
Int J App Pharm, Vol 17, Issue 4, 2025, 193-200Original Article
OPTIMIZATION OF SODIUM BICARBONATE-CITRIC ACID CONCENTRATION IN APHRODISIAC EFFERVESCENT GRANULE DOSAGE FORM OF A COMBINATION OF JAVANESE CHILI EXTRACT AND RED GINGER EXTRACT AND EVALUATION OF ITS PHYSICAL PROPERTIES
RIZA MAULANA, ARIFAH SRI WAHYUNI, MUHAMMAD DAI*
Faculty of Pharmacy, Universitas Muhammadiyah Surakarta, Jl. A. Yani No 1, Pabelan, Surakarta, Indonesia
*Corresponding author: Muhammad Dai; *Email: muhammad.dai@ums.ac.id
Received: 27 Dec 2024, Revised and Accepted: 16 May 2025
ABSTRACT
Objective: Javanese chili and red ginger have been recognized as natural ingredients that are potential to act as aphrodisiac agents. The flavonoids and alkaloids in these two natural ingredients can help to increase testosterone hormone. The value of novelty in this study is to combine the two active ingredients into one effervescent granule dosage form that, to be best of our knowledge, has not been done in previous similar studies. This study aims to optimize the concentration of citric acid and sodium bicarbonate in the effervescent granule dosage form of a combination of Javanese chili extract and red ginger extract to obtain an acceptable physical properties.
Methods: The research started from the extraction process of Javanese chili and red ginger until obtaining the dry extract, to the formula optimization stage and evaluation of physical properties, including organoleptical evaluation, granule flow speed, pH, and dispersion time of effervescent granules. The optimization process was carried out through the D-Optimal method with the help of Design Expert version 13 software.
Results: The results from the extraction of Javanese chili and the extraction of red ginger obtained 2.23% and 18.85%, respectively. The optimization results obtained the optimum formula of sodium bicarbonate: citric acid concentration ratio at 1225.08 and 280.92 mg, respectively. Furthermore, the results of statistical analysis using one sample t-test with SPSS software showed granule flowability at 13.78±0.94 g/sec (p value= 0.280; p>0.05), pH 6.02±0.09 (p value= 0.016; p<0.05), and dispersion time at 76.99±8.37 sec (p value= 0.245; p>0.05).
Conclusion: These results showed that granule flowability and dispersion time parameters were not significantly different between the predicted and verified values; however, for pH it was significantly different although the pH obtained still met the pH requirements of effervescent dosage form.
Keywords: Aphrodisiacs, Blueberry, Effervescent, Javanese chili, Red ginger
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijap.2025v17i4.53539 Journal homepage: https://innovareacademics.in/journals/index.php/ijap
INTRODUCTION
Erectile dysfunction refers to the persistent inability to achieve and maintain an erection sufficient for satisfactory sexual performance [1, 2]. The prevalence of erectile dysfunction is assumed to increase globally in which a number of previous studies predicted that erectile dysfunction will affect 322 million men worldwide by 2025, a 111% increase from 1995 [3].
Aphrodisiacs, as defined, are organic compounds or medications recognized for their capacity to enhance sexual arousal. The natural sources containing alkaloids, essential oils, flavonoids, and terpenoids, have the capability to stimulate androgen hormones that can induce masculinizing effects, stimulate spermatogenesis in the testes, enhance aggression, contribute to bone and muscle growth, and influence sexual behavior. One type of androgen hormone is testosterone [4–7] in which the higher the testosterone level, the higher the quality of sperm produced [8].
Recent studies have explored the potential of red ginger (Zingiber officinale) and Javanese chili (Piper retrofractum Vahl) in pharmaceutical applications, particularly as aphrodisiacs. The content of compounds in Javanese chili that act as aphrodisiacs is piperine where this substance has the potential to contain natural testosterone. In addition, Javanese chili contains alkaloids, flavonoids, and terpenoids, also known to have the potential to increase androgen hormone activity [5, 9-11]. Meanwhile, the active compounds contained in red ginger that act as aphrodisiacs are flavonoids and alkaloids. Flavonoids act to insscrease dehydroepiandrosterone levels, which can increase testosterone levels and encourage sexual behavior in men. Alkaloids, meanwhile, are known to have a role in inducing vasodilation, resulting in erection [5, 12].
Effervescent drug delivery systems, including granule form, can enhance drug dissolution, bioavailability, and patient compliance, particularly for those with swallowing difficulties [18, 19]. The utilization of effervescent dosage form for aphrodisiacs has been widely used in previous studies to cover the bitter and unpleasant taste of bitter melon and garlic extracts with the result of increasing the libido of white mice [11]. In common, effervescent granule formulations use a combination of citric acid and tartric acid that can affect the dissolution time [20–22]. This study in turn aims to optimize the concentration of citric acid and sodium bicarbonate in effervescent granule dosage form of a combination of Javanese chili extract and red ginger extract.
MATERIALS AND METHODS
Materials
This study used a rotary evaporator, waterbath, oven (Memmert), moisturizer balance, funnel flow properties, and pH meter (Ohaus). The materials used included fresh Javanese chili fruit, red ginger, blueberry, tartric acid, citric acid, sodium bicarbonate, lactose, Stevia sweetener, polyvinylpyrrolidone (PVP), and 96% ethanol.
Sample preparation
The materials that need to be prepared included Javanese chili (Piper retrofractum Vahl.), red ginger (Zingiber officinale), and blueberry (Vaccinium corymbosum). The extraction process used maceration method with 70% ethanol solvent (chili and red ginger). The method used was maceration with the ratio of simplisia and solvent at 1: 7.5 and re-maceration for 2 times with the ratio of simplisia and solvent at 1: 5 for 3 d. The filtrate obtained was then evaporated with a rotary evaporator for 1 h and continued on a waterbath for 24 h to obtain a thick extract. Meanwhile, the blueberry extraction process used 95% ethanol solvent with acidified using 0.3% HCl 0.1N with the ratio of simplisia and solvent at 1:10. Furthermore, the drying process of the extract was carried out by modifying research result [20] by adding a mixture of aerosil and lactose (1:1) for the drying process. The mixture obtained was then put into an oven at 60 °C overnight.
Design of effervescent granule formula
Effervescent granules can be formulated via wet granulation method. The wet granulation process was carried out using 3% polyvinylpyrrolidone (PVP) binder by modifying the method from research [21, 22]. A total of 3 g of PVP was dissolved in 100 ml of 96% ethanol and added to the powder mixture in the acidic part. The acidic part of the preparation-a mixture of citric acid, tartric acid, the three types of extracts, lactose, and Stevia sweetener-was sieved by means of mesh 20 and dried in an oven at 60 °C for 2 h. Subsequently, the base part, i. e. sodium bicarbonate, was added at a controlled room temperature at 20ᵒC and humidity of 45% [23, 24]. Table 1 presents the design of the effervescent granule formula. Research result [5] mentioned that red ginger extract and Javanese chili extract with a dose of 30 mg each produced high aphrodisiac activity, as characterized by a high frequency of occurrence of Introduction, Climbing, and Coitus in male rats.
Table 1: Design of effervescent granule formula
| Ingredients | Formulas (mg) | ||||
| F1 | F2 | F3 | F4 | F5 | |
| Red ginger extract | 30 | 30 | 30 | 30 | 30 |
| Javanese chili extract | 30 | 30 | 30 | 30 | 30 |
| Blueberry extract | 250 | 250 | 250 | 250 | 250 |
| Lactose | 1000 | 1000 | 1000 | 1000 | 1000 |
| Stevia | 1000 | 1000 | 1000 | 1000 | 1000 |
| Sodium bicarbonate | 1036 | 1100 | 1164 | 1228 | 1292 |
| Citric acid | 470 | 406 | 342 | 278 | 214 |
| Tartric acid | 684 | 684 | 684 | 684 | 684 |
| PVP (3%) | q. s | q. s | q. s | q. s | q. s |
| Total weight | 4500 | 4500 | 4500 | 4500 | 4500 |
Optimization of effervescent granule dosage form
Optimization was carried out through the D-Optimal method with the help of Design Expert software version 13. This process was carried out to find the optimum formula based on variations in citric acid and sodium bicarbonate concentrations. The response parameters used included granule flowability, pH, and dispersion time. Table 2 presents the determination of the lower and upper limits of citric acid and sodium bicarbonate based on the results of lab data orientation and table 3 depicts the optimization design using 11 runs.
Table 2: Concentration variation of sodium bicarbonate-citric acid
| Ingredients | Concentration (mg) | |
| Low | High | |
| Sodium bicarbonate | 1036 | 1292 |
| Citric acid | 214 | 470 |
Table 3: Optimization design of effervescent granule formula
| Formulas | Runs | Concentration (mg) | |
| Citric acid | Sodium bicarbonate | ||
| 4 | 470 | 1036 | |
| 1 | 5 | 470 | 1036 |
| 8 | 470 | 1036 | |
| 2 | 9 | 406 | 1100 |
| 3 | 1 | 342 | 1164 |
| 2 | 342 | 1164 | |
| 4 | 11 | 278 | 1228 |
| 3 | 214 | 1292 | |
| 5 | 6 | 214 | 1292 |
| 7 | 214 | 1292 | |
| 10 | 214 | 1292 |
Physical properties evaluation of effervescent granule Organoleptical properties
This test included the observation of the shape, color, odor, and taste of the effervescent dosage form after the granule dispersion. Testing can be visually done to assess odor, color, and taste using the five senses [25, 26].
Granule flowability
The granule was slowly poured into a measuring funnel (granule flow tester) through the edge of the funnel. The lid of the funnel was then opened slowly, and the granule was allowed to flow out. The flow rate of the effervescent granule was recorded (direct method). Flow time measurement was carried out to determine the flow speed of the granule. Here, the requirement for a good flow speed is ≥10 sec [22, 26, 27].
Value of pH evaluation
The efficacy of the effervescent dosage form is contingent upon the acidity level, which should ideally approximate neutral acidity (pH 6-7). The pH test was carried out by initially dissolving the effervescent granule in 200 ml of distilled water until completely being dispersed and it was then measured by means of a pH meter [28, 29].
Dispersion time
Dispersion time is the time required to disperse the effervescent granule in an appropriate medium purposely to make no part of the granule left behind or settled. The test method for granule dispersion time was by putting 5 g of granules into 200 ml of distilled water. The calculation of dispersion time was carried out from the moment the granule was immersed in distilled water until all the granules were dissolved and the bubbles around the container disappeared. The ideal dispersion time of effervescent dosage form is <5 min [30, 31].
Data analysis
Data analysis was carried out by means of Design Expert software version 13 and SPSS software to obtain the optimum formula of effervescent granule dosage form tested with the response parameters of granule flow speed, pH of effervescent solution, and dispersion time of effervescent granules.
RESULTS AND DISCUSSION
Sample extraction
Table 4 shows the extraction results where the moisture content for the three dried extracts was found below<10%. A good dry extract was characterized by a moisture content of <10% [32]. Moisture content significantly impacts the stability of pharmaceutical formulations, including effervescent granules. High moisture levels can lead to degradation, color changes, and loss of active ingredients. While, low moisture content contributes to the stability of effervescent granules [33–35].
Extraction was carried out until dry extract was obtained to facilitate the mixing process of the effervescent granule formula. It is important that the mixing process of effervescent granules is dry to prevent any acid-base reactions. The presence of water particles in the system during the mixing process can trigger a reaction. In addition, temperature and humidity are other factors that need to be considered in which they need to be controlled during the manufacturing process. Maceration remains competitive due to its energy productivity and easier scale-up; besides, this method can yield higher phenolic compound content [36, 37].
Table 4: Extraction results of the three types of extracts
| Parameters | Extracts | ||
| Javanese chili | Red ginger | Blueberry | |
| Simplisia weight (g) | 900.18 | 800.07 | 756.34 |
| Thick extract weight (g) | 94.62 | 42.91 | 97.49 |
| Dry extract weight (g) | 155.78 | 92.66 | 148.99 |
| Yield (%) | 17.31 | 11.58 | 19.7 |
| Moisture content (%) | 3.87 | 2.69 | 4.97 |
Blueberries contain high amounts of flavonoids and polyphenols as well as anthocyanins that can inhibit inflammation and act as good antioxidant agents. Blueberry extract was carried out under acidic condition to keep the anthocyanin glycone chain intact to maintain the resulting color. The anthocyanin color in blueberries is used as a natural colorant in effervescent granule dosage form [16, 17, 38].
Organoleptical testing
The effervescent granule visually is purple-the color of blueberry. Blueberry can act as a natural colorant that can be utilized in the pharmaceutical formulation and food dosage form. The effervescent granule was then dispersed in 200 ml of distilled water and it could produce a clear purple with a very small amount of brown extract granules. The resulting taste was sweet and pleasant with blueberry aroma and was able to cover the unpleasant taste of Javanese chili and red ginger. The odor produced has a typical effervescent aroma with a slight blueberry odor [39, 40].
Effervescent granule formula optimization
Research has shown that red ginger extract exhibited an aphrodisiac activity in animal models. Similarly, Javanese chili extract has been found to significantly increase libido in male mice, with higher doses correlating strongly to enhanced sexual behaviors [13–15]. These findings suggest that red ginger and Javanese chili are potential as natural aphrodisiacs and warrant further investigation for pharmaceutical applications.
D-optimal design is a powerful method for experiment optimization minimizing the determinant of the covariance matrix of estimated parameter. It outperforms any existing methods in accuracy and precision for apparent diffusion coefficient imaging [41]. The optimization process was carried out by evaluating the values of effervescent granule flowability, pH, and dispersion time as response parameters. Table 6 presents the test results on 11 runs.
Table 6: Granule effervescent optimization data
| Formulas | Runs | Granule flowability* (g/sec) | pH* | Dispersion time* (sec) |
| F1 | 4 5 8 |
13.94±1.14 9.7±2.13 11.44±2.31 |
6.23±0.52 6.22±1.28 5.33±0.14 |
65.26±2.33 70.07±3.12 80.09±2.41 |
| F2 | 9 | 14.99±1.33 | 5.98±0.67 | 117.59±1.12 |
| F3 | 1 2 |
17.06±2.17 13.99±1.62 |
6.1±0.06 6.19±0.19 |
102.77±0.45 118.18±0.77 |
| F4 | 11 | 15.73±2.03 | 5.78±1.88 | 58.81±1.67 |
| F5 | 3 6 7 10 |
9.34±0.81 10.12±1.08 10.84±1.24 11.15±0.35 |
6.16±0.69 5.33±1.17 4.75±0.11 4.67±0.93 |
85.94±2.13 92.98±2.49 100.16±1.22 80.67±2.67 |
*Data are presented as mean±standard deviation (SD); n = 3.
Table 7: Responses model equation
| Responses | Equation | Model | p-value | Lack of fit |
| Granule flowability | Y=(-)0.014A-0.256B+0.0003AB | Quadratic | 0.004 | 0.688 |
| pH | Y=0.0033A-0.024B+0.000027AB | Quadratic | 0.0004 | 0.631 |
| Dispersion time | Y=0.961A-45.952B+0.055AB-0.000022AB(A-B) | Cubic | 0.0026 | 0.1012 |
The response model for the response parameters of granule flowability and pH was quadratic, while for disperse time was cubic. The p-value for all parameters was found at<0.05, making it significant. A significant p-value indicates that the varied variables have a real effect on the response. On the other hand, the lack of fit value for all responses was found at>0.05, making it insignificant. This indicated that the selected model can be used to optimize variables on the response and the discrepancy between the value of the test results and the predicted value of the software was not significantly different. In other words, there was a match between the response data and the model.
As shown in table 7, the granule flowability response equation showed that sodium bicarbonate and citric acid basically had a negative effect on granule flowability. Here, the negative effect of citric acid on granule flowability was more dominant as indicated by a larger coefficient value than the sodium bicarbonate coefficient. Granulation process, therefore, was needed to improve the flow properties of granules. Wet granulation was carried out at a temperature of 60ºC so as not to degrade the extract mixed into the acidic part in view of the influence of heat and humidity. PVP material here was used as a binder to be added to the acid part to initially form granules before being mixed with the base part. PVP can be used to improve the flow properties of effervescent granules. The interaction of sodium bicarbonate and citric acid in the preparation was able to increase the flow speed of the granule as indicated by the “+” sign in the response equation although with a small effect. As illustrated in graph 1(A), the effect of sodium bicarbonate and citric acid together could increase the flowability of effervescent granules until the optimal point, after which the granule flowability would decrease. This could also be due to the smaller amount of granulated citric acid affecting the response of the granule [42, 43]. This indicates an optimal concentration of sodium bicarbonate and citric acid to produce the highest granule flowability value.
Granule flowability is crucial in effervescent formulations, determining processability and final product quality. Good flow properties ensure uniform dosing and efficient manufacturing. Typically, wet granulation yields better flowability compared to direct compression. The evaluation of granule characteristics, including flowability, is deemed essential to optimize formulations and predict tablet properties. Porous particles improve flowability and dissolution rates compared to dense particles [43-45].
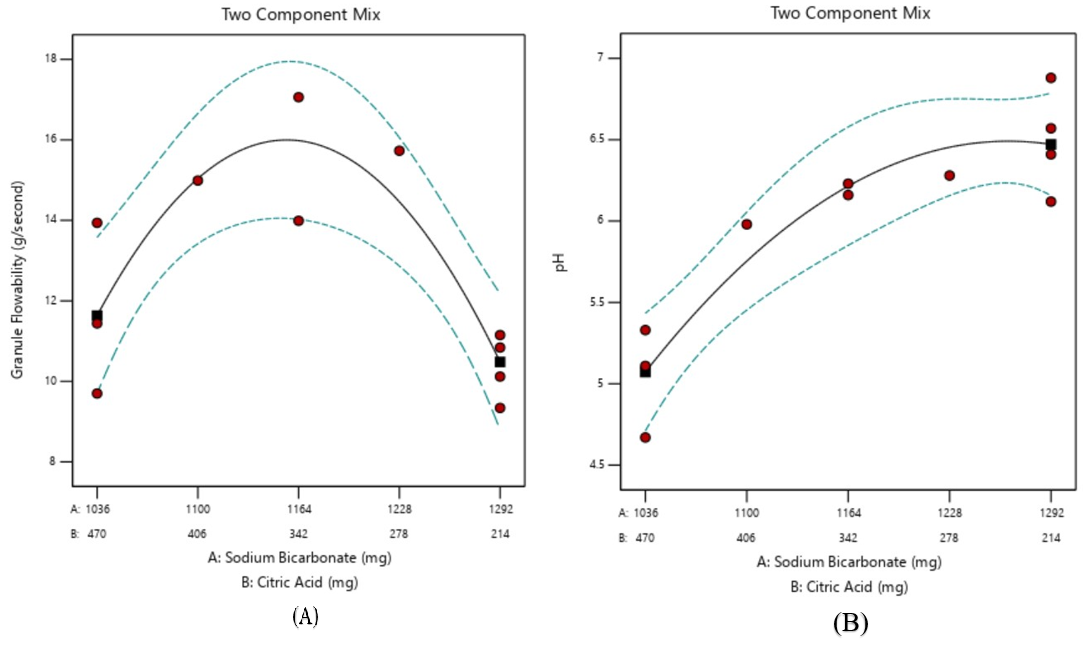
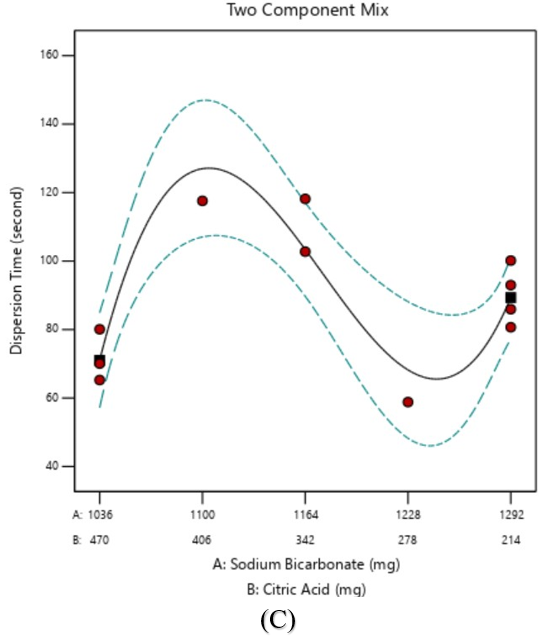
Fig. 1: Equation graphs of (A) granule flowability; (B) pH; (C) dispersion time
Furthermore, as shown in table 7, the optimization results of the pH response obtained the equation, indicating that sodium bicarbonate increased the pH value, while citric acid decreased the pH of the effervescent solution. This has been in accordance with the acid-base principle of both ingredients. Fig. 1(B) shows that the interaction of the two ingredients could increase the pH value to the optimal point, as characterized by the highest pH value close to neutral pH. This was because the proportion of the base part in the effervescent system was found greater than the acid part. The commonly used acid-base systems in effervescent dosage form is in the the ratio of citric acid: tartric acid: sodium bicarbonate with a portion of 1:2:3.4 in order [46].
This acid-base system will affect the solubility of effervescent granules when being in contact in water, later on affecting the pH formed. The ideal pH value for effervescent solutions is 6-7. Setting the pH is critical because, if the pH value is too acidic or alkaline, it can cause stomach irritation and a bitter taste. The acid base in this effervescent granule dosage form gives a sparkling taste like soda, while the base gives the effect of the emergence of air bubbles in water due to reacting with acid [46, 47].
The evaluation of pH value is crucial in effervescent dosage forms in view of its impact on various aspects of formulation and performance. The pH affects the effervescence process, which is essential for rapid drug dissolution and enhanced bioavailability. It determines the reaction between acid and base components, leading to carbon dioxide generation and tablet disintegration. pH also plays a role in taste masking, which is important for patient acceptability. Also, pH evaluation is deemed critical for ensuring stability and consistent product performance as effervescent formulations are sensitive to environmental conditions. The optimal pH can affect the solubility and release the kinetics of the active ingredients. A careful pH control therefore is necessary to achieve desired physicochemical properties, dissolution time, and overall quality of effervescent dosage forms. The stability of anthocyanins, natural pigments responsible for vibrant colors in fruits and vegetables, is significantly determined by pH levels. Lower pH values can enhance anthocyanin stability, while the higher one can lead to degradation and color loss. The pH of effervescent products is an important parameter assessed during formulation and stability testing [48–52].
The optimization results of the dispersion time response showed that the effect of increasing sodium bicarbonate concentration could shorten the dispersion time though with the more dominant influence of citric acid where the increasing concentration of effervescent granule dispersion time required became longer. The interaction of sodium bicarbonate and citric acid will directly affect the required effervescent granule dispersion time-dependent upon the proportion of acid and base in the effervescent system. Fig. 1(C) illustrates the effect of varying the concentration of these two ingredients where the model obtained was cubic. The dispersion time can be shorter or longer dependent upon the amount of sodium bicarbonate and citric acid [48, 53, 54]. The cubic model showed that changes in the amount of sodium bicarbonate-citric acid could cause an increase or decrease in dispersion time at a certain concentration.
The evaluation of dispersion time is crucial for ensuring the optimal performance of effervescent formulations. Studies have shown that effective formulations should have dispersion times less than 3 min. Dispersion time can be determined by a number of factors such as the type and quantity of effervescent agents and disintegrants. Dispersion time evaluation helps in determining the suitability of medications for administration through enteral feeding tubes, potentially improving safety and efficacy in clinical settings. Typically, commercial effervescent tablets disintegrate within 1-2 min with expired tablets showing shorter disintegration times than unexpired ones [48, 53, 55, 56].
Effervescent powder formulations generally use a combination of citric acid and tartric acid. If tartric acid is used as a single acid, the resulting powder will easily lose its strength and clump. While, citric acid alone will produce a sticky mixture and is difficult to become a powder. The base component in the effervescent powder has a similar function as the acid component that is to facilitate solubility. The most widely used base source is sodium bicarbonate that reacts by releasing Na+ions, which will then react with water and acidic sources to form sodium bicarbonate salts, thereby reducing H+activity, causing the solution to be more alkaline. Sodium bicarbonate is the largest source of carbonate with a very large solubility in water, free flowing, and non-hygroscopic. It will give rise to CO2 gas when reacted with acid. To produce the effervescent reaction, it takes three molecules of sodium bicarbonate to neutralize one molecule of citric acid and two molecules of sodium bicarbonate to neutralize one molecule of tartric acid. The more the addition of acid and base sources, the faster the dissolution time. This is because the more acidic and basic sources used, the more carbon dioxide produced [56–59].
The optimization of citric acid and sodium bicarbonate is crucial in effervescent formulations as these components react to form carbon dioxide, facilitating tablet disintegration and drug dissolution. A number of studies have explored the mechanistic effect of sodium bicarbonate and citric acid in effervescent formulations. These compounds generate carbon dioxide when combined, which can enhance drug release and permeation. In effervescent buccal discs, this reaction can lead to faster drug release and improve bioavailability compared to non-effervescent formulations. Similarly, effervescent tablets of bilastine showed improved dissolution properties when formulated with sodium bicarbonate and citric acid [57–59].
The optimization of citric acid and sodium bicarbonate ratios is crucial for developing effective effervescent formulations. Some studies have explored various molar ratios, including 1:3.11, 1:3, and 1:2.93 for Moringa leaves effervescent granules. For bismuth sub-citrate effervescent tablets, a 1:3 ratio was found optimal. The concentration ratio affects several important parameters such as disintegration time, pH, CO2 production, and organoleptic properties. These studies demonstrated that optimizing citric acid and sodium bicarbonate concentrations is essential for achieving desired physicochemical properties and consumer acceptability in effervescent formulations [52, 59, 60].
Determination of responses criteria
The response criteria for the flow speed of the effervescent granule was chosen maximize so that the granule obtained could be easy to flow, making it also easier for both the packaging process and consumption. The pH value was chosen maximize to enter the ideal range of pH of the effervescent solution, i. e. 6-7 [28, 29]. Whereas, the dispersion time response was chosen minimize so that it could take a shorter time for the effervescent granule to dissolve in water. Setting the goal of each response also adjusts to the range of lower and upper limit values obtained from the test results to match the ideal value of each response. Importance is needed as an indicator if there is a response that is more decisive than other responses in a dosage system. In this study, importance was made equal as all tested responses had an equally important role to achieve optimal granule and effervescent solution conditions. Table 8 attaches the determination of response criteria.
Table 8: Responses criteria
| Responses | Units | Goal | Lower limit | Upper limit | Importance |
| Granule flowability | g/sec | Maximize | 9.34 | 17.06 | +++ |
| pH | - | Maximize | 4.67 | 6.88 | +++ |
| Dispersion time | Second | Minimize | 58.81 | 118.18 | +++ |
The optimum formula of effervescent granule dosage form was obtained at a concentration of sodium bicarbonate at 1225.08 mg and citric acid at 280.92 mg. This combination produced the highest desirability value of 0.767. The desirability value indicates the ability of the model to optimize the product in accordance to the specified criteria; a good desirability value is more than 0.5 [61]. A desirability value close to 1 indicates the product produced is close to perfect and more likely to become the optimum formula [62, 63].
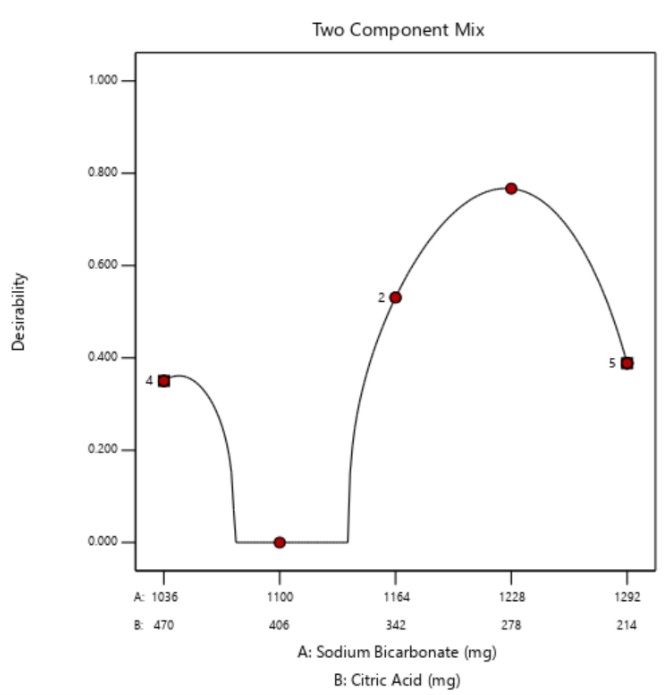
Fig. 2: The relationship between sodium bicarbonate-citric acid concentration variation and desirability value
Verification of optimum formula
The optimum formula verification stage needs to be done to test the optimum formula prediction from the software. The predicted optimum formula was created and tested according to the response parameters. Replication was carried out 2 times so that 3 data were obtained for each response. The data obtained were then entered into the Design Expert version 13 software, and compared with the predicted value [60]. The test results (observed mean) showed that the response values were within the 95% Prediction Interval (PI) range, as listed in table 9. The observed mean data also showed that the response values were within the 95% Confidence Interval range, except the pH response. The 95% Prediction Interval (PI) describes the range of individual predicted values generated by observations at the 95% confidence level [64]. Confidence Interval (CI) are the essential statistical tools for estimating population parameters from sample data. They provide a range likely to include the true population value with a specified level of certainty, typically 95% [65]. Further, the optimum formula obtained from the Design Expert was verified and analyzed with the help of the SPSS software. The normality test showed that the data were normally distributed enabling it to use the one sample t-test with 95% confidence level to compare the verification data with the predicted value [66]. The results revealed that for granule flowability and dispersion time parameters (p value= 0.280 and 0.245, respectively; p>0.05), indicating that the prediction and verification results were not significantly different. However, pH (p value= 0.016; p<0.05) showed that the prediction and verification results were significantly different although it still met the pH requirements of effervescent dosage form. Based on the results of the one sample t-test, the standard error value for the pH parameter was 5.3%. When applying Design of Experiments (DOE), like Design Expert software, to optimize formulations, the prediction errors of less than 10% are considered acceptable in predicting responses [67, 68].
Table 9: Verification results of optimum formula
| Responses | Units | Predicted mean | Observed mean | PI 95% value | CI 95% value | ||
| Low | High | Low | High | ||||
| Granule flowability | g/sec | 14.59 | 13.78 | 12.02 | 17.15 | 12.96 | 16.21 |
| pH | - | 6.45 | 6.02 | 5.97 | 6.93 | 6.15 | 6.75 |
| Dispersion time | second | 69.11 | 76.99 | 44.87 | 93.36 | 49.27 | 88.96 |
Effervescent Drug Delivery Systems (EDDS) offer advantages such as improved bioavailability and patient compliance, but they face challenges in scaling up production. Key issues include sticking, high hygroscopicity, and compactibility problems during manufacturing. Controlling humidity and temperature during manufacturing is deemed crucial for maintaining product quality across different scales. Herbal aphrodisiacs have gained popularity as perceived safer alternatives to synthetic drugs though they may still cause adverse effects, particularly when overdosed or combined with other medications. The formulation of drugs as effervescent granules, such as ibuprofen, has shown a promise in increasing dissolution rates and rapid onset of action. This dosage form could potentially be applied to aphrodisiacs in effervescent granule form, combining the benefits of EDDS with the therapeutic effects of sexual enhancers [43, 69–71].
CONCLUSION
The optimum formula of effervescent granule dosage form was obtained at a concentration of sodium bicarbonate 1225.08 mg and citric acid 280.92 mg with good physical properties including the value of granule flowability of 13.78±0.94 g/sec, pH 6.02±0.09, and dispersion time of 76.99±8.37 sec, which are in accordance with the requirements for the acceptability of effervescent dosage form.
ACKNOWLEDGEMENT
The authors acknowledge the Ministry of Education, Culture, Research, and Technology of the Republic of Indonesia for providing support through grant contract agreement number 007/lL6/PB/AL.04/2024,196.39/A.3-III/lRI/VI/2024.
FUNDING
Nil
AUTHORS CONTRIBUTIONS
All authors have made an equally contribution. Riza Maulana was in charge of conducting formulation and evaluation to the physical properties of effervescent granules. Arifah Sri Wahyuni was responsible for conducting extraction process of Javanese chili, red ginger, and blueberry. Meanwhile, Muhammad Da'i conducted data analysis and manuscript review.
CONFLICT OF INTERESTS
All authors declare no conflict of interest.
REFERENCES
ReferencesLiu Q, Zhang Y, Wang J, Li S, Cheng Y, Guo J. Erectile dysfunction and depression: a systematic review and meta-analysis. J Sex Med. 2018;15(8):1073-82. doi: 10.1016/j.jsxm.2018.05.016, PMID 29960891.
Wati W, Rahman EY, Rosida L, Sutapa H, Panghiyangani R. Literature review: relationship between age severity of benign prostate hyperplasia (BPH) and incidence of erectile dysfunction. Homeostasis. 2021 Apr 30;4(1):237–44.
Goldstein I, Goren A, Li VW, Tang WY, Hassan TA. Epidemiology update of erectile dysfunction in eight countries with high burden. Sex Med Rev. 2020;8(1):48-58. doi: 10.1016/j.sxmr.2019.06.008.
Wahyuni AS, Wahyuningtyas N, Arifiyanti. Aphrodisiac activity of essential oil from Syzygium aromaticum (L.) Merr. and perry. Pharmacon J Farm Indones. 2010;11(2):43-6.
Sri Wahyuni A, Maulana R, Maryati M, Da’i M. Exploration of indonesian herbal plants as aphrodisiac agents and testing their activity in animal testing. Res J Pharm Technol. 2024;17(8):3969-75. doi: 10.52711/0974-360X.2024.00616.
Salleh M, Ahmad F. Phytopharmacological investigations of Piper retrofractum Vahl. a review. Agric Conspec Sci. 2020;85(3):193-202.
Sharma M, Arya D, Bhagour K, Gupta RS. Natural aphrodisiac and fertility enhancement measures in males: a review. Curr Med Res Pract. 2017;7(2):51-8. doi: 10.1016/j.cmrp.2017.02.007.
Rachmawati L, Ismaya AP. Correlation between testosterone hormone libido and sperm quality in bligon kejobong and peranakan etawah goats. Bul Peternak. 2014;38(1):8-15.
Muslichah S. Aphrodisiac potential of active ingredients of java chili fruit (Piper retrofactrum Vahl.) in male wistar rats. J Agrotek. 2011;5(2):11-20.
Himayani R. The relationship of giving Javanese chili extract Piper retrofractum to the number of spermatozoa of adult male mice. J Kedokt Kesehat Univ Lampung. 2012;2(1):73-6.
Listiana O, Nurhidayati LG, Fia NS. Test of aphrodisiac activity of effervescent tablet preparations of combination extracts of bitter melon (Momordica charantia L.) and garlic (Allium sativum L.) in male white mice (Mus musculus L.). USADHA J Integr Obat Tradis. 2023;2(3):30-6.
Oktadiana I. Preformulation and evaluation of capsule preparations from red ginger (Zingiber officinale) as antidisminorhea. MEDFARM J Farm Kesehat. 2022;11(1):93-100. doi: 10.48191/medfarm.v11i1.99.
Jadhav S, Gangurde A. A bird eye view on effervescent drug delivery system. Int J Drug Deliv Technol. 2023;13(3):1046-58. doi: 10.25258/ijddt.13.3.45.
Vanhere KG, Derle DV, Khatale SB, Nangude SL. A comprehensive review on effervescent tablets. J Drug Delivery Ther. 2023;13(7):141-50. doi: 10.22270/jddt.v13i7.6120.
Yulianti DA, Sutoyo S. Formulation of effervescent tablets of katuk leaf extract (Sauropus androgynous L. Merr.) with variation of acid and base concentration. J Farm Sains Terap. 2021;8:34-40. doi: 10.36858/ipj.v2i1.44
Septianingrum NM, Hapsari WS, Amin MK. Formulation and test of effervescent powder preparation of okra extract (Abelmoschus esculentus) as nutridrink in patients with diabetes. Media Farm. 2019;16(1):11-20.
Rani KC, Parfati N, Muarofah D, Sacharia SN. Effervescent granule formulation of herba meniran (Phyllanthus niruri L.) with variations of suspending agent xanthan gum Cmc-Na, and combination of Cmc-Na-Microcrystalline cellulose Rc-591. J Sains Farm Klin. 2021;7(1):39-51.
Dani DH, Naqvi SB, Akram M, Khaliq SA, Nasiri MI. Design formulation optimization and stability studies of paracetamol effervescent tablets. Pak J Pharm Sci. 2023;36(1):247-54. doi: 10.36721/PJPS.2023.36.1.SP.247-254.1.
Diyya AS, Thomas NV. Formulation and evaluation of metronidazole effervescent granules. Int J Pharm Sci Res. 2018;9(6):2525-9. doi: 10.52711/2231-5691.2024.00055.
Sholikhah AM, Amal S, Susilowati F. Effervescent tablet formulation of kersen leaf (Muntingia calabura L.) with variation of effervescent mix concentration. Pharmasipha. 2018;2(2):37-42. doi: 10.21111/pharmasipha.v2i2.2779.
Azahra AS, Prabowo A, Yasmin AA. Development and evaluation of effervescent powder formulations combining red ginger extract and honey. IJHMS. 2024;2(2):41-5. doi: 10.46336/ijhms.v2i2.101.
Ministry of Health of Indonesia Republic. Indonesian pharmacopoeia. VI. Jakarta; 2020.
Kailaku SI, Sumangat J. Hernani. Formulation of antioxidant rich effervescent granule from gambir leaf extract. J Pascapanen. 2012;9(1):27-34.
Syaputri FN, Saila SZ, Tugon TD, R AP, Lestari D. Formulation and test of physical characteristics of effervescent granule preparations of ethanol extract of red betel leaf (Piper crocatum Ruiz) as antidiabetics. J Ilmu Kefarmasian. 2023;4(1):191-8.
Putri N, Slamet N, Wicita P, Imran A. Effervescent granule from combination of telang flower (Clitoria ternatea) and kalamansi orange (Citrus microcarpa) as alternative health drink. J Exp Clin Pharm. 2021;1(1):16-23.
Gopalan S, Gozali D. Article review: formulation and evaluation of effervescent granule and tablet preparations by wet granulation method. Farmaka. 2018;16(1).
Ministry of Health of Indonesia Republic. Indonesian Herbal Pharmacopoeia. II. Jakarta; 2017.
Dhondale MR, Thakor P, Nambiar AG, Singh M, Agrawal AK, Shastri NR. Co-crystallization approach to enhance the stability of moisture sensitive drugs. Pharmaceutics. 2023;15(1):189. doi: 10.3390/pharmaceutics15010189, PMID 36678819.
Jutkus RA, Li N, Taylor LS, Mauer LJ. Effect of temperature and initial moisture content on the chemical stability and color change of various forms of vitamin C. Int J Food Prop. 2015;18(4):862-79. doi: 10.1080/10942912.2013.805770.
Nadendla RR, Kanna S, Satyanarayana J. Design and evaluation of rivaroxaban effervescent granules. Indian Drugs. 2024 Mar 1;61(3):61-4. doi: 10.53879/id.61.03.14149.
Debiasi BW, Rodrigues PG, Torres MP, Bonacorsi C, Andrighetti CR, Ribeiro EB. Comparison between maceration and ultrasound assisted extraction of white bracts with flowers of Bougainvillea spectabilis willd. Sci Elec Arch. 2021;14(2):47. doi: 10.36560/14220211297.
Oprescu EE, Enascuta CE, Radu E, Ciltea Udrescu M, Lavric V. Does the ultrasonic field improve the extraction productivity compared to classical methods maceration and reflux distillation? Chem Eng Process Process Intensif. 2022 Sep 1;179:109082. doi: 10.1016/j.cep.2022.109082.
Atnip AA, Sigurdson GT, Bomser J, Giusti MM. Time concentration and pH-dependent transport and uptake of anthocyanins in a human gastric epithelial (NCI-N87) cell line. Int J Mol Sci. 2017;18(2):446. doi: 10.3390/ijms18020446, PMID 28218720.
Quina FH, Bastos EL. Chemistry inspired by the colors of fruits flowers and wine. An Acad Bras Cienc. 2018;90(1 Suppl 1):681-95. doi: 10.1590/0001-3765201820170492, PMID 29742202.
Bhatt U, Garishma SV. Therapeutic protective and industrial significances of anthocyanins: a review. Avicenna J Med Biochem. 2022;11(2):x-x. doi: 10.34172/ajmb.2338.
Olas B, Bialecki J, Urbanska K, Brys M. The effects of natural and synthetic blue dyes on human health: a review of current knowledge and therapeutic perspectives. Adv Nutr. 2021 Nov 1;12(6):2301-11. doi: 10.1093/advances/nmab081, PMID 34245145.
Duan Y, Tarafdar A, Chaurasia D, Singh A, Bhargava PC, Yang J. Blueberry fruit valorization and valuable constituents: a review. Int J Food Microbiol. 2022;381:109890. doi: 10.1016/j.ijfoodmicro.2022.109890, PMID 36095867.
Panjaitan EN, Saragih A, Purba D. Gel formulation of red ginger rhizome extract (Zingiber officinale Roscoe) gel formulation of red ginger (Zingiber officinale roscoe) extract. J Pharm Pharmacol. 2012;1(1):9-20.
Rullyansyah S, Muzakky F, Samlan K, Hanistya R, Maulidiyanti ET. Phytochemical screening and evaluation of red ginger extracts on aphrodisiac activity. Gac Med Caracas. 2022;130 Supll 1:S143-8. doi: 10.47307/GMC.2022.130.s1.26.
Erjon SY, Lely N, Sari E. Effects of Javanese chili extract (Piper retrofractum Vahl) on libido enhancement of male white mice. Fitofarmaka J Ilm Farm. 2020;10(2):133-9. doi: 10.33751/jf.v10i2.2176.
Alipoor M, Maier SE, Gu IY, Mehnert A, Kahl F. Optimal experiment design for monoexponential model fitting: application to apparent diffusion coefficient imaging. BioMed Res Int. 2015;2015:138060. doi: 10.1155/2015/138060, PMID 26839880.
Adi Dako O, Kumadoh D, Egbi G, Okyem S, Addo PY, Nyarko A. Strategies for formulation of effervescent granules of an herbal product for the management of typhoid fever. Heliyon. 2021 Oct 9;7(10)::e08147:1–8. doi: 10.1016/j.heliyon.2021.e08147.
Al Mousawy J, Al Hussainy Z, Alaayedi M. Formulation and evaluation of effervescent granules of ibuprofen. Int J App Pharm. 2019;11(6):66-9. doi: 10.22159/ijap.2019v11i6.34912.
Vasiljevic I, Turkovic E, Aleksic I, Parojcic J. Mathematical approaches for powders and multiparticulate units processability characterization in pharmaceutical development. Arh Farm. 2022;72(6):637-60. doi: 10.5937/arhfarm72-40961.
Nikam V, Bhosale A. Design development and physicochemical evaluation of effervescent tablets of antihistamine drug. Int J Drug Deliv Technol. 2023;13(4):1520-6. doi: 10.25258/ijddt.13.4.61.
Parajuli Baral K. Formulation and evaluation of quality parameters of effervescent granules from the potent antioxidant between two variants of the adaptogenic herb Ocimum tenuiflorum L. Sci World J. 2023 Apr 25;2023:1-8. doi: 10.1155/2023/2050846.
Forestry D, Abdurrahman, Ramadhan H. Effervescent tablet formulation ethanol extracts 70% kelakai root (Stenochlaena palutris (Burm. F.) Bedd.) with variation concentration of gas generating agent. Int J Appl Pharm. 2022;14( 2):10-6. doi: 10.22159/ijap.2022.v14s2.44740.
Zhou M, Wang Y, Wu F, Shen L, Lin X, Feng Y. Development on porous particles of pueraria lobatae radix for improving its compactibility and dissolution. RSC Adv. 2018;8(43):24250-60. doi: 10.1039/c8ra04125c, PMID 35539169.
Jamei R, Babaloo F. Tandfonline; 2017. International journal of food properties stability of blueberry (cornus mas-yulyush) anthocyanin pigment under ph and co-pigment treatments. Available from: https://www.com/action/journalinformationjournalcode=ljfp20. [Last accessed on 23 Feb 2025].
Verma D, Sharma N, Malhotra U. Structural chemistry and stability of anthocyanins. Pharma Innovation. 2023;12(7):1366-73. doi: 10.22271/tpi.2023.v12.i7p.21416.
Aulifa DL, Wibowo DP, Safitri N, Budiman A. Formulation of effervescent granules from red ginger (Zingiberis officinale roscoe var. rubrum) extract and its antioxidant activity. Int J Appl Pharm. 2022;14(1):112-5. doi: 10.22159/ijap.2022v14i1.43377.
Arote R, Malpure P, Talele G, Chaudhary J, Chaudhary R, Dhamane P. A review on Moringa olifera effervescent tablet. Asian J Pharm Res Dev. 2024;12(6):168-71. doi: 10.22270/ajprd.v12i6.1489.
Oza N, Sagar S, Khodakiya A, Sahu A. A statistical approach to development of taste masked effervescent tablets of sildenafil citrate containing kyron T134. Int J App Pharm. 2020;12(4):135-43. doi: 10.22159/ijap.2020v12i4.37624.
Pramono YB, Nurwantoro N. Evaluasi kadar gula kadar air kadar asam dan pH pada pembuatan tablet effervescent buah nangka. J Teknol Pang. 2019;3(1):36-41. doi: 10.14710/jtp.2019.20519.
Meisner M, Duda P, Szulc Musiol B, Sarecka Hujar B. Characteristics of commercial effervescent tablets using selected pharmacopeial and novel analytical methods. Appl Sci. 2023;13(5):3171. doi: 10.3390/app13053171.
Kholidah S, Yuliet KA. Formulation of ginger effervescent tablets (Zingiber officinale roscoe) with variation of acid and base source concentration. Online J Nat Sci. 2014;3:216-29.
Dubray C, Maincent P, Milon JY. From the pharmaceutical to the clinical: the case for effervescent paracetamol in pain management a narrative review. Curr Med Res Opin. 2021;37(6):1039-48. doi: 10.1080/03007995.2021.1902297, PMID 33819115.
Jaipal A, Pandey MM, Charde SY, Sadhu N, Srinivas A, Prasad RG. Controlled release effervescent buccal discs of buspirone hydrochloride: in vitro and in vivo evaluation studies. Drug Deliv. 2016;23(2):452-8. doi: 10.3109/10717544.2014.917388, PMID 24892624.
Taymouri S, Mostafavi A, Javanmardi M. Formulation and optimization of effervescent tablet containing bismuth sub-citrate. J Reports Pharm Sci. 2019 Jul 1;8(2):236–44.
Iwansyah AC, Fauzi H, Cahyadi W, Hariadi H, Indriati A, Wardhani R. Development physiochemical and sensory evaluation of a new effervescent tablet formulation based on Moringa oleifera leaves extract. Int J Food Eng. 2023;19(3-4):133-41. doi: 10.1515/ijfe-2022-0170.
Laili N, Komala M, Maulida H, Suprapto. Optimization of sago amylum concentration (Metroxylon rumphii) as co-processed in theophylline tablets. Pharmacon J Farm Indones. 2017;14(2):72-80.
Ramadhani RA, Riyadi DH, Triwibowo B, Kusumaningtyas RD. Review pemanfaatan design expert untuk optimasi komposisi campuran minyak nabati sebagai bahan baku sintesis biodiesel. J Tek Kim Ling. 2017;1(1):11-6. doi: 10.33795/jtkl.v1i1.5.
Asyhari HF, Cabral KB, Wikantyasning ER. Optimization of soursop (Annona muricata L.) leaf extract in nanoemulgel and antiacnes activity test against propionibacterium acnes staphylococcus aureus staphylococcus epidermidis bacteria. Pharmacon. 2023;20(2):216-25. doi: 10.23917/pharmacon.v20i2.23308.
Peter EL, Sesaazi CD. D-optimal mixture design optimized solid formulation containing fruits extracts of Momordica charantia and Abelmoschus esculentus. PLOS One. 2022;17(6):e0270547. doi: 10.1371/journal.pone.0270547, PMID 35749521.
Hazra A, Gogtay N. Biostatistics series module 1: basics of biostatistics. Indian J Dermatol. 2016;61(1):10-20. doi: 10.4103/0019-5154.173988, PMID 26955089.
Rahmana AY, Nurwaini S. Optimization of ethanol extract preparation gel formula from the combination of lime peel and neem leaves: optimum physical properties and antibacterial activity. 2024;21(1):9–18. doi: 10.23917/pharmacon.v21i0.23586.
Suksaeree J, Monton C. Applying design of experiments on the mechanical properties of mefenamic acid loaded transdermal films. Trends Sci. 2023;20(10):6-13. doi: 10.48048/tis.2023.7065.
Ghasemian E, Motaghian P, Vatanara A. D-optimal design for preparation and optimization of fast dissolving bosentan nanosuspension. Adv Pharm Bull. 2016;6(2):211-8. doi: 10.15171/apb.2016.029, PMID 27478783.
Zheng X, Wu F, Hong Y, Shen L, Lin X, Feng Y. Improvements in sticking hygroscopicity and compactibility of effervescent systems by fluid bed coating. RSC Adv. 2019;9(54):31594-608. doi: 10.1039/c9ra05884b, PMID 35527953.
Jang EH, Park YS, Kim MS, Choi DH. Model based scale up methodologies for pharmaceutical granulation. Pharmaceutics. 2020;12(5):453. doi: 10.3390/pharmaceutics12050453, PMID 32423051.
Brunetti P, LO Faro AF, Tini A, Busardo FP, Carlier J. Pharmacology of herbal sexual enhancers: a review of psychiatric and neurological adverse effects. Pharmaceuticals (Basel). 2020;13(10):309. doi: 10.3390/ph13100309, PMID 33066617.