
Int J App Pharm, Vol 17, Issue 3, 2025, 322-327Original Article
BIOCOMPATIBILITY OF ASTAXANTHIN GEL: CHARACTERIZATION, ANTIOXIDANT, AND SKIN SAFETY ASSESSMENT
DWI ANDRIANI1*, AGNI F. PARGAPUTRI1, SYAMSULINA REVIANTI1, KRISTANTI PARISIHNI1, ANGELICA KRESNAMURTI2, LISA SOEGIANTO3
1Department of Oral Biology, Faculty of Dentistry, Universitas Hang Tuah, Surabaya, East Java-60111, Indonesia. 2Faculty of Pharmacy, Universitas Hang Tuah, Surabaya, East Java-60111, Indonesia. 3Faculty of Pharmacy, Widya Mandala Catholic University, Surabaya, East Java-60111, Indonesia
*Corresponding author: Dwi Andriani; *Email: dwi.andriani@hangtuah.ac.id
Received: 10 Dec 2024, Revised and Accepted: 14 Feb 2025
ABSTRACT
Objective: Astaxanthin is a natural compound that possesses a strong antioxidant activity. This substance demonstrates huge potential for both maintenance and therapy. Previous studies suggested this substance could be used as gel therapy for oral cavity ulcers. This research aims to explore the characterization and antioxidant properties of gel formulations or conducted skin safety assessments to evaluate the biocompatibility of astaxanthin gel.
Methods: Characterization assessment of astaxanthin gel at concentrations of 0.1%, 0.5%, 1%, and 5%, measuring material viscosity, pH, homogeneity, spreadability, adhesiveness, and organoleptic qualities. Antioxidant assessment was carried out in vitro using the DPPH technique by observing the value of antioxidant inhibition (IC50). The acute skin sensitivity and irritation tests were performed in vivo, and the effects were for 14 days. The result of observing clinical changes and the death of rats were noted. The irritation test is observed from the redness on the skin and scored using the Draize-FHSA scoring system and primary irritation index (PII).
Results: Astaxanthin gel 0.1%, 0.5%,1%, and 5% have a viscosity value of 3250-3500, pH 7.7-7.85, homogeneous, with spreadability of 3.1-3.5 cm. Astaxanthin gel 1% has the maximum viscosity, pH range, and spreadability with adhesion duration is 3 min and 7 seconds. The IC50 test showed a value of<50 ppm. The gel exhibits no alterations in the skin, fur, eyes, or behavior of rats. The redness and edema score results in all groups are zero, indicating no irritation.
Conclusion: The outcomes of this experiment show that 1% astaxanthin gel has the highest antioxidant impact compared to other concentrations. The acute skin toxicity and irritation tests revealed that this gel is acceptable in all concentrations.
Keywords: Astaxanthin, Antioxidant, Characterization gel, Acute dermal toxicity, Edema test
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijap.2025v17i3.53399 Journal homepage: https://innovareacademics.in/journals/index.php/ijap
INTRODUCTION
The notion of biocompatibility has gained significant recognition, particularly in the domains of medicine and dentistry. Material is biocompatible and must not negatively impact the recipient [1]. The dental material we use for oral wound healing therapy is in gel form. Topical gels are adaptable owing to their capacity to integrate solid and liquid elements. They must be transparent, homogeneous, inert, non-adhesive, stable, non-irritating, possess appropriate viscosity, and exhibit antibacterial properties, assuring no interaction with other components [2]. Given the numerous benefits of this formulation, it is prudent to construct a gel with astaxanthin as the primary ingredient, owing to its notable antioxidant and anti-inflammatory properties.
Astaxanthin, a xanthophyll derivative of carotenes, is a prominent carotenoid group that provides several health benefits due to its antioxidant capabilities and interaction with cell membranes. Astaxanthin is a carotenoid generated by bacteria, microalgae, and yeast, primarily by Haematococcus pluvialis [3]. Astaxanthin exhibited stronger antioxidant activity rather than such carotenoids as lycopene, lutein, and α,β-carotene. Xanthophylls and carotenes are two important types of carotenoids that provide various health benefits [4]. It exerts advantageous benefits on human wellness, such as reducing oxidative stress, inhibiting low-density lipoprotein (LDL) oxidation, enhancing immunological response, and possessing anti-inflammatory and anti-aging qualities. Moreover, due to its primary function as a scavenger of ROS, or reactive oxygen species, and its antioxidant capabilities, astaxanthin is tenfold more potent compared to other defenders such as canthaxanthin, lutein, and zeaxanthin [5].
Research on the benefits of astaxanthin has been extensively studied. According to Aripin et al. research on the administration of astaxanthin, which is generated by Haematococcus pluvialis species, a concentration of 0,5% and 1% astaxanthin gel results in decreased ulcer diameter in traumatic ulcer rats model [6]. A previous study conducted by Andriani et al. found Astaxanthin gel 0.5% and 1% had a therapeutic impact in raising the number of neutrophils, macrophages, and fibroblasts on day 3, as well as fibroblast and collagen density on day 7 of the healing phase of an oral traumatic ulcer [7]. Nonetheless, we have not yet performed safety testing of the gel on the dermis. It is now obligatory to guarantee public confidence and detect risk issues. Consequently, toxicological screening is crucial for determining the possibility of intoxication [8].
Understanding astaxanthin's significant antioxidant properties and the fact that antioxidants can neutralize free radicals and reduce the possibility of oxidant damage, an antioxidant test utilizing astaxanthin incorporated in gel form is required. Antioxidant testing in gel formulations is essential, and toxicity testing is also vital to assess the safety of the gel for therapeutic usage. Therefore, this study was conducted to evaluate the characterization of the gel and measure the antioxidant value, acute skin sensitivity, and irritation test of astaxanthin gel with concentrations of 0.1%, 0.5%, 1%, and 5%.
MATERIALS AND METHODS
This research is an experimental laboratory (with in vitro and in vivo tests) with a one-shot case study design (post-test only group). The in vitro testing in this study is the antioxidant test using the DPPH method. In contrast, the in vivo test is conducted to assess the toxicity of the astaxanthin gel formulation at concentrations of 0.1%, 0.5%, 1%, and 5%. Animal handling is carried out following ethical guidelines for animal research. Ketamine and xylazine are used to anesthetize the rat both before its termination and before its fur is trimmed. This research has received ethical approval from the Research Ethics Committee of the Faculty of Dentistry, Hang Tuah University (No. EC/080/KEPK-FKGUHT/IX/2024).
Materials
Astaxanthin derived from green microalgae Haematococcus pluvialis in powder form, obtained from Indonesia (PT. Evergen). The powder was placed into plastic food storage bags weighing around 250g and frozen at-20 °C till usage.
Gel preparations for test
The gel base was made based on Sagar et al. (2020) [9]. Weigh the HPMC, add it to water, and stir until it dissolves and a gel forms. Then, add propylene glycol. Weigh the hyaluronic acid, dissolve it in water, and mix it into the gel. Weigh the astaxanthin and incorporate it into the gel divided into 0.1%, 0.5%, 1% and 5%. Dissolve the methylparaben in glycerin, then add it to the gel. Finally, check the characterization of the gel. The last stage is that the gel is packed in a tightly closed container and stored at room temperature.
Characterization of gels
Characterization evaluation of astaxanthin gel at concentrations of 0.1%, 0.5%, 1%, and 5% was performed with five replications for each concentration. The assessments comprise:
Assessment of viscosity of the gel substance utilizing a viscometer (Ametek Brookfield, USA). Fifty milliliters of gel was introduced into a beaker, after which the viscometer spindle was positioned at the gel's center and activated at a speed of 100 rpm. The scale measurement is recorded once the rotation is stable.
pH assessment utilizing a pH meter (Amtast AMT20, Indonesia). Fifty milliliters of gel is deposited into a beaker, after which the pH meter's probe is positioned in the gel's center and activated; the pH measurement of the gel is subsequently documented.
Assessments of homogeneity, spreadability, and adhesion through visual examination of the gel formulation, focusing on consistent coloration and the absence of particulates. 0.5 g of gel was applied to a glass microscope slide and permitted to rest for 3 min. The spreadability was assessed both transversely and longitudinally and subsequently averaged. Concurrently, adhesion was evaluated by positioning 0.5 g of gel on a glass slide, overlaying it with another glass slide, and exerting a 1 kg weight for 3 min. The adhesion assessment was predicated on the duration necessary for the separation of the two glass slides.
The organoleptic evaluation included assessing the gel's color and odor. The color test is performed visually, whereas the odor test is performed by olfactory evaluation of the gel.
Antioxidant test
Antioxidant assay against free radicals utilizing the technique known as the DPPH (2,2-diphenyl-1-picrylhydrazyl) method, comprising five sample duplicates for each test. The antioxidant activity was assessed by generating a 0.06 mmol solution of 2.2-diphenyl-1-picrylhydrazyl (DPPH) and then combining DPPH with methanol. The test solution was produced by soaking 10 mg of each specimen in methanol. The material was thereafter serially diluted with methanol p. a. Aquercetin solution served as the standard. Subsequently, 3.5 ml of DPPH solution was incorporated into 1 ml of methanol. The absorption value of the solution was quantified with a UV-Vis spectrophotometer at a wavelength of 517 nm. The antioxidant activity of the sample was assessed by introducing 4 ml of DPPH solution to 1 ml of each sample concentration. The solution was subsequently assessed for absorbance at the highest point wavelength. The absorbance value is afterward input into the formula for calculating the % inhibition of free radicals. IC50 Calculation to determine the sample concentration that can inhibit 50% of DPPH free radicals. This value is calculated by putting the computation results into linear regression [5].
Acute dermal test
This test is based on OECD (2017) Test Guideline 402 for assessing acute skin toxicity using up to 3 Rats for each test. The treatment and control doses were evaluated on eighteen healthy (three per group), male wistar rats to avoid hormonal intervention, weighing 150-200g in each group. Before being treated, the rats were acclimatized for 7 d. Rats to be treated had their back fur shaved in 3x3 cm sections with a shaver and rested about an entire day before starting treatment. The test substance was administered by pouring it over shaved flesh. To prevent the liquid from evaporating, the treated skin region was covered with plastic, and the outermost layer was wrapped in gauze and plastered. Behavioral changes and physiological responses were observed at one, two, three, and four hours. Behavioral modifications and physiological responses were recorded at 1 h, 2 h, 3 h, 4 h, and 24 h following treatment. Throughout the 24 h monitoring time, the plaster cast was removed, the skin was washed with water, and the alterations to the skin were documented. Monitoring was conducted until the fourteenth day post-treatment by quantifying the number of deceased rats and documenting clinical complaints [10].
Irritation test
The test was carried out on fifteen healthy male Wistar rats weighing 150-200g (three per group). The fur on the animal's dorsal half was carefully shaved in 3x3 cm sections about 24 h before testing began. The assessment material was administered to the skin of the subject animal, with an untreated area functioning as a control. The test material was administered to a small skin region (about 6 cm²) and covered with gauze, secured with non-irritating tape. The test material was initially administered on gauze, which was subsequently positioned on the skin. Throughout the exposure duration, the gauze was maintained in gentle contact with the skin using a suitable semi-occlusive dressing. Animals were observed for 14 d post-patch removal to evaluate the reversibility of effects. The animals were evaluated for erythema and swelling and scored at 60 min, 24 h, 48 h, and 72 h post-patch removal. Following the initial test on one animal, the test location was assessed immediately following the patch's removal. The scoring utilizes the Draize-FHSA Scoring system to assess redness and swelling scores at 24 h, 48 h, and 72 h, which are subsequently gathered and averaged to determine the primary irritation index (PII) [11]. Irritation results based on classification according to the primary irritation index (PII) [12].
RESULTS AND DISCUSSION
The previous study evaluated the anti-inflammatory properties of astaxanthin gel [6], and the current formulation we have refined for enhanced stability. The combination of mucoadhesive polymeric substances, including HPMC, HPEC, Carbomer 934, Na-CMC, benzocaine, gum tragacanth, and chitosan, serves in the formulation of medicament gels for the oral mucosa to extend the medication's contact duration that can increase formation of the protective layer, and giving moisture effects [13]. This characterization test is performed to assess the formulation by analyzing viscosity, homogeneity, spreadability, adhesiveness, pH to ascertain the gel's acidity and organoleptic qualities to verify the color and odor are acceptable to individuals.
Table 2 shows the results of the characterization assessment of astaxanthin gel. The table indicates that the viscosity of the gels ranges from 3250-3500 cP (Centipoise) and 1% astaxanthin gel exhibits the maximum viscosity. Viscosity is a material feature of the formulation related to the gel's thicknesses. The greater viscosity of a formulation impedes medication dispersion [14]. Nevertheless, in terms of its value, the gel maintains a comparable viscosity and exhibits a spreading ability with a diameter range that is nearly the same. In-situ gel formation, influenced by temperature, pH, and UV irradiation, is attracting interest for its ability to decrease daily dosages, enhance biocompatibility, and offer cost-effectiveness [15].
The pH measurements range from 7.5 to 7.85 and it approached neutral pH. The best result of this study at 1% and 5% astaxanthin gel. The physiological pH of the oral cavity in healthy individuals varies from 6.8 to 7.8 [16]. The gel analysis outcomes indicate that the 1% and 5% astaxanthin gels are deemed reliable, although the 0.1% and 0.5% amounts exceed that threshold marginally. All sample gels exhibit homogeneity with a spreadability range of 3.1-3.5 cm, with 0.5% astaxanthin the widest, whereas optimal adhesion is achieved with 1% astaxanthin, lasting for 187 seconds or 3 min and 7 seconds. The mucoadhesion measurement is essential for the formulation and topical administration of the product to the oral mucosa. The effectiveness of gel formulation may be improved by increasing the stability of the formulation. However, quantitative measurement is challenging due to the reduced adhesive bonding strength [17]. The incorporation of astaxanthin results in a modification of color and the distinctive odor associated with astaxanthin and it remains appropriate.
Table 1: Draize-FHSA scoring system and primary irritation index (PII) [11, 12]
| Draize-FHSA scoring system | |
| Erythema and eschar formation | Score |
| No erythema present | 0 |
| Minimal erythema (seldom noticeable) | 1 |
| Finely delineated erythema | 2 |
| Moderate to severe erythema | 3 |
| Severe erythema (intense redness) to minimal eschar formation (deep tissue damage) | 4 |
| Edema formation | Score |
| No edema present | 0 |
| Minimal edema (seldom noticeable) | 1 |
| Slightly edema (boundaries of the area distinctly elevated) | 2 |
| Moderate edema (around 1 mm elevation) | 3 |
| Severe edema (elevated over 1 mm and surpassing the region of exposure) | 4 |
| Primary irritation index (PII) and classification of skin irritancy: | |
| Classification | PII |
| Insignificant | 0–0.4 |
| Slighly irritation | 0.5–1.9 |
| Moderate irritation | 2–4.9 |
| Severe irritation | 5–8 |
Table 2: Characterization of astaxanthin gel result
| Sample number | Sample size | Sample | Viscosity (cP) | pH | Homogeneity | Adhesion (s) | Spreadability (cm) | Color | Odor |
| Mean±SD | Mean±SD | Mean ±SD | Mean ±SD | ||||||
| I | 5 | Astaxanthin 0.1% | 3250±53.86a | 7.9±0.05a | Homogeneous | 63± 2.17a | 3.2±0.03a | Transparent with slight red | Slight fishy |
| II | 5 | Astaxanthin 0.5% | 3400±146.80a | 7.8±0.09a | Homogeneous | 158±1.58b | 3.5±0.04b | Transparent with slight red | Slight fishy |
| III | 5 | Astaxanthin 1% | 3500±70.00b | 7.7±0.05b | Homogeneous | 187±1.79c | 3.1±0.04a | Transparent with midly red | Slight fishy |
| IV | 5 | Astaxanthin 5% | 3300±50.87a | 7.7±0.03c | Homogeneous | 167±1.58d | 3.3±0.04c | Transparent with midly red | Slight fishy |
| p-Value (One-way ANOVA) | 0.009* | 0.038* | - | 0.000* | 0.000* | - | - |
Describe as: cP = centipoise, s = second, cm = centimetre, *statistically significant at α=0.05, abcdThe identical superscript denotes no distinction between groups (Posthoc LSD), n=3
A correlation exists between antioxidants and the process of wound healing. A study indicated an elevation in antioxidants in patients with oral cavity ulcers, namely Recurrent Aphthous Stomatitis (RAS) [18]. An antioxidant substance is required to counteract the oxidant; thus, this study utilized astaxanthin synthesized in gel form as the antioxidant. Numerous antioxidant assessment methods, including the 2.2-diphenyl-1-picrylhydrazyl (DPPH) assay, the 2.2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) assay, and the dichloro-dihydro-fluorescein diacetate (DCFH-DA) assay. We performed the DPPH assay approach to validate our antioxidant activity [19]. Astaxanthin exhibits antioxidant properties that are sixty-five times stronger than those of vitamin C and fifty times more powerful than vitamin E, as well as other carotenoids like coenzyme Q [20]. Consequently, this study mentioned vitamin C as a standard.
Table 3 and fig. 2 show the results of the antioxidant test with astaxanthin gel. According to the results of the antioxidant activity tests, the IC50 value for sample 2 is 0.96059 ppm, sample 3 is 0.6739 ppm, sample 4 is 0.19466 ppm and sample 5 is 0.63831 ppm. Jumina et al. (2019) categorize antioxidant strength into four tiers: highly potent (IC50<50 μg/ml), potent (IC50: 50–100 μg/ml), intermediate (IC50: 101–150 μg/ml), and weak (IC50: 250–500 μg/ml) [21] As a consequence, the preparation of astaxanthin gel is in the<50 ppm range, indicating that it could potentially prove to be a highly potent antioxidant. Our result showed astaxanthin gel 1% has the best antioxidant activity compared to the other concentrations due to the smallest number of IC50. Antioxidant activity is quantified by the IC50 value (Inhibition Concentration 50). A smaller IC50 value indicates more free radical scavenging activity [22]. According to studies by Andriani et al. (2024), the 1% astaxanthin gel has the most effective anti-inflammatory properties [7]. This result can be linked to the higher antioxidant levels identified in this study's results.
Table 3: IC50 value antioxidant testing using the DPPH method
| Sample number | Sample | Regression equation | IC50 (ppm) |
| I | Vitamin C | Y=8.0201 X+9.0272 R = 0.9998 |
5.10875 |
| II | Astaxanthin 0.1% | Y = 51.662 X+0.3742 R = 0.7817 |
0.96059 |
| III | Astaxanthin 0.5% | Y = 71.241 X+1.9905 R= 0.664 |
0.6739 |
| IV | Astaxanthin 1% | Y = 251.98 X+0.9488 R= 0.9123 |
0.19466 |
| V | Astaxanthin 5% | Y = 74.603 X+2.3799 R= 0.1961 |
0.63831 |
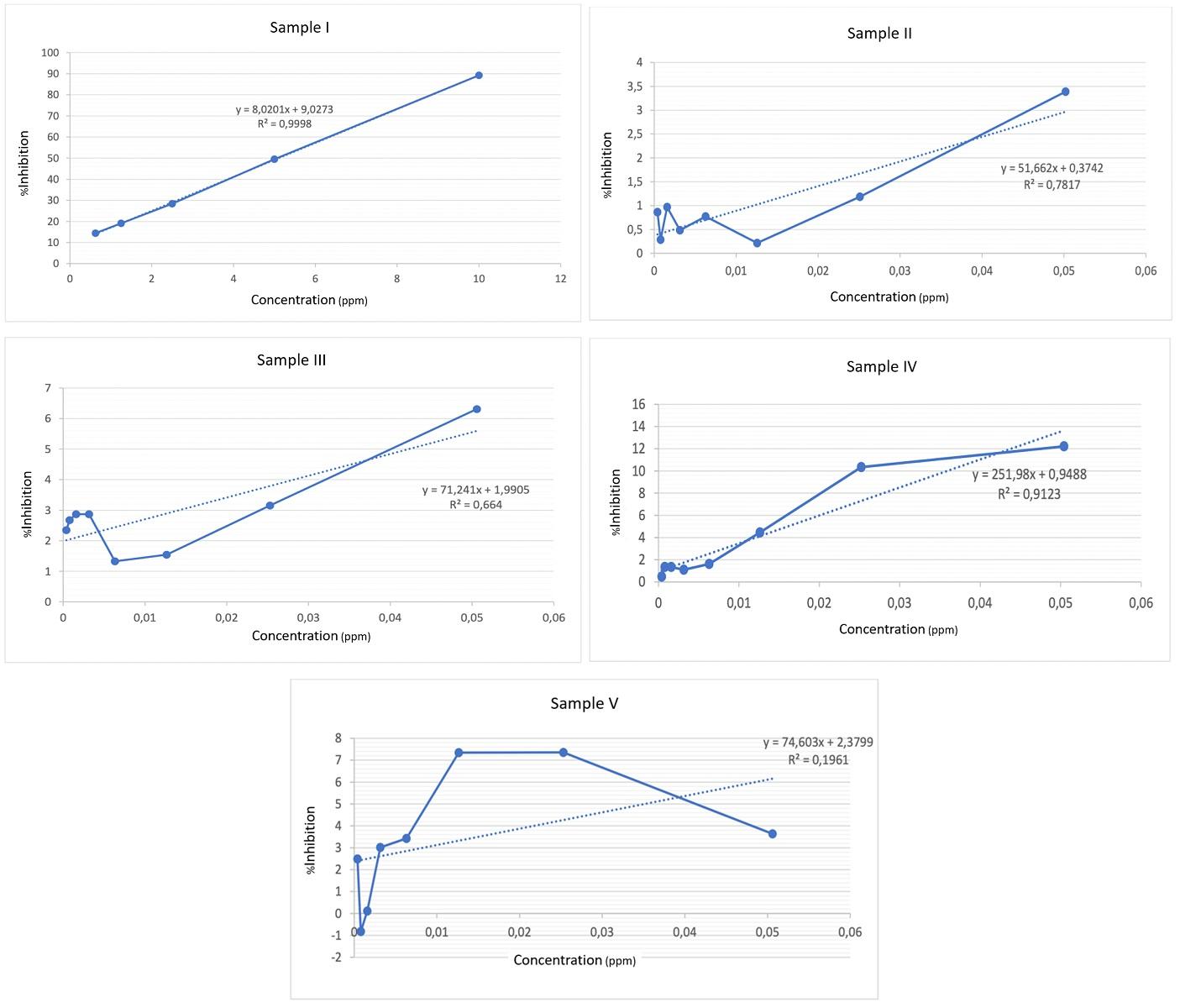
Fig. 2: Graph of linear regression for antioxidant testing
Crude Astaxanthin from green alga Haematococcus pluvialis extract proved to have antioxidant and hepatoprotective activity [23]. The high antioxidant content reduces oxidative stress, a condition where oxidants outweigh antioxidants, leading to molecular damage and various diseases like diabetes mellitus [24]. Elevated ROS production in diabetic wounds leads to dysfunctional wound healing, elevated levels of cell senescence and death, lipid peroxidation, oxidative stress, protein alteration, and DNA damage are all symptoms of this condition [25]. Astaxanthin mechanism as an antioxidant stimulates the Nrf2 or HO-1 antioxidant pathway by producing modest levels of reactive oxygen species (ROS) and also regulates the antioxidant enzymes catalase, superoxide dismutase 2, and glutathione peroxidase 1, as well as proteins targeting Nrf2 HO-1 [26]. Astaxanthin’s benefits may be linked to the Nrf2 signaling pathway, which activates antioxidant enzymes by nuclear translocation, mitigating oxidative stress, reducing oxidative stress, and protecting cells from damage [27]. Astaxanthin exerts antioxidant effects on cells via this route, which can facilitate the acceleration of wound healing.
Table 4: In vivo acute dermal toxicity results
| Group | Sample size | Material | Skin, fur, eyes | Behaviour | Mean± SD body weight (g) | Mortality | Result | |
| Day-0 | Day-4 | |||||||
| Healthy Control (C1) | 3 | No Material | Normal | No Change | 163.67±5.51 | 183.33±11.02 | No | Non-toxic |
| Gel base control (C2) | 3 | Gel Base | Normal | No Change | 154.33±5.13 | 180±15.39 | No | Non-toxic |
| Treatment 1 (T1) | 3 | Astaxanthin 0.1% Gel | Normal | No Change | 158.33±1.53 | 186±12.29 | No | Non-toxic |
| Treatment 2 (T2) | 3 | Astaxanthin 0.5% Gel | Normal | No Change | 159.33±0.58 | 182±15.39 | No | Non-toxic |
| Treatment 3 (T3) | 3 | Astaxanthin 1% Gel | Normal | No Change | 168.00±5.00 | 191±10.41 | No | Non-toxic |
| Treatment 4 (T4) | 3 | Astaxanthin 5% Gel | Normal | No Change | 168.67±7.51 | 187.67±10.79 | No | Non-toxic |
N=6
The results of the acute skin sensitivity are shown in table 4. There is no change in skin, fur, eyes, or behavior in rats after treatment. Weight gain was recorded on day 14 and the rats survived. This indicates that the formulation of this gel is non-toxic and safe to use. The results of the irritation testing based on the scoring from the Draize-FHSA Scoring system and primary irritation index, our research results show no erythema (0) and no edema (0) at 1, 24, 48 and 72 h, indicating that all formulations of this gel are safe and does not irritate the skin.
Cosmetic chemicals with less harmful or harmless effects are not permitted for industrial applications. Industrial safety assessors must evaluate skin and mucous membrane irritation, sensitization potential, and genotoxicity [28]. One of the skin sensitivities caused by medication is allergy contact dermatitis. Allergy contact dermatitis is a skin hypersensitivity reaction triggered by exposure to external chemicals or physical agents, manifesting in acute, subacute, and chronic forms, with aute presentations marked by erythema, edema, and crusting [29]. Our research results show alterations in skin, behavior, and body weight and no mortality in the rats, suggesting that the gel is non-toxic. Acute dermal toxicity tests and irritation tests were conducted to ensure the safety of astaxanthin gel as a candidate for oral ulcer healing therapy, and our results verify that this gel is safe.
Herbal products and formulas are widely used in various fields due to their safety or low toxicity, with the use of oral toxicities being critical for not only determining potential dosages but also assessing potential clinical effects [30, 31]. The presence of antioxidant effects and the safety of this astaxanthin gel formulation make 1% astaxanthin gel recommended for oral cavity wounds, especially in the condition of diabetes mellitus. Preclinical evaluations regarding the characterization and safety of the substance have been performed. Additional testing is required to assess the material's stability and antibacterial properties to evaluate its durability and effectiveness. Clinical trials of the substance are necessary to assess its potential as a therapeutic agent for the treatment of oral cavity ulcers.
CONCLUSION
According to this research, the 1% astaxanthin gel provides a great characterization of the gel and the highest level of antioxidants. Astaxanthin gel is safe and causes no edema or erythema. This gel is potentially applied as an antioxidant for oral cavity wound treatment. Further investigation is needed to determine the effect of this gel on wound healing.
ACKNOWLEDGMENT
The Ministry of Research, Technology, and Higher Education of Indonesia funds this research.
AUTHORS CONTRIBUTIONS
Dwi Andriani contributed to developing concepts, identifying issues, research methodologies, and project management. Agni F. Pargaputri participated in data analysis, authored the review, and performed editing. Syamsulina Revianti and Kristanti Parisihni contributed to the article's oversight, data verification, and manuscript preparation. Angelica Kresnamurti and Lisa Soegianto contributed to data curation, visualization, and analysis.
CONFLICT OF INTERESTS
We declare no conflict of interest
REFERENCES
Vazquez TA, Grube BD. Biocompatibility in dentistry: amini review. Mod Res Dent. 2021 Jun 15;6(4):49-54.
Sharma MU, Arjariya S, Chouksey R, Sharma N. A review: formulation and evaluation of pharmaceutical gel. J Pharm Neg Results. 2022 Oct 3:1344-62.
Davinelli S, Nielsen ME, Scapagnini G. Astaxanthin in skin health repair and disease: a comprehensive review. Nutrients. 2018;10(4):522. doi: 10.3390/nu10040522, PMID 29690549.
Nurdianti L, Setiawan F, Indra AR, Mudhakir D, Anggadiredja K. Nanoemulsion based gel formulation of astaxanthin for enhanced permeability: potential as a transdermal drug delivery system. Int J Pharm Sci Rev Res. 2018;52(2):55-9.
Tejaputri NA, Arsianti A, Qorina F, Fithrotunnisa Q. Phytochemical analysis and antioxidant properties by dpphradical scavenger activity of ruelliabrittonianaflower. Int J Appl Pharm. 2019;11(6):24-8.
Aripin AN, Andriani D, Ashrin MN. The effect of astaxanthin (Haematococcus pluvialis) on diameter measurement in traumatic ulcer model. J Kedokteran Gigi Univ Padjadjaran. 2022;34(3):209-15.
Andriani D, Pargaputri AF, Revianti S, Parisihni K, Ashrin MN. Potency astaxanthin (Haematococcus pluvialis) as anti-inflammatory gel to oral traumatic ulcer. Mal J Med Healthsci. 2024 Jun;20 Suppl 5:5-10.
Pal D, Halder A, Das D, Sekhar Maji HS. Promising pharmacological activities of Ziziphus herbal extract and its formulations. Res J Pharm Technol. 2023;16(7):3300-4. doi: 10.52711/0974-360X.2023.00544.
Sagar H, Jha KK, Sharma S, Kumar A. Therapeutic study of garlic gel formulation for tongue ulcer healing. J Adv Pharmacogn. 2020;1(1):9-29.
Mielke H, Strickland J, Jacobs MN, Mehta JM. Biometrical evaluation of the performance of the revised OECD test guideline 402 for assessing acute dermal toxicity. Regul Toxicol Pharmacol. 2017 Oct;89:26-39. doi: 10.1016/j.yrtph.2017.07.007, PMID 28709685.
Barel AO, Paye M, Maibach HI. Handbook of cosmetic science and technology. 4th ed. Boca Raton: CRC Press; 2014. p. 123-5.
Han J, Lee GY, Bae G, Kang MJ, Lim KM. Chemskin reference chemical database for the development of an in vitro skin irritation test. Toxics. 2021;9(11):314. doi: 10.3390/toxics9110314, PMID 34822705.
Wahyuni IS, Sufiawati I, Shafuria A, Nittayananta W, Levita J. Formulation and evaluation of mucoadhesive oral care gel containing Kaempferia galanga extract. Pharmaceutics. 2024 Mar 19;16(3):421. doi: 10.3390/pharmaceutics16030421, PMID 38543315.
Safitri FI, Nawangsari D, Febrina D. Overview: application of carbopol 940 in gel. Adv Health Sci Res. International Conference on Health and Medical Sciences (AHMS 2020). Vol. 34; 2021 Jan 27. p. 80-4.
Gupta CH, Juyal VI, Nagaich UP. Formulation and optimization of thermosensitive in-situ gel of moxifloxacin hydrochloride for ocular drug delivery. Int J Appl Pharm. 2018;10(3):123-30.
Loke C, Lee J, Sander S, Mei L, Farella M. Factors affecting intra oral pH a review. J Oral Rehabil. 2016 Oct;43(10):778-85. doi: 10.1111/joor.12429, PMID 27573678.
Rençber S, Kose FA, Karavana SY. Development of novel mucoadhesive gels containing nanoparticle for buccal administration of dexamethasone. Braz J Pharm Sci. 2022 Nov 4;58:e20041.
Jesija JS, Gopal S, Skiel HP. Recurrent aphthous stomatitis: an assessment of antioxidant levels in plasma and saliva. J Clin Diagn Res. 2017;11(9):ZC64-7. doi: 10.7860/JCDR/2017/29065.10624, PMID 29207836.
Fadilah NI, Phang SJ, Kamaruzaman N, Salleh A, Zawani M, Sanyal A. Antioxidant biomaterials in cutaneous wound healing and tissue regeneration: a critical review. Antioxidants (Basel). 2023 Mar 23;12(4):787. doi: 10.3390/antiox12040787, PMID 37107164.
Ekpe L, Inaku K, Ekpe V. Antioxidant effects of astaxanthin in various diseases—a review. J Mol Pathophysiol. 2018;7(1):1-6. doi: 10.5455/jmp.20180627120817.
Jumina J, Siswanta D, Zulkarnain AK, Triono S, Priatmoko P, Yuanita E. Development of C-arylcalix (4) resorcinarenes and C-arylcalix [4] pyrogallolarenes as antioxidant and UV-B protector. Indones J Chem. 2019;19(2):273-84.
Nandini P, Bhikshapathi DV. Preparation and evaluation of entrectinibplga nanobubbles by central composite design. Int J Appl Pharm. 2025;17(1):304-15.
Rather AH, Rao R. Evaluation of antioxidant and hepatoprotective activity of biomass and crude astaxanthin extract of green alga haematococcus pluvialis. Res J Pharm Technol. 2021;14(4):1933-7.
Sies H. Oxidative stress: concept and some practical aspects. Antioxidants (Basel). 2020;9(9):852. doi: 10.3390/antiox9090852, PMID 32927924.
Zhang W, Chen L, Xiong Y, Panayi AC, Abududilibaier A, HU Y. Antioxidant therapy and antioxidant-related bionanomaterials in diabetic wound healing. Front Bioeng Biotechnol. 2021 Jun 24;9:707479. doi: 10.3389/fbioe.2021.707479, PMID 34249895.
Andriani D, Roestamadji RI, Rahayu RP. Astaxanthin is a promising therapy for wound healing in diabetic conditions: a review. J Int Dent Med Res. 2023;16(4):1824-9.
Ashrafizadeh M, Ahmadi Z, Yaribeygi H, Sathyapalan T, Sahebkar A. Astaxanthin and Nrf2 signaling pathway: a novel target for new therapeutic approaches. Mini Rev Med Chem. 2022;22(2):312-21. doi: 10.2174/1389557521666210505112834, PMID 33964864.
Bialas I, Zelent Kraciuk S, Jurowski K. The skin sensitisation of cosmetic ingredients: review of actual regulatory status. Toxics. 2023;11(4):392. doi: 10.3390/toxics11040392, PMID 37112619.
Adhikari A, Barakoti H, Dey BK. Evaluation of acute dermal irritation, sensitization and acute dermal toxicity of leather cream in laboratory animals. Indo Glob J Pharm Sci. 2021;11(3):1-6.
Monisha A, Bhuvaneshwari S, Velarul S, Sathiya Vinotha AT, Umamageswari MS, Vijayamathy A. Acute toxicity study of arsenicum album in wistar albino rats. Int J Pharm Pharm Sci. 2024 Dec;16(12):37-41.
Sarkar S, Roy Chowdhury P, Choudhuri D. Evaluation of acute and sub-acute toxicity of methanolic leaf extract of bambusa vulgaris on male wistar rats. Asian J Pharm Clin Res. 2024;17(5):72-7. doi: 10.22159/ajpcr.2024.v17i5.50325.