
Int J App Pharm, Vol 17, Issue 2, 2025, 225-239Original Article
DEVELOPMENT AND OPTIMIZATION OF FAST-DISSOLVING BILASTINE SUBLINGUAL FILMS USING STATISTICAL DESIGN
JASMINE LIBERATA FERNANDES1, PRASHANT JIVAJI BHIDE1, SHAILA ANGELA LEWIS2*
1Department of Pharmaceutics, Goa College of Pharmacy, 18th June Road, Panaji, Goa-403001, India. 2Department of Pharmaceutics, Manipal College of Pharmaceutical Sciences, Manipal Academy of Higher Education (MAHE), Manipal-576104, Karnataka, India
*Corresponding author: Shaila Angela Lewis; *Email: s.lewis@manipal.edu
Received: 22 Nov 2024, Revised and Accepted: 05 Feb 2025
ABSTRACT
Objective: This present research study was undertaken with the objective of formulating a fast-dissolving sublingual film of pre-solubilized bilastine. The aim was to overcome the need for acidic gastric media for the solubilization of bilastine, eliminating the concern of reduced bioavailability due to interaction with food and providing a faster onset of action, owing to the high vascularity in the sublingual region.
Methods: Solubility enhancement of bilastine was achieved by inclusion-complex formation of bilastine and Hydroxypropyl Beta-Cyclodextrin (2-HP-β-CD). Fast-dissolving sublingual films were formulated by incorporating bilastine-2-HP-β-CD in the film prepared using the solvent casting method, wherein the sublingual film formulation was optimized using the Design of Experiments (DoE) approach by applying 23 factorial designs. The optimized sublingual film was evaluated.
Results: Evaluation tests revealed that the optimized sublingual film possessed adequate flexibility and tensile strength. It exhibited a faster in vitro drug release of 97.41%, which was achieved in 7 min compared to a 35.77% in vitro drug release demonstrated by the pure drug-containing film. This indicates a 2.7-fold enhancement in dissolution with an average disintegration time of 41.66±0.57 s. The ex vivo permeation studies showed an in vitro drug release of 95.21%, indicating good permeation.
Conclusion: Based on the research findings obtained, it is possible to conclude that the developed quick-dissolving sublingual film of bilastine possessed an enhanced dissolution profile and exhibited a faster onset of action. These results demonstrate the potential to avoid food interactions and improve patient compliance. Incorporating bilastine into sublingual films offers superior benefits over conventional oral formulations, such as circumventing hepatic first-pass metabolism and providing quick relief. Thus, it serves as a potential alternative for the treatment of chronic urticaria and allergic rhinitis.
Keywords: Bilastine, Fast dissolving sublingual film, HP-β-CD inclusion complex, Design of experiments, Full factorial design
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijap.2025v17i2.53250 Journal homepage: https://innovareacademics.in/journals/index.php/ijap
INTRODUCTION
Chronic urticaria is an unsettling and debilitating allergic condition of the skin. They can be of long duration and result not only in a substantial reduction in quality of life but also a negative socioeconomic impact due to work productivity impairment [1, 2]. Bilastine is a new-generation, very specific histamine H1 receptor antagonist that was very recently granted approval as the symptomatic therapy for Chronic Urticaria (CU) and Allergic Rhinitis (AR). Bilastine possesses poor aqueous solubility and high lipophilicity (belonging to the Biopharmaceutical Classification System (BCS) class II) [3–5]. Bilastine dissolves well in an acidic environment; therefore, it is prescribed 1 h before food or 2 h after food. The presence of food significantly reduces the bioavailability. The presence of food significantly reduces the bioavailability. Studies report that in the presence of high-fat meals, bioavailability is reduced by up to 30%, while in low-fat meals, bioavailability is reduced to as low as 25% [6]. This is one of the major issues that affect the efficacy of bilastine in conventional tablet formulations available on the market, and it may also result in poor patient compliance. The patients are required to take bilastine either before meals on an empty stomach or must wait for two hours after meals for effective therapy, causing discomfort to patients and hence resulting in poor patient compliance. This serves as a significant challenge in the therapy of allergic conditions by bilastine. If bilastine is administered in a pre-solubilized form in a suitable dosage form and via suitable route of administration, it avoids the need for acidic gastric media for solubilization of the drug and hence eliminates the problem of reduced bioavailability as a result of interaction with food. Low aqueous solubility presents as one of the major critical concerns commonly encountered in the development of new chemical entities and generics. It is estimated that over 40% of the New Chemical Entities (NCEs) designed and introduced by the pharmaceutical industry sector are essentially practically water-insoluble. Solubility poses a tricky challenge for formulation scientists, as these low aqueous soluble drugs possessing slow drug absorption cause insufficient and erratic bioavailability and gastrointestinal mucosal toxicity [7, 8]. Various studies have been executed over the years in an effort to enhance the bioavailability and increase the aqueous solubility of BCS Class II drugs. Novel drug delivery systems employing nanotechnology have been immensely successful in overcoming these limitations. However, these platforms very often fail to transform into a translative product due to several challenges such as feasibility, difficulty scaling up, complex development processes, reproducibility issues, and, very importantly, safety and stability concerns. Hence, simpler dosage forms that can be easily developed, scaled up, and commercialized are a need of the hour [9-13]. Various non-nano-formulation approaches can be employed for solubility enhancement of the BCS Class II drug, such as pH adjustment technique, complex formation, solid dispersion, etc. Complexing a drug with cyclodextrin not only increases the solubility but also aids in taste masking, increased permeation from the polymer matrix, and stability [8]. Quick-dissolving films were introduced in the realm of clinical drug therapy as an alternative to tablets, capsules, and liquid orals, especially for paediatrics and geriatrics patient populations during the latter half of the 1970s, owing to their difficulty in swallowing conventional standard oral dosage forms. The orally fast disintegrating films are solid dosage forms that disintegrate and solubilize quickly within seconds in the buccal cavity without the requirement of water [14]. For drugs needing rapid absorption and fast onset of action, sublingual delivery of a drug serves as a very promising route of administration, leading to an overall increased bioavailability. This is owing to the existence of thin mucosa and considerably higher permeability in the sublingual region, which permits the direct entry of the drug into the bloodstream, bypassing the pre-systemic metabolism [15]. In allergic conditions, a quick-acting formulation is required for a quick onset of action, and symptomatic relief in allergic manifestations is desired. Hence, we formulated rapidly disintegrating sublingual films of bilastine using 2-hydroxypropyl beta-cyclodextrin (2-HP-β-CD) to overcome the limitations of poor aqueous solubility and food-drug interactions for faster onset of action and improved patient compliance.
MATERIALS AND METHODS
Materials
Bilastine was provided as a kind gift by Symed Labs Ltd. Hyderabad, Telangana. 2-Hydroxypropyl-Beta-Cyclodextrin, Beta-Cyclodextrin, and Croscarmellose Sodium were generously obtained as gifts from Signet Chemical Corporation Pvt. Ltd, Mumbai. Citric acid monohydrate, Glycerin, Sodium dihydrogen orthophosphate dihydrate, Sodium hydroxide, Methanol, and Calcium chloride were purchased from Molychem, Mumbai. HPMC E5 was received as a generous contribution from Colorcon Asia Ltd, Goa. All other excipients, solvents, and chemical reagents utilized for the study were of analytical grade.
Methods
Preliminary studies
Compatibility studies between drug and excipients
The IR spectra of the bilastine and a physical mixture of bilastine and excipients were taken on an FT-IR coupled with an ATR (IR affinity-1S, Shimadzu). The samples were analyzed from 4000-400 cm-1, and the resulting spectra were compared against the standard FTIR spectrum of the pure drug to determine any possible interactions or changes in the characteristic peaks of the sample drug.
Selection of a solubilizing agent
0.01 M of stock solution of Beta-cyclodextrin (βCD) and 2-Hydroxypropyl-Beta-Cyclodextrin (HPβCD) was prepared individually in distilled water. Molar solutions ranging from 0.002 to 0.01 M βCD and 2-HPβCD were prepared by adequately diluting in distilled water. Vials with screw caps were filled with 5 ml of each molar solution, and the surplus amount of the drug was added to each vial individually. Using a laboratory shaker, the vials were shaken for a period of about 48 h at room temperature. The supernatant solution was subsequently filtered through a 0.45 μm PTFE membrane. The filtered solutions were analyzed spectrophotometrically (LabIndia, UV 3092) at 273 nm to assess the concentration of bilastine present [16].
Optimization of solubilizing agent
10 ml of distilled water containing citric acid was poured into the screw-capped vials, and then 2-HPβCD in the concentration range of 100-600 mg was added separately to these vials and kept on a magnetic stirrer till a transparent solution was attained. Then, the surplus amount of the bilastine was added to each vial. A laboratory shaker was then used to agitate the vials at room temperature for 48 h. The supernatant solutions were subsequently filtered through a 0.45 µm PTFE membrane. The filter-treated solutions were analyzed spectrophotometrically to determine the concentration of the drug present (LabIndia, UV 3092). The absorbance of the resultant solutions was scanned at 273 nm.
Screening of film former, plasticiser, and super disintegrant
Various types of film formers of natural and synthetic origin were evaluated for their film-forming capabilities. Among the natural polymers, sodium alginate, Gellan gum, and pectin, and among synthetic polymers, HPMC E5, HPMC E15, and HPMC E50 were evaluated for their film-forming capabilities. The use of PEG-400, glycerin, and dibutyl phthalate as a plasticising agent was evaluated. Super disintegrants like croscarmellose sodium, crospovidone, and sodium starch glycolate were evaluated. The film formers were soaked separately in the required quantity of distilled water overnight, following which the film formers, plasticisers, and disintegrants were solubilized in distilled water in various fixed amounts using a magnetic stirrer set at 100 rpm. The prepared solution was poured onto a Petri plate, which was then placed in a hot air oven and dried overnight at 45 °C to obtain the sublingual films. The films were carefully examined for any imperfections, cuts, peel-ability without rupturing, tackiness, folding endurance, and disintegration time. These parameters were assessed to decide the best polymer, plasticiser, super disintegrant, and the required concentration range of polymer, plasticiser, and super disintegrant for film formulation [17, 18].
Folding endurance
Folding endurance was determined by the amount of times the film sample could be repeatedly folded at the same location until it fractured or formed visible cracks in the film [14, 18].
Disintegration test
The petri-plate technique was carried out to evaluate the disintegration time of the films. 10 ml of simulated salivary fluid (phosphate buffer pH 6.8) was filled in a Petri plate, and a film bearing a dimension of about (2×2 cm2) was kept in it. The duration taken by the film to completely disintegrate was noted [17, 18].
Optimization of quick-dissolving sublingual film
Optimization of the sublingual fast-dissolving film was done using the Design of Experiments (DoE) approach. A 23 factorial design from Design Expert® Software (Version 13, Stat-Ease, Inc, Minneapolis, MN) was applied to optimize the concentration of excipients utilized for the sublingual film formulation. Film former, plasticiser, and super disintegrant concentration were adopted as the independent variable. The impact of these independent variables on the dependent responses, such as tensile strength, folding endurance, and disintegration time, was examined. Five center points were selected in 23 factorial designs as these designs use only two levels, i. e., high and low concentration. The incorporation of center points allows midpoint concentrations to be introduced and accounted for, ensuring reliability in the experimental data obtained. Utilising Design Expert® Software, the data acquired were statistically subjected to one-way ANOVA to test the significance of the model, where p<0.05 was considered to be significant. The optimum formulation was determined based on the software’s suggested solutions depending upon the possession of desirable properties such as adequately high tensile strength, high folding endurance, and low disintegration time. This was then formulated into a sublingual fast-dissolving film, which was evaluated for various parameters [19].
Preparation of quick-dissolving sublingual film of bilastine
Preparation of polymer solution
The polymer Hydroxypropyl Methyl Cellulose E5 (HPMC E5) was soaked for 24 h in 4 ml distilled water to prepare the polymeric solution.
Preparation of bilastine liquid dispersion
To 6 ml of distilled water, the required amount of glycerine was added, and this solution was stirred on a magnetic stirrer at 350 rpm for 5 min, following which the required quantity of 2-HP-β-CD was added and stirred for 1 h at 350 rpm to obtain a homogenous solution. Finally, the drug was added slowly, part by part, and the mixture was stirred for a period of 2 h at 350 rpm until a clear solution was obtained.
Preparation of bilastine-loaded sublingual quick-dissolving films
The bilastine liquid dispersion was slowly added, part by part, to the polymer solution along with the remaining excipients, which were kept on the magnetic stirrer at 120 rpm, and mixing was continued for one hour. After achieving a homogenous solution, it was transferred into identical Petri dishes and spread evenly throughout the Petri dishes, after which they were kept in the vacuum oven at 40 °C overnight for drying. After drying, the dried films were packaged in aluminum foil and placed in the desiccator [18, 20, 21].
Evaluation of the optimized bilastine liquid dispersion
FT-IR spectroscopy
FT-IR was conducted to provide confirmatory evidence of the encapsulation of bilastine with 2-HP-β-CD molecules. The IR spectrum of the bilastine liquid dispersion was obtained using FT-IR coupled with an ATR (Shimadzu, IRAffinity-1S). The samples were recorded from 4000-400 cm-1, and the resulting spectrum was compared against the reference IR spectrum of pure drug [14].
X-Ray diffraction study
X-ray diffractogram of the prepared liquid dispersion of bilastine was scanned employing a diffractometer (Bruker, D8 Advance A25). XRD analysis was conducted to determine any alterations in the crystallinity of the encapsulated drug in the liquid dispersion containing 2-2-HP-β-CD. The appropriately dried and pulverized bilastine liquid dispersion was evenly spread on an amorphous silica holder attached to the goniometer. The diffractometer was set to run at an accelerating voltage of 40 mV and a fixed tube current of 40 mA, and recording was taken from 5-40⁰ with a B-B geometry [14].
Total drug content
1 ml of the liquid dispersion containing 20 mg of bilastine was placed in a typical volumetric flask. This dispersion was suitably diluted with phosphate buffer pH 6.8, and drug content was analyzed by reading the absorbance at 273 nm with a UV-visible spectrophotometer (LabIndia UV 3092)[14].
Evaluation of quick-dissolving sublingual films of bilastine
General appearance
The films were carefully evaluated for size, colour, odour, surface texture, and any visibly noticeable physical imperfections [18, 21].
SEM study
The morphology of the surface of optimized sublingual film formulation was evaluated with a scanning electron microscope (Carl Zeiss) [20, 22].
Thickness
The thickness of the optimized film was examined with a digitized Vernier Caliper (Mitutoyo Japan, Digimatic Caliper), having an accuracy of 0.01 mm. The thickness was evaluated at three separate locations of the film, and the mean with standard deviation was calculated [23].
Weight variation
Three random film samples (2×2 cm2) were weighed individually on an analytical weighing balance (JB 1603-L-C, Mettler Toledo, USA). The mean with standard deviation was calculated [18, 24].
Surface pH
This test was conducted to study the pH of the surface of the sublingual film because changes in the pH of the film can result in irritation to the buccal mucosa. The surface pH measurement of the film was performed by wetting the surface of the film with 500 microlitres of distilled water and then using a calibrated pH meter (Genial, BioEra Life Sciences) to determine the pH. This study was done in triplicate (n=3), and the mean with standard deviation was determined [18, 25].
Drug content uniformity
The content uniformity of the film was evaluated by solubilizing the film in a beaker comprising 100 ml of phosphate buffer pH 6.8 (simulated salivary fluid) and stirring over a magnetic stirrer for 1 h. Upon filtration and appropriate dilution, absorbance was read at 273 nm with the aid of a UV spectrophotometer (LabIndia, UV 3092). The study was conducted in triplicate (n=3), and the mean with standard deviation was computed [18, 25–27].

Folding endurance
Folding endurance is defined as the amount of folds necessary to snap the film by continuously folding the film at the same location until the film fractures or develops noticeable cracks. This demonstrates the brittleness of the film. The test required folding a 2×2 cm2 size film continuously at the same spot until it broke or developed any visible cracks. The study was conducted in triplicate (n=3), and the mean with standard deviation was determined [14, 18, 27, 28].
Tensile strength
A tensile strength study was executed to ascertain the strength and elasticity of the optimized film formulation. It is known as the maximum stress exerted to a point at which the film snaps. A laboratory-designed in-house tensile strength tester was employed to measure the tensile strength of the film. To test the tensile strength, the film strip was clamped longitudinally in the tensile grip of the tester such that one end of the 2×2 cm2 size film strip was fixed to the suspending pan, and the opposite end was clamped at the stationary end. The weights were added gradually in increasing order until the film was broken. The below formula was used to measure the tensile strength of the optimized film formulation. The study involved analysis of 3 samples of the film (n=3), and the mean with standard deviation was computed [18, 25–27, 29].
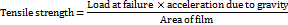
Disintegration test
The duration taken by a film to entirely disintegrate was assessed using the petri-plate method. The disintegration study was conducted by keeping a film measuring 2×2 cm2 in a glass petri dish consisting of 10 ml of pH 6.8 phosphate buffer (simulated salivary fluid) as the test medium, and the medium’s surface was left uninterrupted, that is, without being stirred. The duration taken for the entire film to disintegrate was noted. The study was conducted in triplicate (n=3), and the mean with standard deviation was recorded [18, 30].
Percent elongation
On exerting stress to the film (2×2 cm2), it leads to elongation or stretching of the film, which is known as strain and can be expressed as the deformation of the film prior to its breakage under stress. It is calculated by noting the difference in the sublingual film length both prior to and after the application of stress. Percent elongation can be calculated by using the formula given below. The study was done in triplicate (n=3), and the mean with standard deviation was recorded [14, 18].
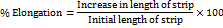
Percentage moisture loss
This study was conducted to ascertain the structural integrity and physical stability of the optimized sublingual film. Films measuring (2x2 cm2) were sampled and accurately measured on an analytical weighing balance. After completion of the weighing, the films were carefully stored for 72 h in a desiccator comprising fused anhydrous calcium chloride. The films were reweighed at the end of 72 h, and the percentage of moisture loss was determined with the formula given below. The study was conducted in triplicate, and the mean with standard deviation was calculated [14, 18, 25–27].
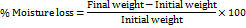
Percent moisture content
Films measuring (2×2 cm2) were sampled, placed in a porcelain dish, and accurately measured on a weighing balance. 3 of these film samples were weighed and kept in a hot air oven at 105 ⁰C for 2 h. On completion of the specified time, the films were reweighed, and the formula given below was used to determine the percent moisture content. The study was executed in triplicate, and the mean with standard deviation was recorded [18].
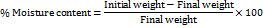
DSC
DSC (TA instruments, Q20, Germany) was used to conduct a thermal analysis of the optimized film formulation. The thermogram was subjected to DSC analysis to track any shifts, disappearances, or appearances of new peaks. The required amount of the sample was kept in a DSC pan, and the DSC machine was run at 20 °C/min [14, 18, 21].
In vitro dissolution test
The dissolution test for the optimized film formulation was performed with phosphate buffer pH 6.8 (simulated salivary fluid) as the dissolution medium. The study was conducted by placing the film in a beaker consisting of 30 ml of the dissolution medium. The medium was regulated at 37±0.5 °C by continual stirring at 100 rpm with a magnetic stirrer. Sample aliquots of 5 ml were collected at predefined time intervals and filtered through 0.45µm Whatman filter paper. The collected samples were replenished with fresh dissolution medium to regulate sink condition. The drug content of the sample solution was analyzed by measuring absorbance at 273 nm after appropriate dilutions by a UV-visible spectrophotometer (LabIndia, UV 3092). The study was executed in triplicates (n=3), and the cumulative percentage of drug release was determined [18, 21].
Ex-vivo permeation test
A Franz diffusion cell was employed for the ex-vivo permeation test of the optimized film (2×2 cm2) on porcine sublingual mucosa obtained from the slaughterhouse. Porcine buccal mucosa shows a close resemblance to the human buccal mucosa with regards to its morphological structure, enzymatic activity, composition of lipids, and, most importantly, its permeability characteristics. As a result, it is widely acknowledged as a standard animal model for carrying out permeability studies. Lately, this tissue has gained preference for its use in permeability studies as it is easily available and economically feasible, with fewer ethical restrictions in comparison to human buccal mucosa [31, 32]. In this test, the receptor compartment consisted of phosphate buffer pH 6.8(simulated salivary fluid) as the diffusion medium. The medium was regulated at 37±0.5 °C by consistent stirring at 100 rpm with a magnetic stirrer. The film was adhered to the porcine sublingual mucosa membrane in the middle of the receptor and donor compartments. At predefined time points, samples of 5 ml were collected from the diffusion medium. The samples collected were substituted with fresh phosphate buffer pH 6.8 to sustain sink conditions every time a sample was withdrawn for analysis. Aliquots were collected, filtered, and analyzed by measuring absorbance at 273 nm after appropriate dilutions by a UV-visible spectrophotometer (LabIndia, UV 3092) [23].
Short-term stability studies
The optimized sublingual films were evaluated for their short-term stability at room temperature for a period of 90 d. At 0, 30, 60, and 90 d, films were sampled and evaluated for physical appearance, surface pH, disintegration time, folding endurance, and percent drug content after 0, 30, 60, and 90 d [23].
RESULTS AND DISCUSSIONS
Preliminary studies
Compatibility studies between the drug and the excipients
FT-IR spectral analysis was employed to detect any possible interactions between bilastine and the various excipients used in the film formulation. The spectra were scanned across the 4000-600 cm-1 frequency range. The FT-IR spectra of bilastine and the physical mixture of bilastine and the excipients employed in the formulation showed that the major peaks at the characteristic absorption bands of pure bilastine are present, indicating that there was a lack of significant interaction of bilastine with excipients used in the film formulation. Hence, it can be interpreted that bilastine and the excipients are compatible and safe to be employed in the film formulation.
Selecting the suitable solubilizing agent for bilastine
HPβCD was chosen as an appropriate solubilizing agent as bilastine was present in higher concentrations in 2-HPβCD in comparison to βCD and natural cyclodextrins such as βCD possess poor aqueous solubility than their derivatives such as 2-HPβCD.
Optimization of solubilizing agent
Bilastine exhibited a maximum solubility of 9.788 mg/ml in 30 mg/ml of 2-HP-β-CD. Hence, 30 mg/ml was chosen as the concentration of solubilising agent required for the effective solubilization of bilastine in the formulation.
Table 1: The concentration of bilastine in CDs
| Concentration of Cyclodextrin (mmol) | Concentration of drug in βCD (μg/ml) | Concentration of drug in 2-HPβCD (μg/ml) |
| 0.002 | 84.92 | 107.40 |
| 0.004 | 101.80 | 132.80 |
| 0.006 | 106.00 | 175.00 |
| 0.008 | 110.9 | 186.30 |
| 0.01 | 118.7 | 203.20 |
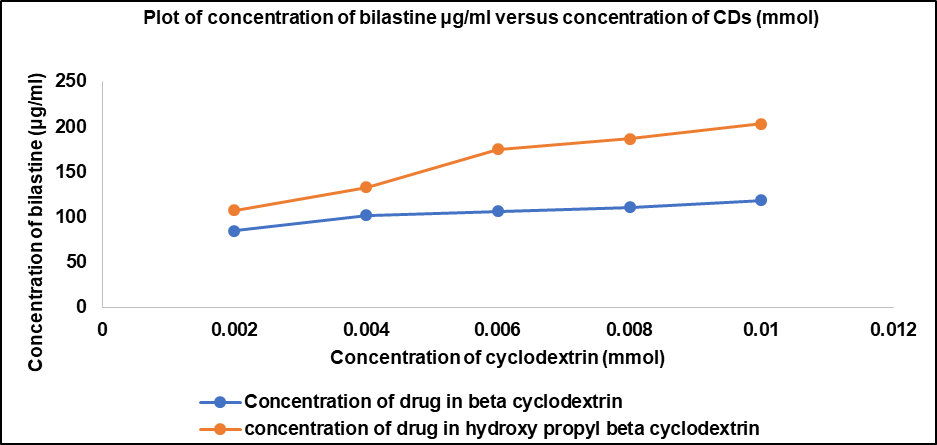
Fig. 1: Plot of concentration of bilastine (μg/ml) versus concentration of CDs (mmol)
Table 2: The concentration of bilastine in 2-HβCD
| S. No. | pH 6.8 | 2-HPβCD (mg/ml) | Drug (mg/ml) |
| 1 | 10 | 6.169 | |
| 2 | 15 | 7.859 | |
| 3 | 20 | 8.352 | |
| 4 | 25 | 9.197 | |
| 6 | 30 | 9.788 |
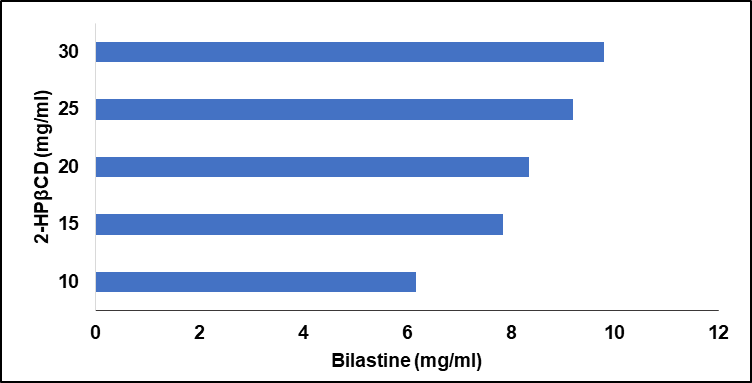
Fig. 2: A clustered bar graph depicting the concentration of bilastine (mg/ml) in different concentrations of 2-HPβCD (mg/ml)

Fig. 3: Screening studies
Table 3: Screening study results of film formers
| S. No. | Polymers utilized | Concentration range %w/v | Film forming capacity | Appearance |
| 1 | Sodium Alginate | 2 | Poor | Rough, translucent |
| 2 | Gellan gum | 2 | Satisfactory | Slightly rough, translucent |
| 3 | Pectin | 2 | Good | Smooth, translucent |
| 4 | HPMC E5 | 2 | Excellent | Smooth, transparent |
| 5 | HPMC E15 | 2 | Very good | Smooth, transparent |
| 6 | HPMC E50 | 2 | Good | Smooth, transparent |
Table 4: Screening study results for super disintegrants
| S. No. | Super disintegrants utilised | Quantity taken | Disintegration time (s)* |
| 1 | Crospovidone | 20 mg | 62.00± 2.94 |
| 2 | Croscarmellose sodium | 20 mg | 37.00±0.81 |
| 3 | Sodium starch glycolate | 20 mg | 45.33± 1.24 |
*Experiment was performed in triplicates (n=3) and each value is expressed as mean±SD.
Various types of film formers from natural as well as synthetic origins were evaluated for their film-forming capabilities, and HPMC E5 was selected as a film-forming agent due to its desired properties. The use of PEG-400, glycerin, and polyethylene glycol as a plasticising agent was evaluated, and glycerin was selected as a suitable plasticising agent. Super disintegrants like crospovidone, croscarmellose sodium, and sodium starch glycolate were evaluated, and croscarmellose sodium was selected as a suitable super disintegrant. After the selection of the film former, plasticiser, and super disintegrant, different concentrations of these components were examined to determine the lower and upper limits of the different concentrations of the independent variables essential for employing the factorial design. Based on the trials conducted, HPMC E5 gave films with the most desired properties at the concentration range of 1.5-2.5 % w/v. Glycerin was found to be a suitable plasticiser, producing films with good flexibility and optimum plasticity in the concentration range of 0.8-1.4%w/v, and croscarmellose sodium was found to demonstrate optimum disintegration at the concentration range of 10-30 mg.
Optimization of quick-dissolving sublingual film using statistical design
Quick dissolving sublingual film of bilastine was formulated employing the solvent casting method of film formation. HPMC E5 as film former, Glycerin as plasticiser, and Croscarmellose sodium as super disintegrating agent were utilized for the formulation development based on the preliminary tests performed. Films were formulated using varied concentrations of HPMC E5, Glycerin, and croscarmellose sodium, as recommended by the experimental design, and were optimized by statistically and graphically analyzing the data with the Design Expert® 13 software.
Table 5: Optimisation of quick dissolving film formulations of bilastine
| Run | Factor 1 | Factor 2 | Factor 3 | Response 1 | Response 2 | Response 3 |
| A: Concentration of film former | B: Concentration of plasticiser | C: Concentration of superdisintegrant | Tensile strength | Folding endurance | Disintegration time | |
| mg | mg | mg | g/sq. cm | s | ||
| 1 | 150 | 140 | 10 | 95.55 | 170 | 37 |
| 2 | 250 | 140 | 30 | 149.45 | 191 | 55 |
| 3 | 200 | 110 | 20 | 120.05 | 162 | 48 |
| 4 | 250 | 140 | 10 | 151.9 | 204 | 63 |
| 5 | 250 | 80 | 10 | 124.95 | 186 | 54 |
| 6 | 200 | 110 | 20 | 105.35 | 170 | 41 |
| 7 | 200 | 110 | 20 | 107.8 | 168 | 43 |
| 8 | 200 | 110 | 20 | 110.25 | 163 | 40 |
| 9 | 150 | 80 | 30 | 78.4 | 132 | 29 |
| 10 | 150 | 80 | 10 | 88.2 | 138 | 35 |
| 11 | 250 | 80 | 30 | 122.5 | 177 | 44 |
| 12 | 150 | 140 | 30 | 93.1 | 168 | 31 |
| 13 | 200 | 110 | 20 | 102.9 | 154 | 39 |
The 23 factorial design having 5 center points was utilised to determine the ideal concentration of film former, plasticiser, and super disintegrating agent to attain desirable film characteristics (table 1). The obtained design matrix of 13 runs displayed the tensile strength, folding endurance, and disintegration time in the range of 78.4-151.9 g/cm2, 132-204 times, and 29-63 s, respectively. Residual analysis (R²) and Polynomial equations were obtained on the basis of best-fitted models, with the positive sign in a polynomial equation suggesting an increment in the response owing to the relevant factor.
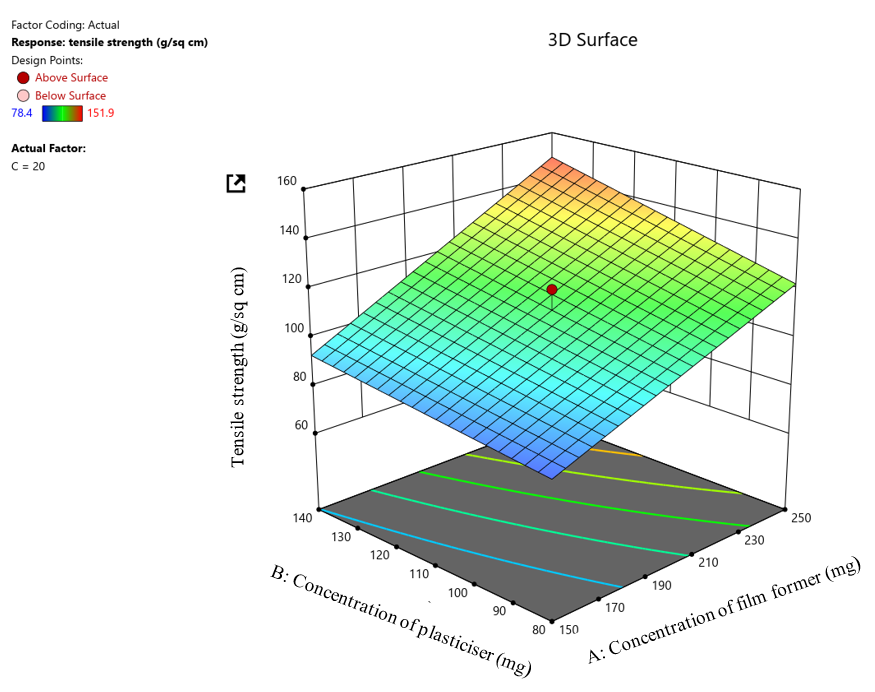
Fig. 4: 3D surface plot for tensile strength
Table 6: Statistical data table for tensile strength response
| Source | Sum of squares | df | mean square | F-value | p-value | |
| Model | 5530.55 | 3 | 1843.52 | 60.28 | <0.0001 | significant |
| A-concentration of film former | 4682.70 | 1 | 4682.70 | 153.11 | <0.0001 | |
| B-concentration of plasticiser | 721.05 | 1 | 721.05 | 23.58 | 0.0009 | |
| AB | 126.80 | 1 | 126.80 | 4.15 | 0.0722 | |
| Residual | 275.25 | 9 | 30.58 | |||
| Lack of Fit | 99.98 | 5 | 20.00 | 0.4563 | 0.7939 | not significant |
| Pure Error | 175.27 | 4 | 43.82 | |||
| Cor total | 5805.80 | 12 |
The significant impact of independent variables/factors on dependent variables/responses was assessed by using ANOVA. ANOVA test results for tensile strength revealed that the. The model F-value obtained was 60.28, which indicates that the model is significant. P-values obtained were all under 0.0500, signifying the model terms are significant. Therefore, A (film former concentration) and B (plasticiser concentration) are significant model terms. value larger than 0.1000 implied that the model terms are insignificant. The Lack of Fit F-value obtained was 0.46, indicating the Lack of Fit is insignificant relative to the pure error. The Predicted R² of 0.9171 is in reasonable agreement with the Adjusted R² of 0.9368; i. e. the difference is less than 0.2
Based on ANOVA results, the polynomial equation was generated.
In a polynomial equation, a positive sign denotes a synergistic effect, and a negative sign suggests an antagonistic effect. The polynomial equation for tensile strength response is given as:
Tensile Strength =+111.57+24.19A+9.49B+3.98AB
Where, A is the film former concentration, B is the Plasticiser concentration, and AB is an interaction term. From the equation, it can be interpreted that both film former and plasticiser concentration positively impact the tensile strength response, i. e., with an increase in film former and plasticiser concentration, the tensile strength response also increases. However, the film former has a stronger influence than the plasticiser concentration on the tensile strength response. The positive interaction term indicates a synergistic effect of film former and plasticiser by 3.98 units in addition to their individual effects on the tensile strength response. A Pareto chart depicting the effect of independent variables (A=film former concentration and B= plasticiser concentration) on tensile strength response was generated and is available in the supplementary data. It is a graphical tool for managing model choice in two-level factorial designs. The Pareto chart illustrated the effect of independent variables such as film former and plasticiser concentration on the dependent response, i. e., Tensile strength. If the factor exceeds the t value limit, it is suggested to exert some effect on the dependent response. Similarly, if the factor exceeds the Bonferroni limit, it is suggested that the factor exerts a significantly greater effect on the dependent response. From the fig. generated, it is observed that both the film former and plasticiser concentration exerts a considerably greater effect on the dependent tensile strength response, as it is seen to exceed both the t-value and Bonferroni limits. Additionally, it is also visible that the concentration of film former possessed a superior effect compared to the concentration of plasticiser on the tensile strength response.
3D Surface plot depicting the effect of independent variables (A=film former concentration and B=plasticiser concentration) on tensile strength response. The 3D Surface plot depicts the impact of independent factors, such as the concentration of film former and plasticizer, on the dependent response of tensile strength. It is seen from the 3D surface plot (fig. 2 A) that as the concentration of film former increases, the tensile strength of the film also increases. Similarly, as the concentration of plasticiser is increased, the tensile strength of the film also increases but to a lesser extent in comparison to the effect exerted by the concentration of film former on the tensile strength response.
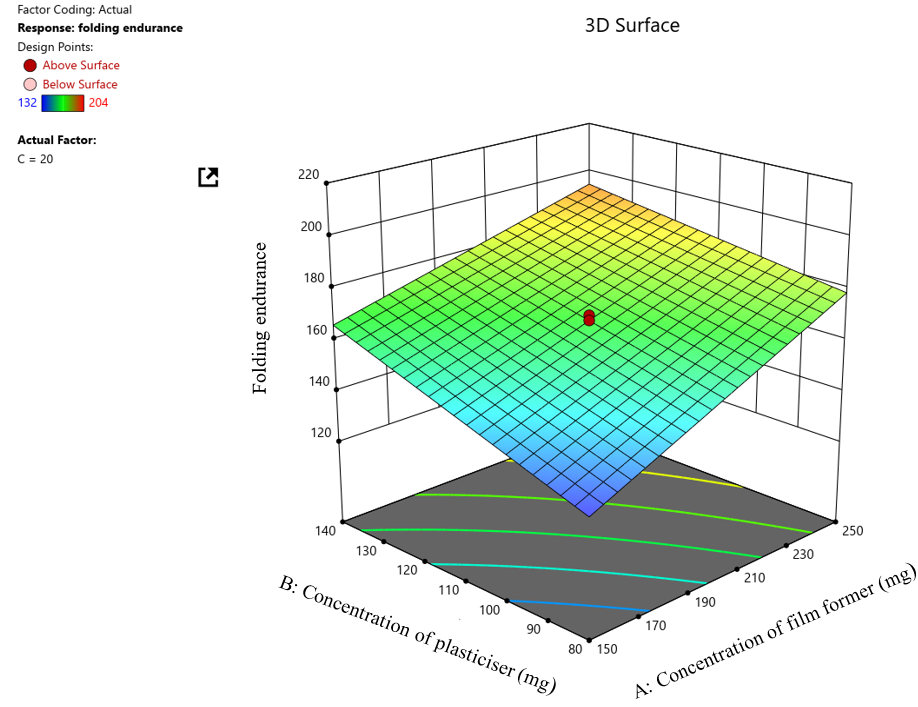
Fig. 5: 3D Surface plot for folding endurance
Table 7: Statistical data table for folding endurance response
| Source | Sum of squares | df | mean square | F-value | p-value | |
| Model | 4224.50 | 3 | 1408.17 | 27.17 | <0.0001 | significant |
| A-concentration of film former | 2812.50 | 1 | 2812.50 | 54.27 | <0.0001 | |
| B-concentration of plasticiser | 1250.00 | 1 | 1250.00 | 24.12 | 0.0008 | |
| AB | 162.00 | 1 | 162.00 | 3.13 | 0.1109 | |
| Residual | 466.42 | 9 | 51.82 | |||
| Lack of Fit | 311.22 | 5 | 62.24 | 1.60 | 0.3338 | not significant |
| Pure Error | 155.20 | 4 | 38.80 | |||
| Cor total | 4690.92 | 12 |
ANOVA test results for folding endurance revealed a Model F-value of 27.17,which implied the model’s significance. P-values obtained were below 0.0500, indicating the model terms are significant. Thus, A (concentration of film former) and B (concentration of plasticiser) are significant model terms. The Lack of Fit F-value obtained was 1.60,which suggested that the Lack of Fit is insignificant relative to the pure error. The Predicted R² of 0.7873 is in reasonable agreement with the Adjusted R² of 0.8674, i. e., the difference is less than 0.2.
In a polynomial equation, a positive sign is suggestive of a synergistic effect, and a negative sign denotes an antagonistic effect. The polynomial equation for folding endurance response is given as:
Folding endurance =+167.92+18.75A+12.50 B – 4.50 AB
Where A is the film former concentration, B is the plasticiser concentration, and AB is an interaction term. From the equation, it can be interpreted that both film former and plasticiser concentration positively impact the folding endurance response, i. e., with an increase in film former, the structural integrity of the film enhances increasing the folding endurance response. Similarly, with an increase in plasticiser concentration, the flexibility of the film improves, causing folding endurance to increase. It is also noted that the film former has a stronger influence than the plasticiser concentration on the folding endurance response. However, the negative interaction term indicates an antagonistic effect when the film former and plasticiser concentration increases together. The increase in folding endurance is lesser than expected on the basis of their individual effects. This implies that higher concentration levels of both film former and plasticiser may negatively impact the folding endurance, structural integrity, and flexibility of the film. The Pareto chart depicted the impact of independent variables such as film formers and plasticisers concentration on the dependent response, i. e., Folding endurance. From the fig. generated, it is observed that both the film former and the plasticiser concentration exerts a significantly greater effect on the dependent Folding endurance response as it is seen to exceed both the t-value and Bonferroni limits. Additionally, it is also visible that the concentration of film former possessed a superior effect compared to the concentration of plasticiser on the folding endurance response.
The 3D Surface plot depicts the impact of independent factors such as concentration of film former and plasticiser on the dependent response of folding endurance. It is seen from the 3D surface plot (fig. 2 B) that as the concentration of film former increases, the folding endurance of the film also increases. Similarly, as the concentration of plasticiser is increased, the folding endurance of the film also increases but to a lesser extent in comparison to the effect exerted by the concentration of film former on the folding endurance response.
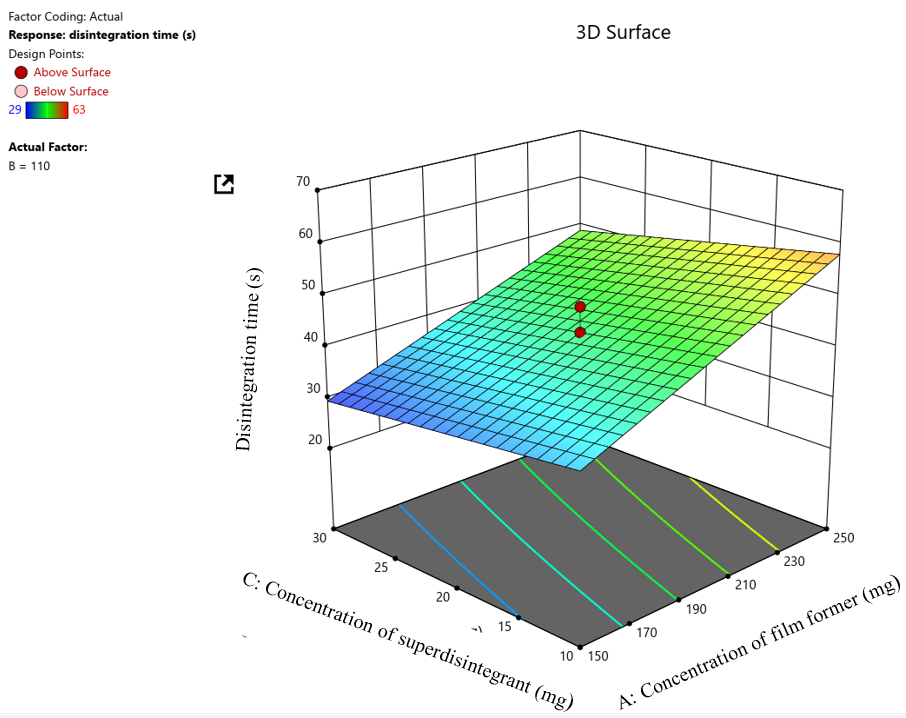
Fig. 6: 3D Surface plot for disintegration time
ANOVA test results for disintegration time revealed a Model F-value of 14.08, which implied the model’s significance. P-values obtained were below 0.0500, indicating that the model terms are significant. Hence, A (Concentration of film former) and C (concentration of super disintegrating agent) are significant model terms. The Lack of Fit F-value obtained was 0.41, which suggested that the Lack of Fit is insignificant relative to the pure error. The Predicted R² of 0.8994 is in reasonable agreement with the Adjusted R² of 0.8841, i. e., the difference is less than 0.2. In a polynomial equation, a positive sign is suggestive of a synergistic effect, and a negative sign is an implication of an antagonistic effect. The polynomial equation for disintegration time response is given as:
Disintegration time =+43.00+10.50 A+3.00 B – 3.75 C+2.00 AB – 0.7500 AC+0.2500 BC+0.2500 ABC
Table 8: Statistical data table for disintegration time response
| Source | Sum of squares | df | Mean square | F-value | p-value | |
| Model | 1104.00 | 7 | 157.71 | 14.08 | 0.0051 | significant |
| A-concentration of film former | 882.00 | 1 | 882.00 | 78.75 | 0.0003 | |
| B-concentration of plasticiser | 72.00 | 1 | 72.00 | 6.43 | 0.0522 | |
| C-concentration of superdisintegrant | 112.50 | 1 | 112.50 | 10.04 | 0.0248 | |
| AB | 32.00 | 1 | 32.00 | 2.86 | 0.1518 | |
| AC | 4.50 | 1 | 4.50 | 0.4018 | 0.5540 | |
| BC | 0.5000 | 1 | 0.5000 | 0.0446 | 0.8410 | |
| ABC | 0.5000 | 1 | 0.5000 | 0.0446 | 0.8410 | |
| Residual | 56.00 | 5 | 11.20 | |||
| Lack of Fit | 5.20 | 1 | 5.20 | 0.4094 | 0.5571 | not significant |
| Pure Error | 50.80 | 4 | 12.70 | |||
| Cor total | 1160.00 | 12 |
Where A is the film former concentration, B is the plasticiser concentration, C is the disintegrant concentration, and ABC is an interaction term. From the equation, it can be interpreted that an increase in the film former and plasticiser concentration increases the disintegration time of the film. This is due to the fact that as the concentration of film former increases, the structural integrity and thickness of the film also increase, resulting in longer film disintegration time. Similarly, as the concentration of plasticiser increases, films become stickier and more cohesive, causing a delay in the disintegration of the film. It is also observed that the disintegrant concentration has an antagonistic effect implied by the negative sign, i. e., with an increase in the super disintegrant concentration, there is a decrease in the disintegration time response. This will result in the rapid disintegration of the films. The interaction terms demonstrated how the different combinations of the three factors affect the disintegration time response.+2.00AB interaction term indicates a synergistic effect of film former and plasticiser concentration on disintegration time response. This implies that the combined increase in the concentration of film former and plasticiser, in addition to their individual effects, causes a further increase in the disintegration time of the film. The –0.7500A Cinteraction term indicates an antagonistic effect of the concentration of film former and the concentration of the super disintegrant on the disintegration time response. This implies that the combined increase in the film former and the superdisintegrant concentration results in a slight reduction in the film disintegration time. The +0.2500BC interaction term indicates a slight synergistic effect of the concentration of plasticiser and superdisintegrant on the disintegration time response, implying that the film will require slightly more time to disintegrate when both the concentration of plasticiser and superdisintegrant is increased. In this interaction term, the slight delay in disintegration may be due to the increase in plasticiser concentration, which makes the films sticky and cohesive. Lastly, the+0.2500ABC interaction term implies a slight synergistic effect of all three involved factors on the disintegration time response, leading to a slightly longer disintegration time.
Pareto chart depicting the effect of independent variables (A=film former concentration and C= superdisintegrant concentration) on disintegration time response.
Pareto chart depicted the effect of independent variables such as film former and plasticiser concentration on the dependent response i. e., Disintegration time. From the fig. generated, it is observed that the concentration of film former exerts a significantly greater effect on the dependent tensile strength response as it is seen to exceed both the t-value and Bonferroni limits, but the concentration of super disintegrant only exceeded the t-value limit indicating that the concentration of superdisintegrant exerts some effect on the disintegration time response, but this effect is not as significant as the effect exerted by the concentration of the film former.
3D Surface plot depicting the effect of independent variables (A=film former concentration and C=superdisintegrant concentration) on Disintegration time response. The 3D Surface plot depicts the impact of independent factors, such as the concentration of film former and superdisintegrant, on the disintegration time response. It is seen from the 3D surface plot (Fig. 2C) that as the concentration of film former increases, the disintegration time of the film also increases, indicating that the film will take a longer time to disintegrate. As the concentration of superdisintegrant increases, the disintegration time of the film decreases. The 3D surface plot also reveals that the effect exerted by the concentration of film former was significantly greater than that of the concentration of superdisintegrant on disintegration time response.
Evaluation of bilastine liquid dispersion
X-ray diffraction of the optimized liquid dispersion
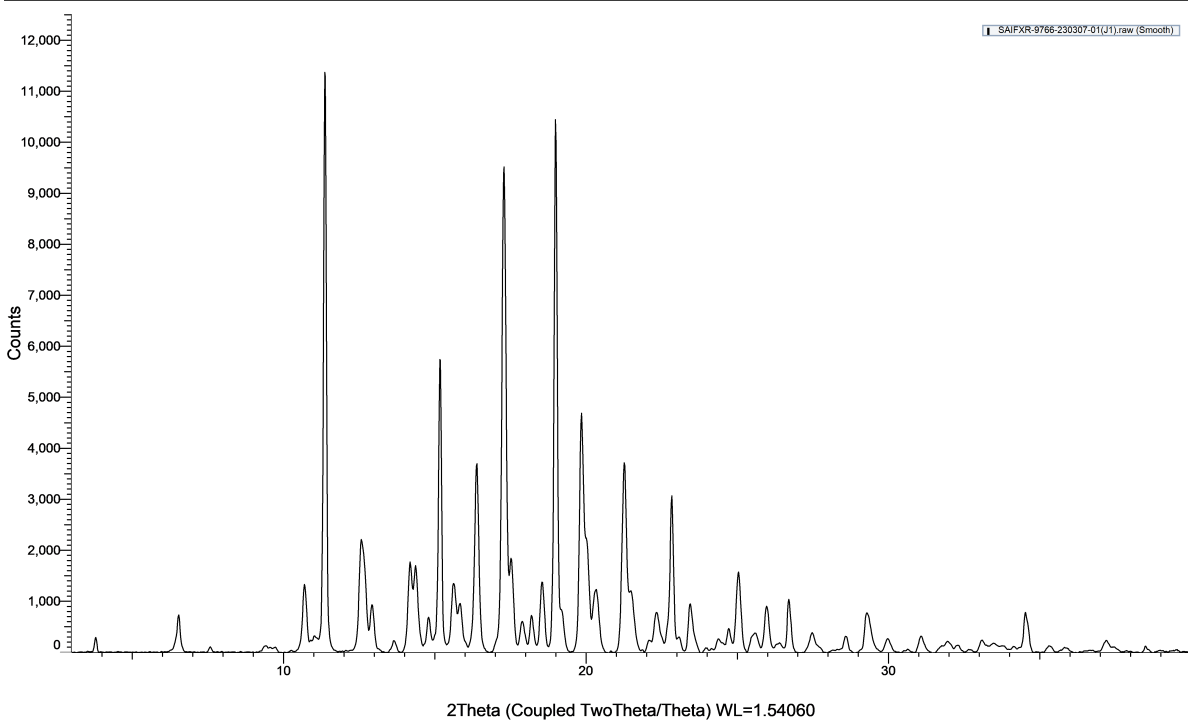
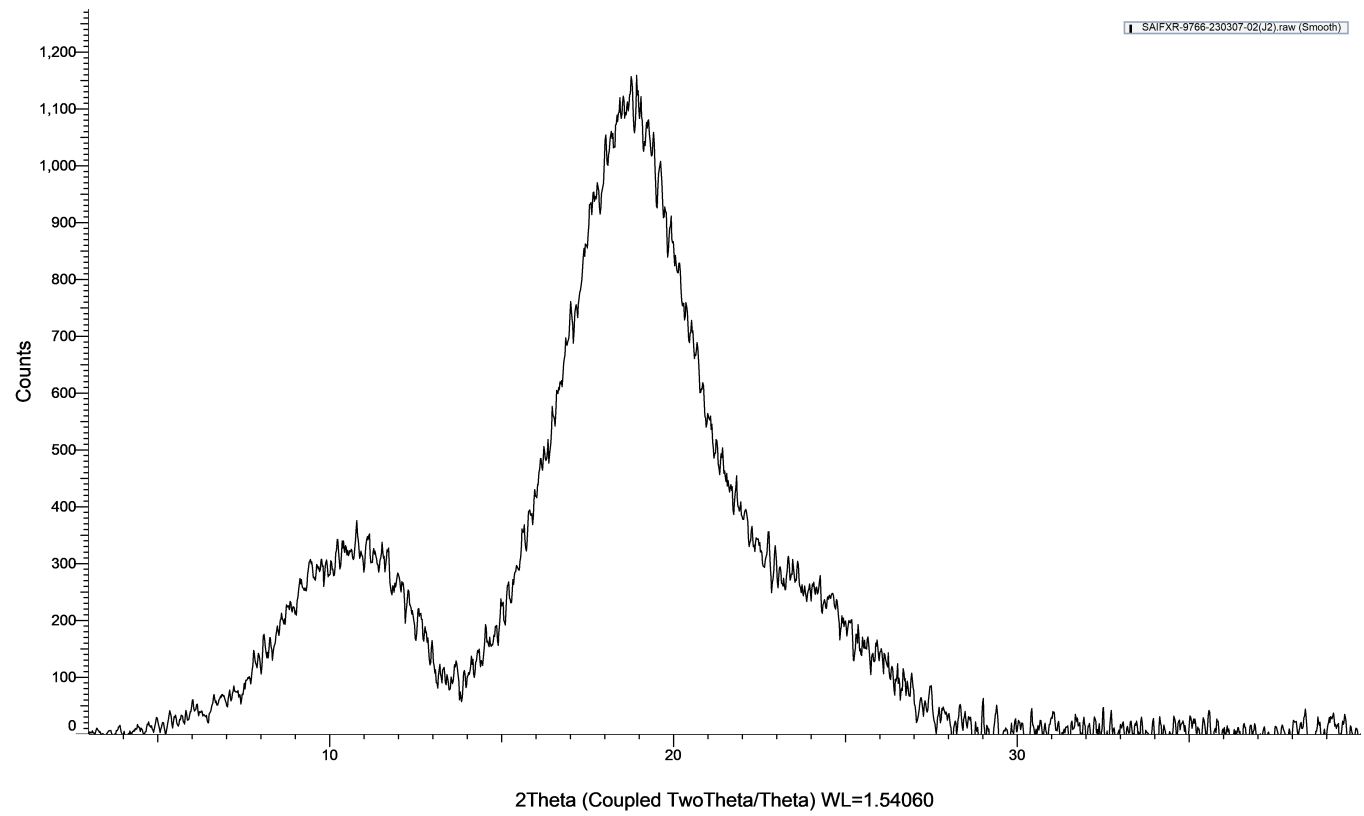
The diffractogram of pure bilastine displayed intense crystalline peaks, while the diffractogram of optimized liquid dispersion did not show any sharp peaks of bilastine. It was also observed that there was a disappearance of major characteristic peaks of bilastine and a reduction in peak intensity, along with a significant broadening of the remaining observed peaks, confirming the transformation of bilastine from crystalline insoluble form to an amorphous form possessing superior aqueous solubility.
FT-IR spectroscopy
FT-IR spectroscopy has been established as an efficient tool in identifying the characteristic functional groups available in a material by relating to the characteristic peaks obtained by scanning the spectrum of the material under investigation in the IR range. The drug was characterized by FTIR spectroscopy, and the spectrum was recorded using an FT-IR Spectrophotometer. The major peaks present in the FT-IR spectra help in the identification of the drug compound. The characteristic peaks of pure bilastine were observed at 3080.32 (O-H stretching (Carboxylic acid)), 2974.23, 2927.94, 2856.58 (C-H stretching (Alkane)), 1688.78 (C=O stretching (Conjugated ketone)), 1456.26 (OH bending (Carboxylic acid)), 1253.73 (C-N stretching (Aromatic amine)) and 1118.71 (C-O peak (Aliphatic ether))
In the FT-IR spectrum of the optimized bilastine liquid dispersion shown in Figure 4, it can be observed that the distinct peaks of pure bilastine (3080.32, 2974.23, 2927.94, 2856.58 cm-1) are absent, which provides confirmatory evidence of the encapsulation of bilastine within 2-HP-β-CD. It can also be seen that the absorption bands significantly widen and move to lower wave numbers.
Total drug content of bilastine in optimized liquid dispersion
The drug content of the optimized liquid dispersion was determined to be 9.78±0.14 mg/ml, which was equivalent to the amount of bilastine incorporated into the liquid dispersion (i. e., 10 mg/ml).
General Appearance
The prepared films were translucent with a smooth texture and devoid of any visible physical deformities.
Thickness
The optimized film formulation displayed a thickness of about 155.3±12 μm. Results indicate satisfactory thickness for sublingual administration and uniformity of the film, which adheres to the reported range of 5-200 μm.
A
B
Fig. 7: A) XRD diffractogram of pure bilastine B) XRD diffractogram of bilastine liquid dispersion
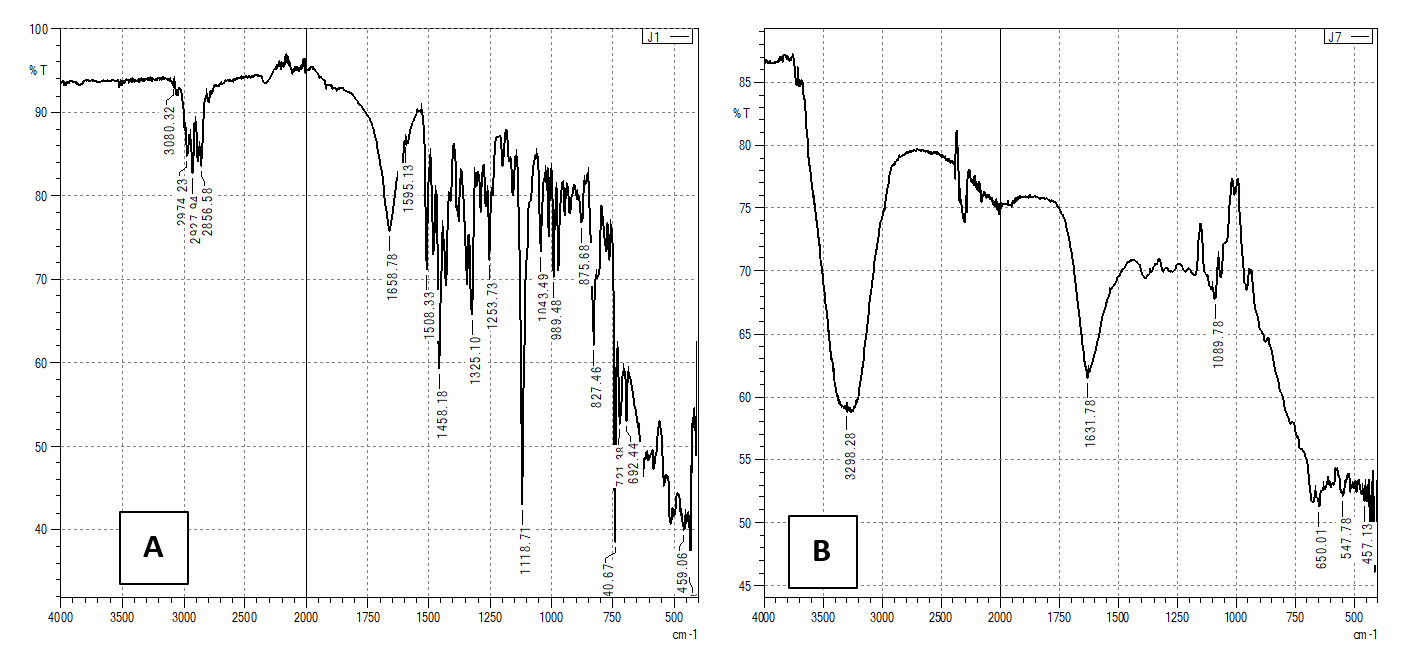
Fig. 8: A) FT-IR spectrum of pure drug bilastine and B) FT-IR spectrum of optimised liquid dispersion of bilastine
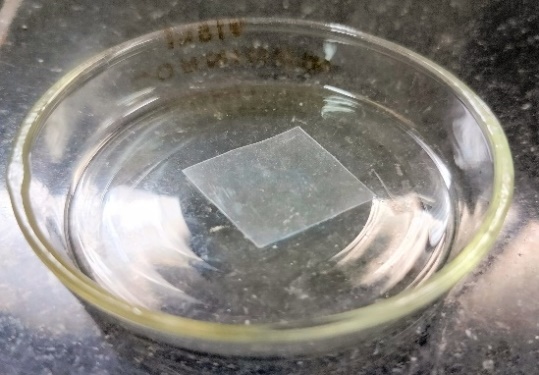
Fig. 9: Optimized bilastine loaded fast-dissolving sublingual film
Weight variation
The weight variation test can assist in confirming the precision of the dose distribution in the sublingual film. The test was conducted in triplicate. The mean and standard deviation weight of the optimized film (2×2 cm2) was found to be 42.08±0.51 mg demonstrating the uniformity in the film.
Surface pH
The optimized film exhibited a pH of 6.73±0.02, which was found to be in the range limit of normal saliva pH range (6.2 to 7.6). Hence, the results indicate that there was no risk of irritation to the oral mucosa upon administration.
Drug content uniformity
The percent drug content of the optimized film was discovered to be 95.01±1.07%, suggesting that there was a uniform distribution of the drug throughout the film.
Folding endurance
The physical integrity and flexibility of thin polymeric sublingual films can be assessed by their folding endurance. The folding endurance of the optimized film formulation was discovered to be 169±1.00, which indicated good flexibility.
Tensile strength
Films should have enough toughness and structural integrity to withstand the stress that can be generated during manufacturing and handling, as determined by tensile strength. The obtained tensile strength of the optimized film was 111.06±1.41 g/cm2. The overall result indicated the ability of the film to endure high strain before rupture.
Disintegration test
The in vitro disintegration time of the optimized film was found to be 41.66±0.57 s which indicated rapid disintegration of the film, thereby suggesting faster drug release and, subsequently, faster submucosal absorption upon administration.
Percent elongation
The films which exhibit an overall high tensile strength but low percent elongation is regarded as fragile and rigid. Hence, it is necessary that a film has an adequate percentage of elongation. The obtained percentage elongation of the optimized sublingual film was 23.33±2.88%.
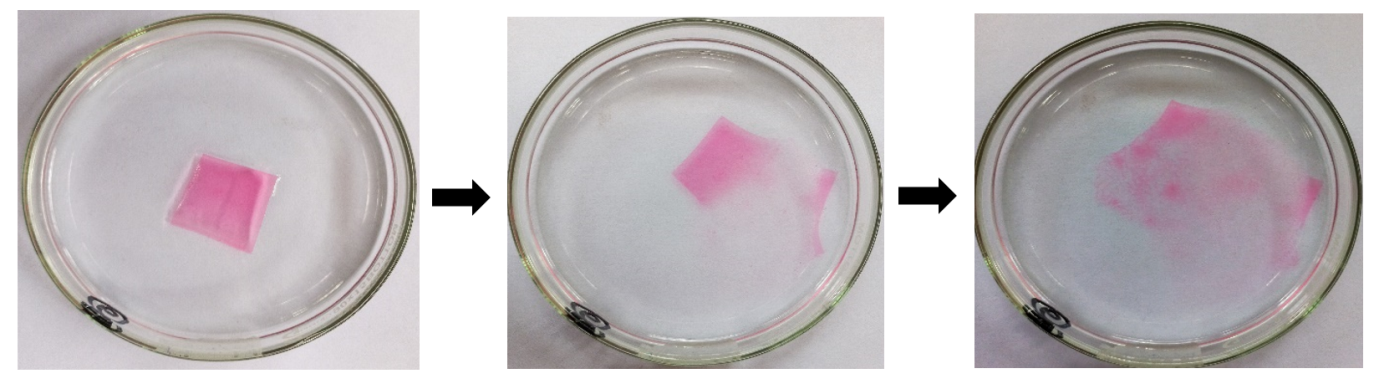
Fig. 10: Disintegration of the film (coloured with the aid of amaranth to visualize disintegration)
Percentage moisture loss
The optimized sublingual film was found to have a percent moisture loss of 1.21±0.35%, demonstrating the film’s overall good stability.
Percent moisture content
The percent moisture content of the optimized film was found to be 3.06±0.14% which indicated that the optimized film had sufficient stability.
Differential scanning calorimetry (DSC)
The optimized quick-dissolving sublingual film thermogram showed the disappearance of the pure drug peak, and the endotherm is observed to broaden and slightly shift to a lower temperature. The absence of a sharp, intense peak of bilastine at 202.10 °C indicates the thermal transition effect, which is responsible for the transformation of the crystalline form of the drug to its amorphous forms. The broadening of the peak further confirms the drug’s amorphous drug nature.
SEM
The morphology of the surface of the pure bilastine incorporated in the film and the optimized sublingual film containing bilastine were examined with SEM. The SEM image of the pure bilastine-loaded film in fig. 12A revealed the presence of the drug in the form of irregularly shaped crystals, indicating the highly crystalline nature of the pure drug bilastine, which owes to its poor aqueous solubility. Fig. 6B shows the SEM photograph of an optimized sublingual film containing bilastine, which depicted the presence of bilastine dispersed on the carrier surface, indicating the complex formation of bilastine-2-HPβCD suggesting the change in the crystal nature of bilastine from highly crystalline insoluble form to an amorphous form possessing superior aqueous solubility. This result is in agreement with the XRD pattern of the liquid dispersion of bilastine, confirming the change of the crystalline form of the drug to the amorphous form, thus improving its dissolution rate. The SEM photograph of optimized sublingual fast-dissolving Bilastine film also reveals the presence of suspended irregularly shaped particles of super disintegrating agent.
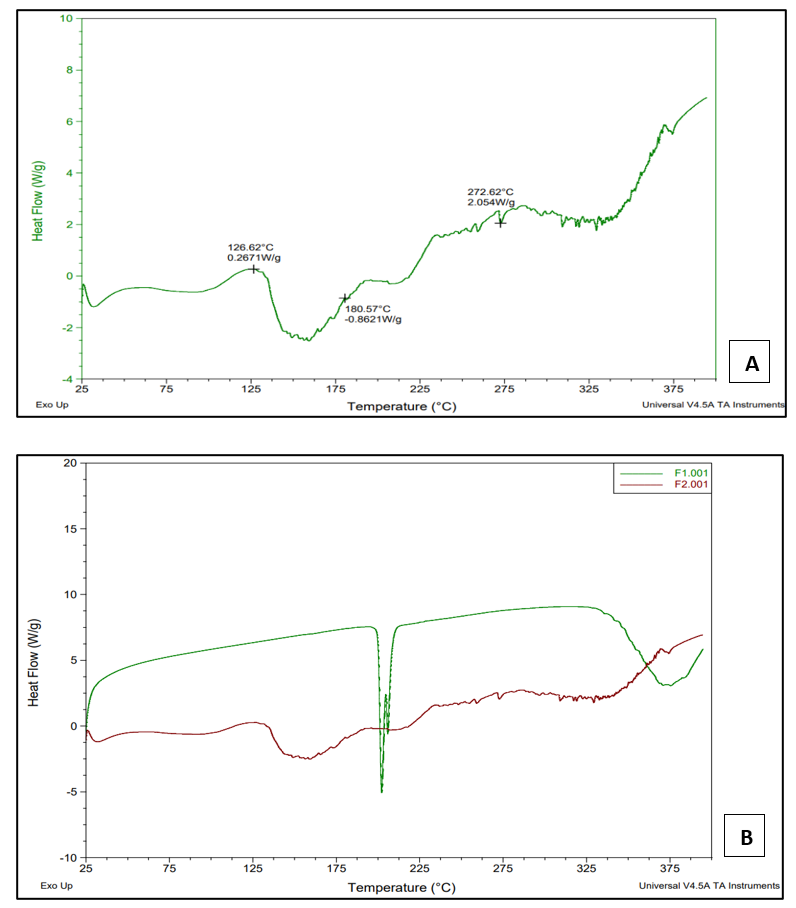
Fig. 11: A) DSC thermogram of optimized sublingual film of bilastine B) Overlay plot of DSC thermogram of pure bilastine and optimized sublingual film of bilastine
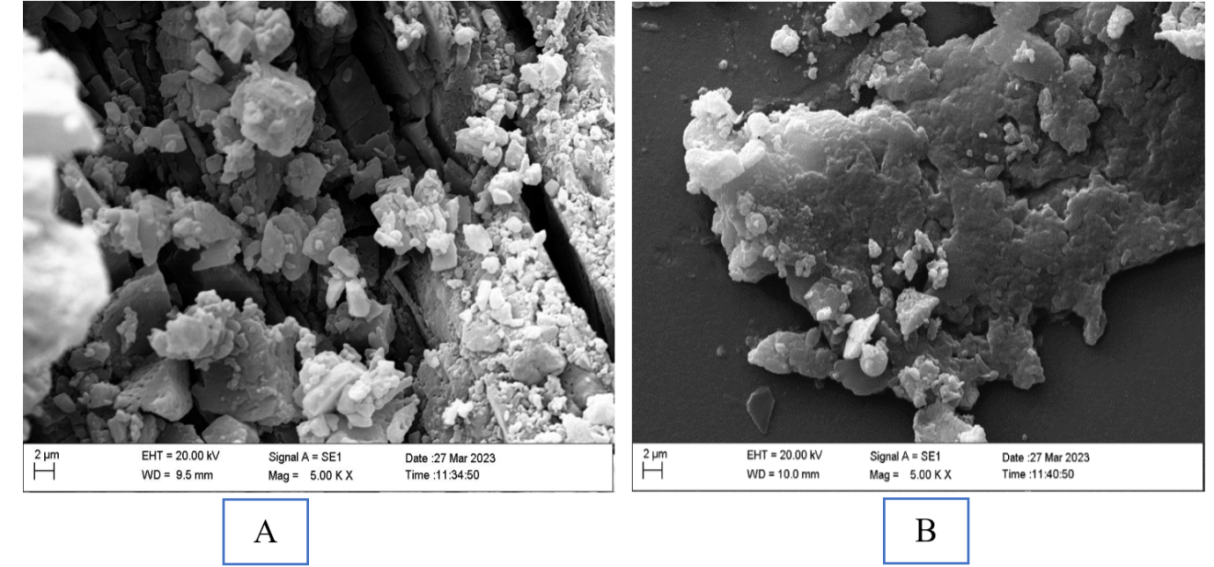
Fig. 12: SEM photograph of A) Pure bilastine loaded film and B) Optimised sublingual film containing bilastine
In vitro drug release
A comparative study on the in vitro drug release was done between pure bilastine in film and optimized fast-dissolving sublingual film of bilastine. The obtained data (table 2) was subjected to an unpaired t-test using GraphPad to determine the statistical significance. The calculated two-tailed P value was 0.0076, which is lower than the significance level of 0.05, indicating a considerable and statistically significant difference between the % CDR of the pure drug and the % CDR of the optimized film. The data obtained revealed that the drug release from the optimized film was significantly higher (97.41%) than that of pure bilastine in film (35.77%). This suggested that the drug’s dissolution profile demonstrated a 2.7-fold enhancement as an outcome of solubility improvement owing to the inclusion complex formation with 2-HP-β-CD.
Table 9: In vitro drug release values for films containing pure drug and optimized sublingual films of bilastine
| Time (s) | Film containing pure bilastine (%) (n=3)* | Optimised fast-dissolving film of bilastine (%) (n=3)* |
| 0 | 0 | 0 |
| 1 | 9.10±0.15 | 26.29±0.07 |
| 2 | 15.15±0.12 | 49.4±0.20 |
| 3 | 22.26 ±0.10 | 64.93±0.37 |
| 4 | 25.1±0.25 | 74.77±0.11 |
| 5 | 28.3±0.22 | 89.23±0.23 |
| 6 | 34.70±0.22 | 96.23±0.13 |
| 7 | 35.77±0.06 | 97.41±0.30 |
*Each experiment was carried out in triplicates; values are represented as mean±SD
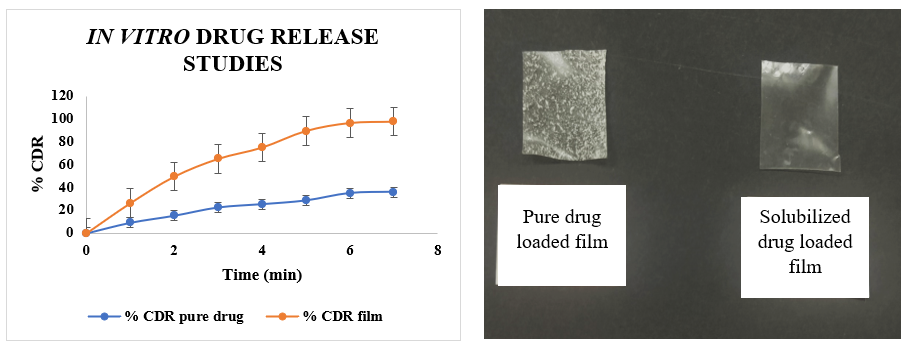

Fig. 13: Comparison between in vitro drug release values for film containing pure bilastine and optimized sublingual bilastine film
Table 10: Drug release kinetic modelling data obtained
| Mathematical drug release model | Linear coefficient (R2 Value) |
| Zero order model | 0.9366 |
| First order model | 0.9602 |
| Higuchi model | 0.9815 |
| Korsmeyer-Peppas’ model | 0.9857 |
| Hixon Crowell model | 0.9854 |
Drug release kinetics
Based on the value of the correlation coefficient(R2), the best-fitted model was selected. The kinetic analysis revealed that the R2 Value obtained was highest for the Korsmeyer-Peppas’ model, indicating that the formulation exhibited a diffusion mechanism of drug release with an n value of 0.7093, suggesting it showed the non-fiction type of diffusion from polymeric delivery systems. Additionally, the correlation coefficient value was high for the Hixon Crowell model, suggesting that there was a change in the surface area of the polymeric film matrix during dissolution, which has an impact on the release of the drug from the optimized sublingual film formulation.
Ex-vivo permeation test
Ex-vivo permeation of optimized fast-dissolving film formulation of bilastine through the porcine sublingual mucosa was discovered to be 95.21% in 7 min, which suggested good tissue permeability of the bilastine from the optimized fast-dissolving sublingual formulation. Thus, provides confirmatory evidence that a significant amount of bilastine from the optimized sublingual film is permeated through the sublingual mucosa, which suggests quicker onset action owing to high vascularity in the sublingual region of humans, which is desired to provide symptomatic relief from symptoms of chronic urticaria and allergic rhinitis in patients.
Short-term stability study
The stability study of the optimized quick-dissolving sublingual film was conducted at room temperature for a duration of 90 d. The films did not show any considerable change in the physical appearance, surface pH, disintegration time, folding endurance and percent drug content noted over a period of 90 d stored at room temperature.
Table 11: Results of stability study for the optimized fast-dissolving sublingual film
| No. of days | Storage at room temperature |
| Appearance | |
| 0 | Translucent |
| 30 | Translucent |
| 60 | Translucent |
| 90 | Translucent |
*value are expressed as mean±SD, n=3
CONCLUSION
The present research study involved the development of bilastine quick-dissolving sublingual films using 2-HP-β-CD. By the inclusion complex formation of 2-HP-β-CD with bilastine, the solubility of this poorly aqueous soluble drug was enhanced by an impressive 2.7-fold. The rapid disintegration of the sublingual bilastine film within 41 sec indicated accelerated drug release, enabling a quicker onset of action due to faster absorption of the drug via sublingual mucosa owing to high vascularity in the sublingual region. This formulation is also expected to prevent any food-drug interactions resulting in extremely poor bioavailability as bilastine is given sublingually in a pre-solubilized form, avoiding the need for acidic gastric media for the dissolution of bilastine. Moreover, as patients are not required to wait one hour before food or two hours after food to take the formulation, an increase in patient compliance is anticipated due to the convenience of administration and use of these sublingual films. This formulated quick-dissolving sublingual film of bilastine can serve as a promising alternative for treating chronic urticaria and allergic rhinitis and provide quick relief from these allergic manifestations. However, before this can be achieved, there’s a long road ahead. Further studies are required to carry out ICH-guided accelerated and long-term stability studies to ensure the stability of the formulation over extended periods. Furthermore, carrying out clinical trials to demonstrate the therapeutic efficacy and safety of the formulation is essential to gain regulatory approval.
ACKNOWLEDGMENT
The authors are grateful to Goa College of Pharmacy, Goa, and Manipal College of Pharmaceutical Sciences for providing the necessary facilities and support required to carry out this experimental research study. The authors also acknowledge Miss. Cleona Elizabeth DCruz, Miss. Parveen Farooqui and Dr. Rupesh Kalidas Shirodkar, Department of Pharmaceutics, Goa College of Pharmacy, for their kind help.
FUNDING
This research work did not receive any specific grant from funding agencies in public, commercial, or not-for-profit sectors.
AUTHORS CONTRIBUTIONS
Jasmine Liberata Fernandes: Validation, Formal analysis, Investigation, Data Curation, Writing-Original Draft, Writing-Review and Editing, Visualization, Funding acquisition, Prashant Jivaji Bhide: Conceptualization, Methodology, Resources, Supervision, Project administration. Shaila Angela Lewis: Resources, Writing-Review and Editing, Validation, Formal analysis, Visualization.
CONFLICT OF INTERESTS
Declared none
REFERENCES
Sachdeva S, Gupta V, Amin SS, Tahseen M. Chronic urticaria. Indian J Dermatol. 2011 Nov;56(6):622-8. doi: 10.4103/0019-5154.91817, PMID 22345759.
Dias GA, Pires GV, Valle SO, Dortas SD Junior, Levy S, França AT. Impact of chronic urticaria on the quality of life of patients followed up at a university hospital. An Bras Dermatol. 2016 Nov-Dec;91(6):754-9. doi: 10.1590/abd1806-4841.20165071, PMID 28099596.
Ridolo E, Montagni M, Bonzano L, Incorvaia C, Canonica GW. Bilastine: new insight into antihistamine treatment. Clin Mol Allergy. 2015 Apr 15;13(1):1. doi: 10.1186/s12948-015-0008-x, PMID 25878559.
Carter NJ. Bilastine: in allergic rhinitis and urticaria. Drugs. 2012 Jun 18;72(9):1257-69. doi: 10.2165/11209310-000000000-00000, PMID 22686617.
Church MK, Tiongco Recto M, Ridolo E, Novak Z. Bilastine: a lifetime companion for the treatment of allergies. Curr Med Res Opin. 2020 Mar;36(3):445-54. doi: 10.1080/03007995.2019.1681134, PMID 31612732.
Church MK. Safety and efficacy of bilastine: a new H(1)-antihistamine for the treatment of allergic rhinoconjunctivitis and urticaria. Expert Opin Drug Saf. 2011 Sep;10(5):779-93. doi: 10.1517/14740338.2011.604029, PMID 21831011.
Bhalani DV, Nutan B, Kumar A, Singh Chandel AK. Bioavailability enhancement techniques for poorly aqueous soluble drugs and therapeutics. Biomedicines. 2022 Aug 23;10(9):2055. doi: 10.3390/biomedicines10092055, PMID 36140156.
Savjani KT, Gajjar AK, Savjani JK. Drug solubility: importance and enhancement techniques. ISRN Pharm. 2012;2012:195727. doi: 10.5402/2012/195727, PMID 22830056.
Alhamhoom Y, Sharma A, Nanjappa SH, Kumar A, Alshishani A, Ahmed MM. Development and evaluation of solid dispersion based sublingual films of nisoldipine. Pharmaceuticals (Basel). 2023 Nov 9;16(11):1589. doi: 10.3390/ph16111589, PMID 38004454.
Yusuf A, Almotairy AR, Henidi H, Alshehri OY, Aldughaim MS. Nanoparticles as drug delivery systems: a review of the implication of nanoparticles physicochemical properties on responses in biological systems. Polymers (Basel). 2023 Mar 23;15(7):1596. doi: 10.3390/polym15071596, PMID 37050210.
Thapa RK, Kim JO. Nanomedicine based commercial formulations: current developments and future prospects. J Pharm Investig. 2023;53(1):19-33. doi: 10.1007/s40005-022-00607-6, PMID 36568502.
Shah JN, Shah KN, Mehta TA. Hydroxy propyl β-cyclodextrin complexation of promethazine hydrochloride for the formulation of fast dissolving sublingual film: in vitro and in vivo evaluation. J Pharm Investig. 2015 Feb 1;45(1):91-9. doi: 10.1007/s40005-014-0150-3.
Ulucan Karnak F, Kuru CI. Advantages of nanodrug targeting than conventional dosage system. Nanotechnology for Drug Delivery and Pharmaceuticals; 2023. p. 295-310. doi: 10.1016/B978-0-323-95325-2.00003-1.
Mary DC ruz CE, Bhide PJ, Kumar L, Shirodkar RK. Novel nano spanlastic carrier system for buccal delivery of lacidipine. J Drug Deliv Sci Technol. 2022 Feb;68:103061. doi: 10.1016/j.jddst.2021.103061.
Singh H, Singla YP, Narang RS, Pandita D, Singh S, Narang JK. Frovatriptan loaded hydroxy propyl methyl cellulose/treated chitosan based composite fast dissolving sublingual films for management of migraine. J Drug Deliv Sci Technol. 2018 Oct;47:230-9. doi: 10.1016/j.jddst.2018.06.018.
Ghosh A, Biswas S, Ghosh T. Preparation and evaluation of silymarin β-cyclodextrin molecular inclusion complexes. J Young Pharm. 2011 Jul;3(3):205-10. doi: 10.4103/0975-1483.83759, PMID 21897659.
Dinge A, Nagarsenker M. Formulation and evaluation of fast dissolving films for delivery of triclosan to the oral cavity. AAPS Pharm Sci Tech. 2008;9(2):349-56. doi: 10.1208/s12249-008-9047-7, PMID 18431674.
Farooqui P, Gude R. Formulation development and optimisation of fast dissolving buccal films loaded glimepiride solid dispersion with enhanced dissolution profile using central composite design. Int J Pharm Pharm Sci. 2023 Jun 1;15(6):35-54. doi: 10.22159/ijpps.2023v15i6.47992.
El Said IA, Aboelwafa AA, El Gazayerly ON. Optimization of taste masked dapoxetine oral thin films using factorial design: in vitro and in vivo evaluation. Pharm Dev Technol. 2021 Jun;26(5):522-38. doi: 10.1080/10837450.2021.1894445, PMID 33663316.
Al Nemrawi NK, Dave RH. Formulation and characterization of acetaminophen nanoparticles in orally disintegrating films. Drug Deliv. 2016;23(2):540-9. doi: 10.3109/10717544.2014.936987, PMID 25013958.
Chavan DU, Marques SM, Bhide PJ, Kumar L, Shirodkar RK. Rapidly dissolving felodipine nanoparticle strips formulation using design of experiment and characterisation. J Drug Deliv Sci Technol. 2020 Dec;60:102053. doi: 10.1016/j.jddst.2020.102053.
Chonkar AD, Bhagawati ST, Udupa N. An overview on fast dissolving oral films. Asian J Pharm Technol. 2015;5(3):129. doi: 10.5958/2231-5713.2015.00020.3.
Koland M, Sandeep V, Charyulu N. Fast dissolving sublingual films of ondansetron hydrochloride: effect of additives on in vitro drug release and mucosal permeation. J Young Pharm. 2010 Jul;2(3):216-22. doi: 10.4103/0975-1483.66790, PMID 21042474.
Londhe V, Shirsat R. Formulation and characterization of fast dissolving sublingual film of iloperidone using box behnken design for enhancement of oral bioavailability. AAPS Pharm Sci Tech. 2018 Apr;19(3):1392-400. doi: 10.1208/s12249-018-0954-y, PMID 29396734.
Rekha SM, Shaheda Sultana SK, Mahathi K, Parveen P, Prathima B, Seetha Devi A. Formulation and evaluation of fast dissolving buccal film containing isradipine solid dispersion. American Journal of PharmTech Res. 2015;5(2).
Kumar GP, Phani AR, Prasad RG, Sanganal JS, Manali N, Gupta R. Polyvinylpyrrolidone oral films of enrofloxacin: film characterization and drug release. Int J Pharm. 2014 Aug 25;471(1-2):146-52. doi: 10.1016/j.ijpharm.2014.05.033, PMID 24858388.
Bharti K, Mittal P, Mishra B. Formulation and characterization of fast dissolving oral films containing buspirone hydrochloride nanoparticles using design of experiment. J Drug Deliv Sci Technol. 2019 Feb;49:420-32. doi: 10.1016/j.jddst.2018.12.013.
Dhimmar B, Pokale R, Rahamathulla M, Hani U, Alshahrani MY, Alshehri S. Newfangled topical film forming solution for facilitated antifungal therapy: design development characterization and in vitro evaluation. Polymers (Basel). 2023 Feb 17;15(4):1003. doi: 10.3390/polym15041003, PMID 36850286.
Torgal T, Borkar S, Bhide P, Arondekar A. Formulation development and evaluation of fast dissolving films of ebastine. Int J Curr Pharm Sci. 2020 Sep 17;12(5):111-5. doi: 10.22159/ijcpr.2020v12i5.39782.
Takeuchi Y, Kawamoto M, Tahara K, Takeuchi H. Design of a new disintegration test system for the evaluation of orally disintegrating films. Int J Pharm. 2018 Dec 20;553(1-2):281-9. doi: 10.1016/j.ijpharm.2018.10.049, PMID 30366069.
Pinto S, Pintado ME, Sarmento B. In vivo ex vivo and in vitro assessment of buccal permeation of drugs from delivery systems. Expert Opin Drug Deliv. 2020 Jan;17(1):33-48. doi: 10.1080/17425247.2020.1699913, PMID 31786958.
Kulkarni U, Mahalingam R, Pather I, LI X, Jasti B. Porcine buccal mucosa as in vitro model: effect of biological and experimental variables. J Pharm Sci. 2010 Mar;99(3):1265-77. doi: 10.1002/jps.21907, PMID 19739112.