
Int J App Pharm, Vol 17, Issue 4, 2025, 453-461Original Article
A NOVEL TOPICAL GEL OF THE FRESH YOUNG COCONUT HUSK ETHANOLIC EXTRACT DEDICATED FOR DIABETIC WOUND HEALING
CHRISTOFORI MARIA RATNA RINI NASTITI1*, YOHANES DWIATMAKA2, CHRISTINE PATRAMURTI3, MICHAEL RAHARJA GANI3, FLORENTINUS DIKA OCTA RISWANTO3
1Division of Pharmaceutics and Pharmaceutical Technology, Faculty of Pharmacy, Sanata Dharma University, Campus 3 Paingan, Maguwoharjo, Depok, Sleman, Yogyakarta-55282, Indonesia. 2Division of Pharmaceutical Biology, Faculty of Pharmacy, Sanata Dharma University, Campus 3 Paingan, Maguwoharjo, Depok, Sleman, Yogyakarta-55282, Indonesia. 3Division of Pharmaceutical Analysis and Medicinal Chemistry, Faculty of Pharmacy, Sanata Dharma University, Campus 3 Paingan, Maguwoharjo, Depok, Sleman, Yogyakarta-55282, Indonesia
*Corresponding author: Christofori Maria Ratna Rini Nastiti; *Email: ratnarini@usd.ac.id
Received: 27 Dec 2024, Revised and Accepted: 09 May 2025
ABSTRACT
Objective: This study aimed to develop and to optimize the formulation of novel Fresh-Young Coconut-Husk-Ethanolic-Extract (FYCHEE) gels by Response Surface Methodology (RSM) of a Box Behnken Design (BBD), and to investigate the activity of novel FYCHEE topical gels on accelerating the Diabetic Wound Healing (DWH).
Methods: The FYCHEE was prepared by maceration using 96% ethanol and was standardized. Gels were formed by incorporating the FYCHEE into the gel-forming system containing Diethylene-Glycol Monoethyl-Ether (DGME), Carbopol 940, and Triethanolamine (TEA). Sixteen experimental runs were set using BBD. Physical properties (viscosity, pH and spread ability) were further characterized. The data were analyzed and optimized using “rsm” package of the R software. The predicted model of DGME, Carbopol 940 and TEA composition was further validated. The in vivo study of the activity of FYCHEE gels on accelerating DWH was conducted on groups of male Wistar rats in 12 d of topical application of FYCHEE gels containing 5%, 10%, 20% extracts.
Results: The FYCHEE gels performed light-brown semisolid texture with varied physical characteristics. The RSM generated visualization of response surface plots based on three model equations. The novel FYCHEE gels with optimum responses have been well developed (DGME: Carbopol 940: TEA = 8:0.8:0.6). The formulation based on predicted composition showed 1.194±0.136 Pa. s (viscosity), 5.715±0.213 (pH) and 4.997±0.265 cm (spread-ability). The FYCHEE gels showed significant results on accelerating the wound closure in 12 d, with percentage of (88.798±4.697) %, (91.685±3.124) %, and (93.060±1.687) % for FYCHEE of 5%, 10%, and 20%, respectively. These results were similar to the positive control (p>0.05).
Conclusion: The novel FYCHEE gels were successfully fabricated with standardized FYCHEE. The RSM of the BBD was applied satisfactorily to optimize the formula. Further study of FYCHEE gels on accelerating DWH revealed promising results of the FYCHEE gels to be potentially developed as diabetic wound healing topical preparation.
Keywords: Formula, Optimization, Ethanolic extract, Coconut husk, Topical gel
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijap.2025v17i4.53525 Journal homepage: https://innovareacademics.in/journals/index.php/ijap
INTRODUCTION
Diabetic condition due to the uncontrolled glucose levels may get unmanageably complicated by impaired wound healing, which may cause fatal condition of tissue death and gangrene infection leading to amputation [1, 2]. Many efforts have been done to develop a wide series of wound healing therapies to overcome this condition, including exploring the pharmacological benefits of natural products [3]. A number of plants and phytoconstituents which were potential for Diabetic Wound Healing (DWH) has been comprehensively reviewed and studied [4–6]. However, the potential of coconut on DWH has not been thoroughly investigated. Coconut (Cocos nucifera L) is known as a multi-benefit tropical fruit in Indonesia [7]. Empirically, young coconut water is used as an antidote of some toxicity cases [8]. In terms of healing the diabetic wound, the virgin coconut oil and the liquid smoke of coconut shells appear to be the only materials from coconut which has been widely reported [9–11]. The husk of coconut has a high content of tannin. It was suggested that total tannin in the extract of husk was up to 141.5 ±5.08 mg Tannic Acid Equivalents (TAE)/g [12]. Tannin shows astringency, antioxidant and anti-inflammatory effects; therefore, the extract of the young coconut husk is potential to develop as a wound-healing agent [13, 14].
To be practically applicable, a topical formulation needs to be carefully designed for the extract of the young coconut husk. A topical hydrogel system is favorable as it provides many valuable features for wound healing, such as: high hydration and cooling effect, firm adhesion, and ease of application [15]. A number of gel-forming systems have been explored in order to provide suitable delivery systems for diabetic wound healing [16]. However, to the best of our knowledge, topical gel formulation which incorporates the plant extracts has not been extensively studied. As the extract of the young coconut husk is acidic due to the content of tannic acid, the quality of the gel product may be significantly affected by this property. Therefore, material selection of the gel-forming system must focus on the capability of the product to withstand the acidic environment and to create a supporting system for the stability of the extract. In addition to that, viscosity and the spread-ability of the gel are two essential physical properties as these properties may affect the physical stability and ease of product application as well as the filling process in manufacturing area [17–19]. These properties are significantly governed by the gel-forming system in the formulation.
Response Surface Methodology (RSM) in the Box Behnken Design (BBD) is a promising tool for the formulator in designing the formula to predict the composition with the optimum responses. Multiple responses optimization is meticulously facilitated in BBD to investigate more than two independent variables at three different levels [20]. BBD uses fewer number of experiments than any other optimization, such as central composite design, resulting in efficiency in carrying out the experiments [21].
To ensure the effect of accelerating the wound recovery, it is imperative to evaluate the activity of the topical hydrogel containing Fresh Young Coconut Husk Ethanolic Extract (FYCHEE) on the wound contraction. Punch biopsy is one of wound models which can be applied to assess the effectivity of wound healing topical preparation [22].
This study aimed to provide good quality of FYCHEE and to optimize the formula by applying the response surface methodology in BBD. The optimization focused on the composition of the gel-forming system containing Diethylene Glycol Monoethyl Ether (DGME), Carbopol 940 and Triethanolamine (TEA), which provides the optimum responses of viscosity, pH and spread-ability. The computational optimization was assisted by an open-source R software package called “rsm” [23] which was evident in implementing BBD [18, 24]. Furthermore, an in vivo study was conducted with the objectives to confirm the potential effects of the FYCHEE gel in accelerating DWH.
MATERIALS AND METHODS
Materials
Fresh young coconut husk; Diethylene Glycol Monoethyl Ether (DGME; Sigma Aldrich, Singapore; pharmaceutical grade); ethanol 96% (grade A for extraction; General Labora, Indonesia); Carbopol 940, Triethanolamine (TEA), methylparaben (nipagin), Butyl Hydroxy Toluene (BHT), these materials are technical grade, purchased from General Labora, Indonesia), demineralized water.
Methods
Young coconut husk extraction and characterization
Fresh young coconuts were thoroughly washed and longitudinally cut into 2 (two) pieces. The shell and green outer skin were removed. Each piece was then horizontally divided into 4 (four) parts. Every part was sliced at 1 cm long and then grated with an electric grater (Bison, Indonesia). The grated husk was then mechanically macerated in 96% ethanol (in ratio 1:10) by using an orbital shaker (Innova 2100, Germany), with the speed of 150 rpm at room temperature for 4 h. The liquid extract was then vacuum-filtered and undergone solvent evaporation by a vacuum rotary evaporator (Buchi, Switzerland) at 35 °C and 45 mmHg pressure, resulting in the semi-concentrated extract. The semi-concentrated extract was further evaporated to obtain the dry extract.
To characterize the extract, the organoleptic observation was carried out, followed by the yield calculation of the semi-concentrated and dry extracts. Water content was determined on the semi-concentrated extract, while for the dry extract, Loss on Drying (LOD) was examined. In order to facilitate the ease of mixing, the semi-concentrated extract was selected for topical gel formulation (at concentration of 10% w/w gel).
FYCHEE gel formulation
The fabrication of the FYCHEE gels was initiated by a gelation process of Carbopol 940 in demineralized water containing preservative, which was then kept aside at room temperature for 24 h. The half-made gel was then added with TEA, BHT and DGME and homogenously mixed by an overhead stirrer (IKA Works, Malaysia) at 650 rpm for 3 min to obtain a viscous gel system. The FYCHEE was further incorporated into the gel system and continuously mixed at 650 rpm for 2 min. The gel was then contained in a light-resistant glass jar and kept at 2-8 °C for 24 h prior to physical characterization.
Table 1: The basic formula of the FYCHEE gel
| Ingredients | Weight (g) |
| Carbopol 940 | 1 |
| Water | 78 |
| Nipagin | 0.1 |
| TEA | 0.4 |
| BHT | 0.1 |
| Ethanol | 0.4 |
| DGME | 10 |
| FYCHEE | 10 |
The composition of the excipients was organized based on the BBD stated below.
Experimental design
The BBD model allowed 16 runs of experiments with three different independent variables selected, which were DGME, Carbopol 940, and TEA, in three levels. The dependent variables optimized were viscosity (Pa. s), pH and the spread-ability (cm). The experimental variables for the response surface methodology are shown at table 2.
Table 2: Experimental variables in three levels
| Variables | Levels | ||
| Low | Medium | High | |
| x1: DGME (g) | 8 | 10 | 12 |
| x2: Carbopol 940 (g) | 0.8 | 1 | 1.2 |
| x3: TEA (g) | 0.2 | 0.4 | 0.6 |
Physical characterization of FYCHEE gels
Organoleptic characterization and pH confirmation
Visual organoleptic characterization was carried out for the extracts and the FYCHEE gels. This characterization involved color, odor, and consistency. To confirm the pH of the semi-concentrated extract and FYCHEE gels, a calibrated-pH meter (WTW pH 3110 SET2, Germany) was used. In brief, the probe was directly immersed in the samples for 30 seconds and the pH could be read on the display. The gels should have delicate, light-brown semisolid consistency, with pH of 4.5-5.9.
Determination of viscosity
A Merlin VR viscometer (Rheosys, USA) was used to determine the viscosity of the FYCHEE gels, with cone and plate mode 2°/30 mm, operating at 50 rpm, at room temperature. The measurement was replicated, with a delay time of 20 seconds, zero-shear time of 20 seconds and the integration time of 10 seconds [17]. The viscosity of 0.5-1.5 Pa. s was preferable.
Spread-ability measurement
The spreading diameter of 1 (one) g of the FYCHEE gel, which was sandwiched in between two glass plates horizontally for 1 minute, with the upper plate loaded with metals thereby weighing 125g [25], was measured by a ruler. The spreading diameter was the average diameter measured in 4 directions passing the center point. The diameter should be 4-7 cm.
Statistical analysis of the BBD
The BBD model of 16 runs and the response surface methodology were designed with the aid of R open-source software version 4.4.1 and Rstudio version 2024.04.2 Build 764 equipped with “rsm” package [23]. The optimization of multiple responses and the desirability analysis were executed to obtain a composition of Carbopol 940, triethanolamine, and DGME which resulted in optimum responses of physical characteristics (pH, viscosity, and spreadability). Statistical analysis was carried out with 95% confidence interval.
Stability evaluation of the FYCHEE gels
In-use stability of FYCHEE gels (E5%, E10%, E20%) was carried out parallel with the in vivo activity study. FYCHEE gels were stored at two different storage conditions: cold storage (2-8 °C) and at room temperature. (28-33 °C). The observation was based on the alteration of viscosity, spread-ability and pH in 14 d. The data was statistically analyzed using the paired t test for the condition on day 1 and the condition on day 14.
The in vivo study of the activity of FYCHEE gels on accelerating DWH
Experimental design
The study was conducted following the approval of animal ethical clearance by the Ethic Committee of Faculty of Medicine, Public Health and Nursing, Gadjah Mada University, Indonesia on October 1st, 2024, number KE/FK/1523/EC/2024.
Healthy male Wistar rats (2.5-3 mo; 200g-250g BW) were acclimatized under 12h light/dark cycles at room temperature with 70% RH and caged individually, fed by standard nutritious pellets. The rats were allowed to drink clean fresh water ad libitum.
The induction of diabetic condition was carried out by injecting Streptozotocin (45 mg/kg BW) and Nicotinamide (110 mg/kg BW) intraperitoneally. After 72 h, the diabetic condition of the rats (blood glucose level at ≥ 250 mg/dL) was confirmed by measuring the blood glucose level using Glucose-Oxidase Peroxidase (GOD-PAP).
The groups of rats were then divided into 6 (six) groups @ 3 rats.
The first group was the Untreated Non-Diabetic Group (UTNDG), which was set as a reference of wound healing normal response. The second group involved the diabetic rats with untreated wounds (Untreated Diabetic Group; UTDG). The group of Positive Control (PC) was treated by the commercial product (Lukajel®). The other three groups were the group treated with FYCHEE gels containing 5% extract (E5%), 10% extract (E10%), and 20% extract (E20%).
The hairs on the dorsal surface were removed to provide hairless space for observation. All rats were then anaesthetized by intramuscular injection of ketamine HCl (50 mg/kg BW) and xylazine (10 mg/kg BW) prior to punch biopsy (diameter of 5 mm). Punch biopsy was carried out two times on each side of the rats and the wound was kept open for 24 h to let the exudates dry. Approximately 200 mg of the topical preparation was then applied on the wound with the aid of a metal mini spatula, twice a day for 11 consecutive days.
The observation of the wound contraction was carried out every three days, on day 3, day 6, day 9, and day 12. The wounds were captured perpendicularly using a digital camera (Samsung Galaxy S23, South Korea) at around 20 cm height, with the scaling aid of millimeter-scale paper. The area of the wounds was further analyzed using ImageJ software (National Health Institute, U. S). The percentage of wound contraction was calculated by: [(wound area on the day 0 – wound area on the day X): wound area on the day 0] [22, 26].
On day 9, after being visually observed, one rat of each group was sacrificed, and the wounded skin tissues were then removed and fixed in 10% formalin for histological observation.
Fixed tissues were further embedded in paraffin, sectioned at the thickness of 5µm using a microtome, then stained with hematoxylin and eosin. The sections were examined under a light microscope for the evaluation of histopathological parameters. Three parameters were selected those are the number of inflammatory cells (represented by neutrophils), the epithelium generation, and the number of vessels generated (angiogenesis).
Data presentation and statistics analysis
The data are presented as the mean±SD for physical characteristics with n=3 replications/formula and the mean±SD for wound healing activity study with n=5 wounds/group. Sample size was justified according to Arifin et al. [27] with the formula of 10/k+1 as the minimum number of animals per group. Normally distributed data were analyzed using either ANOVA or unpaired t test. For the non-parametric data, a Wilcoxon test and Kruskal Wallis applied. Significant differences were considered if p<0.05. All data were statistically analyzed using Analysis-ToolPak on the MS Excel 2019 (Microsoft, USA). Statistical analysis was carried out with 95% confidence interval. In terms of the activity of accelerating wound contraction, comparisons were made among gels and between the gels and control groups of the diabetic-induced rats.
RESULTS
Coconut husk extraction and standardization
The organoleptic observation revealed that the semi-concentrated extract was brown and transparent liquid, while the dry extract showed a sticky-brown semisolid texture (fig. 1).

Fig. 1: Visual appearance of the FYCHEE: a. Semi-concentrated extract; b. Dry extract
The yield of the semi-concentrated extract was 5.03±0.21 ml per 500 ml solvent with water content of (86.88±0.006) %. The pH of the semi-concentrated extract was 4.59. In terms of the dry extract, the yield was 5.07±0.590 % per 50g husk and the LOD was 13.81±0.866 %.
Table 3: Design of experimental results for DGME, carbopol, and TEA composition in the FYCHEE gel formulation
| Run order | Standard order | Independent variables | Dependent variables | ||||
| DGME (x1) | Carbopol (x2) | TEA (x3) | Viscosity (y1) | pH (y2) | Spread ability (y3) | ||
| 1 | 6 | 12 | 1.0 | 0.2 | 0.316 | 4.403 | 4.750 |
| 2 | 3 | 8 | 1.2 | 0.4 | 0.674 | 4.314 | 4.750 |
| 3 | 1 | 8 | 0.8 | 0.4 | 0.326 | 4.686 | 5.400 |
| 4 | 16 | 10 | 1.0 | 0.4 | 0.364 | 4.436 | 5.225 |
| 5 | 8 | 12 | 1.0 | 0.6 | 0.810 | 5.091 | 4.537 |
| 6 | 2 | 12 | 0.8 | 0.4 | 0.412 | 4.950 | 5.850 |
| 7 | 9 | 10 | 0.8 | 0.2 | 0.138 | 4.302 | 6.000 |
| 8 | 11 | 10 | 0.8 | 0.6 | 0.689 | 5.123 | 5.400 |
| 9 | 4 | 12 | 1.2 | 0.4 | 0.526 | 4.629 | 5.400 |
| 10 | 15 | 10 | 1.0 | 0.4 | 0.445 | 4.471 | 5.650 |
| 11 | 7 | 8 | 1.0 | 0.6 | 1.295 | 5.180 | 4.500 |
| 12 | 12 | 10 | 1.2 | 0.6 | 1.197 | 5.269 | 3.887 |
| 13 | 5 | 8 | 1.0 | 0.2 | 0.357 | 4.488 | 5.500 |
| 14 | 14 | 10 | 1.0 | 0.4 | 0.610 | 4.529 | 5.300 |
| 15 | 10 | 10 | 1.2 | 0.2 | 0.096 | 4.656 | 5.425 |
| 16 | 13 | 10 | 1.0 | 0.4 | 0.869 | 4.352 | 5.475 |
Topical gel fabrication, design of experiment, and physical characterization
Sixteen runs of the Box Behnken model were executed with DGME, Carbopol 940, and TEA as the independent variables. The FYCHEE gels were then physically characterized in terms of viscosity, pH, and spread-ability as the dependent variables. The visual appearance of the topical gels was light brown semisolid with varied values of the observed physical properties (table 3).
Response surface methodology
Responses in terms of viscosity, pH and spreadability in uncoded units were evaluated using RSM (table 4). Contour plots and response surfaces were generated and depicted in fig. 2.
The acceptance criteria for optimization were in the range of 0.5-1.5 Pa. s for viscosity, 4.5-5.9 for pH and 4-7 cm for the spread ability. Based on the desirability analysis, with the composite desirability value of 0.673, it was predicted that a composition of DGME: Carbopol 940: TEA = 8: 0.8: 0.6 might result in optimum responses.
The predicted responses were 0.827 Pa. s for the viscosity, 5.304 for the pH and 4.988 cm for the spread-ability. Validation of the prediction was further carried out and the observed values were closed to the prediction and met the acceptance criteria (table 5). The visual appearance of topical gels of the ethanolic extract of fresh young coconut husk was transparent, light-brown semisolid (fig. 3).
Table 4: Results of RSM modeling for viscosity, pH, and spread-ability
| Responses | R2 | Equation |
| Viscosity (Pa. s) | 0.896 | y1 =-3.464+0.028 x1+6.972 x2-0.412 x3-0.146 x1x2-0.278 x1x3+3.438 x2x3+0.009 x12-3.152 x22+2.096 x32 |
| pH | 0.840 | y2 = 9.416-0.382 x1-6.038 x2-2.279 x3+0.032 x1x2-0.003 x1x3-1.300 x2x3+0.019 x12+3.059 x22+6.703 x32 |
| Spread-ability (cm) | 0.840 | y3 = 5.785+0.749 x1-8.241 x2+6.477 x3+0.125 x1x2+0.492 x1x3-5.863 x2x3-0.052 x12+3.672 x22-9.534 x32 |

Fig. 2: Visualization of response surface plot for viscosity (y1), pH (y2), and spread-ability (y3)

Fig. 3: Visual appearance of optimized topical gels of ethanolic extract of fresh young coconut husk
Table 5: Desirability analysis for topical gel formulation containing young coconut husk ethanolic extract
| Responses | Optimization setting | Composite desirability | Prediction | Validation results | ||
| Lower | Target | Upper | ||||
| Viscosity (Pa. s) | 0.5 | 1.0 | 1.5 | 0.673 | 0.827 | 1.194±0.136 |
| pH | 4.5 | 5.2 | 5.9 | 5.304 | 5.715±0.213 | |
| Spread-ability (cm) | 4.0 | 5.5 | 7.0 | 4.988 | 4.997±0.265 | |
Note: validation data are presented as x̅±SD with 3 replicates
Stability evaluation of the FYCHEE gels
FYCHEE gels were successfully developed with noticeable physical properties. The viscosity of FYCHEE containing 5% extract was the highest among three formulations, with the consequence of being the least spreadable (table 6), whereas the characteristics of viscosity and spread-ability of FYCHEE with 10 and 20% extract were comparable. Focusing on the pH, since the pH of the extract was acidic, the higher the concentration tends to give more acidic gels, although FYCHEE 5% and 10% were similar.
Table 6: Properties of FYCHEE gel 24h after fabrication
| FYCHEE concentration (%) | Viscosity (Pa. s) | Spread-ability (cm) | pH |
| 5 | 1.442±0.0199 | 4.050±0.0577 | 4.790±0.0290 |
| 10 | 0.951±0.0147 | 4.925±0.0957 | 4.949±0.0156 |
| 20 | 0.850±0.005 | 5.025±0.0957 | 4.493±0.007 |
Note: Data are presented as x̅±SD with 3 replicates
In 14 d of storage, the FYCHEE gel spread-ability increased on both cold and at room temperature condition as a result of decreasing viscosity (table 7). In terms of pH, the values were slightly decreased. The stability of the FYCHEE was moderate with the viscosity shift at around 18% for cold storage and approximately 25% at room temperature.
Table 7: Stability of FYCHEE gel after 14 d of storage
| FYCHEE (%) | Cold storage condition | Room temperature storage condition | |||
| Viscosity shift (%) | Spread-ability shift (%) | Viscosity shift (%) | Spread-ability shift (%) | pH shift | |
| 5 | 16.596±1.3723 | 30.384±5.1061 | 34.98±9.3683 | 37.55±2.2036 | 1.846±0.8329 |
| 10 | 27.548±4.1368 | 31.267±7.7530 | 26.908±5.8970 | 15.88±8.4454 | 2.765±0.4536 |
| 20 | 22.302±4.3265 | 24.015±6.4347 | 21.34±5.1532 | 20.21±6.3966 | 2.951±0.1734 |
Note: Data are presented as x̅±SD with 3 replicates
The in vivo study of the activity of FYCHEE gels on accelerating diabetic wound healing
Fig. 4A depicts visual appearance of wound contraction over time. The skin with normal condition (Untreated Non-Diabetic Group; UTNDG) showed natural recovery of wound healing without any treatments over 12 d, whereas the diabetic wounds, as shown on the Untreated Diabetic Group (UTDG) were getting exaggerated due to massive inflammation on the first stage of wound healing. Rapid increase of contraction evidenced on day 9 on the FYCHEE gels and PC (table 9). FYCHEE gels provided significant recovery profile from day 3 to day 12, like the positive control (p>0.05). Different concentrations of FYCHEE in gels unaffected the wound contraction on the day 12 (p>0.05).

Fig. 4: In vivo study of DWH acceleration by FYCHEE: A. Macroscopic appearance of the wound contraction on day 3, 6, 9, 12. B. Percentage of wound contraction on each day of observation. C. Wound closure tendency over 12 d. UTNDG: Untreated Non-Diabetic Group; UTDG: Untreated Diabetic Group; PC: Positive Control group; E5%: wound applied with FYCHEE 5% gel; E10%: wound applied with FYCHEE 10% gels; E20%: wound applied with FYCHEE 20% gels
Table 9: Diabetic wound healing process
| Days | Wound contraction (%) | |||||
| UTNDG | UTDG | PC | E5% | E10% | E20% | |
| Day 3 | 14.34±8.975 | -20.68±.057 | 25.95±3.552 | 21.16±5.912 | 30.19±3.566 | 49.08±10.720 |
| Day 6 | 68.24±2.706 | -15.16±9.417 | 44.76±18.776 | 39.23±16.555 | 35.64±9.012 | 58.76±5.492 |
| Day 9 | 78.59±7.391 | 38.00±15.696 | 87.65±2.724 | 81.68±3.756 | 74.61±9.100 | 77.09±7.799 |
| Day 12 | 95.79±1.214 | 75.76±8.370 | 90.58±3.223 | 88.80±4.697 | 91.69±3.124 | 93.06±1.687 |
Note: UTNDG: Untreated Non-Diabetic Group; UTDG: Untreated Diabetic Group; PC: Positive Control group; E5%: wound applied with FYCHEE 5% gel; E10%: wound applied with FYCHEE 10% gels; E20%: wound applied with FYCHEE 20% gels. Data are presented as x̅±SD with 5 replicates
The groups of treated diabetic wounds showed significant recovery from day 9 (fig. 4B and fig. 4C). Moreover, there is strong evidence that the FYCHEE gels accelerated the diabetic wound contraction showing by the significant higher percentage over time compared to the untreated diabetic wound control group. FYCHEE, with 20% extract, managed to contract the wound at almost 100% on the day 12, showing a comparable result to the positive control.
Reepithelization of the treated wounds on the day 9 was confirmed by the H and E images of the wound-fixed tissue (fig 5). Epithelia layer appeared on top of skin of normal wound (UTNDG; 5A). However, fig 5B depicted that the epithelial layer was still unable to grow on the untreated diabetic group (UTDG) on day 9. On the positive control (PC; 5C) the epithelial layer appeared to grow well, and similar appearance was also noticed at the FYCHEE gels in all concentrations (fig 5D-5F).
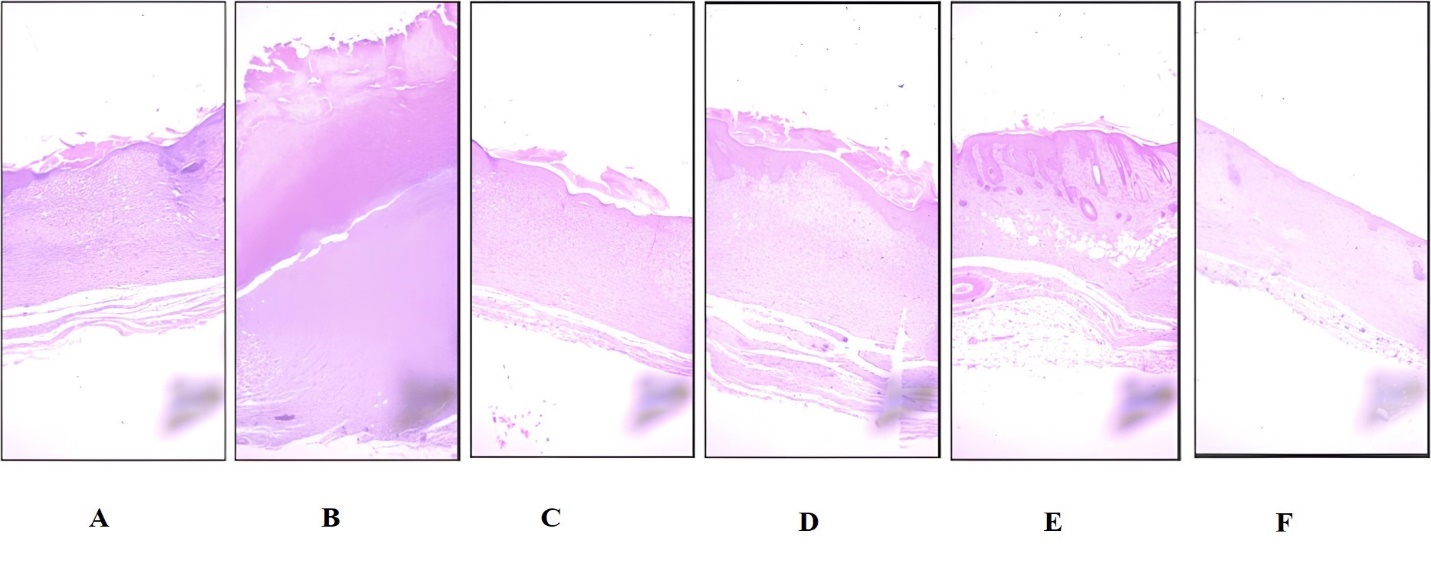
Fig. 5: Re-epithelization progress on day 9 of diabetic wound healing. A. Untreated Non-Diabetic Group (UTNDG); B. Untreated Diabetic Group (UTDG); C. Positive Control group (PC); D. Wound applied with FYCHEE 5% gel (E5%); E. Wound applied with FYCHEE 10% gels (E10%); F. wound applied with FYCHEE 20% gels (E20%)
Fig. 6 illustrates the condition of wound tissue on day 9. The untreated non-diabetic (UTNDG) wound showed only few neutrophils without angiogenesis (fig. 6A), however, massive inflammation was still evidenced on the untreated diabetic group (UTDG; fig. 6B), shown by the huge amounts of neutrophils as the inflammation cell marker. Interestingly, the angiogenesis was also enormously increased, indicating the natural effort of homeostatic response to the wound (see inserted fig. on fig 6B). The wounds treated with FYCHEE gels revealed less neutrophils compared to the UTDG, but similar to the control group (fig.6C), although the vascularization was still ongoing (fig 6D-F). The appearance of neutrophils was decreasing on the wound applied with FYCHEE gels with the least was on the FYCHEE 20% gels.
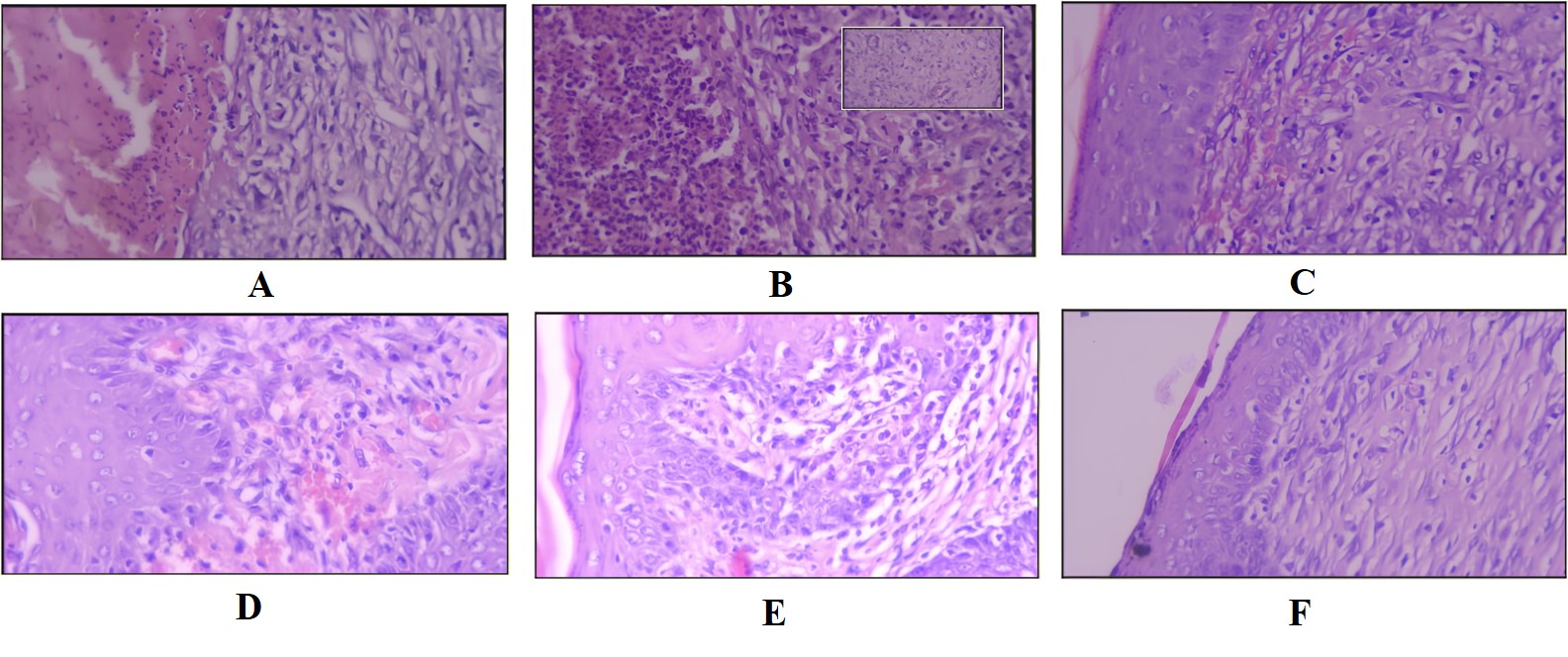
Fig. 6: Histological images focused on the neutrophils and the angiogenesis on the day 9 of diabetic wound healing. A. Untreated Non-Diabetic Group (UTNDG); B. Untreated Diabetic Group (UTDG); C. Positive Control group (PC); D. Wound applied with FYCHEE 5% gel (E5%); E. Wound applied with FYCHEE 10% gels (E10%); F. wound applied with FYCHEE 20% gels (E20%)
DISCUSSION
Plant-based diabetic wound healing formulation has recently gained much interest due to the biocompatibility and abundant renewable resource of the active ingredients. This current study highlights the significance of novel topical gel formula optimization for delivering FYCHEE, which is potential as diabetic wound healing accelerator. DGME, Carbopol 940, TEA were selected as the experimental independent variables on providing the optimum responses of pH, viscosity and spreadability. This study also aimed to evaluate the capacity of FYCHEE gels to accelerate diabetic wound healing.
The ethanolic extract of fresh young coconut husk was successfully collected using mechanical maceration for 4 h. The solvent underwent massive evaporation to generate semi-concentrated and dry extracts. The standardization had been carried out in terms of yield, solvent reduction and loss on drying. The pH of the semi-concentrated extract was also confirmed, which was approximately 4.5. The semi-concentrated extract was further selected to be applied in the formulation as it was easier to mix with other components.
Carbopol 940 is a common gel-forming agent, which the viscosity can be enhanced by neutralizing the pH using an alkalizing agent such as TEA. The addition of TEA will ionize the carboxylic groups therefore it creates repulsion in between branches, hence forming the 3D structure of gel [28]. The combination of Carbopol 940-TEA provides a rigid transparent form of gel [29] which is expected to be able to deliver the ethanolic extract of fresh young coconut husk dedicated for diabetic wound healing. The addition of DGME, a stabilizer, is found to increase the viscosity of the gel system and stabilizes it. The application of those materials may influence the physical characteristics of the gel formulation especially on pH, viscosity, and spread-ability. Therefore, it is imperative to optimize the formula to provide targeted responses based on acceptance criteria.
Acidic pH of the products must be scrutinized as it will provide a supporting atmosphere for the stability of extract and encourage the wound repairing acceleration. An acidic wound microenvironment with pH 4 has been suggested for a better scheme of wound healing therapy [30]. In this study, targeted pH was on the range of 4.5-5.9. Viscosity of product also plays an important role on the formulation, as the optimum viscosity will assist the manufacturing handling and ease the application on the wound [17, 19]. Spread ability, which is represented by the diameter of the gel applied under 125g weight [25], as a function of viscosity, will also contribute to the optimum way of application the semisolid preparation. Viscosity of 0.5-1.5 Pa. s and the spread-ability of 4-7 cm were targeted based on our previous study [22] and some preliminary experiments.
Response surface methodology of BBD has been satisfactorily applied to optimize the formula with 3 (three) factors of the gel-forming system, which were DGME, Carbopol 940 and TEA (x1, x2, x3) in three different levels of low, medium, and high. The observed responses which were the physical characteristics of the formula involved viscosity, pH and spread ability, served as y1, y2, and y3, respectively. Those three factors in three levels were modelled using the “rsm” package of the R open-source software program to create 16 experimental runs with various compositions, hence various values of each type of response. The model equations of viscosity, pH, and spread-ability were y1 =-3.464+0.028 x1+6.972 x2-0.412 x3-0.146 x1x2-0.278 x1x3+3.438 x2x3+0.009 x12-3.152 x22+2.096 x32(R2 = 0.896), y2 = 9.416-0.382 x1-6.038 x2-2.279 x3+0.032 x1x2-0.003 x1x3-1.300 x2x3+0.019 x12+3.059 x22+6.703 x32(R2 = 0.840), and y3 = 5.785+0.749 x1-8.241 x2+6.477 x3+0.125 x1x2+0.492 x1x3-5.863 x2x3-0.052 x12+3.672 x22-9.534 x32 (R2 = 0.840), respectively. Those equations then were considered to provide response surface plots. Response surface plots of combination of two factors resulted in various surface profiles (fig. 2). Three perspective plots of each response showed the different properties of each surface.
In this current study, it was found that the composite desirability of 0.673. This value was generated from the consideration of three responses namely viscosity, pH, and spread ability. Response of viscosity was set as a target response with the lower, target, and upper of 0.5, 1.0, and 1.5 Pa. s, respectively. Response of pH was set as a target response with the lower, target, and upper of 4.5, 5.2, and 5.9, respectively. Response of spread-ability was set as a target response with the lower, target, and upper of 4.0, 5.5, and 7.0 cm, respectively. Desirability analysis with a value between 0-1 is a statistical tool to optimize the formula based on the target setting of each response [31]. The most expected responses will generate the desirability values close to 1 [32].
It is important to verify the RSM prediction by performing an empirical study of validation of the experimental design. In this current study, the optimized composition of DGME, Carbopol 940, and TEA were applied in the formulation of the topical gel. Three predicted responses were then evaluated. The results of the desirability analysis as well as the validation study were presented in table 5. The visual appearance of topical gels obtained from the optimized condition was also presented in fig. 3. It was found that the validation of the prediction and the observed values were closed and met the acceptance criteria.
The in-use stability results showed that the percentage of alteration of physical properties such as viscosity, which would impact the spread-ability was quite high. The viscosity of the gels reduced over time by around 25% both at cold storage and at room temperature. FYCHEE gels were formulated with quite low viscosity, as it considered to be able to facilitate the release of the active ingredients faster [33, 34]. However, this consideration might cause the risk of the instability of gels on the storage. The high viscosity of Carbopol 940 as gel gel-forming agents would be well performed at neutral pH [35]. The lower the pH of the system would reduce the viscosity [36]. On the other side, the stability of tannin as polyphenol molecules would be endangered in alkaline atmosphere [37]. Furthermore, the acidic pH of wound healing topical preparation was convincingly emphasized to manage faster recovery of wound healing [30]. Incorporating such acidic FYCHEE appeared to be challenging as it undeniably decreased the pH of the system overtime, resulting in the decrease of viscosity of gels. Moderate results of stability evaluation indicated that the formulation study was in the right direction, but the development must be progressing on how to enhance the stability of FYCHEE gels in storage. Exploring the potential of other gel forming agents and suggesting an encapsulation technique for the extract may be of interest for the future research to enhance the stability of the gels.
Although the in-use stability showed moderate results, an in vivo study of diabetic wound healing acceleration revealed that the FYCHEE gels successfully accelerated the wound contraction (table 9 and fig. 4). Wounds contracted at almost 100% on day 12. E5% results were comparable to PC, whereas E10% and E20% showed higher percentage. The astringency effect of tannin as the main component aided with the anti-inflammatory effect of other flavonoids was predicted to be the main roles on diabetic wound contraction acceleration, supported with gel formulation as the prominent vehicle [14, 22, 38, 39].
The novel FYCHEE gels were successfully formulated. Further development of the formulations, especially on the enhancement of gel stability, needs to be done to ensure the quality of FYCHEE gels. Moreover, future studies of long-term stability, clinical trials, and scaling up of commercialization are encouraged to emphasize the FYCHEE contribution to the alternative solution of diabetic wound treatment, with lateral objective of increasing the economic value of the young coconut husk.
CONCLUSION
The “rsm” package of the R open-source program could satisfactorily assisted in conducting the response surface methodology of BBD to optimize the FYCHEE gel formula. A predicted composition of DGME: Carbopol 940: TEA of 8: 0.8: 0.6 could yield the topical gel of ethanolic extract of fresh young coconut with optimized responses which met the target of formula (1.194±0.136 Pa. s for the viscosity; 5.715±0.213 for the pH; 4.997±0.265 for the spread ability) with the composite desirability of 0.673. The FYCHEE gels evidenced to accelerate the diabetic wound on diabetes-induced Wistar rats with similar results to the positive control.
FUNDING
This research was funded by the Directorate of Research, Technology, and Community Services, the Directorate General of Higher Education, Research, and Technology, the Indonesian Ministry of Education, Culture, Research, and Technology (No. 107/E5/PG.02.00. PL/2024, 11th June 2024)
AUTHORS CONTRIBUTIONS
C. M. R. R. N. and F. D. O. contributed to conceptualization, data acquisition, data analysis and interpretation, supervision as well as article writing and critical reviewing. Y. D., C. P., M. R. G. contributed to data acquisition, data analysis, and critical reviewing.
CONFLICT OF INTERESTS
Declared none
REFERENCES
Burgess JL, Wyant WA, Abdo Abujamra BA, Kirsner RS, Jozic I. Diabetic wound healing science. Medicina (B Aires). 2021;57(10):1072. doi: 10.3390/medicina57101072, PMID 34684109.
Jalilian M, Ahmadi Sarbarzeh P, Oubari S. Factors related to severity of diabetic foot ulcer: a systematic review. Diabetes Metab Syndr Obes. 2020 May 25;13:1835-42. doi: 10.2147/DMSO.S256243, PMID 32547145.
Prabhakar PK, Singh K, Kabra D, Gupta J. Natural SIRT1 modifiers as promising therapeutic agents for improving diabetic wound healing. Phytomedicine. 2020 Sep 1;76:153252. doi: 10.1016/j.phymed.2020.153252, PMID 32505916.
Shedoeva A, Leavesley D, Upton Z, Fan C. Wound healing and the use of medicinal plants. Evid Based Complement Alternat Med. 2019;2019(1):2684108. doi: 10.1155/2019/2684108, PMID 31662773.
Sultana A, Borgohain R, Rayaji A, Saha D, Kumar Das BK. Promising phytoconstituents in diabetes-related wounds: mechanistic insights and implications. Curr Diabetes Rev. 2024;21(2):e270224227477. doi: 10.2174/0115733998279112240129074457, PMID 38424430.
Chrismaurin F, Dwiastuti R, Chabib L, Yuliani SH. The effect of olive oil, tween 60 and span 20 on physical characteristics of quercetin nanoemulgel. Int J App Pharm. 2023;15(1):212-7. doi: 10.22159/ijap.2023v15i1.46423.
Alouw JC, Wulandari S. Present status and outlook of coconut development in Indonesia. IOP Conf S Earth Environ Sci. 2020;418(1):12035. doi: 10.1088/1755-1315/418/1/012035.
Rethinam P, Krishnakumar V. Health benefits of coconut water. In: Coconut water: a promising natural health drink distribution processing and nutritional benefits. Springer; 2022. p. 385-455. doi: 10.1007/978-3-031-10713-9_9.
Soliman AM, Lin TS, Ghafar NA, Das S. Virgin coconut oil and diabetic wound healing: histopathological and biochemical analysis. Eur J Anat. 2018;22(2):135-44.
Yuniati R, Subchan P, Riawan W, Khrisna MB, Restiwijaya M, Dyan NS. Topical ozonated virgin coconut oil improves diabetic ulcer wound healing in diabetic mice model. J Phys Conf S. 2020;1524(1):12127. doi: 10.1088/1742-6596/1524/1/012127.
Surboyo MD, Arundina I, Rahayu RP, Mansur D, Bramantoro T. Potential of distilled liquid smoke derived from coconut (Cocos nucifera L) shell for traumatic ulcer healing in diabetic rats. Eur J Dent. 2019;13(2):271-9. doi: 10.1055/s-0039-1693527, PMID 31487751.
Okon OE, Ajienka JA, Ikiensikimama SS, Akaranta OE. Phytochemical characterization of selected agro waste extracts as kinetic inhibitors in methane hydrates formation. Results Eng. 2024 Sep;23:102429. doi: 10.1016/j.rineng.2024.102429.
Chen Y, Tian L, Yang F, Tong W, Jia R, Zou Y. Tannic acid accelerates cutaneous wound healing in rats via activation of the ERK 1/2 signaling pathways. Adv Wound Care. 2019;8(7):341-54. doi: 10.1089/wound.2018.0853, PMID 31737421.
Baldwin A, Booth BW. Biomedical applications of tannic acid. J Biomater Appl. 2022;36(8):1503-23. doi: 10.1177/08853282211058099, PMID 34991392.
Gounden V, Singh M. Hydrogels and wound healing: current and future prospects. Gels. 2024;10(1):43. doi: 10.3390/gels10010043, PMID 38247766.
Bardill JR, Laughter MR, Stager M, Liechty KW, Krebs MD, Zgheib C. Topical gel based biomaterials for the treatment of diabetic foot ulcers. Acta Biomater. 2022 Jan 15;138:73-91. doi: 10.1016/j.actbio.2021.10.045, PMID 34728428.
Rini Nastiti CM, Dwiastuti R, Riswanto FD. Novel quercetin nanoemulgel optimization: gelling agents evaluation and the application of response surface methodology. Int J App Pharm. 2023;15(1):72-8. doi: 10.22159/ijap.2023v15i1.46585.
Nastiti CM, Riswanto FD. Analytical method validation and formula optimization of topical nanoemulsion formulation containing resveratrol. Indones J Chem. 2021;21(5). doi: 10.22146/ijc.63730.
Pal R. Modeling the viscosity of concentrated nanoemulsions and nanosuspensions. Fluids. 2016;1(2):11. doi: 10.3390/fluids1020011.
Riswanto FD, Rohman A, Pramono S, Martono S. Application of response surface methodology as mathematical and statistical tools in natural product research. J App Pharm Sci. 2019;9(10):125-33. doi: 10.7324/JAPS.2019.91018.
Ferreira SL, Bruns RE, Ferreira HS, Matos GD, David JM, Brandao GC. Box behnken design: an alternative for the optimization of analytical methods. Anal Chim Acta. 2007;597(2):179-86. doi: 10.1016/j.aca.2007.07.011, PMID 17683728.
Nastiti CM, Michelina E, Wijayanti FR, Gani MR. Evaluation of diabetic wound healing activity of novel quercetin topical preparations. J Pharm Sci Community. 2024;21(1):51-9. doi: 10.24071/jpsc.007288.
Lenth RV. Response surface methods in R using rsm. J Stat Softw. 2010;32(7):1-17. doi: 1018637/jss.v032.i07.
Riswanto FD, Desra A, Sari RM, Thomas V, Rohman A, Pramono S. Employing an R software package rsm for optimizing of genistein daidzein and glycitein separation and its application for soy milk analysis by HPLC method. Indones J Chem. 2020;20(5):1184-98. doi: 10.22146/ijc.51669.
Garg A, Aggarwal D, Garg S, Singla KA. Spreading of semisolid formulations: an update. Pharm Technol. 2002;26(9):84-105.
Sabat PK, Pradhan SP, Patro R. Evaluation of excisional and incisional wound healing activity of electrohomeopathic drug (spagyric essence) green electricity in rats. Int J Pharm Pharm Sci. 2020;12(10):72-5. doi: 10.22159/ijpps.2020v12i10.38674.
Arifin WN, Zahiruddin WM. Sample size calculation in animal studies using resource equation approach. Malays J Med Sci. 2017;24(5):101-5. doi: 10.21315/mjms2017.24.5.11, PMID 29386977.
Migliozzi S, Angeli P, Mazzei L. Gelation kinetics of non-aqueous carbopol dispersions. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2019;577:84-95. doi: 10.1016/j.colsurfa.2019.05.051.
Kaur D, Raina A, Singh N. Formulation and evaluation of carbopol 940 based glibenclamide transdermal gel. Int J Pharm Pharm Sci. 2014;6(8):434-40.
Sim P, Strudwick XL, Song Y, Cowin AJ, Garg S. Influence of acidic pH on wound healing in vivo: a novel perspective for wound treatment. Int J Mol Sci. 2022;23(21):13655. doi: 10.3390/ijms232113655, PMID 36362441.
Patel MN, Kothari CS. Multivariate approaches for simultaneous determination of avanafil and dapoxetine by UV chemometrics and HPLC-QbD in binary mixtures and pharmaceutical product. J AOAC Int. 2016;99(3):649-63. doi: 10.5740/jaoacint.15-0259.
Amdoun R, Khelifi L, Khelifi Slaoui M, Amroune S, Asch M, Assaf Ducrocq C. The desirability optimization methodology a tool to predict two antagonist responses in biotechnological systems: case of biomass growth and hyoscyamine content in elicited datura starmonium hairy roots. Iran J Biotechnol. 2018;16(1):e1339. doi: 10.21859/ijb.1339, PMID 30555836.
Aly UF. Preparation and evaluation of novel topical gel preparations for wound healing in diabetics. Int J Pharm Pharm Sci. 2012;4(4):76-7.
Morsy MA, Abdel Latif RG, Nair AB, Venugopala KN, Ahmed AF, Elsewedy HS. Preparation and evaluation of atorvastatin-loaded nanoemulgel on wound healing efficacy. Pharmaceutics. 2019;11(11):609. doi: 10.3390/pharmaceutics11110609, PMID 31766305.
Rowe RC, Sheskey P, Quinn M. Handbook of pharmaceutical excipients. Libros Digitales Pharmaceutical Press; 2009.
Agarwal M, Joshi YM. Signatures of physical aging and thixotropy in aqueous dispersion of carbopol. Phys Fluids. 2019;31(6):63107. doi: 10.1063/1.5097779.
Makkar HP, Becker K. Effect of pH temperature and time on inactivation of tannins and possible implications in detannification studies. J Agric Food Chem. 1996 Jan 1;44(5):1291-5. doi: 10.1021/jf9506287.
Antonia SL, Laynne HC, Davi S, Livio CC, Jose AD. Incorporation of tannic acid in formulations for topical use in wound healing: a technological prospecting. Afr J Pharm Pharmacol. 2015;9(26):662-74. doi: 10.5897/AJPP2015.4361.
Veronica E, Dwiastuti R. Formulation and evaluation of wound healing gel of white lead tree (Leucaena Leucocphala (lam.) de Wit.) leaves extract. Int J Appl Pharm. 2022 Jan 7;14(1):275-80. doi: 10.22159/ijap.2022v14i1.42126.