical performance. Further studies are needed to directly assess the effect of this formulation on dentine collagen stabilization in clinical settings.
The findings of this study highlight the potential of nanoparticle-enhanced endodontic sealers in improving key clinical properties, such as setting time, flow, solubility, dimensional stability, and bioactivity. Future research should explore in vivo applications and assess the direct effects on dentine collagen stabilization and antimicrobial efficacy under clinical conditions. Additionally, limitations such as the absence of direct in vivo validation and the lack of quantitative measures of bioactivity on dentine collagen are acknowledged. Future studies should aim to incorporate animal models and advanced imaging techniques to evaluate these parameters and validate the sealer's clinical efficacy. Additionally, understanding the interactions of these nanoparticles with dentine at a molecular level could inform the development of next-generation sealers with even greater efficacy. The promising results suggest that integrating calcium hydroxide and PAC-PLGA NPs in endodontic sealers could become a viable strategy to overcome the limitations of traditional sealers, ultimately enhancing patient outcomes in endodontic therapy.
CONCLUSION
The incorporation of PAC NPs encapsulated in PLGA into a calcium hydroxide-based sealer resulted in significant improvements in physical properties, including flow, setting time, solubility, and dimensional stability. The material's ability to maintain a highly alkaline pH suggests that it can continue to promote periapical healing and inhibit bacterial growth over extended periods. Although dentine collagen stabilization was not directly assessed, the sustained release of PACs offers potential for future improvements in collagen preservation and long-term treatment success. Future research should focus on optimizing the release kinetics of bioactive agents and assessing the material's performance in vivo to further validate its clinical efficacy. In vivo studies should include evaluations of dentine collagen preservation using animal models and assessments of long-term sealing integrity under dynamic intraoral conditions. Advanced imaging techniques and histological analyses could provide deeper insights into the material's interaction with biological tissues, aiding in the development of next-generation endodontic sealers.
FUNDING
Nil
AUTHORS CONTRIBUTIONS
Conceptualization, V. S., G. S. and R. N. C.; methodology, V. S., G. S., R. N. C. and M. N. H.; software, V. S.; validation, V. S., G. S., R. N. C. and M. N. H.; formal analysis, V. S., G. S., R. N. C. and M. N. H.; investigation, V. S., G. S., R. N. C. and M. N. H.; resources, V. S.; data curation, V. S.; writing—original draft preparation, V. S., G. S., R. N. C. and M. N. H.; writing—review and editing, V. S., G. S., R. N. C. and M. N. H.; visualization, V. S.; supervision, V. S., G. S., R. N. C. and M. N. H.; project administration, V. S. and M. N. H.; funding acquisition, V. S. All authors have read and agreed to the published version of the manuscript.
CONFLICT OF INTERESTS
Declared none
REFERENCES
Foreman PC, Barnes IE. Review of calcium hydroxide. Int Endod J. 1990 Nov;23(6):283-97. doi: 10.1111/j.1365-2591.1990.tb00108.x, PMID 2098345.
Mohammadi Z, Dummer PM. Properties and applications of calcium hydroxide in endodontics and dental traumatology. Int Endod J. 2011 Aug;44(8):697-730. doi: 10.1111/j.1365-2591.2011.01886.x, PMID 21535021.
Kim D, Kim E. Antimicrobial effect of calcium hydroxide as an intracanal medicament in root canal treatment: a literature review-part I. In vitro studies. Restor Dent Endod. 2014 Nov;39(4):241-52. doi: 10.5395/rde.2014.39.4.241, PMID 25383341.
Siqueira JF Jr, Lopes HP. Mechanisms of antimicrobial activity of calcium hydroxide: a critical review. Int Endod J. 1999 Sep;32(5):361-9. doi: 10.1046/j.1365-2591.1999.00275.x, PMID 10551109.
Pashley DH, Tay FR, Yiu C, Hashimoto M, Breschi L, Carvalho RM. Collagen degradation by host-derived enzymes during aging. J Dent Res. 2004;83(3):216-21. doi: 10.1177/154405910408300306, PMID 14981122.
Siqueira JF Jr, Rocas IN, Ricucci D, Hulsmann M. Causes and management of post-treatment apical periodontitis. Br Dent J. 2014;216(6):305-12. doi: 10.1038/sj.bdj.2014.200, PMID 24651336.
Chopra V, Davis G, Baysan A. Physico-chemical properties of calcium-silicate vs. resin based sealers-a systematic review and meta-analysis of laboratory-based studies. Materials (Basel). 2021;15(1):229. doi: 10.3390/ma15010229, PMID 35009375.
Capuano N, Amato A, Dell’Annunziata F, Giordano F, Folliero V, Di Spirito F. Nanoparticles and their antibacterial application in endodontics. Antibiotics (Basel). 2023;12(12):1690. doi: 10.3390/antibiotics12121690, PMID 38136724.
Javidi M, Zarei M, Naghavi N, Mortazavi M, Nejat AH. Zinc oxide nano-particles as sealer in endodontics and its sealing ability. Contemp Clin Dent. 2014;5(1):20-4. doi: 10.4103/0976-237X.128656, PMID 24808690.
Sabatini C, Pashley DH. Mechanisms regulating the degradation of dentin matrices by endogenous dentin proteases and their role in dental adhesion. A review. Am J Dent. 2014 Aug;27(4):203-14. PMID 25831604.
Aguiar TR, Vidal CM, Phansalkar RS, Todorova I, Napolitano JG, McAlpine JB. Dentin biomodification potential depends on polyphenol source. J Dent Res. 2014 Apr;93(4):417-22. doi: 10.1177/0022034514523783, PMID 24574140.
Bedran Russo AK, Pauli GF, Chen SN, McAlpine J, Castellan CS, Phansalkar RS. Dentin biomodification: strategies, renewable resources and clinical applications. Dent Mater. 2014 Jan;30(1):62-76. doi: 10.1016/j.dental.2013.10.012, PMID 24309436.
Makadia HK, Siegel SJ. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers (Basel). 2011 Sep 1;3(3):1377-97. doi: 10.3390/polym3031377, PMID 22577513.
Shariati A, Chegini Z, Ghaznavi-Rad E, Zare EN, Hosseini SM. PLGA-based nanoplatforms in drug delivery for inhibition and destruction of microbial biofilm. Front Cell Infect Microbiol. 2022 Jun 21;12:926363. doi: 10.3389/fcimb.2022.926363, PMID 35800390.
Cao L, Xie X, Yu W, Xu HH, Bai Y, Zhang K. Novel protein-repellent and antibacterial polymethyl methacrylate dental resin in water-aging for 6 mo. BMC Oral Health. 2022 Oct 29;22(1):457. doi: 10.1186/s12903-022-02506-6, PMID 36309721.
Han B, Jaurequi J, Tang BW, Nimni ME. Proanthocyanidin: a natural crosslinking reagent for stabilizing collagen matrices. J Biomed Mater Res A. 2003;65(1):118-24. doi: 10.1002/jbm.a.10460, PMID 12635161.
Castellan CS, Pereira PN, Grande RH, Bedran Russo AK. Mechanical characterization of proanthocyanidin-dentin matrix interaction. Dent Mater. 2010;26(10):968-73. doi: 10.1016/j.dental.2010.06.001, PMID 20650510.
Liu R, Fang M, Xiao Y, Li F, Yu L, Zhao S. The effect of transient proanthocyanidins preconditioning on the cross-linking and mechanical properties of demineralized dentin. J Mater Sci Mater Med. 2011 Nov;22(11):2403-11. doi: 10.1007/s10856-011-4430-4, PMID 21979164.
Mazzoni A, Angeloni V, Apolonio FM, Scotti N, Tjäderhane L, Tezvergil Mutluay A. Effect of carbodiimide (EDC) on the bond stability of etch-and-rinse adhesive systems. Dent Mater. 2013;29(10):1040-7. doi: 10.1016/j.dental.2013.07.010, PMID 23916318.
Xu HH, Weir MD, Sun L, Takagi S, Chow LC. Effects of calcium phosphate nanoparticles on Ca-PO4 composite. J Dent Res. 2007;86(4):378-83. doi: 10.1177/154405910708600415, PMID 17384036.