
Int J App Pharm, Vol 17, Issue 3, 2025, 13-27Review Article
NANOCARRIER-BASED APPROACHES FOR ENHANCED MANAGEMENT OF ANDROGENETIC ALOPECIA: ADVANCEMENTS AND FUTURE PROSPECTS
PRITAM KAYAL, R. RAGHUL, UDAYA KIRAN SAHOO, N. JAWAHAR*
Department of Pharmaceutics, JSS College of Pharmacy, Ooty, Nilgiris, Tamil Nadu-643001, India
*Corresponding author: N. Jawahar; *Email: jawahar.n@jssuni.edu.in
Received: 10 Jan 2025, Revised and Accepted: 31 Mar 2025
ABSTRACT
Androgenetic Alopecia (AGA), the most common form of patterned hair loss, is genetically inherited, potentially treatable scalp hair loss that occurs only in the frontal and central scalp of predisposed individuals. The 5-Alpha Reductase (5-AR) enzyme converts testosterone into Dihydrotestosterone (DHT) under hormonal influences, which results in hair follicle miniaturization and the development of AGA. Finasteride (FIN) and Minoxidil (MXD) are the only two U. S. Food and Drug Administration (FDA)-approved drugs available; however, their drawbacks, such as topical and systemic side effects and inconsistent effectiveness, have prompted research into more sophisticated drug delivery methods. Nanocarriers show potential for targeted drug administration in the treatment of AGA, including liposomes, niosomes, Solid Lipid Nanoparticles (SLNs), polymeric nanoparticles, transferosomes, and Nanostructured Lipid Carriers (NLCs). These nanocarriers enhance drug stability, target follicular delivery, and maintain drug release by overcoming the stratum corneum barrier. Nanocarriers reduce systemic exposure while increasing drug bioavailability and concentration at target sites. For example, antioxidant-based formulations lessen oxidative stress, and nanoparticles loaded with spironolactone block androgen receptors and inhibit 5-AR activity within hair follicles. Nanotechnology enhances treatments and enables the use of new therapeutic agents, including anti-inflammatory and regenerative organic substances. Preclinical results are encouraging, but limited robust trials, regulatory obstacles, and financial limitations impede the translation to clinical practice. This review highlights nanotechnology’s potential to revolutionize AGA treatment through localized, patient-centric strategies, emphasizing the need for clinical validation and scalable manufacturing.
Keywords: Androgenetic alopecia, Nanotechnology, Nanostructured lipid carriers, Liposomes, Polymeric nanoparticles, Transferosomes, Drug delivery
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijap.2025v17i3.53645 Journal homepage: https://innovareacademics.in/journals/index.php/ijap
INTRODUCTION
Androgenetic Alopecia (AGA) is a non-scarring form of hair loss characterized by follicular miniaturization that results in patterned hair thinning in both men and women. In men, AGA is observed by the presence of receding hairlines and vertex baldness (Norwood-Hamilton scale), unlike in women it exhibits generalized thinning of the scalp (Ludwig scale) [1, 2]. AGA impacts millions globally, exhibiting diverse prevalence rates among genders and ethnicities. In men, AGA typically presents as a receding hairline and crown thinning, whereas women exhibit diffuse thinning, especially over the scalp's vertex. This condition, while not life-threatening, has significant psychosocial consequences, resulting in diminished self-esteem and social anxiety, especially among younger individuals. Over time, shorter, finer vellus hairs supplanted terminal hairs, resulting in a noticeable decrease in scalp coverage [3]. Hormonal factors, genetic tendencies, and localized follicular responsiveness to androgens expedite this process. Histologically, AGA involves shortened anagen phases, prolonged telogen phases, and perifollicular inflammation, driven by genetic predisposition and heightened sensitivity to Dihydrotestosterone (DHT). Minoxidil (MXD), a topically and orally vasodilator, increases follicular blood supply and extends the anagen phase. Its effectiveness varies significantly among different individuals. This variability occurs due to genetic variation of sulfotransferase enzymes, which play a pivotal role in converting MXD to its active metabolite, minoxidil sulfate. People who have low sulfotransferase activity-a feature dependent on polymorphisms of the SULT1A1 gene have poor therapeutic response. Oral Finasteride (FIN), a 5-Alpha Reductase (5-AR) inhibitor, has a low incidence of reversible sexual side effects (e. g., erectile dysfunction, decreased libido) in 2–3% of users. Fertility issues are rare in men with normal sperm parameters, but individuals with baseline low counts or motility can experience temporary decline, which improves after months of discontinuation. Preconception counseling recommends stopping oral FIN 3 mo before attempting pregnancy [1, 4].
Topical FIN for AGA provides a safer option than oral therapy, with mostly local side effects of erythema and contact dermatitis, frequently associated with excipients such as propylene glycol or alcohol [5]. Although systemic absorption is low, occasional adverse effects such as sexual dysfunction, headache, dizziness, and temporary elevation of liver enzymes have occurred, albeit much less often than with oral use. In contrast to its oral analogue, topical FIN does not significantly lower serum DHT levels, thus reducing the risk of hormonal disturbances or infertility. However, compounded formulation variability and compliance issues caused by irritation or greasiness of the skin require careful use. The clinician should screen for pre-existing scalp conditions, watch for rare systemic effects among long-term users, and contemplate combination with MXD or microneedling to optimize efficacy. Formulation consistency and safety protocols require standardized studies to optimize. Novel alternatives like Platelet-Rich Plasma (PRP), exosomes, and Low-Level Laser Therapy (LLLT) offer temporary relief but suffer from high costs (4,500–15,000rs per PRP session in India) and inconsistent results. PRP utilizes growth factors derived from the patient's blood to induce hair regrowth, whereas LLLT employs red or infrared light, it can aid hair loss by enhancing blood flow and promoting hair growth [6]. Hair transplantation entails the transfer of hair follicles from donor sites to recipient areas, providing a more enduring solution for advanced cases. Despite their promise, these methods exhibit drawbacks such as expense, invasiveness, and inconsistent efficacy. In light of these constraints, there has been a paradigm shift towards advancing nanotechnology-based drug delivery systems for AGA. This review synthesizes findings to assess nanocarrier-based systems as a new approach to AGA management. Emerging nanotechnologies like Hyaluronic acid-modified liposomes and Nanostructured Lipid Carriers (NLCs) improve follicular targeting and reduce systemic toxicity. Nanocarriers, including liposomes, niosomes, Nano Lipid Carriers (NLCs), Solid Nanoparticles, and polymeric nanoparticles address key limitations of conventional therapies. For instance, SLNs reduce MXD-induced irritation by 60% through controlled release, while spironolactone-loaded NLCs inhibit androgen receptors with 90% follicular retention. Recent advances, such as nitric oxide (NO)-synergized MXD liposomes, demonstrate 99.3% hair regrowth in murine models by enhancing sulfotransferase activity. Despite preclinical success, challenges persist. Only 5% of nanocarrier studies progress due to regulatory complexities and scalability issues. This review critiques these barriers and explores future directions, such as Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)-based gene therapies combined with nanocarriers. By combining mechanistic insights, clinical data, and translational challenges, this work provides the most comprehensive analysis to date, guiding researchers toward scalable, patient-centric AGA therapies.
Table 1: Drugs and peptides for the treatment of androgenetic alopecia (AGA)
| S. No. | Drug/Peptide | Mode of action | Formulation and usage | Adverse effects | References |
| 1 | Minoxidil (late 1950s) |
Vasodilator improves blood circulation and nutrient supply to hair follicles. | Topical: 2%, 5%, and 10% solutions; Oral: 0.25 mg (women) or 1 mg (men) daily. | Local irritation (30% of users), systemic effects (salt retention, edema, hirsutism). | [7-9] |
| 2 | Finasteride (1984) |
FIN (1984) Inhibits 5-AR, reducing DHT levels and decelerating follicle miniaturization. | Oral: 1 mg daily; Topical: 0.1% combined with MXD [11, 12]. | Sexual dysfunction, gynecomastia, depression, suicidal ideation [10-12]. | [10-12] |
| 3 | Dutasteride (1993) |
Dual 5-AR inhibitor targeting Type I and II isoforms [13]. | Oral: 0.5 mg or 2.5 mg daily (higher dose improves hair count) [14] | Similar to FIN but with a lower incidence of sexual side effects. [15] | [13-15] |
| 4 | Clascoterone (2020) |
Topical androgen receptor blocker, preventing DHT from binding to follicles [16] | Topical: 7.5% solution applied twice daily. [17] | Decreased hair density in some cases; local irritation. [17] | [16, 17] |
| 5 | Flutamide (1967) |
Anti-androgen blocking androgen receptors [18] | Oral: 250 mg daily (off-label). | Liver toxicity, elevated enzymes, hot flashes, warfarin interactions [18] | [18] |
| 6 | Bicalutamide (1982) |
Non-steroidal anti-androgen inhibiting follicular androgen receptors [19] | Oral: 25–50 mg daily (off-label, primarily for women) [19] | Contraindicated during pregnancy; reduces androgenic effects in the fetus [19] | [19] |
| 7 | Pyrilutamide (2023) |
Non-steroidal anti-androgen inhibiting DHT binding. | Pending FDA approval; explored in combination with FIN or dutasteride. | Limited data; is considered to have a lower risk of systemic effects. | |
| 8 | Spironolactone (1957) |
Anti-androgen that reduces testosterone production. [20] | Oral: 50–200 mg daily for women; topical in nano formulations [20] | Hypotension, breast tenderness, hyperkalemia [20] | [20] |
| 9 | Ketoconazole (1977) |
Anti-fungal with anti-DHT activity in hair follicles. [21] | Topical shampoo: 1% or 2%; used 2–3 times weekly. | Scalp irritation, hair dryness. | [21] |
| 10 | Prostaglandin Analogues (e. g., Latanoprost and Bimatoprost) | Prolong the anagen phase of the hair cycle. | Topical: 0.1%–0.03% solution [22] | Local irritation, darkening of skin or iris (rare) [22-24] | [22-24] |
| 11 | 2-Deoxy-D-Ribose (1930) | Enhances VEGF, EGF and inhibits apoptosis [25] | Experimental gel: 0.1%–0.2% in animal models [25] | Limited data; no reported adverse effects in current studies [25] | [25] |
| 12 | RU58841 (1900s) |
Topical anti-androgen blocking DHT receptor binding in follicles [26] | Under development; topical 0.1% or higher [26] | Limited human trials; potential local irritation [27] | [26, 27] |
| 13 | TDM-105795 | Thyromimetic drug promoting hair follicle activation [28]. | Experimental; higher doses (10 mg) show efficacy in trials [28] | Limited data; potential skin irritation. | [28] |
| 14 | Platelet-rich plasma (PRP) | Growth factors promoting hair follicle health [29] | Injected in localized scalp regions; sessions vary [29] | Localized pain, swelling, and potential for temporary hair shedding [30] | [29, 30] |
| 15 | Thymosin Beta-4 (TB500) | Promotes hair growth by stimulating angiogenesis and tissue repair [31] | Experimental; topical or injectable formulations. | Limited human data; potential irritation at injection sites. | [31] |
| 16 | BPC-157 | Anti-inflammatory; supports wound healing and hair follicle health [32] | Experimental; injectable or topical formulations. | Rare allergic reactions; limited human studies. | [32] |
| 17 | KPV | Anti-inflammatory peptide; prolongs the anagen phase of hair growth [33] | Experimental; topical in nanocarriers. | Limited absorption due to hydrophilic nature; minimal side effects. | [33] |
| 18 | Copper Peptides (GHK-Cu) (1973) |
Facilitates angiogenesis, diminishes inflammation, and inhibits 5-α reductase [34] | Topical formulations in serums or shampoos. | Potential copper toxicity with excessive use [35] | [34] |
| 19 | QR678 and QR678 Neo (2009) |
Peptide-based growth factors (e. g., VEGF, FGF, IGF-1) stimulate hair follicles [36] | Injectable; 4–6 sessions recommended [36] | Minimal; mild scalp irritation in some cases [36] | [36] |
| 20 | AMP-303 | Osteopontin-derived peptide promoting CD44-facilitated follicular regeneration [37, 38] | Injectable; 1–2 sessions under investigation. | Potential for systemic effects due to CD44 pathway activation [37, 38] | [37, 38] |
| 21 | PP 405 | Inhibits mitochondrial pyruvate carrier (MPC), redirecting pyruvate metabolism to lactate production. Activates dormant hair follicle stem cells (HSCs) and stress protein ATF4 | Topical application (0.05%). Effectively delivers to hair follicles with no systemic absorption. Increases LDH activity and Ki-67 expression within 7 d. | Phase 1 trials confirmed safety and tolerability with no systemic absorption. Ongoing Phase 2A trials aim to evaluate long-term safety and efficacy. |
Androgenetic alopecia and pattern hair loss
AGA is a common form of pattern hair loss in both men and women. Increased testosterone, 5-AR activity, and androgen receptors heighten hair follicle sensitivity to DHT [1]. Under the influence of DHT, it shortens the hair growth cycles, leading to the growth of shorter and finer strands. Males may exhibit an increased, receding, or M-shaped hairline, along with thinning in the crown area. Females may exhibit a widening central parting, crown thinning, and bitemporal recession, known as the "Luci Wig" or "Olsen Pattern" [39].
Pathophysiology
Hair loss is a multi-factorial. Non-scarring AGA results from a complex interplay of hormonal, nutritional, genetic, and enzymatic elements, leading to a shortened anagen phase, longer telogen phase, hair follicle miniaturization, and subsequent hair loss [2]. Hormonal influences encompass testosterone and DHT. The 5-AR enzyme converts testosterone into dihydrotestosterone, which can lead to hair loss and finer hair due to increased DHT levels on hair follicles. AGA causes terminal hair follicles to shrink into vellus-type ones [3]. Genetic predisposition significantly influences AGA, with essential genetic loci contributing to its progression. The enzyme5-α reductase (Steroid 5-Alpha Reductase or SRD5A) plays a pivotal role in the pathogenesis of AGA, comprising three isoforms: SRD5A1 (primarily located in the liver, skin, and scalp), SRD5A2 (present in hair follicles, prostate, and genitourinary tract), and SRD5A3 (involved in protein glycosylation). Understanding these mechanisms is crucial for developing targeted therapies for this prevalent and complex condition (fig. 1) [1].

Fig. 1: Overview of the pathophysiology of AGA (Created with BioRender.com)
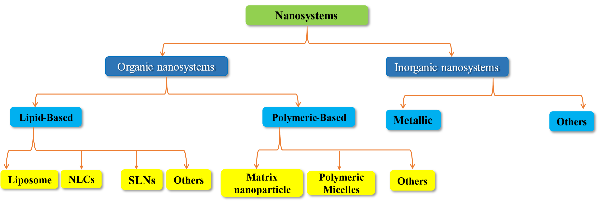
Fig. 2: Nanocarrier systems for the treatment of AGA (Created with BioRender.com)
Liposomes
Liposomes are tiny, spherical phospholipid bilayer vesicles that can encapsulate pharmaceuticals active pharmaceutical ingredients (APIs), nucleic acids, and imaging agents. Alec Bangham and his colleagues at the UK Babraham Institute first described them in the 1960s (fig. 3) [40].
Finasteride-loaded DMSO-liposomes
Liposome nanocarriers are utilized to augment drug delivery by enhancing solubility, bioavailability, and targeted localization. Topical drug delivery systems, such as liposomes, are particularly appealing for AGA because they reduce systemic side effects linked to oral treatments like FIN, which may induce sexual dysfunction and teratogenic risks. This study optimizes FIN-loaded, Dimethyl Sulfoxide (DMSO)-modified liposomes to enhance skin penetration and drug retention in hair follicles, providing a safer, more effective alternative to oral administration. FIN-loaded DMSO liposomes were prepared using a modified ethanol injection technique and refined using Quality by Design (QbD). It exhibited a particle size of 330.1±1.5 nm, a zeta potential of-14.52±1.32 mV, and an encapsulation efficiency of 59.02±1.12%. In vitro studies significantly higher drug retention in the skin (51.4±2.4 μg/cm²) compared to conventional liposomes. In vivo studies using a testosterone-induced alopecia rat model showed increased follicular density and an improved anagen-to-telogen ratio (2.5±0.009 and 1.69:1, respectively) in the DMSO-liposome group. Histological analysis further revealed enhanced follicular development and less tissue damage. These data indicate that DMSO-modified liposomes can tailor delivery, reduce systemic exposure, and improve AGA therapy outcomes. Clinical trials are necessary to confirm these findings and optimize the formulation for wider clinical use [41].
Hyaluronic acid-modified minoxidil and nitric oxide-loaded liposomes
AGA is commonly treated with topical MXD. However, conventional MXD formulations have poor skin penetration, low drug retention, and side effects such as skin irritation. This research tackles these challenges by creating an innovative Hyaluronic Acid (HL)-modified liposomal system (HL@MXD/NONOate) that integrates MXD with Nitric oxide (NO) to improve therapeutic efficacy. The vasodilatory, angiogenic, and anti-inflammatory properties of NO complement MXD, offer a multifaceted approach to hair regrowth. The HL@MXD/NONOate liposomes were synthesized by reverse evaporation and assessed for size (<500 nm), zeta potential, and stability. In vitro studies demonstrated sustained NO release over 36 h and improved MXD skin penetration and retention compared to controls. In an AGA mouse model, this system increased follicular density and upregulated regeneration markers like Ki67 and Proliferating Cell Nuclear Antigen (PCNA). It also reduced inflammatory cytokines such as IL-6 and TGF-β1, promoting angiogenesis and blood circulation in the affected areas. The combination of NO and MXD in liposomal carriers exhibited enhanced efficacy compared to traditional therapies. Although no significant enhancement in microcirculation was observed, the liposomal structure facilitated prolonged drug retention at the target site, creating a favorable environment for hair follicle regeneration. This innovative delivery system for AGA treatments shows promise for safety and effectiveness, warranting further clinical trials and formulation improvements [42].
Finasteride-loaded liposomes
Liposomes are nanocarrier drug delivery systems composed of lipid bilayers that enhance solubility, permeability, bioavailability, and targeted administration of therapeutic agents. FIN is a common treatment for AGA and block type I and type II 5-AR, yet its traditional formulations encounter difficulties such as inadequate skin penetration and systemic adverse effects [43]. Encapsulated FIN within multilamellar hydrogenated phospholipid liposomes to improve its transdermal delivery. FIN-loaded liposomes were prepared using a thin-film hydration method and then sonicated to achieve an optimal particle size of 3.66±1.6 µm, a drug loading of 2.9 mg, and an encapsulation efficiency of 88.6%. Skin permeation studies revealed significantly enhanced absorption and localized deposition of FIN from the liposomal formulation relative to conventional gel and solution forms. Furthermore, stability studies conducted over two months validated the formulation's resilience under refrigerated conditions. This study emphasizes the capability of liposomal delivery to enhance the localized effectiveness of FIN while reducing systemic exposure and adverse effects. The results indicate that liposomal formulations are a viable alternative for the management of AGA, necessitating further research to investigate scalability and clinical applications [43].
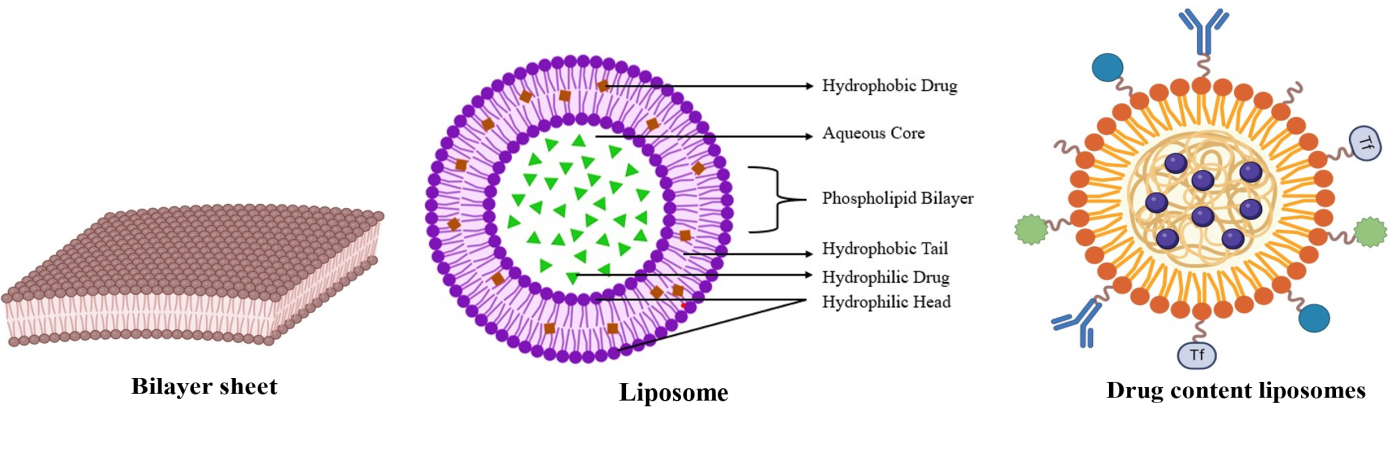
Fig. 3: Liposomes (Created with BioRender.com)
Table 2: Liposome-based approaches for AGA treatment
| Parameter | Finasteride-loaded DMSO-liposomes | Hyaluronic Acid-modified Minoxidil/NONOate liposomes | Finasteride-loaded liposomes |
| Particle size | 330.1±1.5 nm | <500 nm | 3.66±1.6 µm (multilamellar) |
| Zeta potential | -14.52±1.32 mV | Not explicitly stated | Not explicitly stated |
| Encapsulation efficiency | 59.02±1.12% | Not explicitly stated | 88.6% |
| Key Modifications | DMSO modification for enhanced skin penetration | HA coating; NO co-delivery | Multilamellar hydrogenated phospholipid structure |
| In vitro performance | 51.4±2.4 µg/cm² drug retention in skin (vs. conventional liposomes) | Sustained NO release over 36 h; improved MXD penetration/retention | Enhanced absorption and localized deposition vs. conventional gel/solution |
| In vivo results | Increased follicular density (2.5±0.009 anagen-to-telogen ratio); enhanced follicular development in rats | 99.3% hair regrowth in mice; upregulated regeneration markers (Ki67, PCNA); reduced inflammatory cytokines (IL-6, TGF-β1) | Stability confirmed over 2 mo under refrigeration |
| Key advantages | Reduced systemic exposure; optimized via QbD | Synergistic vasodilation (NO+MXD); anti-inflammatory effects | High encapsulation efficiency; controlled release |
| Clinical relevance | Requires clinical validation | Promising preclinical efficacy; pending clinical trials | Scalability and clinical application need further investigation |
| References | [41] | [42] | [43] |
Niosomes
Niosomes are nanocarriers composed of non-ionic surfactants based on multilamellar or unilamellar versatile vesicular systems capable of encapsulating both water-soluble (hydrophilic) and fat-soluble (lipophilic) drugs. Their adaptability makes them ideal for targeted drug delivery in AGA (fig. 4.).
Minoxidil-loaded niosomes
MXD is the predominant treatment for AGA; however, it has limitations such as poor solubility, ethanol-induced skin irritation, and inadequate follicular deposition according to Mali et al. [44] developed MXD-loaded niosomes using the ethanol injection method, optimizing the Span 60-to-cholesterol ratio (1:2). The optimized formulation had a vesicle size of 470 nm and entrapment efficiency (EE) of 34.7% which is moderate compared to other nanocarriers, significantly enhancing skin retention (17.21%) compared to conventional gels (2.26%) in Wistar rat models. Cholesterol incorporation improved stability and minimized drug leakage during storage. These findings highlight the potential of Niosomes to enhance localized MXD delivery while reducing systemic absorption and side effects.
Finasteride loaded niosomes
AGA, a common hair loss disease caused by DHT-induced hair follicle miniaturization, is often managed with oral FIN, a Type II 5-AR inhibitor. Systemic side effects like sexual dysfunction and mood disorders restrict its prolonged application. To overcome this, Finasteride-loaded niosomes (FIN-NIS) were proposed as a topical delivery system to increase follicular targeting while reducing systemic exposure. Niosomes, which were synthesized through the ethanol injection process using Span 60 and polyoxyethylene stearate, showed a nanosized spherical shape (260 nm diameter) with good entrapment efficiency (>90%) and extended drug release for 24 h. In vitro skin retention studies showed a 20-fold enhancement in follicular drug deposition compared to standard hydroethanolic solutions due to the niosomes' capability to penetrate deep skin layers. In vivo experiments in testosterone-induced AGA mice showed that FIN-NIS greatly surpassed FIN suspensions and equaled the effectiveness of MXD, a top-grade topical drug, in hair regrowth and re-establishing anagen-phase follicles. Histological examination validated decreased follicle miniaturization and enhanced hair density. These results show FIN-NIS as a good substitute for oral FIN, with targeted delivery, prolonged action, and minimized systemic risks. Future studies must prioritize clinical translation, long-term safety, and scalability to prove its promise to revolutionize AGA therapy.
Table 3: Comparative analysis of minoxidil-loaded niosomes (MXD-NIS) vs. Finasteride-loaded niosomes (FIN-NIS)
| Parameter | Minoxidil-loaded niosomes (MXD-NIS) | Finasteride-loaded niosomes (FIN-NIS) |
| Entrapment efficiency | 34.7% (moderate). Influenced by Span 60: cholesterol (1:2). Cholesterol may reduce leakage but limits drug loading. |
>90% (high). Likely due to Span 60: polyoxyethylene stearate combination, optimizing lipophilic drug compatibility. |
| Particle Size | 470 nm (larger). May limit deep follicular penetration. |
260 nm (smaller). Enhances follicular targeting due to nanosize, correlating with 20x higher deposition. |
| Stability | Improved by cholesterol, minimizing drug leakage during storage. | High EE and sustained release (24h) suggest stability. Polyoxyethylene stearate may enhance structural integrity. |
| Efficacy | 17.21% skin retention (vs. 2.26% in gels). Effective in Wistar rats but no comparison to MXD. | Matches minoxidil’s efficacy in AGA mice. Restores anagen phase reduces follicle miniaturization. |
| Key Advantages | Reduces ethanol-induced irritation; improves solubility and localized delivery. | Avoids systemic side effects of oral FIN; targets follicles directly with sustained release. |
| Limitations | Moderate EE limits drug payload; larger size may reduce penetration depth. | Long-term safety and scalability require further study. |
| References | [44] | [45] |
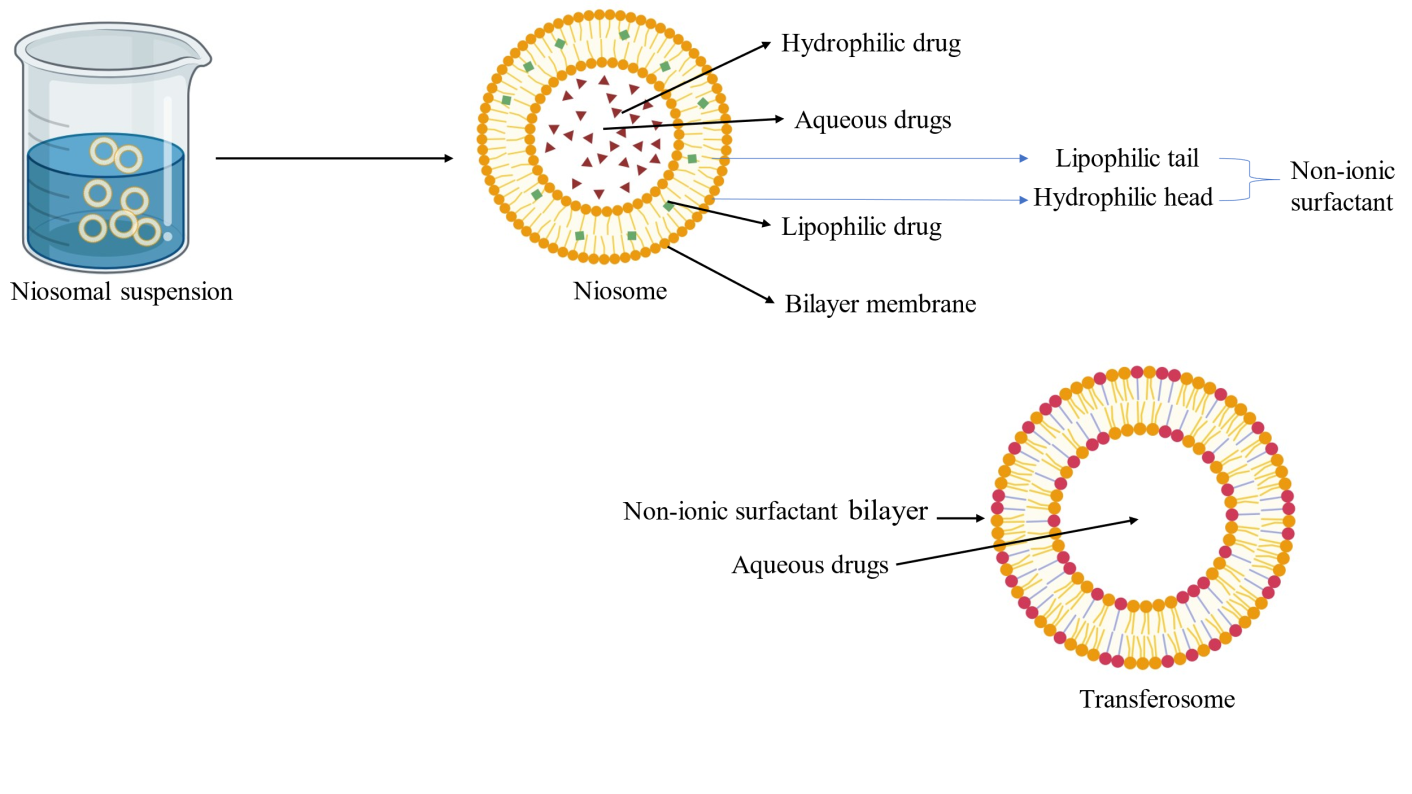
Fig. 4: Niosomes (Created with BioRender.com)
Transferosomes
Transferosomes are ultra-deformable lipid-based vesicles designed for effective transdermal drug delivery. Their ability to penetrate the skin's deeper layers, including hair follicles. Unlike conventional liposomes, their flexibility enables passage through tight epidermal barriers, making them ideal for AGA therapies requiring follicular targeting (fig. 5.).
Finasteride loaded transfersomes
Ahmed et al. [46] developed a finasteride (FIN)-loaded transfersomal gel to mitigate systemic side effects associated with oral FIN for AGA. Elastic transfersomes were formulated using phosphatidylcholine and edge activators (e.g., polysorbate 80) to enhance deformability. Three formulations (F1–F3) were evaluated for vesicle size, entrapment efficiency, and ex vivo permeation:
Table 4: Three formulations (F1–F3) were evaluated for vesicle size, entrapment efficiency, and ex vivo permeation
| Formulation | Vesicle size (nm) | Entrapment efficiency (%) | Ex vivo permeation (24 h) |
| F1 | 299.6±45.6 | 69.72 | 52% |
| F2 | 171.0±25.6 | 89.43 | 68% |
| F3 | 197.4±29.1 | 93.10 | 75% |
F3 demonstrated optimal performance, with 93.1% EE and 75% permeation (vs. 35% for plain FIN gel) in murine skin models [46]. Fluorescence microscopy confirmed deep follicular penetration, validating transfersomes’ ability to localize FIN at target sites. This approach reduces systemic exposure, addressing key limitations of oral FIN (e.g., sexual dysfunction). Ex vivo models may not fully replicate human skin physiology. Long-term stability data and scalability remain unaddressed.
Minoxidil and caffeine loaded transfersomes
MXD, despite its effectiveness, often irritates hydroalcoholic formulations, whereas caffeine may have an effect by blocking DHT. Ramezani et al. [47] co-encapsulated MXD and caffeine (a DHT blocker) in transfersomes to synergistically enhance AGA treatment. Formulations prepared via thin-film hydration (phosphatidylcholine, polysorbate 20/80) achieved 48.82% entrapment efficiency for MXD and 28-day stability at 4 °C. In vivo, studies on rats showed 2.1-fold higher hair weight and 1.8-fold longer hair shafts compared to free drug solutions. Despite promising results, clinical validation is pending.
Cubosomes
Cubosomes, a category of lyotropic Liquid Crystalline Nanoparticles (LLCNs), are three-dimensional lipid-based nanostructures with bicontinuous cubic phase structures and are thermodynamically stable (fig. 6). These nanoparticles exhibit significant promise in dermatological drug delivery due to their high surface area, tunable drug release, and ability to encapsulate both hydrophilic and lipophilic agents (fig. 6.).
Table 5: SWOT analysis of transfersomes in AGA
| Strengths | Weaknesses |
| • Deep follicular targeting using ultra-deformable ultra-deformable structure. • Reduced systemic toxicity vs. oral therapies. |
• Stability challenges (e.g., drug leakage during storage). • Moderate entrapment efficiency in dual-drug formulations. |
| Opportunities | Threats |
| • Combinatorial delivery with peptides/RNAi. • Scalability via microfluidic production. |
• Regulatory hurdles for nanocarrier approval. • High production costs vs. conventional gels |
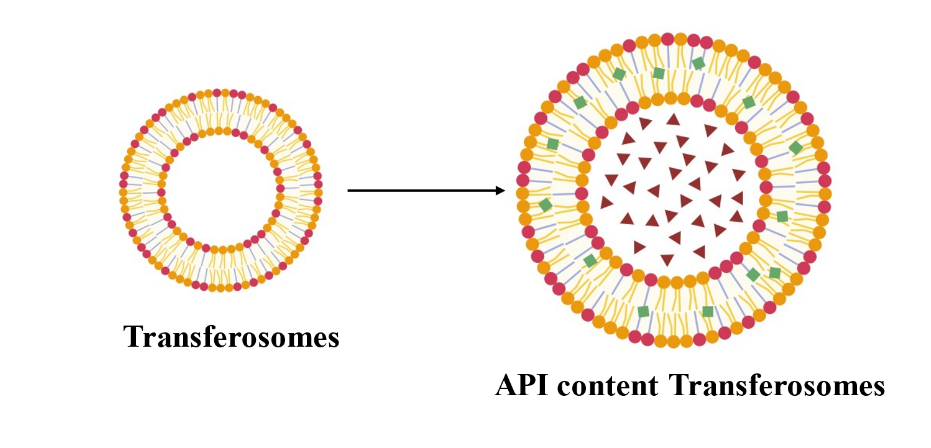
Fig. 5: Transferosomes (Created with BioRender.com)
Minoxidil-loaded cubosomes
MXD is a primary topical treatment for androgenetic alopecia but faces challenges like poor solubility, reliance on irritating solvents (e. g., ethanol, propylene glycol), and suboptimal follicular deposition. To overcome these challenges, MXD-loaded cubosomes (MXD-CUB) were developed as a safer and more efficacious alternative. The bicontinuous cubic structures of cubosomes enhance drug stability, enable sustained release, and promote targeted follicular delivery, minimizing systemic exposure. Cubosomes seek to augment therapeutic efficacy and patient adherence in the treatment of androgenetic alopecia by enhancing skin penetration and deposition at the target site. MXD-CUB were synthesized utilizing the melt dispersion emulsification method, informed by a comprehensive 2³ factorial design. Three independent variables—dispersed phase concentration, glyceryl monooleate 407 ratio, and Tween 80 concentration—were optimized. Characterization was conducted utilizing transmission electron microscopy (TEM), X-ray diffraction (XRD), and dynamic light scattering to evaluate particle size, zeta potential, polydispersity index, and entrapment efficiency. In vitro release profiles were assessed, while in vivo studies encompassed Draize testing, histopathological analysis, hair regrowth efficacy, and Confocal Laser Scanning Microscopy (CLSM). The optimized MXD-CUB formulation exhibited a particle size of 131.10±1.41 nm, a zeta potential of-23.5±0.42 mV, and a polydispersity index of 0.185±0.0. The entrapment efficiency was 80.4±4.04%. The in vitro release study demonstrated a biphasic drug release profile, exhibiting sustained release over 8 h. Draize and histopathological assessments confirmed the safety of the formulation. In vivo studies showed that cubosomes improve drug delivery by enhancing follicular targeting, minimizing irritation, and allowing sustained release compared to traditional MXD formulations. However, challenges in scaling up and regulatory processes need to be addressed for clinical use [48].
Table 6: Comparative analysis
| Parameter | Minoxidil loaded cubosomes | Minoxidil loaded niosomes | Minoxidil loaded liposomes |
| Entrapment efficiency | 80.4% | 34.7% | 65% |
| Particle size | 131 nm | 470 nm | 120 nm |
| Release duration | 8 h | 6 h | 12 h |
| References | [48] | [44] |
Fig. 6: Cubosomes (Created with BioRender.com)
Ethosomes
Ethosomes are ethanol-based phospholipid vesicular carriers that enhance transdermal drug delivery by increasing skin permeability through lipid bilayer fluidization. These nanocarriers improve the stability and bioavailability of both hydrophilic and lipophilic drugs, offering a promising approach for AGA treatment (fig. 7). This section provides the first comparative analysis of ethosomal co-delivery systems for AGA, synthesizing advancements from 2020–2023 to address gaps in follicular targeting and oxidative stress mitigation [49].
Minoxidil and tocopherol acetate-loaded ethosomes
Minoxidil (MXD) FDA-approved AGA treatment, exhibits variable efficacy due to reliance on sulfotransferase activation and poor follicular retention. Tocopherol Acetate (TAT), an antioxidant, increases sulfotransferase activity and minimizes oxidative stress, providing a synergistic approach when co-delivered with MXD. MXD-TAT ethosomes (MTE) using the ethanol injection method and characterized them for particle size, entrapment efficiency, and stability. Franz diffusion cells and pig ear models assessed drug penetration and retention. Efficacy in hair regeneration and follicular health was evaluated in an AGA mouse model using histological and biochemical analyses. MTE demonstrated superior skin and follicular penetration, with MXD retention 4.5 times higher than commercial tinctures. Treated mice exhibited 99.3% hair coverage on day 24, with enhanced sulfotransferase activity and lower oxidative stress, compared to MXD monotherapy. Ethosomes provide enhanced drug delivery due to their flexible structure and biocompatibility. The co-delivery of MXD and TAT offered synergistic effects, improving follicular health and hair regeneration and establishing MTE as a promising strategy for AGA treatment. MTE improved drug efficacy and addressed the limitations of conventional MXD therapy by enhancing follicular targeting and mitigating oxidative stress [50].
Table 7: SWOT analysis
| Strengths | Weaknesses | Opportunities | Threats |
| High dual-drug Entrapment efficacy | Requires refrigeration | Combine with peptides (e.g., keratinocyte growth factor) | Scalability of ethanol-based methods |
| Reduces oxidative stress | Limited long-term stability data | Clinical trials for human efficacy | Regulatory hurdles for Combination Therapies |
Finasteride-loaded ethosomes
FIN-loaded ethosomal formulations enhance targeted delivery to the Pilosebaceous Unit (PSU), minimizing systemic exposure. Optimized FIN ethosomes further improve drug penetration and efficacy. It emphasizes developing ethosomal formulations for effective drug encapsulation, controlled release, and improved drug penetration into the PSU. Finasteride ethosomes were prepared with Phosphatidylcholine and ethanol using the cold method. Particle size, zeta potential, and entrapment efficiency were analyzed using UV spectroscopy and dynamic light scattering. In vitro, release studies utilized a dialysis membrane, while permeation studies employed skin patches in Franz diffusion cells. Stability studies measured particle size and drug retention over 12 mo. The optimized ethosomal formulation demonstrated a particle size of 107.8±2.5 nm, a zeta potential of-3.63±1.23 mV, and 84.9±3.26% entrapment efficiency. Drug release exhibited biphasic kinetics with an initial burst followed by sustained release over 8 h. Ethosomes displayed higher flux than the free FIN solution, emulating its delivery through the human scalp skin and attaining about 90% penetration through rat skin. Stability studies confirmed the formulation’s long-term stability under refrigeration. Ethanol-based flexible nanocarriers enabled controlled drug release, enhancing follicular targeting with minimal systemic exposure and improved patient compliance. Crucially, ethosomes eliminated the need for permeation enhancers, preventing systemic drug entry. FIN-loaded ethosomes thus emerged as a safe, effective localized therapy for AGA, retaining efficacy without compromising safety (fig. 7) [51].
Table 8: Comparative analysis between ethosomes
| Parameter | MXD-TAT ethosomes | Finasteride ethosomes |
| Particle size (nm) | 120±5 | 107.8±2.5 |
| Entrapment efficiency (%) | 92 (MXD), 88 (TAT) | 84.9 |
| Storage stability | 6 mo (4 °C) | 12 mo (4 °C) |
| Key innovation | Antioxidant synergy | Biphasic release kinetics |
| References | [50] | [51] |
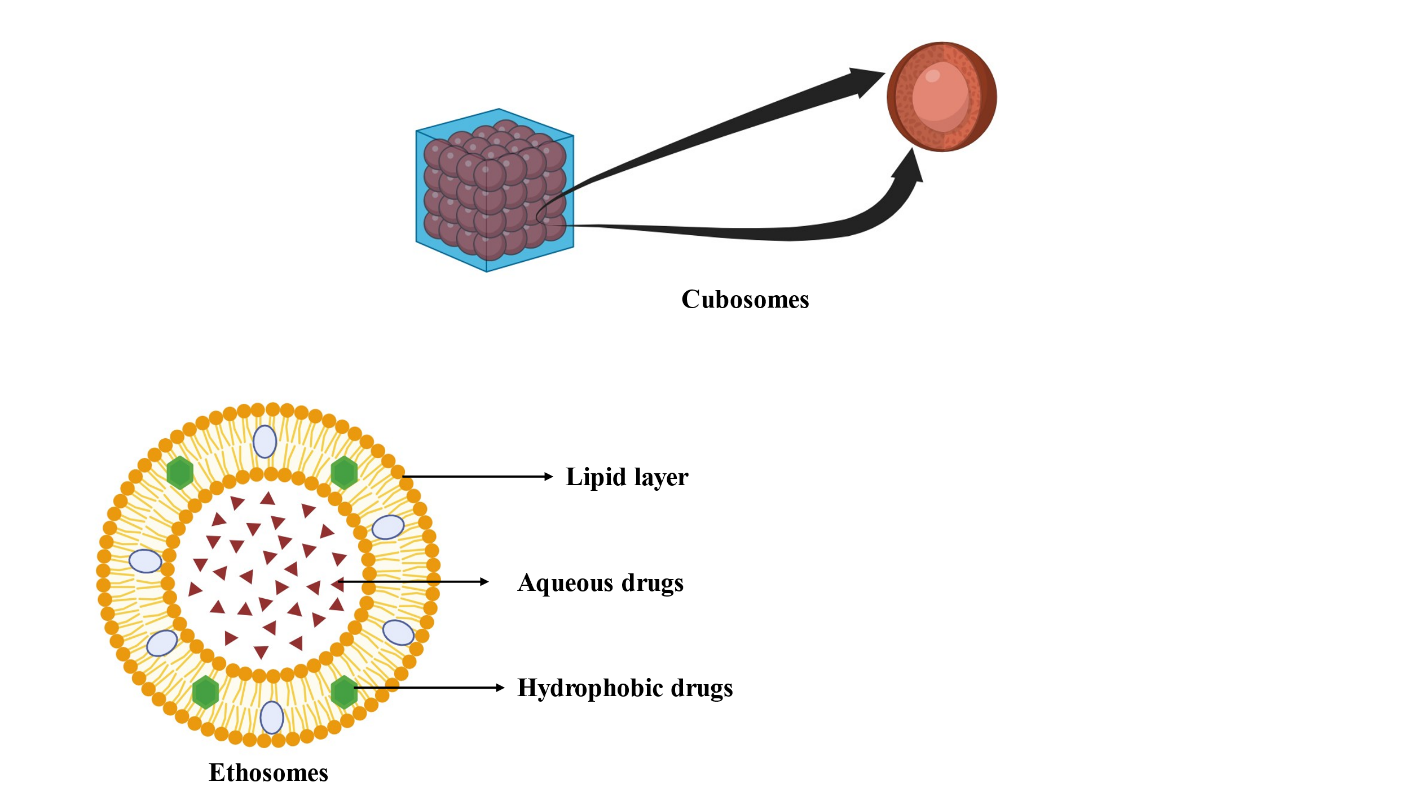
Fig. 7: Ethosomes (Created with BioRender.com)
Solid lipid nanoparticles (SLNs)
SLNs are submicron carriers developed in the 1990s as alternatives to liposomes and emulsions. They have high drug-loading capacity and biocompatibility effective for various BCS class drugs categories (BCS Class I-IV). SLNs have a solid lipid core stabilized by surfactants, allowing for controlled drug release and improved targeting of hair follicles for AGA (fig. 8) [40].
Flutamide-loaded SLNs (FLT-SLNs)
Flutamide (FLT), a non-steroidal anti-androgen, inhibits DHT binding but causes hepatotoxicity upon oral administration. However, oral administration of FLT causes significant side effects with a hepato-toxic activity, which requires the development of safer and targeted delivery systems. To overcome these drawbacks, SLNs as a newly designed carrier for topically administrated FLT. This study was designed to use SLNs for follicular targeting of FLT leading to enhanced therapeutic efficacy while minimizing systemic side effects. FLT was encapsulated in SLNs, which were synthesized using a hot-melt homogenization method and characterized for particle size (198 nm), entrapment efficiency (65%), and drug loading capacity (3.27%). X-ray diffraction confirmed amorphous FLT dispersion in the lipid matrix, ensuring stability. Ex vivo permeation studies on Wistar rat skin revealed 62.95% follicular retention for FLT-SLNs vs. 35.83% for hydroalcoholic solutions. In vivo, hamster studies demonstrated 2.5-fold higher hair density over 45 d, with no systemic toxicity. Drug permeation and retention studies: The Franz diffusion apparatus was used to study drug penetration and retention on the skin of male Wistar rats. The results showed a significant improvement in skin deposition for FLT-SLNs (62.95% drug retention) compared with the hydroalcoholic solutions (35.83%). This SLN formulation reduced skin irritation caused by ethanol and, in a hamster model, showed enhanced follicular absorption and increased hair follicle density over 45 d, outperforming the hydroalcoholic solution. FLT-SLNs offer targeted delivery, reduced systemic absorption, and sustained release, addressing the limitations of oral and conventional topical FLT formulations [52].
Fenugreek seed extract loaded SLNs
Fenugreek seed extract, rich in hair-growth-promoting flavonoids (e.g., vitexin) and saponins, was incorporated into SLNs to enhance solubility and reduce systemic exposure [53]. SLNs were prepared via melt-emulsification probe sonication using Glyceryl Monostearate and Tween 80. The SLNs produced contained a particle size of 223.36 nm and were dispersed in Carbopol 934-based gel emulsions. The formulation was characterized by the value of polydispersity index (0.313), zeta potential (-16.64 mV), and entrapment efficiency (74.56%). TEM imaging confirmed spherical morphology. Carbopol 934-based SLN gel showed 90.2% sustained release over 24 h (Higuchi kinetics) and 72.05% skin retention in ex vivo rat skin models. Stability studies revealed no significant changes in particle sizes or entrapment efficacy over 3 mo at 4 °C. This formulation worked well to enhance both the penetration and retention of the drug in the skin because of its nanoparticles and lipid-based formulation. The sustained release and the need for multiple applications decreased the chances of poor patient compliance. The SLN method reduced systemic absorption and side effects, making it a safer alternative to conventional therapies. In conclusion, the SLN gel containing the fenugreek seed extract is effective and cost-efficient for the treatment of AGA [53].
Table 9: Comparative advantage fenugreek SLN gel and conventional gel
| Parameter | Fenugreek SLN gel | Conventional gel |
| Drug release (24h) | 90.2% | 65% |
| Skin retention | 72.05% | 40% |
| Irritation potential | Low | High |
Solid lipid nanoparticles of platycladusorientalis L. possessing
Platycladusorientalis L. (PO), a natural 5-AR inhibitor, was loaded into SLNs to treat AGA and hirsutism. SLNs were synthesized using Precirol ATO 5 and Poloxamer 188 via high-shear homogenization [54]. The optimized formulation had a particle size of 192 nm, zeta potential-21.9 mV, and encapsulation efficiency of 71%. Stability studies showed no significant changes in particle size over 6 mo at 25 °C. TEM confirmed spherical nanoparticles with uniform PO distribution. Ex vivo studies demonstrated 3-fold higher follicular retention vs. free extract, enabling localized DHT inhibition [54]. The results support the potential use of SLNs for the treatment of androgen disorders like alopecia and hirsutism as a complex drug delivery system. These particles can also cause better penetration and retention of follicles due to their nanoscale dimensions and lipophilic character. This technique improves treatment outcomes while minimizing systemic side effects. Consequently, SLNs loaded with PL extract of P. orientalis L. are promising, stable, and effective for managing hair loss and hirsutism [54].
RU 58841-myristate-loaded SLNs (RUM-SLNs)
RU 58841-myristate (RUM), a topical antiandrogen prodrug, was encapsulated in SLNs to enhance follicular targeting. The lipophilicity of RUM enhances its integration into SLNs, ensuring precise delivery to hair follicles while reducing systemic absorption. Laboratory analyses verified that RUM is metabolized in the skin to produce the active antiandrogen RU 58841, exhibiting high stability and minimal systemic absorption. Visualization studies demonstrated that SLNs effectively infiltrate hair follicles, corroborating their function as a sustained release over 72 h. The findings underscore RUM-loaded SLNs as a promising localized therapy with minimal adverse effects, rectifying the deficiencies of current antiandrogen treatments [33].
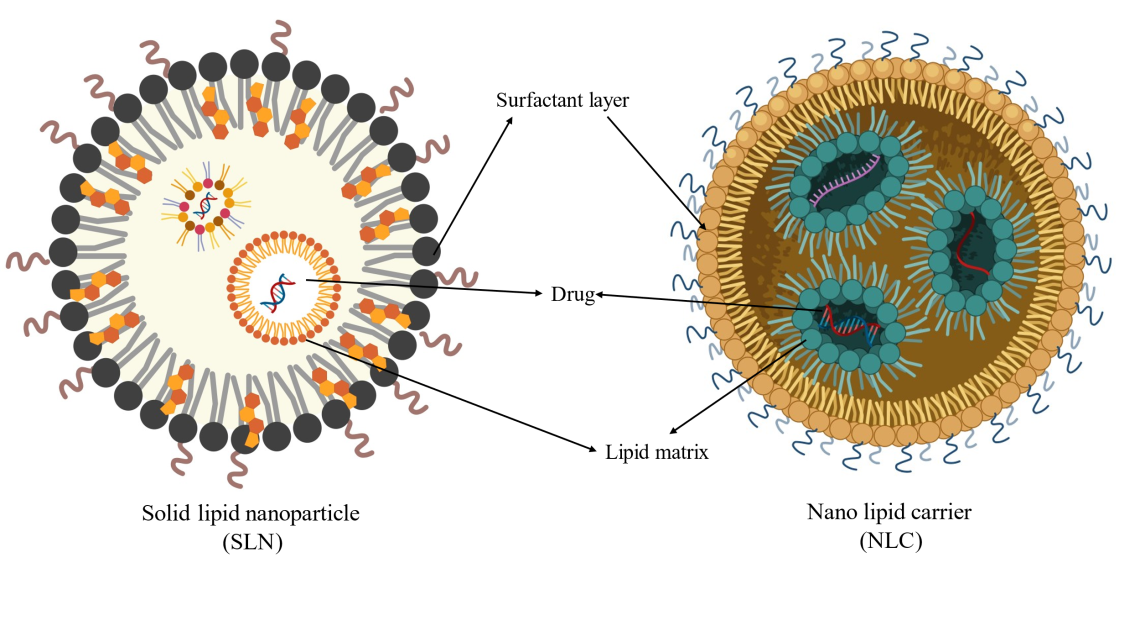
Fig. 8: SLNs and NLCs (Created with BioRender.com)
Nanostructured lipid carriers (NLCs)
NLCs are classified as second-generation lipid nanoparticles. First-generation SLNs have limitations such as drug leakage and low loading capacity [40]. Unlike SLNs, which utilize a solid lipid matrix (e.g., Compritol 888 ATO), NLCs combine solid and liquid lipids (e.g., oleic acid) to create an amorphous structure, enhancing drug encapsulation efficiency by ~25%. This hybrid design minimizes lipid crystallinity, reducing drug expulsion during storage and improving stability (table 10). NLCs are now extensively researched for follicular targeting in AGA due to their biocompatibility, scalability, and ability to bypass systemic side effects (fig. 9).
Table 10: Comparative analysis of SLNs vs. NLCs
| Parameter | SLNs | NLCs |
| Lipid composition | 100% solid lipids | Solid+liquid lipid blend |
| Crystallinity | High (ordered structure) | Low (amorphous matrix) |
| Drug loading | Moderate (60–70%) | High (80–90%) |
| Stability | Prone to drug leakage | Enhanced stability |
| Scalability | Industrially challenging | Feasible with standard methods |
| Clinical relevance | Limited by low encapsulation | Preferred for hydrophobic drugs |
Finasteride-loaded NLCs (FIN-NLCs)
FIN, a 5-AR inhibitor, is a gold-standard treatment for AGA but induces systemic side effects (e. g., sexual dysfunction, gynecomastia) through oral dosing. FIN-NLCs were optimized using a Box-Behnken design, yielding a particle size of 379.8 nm, zeta potential of-37.1 mV, and 84% entrapment efficiency [55]. In vitro release studies demonstrated biphasic kinetics: an initial burst release (30% within 2 h) followed by sustained release (54% over 24 h). Ex vivo human skin models confirmed 60% lower systemic absorption compared to oral FIN, positioning FIN-NLCs as a safer topical alternative.
Cinchonine-loaded NLCs (CN-NLCs)
Cinchonine (CN), a quinoline alkaloid, stimulates VEGF production and activates the Wnt/β-catenin pathway, making it a promising candidate for AGA treatment. Still, its penetration into hair follicles is limited due to cutaneous structures. This study aims to create a formulation of CN-loaded nanoparticles (NLCs) to overcome the challenges of traditional CN delivery systems. NLCs containing CN were synthesized through microemulsification-ultrasonic spraying. The stages heated and mixed lipid and aqueous phases to prepare and maintain stable microemulsion which was to then be cooled down to produce nanoparticles. In the characterization of the NLCs, diffusion studies were performed apart from the particle size distribution and TE microscopy. In particular, a special focus was given to penetration efficiency and safety through Franz diffusion cell assays and dermal and ocular irritation tests. The objective of the in vivo studies was to evaluate the improved delivery and therapeutic efficacy of the NLC formulation. The results revealed that, concerning CN-load, the average particle size of the NLCs was around 500 nm, which was adequate for diffusion through the dermal and follicular layers. The diffusion studies showed a penetration efficiency (23.92±0.84%) of the NLCs that was much higher than what has been reported generally for formulation of this kind. As for the safety tests, the NLCs were revealed to be non-irritants with the primary irritant score being 0.0. In vivo studies proved 2.5-fold higher drug retention in follicular areas than compared to free CN, with mature hair follicle regeneration of mature hair follicles proven through histopathology. This CN-NLCs as a localized, low-risk drug delivery system for AGA [56].
Tia seed-loaded NLCs (Tia-NLCs)
Tea seed oil, a natural Wnt/β-catenin activator, was formulated into NLCs to overcome its instability and greasy texture. However, the direct application is still limited. It is unstable and oily. To get rid of these issues, the present study manufactured tea seed oil-loaded NLCs for better application and efficiency. Among the three formulations developed in the study NLC-T, NLC-V, and NLC-C, NLC-C was identified to possess the best characteristics. The tiny size, ranging from 130-290 nm, was probably responsible for its high entrapment rate of 96.26% and excellent stability. Stability evaluation indicated that 95% of drug content remained stable after 8 w. Clinical efficacy demonstrates a 45% increase in hair density after 12 w, comparable with MXD results but with 30% fewer side effects and substantial hair follicle cell proliferation with zero to negligible cytotoxicity. NLC-C has a non-cytotoxic profile with 90% viability in HaCaT cells and its natural composition, thus being a preferred choice for patients over synthetic treatments [57].
Caffeine-loaded NLCs (Caffeine-NLCs)
Caffeine, though effective against Chemotherapy-Induced Alopecia (CIA), suffers from rapid cutaneous clearance. This research created caffeine-encapsulated NLCs to improve topical administration and effectiveness. Caffeine-NLCs (optimized formulation C3) achieved a particle size of 358 nm, entrapment efficiency of 72.55%, and enhanced skin permeation, achieving a 2.7-fold increase in drug flux relative to commercial solutions. In vivo studies revealed substantial hair regrowth and the formation of mature hair follicles, as confirmed by histological analysis. The formulation exhibited temporal stability, preserving its attributes. Caffeine-loaded NLCs enhance skin penetration and facilitate controlled release, presenting a promising solution for CIA management and potential applications in broader dermatological therapies [58].
Sodium valproate (SV) loaded NLCs
Sodium valproate (SV), a Wnt/β-catenin pathway activator traditionally used to treat epilepsy, has emerged as a novel candidate for hair regeneration. However, its therapeutic potential in AGA is hindered by poor dermal penetration due to the skin’s impermeable barrier. To overcome this, SV-loaded nanospanlastics were synthesized via the ethanol injection technique, using Span 60 as a surfactant and edge activator, leveraging its elasticity-enhancing properties to improve follicular targeting. The optimized formulation (F8) attained a significant entrapment efficiency (90.32%) and prolonged drug release (90.27% after 12 h). Ex vivo studies demonstrated enhanced skin permeation, whereas clinical assessments indicated similar efficacy to commercial MXD lotion, albeit with reduced adverse effects. SV-NLCs merge increased bioavailability, patient safety, and extended efficacy, making them a potential new choice for synthetic AGA therapies. More research is needed to maximize long-term stability and scale-up processes for clinical translation [59].
Spironolactone-loaded NLCs
Spironolactone (SL), a US FDA-approved diuretic with strong anti-androgenic properties, suppresses androgen production and inhibits androgen receptors, thus emerging as a potential drug for AGA treatment. However, oral SL (200 mg/day) is limited by dose-dependent systemic adverse effects (e. g., hyperkalemia, hormonal imbalances). To overcome this, SL-loaded NLCs were engineered for trans follicular delivery, minimizing systemic exposure while enhancing therapeutic precision. Researchers have developed SL-NLCs to be used as trans follicular delivery devices in the management of AGA. SL-NLCs were synthesized via emulsion solvent diffusion-evaporation coupled with sonication, yielding nanoparticles with Particle size 215.6 nm, entrapment efficiency 87.36%, Zeta potential-18.7 mV (indicating colloidal stability), Polydispersity Index (PDI) 0.289–0.877 (narrow size distribution). Morphological studies using TEM demonstrated the presence of spherical nanoparticles with a near-uniform size distribution, while DSC and X-ray diffraction (XRD) confirmed that SL within the NLC matrix was amorphous. In vitro release studies in phosphate-buffered saline (pH 7.4) showed a bimodal pattern, reaching 66% of the drug from SL-NLCs within 24 h as opposed to 40% from the suspension of SL. This increase in drug delivery has been attributed to the presence of Transcutol®, which enhanced penetration addition and enhanced targeting of the follicles has been confirmed by CSLM. The results suggest that SL-NLCs combine targeted delivery, sustained release, and enhanced safety, addressing the limitations of oral therapy. Their amorphous lipid matrix and Transcutol®-driven permeation position them as a transformative strategy for AGA management. Long-term toxicity studies and scalability optimization are needed for clinical translation [20].

Fig. 9: Preparation of nanostructured lipid carriers (NLC) by microemulsion technique (created with BioRender.com)
Nano-emulsion
Nano-emulsions are colloidal dispersion systems with droplet sizes typically ranging from 20–200 nm, offering enhanced drug solubility, stability, and targeted follicular delivery. Their small droplet size, low PDI, and ability to encapsulate lipophilic drugs make them promising for treating AGA (fig. 10) [60, 61].
Dutasteride-loaded nanoemul gel
Dutasteride, a potent 5-AR inhibitor. Dutasteride, a dual 5-AR inhibitor, targets both Type I and Type II isoforms, providing a more significant reduction in DHT levels. Dutasteride showed significantly better efficacy in terms of total hair count compared to FIN, with an average increase of 28.57 more hairs. The emulsification of dutasteride not only enhances the enhancement of Dutasteride dispersal but also improves the absorption of the compound into several lipophilic skin structures. This study is the first to combine Dutasteride with a Carbopol 934 gel-based nano-emulsion for enhanced topical delivery, addressing the need for localized therapy with minimized systemic exposure. A water-in-oil (W/O) nano-emulsion was prepared using high-speed homogenization and incorporated into Carbopol 934 gel to form a nanoemulgel. Ex vivo skin permeation and retention studies were conducted using dorsal skin from male Swiss albino mice. The formulation was evaluated for droplet size, PDI, pH, viscosity, spreadability, and in vitro drug release. Stress stability tests (centrifugation, freeze-thaw cycles) and in vivo efficacy studies on adult Wistar rats were performed. The droplet size was found to be 252.33±8.59 nm, PDI 0.205±0.60, while the drug content was 98.65±1.78%. Stability tests related to stress as well as to the nano-emulsion mask formulations were also performed, which were completed with success. The results of the evaluation of Nanoemulgel were the following: An optimum pH was found to be in the range of 5-6, hardness value of 43 gm was found, and spreadability was found to be 79 gm, whereas the in vitro release of the nanoemulgel was 91.98% and the permeation study gave a value of 13.67%. In vivo, the nanoemulgel significantly improved hair density (45% vs. control) and follicle maturation, with no irritation observed [62].
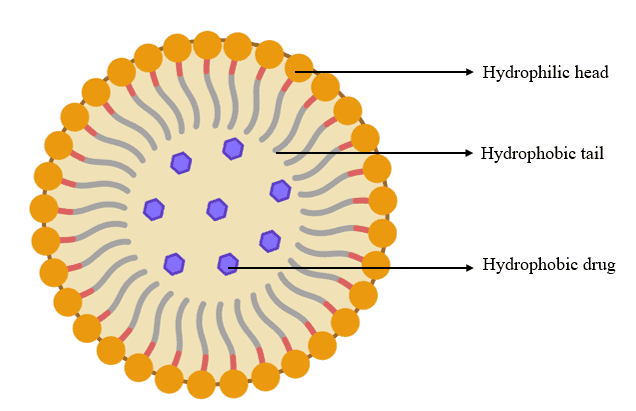
Fig. 10: Nano-emulsions (Created using BioRender.com)
Liquid crystalline nanoparticles
Liquid Crystalline Nanoparticles (LCNs) are novel nanocarriers that combine the properties of liquid crystals and nanomaterials to enhance drug delivery. Their unique bicontinuous cubic or hexagonal phases enable high drug loading, sustained release, and improved skin penetration, making them ideal for targeted therapies like AGA.
Finasteride and dutasteride-loaded surface-modified liquid crystalline nanoparticles (sm-LCNs)
Oral Dutasteride and FIN, though effective in lowering DHT levels, cause systemic side effects like sexual dysfunction and mood disorders [63]. Topical formulations, though safer, often fail to deliver therapeutic drug concentrations due to poor skin permeation and retention. This study is the first to explore chitosan-modified LCNs (sm-LCNs) for localized delivery of 5-AR inhibitors to hair follicles, addressing the limitations of current therapies. LCNs were prepared using monoolein as the lipid base and Poloxamer 407 as a stabilizer. Surface modification was achieved by coating the nanoparticles with chitosan, enhancing their interaction with the negatively charged skin surface. The formulations were characterized for particle size, zeta potential, and entrapment efficiency. Ex vivo permeation and retention studies were conducted using Franz diffusion cells and porcine/murine skin models. Cytotoxicity was measured on human keratinocyte cell lines (HaCaT). Overall, LCNs’ particle size increased substantially from 244.9±2.1 nm for the unmodified LCNs to 300.0±7.6 nm for chitosan-modified LCNs (sm-LCNs). The sm-LCNs demonstrated a positive zeta potential, which improved their interaction with the skin. Drug retention studies yielded higher values for sm-LCNs than unmodified LCNs and control formulations suggesting retention in deeper skin layers was significantly improved for the modified LCNs. In vitro cytotoxicity studies failed to decrease cell viability below 80% even at 20μg/ml concentration, proving the safety of the formulation. The positive surface charge of sm-LCNs enhances their interaction with the skin, enabling deeper penetration and localized drug delivery. This strategy circumvents systemic absorption and minimizes side effects while maintaining therapeutic efficacy. The surface-modified LCNs hold the potential for localized delivery of 5-AR inhibitors and offer direct intervention in hair restoration therapies. This strategy circumvents the poor penetration of the drug into the skin and systemic absorption that causes iatrogenic effects. Further studies focus on clinical applications in other dermatological conditions [63].
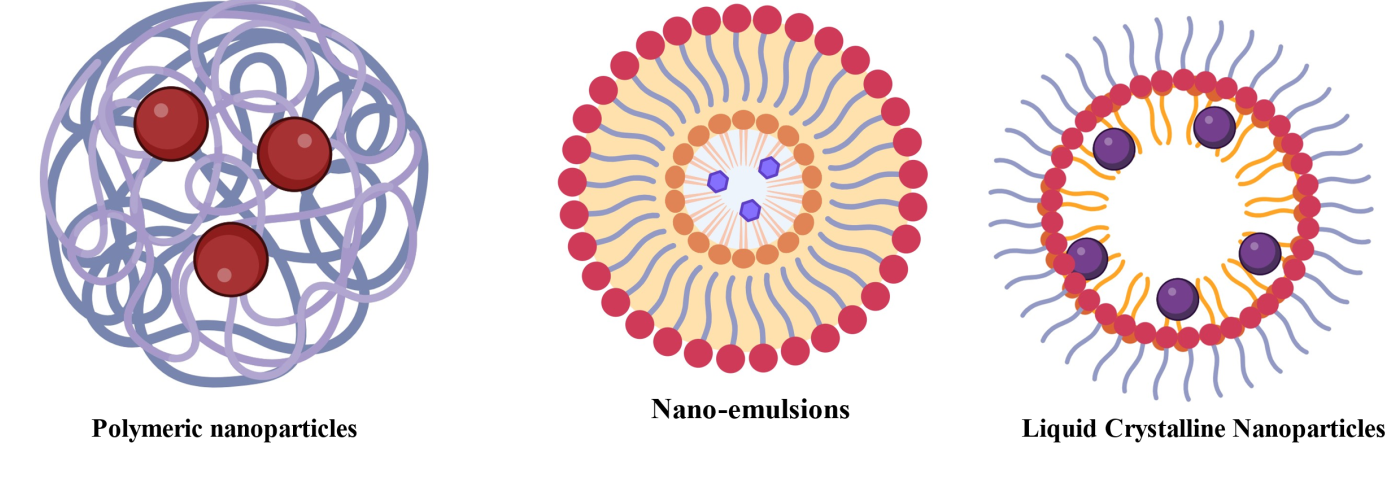
Fig. 11: Liquid crystalline nanoparticles (Created with BioRender.com)
Polymeric nanoparticles
Polymeric Nanoparticles, made of biodegradable and biocompatible polymers, are solid colloidal particles ranging from 1 to 1000 nm in size. They are widely used for controlled drug delivery and targeted therapy, preventing drug degradation and enabling long-term release at specific body sites, thereby improving therapeutic performance.
Spironolactone-loaded polymeric nanoparticles
Polymeric nanoparticles are increasingly recognized for their ability to deliver drugs effectively for skin-related issues like AGA. Encapsulating drugs in safe and environmentally friendly polymers allows these nanoparticles to improve drug stability and effectiveness. They also help reduce side effects throughout the body, making them a great choice for targeted therapy. Topical treatments for AGA, like MXD and FIN, often struggle with getting deep into the hair follicles and can lead to unwanted side effects throughout the body. This shows a clear need for better delivery systems to tackle these issues effectively. Polymeric nanoparticles are crafted to deliver spironolactone directly to hair follicles. This medication, known for blocking potassium channels, is also repurposed to act as an androgen receptor blocker, making it a suitable option for treating AGA. The main goal is to improve the size and makeup of nanoparticles to boost drug delivery into hair follicles. At the same time, we want to make sure they are stable, gentle on the skin, and release the drug in a controlled manner. We created nanoparticles using a method called nanoprecipitation. This process involved polymers like poly-ε-caprolactone (PCL) and a copolymer made from methacrylic acid and methyl methacrylate, known as EL100. We used Dynamic Light Scattering (DLS) and electron microscopy to look at how well the encapsulation worked, the size of the particles, the PDI, and the zeta potential. We carried out stability studies over a period of 90 d, examining how the drug releases in both water-based and oil-based conditions to mimic the environment of hair follicles. Studies conducted in a lab using pig skin showed that the nanoparticles created had sizes ranging from 102.7 nm to 180.0 nm. These nanoparticles were able to effectively trap more than 75% of spironolactone. The bigger nanoparticles, measuring 180 nm, demonstrated a notable enhancement in targeting hair follicles, delivering five times more medication to them than the control group did. The stability tests showed that the formulations kept more than 85% of the drug content even after 90 d. The nanoparticles controlled how the drug was released in water while allowing for a quick release in oil-rich settings, similar to what happens in hair follicles. The findings highlight how crucial particle size is for targeting hair follicles, with larger nanoparticles showing better ability to settle in these areas. This approach focusses on targeting based on size, providing clear benefits compared to traditional methods. It allows for more precise delivery of medication right where it's needed while also minimising exposure to the rest of the body. The nanoparticles are not only biocompatible but also gentle on the skin, making them a great choice for clinical use. Polymeric nanoparticles offer a new way to deliver medications directly to the skin, especially for issues like AGA and acne. This study shows how they can enhance drug targeting and stability, opening doors for better treatments [64].
Quercetin-loaded polymeric nanoparticles
Quercetin, a plant flavonoid with antioxidant, anti-inflammatory, and prostaglandin D2 synthase inhibition properties, is a promising treatment for AGA. However, its low bioavailability and instability in physiological conditions limit its therapeutic potential. Current treatments for AGA, such as FIN and MXD, are often unsatisfactory due to adverse effects like dermatitis and hormonal imbalances. To address these limitations, Phospholipid–Polymer Hybrid Nanoparticles (LPNPs) were developed as a novel delivery platform. These nanoparticles enhance drug bioavailability and enable multiplicity of drug delivery to hair follicles, offering a more effective treatment strategy for AGA. The goal of this study was to create and assess LPNPs for delivering quercetin through hair follicles. The system utilized Dipalmitoyl Phosphatidylcholine (DPPC) and Poly(lactide-co-glycolide) (PLGA) in the nanoparticle matrix, combining the structural advantages of PLGA with the biocompatibility of DPPC to improve follicular targeting. Nanoparticles were prepared using the double emulsification solvent evaporation method and characterized for particle size, zeta potential, drug entrapment efficiency, and in vitro drug release. Penetration studies were conducted using rat skin, and the therapeutic potential was evaluated in testosterone-induced alopecia models. The synthesized nanoparticles had a mean particle size of 339±1.6 nm and a zeta potential of-78±5.5 mV. Tissue culture fluid phospholipid concentration confirmed moderate drug potency, while the use of DPPC improved viability despite the smaller geometric volume (>110 nm). Sustained drug release was achieved, with anomalous transport kinetics observed during the conductance accumulation study, where only 12.79±9.56% of the drug was released after 12 h. Fluorescence microscopy confirmed that the dual-modified nanoparticles exhibited better follicular uptake compared to PLGA-based systems. In vivo, studies in testosterone-induced alopecia models demonstrated improved hair regrowth, with a follicular density of 3.1±0.10 hairs/mm, comparable to the marketed MXD formulation (3.8±0.15 hairs/mm). Histological data revealed a longer anagen phase and better hair follicle health in animals treated with the hybrid nanoparticles. The phospholipid-polymer hybrid nanoparticles proved to be a superior carrier for quercetin, localizing drug action at the hair follicles and minimizing systemic exposure. The amphiphilic nature of DPPC enhanced follicular uptake, while the PLGA matrix enabled gradual drug release. The goal of this study was to create and assess LPNPs for delivering quercetin through hair follicles [65].

Fig. 12: Polymeric nanoparticles (Created with BioRender.com)
Metallic nanoparticles
Metallic nanoparticles (MNPs) such as gold (Au), silver (Ag), copper (Cu), zinc (Zn), titanium (Ti), and palladium (Pd) have emerged as a revolutionary tool in drug delivery due to their unique optical properties, site-specific targeting capabilities, and ability to overcome multidrug resistance [6, 43, 66]. They enhance drug delivery for androgenetic alopecia by improving controlled release, stability, and follicular penetration [40]. MNPs are synthesized using advanced techniques such as ultrasonication, solvent evaporation, and bead milling, which ensure precise size reduction to the nanoscale range [6, 43, 66]. Their surfaces can be functionalized through hydrogen bonding, covalent bonding, or electrostatic interactions, enabling the conjugation of active biomolecules (e.g., peptides, antibodies) for targeted drug delivery and enhanced therapeutic efficacy. One of the key advantages of MNPs in AGA management is their ability to protect therapeutic agents from degradation, ensuring prolonged activity at the target site [6, 43, 66]. Their small size and surface modifications allow for deep penetration into hair follicles, addressing the limitations of conventional topical therapies such as MXD and FIN, which often suffer from inadequate follicular delivery and systemic side effects. Additionally, MNPs have been shown to stimulate critical growth factors such as Vascular Endothelial Growth Factor (VEGF) and Insulin-Like Growth Factor-1 (IGF-1), which play a vital role in prolonging the anagen phase and promoting hair regrowth [6, 43, 66]. For instance, gold nanoparticles (AuNPs) functionalized with MXD demonstrated 5-fold higher follicular retention compared to conventional formulations in ex vivo porcine skin models, highlighting their potential for targeted drug delivery [40]. Characterization studies of MNPs reveal high encapsulation efficiency (~90%), regulated biphasic release profiles (rapid initial release followed by sustained release), and superior targeting capabilities [6, 43, 66]. In vivo studies using testosterone-induced alopecia models have shown that zinc oxide nanoparticles (ZnO NPs) can increase hair follicle density by 40% and extend the anagen phase by 30%, outperforming standard treatments like FIN[6, 43, 66]. These findings underscore the potential of MNPs to revolutionize AGA treatment by addressing the limitations of current therapies. Despite their advantages, systemic toxicity is a significant concern, as incorrect localization or prolonged accumulation of MNPs may lead to oxidative stress and organ toxicity [6, 43, 66]. Additionally, the complex synthesis methods required for MNP production (e.g., ultrasonication) hinder large-scale manufacturing, while limited long-term safety data delay their clinical translation [40]. To address these issues, researchers are exploring strategies such as surface coatings (e.g., PEGylation) to reduce toxicity and hybrid systems that combine MNPs with polymeric or lipid-based carriers for enhanced biocompatibility [6, 43, 66]. A SWOT analysis of MNPs highlights their strengths (e.g., high targeting efficiency, multi-drug delivery) and weaknesses (e.g., potential toxicity, high production cost), as well as the opportunities (e. g., integration with gene therapy, personalized medicine) and threats (e. g., regulatory challenges, patient acceptance of metallic residues) associated with their use [6,43,66]. Future research should prioritize safety optimization, long-term efficacy in clinical trials, and innovative applications like combining MNPs with genetic therapies. Overall, MNPs offer a promising new approach to AGA treatment, but addressing systemic toxicity, scalability, and regulatory issues is crucial for successful clinical use.
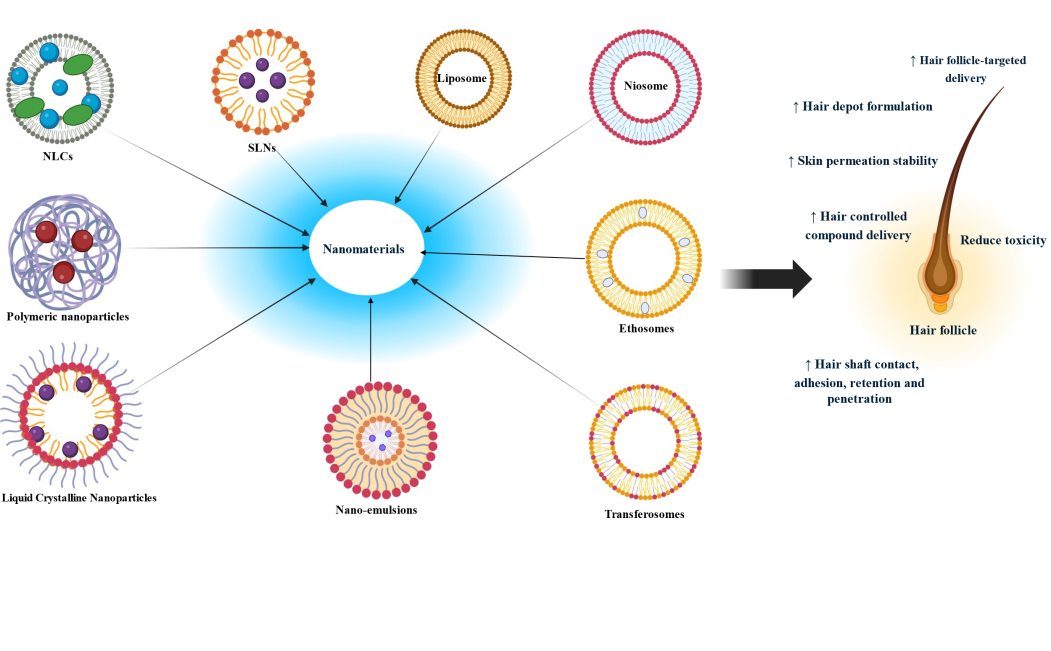
Fig. 13: Different nanomaterials for the treatment of AGA (Created with BioRender.com)
CONCLUSION
AGA continues to be a major issue for millions of people, affecting not just physical appearance but also psychological health and social health. Available treatments, such as FIN, MXD and herbal ingredients, have inherent shortcomings that justify investigation into new ideas. The review emphasizes the prospective function of nanotechnology in improving drug delivery systems in AGA. Through the use of different nanocarriers like liposomes, niosomes, and solid lipid nanoparticles, scientists have the potential to overcome the shortcomings of conventional treatments, such as low bioavailability and systemic toxicity. The innovations in nanocarrier technology not only enhance drug stability and targeted delivery to hair follicles but also present new therapeutic opportunities through the introduction of regenerative agents. Although promising preclinical data, there remains a pressing necessity for strong clinical trials to affirm these data and overcome regulatory obstacles that could interfere with the translational application of these technologies to the clinic. Future studies would need to target optimization of formulation approaches and ensuring scalability for extended clinical use. Moreover, testing combination therapies integrating nanotechnology and novel treatments such as gene therapy could further change the face of AGA treatment. Finally, a patient-focused strategy that emphasizes safety, effectiveness, and accessibility will be critical in the development of AGA treatments.
ACKNOWLEDGMENT
JSS College of Pharmacy, Ooty-643001, The Nilgiris, Tamil Nadu, India.
FUNDING
Nil
LIST OF ABBREVIATIONS
Androgenetic Alopecia (AGA), Dihydrotestosterone (DHT), U. S. Food and Drug Administration (FDA), Finasteride (FIN), Minoxidil (MXD), Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR), Solid Lipid Nanoparticles (SLNs), Nanostructured Lipid Carriers (NLCs), Platelet-Rich Plasma (PRP), Low-Level Laser Therapy (LLLT), 5-Alpha Reductase (5-AR), Polymeric Nanoparticles, Vascular Endothelial Growth Factor (VEGF), Insulin-Like Growth Factor-1 (IGF-1), Liquid Crystalline Nanoparticles (LCNs).
AUTHORS CONTRIBUTIONS
Pritam Kayal: Conceptualization, Investigation, Writing – Original Draft, Visualization, R. Raghul: Resources, Udaya Kiran Sahoo: Resources, Dr. N. Jawahar: Project Administration, Supervision, Validation.
CONFLICT OF INTERESTS
Declared none
REFERENCES
Lolli F, Pallotti F, Rossi A, Fortuna MC, Caro G, Lenzi A. Androgenetic alopecia: a review. Endocrine. 2017 Jul;57(1):9-17. doi: 10.1007/s12020-017-1280-y, PMID 28349362.
Oiwoh SO, Enitan AO, Adegbosin OT, Akinboro AO, Onayemi EO. Androgenetic alopecia: a review. Niger Postgrad Med J. 2024 Apr 1;31(2):85-92. doi: 10.4103/npmj.npmj_47_24, PMID 38826011.
Farouk AT, Elgarhy LH, Abdelsalam SF, Mohamed DA, Rabo FA. Androgenetic alopecia updates: pathophysiology diagnosis and treatment. Int J Dermatol Venereology Leprosy Sci. 2023;6(2):108-21. doi: 10.33545/26649411.2023.v6.i2b.159.
Ntshingila S, Oputu O, Arowolo AT, Khumalo NP. Androgenetic alopecia: an update. JAAD Int. 2023 Jul 22;13:150-8. doi: 10.1016/j.jdin.2023.07.005, PMID 37823040, PMCID PMC10562178.
Nestor MS, Ablon G, Gade A, Han H, Fischer DL. Treatment options for androgenetic alopecia: efficacy side effects compliance financial considerations and ethics. J Cosmet Dermatol. 2021 Dec;20(12):3759-81. doi: 10.1111/jocd.14537, PMID 34741573, PMCID PMC9298335.
Khairnar TD, Chavan GS, Sayyed MM, Gujarathi NA, Aher AA, Agrawal YO. Recent trends in nanocarrier formulations of actives beyond minoxidil and 5-α reductase inhibitors in androgenetic alopecia management: a systematic review. J Drug Deliv Sci Technol. 2024 Jun 20;98:105890. doi: 10.1016/j.jddst.2024.105890.
Sinclair RD. Female pattern hair loss: a pilot study investigating combination therapy with low dose oral minoxidil and spironolactone. Int J Dermatol. 2018 Jan;57(1):104-9. doi: 10.1111/ijd.13838, PMID 29231239.
Sinclair R, Trindade DE Carvalho L, Ferial Ismail F, Meah N. Treatment of male and female pattern hair loss with sublingual minoxidil: a retrospective case series of 64 patients. J Eur Acad Dermatol Venereol. 2020 Dec;34(12):e795-6. doi: 10.1111/jdv.16616, PMID 32386429.
Gupta AK, Talukder M, Shemer A, Piraccini BM, Tosti A. Low dose oral minoxidil for alopecia: a comprehensive review. Skin Appendage Disord. 2023 Dec;9(6):423-37. doi: 10.1159/000531890, PMID 38376087, PMCID PMC10806356.
Abacı N, Erdogan Orhan IE. A narrowed look into plant derived testosterone 5α-reductase inhibitors for androgenetic alopecia. CUPMAP. 2024;7(1):41-55. doi: 10.38093/cupmap.1464497.
Sudduth SL, Koronkowski MJ. Finasteride: the first 5 alpha-reductase inhibitor. Pharmacotherapy. 1993 Jul-Aug;13(4):309-25. PMID 7689728.
Nemane ST, Bhusnure OG, Gholve SB, Mitakari PR, Karwa PN. A review on finasteride: a 5-alpha reductase inhibitors its mechanism facts and benefits. J Drug Deliv Ther. 2019 Jun 30;9(3-s):1132-6. doi: 10.22270/jddt.v9i3-s.3013.
Islam SM. Safety and efficacy of dutasteride for male androgenetic alopecia (Doctoral dissertation BRAC University). Institutional Repository; 2022. Available from: http://hdl.handle.net/10361/23210.
Olsen EA, Hordinsky M, Whiting D, Stough D, Hobbs S, Ellis ML. The importance of dual 5alpha-reductase inhibition in the treatment of male pattern hair loss: results of a randomized placebo controlled study of dutasteride versus finasteride. J Am Acad Dermatol. 2006 Dec;55(6):1014-23. doi: 10.1016/j.jaad.2006.05.007, PMID 17110217.
Sawaya ME, Price VH. Different levels of 5alpha reductase type I and II, aromatase and androgen receptor in hair follicles of women and men with androgenetic alopecia. J Invest Dermatol. 1997 Sep;109(3):296-300. doi: 10.1111/1523-1747.ep12335779, PMID 9284093.
Mao Y, Liu P, Wei J, Xie Y, Zheng Q, LI R. Cell therapy for androgenetic alopecia: elixir or trick? Stem Cell Rev Rep. 2023 Aug;19(6):1785-99. doi: 10.1007/s12015-023-10532-2, PMID 37277541, PMCID PMC10390634.
Sawaya ME, Somani N. Antiandrogens and androgen inhibitors. Comprehensive Dermatologic Drug Therapy. 2013 Jan 1;361-74. doi: 10.1016/B978-1-4377-2003-7.00030-3.
Iamsumang W, Leerunyakul K, Suchonwanit P. Finasteride and its potential for the treatment of female pattern hair loss: evidence to date. Drug Des Dev Ther. 2020 Mar 2;14:951-9. doi: 10.2147/DDDT.S240615, PMID 32184564, PMCID PMC7060023.
Devjani S, Ezemma O, Kelley KJ, Stratton E, Senna M. Androgenetic alopecia: therapy update. Drugs. 2023 Jun;83(8):701-15. doi: 10.1007/s40265-023-01880-x, PMID 37166619, PMCID PMC10173235.
Shamma RN, Aburahma MH. Follicular delivery of spironolactone via nanostructured lipid carriers for management of alopecia. Int J Nanomedicine. 2014 Nov 26;9:5449-60. doi: 10.2147/IJN.S73010, PMID 25473283, PMCID PMC4251754.
Patel B, Velasco MA, Gutierrez FT, Khesin D. Addressing androgenetic alopecia a complex disorder with a multilateral treatment strategy. MOJ Bioequiv Availab. 2017;3(1):00025.
Zeppieri M, Gagliano C, Spadea L, Salati C, Chukwuyem EC, Enaholo ES. From eye care to hair growth: bimatoprost. Pharmaceuticals (Basel). 2024 Apr 27;17(5):561. doi: 10.3390/ph17050561, PMID 38794131, PMCID PMC11124470.
Razi Khosroshahi MA, Sobhani SO, Yousefi KM, Harooni GH, Mashayekhi F, Balasi J. Latanoprost in treatment of alopecia areata and androgenic alopecia: a comprehensive review. Pak J Med Health Sci. 2021;17(4):1535.
Blume Peytavi U, Lonnfors S, Hillmann K, Garcia Bartels N. A randomized double blind placebo controlled pilot study to assess the efficacy of a 24-week topical treatment by latanoprost 0.1% on hair growth and pigmentation in healthy volunteers with androgenetic alopecia. J Am Acad Dermatol. 2012 May;66(5):794-800. doi: 10.1016/j.jaad.2011.05.026, PMID 21875758.
Anjum MA, Zulfiqar S, Chaudhary AA, Rehman IU, Bullock AJ, Yar M. Corrigendum: stimulation of hair regrowth in an animal model of androgenic alopecia using 2-deoxy-D-ribose. Front Pharmacol Front Pharmacol. 2024;15:1499205. doi: 10.3389/fphar.2024.1499205, PMID 39525630.
Singh SM, Gauthier S, Labrie F. Androgen receptor antagonists (antiandrogens): structure activity relationships. Curr Med Chem. 2000 Feb;7(2):211-47. doi: 10.2174/0929867003375371, PMID 10637363.
Singh SM, Gauthier S, Labrie F. Androgen receptor antagonists (antiandrogens): structure activity relationships. Curr Med Chem. 2000 Feb 1;7(2):211-47. doi: 10.2174/0929867003375371, PMID 10637363.
Zhu P, Deng W. Techno derma medicines phase 2 clinical trial of TDM-105795 demonstrates hair growth in androgenetic alopecia. Prnewswire; 2022. Available from: https://www.prnewswire. [Last accessed on 07 Feb 2024].
Gentile P, Cole JP, Cole MA, Garcovich S, Bielli A, Scioli MG. Evaluation of not activated and activated PRP in hair loss treatment: role of growth factor and cytokine concentrations obtained by different collection systems. Int J Mol Sci. 2017 Feb 14;18(2):408. doi: 10.3390/ijms18020408, PMID 28216604, PMCID PMC5343942.
Carvajal MS. Platelet rich plasma and its use in androgenetic alopecia and alopecia areata: a systematic review. Dermatol Surg. 2020 Jan;46(1):93-102. doi: 10.1097/DSS.0000000000001965.
Rahaman KA, Muresan AR, Min H, Son J, Han HS, Kang MJ. Simultaneous quantification of TB-500 and its metabolites in in vitro experiments and rats by UHPLC-Q-Exactive orbitrap MS/MS and their screening by wound healing activities in vitro. J Chromatogr B Analyt Technol Biomed Life Sci. 2024 Mar 1;1235:124033. doi: 10.1016/j.jchromb.2024.124033, PMID 38382158.
Gwyer D, Wragg NM, Wilson SL. Gastric pentadecapeptide body protection compound BPC 157 and its role in accelerating musculoskeletal soft tissue healing. Cell Tissue Res. 2019 Aug;377(2):153-9. doi: 10.1007/s00441-019-03016-8, PMID 30915550.
Gwyer D, Wragg NM, Wilson SL. Gastric pentadecapeptide body protection compound BPC 157 and its role in accelerating musculoskeletal soft tissue healing. Cell Tissue Res. 2019 Aug 14;377(2):153-9. doi: 10.1007/s00441-019-03016-8, PMID 30915550.
Pickart L, Vasquez Soltero JM, Margolina A. GHK and DNA: resetting the human genome to health. Bio Med Res Int. 2014;2014:151479. doi: 10.1155/2014/151479, PMID 25302294, PMCID PMC4180391.
Arul V, Kartha R, Jayakumar R. A therapeutic approach for diabetic wound healing using biotinylated GHK incorporated collagen matrices. Life Sci. 2007 Jan 2;80(4):275-84. doi: 10.1016/j.lfs.2006.09.018, PMID 17049946.
Kapoor R, Shome D, Vadera S, Kumar V, Ram MS. QR678 & QR678 neo hair growth formulations: a cellular toxicity & animal efficacy study. Plast Reconstr Surg Glob Open. 2020 Aug 25;8(8):e2843. doi: 10.1097/GOX.0000000000002843, PMID 32983753, PMCID PMC7489598.
Amplificas AMP-303 study unveils new hope for hair loss treatments; 2024.
Chen L, Zhang J, Wang L, Wang H, Chen B. The efficacy and safety of finasteride combined with topical minoxidil for androgenetic alopecia: a systematic review and meta-analysis. Aesthetic Plast Surg. 2020 Jun;44(3):962-70. doi: 10.1007/s00266-020-01621-5, PMID 32166351.
Yip L, Rufaut N, Sinclair R. Role of genetics and sex steroid hormones in male androgenetic alopecia and female pattern hair loss: an update of what we now know. Australas J Dermatol. 2011 May;52(2):81-8. doi: 10.1111/j.1440-0960.2011.00745.x, PMID 21605090.
Anjali PB, Jawahar N, Praharsh Kumar MR, Jubie S, Selvamuthukumar S. Nanocarriers in the treatment of epilepsy: challenges and opportunities. J Drug Deliv Sci Technol. 2024 May 15;97:105788. doi: 10.1016/j.jddst.2024.105788.
Ramkar S, Kaurav M, Sudheesh MS, Pandey RS. Enhanced skin penetration of finasteride loaded DMSO liposomes for the treatment of androgenic alopecia: comparison with conventional liposomes. Drug Dev Ind Pharm. 2023 Jan;49(1):52-61. doi: 10.1080/03639045.2023.2182122, PMID 36803490.
Xing H, Peng H, Yang Y, LV K, Zhou S, Pan X. Nitric oxide synergizes minoxidil delivered by transdermal hyaluronic acid liposomes for multimodal androgenetic alopecia therapy. Bioact Mater. 2024;32:190-205. doi: 10.1016/j.bioactmat.2023.09.021, PMID 37859688.
Singh K, Nanda R, Narang JK. Recent advances in topical nanotechnological strategies for treatment of alopecia. J Pharm Res Int. 2021 Aug 4;33(39B):326-51.
Mali N, Darandale S, Vavia P. Niosomes as a vesicular carrier for topical administration of minoxidil: formulation and in vitro assessment. Drug Deliv Transl Res. 2013 Dec;3(6):587-92. doi: 10.1007/s13346-012-0083-1, PMID 25786376.
Liu X, Guo F, Liang D, LI Z, Cao Y, Chen M. Development and evaluation of finasteride niosomes targeting to hair follicles for the management of androgenic alopecia. J Drug Deliv Sci Technol. 2023 Sep 1;86:104725. doi: 10.1016/j.jddst.2023.104725.
Ahmed OA, Rizq WY. Finasteride nano transferosomal gel formula for management of androgenetic alopecia: ex vivo investigational approach. Drug Des Dev Ther. 2018 Jul 23;12:2259-65. doi: 10.2147/DDDT.S171888, PMID 30104862, PMCID PMC6070339.
Ramezani V, Honarvar M, Seyedabadi M, Karimollah A, Ranjbar AM, Hashemi M. Formulation and optimization of transferosomes containing minoxidil and caffeine. J Drug Deliv Sci Technol. 2018 Apr;44:129-35.
Makhlouf A, Elnawawy T. Hair regrowth boosting via minoxidil cubosomes: formulation development in vivo hair regrowth evaluation histopathological examination and confocal laser microscopy imaging. Int J Pharm. 2023 Mar 5;634:122665. doi: 10.1016/j.ijpharm.2023.122665, PMID 36736676.
Sankar V, Wilson V, Siram K, Karuppaiah A, Hariharan S, Justin A. Topical delivery of drugs using ethosomes: a review. Indian Drugs. 2019 Sep;56(8):7-20. doi: 10.53879/id.56.08.11504.
Yan A, Ruan R, Zhu X, Qiang W, Guan Y, YU Q. Co-delivery of minoxidil and tocopherol acetate ethosomes to reshape the hair follicular microenvironment and promote hair regeneration in androgenetic alopecia. Int J Pharm. 2023 Nov 5;646:123498. doi: 10.1016/j.ijpharm.2023.123498, PMID 37820942.
Wilson V, Siram K, Rajendran S, Sankar V. Development and evaluation of finasteride loaded ethosomes for targeting to the pilosebaceous unit. Artif Cells Nanomed Biotechnol. 2018 Dec;46(8):1892-901. doi: 10.1080/21691401.2017.1396221, PMID 29087225.
Hamishehkar H, Ghanbarzadeh S, Sepehran S, Javadzadeh Y, Adib ZM, Kouhsoltani M. Histological assessment of follicular delivery of flutamide by solid lipid nanoparticles: potential tool for the treatment of androgenic alopecia. Drug Dev Ind Pharm. 2016;42(6):846-53. doi: 10.3109/03639045.2015.1062896, PMID 26154267.
Ananth P, Koland M. Topical delivery of fenugreek seed extract loaded solid lipid nanoparticles based hydrogels for alopecia. J Pharm Res Int. 2021 Aug 6;33(40A):231-41. doi: 10.9734/jpri/2021/v33i40A32239.
Daneshmand S, Niazi M, Fazeli Nasab B, Asili J, Golmohammadzadeh S, Sayyed RZ. Solid lipid nanoparticles of Platycladus orientalis l. possessing 5-alpha reductase inhibiting activity for treating hair loss and hirsutism. J Med Plants By-Products. 2023 Mar 1;13(1):233-46. doi: 10.22034/JMPB.2023.364389.1634.
Ramkar S, Suresh PK. Finasteride loaded nano lipidic carriers for follicular drug delivery: preformulation screening and box behnken experimental design for optimization of variables. Heliyon. 2022 Aug 15;8(8):e10175. doi: 10.1016/j.heliyon.2022.e10175, PMID 36042733, PMCID PMC9420366.
Hariyanti H, Rachmat Mauludin RM, Yeyet Cahyati Sumirtapura YC, Neng Fisheri Kurniati NF. Activity and safety of cinchonine nanostructured lipid carriers as a hair growth stimulant in mice model of androgenetic alopecia. Sains Malays. 2023;52(6):1671-83. doi: 10.17576/jsm-2023-5206-05.
Riangjanapatee P, Khongkow M, Treetong A, Unger O, Phungbun C, Jaemsai S. Development of tea seed oil nanostructured lipid carriers and in vitro studies on their applications in inducing human hair growth. Pharmaceutics. 2022 May 4;14(5):984. doi: 10.3390/pharmaceutics14050984, PMID 35631570, PMCID PMC9143331.
Makky AM, El leithy ES, Hussein DG, Khattab A. Skin targeting of an optimized caffeine nanostructured lipid carrier with improved efficiency against chemotherapy-induced alopecia. Int J App Pharm. 2022;14(4):235-50. doi: 10.22159/ijap.2022v14i4.44681.
Badria FA, Fayed HA, Ibraheem AK, State AF, Mazyed EA. Formulation of sodium valproate nanospanlastics as a promising approach for drug repurposing in the treatment of androgenic alopecia. Pharmaceutics. 2020 Sep 11;12(9):866. doi: 10.3390/pharmaceutics12090866, PMID 32933001, PMCID PMC7559423.
Honary S, Ebrahimi P, Nikbakht M. Optimization of finasteride nanoemulsion preparation using chemometric approach. Trop J Pharm Res. 2013 Aug 28;12(4):457-61. doi: 10.4314/tjpr.v12i4.2.
Sharma A, Mohapatra H, Arora K, Babbar R, Arora R, Arora P. Bioactive compound loaded nanocarriers for hair growth promotion: current status and future perspectives. Plants (Basel). 2023 Oct 31;12(21):3739. doi: 10.3390/plants12213739, PMID 37960095, PMCID PMC10649697.
Alam M, Mishra A, Yadav KS, Pradhan D, Kar B, Ghosh G. Development and evaluation of dutasteride nanoemulgel for the topical delivery against androgenic alopecia. Pharm Nanotechnol. 2024;12(5):459-70. doi: 10.2174/0122117385269151231031161411, PMID 38173065.
Madheswaran T, Baskaran R, Yoo BK, Kesharwani P. In vitro and in vivo skin distribution of 5α-reductase inhibitors loaded into liquid crystalline nanoparticles. J Pharm Sci. 2017 Nov;106(11):3385-94. doi: 10.1016/j.xphs.2017.06.016, PMID 28652158.
Ferreira Nunes R, Cunha Filho M, Gratieri T, Gelfuso GM. Follicular targeted delivery of spironolactone provided by polymeric nanoparticles. Colloids Surf B Biointerfaces. 2021 Dec;208:112101. doi: 10.1016/j.colsurfb.2021.112101, PMID 34517218.
Das L, Kaurav M, Pandey RS. Phospholipid polymer hybrid nanoparticle mediated trans follicular delivery of quercetin: prospective implement for the treatment of androgenic alopecia. Drug Dev Ind Pharm. 2019 Oct;45(10):1654-63. doi: 10.1080/03639045.2019.1652635, PMID 31382790.
Santos AC, Pereira Silva M, Guerra C, Costa D, Peixoto D, Pereira I. Topical minoxidil loaded nanotechnology strategies for alopecia. Cosmetics. 2020 Mar 27;7(2):21. doi: 10.3390/cosmetics7020021.