
Int J App Pharm, Vol 17, Issue 5, 2025, 444-453Original Article
STANDARDIZATION OF AN AYURVEDIC POLYHERBAL LIQUID FORMULATION WITH IMPROVED PALATABILITY FOR THE MANAGEMENT OF ANEMIA
KOPPERUNDEVI RAMACHANDRAN1, SHALINI KARUNANITHI2, DAMODHARAN NARAYANASAMY3*
1,3Department of Pharmaceutics, SRM College of Pharmacy, SRM Institute of Science and Technology, Kattankulathur-603203, Chengalpattu, India. 2Department of Pharmacognosy, SRM College of Pharmacy, SRM Institute of Science and Technology, Kattankulathur-603203, Chengalpattu, India
*Corresponding author: Damodharan Narayanasamy; *Email: damodhan@srmist.edu.in
Received: 16 Dec 2024, Revised and Accepted: 17 Jun 2025
ABSTRACT
Objective: This study aimed to develop and standardize a palatable Ayurvedic polyherbal oral liquid formulation for anaemia treatment. By integrating taste-masking techniques, the aim is to enhance iron absorption, reduce side effects, and improve patient adherence.
Methods: Four iron-rich Ayurvedic herbs, namely Punica granatum, Vitis vinifera, Boerhaavia diffusa, and Terminalia bellirica, were extracted and formulated with taste-masking excipients. The syrup formulation involved hydroalcoholic extraction, freeze-drying, and stability testing under accelerated and long-term conditions. Standardization parameters included organoleptic evaluation, physicochemical assessment, microbiological testing, and heavy metal residue analysis.
Results: The hydroalcoholic extraction of Terminalia bellirica fruit yielded 17.3%, while Boerhavia diffusa root extract yielded 9.3%. Freeze-dried fruit juices of Vitis vinifera and Punica granatum yielded 8.6% and 7.3%, respectively. A polyherbal syrup was formulated and optimized through six trials, balancing taste, viscosity (120–146 cP), and stability. The final formulation contained 12.5 mg iron per 5 ml, with synergistic herbal components enhancing bioavailability. Sensory evaluations confirmed improved palatability and stability studies demonstrated a prolonged shelf life. Heavy metal analysis confirmed compliance with safety standards, ensuring the formulation's suitability for anaemia management.
Conclusion: This Ayurvedic polyherbal liquid formulation, with its enhanced taste profile and iron-rich content, offers a novel and patient-friendly approach to anaemia management. Its improved palatability may enhance patient compliance, particularly in populations struggling with conventional iron supplements. Moreover, its formulation aligns with the growing demand for natural, well-tolerated therapeutic alternatives, underscoring its potential for commercialization. Further clinical studies are recommended to validate its efficacy, long-term safety, and market viability for broader therapeutic application.
Keywords: Anaemia, Ayurvedic polyherbal formulation, Iron deficiency, Taste-masking, Hemoglobin enhancement
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijap.2025v17i5.53419 Journal homepage: https://innovareacademics.in/journals/index.php/ijap
INTRODUCTION
A common global public health concern, anaemia primarily affects mothers and children in developing nations [1]. Anaemia remains a significant global health concern, particularly among women and children. Despite some reductions in moderate and severe cases, progress toward the World Health Assembly's goal of halving anaemia prevalence by 2030 has been insufficient [2]. Iron deficiency is a leading cause of anaemia, impairing blood oxygen transport and leading to symptoms such as weakness, fatigue, and weakened immunity [3]. In severe cases, it compromises immune function, increases susceptibility to infections, and negatively impacts cognitive development, potentially affecting long-term learning and academic performance [4]. Pregnant women with anaemia face heightened risks, including low birth weight, premature delivery, and maternal mortality, further underscoring its public health significance [5]. Individual health and quality of life are significantly impacted by anaemia, especially when it results from iron deficiency [6]. Traditional iron supplements are commonly prescribed for anaemia management; however, poor adherence due to their metallic taste, particularly among children and elderly patients, remains a challenge [7]. Additionally, iron absorption can be hindered by dietary factors, inflammation, or underlying health conditions, limiting treatment efficacy [8]. These limitations highlight the need for alternative formulations that improve palatability, enhance compliance, and address iron deficiency effectively. Ayurvedic medicine, with its holistic approach, offers a promising alternative for anaemia management. Ayurveda emphasizes treating diseases by restoring balance and preventing recurrence through natural therapeutic agents [9]. India’s vast biodiversity includes over 15,000 medicinal plants, many of which have demonstrated haematinic properties [10]. By combining iron-rich herbs with taste-masking strategies, alternative strategies like polyherbal formulations that include Ayurvedic principles may provide a workable solution that improves patient acceptance and therapeutic efficacy. With its comprehensive and customized approach, Ayurvedic medicine offers a viable treatment option for anaemia (Pandurog) [11, 12]. Traditionally utilized for their haematinic and tonic qualities, a variety of Ayurvedic herbs can be successfully blended in a polyherbal preparation to promote hemoglobin production and enhance general health [13, 14]. Polyherbal formulations, incorporating iron-rich herbs and taste-masking strategies, present a potential solution for improving patient acceptance and therapeutic efficacy. By leveraging synergistic interactions, these formulations may enhance iron absorption, minimize side effects, and improve compliance [15]. This study explores the potential of a proprietary Ayurvedic polyherbal liquid formulation designed to mask the metallic taste of iron while maintaining its therapeutic benefits. By addressing palatability issues and optimizing bioavailability, this approach aims to improve patient adherence, ensuring a more effective and sustainable solution for anaemia treatment.
MATERIALS AND METHODS
Preparation of extract
Terminalia bellirica (TB) fruit and Boerhavia diffusa (BD) roots were collected from Rettanai, Villipuram District-604306 and authenticated by Dr. K. N. Sunil Kumar, Research Officer and Head, Department of Pharmacognosy and Dr. P. Elankani, Research Officer, Scientist-III Siddha Central Research Institute, Arumbakkam, Chennai-600106 (Specimen Number: 852.15052401-04) begins with cleaning and drying the selected plant parts, which are then pulverized into coarse powder. For extraction, approximately 100 g of the powder is weighed and packed into a Soxhlet apparatus. A solvent mixture of ethanol and purified water in a 50:50 ratio is prepared, optimizing the extraction of both polar and nonpolar compounds. The Soxhlet extraction runs for 8 h, allowing continuous solvent circulation through the plant material, which helps dissolve and concentrate bioactive compounds. Following extraction, the hydroalcoholic extract is collected and concentrated using a rotary evaporator to remove excess solvent, yielding a potent extract. This procedure is then scaled up, repeating the extraction for 500 g of plant material to obtain a larger batch, ideal for further in vitro and in vivo pharmacological testing [16, 17].
Freeze-dried extract of VV and PG fruit juice
Fresh juice is first extracted from the whole grapes and the pulp of pomegranate fruits (fig. 1). This juice is then subjected to freeze drying, preserving active compounds by removing water at low temperatures. For freeze-drying, a coolant temperature of-8 °C is used, with a circulation flow rate set to 3000 ml/min to ensure consistent and uniform cooling throughout the process. The freeze-drying operation is maintained for 20 min, which allows for effective dehydration while minimizing the potential degradation of heat-sensitive compounds in the juice. The final yield of the freeze-dried juice concentrate achieves a concentration of 8% Brix (8 g of soluble solids such as sugars, polyphenols, and other phytochemicals, are present per 100 ml of extract, ensuring consistency in formulation, a measure of sugar content, indicating that the extract retains a high concentration of the fruit's natural components. This freeze-dried extract can then be used in further pharmacological studies to evaluate its potential bioactivity [18].

Fig. 1A: Synergistic blend of fruits used in polyherbal formulation for medicinal benefits
Pre-formulation studies
Each extract with excipients, prepared and loaded at 40 °C/75% RH, was stored for about 30 d. The physical parameters, such as description, pH, and solubility, were then checked [19]. The formulation composition of ingredients used is provided in table 1 for F1-F6, respectively. The manufacturing process begins by weighing and transferring a sorbitol solution into a stainless steel (S. S.) mixing vessel under mechanical stirring. Potassium sorbate is dissolved in purified water, filtered through a #200 nylon mesh, and added to the vessel. Freeze-dried powders of Vitis vinifera and Punica granatum were dissolved, filtered, and transferred similarly. Kassishaka Suddha was dissolved in purified water, followed by the addition of Polyoxyl 40 hydrogenated castor oil, which was previously dissolved in hot water. To this mixture, extracts of Boerhaavia diffusa and Terminalia bellirica were added, and the mixture was well mixed, filtered, and then transferred to the vessel. Hydroxyethyl cellulose is dispersed in glycerin, filtered through #100 mesh, and added to the mixture. Disodium EDTA, citric acid, and sodium citrate are each dissolved in purified water, filtered, and incorporated. The syrup is milled in a colloidal mill, then filtered, and taste-masking agents and grape flavor are blended in. The final volume is adjusted with purified water, thoroughly mixed, filtered through #200 mesh, and filled into 200 ml amber PET bottles. After initial analysis, the syrup is loaded in a stability chamber under accelerated (40 °C, 75% RH) and long-term (30 °C, 75% RH) conditions for stability testing.
Table 1: Formulation composition of different variants (F1–F6)
| Name of the ingredients | Input in percentage (%) | |||||
| F1 | F2 | F3 | F4 | F5 | F6 | |
| Punica granatum Fr. | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 |
| Vitis vinifera Fr. | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 |
| Boerhaaviadiffusa Rt. | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 |
| Terminalia bellirica Fr. | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 |
| Kassisaka Suddha | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 |
| Sobitol non-crystallizing | 50.00 | 50.00 | 50.00 | 50.00 | 50.00 | 50.00 |
| Glycerin | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| Potassium Sorbate | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 |
| Citric Acid | 0.10 | 0.30 | 0.35 | 0.35 | 0.35 | 0.35 |
| Xanthan Gum | 0.10 | 0.10 | - | - | - | - |
| Disodium EDTA | 0.05 | 0.05 | 0.50 | 0.05 | 0.05 | 0.05 |
| Polyoxyl 40 hydrogenated Castor Oil | 1.00 | 1.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Sucralose | - | 0.07 | 0.20 | 0.10 | 0.10 | 0.10 |
| Sodium Alginate | - | - | 0.50 | 0.50 | - | - |
| Hydroxy Ethyl cellulose | - | - | - | - | 1.00 | 0.50 |
| Purified Water | q. s | q. s | q. s | q. s | q. s | q. s |
Preparation of polyherbal liquid formulation
The preparation of the polyherbal liquid formulation begins with setting up clean, calibrated equipment, including an electronic weighing balance, stainless steel (S. S.) mixing vessels, a Remi mechanical stirrer, a colloidal mill, and an induction heater. The process starts by weighing and adding purified water as the primary solvent in the S. S. mixing vessel, followed by sorbitol and glycerin. With continuous stirring, citric acid and sodium citrate are added to adjust the pH. Next, hydroxyethyl cellulose is gradually incorporated to achieve the desired viscosity, and potassium sorbate and disodium EDTA are added as preservatives and stabilizers. Polyoxyl 40 hydrogenated castor oil is included as a solubilizer, and sucralose is added for sweetness. The plant extracts and Kassishaka Suddha (ferrous sulfate) were then introduced and homogenized using a colloidal mill for even dispersion. The grape flavor was added for taste, and the formulation was stirred continuously, with heating if necessary, to ensure complete dissolution and a stable mixture. Finally, the formulation is transferred to labeled storage containers and kept under specified conditions, resulting in a uniform polyherbal liquid formulation suitable for Ayurvedic applications.
Excipients used in the formulation
The formulation comprises various excipients, each serving a specific functional purpose. Sorbitol non-crystallizing acts as a solvent, while glycerin serves as a co-solvent and viscosity modifier. Potassium sorbate is included as a preservative, and citric acid functions as a pH modifier to maintain stability. Viscosity is adjusted using xanthan gum, hydroxypropyl methylcellulose, hydroxypropyl cellulose, and hydroxyethyl cellulose, each contributing to the formulation’s consistency. Disodium EDTA acts as a sequestering agent, and polyoxyl 40 hydrogenated castor oil, along with polysorbate 80, are used as solubilizers to ensure uniform dispersion of ingredients. Sucralose provides sweetness without impacting calorie content, and purified water serves as the primary vehicle for the formulation. Together, these excipients ensure the formulation's stability, efficacy, and desirable sensory properties.
Viscosity test
The viscosity of the polyherbal formulation solution can be determined using a rotational viscometer such as the Brookfield DV-II+Pro. First, the formulation should be homogenized and allowed to stand to remove air bubbles, ensuring accurate readings. The sample is maintained at a constant temperature (typically 25±0.5 °C) using a water bath, as viscosity is temperature-dependent. A suitable spindle, LV2, is selected based on the expected viscosity range, and the sample is poured into a beaker to the required level. The spindle is immersed in the solution, and the viscometer is set to an appropriate speed (20 or 50 rpm). Once the reading stabilizes (after 30–60 seconds), the viscosity is recorded in centipoise (cP). Multiple readings are taken at different speeds to account for shear dependency in non-Newtonian fluids. The process is repeated thrice to ensure accuracy, and the mean viscosity is reported along with the temperature. This method follows the guidelines of USP<912>Viscosity.
Quantitative estimation of iron content in polyherbal formulation by atomic absorption spectroscopy (AAS)
The iron content in the polyherbal formulation was estimated using AAS at a wavelength of 248.3 nm. Standard iron solutions (0.5–5 ppm) were prepared to construct a calibration curve. The sample was digested using a nitric and perchloric acid mixture, filtered, and diluted to a known volume with deionized water. After calibrating the AAS with the standards, the digested sample's absorbance was measured, and the iron concentration was determined using the calibration curve, with reagent blanks ensuring baseline correction. The iron content was calculated in mg/g based on the dilution factor and sample weight. The analysis was performed in triplicate for accuracy. This method complies with AOAC Official Method 999.11 and validated protocols, providing a reliable quantification of iron in the formulation.
Taste evaluation
The sensory evaluation was conducted using a simple score method, allowing participants to rate specific attributes of the sample, such as taste, texture, aroma, and overall acceptability. The data collected from this scoring were then statistically analyzed using the Randomized Complete Block Design to control for variability among the participants. Specifically, the Friedman test was applied, a non-parametric method suitable for analyzing ranked or ordinal data across multiple conditions or treatments. Following this initial analysis, an Analysis of Variance was performed to identify significant differences between samples. A post hoc test, the Least Significant Difference test, was used at a 95% confidence level to pinpoint which specific samples differed from each other. Statistical analysis was performed using SPSS version 1, ensuring robust and reliable interpretation of the sensory data.
Heavy metal test
The analysis of heavy metals and trace elements involves precise preparation and measurement techniques to ensure accurate quantification. Arsenic (As), Cadmium (Cd), Lead (Pb), and Mercury (Hg) were specifically chosen as they are the most commonly monitored contaminants in herbal formulations, primarily due to potential environmental exposure from soil, water, and agricultural practices. These metals can pose significant health risks if present above permissible limits. The process begins with sample preparation, where a specific amount of the formulation is digested using a mixture of concentrated nitric acid and perchloric acid, followed by dilution with deionized water. Calibration standards for each element, prepared using certified reference materials, are used to establish a range of concentrations for accurate measurement. For heavy metals such as arsenic, cadmium, lead, and mercury, techniques like Inductively Coupled Plasma Mass Spectrometry or AAS are employed, while trace elements like iron, copper, and zinc are analyzed using Inductively Coupled Plasma Optical Emission Spectrometry or Flame AAS. Quality control measures, including reagent blanks, matrix-matched standards, and spiked samples, are implemented to validate the accuracy and precision of the results. The data is analyzed by calculating concentrations using calibration curves and expressed in ppm or µg/g. Finally, the results are compared against regulatory guidelines to ensure compliance, and the findings are reported in tabular form for evaluation.
Stability testing of a polyherbal oral liquid formulation: accelerated and long-term assessments
The stability testing for the polyherbal oral liquid formulation developed as an Ayurvedic proprietary medicine aims to assess both accelerated and long-term stability. For the accelerated stability study, samples are stored at 40 °C±2 °C and 75% RH±5%, with testing at initial (0 mo), 3rd, and 6th mo. These conditions align with ICH guidelines for accelerated stability testing, which are widely accepted for assessing the long-term stability of herbal and Ayurvedic preparations. Given that Ayurvedic formulations often contain bioactive phytochemicals sensitive to temperature and humidity, these conditions help predict potential degradation pathways, ensuring product efficacy and shelf life. Additionally, the high humidity conditions mimic tropical climates where Ayurvedic medicines are commonly used, making the findings more relevant for real-world storage conditions. Long-term stability testing is conducted at 30 °C±2 °C and 75% RH±5%, with evaluations at initial (0 mo), 3rd, 6th, 9th, 12th, 18th, and 24th mo. Stability parameters to be analyzed include the description of the formulation, pH, water-soluble extractive value, weight per ml, HPTLC profile, and the assay of iron content using AAS. These tests provide insights into the formulation's stability over time and under various conditions. The stability study on the polyherbal oral liquid formulation aimed to evaluate the product’s stability under both accelerated and long-term storage conditions, ensuring its quality, efficacy, and safety over time. The product was stored at 40 °C ±2 °C with 75% RH ±5% to simulate accelerated aging. Testing was conducted at 0 (initial), 3, and 6 mo. This setup provides insight into the formulation's potential shelf life by observing the changes that occur under higher temperature and humidity conditions. The product was also tested under more moderate storage conditions at 30 °C ±2 °C with 75% RH ±5%, which aligns with typical storage environments. Testing intervals were more extended, including 0 (initial), 3, 6, 9, 12, 18, and 24 mo. This approach allows for a comprehensive evaluation of the formulation’s stability over a two-year period, reflecting its behavior in real-world conditions.
Preparation and evaluation of microbial cultures for antimicrobial effectiveness testing
Prepare cultures of the specified microorganisms Escherichia coli (NCIM 2065), Staphylococcus aureus (NCIM 2079), Pseudomonas aeruginosa (NCIM 2200), Candida albicans (NCIM 3471), Aspergillusbrasiliensis (NCIM 1196) in the required growth media using Soybean Casein Digest Agar for bacterial cultures and Sabouraud Dextrose Agar for fungal cultures. Each microorganism's inoculum is then standardized in a 0.9% saline solution to ensure an appropriate concentration according to USP<51>guidelines. Aseptically introduce this inoculum to the formulation containing 0.2% potassium sorbate and mix thoroughly to ensure an even distribution of microorganisms. The inoculated samples are then placed in a BOD incubator, with temperatures set to 20-25 °C for fungi and 30-35 °C for bacteria, to foster optimal microbial growth conditions. At predetermined intervals (such as 7, 14, and 28 d), samples are withdrawn aseptically and plated on SCDA or SDA to assess microbial counts. Finally, microbial reduction is calculated and analyzed over time using standard statistical methods to confirm the formulation’s antimicrobial effectiveness.
RESULTS
Extract percentage yield
The extraction yields from the selected plant materials varied based on the type of plant part and extraction method used. The hydroalcoholic extraction of Terminalia bellirica fruit produced a yield of 17.3%, indicating a relatively high extraction efficiency, likely due to the presence of abundant bioactive compounds in the fruit. Similarly, the hydroalcoholic extraction of Boerhavia diffusa root resulted in a yield of 9.3%, which reflects the compound concentration typical in root tissues. Additionally, freeze-drying of fruit juices was conducted for Vitis vinifera (grape) and Punica granatum (Pomegranate), yielding 8.6% and 7.3%, respectively. The relatively lower yields in freeze-dried fruit juices compared to hydroalcoholic extracts could be attributed to the different extraction processes and the nature of compounds present in the fruit juices. These extraction yields provide valuable insights into the efficiency of the methods used and the potential bioactive content available for further analysis.
Formulation development and optimization
Each plant is selected for its specific therapeutic role, such as Punica granatum for managing Raktapitta (bleeding disorders) or Boerhaavia diffusa for its rejuvenating properties in Pandu (anemia). The dosages align with Ayurvedic Pharmacopoeia of India (API) standards to ensure safety and efficacy in table 2, respectively. This table highlights the importance of traditional Ayurvedic plants in treating anemia and related conditions.
The initial trial focuses on balancing key active ingredients, including herbal extracts like Punica granatum and Boerhaavia diffusa, with excipients for stability and palatability. Glycerin and sorbitol serve as sweeteners and stabilizers, while potassium sorbate and EDTA are included as preservatives as observed in Trail-1, respectively. This trial modifies the proportions of grape juice and citric acid to improve flavor and antioxidant potential. Sucralose is added to enhance sweetness without increasing caloric content, making the formulation more acceptable for patients, as mentioned in Trail-2, respectively. Further refinement includes the addition of sodium alginate for better viscosity and uniform dispersion of active ingredients. Increased citric acid concentration enhances the taste and stabilizes the pH, as mentioned in Trail-3, respectively. Table 3 presents a scaled-up formulation designed for batch production. Ingredients like sorbitol, glycerin, and citric acid are optimized to ensure long-term stability, while hydroxyethyl cellulose provides improved texture and mouthfeel, as mentioned in Trail-4, respectively.
Table 2: Traditional medicinal plants and their uses with dosage references
| Name of the plant | Dose | Use* | Reference |
| Dadima (Punica granatum) Fr. Juice | 2.1 gm-4.5 gm (15 ml to 30 ml)/d | Raktspitta | API, Part-I, Vol.2, Page no.2 0 to26 |
| Punarnava (Boerhaavia diffusa) Rt. Extract | 1 to 3 gm/d | Pandu, Hrdroga | API, Part-I, Volume-III, Page No.33-34 |
| Grapes (Vitis vinivera) Fr. Juice | 5 gm to 10 gm/d | Raktspitta, Pandu, | API, Part I, Vol. 3, Page 45 to 46 |
| Bibitaki (Terminalia bellirica) Fr. Extract | 3 to 6 gm/d | Vibandha | API, Part-I, Volume-I, Page No.33-34 |
| KasisakaSuddha (Ferrous Sulphate) | 60 to 250 mg/d | Pandu, | API, Part-I, Volume-VII, Page No.19-21 |
*API-Ayurvedic Pharmacopoeia of India
Table 3: Comparative analysis of formulation trials: ingredient composition across different trials and finalized formulation
| Name of the ingredients | Trial 1 (Each 5 ml) | Trial 2 (Each 5 ml) | Trial 3 (Each 5 ml) | Trial 4 (Each 5 ml) |
Trial 5 (Each 5 ml) | Trial 6 (Each 5 ml) finalized |
| Dadima (Punica granatum) Fr. Juice | 500.00 mg | 500.00 mg | 500.00 mg | 500.00 mg | 500.00 mg | 500.00 mg |
| Punarnava (Boerhaavia diffusa) Rt. Extract | 500.00 mg | 500.00 mg | 500.00 mg | 500.00 mg | 500.00 mg | 500.00 mg |
| Grapes (Vitis vinivera) Fr. Juice | 500.00 mg | 100.00 mg | 100.00 mg | 100.00 mg | 100.00 mg | 100.00 mg |
| Bibitaki (Terminalia bellirica) Fr. Extract | 500.00 mg | 500.00 mg | 500.00 mg | 500.00 mg | 500.00 mg | 500.00 mg |
| KasisakaSuddha (Ferrous Sulphate) | 12.50 mg | 12.50 mg | 12.50 mg | 12.5 mg | 12.50 mg | 12.50 mg |
| Sobitol non-crystallizing | 2500.00 mg | 2500.00 mg | 2500.00 mg | 2500.00 mg | 2500.00 mg | 2500.00 mg |
| Glycerin | 250.00 mg | 250.00 mg | 250.00 mg | 250.00 mg | 250.00 mg | 250.00 mg |
| Potassium Sorbate | 10.00 mg | 10.00 mg | 10.00 mg | 10.00 mg | 10.00 mg | 10.00 mg |
| Citric Acid | 5.00 mg | 15.00 mg | 17.50 mg | 17.50 mg | 17.50 mg | 17.50 mg |
| Xanthan Gum | 5.00 mg | 5.00 mg | - | - | - | - |
| Sodium Alginate | - | - | 50.00 mg | 25.00 mg | - | - |
| Hydroxy Ethyl Cellulose | - | - | - | - | 50.00 mg | 25.00 mg |
| Disodium EDTA | 0.25 mg | 0.25 mg | 0.25 mg | 0.25 mg | 0.25 mg | 0.25 mg |
| Polyoxyl 40 hydrogenated castor oil | 100.00 mg | 100.00 mg | 100.00 mg | 100.00 mg | 100.00 mg | 100.00 mg |
| Sucralose | - | 0.35 mg | 10.00 mg | 5.00 mg | 5.00 mg | 5.00 mg |
| Purified water | q. s. | q. s. | q. s. | q. s. | q. s. | q. s. |
In this trial, the composition is fine-tuned by increasing the concentration of sucralose and introducing hydroxyethyl cellulose for enhanced viscosity and taste masking. This marks a near-final iteration, balancing efficacy with patient compliance, as mentioned in Trail-5, respectively. The finalized formula achieves the desired taste, texture, and stability balance. The inclusion of "Fantasy Fruit masking" flavor ensures palatability, while the active herbal components maintain their therapeutic efficacy. This formulation is ready for clinical validation and scale-up, as mentioned in Trail-6, respectively. Fig. 2 showcases the finalized formulation's appearance, highlighting its deep red color due to the iron-rich and polyphenolic content. The homogeneity ensures consistent dosing, while the viscosity reflects its ease of administration. This visually demonstrates the formulation's readiness for therapeutic application.
Flavor selection studies emphasize the importance of patient acceptability in polyherbal formulations. The trials determined that "Fantasy Fruit masking" was most effective in masking the metallic taste of iron, followed by other options like orange and strawberry, as mentioned in table 4, respectively.
Evaluation of syrup taste using time-intensity sensory analysis
The taste of the syrup was evaluated using a time-intensity method with 10 human volunteers. Various market samples and an iron solution equivalent to a normal 5 ml dose were held in the mouth for 60 seconds. Volunteers were asked to assess the taste, focusing on bitterness and metallic notes at intervals of 2, 10, and 60 seconds. Bitterness intensity was rated on a numerical scale compared to the pure drug (12.5 mg of ferrous sulfate in a sorbitol and water solution): 3 for strong bitterness, 2 for moderate bitterness, 1 for slight bitterness, X for threshold bitterness, and 0 for no bitterness. Volunteers were instructed not to swallow the granules placed on their tongue and to rinse their mouths thoroughly with distilled water after each test.

Fig. 2: The final polyherbal formulation for anaemia treatment is shown. The formulation appears as a deep red liquid, characteristic of its iron-rich content and the inclusion of natural pigments from herbal constituents. The beaker (left) displays the prepared bulk formulation, while the Petri dish (right) highlights the homogeneity and viscosity, ensuring uniform dosing. The rich coloration indicates the presence of bioactive compounds, which are hypothesized to support hematopoiesis and enhance iron bioavailability, contributing to its efficacy as a hematinic agent.
Table 4: Flavour selected based on the taste evaluation studies
| Name of the flavours | Concentration range used for the trials |
| Orange Flavour | 0.05% to 0.3 % |
| Strawberry flavour | 0.05 to 0.2 % |
| Fantasy Fruit masking (Guividon) | 0.20 % |
| Grape Flavour | 0.30% |
Viscosity evaluation and optimization of polyherbal formulation
The viscosity of the polyherbal formulation solution was assessed across six trials, with values ranging from 120 to 150 cP in Trial 1 and 120 to 146 cP in Trial 3. Trial 1 exhibited a dark brown syrup with significant bitterness and a metallic taste. In Trial 3, bitterness was reduced, but the metallic taste persisted, and precipitation was observed after a week. Subsequent trials introduced stabilizing agents such as Hydroxy Ethyl Cellulose (Trial 5) and other adjustments to improve rheological properties and stability. The finalized formulation (Trial 6) demonstrated enhanced stability, optimized viscosity, and acceptable taste, making it suitable for therapeutic applications.
Evaluation of the formulation for anaemia treatment
The iron content in the polyherbal formulation was determined to be 12.5 mg/5 ml, aligning with the formulation's design specifications. This concentration is adequate to address iron deficiency anaemia, as it falls within the therapeutic range recommended for iron supplementation in mild to moderate cases of anaemia. The formulation also contains synergistic ingredients such as Punica granatum (Pomegranate) and Boerhaaviadiffusa, which enhance iron absorption and provide antioxidant support to combat oxidative stress associated with anaemia. The presence of non-crystallizing sorbitol and citric acid aids in improving the bioavailability of iron by preventing its precipitation and enhancing solubility in the gastrointestinal tract. Further, the optimized formulation demonstrated excellent stability, with a viscosity of 120–146 cP and a pH of 5.1–4.56, ensuring compatibility with the gastrointestinal environment. Sensory modifications, including the addition of sucralose and citric acid, significantly reduced the metallic taste, improving patient compliance. The high specific gravity (1.135–1.150) ensures a consistent dosage delivery. Overall, the formulation provides a promising solution for managing anaemia.
Stability studies
The accelerated stability testing at the 3-and 6-month points offers a rapid assessment of how the product might behave over a longer period, providing preliminary evidence of stability under stress conditions. If the formulation remains stable under these accelerated conditions, it suggests it will likely retain stability under normal storage conditions over its intended shelf life. Long-term storage testing, spanning up to 24 mo, provides a comprehensive view of the formulation’s durability under typical conditions, affirming that it can be safely stored and used without compromising its efficacy or safety. Overall, stability under both accelerated and long-term conditions supports the product’s extended shelf life and consistent performance, confirming its suitability as an Ayurvedic proprietary formulation for prolonged use
Heavy metals
The heavy metals and trace elemental analysis conducted for the selected polyherbal formulation revealed that all were under the permissible limits as per the Indian Pharmacopoeia. The Arsenic, Cadmium, and Lead were in the permissible levels and the presence of Mercury in the formulation was not detected and listed in table 5.
Antimicrobial test
The antimicrobial efficacy of the formulated polyherbal Ayurvedic oral liquid was assessed against a panel of microorganisms, including both bacterial and fungal species. The study focused on evaluating the microbial challenge count and the inoculation concentrations to establish the product's microbial safety and efficacy. Table 6 provides the initial microbial load (CFU/ml) of test organisms used in the study. The microorganisms selected are commonly associated with microbial contamination and represent both Gram-positive and Gram-negative bacteria, along with fungal species. The microbial loads were carefully quantified to ensure consistency in testing.
Table 5: Heavy metal analysis of formulations
| Elements (ppm) | F1 | F2 | F3 | F4 | F5 | F6 |
| Arsenic (AS) | 0.056±0.001 | BDL | BDL | BDL | BDL | BDL |
| Cadmium (Cd) | 0.031±0.002* | 0.035±0.002* | 0.027±0.003* | 0.017±0.003* | 0.026±0.003* | 0.036±0.004* |
| Lead (Pb) | 1.042±0.005* | 0.971±0.009* | 0.989±0.003* | 0.881±0.007* | 0.407±0.017* | 0.768±0.121* |
| Mercury (Hg) | BDL | BDL | BDL | BDL | BDL | BDL |
| Aluminium (Al) | 0.692±0.004* | 0.761±0.002* | 0.863±0.006* | 0.941±0.129* | 1.619±0.041* | 1.072±0.003” |
| Calcium (Ca) | 0.752±0.005* | 0.645±0.006* | 0.978±0.007* | 0.874±0.009* | 0.909±0.005* | 1.125±0.478* |
| Chromium (Cr) | 0.161±0.006* | 0.153±0.112* | 0.318±0.04* | 0.502±0.051* | 0.151±0.033* | 0.297±0.009* |
| Copper (Cu) | 0.418±0.051* | 0.448±0.028* | 0.394±0.006* | 0.402±0.049* | 0.415±0.012* | 0.384±0.015* |
| Cobalt (Co) | 0.264±0.007* | 0.314±0.006* | 0.102±0.018* | 0.191±0.004* | 0.221±0.013* | 0.209±0.012* |
| Iron (Fe) | 5.152±0.412* | 4.293±0.038* | 2.069±0.021* | 2.77±0.035* | 6.981±0.027* | 4.561±0.025* |
| Potassium (K) | 0.848±0.003* | 0.762±0.005* | 1.288±0.478* | 1.089±0.001* | 1.995±0.008* | 2.0769±0.012* |
| Magnesium (Mg) | 2.209±0.008* | 3.871±0.015* | 2.969±0.004* | 4.391±0.395* | 3.121±0.027* | 3.056±0.056* |
| Nickel (Ni) | 1.565±0.014* | 1.663±0.006* | 1.039±0.001* | 1.106±0.012* | 2.545±0.008* | 3.028±0.009* |
| Sodium (Na) | 2.911±0.006* | 1.913±0.062* | 2.893±0.028* | 3.837±0.013* | 2.614±0.008* | 1.848±0.017* |
All values are expressed as mean±standard deviation (SD) (n = 3). "BDL" indicates that the element was below detectable limit in the respective formulation. Statistical analysis was performed using one-way ANOVA followed by Tukey’s post-hoc test using GraphPad Prism 8.4.3. p-values* indicate significant differences within a row (p < 0.05).
Table 6: Sample preparation and initial challenge count for test organisms
| S. No. | Test organism | Initial challenge count (CFU/ml) |
| Escherichia coli | 1.68 x 108 | |
| Staphylococcus aureus | 1.75 x 108 | |
| Pseudomonas aeruginosa | 1.50 x 108 | |
| Candida albicans | 1.74 x 108 | |
| Aspergillusbrasiliensis | 7.80 x107 |
A consistent inoculum volume of 0.1 ml was used for all microorganisms, ensuring uniformity across the test. Each test was conducted with 20 g of the product. The final microbial concentrations are expressed in CFU/g and Log10 values to enable comparative analysis. The inclusion of an uninoculated sample (control) ensures baseline reference for product sterility. Table 7 details the method for preparing the test samples, specifying the quantity of product and inoculum used, as well as the final microbial concentration after inoculation. The Log10 values reflect the microbial load, which is critical for understanding the impact of the product on each organism, highlighting potential antimicrobial activity or stability.
Table 7: Sample preparation and inoculation concentrations for test organisms
| S. No. | Test organism | Quantity taken | Final concentration after inoculum | ||
| Inoculum (ml) | Product (g) | CFU/g | Log10 | ||
| E. coli | 0.1 | 20 | 8.40 x 105 | 5.92 | |
| S. aureus | 0.1 | 20 | 8.75 x 105 | 5.94 | |
| P. aeruginosa | 0.1 | 20 | 7.50 x 105 | 5.88 | |
| C. albicans | 0.1 | 20 | 8.70 x 105 | 5.94 | |
| A. brasiliensis | 0.1 | 20 | 3.90 x 105 | 5.59 | |
| Un un-inoculated sample | NA | 20 | NA | NA | |
Each test organism was inoculated using 0.1 ml of standardized culture into 20 g of the formulation. Final microbial concentrations are expressed as colony-forming units per g (CFU/g) and corresponding Log₁₀ values. The uninoculated control was used to confirm product sterility. Each microbial strain was inoculated in triplicate. CFU: Colony Forming Units; NA: Not Applicable. Final concentrations were calculated based on standardized inoculum volumes and are expressed in CFU per g of product.
Initial analysis (0 H)
The initial microbial challenge for each organism showed high inoculum concentrations (105-108 CFU/ml) to evaluate the preservative's potency. Log reductions in CFU counts immediately after the inoculation were generally low (0.18-1.04 log10), indicating minimal preservative impact on microbial counts at this early stage, as expected. Table 8 records the microbial load immediately after inoculation, providing baseline data to assess the preservative impact.
Table 8: Initial analysis (0 H) microbial counts and log reduction for test organisms
| S. No. | Test organism | Final concentration of the inoculum | Observed count | Log reduction in Log10 (A-B) | ||
| CFU/g | Log10 value (A) | CFU/g | Log10 value(B) | |||
| E. coli | 8.40 x 105 | 5.92 | 1.33 x 105 | 5.12 | 0.80 | |
| S. aureus | 8.75 x 105 | 5.94 | 2.32 x 105 | 5.37 | 0.57 | |
| P. aeruginosa | 7.50 x 105 | 5.88 | 6.90 x 105 | 4.84 | 1.04 | |
| C. albicans | 8.70 x 105 | 5.94 | 5.70 x 105 | 5.76 | 0.18 | |
| A. brasiliensis | 3.90 x 105 | 5.59 | 7.70 x 105 | 4.89 | 0.70 | |
| Un inoculated sample | NA | NA | <10 | NA | NA | |
Initial microbial challenge counts were calculated immediately after inoculation (0 h). Log₁₀ reductions represent the difference between the logarithmic values of the inoculum concentration (A) and the observed count (B). Slight reductions at this stage are expected prior to preservative activity onset. Control samples confirmed product sterility. Data expressed as mean of triplicates (n = 3).
14 d analysis
After 14 d, a significant reduction in CFU/ml was observed across all microbial strains, such as E. coli, S. aureus, P. aeruginosa, and C. albicans demonstrated complete inhibition, with counts falling below the detection limit (<10 CFU/ml), corresponding to log reductions of approximately 4.88-4.94 as discussed in table 9 respectively. This indicates the strong efficacy of potassium sorbate against these bacteria and yeast within two weeks. A. brasiliensis exhibited a moderate reduction, from an initial count of 3.90 x 105 to 3.90 x 103 CFU/ml, resulting in a 2.00 log reduction. While effective, this result suggests a relatively higher resistance of A. brasiliensis to the preservative compared to the other organisms.
28 d analysis
At 28 d, results confirmed sustained preservative efficacy of E. coli, S. aureus, P. aeruginosa, and C. albicans remained below the detection limit (<10 CFU/ml), maintaining their log reductions from the 14 d analysis with no re-growth as discussed in table 10 respectively. A. brasiliensis exhibited an additional decrease, reaching<10 CFU/ml, thus achieving a total log reduction of 4.59, indicating the preservative’s continued effect and eventual control of fungal growth.
Table 9: Microbial counts and log reduction for test organisms at 14 d analysis
| S. No. | Test organism | Final concentration of the inoculum | Observed count | Log reduction in Log10 (A-B) |
| CFU/g | Log10 value (A) | CFU/g | ||
| E. coli | 8.40 x 105 | 5.92 | <10 | |
| S. aureus | 8.75 x 105 | 5.94 | <10 | |
| P. aeruginosa | 7.50 x 105 | 5.88 | <10 | |
| C. albicans | 8.70 x 105 | 5.94 | <10 | |
| A. brasiliensis | 3.90 x 105 | 5.59 | 3.90 x 103 | |
| Un inoculated sample | NA | NA | <10 |
Log₁₀ value of 1.00 was assigned for samples with microbial counts<10 CFU/g to facilitate log reduction calculations in accordance with USP<51>guidelines. All values represent the mean of three replicates (n = 3). A log reduction ≥3 is indicative of effective preservative activity. The uninoculated sample confirmed formulation sterility.
Table 10: Microbial counts and log reduction for test organisms at 28 d analysis
| S. No. | Test organism | Final concentration of the inoculum | Observed count | Log reduction in Log10 (A-B) | ||
| CFU/g | Log10 value (A) | CFU/g | Log10 value (B) | |||
| E. coli | 8.40 x 105 | 5.92 | <10 | 1 | 4.92 | |
| S. aureus | 8.75 x 105 | 5.94 | <10 | 1 | 4.94 | |
| P. aeruginosa | 7.50 x 105 | 5.88 | <10 | 1 | 4.88 | |
| C. albicans | 8.70 x 105 | 5.94 | <10 | 1 | 4.94 | |
| A. brasiliensis | 3.90 x 105 | 5.59 | <10 | 1 | 4.59 | |
| Un inoculated sample | NA | NA | <10 | NA | NA | |
Microbial counts<10 CFU/g were assigned a log value of 1.00 for conservative calculation of log₁₀ reductions, following USP<51>guidelines. All values represent the mean of triplicates (n = 3). A reduction of ≥3 log₁₀ is considered acceptable preservative efficacy. The uninoculated sample confirmed the absence of contamination.
All bacterial and yeast strains showed a log reduction greater than the minimum requirement (1.0 log reduction at 14 d with no increase at 28 d) as mentioned in table 11, respectively. The fungal strain A. brasiliensis displayed delayed susceptibility, showing a 2.00 log reduction by day 14 and further reduction by day 28, meeting the acceptance criteria by maintaining reduced counts over time.
Table 11: Log reductions and acceptance criteria compliance for test organisms
| S. No. | Test organism | Log reduction | Acceptance criteria | |
| After 14 d | After 28 d | |||
| E. coli | 4.92 | 4.92 | Not less than 1.0 log reduction from the initial count at 14 d, and no increase from the 14 d count at 28 d. | |
| S. aureus | 4.94 | 4.94 | ||
| P. aeruginosa | 4.88 | 4.88 | ||
| C. albicans | 4.94 | 4.94 | “No increase” from the initial calculated count at 14 and 28 d. | |
| A. brasiliensis | 2.00 | 4.59 | ||
All values represent log reductions (log₁₀) in colony-forming units (CFU/g). Results were evaluated as per the USP<51>Antimicrobial Effectiveness Test guidelines. The polyherbal formulation met the required microbial reduction criteria across all test organisms.
The antimicrobial efficacy of various formulations, tested using the agar well diffusion method, is represented in fig. 3 respectively. The clear zones of inhibition around the wells indicate the effectiveness of the samples against microbial growth. The observed inhibition zones validate the presence of antimicrobial properties in the test formulations. This supports their potential for application in microbial contamination control, though quantitative analysis is recommended to compare their relative efficacy accurately. Further studies could refine the most effective formulations based on these qualitative results. Stability and consistency in antimicrobial activity across storage conditions should also be evaluated.
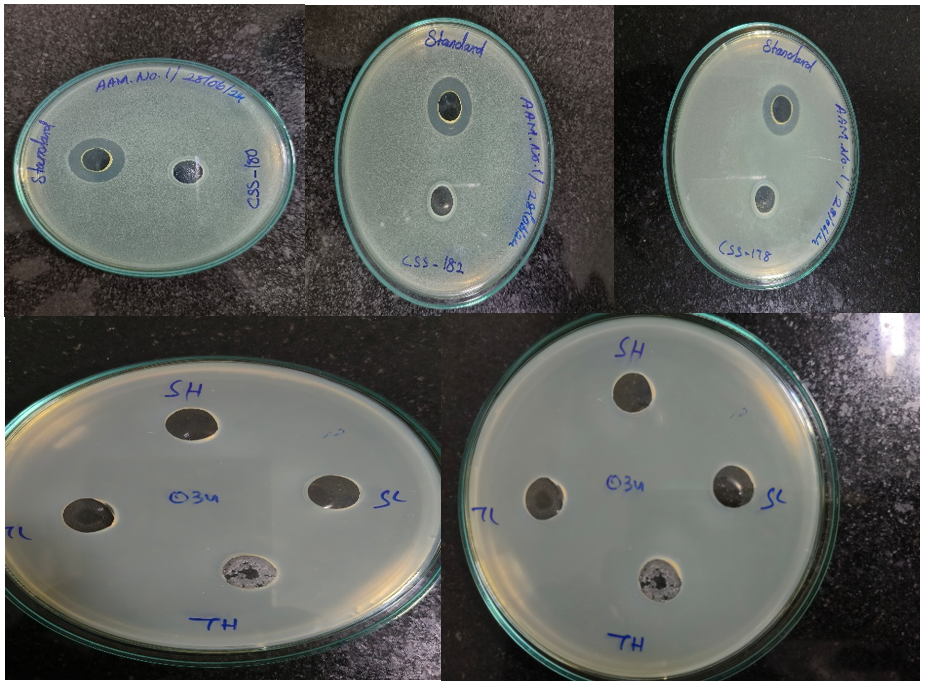
Fig. 3: The fig. illustrates antimicrobial activity assessed through the agar well diffusion method, with zones of inhibition indicating the effectiveness of tested samples against microbial growth. The wells labelled as "Standard" consistently exhibit large zones of inhibition, serving as a positive control to validate the assay. Test samples, including those labelled CSS-50, CSS-182, and CSS-178, show varying levels of antimicrobial activity, as evidenced by the differing sizes of inhibition zones. In the lower plates, samples labelled "SH," "SL," "TH," and "TL" also display antimicrobial activity, with zone sizes suggesting variability in potency, possibly due to differences in concentrations or formulations. The variation in inhibition zones among the test samples highlights differences in antimicrobial efficacy, suggesting some formulations or compounds are more effective than others. These findings demonstrate the presence of antimicrobial properties in the tested samples, warranting further quantitative analysis to compare their relative effectiveness accurately
Table 12: Recovery analysis of test organisms using sample and control tubes
| Test organism | Inoculum added final conc. (CFU/ml) | Sample tube | Control tube | ||||||
| P1 | P2 | Avg. | % of Recovery | P1 | P2 | Avg. | % of Recovery | ||
| E. coli | 84 | 65 | 73 | 69 | 82 | 73 | 79 | 76 | 90 |
| S. aureus | 88 | 67 | 71 | 69 | 78 | 72 | 82 | 77 | 88 |
| P. aeruginosa | 75 | 60 | 64 | 62 | 83 | 70 | 60 | 65 | 87 |
| C. albicans | 87 | 76 | 72 | 74 | 85 | 83 | 77 | 80 | 92 |
| A. brasiliensis | 78 | 65 | 55 | 60 | 77 | 65 | 69 | 67 | 86 |
CFU-Colony Forming Unit; P1-Plate 1; P2-Plate 2
Test suitability
The test suitability results indicate the recovery rates of various microbial organisms when inoculated in both sample and control tubes. This helps assess whether the test conditions are appropriate for supporting microbial growth, a crucial step in confirming the validity of microbial testing. For each test organism, two plates (P1 and P2) were used to measure colony-forming units (CFUs) and determine the average percentage of recovery. In the sample tube, the average recovery percentages ranged from 60% to 74% across the organisms, while in the control tube, recovery percentages were consistently higher, ranging from 76% to 92%. E. coli had a recovery of 69% in the sample and 90% in the control (table 12). S. aureus showed 69% in the sample and 88% in the control. P. aeruginosa exhibited 62% in the sample and 87% in the control. C. albicans had 74% recovery in the sample and 92% in the control. A. brasiliensis showed 60% recovery in the sample and 86% in the control. The lower recovery in sample tubes compared to control tubes indicates the effectiveness of the sample conditions in inhibiting microbial growth, which validates the preservation method’s ability to control contamination within the formulation. The control tubes showing higher recovery confirm that the testing environment is suitable for microbial growth, verifying that any reduction in CFUs in the sample tube is due to the formulation’s inhibitory effect, not testing limitations.
DISCUSSION
The standardization results are pivotal in ensuring that the polyherbal liquid formulation adheres to quality benchmarks that align with therapeutic efficacy and safety [20]. Standardization minimizes batch-to-batch variations, ensuring that every dose delivers consistent therapeutic effects [21]. This is especially critical for polyherbal formulations, as variations in phytochemical content due to plant origin, extraction methods, or processing can affect outcomes. The organoleptic properties, including taste, color, and aroma, are crucial for user acceptability. A pleasant taste and smell can significantly improve adherence to the treatment, especially in populations such as children and elderly patients who may otherwise struggle with bitter or unpalatable medications [22, 23]. Similarly, the physicochemical properties, such as pH and viscosity, ensure stability, bioavailability, and ease of administration. For instance, maintaining an optimal pH prevents the degradation of active compounds [24], while appropriate viscosity aids in uniform dosing and absorption [25]. These properties also contribute to the formulation’s shelf life, offering long-term usability. Furthermore, safety considerations such as the absence of heavy metals, microbial contamination, and harmful additives reinforce its acceptability as a therapeutic option [26]. By ensuring that the formulation meets stringent safety parameters, it positions itself as a reliable alternative in the market. The polyherbal liquid formulation offers distinct advantages over existing Ayurvedic and allopathic treatments for anaemia such as allopathic iron supplements focus primarily on hemoglobin restoration, they often come with side effects like gastrointestinal discomfort (e. g., constipation or dark-colored stools) [27]. The polyherbal approach addresses not just hemoglobin levels but also symptoms like fatigue, poor immunity, and reduced vitality, providing a more comprehensive treatment. Although, traditional ayurvedic treatments, such as powders or capsules, may have better absorption rates [28], the liquid formulation ensures quicker and more efficient absorption of active ingredients, which is particularly beneficial for patients with compromised digestive systems. The liquid form makes the formulation more suitable for populations with swallowing difficulties, such as children or elderly individuals. Its palatable taste further enhances compliance [29, 30], a critical factor in managing chronic conditions like anaemia. The absence of synthetic additives and the use of natural ingredients make the formulation less likely to cause side effects. This positions it as a safer, patient-friendly alternative to allopathic treatments, particularly for long-term use. The polyherbal formulation’s hematinic properties can be attributed to the synergistic action of its phytochemical components, which target multiple aspects of hematopoiesis and iron metabolism [31]. Emblicaofficinalis (Amla) is a rich source of vitamin C, a crucial co-factor in enhancing non-heme iron absorption in the gastrointestinal tract [32, 33]. This ensures efficient utilization of dietary iron, addressing one of the key challenges in anaemia management. Withaniasomnifera (Ashwagandha) known for its adaptogenic and rejuvenating properties [34], improves energy levels, reduces fatigue, and stimulates erythropoiesis [35]. Research suggests it may also modulate stress-induced suppression of hematopoiesis, thereby enhancing overall vitality [36, 37]. Tinosporacordifolia (Guduchi) is known for its immunomodulatory and anti-inflammatory properties, which may support the bone marrow microenvironment and improve red blood cell production [38-40]. Specifically, improved palatability and appropriate viscosity enhance patient compliance, particularly in pediatric and geriatric populations. In pediatric patients, better taste masks bitterness and encourages adherence to prescribed regimens, reducing the risk of missed doses. In geriatric patients, optimized viscosity prevents swallowing difficulties, ensuring safer and more effective medication intake. Additional herbs may contribute through mechanisms such as antioxidant activity, reduction of oxidative stress, and support of erythropoietin production. These actions collectively promote a favorable environment for red blood cell formation and maturation. This multi-faceted approach addresses both the symptoms and underlying causes of anaemia, ensuring faster recovery and long-term health benefits. Thus, hypotheses regarding the formulation’s effect on pathways like erythropoietin signaling or regulation of iron metabolism enzymes provide avenues for further research.
LIMITATION
Despite promising findings, the study has some limitations. Large-scale clinical trials are necessary to confirm efficacy and safety across diverse populations, including pediatric, geriatric, and pregnant individuals. Additionally, standardized dosage guidelines for different demographic groups remain to be established. While the formulation is hypothesized to enhance hematopoiesis and iron metabolism, detailed molecular studies are required to elucidate the specific mechanisms involved. Another key limitation is the need for long-term safety assessments, as prolonged use may have unforeseen effects that warrant further investigation. Furthermore, interindividual variability due to genetic, dietary, and health-related factors has not been fully explored, necessitating broader population-based studies. Lastly, while the formulation’s benefits over existing Ayurvedic and allopathic treatments have been discussed, direct comparative clinical studies are essential to substantiate these claims and establish its potential as a superior or complementary therapeutic option.
FUTURE PERSPECTIVES
Future research should focus on conducting large-scale randomized controlled trials to validate the formulation’s efficacy compared to standard iron supplements. Mechanistic studies investigating the phytochemical interactions involved in iron metabolism and erythropoiesis will further enhance scientific understanding. Additionally, diversifying product formulations—such as developing effervescent tablets or gummies—could increase patient accessibility and compliance. Given the growing demand for natural therapeutic alternatives, exploring international regulatory approvals could facilitate global commercialization. Investigating the formulation’s interaction with dietary factors will also be crucial in optimizing iron absorption and overall effectiveness. These advancements will be instrumental in positioning the formulation as a viable and patient-friendly solution for anemia management.
CONCLUSION
The result suitability demonstrates that the recovery of growth does not differ by a factor greater than 2 achieved with 1:10 product dilution in 0.9 normal saline. The antimicrobial Effectiveness test of the formulation with preservatives meets the acceptance criteria for all the test organisms. This study successfully developed and standardized an Ayurvedic polyherbal oral liquid formulation for the treatment of anaemia. The formulation demonstrated promising hematinic activity in preliminary evaluations, meeting quality standards for organoleptic, physicochemical, and microbiological properties. These findings suggest that this formulation could serve as a viable, natural alternative to conventional anaemia treatments. Future clinical studies are warranted to establish its effectiveness and safety in larger populations, which could support its integration into public health strategies for anaemia management.
FUNDING
Nil
AUTHORS CONTRIBUTIONS
KR: Conceptualization, methodology, data curation, formal analysis, writing original draft preparation. SK: Investigation, validation, phytochemical analysis, manuscript review, and editing. DN: Supervision, project administration, funding acquisition, final manuscript review, and approval.
CONFLICT OF INTERESTS
The authors declare no potential conflict of interest
REFERENCES
Aggarwal A, Goyal S, Aggarwal S. Iron deficiency anemia among adolescents: a global public health concern. International Journal of Advanced Community Medicine. 2020 Apr 1;3(2):35-40. doi: 10.33545/comed.2020.v3.i2a.148.
Stevens GA, Paciorek CJ, Flores Urrutia MC, Borghi E, Namaste S, Wirth JP. National regional and global estimates of anaemia by severity in women and children for 2000-19: a pooled analysis of population representative data. Lancet Glob Health. 2022 May 1;10(5):e627-39. doi: 10.1016/S2214-109X(22)00084-5, PMID 35427520.
Leung AK, Lam JM, Wong AH, Hon KL, Li X. Iron deficiency anemia: an updated review. Curr Pediatr Rev. 2024 Aug 1;20(3):339-56. doi: 10.2174/1573396320666230727102042, PMID 37497686.
Hassan TH, Badr MA, Karam NA, Zkaria M, El Saadany HF, Abdel Rahman DM. Impact of iron deficiency anemia on the function of the immune system in children. Medicine. 2016 Nov 1;95(47):e5395. doi: 10.1097/MD.0000000000005395, PMID 27893677.
Shand AW, Kidson Gerber GL. Anaemia in pregnancy: a major global health problem. Lancet. 2023 May 13;401(10388):1550-1. doi: 10.1016/S0140-6736(23)00396-3, PMID 37088094.
Young MF, Luo H, Suchdev PS. The challenge of defining the global burden of iron deficiency anaemia. Lancet Haematol. 2023 Jul 31;10(9):e702-4. doi: 10.1016/S2352-3026(23)00168-0, PMID 37536355.
Pantopoulos K. Oral iron supplementation: new formulations old questions. Haematologica. 2024 Apr 11;109(9):2790-801. doi: 10.3324/haematol.2024.284967, PMID 38618666.
Allen LH. Iron supplements: scientific issues concerning efficacy and implications for research and programs. J Nutr. 2002 Apr 1;132(4)Suppl:813S-9S. doi: 10.1093/jn/132.4.813S, PMID 11925487.
Maurya H, Kumar T. Formulation standardization and evaluation of polyherbal dispersible tablet. Int J Appl Pharm. 2019 Jan;11(1):158-67. doi: 10.22159/ijap.2019v11i1.30113.
Parasuraman S, Thing GS, Dhanaraj SA. Polyherbal formulation: concept of ayurveda. Pharmacogn Rev. 2014 Jul;8(16):73-80. doi: 10.4103/0973-7847.134229, PMID 25125878.
Srikanth N. Management of anaemia through ayurveda. CCRAS Ministry of Ayush; 2019. p. 1-94.
Verma SK, Pandey M, Sharma A, Singh D. Exploring ayurveda: principles and their application in modern medicine. Bull Natl Res Cent. 2024 Aug 5;48(1):77. doi: 10.1186/s42269-024-01231-0.
Kaushik P, Ahlawat P, Singh K, Singh R. Chemical constituents pharmacological activities and uses of common ayurvedic medicinal plants: a future source of new drugs. AdVTradit Med. 2023 Sep;23(3):673-714. doi: 10.1007/s13596-021-00621-3.
Divya Sree MS, Rajasekaran S. Ethnopharmacological review of traditional medicinal plants found as an astounding remedy to anemia. Int J Pharm Sci Rev Res. 2021 Des 20;71(2):66-71. doi: 10.47583/ijpsrr.2021.v71i02.011.
Anwar S, Kausar MA, Parveen K, Zahra A, Ali A, Badraoui R. Polyherbal formulation: the studies towards identification of composition and their biological activities. J King Saud Univ Sci. 2022 Oct 1;34(7):102256. doi: 10.1016/j.jksus.2022.102256.
Mony RS, Baskaran SK, Perumalsamy M, Kannappan P. Effect of hydro alcoholic extracts of Boswellia serrata and Terminalia Bellerica against Cyclo-oxygenase and lipoxygenase enzymes an in vitro approach. Int J Pharmacogn Phytochem Res. 2017;9(8):1067-73. doi: 10.25258/phyto.v9i08.9613.
Thajudeen KY, Alsayari A, NajibUllah SN, Salam S, Elayadeth Meethal M, Uoorakkottil I. Validation optimization and hepatoprotective effects of Boeravinone B and caffeic acid compounds from Boerhavia diffusa Linn. Separations. 2022 Jul 18;9(7):177. doi: 10.3390/separations9070177.
Safiei NZ, Ngadi N, Johari A, Zakaria ZY, Jusoh M. Grape juice concentration by progressive freeze concentrator sequence system. J Food Process Preserv. 2017 Feb;41(1):e12910. doi: 10.1111/jfpp.12910.
Shehzad M, Rizwani GH, Ishaq S, Yaqoob MA, Shareef H. Pharmaceutical evaluation of graphirine syrup formulated from some common indigenous herbs of Pakistan. Pak J Pharm Sci. 2020 Jan 3;33(1Suppl):317-23. doi: 10.36721/PJPS.2020.33.1, PMID 32122864.
Mohapatra P, Shirwaikar A, Ram HA. Standardization of a polyherbal formulation. Pharmacogn Mag. 2008;4(13):65.
Patnala S, Kanfer I. Quality control extraction methods and standardization: interface between traditional use and scientific investigation. In: Henkel R, Agarwal A, editors. Herbal Medicine in Andrology. 2021 Jan 1;175-87. doi: 10.1016/B978-0-12-815565-3.00006-0.
Sohi H, Sultana Y, Khar RK. Taste masking technologies in oral pharmaceuticals: recent developments and approaches. Drug Dev Ind Pharm. 2004 Jan 1;30(5):429-48. doi: 10.1081/DDC-120037477, PMID 15244079.
Mu Y, Zhao L, Shen L. Medication adherence and pharmaceutical design strategies for pediatric patients: an overview. Drug Discov Today. 2023 Nov;28(11):103766. doi: 10.1016/j.drudis.2023.103766, PMID 37708932.
Vazquez Blanco S, Gonzalez Freire L, Davila Pousa MC, Crespo Diz C. pH determination as a quality standard for the elaboration of oral liquid compounding formula. Farm Hosp. 2018;42(6):221-7. doi: 10.7399/fh.10932, PMID 30381041.
Atteback M, Hedin B, Mattsson S. Formulation optimization of extemporaneous oral liquids containing naloxone and propranolol for pediatric use. Sci Pharm. 2022 Feb 22;90(1):15. doi: 10.3390/scipharm90010015.
Debnath M, Paul N, Bhattacharya S, Biswas M, Haldar PK. Formulation and assessment of microbial and heavy metal contents of vidangadilouham: a classical ayurvedic formulation. Int J Herb Med. 2020;8(4):101-2.
Johnson Wimbley TD, Graham DY. Diagnosis and management of iron deficiency anemia in the 21st century. Ther Adv Gastroenterol. 2011 May 21;4(3):177-84. doi: 10.1177/1756283X11398736, PMID 21694802.
Jayaweera JA. Introduction to ayurvedic formulations: exploring the classical concepts with modern science. In: Amalraj A, Kuttappan A, Varma K, editors. Chemistry biological activities and therapeutic applications of medicinal plants in ayurveda; 2022 Nov. p. 1-21. doi: 10.1039/9781839166211-00001.
Shahiwala A. Formulation approaches in enhancement of patient compliance to oral drug therapy. Expert Opin Drug Deliv. 2011 Nov 1;8(11):1521-9. doi: 10.1517/17425247.2011.628311, PMID 21995544.
Chiclana Rodriguez B, Garcia Montoya E, Romero Obon M, Rouaz El Hajoui K, Nardi Ricart A, Sune Pou M. Palatability and stability studies to optimize a carvedilol oral liquid formulation for pediatric use. Pharmaceutics. 2023 Dec 25;16(1):30. doi: 10.3390/pharmaceutics16010030, PMID 38258041.
Kotmire S, Desai A, Chougule N. The advances in polyherbal formulation. J Pharmacogn Phytochem. 2024;13(1):210-21. doi: 10.22271/phyto.2024.v13.i1c.14828.
Chambial S, Dwivedi S, Shukla KK, John PJ, Sharma P. Vitamin C in disease prevention and cure: an overview. Indian J Clin Biochem. 2013 Oct;28(4):314-28. doi: 10.1007/s12291-013-0375-3, PMID 24426232.
Bhoot HR, Zamwar UM, Chakole S, Anjankar A. Dietary sources bioavailability and functions of ascorbic acid (vitamin C) and its role in the common cold tissue healing and iron metabolism. Cureus. 2023 Nov 23;15(11):e49308. doi: 10.7759/cureus.49308, PMID 38146585.
Priyanka G, Anil Kumar B, Lakshman M, Manvitha V, Kala Kumar B. Adaptogenic and immunomodulatory activity of ashwagandha root extract: an experimental study in an equine model. Front Vet Sci. 2020 Sep 29;7:541112. doi: 10.3389/fvets.2020.541112, PMID 33134345.
Tamoli S, Gokarn V, Ibrahim M, Ahmad S. Comparative investigation of ashwagandha FMB extract and standardized extract for their antioxidant anti-inflammatory and immunomodulatory potential. Ann Phytomedicine. 2022;11(1):405-12. doi: 10.54085/ap.2022.11.1.46.
Devarasetti AK, Bharani KK, Khurana A, Anand S, Kollipaka R, Saranu VD. Adaptogenic ashwagandha root extract modulates inflammatory markers in feline stress management: a double blind placebo controlled clinical trial. J Appl Anim Res. 2024 Dec 31;52(1):2335921. doi: 10.1080/09712119.2024.2335921.
Nian Q, Liu R, Zeng J. Unraveling the pathogenesis of myelosuppression and therapeutic potential of natural products. Phytomedicine. 2024 Jun 8;132:155810. doi: 10.1016/j.phymed.2024.155810, PMID 38905848.
Sharma H, Rao PS, Singh AK. Fifty years of research on Tinospora cordifolia: from botanical plant to functional ingredient in foods. Trends Food Sci Technol. 2021 Dec 1;118:189-206. doi: 10.1016/j.tifs.2021.10.003.
Kumar S, Tambuwala MM, Mishra Y, Mishra V. Current biological and pharmacological updates on Tinospora cordifolia. Excli J. 2024 May 21;23:811-5. doi: 10.17179/excli2024-7170, PMID 39165586.
Gupta A, Gupta P, Bajpai G. Tinospora cordifolia (Giloy): an insight on the multifarious pharmacological paradigms of a most promising medicinal ayurvedic herb. Heliyon. 2024 Feb 29;10(4):e26125. doi: 10.1016/j.heliyon.2024.e26125.