
Int J App Pharm, Vol 17, Issue 3, 2025, 370-382Original Article
TERCONAZOLE-LOADED MICRO-SPONGES: PREPARATION, CHARACTERIZATION, AND OPTIMIZATION FOR SOLUBILITY AND DISSOLUTION RATE ENHANCEMENT
MAHA MAHDI ALI1, MANAR ADNAN TAMER2, SABA ABDULHADI JABER2*
1Department of Pharmaceutics, College of Pharmacy, University of Hilla, Babylon, Iraq. 2Department of Pharmaceutics, College of Pharmacy, University of Baghdad, Baghdad, Iraq
*Corresponding author: Saba Abdulhadi Jaber; *Email: sabahadeejabir77@gmail.com
Received: 10 Dec 2024, Revised and Accepted: 14 Feb 2025
ABSTRACT
Objective: Terconazole is a potent antifungal agent characterized by insufficient aqueous solubility; which is a significant challenge for formulation development and therapeutic efficacy. The current research aimed to develop and optimize an innovative carrier system using micro-sponges to improve the solubility and dissolution rate of Terconazole.
Methods: Fifteen formulations of Terconazole-loaded micro-sponges were prepared by quasi-emulsion solvent diffusion technique, with various parameters such as polymer type and concentration, emulsifying agent concentration, plasticizer percentage, and pore inducer amount being systematically investigated. The impact of these parameters on particle size, production yield, loading efficiency, saturation solubility, and In vitro dissolution profiles was thoroughly assessed.
Results: The results indicated that formula F15, comprising 0.1g Eudragit L100, 25 mg Poly Vinyl Alcohol (PVA), 0.1 ml glycerol, and 1.5g Pre-Gelatinized Starch (PGS), exhibited the smallest particle size, higher production yield and loading efficiency, achieving a remarkable nine-fold enhancement in saturation solubility (752.26±19.84µg/ml) compared to pure Terconazole of 83.42±3.39µg/ml. furthermore, the percentage of Terconazole released after one hour from F15 was 92.85%, significantly higher than the 33.54% from its pure powder. Scanning Electron Microscope (SEM) analysis revealed highly porous structures of the micro-sponges, while Fourier Transform Infra-Red (FTIR) studies showed no evidence of chemical interaction, and Differential Scanning Calorimetry (DSC) indicated no change in Terconazole’s nature during micro-sponges production.
Conclusion: Overall, the findings suggest that micro-sponges represent a promising system for enhancing the saturation solubility and dissolution rate of poorly water-soluble Terconazole, potentially improving its bioavailability and therapeutic outcomes in clinical settings, especially ocular medications. The implications of this study extend beyond Terconazole, offering valuable insights and methodologies that can be applied to improve the solubility and bioavailability of a wide range of pharmaceutical compounds.
Keywords: Terconazole, Solubility, Dissolution rate, Micro-sponges, Eudragit
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijap.2025v17i3.53373 Journal homepage: https://innovareacademics.in/journals/index.php/ijap
INTRODUCTION
Solubility is the most critical pre-formulation property, which significantly influences formulation development and therapeutic performance [1].
The main challenge associated with drug delivery systems is that most medications are poorly aqueous soluble, which creates many difficulties during formulating them in traditional dosage forms [2].
Micro-sponges technology was developed by Won in 1987, which is currently considered a revolutionary manner for delivering drugs in a unique, versatile structured form [3].
Micro-sponges are actually polymeric porous microspheres, with a large porous sponge-like structure and very tiny spherical shape particles. The size of micro-sponges is varied, usually from 5 to 300μm in diameter. A typical 25µm sphere can have up to 25×104 pores, with internal structure equivalence to 10 feet per pore in length, providing a total pore volume of about 1 ml/g for extensive retention of pharmaceutical active ingredients [4].
Micro-sponges are designed to deliver a drug efficiently at a minimum dose, which improves the drug stability and reduces the adverse effects. In addition, they can enhance the solubilization and dissolution rate of poorly aqueous soluble drugs and also modify the drug release profile in a controlled manner [5].
Previously, the micro-sponges drug delivery system was mostly used for topical administration, while nowadays as per current information according to many researchers; it is used for drug delivery via oral, parental, and ophthalmic routes.
Micro-sponges are predominantly composed of polymers dispersed in aqueous media, along with an appropriate emulsifying agent. For the enhancement of drug dissolution and controlling of drug release in Micro-sponges, Eudragit are frequently reported as a polymer of choice [6].
The quasi-emulsion solvent diffusion technique is a prevalent method for micro-sponge engineering, involving the dissolution of a water-insoluble polymer in a water-immiscible solvent, which is then emulsified with a hydrophilic plasticizer aqueous phase. The organic solvent gradually diffuses out while stirring, forming spherical scaffold structures. Key factors influencing the efficacy of these micro-carriers include the active drug-to-polymer ratio, surfactant, and plasticizer. Eudragit polymers are extensively utilized in the fabrication of micro-sponges [7].
Eudragit polymers are biologically inert, non-toxic, and non-biodegradable polymers. They encompass various types of pH-dependent and pH-independent coating polymers with a wide range of apparent viscosity; this versatility in properties confers a wide scope in the preparation of micro-sponges and enhancement of desired attributes such as particle size, loading efficiency, and saturation solubility [8].
Corneal pathologies significantly contribute to visual impairment, with various ocular regions vulnerable to fungal infections. Fungal keratitis is a notable condition caused by various fungal species, predominantly Candida. Despite being the leading cause of ocular fungal infections, Candida's resistance to common antifungal agents poses challenges. Historically, amphotericin B was the primary treatment for fungal keratitis, but it often led to diminished visual acuity. In complex cases, corneal grafts became necessary. Alternative methods for applying topical amphotericin B have been suggested, particularly the use of topical azoles. Fluconazole, voriconazole, and terconazole proved to have less toxicity to corneal epithelia, demonstrating better ocular tolerability than amphotericin B [9].
Terconazole is a potent antifungal agent over a wide range of yeasts and mycelium-forming fungi. It inhibits the fungal cytochrome P-450 dependent ergosterol synthesis, which is a vital component of a fungal cell membrane, leading to disruption of the fungal structure, function, and growth [10]. Terconazole was the first triazole marketed for the topical management of vaginal candidiasis (0.4% and 0.8% creams and 80 mg suppositories).
Chemically Terconazole (C26H31Cl2N5O3) is 1-[4-[[2-(2,4-dichlorophenyl)-2-(1H-1,2,4-triazol-1-ylmethyl)-1,3dioxolan-4-yl]methoxy]phenyl]-4-1-methylethyl)piperazine [11]. It is a weak base with a molecular weight of 532.47 Daltons. It has pka and log P of 8.41 and 5.37, respectively [12].
Terconazole, which belongs to Biopharmaceutical Classification System (BSC) Class-II, has insufficient aqueous solubility. Unfortunately, Terconazole suffers from a limit dissolution rate, poor ocular absorption, and rapid drainage from ocular tissues, which collectively restrict its ocular bioavailability despite its good efficacy [13]. Consequently, an effective delivery system is essential to overcome these drawbacks and improve its therapeutic activities. Thus, micro-sponges may provide significant improvements in the in vitro and in vivo performance of poorly water-soluble drugs and, therefore, may serve as effective vehicles for ocular drug delivery [14].
Moreover, it is noteworthy that micro-sponge carriers have not been utilized in previous literature for Terconazole loading despite being regarded as a promising method for enhancing drug solubility and bioavailability.
Various studies have explored different carrier systems to improve the bioavailability and therapeutic efficacy of Terconazole, for ocular applications. Terconazole was loaded onto mesoporous silica microparticles, specifically Syloid® 244 FP, modified with Poly(Lactic-co-Glycolic Acid) to enhance its solubility and dissolution rate. The optimal formulation showed a significant increase in mean residence time, Cmax, and AUC0–24-values in rabbit eyes, indicating improved ocular bioavailability [12]. Silica/Chitosan Nanoparticles (SCNs) were developed using tetraethyl ortho silicate and chitosan HCl, with cyclodextrins as cryoprotectants. The optimized SCNs demonstrated excellent mucoadhesive properties and increased Cmax and AUC0–24 values, suggesting enhanced ocular bioavailability compared to Terconazole suspension [9]. While these innovative carriers show promise in enhancing the solubility and dissolution rate of Terconazole for ocular delivery, challenges such as formulation stability, scalability, and long-term safety need to be addressed.
The objective of this study was to develop an optimized formulation of Terconazole-loaded micro-sponges for improving Terconazole solubility and dissolution rate, which is the limitation of ocular delivery system developments.
MATERIALS AND METHODS
Materials
Terconazole powder was provided from Nosch Labs Private Limited, India. Eudragit polymer S100 powder was supplied from Röhm GMBH Weiterstdt, Germany. Eudragit polymers (L100 powder and E100 granules) and PGS powder were obtained as a gift sample from Samara Drug Industry, Iraq. Eudragit polymers (RS100 and RL100) powders were supplied from Vikram Thermo Limited, India. PVA powder was received from Panreac, Espana. Glycerol was supplied from BDH Chemicals Limited, England. All other ingredients used in this research were of analytical grade.
Preparation of terconazole-loaded micro-sponges
Terconazole-loaded micro-sponges were prepared via quasi-emulsion solvent diffusion technique, using different polymers, as shown in table 1.
Firstly, the organic (inner) phase was prepared by dissolving one type of Eudragit polymers (S100, L100, RS100, RL100, or E100) and glycerol as plasticizer, in 2.5 ml of ethanol, which can dissolve all used polymers as well as Triconazole. On the other hand, 100 ml of the aqueous (outer) phase was prepared by dissolving PVA, as surfactant, in distilled water at 70 °C, until it dissolved completely and then allowed to cool down to room temperature.
1g of Terconazole was added to the organic phase and dissolved under ultrasonication (ultrasonic power 100W and frequency 40KHZ) at 35 °C for 15 min, until a clear solution was obtained. The resulting solution was then poured, drop by drop, into the aqueous phase. The mixture was stirred at 500rpm for 1h at room temperature. Hence, micro-sponges were created by expelling ethanol from the solution through evaporation. The prepared mixture was left in a refrigerator for one day in order to complete precipitation of the micro-sponges. Subsequently, the performed mixture was filtered to separate the micro-sponges, and then washed several times with distilled water. The formed Terconazole-loaded micro-sponges were dried in an oven at 40 °C for 12h and stored for subsequent investigations [15, 16].
Table 1: The composition of different micro-sponges formulas prepared by quasi-emulsion solvent diffusion technique
| Formula code | *Terconazole (g) | Inner phase | Outer phase | PGS (mg) | |||||
| Eudragit type | Polymer (mg) | Glycerol (µl) | *Ethanol (ml) | PVA (mg) | *Water (ml) | ||||
| F1 | 1 | S100 | 125 | 250 | 2.5 | 25 | 100 | - | |
| F2 | 1 | L100 | 125 | 250 | 2.5 | 25 | 100 | - | |
| F3 | 1 | RS 100 | 125 | 250 | 2.5 | 25 | 100 | - | |
| F4 | 1 | RL 100 | 125 | 250 | 2.5 | 25 | 100 | - | |
| F5 | 1 | E100 | 125 | 250 | 2.5 | 25 | 100 | - | |
| F6 | 1 | L100 | 75 | 250 | 2.5 | 25 | 100 | - | |
| F7 | 1 | L100 | 100 | 250 | 2.5 | 25 | 100 | - | |
| F8 | 1 | L100 | 150 | 250 | 2.5 | 25 | 100 | - | |
| F9 | 1 | L100 | 100 | 125 | 2.5 | 25 | 100 | - | |
| F10 | 1 | L100 | 100 | 500 | 2.5 | 25 | 100 | - | |
| F11 | 1 | L100 | 100 | 500 | 2.5 | 5 | 100 | - | |
| F12 | 1 | L100 | 100 | 500 | 2.5 | 50 | 100 | - | |
| F13 | 1 | L100 | 100 | 500 | 2.5 | 25 | 100 | 0.5 | |
| F14 | 1 | L100 | 100 | 500 | 2.5 | 25 | 100 | 1 | |
| F15 | 1 | L100 | 100 | 500 | 2.5 | 25 | 100 | 1.5 | |
| *Value were kept constant, PVA is Poly Vinyl Alcohol and PGS is Pre-Gelatinized Starch, The specific ranges for polymer, plasticizer, emulsifying agent, and pore inducer concentrations were chosen based on preliminary experiments, in addition to using what was mention in previous literature. | |||||||||
Evaluation of terconazole-loaded micro-sponges
Particle size analysis
The particle size of the prepared micro-sponges was measured with an Olympus BX51 optical microscope, by using a calibrated ocular and stage micrometer under a regular polarized light. A tiny amount of sample was spread on a clean glass slide and observed under 100X lens magnification. The average particle size was calculated using the following equation [16]:
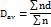
Where:
Dav is the average diameter of at least 100 particles (μm).
n is the number of particles per group.
d is the middle value of particles diameter (μm).
Determination of the micro-sponges production yield
The production yield of micro-sponges was obtained by comparing the accurate weight of the final formed micro-sponges with the initial weight of the combined raw components. The percentage yield was calculated by the following equation [17]:
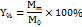
Where:
Y% is the percentage of Micro-sponges production yield.
M0 is the theoretical total mass of Terconazole and polymer used.
Mm is the mass of the formed micro-sponges.
Determination of terconazole loading efficiency
The amount of Terconazole loaded into the micro-sponges pores was quantified spectrophotometrically.
Initially, an accurate weight (10 mg) of Terconazole-loaded micro-sponges sample was crushed in a mortar; and then kept in 100 ml of Phosphate Buffer Solution (PBS) pH7.4 for 24h, to extract the Terconazole.
Subsequently, the solution was filtered through a 0.45μm cellulose membrane filter. After that, the filtrate was appropriately diluted with PBS (pH7.4), and spectrophotometric absorbance was taken at the maximum wave length of Terconazole.
The Terconazole content was determined from the standard calibration curve. The loading efficiency of Terconazole was estimated using the following equation [8, 18]:
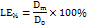
Where:
LE% is the percentage of Terconazole loading efficiency.
D0 is the Theoretical Terconazole amount that was fed initially to prepare a formula.
Dm is the amount of free Terconazole that leaks from the micro-sponges formula.
Saturation solubility study
The saturated solubility determination was achieved by using the shake flask method. This study was performed, at room temperature, for the unprocessed pure Terconazole and all the prepared micro-sponges formulas.
To gain maximum solubility, an excess amount of the sample was dispersed in 10 ml of PBS (pH7.4). The suspension was sonicated for 15 min and then stirred in a water bath shaker at a constant temperature of 24±0.5 °C. After 72h, which was sufficient to achieve an equilibrium state, the suspension was filtered through a 0.45μm cellulose membrane filter. The filtrate was collected and suitably diluted with the same buffer solvent.
The absorbance of Terconazole was analyzed by a UV-Visible Spectrophotometer, at the previously scanned λmax in a particular BPS (pH7.4) of 245 nm. Finally, the concentration of the dissolved Terconazole was determined using a standard calibration curve [19].
The saturation solubility of pure Terconazole was also determined at 37±0.5 °C for sink condition.
In vitro dissolution study
The in vitro dissolution rate of Terconazole from the optimized micro-sponges formula was compared with pure Terconazole powder, using United States Pharmacopeia (USP) apparatus-II (paddle assembly). 900 ml of PBS (pH7.4) was used as a dissolution medium close to lachrymal fluid. Keeping temperature at 37±0.5 °C and stirring rate at 50rpm. An accurately measured amount of micro-sponges equivalent to 10 mg of Terconazole was used. An aliquot of 10 ml of dissolution fluid was collected at regular predicated intervals (10, 20, 30 45, 60, 120, and 180 min) and immediately replaced with 10 ml of the fresh dissolution medium to maintain a constant volume. The samples were filtered through a 0.45μm filter, suitably diluted, and assayed at λmax of Terconazole using a UV-Visible spectrophotometer [8, 20].
Kinetic modeling of drug release from micro-sponge
In order to elucidate the mechanism underlying the release of Terconazole from the formulations, the in vitro release data were subjected to fitting with various kinetic models of release. The models employed include: zero order, first order, Higuchi model, Hixon-Crowell model, and Korsmeyer-Peppas. The model exhibiting the highest correlation coefficient was designated as the most appropriately fitting model [21].
SEM study
SEM technique was employed to survey the morphology and surface topography of the optimized micro-sponges formula. The sample was coated with gold-palladium, and then scanned using SEM (Inspect S50-FEI Company, Netherlands) under an argon atmosphere at room temperature [22].
Compatibility studies
Compatibility studies were carried out using thermal powerful analytical techniques, to determine the possibility of physicochemical interaction between Terconazole and a polymer used in the preparation of the optimized micro-sponges formula.
FTIR analysis
FTIR spectra of pure Terconazole, Eudragit-L100, a physical mixture of Terconazole and Eudragit-L100 at a ratio 1:1, and an optimized micro-sponges formula were recorded to achieve compatibility. An accurately weighed sample of 1 mg was grounded finely, mixed thoroughly with 100 mg of potassium bromide, and then scanned in the range from 400 cm-1 to 4000 cm-1 of 2 cm-1 resolution, using the FTIR spectroscopy-8300 Shimadzu model [23].
DSC analysis
DSC thermogram can be used to confirm the crystallinity of Terconazole, especially in the optimized formula, and also to ascertain compatibility. It was performed for the same FTIR samples by using a DSC analyzer-60plus Shimadzu model. An accurately weighed sample of 5 mg was placed in an aluminum pan, sealed hermetically, and scanned at a heating rate of 10 °C/min covering a temperature range of 0-350 °C, under a nitrogen atmosphere with a flow rate of 20 ml/min [24].
Statistical analysis
All the experimental studies were repeated in triplicate. All factors and evaluation parameters were statistically calculated individually. Results were presented as a means of three samples±standard deviation and were analyzed according to the One-Way Analysis of Variance (ANOVA) test using Microsoft Excel Program 2013. Differences were considered to be statistically significant at the level of p<0.05.
RESULTS AND DISCUSSION
Preparation of Terconazole-loaded micro-sponges
Quasi-emulsion solvent diffusion technique is a simple, safe, cost-effective, and reproducible procedure, involved in the preparation of Terconazole-loaded micro-sponges [25].
Micro-sponges were formed by rapid diffusion of ethanol into the inner phase, resulting in reduced solubility of hydrophobic Eudragit polymer in the droplets. Immediate mixing of ethanol and water at the droplet interface resulted in the precipitation of Eudragit, thus forming a shell surrounding the dissolved Terconazole with ethanol. Ethanol diffusion resulted in the solidification of finely dispersed droplets in the aqueous solution [8].
Different variables were studied for their effect on the prepared Terconazole-loaded micro-sponges, to select the optimum formula that achieves the aim of this study.
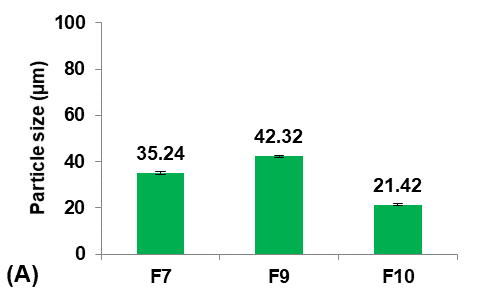
The saturation solubility of pure Terconazole powder in PBS (pH7.4) at 24±0.5 °C was 83.42±3.59µg/ml, which is similar to the result reported previously [26].
Effect of polymer type of inner phase
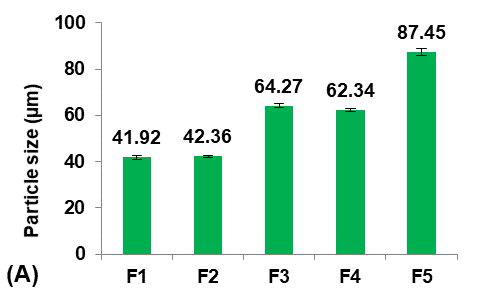
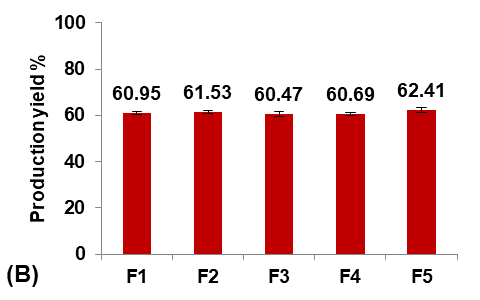
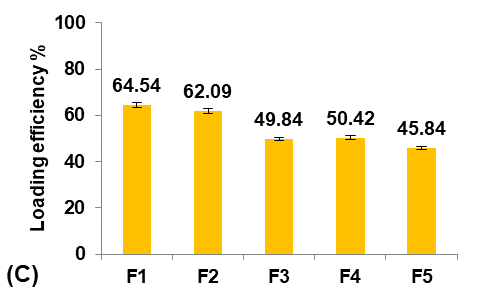
Initially, five formulations were prepared using different Eudragit polymers to study their effect on the Terconazole-loaded micro-sponges, as shown in fig. 1.
The type of Eudragit has a considerable impact on the nature of micro-sponges formulations [27]. It was observed that the average particle size of micro-sponges was inversely proportional to the apparent viscosity of Eudragit polymers, as shown in the following order:
Eudragit polymers S100<L100<RS100<RL100<E100 as found in the micro-sponges formulas (F1-F5) respectively.
The apparent viscosities of Eudragit polymers are 50-200mpa. s for Eudragit-S100, 60-120mpa. s for Eudragit-L100, 1-15mpa. s for Eudragit-RS100 and-RL100, and 3-6mpa. s for Eudragit-E100 [28].
This result was attributed to the viscosity difference between the inner and the outer phases. Since, when the difference between the viscosity of the Eudragit dispersed phase and the aqueous dispersion medium was decreased, the emulsion was barely broken down into small droplets, and micro-sponges with larger particle sizes were formed [29].
It was shown that as the particle size decreased, the loading efficiency of Terconazole increased significantly (p<0.05). Further reduction in particle size increases their surface area with more active sites available for drug uptake, leading to improved loading efficiency [30].
 |
 |
 |
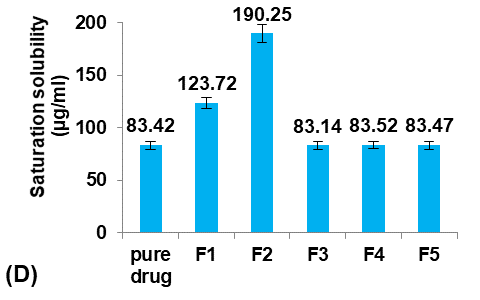 |
Fig. 1: Effect of polymer type of inner phase on: A) particle size (µm), B) production yield %, C) loading efficiency %, and D) saturation solubility (µg/ml) of Terconazole-loaded micro-sponges formulas F1-F5, which prepared by using Eudragit polymers-S100,-L100,-S100,-RL100, and-S100 respectively (all data expressed as mean±SD, n=3)
Moreover, the apparent viscosity of Eudragit polymers also has a noticeable effect on the loading efficiency. As the viscosity of the inner phase increased, the movement of Terconazole out of the dispersed droplets reduced, resulting in a greater amount of Terconazole entrapment [8].
The saturation solubility of Terconazole in PBS (pH7.4) was significantly improved (p<0.05) in micro-sponges formulas F1 and F2, prepared from Eudragit-S100 and-L100, respectively. Formulas F3-F5, prepared from Eudragit-RS100,-RL100, and-E100 respectively, gave no significant difference (p>0.05) in the saturation solubility of Terconazole.
The reason behind these results is that the Terconazole release occurs after complete swelling and degradation of the Eudragit matrix. Eudragit-S100,-L100, and-E100 are pH-dependent coating polymers that dissolve in definite pH media with the formation of their polymeric salt as follows:
Eudragit-S100 dissolves above pH7, Eudragit-L100 dissolves above pH6 and Eudragit-E100 dissolves below pH5, while Eudragit-RS100 and-RL100 are pH-independent polymers [31].
Formula F2 of Eudragit-L100 gave the best improvement in the solubility of Terconazole, in comparison with Formula F1 of Eudragit-S100. This result is because increasing Eudragit viscosity increases the adhesion of Terconazole molecules with polymeric molecules and reduces their mobility outside of the formed micro-sponges [17].
The production yield of micro-sponges was not significantly affected (p>0.05) by using different types of Eudragit polymers.
Formula F2, with acceptable particle size, production yield, and loading efficiency, and best solubility improvement, was selected for further optimization studies.
Effect of polymer concentration
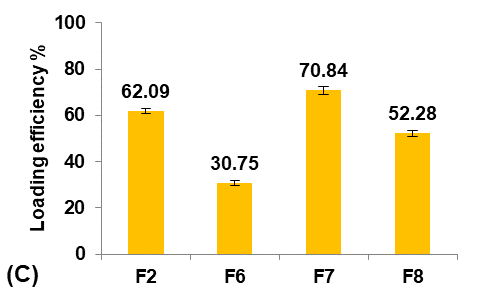
Different concentrations of Eudragit-L100 (30, 40, 50, and 60 mg/ml ethanol) were used to prepare formulas F6, F7, F2, and F8, respectively, to study their effect on the Terconazole-loaded micro-sponges, as illustrated in fig. 2.
At a decreased concentration of Eudragit-L100, below 30 mg/ml, the finely dispersed spherical quasi-emulsion droplets were observed in a solvent under agitation. Still, as the agitation was discontinued, emulsion droplets adhered together and aggregated. The result suggests that the Eudragit-L100 concentration of the inner phase needs to be controlled within an appropriate range to maintain the optimal viscosity that affects the formation of quasi-emulsion droplets at the initial stage and the solidification of Terconazole and Eudragit-L100 in the droplets. The good micro-sponges were produced only when 30 mg/ml of Eudragit-L100 was used.
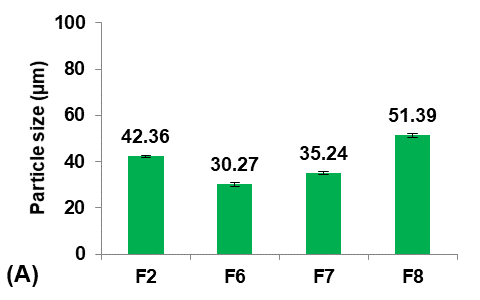 |
 |
 |
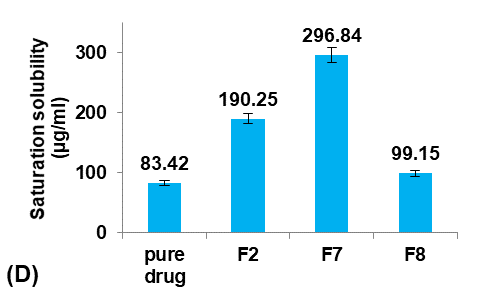 |
Fig. 2: Effect of polymer concentration on: A) particle size (µm), B) production yield %, C) loading efficiency %, and D) saturation solubility (µg/ml) of Terconazole-loaded micro-sponges formulas F2 and F6-F8, which prepared by using 50, 30, 40, and 60 mg/ml of Eudragit-L100, respectively, (all data expressed as mean±SD, n=3)
It was observed that decreasing the concentration of Eudragit-L100 led to a significant decrease (p<0.05) in the average particle size of micro-sponges. This occurred because the proportion of polymer available per micro-sponges was decreased, and thus a smaller particle size was obtained. This provides an extensive surface area for high entrapment [32].
Production yield and loading efficiency increased significantly (p<0.05) with decreasing particle size, as shown in formulas F2, F7, and F8. At the lowest concentration of Eudragit-L100 (Formula F6), production yield and loading efficiency decreased significantly (P<0.05) despite the decrease in particle size due to the reduction in the Eudragit-L100 fraction available for Terconazole encapsulation [8]. Therefore, formula F6 was rejected from the study.
The saturation solubility of Terconazole in PBS (pH7.4) was significantly improved (p<0.05) by reducing the concentration of Eudragit-L100. This result was due to an increased ratio of Terconazole to Eudragit-L100, which resulted in a decreased matrix wall thickness of the micro-sponges pores, and thus a more extensive diffusion pathway and ultimately more Terconazole release [20].
Formula F7, with lower particle size, higher production yield and loading efficiency, and best solubility improvement, was selected for further optimization studies.
Effect of plasticizer percentage
Glycerol was used as a plasticizer to overcome the problem of coalescence during solvent evaporation [33].
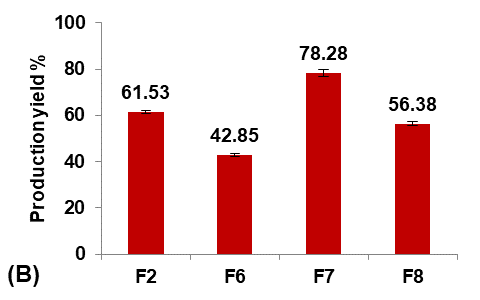
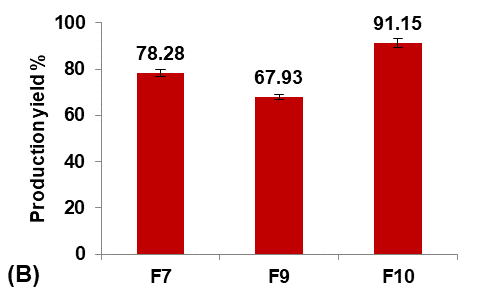
Different glycerol percentages of (5, 10, and 20%v/v ethanol) were used to prepare formulas F9, F7, and F10, respectively, to study their effect on the Terconazole-loaded micro-sponges, as shown in fig. 3.
The results indicated that increasing the percentage of glycerol led to a significant decrease (P<0.05) in the average particle size with a significant increase (P<0.05) in production yield and loading efficiency, in addition to a significant improvement (P<0.05) in the saturation solubility of Terconazole in PBS (pH7.4).
These results were attributed to a higher stabilizing effect of glycerol on small emulsion droplets, as it reduces the phase tension between the droplets and the dispersion medium, preventing them from aggregating into larger particles. Therefore, micro-sponges with smaller particles and larger surface areas were formed [34].
Formula F10, with lower particle size, higher production yield and loading efficiency, and best solubility improvement, was selected for further optimization studies.
Effect of emulsifying agent concentration
PVA was employed as a non-ionic emulsifying agent in the preparation of micro-sponges to maintain the viscosity of the aqueous outer phase [35].
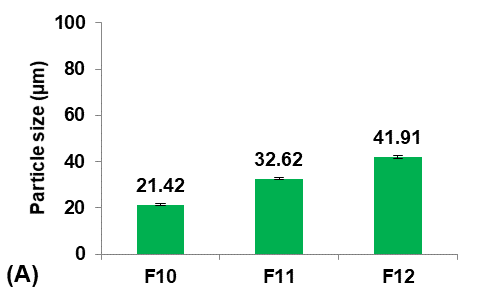
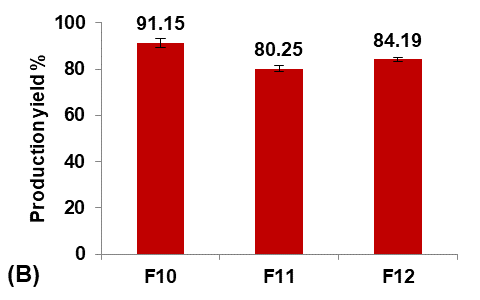
Different concentrations of PVA (5, 25, and 50 mg/100 ml water) were used to prepare formulas F11, F10, and F12, respectively, to study their effect on the Terconazole-loaded micro-sponges, as illustrated in fig. 4.
It was observed that increasing the PVA concentration (from 5 to 25 mg/100 ml) gave better stability against the coalescence of the emulsion and led to a significant decrease (p<0.05) in the average particle size.
On the other hand, increasing the PVA concentration (from 25 to 50 mg/100 ml) also resulted in a significant increase (p<0.05) in the average particle size, which was attributed to the lower viscosity difference between the inner and outer phases. This would result in larger emulsion droplets and consequently larger micro-sponges with a lower active surface area [16].
Production yield, loading efficiency, and saturation solubility of Terconazole in PBS (pH7.4) decreased significantly (p<0.05) with increasing particle size.
Formula F10, with lower particle size, higher production yield and loading efficiency, and best solubility improvement, was selected for further optimization studies.
 |
 |
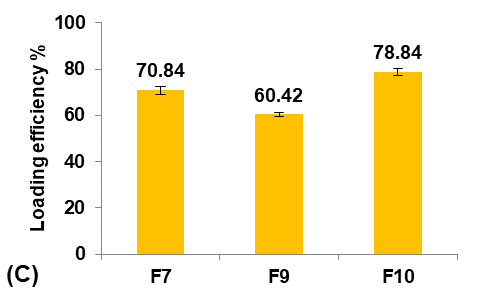 |
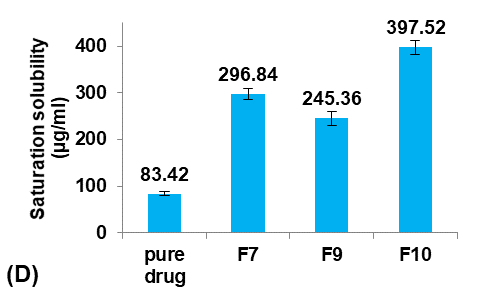 |
Fig. 3: Effect of plasticizer percentage on: A) particle size (µm), B) production yield %, C) loading efficiency %, and D) saturation solubility (µg/ml) of Terconazole-loaded micro-sponges formulas F7, F9, and F10, which contained of 10, 5, and 20% glycerol, respectively, (all data expressed as mean±SD, n=3)
 |
 |
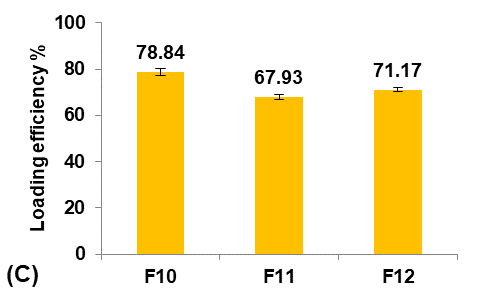 |
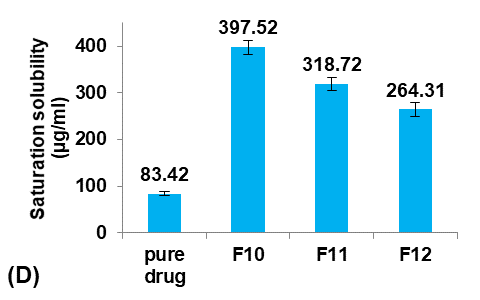 |
Fig. 4: Effect of emulsifying agent concentration on: A) particle size (µm), B) production yield %, C) loading efficiency %, and D) saturation solubility (µg/ml) of Terconazole-loaded micro-sponges formulas F10-F12, which contained of 25, 5, and 50 mg PVA/100 ml water, respectively (all data expressed as mean±SD, n=3)
Effect of pore inducer amount
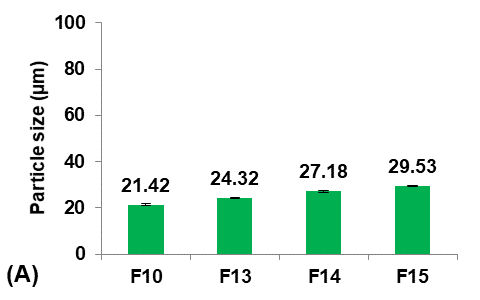
PGS was introduced as a pore inducer in the preparation of micro-sponges to study its effect on Terconazole solubility. First, Terconazole was triturated with PGS and dispersed in 3 ml of ethanol. This dispersion was allowed to dry completely and then incorporated into the organic phase, after which the general preparation method was carried out [31].
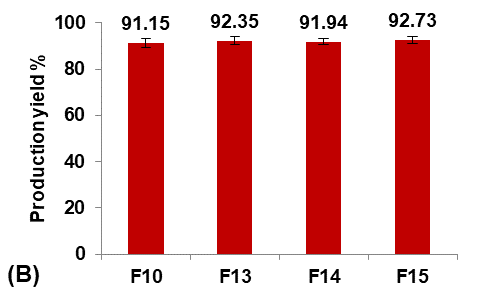
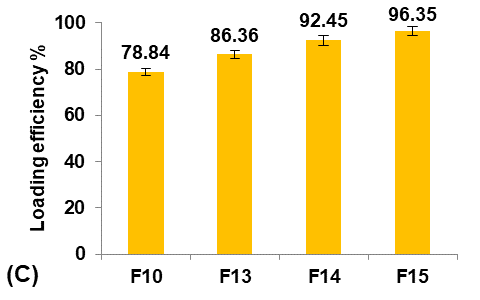
Formulas F13-F15 (containing 0.5, 1, and 1.5 gm of PGS, respectively) were prepared for comparison with the selected formula F10, as shown in fig. 5.
 |
 |
 |
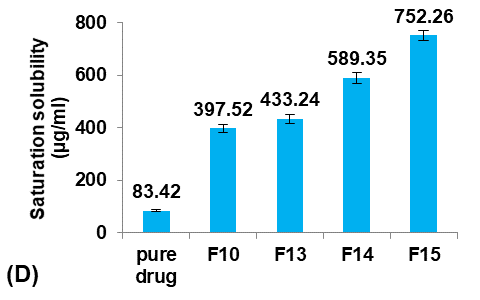 |
Fig. 5: Effect of pore inducer amount concentration on: A) particle size (µm), B) production yield %, C) loading efficiency %, and D) saturation solubility (µg/ml) of Terconazole-loaded micro-sponges formulas F10 and F13-F15, which contained of 0, 0.5, 1, and 1.5 mg PGS, respectively, (all data expressed as mean±SD, n=3)
It was observed that increasing the amount of PGS significantly increased (p<0.05) the particle size, entrapment efficiency, and saturation solubility, with no significant difference (p>0.05) in the production yield.
These results can be attributed to increased pores and channels within the particles, which provided a large surface area for Terconazole to be evenly absorbed into the micro-sponges, resulting in increased loading efficiency. On the other hand, the PBS (pH7.4) solution can pass through the pores of the micro-sponges, releasing the drug carried onto their surface by osmotic effect, resulting in enhanced solubility of Terconazole molecules [15].
Formula F15 possessed a higher percentage of uniform spherical particles during optical microscopy analysis.
Table 2 summarizes the statistical significance (p-value) of the different effects on each evaluation parameter of Terconazole-loaded micro-sponges formulas.
From all these studies, F15 was considered the optimized Terconazole-loaded micro-sponges formula.
In vitro dissolution study
The in vitro dissolution profile (fig. 6) detects that formula F15 gave a better release of Terconazole than its pure powder because of the high thermodynamic activity of Terconazole molecules. The percentage of Terconazole release from micro-sponges formula F15 reached 92.85% within one hour, while Terconazole powder had only 33.54% content dissolved at that time.
In comparison, 70% of Terconazole was released after 3 h from the optimized formula prepared as Cyclodextrin Stabilized Freeze-Dried Silica/Chitosan Nanoparticles as reported previously [10].
Micro-sponges enhance the solubility of terconazole by trapping its molecules within their pores. Terconazole is effectively reduced to microscopic particles and increased surface area results in improved dissolution rate.
These results are in good accordance with Noyes–Whitney equation which states that the decrease in drug particle size caused an increase in the surface area and consequently enhanced the contact between particles and the dissolution medium, leading to an increased dissolution rate [8, 36].
On the other hand, the porous structure of micro-sponges allows easy penetration and access of dissolution medium to the entrapped drug molecules, as the pores provide channels for drug release. The results indicated that most of the Terconazole molecules are adsorbed on the surface of the micro-sponges and thus have the potential to undergo rapid solubilization and then quick drug release [20, 32].
Table 2: The statistical significance (p-value) of the different effects on each evaluation parameter of terconazole-loaded micro-sponges formulas
| Effects | Evaluation parameters |
| Particle size | |
| Eudragit type (Increase the apparent viscosity) | Significant decrease (p<0.05) |
| Decrease Eudragit-L100 concentration | Significant decrease (p<0.05) |
| Increasing glycerol percentage | Significant decrease (p<0.05) |
| Increasing PVA concentration | Significant increase (p<0.05) |
| Increasing PGS amount | Significant increase (p<0.05) |
| PVA is poly vinyl alcohol and PGS is pre-gelatinized starch |
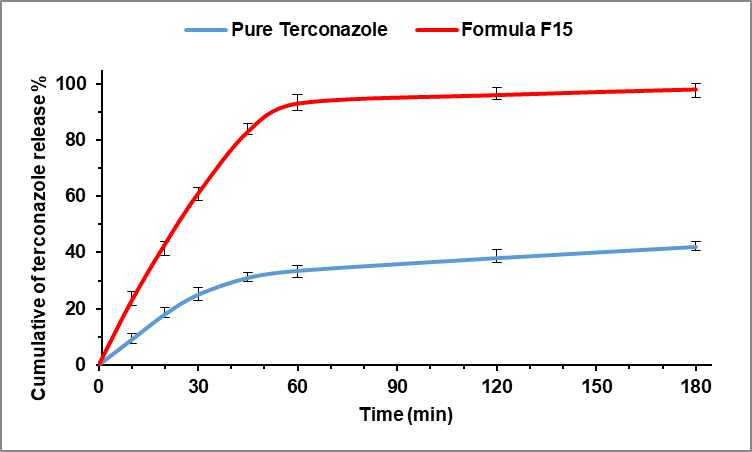
Fig. 6: Dissolution profile of Terconazole from micro-sponges formula F15 and pure Terconazole in PBS (pH 7.4) at 37±0.5 °C (all data expressed as mean±SD, n=3)
Kinetic modeling of drug release from micro-sponge
The release kinetics of Terconazole from micro sponge formulation F15 predominantly conform to the Hixon-Crowell release model, as indicated by the superior (R²) values obtained. The findings demonstrated that the release exponent "n" value for F15 micro-sponges is greater than 0.5 and less than 1, signifying a non-Fickian (anomalous) release profile. Consequently, it is posited that this formulation delivers its active ingredient through a mechanism involving a combination of diffusion and erosion [21, 32].
SEM study
The SEM micrograph of the optimized micro-sponges formula F15 is presented in fig. 7. The image shows that the particles formed were mostly spherical with a finely porous sponge-like structure. A rough texture topography containing fine particles of Terconazole crystals adhered to the porous surface is also visible [31].
Compatibility studies
FTIR analysis
FTIR spectra are presented in fig. 8: A-D. Terconazole spectrum shows characteristic absorption bands at 1514 cm-1 (C=C stretching of aromatic ring), 1273 cm-1 (aromatic ether stretching), 1215 cm-1 (C-O stretching), 1149 cm-1 (C-O-C stretching) and 650 cm-1(C-Cl bond) [38].
FTIR spectra of the physical mixture and formula F15 revealed no change in functional group peaks of Terconazole, confirming that there is no significant change in the chemical integrity of Terconazole as well as no chemical interaction or complexation between Terconazole and Eudragit-L100. The lack of interaction signals in FTIR spectra implies that the molecular structures of the drug and polymer remain intact, supporting the formulation's stability [20, 39].

Fig. 7: SEM image of micro-sponges formula F15
DSC analysis
The DSC thermograms are presented in fig. 9: A-D. The terconazole curve shows a sharp characteristic endothermic peak at 126 °C, corresponding to its melting point, which indicates that Terconazole is used in a pure crystalline state [9].
DSC curves of the physical mixture and formula F15 show the typical peak of Terconazole crystals. The disappearance of Eudragit-L100 signal in the formula F15 is primarily due to the lower amount of Eudragit-L100 used compared to Terconazole, as well as the amorphous nature of Eudragit-L100.
These results confirmed the compatibility between Terconazole and Eudragit-L100, which indicates that the micro-sponges production process did not change the nature of Terconazole in micro-sponges, as well as it suggests that the active ingredient remains unaltered and effective over time [8].
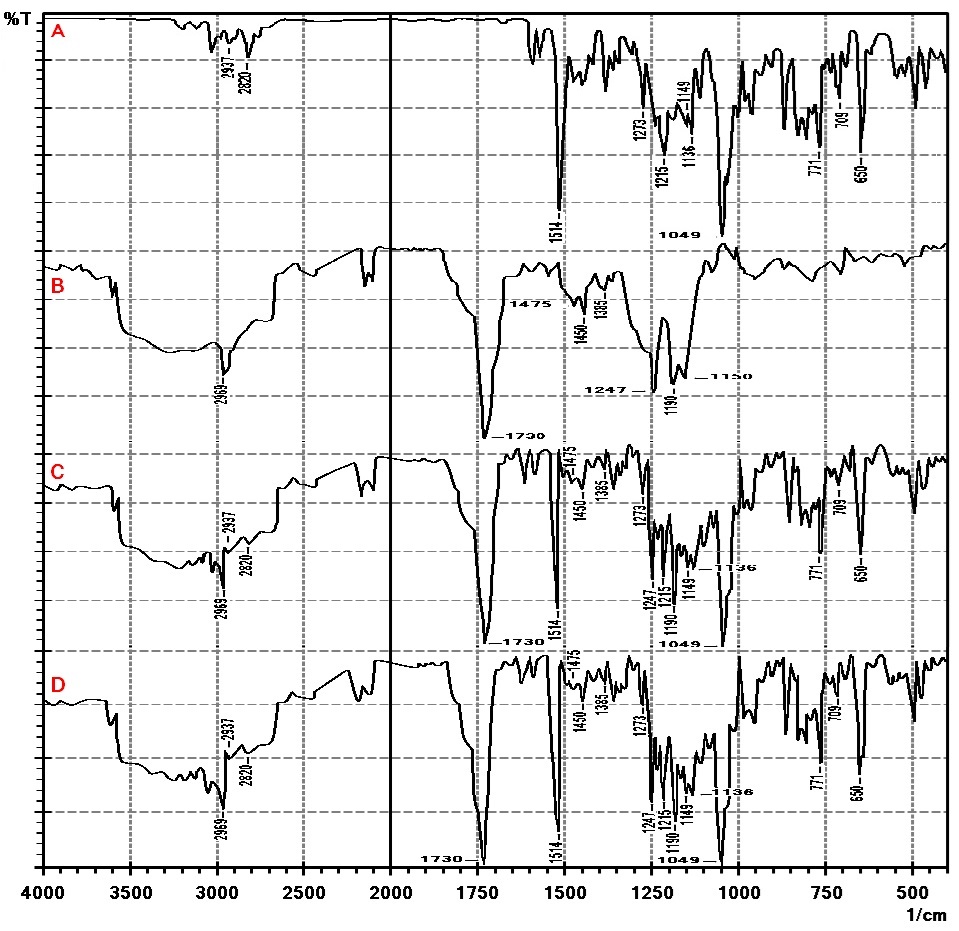
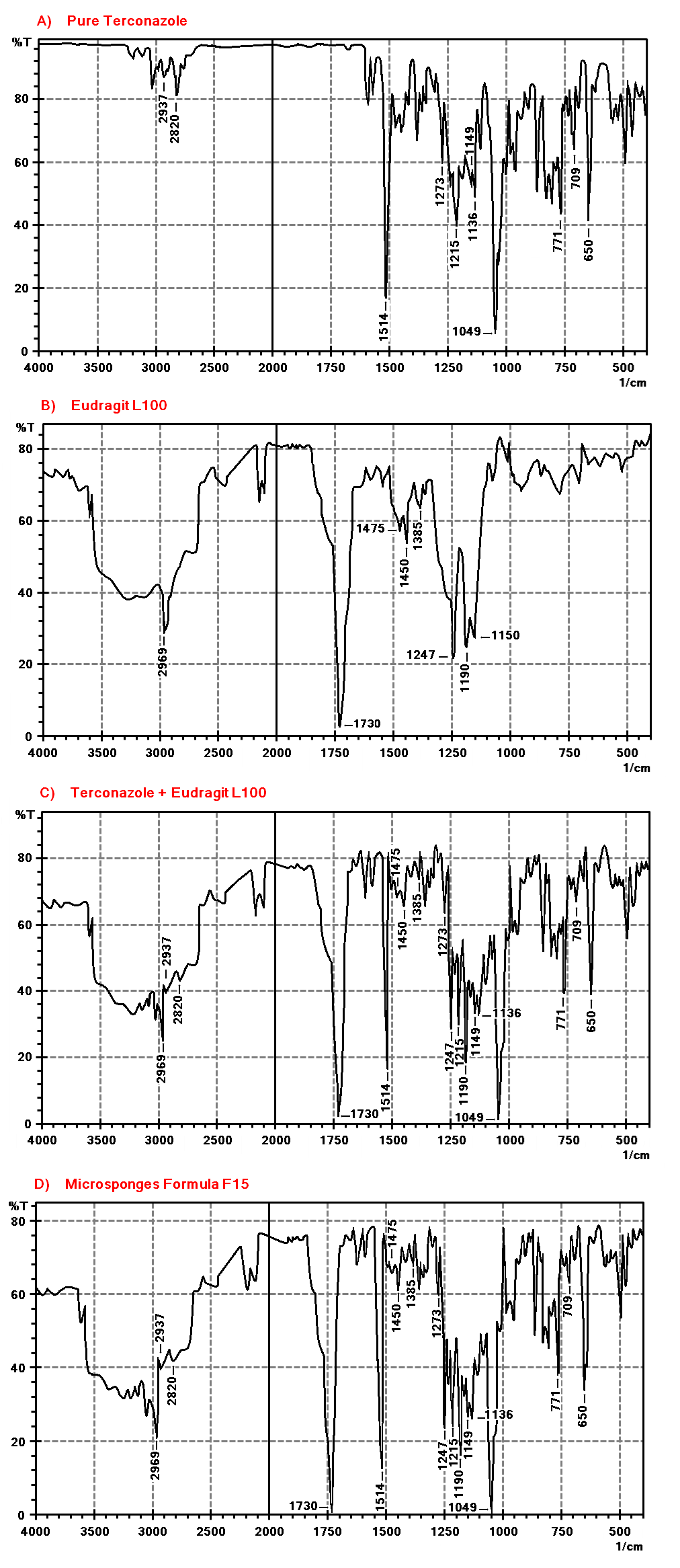



Fig. 8: FTIR spectra of A) Terconazole, B) Eudragit-L100, C) physical mixture, and D) formula F15
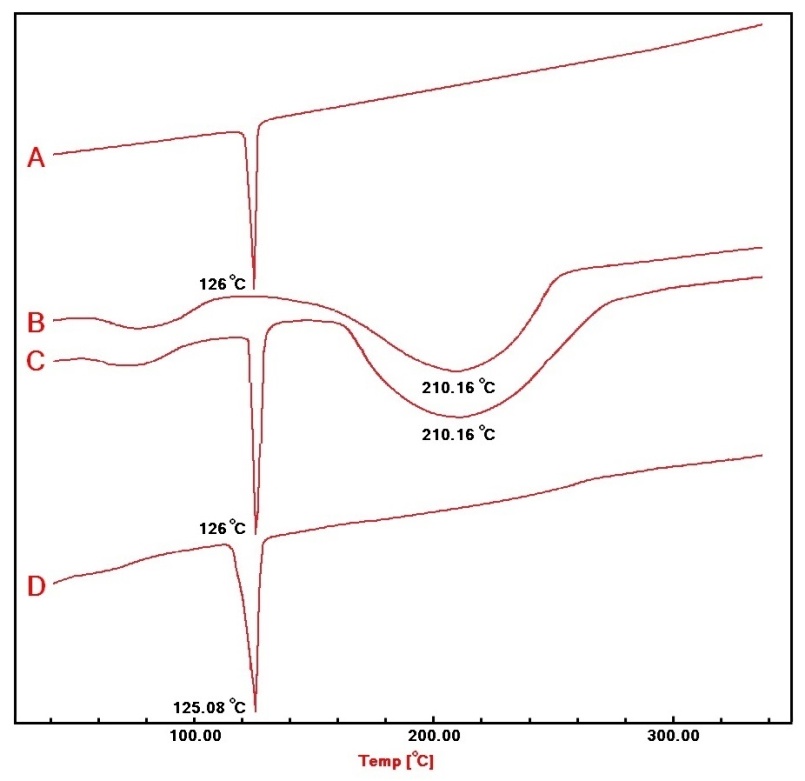
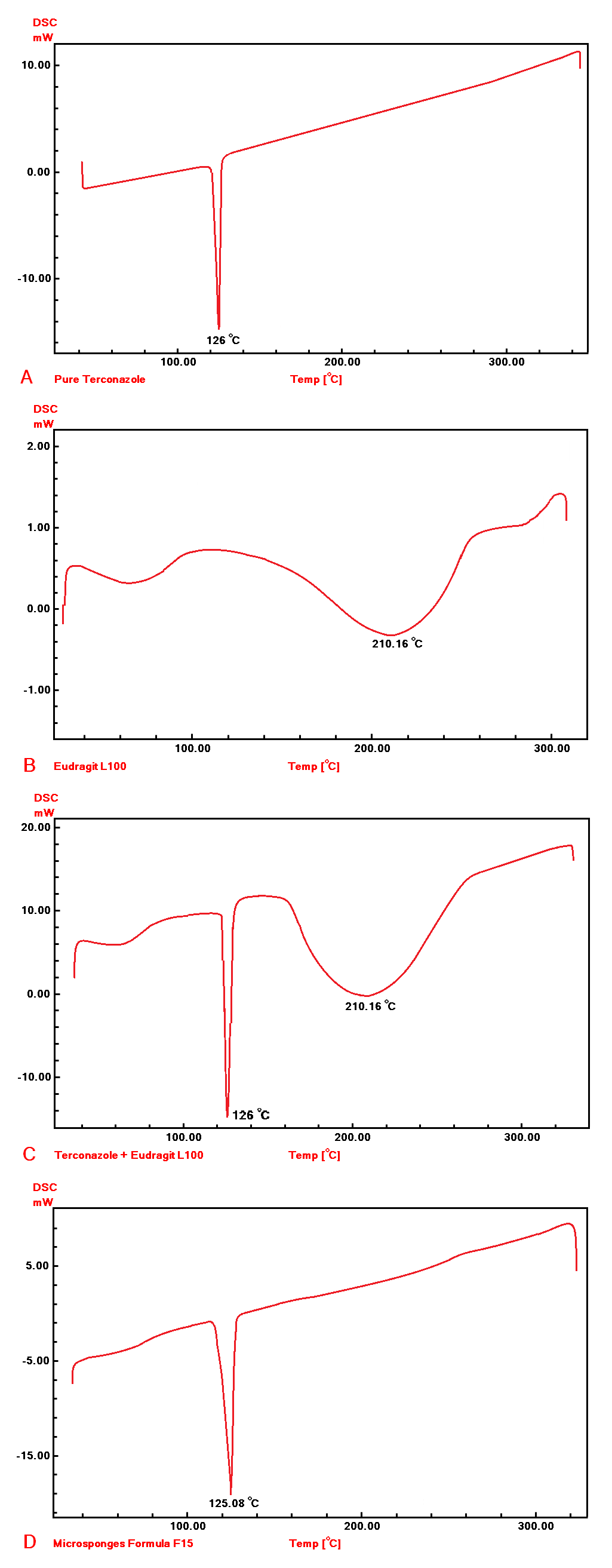
Fig. 9: DSC thermograms of A) Terconazole, B) Eudragit-L100, C) physical mixture, and D) formula F15
CONCLUSION
The investigation into Terconazole-loaded micro-sponges signifies a noteworthy progression in the formulation of drugs with poor aqueous solubility. The study validates the quasi-emulsion solvent diffusion technique as an effective method for micro-sponge preparation, with Eudragit-L100 identified as the most proficient polymer for enhancing Terconazole's solubility and dissolution rate. Optimization of formulation parameters notably improved micro-sponge characteristics, achieving a nine-fold increase in saturation solubility and superior dissolution profiles. The research establishes a foundational understanding for advancing ocular drug delivery systems and emphasizes the necessity of in vivo studies to assess the therapeutic efficacy of the developed micro-sponges.
FUNDING
Nil
AUTHORS CONTRIBUTIONS
Maha Mahdi Ali designed the research work, performed the experiment, and analyzed the results. Manar Adnan Tamer contributed to the preparation and revision of the manuscript and provided guidance. Saba Abdulhadi Jaber monitored the research outcomes and finalized the paper for submission.
CONFLICT OF INTERESTS
Declared none
REFERENCES
Bhalani DV, Nutan B, Kumar A, Singh Chandel AK. Bioavailability enhancement techniques for poorly aqueous soluble drugs and therapeutics. Biomedicines. 2022;10(9):2055. doi: 10.3390/biomedicines10092055, PMID 36140156.
Rocha B, DE Morais LA, Viana MC, Carneiro G. Promising strategies for improving oral bioavailability of poor water soluble drugs. Expert Opin Drug Discov. 2023;18(6):615-27. doi: 10.1080/17460441.2023.2211801, PMID 37157841.
Yousif NZ, Salman ZD. Microsponge as a strategy for effective drug delivery system. Al Mustansiriyah. J Pharm Sci. 2023;23(3):322-35. doi: 10.32947/ajps.v23i3.1051.
Biharee A, Bhartiya S, Yadav A, Thareja S, Jain AK. Microsponges as drug delivery system: past present and future perspectives. Curr Pharm Des. 2023;29(13):1026-45. doi: 10.2174/1381612829666230404082743, PMID 37013425.
Mandal S, Km Bhumika B, Kumar M, Hak J, Vishvakarma P, Sharma UK. A novel approach on micro sponges drug delivery system: method of preparations, application, and its future prospective. Ind J Pharm Edu Res. 2023;58(1):45-63. doi: 10.5530/ijper.58.1.5.
Mandal S, Km Bhumika B, Kumar M, Hak J, Vishvakarma P, Sharma UK. A novel approach on micro sponges drug delivery system: method of preparations application and its future prospective. Ind J Pharm Edu Res. 2023;58(1):45-63. doi: 10.5530/ijper.58.1.5.
Qureshi S, Alavi SE, Mohammed Y. Microsponges: development, characterization, and key physicochemical properties. Assay Drug Dev Technol. 2024;22(5):229-45. doi: 10.1089/adt.2023.052, PMID 38661260.
A Nief R, A Hussein A. Preparation and evaluation of meloxicam microsponges as transdermal delivery system. Iraqi J Pharm Sci. 2017;23(2):62-74. doi: 10.31351/vol23iss2pp62-74.
Zaghloul N, El Hoffy NM, Mahmoud AA, Elkasabgy NA. Cyclodextrin stabilized freeze dried silica/chitosan nanoparticles for improved terconazole ocular bioavailability. Pharmaceutics. 2022;14(3):470. doi: 10.3390/pharmaceutics14030470, PMID 35335847.
Zaghloul N, El Hoffy NM, Mahmoud AA, Elkasabgy NA. Cyclodextrin stabilized freeze dried silica/chitosan nanoparticles for improved terconazole ocular bioavailability. Pharmaceutics. 2022;14(3):470. doi: 10.3390/pharmaceutics14030470, PMID 35335847.
Jaklova Dytrtova J, Kovac I, Navratil T, Jakl M. Interactions of triazole terconazole with copper and zinc cations. Monatshefte Fur Chemie Chemical Monthly. 2023;154:1071-81. doi: 10.1007/s00706-023-03074-3.
Zaghloul N, Mahmoud AA, Elkasabgy NA, El Hoffy NM. PLGA-modified Syloid® based microparticles for the ocular delivery of terconazole: in vitro and in vivo investigations. Drug Deliv. 2022;29(1):2117-29. doi: 10.1080/10717544.2022.2092239, PMID 35838555.
Nemr A, El Mahrouk G, Badie H. A comprehensive review on bilosome: a nano vesicular drug delivery system to enhance bioavailability of the drug through different routes of administration. Bulletin of Pharmaceutical Sciences Assiut University. 2024;47(1):41-64. doi: 10.21608/bfsa.2023.233276.1888.
Biharee A, Bhartiya S, Yadav A, Thareja S, Jain AK. Microsponges as drug delivery system: past present and future perspectives. Curr Pharm Des. 2023;29(13):1026-45. doi: 10.2174/1381612829666230404082743, PMID 37013425.
Zaki Rizkalla CM, latif Aziz R, Soliman II. In vitro and in vivo evaluation of hydroxyzine hydrochloride microsponges for topical delivery. AAPS Pharm Sci Tech. 2011;12(3):989-1001. doi: 10.1208/s12249-011-9663-5, PMID 21800216.
Saad A, Sabri L. Study the variables affecting formulation of ethylcellulose-based microsponges loaded with clobetasol. Iraqi J Pharm Sci. 2023;32(1):225-34. doi: 10.31351/vol32issSuppl.pp225-234.
Abdalla KF, Osman MA, Nouh AT, El Maghraby GM. Microsponges for controlled release and enhanced oral bioavailability of carbamazepine. J Drug Deliv Sci Technol. 2021 Oct;65:102683. doi: 10.1016/j.jddst.2021.102683.
Rathnam G, Sangeetha G. Formulation and evaluation of dorzolamide hydrochloride microsponges loaded in situ gel for ocular administration. World J Bio Pharm Health Sci. 2024;18(2):332-42. doi: 10.30574/wjbphs.2024.18.2.0287.
Mohammed BS, Al Gawhari FJ. Preparation of posaconazole nanosponges for improved topical delivery system. Int J Drug Deliv Technol. 2022;12(1):8-14. doi: 10.25258/ijddt.12.1.2.
Abd Alhammid SN. Enhancement of the solubility and the dissolution rate of candesartan cilexetil using microsponge technology. Asian J Pharm Clin Res. 2018;11(9):385-90. doi: 10.22159/ajpcr.2018.v11i9.26816.
Ambikar RB, Bhosale AV. Development and characterization of diclofenac sodium loaded eudragit RS100 polymeric microsponge incorporated into in situ gel for ophthalmic drug delivery system. Int J Pharm Pharm Sci. 2021;13(9):63-9. doi: 10.22159/ijpps.2021v13i9.42405.
Jafar M, Ahmad Khan MS, Akbar MJ, AlSaihaty HS, Alasmari SS. Obliteration of H. pylori infection through the development of a novel thyme oil laden nanoporous gastric floating microsponge. Heliyon. 2024;10(8):e29246. doi: 10.1016/j.heliyon.2024.e29246, PMID 38638985.
Katta N, Madhava A, Sudheer P. Mesalamine loaded microsponges as a potential strategy for colon specific anti-inflammatory therapy: design and evaluation. Int J Pharm Investig. 2024;14(3):786-93. doi: 10.5530/ijpi.14.3.88.
Pawar AR, Shete NA, Jadhav PV, Deshmukh VK, Mehetre JS. Enhancement of aqueous solubility dissolution profile and oral bioavailability of pentoxifylline by microsponges. Pharm Fronts. 2021;3(4):e200-7. doi: 10.1055/s-0041-1740242.
Halder S, Behera US, Poddar S, Khanam J, Karmakar S. Preparation of microsponge drug delivery system (MSDDS) followed by a scale up approach. AAPS Pharm Sci Tech. 2024;25(6):162. doi: 10.1208/s12249-024-02874-y, PMID 38997615.
Elnaggar YS, Talaat SM, Bahey El Din M, Abdallah OY. Novel lecithin integrated liquid crystalline nanogels for enhanced cutaneous targeting of terconazole: development in vitro and in vivo studies. Int J Nanomedicine. 2016;11:5531-47. doi: 10.2147/IJN.S117817, PMID 27822033.
Tyagi G, Choudhary S. Preparation of flutrimazole micro sponge gel by quasi-emulsion solvent diffusion method. Asian J Pharm Res Dev. 2024;12(3):43-9. doi: 10.22270/ajprd.v11i3.1393.
Senarat S, Phaechamud T, Mahadlek J, Tuntarawongsa S. Fluid properties of various Eudragit® solutions in different solvent systems for periodontal pocket injection. Mater Today Proc. 2022;65(4):2399-406. doi: 10.1016/j.matpr.2022.05.527.
Othman MH, Zayed GM, Ali UF, Abdellatif AA. Colon specific tablets containing 5-fluorouracil microsponges for colon cancer targeting. Drug Dev Ind Pharm. 2020;46(12):2081-8. doi: 10.1080/03639045.2020.1844730, PMID 33135492.
Ali MM, Mahmod WS, Ali AM. Study the effect of polymers type on preparation and characterization of etoposide loaded gold nanoparticles. Int J Drug Deliv Technol. 2022;13(2):713-21. doi: 10.25258/ijddt.13.2.39.
Nikam A, Sahoo PR, Musale S, Pagar RR, Paiva Santos AC, Giram PS. A systematic overview of Eudragit® based copolymer for smart healthcare. Pharmaceutics. 2023;15(2):587. doi: 10.3390/pharmaceutics15020587, PMID 36839910.
Rajab NA, Jawad MS. Formulation and in vitro evaluation of piroxicam microsponge as a tablet. Int J Pharm Pharm Sci. 2016;8(2):104-14.
Eslavath RN, Bakshi V, Jadi RK. Formulation development and in vitro release studies of tenofovir-containing microsponges. Innosc Theranostics and Pharmacological Sciences. 2019;2(2):16-24. doi: 10.36922/itps.v2i2.545.
Chong Kook K, MI Jung K, Kyoung Hee O. Preparation and evaluation of sustained release microspheres of terbutaline sulfate. International Journal of Pharmaceutics. 1994;106(3):213-9. doi: 10.1016/0378-5173(94)90004-3.
Shirodkar S, Pissurlenkar R. Formulation and characterisation of cilnidipine microsponge loaded hydrogels for antihypertensive activity. Drug Deliv Lett. 2023;13(1):48-68. doi: 10.2174/2210303113666221207142644.
Thiresssa B, Prasad AR, Haroled PP. Formulation and evaluation of lornoxicam MDS using eudragit RS 100 and eudragit RSPO. Asian J Pharm Clin Res. 2018;11(10):217-21.
Song M, LI N, Sun S, Tiedt LR, Liebenberg W, DE Villiers MM. Effect of viscosity and concentration of wall former emulsifier and pore inducer on the properties of amoxicillin microcapsules prepared by emulsion solvent evaporation. Farmaco. 2005;60(3):261-7. doi: 10.1016/j.farmac.2004.11.009, PMID 15784247.
Mohsen AM. Cationic polymeric nanoparticles for improved ocular delivery and antimycotic activity of terconazole. J Pharm Sci. 2022;111(2):458-68. doi: 10.1016/j.xphs.2021.09.019, PMID 34547306.
Kumari P, Mishra SK. A comprehensive review on novel microsponges drug delivery approach. Asian J Pharm Clin Res. 2016;9(1):25-30.