
Int J App Pharm, Vol 17, Issue 4, 2025, 529-540Original Article
EVALUATION OF ORGAHEALTM TURMERIC CURCUMIN WITH BOSWELLIA, GINGER AND BLACK PEPPER CAPLET ROLE IN MODULATING INFLAMMATION AND JOINT FUNCTION
RAJENDRAN A.1, R. SUDESH RAJ1*, TEJAS M.2, S. JAYAKUMAR1, S. REETHI1, S. SNEHA1
1Life Care Phyto Labs, Iyyappanthangal, Chennai, Tamil Nadu, India. 2Sri Ramachandra Medical College and Research Institute, Porur, Chennai, Tamil Nadu, India
*Corresponding author: R. Sudesh Raj; *Email: sudesh@lifecarephytolabs.com
Received: 22 Nov 2024, Revised and Accepted: 26 Apr 2025
ABSTRACT
Objective: This study evaluated the antioxidant, anti-inflammatory, and anti-arthritic properties of the OrgahealTM Turmeric curcumin with Boswellia (boswellic acids), ginger (gingerols), and black pepper (piperine) caplet (OTC).
Methods: Antioxidant activity was assessed using standard assays, while anti-inflammatory effects were tested via protein denaturation models. Cell viability, (NO) production, cytokine levels (IL-10, IL-6, TNF-α), reactive oxygen species (ROS), and endogenous antioxidant enzyme activity were evaluated in RAW 264.7 and SW982 cell lines. Statistical significance was set at p<0.05.
Results: TAE exhibited significant antioxidant activity, effectively scavenging radicals and inhibiting lipid peroxidation. It inhibited protein denaturation with IC50 values of 2.76 mg/ml (BSA) and 2.73 mg/ml (egg albumin). In RAW 264.7 cells, TAE maintained viability up to 50 µg/ml, reduced NO production, and restored antioxidant enzyme activity. In SW982 cells, it reduced IL-1β-induced ROS, improved antioxidant levels, and demonstrated anti-arthritic potential by decreasing TNF-α and IL-6 while increasing IL-10.
Conclusion: OrgahealTM Turmeric curcumin with boswellic acids, gingerols, and piperine caplet (OTC), demonstrates significant antioxidant, anti-inflammatory, and anti-arthritic properties. These findings suggest its potential as a complementary therapeutic agent for managing oxidative stress, inflammation, and arthritis-related conditions, offering a natural alternative for chronic inflammatory disease management.
Keywords: TAE, Antioxidant activity, Anti-inflammatory, Anti-arthritic activity
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijap.2025v17i4.53281 Journal homepage: https://innovareacademics.in/journals/index.php/ijap
INTRODUCTION
Joint diseases, including arthritis, are prevalent and debilitating conditions that significantly impact the quality of life for millions worldwide. These conditions are characterized by inflammation, pain, stiffness, and reduced joint function. The most prevalent types are Osteoarthritis (OA) and Rheumatoid Arthritis (RA). OA is a degenerative joint condition that mainly impacts cartilage, causing pain, swelling, and restricted movement. It is commonly linked to aging, joint injuries, obesity, or genetic predispositions [1].
In contrast, RA is an autoimmune condition where the immune system targets synovial lining of the joints. This leads to significant inflammation, joint damage, and systemic symptoms like fatigue and fever [2, 3]. Both OA and RA lead to chronic discomfort and reduced mobility, with RA potentially affecting multiple joints and other organs.
The OTC is a proprietary blend meticulously crafted to harness the antioxidant, anti-inflammatory, and anti-arthritic properties of organic extracts, including Turmeric (Curcuma longa), Boswellia (Boswellia serrata), Ginger (Zingiber officinale), and Black Pepper (Piper nigrum). This unique formulation capitalizes on the individual and synergistic benefits of its components to promote joint health and alleviate arthritis symptoms.
Curcumin, the primary active compound in turmeric, is renowned for its robust anti-inflammatory and antioxidant properties, making it highly effective in managing joint inflammation associated with conditions like OA [4]. It inhibits key inflammatory enzymes and modulates the activity of pro-inflammatory cytokines, thereby reducing inflammation. Similarly, Boswellia extract contains boswellic acids, which target specific inflammatory pathways, effectively diminishing inflammation and joint pain [5]. Ginger extract, abundant in gingerol, offers significant anti-inflammatory benefits by suppressing inflammatory cytokines and enzymes [6]. While piperine, an alkaloid found in black pepper, enhances the bioavailability of curcumin. This ensures optimal absorption and maximizes the therapeutic effects of the formulation [7].
By combining these powerful ingredients, the TAE provides a holistic and synergistic solution for managing arthritis symptoms. Curcumin acts as a potent inhibitor of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), a key transcription factor involved in the expression of pro-inflammatory cytokines. Boswellic acids complement curcumin by targeting 5-lipoxygenase and reducing leukotriene production, critical contributors to inflammation and pain in arthritis [8]. Gingerols further bolster this anti-inflammatory action by inhibiting cyclooxygenase and lipoxygenase pathways, common therapeutic targets in pain and inflammation management [9]. The inclusion of piperine enhances the bioavailability of curcumin by inhibiting its rapid metabolism in the liver, ensuring sustained therapeutic levels in the bloodstream. By integrating these compounds into a single formula, TAE offers a multifaceted approach to managing arthritis symptoms, addressing inflammation, oxidative stress, and pain through complementary mechanisms.
While conventional arthritis treatments such as NSAIDs and DMARDs offer some relief, they often come with drawbacks, including gastrointestinal issues, cardiovascular risks, and long-term side effects [10]. Moreover, these treatments generally focus on managing symptoms rather than addressing the underlying causes of arthritis, such as chronic inflammation and oxidative stress. OTC fills these gaps by offering a natural, side-effect-friendly alternative that not only alleviates symptoms but also promotes overall joint health. Its ingredients are well-tolerated and ideal for individuals seeking a safer, more holistic approach to managing arthritis.
In essence, the OTC is more than just a supplement. It’s a comprehensive strategy for improving joint health and overall well-being. Combining potent botanicals with scientifically supported anti-inflammatory and antioxidant properties, it provides relief from pain and stiffness while supporting long-term joint function. For those struggling with arthritis and seeking natural, effective solutions, this formula offers a promising pathway to enhanced quality of life [11].
MATERIALS AND METHODS
Chemicals and reagents
DPPH and ABTS were purchased from SRL Chemicals, India. BHT was procured from SRL Chemicals, India. Thiobarbituric acid, ferrous sulfate, nitro blue tetrazolium, NADH disodium salt, and Phenazine methosulfate, D-PBS (#TL1006) were obtained from Himedia, India. Griess reagent and 2, 4, 6-Tris (2-pyridyl)-s-triazine (TPTZ) were purchased from Sigma, USA. DMSO and LPS were supplied by Sigma, USA. FBS, DMEM (High Glucose), L-glutamine (200 mmol solution), and MTT reagent were sourced from Himedia, India. H2DCFDA was procured from Life Technologies, Invitrogen, USA. NO estimation kit (#CCK061) was purchased from Himedia, India. ELISA kits for Human catalase, Human SOD1, Human glutathione peroxidase, and Human glutathione were procured from KRISHGEN BioSystems, India, and Elabscience, USA.
Preparation of extract
The key materials of OTC were sourced from Life Care Phyto Labs, Iyyappanthangal, Chennai, Tamil Nadu, India. About 10 g of OTC powder is extracted with 100 ml of distilled water. The mixture was heated at 80 °C for 1 h. The mixture was cooled and centrifuged at 8,000 rpm to get a clear supernatant. The supernatant was dried at room temperature to obtain an aqueous extract of TAE. The dried extract was stored at 4 °C for further use (fig. 1). Aqueous extraction was chosen over ethanol for its safety, absence of residual solvents, and ability to selectively isolate water-soluble bio-actives like curcumin. This method is eco-friendly, non-toxic, and enhances bioavailability, making it ideal for therapeutic formulations.
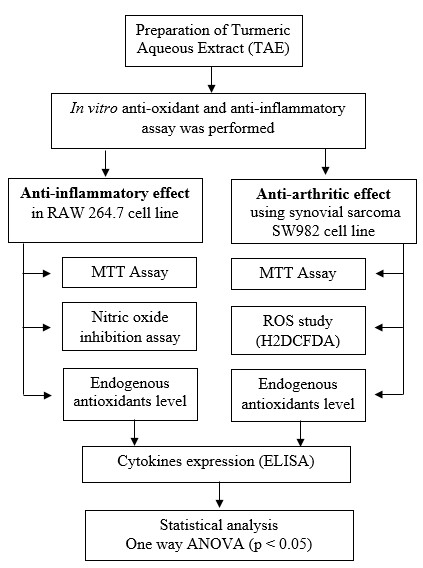
Fig. 1: Experimental workflow for assessing antioxidant, anti-inflammatory, and anti-arthritic effects of TAE
In vitro antioxidant assay
The antioxidant potential of TAE was assessed using various standard assays. For the DPPH radical scavenging assay, 500 µM DPPH in methanol was combined with various concentrations of the extract and ethanol to complete a 1 ml volume. The mixture was incubated in the dark for 30 min, and absorbance was measured at 517 nm; the control contained only DPPH and ethanol [12], calculated with the formula:
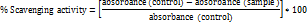
The hydrogen peroxide scavenging assay included ferrous ammonium sulfate, hydrogen peroxide, and 1, 10-phenanthroline, with readings at 510 nm, and a control made up of the reagents without the extract [13]. The FRAP assay was based on the reduction of the Fe³⁺-TPTZ complex to Fe²⁺-TPTZ in a reaction mixture containing FRAP reagent and extract, incubated at 37 °C for 15 min, with readings taken at 593 nm. A control without the extract was used for comparison [14]. The hydroxyl radical scavenging activity was measured using a reaction mixture of iron-EDTA, DMSO, and ascorbic acid, heated at 80–90 °C, terminated with TCA, and read at 412 nm [15]. In the NO scavenging assay, sodium nitroprusside in phosphate buffer saline (pH 7.4) was mixed with the extract, incubated at 25 °C for 1 h, and treated with Griess reagent, with absorbance measured at 546 nm. The control excluded the extract [16]. Lipid peroxidation inhibition was tested using egg homogenate and FeSO₄ to induce peroxidation, followed by the addition of a TBA-containing reagent and butanol, with absorbance recorded at 532 nm. The control lacked the sample [17].
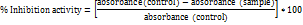
Lastly, superoxide radical scavenging assay, a reaction mixture of phosphate buffer, NADH, NBT, and PMS was incubated at 25 °C for 5 min, and absorbance was read at 560 nm; the control contained the reagents without the sample [18]. Each assay calculated scavenging or inhibition activity based on absorbance changes relative to controls. For all assays, controls without the extract were used to establish a baseline for antioxidant activity.
Anti-inflammatory assay
The study assessed the anti-inflammatory effects of test compounds using two protein denaturation assays. In the BSA denaturation assay, 0.5% BSA was mixed with varying concentrations of the test compounds, incubated for 20 min, and then heated for 15 min. After cooling, turbidity was measured at 660 nm with a multi-well plate reader. Controls included a blank with buffer alone and true blanks containing test compounds without BSA to eliminate interference [19]. In the egg albumin denaturation assay, 200 µl of fresh egg albumin was mixed with phosphate buffer and 2 ml of either the test compounds or diclofenac sodium. Absorbance was measured at 660 nm, and the inhibition (%) was calculated. Controls consisted of a buffer-only blank and a reaction mixture without the test compound to establish the baseline [20].
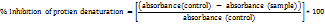
Anti-inflammatory effect in macrophage RAW 264.7 cell line
Maintenance of cell lines
The Raw 264.7 (Mouse Monocyte/Macrophage cell line) is purchased from NCCS, Pune, India. The RAW 264.7 cells were maintained in DMEM High Glucose media supplemented with 10 % FBS along with the 1% antibiotic-antimycotic solution and 1% L-Glutamine (200 mmol) in atmosphere of 5% CO2, 18-20% O2 at 370 °C temperature maintained in the CO2 incubator and sub-cultured for every 2 d.
MTT assay
To conduct a cell viability test, cells were seeded into a 96-well plate and incubated. Then, different concentrations of TAE and LPS were added, followed by an additional 24h incubation period [21]. After incubating the cells, MTT reagent was added to the covered plate and incubated for 3h. DMSO was added to dissolve the formazan crystals. Absorbance was measured at 570 nm to assess cell viability [22, 23].
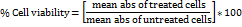
NO inhibition assay
To evaluate the NO inhibition, 200 μl of cell suspension was seeded into a 96-well plate and grown for 1d. Afterward, treat the cells with various concentrations of TAE and 1 µg/ml of LPS and incubate them for 1d in a CO2 atmosphere. Following the incubation period, collect the culture supernatant and measure the NO levels using a commercial kit. EZAssay TMNO Estimation Kit.
Endogenous antioxidants level
Cells (2 × 105 cells density) in a 6-well plate and incubated for 1 d. After treatment, the cells were washed with PBS, trypsinized using 300 µl of Trypsin-EDTA solution, and collected into Eppendorf tubes. These tubes were then centrifuged for 5 min; the supernatant was again centrifuged for 20 min. If the cell concentration exceeded 1 M/ml, the suspension was diluted with PBS and subjected to freeze-thaw cycles to release intracellular components. The enzyme levels were measured using the Mouse Glutathione (GSH) GENLISA™ ELISA, Mouse Catalase (CAT) GENLISA™ ELISA, Mouse Superoxide Dismutase Cu-Zn (SOD1) ELISA, and Elabscience® Mouse GPX1 (Glutathione Peroxidase 1) ELISA kits.
Anti-arthritic effect using synovial sarcoma SW982 cell line
Maintenance of cell lines
The SW982 (Synovial Sarcoma) is purchased from NCCS, Pune, India. The SW982 cells were maintained in DMEM High Glucose media supplemented with 10 % FBS along with the 1% antibiotic-antimycotic solution in an atmosphere of 5% CO2, 18-20% O2 at 370 °C temperature maintained in the CO2 incubator and subcultured for every 2 d.
MTT assay
To conduct a cell viability test, 200μl of cell suspension was seeded in a 96-well plate and incubated for 1d, add the test agents (TAE) to the appropriate wells and incubate for another 1d. Then, remove spent media and add MTT reagent to each well. Cover the plate with aluminum foil and incubate for 3h. After incubation, add 100μl of DMSO to each well after removing the MTT reagent. Measure the absorbance at 570 nm using a plate reader and calculate cell viability (%) using the formula.
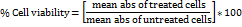
ROS study (H2DCFDA) by flow cytometry
Cells seeded in a 96-well plate and incubated for 24h. They are divided into four groups: control, IL-1β treated, TAE treated, and a combination of TAE and IL-1β treated. After incubation, the cells are collected, PBS-washed, treated, and harvested with Trypsin-EDTA. Following centrifugation, the cells were PBS-washed twice and resuspended to detect reactive oxygen species. After incubating, the cells were centrifuged again, resuspended in DPBS, and using flow cytometry, it was analyzed to assess oxidative stress level [24].
Endogenous antioxidant levels
Cells cultured in a 6-well plate and incubated with CO2 for 1d, were treated with four groups: control, IL-1β, TAE, and TAE+IL-1β. The medium was removed, cells washed, trypsinized, and harvested, supernatant was collected and intracellular components were released through freeze-thaw cycles. Enzyme levels in the supernatant were determined according to Human CAT GENLISA™ ELISA, Human GPX1 ELISA, GSH GENLISA™ ELISA, Elabscience® Human SOD1 ELISA kit instructions.
Cytokines expression (ELISA)
Cells seeded in a 6-well plate and incubated for 24h. The spent medium was removed, and the cells were divided into control, IL-1β treated, TAE-treated, and a combination of IL-1β and TAE-treated. The protocol included adding standards and samples to wells containing immobilized antibodies, followed by incubation. The wells were then washed and treated with biotinylated antibodies, HRP-conjugated streptavidin, and TMB substrate. Absorbance was measured at 450 nm to quantify cytokine levels using the RayBio® Human IL-10 ELISA Kit, Human IL-6 ELISA Kit, and Human TNF-alpha ELISA Kit.
Statistical analysis
Statistical results are presented as mean±SD. Differences between groups were analyzed using one-way ANOVA, with Tukey's HSD test for multiple comparisons. A p-value less than 0.05 is considered statistically significant. Data analysis was conducted using IBM SPSS Statistics for Windows, Version 29.0.2.0.
RESULTS
In vitro antioxidant assay
TAE enriched with curcumin, boswellic acids, gingerols, and piperine, demonstrates a broad spectrum of antioxidant activities, albeit with varying degrees of potency. It effectively scavenges DPPH radicals, nitric oxide, superoxide radicals, and hydrogen peroxide radicals, while also inhibiting lipid peroxidation and significantly increasing FRAP assay absorbance from 0.25 to 0.93 at 593 nm. However, it lacks activity against hydroxyl radicals (table 1). Compared to the standards ascorbic acid, curcumin, lipoic acid, and gallic acid, TAE exhibits lower potency, indicating milder antioxidant effects. The absence of hydroxyl radical scavenging is attributed to the inherent reactivity of its bioactive compounds, as curcumin and piperine lack the structural features required to neutralize these highly reactive radicals. These results suggest that TAE, with its bioactive compounds, holds potential as a multi-functional antioxidant supplement, particularly in applications where moderate antioxidant effects are desirable or when used in combination with other antioxidants to enhance efficacy (fig. 2).
Anti-inflammatory effect in bovine serum albumin and egg albumin
The anti-inflammatory activity of the TAE was assessed by its ability to inhibit the denaturation of BSA and egg albumin proteins. Although TAE shows anti-inflammatory properties, it’s less potent than the standard drug diclofenac (fig. 3). Despite its lower potency, TAE, which contains curcumin, boswellic acids, gingerols, and piperine, could be beneficial as a natural, milder anti-inflammatory agent or when combined with other anti-inflammatory compounds.
Table 1: In vitro antioxidant activity of TAE and standard drug. The IC50 values of MCT oil and standard for various radicals scavenging activity
| Assay | Activity present/absent | IC50 value | |
| TAE | Standard | ||
| DPPH scavenging | Present | 737.11µg/ml | 4.74µg/ml(Ascorbic acid) |
| NO scavenging | Present | 748.27µg/ml | 24.58 µg/ml (curcumin) |
| Superoxide radical scavenging | Present | 812.06µg/ml | 2.80µg/ml(Ascorbic acid) |
| Lipid peroxidation inhibition | Present | 138.38µg/ml | 108.69µg/ml(Lipoic acid) |
| Hydrogen peroxide radical scavenging | Present | 691.66 µg/ml | 49.33 µg/ml(Gallic acid) |
| Hydroxyl radical scavenging | Absent | - | - |
| FRAP | Present | OD increased from 0.25 to 0.93 at 593 nm | OD increased from 0.31 to 0.98 at 593 nm (Ascorbic acid) |
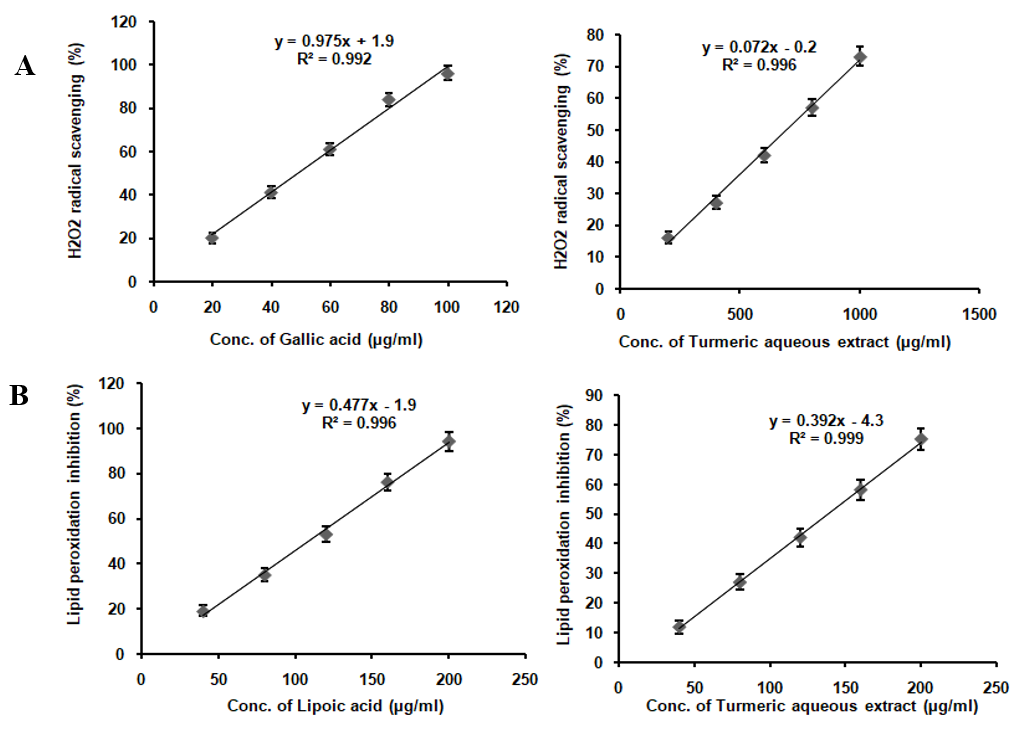
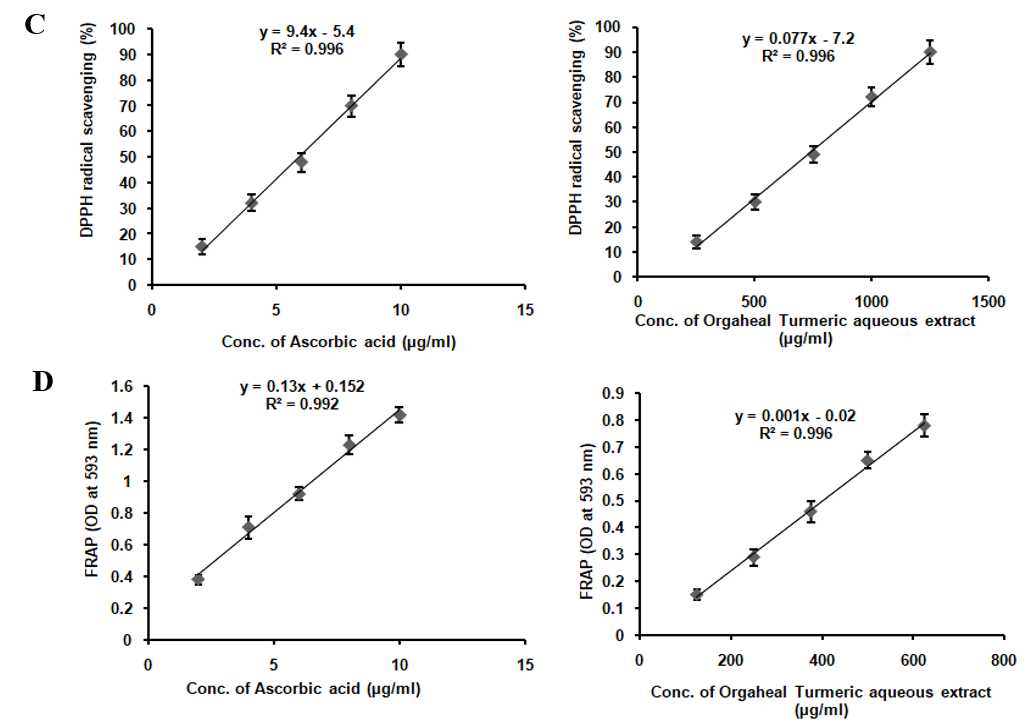
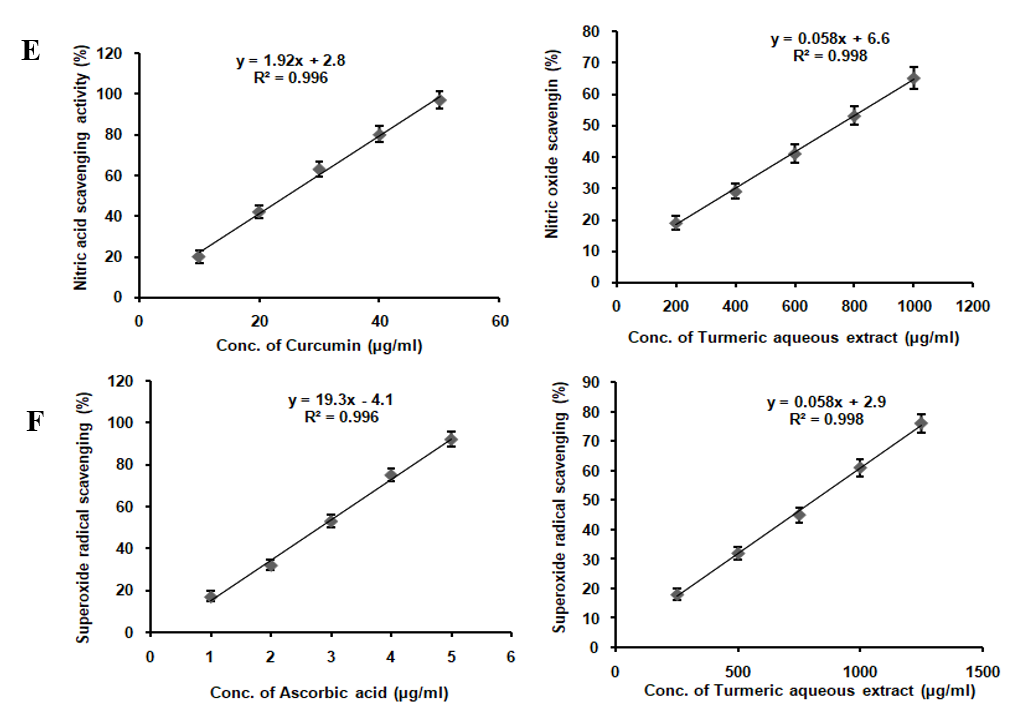
Fig. 2: In vitro antioxidant assay of TAE (A) DPPH scavenging assay, (B) FRAP activity, (C) Hydrogen peroxide radical scavenging, (D) Lipid peroxidation inhibition, (E) NO scavenging assay, (F) Superoxide scavenging assay
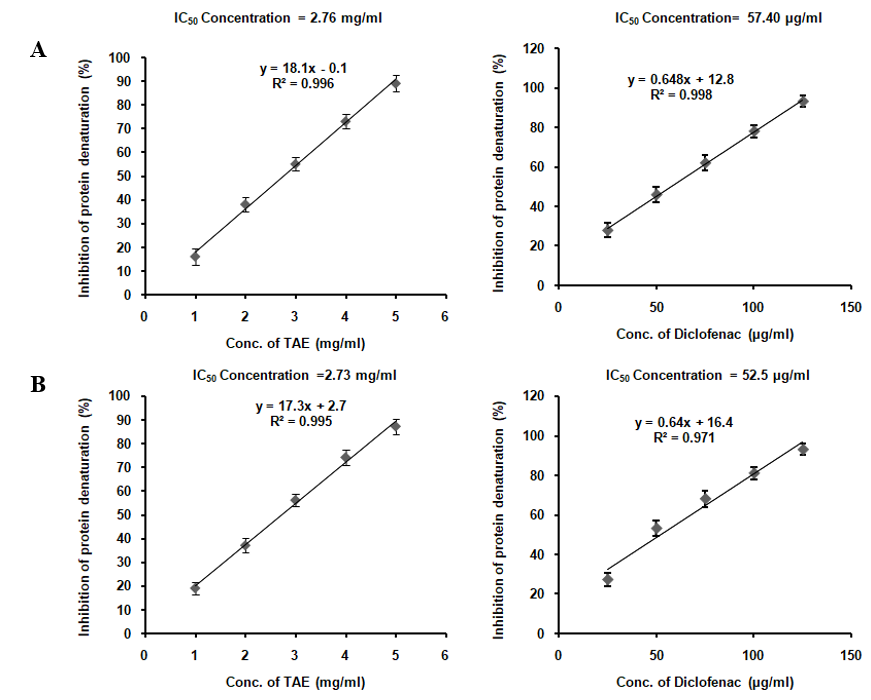
Fig. 3: Protein denaturation inhibition in (A) BSA and (B) egg albumin using TAE
Anti-inflammatory effect in macrophage RAW 264.7 cell line (MTT assay)
Treatment with TAE, maintained the viability of RAW 264.7 cell line up to a concentration of 50 µg/ml, as depicted in fig. 4, without altering cell morphology. The selection of 25 µg/ml and 50 µg/ml was based on being the highest concentrations that did not affect cell viability. When exposed to 1 µg/ml LPS, cell viability decreased to 52%, and TAE was evaluated for its protective effect against this LPS-induced toxicity. Additionally, TAE at various concentrations improved cell viability against H2O2-induced toxicity in a neuronal cell line (fig. 5). Under oxidative stress induced by H2O2, cells treated with TAE retained normal morphology. The combination of these bioactive thus yields a comprehensive cellular defense mechanism, making TAE an effective option in mitigating oxidative and inflammatory stress in cell lines. Statistical analysis was performed using one-way ANOVA followed by Tukey's HSD test [F (5, 30) = 2110.308, p<0.001].
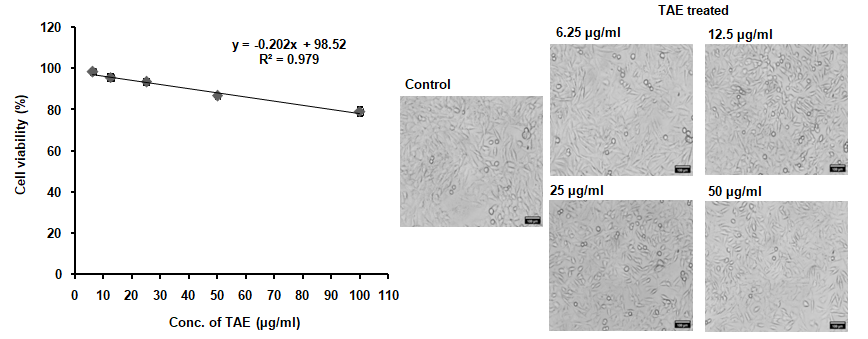
Fig. 4: Non-toxic concentrations and protective effects against LPS-induced inflammation in RAW 264.7 cells
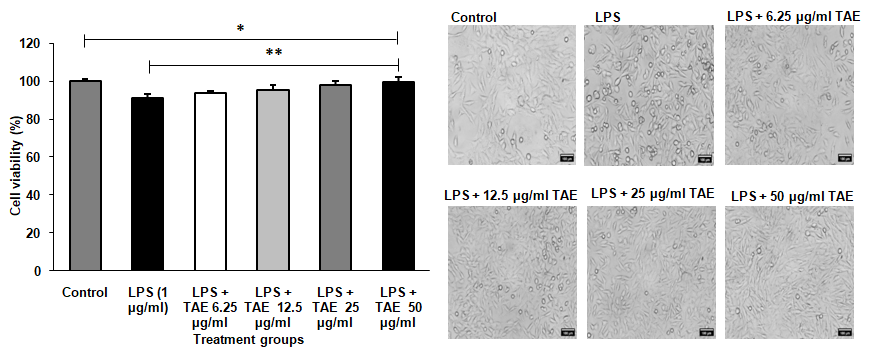
Fig. 5: Protection against lipopolysaccharide (LPS)-induced inflammation in RAW 264.7 macrophage cell line. The results are shown as mean±SD (n=3), with *P<0.001 indicating significance compared to the control group and **P<0.001 indicating significance between groups
NO inhibition assay
The NO levels in RAW 264.7 cell lines assessed the following treatment with lipopolysaccharide at 1 µg/ml significantly elevated NO production compared to the control. When treated with TAE, at varying concentrations alongside LPS, a dose-dependent reduction in NO production was observed (fig. 6). Notably, the highest reduction, bringing NO levels close to the control group's levels, was achieved with 50 µg/ml of TAE. Statistical analysis using one-way ANOVA followed by Tukey's HSD test confirmed these findings with F (5, 30) = 340976.487, p<0.001.
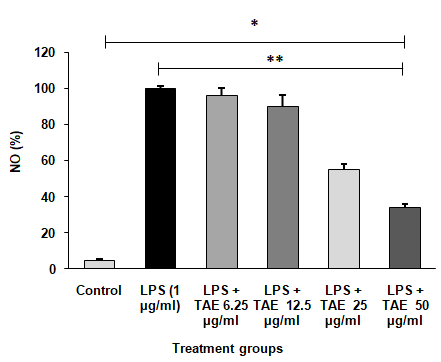
Fig. 6: Inhibitory effect of TAE on LPS-induced NO production. The data are shown as mean±SD (n=3), with *P<0.001 indicating significance compared to the control group and **P<0.001 indicating significance between different groups
Endogenous antioxidants level
The study found that LPS treatment significantly reduced antioxidant enzyme levels in RAW 264.7 cells, indicating oxidative stress. Specifically, CAT, GPx, GSH, and SOD levels dropped drastically (table 2). Co-treatment with the TAE at 25µg/ml and 50 µg/ml significantly restored these enzyme levels in a dose-dependent manner (fig. 7). At higher concentrations, TAE, almost normalized the levels of these enzymes, indicating its potential as a natural therapeutic agent for reducing inflammation and oxidative damage. Statistical analysis was conducted using one-way ANOVA followed by Tukey’s HSD test, with significant results observed for GPX [F (3, 8) = 2224.803, p<0.001], GSH [F (3, 8) = 543.828, p<0.001], CAT [F (3, 8) = 951.473, p<0.001], and SOD [F (3, 8) = 395.419, p<0.001].
Table 2: Levels of endogenous antioxidants in TAE
| Culture condition | CAT (ng/ml) | GPx (pg/ml) | GSH (µg/ml) | SOD1(ng/ml) |
| Control | 3.83±0.13 | 1868.48±46.17 | 1181.86±50.58 | 9.73±0.28 |
| LPS 1 µg/ml | 0.17±0.02 | 239.92±14.64 | 307.78±15.64 | 2.44±0.18 |
| LPS+TAE-25 µg/ml | 1.88±0.06 | 403.42±16.04 | 578.54±14.27 | 4.84±0.37 |
| LPS+TAE-50µg/ml | 3.09±0.11 | 1258.43±22.98 | 931.75±16.20 | 7.83±0.19 |
Data shown as mean±SD (n=3). LPS: Lipopolysaccharide (1 µg/ml); TAE: Turmeric Aqueous Extract. CAT: Catalase, GPx: Glutathione Peroxidase, GSH: Glutathione, SOD1: Superoxide Dismutase 1.
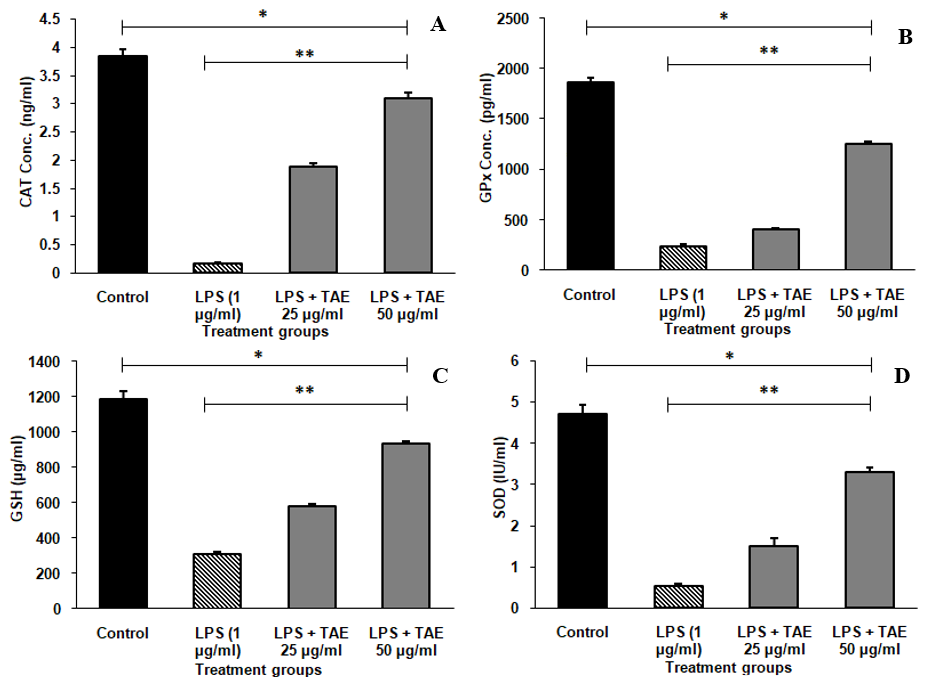
Fig. 7: Endogenous antioxidant levels of TAE in Raw 264.7 cells (A) Catalase, (B) Glutathione peroxidase, (C) Glutathione and (D) Superoxide dismutase in SH-SY5Y Cell line, The data are expressed as mean±SD (n=3). Significance is indicated by *P<0.001 compared to the control group and **P<0.001 for comparisons between groups
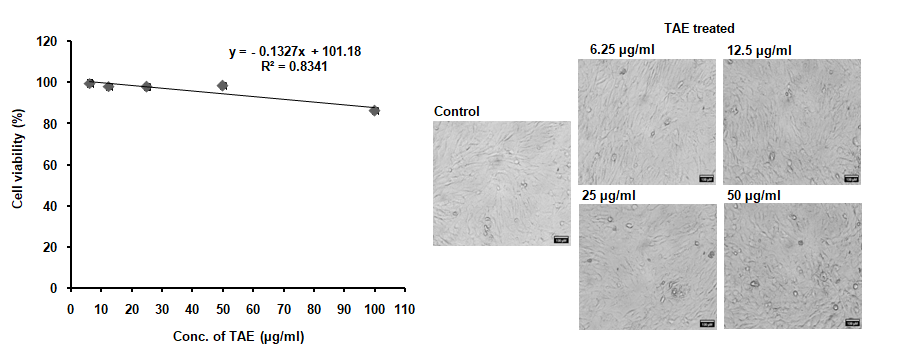
Fig. 8: Nontoxic concentration of TAE against SW982 cell line
Anti-arthritic effect using synovial sarcoma SW982 cell line (MTT assay)
Treatment of SW982 synovial cells with TAE, at concentrations did not affect cell viability until 50 µg/ml. The cell morphology remained unchanged, as illustrated in fig. 8. These findings suggest that TAE with its bioactive compounds may be a safe therapeutic option for conditions related to synovial cells, such as arthritis, without adversely affecting cell health at the tested concentrations.
ROS study (H2DCFDA) by flow cytometry
Treatment with IL-1β increased ROS production in synovial SW982 cells, while TAE treatment reduced ROS levels (fig. 9). The study evaluated ROS generation in synovial sarcoma SW982 cells treated with IL-1β, using flow cytometry to measure DCF intensity. IL-1β treatment significantly increased ROS levels, as indicated by the elevated percentage of cells expressing DCF intensity compared to the control group, demonstrating its pro-oxidant effect. However, co-treatment with TAE, at concentrations of 25µg/ml and 50µg/ml, notably decreased ROS levels. The IL-1β+TAE (25 µg/ml) and IL-1β+TAE (50 µg/ml) groups show a significant reduction in ROS levels compared to the IL-1β group, with 50 µg/ml concentration exhibiting the lowest ROS levels, indicating a dose-dependent antioxidant effect of TAE. The overlay of representative histograms further supported these findings (fig. 10), showing distinct peaks for each treatment group: minimal ROS levels in the untreated group, a pronounced peak shift in the IL-1β group, and progressively smaller peaks in the IL-1β+TAE groups, correlating with reduced ROS generation. For statistical analysis, using one-way ANOVA and post-hoc analysis (Turkey’s HSD test) [F (3, 12) = 125495.693, p<0.001].
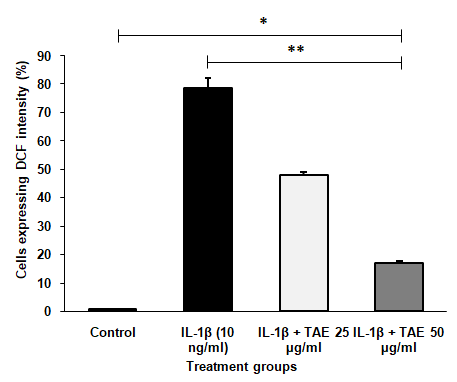
Fig. 9: ROS level determination using DCF intensity by flow cytometry. The data are presented as mean±SD (n=3), with *P<0.001 indicating significance compared to the control group and **P<0.001 indicating significance between groups
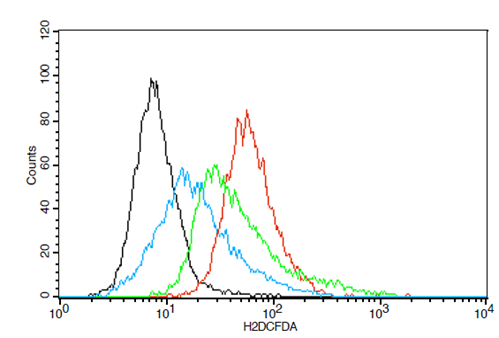
Fig. 10: Overlay of representative histogram obtained using flow cytometry on level of Reactive oxygen species using TAE in SW982 cell line
Endogenous antioxidants level
The study investigated the impact of TAE on endogenous antioxidant levels in IL-1β-treated synovial sarcoma SW982 cells, using ELISA assays. IL-1β treatment significantly reduced antioxidant levels, indicating increased oxidative stress (table 3). However, co-treatment with TAE restored these antioxidant levels in a dose-dependent manner. At 25µg/ml, TAE partially elevated antioxidant levels, while at 50 µg/ml, it further increased CAT, GPx, GSH, and SOD1 levels significantly (fig. 11). These findings suggest that TAE, effectively mitigates IL-1β-induced oxidative stress, highlighting its potential as a therapeutic agent for oxidative stress-related conditions such as arthritis. For statistical analysis, using one-way ANOVA and post-hoc analysis (Turkey’s HSD test) CAT [F (3, 8) = 103.861, p<0.001], SOD [F (3, 8) = 334.543, p<0.001], GSH [F (3, 8) = 406.478, p<0.001], GPx [F (3, 8) =1076.411, p<0.001.
Cytokines expression
The cytokine levels in IL-1β-treated SW982 cells were assessed using ELISA assays in TAE (table 4). Co-treatment with TAE, however, counteracted these effects in a dose-dependent manner. At concentrations of 25 µg/ml and 50 µg/ml, TAE reduced IL-6 levels and TNF-α levels and increased IL-10 levels (fig. 12). This modulation can be attributed to the synergistic anti-inflammatory action of curcumin and boswellic acids, along with the bioavailability-enhancing properties of piperine, which likely enhance the overall efficacy of TAE. These results indicate that TAE effectively regulates inflammation by decreasing pro-inflammatory cytokines and promoting anti-inflammatory cytokines. The combination of these bioactive compounds contributes to its robust anti-inflammatory properties, making TAE a promising natural agent for managing inflammatory conditions such as arthritis. Statistical analysis was conducted using one-way ANOVA followed by Tukey’s HSD test. The results were significant for TNF-α [F (2, 12) = 462272.586, p<0.001], IL-10 [F (3, 12) = 15397.375, p<0.001], and IL-6 [F (3, 12) = 660596.086, p<0.001].
Table 3: Endogenous antioxidants level in Catalase, Glutathione peroxidase, Glutathione and Superoxide dismutase in SW982
| Culture condition | CAT (ng/ml) | GPx (pg/ml) | GSH (ng/ml) | SOD1(pg/ml) |
| Control | 8.07±0.89 | 916.51±28.51 | 19.66±1.12 | 2962.18±149 |
| IL-1β 10 ng/ml | 1.36±0.25 | 110.03±7.18 | 2.45±0.24 | 114.34±5.55 |
| IL-1β+TAE-25 µg/ml | 3.63±0.21 | 329.62±20.04 | 7.17±0.33 | 1332.24±78.50 |
| IL-1β+TAE-50 µg/ml | 5.45±0.17 | 677.22±12.94 | 11.49±0.41 | 813.65±51.57 |
Data are expressed as mean±standard deviation (SD) (n=3), CAT: Catalase, GPx: Glutathione Peroxidase, GSH: Glutathione, SOD1: Superoxide Dismutase 1.
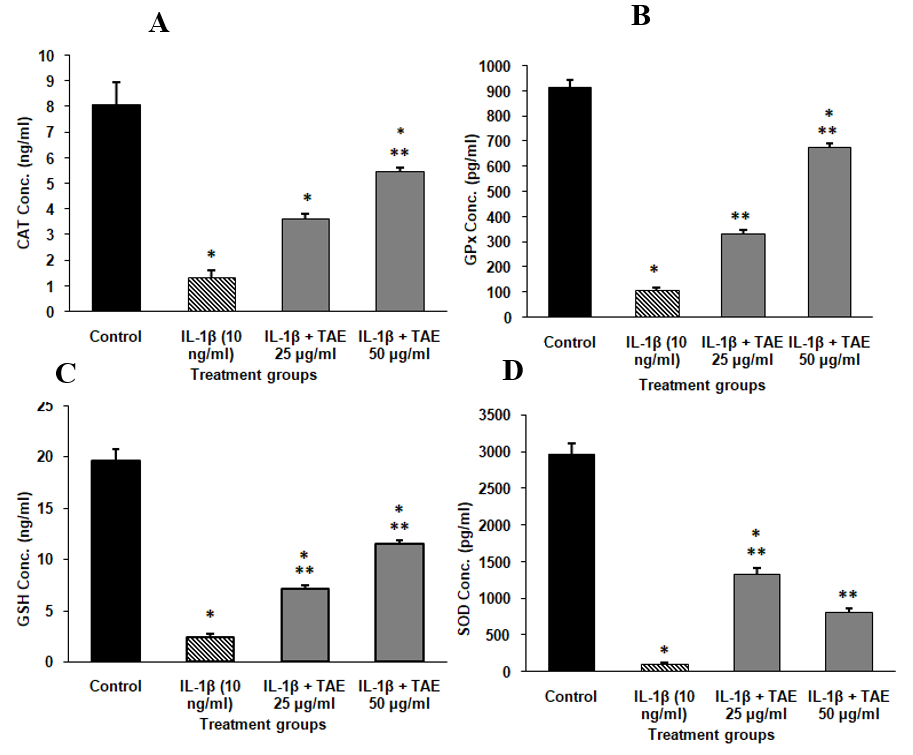
Fig. 11: Endogenous antioxidant levels in SW982 Cell line (A) Catalase, (B) Glutathione peroxidase, (C) Glutathione, and (D) Superoxide dismutase in SH-SY5Y Cell line. The data are shown as mean±SD (n=3), with *P<0.001 indicating significance compared to the control group and **P<0.001 indicating significance between groups
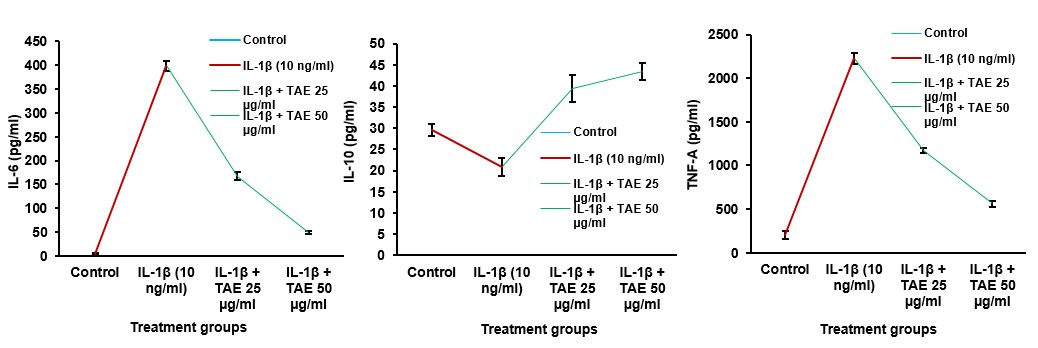
Fig. 12: Cytokine modulation through TAE: effects on IL-6, IL-10, and TNF-α levels
Table 4: Cytokine levels of TAE in SW982 cell lines
| Culture condition | IL-6 (ng/ml) | TNF-alpha (pg/ml) | IL-10 (ng/ml) |
| Control | 4.40±1.56 | 204.10±43.07 | 29.6±1.36 |
| IL-1β 10 ng/ml | 398.79±10.78 | 2225.65±57.83 | 20.8±2.1 |
| IL-1β+TAE-25 µg/ml | 167.09±8.50 | 1172.79±27.16 | 39.3±3.2 |
| IL-1β+TAE-50 µg/ml | 48.56±2.77 | 560.95±35.93 | 43.4±1.9 |
Data presented as mean±SD (n=3). Statistical significance is indicated by *P<0.001 compared to the control group and **P<0.001 between different groups
DISCUSSION
The findings from this study highlight the potential therapeutic benefits of the OTC in managing the multifaceted pathophysiology of joint diseases. While previous research has often focused on ethanol extracts like ethanol extract of ginger and turmeric, which exhibit high antioxidant capacities [25], our study focused on the aqueous extract of TAE. Aqueous extraction was chosen for its safety, absence of residual solvents, and ability to isolate water-soluble bioactives like curcumin. This eco-friendly method enhances bioavailability and eliminates ethanol-based solvent risks, making it ideal for long-term use. TAE demonstrated strong antioxidant activities, including DPPH scavenging, NO inhibition, and FRAP, although it showed limited effects against hydroxyl radicals due to the structural properties of curcumin and piperine. While TAE's IC50 values were higher than standard antioxidants, this likely reflects inherent differences in bioactive concentrations. Despite this, TAE remains a safer, long-term alternative for managing oxidative stress without the risks of synthetic compounds.
TAE's anti-inflammatory effects were assessed by its ability to inhibit the denaturation of bovine serum albumin (BSA) and egg albumin, with IC50 values of 2.76 mg/ml and 2.73 mg/ml, respectively, which are higher than those for the standard drug, diclofenac (57.40 µg/ml for BSA and 52.5 µg/ml for egg albumin). This suggests that while TAE exhibits moderate anti-inflammatory activity, its efficacy is lower compared to diclofenac, a well-established anti-inflammatory agent. The inhibition of albumin denaturation serves as a reliable indicator of anti-inflammatory activity, as protein denaturation is a key factor in inflammatory responses. In comparison, previous studies, such as one by [26], demonstrated the efficacy of curcumin in reducing inflammation by inhibiting COX-2 and TNF-α. Our findings align with these results, showing that TAE can also modulate inflammatory responses, possibly through different mechanisms. This underscores the value of turmeric-derived compounds, whether in ethanol or aqueous forms, as natural anti-inflammatory agents.
The efficacy of turmeric-derived compounds in mitigating oxidative stress and inflammation in macrophages and neuronal cells. One study showed that curcumin stimulates the Nrf2-Keap1 signaling pathway, leading to increased antioxidant enzyme activity and decreased oxidative damage. The effects varied with dosage: low and moderate doses of curcumin lowered ROS, whereas high doses produced negative effects [27]. In our study, the effects of TAE on RAW264.7 macrophages, a common model for studying inflammatory responses preserved cell viability and morphology even at higher concentrations, suggesting a favorable safety profile. This is significant, as high doses of certain compounds, like curcumin, have been shown to reduce cell viability. TAE's ability to maintain cell health while reducing LPS-induced NO production highlights its potential to manage inflammation without compromising cellular integrity.
Further, previous study demonstrated that curcumin reduced cell survivability, TNF-α protein production were measured in SW982 cells stimulated with IL-1β, IL-6, or TNF-α, confirming its beneficial effects in an RA in vitro model [29]. In contrast, our findings with SW982 synovial sarcoma cells, TAE effectively counteracted IL-1β-induced oxidative stress, significantly reducing ROS levels and restoring antioxidant enzyme activities (CAT, GPx, GSH and SOD1). This combined with its ability to modulate cytokine levels by reducing IL-6 and TNF-α while increasing the anti-inflammatory cytokine IL-10, highlights the rationale for selecting IL-10, IL-6, and TNF-α as markers due to their critical roles in inflammation and immune modulation. IL-6 and TNF-α are the key pro-inflammatory cytokines involved in the pathogenesis of arthritis, contributing to synovial inflammation, joint destruction, and pain. Conversely, IL-10 is an anti-inflammatory cytokine that counteracts pro-inflammatory mediators, promoting immune regulation and tissue repair. The findings from our study on the TAE align closely with the outcomes of the comparative study on turmeric, ginger, and black pepper supplementation in patients with chronic knee OA. Both studies underscore the potential of herbal combinations to modulate inflammatory pathways effectively. The earlier clinical study [30] demonstrated that co-supplementation with turmeric extract, black pepper, and ginger significantly reduced prostaglandin E2 (PGE2) levels, achieving comparable efficacy to Naproxen without significant inter-group differences. Similarly, our in vitro study revealed that TAE, enriched with curcuminoids, boswellic acids, gingerols, and piperine, exhibited potent anti-inflammatory, antioxidant, and anti-arthritic effects.
Notably, TAE reduced pro-inflammatory markers like TNF-α and IL-6 while enhancing IL-10 levels, alongside improving antioxidant enzyme activity and mitigating oxidative stress. These synergistic effects likely stem from the bio enhancing properties of piperine and the complementary mechanisms of curcuminoids, gingerols, and boswellic acids. While the clinical study focused on PGE2 modulation, our findings provide a broader mechanistic insight into the cellular pathways involved, strengthening the evidence for these herbal formulations as natural alternatives for managing inflammatory conditions like OA. Similarly, another study explored the effects in oleuropein on IL-1β-induced oxidative stress and inflammation in SW982 synovial fibroblast cells. The study demonstrated that oleuropein reduced IL-1β-induced ROS levels, decreased IL-6 and TNF-α, and modulated the antioxidant enzymes expressions [31].
These findings align with our results on TAE, suggesting OTC can effectively reduce oxidative stress and inflammation in synovial cells, highlighting their potential in treating conditions like arthritis. The preservation of cell viability and morphology at concentrations up to 50 µg/ml further supports its potential as a safe therapeutic option. These findings indicate TAE shows promising therapeutic potential in treating inflammatory conditions, especially joint diseases, for its antioxidant and anti-inflammatory properties and cell viability preservation. Despite these promising findings, there are limitations to this study. Most notably, the study relied solely on in vitro models, which, while useful for understanding cellular mechanisms, may not fully replicate the complexity of in vivo systems. The absence of in vivo studies limits the ability to assess the pharmacokinetics, bioavailability, and systemic effects of OTC, which are crucial for therapeutic applications. Future studies incorporating animal models or clinical trials are necessary to validate these findings and further establish OTC efficacy and safety profile in the context of joint diseases.
CONCLUSION
The OrgahealTM Turmeric Curcumin with Boswellia, Ginger and Black Pepper caplet (OTC) enriched with its bioactive compounds such as curcumin, boswellic acids, gingerols, and piperine, demonstrates significant potential for its anti-inflammation, antioxidant, and anti-arthritic properties. TAE effectively scavenges DPPH, nitric oxide, superoxide, and hydrogen peroxide radicals, inhibits lipid peroxidation, and enhances FRAP activity, suggesting potential as a supportive antioxidant therapy. It also inhibits the denaturation of bovine serum albumin (BSA) and egg albumin, offering a milder, natural alternative to traditional anti-inflammatory drugs for long-term use. For anti-arthritic properties, TAE preserves cell viability and morphology in both RAW264.7 macrophages and synovial sarcoma SW982 cells, reduces LPS-induced NO production, and restores key antioxidant enzyme levels. It also decreases IL-1β-induced ROS levels and modulates cytokine production by increasing IL-10. Given its favorable safety profile and multifaceted bioactivity, OTC stands as a promising candidate for natural therapeutic interventions targeting oxidative stress, inflammation, and joint diseases. Translational relevance is high, as OTC combination of bioactive compounds could be developed into an adjunctive therapy for chronic inflammatory conditions, including arthritis and other degenerative diseases. To accelerate its clinical applicability, further in vivo studies are needed to validate its effectiveness and safety in animal models. Additionally, clinical trials can confirm OTC safety and efficacy. It holds potential as a natural supplement or topical for inflammation, oxidative stress, and joint disorders, with fewer side effects than traditional treatments.
ABBREVIATIONS
OTC- OrgahealTM Turmeric Curcumin with Boswellia, Ginger & Black Pepper caplet, TAE- Turmeric aqueous extract), OA-Osteoarthritis, RA-Rheumatoid arthritis (RA), DPPH-2, 2-Diphenyl-1-picrylhydrazyl, ABTS-2, 2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid), BHT – Butylated Hydroxy Toluene, TBA-Thiobarbituric Acid, FeSO₄-Ferrous Sulfate, NBT-Nitro Blue Tetrazolium, NADH-Nicotinamide Adenine Dinucleotide, disodium salt, PMS – Phenazine Methosulfate, D-PBS-Dulbecco's Phosphate-Buffered Saline, TPTZ-2, 4, 6-Tris(2-pyridyl)-s-triazine, DMSO-Dimethyl Sulfoxide, LPS-Lipopolysaccharides, FBS-Fetal Bovine Serum, DMEM-Dulbecco's Modified Eagle Medium, High Glucose, MTT-3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, H2DCFDA-2', 7'-Dichlorofluorescein Diacetate, NO-Nitric Oxide, CCK-Cell Counting Kit, ELISA-Enzyme-Linked Immunosorbent Assay, SOD1-Superoxide Dismutase 1, and GSH-Glutathione.
ACKNOWLEDGMENT
We would like to express our sincere gratitude to Mr. Prateek Kevlani, Founder of the Indian Institute of Organics, for his invaluable support and sponsorship, which made this research possible. His commitment to promoting advancements in this field has been instrumental in the successful completion of our study.
FUNDING
This research was funded by the Indian Institute of Organics, under the sponsorship of Mr. Prateek Kevlani, Founder of the institute.
AUTHORS CONTRIBUTIONS
Rajendran A, R Sudesh raj, Tejas M, and S Jayakumar were responsible for conceptualization and editing. S Reethi and S Sneha contributed to data collection, analysis, and the writing of the original draft. All authors reviewed and approved the final manuscript.
CONFLICT OF INTERESTS
The authors declare no conflict of interest.
REFERENCES
Hunter DJ, Bierma Zeinstra S. Osteoarthritis. Lancet. 2019 Apr 27;393(10182):1745-59. doi: 10.1016/S0140-6736(19)30417-9, PMID 31034380.
Selvarajan C, Ganesan N. A review on macrophages and the impact of proteasome inhibitors on rheumatoid arthritis. Int J Pharm Pharm Sci. 2024;16(5):48-53. doi: 10.22159/ijpps.2024v16i5.50845.
Josef S Smolen, Daniel Aletaha, Iain B MC Innes. Department of Error. Lancet X. 2016;388(10055):1984. doi: 10.1016/S0140-6736(16)30794-2, PMID 27296018.
Gupta SC, Sung B, Kim JH, Prasad S, LI S, Aggarwal BB. Multitargeting by turmeric the golden spice: from kitchen to clinic. Mol Nutr Food Res. 2013 Sep;57(9):1510-28. doi: 10.1002/mnfr.201100741, PMID 22887802.
Sengupta K, Alluri KV, Satish AR, Mishra S, Golakoti T, Sarma KV. A double blind randomized placebo placebo-controlled study of the efficacy and safety of 5-Loxin for treatment of osteoarthritis of the knee. Arthritis Res Ther. 2008;10(4):R85. doi: 10.1186/ar2461, PMID 18667054, PMCID PMC2575633.
Mashhadi NS, Ghiasvand R, Askari G, Hariri M, Darvishi L, Mofid MR. Anti-oxidative and anti-inflammatory effects of ginger in health and physical activity: review of current evidence. Int J Prev Med. 2013 Apr;4 Suppl 1:S36-42. PMID 23717767, PMCID PMC3665023.
Srinivasan K. Black pepper and its pungent principle-piperine: a review of diverse physiological effects. Crit Rev Food Sci Nutr. 2007;47(8):735-48. doi: 10.1080/10408390601062054, PMID 17987447.
Sethi V, Garg M, Herve M, Mobasheri A. Potential complementary and synergistic effects of curcumin and boswellic acids for management of osteoarthritis. Ther Adv Musculoskelet Dis. 2022 Sep 22;14:1759720X221124545. doi: 10.1177/1759720X221124545, PMID 36171802, PMCID PMC9511324.
Yucel C, Karatoprak GS, Acıkara OB, Akkol EK, Barak TH, Sobarzo Sanchez E. Immunomodulatory and anti-inflammatory therapeutic potential of gingerols and their nanoformulations. Front Pharmacol. 2022 Sep 5;13:902551. doi: 10.3389/fphar.2022.902551, PMID 36133811, PMCID PMC9483099.
Ben Mrid R, Bouchmaa N, Ainani H, El Fatimy R, Malka G, Mazini L. Anti-rheumatoid drugs advancements: new insights into the molecular treatment of rheumatoid arthritis. Biomed Pharmacother. 2022 Jul;151:113126. doi: 10.1016/j.biopha.2022.113126, PMID 35643074.
Faienza MF, Giardinelli S, Annicchiarico A, Chiarito M, Barile B, Corbo F. Nutraceuticals and functional foods: a comprehensive review of their role in bone health. Int J Mol Sci. 2024 May 28;25(11):5873. doi: 10.3390/ijms25115873, PMID 38892062, PMCID PMC11172758.
Brand Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT. 1995;28(1):25-30. doi: 10.1016/S0023-6438(95)80008-5.
Mukhopadhyay D, Dasgupta P, Sinha Roy D, Palchoudhuri S, Chatterjee I, Ali S. A sensitive in vitro spectrophotometric hydrogen peroxide scavenging assay using 1,10-phenanthroline. Free Radic Antioxid. 2016 Jan 10;6(1):124-32. doi: 10.5530/fra.2016.1.15.
Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: the FRAP assay. Anal Biochem. 1996 Jul 15;239(1):70-6. doi: 10.1006/abio.1996.0292, PMID 8660627.
Pavithra K, Vadivukkarasi S. Evaluation of free radical scavenging activity of various extracts of leaves from kedrostis foetidissima (Jacq.) cogn. Food Sci Hum Wellness. 2015 Mar 1;4(1):42-6. doi: 10.1016/j.fshw.2015.02.001.
Boora F, Chirisa E, Mukanganyama S. Evaluation of nitrite radical scavenging properties of selected zimbabwean plant extracts and their phytoconstituents. J Food Process. 2014 Apr 6;2014:1-7. doi: 10.1155/2014/918018.
Ruberto G, Baratta MT. Antioxidant activity of selected essential oil components in two lipid model systems. Food Chem. 2000 May 1;69(2):167-74. doi: 10.1016/S0308-8146(99)00247-2.
Chen YT, Zheng RL, Jia ZJ, JU Y. Flavonoids as superoxide scavengers and antioxidants. Free Radic Biol Med. 1990;9(1):19-21. doi: 10.1016/0891-5849(90)90045-k, PMID 2170243.
PP Gunathilake KD, Ranaweera KK, Rupasinghe HP. In vitro anti-inflammatory properties of selected green leafy vegetables. Biomedicines. 2018 Nov 19;6(4):107. doi: 10.3390/biomedicines6040107, PMID 30463216, PMCID PMC6316011.
Alsahli MA, Almatroodi SA, Almatroudi A, Khan AA, Anwar S, Almutary AG. 6-gingerol a major ingredient of ginger attenuates diethylnitrosamine induced liver injury in rats through the modulation of oxidative stress and anti-inflammatory activity. Mediators Inflamm. 2021 Jan 19;2021:6661937. doi: 10.1155/2021/6661937, PMID 33531877, PMCID PMC7837795.
Alley MC, Scudiero DA, Monks A, Hursey ML, Czerwinski MJ, Fine DL. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988 Feb 1;48(3):589-601. PMID 3335022.
Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55-63. doi: 10.1016/0022-1759(83)90303-4, PMID 6606682.
Kurniawan DW, Aini Gumilas NS, Arramel, Hartati, Novrial D, Tarwadi. Preparation characterization and toxicity study of andrographis paniculata ethanol extract poly-lactic-co-glycolic acid (PLGA) nanoparticles in raw 264.7 cells. Int J Appl Pharm. 2024;16(4):78-83. doi: 10.22159/ijap.2024v16i4.50798.
WU D, Yotnda P. Production and detection of reactive oxygen species (ROS) in cancers. J Vis Exp. 2011 Nov 21;(57):3357. doi: 10.3791/3357, PMID 22127014, PMCID PMC3308605.
Erdogan U. Antioxidant activities and chemical composition of essential oil of rhizomes of Zingiber officinale (Ginger) and Curcuma longa L.(Turmeric). Int J Second Metabolite. 2022 May 15;9(2):137-48. doi: 10.21448/ijsm.993906.
Jurenka JS. Anti-inflammatory properties of curcumin a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern Med Rev. 2009 Jun;14(2):141-53. Erratum in: Altern Med Rev. 2009 Sep;14(3):277. PMID 19594223.
Lin X, Bai D, Wei Z, Zhang Y, Huang Y, Deng H. Curcumin attenuates oxidative stress in RAW264.7 cells by increasing the activity of antioxidant enzymes and activating the Nrf2-Keap1 pathway. Plos One. 2019 May 21;14(5):e0216711. doi: 10.1371/journal.pone.0216711, PMID 31112588, PMCID PMC6528975.
Deng T, XU J, Wang Q, Wang X, Jiao Y, Cao X. Immunomodulatory effects of curcumin on macrophage polarization in rheumatoid arthritis. Front Pharmacol. 2024 Feb 28;15:1369337. doi: 10.3389/fphar.2024.1369337, PMID 38487171, PMCID PMC10938599.
Allegra A, Mirabile G, Ettari R, Pioggia G, Gangemi S. The impact of curcumin on immune response: an immunomodulatory strategy to treat sepsis. Int J Mol Sci. 2022 Nov 25;23(23):14710. doi: 10.3390/ijms232314710, PMID 36499036, PMCID PMC9738113.
Heidari Beni M, Moravejolahkami AR, Gorgian P, Askari G, Tarrahi MJ, Bahreini Esfahani N. Herbal formulation turmeric extract, black pepper and ginger versus naproxen for chronic knee osteoarthritis: A randomized double blind controlled clinical trial. Phytother Res. 2020 Aug;34(8):2067-73. doi: 10.1002/ptr.6671, PMID 32180294.
Castejon ML, Rosillo MA, Montoya T, Gonzalez Benjumea A, Fernandez-Bolanos JG, Alarcon-de-la-Lastra C. Oleuropein downregulates IL-1β-induced inflammation and oxidative stress in human synovial fibroblast cell line SW982. Food Funct Food Funct. 2017;8(5):1890-8. doi: 10.1039/c7fo00210f, PMID 28426090.