
Int J App Pharm, Vol 17, Issue 3, 2025, 260-269Original Article
IMPLEMENTING CENTRAL COMPOSITE DESIGN FOR THE FABRICATION OF SELECTIVE SEROTONIN RE-UPTAKE INHIBITOR BASED IN SITU FORMING BIODEGRADABLE DRUG DELIVERY SYSTEM
SRUTHI S.1, GOPINATH S.*2, MERITON STANLEY A.3, SATHEESH KUMAR S.4, RISHAANTH M.5
1,2*,5Department of Pharmaceutics, Sri Ramachandra Institute of Higher Education and Research (DU), Porur, Chennai, India. 3Department of Community Medicine, Sri Ramachandra Medical College and Research Institute, SRIHER (DU), Porur, Chennai, India. 4Department of Pharmaceutics, SNS college of Pharmacy and Health Sciences, SNS Kalvinagar, Kurumbalayam, Saravanampatti, Coimbatore, India
*Corresponding author: Gopinath S.; *Email: drsgopinathsriramachandra@gmail.com
Received: 10 Dec 2024, Revised and Accepted: 14 Feb 2025
ABSTRACT
Objective: The current study aims to develop and optimize an injectable fluvoxamine-loaded biodegradable implant utilizing PLGA 50:50 as the polymer and PEG 6000 as the surfactant to tailor depression therapy.
Methods: A systematic preformulation study assessed the physical and chemical properties of Fluvoxamine, including solubility, melting point, and compatibility through FTIR, DSC, and PXRD. A central composite design was used to optimize the implant formulation, evaluating critical parameters such as drug release profiles at 2 and 6 h. Surface morphology was analyzed using SEM to confirm the structural integrity of the implants.
Results: Studies on preformulation validated the stability and compatibility of the medication. Central Composite design showed that an increment of PEG concentration demonstrated a significant negative effect on Y1 response, while PLGA concentration had a positive impact on Y2. The optimized formulation showed experimental values of 2.60% for the initial burst after 2 h and 19.96% for the release after 6 h. SEM analysis revealed uniform implant morphology, supporting consistent drug delivery.
Conclusion: An optimized Fluvoxamine-loaded biodegradable implant formulation with minimum burst release and maximum sustained release was developed as an innovative solution for MDD management, addressing the challenges of conventional therapies. This approach offers improved bioavailability, patient compliance, and therapeutic outcomes, paving the way for advanced treatment strategies in depression management.
Keywords: Major depressive disorder (MDD), Biodegradable polymeric implant, Chronic Illness, Optimization, Central composite design, Fluvoxamine maleate
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijap.2025v17i3.53413 Journal homepage: https://innovareacademics.in/journals/index.php/ijap
INTRODUCTION
Major Depressive Disorder (MDD) is a major global health concern. It was the third most common cause of sickness globally in 2008 and is expected to overtake all other causes by 2030. Important symptoms such as persistently depressed mood, anhedonia, guilt feelings, loss of energy, cognitive impairments, changes in appetite, psychomotor problems, sleep disturbances, and suicidal thoughts are necessary for the diagnosis of major depressive disorder (MDD) [1]. In addition to being a psychiatric problem, depression is tightly linked to neurological dysfunctions and frequently coexists with other long-term illnesses such as diabetes, cancer, stroke, and cardiovascular disease [2, 3].
Comorbid illnesses such as OCD, substance use disorders, anxiety disorders and Post Traumatic Stress Disorder (PTSD) are frequently present in patients with depression, making therapy and management more difficult. Low bioavailability and irregular absorption are two reasons why traditional anti-depressant treatments, such as oral tablets and injectable drugs, sometimes do not work as well [4]. In situ forming implant drug delivery systems ensure that the medication is delivered consistently over a specified period, eliminating concerns regarding proper and regular administration. One should also remember the challenges faced by individuals suffering from depression, such as impaired cognition or memory difficulties, which make it challenging to take medications daily. Patients with depression often experience significant medication side effects well before the beneficial treatment effects begin to emerge. In a study of patients with depression, demyttenaere and colleagues found that although the average use of medication over time may approximate the correct number of pills per day, many patients take their medication irregularly, alternating between taking too many pills on some days and fewer pills on other days. Using a computerized system to evaluate pill-taking behaviour, these investigators found that 31 percent of patients had at least one three-day drug holiday over a nine-week study period [5] Most studies have also found that depot antipsychotics produce better adherence to treatment and reduce rates of relapse, resulting in a favourable attitude from many patients [6]. Therefore, in situ forming implant drug delivery systems enhance the comfort and ease of use for patients undergoing treatment for depression [7]. Biodegradable implants are one of the most innovative drug delivery techniques, which offer a ground-breaking solution providing a prolonged and sustained release eliminating the need for frequent dosing. The biocompatible materials used to make these implants change into a gel-like substance when they come into touch with physiological fluids also lowers the possibility of side effects and peak drug concentrations by enabling a regulated and prolonged release of the therapeutic ingredient.
Compared to traditional antidepressant treatments, biodegradable implants have several advantages. They are minimally invasive, decompose naturally, are customizable, and provide better bioavailability and targeted drug delivery; additionally, they make the treatment more patient-friendly and cost-effective by removing the need for surgical removal after use.
A drug release profile from a biodegradable implant normally shows a bi-or triphasic release profile. Starting with a burst release followed by a slow release or a constant drug release rate, and subsequently, the last phase with a rapid release. A burst release is a drawback of monolithic implants. It may cause side effects due to rapidly increasing drug levels in a short period. Burst release is undesirable for biodegradable implants. This study also focuses on reducing the initial burst release. The ultimate objective of this research is to develop a novel drug delivery system with enhanced bioavailability, low initial burst and reduced frequency of dosing for the treatment of depression.
Thereby improving the quality of life of patients and caregivers while streamlining treatment regimens. This innovative method has the potential to revolutionize the treatment of MDD by overcoming the drawbacks of traditional therapies and providing a more effective, practical, and patient-centred approach to a condition that affects millions of people globally.
MATERIALS AND METHODS
Fluvoxamine was purchased from TCI Chemicals, Chennai. PLGA 50/50 was purchased from Nomisma Healthcare, Vadodara Gujarat. Polyethylene Glycol 6000 and N-Methyl Pyrrolidone (NMP) were purchased from Sisco Research Laboratories (P) Ltd., Taloja, Maharashtra. All the chemicals and reagents used were of analytical grade.
Preformulation study
Organoleptic evaluation
The examination assessed a pure substance's colour, physical state, and taste as it’s organoleptic qualities. These metrics offer a preliminary understanding of the physical properties of the drug, which can impact the formulation procedure.
Determination of melting point
A digital melting point device (DB-31354, Decibels Instruments, Perfit, India) was used to ascertain the drug's melting point. A capillary tube with a small amount of the drug was heated in a bath. Melting and liquefaction temperatures were noted. The identity and purity of the medication were verified by comparing the data with existing literature. This method is essential to formulation since it aids in the selection of excipients and the formulation itself, particularly in formulations that are heat-sensitive [8].
Solubility
To determine its compatibility and profile, fluvoxamine maleate's solubility was evaluated in a range of solvents. An exact 1 mg of the drug was dissolved in several solvents, and the solubility of the drug was visually assessed and contrasted with data from the literature. For preformulation investigations, the solubility profile is essential because it affects drug bioavailability and formulation strategy, which improves therapeutic efficacy.
FTIR spectroscopy
The study examined drug’s functional groups and compatibility with excipients using Attenuated Total Reflectance-Fourier Transform Infrared (ATR-FTIR) spectroscopy. In preformulation investigations, ATR-FTIR is an essential analytical method that aids in determining possible chemical incompatibilities between medications and excipients. The medicine and excipients, such as PLGA 50/50 and PEG 6000 were both analysed for FTIR spectra to find any interactions that would compromise the stability or functionality of the formulation. Preparing the drug-excipient physical mixture with potassium bromide powder in a 1:1:1 weight ratio was another evaluation. The functional group found in the components and their physical combination were thoroughly described by the analysis. It can be used to identify additional peaks or variations in peak intensities due to the presence of unique absorption bands, which may indicate chemical interactions [9]. Successful therapeutic formulation development requires an understanding of these interactions since incompatibilities can lead to decreased stability, poor bioavailability or modified release kinetics [10].
Differential scanning calorimetry analysis
Utilizing Differential Scanning Calorimetry (DSC) (NETZSCH DSC 204F1 Phoenix), the study examined the drug's, excipient's, and mixture's thermal characteristics. Plotting the findings downward, the results assessed the drug's compatibility with the excipients and it’s heat stability. To ensure formulation stability, DSC analysis is essential during the preformulation stage. It offers information about the drug's and the excipients' thermal stability [11].
Powder X-ray diffraction analysis
Using a Bruker D8 Advance X-ray diffractometer, Powder X-ray Diffraction (PXRD) was used to analyze the crystalline structure of the sample. CuKα radiation analysis is used to better understand the crystalline nature of the drug and possible formulation adjustments that may affect it’s bioavailability and rate of dissolution [12].
Method of preparation
To formulate a polymer solution, PLGA 50/50 was dissolved in 2-NMP to create the biodegradable implant. After adding, polyethylene glycol 6000 was incorporated and vortexed. Then medicament was added. The implant's viability was evaluated after infusing the medication into PBS with mild constant stirring. In order to ensure homogeneity and successful implant formation for consistent drug release and therapeutic efficacy, syringeability was assessed for each formulation [13].
Optimization of the formulation
Utilizing the resources at hand and taking into account every aspect impacting the experiment, the implant composition was optimized. The polymer concentration, polyethene glycol concentration, and solvent concentration were the three independent factors that were compared to the dependent variables, such as the drug release profile at the second and sixth hours. X1, X2, and X3 at different levels were developed in implant formulations using a core composite architecture [14]. The selected polymer range ensures a balance between achieving sufficient matrix density for controlled release and avoiding high viscosity that could hinder injectability or cause premature precipitation [15]. The range of PEG fine-tunes the initial burst release and enhances drug dispersion within the matrix. Additionally, the chosen solvent concentration guarantees complete solubilization of PLGA while maintaining a manageable viscosity for injectability. The program produced sixteen runs that were carried out in a random order, guaranteeing the stability and efficacy of the formulation. Table 1 provides an exhaustive list of variable levels employed in the Central Composite Design (CCD). Table 2 provides the actual composition of each formulation batch (F1-F16). Experiments were performed in triplicate to ensure reproducibility [16, 17].
Surface morphological evaluation
Formulations were prepared and injected into phosphate-buffered saline (PBS) for processing; after 48 h of freeze-drying the samples were cut into smaller pieces and coated with a thin layer of gold to increase conductivity. The samples were then placed on a sample holder for SEM imaging (JEOL JSM 6390). The SEM pictures revealed information about the surface morphology and structural properties of the freeze-dried formulations, allowing to better understand the effects of the freeze-drying procedure as well as the impact of gold coating on the sample’s surfaces. This information was critical for optimizing the preparation procedure [18, 19].
Table 1: Variables used in CCD
| Independent variable/Factors | Low level | High level |
| Amount of Polymer | 30.00 | 40.00 |
| Amount of Surfactant | 5.00 | 15.00 |
| Amount of Solvent | 8.00 | 9.00 |
| Dependent variable/Constants | Desirability constraints | |
| Y1(Initial drug release) | Minimize | |
| Y2 (Parameter for sustained release) | Maximize |
Table 2: Composition of different batches of formulations
| Run | Batch | X1: Conc of PLGA (mg) | X2: Conc of PEG (mg) | X3: Conc of NMP (ml) | Y1 %: release after 2h | Y2 %: release after 6h |
| 1 | F1 | 40 | 15 | 8 | 2.74 | 21.85 |
| 2 | F2 | 35 | 10 | 8.5 | 4.85 | 17.92 |
| 3 | F3 | 35 | 18.409 | 8.5 | 1.36 | 16.06 |
| 4 | F4 | 35 | 1.59104 | 8.5 | 22.32 | 14.61 |
| 5 | F5 | 40 | 5 | 8 | 15.39 | 20.36 |
| 6 | F6 | 35 | 10 | 8.5 | 6.92 | 17.58 |
| 7 | F7 | 30 | 5 | 8 | 11.17 | 15.61 |
| 8 | F8 | 30 | 15 | 9 | 2.46 | 10.25 |
| 9 | F9 | 35 | 10 | 9.3409 | 5.40 | 17.33 |
| 10 | F10 | 35 | 10 | 7.6591 | 6.89 | 13.56 |
| 11 | F11 | 40 | 5 | 9 | 13.28 | 19.94 |
| 12 | F12 | 30 | 15 | 8 | 3.00 | 11.71 |
| 13 | F13 | 30 | 5 | 9 | 10.30 | 12.54 |
| 14 | F14 | 40 | 15 | 9 | 2.18 | 18.15 |
| 15 | F15 | 43.409 | 10 | 8.5 | 6.70 | 24.20 |
| 16 | F16 | 26.591 | 10 | 8.5 | 5.12 | 9.09 |
Statistical analysis
To get desirable drug release profiles, the study sought to identify the ideal concentrations of three independent variables: polymer concentration (X1), Polyethylene glycol concentration (X2) and solvent concentration (X3). A polynomial equation (Y=β0+β1X1+ β2X2+β11X12+β22X22+β12X1X2) and a generalized response surface model were used to model the response function (Y), with the coefficients denoting the linear and quadratic impacts of the independent variables as well as the interaction effects among the variables. To determine how the surface factor affect the drug release properties of the formulation, the expected response (Y) was examined.
To determine the significant impacts of the independent variables on the response regression coefficients, analysis of variance (ANOVA) was utilized. All significant independent variable effects (p<0.05) were included in the reduced model. The reaction was predicted more accurately by the reduced model, which kept important influences. To see how the factors interacted and affected the drug release profiles, three-dimensional response surface plots were developed.
By imposing particular limitations on the in vitro drug release at the second and sixth hours, Design-Expert® software performed a numerical optimization analysis and produced the best in situ implant formulation that satisfied the required release parameters. To verify the theoretical predictions, the improved formulation was subjected to in vitro drug release tests. These investigations validated the model's accuracy and the optimized formulation's ability to produce the desired drug release profile.
RESULTS AND DISCUSSION
Organoleptic evaluation
Fluvoxamine maleate's authenticity and high quality were validated by the organoleptic examination since it’s physical attributes, such as colour, state, and taste matched descriptions found in the literature. These results, which are shown in table 3, employed organoleptic evaluations as a first step in psychotropic medication quality control. This assessment makes sure the content satisfies the requirements needed for the development of pharmaceuticals.
Table 3: Organoleptic evaluation of fluvoxamine maleate
| S. No. | Evaluation parameter | Method used | Observed results |
| 1. | Colour | Self-Observed | White |
| 2. | State of Matter | Optical Microscopy | Crystalline |
| 3. | Odour | Self-Observed | Bitter |
| 4 | Taste | Self-Observed | Bitter |
Determination of melting point
It was determined that fluvoxamine maleate had a melting point of 124±3 °C, which verified it’s identification and purity, which is shown in table 4. This finding highlighted the significance of a constant melting point range in assessing the purity of drugs in pharmaceutical compounds.
Solubility
Fluvoxamine maleate's solubility profile (table 5) shows that it is more soluble in phosphate buffer saline (pH 7.4) than it is in distilled water, which is important for both it’s bioavailability and therapeutic effectiveness. This implies that the formulation of fluvoxamine maleate in sophisticated delivery systems may be successful.
Table 4: Melting point of fluvoxamine maleate
| S. No. | Melting point (°C) | Mean±SD (°C) |
| 1 | 122 | 124±3 |
| 2 | 124 | |
| 3 | 126 |
*value are mean±SD of data from 3 experiments.
Table 5: Qualitative solubility of fluvoxamine in various solvents
| S. No. | Solvent (1 ml) | Solubility of the drug (1 mg) |
| 1 | Distilled water | sparingly soluble |
| 2 | Phosphate buffer saline pH 7.4 | soluble |
| 3 | Methanol | Highly soluble |
| 4 | Ethanol | Highly soluble |
| 5 | HCL | soluble |
| 6 | Chloroform | Insoluble |
FTIR analysis
Fluvoxamine maleate's integrity and purity were verified by FTIR analysis, which showed all of the major diagnostic peaks (table 6). The pure drug's spectrum matched published data, proving it was devoid of contaminants. Additionally, the compatibility of fluvoxamine with PEG 6000 and PLGA 50/50 excipients were evaluated by FTIR analysis. The shifts in PLGA and it’s physical mixture indicate minimal interaction, maintaining the polymer matrix's integrity for drug encapsulation. The stable C-H stretching in PLGA and PEG confirms the aliphatic chain’s stability, while unchanged NH and imine stretching in fluvoxamine and the physical mixture affirm the preservation of essential functional groups crucial for the drug's therapeutic efficacy. Likewise, the stability of aromatic rings and CH2 rocking and twisting suggests a lack of significant chemical interactions, thus maintaining the structural integrity of both the drug and polymer. These findings imply weak or negligible interactions, such as hydrogen bonding or van der Waals forces, which are advantageous for the physical stability of the matrix and enable controlled drug release. Consequently, the observed peak shifts corroborate the drug-polymer system's compatibility, essential for achieving an effective and predictable delivery mechanism (fig. 1). This shows that these excipients can be used to safely synthesize fluvoxamine without running the risk of degradation or loss of efficacy.
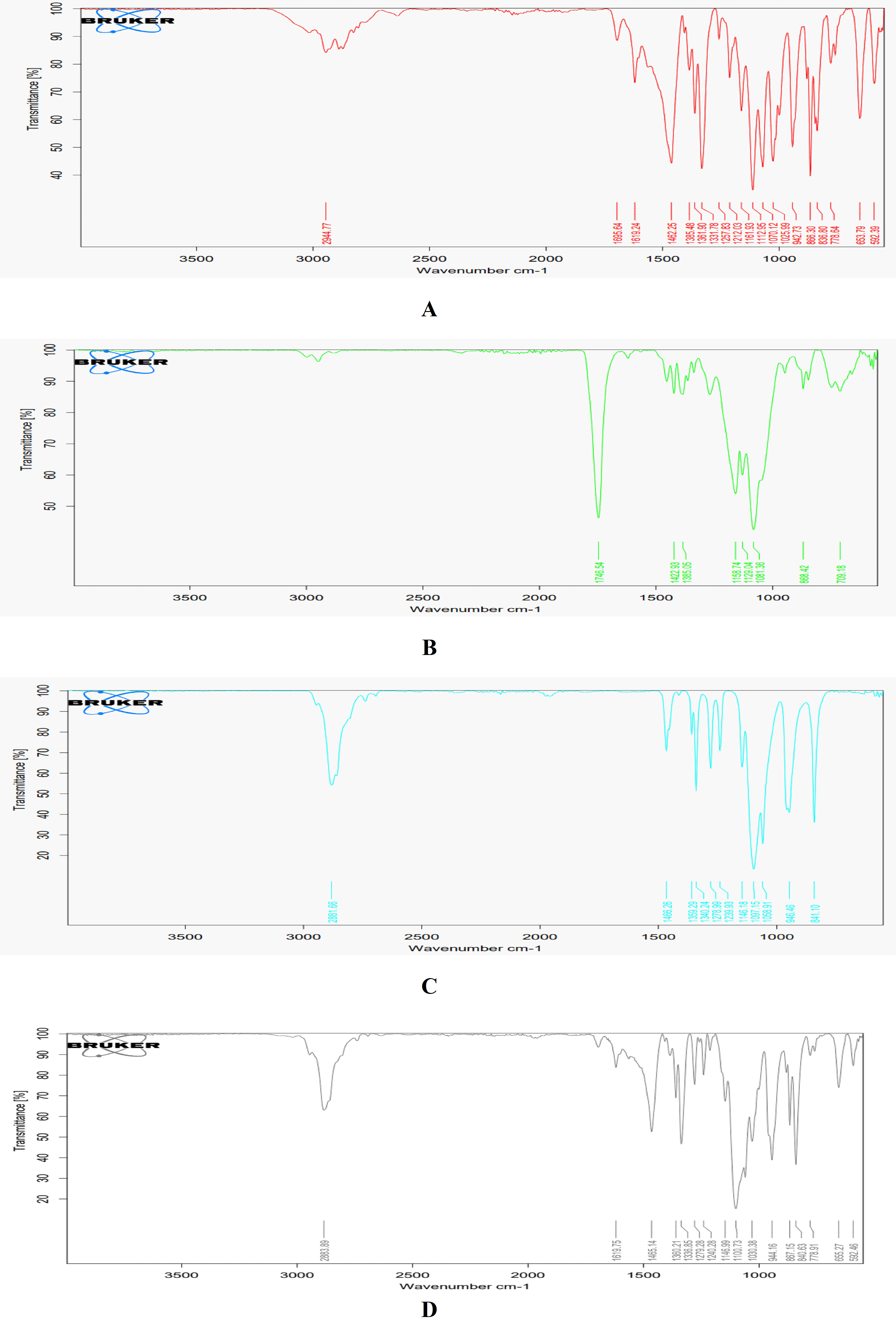
Fig. 1: FTIR spectra of fluvoxamine maleate B: FTIR spectra of PLGA 50:50, C: FTIR spectra of PEG 6000, D: FTIR spectra of the physical mixture
Table 6: Comparative FTIR spectra for fluvoxamine, PLGA 50:50, PEG 6000, physical mixture
| Functional group | Fluvoxamine | PLGA 50:50 | PEG 6000 | Physical mixture | Observation |
| C=O stretching | 1619.24 (1820-1600) | 1764.54 (1820-1600) | Not Observed | 1619.75 (1820-1660) | The slight shift in PLGA and physical mixture indicates compatibility with the polymer matrix. |
| C-H stretching | Not Observed | Observed (2950-3000) | 2881.66 (3650-3600) | 2883.89 (3400-2400) | C-H stretching in PLGA and PEG indicates the presence of aliphatic chains. |
| NH Stretch/bend | 3100 (3500-3100), 1640-1550 (Small Peaks) | Not Observed | Not Observed | 3100 (Small Peak), 1640-1550 (Small Peak) | Consistent presence of NH stretching in Fluvoxamine and the physical mixture, indicating no interaction. |
| C-O-C stretch | 1112.95 (1300-1000) | Not Observed | 1359.29 (1350-1150) | 1100.73 (1300-1000) | Similar peaks suggest no significant interaction affecting ether groups. |
| Aromatic ring | 1462.95 (1600-1450) | Not Observed | Not Observed | 1465.14 (1600-1450) | The aromatic ring in Fluvoxamine is preserved in the physical mixture. |
| Imine (C=N) stretch | 1695.64 (1690-1640) | Not Observed | Not Observed | 1695.64 (1690-1640) | Imine stretch remains unaffected in the physical mixture. |
| C-F stretch | 1331.78 (1400-1000) | Not Observed | Not Observed | 1336.85 (1400-1000) | A slight shift indicates minimal interaction with excipients. |
| CH2 rocking | Not Observed | Not Observed | 841.1 | 840.63 | The presence of CH2 rocking in PEG and physical mixture. |
| CH2 twisting | Not Observed | Not Observed | 946.46 | 944.16 | The consistency of CH2 twisting suggests stable interactions. |
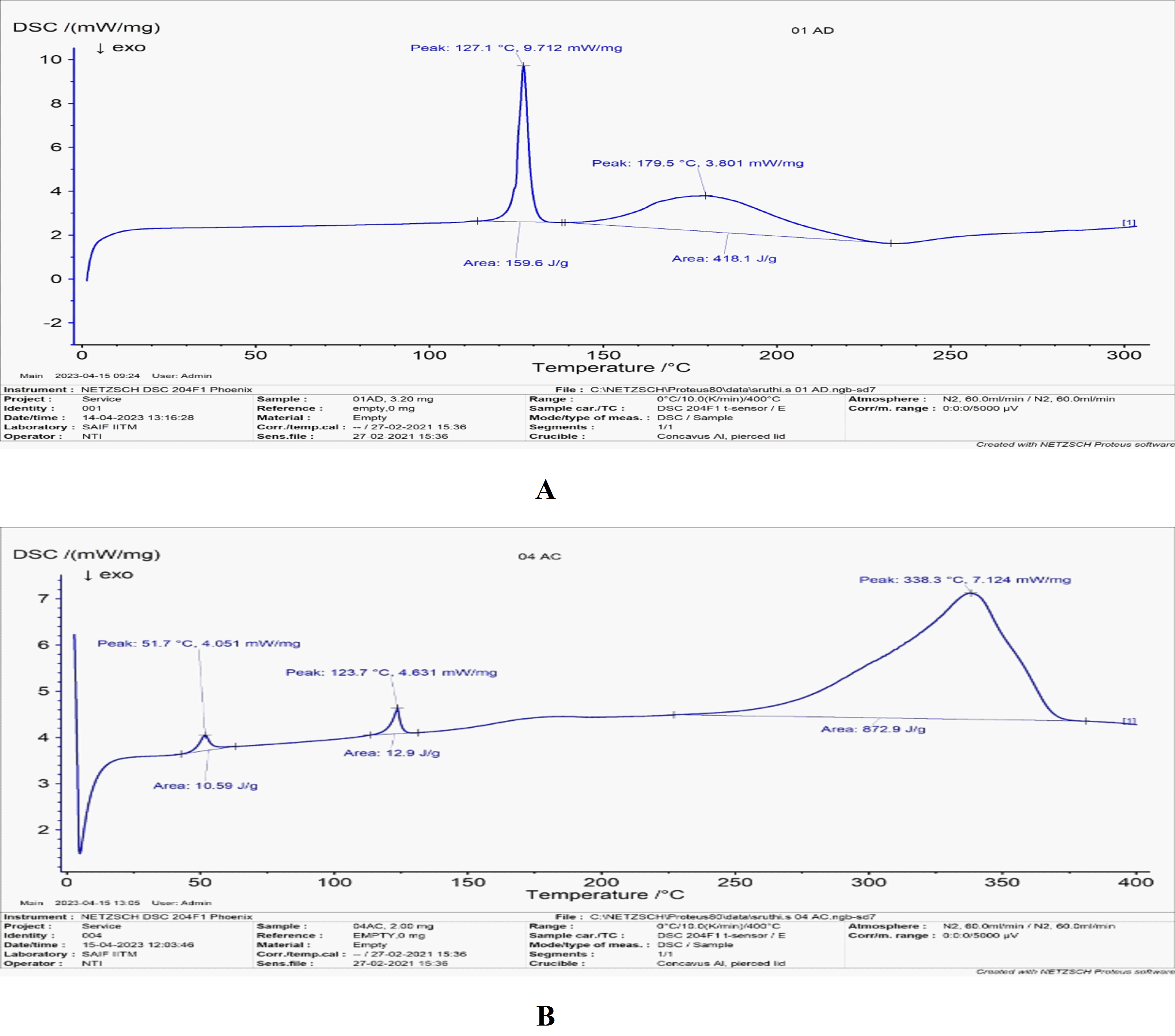
Fig. 2A: DSC thermogram of fluvoxamine maleate, B: DSC Thermogram of the physical mixture
DSC analysis
The DSC thermogram of fluvoxamine maleate (FLM) demonstrated a strong endothermic peak at 127.1 °C, confirming it’s crystalline nature and purity (fig. 2A). A secondary melting peak observed during heating suggests the presence of polymorphism, which may influence the drug's stability and bioavailability. Similarly, previous studies have reported an endothermic peak for FLM at approximately 127.2 °C, supporting the consistency of these findings. Moreover, the DSC analysis of FLM in combination with PLGA 50/50 and PEG 6000 revealed a slight increase in the initial melting temperature to 123.7 °C, indicating potential compatibility with these excipients (fig. 2B). Ghaderi et al. [20] research has highlighted the impact of heating rate variations on DSC thermograms, influencing the temperature range and curve shape. This suggests that additional DSC techniques, such as multiple scan methods at different heating rates and kinetic parameter calculations, may provide deeper insights into drug-excipient interactions.
XRD analysis
The X-ray diffraction (XRD) analysis confirmed the highly crystalline nature of Fluvoxamine Maleate (FLM), as evidenced by sharp peaks between 5° and 50° (2θ), with prominent reflections at 8.299, 9.399, 16.657, 18.930, 20.396, 20.836, 23.842, 24.842, 25.089, 33.740, and 42.538 (fig. 3A). These peaks align with previously reported findings, confirming the crystalline state of FLM. However, upon physical mixing with a polymer, a notable reduction in peak intensity was observed at corresponding 2θ values (fig. 3B), suggesting partial disruption of the crystalline structure due to drug-polymer interactions. Choi et al., [21] reported that decreased peak intensity and peak shifts in polymer-based solid dispersions indicating a transition from a crystalline to an amorphous state, which plays a crucial role in enhancing drug solubility and dissolution. The reduced crystallinity of the FLM-polymer mixture suggests molecular dispersion of the drug within the polymer matrix, potentially improving it’s release characteristics and bioavailability. This is particularly relevant in the formulation of in situ forming biodegradable drug delivery systems, where controlled drug release and enhanced solubility are critical for therapeutic efficacy.
Preparation of polymer solution and feasibility of implant formation
Fluvoxamine maleate generates an injectable formulation in NMP, which, upon administration into PBS, initiates solidification within half an hour, resulting in an in-situ implant. It was observed that the introduction of the polymer matrix into the PBS buffer, accompanied by gentle stirring, gradually induces phase inversion, leading to the precipitation of the polymer. A dense mass of depot material was established. The underlying mechanism for the formation of the implant is attributed to the swift diffusion of NMP into the aqueous medium. This phenomenon prompts the precipitation of the water-insoluble polymer and drug, culminating in the formation of a depot. Formulation, when tested for syringeability, demonstrated excellent syringeability with low extrusion force, uniform flow, and minimal needle clogging.
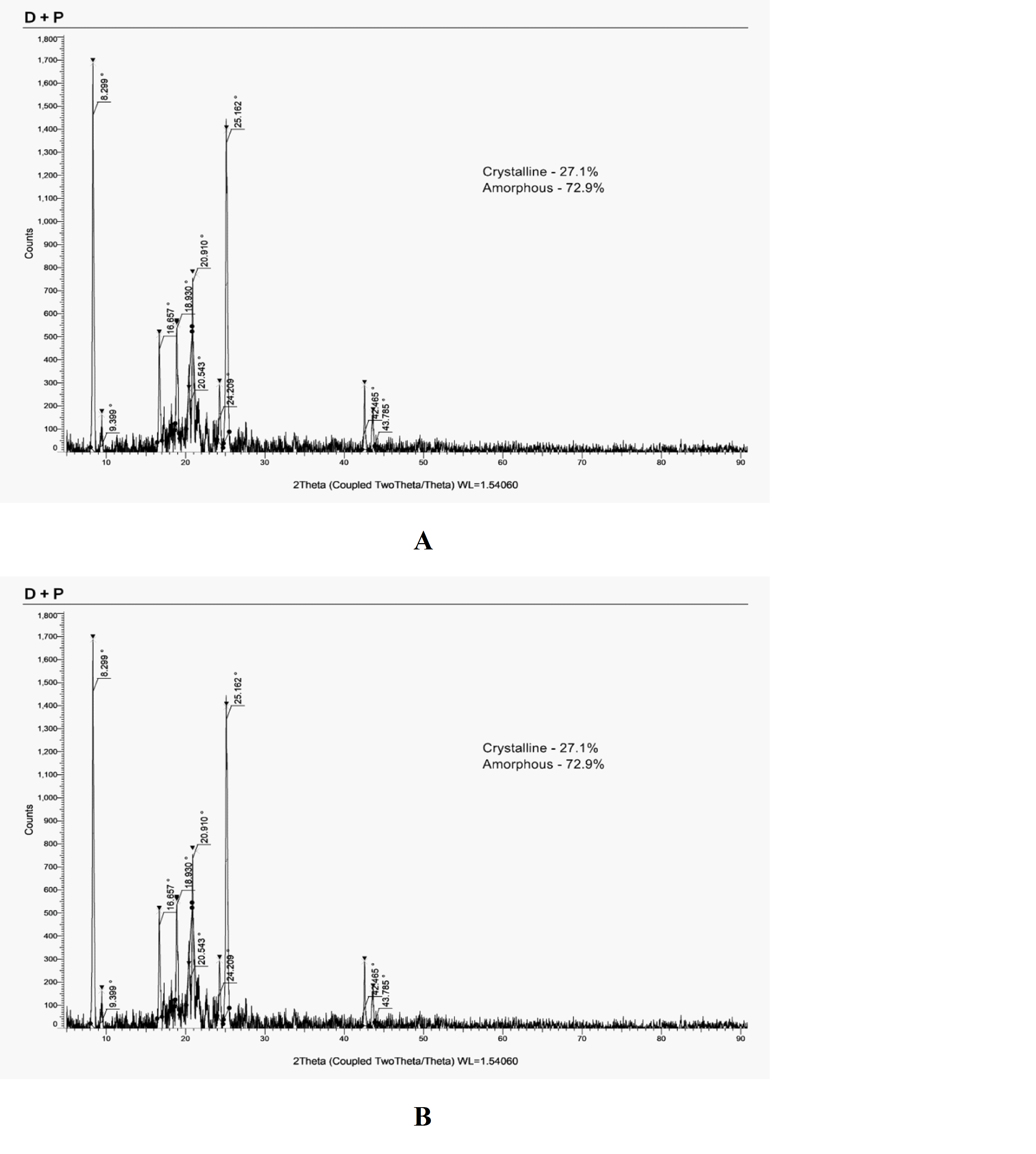
Fig. 3A: PXRD of fluvoxamine maleate, B: PXRD of the physical mixture
Surface morphological evaluation
The SEM analysis of freeze-dried formulations revealed a porous, micro void-like surface morphology, enhancing drug dispersion and dissolution rates (fig. 4). The mean particle size was 810 nm, with a percentage porosity of 1.709%, indicating a relatively dense implant structure that aids in sustained drug release by minimizing rapid diffusion and initial burst effects while improving mechanical stability. The ISFI delivery system exhibited low porosity and a spongy texture, primarily influenced by hydrogen bonding between NMP and PEG, which significantly affected the drug release profile by restricting NMP diffusion into the release medium. This controlled porosity contributes to a slower drug release rate, beneficial for sustained drug delivery. Similar studies have reported that ISFIs containing DMSO and NMP formed porous, foam-like structures, whereas denser, less porous depots were observed with different solvent compositions. The findings from this study align with research of Zhang et al. [22], demonstrating that solvent selection and porosity play crucial roles in controlling the structural characteristics and release kinetics of ISFIs.

Fig. 4: SEM evaluation of the formulation
Analytical statistics
In situ forming implant (ISFI) drug delivery systems, controlling burst and sustained release is essential for effective therapy. Burst release denotes rapid drug discharge post-administration, often due to surface diffusion or weak drug-polymer interactions. This phenomenon can result in toxicity, diminished efficacy, and erratic delivery. Reducing burst release is critical for safety, consistent therapeutic levels, and smoother drug delivery transitions. In contrast, sustained release, facilitated by polymer degradation and diffusion, guarantees extended therapeutic effects, lowers dosing frequency, improves patient adherence, and stabilizes drug concentrations. Achieving an optimal balance necessitates the refinement of polymer characteristics, drug-polymer interactions, solvent systems, and excipients to modulate release kinetics, ensuring ISFI systems deliver safe, effective, and reliable long-term therapy.
The impact of polymer concentration (X1), polyethene glycol concentration (X2), and solvent concentration (X3) on the drug release patterns at the second hour (Y1) and sixth hour (Y2) were evaluated using the Central Composite Design (CCD) approach in this study. The experimental data was analysed using Design Expert software, and the results showed that a linear model fit the data best because it could maximize the adjusted and anticipated R2 values.
Model fitting and ANOVA examination
ANOVA was used to validate the linear model, which demonstrated the considerable influence of factors on drug release profiles. Responses were considerably impacted by the concentrations of PEG and PLGA. The model's accuracy and predictive power were confirmed by high R² values and a small gap between adjusted and anticipated R² values (table 7). The expected vs. actual graphs for each response are displayed in fig. 5A and 5B. Strong signal-to-noise ratio and adequate precision values for Y1 and Y2 (45.145 and 15.308) demonstrated the model's dependability in navigating the design space.
For both y1 and y2 the statistical significance of interaction terms (2FI) was low, as indicated by high sequential p-values (0.0997 for y1 and 0.7439 for y2), suggesting that these terms do not meaningfully enhance the model's predictive power. Quadratic effects were also excluded in both cases due to high sequential p-values (0.8381 for y1 and 0.7048 for y2), lower predicted R2 values (0.9604 for y1 and 0.2086 for y2), and the potential for overfitting. The linear model was selected as the best fit for both responses, supported by statistically significant p-values (1.31×10−10 times fory1 and 2.47×10−5 for y2) and strong adjusted R2 (0.9764 for y1 and 0.8197 for y2) and predicted R2 values (0.9691 for y1 and 0.7372 for y2).
Adequacy of precision measures the signal-to-noise ratio in the model, assessing whether the model provides a reliable predictive response. High adequacy of precision ensures that the model's predictions are consistent and not overly influenced by random variation. Adequacy of precision, although not explicitly provided, is inferred to be acceptable for both models based on their robustness and validation metrics. This ensures that the linear models are reliable for predictions while maintaining simplicity by excluding insignificant interaction and quadratic terms.
Table 7: Anova analysis of response Y1 and Y2
| Variables | Initial drug release after 2 h (Y1) | Drug release after 6 h (Y2) |
Intercept A-Plga B-peg C-Nmp R 2 Adjusted R 2 Predicted R 2 Adeq Precision Std. Dev. |
Coefficient estimate | P-value |
1.75 0.0596 -0.8045 -0.0779 0.9811 0.9764 0.9691 45.1446 0.1199 |
˂0.0001 0.0910 ˂0.0001 0.0334 |
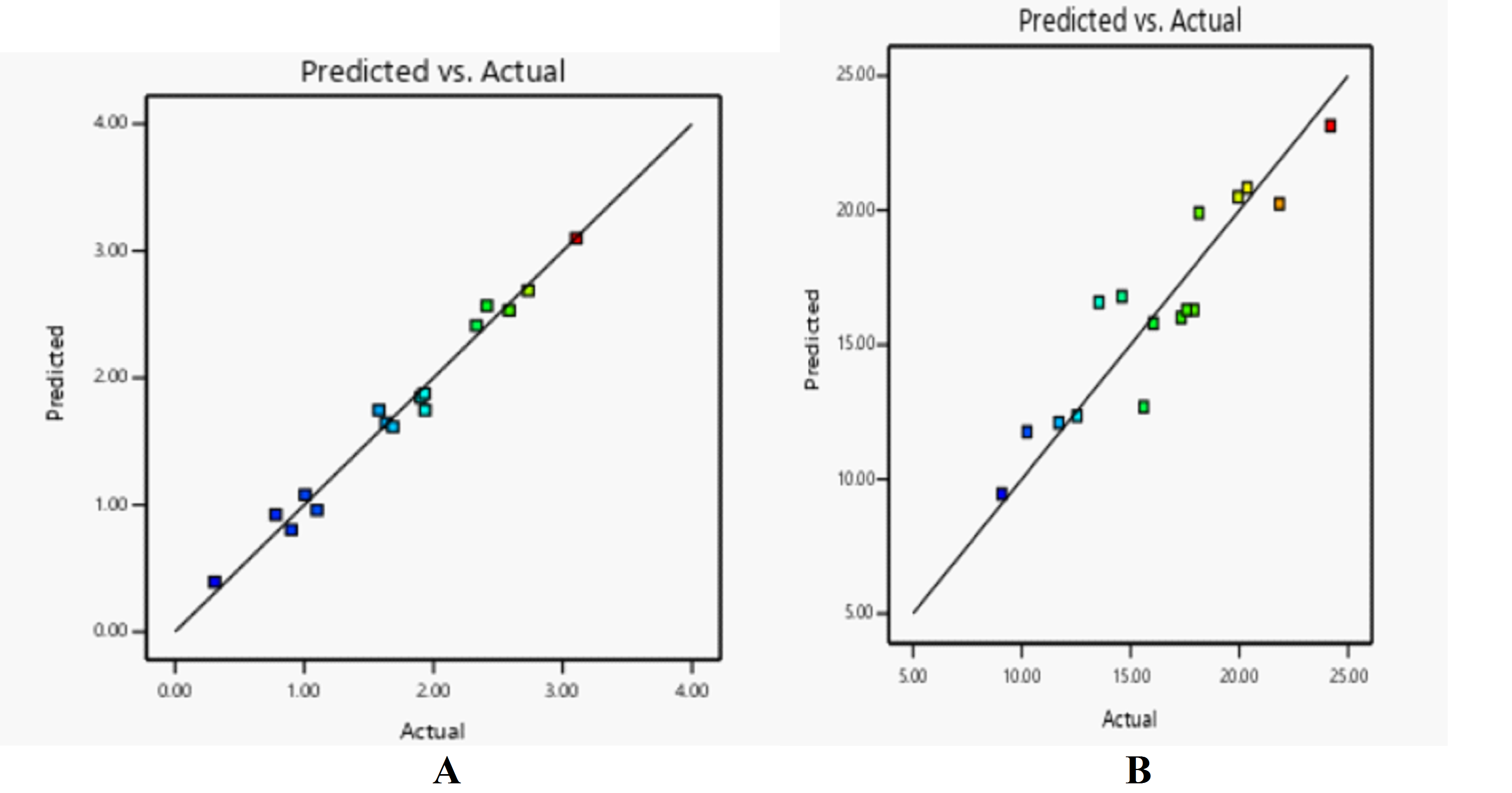
Fig. 5: Predicted vs. actual plot for A: initial burst release, B: drug release after 6h
Impact on initial burst release (y1)
Final equation in terms of actual factors
Ln (y1) = 4.26159+0.0119204 *A+-0.160892 *B+-0.155816 *C
Where variables; A-PLGA50/50, B-PEG 6000, C-2-NMP and 4.26159 as the intercept. The equation describes how the natural logarithm of the response variable y1 changes concerning the amounts of PLGA 50/50, PEG 6000, and 2-NMP. Positive coefficients (like for PLGA 50/50) indicate a positive relationship with y1, while negative coefficients (like for PEG 6000 and 2-NMP) indicate a negative relationship with y1.
A 208.06 F-value supported the model's relevance, with solvent and PEG concentrations showing the greatest effects. Due to it’s plasticizing impact, increasing PEG content decreased early burst release, improving solubilization and homogeneous drug dispersion inside the PLGA matrix, as demonstrated by the 3D response surface plot (fig. 6A). Drug particle adsorption onto the polymer surface was reduced as a result of this reduction. [23].
Effect on drug release after six hours (y2)
Final equation
y2=−8.73363+0.814271⋅A−0.0593314⋅B−0.338239⋅C
The observed positive coefficient for PLGA 50/50 (A) suggests that increasing it’s concentration leads to the formation of a more porous polymer matrix, which enhances drug release over time, as supported by the response surface plot (fig. 6B). This correlates with the findings of Bode et al. [24] who demonstrated that higher PLGA concentrations contribute to a more structured implant, facilitating sustained drug release. Conversely, the negative coefficient for PEG 6000 (B) and NMP indicates that increasing these excipients reduces drug release, a trend consistent with prior studies showing that these additives regulate polymer swelling and modulate release kinetics by restricting solvent diffusion. The significant F-value (23.74) further reinforces the statistical relevance of polymer concentration in controlling drug release rates. Additionally, the observed impact of formulation composition on implant porosity and drug dispersion suggests that fine-tuning PLGA and excipient ratios can optimize in-situ forming implant performance for sustained drug delivery applications.
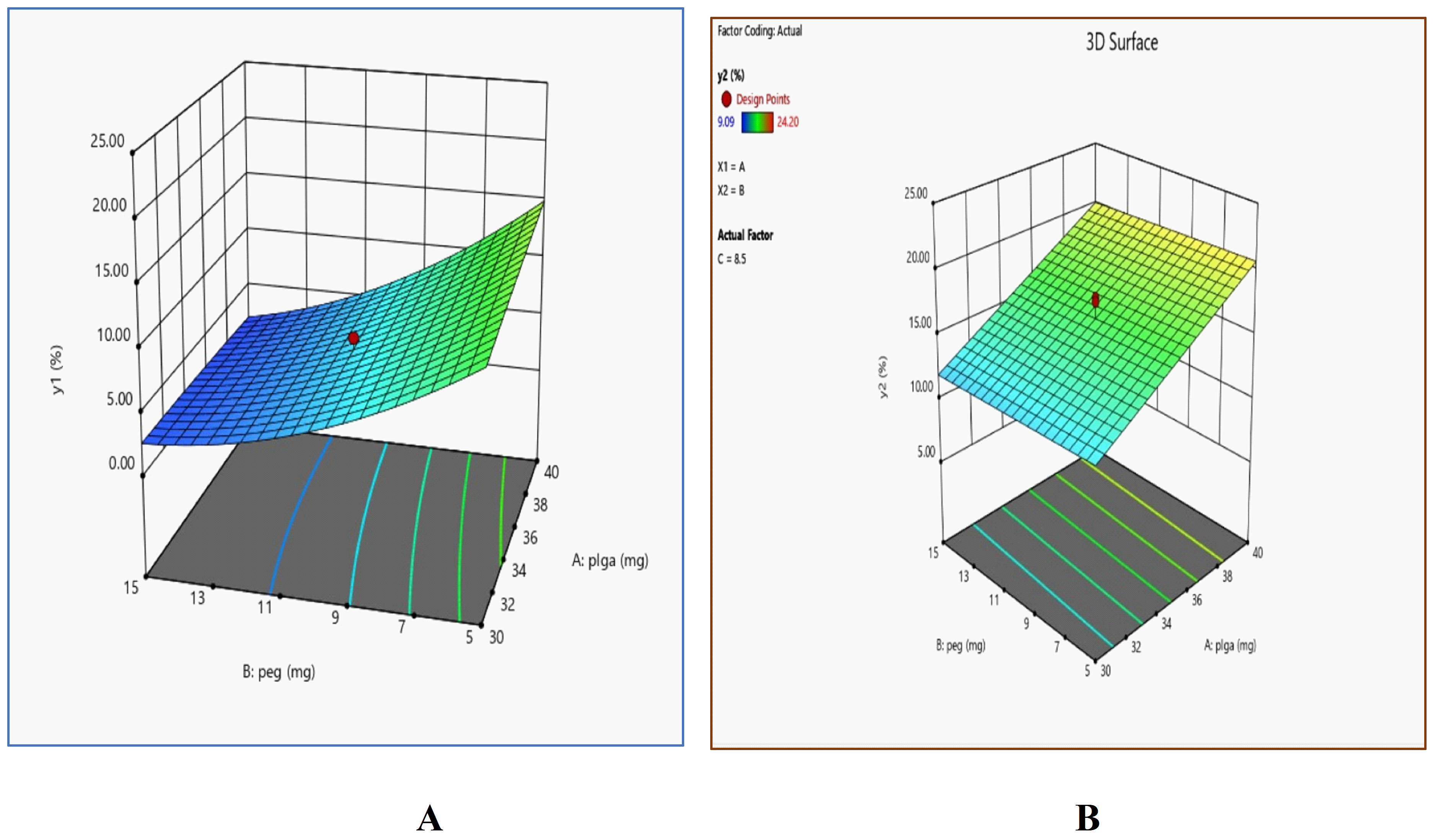
Fig. 6: 3D surface response plots showing the effect of PLGA (mg) and PEG (mg) concentrations on the dependent variables: A Y1 (%) and B Y2 (%)
Determination of optimum formulation
A formulation that maximizes drug release after six hours and minimizes initial burst release was selected. It was estimated that the mixture, 40 mg of PLGA, 15 mg of PEG, and 9 ml of N-methyl pyrrolidone, would yield 2.53% drug release after two hours and 19.93% drug release after six hours. These predictions were confirmed by in vitro drug release tests, which showed a 2.60% initial burst release and a 19.96% six-hour drug release, both of which matched the expected values and fell within the 95% prediction intervals (table 8).
The efficacy of the Design of Experiments methodology in enhancing drug delivery formulations is emphasized by this validation.
Table 8: A comparison between the projected and observed response values of the formulation prepared under optimal conditions
| Response | Predicted values | Observed values | Prediction error (%) |
| Initial burst release after 2 h (%) | 2.53% | 2.60% | 2.69% |
| In vitro drug release after 6 h (%) | 19.93% | 19.96 % | 0.15% |
CONCLUSION
This study highlights the potential of biodegradable implants as a novel approach to treating MDD. Using fluvoxamine maleate as a model drug, the formulation with PLGA 50/50 and PEG 6000 ensures sustained drug release, reducing the need for frequent dosing and improving patient adherence. By minimizing burst release and eliminating the need for surgical removal, this technology offers a safer and more convenient alternative to traditional antidepressant therapy. While the benefits are clear, future studies should focus on in vivo trials to confirm efficacy and safety. Additionally, challenges such as large-scale production, cost-effectiveness, and patient acceptance must be addressed to ensure widespread clinical adoption. Overcoming these hurdles could pave the way for broader applications in managing other chronic and psychological disorders. With continued research and optimization, biodegradable implants could revolutionize the treatment landscape for depression, enhancing patient outcomes and quality of life.
FUNDING
Nil
AUTHORS CONTRIBUTIONS
Conceptualization, design, supervision and review of manuscript draft: Dr. S Gopinath. Methodology framework, Supervision and review of manuscript: Dr. Meriton Stanley A, Dr. Satheeshkumar. Sample preparation, Data curation, Analysis and writing-original draft: Sruthi. S. Contribution of reagents and tools: Rishaanth M.
CONFLICT OF INTERESTS
Declared none
REFERENCES
Sabic D, Sabic A, Bacic Becirovic A. Major depressive disorder and difference between genders. Mater Sociomed. 2021;33(2):105-8. doi: 10.5455/msm.2021.33.105-108, PMID 34483737.
Yuan S, MA T, Zhang YN, Wang N, Baloch Z, MA K. Novel drug delivery strategies for antidepressant active ingredients from natural medicinal plants: the state of the art. J Nanobiotechnology. 2023;21(1):391. doi: 10.1186/s12951-023-02159-9, PMID 37884969.
Roy Byrne PP, Davidson KW, Kessler RC, Asmundson GJ, Goodwin RD, Kubzansky L. Anxiety disorders and comorbid medical illness. Gen Hosp Psychiatry. 2008;30(3):208-25. doi: 10.1016/j.genhosppsych.2007.12.006, PMID 18433653.
Huang X, Brazel CS. On the importance and mechanisms of burst release in matrix controlled drug delivery systems. J Control Release. 2001;73(2-3):121-36. doi: 10.1016/s0168-3659(01)00248-6, PMID 11516493.
Dankert ME, Brensinger CM, Ralph LN, Seward DA, Bilker WB, Siegel SJ. Psychiatric health care provider attitudes towards implantable medication. Psychiatry Res. 2010;177(1-2):167-71. doi: 10.1016/j.psychres.2008.12.012, PMID 20378184.
Makadia HK, Siegel SJ. Poly lactic co glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers (Basel). 2011;3(3):1377-97. doi: 10.3390/polym3031377, PMID 22577513.
Demyttenaere K, Mesters P, Boulanger B, Dewe W, Delsemme MH, Gregoire J. Adherence to treatment regimen in depressed patients treated with amitriptyline or fluoxetine. J Affect Disord. 2001;65(3):243-52. doi: 10.1016/s0165-0327(00)00225-1, PMID 11511404.
Jain N, Verma A, Jain N. Preformulation studies of niosomal gel containing dipivefrin hydrochloride for antiglaucomatic activity. Int J Pharm Pharm Sci. 2024;16(2):17-23. doi: 10.22159/ijpps.2024v16i2.49174.
Ambati T, Nizampuram V, Selvaganesh S, SR, Nesappan T. Efficacy of copper oxide nanoparticles using Piper longum and piper betle. Bioinformation. 2023;19(9):964-70. doi: 10.6026/97320630019964, PMID 37928485.
Hattali Ws Al, Samuel BA, Philip AK. Enhancing fluconazole solubility and bioavailability through solid dispersion techniques: evaluation of polyethylene glycol 6000 and sodium carboxymethylcellulose systems using fiber optics. Int J Pharm Pharm Sci. 2024;16(12):51-9. doi: 10.22159/ijpps.2024v16i12.52739.
Rojek B, Wesolowski M. DSC supported by factor analysis as a reliable tool for compatibility study in pharmaceutical mixtures. J Therm Anal Calorim. 2019;138(6):4531-9. doi: 10.1007/s10973-019-08223-7.
Majumdar R, Kar PK. Biosynthesis characterization and anthelmintic activity of silver nanoparticles of clerodendrum infortunatum isolate. Sci Rep. 2023;13(1):7415. doi: 10.1038/s41598-023-34221-9, PMID 37150767.
Avachat AM, Kapure SS. Asenapine maleate in situ forming biodegradable implant: an approach to enhance bioavailability. Int J Pharm. 2014;477(1-2):64-72. doi: 10.1016/j.ijpharm.2014.10.006, PMID 25305379.
Hatefi A, Amsden B. Biodegradable injectable in situ forming drug delivery systems. J Control Release. 2002;80(1-3):9-28. doi: 10.1016/s0168-3659(02)00008-1, PMID 11943384.
Ahmed TA, Alharby YA, El Helw AR, Hosny KM, El Say KM. Depot injectable atorvastatin biodegradable in situ gel: development optimization in vitro and in vivo evaluation. Drug Des Dev Ther. 2016 Jan 20;10:405-15. doi: 10.2147/DDDT.S98078, PMID 26855565.
Nadendla RR, Priyanka PV. Optimizing transdermal patch formulation for enhanced delivery of rivaroxaban: a comprehensive design of experiments approach. Int J Pharm Pharm Sci. 2024;16(12):8-20. doi: 10.22159/ijpps.2024v16i12.51075.
Rebbapragada D, Kalyanaraman R. Statistical optimization of medium components by plackett burman design and response surface methodology for enhanced antioxidant activity by xylaria feejeensis HMJAU22039. Int J Pharm Pharm Sci. 2016;8(11):159. doi: 10.22159/ijpps.2016v8i11.14257.
Rezaeian Shiadeh SN, Hadizadeh F, Khodaverdi E, Gorji Valokola M, Rakhshani S, Kamali H. Injectable in-situ forming depot based on PLGA and PLGA-PEG-PLGA for sustained release of risperidone: in vitro evaluation and pharmacokinetics in rabbits. Pharmaceutics. 2023;15(4):1229. doi: 10.3390/pharmaceutics15041229, PMID 37111714.
Farooqui P, Gude R. Formulation development and optimisation of fast dissolving buccal films loaded glimepiride solid dispersion with enhanced dissolution profile using central composite design. Int J Pharm Pharm Sci. 2023;15(6):35-54. doi: 10.22159/ijpps.2023v15i6.47992.
Ghaderi F, Nemati M, Siahi Shadbad MR, Valizadeh H, Monajjemzadeh F. Thermal stability and kinetic study of fluvoxamine stability in binary samples with lactose. Adv Pharm Bull. 2017;7(1):43-51. doi: 10.15171/apb.2017.006, PMID 28507936.
Choi MJ, Woo MR, Choi HG, Jin SG. Effects of polymers on the drug solubility and dissolution enhancement of poorly water-soluble rivaroxaban. Int J Mol Sci. 2022;23(16):9491. doi: 10.3390/ijms23169491, PMID 36012748.
Zhang X, Yang L, Zhang C, Liu D, Meng S, Zhang W. Effect of polymer permeability and solvent removal rate on in situ forming implants: drug burst release and microstructure. Pharmaceutics. 2019;11(10):520. doi: 10.3390/pharmaceutics11100520, PMID 31658642.
Ahmed TA, Alharby YA, El Helw AR, Hosny KM, El Say KM. Depot injectable atorvastatin biodegradable in situ gel: development optimization in vitro and in vivo evaluation. Drug Des Dev Ther. 2016;10:405-15. doi: 10.2147/DDDT.S98078, PMID 26855565.
Bode C, Kranz H, Kruszka A. In situ forming PLGA implants: how additives affect swelling and drug release. J Drug Deliv Sci Technol. 2019 Oct;53:101180. doi: 10.1016/j.jddst.2019.101180.