
Int J App Pharm, Vol 17, Issue 2, 2025, 39-52Review Article
THE NUTRACEUTICAL MARKET: INTELLECTUAL PROPERTY, CLINICAL RESEARCH, AND FUTURE INSIGHTS
BISHOP ADHIKARI1 , D. NAGASAMY VENKATESH2*
, D. NAGASAMY VENKATESH2*
1Department of Pharmaceutical Regulatory Affairs, JSS College of Pharmacy, (JSS Academy of Higher Education and Research, Mysuru), Ooty-643001, Nilgiris, Tamil Nadu, India. 2Department of Pharmaceutics, JSS College of Pharmacy, (JSS Academy of Higher Education and Research, Mysuru), Ooty-643001, Nilgiris, Tamil Nadu, India
*Corresponding author: D. Nagasamy Venkatesh; *Email: nagasamyvenkatesh@jssuni.edu.in
Received: 26 Nov 2024, Revised and Accepted: 04 Feb 2025
ABSTRACT
The global nutraceuticals industry is a dynamic and rapidly evolving sector with diverse products, each offering numerous health benefits that significantly impact behaviours and market trends. The evaluation of patents within this industry reveals the dual nature of intellectual property rights as both a tool for fostering innovation and a potential barrier. However, with this growing recognition of these nutraceutical products face the challenges of ensuring product integrity, particularly in addressing issues such as adulteration and substandard quality. Key aspects include its market potential, nutraceutical company challenges, adulteration, intellectual property rights, and identification techniques that will ensure public access to safe and effective products. In addressing the core relationships between intellectual property rights, regulations, and safe and effective products, this paper identifies several critical pathways for advancing industry practices like strengthening regulatory oversight, encouraging innovation through intellectual property rights, promoting ethical practices investing in research and development, and enhancing international collaboration. Also, it focuses on fostering a regulatory environment that promotes innovation and accessibility to its stakeholders who can ensure that nutraceutical products contribute meaningfully to public health objectives. There are several parameters like high-cost research and development, complex regulatory landscapes, consumer skepticism, and technologies like artificial intelligence and machine learning to streamline product development and help tailor strategies to address its specific needs. The insights of collaboration and innovation among international organizations are crucial to improving global health and well-being positively by shaping the future of the nutraceutical sector. Therefore, by addressing these collaboration relationships between governments, industry stakeholders, researchers, and civil society. Also, it is necessary to address the core relationship between intellectual property rights, regulation, and promotion of safe and effective nutraceutical products in this rapidly evolving industry.
Keywords: Nutraceuticals, Intellectual property rights (IPR), Clinical trials regulations (CTR), Public health, Industry, Regulated markets
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijap.2025v17i2.53278 Journal homepage: https://innovareacademics.in/journals/index.php/ijap
INTRODUCTION
The nutraceuticals industry is revolving as a potential and consumer-centric market. Which encompasses functional foods, dietary supplements, and bioactive compounds that are gaining recognition for their role in preventing chronic disease and promoting overall well-being. However, achieving the full potential of these products requires addressing critical issues such as product quality, safety, and efficacy. Also, parameters such as adulteration, substandard quality, and inconsistent bioavailability remain significant challenges, which necessitate robust regulatory oversight and significant rigor [1]. Even as the nutraceutical industry became more standardized in the late 20th century according to research and due to more effective regulations at a global level [2]. But, there are some emerging trends and gaps in the nutraceuticals landscape like lack of harmonized international standards, emerging technologies like wearable devices and digital health applications, tools like real-time feedback and personalized recommendations, also analytic techniques like mass spectrometry and nuclear magnetic resonance and high-performance liquid chromatography, these tools can enable differentiation in a competitive market. There is effective collaboration among governments, industries, and academia is crucial in developing globally consistent regulatory standards and fostering innovation. Numerous health benefits of nutraceutical products, it is evident that people are increasingly inclined towards seeking more natural and comprehensive health solutions through nutraceuticals. Nutraceuticals also have the potential to be incredibly beneficial for a variety of health concerns, such as enhancing the immune system and promoting heart health. Several factors must be taken into account also including the fact that most nutraceuticals are produced from naturally occurring sources with complex compositions that can affect human systemic circulation [3]. In this scenario, clinical trials play a crucial role in pinpointing the correct dosage that can offer maximum health benefits while reducing potential risks [4]. Nutraceutical products need the potential to safeguard innovations, promote research and development, and provide competitive advantages in a market that would deliver diverse goods and services [5]. It may also result in the development of effective products that combine strengths from other areas of nutraceutical science [6]. Most countries, such as the United States of America (USA), Europe, and Japan have stringent requirements for clinical manufacturing applications. This demonstrates a high level of regulatory oversight aimed at ensuring that consumers can trust the health claims made by manufacturing companies [7]. There are different exclusivities associated with IPR that have limited access to affordable products, especially in developing markets like stringent labeling requirements, post-marketing surveillance, ethical consideration, Artificial Intelligence (AI) and Machine Learning (ML) streamlining in the R and D process, and enhanced regulatory compliance. However, all the aspects can optimize clinical trial designs predict consumer behavior, and facilitate market entry. Such measures ultimately enhance the purchasing power for safe and effective products in the consumer segment. As the industry progresses, technological advancements, scientific knowledge, and regulatory standards are expected to influence its market possibilities more in the future [8]. This paper version aligns with consumer-centric approaches that prioritize transparency, safety, and efficacy with a focus on emerging areas like personalized nutrition, sustainability, and consumer education. Also how intellectual property rights and clinical research can contribute meaningfully to sustainable growth [5].
Critical issues in public health and intellectual property for global nutraceuticals
The global nutraceutical industry encounters several challenges that hinder public health, clinical practices, and IPR [9]. Public health experts around the world have been advocating for more rigorous clinical trials of nutraceutical products [10]. Furthermore, these actions support innovative techniques such as food security and technological advancements that prioritize the holistic well-being of consumers by promoting sustainable product development [11]. The industry has developed a patent position framework that is intended to offer an improved approach to new inventions [12]. The various challenges encountered in identifying data for their research project under this nutraceutical clinical research. These challenges include issues like lack of time, data misappropriation, lack of control over data, skill deficiencies, absence of compensation, unfavorable policies and legal norms, inadequate infrastructure, and interoperability issues [13, 14]. Helal N V et al., in their research case studies, have been discussing research, innovation, and patenting of formulations over the past 15 y in the field of nutraceuticals. However, there have been no significant advancements yet. They are examining market dynamics, and regulatory challenges, and providing solutions along with future directions for nutraceutical products [15]. The detailing of the composition and dietary supplements that claim to have therapeutic effects on medical conditions but lack scientific evidence or have questionable reliability always in this industry [16]. The latest updates from the Food and Drug Administration (FDA) have made it mandatory for nutraceutical companies to provide clinical data to support any health claims made on their marketed products [17]. According to the recent global survey done by Nutraceuticals Insights 2022 only 45% of respondents could accurately define nutraceuticals and 30 % expressed doubts about the safety and efficacy of such products. Most of the product awareness campaigns have primarily targeted urban populations leaving rural and underserved communities with limited knowledge about the potential benefits and risks of nutraceuticals. One of the primary reasons for consumer skepticism is the prevalence of unsubstantiated health claims. The study conducted by the research team revealed that 20% of nutraceuticals sold online contain misleading information about their therapeutic effects [7]. For example, the Food and Drug Administration's 2020 warning about adulated dietary supplements in the US led to the recall of over 150 products in 2018 Major nutraceutical companies faced backlash due to their popular products and supplements containing traces of harmful bacteria. But after that, those companies have taken effective multi-pronged strategies like conducting independent third-party audits, transparent marketing campaigns, and collaborating with regulatory authorities, this approach has increased their sales by 25% within two years as reported by the market analytics 2021. The companies Amway® and Herbalife® have invested a larger number of revenue in clinical trials to validate the efficacy and the safety of their market products for different chronic and non-chronic diseases. Europe has programs called “ Know your nutraceuticals” to educate millions of consumers about how to identify genuine products, also these campaigns have emphasized the importance of checking for regulatory approval and verifying third-party certifications to understand product labels and claims [81]. The top companies can truly distinguish themselves in the market by showcasing the clinical trial data of their unique formulations in comparison to their competitors' products, all while safeguarding their intellectual property [18]. Strategies like identifying public health concerns have an emphasis on effective tracking of dietary trends and deficiencies. Next setting regulatory standards and safety guidelines need to mandate FDA or EU clinical trials for these products. Approaches like addressing global malnutrition promote solutions to fortified foods to address vitamin and mineral deficiencies [82]. In most cases monitoring the product quality and adulteration can be justified with innovative detectors like mass spectroscopy and HPLCs. The partnerships on research and evidence-based claims validated the clinical efficacy of products eg. Omega-3 supplements in cardiovascular health. To promote ethical marketing practices ensuring truthfulness in dietary supplement advertisements. Joint initiatives to address consumer concerns and maintain transparency. Raise awareness about the safe use of nutraceuticals through campaigns and training programs that help in prescribing dietary supplements effectively [83].
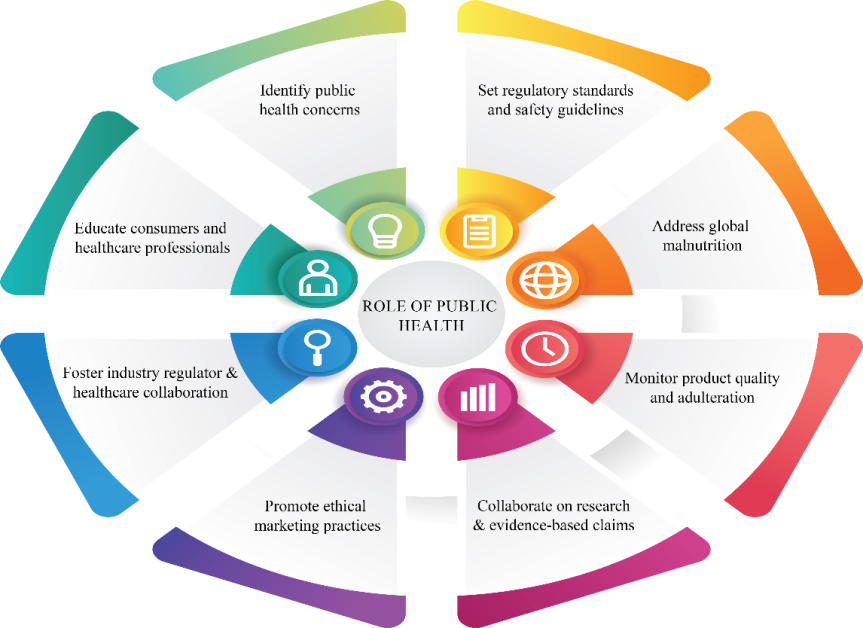
Fig. 1: The role of public health in nutraceutical integration
Intellectual property and clinical validation barriers for the global nutraceutical market
The interplay between Intellectual Property Rights and market access is one critical factor influencing the success of the worldwide nutraceutical market. IPR has served as double-edged facilities for any product or service like fostering innovation and protecting investments. This two active access will help to attract global commercialization, enabling relationships between IPR and market access. Also, the innovative research and development in this sector shows the many challenges of innovative research and development, brand trust, strategic collaboration, and facilitating global market entry [19, 20]. There is recent studies reveal that 58% of medium-sized businesses are facing profit constraints in the market due to these challenges [21]. Most of these limitations are primarily caused by problems related to inventory management, storage, and supply chain, which arise from non-compliance with regulations for those small-scale companies [22]. In IPR systems, development is crucial for the nutraceutical industry to stay competitive and offer innovative products with health benefits for future consumption towards the customer base [23-25]. Also, the challenges of biopiracy concerns and insufficient equity capital make the industry highly disrupted and competitive [26]. The shortage of human capital in the nutraceutical production sector poses challenges, and companies may find it challenging to protect patents and innovations in the long run [27]. Innovation is complicated due to high costs and legal complexity, prolonged litigation, licensing fees, and regional disparities in enforcement to enter the international markets for any of those products from small-scale companies [28, 29]. There are also disputes over contractual rights and the establishment of definitive IPR for independent firms adds to the complexity of their operational activity [30]. A public-private partnership can be a means of balancing public health objectives with the protection of intellectual property rights when it comes to clinically validated products [10].
Fig. 2(A): The interaction between IPR and market access
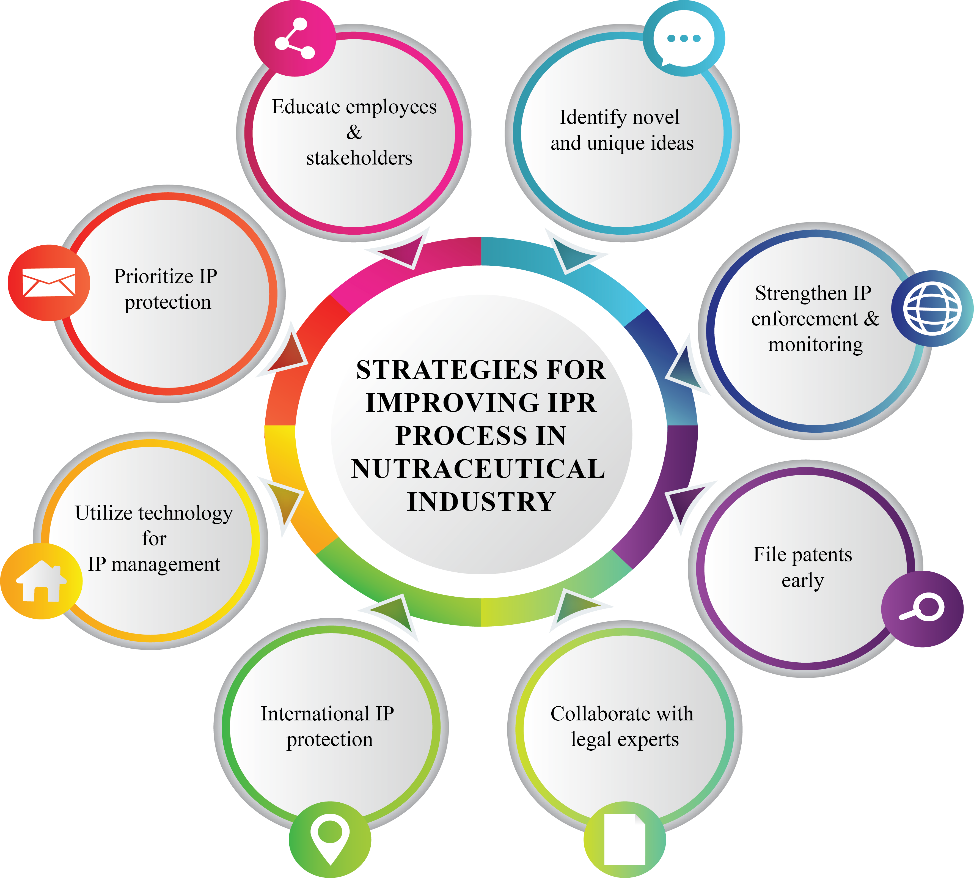
Fig. 2(B): Companies should adopt strategic approaches for the development of new nutraceutical products
The regular training programs to ensure employees understand IPR policies and best practices. The outcome of 95% compliance in employee knowledge assessments by the end of each year. Establish in-house R and D teams dedicated to patentable innovations and product differentiation, the 15% annually through consistent ideation sessions to increase new patent applications. Implementation of AI tools to track potential IP infringements globally and manage legal claims effectively, which reduces the occurrence of IP infringements by 25% within two years [84]. The proactive IP filing approach with dedicated legal support for expedited patent applications the measurable 80% of patent filings completed within three months of innovation identification. Partner with IP law firms to handle patent disputes and streamline the documentation process by resolving 90% of IP disputes in favour of the company within six months of initiation. International IP protection for region-specific regulations with secure IP protection in 5 new global markets every year. Employ cloud-based IP management software to organize and monitor patents trademarks and copyrights. To achieve 100% digitalization of IP assets within the next two years. Allocate a dedicated budget for IP-related activities and enforcement actions. The IP protection investments by 20% annually to safeguard critical assets [85].
The study designs intellectual property strategies for breakthroughs in nutraceuticals
The nutraceuticals study design has been covered with three factors credibility, protection innovation, and securing market advantages [29, 31]. Effective nutraceutical study design begins with rigorous preclinical research [32]. These pre-clinical results help to identify the specific compounds that can offer the best advantages and ensure consumer safety [33]. There is study design will depend on regulatory standards and demonstrate randomized control trials, which are crucial to optimal dosage and delivery mechanisms to maximize the effects of nutraceutical products [34]. In USA and Europe, nutraceuticals are commonly protected by patents that cover their compositions of matter, methods of use, or manufacturing techniques [35, 36]. Amanda Starling-Windh et al., mention how nano-delivery systems can enhance natural or pharmaceutical products. This type of innovation can provide an overview of the trends in intellectual property (IP) and the geography of nutraceutical drug delivery systems. The document contains chapters that discuss various aspects of IP protection in drug delivery systems. It highlights the importance of thorough IP monitoring, technology transfer, and patent strategies. It focuses on the importance of clinical trial data in securing strong patent applications and shows how improvements to IP practices have stimulated innovation for at least 30 y [37]. The global patent cooperation treaty (PCT) is streamlining the process of securing global IP protection and rights, which mitigation the risk of IP theft in emerging markets [38].
Randomized Controlled Trials (RCTs) are the gold standards for establishing the efficacy and safety of interventions in health and medicine. In the nutraceuticals industry RCTs will play a crucial role in therapeutic claims, unique challenges, funding constraints, regulatory inconsistencies, sample size difficulties, and public perception. RCTs are designed to minimize bias and confounding variables, making them an indispensable tool for assessing the true impact of nutraceutical products. The scientific validation through RCTs enhances consumer confidence in nutraceuticals differentiating credible products from those lacking evidence. Many regulatory agencies, such as the FDA and EFSA, require RCT data for health claim approval. Now, investors are more likely to support nutraceutical companies with clinically validated products, as RCTs reduce the risk of consumer backlash and regulatory non-compliance [86]. There are unique challenges in conducting RCTs for nutraceuticals compared to pharmaceutical trials. Conducting RCTs is expensive and requires millions of dollars for mid-large scale production. Most nutraceuticals often have thinner profit margins making it difficult to justify the high upfront costs of RCTs. Most of the RCTs vary widely across regions, creating confusion and increasing the complexity of trial design. Many nutraceuticals straddle the line between food and medicine leading to ambiguities in trial requirements [87]. It can be challenging especially when the product is perceived as low risk but lacks initial safety data. To detect meaningful effects, nutraceutical trials often require larger sample sizes, especially for interventions with subtle or long-term benefits. Also, recruitment challenges like products perceived as “natural” and safe can be difficult as they may not see the need for rigorous testing [88]. In outcome, variabilities like quality of life improvements and the placebo effect are particularly strong in nutraceutical trials. The most common challenge in this trial is that participant dropouts are more common in long-term studies potentially skewing results. Addressing these challenges of RCTs requires the most innovative approaches that balance scientific rigor with practical considerations. The harmonizing standards, adaptive trial designs, optimizing trial design, and leveraging technology like digital recruitment and wearable devices to improve the metabolic health of consumers [89]. The study like Omega 3 supplements on cardiovascular health enrolled over 20,000 participants with the active procedure to be followed by its inclusion in clinical guidelines. The future of RCTs will be high demand for high-quality industry growth of nutraceuticals with decentralized trials, integration with real-world evidence, policy innovations, and AI and big data improving the efficiency of nutraceuticals RCTs [90].
Techniques for good manufacturing practices and intellectual property protection for the nutraceutical sector
Good manufacturing practices (GMP) ensure product quality and safety and IP protection will provide the framework for innovation processes and proprietary formulations [27]. Several techniques need to be justified under GMP like SOPs, critical aspects of raw materials, different control environments, quality, and total quality management with lean manufacturing [39, 40]. The efforts with a systematic approach to control consumer confidence and regulatory compliance, as operational efficiencies will be improved the lower production costs and increase profitability [41]. IP protection is crucial in terms of formulations and delivery mechanisms, brand protection, market differentiation, confidential information, and IP portfolio management [42, 43]. Enforcing IP rights will be more innovative, especially with research instructions collaborating with nutraceutical companies with licensing agreements [44, 45]. With these combined strategies, nutraceutical companies can evolve and highly maintain competitive advancements in the regulated market [46-48].
The rapid growth necessitates strict adherence to GMP and robust intellectual property protection to ensure product safety, quality, and competitiveness. While GMP ensures product integrity and compliance with regulatory requirements, IP protection safeguards innovative ideas, formulations, and branding. The key elements of GMP in the nutraceutical sector must establish detailed SOPs for all manufacturing processes and finished products with quality control and quality assurance to ensure compliance with GMP standards, manufacturing facilities, and equipment design to prevent contamination, conduct regular GMP training for its employees, the recommendations for the documentation and record-keeping for the manufacturing process, including raw material sourcing, batch production, and distribution. The techniques for ensuring GMP adherence will depend on automation and digitalization which can improve packaging systems, reduce human error, and improve GMP compliance. Risk-based approaches like different assessment tools help identify and mitigate potential GMP violations before they occur [91]. Most of the successful examples of GMP adherence like Amway’s® Nutrilite® brand have conducted over 500000 quality checks annually ensuring its products meet global standards. Nestle® Health Science has implemented GMP practices across its global manufacturing facilities. Herbal Life® maintains a comprehensive GMP program, in-process testing, and post-market surveillance. According to a 2022 report global food safety initiative is approximately 85%, and the U. S. FDA issued 35 warning letters to dietary supplement manufacturing in 2021 for GMP violations, underscoring the importance of adherence. The recent World Intellectual Property Organization reported a 15 % increase in nutraceutical patent filings in 2022. In a 2021 survey by the International Trademark Association (INTA) 60% of nutraceutical companies identified IP protection as a top priority for sustaining market competitiveness. The opportunities of GMP can be improved with the help of blockchain technology, AI streamlining GMP, industry-wide collaborations with IP enforcement mechanisms, and education consumers can drive demand for quality nutraceuticals. According to a new study in 2022 by Deloitte nutraceuticals companies investing in GMP and IP protection saw a 20% higher market growth rate compared to those that did not [92].
Yakult Honsha® is the company that has successfully protected its trade secrets related to probiotic formulations, which have impacted a competitive advantage in the global probiotic drink market for decades. Cargill® the company has enforced its patents for stevia-based sweetener technology, which helps them significant revenue growth from its products. Abbott Laboratories has filed a lawsuit against a competitor for patent infringement on its Similac infant formula, which won the case, safeguarding its proprietary formula and reinforcing its position in the infant nutrition market. Nestle® Health Science defended trademarks and patents for its Boost and Resource brands in various international jurisdictions which strengthened brand recognition and market share globally [93]. Amway® Nutrilite® enforced trademark and patents against the counterfeiters attempting to sell imitation Nutrilite products, which reduced this activity and improved customer trust in Nutrilite as a premium nutraceutical brand. Herbalife® Nutrition took legal action against unauthorized sellers using its trademarks for counterfeit products which minimized unauthorized sales and improved brand reputation. DSM nutritional products litigated against IP violations for its omega-3 production patents which reserved its leadership in the market by blocking competitors from using patented methods [94]. GlaxoSmithKline® protected its patented formulations for Horlicks® from infringement which ensured market dominance for these popular products in the nutraceuticals segment. GNC patented its delivery systems for protein and multivitamin supplements and defended these patents in court and its products in a crowded market and ensured sustained customer loyalty. BASF will field patents and enforce legal rights for omega-3 encapsulation technologies to maintain a strong position in the encapsulated supplement market [95].
The promising market potential of nutraceuticals in global health
According to market research data, the majority of nutraceutical products are sold by companies that are labelled as 100% natural [49]. From the market perspective, sectors such as dietary or nutraceutical products, as well as all fortified products, are commonly referred to as naturally safe and categorized as nutrients [50-52]. However, this view may seem ambiguous when considering customer health implications. Recent research has revealed that a significant 80% of consumers worldwide prefer nutraceutical products for their nutrients over pharmaceutical ones [53, 54]. However, a pertinent question arises: How can we ensure that these products are free from adulteration? This issue is compounded by the fact that many of these products do not adhere to regulatory guidelines and are subject to minimal regulatory scrutiny in the nutraceutical industry. As the demand for natural and sustainable products continues to rise, nutraceuticals are poised to play a crucial role in shaping the future of healthcare [55].
Enhancing standards: analytical and safety challenges in nutraceuticals
Nutraceuticals are one of the most falling regulatory grey areas between foods and pharmaceuticals. Analytical testing is a more vital part in terms of product quality, safety, consistency, and achieving precise and accurate measurements because nutraceutical compounds are complex [56]. There are certainly some of the challenges this industry always facing like the diversity of bioactive compounds, lack of standardized testing protocols, analytical method development for complex matrices, quality control, and stability testing, and ensuring bioavailability and efficacy. Are diverse range of safety factors hindering nutraceutical production like the risk of contamination, adulteration and mislabelling, exact therapeutic doses, inadequate clinical and toxicology data, and compliance, and regulatory standards, rather than pre-market safety assessments remain a critical need for this industry [22]. But there both regulated and non-regulated countries are facing challenges in the nutraceutical industry, including patent violations or counterfeiting. In the nutraceuticals industry, it is essential to implement strategies to recognize innovators and enhance procedures, as this is paramount for progress [16]. Pharmaceutical products go through thorough analysis using advanced techniques such as HPLC and other validation processes like pre-clinical and clinical studies. The same level of analytical rigor should be implemented for nutraceuticals to improve their quality [55]. Any fault products can be identified efficiently through cross-sectional observation and official tests covering weight variation, disintegration, and evaluation techniques. Also, the concern for safety and cleanliness involves managing heavy metal contaminations in nutraceutical raw materials [7].
The articles selected for this review were sourced from specialized databases, including Elsevier, PubMed, and Cambridge, covering 2016 to 2024. The keywords used for the search included "Nutraceuticals", "Intellectual Property Rights", "Clinical Trials Regulations", "Public Health", "Industry" and "Regulated Markets". Also, the additional resources were drawn from Springer, Wiley, internet sources, and online publications such as The Lancet, Medscape, Elsevier Science Direct, and StatPearls.
Fig. 3: Establishing optimal product safety protocols for future company use cases in new product development
Balancing community interests and product integrity
The rapid growth of this sector has raised a complex issue like product integrity, and community interests, including public health, cultural practices, and environmental sustainability [55]. To maintain this all the factors need the best safety and efficacy policy is essential to building a sustainable and ethical nutraceuticals market. In most cases, small and medium-sized businesses focusing on traditional or local products may face a meet the standards due to financial or logistic constraints [56, 57]. Traditional knowledge is a cornerstone of this industry because many products are derived from Indigenous practices and ethnobotanical sources [58]. To balance product integrity with community interest nutraceuticals companies should adopt sustainable sourcing practices that minimize ecological impact while supporting biodiversity [59]. The high cost of premium nutraceuticals is always inaccessible to low-income groups which contributes to health inequities. Some multiple strategies and benefits foster balancing community interest and product integrity as like adoption of rigorous standards with flexibility, consumer education, integration through benefit-sharing models, enabling pricing and distribution models, and collaboration with regulatory bodies these are essential for the nutraceutical industry’s long-term success [60].
Advancing nutraceutical literacy for healthcare providers
Advancing nutraceutical literacy among healthcare providers is essential to bridging the gaps with factors like knowledge gaps, informed patient outcomes, and supporting informed decision-making in integrative and preventive healthcare [61]. The knowledge gaps have led to inconsistent recommendations, overlooking drug-nutrient interactions to recognize conditions that may benefit from nutraceuticals. There are certain barriers to nutraceutical literacy limited education in medical training of nutraceuticals, lack of standardized information and research, the complexity of nutraceutical regulations, and challenges in accessing reliable resources, also making it difficult to find high-quality data on dietary supplements and other natural products [62]. Strategies that can help advance nutraceutical literacy as medical and nursing curricula, develop continuing education programs, and create accessible resources such as databases of nutraceuticals, digital technology applications, and clinical practice guidelines to give informed advice based on current best practices [63]. The evidence-based practice as personalized medicine, naturopathic practitioners, well-rounded patient care, understanding of the science of nutraceuticals, funding activity, regulatory complexity, and understanding of long-term safety and efficacy. All aspects provide several outreach for extended beyond individual patient care [64].
Ensuring purity in nutraceuticals: tackling adulteration for public health
Nutraceuticals rapid growth has also brought challenges, particularly in the issues of adulteration. The adulteration has direct and indirect consequences on public health intended health benefits, maintaining consumer trust, and harmful substances.
The rapid expansion of this industry has also increased the prevalence of adulteration and mislabelling, posing significant risks to consumer health and product integrity. Product adulteration refers to the addition of non-declared substances, contaminants, or inferior-quality materials to a product. These practices undermine the efficacy, safety, and trustworthiness of nutraceutical products. A report of 2022 by per World Health Organization found that 30% of dietary supplements sold globally contain undisclosed or banned substances, also in 2016 to 2022 of US FDA identified over 700 tainted supplements primarily in weight loss, sexual enhancement, and muscle-building products [96]. The published study in 2020 revealed that 21% of herbal supplements tested in North America contained pharmaceutical contaminants. The global economic loss due to counterfeit and adulterated nutraceuticals is estimated to exceed $25 billion annually. India alone counterfeit dietary supplements caused losses exceeding $ 2 billion in 2021 as per the Indian Council for Research on International Economics Relations [96]. In the years 2018 and 2021 over 50,000 adverse events linked to contaminated supplements were reported to the FDA, in 2019 outbreak in Europe linked to adulterated weight loss supplements caused severe liver damage in 40% of affected individuals leading to multiple fatalities. A study has been conducted in Southeast Asia found that 15% of Ayurvedic supplements contain lead levels exceeding safe limits [97].
There are different types of adulteration in nutraceuticals as pharmaceutical adulteration, chemical and heavy metal contamination, synthetic and cheap fillers, and botanical adulteration and mislabelling [65]. Adulterated nutraceuticals have several health risks for consumers such as acute toxicity liver damage, and seizures, also there are long-term health effects such as neurological impairment and increased risk of cancer. Strategies for tackling adulteration in nutraceuticals need advanced analytical methods, establishing supplier audits and verification, certification, labelling, and blockchain technology, all the aspects will be identified under mandatory product testing and registration, strict labelling requirements, and harmonizing global standards to exceed maximum quality and promote consumer safety [56]. Most commitments to purity not only support public health but also reinforce the industry's role in promoting wellness through safe, effective products. Recent research has revealed that approximately 80% of consumers worldwide prefer nutraceutical products for their nutrients over pharmaceutical products. However, the concern remains whether these products are free from adulteration. This is because many of these products do not adhere to regulatory guidelines established by authorities, resulting in minimal regulatory control over the nutraceutical industry in various countries [40]. This sector will be saturated with the help of the e-pharmacy domain. In most cases, all nutraceutical products are safely shipped and delivered to the consumer's end. Consumers are often unaware of whether they are consuming natural or adulterated products. Nutraceutical products may be adulterated at various stages, including manufacturing, raw material collection, and storage. This can lead to contamination by microbial agents, heavy metals, and pesticides [66]. These factors will have a significant impact on health, potentially causing liver damage, bone disease, and even life-threatening conditions. Logic is maintained by managing the management of raw materials and finished products, which are subject to clinical specifications. The statistical method plays a crucial role in ensuring the stability of any activity [67]. It is essential for the success of a product in the marketplace. Recent studies have indicated that a majority of nutraceuticals are found to contain heavy metal contaminants because they have not undergone proper clinical testing. Some risks can be intentionally used to manipulate nutritional products for profit, however, the pharmacological effects on the human body from such product use remain uncertain. A multitude of naturally occurring elements have played a crucial role in the creation of new products and the treatment of various conditions, including metabolic metabolism, physical disease, skin disorders, and some types of cancer[68]. It should primarily be an indication of industry norms and regulatory frameworks, as well as licensing regimes. Therefore, the government and healthcare professionals must establish adequate regulations and guidelines, as well as implement targeted pre-and post-marketing monitoring to protect and provide optimal community support [69].
There are current regulatory approaches to tackling adulteration like the WHO and FAO established international food safety standards, including for dietary supplements. ISO 22000 outlines specific requirements for food safety management systems applicable to the nutraceuticals sector. The US FDA has been compliant and is mandatory for U. S.-based manufacturers. In European Food Safety Authority (EFSA) oversees product approval and monitors contaminants through its Rapid Alert System for Food and Feed (RASFF). India the food safety standards authority of India mandates product registration and adherence to stringent quality standards [98]. A 2020 study by the University of Guelph found that DNA barcoding reduced adulteration rates by 25 % in monitored samples. In India 18% of tested products were found to be adulterated, leading to the suspension of several manufacturing licenses. To address these challenges variations in safety standards and enforcement mechanisms across countries create loopholes for adulterators. Regulatory agencies often face budget limitations which also hinder their ability to conduct comprehensive testing and surveillance [99]. Also lack the knowledge to identify adulterated products, particularly in online marketplaces. The globalized nature of supply chains makes it difficult to trace contamination sources. Improved regulation of the active recommendation-like framework for global nutraceutical quality standards, should be adopted to detect contaminants more effectively, imposing harsher penalties on manufacturing found guilty of adulteration can deter malpractice. Public awareness initiatives should be launched to educate consumers about the risks of adultery [100].
Table 1: Clinical advancements in diabetes research for justifying the role of nutraceuticals in therapeutic applications
| Clinical trail ID no/Drug | Sponsor name | Enrolled candidate | Sexes eligible for Study | Disease/treatment given | Phase | Country | Study type | Study start/End date | Ages eligible for study | Study plan | References |
| NCT05753436/Curcumin | Ain Shams University | 72 | All (Male and Female) | Diabetes Mellitus, Type 2 | Phase 2 | Egypt | Interventional | 2023-07-01/2024-05 | 40 y | Randomized | [79] |
| NCT03262363/Curcumin | Unidad de Investigation Medica an Enfermedades Renales | 176 | All | Diabetes Mellitus, Type 2 | Phase 2 Phase 3 |
Mexico | Interventional | 2017-08-18/2018-05-28 | 18 y to 90Y (Adult, Older Adult) |
Randomized | |
| NCT02908152/Curcumin | National Nutrition and Food Technology Institute | 50 | All | Type 2Diabetes | Phase2 Phase 3 |
Iran | Interventional | 2017-02-10/2017-10-10 | 30 y to 65Y (Adult, Older Adult) |
Randomized | |
| NCT02216552/Resveratrol | University of Manitoba | 10 | All | Type 2Diabetes | Phase 2 Phase 3 |
Canada | Interventional | 2015-08/2017-03-20 | 13 y to 18 y (Child, Adult) |
Randomized | |
| NCT01881347/Resveratrol | Boston University | 54 | All | Type 2 Diabetes Mellitus | Not Applicable | United States | Interventional | 2013-01-01/2016-07 | 21 Y to 75 Y (Adult, Older Adult) |
Randomized | |
| NCT01038089/Resveratrol | Boston University | 20 | All | Type 2 Diabetes Mellitus | Not Applicable | United States | Interventional | 2010-01/2010-12 | 21 Y to 80 Y (Adult, Older Adult) |
Non-Randomized | |
| NCT00951912/Genistein | Sun Yat-sen University | 165 | Female | Type 2 Diabetes Mellitus | Not Applicable | Guangdong,China | Interventional | 2009-08/2011-09 | 30 Y to 70 Y (Adult, Older Adult) |
Randomized | |
| NCT01839344/Quercetin | Bastyr University | 19 | All | Type 2 Diabetes Mellitus | Phase 2 | United States | Interventional | 2013-05/2015-03 | 18 Y to 75 Y (Adult, Older Adult) |
Randomized | |
| NCT02263352/lycopene | Panineeya Mahavidyalaya Institute of Dental Sciences and Research Centre | 40 | All | Type 2 Diabetes Mellitus | Phase 4 | India, Hyderabad | Interventional | 2013-01/2013-08 | 35 Y to 50 Y (Adult) |
Randomized | |
| NCT04299763/Beta-Glucans | Centro de EstudiosenAlimentación y Nutrición, Chile | 37 | All | Type 2 Diabetes Mellitus | Phase 2 | Chile, Talca | Interventional | 2018-06-19/2019-01-18 | 30 to 45 y(Child, Adult, Older Adult) | Randomized | |
| NCT01003236/Silymarin | Shiraz University of Medical Sciences | 60 | All | Type II diabetes | Phase 2 | Iran, Shiraz | Interventional | 2010-10/2011-11 | 30 Y to 70 Y (Adult, Older Adult) |
Randomized | |
| NCT00425009/Berberine | Shanghai Jiao Tong University School of Medicine | 70 | All | Type II diabetes | Phase 1 Phase 2 |
China, Shanghai | Interventional | 2004-01/2004-12 | 25 Y to 75 Y (Adult, Older Adult) |
Randomized | |
NCT04009889/Probiotic Blend (Lactobacillus rhamnoses GG+Lactobacillus jensenii) |
I-Health, Inc. | 170 | Female | Gestational Diabetes Mellitus in Pregnancy | Not Applicable | Germany, Kiel | Interventional | 2018-04-05/2020-06-12 | 18 Y and older (Adult, Older Adult) | Randomized | |
| NCT05296759/Amino Acids | Alexandria University | 30 | All | Diabetic Neuropathies | Phase 4 | Egypt | Interventional | 2021-02-01/2022-03-08 | (Child, Adult, Older Adult) | Non-Randomized | |
| NCT03749642/Diabetic Kidney Disease | AziendeChimicheRiunite Angelini Francesco S. p. A | 240 | All | Diabetic Neuropathy | Phase 2 | Czechia, France, United Kingdom, Poland, France | Interventional | 2018-11-22/2020-06-06 | 18 Y to 75 Y (Adult, Older Adult) |
Randomized | |
| NCT03325322/Fisetin | Mayo Clinic | 30 | All | Diabetic Nephropathies | Phase 2 | United States | Interventional | 2018-01-02/2026-12 | 40 Y to 80 Y (Adult, Older Adult) | Randomized |
Navigating IPR enforcement challenges for nutraceuticals in developing regions
Nutraceuticals, which are relatively new and have not yet been regulated by government, are classified as emerging nutraceuticals. The products that are regulated by the government are known as nutraceuticals, which are long-term products [70]. Arunabha Ray et al. discuss nutraceutical regulations across the United States, European Union, Japan, China, and India, highlighting key legislative acts like the Dietary Supplement Health and Education Act (DSHEA) in the U. S. and the EU’s Directive 2002/46/EC. Each region has unique rules for product claims, approvals, and oversight by entities such as the China Food and Drug Administration (CFDA) and the Food Safety and Standards Authority of India (FSSAI) [71]. The analysis underscores the importance of aligning with evolving regulations for0 consumer safety and effective market access [65]. The article highlights the necessity of evaluating, analysing, and authoring product trials, licensing, and formulating accurate health claims to gain access to significant portions of the Indian and international nutraceutical market [72]. The continuous evolution of nutraceutical regulations highlights the importance of consistent updates and advancements to ensure the safe and effective utilization of these products for consumers [65].

Fig. 4: Challenges faced by nutraceutical companies in recent times for product development
Standardized regulations are important in addressing the close relationship between healthy foods, nutraceuticals, and medicines through implementing new IPR rules to meet consumer needs. The considerable size and growth potential of the global nutraceutical market are highlighted, with the US, Japan, and Europe emerging as key players. The scientific basis and market importance of nutraceutical products for future opportunities in global health and wellness innovation [37].
A focus will be given to investigating how much the level of external openness within research and development operations is associated with the innovation power and inventiveness of these companies [73]. The study examines product and process inventions, as well as the percentage of transactions from new products, as indicators of innovation performance. As international trade expands, it is apparent that non-conformist IPR and indigenous knowledge systems conflict [74]. The surge in efforts in medicinal and biotechnology, as well as a growing interest in natural remedies and supplements, has led to the commercialization of bio-resources and traditional knowledge [75]. The uncovers critical features of open innovation that bring about societal advantages, providing valuable insights for organizations and policymakers. It also uses a typology of the literature and an analysis of benefits gaps to help develop effective policy measures [76]. The need for responsible innovation strategies emphasizes that effective regulatory policies must address critical innovation issues [77, 78]. Legal rights have been facing the challenges of global infringements, high costs, and technological barriers. Most of the threats need to be justified with new business strategies and the economic trust of consumers. Also integration of the IP audits, training programs, legal partnerships, legislation, global cooperation, and awareness campaigns. The future will be trends in intellectual property (IP) management on AI in IP management, sustainable considerations, and global harmonization [75]. It highlights the significance of clear joint venture agreements to determine patent ownership. Additionally, it stresses the importance of monitoring the market for potential patent infringements [73].
Thinking and navigating the nutraceutical landscape
In today's rapidly evolving world, the interaction between academia and industry plays a crucial role in driving innovation, economic growth, and societal progress. In many parts of the world aging populations are looking towards improving their quality of life, managing chronic diseases, and also reducing medical interventions. The development in food science and biotechnology has enabled the formulation of advanced nutraceuticals, there are products like probiotics, prebiotics, and products with enhanced bioavailability [63]. In most cases regulatory aspects in different countries like the USA are not required to undergo pre-market approval, in Europe requires scientific substantiation before products can advertise benefits, in Japan foods with function claims have more flexible requirements, in India they have recently updated aim to define standards for ingredient safety, labelling, and permissible health claims [66]. Most nutraceutical products have been facing unique challenges like the variability of natural ingredients, the high cost of clinical trials, and insufficient regulatory limitations on health claims. Standardizing formulations and dosages for clinical research is crucial for nutraceutical research but it’s challenging. In product development, there should be a need the focus on encapsulation techniques, nanoemulsions, and fermentation. The advanced delivery systems are re-shaping the nutraceuticals market under the category of innovation like gummies and chewable for younger populations, powders, and functional beverages to add supplements to food or drinks, sublingual and transdermal delivery which have been used as nutraceuticals particularly those sensitive to stomach [57]. Navigating the nutraceuticals landscape has global demand and opportunities in emerging markets, where interest in preventive health and wellness is growing [75].
Table 2: Disease-specific intellectual properties filed under the nutraceuticals category over the past decade
| Id | Title | Assignee | Inventor/author | Priority date | Filing/creation date | Publication date | Grant date | Used disease | References |
| US-10842797-B2 | Nutraceutical co-crystal compositions | University Of South Florida | Michael john zaworotko et al. | 06-06-2007 | 24-05-2019 | 24-11-2020 | 24-11-2020 | Vitamin E deficiency | [80] |
| EP-3057604-B1 | Nanoparticle compositions and methods as carriers of nutraceutical factors across cell membranes and biological barriers | Nanosphere health sciences Inc. | Richard clark kaufman | 14-10-2013 | 14-10-2014 | 09-06-2021 | 09-06-2021 | Improved antioxidant deficiency | |
| US-2019201363-A1 | Oils with anti-inflammatory activity containing natural specialized pro-resolving mediators and their precursors | Solutex Na Llc | Gerhardus Lucas Bannen Berget al., | 10-05-2012 | 08-03-2019 | 04-07-2019 | Not yet granted | Ameliorating Anti-inflammatory activity | |
| US-11813272-B2 | Active agents and methods of their use for the treatment of metabolic disorders and non-alcoholic fatty liver disease | Flagship Pioneering Innovations V, Inc. | Steven John Taylor et al., | 05-06-2018 | 17-11-2020 | 17-11-2020 | 14-11-2023 | Liver Diseases | |
| US-10842752-B2 | Pharmaceutical or nutraceutical composition with sustained release characteristics and with resistance against the influence of ethanol | Evonik Operations Gmbh | Priyanka Bansilal Haksar et al., | 27-08-2012 | 18-10-2012 | 24-11-2020 | 24-11-2020 | Gastro-resistant activity | |
| TW-I432147-B | Dietary supplements for the promotion of growth, repair, and maintenance of bone and joints | Novus Int Inc | Jeremy D Moore et al., | 06-06-2007 | 06-06-2008 | 01-04-2014 | 01-04-2014 | Promoting growth, repair, and maintenance of bones | |
| US-8114445-B2 | Dietary supplements for promoting wellness and weight loss and methods of administering the same | Reliv International Inc. | Carl W. Hastings | 07-11-2008 | 05-11-2009 | 14-02-2012 | 14-02-2012 | Weight Loss | |
| EP-2303311-B1 | Angiogenin for use in treating skeletal muscle disorders | Agriculture Victoria Services Pty Ltd, Saputo Dairy Australia Pty Limited | Matthew Donaghet al., | 14-05-2008 | 14-05-2009 | 01-08-2018 | 01-08-2018 | Skeletal Muscle disorders | |
| CA-2377627-C | Cartilage-enhancing food supplements and methods of preparing the same | Joint Juice Incorporated, Kevin R. Stone, Premier Nutrition Corporation, Post Holdings, Inc. | Kevin R. Stone | 22-06-1999 | 21-06-2000 | 07-06-2011 | 07-06-2011 | Improving cartilage | |
| AU-2003270537-B2 | Methods and compositions for the production of flavonoid and isoflavonoid nutraceuticals | The Samuel Roberts Noble Foundation, Inc. | Bettina Deavours et al., | 10-09-2002 | 10-09-2003 | 20-03-2008 | 20-03-2008 | Anti-cancer activity | |
| US-7014872-B2 | Herbal nutraceutical formulation for diabetics and process for preparing the same | Council Of Scientific And Industrial Research | PalpuPushpangadan et al. | 26-03-2002 | 26-03-2002 | 21-03-2006 | 21-03-2006 | Diabetics Activity | |
| US-2022168324-A1 | Cancer therapy | Ned Biosystems, Inc. | Rebecca Lambert BENT | 12-04-2013 | 02-07-2021 | 02-06-2022 | Not Granted Yet | Cancers of epithelial origin | |
| US-10517322-B1 | Dietary supplement formulations for promoting sleep | Life Kitchen, LLC | James Wukjae Lee | 19-03-2018 | 18-03-2019 | 31-12-2019 | 31-12-2019 | Improving Sleep Conditions | |
| US-11026441-B2 | Avocado flesh and/or skin extract rich in polyphenols and cosmetic, dermatological, and nutraceutical compositions comprising the same | LaboratoiresExpanscience | Alex Saunois et al., | 22-12-2010 | 30-08-2018 | 08-06-2021 | 08-06-2021 | Skin Enhancing Activity | |
| US-9861611-B2 | Formulations of water-soluble derivatives of vitamin E and soft gel compositions, concentrates, and powders containing the same | Virun, Inc. | Philip J. Bromley | 18-09-2014 | 25-09-2015 | 09-01-2018 | 09-01-2018 | Improved Nutrition Activity |
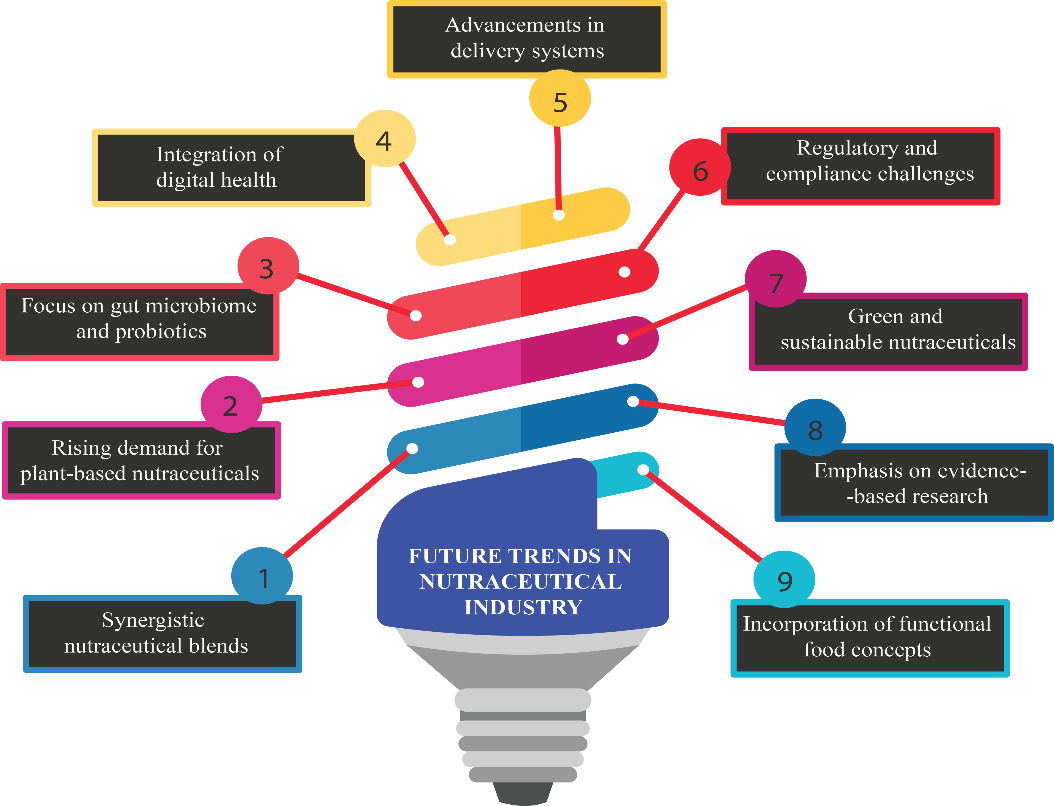
Fig. 5: Nutraceutical companies must embrace emerging trends for future success
Ethical and regulatory considerations
The lack of robust regulatory frameworks in an under regulated market exacerbates the risk of malpractices such as mislabelling, adulteration, and misleading health claims. Ethical consumer practices in the nutraceuticals industry depend on the three possible reach profitability vs ethical responsibility, quality assurance, and ethical business models which can lead an initiative to provide affordable supplements for underprivileged communities while ensuring GMP compliance. Also, most unregulated markets face challenges like adulteration and contamination, mislabelling, and market entry of substandard products, which undermine consumer trust [101]. In 2021, a study revealed that over 30% of nutraceuticals in an emerging market were mislabelled with some containing unlisted pharmaceutical ingredients. The global disparities in regulations in the USA manufacturers are not required to prove efficacy before marketing leaving room for unsubstantiated claims, Europe has mandated scientific validation of health claims offering a more robust regulatory framework and Asia and Africa lack dedicated regulatory bodies for nutraceuticals creating significant risks for consumers. In between of that harmonizing global regulatory standards can help address disparities and ensure product quality worldwide. Organizations like the World Health Organization (WHO) can facilitate knowledge sharing and capacity building for under regulated markets [102]. Also, there are strategies like strengthening those regulations like mandatory product testing, clear labelling requirements, and establishing independent regulatory bodies and public awareness campaigns this can result in a market dominated by high-quality products. Quantitatively insights according to a 2020 report up to 25% of nutraceutical products worldwide were found to contain contaminants or unlisted ingredients posing serious health risks for active consumer trust and economic impact which lacks trust in the efficacy and safety of nutraceuticals [103]. By prioritizing ethical responsibility and regulatory compliance, the nutraceutical industry can achieve sustainable growth and continue to contribute to global health and wellness [104].
Future potential and challenges for nutraceuticals
The nutraceuticals industry holds significant promise for advancing public health and addressing chronic disease burdens. Adopting international standards, promoting mutual recognition agreements, and streamlining labelling requirements will demonstrate progress toward harmonization. The incentivize private investments, expand public funding, integrate academic research, and promote open science initiatives can accelerate innovation through R and D outcomes on natural health products and supplements. The collaboration like developing cross-sector partnerships, training healthcare providers, and incorporating nutraceuticals in preventive care in disease prevention programs [105]. Ethical actions need to justify these gaps strengthen ethical marketing practices, invest in sustainable sourcing, and certify supply chains to promote product safety and sustainability. The rise of plant-based nutraceuticals demonstrates a shift towards more sustainable options. Addressing the market inquiries with the recommendations of subsidized nutraceuticals programs, capacity building in regulations, expand distribution networks example the WHO has assisted African nations in drafting nutraceutical regulations [106]. Embracing digital technologies like leveraging AI and Big data, developing digital platforms, and promoting telehealth integration to help all nutraceutical companies and products expand their market reach. By adopting these actionable recommendations the nutraceutical sector can balance profitability with public health priorities ensuring long-term success and impact [107]. Also rather countries like China, India, and Brazil have growth opportunities, because of their rising middle-class populations which shows the shift toward Westernized health practice. India’s nutraceuticals market is expanding to understand and cater to the cultural and regulatory prospects to benefit from expanding demand. The active technology transfer in food science, biotechnology, and material science enables the development of more advanced nutraceutical products [53]. Innovations like sublingual sprays, transdermal patches, and functional beverages make it easier for active consumer groups to integrate nutraceuticals into their daily routines. Investment in clinical research will be vital to substantiating health claims and fostering collaboration between nutraceutical companies and the healthcare sector. Most company needs to be focused on health outcomes and targeting niche markets which can help companies establish a strong position in the nutraceuticals landscape [60]. The best ethical business models in this sector will also support long-term industry credibility and consumer trust. Also, there is conventional medicine can be improved by regular validation in clinical applications and the application of reliable therapeutic agents [49]. To realize the market potential in the national and international markets, critical challenges related to nutraceutical products must be addressed [76-78].
CONCLUSION
A sustainable, ethical, and innovation-driven nutraceuticals industry is within reach, provided that stakeholders prioritize collaboration and long-term planning. By focusing on the multifaced reach actions, consumer engagements are merely profit-oriented to becoming a key player in global public health initiatives. The place should be addressed with the help of the best ecosystems with incentivize ethical practices and technological advances. The road ahead demands collective action. The regulators, manufacturers, researchers, and healthcare providers must unite in developing this industry that not only meets market demands but also improves lives across all socioeconomic strata. A best-harmonised evidence-based and transparent nutraceutical industry can act as a cornerstone for healthier global populations and sustainable economic growth. Also, this journey towards the nutraceuticals market or product development realizing this potential is shaped impact on public health activity. However, careful planning and execution, short-term problem solving, and embracing new tools such as data analytics and artificial intelligence can also prove invaluable in transforming nutraceuticals into opportunities for growth and success. There are regulatory approaches that can create complexities for companies seeking to operate globally. Without any harmonized regulatory framework most companies need to invest additional resources in their product development and labelling to meet each region's specific requirements. There is scientific validation including high research costs, adulteration, bioavailability, ethical and environmental considerations, unrealistic expectations practices, clear labelling, and collaboration with healthcare providers, these can become a cornerstone of the preventive healthcare approach also the holistic vision of healthcare in the years to come. The future of nutraceuticals is filled with promise and complexity. These aspects might involve reconsidering the patent system to promote innovation and ensure that crucial products remain accessible to everyone. Collaborating can guarantee that all individuals can access the advantages of this swiftly expanding sector, and can enhance public health results for future generations.
ACKNOWLEDGEMENT
The authors would like to express their gratitude to the Department of Science and Technology-Fund for Improvement of Science and Technology Infrastructure (DST-FIST), Promotion of University Research and Scientific Excellence (DST-PURSE), and the Department of Biotechnology Boost to University Interdisciplinary Pharmaceutical Regulatory Affairs Science Departments for Education and Research program (DBT-BUILDER) for the facilities provided in our department. Additionally, I thank the following individuals for their expertise and assistance throughout all aspects of my review study and for their help in writing the manuscript. I also extend my heartfelt thanks to JSS Academy of Higher Education, Mysuru, and JSS College of Pharmacy, Ooty, for providing their pool of resources to support and enhance the quality of my review.
FUNDING
Nil
AUTHORS CONTRIBUTIONS
Bishop Adhikari: Conceptualization, Data acquisition, Analysis, Interpretation, Writing-original draft, and Evaluation. D. Nagasamy Venkatesh: Review and editing, Supervision, Critical evaluation, and Validation.
CONFLICT OF INTERESTS
The author is reporting no conflict of interest.
REFERENCES
Puri V, Nagpal M, Singh I, Singh M, Dhingra GA, Huanbutta K. A comprehensive review on nutraceuticals: therapy support and formulation challenges. Nutrients. 2022 Nov 3;14(21):4637. doi: 10.3390/nu14214637, PMID 36364899, PMCID PMC9654660.
Chandra S, Saklani S, Kumar P, Kim B, Coutinho HD. Nutraceuticals: pharmacologically active potent dietary supplements. Bio Med Res Int. 2022 Jul 4;2022:2051017. doi: 10.1155/2022/2051017, PMID 35832855, PMCID PMC9273442.
Al Ali M, Alqubaisy M, Aljaafari MN, AlAli AO, Baqais L, Molouki A. Nutraceuticals: transformation of conventional foods into health promoters/disease preventers and safety considerations. Molecules. 2021 Apr 27;26(9):2540. doi: 10.3390/molecules26092540, PMID 33925346, PMCID PMC8123587.
Sachdeva V, Roy A, Bharadvaja N. Current prospects of nutraceuticals: a review. Curr Pharm Biotechnol. 2020;21(10):884-96. doi: 10.2174/1389201021666200130113441, PMID 32000642.
Pentu N, Bindu SB, Rao TR. Flavonoids: an era of nutraceuticals turning into medicinal agents. Asian J Pharm Clin Res. 2024 Jun;17(6):9-17. doi: 10.22159/ajpcr.2024.v17i6.50514.
Marimuthu A, Seenivasan R, Pachiyappan JK, Nizam I, Ganesh G. Synergy of science and tradition: a nanotechnology driven revolution in natural medicine. Int J App Pharm. 2024;16(6):10-20. doi: 10.22159/ijap.2024v16i6.50767.
Chopra AS, Lordan R, Horbanczuk OK, Atanasov AG, Chopra I, Horbanczuk JO. The current use and evolving landscape of nutraceuticals. Pharmacol Res. 2022 Jan;175:106001. doi: 10.1016/j.phrs.2021.106001, PMID 34826602.
Bansal R, Dhiman A. Nutraceuticals: a comparative analysis of regulatory framework in different countries of the world. Endocr Metab Immune Disord Drug Targets. 2020;20(10):1654-63. doi: 10.2174/1871530320666200519084415, PMID 32427089.
Puttasiddaiah R, Lakshminarayana R, Somashekar NL, Gupta VK, Inbaraj BS, Usmani Z. Advances in nanofabrication technology for nutraceuticals: new insights and future trends. Bioengineering (Basel). 2022 Sep 16;9(9):478. doi: 10.3390/bioengineering9090478, PMID 36135026, PMCID PMC9495680.
Kartal M. Intellectual property protection in the natural product drug discovery traditional herbal medicine and herbal medicinal products. Phytother Res. 2007 Feb;21(2):113-9. doi: 10.1002/ptr.2036, PMID 17117452.
Wrobel K, Zastawna B, Milewska AJ, Marczak M, Kozlowski R. Comparison between the American and the European systems of monitoring adverse effects of dietary supplements and their usefulness on the polish market. Int J Environ Res Public Health. 2023 Jan 4;20(2):902. doi: 10.3390/ijerph20020902, PMID 36673658, PMCID PMC9859348.
Wierzejska RE. Dietary supplements for whom? The current state of knowledge about the health effects of selected supplement use. Int J Environ Res Public Health. 2021 Aug 24;18(17):8897. doi: 10.3390/ijerph18178897, PMID 34501487, PMCID PMC8431076.
Ashouri S, Mention AL, Smyrnios KX. Anticipation and analysis of industry convergence using patent level indicators. Scientometrics. 2021;126(7):5727-58. doi: 10.1007/s11192-021-04025-7.
Lordan R, Rando HM, COVID-19 Review Consortium, Greene CS. Dietary supplements and nutraceuticals under investigation for COVID-19 prevention and treatment. M Systems. 2021 May 4;6(3):e00122-21. doi: 10.1128/mSystems.00122-21, PMID 33947804, PMCID PMC8269209.
Helal NA, Eassa HA, Amer AM, Eltokhy MA, Edafiogho I, Nounou MI. Nutraceuticals novel formulations: the good the bad the unknown and patents involved. Recent Pat Drug Deliv Formul. 2019;13(2):105-56. doi: 10.2174/1872211313666190503112040, PMID 31577201, PMCID PMC6806606.
Jargin S. Drugs and dietary supplements with unproven effects in research and practice. J Complement Med Res. 2019;10(1):27-37. doi: 10.5455/jcmr.20181223075028.
Chakrabartty I, Mohanta YK, Nongbet A, Mohanta TK, Mahanta S, Das N. Exploration of lamiaceae in cardio vascular diseases and functional foods: medicine as food and food as medicine. Front Pharmacol. 2022 Jun 14;13:894814. doi: 10.3389/fphar.2022.894814, PMID 35774598, PMCID PMC9237463.
Dwyer JT, Coates PM, Smith MJ. Dietary supplements: regulatory challenges and research resources. Nutrients. 2018 Jan 4;10(1):41. doi: 10.3390/nu10010041, PMID 29300341, PMCID PMC5793269.
Lozda R. Regulatory issues of voluntary certification of food supplements in Russia. Ther Innov Regul Sci. 2020 Jan;54(1):177-83. doi: 10.1007/s43441-019-00043-z, PMID 32008230.
Intrasook J, Tsusaka TW, Anal AK. Trends and current food safety regulations and policies for functional foods and beverages containing botanicals. J Food Drug Anal. 2024 Jun 15;32(2):112-39. doi: 10.38212/2224-6614.3499, PMID 38934687, PMCID PMC11210467.
Sadgrove NJ. Honest nutraceuticals cosmetics therapies and foods (NCTFs): standardization and safety of natural products. Crit Rev Food Sci Nutr. 2022;62(16):4326-41. doi: 10.1080/10408398.2021.1874286, PMID 33480270.
Pagani E, Ropke CD, Soares CM, Perez SA, Benevides PJ, Barbosa BS. Technology readiness level roadmap for developing innovative herbal medicinal products. Pharmaceuticals (Basel). 2024 May 29;17(6):703. doi: 10.3390/ph17060703, PMID 38931370, PMCID PMC11206302.
Jagim AR, Harty PS, Erickson JL, Tinsley GM, Garner D, Galpin AJ. Prevalence of adulteration in dietary supplements and recommendations for safe supplement practices in sport. Front Sports Act Living. 2023 Sep 29;5:1239121. doi: 10.3389/fspor.2023.1239121, PMID 37841887, PMCID PMC10570429.
Ubaid M, Salauddin SMA, Shadani MA, Kawish SM, Albratty M, Makeen HA. Daidzein from dietary supplement to a drug candidate: an evaluation of potential. ACS Omega. 2023 Aug 27;8(36):32271-93. doi: 10.1021/acsomega.3c03741, PMID 37780202, PMCID PMC10538961.
Xia N. Intellectual property protection for traditional medical knowledge in China’s context: a round peg in a square hole? Med Law Rev. 2023 Aug 25;31(3):358-90. doi: 10.1093/medlaw/fwad006, PMID 37018625, PMCID PMC10452053.
Sharma A, Ranout AS, Nadda G. Insights into cultivation strategies bioactive components therapeutic potential patents and market products of Ophiocordyceps sinensis: a comprehensive review. S Afr J Bot. 2024 Aug;171:546-70. doi: 10.1016/j.sajb.2024.06.036.
Chen X, Shi J, Lai Y, Xue Y, Ung CO, HU H. Systematic analysis of randomised controlled trials of Chinese herb medicine for non-alcoholic steatohepatitis (NASH): implications for future drug development and trial design. Chin Med. 2023 May 19;18(1):58. doi: 10.1186/s13020-023-00761-5, PMID 37208742, PMCID PMC10199512.
Zhang C, Rao A, Chen C, LI Y, Tan X, Long J. Pharmacological activity and clinical application analysis of traditional Chinese medicine ginger from the perspective of one source and multiple substances. Chin Med. 2024 Jul 12;19(1):97. doi: 10.1186/s13020-024-00969-z, PMID 38997763, PMCID PMC11241951.
Farooqi MA, Kang CU, Choi KH. Organ on chip: advancing nutraceutical testing for improved health outcomes. ACS Omega. 2023 Aug 21;8(35):31632-47. doi: 10.1021/acsomega.3c03155, PMID 37692213, PMCID PMC10483668.
Paul Chima UO, Ugwu CN, Alum EU. Integrated approaches in nutraceutical delivery systems: optimizing ADME dynamics for enhanced therapeutic potency and clinical impact. RPS Pharmacy and Pharmacology Reports. 2024;3(4):rqae024. doi: 10.1093/rpsppr/rqae024.
Singh S, Chib S, Akhtar MJ, Kumar B, Chawla PA, Bhatia R. Paradigms and success stories of natural products in drug discovery against neurodegenerative disorders (NDDs). Curr Neuropharmacol. 2024;22(6):992-1015. doi: 10.2174/1570159X21666230105110834, PMID 36606589, PMCID PMC10964107.
Dwyer JT, Coates PM. Why Americans need information on dietary supplements. J Nutr. 2018 Aug 1;148 Suppl 2:1401S-5S. doi: 10.1093/jn/nxy081, PMID 31505678, PMCID PMC6857605.
Jalil B, Rollinger JM, Atanasov AG, Singla RK, Kinghorn AD, Heinrich M. Core publications in drug discovery and natural product research. Front Nat Prod. 2024 Nov;3:1493720. doi: 10.3389/fntpr.2024.1493720.
Rivero Segura NA, Zepeda Arzate EA, Castillo Vazquez SK, Fleischmann Dela Parra P, Hernandez Pineda J, Flores Soto E. Exploring the geroprotective potential of nutraceuticals. Nutrients. 2024 Aug 24;16(17):2835. doi: 10.3390/nu16172835, PMID 39275153, PMCID PMC11396943.
Coates PM, Bailey RL, Blumberg JB, El Sohemy A, Floyd E, Goldenberg JZ. The evolution of science and regulation of dietary supplements: past present and future. J Nutr. 2024 Aug;154(8):2335-45. doi: 10.1016/j.tjnut.2024.06.017, PMID 38971530, PMCID PMC11375470.
Choudhury SD, Kumar P, Choudhury D. Bioactive nutraceuticals as G4 stabilizers: potential cancer prevention and therapy a critical review. Naunyn Schmiedebergs Arch Pharmacol. 2024 Jun;397(6):3585-616. doi: 10.1007/s00210-023-02857-z, PMID 38019298.
Starling Windhof A, Srinivasan A, Tomeo T, Clark AM, Mattei P. Trends in the intellectual property (IP) landscape of drug delivery systems. In: Shegokar R, editor. Nanopharmaceuticals. Elsevier; 2020. p. 201-30. doi: 10.1016/B978-0-12-817778-5.00010-5.
LI L, Wang L, Zhang L. Therapeutic potential of natural compounds from herbs and nutraceuticals in alleviating neurological disorders: targeting the Wnt signaling pathway. J Agric Food Chem. 2024 Feb 7;72(5):2411-33. doi: 10.1021/acs.jafc.3c07536, PMID 38284360.
Sarkar R, Nayak SL, Suthar MK, Das M. Nutraceutical formulations from medicinal plants: a potential therapeutic agent. In: Nandave M, Joshi R, Upadhyay J, editors. Ethnopharmacology and OMICS advances in medicinal plants. Vol. 1. Singapore: Springer; 2024. p. 391-417. doi: 10.1007/978-981-97-2367-6_19.
Sato K, Kodama K, Sengoku S. Driving innovation through regulatory design and corporate behaviour: a case study of functional food industry in Japan. Foods. 2024 Oct 18;13(20):3302. doi: 10.3390/foods13203302, PMID 39456364, PMCID PMC11508179.
Ayurveda CD. Bridging innovation for human and animal health. J Res Ayurvedic Sci. 2024;8(1):9-11. doi: 10.4103/jras.jras_83_23.
Al Worafi YM. Medical and health sciences in developing countries: research topic appropriateness. In: Al Worafi YM, editor. Handbook of medical and health sciences in developing countries. Berlin: Springer; 2024. p. 1-41. doi: 10.1007/978-3-030-74786-2_366-1.
Meti BS, Bhat S, Shivaleela VB, Jigajinni SK. Food business management and bioentrepreneurship. In: Yaradoddi JS, Meti BS, Mudgulkar SB, Agsar D, editors. Frontiers in food biotechnology. Singapore: Springer; 2024. p. 399-425. doi: 10.1007/978-981-97-3261-6_22.
Ahmed N, Sheikh MA, Ubaid M, Chauhan P, Kumar K, Choudhary S. Comprehensive exploration of marine algae diversity bioactive compounds health benefits regulatory issues and food and drug applications. Measurement: Food. 2024 Jun;14:100163. doi: 10.1016/j.meafoo.2024.100163.
Wells ML, Potin P, Craigie JS, Raven JA, Merchant SS, Helliwell KE. Algae as nutritional and functional food sources: revisiting our understanding. J Appl Phycol. 2017;29(2):949-82. doi: 10.1007/s10811-016-0974-5, PMID 28458464, PMCID PMC5387034.
Chandrasekhar T, Riazunnisa K, Vijaya Lakshmi D, Anu Prasanna V, Veera Bramhachari P. Exploration of bioactive functional molecules from marine algae: challenges and applications in nutraceuticals. In: Veera Bramhachari P, Berde CV, editors. Marine bioactive molecules for biomedical and pharmacotherapeutic applications. Singapore: Springer; 2023. p. 187-96. doi: 10.1007/978-981-99-6770-4_10.
The Lancet. Dietary supplement regulation: FDA’s bitter pill. Lancet. 2019 Feb 23;393(10173):718. doi: 10.1016/S0140-6736(19)30406-4, PMID 30799001.
Cadwallader AB, Council ON Science and Public Health A. Which features of dietary supplement industry product trends and regulation deserve physicians attention? AMA J Ethics. 2022 May 1;24(5):E410-418. doi: 10.1001/amajethics.2022.410, PMID 35575573.
Jayaweera JA. Current trends and technologies in nutraceutical industry. In: Amalraj A, Kuttappan S, Varma AC, Matharu A, editors. Herbs spices and their roles in nutraceuticals and functional foods. Elsevier; 2023. p. 347-60. doi: 10.1016/B978-0-323-90794-1.00008-9.
Resnik DB. Proportionality in public health regulation: the case of dietary supplements. Food Ethics. 2018 Dec;2(1):1-16. doi: 10.1007/s41055-017-0023-3, PMID 30911602, PMCID PMC6430238.
Dama A, Shpati K, Daliu P, Dumur S, Gorica E, Santini A. Targeting metabolic diseases: the role of nutraceuticals in modulating oxidative stress and inflammation. Nutrients. 2024 Feb 10;16(4):507. doi: 10.3390/nu16040507, PMID 38398830, PMCID PMC10891887.
Mittal RK, Mishra R, Sharma V, Purohit P. Bioactive exploration in functional foods: unlocking natures treasures. Curr Pharm Biotechnol. 2024;25(11):1419-35. doi: 10.2174/0113892010282580231120041659, PMID 38031768.
Bansal P, Maithani M, Gupta V, Kaur G, Bansal R. Future prospective of nutraceutical and functional food with herbs and spices. In: Amalraj A, Kuttappan S, Varma AC, Matharu A, editors. Herbs spices and their roles in nutraceuticals and functional foods. Elsevier; 2023. p. 361-81. doi: 10.1016/B978-0-323-90794-1.00015-6.
Aguilar Perez KM, Ruiz Pulido G, Medina DI, Parra Saldivar R, Iqbal HM. Insight of nanotechnological processing for nano fortified functional foods and nutraceutical opportunities challenges and future scope in food for better health. Crit Rev Food Sci Nutr. 2023;63(20):4618-35. doi: 10.1080/10408398.2021.2004994, PMID 34817310.
Ahmed Z, Chen J, Tufail T, Latif A, Arif M, Ullah R. Fundamental opportunities and challenges of nutraceutical noodles enriched with agri food by products. Trends Food Sci Technol. 2024 Jan;143:104299. doi: 10.1016/j.tifs.2023.104299.
Sarma N, Upton R, Rose U, Guo DA, Marles R, Khan I. Pharmacopeial standards for the quality control of botanical dietary supplements in the United States. J Diet Suppl. 2023;20(3):485-504. doi: 10.1080/19390211.2021.1990171, PMID 34699287.
Garza Juarez A, Perez Carrillo E, Arredondo Espinoza EU, Islas JF, Benitez Chao DF, Escamilla Garcia E. Nutraceuticals and their contribution to preventing noncommunicable diseases. Foods. 2023 Aug 30;12(17):3262. doi: 10.3390/foods12173262, PMID 37685194, PMCID PMC10486909.
Allen LH, Miller JW, DE Groot L, Rosenberg IH, Smith AD, Refsum H. Biomarkers of nutrition for development (BOND): vitamin B-12 review. J Nutr. 2018 Dec 1;148 Suppl 4:1995S-2027S. doi: 10.1093/jn/nxy201, PMID 30500928, PMCID PMC6297555.
Dominguez Diaz L, Fernandez Ruiz V, Camara M. The frontier between nutrition and pharma: the international regulatory framework of functional foods food supplements and nutraceuticals. Crit Rev Food Sci Nutr. 2020;60(10):1738-46. doi: 10.1080/10408398.2019.1592107, PMID 30924346.
Lordan R, Rando HM, COVID-19 Review Consortium Greene CS. Dietary supplements and nutraceuticals under investigation for COVID-19 prevention and treatment. M Systems. 2021;6(3):e00122-21. doi: 10.1128/mSystems.00122-21, PMID 33947804.
Ward WE, Chilibeck PD, Comelli EM, Duncan AM, Phillips SM, Robinson LE. Research in nutritional supplements and nutraceuticals for health physical activity and performance: moving forward 1. Appl Physiol Nutr Metab. 2019 May;44(5):455-60. doi: 10.1139/apnm-2018-0781, PMID 30794435.
Teoh SL, Ngorsuraches S, Lai NM, Bangpan M, Chaiyakunapruk N. Factors affecting consumers decisions on the use of nutraceuticals: a systematic review. Int J Food Sci Nutr. 2019 Jun;70(4):491-512. doi: 10.1080/09637486.2018.1538326, PMID 30634867.
Kaptur B, Peterman N, Lee J. Nutraceuticals and dietary supplements: disparities in usage and potential for harm. Nutr Health. 2022 Sep;28(3):325-30. doi: 10.1177/02601060221099690, PMID 35521933.
Darnton Hill I. Public health aspects in the prevention and control of vitamin deficiencies. Curr Dev Nutr. 2019 Jun 21;3(9):nzz075. doi: 10.1093/cdn/nzz075, PMID 31598578, PMCID PMC6775441.
Sattigere VD, Ramesh Kumar P, Prakash V. Science based regulatory approach for safe nutraceuticals. J Sci Food Agric. 2020 Nov;100(14):5079-82. doi: 10.1002/jsfa.9381, PMID 30264462.
Bansal K, Sundram S, Malviya R. Herbal components inspiring current lifestyle disease treatment: role of nutraceuticals. Curr Drug Res Rev. 2024;16(2):111-27. doi: 10.2174/2589977515666230512142020, PMID 37183457.
Frankos VH, Street DA, O Neill RK. FDA regulation of dietary supplements and requirements regarding adverse event reporting. Clin Pharmacol Ther. 2010 Feb;87(2):239-44. doi: 10.1038/clpt.2009.263, PMID 20032973.
Sniffen JC, MC Farland LV, Evans CT, Goldstein EJ. Choosing an appropriate probiotic product for your patient: an evidencebased practical guide. Plos One. 2018 Dec 26;13(12):e0209205. doi: 10.1371/journal.pone.0209205, PMID 30586435, PMCID PMC6306248.
Ghosh P, Ghosh PK. Regulatory guidelines and protocols for food fortification and enrichment by liposomes. In: Anandharamakrishnan C, Dutta S, editors. Liposomal encapsulation in food science and technology. Elsevier; 2023. p. 255-68. doi: 10.1016/B978-0-12-823935-3.00002-3.
Wadekar R, Mandal SC, Patil K. Intellectual property rights naturally derived bioactive compounds and resource conservation. In: Dhara AK, Mandal SC, editors. Role of herbal medicines. Singapore: Springer; 2023. p. 559-71. doi: 10.1007/978-981-99-7703-1_28.
Resnik DB. Proportionality in public health regulation: the case of dietary supplements. Food Ethics. 2018 Dec;2(1):1-16. doi: 10.1007/s41055-017-0023-3, PMID 30911602, PMCID PMC6430238.
Arboleda AM. Consumers perspectives regarding the nutraceutical nature of spirulina: perceptions of the nourishing naturalness and tastiness tablets vs flakes. J Int Food Agribusiness Mark. 2025;37(1):50-71. doi: 10.1080/08974438.2023.2270612.
Huttner S, Johansson A, Gonçalves Teixeira P, Achterberg P, Nair RB. Recent advances in the intellectual property landscape of filamentous fungi. Fungal Biol Biotechnol. 2020 Nov 12;7(1):16. doi: 10.1186/s40694-020-00106-z, PMID 33292599, PMCID PMC7664042.
Durazzo A, Sorkin BC, Lucarini M, Gusev PA, Kuszak AJ, Crawford C. Analytical challenges and metrological approaches to ensuring dietary supplement quality: international perspectives. Front Pharmacol. 2022 Jan 11;12:714434. doi: 10.3389/fphar.2021.714434, PMID 35087401.
Nwosu OK, Ubaoji KI. Nutraceuticals: history classification and market demand. In: Egbuna C, Dable Tupas G, editors. Functional foods and nutraceuticals. Berlin: Springer; 2020. p. 13-22. doi: 10.1007/978-3-030-42319-3_2.
Agarwal S, Chauhan ES. Nutraceutical potential of vermicelli prepared by gluten free composite flour. Int J Curr Pharm Sci. 2022 Jan;14(1):83-6. doi: 10.22159/ijcpr.2022v14i1.44116.
Dimple KA, Kumar A, Kumar V, Tomer V. Traditional medicinal systems for treatment of diabetes mellitus: a review. Int J Pharm Pharm Sci. 2018;10(5):25374. doi: 10.22159/ijpps.2018v10i5.25374.
Surini S, Wardani MR, Sagita E. Evaluating of effervescent tablets containing grape seed (Vitis vinifera l.) extract as a nutraceutical. Int J App Pharm. 2017;9(1). doi: 10.22159/ijap.2017.v9s1.76_83.
Clinical trials. Library of medicine. Available from: https://clinicaltrials.gov/.bethesda:national. [Last accessed on 24 Nov 2024].
https://patents.googlescholarofpatents:google.google.com. [Last accessed on 24 Dec 2024].
Nutritional outlook. Intellectual property in nutraceuticals. Available from: https://www.nutritionaloutlook.com/view/intellectual-property-nutraceuticals-patents-trademarks-and-trade-secrets:patentstrademarks. [Last accessed on 4 Jan 2025].
Kearney. Nutraceuticals in India: capitalizing on the shift toward preventive healthcare. Available from: https://www.kearney.com/industry/health/article/nutraceuticals-in-india.capitalizing-on-the-shift-toward-preventive-healthcare. [Last accessed on 4 Jan 2025].
View G. India nutraceuticals market report. Available from: https://www.grandviewresearch.com/industry-analysis/india-nutraceuticals-market-report. [Last accessed on Jan 2025].
Food Research Lab. Nutraceutical product development solutions. Available from: https://www.foodresearchlab.com/what-we-do/new-product-developmentservice/nutraceutical-product-development-solutions. [Last accessed on 5 Jan 2025].
Solution buggy. Essential guide to nutraceutical manufacturing. Available from: https://www.solutionbuggy.com/blog/essential-guide-to-nutraceutical-manufacturing. [Last accessed on 5 Jan 2025].
Evans M, Lewis ED, Antony JM, Crowley DC, Guthrie N, Blumberg JB. Breaking new frontiers: assessment and re-evaluation of clinical trial design for nutraceuticals. Front Nutr. 2022 Sep 23;9:958753. doi: 10.3389/fnut.2022.958753, PMID 36211523, PMCID PMC9540398.
Shi J, HU H, Harnett J, Zheng X, Liang Z, Wang YT. An evaluation of randomized controlled trials on nutraceuticals containing traditional Chinese medicines for diabetes management: a systematic review. Chin Med. 2019 Nov 29;14:54. doi: 10.1186/s13020-019-0276-3, PMID 31798675, PMCID PMC6884840.
DE Bles NJ, Gast DA, van Der Slot AJ, Didden R, Van Hemert AM, Rius-Ottenheim N. Lessons learned from two clinical trials on nutritional supplements to reduce aggressive behaviour. J Eval Clin Pract. 2022 Aug;28(4):607-14. doi: 10.1111/jep.13653, PMID 35040231, PMCID PMC9543803.
Prescott SL, Logan AC, D Adamo CR, Holton KF, Lowry CA, Marks J. Nutritional criminology: why the emerging research on ultra processed food matters to health and justice. Int J Environ Res Public Health. 2024 Jan 23;21(2):120. doi: 10.3390/ijerph21020120, PMID 38397611, PMCID PMC10888116.
Gast DA, Didden R, Westera JJ, Van DE Rest O, Van Hemert AM, Giltay EJ. Dietary supplements for aggressive behaviour in people with intellectual disabilities: a randomised controlled crossover trial. J Appl Res Intellect Disabil. 2023 Jan;36(1):122-31. doi: 10.1111/jar.13041, PMID 36224110, PMCID PMC10092216.
US Food and Drug Administration (FDA). Current good manufacturing practices (CGMPs) for food and dietary supplements. Available from: https://www.fda.gov/food/guidance-regulation-food-and-dietary-supplements/current-good-manufacturing-practices-cgmps-food-and-dietary-supplements. [Last accessed on 06 Jan 2025].
Sagacious research. Nutraceuticals: Insights on Industry Associated IP Rights. Available from: https://sagaciousresearch.com/blog/nutraceuticals-insights-on-industry-associated-ip-rights. [Last accessed on 06 Jan 2025].
Sato K, Kodama K, Sengoku S. Driving innovation through regulatory design and corporate behaviour: a case study of functional food industry in Japan. Foods. 2024 Oct 18;13(20):3302. doi: 10.3390/foods13203302, PMID 39456364, PMCID PMC11508179.
Vlaicu PA, Untea AE, Varzaru I, Saracila M, Oancea AG. Designing nutrition for health incorporating dietary by products into poultry feeds to create functional foods with insights into health benefits risks bioactive compounds food component functionality and safety regulations. Foods. 2023 Nov 1;12(21):4001. doi: 10.3390/foods12214001, PMID 37959120, PMCID PMC10650119.
Gonzalez Diaz C, Vilaplana Aparicio MJ, Iglesias Garcia M. How is functional food advertising understood? An approximation in university students. Nutrients. 2020 Oct 29;12(11):3312. doi: 10.3390/nu12113312, PMID 33137940, PMCID PMC7692513.
Neethirajan S, Ragavan V, Weng X, Chand R. Biosensors for sustainable food engineering: challenges and perspectives. Biosensors (Basel). 2018 Mar 12;8(1):23. doi: 10.3390/bios8010023, PMID 29534552, PMCID PMC5872071.
Pathak R, Gaur V, Sankrityayan H, Gogtay J. Tackling counterfeit drugs: the challenges and possibilities. Pharm Med. 2023 Jul;37(4):281-90. doi: 10.1007/s40290-023-00468-w, PMID 37188891, PMCID PMC10184969.
Sen S, Chakraborty R. Revival modernization and integration of Indian traditional herbal medicine in clinical practice: importance challenges and future. J Tradit Complement Med. 2017;7(2):234-44. doi: 10.1016/j.jtcme.2016.05.006, PMID 28417092.
WU L, Chen W, Wang Z. Traditional Indian medicine in China: the status quo of recognition development and research. J Ethnopharmacol. 2021 Oct 28;279:114317. doi: 10.1016/j.jep.2021.114317, PMID 34111541.
Tandon N, Yadav SS. Contributions of Indian council of medical research (ICMR) in the area of medicinal plants/traditional medicine. J Ethnopharmacol. 2017;197:39-45. doi: 10.1016/j.jep.2016.07.064, PMID 27452657.
Dhokane D, Shaikh A, Yadav A, Giri N, Bandyopadhyay A, Dasgupta S. CRISPR-based bioengineering in microalgae for production of industrially important biomolecules. Front Bioeng Biotechnol. 2023 Oct 26;11:1267826. doi: 10.3389/fbioe.2023.1267826, PMID 37965048, PMCID PMC10641005.
Cadwallader AB. Seven points for athletes to consider before using a dietary supplement. AMA J Ethics. 2022 May 1;24(5):E443-451. doi: 10.1001/amajethics.2022.443, PMID 35575576.
Mohi Ud Din R, Mir RH, Pottoo FH, Sawhney G, Masoodi MH, Bhat ZA. Nanophytomedicine ethical issues regulatory aspects and challenges. In: Beg S, Barkat MA, Ahmad FJ, editors. Nanophytomedicine. Singapore: Springer; 2020. p. 173-92. doi: 10.1007/978-981-15-4909-0_10.
Ganguly D, Choudhury A, Chanda R, Das H, Mitra S, Ghosh R. Nanoparticulate formulation for the treatment of different types of colon disease like ulcerative colitis: a comprehensive review. J Young Pharm. 2023;15(4):585-8. doi: 10.5530/jyp.2023.15.82.
Dwyer JT, Coates PM, Smith MJ. Dietary supplements: regulatory challenges and research resources. Nutrients. 2018 Jan 4;10(1):41. doi: 10.3390/nu10010041, PMID 29300341, PMCID PMC5793269.
Dwyer JT. Have safety and efficacy assessments of bioactives come of age? Mol Aspects Med. 2023 Feb;89:101103. doi: 10.1016/j.mam.2022.101103, PMID 35853784, PMCID PMC9841065.