
Int J App Pharm, Vol 17, Issue 2, 2025, 240-249Original Article
DUAL-DRUG QUANTIFICATION: HPLC METHOD VALIDATION FOR HESPERIDIN AND PIPERINE IN ETHOSOMAL DELIVERY SYSTEMS
PREETI MAAN1,2, SHILPI CHAUHAN2, NISHA GUPTA3, DIMPY RANI1*
1Department of Pharmacy, School of Healthcare and Allied Sciences, GD Goenka University, Sohna, Gurugram-122103, India. 2Lloyd Institute of Management and Technology, Plot No-11, Knowledge Park-II, Greater Noida-201306, Uttar Pradesh, India. 3Mangalmay Pharmacy College, Plot No. 9, Knowledge Park II, Greater Noida-201306, Uttar Pradesh, India
*Corresponding author: Dimpy Rani; *Email: dimpy1990@gmail.com
Received: 13 Nov 2024, Revised and Accepted: 07 Feb 2025
ABSTRACT
Objective: The current study aims to develop and validate a simple, accurate, precise, and robust Reverse Phase-High Performance Liquid Chromatography (RP-HPLC) method to estimate hesperidin and piperine in an ethosomal formulation simultaneously. This method is intended to support the characterization of a novel ethosomal formulation designed to enhance the transdermal delivery of hesperidin and piperine.
Methods: Chromatographic separation was done using a C18 column (250×4.6 mm) featuring a 5 μm particle size, maintained at 25 °C) with a Photodiode Array (PDA) detector. The mobile phase consisted of a mixture of methanol, Acetonitrile (ACN), and water (70:20:10 v/v, pH 3.2). Separation was performed at a flow rate of 0.8 ml/min with a 10 µl injection volume. All the detection was done at an isosbestic wavelength of 291 nm.
Results: Hesperidin and piperine were eluted with retention time of 3.15 and 5.40 min, respectively. The developed RP-HPLC method demonstrated excellent accuracy, precision, and linearity for simultaneous quantification of hesperidin and piperine. According to the guidelines, the optimized ethosomal formulation exhibited a particle size of 208.4±0.3 nm, a Polydispersity Index (PDI) of 0.1294, and high entrapment efficiencies of hesperidin: 94.14±0.13%, and piperine: 79.57±0.11%. In vitro drug release of the ethosomal formulation was found to be 87.27±2.56% for hesperidin and 83.95±5.32% for piperine.
Conclusion: This HPLC method could be used to measure both hesperidin and piperine simultaneously in ethosomes and in other nanoparticle-based formulations. The optimized ethosomal formulation demonstrated desirable characteristics for transdermal drug delivery, including sustained release property and good entrapment efficiency.
Keywords: Hesperidin, Piperine, RP-HPLC, Method development, Method validation, Drug quantification, Ethosomal gel
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijap.2025v17i2.53262 Journal homepage: https://innovareacademics.in/journals/index.php/ijap
INTRODUCTION
Hesperidin, a compound found in citrus fruits (flavonoid glycoside), has anti-inflammatory antioxidant, and anti-allergic properties [1, 2]. Piperine, a pungent alkaloid derived from black pepper, has been reported to have anti-inflammatory activity although it is also used to enhance the bioavailability of various drugs by inhibiting metabolic enzymes and drug transporters (the chemical structures of hesperidin and piperine are given in fig. 1 and 2 respectively) [3-5]. Hesperidin lowers Interleukin (IL)-6 levels in conditions like experimental ulcerative colitis and reduces markers of inflammation, such as IL-1β and Tumour Necrosis Factor-alpha (TNF-α), in a rat model of diabetic neuropathy [6]. It also supports cartilage repair by blocking pro-inflammatory cytokines, including Interferon-γ, IL-2, IL-4, and IL-10. When combined with bee venom, it can reduce inflammation in arthritic rats by preventing T helper type 1 cell differentiation (which lowers TNF-α, IL-2, and IL-12) and promoting T helper type 2 cell differentiation (which increases IL-10 and IL-4) [7]. Piperine inhibits IL-1β induced over expression of inflammatory mediators, suppresses the production of Prostaglandin (PGE) 2 and nitric oxide, and reduces the gene expression and production of Matrix Metalloproteinase (MMP)-3, MMP-13, Inducible Nitric Oxide Synthase (iNOS), and Cyclooxygenase (COX)-2 in human osteoarthritis chondrocytes [8]. Additionally, piperine inhibits the activities of platelet cytosolic phospholipase A2 and thromboxane A2 synthase without affecting COX-1 activity, suggesting distinct mechanisms for inhibiting platelet aggregation and the macrophage inflammatory response [9]. Researchers have explored the synergistic potential of phytochemicals in the combination formulation. The findings suggested that synergistic effect of combined formulation might surpass the additive effects of individual components. These synergistic effects may result from chemical interactions or by simultaneously targeting multiple disease pathways [10, 11]. A recent in silico study highlighted the potential synergy of combined phytoconstituents in addressing inflammatory pathways involved in Rheumatoid Arthritis (RA) effectively [12-14]. This mainly includes the Janus Kinase-Signal Transducer and Activator of Transcription (JAK-STAT) [15], Nuclear Factor-kappa B (NF-κB) [16], and Mitogen-Activated Protein Kinase (MAPK) pathways [17]. Hesperidin and piperine both help reduce inflammation by targeting separate pathways and receptors in the body. The literature study revealed that hesperidin and piperine both reduced inflammation mainly regulating cytokine levels, influencing inflammasomes, and affecting immune cell differentiation. On the other hand, piperine works by lowering COX-2 expression, suppressing NF-κB activation, and acting as a bioenhancer, which helps the body absorb other compounds more effectively. Because they act through different yet complementary mechanisms, combining hesperidin and piperine could lead to a stronger anti-inflammatory effect than either one alone. However, topical use of these compounds for RA treatment poses challenges due to the skin's barrier properties of hesperidin [18].
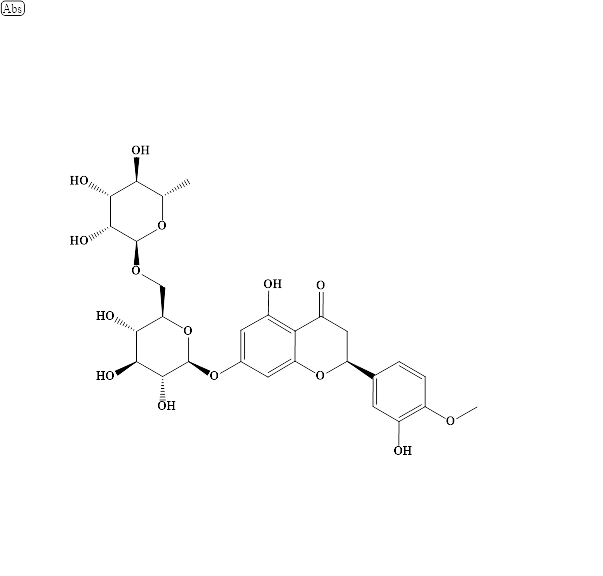
Fig. 1: Chemical structure of hesperidin
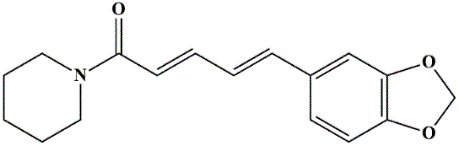
Fig. 2: Chemical structure of piperine
To overcome this problem, a novel lipid-based ethosome has emerged as a promising strategy to enhance the transdermal delivery of these therapeutic agents [19, 20]. Ethosomes are composed of phospholipid, ethanol, and water, and are known to improve drug permeation into the skin's topmost layer, the stratum corneum [21-23]. The phospholipid bilayer structure of ethosomes is similar to a cell membrane, aiding in the effective delivery of drugs into deeper skin layers [24, 25]. Further, the addition of piperine not only increases the biological action of formulation but also enhances the bioavailability of hesperidin. Although, polyherbal products are safe but many adverse effects can be implicated due to the presence of heavy metals, toxic contaminants, pesticides, and various adulterating additives. So, the standardization and quality control of herbal preparation must be a paramount step to ensure the quality and safety of the herbal product [26]. In this view, the present study is aimed to develop and validate the Reverse Phase-High Performance Liquid Chromatography (RP-HPLC) method for the simultaneous estimation of hesperidin and piperine in ethosomal delivery systems. Surprisingly, no study has been reported till now on the simultaneous determination of hesperidin and piperine within lipid-based formulations. Hence, this method is important for ensuring the consistency and quality of herbal formulations containing these bioactive constituents, and provides valuable insights into the quantification of these compounds in ethosomal delivery systems, contributing to the advancement of herbal medicine research [27, 28].
MATERIALS AND METHODS
Materials
Analytical-grade hesperidin (98%) and piperine ≥97% were procured from Otto Kemi Pvt. Ltd. Mumbai, India. Soya lecithin, ethanol, propylene glycol, and cholesterol were purchased from Sigma-Aldrich Chemicals, Bangalore, India whereas HPLC-grade methanol, Acetonitrile (ACN), and Orthophosphoric acid (OPA) were purchased from Merck, Mumbai, India. HPLC-grade water was used for all the experiments. All other reagents and chemicals used in this study were of analytical grade.
Preparation of standard stock solution
A standard stock solution (1000 µg/ml) of hesperidin and piperine was prepared using HPLC-grade methanol by adding 10 ml of each drug to 10 ml of methanol in a 10 ml volumetric flask.
Preparation of a working solution
The stock solution of each drug was diluted to attain a concentration of 100 µg/ml with methanol. Further, a series of dilutions of each drug was prepared to obtain concentrations ranging from 10 µg/ml to 60 µg/ml [29].
Selection of the wavelength
The standard solutions of each drug were diluted with methanol to a concentration of 10 µg/ml. The above solutions were analyzed using an Ultra Violet-Visible (UV-Vis) spectrophotometer (UV-2600, Shimadzu corporation, Kyoto, Japan with a pair of identical quartz cells, 1 cm path length) within 200-400 nm wavelength range. The resulting spectra were examined to identify the maximum absorption wavelength (λmax) for both hesperidin and piperine. A merged overlay of the absorption spectra was then generated to determine a suitable isosbestic point, representing a wavelength where both drugs exhibit equal absorbance. This isosbestic point was selected as the detection wavelength for the HPLC method to ensure accurate and sensitive simultaneous quantification of hesperidin and piperine. Fig. 3 shows the overlay UV spectrum of hesperidin and piperine [30, 31].
RP-HPLC instrumentation
RP-HPLC instrument (Shimadzu Corporation, Kyoto, Japan) with a Photodiode Array (PDA) detector and auto-sampler was employed for the analysis. Chromatographic separation was carried out on an RP-C18 column (250×4.6 mm) featuring a 5 μm particle size. The LC lab solution software (Shimadzu Corporation, Kyoto, Japan) was used for equipment control, data acquisition, and integration of analytical processes.
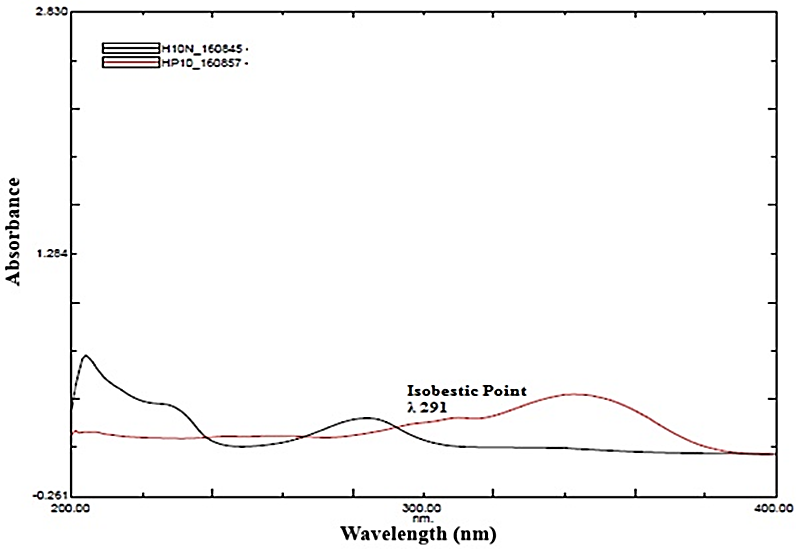
Fig. 3: UV-Vis absorbance spectrum of hesperidin (black) and piperine (red) at 291 nm
Optimization of chromatographic conditions
Before the mobile phase selection, the solubility studies of both drugs were performed with various solvents (water, methanol, ethanol, chloroform, ACN, and phosphate buffer pH 7.4). According to the results, piperine was soluble in methanol and ACN. Though hesperidin was found to be slightly soluble in water, ethanol, and methanol but solubility in methanol was highest amongst all solvents. Hence, the mobile phase was selected as a mixture of methanol, water, and ACN. The pH of the mobile phase was adjusted to 3.20 with OPA. The OPA maintained not only the pH but also enhanced the chromatographic behaviour of both drugs. After the selection of the solvents, multiple experimental runs were carefully conducted with varying concentrations of methanol: ACN: water (40:40:20 v/v, 50:20:20 v/v, and 70:20:10 v/v). Subsequent analysis deciphered that the mobile phase having methanol: ACN: water in the ratio of 70:20:10 v/v offered optimal conditions for achieving superior separation, precise Retention Time (Rt), and peak purity. The method used in this study was isocratic elution. A 0.22 μm nylon membrane was used to filter the mobile phase, followed by sonication with a sonicator (Wensar ultra WUC-4L) to eliminate any particles. The chromatographic separation was performed with a flow rate of 0.8 ml/min and a run time of 10 min for each sample. An injection volume of 10 µl was used, and the column temperature was set at 25 °C [32].
Method validation
The developed HPLC method for simultaneous detection and quantification of hesperidin and piperine in ethosomes was validated in accordance with the International Conference on Harmonization (ICH) standards [33]. According to these guidelines, the following factors were assessed:
System suitability test
A system suitability test was performed to verify the suitability of the chromatographic system for the intended analysis. A standard solution of hesperidin and piperine, each at a concentration of 30 µg/ml was injected six times. The system suitability parameters were determined for Retention Time (Rt) %Relative Standard Deviation (%RSD), and peak area. For the test to be considered successful, the % RSD has to be less than 2% [34].
Specificity
Specificity refers to the ability of a procedure to differentiate the analyte from any potential interfering substances. The HPLC method was developed to rule out the interference of the excipients in the formulation. This was performed by injecting a mixture of excipients (soya lecithin, propylene glycol, and cholesterol) and comparing their chromatogram with those of standard solutions. If no interfering peaks were detected at or near the retention times of hesperidin and piperine, the method would be considered specific [35].
Linearity and range
To determine the linearity and range, triplicate recordings of six different concentrations of hesperidin and piperine (10 µg/ml to 60 µg/ml) were tested. The area of each concentration was recorded and the mean area was calculated. A graph was plotted against the mean peak areas of each concentration of analytes and their respective concentrations. Then, slope, intercepts, and correlation coefficients (R²) were calculated. If the correlation coefficient is greater than 0.99, the data fits the regression line and is considered acceptable. The range of the developed method was determined based on the plot obtained for peak area vs concentration and response factor against concentration for calibration standard. The %RSD of the response factor was determined to establish the range of analyte [32].
Accuracy
The degree to which the experimental value matches the predicted value of the chemical in the analytical process is known as accuracy. The developed HPLC method was validated based on %recovery of hesperidin and piperine. Recovery studies were carried out by the addition of the known concentration of standard drug solution at three concentration levels 50%, 100%, and 150 % in pre analysed sample. To assess this parameter, successive analysis (n=6) of each of three different concentrations (20 µg/ml, 40 µg/ml, and 60 µg/ml) of standard hesperidin and piperine solution were performed and the %recovery was calculated. The average recovery acceptable range should be 90-110% [32, 36].
Precision
The precision of the HPLC method was evaluated by using intra-day and inter-day experiments. Repeatability or intra-day precision was evaluated by measuring six replicates of hesperidin, and piperine solutions in the concentration range of 10 µg/ml to 60 µg/ml on the same day. Intermediate precision or inter-day precision was assessed by analysing the six replicates of hesperidin, and piperine solutions in the same concentration range over three days a week under the similar analytical condition. Afterward, %RSD was calculated for repeatability and intermediate precision, the acceptable range should be less than 2% [32, 37].
Sensitivity
The sensitivity of the developed method was determined by the Limit of Detection (LOD) and Limit of Quantification (LOQ). LOD is the lowest concentration of an analyte detected in a sample and LOQ is the smallest amount that can be measured accurately and precisely using standard tests. The LOD and LOQ estimation for hesperidin and piperine were carried out as per ICH guidelines [32, 38].
Robustness
The robustness of the developed HPLC method was evaluated for the ability of the method to remain unaffected by the modest alterations in chromatographic conditions like flow rate and change in wavelength. The wavelength was varied as ±5 nm and the flow rate as±0.2 ml/min. The effect of such changes on the area under the curve was determined and the %RSD value was evaluated [39, 40].
Preparation of drug-loaded ethosome
Hesperidin and piperine-loaded ethosomes were prepared using the cold method. According to this method, the ethosomal systems consisted of 2-5% soya lecithin, 10-50% ethanol, drugs (hesperidin and piperine equivalent to dose), 0.5% cholesterol, and water up to 100% w/w. Soya lecithin and drugs were dissolved in ethanol using a vortex mixture. Then, double distilled water was slowly added in a fine stream with constant mixing at 700 rpm using a mechanical stirrer in a well-sealed container. The mixing was continued for another 5 min. The entire process was carried out at a constant temperature of 30 °C. Once completed, the mixture was allowed to cool to room temperature [41, 23].
Evaluation of the prepared ethosomes
Blank and drug-loaded ethosomes were prepared with the above-mentioned method and were analyzed for particle size, Polydispersity Index (PDI) values, and zeta potential using zetasizer (ZEN-3600, NANO-25, Malvern Panalytical Ltd., UK) at 25 °C [42, 43]. For the particle size measurement, the ethosomes were suitably diluted with distilled water in order to avoid multiscattering phenomena [41, 43].
%Drug entrapment efficiency (%DEE)
The drug entrapment efficiency (%DEE) of drug-loaded ethosomes was determined. For this, six prepared formulation samples were centrifuged at 10,000 Rotations per Minute (rpm) for 20 min at 4 °C. After centrifugation, the supernatant was carefully collected, filtered, and diluted with methanol. The diluted supernatant was then injected into the HPLC system, which has been pre-calibrated for the detection and quantification of hesperidin and piperine [44]. The %DEE was calculated using the following equation:
%DEE= 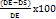
Where DE= theoretical amount of drug added,
DS= drug determined in supernatant.
In vitro drug release
A Franz diffusion cell (Perme Gear, Inc., Hellertown PA) with a cellophane membrane was used to evaluate the release profiles of hesperidin and piperine from ethosomal gels [43]. The cell comprises donor and receptor chambers separated by a cellophane membrane. The receptor chamber was filled with pH 7.4 Phosphate Buffer Saline (PBS) and the donor chamber was filled with a formulation equivalent to 1 g of drug. The setup was placed on a magnetic stirrer with a magnetic bead in the receptor chamber to maintain a constant stirring speed of 100 rpm at 37.0±0.5 °C. At specific time points, 1 ml samples were withdrawn from the receptor chamber and replenished with fresh PBS, ensuring no air bubbles were trapped beneath the membrane. Hesperidin and piperine concentrations in the collected samples were quantified by HPLC. %Drug release was calculated and plotted against time [45].
RESULTS AND DISCUSSION
Selection of UV wavelength
During this analysis, it was observed that hesperidin exhibited absorption maxima at 284 nm and piperine showed absorption maxima at 343 nm the overlay of the spectrum of both drugs displayed an isosbestic point at 291 nm (fig. 3). Based on the results, subsequent method development for simultaneous quantification of each drug was performed at this isosbestic wavelength of 291 nm. This selection allowed for accurate determination of both drugs in the developed RP-HPLC method.
Method development
An RP-HPLC method was developed for the simultaneous quantification of hesperidin and piperine. This process was initiated with mobile phase optimization by evaluating multiple ratios of mobile phase having methanol, ACN, and water acidified with OPA. The mobile was selected on the basis of the distinct chemical properties of hesperidin and piperine. Although, hesperidin is less polar than piperine but it also contains the hydroxyl group. The addition of OPA increases the polarity of hesperidin and also enhanced the interaction of hesperidin with the mobile phase. This leads to the faster elution of hesperidin in the polar mobile phase. The OPA also suppressed the ionization of free unreacted silonol groups in C18 column and reduced peak tailing and improved chromatography. In contrast, piperine remains unaffected by the mild acidity, contributing to its longer retention time compared to hesperidin. The chosen mobile phase composition, with a high percentage of organic solvents (methanol and ACN) increases the overall polarity of the system, thus aiding in the separation of these compounds. Finally, effective chromatographic separation of hesperidin and piperine was achieved with 70:20:10 v/v (pH 3.20) ratio of methanol: ACN: water on C18 column (250 x 4.6 mm, 5 µm particle size) in isocratic elution method at flow rate 0.8 ml/min. Under these conditions, hesperidin and piperine were successfully separated and eluted with distinct Rt. The Rt of hesperidin and piperine with standard solution was found to be 3.16 min and 5.42 min, respectively as depicted in fig. 4 and 5. The Rt of hesperidin and piperine in a mixed standard solution was found to be 3.15 min and 5.40 min as shown in table 1. The chromatogram for hesperidin and piperine in a mixed standard solution is shown in fig. 6.
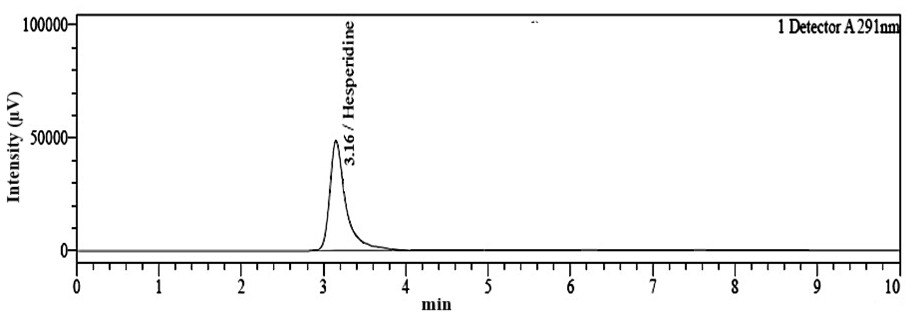
Fig. 4: Chromatogram of hesperidin standard showing the retention time of 3.16 min
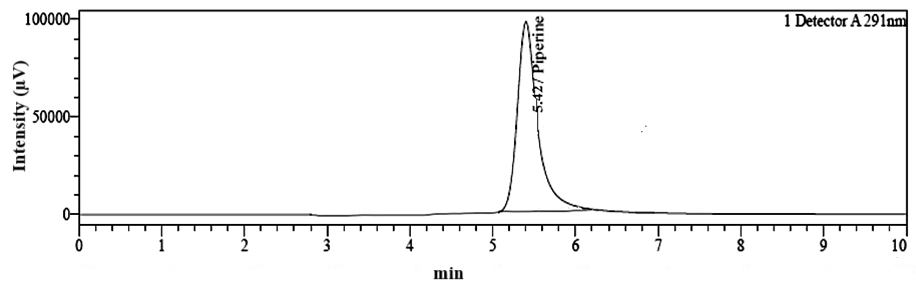
Fig. 5: Chromatogram of piperine standard showing the retention time of 5.42 min
Table 1: The retention time of hesperidin and piperine in mixed standard solution
| S. No. | Name of the phytoconstituent | Retention time (min) | Area |
| 1 | Hesperidin | 3.15 | 606 081 |
| 2. | Piperine | 5.40 | 1 636 613 |
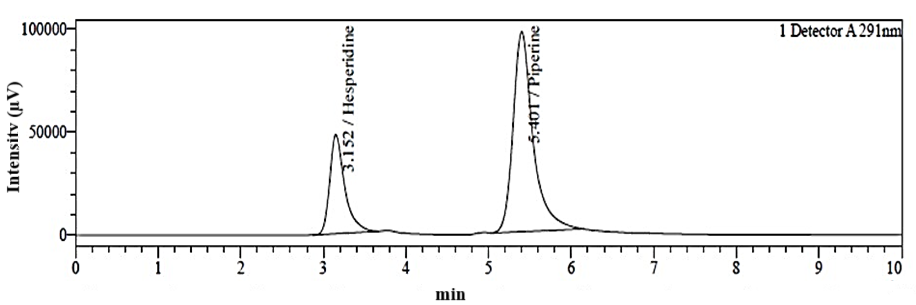
Fig. 6: Simultaneous RP-HPLC chromatogram of hesperidin (Rt=3.15 min) and piperine (Rt=5.40 min) in standard drug solution mixture composed of 30 µg/ml each
Method validation
Specificity
The method’s specificity was assessed by comparing the blank ethosomes samples (without hesperidin or piperine) with the samples spiked with known concentrations of both drugs. The chromatograms showed no interfering peaks of the ethosomes excipients near the retention times of hesperidin and piperine (fig. 6). Hence, the developed RP-HPLC method was found specific for concurrent quantification of hesperidin and piperine.
Linearity and range
The method’s linearity was evaluated by plotting the calibration curves of hesperidin and piperine at various concentrations. Fig. 7 illustrates the calibration curves of hesperidin and piperine. The standard calibration curve showed a linear relationship for both hesperidin and piperine within the concentration range of 10 to 60 µg/ml. The calibration curves exhibited excellent linearity as evidenced by the high value of determination coefficients (R2) obtained. R2 values of 0.9992 and 0.9996 were obtained for hesperidin and piperine. The linear regression equations for each analyte were found as follows: Hesperidin: y = 149846x – 3210.8 and Piperine: y = 413814x – 18380, respectively.
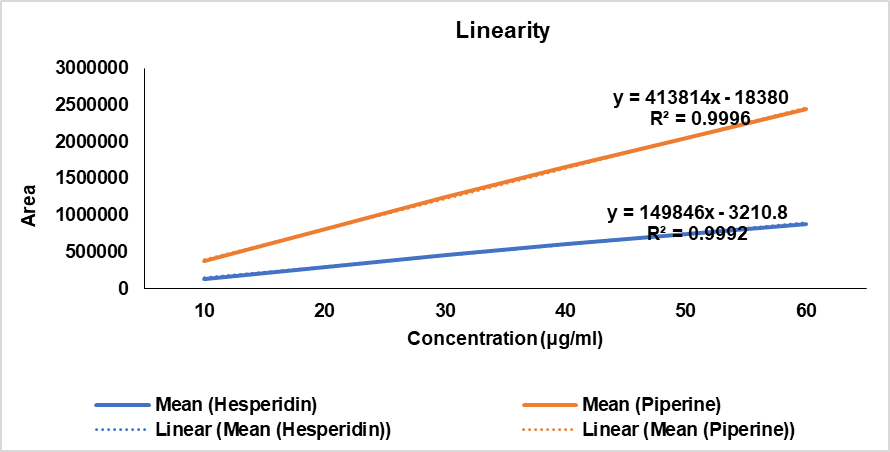
Fig. 7: Calibration curve of standard hesperidin and piperine
Accuracy
Accuracy determines the degree of closeness between the obtained values and the true values. It is expressed as % recovery. The overall % recovery for samples containing 50%, 100%, and 150% concentrations was observed to be in the range of 97% – 100%. The % recovery for hesperidin and piperine was found within the acceptance criteria which suggest the good accuracy of the developed method. It also indicated the suitability of the method for simultaneous estimation of both drugs in the formulation. This approach ensured the validity and reliability of the analytical method to detect drug content in the sample. The results for the accuracy of the developed method are presented in table 2.
Table 2: Accuracy study of hesperidin and piperine
| Level | Concentration label of drugs (µg/ml) | % Recovery of hesperidin* | %RSD of hesperidin | % Recovery of piperine* | % RSD of piperine |
| 50% | 20 | 97.17±0.72 | 0.555 | 98.71±1.12 | 0.628 |
| 100% | 40 | 99.64±0.65 | 1.247 | 100.13±0.57 | 0.676 |
| 150% | 60 | 99.60±0.32 | 0.040 | 98.88±0.40 | 0.436 |
*Data was expressed as mean±SD, n=6
Precision
The precision of the method reflects the repeatability of the method. It was evaluated through intra-day and inter-day evaluation. The results were expressed as %RSD. The %RSD of interday and intraday precision for the developed method was found to be less than 2% which demonstrated excellent precision of the proposed method for both hesperidin and piperine. The results of the precision studies are shown in table 3.
Sensitivity
LOD and LOQ of the method were calculated based on the standard deviation of the response (Sy) of the calibration curve and the slope (S) of the calibration curve at approximate levels of the limit of detection and limit of quantification.
LOD=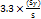
LOQ=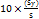
According to the results, hesperidin and piperine have LOD values of 0.46 µg/ml and 0.41 µg/ml, respectively, while their LOQ values were found to be 1.39 µg/ml and 1.23 µg/ml, respectively. The results indicated the method's suitability for detecting and quantifying these analytes at low concentrations within the ethosome formulations.
Table 3: The result of precision studies of validated analytical method
| S. No. | Precision | %Recovery of hesperidin* | %RSD of hesperidin | %Recovery of piperine* | %RSD of piperine |
| 1 | Repeatability | 99.976 | 0.793 | 98.885 | 0.540 |
| 2 | Interday | 98.617 | 0.018 | 98.280 | 0.014 |
| 3 | Intraday | 99.875 | 0.010 | 98.064 | 0.031 |
*Data was expressed as mean, n=6
Robustness
The robustness of the developed RP-HPLC method, which demonstrates its reliability under slightly altered conditions, was tested by intentionally changing the wavelength and flow rate. These variations were tested using a sample of a lower concentration of 40 µg/ml. The effect of variations on drug responses was measured in terms of the %RSD. The %RSD was required to be not more than 2. The results showed that the %RSD for changes in wavelength and flow rate meeting the acceptance criteria as it is below 2 for both parameters. This confirmed that the method was robust. The tests showed that the minor changes did not significantly impact the separation, peak shape, or efficiency of the analysis, highlighting the method’s reliability and suitability for routine use. The robustness results are shown in tables 4 and 5.
Table 4: Robustness data showing the effect of change in wavelength (nm)
| Concentration (µg/ml) | Area at wavelength 286 nm | Area at wavelength 296 nm | Area at wavelength 286 nm | Area at wavelength 296 nm |
| Hesperidin | Piperine | |||
| 40 | 740300 | 442318 | 1424436 | 1975968 |
| 40 | 743106 | 438116 | 1409001 | 2018370 |
| 40 | 744030 | 432284 | 1407882 | 1989808 |
| 40 | 742324 | 433018 | 1404721 | 1994711 |
| 40 | 742881 | 427332 | 1443615 | 1922184 |
| 40 | 742979 | 444804 | 1426907 | 2028057 |
| Mean | 742603.33 | 436312 | 1419427 | 1988183 |
| SD | 1256.16 | 6620.52 | 14995.7 | 37554.02 |
| %RSD | 0.002 | 0.015 | 0.011 | 0.019 |
*n=6
Table 5: Robustness data showing the effect of change in flow rate (ml/min)
| Conc. (µg/ml) | Area at a flow rate of 0.6 ml/min | Area at a flow rate of 1.0 ml/min | Area at a flow rate of 0.6 ml/min | Area at a flow rate of 1.0 ml/min |
| Hesperidin | Piperine | |||
| 40 | 850017 | 509329 | 2325634 | 1429793 |
| 40 | 850327 | 501203 | 2308090 | 1384362 |
| 40 | 855204 | 506549 | 2348997 | 1414830 |
| 40 | 856317 | 506293 | 2917800 | 1406091 |
| 40 | 856377 | 506690 | 2369255 | 1435341 |
| 40 | 854294 | 506400 | 2418669 | 1406202 |
| Mean | 853756 | 506077 | 2448074.17 | 1412769.83 |
| SD | 2882.8 | 2648.81 | 233290.78 | 18429.53 |
| %RSD | 0.003 | 0.005 | 0.095 | 0.013 |
*n=6
Comparison with previously published HPLC method
The comparative analysis of RP-HPLC methods for hesperidin and piperine revealed a notable gap in the literature, especially for their simultaneous quantification in combination formulations. A comparison of previously published HPLC methods with the present developed method was conducted based on mobile phase composition, flow rate, detection wavelength, column specification, limits, and applications, as presented in table 6.
Several RP-HPLC methods have been reported for analysing hesperidin and piperine individually or in combination with other phytochemicals present in various formulations. However, there is no published RP-HPLC method for the simultaneous quantification of hesperidin and piperine, particularly in ethosomal gel formulations. The first three methods primarily focused on hesperidin, have used methanol as the mobile phase with other solvents. The first method is suitable for both plant extracts and ayurvedic formulations, but extensive validation is needed for more complex matrices. The second method employed an ionic liquid-based mobile phase to improve separation of the two drugs in combined tablet formulations, though the high cost of ionic liquids limits its routine use. The third method was designed for liposomal formulations, employed a low flow rate to achieve specificity but has lower throughput, making it less practical for high-volume testing. The last three methods focused on piperine as the analyte, ACN as the mobile phase with other solvents. Methods 4 and 5 utilized ACN and phosphate buffer and are commonly referenced for nanoparticle matrices. Method 6, which is used for dual-drug quantification for quercetin and piperine, is also applicable for nano-structured lipid carriers but offers lower sensitivity compared to the other methods. While prior studies have addressed various limitations such as sensitivity or high costs, the current study fills an important gap by developing and validating an RP-HPLC method for the dual-drug quantification of hesperidin and piperine in ethosomal delivery systems.
The mobile phases used in these methods predominantly feature methanol or ACN combined with water or phosphate buffer, often adjusted with organic acids for pH control. This trend reflected the importance of these components for optimal compound separation. C18 columns are universally preferred due to their robustness and effectiveness in reversed-phase separations for a wide range of applications. The current developed method employed a mobile phase of methanol: ACN: water (70:20:10 v/v, pH 3.20) at a flow rate of 0.8 ml/min and a detection wavelength of 291 nm. This method offers improved accuracy, cost-effectiveness, and robustness according to ICH guidelines and demonstrating acceptable performance within established limits. By optimizing mobile phase composition and flow rates, the method achieves a balanced combination of sensitivity, efficiency, and cost-effectiveness.
The validation results confirmed its precision, simplicity, sensitivity, and reliability for the simultaneous determination of hesperidin and piperine in ethosomes and other lipid-based formulations. This method is suitable for routine quality control analysis and has potential applications for in vitro and in vivo quantification of hesperidin and piperine in other matrices. Furthermore, this novel method will be employed to evaluate a newly developed anti-rheumatic arthritis formulation containing both compounds. This further demonstrated the broader applicability of the developed method.
Applications of the validated analytical method
The validated RP-HPLC method was developed to estimate hesperidin and piperine simultaneously. It was successfully applied to analyse various parameters of the prepared ethosomes, demonstrating its practicality and reliability in characterizing this dual-drug delivery system.
Characterization of the prepared ethosomes
The particle size, PDI, and zeta potential of the prepared ethosomes are the crucial parameters that influence drug loading, release, and stability of ethosomes. These parameters of the prepared ethosomes were analysed using zetasizer. A plot of particle size distribution with the PDI value of drug-loaded ethosome was given in fig. 8. The average particle size of prepared ethosomes was found to be 208.4 nm, with a PDI value of 0.13. The PDI value is a measure of particle size distribution homogeneity. The PDI value of prepared ethosome was found to be less than 0.2 which reflects the monodispersity of the ethosome dispersions. This narrow size distribution and small particle size are desirable for enhancing drug delivery properties, such as increased drug encapsulation, improved cellular uptake, and controlled release.
Table 6: Comparison of hesperidin and piperine in other formulations given in previously published HPLC method
| S. No. | Mobile phase, flow rate and wavelength | Column | Application | Limitations | Reference |
| 1. | Methanol: 0.1% OPA (50:50 v/v), 1 ml/min, 284 nm | Phenomenex Luna C18 Column (250×4.6 mm, 5 μm) | RP-HPLC method for quantification of hesperidin in plant extracts, Ayurvedic formulations, and hesperidin-loaded nanotransferosomes | Application to other matrices requires further validation | [29] |
| 2. | Methanol: water (45:55v/v) with 0.025% ionic liquid, | Lichrospher RP-18 (250×4.6 mm, 5 μm) |
Simultaneous determination of hesperidin and diosmin in combined tablets | Expensive | [30] |
| 3. | Methanol (organic phase): Milli-Q water with 0.1 % v/v OPA (aqueous phase) at a ratio 90:10 v/v), 0.4 ml/min, 275 nm | Shim-Pack Solar C18 column (250×4.6 mm, 5 μm) | Simultaneous estimation of 4-hydroxytamoxifen and hesperidin in liposomal formulation | Low flow rate | [46] |
| 4. | ACN: methanol: water (65:5:30 v/v), 1 ml/min, 353 nm | C18, 5 µm, 250 mm x 4.6 mm | Simultaneous quantification of curcumin and piperine in a microparticle formulation | Less sensitive | [32] |
| 5. | ACN: Phosphate buffer (0.01% OPA; 55:45 v/v) pH 6.0,1 ml/min, 330 nm | Luna C18 Column (250×4.6 mm, 5 μm) | RP-HPLC method for simultaneous estimation of resveratrol and piperine in cubosome and human plasma | Application to other matrices requires further validation | [39] |
| 6. | ACN and water (pH 2.6, adjusted with 2%w/v glacial acetic acid), 1 ml/min, 346 nm |
Hypersil gold C-18 column (150×4.6 mm, 5 μm) | Simultaneous quantification of quercetin and piperine in dual-drug loaded nanostructured lipid carriers by RP-HPLC | [40] |
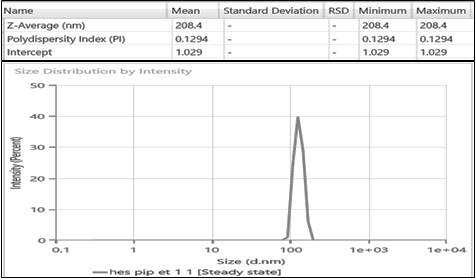
Fig. 8: The particle size distribution of the hesperidin and piperine-loaded ethosomes
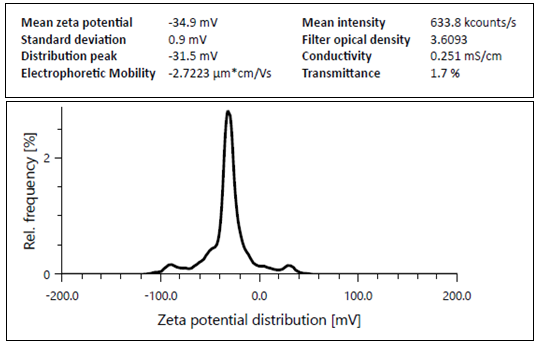
Fig. 9: Zeta potential of optimized ethosome formulation
Zeta potential indicates the degree of repulsion between adjacent similarly charged particles. A high zeta potential reveals stability and ensures that the dispersion will resist aggregation. In general, the dividing line between stable and unstable dispersion is set to a higher or lower value of mV. The zeta potential of the prepared ethosome was measured and was found to be-34.9 mV. A value of more than ±30.0 suggested good stability of the prepared systems with a low probability of aggregation and particle growth. These results ensure the stability of ethosome formulation and avoidance of aggregation of the nanovesicles. Fig. 9 demonstrates the zeta potential of optimized ethosome formulation.
% Drug entrapment efficiency (%DEE)
The amount of hesperidin and piperine successfully encapsulated within the ethosomes i. e., %DEE is a critical parameter reflecting the efficiency of the drug delivery system. The %DEE was determined by centrifuging the formulation for 20 min at 1000 rpm. The supernatant was diluted with methanol and injected to the HPLC system for concurrent estimation of hesperidin and piperine. Blank ethosomes were also prepared and injected in HPLC to check for any interference with the Rt of both drugs. The chromatogram of blank ethosomes and drug-loaded ethosomes are shown in fig. 10 and 11. As per the results, the %DEE values were found to be 94.14% (±1.23) and 79.58% (±2.21) for hesperidin and piperine, respectively.
Fig. 10: Chromatogram of blank ethosomes
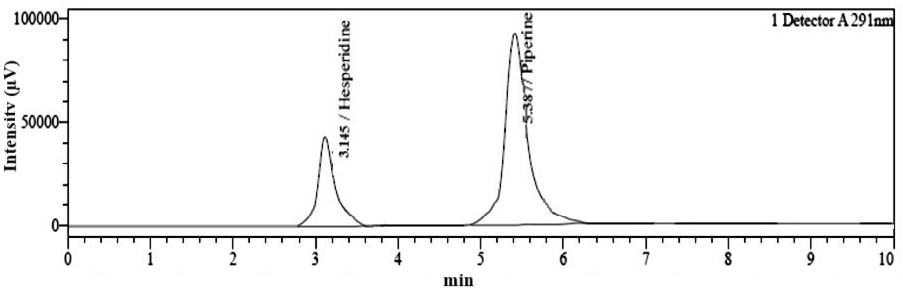
Fig. 11: Simultaneous chromatogram of hesperidin (Rt-3.145) and piperine (Rt-5.387) loaded ethosomes
In vitro drug release study
In vitro drug release profiles of hesperidin and piperine from the developed ethosomal gels were studied using Franz diffusion cells. One g of drug-loaded ethosomal gel was placed in the donor compartment, which was separated from the receptor compartment (containing pH 7.4 PBS) by a cellophane membrane. The receptor compartment was maintained at 37.0±0.5 °C with continuous stirring. The percentage of drug released from the ethosomal gel was calculated. From drug-loaded ethosomes, 87.27±2.56% of hesperidin was released over 24 h while 83.95±5.32% release of piperine was observed within this time. All drug release peaks were well separated and quantified using the validated RP-HPLC method. This method is proven to be effective for the simultaneous detection and quantification of both hesperidin and piperine in the dual-drug-loaded ethosomes, supporting in vitro physicochemical evaluation. A plot of % drug release of hesperidin and piperine from dual drug-loaded ethosomal gel formulations with time is shown in fig. 12.
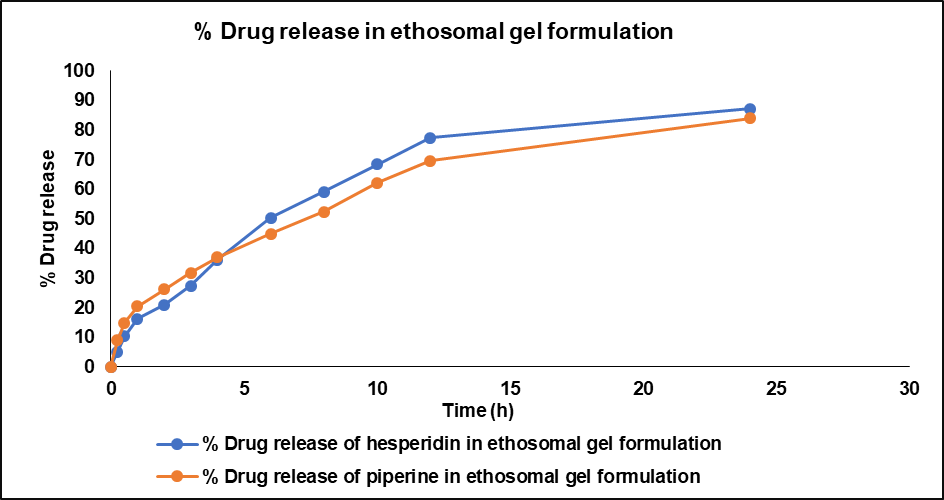
Fig. 12: % drug release of hesperidin and piperine from drug-loaded ethosomal gel formulation through validated simultaneous RP-HPLC method
In vitro drug release studies give us important information about how the active compounds are released from the ethosomal formulation which helps us predict how the drug will be absorbed through the skin in the body. This data provides a basis for further studies on how the drug works in the body and its effectiveness, helping us optimize the formulation for better transdermal delivery.
CONCLUSION
This study presented a conceptualized and rigorously validated RP-HPLC method for the simultaneous estimation of hesperidin and piperine in ethosomes, which is developed according to ICH guidelines. The results demonstrated that the proposed RP-HPLC method is simple, robust, precise, and cost-effective. All critical parameters according to ICH guidelines, such as linearity, accuracy, precision, selectivity, LOD, LOQ, specificity, and robustness were found to be within acceptable limits. It is particularly effective for quantifying hesperidin and piperine in ethosomal gel formulations. However, it would be a valuable tool for routine quality control analysis of these drugs in laboratory analysis as well as in industrial bulk production. Moreover, its adaptability makes it suitable for standardization and quantification of these drugs in herbal formulation and lipid-based nano-formulations. The developed RP-HPLC method delivered exceptional performance, ensuring robust separation, high repeatability, efficiency, and sharp resolution of hesperidin and piperine. Furthermore, the method’s adaptability positions it as a valuable tool not only for laboratory analysis but also for characterizing these bioactives in industrial bulk production. This enhanced its role in maintaining the quality and consistency of polyherbal formulations. Although no RP-HPLC method has been developed for polyherbal matrices that are entirely free from interference by other constituents in the formulation, we hope that the present study can be utilized to assess the in vitro and in vivo pharmacokinetic and pharmacodynamic properties of hesperidin and piperine in animal models.
ACKNOWLEDGMENT
We gratefully acknowledge the Lloyd Institute of Management and Technology, U. P. for their intellectual contributions to this study.
FUNDING
Nil
AUTHORS CONTRIBUTIONS
Preeti Maan: Conducted RP-HPLC experiments and data/evidence collection; Shilpi Chauhan: Critical review and revision of the manuscript; Nisha Gupta: Data collection and writing of the manuscript; Dimpy Rani: Research activity planning including mentorship.
CONFLICT OF INTERESTS
The authors declare no conflicts of interest.
REFERENCES
Hassan RA, Hozayen WG, Abo Sree HT, Al Muzafar HM, Amin KA, Ahmed OM. Naringin and hesperidin counteract diclofenac-induced hepatotoxicity in male wistar rats via their antioxidant, anti-inflammatory and antiapoptotic activities. Oxid Med Cell Longev. 2021 Aug 11;2021:9990091. doi: 10.1155/2021/9990091, PMID 34422219.
Ali YA, Soliman HA, Abdel Gabbar M, Ahmed NA, Attia KA, Shalaby FM. Rutin and hesperidin revoke the hepatotoxicity induced by paclitaxel in male wistar rats via their antioxidant anti-inflammatory and antiapoptotic activities. Evid Based Complement Alternat Med. 2023 May 26;2023:2738351. doi: 10.1155/2023/2738351, PMID 37275575.
El Ghazaly MA, Fadel NA, Abdel Naby DH, Abd El Rehim HA, Zaki HF, Kenawy SA. Potential anti-inflammatory action of resveratrol and piperine in adjuvant induced arthritis: effect on pro-inflammatory cytokines and oxidative stress biomarkers. Egypt Rheumatol. 2020;42(1):71-7. doi: 10.1016/j.ejr.2019.08.003.
Singh S, Kumar P. Piperine in combination with quercetin halt 6-OHDA induced neurodegeneration in experimental rats: biochemical and neurochemical evidences. Neurosci Res. 2018 Aug;133:38-47. doi: 10.1016/j.neures.2017.10.006, PMID 29056550.
Bishnoi M, Chopra K, Rongzhu L, Kulkarni SK. Protective effect of curcumin and its combination with piperine (bioavailability enhancer) against haloperidol-associated neurotoxicity: cellular and neurochemical evidence. Neurotox Res. 2011 Oct;20(3):215-25. doi: 10.1007/s12640-010-9229-4, PMID 21076901.
Nisar A, Jagtap S, Vyavahare S, Deshpande M, Harsulkar A, Ranjekar P. Phytochemicals in the treatment of inflammation associated diseases: the journey from preclinical trials to clinical practice. Front Pharmacol. 2023;14:1177050. doi: 10.3389/fphar.2023.1177050, PMID 37229273.
Ahmed O, Fahim H, Mahmoud A, Eman Ahmed EA. Bee venom and hesperidin effectively mitigate complete freunds adjuvant-induced arthritis via immunomodulation and enhancement of antioxidant defense system. Arch Rheumatol. 2018;33(2):198-212. doi: 10.5606/ArchRheumatol.2018.6519, PMID 30207564.
Tripathi AK, Ray AK, Mishra SK. Molecular and pharmacological aspects of piperine as a potential molecule for disease prevention and management: evidence from clinical trials. Beni Suef Univ J Basic Appl Sci. 2022;11(1):16. doi: 10.1186/s43088-022-00196-1, PMID 35127957.
Son DJ, Akiba S, Hong JT, Yun YP, Hwang SY, Park YH. Piperine inhibits the activities of platelet cytosolic phospholipase A2 and thromboxane A2 synthase without affecting cyclooxygenase-1 activity: different mechanisms of action are involved in the inhibition of platelet aggregation and macrophage inflammatory response. Nutrients. 2014;6(8):3336-52. doi: 10.3390/nu6083336, PMID 25153972.
Van Breda SG, DE Kok TM. Smart combinations of bioactive compounds in fruits and vegetables may guide new strategies for personalized prevention of chronic diseases. Mol Nutr Food Res. 2018;62(1):1700597. doi: 10.1002/mnfr.201700597, PMID 29108107.
Efferth T, Koch E. Complex interactions between phytochemicals. The multi-target therapeutic concept of phytotherapy. Curr Drug Targets. 2011;12(1):122-32. doi: 10.2174/138945011793591626, PMID 20735354.
Zaka M, Sehgal SA, Shafique S, Abbasi BH. Comparative in silico analyses of Cannabis sativa Prunella vulgaris and Withania somnifera compounds elucidating the medicinal properties against rheumatoid arthritis. J Mol Graph Model. 2017 Jun;74:296-304. doi: 10.1016/j.jmgm.2017.04.013, PMID 28472734.
Mohamed Tap F, Abd Majid FA, Ismail HF, Wong TS, Shameli K, Miyake M. In silico and in vitro study of the bromelain phytochemical complex inhibition of phospholipase A2 (PLA2). Molecules. 2018 Jan 19;23(1):73. doi: 10.3390/molecules23010073, PMID 29351216.
Modak D, Paul S, Sarkar S, Thakur S, Bhattacharjee S. Validating potent anti-inflammatory and anti-rheumatoid properties of Drynaria quercifolia rhizome methanolic extract through in vitro in vivo in silico and GC-MS-based profiling. BMC Complement Med Ther. 2021 Mar 12;21(1):89. doi: 10.1186/s12906-021-03265-7, PMID 33711984.
Singh S, Singh S. JAK-STAT inhibitors: immersing therapeutic approach for management of rheumatoid arthritis. Int Immunopharmacol. 2020 Aug;86:106731. doi: 10.1016/j.intimp.2020.106731, PMID 32590315.
Aravilli RK, Vikram SL, Kohila V. Phytochemicals as potential antidotes for targeting NF-κB in rheumatoid arthritis. 3 Biotech. 2017;7(4):253. doi: 10.1007/s13205-017-0888-1, PMID 28721679.
Clark AR, Dean JL. The p38 MAPK pathway in rheumatoid arthritis: a sideways look. Open Rheumatol J. 2012;6:209-19. doi: 10.2174/1874312901206010209, PMID 23028406.
Santiago LA, Agobian GG, DE Sousa LR, DE Oliveira GR, DA Silva Migueis RL, DE Oliveira Santos J. Flavonoids alkaloids and saponins: are these plant derived compounds an alternative to the treatment of rheumatoid arthritis? A literature review. Clin Phytosci. 2021;7:4.
Pandey V, Golhani D, Shukla R. Ethosomes: versatile vesicular carriers for efficient transdermal delivery of therapeutic agents. Drug Deliv. 2015;22(8):988-1002. doi: 10.3109/10717544.2014.889777, PMID 24580572.
LI Y, WU L, WU D, Sun D, Wang T, Zhu X. Mechanism of transdermal permeation promotion of lipophilic drugs by ethosomes. Int J Nanomedicine. 2017 Apr 26;12:2875-82. doi: 10.2147/IJN.S134708.
Yuan F, Quan LD, Cui L, Goldring SR, Wang D. Development of macromolecular prodrug for rheumatoid arthritis. Adv Drug Deliv Rev. 2012 Oct;64(12):1205-19. doi: 10.1016/j.addr.2012.03.006, PMID 22433784.
Ainbinder D, Paolino D, Fresta M, Touitou E. Drug delivery applications with ethosomes. J Biomed Nanotechnol. 2010 Oct 1;6(5):558-68. doi: 10.1166/jbn.2010.1152, PMID 21329048.
Touitou E, Dayan N, Bergelson LD, Godin B, Eliaz M. Ethosomes novel vesicular carriers for enhanced delivery: characterization and skin penetration properties. J Control Release. 2000 Mar 15;65(3):403-18. doi: 10.1016/s0168-3659(99)00222-9, PMID 10699298.
Alharbi WS, Almughem FA, Almehmady AM, Jarallah SJ, Alsharif WK, Alzahrani NM. Phytosomes as an emerging nanotechnology platform for the topical delivery of bioactive phytochemicals. Pharmaceutics. 2021;13(9):1475. doi: 10.3390/pharmaceutics13091475, PMID 34575551.
Ajazuddin M, Saraf S. Applications of novel drug delivery system for herbal formulations. Fitoterapia. 2010 Aug;81(7):680-9. doi: 10.1016/j.fitote.2010.05.001, PMID 20471457.
Mukherjee PK, Bahadur S, Chaudhary SK, Kar A, Mukherjee K. Quality related safety issue evidence based validation of herbal medicine farm to pharma. In: Mukherjee PK, editor. Evidence based validation of herbal medicine. Elsevier; 2015. p. 1-28. doi: 10.1016/B978-0-12-800874-4.00001-5.
Ahmad I, Ahmad Khan MS, Cameotra SS. Quality assessment of herbal drugs and medicinal plant products. In: Meyers RA, editor. Encyclopedia of analytical chemistry. Chichester UK: John Wiley & Sons, Limited; 2014. p. 1-17. doi: 10.1002/9780470027318.a9946.
Huanbutta K, Rattanachitthawat N, Luangpraditkun K, Sriamornsak P, Puri V, Singh I. Development and evaluation of ethosomes loaded with zingiber zerumbet linn rhizome extract for antifungal skin infection in deep layer skin. Pharmaceutics. 2022 Dec 9;14(12):2765. doi: 10.3390/pharmaceutics14122765, PMID 36559259.
Patel CN, Patel NK, Prajapati PH. Development and validation of stability indicating RP-HPLC method for estimation of hesperidin in nanotransferosome and madhiphalarasayana. J Adv Pharm Sci. 2021;3:1-7.
Nasare M, Nagasandhya B, Prasad V, Diwan P. Development and validation of uv spectrophotometric method for simultaneous estimation of hesperidin and diosmin in the pharmaceutical dosage form. ISRN Spectrosc. 2013;2013:534830.
Gupta V, Jain UK. Quantitative analysis of piperine in ayurvedic formulation by UV spectrophotometry. Int J Pharm Sci Res. 2011;2(2):58-61.
Shaikh M, Jadhav AP. Development and validation of RP-HPLC for simultaneous estimation of gallic acid curcumin and piperine in an ayurvedic formulation. Int J Pharm Pharm Sci. 2020;12(6):81-6. doi: 10.22159/ijpps.2020v12i6.33801.
International Conference on Harmonisation. Validation of analytical procedures: text and methodology. Vol. Q2. Geneva: ICH; 2005.
Szymanski M, Mlynarek D, Szymanski A, Matklawska I. Simultaneous determination of diosmin and hesperidin in pharmaceuticals by RPLC using ionic liquids as mobile phase modifiers. Iran J Pharm Res. 2016;15(1):141-8. PMID 27610154.
Sellappan M, Devakumar D. Development and validation of RP-HPLC method for the estimation of escitalopram oxalate and flupentixol dihydrochloride in combined dosage form and plasma. Int J Pharm Pharm Sci. 2021;13(2):61-6. doi: 10.22159/ijpps.2021v13i2.30158.
Ramesh GT, Prasad YR. The development and validation of a fast stability indicating RP-HPLC method for quantification of lumefantrine and its organic impurities using central composite experimental design. Int J App Pharm. 2023;15(3):157-67. doi: 10.22159/ijap.2023v15i3.47698.
Al Bathish MY, Gazy AA, El Jamal MK. RP-HPLC and chemometric methods for the determination of two anti-diabetic mixtures; metformin hydrochloride canagliflozin and metformin hydrochloride gliclazide in their pharmaceutical formulation. Int J Pharm Pharm Sci. 2019;12(2):83-94. doi: 10.22159/ijpps.2020v12i2.35415.
Setyaningsih D, Santoso YA, Hartini YS, Murti YB, Hinrichs WL, Patramurti C. Isocratic high performance liquid chromatography (HPLC) for simultaneous quantification of curcumin and piperine in a microparticle formulation containing Curcuma longa and Piper nigrum. Heliyon. 2021 Mar 24;7(3):e06541. doi: 10.1016/j.heliyon.2021.e06541, PMID 33851044.
Kurangi B, Jalalpure S, Jagwani S. A validated stability indicating HPLC method for simultaneous estimation of resveratrol and piperine in cubosome and human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2019 Aug 1;1122-1123:39-48. doi: 10.1016/j.jchromb.2019.05.017, PMID 31150952.
Chaudhari VS, Borkar RM, Murty US, Banerjee S. Analytical method development and validation of reverse phase high performance liquid chromatography method for simultaneous quantifications of quercetin and piperine in dual drug loaded nanostructured lipid carriers. Spectrochim Acta A Mol Biomol Spectrosc. 2020 Aug 5;235:118312.
Pathan IB, Jaware BP, Shelke S, Ambekar W. Curcumin loaded ethosomes for transdermal application: formulation optimization in vitro and in vivo study. J Drug Deliv Sci Technol. 2018 Apr;44:49-57. doi: 10.1016/j.jddst.2017.11.005.
Swarnakar NK, Jain V, Dubey V, Mishra D, Jain NK. Enhanced oromucosal delivery of progesterone via hexosomes. Pharm Res. 2007 Dec;24(12):2223-30. doi: 10.1007/s11095-007-9409-y, PMID 17828445.
Alam P, Imran M, Jahan S, Akhtar A, Hasan Z. Formulation and characterization of hesperidin loaded transethosomal gel for dermal delivery to enhance antibacterial activity: comprehension of in vitro ex vivo and dermatokinetic analysis. Gels. 2023 Sep 20;9(10):791. doi: 10.3390/gels9100791, PMID 37888364.
Sartori T, Seigi Murakami F, Pinheiro Cruz A, Machado DE Campos A. Development and validation of a fast RP-HPLC method for determination of methotrexate entrapment efficiency in polymeric nanocapsules. J Chromatogr Sci. 2008;46(6):505-9. doi: 10.1093/chromsci/46.6.505, PMID 18647471.
Kumar M, Sharma A, Mahmood S, Thakur A, Mirza MA, Bhatia A. Franz diffusion cell and its implication in skin permeation studies. Drug Deliv. 2023;30:2033-46.
Lobo CL, M M, Shetty A, SA KP, Dubey A. Simultaneous quantification of 4-hydroxytamoxifen and hesperidin in liposomal formulations: development and validation of a RP-HPLC method. Heliyon. 2024 Feb 20;10(4):e25598. doi: 10.1016/j.heliyon.2024.e25598, PMID 38434076.