
Int J App Pharm, Vol 17, Issue 3, 2025, 200-205Original Article
ORAL WOUND HEALING EFFECTS OF GEL COMBINATION OF HYALURONIC ACID AND ASTAXANTHIN: AN ANTIOXIDANT, CLINICAL, AND HISTOLOGICAL EVALUATION
DWI ANDRIANI1,2*, RETNO INDRAWATI ROESTAMADJI3, RETNO PUDJI RAHAYU4, RIMA PARWATI SARI2, NOENGKI PRAMESWARI5
1Doctoral student of Dental Medicine, Faculty of Dental Medicine, Universitas Airlangga, Surabaya, East Java, Indonesia. 2Department of Oral Biology, Faculty of Dentistry, Universitas Hang Tuah, Surabaya, East Java, Indonesia. 3Departmentof Oral Biology, Faculty of Dental Medicine, Universitas Airlangga Surabaya, East Java, Indonesia. 4Department of Oral and Maxillofacial Pathology, Faculty of Dental Medicine, Universitas Airlangga, Surabaya, East Java, Indonesia. 5Department of Orthodontics, Faculty of Dentistry, Universitas Hang Tuah, Surabaya, East Java, Indonesia
*Corresponding author: Dwi Andriani; *Email: dwi.andriani@hangtuah.ac.id
Received: 09 Jan 2025, Revised and Accepted: 06 Mar 2025
ABSTRACT
Objective: This study assesses the efficacy of a combination gel containing 0.2% hyaluronic acid and 1% astaxanthin by conducting antioxidant assays, wound closure differences, and histological assessment by counting fibroblast and collagen density of oral wounds.
Methods: An antioxidant assessment of the combination gel utilizing the DPPH technique and wound healing effects on oral ulcers in diabetes conditions was performed in vivo, with five replications for each. The research comprised four groups: base gel therapy, 0.2% hyaluronic acid gel, 1% astaxanthin gel, and a combined gel of 0.2% hyaluronic acid and 1% astaxanthin. Evaluation occurred after 3, 5, and 7 days of therapy. Subsequently, the rats were euthanized for histological analysis, focusing on fibroblast and collagen density using hematoxylin-eosin and Masson's trichrome staining. The data were analyzed by the Brown-Forsythe followed by posthoc Games-Howell for differential diameter and the Kruskal-Wallis followed by the post hoc Mann-Whitney test (p<0,05) for collagen density.
Results: The IC50 value of the gel combination of 0.2% hyaluronic acid and 1% astaxanthin is 0.06578 ppm. The decrease in diameter was observed on days 3, 5, and 7, with a statistically significant difference among all groups on day 5 (p<0.05). On days 3 and 5, there was an elevation in fibroblast count, which was significantly distinct from the other groups on day 5; however, on day 7, the count was equivalent to that of the Ast1 therapy group. Collagen density grading on days 3, 5, and 7 revealed a score of 4 (indicating high collagen density), with significant differences observed on days 3 and 5 and no significant difference when compared to the Ast1 group (p<0.05).
Conclusion: The gel combination of 0.2% hyaluronic acid and 1% astaxanthin contained potent antioxidants. The combination gel's effect on wound healing acceleration was visible on day 5.
Keywords: Oral ulcer, Antioxidant, Astaxanthin, Hyaluronic acid, Fibroblast, Collagen density, Wound closure
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijap.2025v17i3.53636 Journal homepage: https://innovareacademics.in/journals/index.php/ijap
INTRODUCTION
The effectiveness and efficiency of this combination therapy are significantly affected by the particular interactions among the medications [1]. Consequently, assessing this material formulation is imperative to ascertain the drug's effectiveness. Including supplementary ingredients in the formulation can influence the efficacy of the primary component, whether through additive, synergistic, or agonistic interactions. A combination is classified as synergistic if it yields results that surpass those anticipated from additive or minimal interactions; conversely, if the drug interactions diminish the efficacy of each treatment, the interaction is termed antagonistic. The impact can manifest as buffering, wherein one drug entirely obscures the effect of another, or suppression, where the efficacy of the combined drugs is inferior to that of at least one drug administered alone [1].
Hyaluronic acid exhibits significant anti-inflammatory properties that are conducive to wound healing. In response to tissue injury, Hyaluronic Acid (HA) is synthesized, modulating tissue healing and pathological processes, including activating inflammatory cells, to trigger an innate response to injury and influence the behavior of epithelial cells and fibroblasts [2]. As an anti-inflammatory, High Molecular Weight Hyaluronic Acid (HMW-HA) functions [3]. Numerous studies indicate that hyaluronic acid, when combined with other substances, enhances tissue repair, reduces wound area size, shortens healing duration, decreases pro-inflammatory cytokines, and increases the expression of growth factors and other elements that facilitate accelerated wound healing [2].
Incorporating the antioxidant astaxanthin is essential to augment the healing effects, particularly in chronic wounds. Numerous studies demonstrate antioxidant and anti-inflammatory properties, suggesting its potential efficacy in wound healing therapy for diabetes mellitus [4, 5]. Astaxanthin originates from Haematococcus pluvialis and is an efficacious agent in expediting wound healing [6]. A study reports the therapeutic effect of astaxanthin gel1% was observed to increase the number of neutrophils, macrophages, and fibroblasts on day 3, as well as the density of collagen on day 7, during the repair process of an oral injury ulceration [7].
The combination of these two medicines is expected to produce an additive or synergistic effect, thereby expediting wound healing, particularly in cases of diabetes with prolonged chronic inflammation. Extended inflammation increases Matrix Metalloproteinase (MMP) levels, resulting in the destruction of the extracellular matrix and the degradation of growth factors in the wound, which permits the lesion to transition into a chronic state and delays healing [8]. Hyperglycemia in patients with Diabetes Mellitus (DM) can hinder wound healing and disrupt the function of endothelial cells, which are essential for this process, alongside elevated reactive oxygen species (ROS) due to peripheral nerve injury [9]. The increase in reactive oxygen species (ROS) under hyperglycemic conditions is associated with a rise in pro-inflammatory cytokines (TNF and IL-1b) and a decrease in growth factors (FGF-2 and TGF-b), leading to impaired keratinocyte migration at the wound site, which affects wound re-epithelialization [10]. This condition can hinder wound healing, making the skin more prone to injury and infection.
Therefore, this study assessed the efficacy of combining high molecular weight hyaluronic acid with astaxanthin in enhancing wound healing, including measuring the quantity of antioxidants in the gel, wound diameter, and fibroblast and collagen density.
MATERIALS AND METHODS
This study employs a laboratory experiment utilizing a post-test-only group design. The research sample for antioxidant assessment comprised a gel formulation containing 0.2% hyaluronic acid and 1% astaxanthin. The antioxidant assessment of the gel substance was performed utilizing the DPPH method. The in vivo research sample comprised male Wistar rats (Rattus norvegicus) exhibiting a diabetic mellitus ulcer model. The in vivo trial contains four groups: gel base therapy (Gel B), 0.2% hyaluronic acid (Gel Hya0.2), astaxanthin gel (Gel Ast1), and a combination of both drugs (Gel Hya0.2-Ast1). The research data underwent statistical analysis utilizing SPSS 16.0.
Gel formation
The gel was formulated by preparing a base of hydroxypropyl methylcellulose (HPMC), propylene glycol, and the preservatives methylparaben and propylparaben. This was followed by the incorporation of active components, including hyaluronic acid (Sigma-Aldrich, USA) at a concentration of 0.2% and astaxanthin (PT. Evergen, Indonesia) at a dosage of 1%. For the combo gel, both components were incorporated into a single gel formulation. The gel preparation was carried out at a temperature of 25-30 °C, with a pH of 7-7.8.
Evaluation of antioxidants
The amalgamated gel was subsequently readied for antioxidant evaluation utilizing the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay, following the methodologies established by Arguelles and Sapin (2020) [11] and Indrianingsih et al. (2020) [12]. The antioxidant activity was quantitatively assessed using a UV-Vis spectrophotometer at a wavelength of 517 nm, resulting in an absorbance value. The absorbance value is determined using the equation:
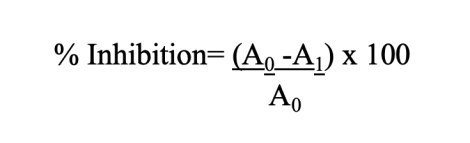
Described as:
A0 = optical density of the control
A1= optical density of the gel sample
As mentioned in the linear regression analysis, the IC50 value was generated to assess the gel's ability to prevent 50% of the DPPH radical scavenging.
Ulcer diabetes mellitus model
Healthy male rats weighing 180-220 g were subjected to a diabetic mellitus model. The rats received acclimatization for 7 d to prepare for receiving treatment of the diabetic mellitus model. Rats were administered a single intraperitoneal dosage of 50 mg/kg body weight of Streptozotocin (STZ) diluted in 0.1 M citrate buffer at pH 4.5. Following induction, the rats were administered 10% dextrose overnight. The condition of diabetes is defined by elevated blood glucose levels ranging from 200 to 300 mg/dl or more. The ulcer was then treated for 3, 5, and 7 d with a sample size of 5 rats. The ethics committee of the Faculty of Dental Medicine, Universitas Airlangga, has authorized this research, Reference Number 1211/HRECC. FODM/X/2023.
Clinical assessment of wound healing
A clinical evaluation by measuring the ulcer's width on the initial formation day before medication, followed by re-measurements on days 3, 5, and 7.
The variation in ulcer diameter is determined using the formula:
![]()
Described as:
DD = Diameter difference
D0 = Initial wound diameter
D1= Final wound diameter
The variation in diameter signifies expedited wound healing. A greater disparity enhances wound healing. The difference diameter data were analyzed using the Shapiro-Wilk normality test (p>0.05) and thereafter subjected to Levene’s homogeneity test (p<0.05). Due to the lack of homogeneity in the data, the difference test was performed with the Brown-Forsythe test followed by the post hoc Games-Howell test.
Histological assessment of wound healing
The rats were subsequently euthanized in compliance with animal research ethics. Tissue sampling was performed via biopsy in the wound area. Sample preparation involved hematoxylin and eosin staining for fibroblast cells, along with the Masson trichrome staining protocol for collagen density assessment. Observations were conducted using an Olympus CX-21 microscope at 400x magnification for a quantitative assessment of fibroblasts, while collagen density was evaluated through scoring at the same 400x magnification. The sample label did not include the sample name and was reviewed by two observers to ensure the authenticity of the observations. The collagen density data was ordinal, and the analysis utilized the non-parametric Kruskal-Wallis Post hoc Mann-Whitney test.
Assessment of collagen density [13]
Score 0: Absence of apparent collagen fibers
Score 1: Collagen fibers are minimal and sparsely distributed, indicating low collagen density
Score 2: Collagen fibers are moderately distributed and are seen fused, reflecting moderate collagen density
Score 3: Collagen fibers are extensively distributed, signifying high collagen density
Score 4: Collagen fibers are optimally attached, demonstrating very high collagen density
RESULTS AND DISCUSSION
Antioxidant testing has been performed, with the % inhibition and IC50 values presented in table 1 and fig. 1. The IC50 value of the gel formulation containing 0.2% hyaluronic acid and 1%astaxanthin is below 50. The gel demonstrates a significantly high antioxidant content. An IC50 value below 50 signifies a potent antioxidant. This result follows the IC50 classification: potent if IC50 ranges less than 10 ppm, strong if IC50 ranges from 10 to 50 ppm, moderate if IC50 is between 50 to 100 ppm, weak if IC50 falls between 100 to 250 ppm, and inactive if IC50 exceeds 250 ppm [14].
Astaxanthin is a potent antioxidant with significant singlet oxygen quenching capabilities and an exceptional capacity to neutralize hydroxyl radicals [15]. Astaxanthin exerts an indirect antioxidant effect by activating the transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2) and enhancing the expression of its target antioxidant genes [16]. Moreover, astaxanthin is a "pure antioxidant" exhibiting superior photostability in human dermal fibroblasts and is twice as efficacious as β-carotene in mitigating lipid peroxidation [15].
Hyperglycemia in diabetes affects immunological function, increasing susceptibility to infection, altering blood circulation, causing tissue necrosis, and potentially resulting in gangrene [17]. This condition also elevates the synthesis of oxidizing agents, including reactive oxygen species and reactive nitrogen species [18]. Oxidative stress is essential for wound healing; nevertheless, high and uncontrolled stress may result in chronic non-healing wounds, usually occurring in diabetes mellitus, which leads to enhanced reactive oxygen species (ROS) generation, results in compromised healing mechanisms, augmented cellular death, lipid peroxidation, protein alteration, and DNA damage [19]. The fact that oxidative stress has a role in the onset and progression of wound healing complications in diabetes mellitus, the application of antioxidants is advised.
Table 1: Results of antioxidant measurement using the DPPH method
| Concentration (ppm) | Average absorbance | % Inhibition | Regression equation | IC50 (ppm) | |
| Sample | DPPH | ||||
| 2530 | 0.2003 | 0.2788 | 39.16805324 | Y = 658.54 X+6.6812 R= 0.9788 |
0.06578 |
| 1265 | 0.2263 | 0.2788 | 23.18114875 | ||
| 632.5 | 0.2353 | 0.2788 | 18.47025496 | ||
| 316.25 | 0.2480 | 0.2788 | 12.434467 | ||
| 158.125 | 0.2603 | 0.2788 | 7.107184018 | ||
| 79.06 | 0.2611 | 0.2788 | 6.779011873 | ||
| 39.53 | 0.2610 | 0.2788 | 6.806282723 | ||
| 19.76 | 0.2633 | 0.2788 | 5.886821117 |
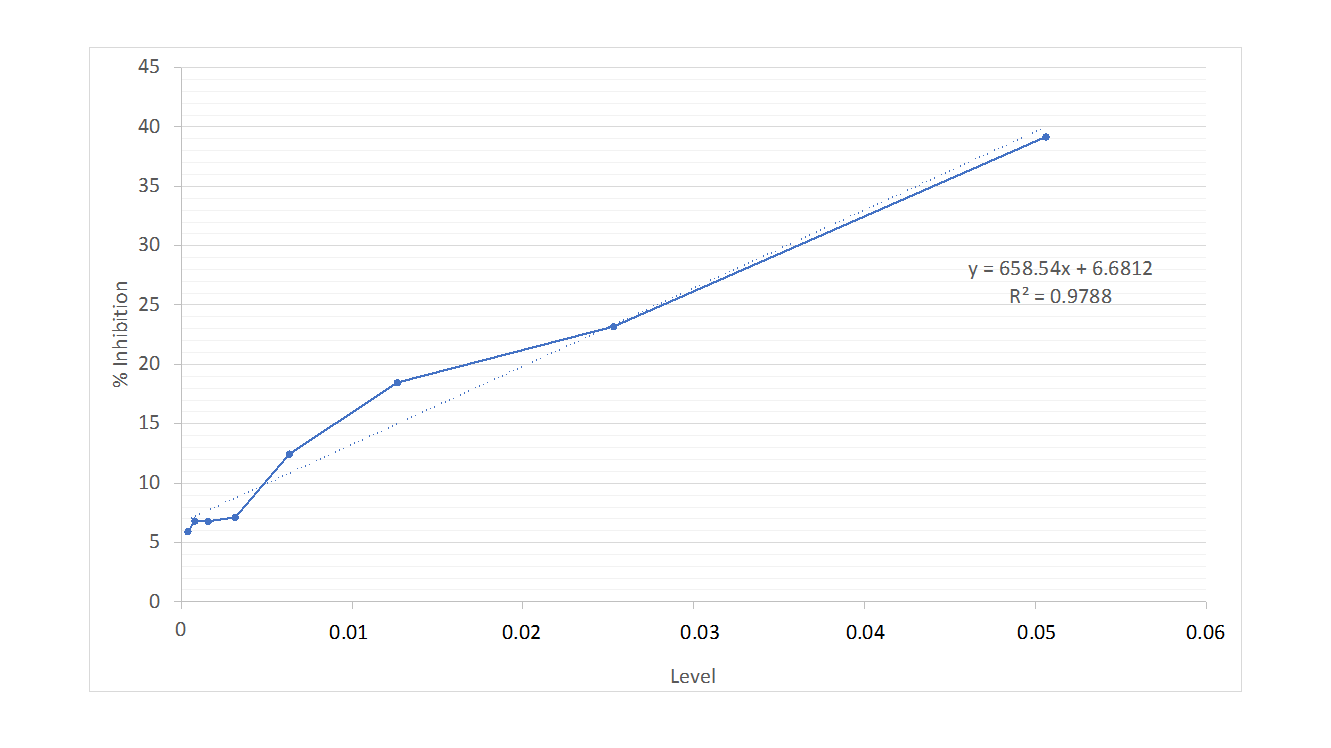
Fig. 1: Antioxidant linear regression graph
The outcomes of the clinical assessment, determined by the difference between the initial and final diameters, are illustrated in table 2 and fig. 2. Data analysis revealed a normal distribution (p>0.05) with no homogeneity (p<0.05), prompting the use of the Brown-Forsythe test followed by post-hoc Games-Howell analysis (p<0.05). A decrease in wound width was noted in the group receiving therapy with active components compared to the gel base. The combination therapy of 0.2% hyaluronic acid and 1% astaxanthin demonstrated the most significant reduction in diameter disparity on day 5, even though on the 7th day there was no substantial difference between Gel Hya0.2 and Gel Ast1.
The wound-healing capabilities of hyaluronic acid have been extensively studied. The study conducted by Yang et al. (2020) indicated a notable decrease in ulcer width by the seventh day following treatment with 0.2% hyaluronic acid [20]. Kawano et al. (2021) showed a 22% reduction in wound area by day 7 with hyaluronic acid therapy [21]. A notable decrease in ulcer diameter, akin to the astaxanthin gel therapy group and the combination gel group, demonstrates this. Astaxanthin is recognized for its antioxidant and anti-inflammatory properties. Meephansan et al. (2017) observed a notable reduction in wounds treated with astaxanthin, achieving complete healing by day 9 [22]. Evidence was observed on days 5 and 7, indicating a decrease in ulcer width with 1% astaxanthin treatment. Both chemicals facilitate the acceleration of wound healing. On the seventh day, the wound healing accelerated effect didn't seem visible in the gel combination of the two components; nevertheless, this impact was clinically observable on the fifth day.
Table 2: Results of the mean analysis of ulcer diameter differences on days 3, 5, and 7
| Group | n | Diameter difference | P-value | ||
| Day 0 to 3 | Day 0 to 5 | Day 0 to 7 | |||
| Mean±Standard Deviation | |||||
| Gel B | 5 | 0.57±0.099a | -0.13± 0.116b | -0.485± 0.322bc | 0.000* |
| Gel Hya0.2 | 5 | 0.205±0.181abc | -0.22± 0.156b | -1.02±0.682abcd | |
| Gel Ast1 | 5 | 0.305±0.078bc | -0.325±0.145b | -1.19±0.338cd | |
| Gel Hya0.2-Ast1 | 5 | -0.209± 0.117bc | -0.95± 0.088c | -1.64± 0.258d |
Described as: n = Replication, -= Reduction of diameter, *Statistically significant at α=0.05 (Brown-Forsythe test), abcdef The identical superscript denotes no distinction between groups
The fibroblast count is presented in table 3 and fig. 3, whilst the collagen density is illustrated in table 4 and fig. 4. The fibroblast count analysis indicated a non-normal distribution of data (p<0.05), prompting the application of a non-parametric Kruskal-Wallis test with subsequent Mann-Whitney post-hoc analysis (p<0.05), which was similarly applied to collagen density. The outcomes of the gel therapy trial incorporating 0.2% hyaluronic acid and 1% astaxanthin demonstrated an elevation in fibroblast levels observable on day 5 and a reduction in collagen quantity evident on day 7. The results align with the collagen density scoring, which yielded a score of 4, signifying a very high collagen density (fig. 4).
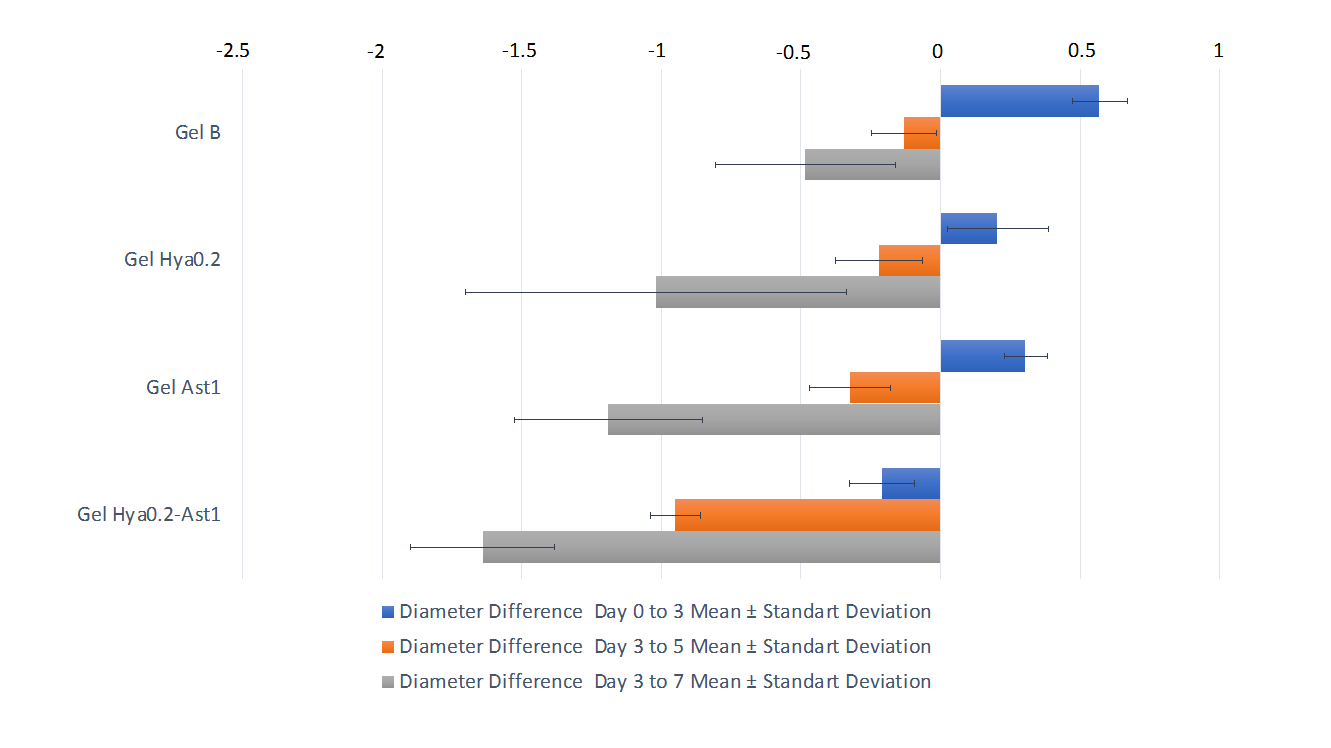
Fig. 2: Graph of the mean difference in ulcer diameter on days 3, 5, and 7
Table 3: The mean of fibroblast count results on days 3, 5, and 7
| Group | n | Fibroblast | P-value | ||
| Day 3 | Day 5 | Day 7 | |||
| Mean±Standard Deviation | |||||
| Gel B | 5 | 10.34±5.21a | 11.28±4.13d | 18.68±8.81f | 0.001* |
| Gel Hya0.2 | 5 | 7.92±2.34bc | 13.08±6.05d | 25.14±7.25f | |
| Gel Ast1 | 5 | 9.86±5.84b | 10.34±7.26d | 4.8±1.74g | |
| Gel Hya0.2-Ast1 | 5 | 16.92±7.83bc | 28.14±1.20e | 5.68±4.00g |
Described as: n = replication, *Statistically significant at α=0.05 (Kruskal-Wallis test), abcdefThe identical superscript denotes no distinction between groups
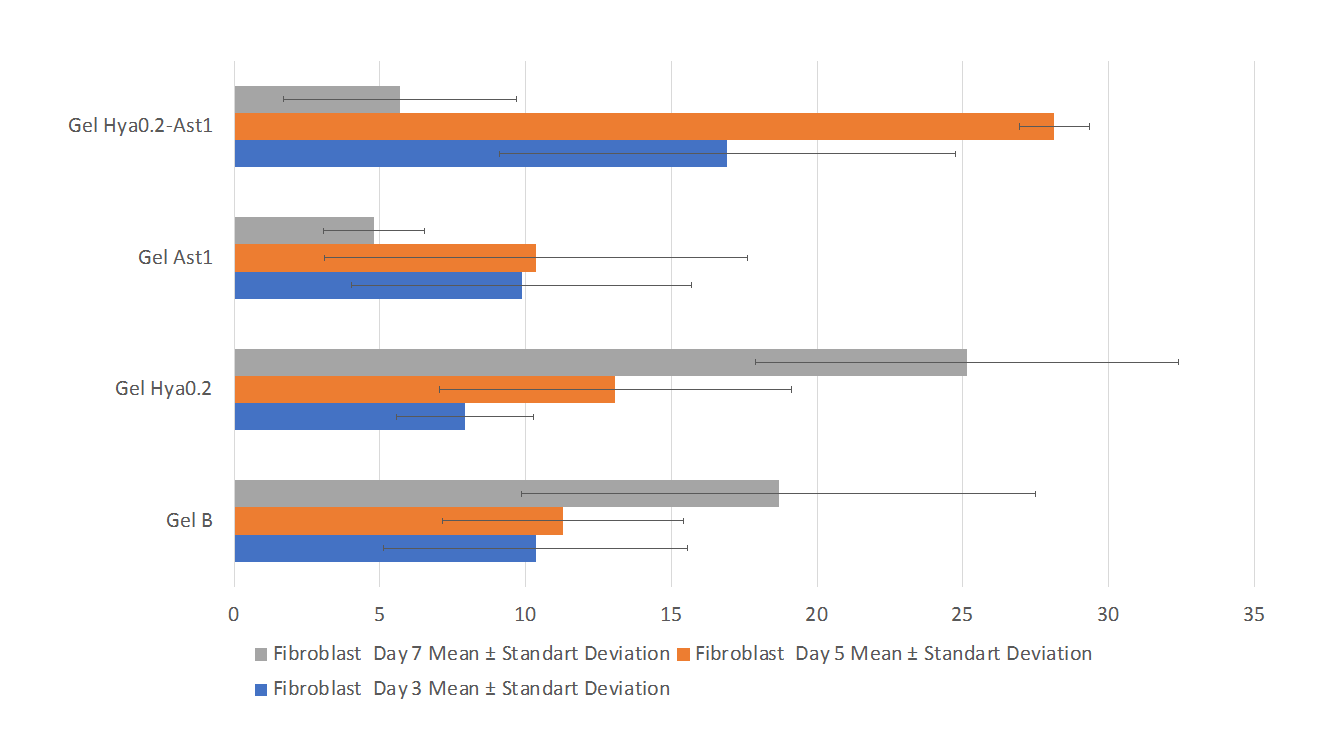
Fig. 2: Graph of the mean of fibroblast count on days 3, 5, and 7
Table 4: Median values and range of collagen density scores on days 3, 5, and 7
| Group | n | Collagen density | |||||
| Day 3 | Day 5 | Day 7 | |||||
| Median±IQD | Min-max | Median±IQD | Min-max | Median±IQD | Min-max | ||
| Gel B | 5 | 1±0.50a | 0-1 | 1±0.50ab | 0-2 | 1±0.50ab | 1-2 |
| Gel Hya0.2 | 5 | 2±0.50b | 1-2 | 2±0.50bc | 1-3 | 3±0.50de | 3-4 |
| Gel Ast1 | 5 | 2±0.50bc | 1-2 | 3±0.50cd | 2-3 | 4±0.50ef | 3-4 |
| Gel Hya0.2-Ast1 | 5 | 4±0.50ef | 3-4 | 4±0.50ef | 3-4 | 4±0.00f | 4-4 |
Described as: n = replication, *Statistically significant at α=0.05 (Kruskal-Wallis test), abcdef The identical superscript denotes no distinction between groups
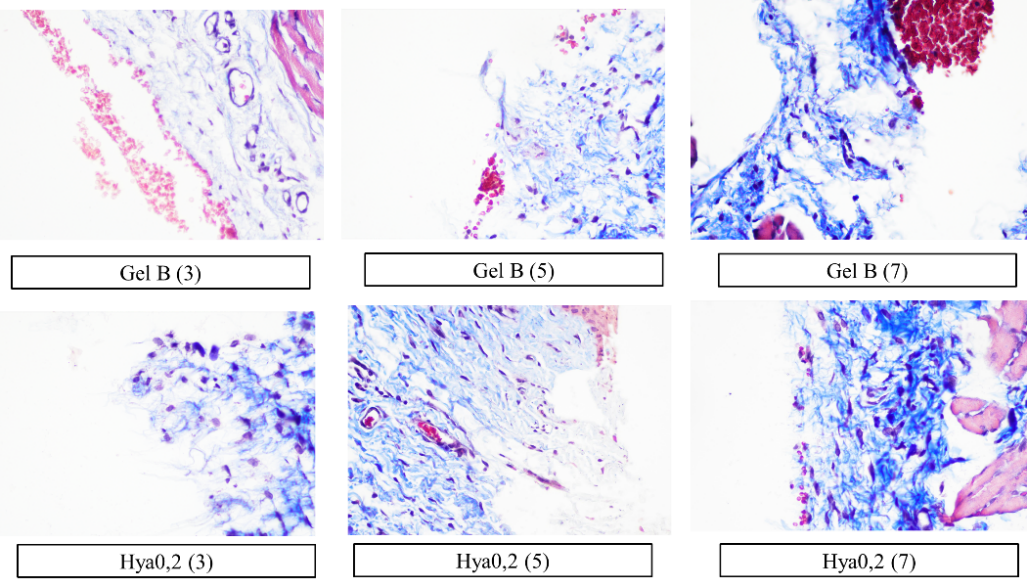
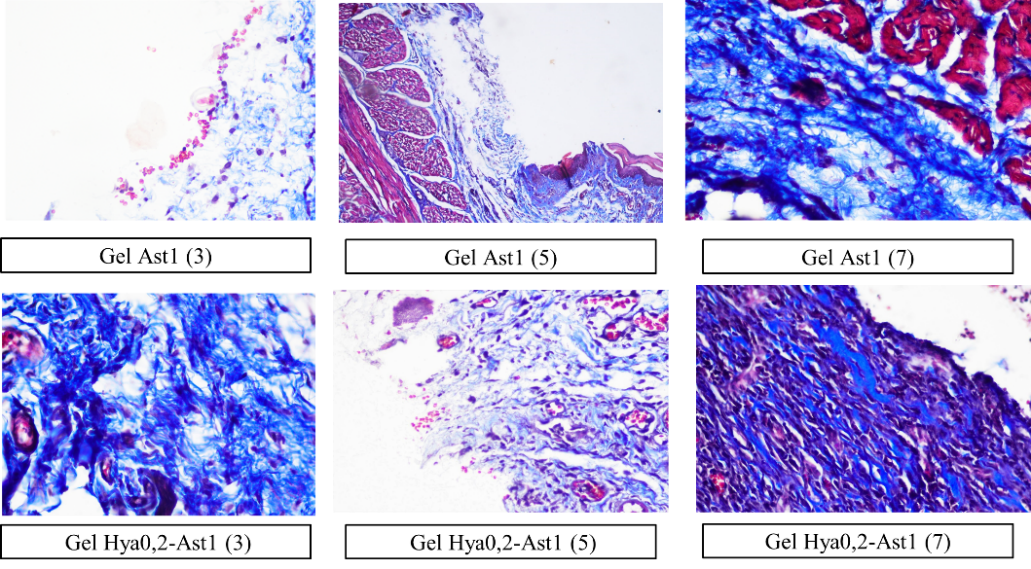
Fig. 4: Histology of collagen density in all groups on days 3, 5, and 7 with a magnification of 400x, the density of collagen is seen from the adhesion of collagen fibers (blue color). The caption in the image indicates the therapy groups, with numbers 3, 5, and 7 in the caption indicating the treatment days
Fibroblasts are crucial in the process of wound healing. The wound healing process comprises four phases: homeostasis, which happens initially; inflammation, lasting from the early stage up to five days; proliferation, which spans from 48 h to 14 d; and remodeling, occurring around two to three weeks post-injury [23]. Fibroblast contributes to the inflammatory phase and enhances the immune response. The inflammatory phase involves proliferation, where fibroblasts appear on day 3, peaking on day 7, and producing the extracellular matrix [24]. Its significance increases during the proliferative phase, contributing to angiogenesis and granulation tissue creation, with fibroblasts becoming predominant in this phase [25]. The 0.2% hyaluronic acid and 1% astaxanthin combination gel demonstrated an expedited wound-healing effect by the 5th day. By the 7th day, the treatment with 0.2% hyaluronic acid gel resulted in an enhanced fibroblast count, but the 1% astaxanthin gel led to a decrease in fibroblasts.
In wound conditions, fibroblasts traverse the extracellular matrix, regulated by chemotactic growth factors, cytokines, and gradients of chemokines, alongside the arrangement of fibrils in the extracellular and provisional matrices. Subsequently, they alter their morphology, adhere, and proliferate, synthesizing granulation tissue components, including collagen, elastin, and proteoglycans [26]. Given the intimate link among fibroblasts, we present results indicating that the elevated fibroblast counts on day 5 in the combination gel correlate with its collagen density. The antioxidant levels in this gel formulation may influence the fibroblast and collagen density results in this study. The use of an antioxidant such as Folic acid, a vitamin exhibiting direct antioxidant properties, enhanced collagen deposition and facilitated wound healing through a reduction of oxidative stress in diabetic mice [27]. Astaxanthin therapy accelerates wound closure through the increase of collagen 1 mRNA, peaking on the 6th day post-injury, and the reduction of iNos, a marker of ROS, on the 3rd day [6].
Fibroblasts appeared to diminish on the seventh day, although collagen density persisted and was comparable to hyaluronic acid and astaxanthin. Comparable research conducted by Andriani et al. (2024) demonstrated a notable disparity in fibroblast count and collagen density on the seventh day when comparing 1% astaxanthin therapy to 0.2% hyaluronic acid therapy [7]. In this investigation, both materials produced identical outcomes. This may result from the different ulcer models and gel formulations. The transformation of fibroblasts into myofibroblasts and other variants influences the quantity of fibroblasts detected in this histological analysis. This research has been limited to a duration of 7 d of treatment, additional investigations are required until the wound is fully healed. Furthermore, molecular assessments, including MMP, TGF-b, and FGF-2, which are integral to the epithelialization process in wound healing, are essential to substantiate the rapid wound healing observed on day 5 due to this combination therapy.
CONCLUSION
The above study demonstrates that the antioxidant content in the combination gel is notable and strong. Clinical results indicated that rapid wound healing was evident by day 5, corroborated by increased fibroblast proliferation and enhanced collagen density. On day 7, the treatment group did not exhibit a significant difference in the effect of faster wound healing. Considering that fibroblasts are essential to the extracellular matrix and facilitate collagen synthesis, an assessment of collagen density indicated that the combined activity of the two substances resulted in elevated collagen density. The impact of accelerated wound healing seems to peak on the fifth day. A molecular assessment such as MMP, TGFb, and FGF-2 is required to elucidate the mechanism by which this combination enhances wound healing.
ACKNOWLEDGEMENT
We express our gratitude to Hang Tuah University for its financial support.
AUTHORS CONTRIBUTIONS
DA assists in the research concept, research execution, and journal writing; RIR provides input to the research concept and journal discussion; RPR assists with the research concept, data analysis, and journal discussion; RPS helps with journal writing; NP helps with journal writing.
CONFLICTS OF INTERESTS
We declare that there exists no conflict of interest in this research.
REFERENCES
Tekin E, Beppler C, White C, Mao Z, Savage VM, Yeh PJ. Enhanced identification of synergistic and antagonistic emergent interactions among three or more drugs. J R Soc Interface. 2016 Jun 30;13(119):20160332. doi: 10.1098/rsif.2016.0332, PMID 27278366.
Marinho A, Nunes C, Reis S. Hyaluronic acid: a key ingredient in the therapy of inflammation. Biomolecules. 2021 Oct 15;11(10):1518. doi: 10.3390/biom11101518, PMID 34680150.
Hu L, Nomura S, Sato Y, Takagi K, Ishii T, Honma Y. Anti-inflammatory effects of differential molecular weight hyaluronic acids on UVB-induced calprotectin-mediated keratinocyte inflammation. J Dermatol Sci. 2022 Jul 1;107(1):24-31. doi: 10.1016/j.jdermsci.2022.06.001, PMID 35717315.
Medoro A, Davinelli S, Milella L, Willcox BJ, Allsopp RC, Scapagnini G. Dietary astaxanthin: a promising antioxidant and anti-inflammatory agent for brain aging and adult neurogenesis. Mar Drugs. 2023 Dec 16;21(12):643. doi: 10.3390/md21120643, PMID 38132964.
Andriani D, Roestamadji RI, Rahayu RP. Astaxanthin is a promising therapy for wound healing in diabetic conditions: a review. J Int Dent Med Res. 2023;16(4):1824-9.
Meephansan J, Rungjang A, Yingmema W, Deenonpoe R, Ponnikorn S. Effect of astaxanthin on cutaneous wound healing. Clin Cosmet Investig Dermatol. 2017 Jul 13;10:259-65. doi: 10.2147/CCID.S142795, PMID 28761364.
Andriani D, Pargaputri AF, Revianti S, Parisihni K, Ashrin MN. Potency astaxanthin (Haematococcus pluvialis) as anti inflammatory gel to oral traumatic ulcer. Malays J Med Health Sci. 2024 Jun 3;20.
Furtado SC, Srinivasan B, Abraham SI. Wound healing concepts: contemporary practices and future perspectives. Int J App Pharm. 2020 Sep 7;12(5):7-15. doi: 10.22159/ijap.2020v12i5.38588.
Burgess JL, Wyant WA, Abdo Abujamra B, Kirsner RS, Jozic I. Diabetic wound-healing science. Medicina (Kaunas). 2021 Oct 8;57(10):1072. doi: 10.3390/medicina57101072, PMID 34684109.
Ko KI, Sculean A, Graves DT. Diabetic wound healing in soft and hard oral tissues. Transl Res. 2021 Oct 1;236:72-86. doi: 10.1016/j.trsl.2021.05.001, PMID 33992825.
Arguelles ED, Sapin AB. Bioactive properties of Sargassum siliquosum J. Agardh (Fucales, Ochrophyta) and its potential as source of skin-lightening active ingredient for cosmetic application. J Appl Pharm Sci. 2020 Jul 4;10(7):51-8. doi: 10.7324/JAPS.2020.10707.
Indrianingsih AW, Rosyida VT, Apriyana W, Hayati SN, Darsih C, Nisa K. Antioxidant and antibacterial properties of bacterial cellulose— indonesian plant extract composites for mask sheet. J Appl Pharm Sci. 2020 Jul 4;10(7):37-42. doi: 10.7324/JAPS.2020.10705.
Hakim RF, Fakhrurrazi F, Andini YR. Effects of broccoli extract (Brassica oleracea var. italica) on collagen images in wound on collagen fibers density in wound healing process. Padj J Dent. 2023;35(2):147-52.
Reviana R, Usman AN, Raya I, Aliyah A, Dirpan A, Arsyad A. Analysis of antioxidant activity on cocktail honey products as female pre-conception supplements. Gac Sanit. 2021 Jan 1;35 Suppl 2:S202-5. doi: 10.1016/j.gaceta.2021.10.021, PMID 34929812.
Santos Sanchez NF, Hernandez Carlos B, Torres Arino A, Salas Coronado R. Astaxanthin and its formulations as potent oxidative stress inhibitors. Pharmacogn Rev. 2021;14(27):8-15. doi: 10.5530/phrev.2020.1.2.
Pereira CP, Souza AC, Vasconcelos AR, Prado PS, Name JJ. Antioxidant and anti‑inflammatory mechanisms of action of astaxanthin in cardiovascular diseases (Review). Int J Mol Med. 2021 Jan 1;47(1):37-48. doi: 10.3892/ijmm.2020.4783, PMID 33155666.
Lang X, Li L, Li Y, Feng X. Effect of diabetes on wound healing: a bibliometrics and visual analysis. J Multidiscip Healthc. 2024 Dec 31;17:1275-89. doi: 10.2147/JMDH.S457498, PMID 38524865.
Ghasemi Dehnoo M, Amini Khoei H, Lorigooini Z, Rafieian Kopaei M. Oxidative stress and antioxidants in diabetes mellitus. Asian Pac J Trop Med. 2020 Oct 1;13(10):431-8. doi: 10.4103/1995-7645.291036.
Zhang W, Chen L, Xiong Y, Panayi AC, Abududilibaier A, Hu Y. Antioxidant therapy and antioxidant-related bionanomaterials in diabetic wound healing. Front Bioeng Biotechnol. 2021 Jun 24;9:707479. doi: 10.3389/fbioe.2021.707479, PMID 34249895.
Yang Z, Li M, Xiao L, Yi Z, Zhao M, Ma S. Hyaluronic acid versus dexamethasone for the treatment of recurrent aphthous stomatitis in children: efficacy and safety analysis. Braz J Med Biol Res. 2020 Jun 26;53(8):e9886. doi: 10.1590/1414-431X20209886, PMID 32609262.
Kawano Y, Patrulea V, Sublet E, Borchard G, Iyoda T, Kageyama R. Wound healing promotion by hyaluronic acid: effect of molecular weight on gene expression and in vivo wound closure. Pharmaceuticals (Basel). 2021 Mar 28;14(4):301. doi: 10.3390/ph14040301, PMID 33800588.
Meephansan J, Rungjang A, Yingmema W, Deenonpoe R, Ponnikorn S. Effect of astaxanthin on cutaneous wound healing. Clin Cosmet Investig Dermatol. 2017 Jul 13;10:259-65. doi: 10.2147/CCID.S142795, PMID 28761364.
Gonzalez AC, Costa TF, Andrade ZA, Medrado AR. Wound healing-a literature review. An Bras Dermatol. 2016;91(5):614-20. doi: 10.1590/abd1806-4841.20164741, PMID 27828635.
Agustiarini V, Fitrya AA, Zerli RS. Second degree burn wound healing activity test of ethanol extract mahogany bark (Swietenia Mahagoni (L.) Jacq.). Int J Appl Pharm. 2022;14 Special Issue 3:31-5.
Cialdai F, Risaliti C, Monici M. Role of fibroblasts in wound healing and tissue remodeling on Earth and in space. Front Bioeng Biotechnol. 2022 Oct 4;10:958381. doi: 10.3389/fbioe.2022.958381, PMID 36267456.
Trinh XT, Long NV, Van Anh LT, Nga PT, Giang NN, Chien PN. A comprehensive review of natural compounds for wound healing: targeting bioactivity perspective. Int J Mol Sci. 2022 Aug 24;23(17):9573. doi: 10.3390/ijms23179573, PMID 36076971.
Zhao M, Zhou J, Chen YH, Yuan L, Yuan MM, Zhang XQ. Folic acid promotes wound healing in diabetic mice by suppression of oxidative stress. J Nutr Sci Vitaminol (Tokyo). 2018;64(1):26-33. doi: 10.3177/jnsv.64.26, PMID 29491269.