
Int J App Pharm, Vol 17, Issue 3, 2025, 28-54Review Article
AN OVERVIEW OF COPD: ITS ADVANCED THERAPEUTIC MANAGEMENT AND CHALLENGES FOR DRUG RELEASE AT THE TARGETED SITE
SUPRAJA A.a , GUNDAWAR RAVIa
, GUNDAWAR RAVIa , TANVI PAINGINKARa
, TANVI PAINGINKARa , GIRISH PAI K.b
, GIRISH PAI K.b , VIRENDRA S. LIGADEc
, VIRENDRA S. LIGADEc , K. SREEDHARA RANGANATH PAId
, K. SREEDHARA RANGANATH PAId , VASANTHARAJU SGa
, VASANTHARAJU SGa , MUDDUKRISHNA BSa*
, MUDDUKRISHNA BSa*
aDepartment of Pharmaceutical Quality Assurance, Manipal College of Pharmaceutical Sciences, Manipal Academy of Higher Education, Manipal-576104, Karnataka, India. bDepartment of Pharmaceutics, Manipal College of Pharmaceutical Sciences, Manipal Academy of Higher Education, Manipal-576104, Karnataka, India. cDepartment of Pharmaceutical Management and Regulatory Affairs, Manipal College of Pharmaceutical Sciences, Manipal Academy of Higher Education, Manipal-576104, Karnataka, India. dDepartment of Pharmacology, Manipal College of Pharmaceutical Sciences, Manipal Academy of Higher Education, Manipal, 576104-Karnataka, India
*Corresponding author: Muddukrishna BS; *Email: krishna.mbs@manipal.edu
Received: 04 Oct 2024, Revised and Accepted: 28 Mar 2025
ABSTRACT
Chronic Obstructive Pulmonary Disorder (COPD) is a diverse lung ailment characterized by persistent respiratory symptoms such as coughing, dyspnea, sputum formation, and worsening that causes increased airflow limitation. Globally, COPD ranks third in 2019 and is responsible for 3.23 million deaths. It is a health problem that is brought on by inflammation in the lungs. The respiratory system comprises the trachea, bronchi, larynx, paranasal sinuses, and two lungs. COPD and other Respiratory Disorders (RDs) have dominated research. This review emphasizes the potential of novel treatment strategies for COPD, highlighting advanced therapeutic approaches. It addresses the ongoing challenges associated with the effective delivery of pharmaceuticals to targeted sites while aiming to achieve optimal therapeutic outcomes with reduced dosages. The Global Initiative for Chronic Obstructive Lung Disease (GOLD) Report 2024 categorizes COPD treatments into pharmacological and non-pharmacological interventions. Pharmacological therapies aim to alleviate symptoms, enhance exercise tolerance, reduce exacerbations, and improve health outcomes. These include antibiotics, Long-Acting Beta-Agonist (LABA), Short-Acting Beta-Agonist (SABA), Long-Acting Muscarinic-Antagonists (LAMA), Short-Acting Muscarinic-Antagonists (SAMA), Inhaled Corticosteroids (ICS), and bronchodilators in various formulations. The report also reviews inhaler types and highlights the role of nanoscale drug delivery systems in detecting drug particle deposition in the respiratory tract, along with the characterization and classification of Nanoparticles (NPs) in nanomedicine. In conclusion, contemporary nanoparticles enhance biodistribution, optimize pharmacokinetics, and promote physiological stimulation. They also reduce toxicity and increase the therapeutic index, thereby facilitating the transformation of medication administration for chronic respiratory disorders.
Keywords: COPD, GOLD, Particle size, Nanoparticles, Inhaled corticosteroids, DPIs, Advanced therapies
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijap.2025v17i3.53675 Journal homepage: https://innovareacademics.in/journals/index.php/ijap
INTRODUCTION
Numerous studies have examined Chronic Obstructive Pulmonary Disorder (COPD) and Respiratory Disorders (RDs). The respiratory system includes the lungs, paranasal sinuses, trachea, bronchi, throat, and larynx. (RDs) impair breathing and affect the lungs and other components. These can range from life-threatening conditions such as pneumonia, pulmonary embolism, tuberculosis, COPD, and lung cancer to milder infections like the flu. RDs can be classified based on their etiology, affected tissues, and symptom patterns [1–3]. Lung disease symptoms are categorized into three types: obstructive (asthma, COPD, bronchitis), tissue-related (fibrosis, pneumonia, COPD, lung cancer), and circulatory issues (pulmonary hypertension, embolism, edema) [4–9]. COPD is a lung disorder marked by persistent cough, shortness of breath, and sputum production. It is caused by chronic bronchitis and emphysema due to alveoli damage, and irritating particles can affect lung flexibility and airflow [10, 11]. COPD is the sixth most common chronic illness globally, causing 90% of adult deaths in low-and middle-income countries. Tobacco smoking is the leading cause, accounting for 70% of cases in high-income and 30% to 40% in low-and middle-income countries [9]. Indoor exhaust affects 25% of smokers, with air pollution and workplace exposure as additional risks. Inhaled toxins cause lung inflammation and tissue damage in COPD patients, worsening the condition even after quitting smoking and disrupting the balance of lung defenses [10–13]. Emphysema and airway diseases cause gas trapping and blockage, leading to COPD [14–16]. Hyperinflation involves increased lung gas volume, causing dyspnea, reduced exercise tolerance, and hospitalizations [17–19]. Abnormalities related to pulmonary gas exchange, such as smooth muscle hypertrophy and intimal hyperplasia, may also manifest [20–23]. COPD can get worse due to respiratory symptom exacerbations, such as worsening dyspnea and declining VA/Q abnormalities [24–26]. Additionally, survival and health may be impacted by multimorbidity [26, 27]. Mast cells induce inflammation and bronchoconstriction by releasing histamine and arachidonic acid metabolites. Cysteinyl leukotrienes increase intracellular calcium via the cysLT1 receptor. In asthma, adenosine reduces cyclic AMP, causing smooth muscle contraction. Airway regulation involves parasympathetic (muscarinic M3 receptor) and sympathetic (β2-adrenergic receptor) fibers [28–36]. The diagnosis of COPD is made using imaging, physiological tests, and spirometry [37]. Pulmonary function tests are primarily conducted using spirometry. This method assesses the air volume inhaled and exhaled, producing pneumotachographs illustrating air movement during breathing [38, 39]. In 2019, COPD was responsible for 3.23 million deaths, ranking as the third leading cause of death globally, according to the WHO [40]. Each year, the Chronic Obstructive Lung Disease (GOLD) publishes a comprehensive report on the management options for COPD, a valuable resource for healthcare professionals. In addition, numerous global and national guidelines have been released, which focus on the specific characteristics of regional healthcare systems [41–50].
Moving on to the briefly discussed developing innovative treatment options, the objectives of pharmacological therapy for COPD are to improve general health, reduce the intensity and frequency of exacerbations, boost exercise tolerance, and lessen symptoms. In individuals with stable COPD who are unwell, influenza vaccines are necessary. Subgroups comprise the maintenance medications for A. bronchodilators: i. β2-Adrenergic Agonists, ii. Muscarinic Antagonists, B. Anti-inflammatory drugs: a. Corticosteroids-i. Inhaled Corticosteroids (ICS), ii. Oral Corticosteroids, iii. Phosphodiesterase Inhibitors and antibiotics with other appropriate dosage formulations, such as single-drug therapy and combination-drug therapy, triple-drug formulations [38, 51, 52]. Routine treatment must be changed in response to acute exacerbations, intermittent bouts of decreasing respiratory function and increasing symptoms associated with COPD [38, 39, 53–57]. An outline of the many types of COPD inhalers available. Several novel and contemporary inhalers [53, 58] were used for DPIs of medication particle deposition in respiratory tract sections [59–61]. The Nanoscale systems with their significance and barriers for delivering drugs to the aimed locations [62]. Information on nanomedicine, including studies on the characterization and classification of various Nanoparticles (NPs) [63, 64].
RDs
The throat, larynx, trachea, bronchi, nasal cavity, paranasal sinuses, and two lungs-bronchioles and alveoli compose the respiratory system. Pathological disorders that harm the lungs and other respiratory system components and make breathing difficult are known as RDs. These include diseases of the trachea, bronchi, bronchioles, alveoli, pleurae, pleural cavity, and respiratory muscles and nerves. From harmless and self-limiting illnesses like the flu, influenza, and pharyngitis to potentially lethal disorders like pneumonia due to bacteria, pulmonary embolism, tuberculosis, COPD, lung cancer, and other severe acute respiratory syndromes, there are many different types of respiratory maladies. RD can be categorized based on the etiology of the diseases, parts or tissues affected, and pattern of symptoms [1–3].
Based on the etiology of diseases
Obstructive lung diseases are caused by excessive contraction of smooth muscles, leading to constriction of bronchi and bronchioles. The main characteristics involved are inflamed and readily collapsible airways, airflow blockage, difficulty exhaling, and frequent hospitalizations. Bronchitis, asthma, and COPD diseases are classic examples of this category. Lung tissue diseases can be characterized by damage to the pleura, a thin tissue layer of the lungs, enabling lungs to expand to regular size, leading to impaired breathing. Pulmonary Fibrosis (scarring of lung tissues), pneumonia (inflamed air sacs), COPD (emphysema), and lung cancer (uncontrolled growth of lung tissues) are examples of lung tissue diseases (fig. 1). Lung circulation diseases occur when the lungs' blood vessels are coagulated, damaged, or swollen, causing an imbalance in the exchange of oxygen and carbon dioxide. Pulmonary hypertension, Pulmonary embolism, and Pulmonary edema all belong to this category [65, 66].
Based on the symptoms
Each lung disease category will have characteristic symptoms. Wheezing, a wet cough, and trouble breathing are symptoms of obstructive lung diseases (bronchitis, COPD, and asthma); symptoms of lung tissue diseases (fibrosis, pneumonia, COPD, lung cancer) include dyspnea with a dry cough; exhaustion, shortness of breath, and chest discomfort are signs of lung circulatory disorders (pulmonary-hypertension, embolism, edema) [4–9].

Fig. 1: Classification of RDs based on the etiology of diseases (BioRender.com)
COPD
Persistent respiratory symptoms, including coughing, dyspnea, sputum production, and exacerbation leading to increasing airflow restriction, are the hallmarks of the heterogeneous lung illness called COPD. It is characterized by damaged alveoli of the lungs, a condition known as emphysema, and/or inflammation of the bronchi/bronchioles termed chronic bronchitis (fig. 2) [10, 11]. With the start of an inflammatory response imposed on by irritant particles in the respiratory tract, COPD is defined by the onset of irreversible inflammation and harm to the air sacs in the lungs, resulting in constriction of the airways.
The lungs become more flexible because of these physiological changes, holding onto more air and obstructing more airflow.
Signs and symptoms
A few of the commonly recognized symptoms include breathing difficulties, particularly when moving, wheezing, chest tightness, and a persistent cough that may produce clear, white, yellow, or greenish mucous or sputum; recurrent respiratory infections; deliberate weight loss (later stages); and swelling in the ankles, legs, or feet.
Shortness of breath-The most defining feature of COPD is its persistent, increasing dyspnoea. Breathlessness symptoms such as wheezing and tightness in the chest might fluctuate during the day or between days and are not always present. Often, chest discomfort occurs after exertion. Low levels of physical exercise are linked to inferior outcomes, and shortness of breath is frequently the cause of limited physical activity [54, 67].
Cough-The initial symptom of COPD is a persistent cough, which could or might not produce phlegm. Diagnosing phlegm coughed up as sputum can be challenging since it might be sporadic and, depending on social or cultural variables, swallowed or spit out. Only up to 30% of patients, meanwhile, also have a productive cough. In some instances, restricted breathing may occur without a cough. When a persistent cough lasts for more than three months and each year for a minimum of two years, it is classified as chronic bronchitis. A persistent productive cough is caused by mucus hypersecretion. Vigorous coughing in those with severe COPD might break ribs or cause momentary unconsciousness [54].
Exacerbations-An abrupt worsening of signs and symptoms that last for a few days is called an acute exacerbation. A frequently observed indicator is air trapping, which makes it difficult to exhale completely. According to research, a pulmonary embolism may occasionally cause these occurrences. Heart failure and pleuritic chest discomfort without symptoms of infection are possible indicators [54].

Fig. 2: Characteristics of COPD include emphysema (destruction of the tiny air sacs-alveoli) and chronic bronchitis (inflammation of bronchi tubes with excess mucous). These are long-term lung conditions causing COPD (BioRender.com)
Causes
About 90% of adult COPD deaths under those over the age-70 occur in nations with low or middle incomes. COPD is the sixth most frequent cause of chronic disease worldwide, as measured by disability-adjusted life years. Tobacco smoking is the primary cause of almost 70% of instances of COPD in high-income nations. Tobacco use accounts for thirty to forty percent of cases in low-and middle-income countries, where indoor exhaust is a significant risk factor for COPD [9]. The leading cause of COPD, which affects up to 25% of cigarette users, is smoking. In addition, occupational contact with dust and fumes, as well as air pollution, can induce COPD [10].
Common risk factors
Smoking-Globally, tobacco use is the primary risk factor for COPD, and research has shown that smokers have an increased risk of the illness in ex-smokers [54, 68]. The main structural element of alveoli-elastin, is broken down by the overproduction of proteases in the lungs due to smoke inhalation. Smoke also affects cilia, preventing mucociliary clearance, which rids the airways of mucus, cell debris, and extra fluid [68]. As per the research, women are more vulnerable than men to the negative consequences of tobacco smoking [69]. Out of 8 million tobacco-related fatalities that occur globally each year, 1.2 million are caused by second-hand smoke, also known as passive smoking [70]. There is also a risk from other forms of tobacco smoke, such as those from pipes, cigars, water pipes, and hookahs [54]. Smoke from water pipes or hookahs seems to be just as toxic as cigarette smoke [71].
Additional lung irritants-Indoor air pollution from poorly ventilated fires is utilized for heating and heating, particularly in developing nations, where one of the leading causes of COPD involves cooking. Coal and biomass, like wood and dried manure, are commonly used to fuel these fires [54]. Individuals diagnosed with COPD are more vulnerable to the harmful consequences of particulate matter exposure, which may result in abrupt exacerbations of infections. Excessive hospitalization risk is linked to black carbon, also called soot, an atmospheric contaminant that causes exacerbations. An elevated mortality rate in COPD is related to prolonged exposure [72]. COPD rates are often more significant in areas with poor outdoor air quality, especially exhaust gas pollution [73].
Age-Most people with COPD are 40 years of age or older when their symptoms first manifest.
Respiratory infections-Two conditions that may make you more susceptible to infection include AIDS and tuberculosis.
Changes in lung development and expansion in the developing fetus-A child’s risk may be increased by diseases that damage the lungs during pregnancy or infancy and alter lung growth and development.
Occupational exposure-The risk of developing COPD due to intense and extended exposure to industrial dust, chemicals, and fumes. Dust from grains and wheat, silica, cadmium, and welding fumes aggravating respiratory symptoms are among the substances identified in the United Kingdom and linked to occupational exposure [54, 74]. In the United States, workplace exposure is thought to be related to around 30% of cases among those who have never smoked. It is believed to be the cause in 10–20% of cases and likely poses a higher risk in nations with insufficient laws [54, 75].
Rare risk factor
Genetics-About 1-5 % of COPD is influenced by genetics [76, 77]. AAT deficiency is a genetic condition that can lead to COPD and lung damage when it runs throughout the family. Smoking or prolonged exposure to dust or fumes can further exacerbate the condition. CHRNA gene mutations and vitamin D insufficiency are possible genetic risk factors [78–80].
The COPD Gene research is a long-term investigation of the epidemiology of the disease that identifies phenotypes and searches for potential gene associations with susceptibility to COPD. As of 2019, whole genome sequencing and the National Heart, Lungs, and Blood Institute collaborate to identify rare genetic determinants [81].
Pathology
With the start of an inflammatory response imposed on by dangerous gases and particles in the respiratory tract, COPD is defined by the onset of irreversible inflammation and harm in the air sacs in the lung while the airways constrict.
These changes in physiological function lead to increased lung compliance, air retention, and airflow obstruction-all of which are signs of COPD. Furthermore, they make it harder for air to pass via the tiny conducting airways [11, 12].
Pathogenesis
A frequent reaction to the inhaled toxins in COPD patients is lung inflammation, particularly in the small airways. This inflammation causes tissue death and impairs defense and healing mechanisms. Even after stopping smoking, these alterations can continue as the condition worsens. Furthermore, there is an imbalance in the lung's antiproteases, oxidants, antioxidants, and proteases [10–13].
Inflammatory cells-COPD is distinguished by increased lung inflammation, caused mainly by airflow restriction, as well as increased neutrophils, macrophages, and T-lymphocytes, all of which produce numerous cytokines and mediators, as opposed to asthma patients, who have a similar pattern [10–13].
Inflammatory mediators-Leukotriene B4, a chemoattractant for draws T cells and neutrophils, is produced by a rise in inflammatory mediators, including macrophages, neutrophils, and epithelial cells. Growth-related oncogene α and its CXC chemokines interleukin Eight and interleukin Eight are chemotactic factors produced by macrophages with epithelial cells. These stimulate pro-inflammatory responses and attract blood cells. Pro-inflammatory cytokines, such as tumour necrosis factor α and interleukins 1β and 6. A cytokine called connective tissue growth factor is released by growth factors such as transforming growth factor β, which can cause fibrosis in the airways either directly or indirectly [10–13].

Fig. 3: Diagram representing the various risk factors involved in developing COPD. Common factors are smoking, additional lung irritants, age, respiratory infections, fetus respiratory system status, and occupational exposure to dust, smoke, and chemicals. Rare factors include genetic factors like AAT deficiency (BioRender.com)
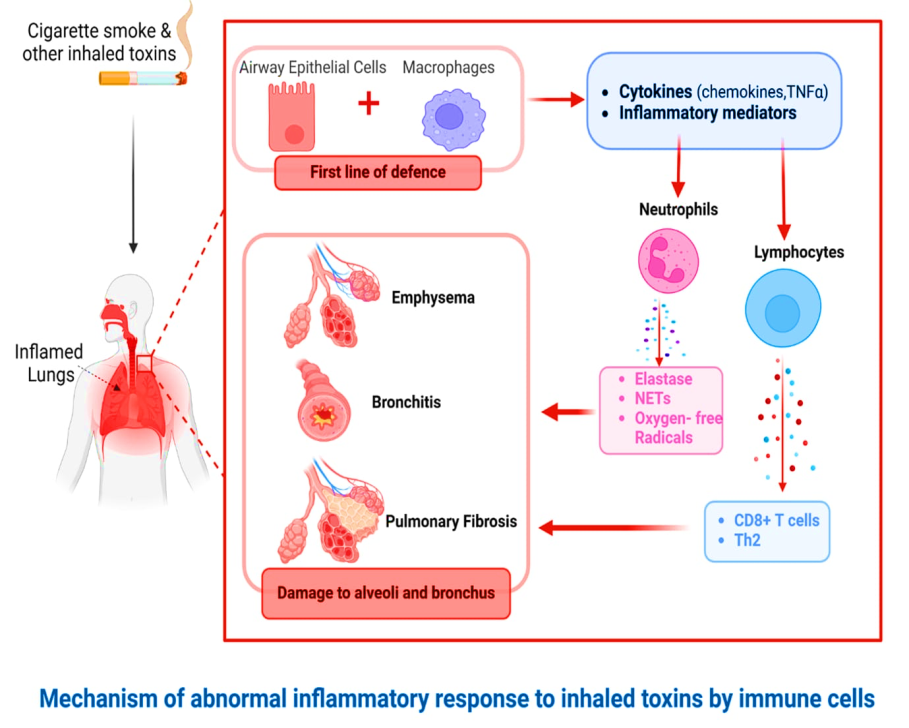
Fig. 4: Pathogenesis of Inflammation in COPD (BioRender.com)
Protease and antiprotease imbalance are caused by increased protease synthesis (or activity) and decreased antiprotease production (or inactivation). Oxidative stress is brought on by both inflammation and cigarette smoke, and it inactivates certain antiproteases and primes many inflammatory cells to produce a combination of proteases. Several metalloproteases found in the matrix MMP-8, MMP-9, and MMP-12 and proteases produced by neutrophils (elastase, cathepsin G, in addition protease 3) as well as macrophages consisting of the cysteine proteases and cathepsins E, A, L, and S are the principal proteases implicated. The main antiproteases linked to the pathogenesis of emphysema include tissue inhibitors of metalloproteases, secretory leucoprotease inhibitors, and α1 antitrypsin [10–13].
Oxidative stress-With COPD, the oxidative load is elevated. Reactive oxygen and nitrogen species emitted by inflammatory cells and cigarette smoke are sources of oxidants. This leads to an imbalance in oxidative stress's antioxidant and oxidant populations. In stable COPD, several oxidative stress indicators are elevated, and during exacerbations, these markers rise even more. Mucus production may be stimulated, or antiproteases may become inactive because of oxidative stress. Furthermore, it can escalate inflammation by facilitating transcription factors (e.g., nuclear factor κB) and consequent generation of pro-inflammatory mediator genes [10–13, 15, 18, 82–84].

Fig. 5: Progression of COPD by the presence of oxidative and proteases (BioRender.com)
Pathophysiology
Airflow obstruction and gas trapping-small airway disease and parenchymal degeneration, including emphysema, which raises airway resistance and lowers lung elastic rebound, combined with COPD. The degree of these variables may change over time. Prolonged inflammation results in altered structure, luminal discharges, narrowing of minor airways, and deterioration of lung parenchyma, which diminishes lung elastic rebound and alveolar attachments to minor airways. Due to these modifications, airways are still less able to be opened during expiration, which can cause mucociliary dysfunction and airflow obstruction. Due to decreased lung growth or more significant airway loss, COPD patients may have fewer tiny airways. This increases the risk of gas entrapment and lung hyperinflation, shortens forced expiratory breathing, and lowers FEV1 and FEV1/FVC ratios [14–16].
Hyperinflation-When the gas volume of the lung is higher than usual, it is called hyperinflation. This condition affects individuals with COPD and causes symptoms including dyspnea, reduced exercise tolerance, increased hospitalization rates, respiratory failure, and poorer mortality. When the highest expiratory flows are created during spontaneous breathing, there is a limitation in expiratory flow and a lack of elastic rebound, which leads to this. Airway abnormalities and emphysematous parenchymal degradation are the causes of this blockage [17–19].
Pulmonary gas exchange abnormalities- Patients with COPD have altered ventilation-perfusion distributions because of structural variations in the pulmonary circulation, airways, and alveoli. Vascular hypoxemia in variable degrees and aberrant pulmonary gas exchange result from this. Acidosis and hypercapnic respiratory failure can result from decreased ventilation brought on by sedatives and hypnotic drugs that decrease ventilatory drive. Pneumatic gas exchange frequently deteriorates throughout the illness, and lung diffusing capacity is further reduced by parenchymal damage associated with emphysema [85, 86].
Pulmonary hypertension-Smokers and COPD patients with mild airflow obstruction might see abnormalities within the pulmonary circulation, such as intimal hyperplasia as well as smooth muscle hypertrophy/hyperplasia. These individuals exhibit endothelial cell failure and inflammatory reactions like those in the respiratory system. Significant pulmonary hypertension, however, is uncommon in people with COPD and is often brought on by pulmonary capillary bed loss from emphysema or hypoxic vasoconstriction. The result of increasing pulmonary hypertension is heart failure on the right side, or "cor pulmonale," which is accompanied by right ventricular hypertrophy. Survival may be hampered by serious complications of pulmonary hypertension [20–23].
Exacerbations- Patients with COPD may experience exacerbation of respiratory symptoms due to unknown reasons, environmental pollutants, and respiratory infections. Increased dyspnea, deteriorating VA/Q abnormalities, increased gas trapping, elevated airway inflammation, and hyperinflation are examples of exacerbations. A result of these circumstances might be arterial hypoxemia. Heart failure, lung, and pneumonia are a few additional conditions that can resemble or worsen COPD exacerbations. It is essential to treat these crises therapeutically [24–26].
Multimorbidity- Patients with COPD frequently have other chronic comorbid illnesses, such as age, inactivity, and smoking, which can harm their health and survival. Limiting airflow, particularly in the case of hyperinflation, can worsen heart failure, ischemic heart disease, osteoporosis, normocytic anaemia, diabetes, and metabolic syndrome, among other heart health issues. Cachexia and atrophy of the skeletal muscles may also be caused by inflammatory mediators in the circulation [26, 27].
Mechanism of action
When mast cells are triggered, histamine and freshly produced phospholipid (arachidonic acid metabolites), along with mediators, are released from stored granules. With the aid of phospholipase A2, arachidonic acid is released from membrane phospholipids during immunologic activation. Prostaglandins and leukotrienes are produced via the fast oxidation of arachidonic acid via the cyclooxygenase (COX) or lipoxygenase (LOX) pathways. In addition to causing inflammation, these mediators also produce bronchoconstriction. The most effective bronchoconstrictors have been identified and are so-called cysteinyl leukotrienes: LTC4, LTD4, and LTE4. They specifically enhance the intracellular calcium content and activate the Gq protein-coupled cysLT1 receptor found on bronchial smooth muscle cells to produce smooth muscle formation. Like dopamine, although to a lesser extent, histamine activates the Gq protein-couple H1 receptor to produce smooth muscle contraction. Now, it has been shown that people with asthma have higher-than-average amounts of adenosine within their lungs. By stimulating the G1 region of the protein-coupled adenosine A1 receptor, adenosine affects bronchial smooth muscle cells by lowering cyclic AMP levels and inducing smooth muscle contraction. In addition, sympathetic and parasympathetic nerve fibers that control contractions and relaxations are also innervated in the smooth muscle of the airways. Smooth muscle relaxation is facilitated by activating the Gs protein-coupled β2-adrenergic receptor by endogenous catecholamines produced by the sympathetic fibers, such as nor-and adrenaline. Conversely, the parasympathetic Fibers’ production of acetylcholine activates the muscarinic M3 receptor that is connected to the Gq protein, increasing intracellular calcium levels and thus causing smooth muscle contraction [28–36].

Fig. 6: Mechanism of action (BioRender.com)
Diagnostic tools of COPD
Diagnosing the condition should be the goal for any patient exhibiting dyspnea, persistent coughing, sputum production, and a history of risk factors for the sickness. To diagnose COPD, forced spirometry must be performed and show that the post-bronchodilator FEV1/FVC<0.7 [37]. Peak expiratory flow measurement has a very high sensitivity, but its specificity is low enough that it cannot be employed as the sole diagnostic test [87]. Forced spirometry measures the following: Three measures are made: (1) forced expiratory volume in one second (FEV1), the amount of air exhaled during the first second of the maneuver; (2) forced vital capacity (FVC), the amount of air forced out at the point of maximal inspiration; and (3) the ratio of these two measurements, or FEV1/FVC. Spirometry measurements are compared to reference values depending on gender, height, and age [88].
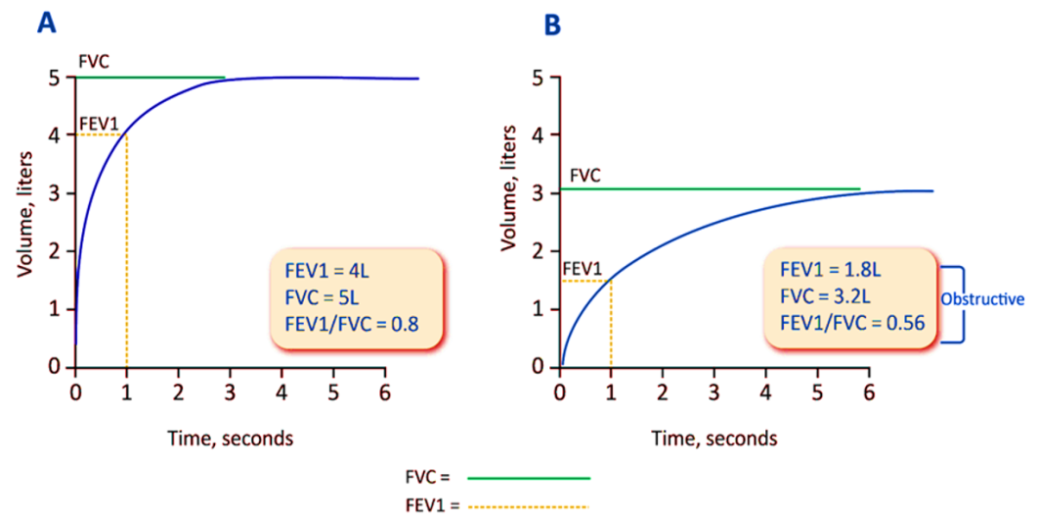
Fig. 7: A: Spirometry-normal tracing, B: Spirometry-Airflow obstruction; Due to airflow obstruction, patients with COPD usually have decreased FEV1 and, to a lesser extent, FVC (because of gas trapping) (BioRender.com)
When considering a diagnosis of COPD, GOLD has advised using post-bronchodilator readings by other national and international standards. Post-bronchodilator beliefs were previously thought that they were more appropriate over confirming a finding for fixed airflow blockage because they were supposed to be more dependable, helpful in deciding out asthma, and could help recognize volume responders alongside bronchodilators within whom the obstruction is confirmed through a rise in FVC administered by the drug [89]. However, it is widely known because a bronchodilator response has minimal relevance in distinguishing between asthma and COPD [90] that obstruction only evident on post-bronchodilator evaluations is rare and that pre-bronchodilator values are repeatable [91, 92]. Physicians might not perform spirometry because it takes longer to get post-bronchodilator values. GOLD suggests pre-bronchodilator spirometry as a preliminary test to ascertain whether an airflow restriction exists in those exhibiting symptoms. Pre-bronchodilator spirometry should be used before undergoing post-bronchodilator spirometry unless COPD is highly likely, around which situation an FVC volume response could indicate FEV1/FVC<0.7. Repeating the tests for pulmonary function after some time may be necessary as a follow-up to ascertain the cause of the patient's symptoms. Post-bronchodilator examination should be utilized to confirm COPD if any pre-bronchodilator readings show a blockage. Individuals with a FEV1 to FVC ratio pre-bronchodilator<0.7, which translates to>0.7 post-bronchodilator, are at a higher risk of developing COPD and should be thoroughly investigated [93].
Some more extra investigations
Additional testing should determine whether lung mechanics, development, or other comorbidities, such as ischemic heart disease, influence the patient's symptoms. This is especially important whenever the degree of airflow obstruction and the indicators noticed are different [38]. Along with these, some physiological tests include lung volumes, Measurement of arterial blood gas, oximetry, and the lungs' ability to diffuse carbon monoxide. Computerized Tomography imaging beneath chest X-ray along with many more like Interstitial lung abnormalities and alpha-1 antitrypsin deficiency [38, 39].
Worldwide growth rate of COPD and global market for inhalers
According to the WHO, in 2019, COPD accounted for 6% of total deaths, making it the “3rd leading cause of death worldwide, causing 3.23 million deaths”. The global market for inhalers, which is estimated to develop at a “compound annual growth rate of 6.1% and reach USD 33,572.9 million by 2023”, is expected to rise [40].
The GOLD annually releases a report on COPD management strategies. Physicians utilize this report, which defines fundamental words and concepts, as a starting point for strategy. Nonetheless, several national and worldwide COPD management guidelines and recommendations have been produced and published in the past, with a greater emphasis on respecting the specific scope, structure, and unique features of local healthcare systems [41–50].
Therapies for COPD
According to the GOLD Report 2024, therapies are classified into two therapies for controlling and treating COPD: I) Pharmacological therapies and II) Non-Pharmacological therapies [38].
Pharmacological therapies
Based on the severity of symptoms and the risk of worsening them, the GOLD Report for 2024 recommends a personalized approach to starting therapy. Breathlessness, exercise restriction, and the frequency of exacerbations while undergoing maintenance therapy are the key symptoms that determine whether to escalate or de-escalate treatment. These recommendations, which offer a systematic approach to treatment, drew some support from data from randomized controlled trials. Nevertheless, these guidelines provide professional guidance according to clinical knowledge because they are intended to help doctors make decisions.
The patient's GOLD group should inform the initial course of pharmacological treatment. Patients present symptom severity as measured by the COPD Assessment Test, the Dyspnea Scale, or the Modified Medical Research Council values. The frequency of exacerbations should be assessed at appropriate intervals (longer for fewer patients, shorter for severe patients). Comorbidities should be reevaluated, along with the treatment's impact and potential side effects.
Review of the inhaler technique, compliance with pharmaceutical and non-pharmacological suggested treatment, current smoking habits, and ongoing contact with hazards should all be part of every clinician session. Promoting physical activity and investigating pulmonary rehabilitation in extreme circumstances are both critical. Each of the following needs to be evaluated independently: lung volume reduction, oxygen therapy, safe support for breathing, and palliative interventions. The action plan should then be modified appropriately. It is recommended that spirometry be performed at least annually. Stopping the patient's bronchodilator medication if they are currently using it while doing spirometry is not recommended.
Table 1: Pharmacological therapies for COPD
| Pharmacological therapies | Reference | ||
| More than two mild exacerbations or more than one that requires hospitalization | “GROUP E” LABA+LAMA* If the blood eos is less than 300, consider LABA+LAMA+ICS*. |
[38] | |
| Moderate exacerbation (0 or 1) (without requiring hospital admission) | “GROUP A” Bronchodilators |
“GROUP B” LABA+LAMA* |
|
| mMRC 0–1, CAT less than 10 | Patients with a mmol RC score of 2 or higher and a CAT score of 10 or more. |
Group A
Bronchodilators can be administered to all Group A patients with short-acting or long-acting administration. Unless a patient experiences dyspnea often, long-acting bronchodilators are advised if they are accessible and cheap [38].
Group B
A combination of LABA and LAMA should be the initial treatment option. According to a randomized controlled trial, using LABA+LAMA for patients with a CAT™ score of ≥ 10 is more effective than using LAMA alone regarding various outcomes, especially for patients who experienced at least one moderate exacerbation the year before the study. Thus, LABA+LAMA is the first pharmacological option that is advised if there are no problems with availability, cost, or adverse effects. The available information does not support prescribing one kind of long-acting bronchodilator over another to patient group B to relieve the first symptoms. The patient's feelings should guide decisions. The prognosis and symptomatology may deteriorate due to complementary disorders [94–96].
Group E
The most effective treatment for COPD exacerbations, assuming no issues with cost, availability, or side effects, is the combination of LABA and LAMA, as stated in a Cochrane systematic review and network meta-analysis. For group E patients, the recommended first-line therapy is LABA+LAMA; however, if an ICS is necessary, it's advised to use LABA+LAMA+ICS due to its superior efficacy. In group E, the text suggests LABA+LAMA+ICS for patients whose eosinophil count is fewer than 300 cells/µl. The following section will examine the relationship between blood eosinophil count and how ICS affects exacerbations. As for starting triple therapy as the principal line of therapy for those suffering from elevated eosinophil levels in those who have just received a diagnosis, there is presently insufficient data to support this practice. Patients should get the same care as those with asthma if they also have COPD [38, 97–99].
When it came to reducing the death rate of COPD patients, neither the SUMMIT research nor the TORCH clinical trial could show that a mixture of LABA and ICS was better than a placebo. The intention-to-treat analysis in the UPLIFT research did not decrease mortality compared to placebo since most individuals took an ICS [100, 101]. Compared to dual inhaled long-acting bronchodilation treatment, fixed-dose inhalation tri combinations (LABA+LAMA+ICS) have been shown in two randomized clinical studies, IMPACT and ETHOS, to reduce all-cause mortality [102, 103].
Bronchodilators
The drugs are known as bronchodilators, which enhance FEV1 and/or modify other spirometry parameters. They work by altering the tone of the smooth muscle within the airways, and rather than altering lung elastic recoil, the improvements in overall expiratory flow result from airway widening. During exercise and rest, bronchodilators often lessen dynamic hyperinflation and enhance exercise efficiency. From the improvement in resting FEV1, it is difficult to estimate the degree of these improvements, particularly in individuals with severe and very severe COPD [38]. While administering an anticholinergic/beta2-agonist by nebulizer offers a subjective advantage during acute episodes, it may not be beneficial when the illness is stable. An order of magnitude increases in dosage. The most common use of bronchodilator drugs in COPD patients is regularly to either avoid or lessen symptoms. In addition, toxicity relates to dose. Regular use of short-acting bronchodilators is generally not advised [104–110].
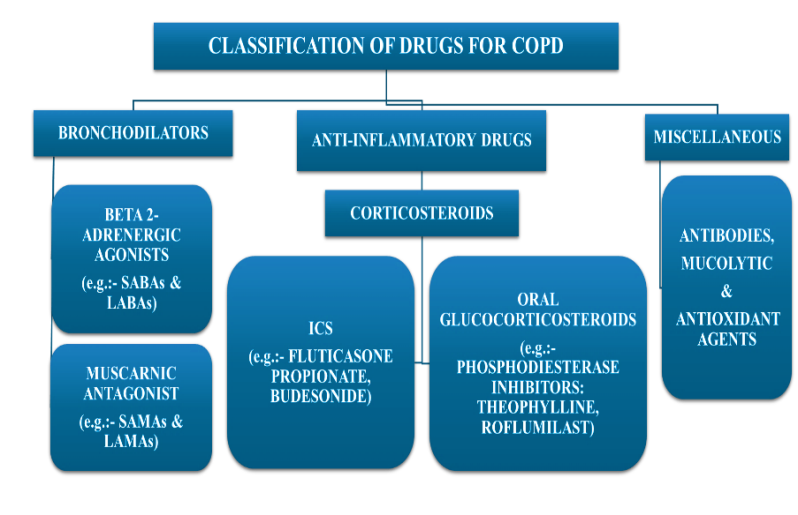
Fig. 8: Classification of drugs for COPD (BioRender.com) [38, 51, 52]
β2- Adrenergic agonist
To provide a functional antagonistic reaction against bronchoconstriction, beta2-agonists primarily work by activating beta2-adrenergic receptors, which raises cyclic AMP. Airway smooth muscle is relaxed as a result. LABA and SABA are the two types of beta2-agonists available. 4–6 h are usually when SABAs start to lose their effects. FEV1 and symptoms are improved when SABA is used regularly as needed. LABAs have an impact that lasts for at least 12 h, and they don't stop subsequent benefits from more SABA treatment when it's required. Salmeterol and formoterol, two twice-daily LABAs, dramatically reduce mortality and the rates when lung function gets worse. They also reduce the rate of exacerbations, hospitalizations, dyspnea, and general health issues. Indacaterol, a once-daily LABA, has been shown to effectively alleviate shortness of breath, enhance overall health, and reduce the frequency of exacerbations. Indacaterol inhalation can cause persistent coughing in certain people. Oladaterol and vilanterol are among the extra once-daily LABAs that enhance lung function and alleviate symptoms [108, 110–120].
Muscarinic antagonists
The drug prevents acetylcholine from constricting the airways by inhibiting M3 muscarinic receptors within the smooth muscle in the airways. Like oxitropium and ipratropium, SAMAs may encourage vagally produced bronchoconstriction. Among the LAMAs are revefenacin, tiotropium, aclidinium, umeclidinium, and glycopyrronium bromide. LAMAs increase how long bronchodilator action lasts [121]. An analysis of RCTs revealed that the short-acting muscarinic antagonist ipratropium had little benefit over the short-acting beta2-agonists regarding lung function, general health, and the requirement for oral steroids. Examples of LAMA drugs that enhance patient well-being and symptoms, reduce hospitalization and exacerbation rates, and boost the efficacy of pulmonary rehabilitation include tiotropium, umeclidinium, and revefenacin. Studies show that tiotropium is more effective than LABA therapy at reducing the frequency of exacerbations [121–127].
Anti-inflammatory agents
Corticosteroids
ICS
Corticosteroid responsiveness of COPD-associated inflammation appears restricted, according to in vitro studies. Furthermore, a few medications, such as macrolides, theophylline, and beta2-agonists, may help COPD patients' corticosteroid sensitivity to some extent [128–130]. Determining the effect's clinical relevance is still premature. According to in vivo data, more studies are required to elucidate the dose-response relationships as well as the long-term (>three years) safety of ICS for individuals with COPD [131]. These are two different treatment choices since using long-acting bronchodilators simultaneously as ICS might alter its effects in COPD patients. COPD patients, both present and former smokers, benefit from ICS treatment as far as both lung function and exacerbation rates; however, the effect size is more significant in heavy smokers compared to moderate or ex-smokers [132, 133].
Oral glucocorticoids
Among the many negative consequences of oral glucocorticoids is steroid myopathy [134], which in those with very severe COPD can worsen respiratory failure, muscle weakness, and reduced functioning. In patients hospitalized or presenting to ERs during acute exacerbations, systemic glucocorticoids have been demonstrated to enhance lung function and dyspnea while decreasing the risk of treatment failure and its recurrence [135]. However, there aren't enough long-term prospective studies on how oral glucocorticoids affect individuals with stable COPD. This indicates that while oral glucocorticoids help treat acute exacerbations of COPD, long-term, daily use of them should be avoided because the significant risk of systemic issues outweighs any potential benefits [136].
Phosphodiesterase-4 (PDE4) inhibitor
By inhibiting intracellular cyclic AMP breakdown, PDE4 inhibitors decrease inflammation. Oral roflumilast, taken once daily, lowers moderate to severe worsening in patients with severe to extremely serious COPD, persistent bronchitis, and exacerbations when combined with systemic corticosteroids. It can, however, affect lung function when used with long-acting bronchodilators or in individuals not adequately managed on fixed-dose LABA+ICS combinations. A higher chance of benefiting from roflumilast is shown among patients who have experienced prior acute exacerbations. No research hasn't been done directly comparing roflumilast with inhaled corticosteroids [137–143].
Long-acting bronchodilator medication combined with ICS
ICS and LABA together improve lung function and overall health more than either drug alone does when used in individuals with moderate to severe COPD who have exacerbations [144, 145]. Clinical studies that used all-cause mortality as their primary endpoint could not show that combination treatment had a statistically meaningful impact on survival [146, 147]. Compared with LABA alone, patients with at least one exacerbation for the year before the study were included in most trials that found a decreased exacerbation rate using a combination with a fixed dose of LABA+ICS [145]. The combination of LABA with ICS in conventional treatment was compared in a comprehensive RCT in an early healthcare setting in the United Kingdom. The study's main objective was to reduce moderate-to-severe deteriorating conditions by 8.4%. However, according to the data, the number of hospital admissions and pneumonia cases remained unchanged. Additionally, the CAT™ score significantly increased [148].
Triple therapy
Research has demonstrated that increasing inhaled medicine to LABA in addition to LAMA together with ICS (triple treatment) improves outcomes reported by patients, pulmonary functioning, and decreases exacerbations when compared to LAMA. separately LABA+ICS and LABA+LAMA[149]. This can be done in several ways [150–160]. An RCT assessing the benefits of LABA+LAMA+ICS found that, despite smoking status, triple treatment enhanced clinical results over dual therapy. A post-hoc analysis supported this finding [161].
In patients with severe airflow obstruction due to COPD and a history of exacerbations, a recent analysis of three clinical trials showed a slight trend towards reduced mortality with triple inhaled therapy compared to non-ICS-based treatments. This suggests a potential benefit of triple therapy in terms of safety outcomes for this specific patient population [162].
Miscellaneous
Antibiotics
Preventive, continuous use of antibiotics did not influence the incidence of COPD exacerbations, according to five-year research examining chemoprophylaxis's efficacy throughout the winter [163-165]. Additional research has demonstrated that using several antibiotics consistently may lower the incidence of exacerbations [166, 167]. Those who were susceptible to exacerbations had a lower chance of experiencing exacerbations following a year of using azithromycin (250 mg/day 500 mg on three occasions per week) either erythromycin (250 mg twice a day) compared to conventional medication [168–170]. Azithromycin use has been connected to longer QTc intervals, a higher prevalence of bacterial resistance, and worse outcomes on hearing tests [170]. Less advantage among smokers who are currently smoking, according to a post-hoc study [171]. There is no proof that long-term azithromycin therapy is safe or helpful in avoiding COPD exacerbations, even after a year of treatment. For patients with recurrent exacerbations and chronic bronchitis, moxifloxacin pulse treatment (400 mg/day over five days once every eight weeks) did not reduce the overall exacerbation rate [172]. Exacerbations were not decreased by long-term doxycycline use, while there could be responder subgroups [173].
Mucolytic and antioxidant agents
Mucolytics like carbocysteine and N-acetylcysteine can be used consistently to help individuals with COPD feel slightly better and experience fewer exacerbations when ICS is not being given to them [174–177]. On the other hand, it has been demonstrated that erdosteine, independent of concomitant ICS therapy, may have a notable impact on (mild) exacerbations. The current data regarding the analyzed demographics, therapeutic dose, and concurrent treatment are too variable to identify the potential target demographic for antioxidant medications in COPD [178].
Put it all up, the most often prescribed medications for the treatment of COPD include inhaled corticosteroids in cases of severe COPD and recurrent exacerbations, long-acting bronchodilators, and selective β2-adrenergic agonists. Bronchodilators benefit patients with moderate to severe COPD, especially those with a long half-life, as they increase exercise tolerance, enhance general health, and reduce exacerbations. Nonetheless, due to potential side effects, inhaled corticosteroids need to be used with caution. Future COPD care seems bright because of ongoing developments in pharmaceutical therapy, which include the creation of novel medication and combination therapies.
Non-pharmacological therapy
Self-management
A conceptual definition of COPD self-management interventions was developed through the Delphi process. It states that these interventions are structured, personalized, and frequently involve multiple components. With the help of these interventions, patients should be able to modify their health-related behaviors for the better and acquire the skills they need to maintain their condition. Patients and healthcare professionals must have ongoing, continuous discussions to provide efficient self-care treatments. Behavior modification strategies evoke motivation, competence, and confidence in patients. Comprehensibility is improved by the employment of literacy-sensitive techniques [179]. It is well-established that self-management enhances the quality of life of individuals with COPD. A 2022 Cochrane review discovered that these interventions enhanced health-related quality of life, reduced hospital admissions for respiratory-related reasons, and had no effect on mortality risks [180]. Past Cochrane reviews and meta-analyses have addressed concerns of elevated mortality, indicating no effect on total mortality. The COMET and PIC-COPD, two separate trials, have demonstrated the possibility of lowering mortality with integrated case management and self-management strategies. Confirming the idea that self-management therapies are unlikely to be harmful are these findings from the most current Cochrane study [181, 182].
According to research, throughout six months, hospitalizations and emergency room visits doubled for individuals who participated in a three-month program designed to help them manage their COPD exacerbations on their own. This shows that self-management techniques could encourage people to use healthcare services more frequently [183]. Generalizing these findings, however, is difficult because of the variety of treatments, application consistency, intervention details, patient groups, duration of follow-up, and outcome measures. A conceptual definition should address these shortcomings, emphasizing the need for iterative patient-provider interactions [184]. The Chronic Respiratory Disease Questionnaire mastery scores of individuals receiving health coaching from respiratory therapists or nurses have shown significant gains in their ability to control their health [185].
Integrated care programs
Because COPD is complicated, several healthcare professionals must work closely together. There is conflicting data about the potential benefits and efficacy of a formal, planned disease management program. Integrated disease care improved quality of life, ability to exercise, hospitalizations, especially inpatient days, but not death, according to a study of 52 studies [186]. This was not supported by a significant multicentre trial conducted in primary care within a well-functioning system, and telemedicine treatments did not yield noteworthy results [187]. Though structured into a codified program, well-organized care may not always be advantageous. A person's integrated care should be tailored to their health literacy and stage of sickness [188, 189].
Physical activity
Both community-based and home-based pulmonary rehabilitation have shown benefits, but encouraging and sustaining physical exercise is essential [190]. A common feature of COPD patients' reduced exercise levels is their diminished quality of living, which raises their chances of hospitalization and mortality [191–193]. Consequently, behavior-targeted therapies to increase physical activity are gaining popularity [194]. Especially for those with poor baseline self-efficacy in COPD, technology-based therapies can improve exercise self-efficacy and encourage healthy lifestyle modifications [195, 196]. Most published research lacks instructions, methodological consistency, and specifics needed to duplicate or modify treatments for use in clinical settings. Research on the effects of community-based physical activity coaching on COPD exacerbation history found no advantages in terms of acute care utilization or survival [197]. Around 12 to 15 mo after the intervention, different research discovered a correlation between it and a lower incidence of acute exacerbations [198]. Studies have demonstrated that non-pharmacological therapies, such as pursed lips and breathing with the diaphragm, enhance exercise tolerance and lung function in people with COPD [199].
Exercise education
Individuals diagnosed with COPD experienced a significant boost in their physical activity levels through exercise training alone or when combined with activity counseling, as revealed by a meta-analysis of RCTs [200]. Strength training yields improved results when paired with intermittent or continuous load training [201]. The recommended limit for resistance workouts is Borg dyspnea, and the tiredness rating is 4 to 6 (average to intense), or 60–80% of an individual's symptom-limited maximal work or heart rate [202, 203]. Exercise regimens that alternate between periods and continuously work well for endurance training. In the latter, the patient completes the same amount of work but breaks it into shorter bursts of intense activity. This tactic is helpful when other comorbidities hinder performance [204, 205]. Exercise ability in COPD patients has been demonstrated to increase with Tai Chi practice, which emphasizes focus and circular body movement. However, Tai Chi's benefits in lowering dyspnea and enhancing quality of life are still up for debate [206]. Since they lessen both resting and dynamic hyperinflation, optimizing bronchodilators can improve exercise training [207]. Although it is helpful for aerobic training, strength training has no positive effects on health or exercise tolerance [208]. Exercise training on the upper extremities improves arm strength and endurance, whereas whole-body vibration training may increase endurance [209, 210]. Exercise that strengthens the inspiratory muscles may help with performance, lessen dyspnea, or promote overall health, although these benefits are not always guaranteed [211–214].
Therapeutic management of COPD acute exacerbation
Acute exacerbations, which can occur at any moment during COPD, are sporadic bouts of worsening pulmonary function and increased respiratory symptoms that call for a change in routine therapy [38, 39, 53–57].
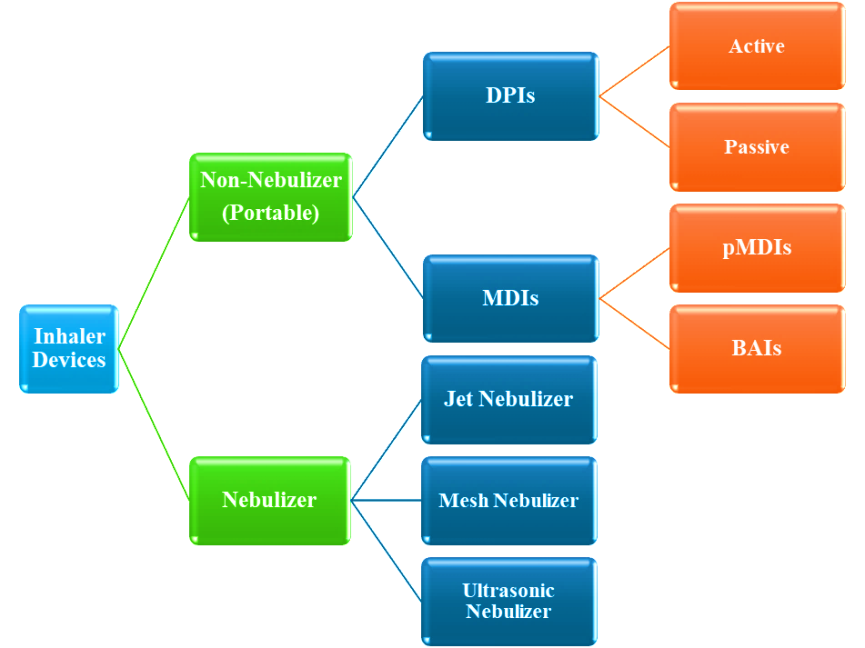
Fig. 9: Types of inhalers used in the treatment of COPD [53, 58]
DPIs
Orally inhalable DPI formulations provide patients with higher compliance, high dose-carrying capacity, and drug stability. Targeting respiratory diseases such as diabetes and pain management, these devices release metered doses of powdered drugs by inhalation. DPIs have been developed to treat both local and systemic abnormal lung diseases, including “asthma” and “chronic obstructive pulmonary disease.” The device, commonly made of polypropylene plastic, delivers the dry powder formulation to the pulmonary tract in a controlled manner.
The dry powder size is critical for inhalations. Since it is typically stored in capsules, the aerodynamic diameter range is 1–5 µm. Less than 2 µm is needed for systemic effects, whereas 2–5 µm is the preferred particle size. Dry powders that can become aerosolized are made by adjusting their shape, density, and porosity. DPIs frequently develop through crystallization and micronization, but both methods have drawbacks regarding particle size, shape, dispersion, and crystallinity. To generate respirable particles, spray drying is employed along with specialized milling procedures to manage humidity during crystallization. For effective delivery of drugs, the pharmaceutical industry must manufacture micron-sized powders on a large scale [215].
Drug deposition and particle size in the lungs
Precautionary measures should be implemented since drug deposition in the lungs can impact how effective an inhalation therapy is therapeutically. The initial stage following the particles' successful inhalation is drug deposition, which can be achieved by various methods, including direct interception, electrostatic deposition, sedimentation, and diffusion (table 2), and impaction, sedimentation, and diffusion.
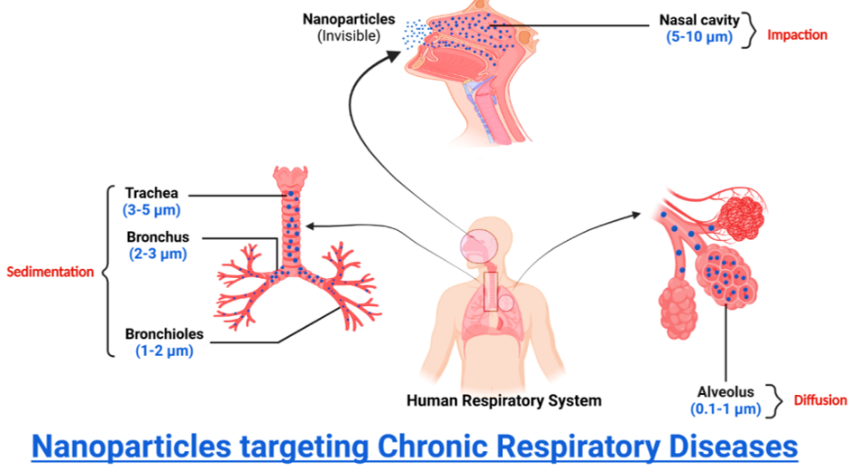
Fig. 10: Mechanisms of particle deposition based on size (BioRender.com)
Table 2: Pathways for particle-size-dependent deposition in the lungs
| Mechanism | Size of particles | Sections of the respiratory tract | Reference (S) |
| Impaction | 5-10 µm | Nasal Cavity(Oropharynx and conducting airways) | [59–61, 216] |
| Sedimentation | 3–5 µm | Trachea | |
| 2-3 µm | Bronchus | ||
| 1-2 µm | Bronchioles | ||
| Diffusion | 0.1-1 µm | Alveolus |
Owing to increased air velocity with turbulent flow, inertial impaction is the primary mechanism for deposition in the oropharynx and conducting airways. Large particles, defined as those with a diameter of more than 5 µm, are pushed out of their original airstream trajectory and eventually strike the upper airway wall because of inertia, which prevents them from reacting to abrupt changes in direction and speed. One may compute the probability (PI) of a particle departing from the airstream using Equation (1) [59–61, 216].
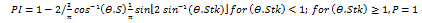 ------------ (Equation 1)
------------ (Equation 1)
Equation (1) indicates that PI = probability of impaction
θ = bending angle (change in the direction of the flow)
Stk = Stokes number, defined as (Equation 2):
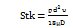 -------------- (Equation 2)
-------------- (Equation 2)
Equation (2) indicates that: ρ = particle density
d = particle diameter
υ = particle velocity
µ = viscosity of fluid
D = airway diameter
The principal difficulty of lung deposition with the dry powder inhaler is the quantity of medication lost because of this mechanism [217].
Sedimentation is the process that impacts particles between 0.1 and 5 µm as they enter the lungs, mainly within the bronchi, bronchioles, as well as alveolar area. The probability of impaction-induced deposition also decreases with velocity. Therefore, sedimentation impacts particles that have made it beyond impaction and into the final five or six lung generations. Gravity causes the particles to start settling, which causes sedimentation. Sedimentation worsens as the particle mass, diameter, residence time, and flow rate rise. When particles with a diameter of 3-5 µm enter the tracheobronchial area, they do so via sedimentation; however, when particles with a diameter of 0.1-3 µm enter the alveolar region, both sedimentation and diffusion are anticipated. Equation (3) expresses the chance that sedimentation (PS) will result in particle deposition [59–61].
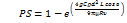 ---------------------- (Equation 3)
---------------------- (Equation 3)
Equation (3) indicates that PS = probability of sedimentation
g = gravitational force
C = Cunningham slip angle correction factor
ρ = particle density
d = particle diameter
L = length of the tube
ø = inclination angle relative to gravity
µ = viscosity of fluid
R = radius of the airways
υ = particle velocity
Different types of inhalers
Here listed below some of the traditionally used inhaled that are:
Breezhaler
Clickhaler
Diskhaler
Diskus
Ellipta
Handinhaler
Jenuair
pMDIs
Respimat
Swinghaler
Turbuhaler
Twincaps
Twisthaler
Rotahaler [218]
Space inhaler
Volumatic device [219]


Fig. 11: Inhalation devices [218, 219]
Presently available inhalers and innovations in inhalation therapy
In recent years, there have been significant advancements and developments in inhalation administration to increase medication availability and distribution, daily regimens, and patient compliance (particularly by lowering the frequency of doses). This has led to advancements in device technology, medication delivery methods, and manufacturing procedures. Lipid-or polymer-based carriers in the formulation and biotech medications [220]. For example, Afrezza® is the only protein DPI (insulin) used to treat type 1 and 2 diabetes. As previously indicated, DPIs present a promising method for delivering biologics, including viruses (like phages), proteins, nucleic acids, and cells (attenuated bacterial cells like BCG for Tuberculosis). Regarding biologics for inhalation, proteins have gotten the most attention; nevertheless, many of the formulation and production processes have been altered to accommodate other biologics, such as nucleic acids and, more recently, phages. DPIs provide benefits such as mucosal immunization and non-invasive injection-free delivery, making them a viable platform for inhaled gene treatments and vaccines [221, 222].
Various DPIs are presently available on the market, and inhalation treatment is constantly evolving to overcome the many obstacles it encounters while maintaining a high level of therapeutic efficacy (fig. 12). Around 500 million people worldwide suffer from various diseases, and out of the 40 varieties of inhaler devices now on the market, DPIs are the most often used variety [223, 224].
Furthermore, fig. 13 gives a detailed breakdown of how to utilize a DPI device step-by-step for a more thorough perspective.
Furthermore, inhalation devices that are more recent and sophisticated can deliver drug doses in the microgram and occasionally nanogram range rather than milligrams (table 2), which results in higher drug deposition. Compared to earlier inhalers, which showed ≤20% lung deposition,>50% lung deposition has been recorded [225, 226]. Relenza® (zanamivir, 5 mg), Tobi Podhaler® (tobramycin, 28 mg), Bronchitol (40 mg, mannitol), and Osmohale® (mannitol) are a few DPIs with large dosages that should be noted [227, 228].

Fig. 12: Presently available inhalers [227]
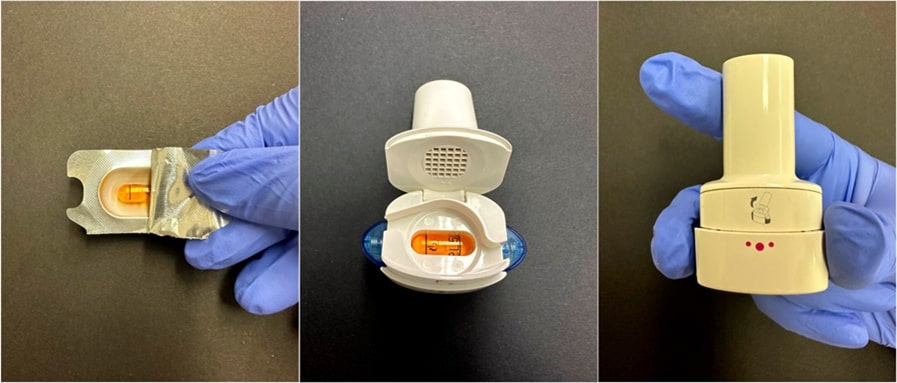
Fig. 13: An explanation in detail on how to use the Breezhaler® DPI device: extraction of the capsules from the blister, insertion into the device through the closure, and pressing of the side buttons to puncture the capsule and release the powder [227]
For respiratory conditions like COPD and asthma to be effectively treated, prescription inhalers must be used as directed. Reduced medication delivery can affect disease control due to ineffective inhalation procedures or inaccurate administration strategies. Spacers, also called holding chamber extension devices, limit inhaled medication, increasing the effectiveness of pMDIs. A spacer can improve medication delivery when used with appropriate breathing techniques. 82.3 % of patients in Indian research reported misusing their inhalers at least once, with MDI users accounting for the most significant percentage of mistakes (94.3%). However, the proportion of errors was reduced (78%) when a spacer was used with MDIs. DPI users made 82.3% of the errors, followed by nebulizer users with 70% [229]. As a result, employing a spacer lessens both the amount of deposition in the oropharyngeal area and the requirement for exact actuation and inhalation synchronization that comes with utilizing a pMDI device alone [230]. Patients who need medical aid, such as elderly patients with COPD and cognitive impairment, or babies and youngsters who might not be able to perform a precise breathing technique or who might not comply, will benefit most from this. Conversely, DPIs don't require a spacer or must be shaken before each usage.
Integrating data analysis and connection in digital smart inhalers transforms respiratory treatment by tracking patient adherence and inhaler usage. These technologies maximize treatment outcomes by enabling patients to control their diseases properly [231]. They are invaluable for people who suffer from obstructive disorders or respiratory conditions like asthma. Personalized treatment regimens are made possible by the inhalers' use of electromechanical sensors and microelectronics to monitor inhaler activation. This is the first line of smart inhalers with embedded sensors, allowing for efficient respiratory condition management [232, 233].
Van Sickle et al.'s study, which provided real-time monitoring and patient adherence insights, validated the effectiveness of digital smart inhalers in redefining respiratory treatment. Utilizing the mobile health service improved asthma outcomes, such as more asthma-free days, greater medication adherence, and better overall asthma management [234].
With the implementation of smart DPIs and related applications, patient involvement and engagement will increase. A more patient-centered healthcare system will result from its successful implementation, assisting individuals in managing their health. Complex business structures and a lack of interoperability standardization are the main obstacles that still need to be addressed [235].
The clinical translation of NPs and inhalation devices entails numerous challenges that must be systematically addressed to optimize patient outcomes. A prominent issue within this context is patient adherence; many individuals encounter significant obstacles in consistently and correctly utilizing their inhalers as prescribed. The factors contributing to non-compliance include a lack of understanding of proper inhalation techniques, physical difficulties associated with device handling, and psychological barriers to managing chronic respiratory conditions [236, 237].
To enhance adherence, it is imperative to implement a comprehensive strategy that incorporates education, technology, and personalized support. Educational initiatives should provide thorough instruction on the correct usage of inhalers, employing visual aids and practical demonstrations to reinforce comprehension [237]. Furthermore, inhalation devices should be designed to focus on user-friendliness, incorporating features such as ergonomic designs, audible feedback mechanisms, and quickly interpretable dosage indicators.
Additionally, establishing ongoing support from healthcare professionals is critical to motivating patients. Regular follow-ups, facilitated through phone communication, text reminders, or specialized mobile applications, can significantly improve adherence by ensuring patients remain cognizant of their medication schedules [231]. Integrating such technological tools provides timely reminders and enables tracking usage patterns, allowing healthcare providers to identify and address potential issues proactively. The likelihood of improved adherence and overall therapeutic success is considerably increased by cultivating a supportive environment and empowering patients in their respiratory health management [238].
Comparative analysis of inhaler types
DPI, pMDI smart inhalers
DPIs
A wide array of DPIs is currently available. To be classified as ‘ideal,’ an inhaler device must encompass several essential criteria: (1) effectiveness ensuring the inhalation of a sufficient fraction of the drug, delivered in breathable-sized particles, regardless of fluctuations in the patient’s inspiratory flow; (2) reproducibility-facilitating the inhalation of a consistent amount of the drug, particularly concerning its breathable fraction; (3) precision allowing for real-time knowledge of the number of doses remaining in the device and confirming the correct execution of inhalation; (4) stability protecting the drug(s) from adverse effects due to variations in temperature and humidity; (5) comfort promoting ease of use across diverse situations, while accommodating multiple doses for long-term application; (6) versatility enabling the administration of various medications; and (7) environmental sustainability ensuring the absence of harmful chemical contaminants. At present, no commercially available DPI fulfills all these criteria. Nevertheless, specific inhaler devices possess features that closely align with the ideal conception of an inhaler in a practical, real-world context [239–241].
pMDIs
A pMDI uses a propellant to deliver a specific dose of medication directly into the lungs. It works well with spacers, which help improve drug delivery and make inhalation easier for patients with coordination issues. pMDIs are small and light, making them convenient for patients who need to take their medications while on the move [242]. Each inhaler can provide multiple doses from a single canister, making it a cost-effective choice for long-term treatment. pMDIs can deliver various medications, including bronchodilators and corticosteroids, allowing healthcare providers to tailor treatments to each patient. Using a pMDI effectively requires good timing between pressing the inhaler and breathing in. This can be difficult for some patients, such as the elderly or young children. The propellants in pMDIs can increase the carbon footprint compared to other inhalation devices, raising concerns about their environmental effects [242, 243].
Smart inhaler
Smart inhalers significantly advance in managing respiratory diseases such as asthma and COPD. These innovative devices are embedded with advanced sensors that meticulously monitor various factors, including medication usage, therapeutic effectiveness, and environmental triggers that could exacerbate symptoms [244]. The comprehensive data collected is transmitted to patients and healthcare providers through user-friendly mobile applications, facilitating effective medication tracking and personalized coaching tailored to individual needs. In addition to these features, smart inhalers send timely reminders to users and provide constructive feedback on inhaler techniques, which is crucial in improving patient medication adherence [245]. Despite these benefits, the higher price point of smart inhalers may pose a barrier to access for some patients, highlighting the need for further large-scale studies to confirm their overall effectiveness and justify their costs. Moreover, while some devices may experience technical malfunctions, user support often resolves these issues. Ongoing research is also critical to address outstanding questions regarding funding strategies and implementing these advanced technologies in diverse healthcare settings [244].
Nano-scale system
A new field of multidisciplinary science called nanoscience has emerged [246]. The field of nanomedicine has gained potential for scientific study due to the introduction of nanotechnology throughout the last two decades [247]. As potential ideal drug delivery systems for poorly soluble, poorly retained, and labile substances, a late-stage advancement in nanoparticulate frameworks, including potent NPs, polymeric NPs, and polymeric self-congregations, is presented in the current matter. The improved solubility, targetability, and binding to tissues, among other new capabilities arising from nanosizing, are illustrated. Furthermore, these capabilities are used to develop an additional drug delivery system [248].
The ability to deliver the treatment over various natural obstacles to the target location is another distinctive feature of nano particulates, and difficulties with NPs formulations are mentioned, accordingly, in fig. 14 and 15.
Nanomedicine formulations
The history of nano-scale development
Nanomedicine is a therapeutic application of technology that enhances the delivery of innovative therapy techniques for inflammatory lung diseases, such as COPD, in future generations [249]. In the past, medicinal plants were used to cure illnesses, but how the drugs were delivered lacked uniformity, control, and precision. In 1955, Jatzkewitz published the first study on polymer-drug conjugate (NP-based) treatment, demonstrating the first instance of medicine and nanotechnology working together to improve medication delivery and effectiveness. Similarly, the identification of liposomes in the 1960s stimulated research on nanocarriers, which stimulated the development of micelles and polymerization drug-loading techniques in the latter part of the 1970s and early 1980s [250, 251]. Over 1000 research publications on nanomedicine, including using NPs and nanocarriers in practical biological contexts, were reported to have been published in Web of Science in 2015. This indicates an exponential growth in the number of publications on this topic of study. This expanding field of study has led to the advancement of conventional medicine delivery through the micro-engineering of NPs, which has been used to treat various chronic inflammation lung conditions [251].

Fig. 14: Relevance of nano-scale system in pulmonary applications (BioRender.com)
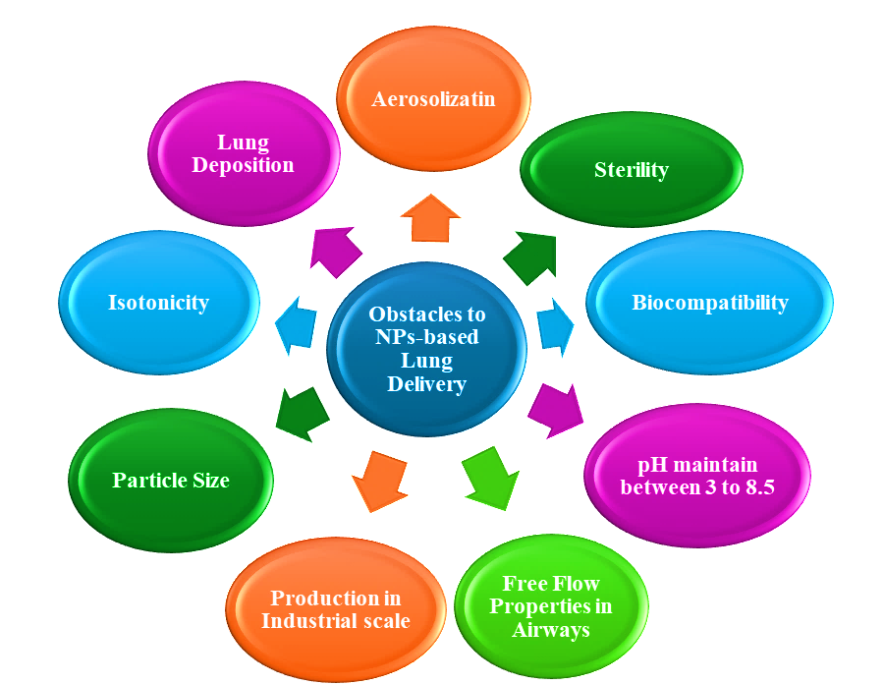
Fig. 15: Obstacles to NPs-based lung delivery (BioRender.com)
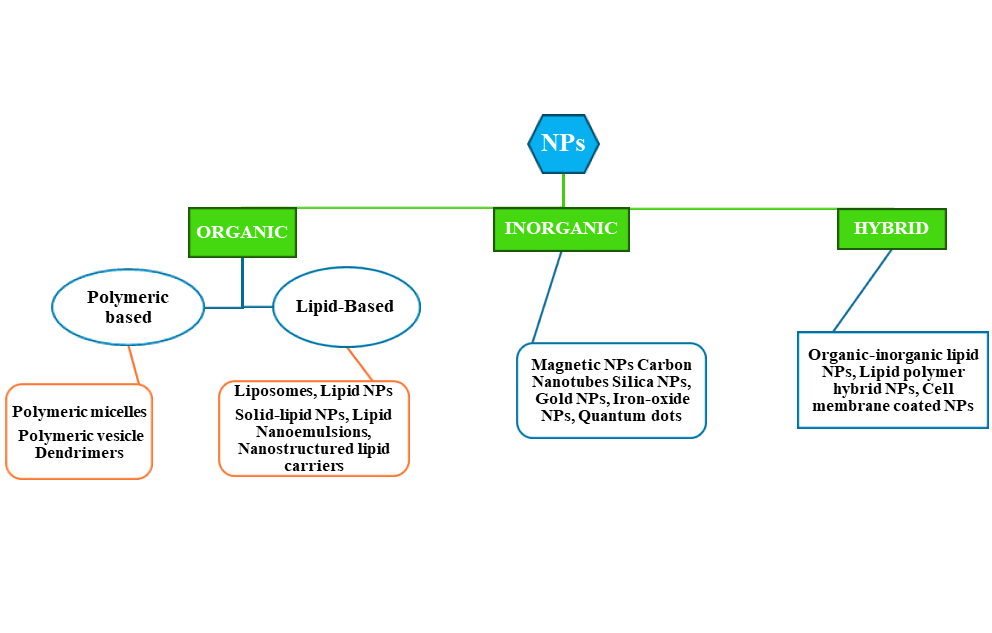
Fig. 16: Several kinds of NPs. The NPs have three different nanoparticle subclasses: A) Organic, B) Inorganic, and C) Hybrid; again, their subgroups are divided into different varieties of NPs(BioRender.com)
Nanomedicine delivery system
As previously stated, present therapy techniques focus on symptomatic alleviation rather than treating the underlying cause of COPD, which is the harm caused by cigarette smoke [252]. This makes it necessary to investigate other treatment options, such as nanoparticle delivery methods. NPs-based formulations encapsulate the drugs, transport them to the desired location, and release their active compounds into the targeted tissues. Among the numerous advantages of this technology-dispersing procedure is the real-time, regulated release of the medicine of choice to the therapeutic target [62]. The Nano formulations contain API and certain other supporting carriers that aid in the dispersion and transportation of Nano formulations with volume, making up for the delivery of drugs throughout the body. The different drug distribution in various routes of administration will also affect other aspects such as pharmacokinetics, ease of administration, toxicity, and infection risk [62].
When it comes to treating severe RDs, nanomedicine has demonstrated enormous therapeutic promise. Examples include polymeric NPs, liposomes, nanotubes, inorganic silver and gold NPs, and micelles. NPs have been used to transport immunomodulatory chemicals, DNA vectors, antibiotics, and vaccine components to their intended target areas. In addition, NPs are preferred because they enable tissue-specific drug delivery and have the capacity to maintain and preserve the medication. As a result, this has improved efficacy over alternative therapy methods and less negative consequences for the patient. Moreover, physical attributes such as size, shape, zeta potential, oxidative potential, and chemical structure can be adjusted to fine-tune a nanoparticle's biological response [253]. A concise overview of the several innovative nanoparticulate systems is shown in fig. 16.
Several kinds of NPs
Organic
Polymer-based
Polymers are macromolecules that consist of repeating units of monomers. Various synthetic groups of polymers have been employed to functionalize and medicate polymer-based NPs. Any polymer NP can be called "polymer-based NPs" but is associated explicitly with nanospheres and nano-capsules. Several materials are available for collecting NPs, including synthetic and characteristic polymers. Regular polymers, including proteins and polysaccharides, are remarkably biocompatible and biodegradable. Different benefits are available from other perspectives when using engineered polymers. Because polyethylene glycol polymers are very bio-inert, for instance, they are frequently utilized for altering the surface of NPs [254]. Using polymeric NPs for medicine delivery aims to maximize beneficial effects while minimizing detrimental ones. This reduces PEGylation and enhances NPs' susceptibility to resistant cells [254, 255]. Because PEG-covered NPs are much-dormant, they are also prepared to enter the respiratory fluid. PEG-covered particles swiftly pierced body fluid at 100 and 200 nm [256]. Due to its electrostatic tendency, polyethyleneimine, a cationic polymer, was employed as an efficient linker to nucleotides in the interim. Because of this characteristic, PEI-based NPs have emerged as a promising option for high-quality transportation for chronic respiratory diseases [255, 256]. Here are some polymer-based NPs enlisted below:
PMs
PMs are nanoscale polymeric capsules whose membranes are often believed to have a hydrophobic bilayer structure like phospholipids [257]. PMs constitute a micelle, even though amphiphilic molecules produced in solution contain macromolecules. "Organized self-assembled bodies formed in liquid and consisting of amphiphilic macromolecules, generally amphiphilic and amphiphilic blocks built of hydrophilic and hydrophobic blocks, di-block or tri-block copolymer," is how the IUPAC defines polymer micelles. Typically, PMs are spherical. PMs are different from typical micelles in the following ways. 1) Greater volume, often with a 10–100 nm diameter. 2) Lower CMC while keeping different macromolecules in balance. 3) The kinetic as well as thermodynamic stability of PMs is better [258]. Furthermore, due to their biological stability and the diversity of polymers found in PMs, it is frequently easier to create the hydrophilic and hydrophobic blocks of PMs. Polyethylene glycol, polyvinyl alcohol, poly-N-vinyl-2-pyrrolidone, poly-acrylic acid, polyacrylamide, polyglycerol, poly amino acids, and polysaccharides are among the substances that provide the hydrophilic shells. The hydrophobic blocks may usually be made from polyesters (like polyglycolic acid, polycaprolactone, and poly-D, L-lactic acid) or polyether (like polyethylene oxide and polypropylene oxide) [259].
Dendrimers
Dendrimers are artificial branching polymeric macromolecules ranging from 10 to 100 nm. They have an outer functional unit layer, an internal recurring unit layer, and a core that allows them to be polyfunctional [260, 261]. In the process of forming, dendrimers go through chemical synthesis by polymerization. Due to the spherical shape and highly adjustable surface of dendrimers, the nanocarrier is more biocompatible and degrades more readily [260, 262].
In one research, Khan et al. used a lipid replacement to encase siRNA in poly(amidoamine) (PAMAM) and poly(propylene imine) dendrimers to treat inflammatory lung disorders such as asthma and COPD. Tie-2 endothelial cells developed in the lung thanks to the combinational method's synergistic impact on the tyrosine-protein kinase receptor. The outcomes demonstrated the significant differences between PAMAM-conjugated dendrimers with C15 cholesterol tails and polyphenylene ethynylene dendrimers containing C15 and C14 cholesterol chains. Moreover, the results obtained in vivo showed that neither the toxicity nor the dendrimer-lipid compounds led to an elevation in proinflammatory cytokines in the mice [262, 263].
Lipid-based
Liposomes
The spherical, nanoscale vesicles known as liposomes have a variety of uses due to their concentric structure, which allows them to combine hydrophilic and hydrophobic drugs in a watery core and hydrophobic phospholipid carbon chains individually. The liposomes' external appearance resembles layers of biological material [264–266]. One of the beneficial characteristics of liposomal arrangements is that they use nontoxic lipids, which are easily absorbed by the body and don't constitute a health risk.
Liposomal arrangements possess favourable characteristics, such as: 1) Nontoxic lipids are employed in liposomal arrangements; these lipids are inexpensive and easily absorbed by the body [267, 268], 2) Easily fabricated in a variety of shapes and types [269], 3) To create liposomes that offer better physical stability, dispersion, and cellular destruction, several kinds of lipid combinations are available [268], 4) Liposomes also enhance the effectiveness of antifibrotic medications Because of their increased permeability, fewer adverse effects, high vascular density, and extended retention duration at infected locations [270, 271]. 5) Different medicinal and diagnostic substances are targeted for lung diseases by encapsulating them in liposomes [270, 271]. 6) simple surface modification using biocompatible and inert polymers that prolong circulation time, lessen opsonization, and shield the medication from the outside environment [272, 273].
SLNs
The colloidal carrier in this lipid form is stable at body and room temperature [274, 275]. For a long time now, SLN has been widely investigated as a possible pneumonic drug delivery system. SLNs are fluid suspensions at the nanoscale composed of physiological lipids, primarily triglycerides and phospholipids. The designs work more satisfactorily and with less harm as a pneumonic drug delivery system since they rely on using physiological components. Phospholipids are essential for breathing and are widely distributed throughout the deep regions of the lungs. Maintaining optimal surface pressure and reducing lung tissue erosion depends on phospholipid-based surfactant proteins introduced onto the alveolar surface [276, 277].
Lipid nanoemulsions
The terms "oil-in-water" (o/w) as well as "water-in-oil" (w/o) refer to heterogeneous dispersions in Nanoemulsions, where droplet sizes fall between 20 and 200 nm. Due to their small size, they have a translucent or somewhat muddy appearance and are well-stabilized against the flocculation coalescence or separate phases [278]. The benefits of NE have drawn scientists to investigate them in several domains, particularly the delivery of medicinal drugs. O/W or W/O Nanoemulsions can improve the oral bioavailability of medications like ezetimibe, ramipril, and curcumin by solubilizing hydrophilic and hydrophobic compounds [279–281]. Because Nanoemulsions are long-lasting, the formulated agents have an extended shelf life. Therapeutic substances are shielded from chemical and enzymatic influences by being encapsulated in Nanoemulsions. They are simple to make, biocompatible, and biodegradable. Specifically, because of the Nanoemulsions' rapid penetration into the skin, low viscosity, and practically translucent nature, dermal "roll-on" formulations that are sprays and gels are aesthetically pleasing and promote patient compliance [282].
NLCs
The second series of lipid carriers is called nanostructured, and they are distinguished through their unstructured solid lipid matrices. These matrices comprise liquid and solid lipids, an aqueous phase, and one or more surfactants. Aqueous phase, liquid and solid lipids, a surfactant, or a mixture of surfactants are combined to create nanostructured lipid carriers, the second generation of hydrophobic carriers. These lipid matrices are unstructured solid fat matrices [283]. NLCs come in three varieties: amorphous, imperfect, and numerous types. Because of its mass production, improved medicine safety, and use of widely accepted safe materials, NLCs are a preferred delivery system choice for the pharmaceutical sector (GRAS). Khurana et al.'s meloxicam NLC gel exhibited enhanced skin penetration, more significant dermal deposition, and a more extended release pattern than the control meloxicam gel [284]. Compared with a drug suspension exhibiting a 4-fold increase in bioavailability, Fathi et al.'s oral simvastatin NLCs showed improved and prolonged reductions in both total cholesterols along non-high-density lipoprotein [285]. In storage, SLN and NLC function differently. Since all SLN's constituents are solid lipids, crystallization creates a hard core that permits drugs to flow freely and be released into dispersion media. The disease lowers the effectiveness of trapping. Unfortunately, an imperfect core arises because NLCs are composed of solid and liquid lipids. Such a core provides adequate space for the medication's integration as well as increased drug loading. As a result, while being stored, the medication cannot escape out of the core [286].
Inorganic
MNPs
MNPs may be used in imaging and drug delivery. These particles can now serve as various operators for appealing reverberation and other complexity imaging when they gather in the targeted area. These MNPs might be directed toward on-site activities via an alluring outdoor space. Like this, using a high-force outer attractive region causes certain NPs primarily interested in metals to become hotter. It is possible to employ elevated temperatures for cell-focused operations. Additionally, MNPs were suggested and evaluated for inward breath near lung conveyance [287–290]. It is now possible to accurately control the dimensions, shape, crystal structure, and magnetic of MNPs. With MNPs, it is possible to modify or substitute the coating materials post-synthesis to regulate the outer surface potential energy, internal chemical groups, and the overall size of the NPs. The research on them is genuinely revolutionary because of MNPs' exceptional physical and chemical characteristics and their capacity to function at the cellular and molecular levels of biological systems. To treat illness intentionally and without endangering healthy tissues, the carriers in these drug delivery systems must be able to release their payloads at specific locations inside the body selectively. MNPs are a potential alternative for selective medication targeting since they may be positioned and concentrated using an external magnetic field. This reduces the number of highly toxic drug side effects that might affect healthy tissues while enabling the delivery of high-dosage drug loads to targeted tissue, such as chemotherapeutic medicines. Furthermore, preclinical and clinical research has demonstrated their safety, and the FDA has recently authorized several formulations for use in medication delivery and clinical imaging. Therefore, producing MNPs as drug conjugates has significant promise for improving cancer and inflammatory disease therapies and diagnostics [291, 292].
Gold NPs (GNPs)
GNPs are possible medication carriers. Cell structure imaging utilizes these. Many studies have been conducted on GNPs in the development of cancer treatments. Magnetic resonance imaging uses gold nanoshells containing silver or copper as a contrast agent [293].
Hybrid
Lipid polymer hybrid NPs
The hybrid NPs demonstrate synergistic effects for specific applications and introduce characteristics of the separate segments. As a result, the development of state-of-the-art combination NPs targeted at therapeutic and diagnostic illnesses has swiftly become a common topic of nanomedicine research. The research institute works on developing new types of programmable crossbreed NPs specifically engineered by amplifying tiny foci into diseased cells, leading to improved efficacy and fewer side effects from chemotherapy. In particular, the phases of crossbreed NPs can simultaneously target unhealthy cells, strengthen the region captured by optical methods, and release healing agents by summoning suffering cells [294]. The versatile nanoscale (<1000 nm) vehicles known as hybrid NPs are the result of self-organization. Half-breed NPs have improved characteristics of controlled release, directed transport, biocompatibility, delayed dispersion period, and productive surface modifications with ligands, which make them widely used in the treatment of lung diseases [295–297]. Three functional components make up hybrid NPs: (i) a hydrophobic polymeric core that contains lipophilic drug conjugates, (ii) a lipid layer encircling the core that functions as a biocompatible barrier and prevents the polymeric Center from building tranquilizers, and (iii) a hydrophilic PEG stealth coat surrounding the lipid shell. The combination of NPs' lipid-PEG shell is crucial in increasing the solidity. The PEG focuses on ligands to provide beneficial gatherings to advance change [298, 299]. Another investigation found that siRNA stacked PEG linked polyethylene imine NPs targeted at tumour integrand receptors to alleviate lung illness. Combining drugs with nanocarriers will finally increase the drug's bioavailability and reduce the effects associated with higher dosages of the drug [300].
DPIs formulation characterization
Dry powder compositions require a particular technological design and manufacturing process overall. The device's formulation-containing compartments should discharge quickly, and the dosage measurement procedure should be repeatable, thanks to the processing of the formulation. The formulation must also be adequate and acceptable for the API to deposit at the proper flow rate and reach the target region. Therefore, the drug and its carriers must be present in the patient's inhaled airstream in an adequate aerodynamic size distribution and suitably dispersed. Aside from managing the inter-particulate pressures, the formulation must also have sufficient flow characteristics. As previously observed, numerous formulations are improved to provide the necessary conditions, and specialist particle engineering methods might be used. Depending on the medication dose, its characteristics, the delivery goals, and the kind of inhaler being used, any of these several approaches can be deemed the most effective [301]. Due to the complexity of the related procedures, extensive general and extra tests are required to guarantee the formulation's effectiveness, caliber, and safety.
The tests carried out on DPI formulations can differ, as mentioned above. Two other studies that might be performed to assess the efficacy, quality, and safety of DPI formulations are stability testing and device-related testing. As previously indicated, defining the particle size is a crucial first step in creating DPIs. When assessing a dry powder formulation's particle size distribution and other attributes, the instrument used most frequently in vitro is the cascade impactor, which is listed in the Pharmacopoeia. They make excipient disruption impossible and allow for a direct evaluation of drug mass and the aerodynamic particle size across various sizes. Optical techniques can also be used to measure particle size. Light dispersion, laser Doppler, or time-of-flight are alternatives to cascade impactors, although laser diffraction is the most widely used equivalent [304]. While laser diffraction may be used to detect particle sizes, it cannot determine aerodynamic diameters; instead, cascade impactors must be used. Parameters such as the FPF and additional size fractions may be defined with NGIs, while other methods, such as laser diffraction, determine the sample's overall particle size distribution without offering any more insights. Nevertheless, since the 1980s, laser diffraction has been a technique [305] for measuring the particle size of nebulized drug solutions. It is considered a quick, exact, flow-rate-independent technique, making it a fantastic substitute for NGIs.
Table 3: Dry powder characterization
| Test | Description | References |
| Particle size determination | The decision is made either by light scattering decay or by cascade impactor. The unit of measurement for particle size is µm. | [60, 227, 302, 303] |
| In vitro Aerodynamic Assessment | It analyses variables such as particle aerodynamic behavior measured by the mass median aerodynamic diameter (MMAD). | |
| Fine Particle Fraction | Deep lung deposition appropriateness is indicated by the DPI, which quantifies the percentage of small particles discharged, usually smaller than 5 µm. | |
| Delivered dose | Ensures that the dosage provided for each actuation follows regulations and is the same as anticipated. | |
| Dose uniformity | Weighs the bottle both before and after a certain number of actuations to guarantee dosage consistency. It is computed how much each weight dosage differs. | |
| Content uniformity | Evaluate the API's consistent distribution throughout formulation. | |
| Moisture content | It uses gas chromatography or Karl-Fischer techniques to determine the moisture content. | |
| Bulk density | It uses techniques like a pycnometer to ascertain the bulk density of the DPI formulation. | |
| Tapped density | How well the powder packs and flows. | |
| Flowability | Examines how the DPI formulation's flow characteristics impact aerosol dispersion and device metering. |
By assessing a formulation's affinity for a solvent media, dissolution testing determines how well the formulation releases and absorbs drugs in the In vitro studies [304]. Evaluating the bioavailability of non-parenteral formulations requires an understanding of their dissolving characteristics. The drug's solubility, dose, particle characteristics, formulation attributes, and epithelium lining fluid composition, which varies throughout the respiratory tract, can all impact the dissolving behavior of various dosage forms. Where drug dissolution is most prominent in the lungs, tiny bronchioles and alveoli are the primary sites of drug absorption. After inhalation, the medicine will dissolve in the respiratory tract's ELF, which comprises an aqueous phase and a surfactant layer [306]. As previously demonstrated, the ELF varies along the respiratory system. The ingredients, thickness, and volume will change depending on the place. A dense coating of mucus gel, measuring 3 to 23 µm, coats the trachea, bronchi, and bronchioles; a skinny layer, detecting around 0.07 µm, covers the alveolar region. It is more difficult to determine how long the particles remain in the air by imitating lung circumstances because of physiological variations caused by the medicine's thinning of the lining fluid as it passes down the respiratory system [307]. ELF is the only fraction of the administered dosage that may breach the alveolar membrane and be absorbed after inhalation. These particles enter the non-ciliated section of the lungs and scatter in it [304]. While several novel techniques for measuring permeability and dissolution have been developed, none have become the industry standard [304, 306, 308, 309]. The flow-through cell apparatus is an additional approach that may be employed to assess the dissolution profile of inhaled formulations of poorly soluble glucocorticoids [310]. As an alternative, the most promising of the three above-mentioned techniques may be employed to examine the dissolving profile of pulmonary formulations: the Franz diffusion cell equipment [304, 311].
Separating the API from the excipients before testing is essential for accurately assessing the dissolution of an inhaled formulation. Due to the lungs' limited fluid capacity and unique functioning, this process presents significant challenges [304]. Unlike the alveolar region, the tracheobronchial section of the lungs is coated with a viscoelastic combination of proteins, glycoproteins, and lipids. However, the composition of this mucus may change in circumstances of diseases like infections [312]. The most accurate method for pulmonary testing is to use genuine SLF for a dissolving medium. In vitro testing necessitates a precise reproduction of in vivo settings. In 1979, Moss created SLF [313]. In research by Hassoun et al., an appropriate SLF was successfully built with precise characteristics, composition, and usage, along with storage instructions. Due to its physical similarities to the fluid that lines the lung, it may be utilized, among other things, for in vitro studies of the dissolution studies of inhaled medications [314].
Characterizing the physico-chemical properties of a powder formulation is also very important. In terms of the physical characteristics of the particle, stability is primarily dependent on its surface. Imaging techniques that demonstrate how a particle's shape changes while kept under certain circumstances include SEM and AFM. SEM uses an electron beam rather than light to scan the particle's surface, giving details about the particle's chemical constitution, electrical behavior, crystalline structure, and surface topography [63, 64].
FUTURE PERSPECTIVE
Chronic respiratory diseases affect individuals across various age demographics; however, the implementation of advanced therapeutic options remains insufficient. Utilizing nanoscale drug delivery systems incorporating engineered NPs presents a viable solution by minimizing toxicity and enhancing the therapeutic index. This is achieved through improved biodistribution and pharmacokinetics of conventional pharmaceuticals.
Nanoscale drug delivery systems circumvent first-pass metabolism, resulting in increased bioavailability and allowing for the administration of reduced drug dosages. These NPs are effectively delivered to the tracheobronchial region, where their substantial surface area facilitates rapid penetration into the alveolar bed. This delivery approach yields local and systemic effects while reducing adverse side effects and ensuring a prompt onset of action. Furthermore, forming aggregates among drug particles enables a sustained release profile, enhancing therapeutic outcomes. Further refinement of the innovative Nano formulation is required before conducting clinical studies to improve its therapeutic efficacy.
CONCLUSION
In conclusion, contemporary NPs represent a substantial advancement in medical therapeutics by significantly enhancing biodistribution, which refers to the efficient delivery and accumulation of therapeutic agents in targeted tissues. These NPs optimize pharmacokinetics by improving drug absorption, distribution, metabolism, and excretion, leading to more effective treatment regimens. Additionally, they promote physiological stimulation, which may enhance the body’s response to therapies. Moreover, these innovative delivery systems reduce toxicity levels associated with traditional medications by ensuring that drugs reach their intended sites of action while minimizing exposure to healthy tissues. As a result, this approach increases the therapeutic index, allowing for higher efficacy with a lower risk of adverse effects. Collectively, these advantages facilitate a transformative advancement in administering medications for chronic RDs, ultimately leading to improved patient outcomes and quality of life.
ACKNOWLEDGEMENT
Thank you to the corresponding author, Dr. Muddukrishna BS., for your constant guidance and support. Thankful to each co-authors shared their thoughts on the article draft, editing, and reviewing. Finally, I thank Manipal College of Pharmaceutical Sciences, Manipal Academy of Higher Education, and the Department of Pharmaceutical Quality Assurances for allowing me to publish this review study.
FUNDING
Nil
ABBREVIATIONS
COPD: Chronic Obstructive Pulmonary Disorder, RDs: Respiratory Disorders, GOLD: Global Initiative for Chronic Obstructive Lung Disease, AAT: Alpha-1 Antitrypsin, AIDS: Acquired Immunodeficiency Syndrome, LABA: Long-Acting Beta-Agonist, SABA: Short-Acting Beta-Agonist, LAMA: Long-Acting Muscarinic-Antagonists, SAMA; Short-Acting Muscarinic-Antagonists, ICS: Inhaled Cortico-Steroids, NPs: Nanoparticles, DPIs: Dry Powder Inhalers, MDIs: Metered Dose Inhalers, pMDIs: Pressurised Metered Dose Inhalers, BAIs: Breath Activated Metered Dose Inhalers, BCG: Bacillus Calmette Guérin, PMs: Polymeric Micelles, SLNs: Solid-Lipid Nanoparticles, NLCs: Nanostructured Lipid Carriers, MNPs: Magnetic Nanoparticles, GNPs: Gold Nanoparticles, NGIs: Next Generation Impactors, ELF: Extracellular Lipid Froth, API: Active Pharmaceutical Ingredient, SLF: Simulating Lung Fluid, SEM: Scanning Electron Microscopy, AFM: Atomic Force Microscopy.
AUTHORS CONTRIBUTIONS
The accountability of each author, including co-authors, is a significant aspect of the work. Supraja Atheriya: Literature review, Conceptualization, Methodology, Data Collecting, and Writing; Muddukrishna BS; Vasantharaju SG; Gundawar Ravi: Editing, Supervision, Evaluation; Tanvi Painginkar; Supraja Atheriya: Scientific figures; Girish Pai K, Virendra S Ligade, K Sreedhara Ranganath Pai: Reviewing, Visualization. The study is significant because of the responsibilities of all authors and co-authors. Every coauthor and author has reviewed and edited the paper.
CONFLICT OF INTERESTS
Declared none
REFERENCES
Bai Y, Liu Y, SU Z, MA Y, Ren C, Zhao R. Gene editing as a promising approach for respiratory diseases. J Med Genet. 2018;55(3):143-9. doi: 10.1136/jmedgenet-2017-104960, PMID 29301855.
COVID. COVID-19 and vascular disease. EBiomedicine. 2020;58:102966. doi: 10.1016/j.ebiom.2020.102966, PMID 32829782.
Sengupta N, Sahidullah M, Saha G. Lung sound classification using cepstral based statistical features. Comput Biol Med. 2016;75:118-29. doi: 10.1016/j.compbiomed.2016.05.013, PMID 27286184.
Restrictive lung disease: practice essentials pathophysiology etiology. Available from: https://emedicine.com/article/301760-overview?form=social.medscape. [Last accessed on 04 Feb 2025].
Martinez Pitre PJ, Sabbula BR, Cascella M. Restrictive lung disease. Preoperative Assess a Case-Based Approach. 2023:101-6.
Restrictive lung disease: overview eMedicine. Medscape. Available from: https://web.archive.org/web/20081219183106.com/article/301760-overview. [Last accessed on 04 Feb 2025].
Restrictive ventilatory defect. Med. Available from: https://oac.jhmi.edu/res_phys/Encyclopedia/RestrictVentDefect/RestrictVentDefect.HTML. [Last accessed on 04 Feb 2025].
Martinez Pitre PJ, Sabbula BR, Cascella M. Restrictive lung disease. Preoperative Assess a Case-Based Approach. 2023:101-6.
Chronic otructive pulmonary disease (COPD). Available from: https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd).
Spurzem JR, Rennard SI. Pathogenesis of COPD. Semin Respir Crit Care Med. 2005;26(2):142-53. doi: 10.1055/s-2005-869535, PMID 16088433.
Singh D, Agusti A, Anzueto A, Barnes PJ, Bourbeau J, Celli BR. Global strategy for the diagnosis management and prevention of chronic obstructive lung disease: the gold science committee report 2019. Eur Respir J. 2019;53(5):1900164. doi: 10.1183/13993003.00164-2019, PMID 30846476.
Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J. Global strategy for the diagnosis management and prevention of chronic obstructive lung disease 2017 report: gold executive summary. Eur Respir J. 2017;49(3):1700214. doi: 10.1183/13993003.00214-2017, PMID 28182564.
Mac Nee W. Pathology pathogenesis and pathophysiology. Br Med J. 2006;332(7551):1202-4. doi: 10.1136/bmj.332.7551.1202.
Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L. The nature of small airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(26):2645-53. doi: 10.1056/NEJMoa032158, PMID 15215480.
Agusti A, Hogg JC. Update on the pathogenesis of chronic obstructive pulmonary disease. N Engl J Med. 2019;381(13):1248-56. doi: 10.1056/NEJMra1900475, PMID 31553836.
MC Donough JE, Yuan R, Suzuki M, Seyednejad N, Elliott WM, Sanchez PG. Small airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med. 2011;365(17):1567-75. doi: 10.1056/NEJMoa1106955, PMID 22029978.
O Donnell DE, Lam M, Webb KA. Spirometric correlates of improvement in exercise performance after anticholinergic therapy in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160(2):542-9. doi: 10.1164/ajrccm.160.2.9901038, PMID 10430726.
Gagnon P, Guenette JA, Langer D. International journal of COPD dovepress pathogenesis of hyperinflation in chronic obstructive pulmonary disease. Int J COPD. 2014;2014:9-187.
O Donnell DE, Laveneziana P. The clinical importance of dynamic lung hyperinflation in COPD. COPD J Chronic Obstruct Pulm Dis. 2006;3(4):219-32. doi: 10.1080/15412550600977478, PMID 17361503.
Iyer KS, Newell JD, Jin D, Fuld MK, Saha PK, Hansdottir S. Quantitative dual energy computed tomography supports a vascular etiology of smoking-induced inflammatory lung disease. Am J Respir Crit Care Med. 2016;193(6):652-61. doi: 10.1164/rccm.201506-1196OC, PMID 26569033.
Kovacs G, Agusti A, Barbera JA, Celli B, Criner G, Humbert M. Pulmonary vascular involvement in chronic obstructive pulmonary disease is there a pulmonary vascular phenotype? Am J Respir Crit Care Med. 2018;198(8):1000-11. doi: 10.1164/rccm.201801-0095PP, PMID 29746142.
Zhang L, Liu Y, Zhao S, Wang Z, Zhang M, Zhang S. The incidence and prevalence of pulmonary hypertension in the COPD population: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2022;17:1365-79. doi: 10.2147/COPD.S359873, PMID 35711174.
Kovacs G, Avian A, Bachmaier G, Troester N, Tornyos A, Douschan P. Severe pulmonary hypertension in COPD: impact on survival and diagnostic approach. Chest. 2022;162(1):202-12. doi: 10.1016/j.chest.2022.01.031, PMID 35092746.
Barbera JA, Roca J, Ferrer A, Felez MA, Diaz O, Roger N. Mechanisms of worsening gas exchange during acute exacerbations of chronic obstructive pulmonary disease. Eur Respir J. 1997;10(6):1285-91. doi: 10.1183/09031936.97.10061285, PMID 9192930.
Celli BR, Fabbri LM, Aaron SD, Agusti A, Brook R, Criner GJ. An updated definition and severity classification of chronic obstructive pulmonary disease exacerbations: the rome proposal. Am J Respir Crit Care Med. 2021;204(11):1251-8. doi: 10.1164/rccm.202108-1819PP, PMID 34570991.
Parker CM, Voduc N, Aaron SD, Webb KA, O Donnell DE. Physiological changes during symptom recovery from moderate exacerbations of COPD. Eur Respir J. 2005;26(3):420-8. doi: 10.1183/09031936.05.00136304, PMID 16135722.
Miller J, Edwards LD, Agusti A, Bakke P, Calverley PM, Celli B. Comorbidity systemic inflammation and outcomes in the eclipse cohort. Respir Med. 2013;107(9):1376-84. doi: 10.1016/j.rmed.2013.05.001, PMID 23791463.
Houghton AM. Matrix metalloproteinases in destructive lung disease. Matrix Biol. 2015;44-46:167-74. doi: 10.1016/j.matbio.2015.02.002, PMID 25686691.
Gharib SA, Manicone AM, Parks WC. Matrix metalloproteinases in emphysema. Matrix Biol. 2018 Mar 23;73:34-51. doi: 10.1016/j.matbio.2018.01.018, PMID 29406250.
Siddiqui NA, Mansour MK, Nookala V. Bullous emphysema. StatPearls. Available from: https://www.ncbi.nlm.nih.gov/books/NBK537243/.
Hendrix AY, Kheradmand F. The role of matrix metalloproteinases in development repair and destruction of the lungs. Prog Mol Biol Transl Sci. 2017;148:1-29. doi: 10.1016/bs.pmbts.2017.04.004, PMID 28662821.
Wang Y, XU J, Meng Y. International journal of COPD dovepress role of inflammatory cells in airway remodeling in COPD. Int J COPD. 2018;13–3341.
Rodrigues SO, Cunha CM, Soares GM, Silva PL, Silva AR, Goncalves DE Albuquerque CF. Mechanisms pathophysiology and currently proposed treatments of chronic obstructive pulmonary disease. Pharmaceuticals. 2021;14(10):979. doi: 10.3390/ph14100979, PMID 34681202.
Burgstaller G, Oehrle B, Gerckens M, White ES, Schiller HB, Eickelberg O. The instructive extracellular matrix of the lung: basic composition and alterations in chronic lung disease. Eur Respir J. 2017;50(1):1601805. doi: 10.1183/13993003.01805-2016, PMID 28679607.
MC Donough JE, Yuan R, Suzuki M, Seyednejad N, Elliott WM, Sanchez PG. Small airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med. 2011;365(17):1567-75. doi: 10.1056/NEJMoa1106955, PMID 22029978.
Rodrigues SO, Cunha CM, Soares GM, Silva PL, Silva AR, Goncalves DE Albuquerque CF. Mechanisms pathophysiology and currently proposed treatments of chronic obstructive pulmonary disease. Pharmaceuticals (Basel). 2021;14(10):979. doi: 10.3390/ph14100979, PMID 34681202.
Buist AS, MC Burnie MA, Vollmer WM, Gillespie S, Burney P, Mannino DM. International variation in the prevalence of COPD (The BOLD Study): a population-based prevalence study. Lancet. 2007;370(9589):741-50. doi: 10.1016/S0140-6736(07)61377-4, PMID 17765523.References
GOLD report global initiative for chronic obstructive lung disease gold. Available from: https://goldcopd/.org/2024-gold-report.
2023 GOLD report global initiative for chronic obstructive lung disease gold. Available from: https://goldcopd/.org/2023-gold-report-2.
Chronic obstructive pulmonary disease (COPD). Available from: https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd).References
Miravitlles M, Soler Cataluna JJ, Calle M, Molina J, Almagro P, Quintano JA. Spanish guidelines for management of chronic obstructive pulmonary disease (GesEPOC) 2017. Pharmacological treatment of stable phase. Arch Bronconeumol. 2017 Jun;53(6):324-35. doi: 10.1016/j.arbres.2017.03.018, PMID 28477954.
GOLD report global initiative for chronic obstructive lung disease GOLD; 2024. Available from: https://goldcopd.org/2024-gold-report/.
Aisanov Z, Avdeev S, Arkhipov V, Belevskiy A, Chuchalin A, Leshchenko I. Russian guidelines for the management of COPD: algorithm of pharmacologic treatment. Int J Chron Obstruct Pulmon Dis. 2018 Jan 8;13:183-7. doi: 10.2147/COPD.S153770, PMID 29386887.
Sliwinski P, Gorecka D, Jassem E, Pierzchala W. Polish respiratory society guidelines for chronic obstructive pulmonary disease. Pneumonol Alergol Pol. 2014;82(3):227-63. doi: 10.5603/PiAP.2014.0030, PMID 24793150.
Pleguezuelos E, Gimeno Santos E, Hernandez C, Mata MD, Palacios L, Pinera P. Recommendations on non-pharmacological treatment in chronic obstructive pulmonary disease from the spanish COPD guidelines (GesEPOC 2017). Arch Bronconeumol. 2018;54(11):568-75. doi: 10.1016/j.arbres.2018.06.001, PMID 30241689.
Zysman M, Chabot F, Housset B, Morelot Panzini C, Devillier P, Roche N. Pharmacological treatment optimisation for stable COPD: an endless story? proposals from the societe de pneumologie de langue francaise. Eur Respir J. 2017;50(4):1701250. doi: 10.1183/13993003.01250-2017, PMID 29025880.
Vukoja M, Kopitovic I, Lazic Z, Milenkovic B, Stankovic I, Zvezdin B. Diagnosis and management of chronic obstructive pulmonary disease in Serbia: an expert group position statement. Int J Chron Obstruct Pulmon Dis. 2019;14:1993-2002. doi: 10.2147/COPD.S214690, PMID 31564847.
Miravitlles M, Vogelmeier C, Roche N, Halpin D, Cardoso J, Chuchalin AG. A review of national guidelines for management of COPD in Europe. Eur Respir J. 2016;47(2):625-37. doi: 10.1183/13993003.01170-2015, PMID 26797035.
Wedzicha JA, Miravitlles M, Hurst JR, Calverley PM, Albert RK, Anzueto A. anagement of COPD exacerbations: a European respiratory society/American thoracic society guideline. Eur Respir J. 2017;49(3):1600791. doi: 10.1183/13993003.00791-2016, PMID 28298398.
Kankaanranta H, Harju T, Kilpelainen M, Mazur W, Lehto JT, Katajisto M. Diagnosis and pharmacotherapy of stable chronic obstructive pulmonary disease: the finnish guidelines. Basic Clin Pharmacol Toxicol. 2015;116(4):291-307. doi: 10.1111/bcpt.12366, PMID 25515181.
COPD medications: types benefits and side effects. Available from: https://www.healthgrades.com/right-care/copd/10-drugs-commonly-prescribed-for-copd.
Sathish MS. Pharm M. Formulation and evaluation of dry powder inhalers of fluticasone propionate for pulmonary delivery. Available from: http://repository-tnmgrmu.ac.in/20122/1/261104619senkathir.pdf.
Desai H, Dalal B. Management options in chronic obstructive pulmonary disease. Clinical Medicine Insights: Therapeutics. 2012;4. doi: 10.4137/CMT.S6563.
Gold Report; 2021. Available from: https://goldcopd.org/wp-content/uploads/2020/11/GOLD-REPORT-2021-v1.1-25Nov20_WMV.pdf.
Singh D, Agusti A, Anzueto A, Barnes PJ, Bourbeau J, Celli BR. Global strategy for the diagnosis management and prevention of chronic obstructive lung disease: the gold science committee report 2019. Eur Respir J. 2019;53(5):1900164. doi: 10.1183/13993003.00164-2019, PMID 30846476.
Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J. Global strategy for the diagnosis management and prevention of chronic obstructive lung disease 2017 report: gold executive summary. Eur Respir J. 2017;49(3):1700214. doi: 10.1183/13993003.00214-2017, PMID 28182564.
Halpin DM, Criner GJ, Papi A, Singh D, Anzueto A, Martinez FJ. Global initiative for the diagnosis, management and prevention of chronic obstructive lung disease. the 2020 gold science committee report on COVID-19 and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2021;203(1):24-36. doi: 10.1164/rccm.202009-3533SO, PMID 33146552.
Magramane S, Papay Z, Turbucz B, Antal I. Formulation and characterization of pulmonary drug delivery systems. Acta Pharm Hung. 2019;89(2):63-83. doi: 10.33892/aph.2019.89.63-83.
Darquenne C. Aerosol deposition in health and disease. J Aerosol Med Pulm Drug Deliv. 2012;25(3):140-7. doi: 10.1089/jamp.2011.0916, PMID 22686623.
Rangaraj N, Pailla SR, Sampathi S. Insight into pulmonary drug delivery: mechanism of drug deposition to device characterization and regulatory requirements. Pulm Pharmacol Ther. 2019 Feb;54:1-21. doi: 10.1016/j.pupt.2018.11.004, PMID 30447295.
Darquenne C. Deposition mechanisms. J Aerosol Med Pulm Drug Deliv. 2020;33(4):181-5. doi: 10.1089/jamp.2020.29029.cd, PMID 32598200.
Jessamine V, Mehndiratta S, DE Rubis G, Paudel KR, Shetty S, Suares D. The application of nanoparticles as advanced drug delivery systems in attenuating COPD. Heliyon. 2024;10(3):e25393. doi: 10.1016/j.heliyon.2024.e25393, PMID 38356590.
Shetty N, Cipolla D, Park H, Zhou QT. Physical stability of dry powder inhaler formulations. Expert Opin Drug Deliv. 2020;17(1):77-96. doi: 10.1080/17425247.2020.1702643, PMID 31815554.
Vernon Parry KD. Scanning electron microscopy: an introduction. III-Vs Review. 2000;13(4):40-4. doi: 10.1016/S0961-1290(00)80006-X.
Gipsman AI, Lapinel NC, Mayer OH. Airway clearance in patients with neuromuscular disease. Paediatr Respir Rev. 2023 Sep;47:33-40. doi: 10.1016/j.prrv.2023.02.002, PMID 36894356.
Restrepo RD. Inhaled adrenergics and anticholinergics in obstructive lung disease: do they enhance mucociliary clearance. Respir Care. 2007;52(9):1159-73. PMID 17716384.
O Donnell DE, Milne KM, James MD, DE Torres JP, Neder JA. Dyspnea in COPD: new mechanistic insights and management implications. Adv Ther. 2020;37(1):41-60. doi: 10.1007/s12325-019-01128-9, PMID 31673990.
Chronic obstructive pulmonary disease (COPD) Straight to the point of care. Cleveland Clinic; 2023.
Han MK, Host MFJ. Gender and early life factors as risks for chronic obstructive pulmonary disease. Clinics in Chest Medicine. 2020;41(3):329-37. doi: 10.1016/j.ccm.2020.06.009.
Tobacco. Available from: https://www.who.int/news-room/fact-sheets/detail/tobacco.references.
Patel MP, Khangoora VS, Marik PE. A review of the pulmonary and health impacts of hookah use. Ann Am Thorac Soc. 2019;16(10):1215-9. doi: 10.1513/AnnalsATS.201902-129CME, PMID 31091965.
Clementi EA, Talusan A, Vaidyanathan S, Veerappan A, Mikhail M, Ostrofsky D. Metabolic syndrome and air pollution: a narrative review of their cardiopulmonary effects. Toxics. 2019;7(1):6. doi: 10.3390/toxics7010006, PMID 30704059.
Pirozzi C, Scholand MB. Smoking cessation and environmental hygiene. Med Clin North Am. 2012;96(4):849-67. doi: 10.1016/j.mcna.2012.04.014, PMID 22793948.
COPD causes occupations and substances. Available from: https://www.hse.gov.uk/copd/causes.htm#substances.
Teresa H. Mosbys pharmacy technician-e-book: principles and practice; 2014. p. 1136.
Brode SK, Ling SC, Chapman KR. Alpha-1 antitrypsin deficiency: a commonly overlooked cause of lung disease. CMAJ. 2012;184(12):1365-71. doi: 10.1503/cmaj.111749, PMID 22761482.
Foreman MG, Campos M, Celedon JC. Genes and chronic obstructive pulmonary disease. Med Clin North Am. 2012;96(4):699-711. doi: 10.1016/j.mcna.2012.02.006, PMID 22793939.
COPD. Causes and risk factors NHLBI, NIH, https://www.nhlbi.nih.gov/health/copd/causes.
Salvi S, Ghorpade D. COPD in never smokers. In: Encyclopedia of respiratory medicine. 2nd ed. Elsevier; 2021. p. 686-701.
Ruvuna L, Sood A. Epidemiology of chronic obstructive pulmonary disease. Clin Chest Med. 2020;41(3):315-27. doi: 10.1016/j.ccm.2020.05.002, PMID 32800187.
Maselli DJ, Bhatt SP, Anzueto A, Bowler RP, DE Meo DL, Diaz AA. Clinical epidemiology of COPD: insights from 10 y of the copdgene study. Chest. 2019;156(2):228-38. doi: 10.1016/j.chest.2019.04.135, PMID 31154041.
Sarath Chandran C, Raj A, Shahin Muhammed TK. Probing the cellular and molecular mechanisms underlying in the pathogenesis of chronic obstructive pulmonary disease. Target Cell Signal Pathw Lung Dis. 2021;7:147-62.
Herr C, Lungarella G, Puttur F. Mechanisms of virus-induced airway immunity dysfunction in the pathogenesis of COPD disease progression and exacerbation. Front Immunol. 2020 Jun 16;11:1205. doi: 10.3389/fimmu.2020.01205.
Bordi L, Vulcano A, Sberna G, Nonis M, Giacomini P, Maggi F. Co-circulation of SARS-CoV-2 and other respiratory pathogens in upper and lower respiratory tracts during influenza season 2022-2023 in lazio region. Microorganisms. 2023;11(9):2239. doi: 10.3390/microorganisms11092239, PMID 37764083.
Rodriguez Roisin R, Drakulovic M, Rodriguez DA, Roca J, Barbera JA, Wagner PD. Ventilation perfusion imbalance and chronic obstructive pulmonary disease staging severity. J Appl Physiol 1985. 2009;106(6):1902-8. doi: 10.1152/japplphysiol.00085.2009, PMID 19372303.
Elbehairy AF, Ciavaglia CE, Webb KA, Guenette JA, Jensen D, Mourad SM. Pulmonary gas exchange abnormalities in mild chronic obstructive pulmonary disease implications for dyspnea and exercise intolerance. Am J Respir Crit Care Med. 2015;191(12):1384-94. doi: 10.1164/rccm.201501-0157OC, PMID 25826478.
Colak Y, Nordestgaard BG, Vestbo J, Lange P, Afzal S. Prognostic significance of chronic respiratory symptoms in individuals with normal spirometry. Eur Respir J. 2019;54(3). doi: 10.1183/13993003.00734-2019.
Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH. Multi-ethnic reference values for spirometry for the 3-95 y age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324-43. doi: 10.1183/09031936.00080312, PMID 22743675.
Walker PP, Calverley PM. The volumetric response to bronchodilators in stable chronic obstructive pulmonary disease. COPD. 2008;5(3):147-52. doi: 10.1080/15412550802092928, PMID 18568838.
Calverley PM, Albert P, Walker PP. Bronchodilator reversibility in chronic obstructive pulmonary disease: use and limitations. Lancet Respir Med. 2013;1(7):564-73. doi: 10.1016/S2213-2600(13)70086-9, PMID 24461617.
Enright PL, Beck KC, Sherrill DL. Repeatability of spirometry in 18,000 adult patients. Am J Respir Crit Care Med. 2004;169(2):235-8. doi: 10.1164/rccm.200204-347OC, PMID 14604836.
Fortis S, Eberlein M, Georgopoulos D, Comellas AP. Predictive value of prebronchodilator and postbronchodilator spirometry for COPD features and outcomes. BMJ Open Respir Res. 2017;4(1):e000213. doi: 10.1136/bmjresp-2017-000213, PMID 29435342.
Buhr RG, Barjaktarevic IZ, Quibrera PM, Bateman LA, Bleecker ER, Couper DJ. Reversible airflow obstruction predicts future chronic obstructive pulmonary disease development in the spiromics cohort: an observational cohort study. Am J Respir Crit Care Med. 2022;206(5):554-62. doi: 10.1164/rccm.202201-0094OC, PMID 35549640.
Oba Y, Keeney E, Ghatehorde N, Dias S. Dual combination therapy versus long-acting bronchodilators alone for chronic obstructive pulmonary disease (COPD): a systematic review and network meta-analysis. Cochrane Database Syst Rev. 2018;12(12):CD012620. doi: 10.1002/14651858.CD012620.pub2, PMID 30521694.
Maltais F, Bjermer L, Kerwin EM, Jones PW, Watkins ML, Tombs L. Efficacy of umeclidinium/vilanterol versus umeclidinium and salmeterol monotherapies in symptomatic patients with COPD not receiving inhaled corticosteroids: the Emax randomised trial. Respir Res. 2019;20(1):238. doi: 10.1186/s12931-019-1193-9, PMID 31666084.
Lange P, Marott JL, Vestbo J, Olsen KR, Ingebrigtsen TS, Dahl M. Prediction of the clinical course of chronic obstructive pulmonary disease using the new gold classification. Am J Respir Crit Care Med. 2012;186(10):975-81. doi: 10.1164/rccm.201207-1299OC.
Lipson DA, Barnhart F, Brealey N, Brooks J, Criner GJ, Day NC. Once daily single inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378(18):1671-80. doi: 10.1056/NEJMoa1713901, PMID 29668352.
Rabe KF, Martinez FJ, Ferguson GT, Wang C, Singh D, Wedzicha JA. Triple inhaled therapy at two glucocorticoid doses in moderate to very severe COPD. N Engl J Med. 2020;383(1):35-48. doi: 10.1056/NEJMoa1916046, PMID 32579807.
W H Kocks J, Wouters H, Bosnic Anticevich S, Van Cooten J, Correia DE Sousa J, Cvetkovski B. Factors associated with health status and exacerbations in COPD maintenance therapy with dry powder inhalers. NPJ Prim Care Respir Med. 2022;32(1):18. doi: 10.1038/s41533-022-00282-y, PMID 35618739.
Vestbo J, Anderson J, Brook RD, Calverley PM, Celli BR, Crim C. The study to understand mortality and morbidity in copd (summit) study protocol. Eur Respir J. 2013;41(5):1017-22. doi: 10.1183/09031936.00087312, PMID 23018908.
Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775-89. doi: 10.1056/NEJMoa063070, PMID 17314337.
Rabe KF, Martinez FJ, Ferguson GT, Wang C, Singh D, Wedzicha JA. Triple inhaled therapy at two glucocorticoid doses in moderate to very severe COPD. N Engl J Med. 2020;383(1):35-48. doi: 10.1056/NEJMoa1916046, PMID 32579807.
Lipson DA, Barnhart F, Brealey N, Brooks J, Criner GJ, Day NC. Once daily single inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378(18):1671-80. doi: 10.1056/NEJMoa1713901, PMID 29668352.
Chrystyn H, Mulley BA, Peake MD. Dose-response relation to oral theophylline in severe chronic obstructive airways disease. BMJ. 1988;297(6662):1506-10. doi: 10.1136/bmj.297.6662.1506, PMID 3147048.
Gross NJ, Petty TL, Friedman M, Skorodin MS, Silvers GW, Donohue JF. Dose response to ipratropium as a nebulized solution in patients with chronic obstructive pulmonary disease a three center study. Am Rev Respir Dis. 1989;139(5):1188-91. doi: 10.1164/ajrccm/139.5.1188, PMID 2523681.
Higgins BG, Powell RM, Cooper S, Tattersfield AE. Effect of salbutamol and ipratropium bromide on airway calibre and bronchial reactivity in asthma and chronic bronchitis PubMed. Eur Respir J. 1991;4(4):415-20. PMID 1830277.
Donohue JF, Anzueto A, Brooks J, Mehta R, Kalberg C, Crater G. A randomized double blind dose ranging study of the novel LAMA GSK573719 in patients with COPD. Respir Med. 2012;106(7):970-9. doi: 10.1016/j.rmed.2012.03.012, PMID 22498110.
Donohue JF, Kalberg C, Shah P, Beerahee M, Mehta R, Gunawan R. Dose response of umeclidinium administered once or twice daily in patients with COPD: a pooled analysis of two randomized double blind placebo controlled studies. J Clin Pharmacol. 2014;54(11):1214-20. doi: 10.1002/jcph.340, PMID 24895108.
Chowdhury BA, Seymour SM, Michele TM, Durmowicz AG, Liu D, Rosebraugh CJ. The risks and benefits of indacaterol the FDA’s review. N Engl J Med. 2011;365(24):2247-9. doi: 10.1056/NEJMp1109621, PMID 22168640.
O Donnell DE, Sciurba F, Celli B, Mahler DA, Webb KA, Kalberg CJ. Effect of fluticasone propionate/salmeterol on lung hyperinflation and exercise endurance in COPD. Chest. 2006;130(3):647-56. doi: 10.1378/chest.130.3.647, PMID 16963658.
Kempsford R, Norris V, Siederer S. Vilanterol trifenatate a novel inhaled long-acting beta2 adrenoceptor agonist is well tolerated in healthy subjects and demonstrates prolonged bronchodilation in subjects with asthma and COPD. Pulm Pharmacol Ther. 2013;26(2):256-64. doi: 10.1016/j.pupt.2012.12.001, PMID 23232038.
Koch A, Pizzichini E, Hamilton A, Hart L, Korducki L, DE Salvo MC. Lung function efficacy and symptomatic benefit of olodaterol once daily delivered via respimat® versus placebo and formoterol twice daily in patients with gold 2-4 COPD: results from two replicate 48-week studies. Int J Chron Obstruct Pulmon Dis. 2014 Jul 5;9:697-714. doi: 10.2147/COPD.S62502, PMID 25045258.
Cazzola M, Rogliani P, Ruggeri P, Segreti A, Proietto A, Picciolo S. Chronic treatment with indacaterol and airway response to salbutamol in stable COPD. Respir Med. 2013;107(6):848-53. doi: 10.1016/j.rmed.2013.02.008, PMID 23490225.
Sestini P, Renzoni E, Robinson S. Short-acting beta2 agonists for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2002 Jul 22:2002(3):CD001495. doi: 10.1002/14651858.CD001495.
Donohue JF, Anzueto A, Brooks J, Mehta R, Kalberg C, Crater G. A randomized, double-blind dose-ranging study of the novel LAMA GSK573719 in patients with COPD. Respir Med. 2012;106(7):970-9. doi: 10.1016/j.rmed.2012.03.012, PMID 22498110.
O Driscoll BR, Kay EA, Taylor RJ, Weatherby H, Chetty MC, Bernstein A. A long-term prospective assessment of home nebulizer treatment. Respir Med. 1992;86(4):317-25. doi: 10.1016/s0954-6111(06)80031-4, PMID 1448587.
Jenkins SC, Heaton RW, Fulton TJ, Moxham J. Comparison of domiciliary nebulized salbutamol and salbutamol from a metered dose inhaler in stable chronic airflow limitation. Chest. 1987;91(6):804-7. doi: 10.1378/chest.91.6.804, PMID 3556051.
Chowdhury BA, Seymour SM, Michele TM, Durmowicz AG, Liu D, Rosebraugh CJ. The risks and benefits of indacaterol the FDA’s review. N Engl J Med. 2011;365(24):2247-9. doi: 10.1056/NEJMp1109621, PMID 22168640.
Donohue JF, Anzueto A, Brooks J, Mehta R, Kalberg C, Crater G. A randomized double-blind dose ranging study of the novel LAMA GSK573719 in patients with COPD. Respir Med. 2012;106(7):970-9. doi: 10.1016/j.rmed.2012.03.012, PMID 22498110.
Vathenen AS, Britton JR, Ebden P, Cookson JB, Wharrad HJ, Tattersfield AE. High-dose inhaled albuterol in severe chronic airflow limitation. Am Rev Respir Dis. 1988;138(4):850-5. doi: 10.1164/ajrccm/138.4.850, PMID 2462383.
Melani AS. Long-acting muscarinic antagonists. Expert Rev Clin Pharmacol. 2015;8(4):479-501. doi: 10.1586/17512433.2015.1058154, PMID 26109098.
Decramer ML, Chapman KR, Dahl R, Frith P, Devouassoux G, Fritscher C. Once daily indacaterol versus tiotropium for patients with severe chronic obstructive pulmonary disease (invigorate): a randomised blinded, parallel group study. Lancet Respir Med. 2013;1(7):524-33. doi: 10.1016/S2213-2600(13)70158-9, PMID 24461613.
Vogelmeier C, Hederer B, Glaab T, Schmidt H, Rutten Van Molken MP, Beeh KM. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med. 2011;364(12):1093-103. doi: 10.1056/NEJMoa1008378, PMID 21428765.
Casaburi R, Kukafka D, Cooper CB, Witek TJ, Kesten S. Improvement in exercise tolerance with the combination of tiotropium and pulmonary rehabilitation in patients with COPD. Chest. 2005;127(3):809-17. doi: 10.1378/chest.127.3.809, PMID 15764761.
Calzetta L, Ritondo BL, Zappa MC, Manzetti GM, Perduno A, Shute J. The impact of long acting muscarinic antagonists on mucus hypersecretion and cough in chronic obstructive pulmonary disease: a systematic review. Eur Respir Rev. 2022;31(164):210196. doi: 10.1183/16000617.0196-2021, PMID 35508331.
Karner C, Chong J, Poole P. Tiotropium versus placebo for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;11(7):CD009285. doi: 10.1002/14651858.CD009285.pub3.
Appleton S, Jones T, Poole P, Pilotto L, Adams R, Lasserson TJ. Ipratropium bromide versus long-acting beta-2 agonists for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006;2006(3):CD006101. doi: 10.1002/14651858.CD006101, PMID 16856113.
Suissa S, Dell Aniello S, Ernst P. Comparative effectiveness and safety of LABA-LAMA vs LABA-ICS treatment of COPD in real-world clinical practice. Chest. 2019;155(6):1158-65. doi: 10.1016/j.chest.2019.03.005, PMID 30922950.
Boardman C, Chachi L, Gavrila A, Keenan CR, Perry MM, Xia YC. Mechanisms of glucocorticoid action and insensitivity in airways disease. Pulm Pharmacol Ther. 2014;29(2):129-43. doi: 10.1016/j.pupt.2014.08.008, PMID 25218650.
Barnes PJ. New anti-inflammatory targets for chronic obstructive pulmonary disease. Nat Rev Drug Discov. 2013;12(7):543-59. doi: 10.1038/nrd4025, PMID 23977698.
Wedzicha JA, Banerji D, Chapman KR, Vestbo J, Roche N, Ayers RT. Indacaterol glycopyrronium versus salmeterol fluticasone for COPD. N Engl J Med. 2016;374(23):2222-34. doi: 10.1056/NEJMoa1516385, PMID 27181606.
Lipson DA, Barnhart F, Brealey N, Brooks J, Criner GJ, Day NC. Once daily single inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378(18):1671-80. doi: 10.1056/NEJMoa1713901, PMID 29668352.
Sonnex K, Alleemudder H, Knaggs R. Impact of smoking status on the efficacy of inhaled corticosteroids in chronic obstructive pulmonary disease: a systematic review. BMJ Open. 2020;10(4):e037509. doi: 10.1136/BMJOPEN-2020-037509, PMID 32300001.
Manson SC, Brown RE, Cerulli A, Vidaurre CF. The cumulative burden of oral corticosteroid side effects and the economic implications of steroid use. Respir Med. 2009;103(7):975-94. doi: 10.1016/j.rmed.2009.01.003, PMID 19372037.
Walters JA, Tan DJ, White CJ, Gibson PG, Wood Baker R, Walters EH. Systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;2014(9):CD001288. doi: 10.1002/14651858.CD001288.pub4, PMID 25178099.
Rice K, Rubins J, Lebahn F, Parenti C, Duane P, Kuskowski M. Withdrawal of chronic systemic corticosteroids in patients with COPD. Am J Respir Crit Care Med. 2000;162(1):174-8. doi: 10.1164/ajrccm.162.1.9909066.
Uzun S, Djamin RS, Kluytmans JA, Mulder PG, Vant Veer NE, Ermens AA. Azithromycin maintenance treatment in patients with frequent exacerbations of chronic obstructive pulmonary disease (Columbus): a randomised double-blind placebo controlled trial. Lancet Respir Med. 2014;2(5):361-8. doi: 10.1016/S2213-2600(14)70019-0, PMID 24746000.
Rabe KF. Update on roflumilast a phosphodiesterase 4 inhibitor for the treatment of chronic obstructive pulmonary disease. Br J Pharmacol. 2011;163(1):53-67. doi: 10.1111/j.1476-5381.2011.01218.x, PMID 21232047.
Calverley PM, Rabe KF, Goehring UM, Kristiansen S, Fabbri LM, Martinez FJ. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet. 2009;374(9691):685-94. doi: 10.1016/S0140-6736(09)61255-1, PMID 19716960.
Martinez FJ, Calverley PM, Goehring UM, Brose M, Fabbri LM, Rabe KF. Effect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): a multicentre randomised controlled trial. Lancet. 2015;385(9971):857-66. doi: 10.1016/S0140-6736(14)62410-7, PMID 25684586.References
Fabbri LM, Calverley PM, Izquierdo Alonso JL, Bundschuh DS, Brose M, Martinez FJ. Roflumilast in moderate to severe chronic obstructive pulmonary disease treated with longacting bronchodilators: two randomised clinical trials. Lancet. 2009;374(9691):695-703. doi: 10.1016/S0140-6736(09)61252-6, PMID 19716961.
Rabe KF, Calverley PM, Martinez FJ, Fabbri LM. Effect of roflumilast in patients with severe COPD and a history of hospitalisation. Eur Respir J. 2017;50(1):1700158. doi: 10.1183/13993003.00158-2017, PMID 28679611.
Albert RK, Connett J, Bailey WC, Casaburi R, Cooper JA, Criner GJ. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365(8):689-98. doi: 10.1056/NEJMoa1104623, PMID 21864166.
Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta(2) agonist in one inhaler versus long-acting beta(2) agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013 Aug 30;2013(8):CD006829. doi: 10.1002/14651858.CD006829.pub2, PMID 22972099.
Nannini LJ, Poole P, Milan SJ, Kesterton A. Combined corticosteroid and long-acting beta(2) agonist in one inhaler versus inhaled corticosteroids alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013;8:CD006826. doi: 10.1002/14651858.CD006826.pub2, PMID 23990350.
Vestbo J, Anderson JA, Brook RD, Calverley PM, Celli BR, Crim C. Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): a double-blind randomised controlled trial. Lancet. 2016;387(10030):1817-26. doi: 10.1016/S0140-6736(16)30069-1, PMID 27203508.
Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775-89. doi: 10.1056/NEJMoa063070, PMID 17314337.
Vestbo J, Leather D, Diar Bakerly N, New J, Gibson JM, MC Corkindale S. Effectiveness of fluticasone furoate vilanterol for COPD in clinical practice. N Engl J Med. 2016;375(13):1253-60. doi: 10.1056/NEJMoa1608033, PMID 27593504.
Brusselle G, Price D, Gruffydd Jones K. The inevitable drift to triple therapy in COPD: an analysis of prescribing pathways in the UK. Int J COPD. 2015 Oct 15;10:2207-17. doi: 10.2147/COPD.S91694.
Singh D, Brooks J, Hagan G, Cahn A, O Connor BJ. Superiority of triple therapy with salmeterol/fluticasone propionate and tiotropium bromide versus individual components in moderate to severe COPD. Thorax. 2008;63(7):592-8. doi: 10.1136/thx.2007.087213, PMID 18245142.
Welte T, Miravitlles M, Hernandez P, Eriksson G, Peterson S, Polanowski T. Efficacy and tolerability of budesonide/formoterol added to tiotropium in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;180(8):741-50. doi: 10.1164/rccm.200904-0492OC, PMID 19644045.
Singh D, Papi A, Corradi M, Pavlisova I, Montagna I, Francisco C. Single inhaler triple therapy versus inhaled corticosteroid plus long-acting β2-agonist therapy for chronic obstructive pulmonary disease (TRILOGY): a double-blind parallel group randomised controlled trial. Lancet. 2016;388(10048):963-73. doi: 10.1016/S0140-6736(16)31354-X, PMID 27598678.
Siler TM, Kerwin E, Singletary K, Brooks J, Church A. Efficacy and safety of umeclidinium added to fluticasone propionate/salmeterol in patients with COPD: results of two randomized double blind studies. COPD. 2016;13(1):1-10. doi: 10.3109/15412555.2015.1034256, PMID 26451734.
Lipson DA, Barnacle H, Birk R, Brealey N, Locantore N, Lomas DA. FULFIL trial: once daily triple therapy for patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;196(4):438-46. doi: 10.1164/rccm.201703-0449OC, PMID 28375647.
Frith PA, Thompson PJ, Ratnavadivel R, Chang CL, Bremner P, Day P. Glycopyrronium once daily significantly improves lung function and health status when combined with salmeterol/fluticasone in patients with COPD: the GLISTEN study a randomised controlled trial. Thorax. 2015;70(6):519-27. doi: 10.1136/thoraxjnl-2014-206670, PMID 25841237.
Hanania NA, Crater GD, Morris AN, Emmett AH, O Dell DM, Niewoehner DE. Benefits of adding fluticasone propionate/salmeterol to tiotropium in moderate to severe COPD. Respir Med. 2012;106(1):91-101. doi: 10.1016/j.rmed.2011.09.002, PMID 22040533.
Jung KS, Park HY, Park SY, Kim SK, Kim YK, Shim JJ. Comparison of tiotropium plus fluticasone propionate/salmeterol with tiotropium in COPD: a randomized controlled study. Respir Med. 2012;106(3):382-9. doi: 10.1016/j.rmed.2011.09.004, PMID 21975275.
Vestbo J, Papi A, Corradi M, Blazhko V, Montagna I, Francisco C. Single inhaler extrafine triple therapy versus long-acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (Trinity): a double-blind parallel-group randomised controlled trial. Lancet. 2017;389(10082):1919-29. doi: 10.1016/S0140-6736(17)30188-5, PMID 28385353.
Lipson DA, Barnhart F, Brealey N, Brooks J, Criner GJ, Day NC. Once daily single inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378(18):1671-80. doi: 10.1056/NEJMoa1713901, PMID 29668352.
Papi A, Vestbo J, Fabbri L, Corradi M, Prunier H, Cohuet G. Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (Tribute): a double-blind parallel group randomised controlled trial. Lancet. 2018;391(10125):1076-84. doi: 10.1016/S0140-6736(18)30206-X, PMID 29429593.
Bardsley S, Criner GJ, Halpin DM, Han MK, Hanania NA, Hill D. Single inhaler triple therapy fluticasone furoate/umeclidinium/vilanterol versus dual therapy in current and former smokers with COPD: impact trial post hoc analysis. Respir Med. 2022 Dec;205:107040. doi: 10.1016/j.rmed.2022.107040, PMID 36470149.
Vestbo J, Fabbri L, Papi A, Petruzzelli S, Scuri M, Guasconi A. Inhaled corticosteroid containing combinations and mortality in COPD. Eur Respir J. 2018;52(6):1801230. doi: 10.1183/13993003.01230-2018, PMID 30209195.
Francis RS, Spicer CC. Chemotherapy in chronic bronchitis. Influence of daily penicillin and tetracycline on exacerbations and their cost. Br Med J. 1960;1(5169):297-303. doi: 10.1136/bmj.1.5169.297, PMID 13824401.
Francis RS, May JR, Spicer CC. Chemotherapy of bronchitis influence of penicillin and tetracycline administered daily or intermittently for exacerbations a report to the research committee of the British Tuberculosis Association by its bronchitis subcommittee. Br Med J. 1961;2(5258):979-85. doi: 10.1136/bmj.2.5258.979, PMID 13894512.
Johnston RN, MC Neill RS, Smith DH, Dempster MB, Nairn JR, Purvis MS. Five-year winter chemoprophylaxis for chronic bronchitis. Br Med J. 1969;4(5678):265-9. doi: 10.1136/bmj.4.5678.265, PMID 4899454.
NI W, Shao X, Cai X, Wei C, Cui J, Wang R. Prophylactic use of macrolide antibiotics for the prevention of chronic obstructive pulmonary disease exacerbation: a meta-analysis. Plos One. 2015;10(3):e0121257. doi: 10.1371/journal.pone.0121257, PMID 25812085.
Herath SC, Poole P. Prophylactic antibiotic therapy for chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev. 2018 Oct 30;10(10):CD009764. doi: 10.1002/14651858.CD009764.pub3.
Uzun S, Djamin RS, Kluytmans JA, Mulder PG, Vant Veer NE, Ermens AA. Azithromycin maintenance treatment in patients with frequent exacerbations of chronic obstructive pulmonary disease (COLUMBUS): a randomised double-blind placebo controlled trial. Lancet Respir Med. 2014;2(5):361-8. doi: 10.1016/S2213-2600(14)70019-0, PMID 24746000.
Seemungal TA, Wilkinson TM, Hurst JR, Perera WR, Sapsford RJ, Wedzicha JA. Long-term erythromycin therapy is associated with decreased chronic obstructive pulmonary disease exacerbations. Am J Respir Crit Care Med. 2008;178(11):1139-47. doi: 10.1164/rccm.200801-145OC, PMID 18723437.
Albert RK, Connett J, Bailey WC, Casaburi R, Cooper JA, Criner GJ. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365(8):689-98. doi: 10.1056/NEJMoa1104623, PMID 21864166.
Han MK, Tayob N, Murray S, Dransfield MT, Washko G, Scanlon PD. Predictors of chronic obstructive pulmonary disease exacerbation reduction in response to daily azithromycin therapy. Am J Respir Crit Care Med. 2014;189(12):1503-8. doi: 10.1164/rccm.201402-0207OC, PMID 24779680.
Sethi S, Jones PW, Theron MS, Miravitlles M, Rubinstein E, Wedzicha JA. Pulsed moxifloxacin for the prevention of exacerbations of chronic obstructive pulmonary disease: a randomized controlled trial. Respir Res. 2010;11(1):10. doi: 10.1186/1465-9921-11-10, PMID 20109213.
Allinson JP, Vlies BH, Brill SE, Law M, Burnside G, Finney LJ. A double blind randomized placebo-controlled trial of long-term doxycycline therapy on exacerbation rate in patients with stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2023;208(5):549-58. doi: 10.1164/rccm.202212-2287OC, PMID 37450935.
Rogliani P, Matera MG, Page C, Puxeddu E, Cazzola M, Calzetta L. Efficacy and safety profile of mucolytic/antioxidant agents in chronic obstructive pulmonary disease: a comparative analysis across erdosteine carbocysteine and N-acetylcysteine. Respir Res. 2019;20(1):104. doi: 10.1186/s12931-019-1078-y, PMID 31133026.
Dal Negro RW, Wedzicha JA, Iversen M, Fontana G, Page C, Cicero AF. Effect of erdosteine on the rate and duration of COPD exacerbations: the restore study. Eur Respir J. 2017;50(4):1700711. doi: 10.1183/13993003.00711-2017, PMID 29025888.
Poole P, Chong J, Cates CJ. Mucolytic agents versus placebo for chronic bronchitis or chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015 Jul 29;7:CD001287. doi: 10.1002/14651858.CD001287.pub5, PMID 26222376.
Cazzola M, Calzetta L, Page C, Jardim J, Chuchalin AG, Rogliani P. Influence of N-acetylcysteine on chronic bronchitis or COPD exacerbations: a meta-analysis. Eur Respir Rev. 2015;24(137):451-61. doi: 10.1183/16000617.00002215, PMID 26324807.
Poole P, Sathananthan K, Fortescue R. Mucolytic agents versus placebo for chronic bronchitis or chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2019 May 20;5(5):CD001287. doi: 10.1002/14651858.CD001287.pub6, PMID 31107966.
Effing TW, Vercoulen JH, Bourbeau J, Trappenburg J, Lenferink A, Cafarella P. Definition of a COPD self-management intervention: international expert group consensus. Eur Respir J. 2016;48(1):46-54. doi: 10.1183/13993003.00025-2016, PMID 27076595.
Schrijver J, Lenferink A, Brusse Keizer M, Zwerink M, Van Der Valk PD, Van Der Palen J. Self-management interventions for people with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2022;1(1):CD002990. doi: 10.1002/14651858.CD002990.pub4, PMID 35001366.
Rose L, Istanboulian L, Carriere L, Thomas A, Lee HB, Rezaie S. Program of integrated care for patients with chronic obstructive pulmonary disease and multiple comorbidities (pic copd+): a randomised controlled trial. Eur Respir J. 2018;51(1):1701567. doi: 10.1183/13993003.01567-2017, PMID 29326330.
Kessler R, Casan Clara P, Koehler D, Tognella S, Viejo JL, Dal Negro RW. Comet: a multicomponent home-based disease management programme versus routine care in severe COPD. Eur Respir J. 2018;51(1):1701612. doi: 10.1183/13993003.01612-2017, PMID 29326333.
Aboumatar H, Naqibuddin M, Chung S, Chaudhry H, Kim SW, Saunders J. Effect of a hospital initiated program combining transitional care and long term self-management support on outcomes of patients hospitalized with chronic obstructive pulmonary disease: a randomized clinical trial. JAMA. 2019;322(14):1371-80. doi: 10.1001/jama.2019.11982, PMID 31593271.
Benzo R, Vickers K, Novotny PJ, Tucker S, Hoult J, Neuenfeldt P. Health coaching and chronic obstructive pulmonary disease rehospitalization. a randomized study. Am J Respir Crit Care Med. 2016;194(6):672-80. doi: 10.1164/rccm.201512-2503OC, PMID 26953637.
Benzo R, MC Evoy C. Effect of health coaching delivered by a respiratory therapist or nurse on self-management abilities in severe copd: analysis of a large randomized study. Respir Care. 2019;64(9):1065-72. doi: 10.4187/respcare.05927, PMID 30914491.
Poot CC, Meijer E, Kruis AL, Smidt N, Chavannes NH, Honkoop PJ. Integrated disease management interventions for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2021;9(9):CD009437. doi: 10.1002/14651858.CD009437.pub3, PMID 34495549.
Kruis AL, Boland MR, Assendelft WJ, Gussekloo J, Tsiachristas A, Stijnen T. Effectiveness of integrated disease management for primary care chronic obstructive pulmonary disease patients: results of cluster randomised trial. BMJ. 2014;349:g5392. doi: 10.1136/bmj.g5392, PMID 25209620.
Cartwright M, Hirani SP, Rixon L. Effect of telehealth on quality of life and psychological outcomes over 12 mo (Whole systems demonstrator telehealth questionnaire study): nested study of patient-reported outcomes in a pragmatic cluster randomised controlled trial. BMJ. 2013 Feb 26;346:f653. doi: 10.1136/bmj.f653.
Gregersen TL, Green A, Frausing E, Ringbæk T, Brondum E, Suppli Ulrik C. Do telemedical interventions improve quality of life in patients with COPD? A systematic review. Int J Chron Obstruct Pulmon Dis. 2016 Apr 21;11:809-22. doi: 10.2147/COPD.S96079, PMID 27143872.
Pitta F, Troosters T, Spruit MA, Probst VS, Decramer M, Gosselink R. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171(9):972-7. doi: 10.1164/rccm.200407-855OC, PMID 15665324.
Yohannes AM, Baldwin RC, Connolly M. Mortality predictors in disabling chronic obstructive pulmonary disease in old age. Age Ageing. 2002;31(2):137-40. doi: 10.1093/ageing/31.2.137, PMID 11937477.
Garcia Aymerich J, Lange P, Benet M, Schnohr P, Anto JM. Regular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: a population-based cohort study. Thorax. 2006;61(9):772-8. doi: 10.1136/thx.2006.060145, PMID 16738033.
Watz H, Pitta F, Rochester CL, Garcia Aymerich J, ZU Wallack R, Troosters T. An official European Respiratory Society statement on physical activity in COPD. Eur Respir J. 2014;44(6):1521-37. doi: 10.1183/09031936.00046814, PMID 25359358.
Mantoani LC, Rubio N, Mckinstry B, Mac Nee W, Rabinovich RA. Interventions to modify physical activity in patients with COPD: a systematic review. Eur Respir J. 2016;48(1):69-81. doi: 10.1183/13993003.01744-2015, PMID 27103381.
Robinson SA, Shimada SL, Quigley KS, Moy ML. A web-based physical activity intervention benefits persons with low self-efficacy in COPD: results from a randomized controlled trial. J Behav Med. 2019;42(6):1082-90. doi: 10.1007/s10865-019-00042-3, PMID 30980223.
Spielmanns M, Gloeckl R, Jarosch I, Leitl D, Schneeberger T, Boeselt T. Using a smartphone application maintains physical activity following pulmonary rehabilitation in patients with COPD: a randomised controlled trial. Thorax. 2023;78(5):442-50. doi: 10.1136/thoraxjnl-2021-218338, PMID 35450945.
Nguyen HQ, Moy ML, Liu IA, Fan VS, Gould MK, Desai SA. Effect of physical activity coaching on acute care and survival among patients with chronic obstructive pulmonary disease: a pragmatic randomized clinical trial. JAMA Netw Open. 2019;2(8):e199657. doi: 10.1001/jamanetworkopen.2019.9657, PMID 31418811.
Wan ES, Kantorowski A, Polak M, Kadri R, Richardson CR, Gagnon DR. Long-term effects of web-based pedometer mediated intervention on COPD exacerbations. Respir Med. 2020;162:105878. doi: 10.1016/j.rmed.2020.105878, PMID 32056676.
Yang Y, Wei L, Wang S, KE L, Zhao H, Mao J. The effects of pursed lip breathing combined with diaphragmatic breathing on pulmonary function and exercise capacity in patients with COPD: a systematic review and meta-analysis. Physiother Theor Pract. 2022;38(7):847-57. doi: 10.1080/09593985.2020.1805834, PMID 32808571.
Lahham A, MC Donald CF, Holland AE. Exercise training alone or with the addition of activity counseling improves physical activity levels in COPD: a systematic review and meta-analysis of randomized controlled trials. Int J Chron Obstruct Pulmon Dis. 2016 Dec 8;11:3121-36. doi: 10.2147/COPD.S121263, PMID 27994451.
Ortega F, Toral J, Cejudo P, Villagomez R, Sanchez H, Castillo J. Comparison of effects of strength and endurance training in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166(5):669-74. doi: 10.1164/rccm.2107081, PMID 12204863.
Horowitz MB, Littenberg B, Mahler DA. Dyspnea ratings for prescribing exercise intensity in patients with COPD. Chest. 1996;109(5):1169-75. doi: 10.1378/chest.109.5.1169, PMID 8625662.
Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM. American college of Sports Medicine position stand quantity and quality of exercise for developing and maintaining cardiorespiratory musculoskeletal and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334-59. doi: 10.1249/MSS.0b013e318213fefb, PMID 21694556.
Vogiatzis I, Nanas S, Roussos C. Interval training as an alternative modality to continuous exercise in patients with COPD. Eur Respir J. 2002;20(1):12-9. doi: 10.1183/09031936.02.01152001, PMID 12166558.
Puhan MA, Busching G, Schunemann HJ, Van Oort E, Zaugg C, Frey M. Interval versus continuous high-intensity exercise in chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med. 2006;145(11):816-25. doi: 10.7326/0003-4819-145-11-200612050-00006, PMID 17146066.
Liu X, FU C, HU W, Hao S, Xie L, WU X. The effect of Tai Chi on the pulmonary rehabilitation of chronic obstructive pulmonary disease: a systematic review and meta-analysis. Ann Palliat Med. 2021;10(4):3763-82. doi: 10.21037/apm-20-940, PMID 33894710.
Ramirez Venegas A, Ward J, Lentine T, Mahler DA. Salmeterol reduces dyspnea and improves lung function in patients with COPD. Chest. 1997;112(2):336-40. doi: 10.1378/chest.112.2.336, PMID 9266866.
Bernard S, Whittom F, Leblanc P, Jobin J, Belleau R, Berube C. Aerobic and strength training in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;159(3):896-901. doi: 10.1164/ajrccm.159.3.9807034, PMID 10051269.
Cardim AB, Marinho PE, Nascimento JF, Fuzari HK, Dornelas DE Andrade A. Does whole-body vibration improve the functional exercise capacity of subjects with COPD? A meta-analysis. Respir Care. 2016;61(11):1552-9. doi: 10.4187/respcare.04763, PMID 27651524.
Velloso M, DO Nascimento NH, Gazzotti MR, Jardim JR. Evaluation of effects of shoulder girdle training on strength and performance of activities of daily living in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2013;8:187-92. doi: 10.2147/COPD.S36606, PMID 23589685.
ReferencesBeaumont M, Forget P, Couturaud F, Reychler G. Effects of inspiratory muscle training in COPD patients: a systematic review and meta-analysis. Clin Respir J. 2018;12(7):2178-88. doi: 10.1111/crj.12905, PMID 29665262.
Beaumont M, Mialon P, LE Ber C, LE Mevel P, Peran L, Meurisse O. Effects of inspiratory muscle training on dyspnoea in severe COPD patients during pulmonary rehabilitation: controlled randomised trial. Eur Respir J. 2018;51(1):1701107. doi: 10.1183/13993003.01107-2017, PMID 29371379.
Chuang HY, Chang HY, Fang YY, Guo SE. The effects of threshold inspiratory muscle training in patients with chronic obstructive pulmonary disease: a randomised experimental study. J Clin Nurs. 2017;26(23-24):4830-8. doi: 10.1111/jocn.13841, PMID 28382660.
Charususin N, Gosselink R, Decramer M, Demeyer H, MC Connell A, Saey D. Randomised controlled trial of adjunctive inspiratory muscle training for patients with COPD. Thorax. 2018;73(10):942-50. doi: 10.1136/thoraxjnl-2017-211417, PMID 29914940.
Kumar KS, Kumaresan C, Anantharaj R. Development and comparison of orally inhalable sustained release formulations for three respiratory drugs for asthma. Adv Chem. 2016;12(25):5679-99. doi: 10.24297/jac.v12i25.4658.
James AC, Stahlhofen W, Rudolf G, Annexe D. Deposition of inhaled particles. Ann ICRP. 1994;24:231-99.
Peng T, Lin S, Niu B, Wang X, Huang Y, Zhang X. Influence of physical properties of carrier on the performance of dry powder inhalers. Acta Pharm Sin B. 2016;6(4):308-18. doi: 10.1016/j.apsb.2016.03.011, PMID 27471671.
Rotahaler (For Cipla Rotacaps)-4NRX (UK). Available from: https://www.4nrx-uk.md/accessories/rota haler.html. [Last accessed on 02 Apr 2025].
Hira D, Koide H, Nakamura S, Okada T, Ishizeki K, Yamaguchi M. Assessment of inhalation flow patterns of soft mist inhaler co-prescribed with dry powder inhaler using inspiratory flow meter for multi inhalation devices. Plos One. 2018;13(2):e0193082. doi: 10.1371/journal.pone.0193082, PMID 29462195.
Ungaro F, Vanbever R. Improving the efficacy of inhaled drugs for severe lung diseases: emerging pulmonary delivery strategies. Adv Drug Deliv Rev. 2014 Aug;75:1-2. doi: 10.1016/j.addr.2014.08.001, PMID 25109852.
Chang RY, Chow MY, Khanal D, Chen D, Chan HK. Dry powder pharmaceutical biologics for inhalation therapy. Adv Drug Deliv Rev. 2021 May;172:64-79. doi: 10.1016/j.addr.2021.02.017, PMID 33705876.
Dierick BJ, Eikholt AA, Van DE Hei SJ, Muris JW, Kerstjens HA, Van Boven JF. Reshaping respiratory care: potential advances in inhaled pharmacotherapy in asthma. Expert Opin Pharmacother. 2024;25(11):1507-16. doi: 10.1080/14656566.2024.2389258, PMID 39099418.
Gaikwad SS, Pathare SR, More MA, Waykhinde NA, Laddha UD, Salunkhe KS. Dry powder inhaler with the technical and practical obstacles and forthcoming platform strategies. J Control Release. 2023 Mar;355:292-311. doi: 10.1016/j.jconrel.2023.01.083, PMID 36739908.
Mehta PP. Dry powder inhalers: a concise summary of the electronic monitoring devices. Ther Deliv. 2021;12(1):1-6. doi: 10.4155/tde-2020-0091, PMID 32873214.
Labiris NR, Dolovich MB. Pulmonary drug delivery. Part I: physiological factors affecting therapeutic effectiveness of aerosolized medications. Br J Clin Pharmacol. 2003;56(6):588-99. doi: 10.1046/j.1365-2125.2003.01892.x, PMID 14616418.
Kumar R, Mehta P, Shankar KR, Rajora MA, Mishra YK, Mostafavi E. Nanotechnology assisted metered dose inhalers (MDIs) for high-performance pulmonary drug delivery applications. Pharm Res. 2022;39(11):2831-55. doi: 10.1007/s11095-022-03286-y, PMID 35552983.
Magramane S, Vlahovic K, Gordon P, Kallai Szabo N, Zelko R, Antal I. Inhalation dosage forms: a focus on dry powder inhalers and their advancements. Pharmaceuticals (Basel). 2023;16(12):1658. doi: 10.3390/PH16121658, PMID 38139785.
Ciphaler® device by Cipla EU ltd-rightbreathe. Available from: https://www.rightbreathe.com/?s=anddevice_type=anddrug_class=anddrug_name=anddevice_type=andcopd_licence=1.
Arora P, Kumar L, Vohra V, Sarin R, Jaiswal A, Puri MM. Evaluating the technique of using inhalation device in COPD and bronchial asthma patients. Respir Med. 2014;108(7):992-8. doi: 10.1016/j.rmed.2014.04.021, PMID 24873874.
DE Pietro M, Gilbert I, Millette LA, Riebe M. Inhalation device options for the management of chronic obstructive pulmonary disease. Postgrad Med. 2018;130(1):83-97. doi: 10.1080/00325481.2018.1399042, PMID 29210318.
Mansour HM, Muralidharan P, Hayes D. Inhaled nanoparticulate systems: composition manufacture and aerosol delivery. J Aerosol Med Pulm Drug Deliv. 2024;37(4):202-18. doi: 10.1089/jamp.2024.29117.mk, PMID 39172256.
Digihaler Support Center. Digihaler inhaler FAQs. Available from: https://www.digihaler.com/support/using-my-inhaler. [Last accessed on 22 Aug 2024].
Pramanik S, Mohanto S, Manne R. Nanoparticle-based drug delivery system: the magic bullet for the treatment of chronic pulmonary diseases. Cite this. Mol Pharm. 2021;18(10):3671-718.
Van SD, Barrett M, Humblet O. Randomized controlled study of the impact of a mobile health tool on asthma SABA use control and adherence. Eur Respir J. 2016;48:PA1018.
Haubermann S, Arendsen LJ, Pritchard JN. Smart dry powder inhalers and intelligent adherence management. Adv Drug Deliv Rev. 2022;191:114580. doi: 10.1016/j.addr.2022.114580, PMID 36273513.
Pothirat C, Chaiwong W, Limsukon A, Phetsuk N, Chetsadaphan N, Choomuang W. Real-world observational study of the evaluation of inhaler techniques in asthma patients. Asian Pac J Allergy Immunol. 2021;39(2):96-102. doi: 10.12932/AP-210618-0348, PMID 30660168.
Poplicean E, Crisan AF, Tudorache E, Hogea P, Mladin R, Oancea C. Unlocking better asthma control: a narrative review of adherence to asthma therapy and innovative monitoring solutions. J Clin Med. 2024;13(22):6699. doi: 10.3390/jcm13226699, PMID 39597843.
Bhattarai A, Shakya R, Bista D. Impact of pharmacist led intervention on adherence to inhalers inhalation technique and disease control among asthma/COPD patients in a resource limited center: an interventional study. Patient Preference Adherence. 2024;18:1395-408. doi: 10.2147/PPA.S460810, PMID 38974680.
Furman EG, Khuzina EA. Age-related features of the choice of inhalers in children and adolescents with bronchial asthma and other respiratory diseases. Meditsinskiy Sovet. 2024;18:92-9.
Dhoble S, Kapse A, Ghegade V, Chogale M, Ghodake V, Patravale V. Design development and technical considerations for dry powder inhaler devices. Drug Discov Today. 2024;29(5):103954. doi: 10.1016/j.drudis.2024.103954, PMID 38531423.
Lavorini F. Easyhaler®: an overview of an inhaler device for day to day use in patients with asthma and chronic obstructive pulmonary disease. Drugs Context. 2019 Jun 5;8:212596. doi: 10.7573/dic.212596, PMID 31210773.
Larsson K, Bjermer L, Svartengren M. The importance of selecting the right type of inhaler for patients with asthma and chronic obstructive pulmonary disease (COPD). Lakartidningen. 2019;116:FF76. PMID 30720857.
Aggarwal B, Gogtay J. Use of pressurized metered dose inhalers in patients with chronic obstructive pulmonary disease: review of evidence. Expert Rev Respir Med. 2014;8(3):349-56. doi: 10.1586/17476348.2014.905916, PMID 24802511.
Mekov E, Petkov R. Smart inhalers: transforming respiratory disease management through artificial intelligence. Gazz Med Ital Arch Sci Med. 2024;183(9):765-8. doi: 10.23736/S0393-3660.24.05533-5.
Chrystyn H, Audibert R, Keller M, Quaglia B, Vecellio L, Roche N. Real life inhaler adherence and technique: time to get smarter. Respir Med. 2019 Oct-Nov;158:24-32. doi: 10.1016/j.rmed.2019.09.008, PMID 31563027.
Abou El Nour KM, Eftaiha A, Al Warthan A. Synthesis and applications of silver nanoparticles. Arab J Chem. 2010 Jul;3(3):135-40. doi: 10.1016/j.arabjc.2010.04.008.
Pandey N, Dhiman S, Srivastava T, Majumder S. Transition metal oxide nanoparticles are effective in inhibiting lung cancer cell survival in the hypoxic tumor microenvironment. Chem Biol Interact. 2016;254:221-30. doi: 10.1016/j.cbi.2016.06.006, PMID 27270449.
Kawashima Y. Nanoparticulate systems for improved drug delivery; 2001. Available from: www.elsevier.com/locate/drugdeliv.
Doroudian M, Armstrong ME, Donnelly SC. Nano-based therapies for acute and chronic lung diseases; 2023. p. 271-86.
Ezike TC, Okpala US, Onoja UL, Nwike CP, Ezeako EC, Okpara OJ. Advances in drug delivery systems challenges and future directions. Heliyon Heliyon. 2023;9(6):e17488. doi: 10.1016/j.heliyon.2023.e17488, PMID 37416680.
Afzal O, Altamimi AS, Nadeem MS, Alzarea SI, Almalki WH, Tariq A. Nanoparticles in drug delivery: from history to therapeutic applications. Nanomaterials. 2022;12(24):4494. doi: 10.3390/nano12244494, PMID 36558344.
DE Rubis G, Paudel KR, Manandhar B, Singh SK, Gupta G, Malik R. Agarwood oil nanoemulsion attenuates cigarette smoke-induced inflammation and oxidative stress markers in bci-ns1.1 airway epithelial cells. Nutrients. 2023;15(4):1019. doi: 10.3390/nu15041019, PMID 36839377.
Saxena J, Bisen M, Misra A, Srivastava VK, Kaushik S, Siddiqui AJ. Targeting COPD with PLGA based nanoparticles: current status and prospects. Bio Med Res Int. 2022;2022:5058121. doi: 10.1155/2022/5058121, PMID 35309178.
Zhang L, GU FX, Chan JM, Wang AZ, Langer RS, Farokhzad OC. Nanoparticles in medicine: therapeutic applications and developments. Clin Pharmacol Ther. 2008;83(5):761-9. doi: 10.1038/sj.clpt.6100400, PMID 17957183.
Yhee JY, Im J, Nho RS. Advanced therapeutic strategies for chronic lung disease using nanoparticle based drug delivery. J Clin Med. 2016;5(9):82. doi: 10.3390/JCM5090082, PMID 27657144.
Schuster BS, Suk JS, Woodworth GF, Hanes J. Nanoparticle diffusion in respiratory mucus from humans without lung disease. Biomaterials. 2013;34(13):3439-46. doi: 10.1016/j.biomaterials.2013.01.064, PMID 23384790.
Lim Soo PL, Eisenberg A. Preparation of block copolymer vesicles in solution. J Polym Sci B Polym Phys. 2004;42(6):923-38. doi: 10.1002/polb.10739.
Vert M, Doi Y, Hellwich KH, Hess M, Hodge P, Kubisa P. Terminology for biorelated polymers and applications (IUPAC recommendations 2012). Pure Appl Chem. 2012;84(2):377-410. doi: 10.1351/PAC-REC-10-12-04.
Pham DT, Chokamonsirikun A, Phattaravorakarn V, Tiyaboonchai W. Polymeric micelles for pulmonary drug delivery: a comprehensive review. J Mater Sci. 2021;56(3):2016-36. doi: 10.1007/s10853-020-05361-4.
Mulhall P, Criner G. Non-pharmacological treatments for COPD. Respirology. 2016;21(5):791-809. doi: 10.1111/resp.12782, PMID 27099216.
Mishra B, Singh J. Novel drug delivery systems and significance in respiratory diseases. In: Targeting chronic inflammatory lung diseases using advanced drug delivery systems. Elsevier; 2020. p. 57-95. doi: 10.1016/B978-0-12-820658-4.00004-2.
Sriram Mishra A, Sugumaran A, Abimanyu S. Nanoparticulate delivery systems for the effective treatment of COPD; 2021. Available from: http://annalsofrscb.ro.
Khan OF, Zaia EW, Jhunjhunwala S, Xue W, Cai W, Yun DS. Dendrimer-inspired nanomaterials for the in vivo delivery of siRNA to lung vasculature. Nano Lett. 2015;15(5):3008-16. doi: 10.1021/nl5048972, PMID 25789998.
Liposomes: a short review. Available from: https://www.researchgate.net/publication/286860779_Liposomes_A_short_review. [Last accessed on 24 Aug 2024].
Pierre MB, Dos Santos Miranda Costa I. Liposomal systems as drug delivery vehicles for dermal and transdermal applications. Arch Dermatol Res. 2011;303(9):607-21. doi: 10.1007/s00403-011-1166-4, PMID 21805180.
Danhier F, Feron O, Preat V. To exploit the tumor microenvironment: passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J Control Release. 2010;148(2):135-46. doi: 10.1016/j.jconrel.2010.08.027, PMID 20797419.
Guo L, Fan L, Ren J, Pang Z, Ren Y, LI J. Combination of TRAIL and actinomycin D liposomes enhances antitumor effect in non-small cell lung cancer. Int J Nanomedicine. 2012 Mar 19;7:1449-60. doi: 10.2147/IJN.S24711, PMID 22619505.
Kellaway IW, Farr SJ. Liposomes as drug delivery systems to the lung. Adv Drug Deliv Rev. 1990;5(1-2):149-61. doi: 10.1016/0169-409X(90)90012-H.
Kraft JC, Freeling JP, Wang Z, HO RJ. Emerging research and clinical development trends of liposome and lipid nanoparticle drug delivery systems. J Pharm Sci. 2014;103(1):29-52. doi: 10.1002/jps.23773, PMID 24338748.
Meng H, XU Y. Pirfenidone loaded liposomes for lung targeting: preparation and in vitro/in vivo evaluation. Drug Des Dev Ther. 2015 Jun 30;9:3369-76. doi: 10.2147/DDDT.S84046, PMID 26185416.
Chennakesavulu S, Mishra A, Sudheer A, Sowmya C, Suryaprakash Reddy C, Bhargav E. Pulmonary delivery of liposomal dry powder inhaler formulation for effective treatment of idiopathic pulmonary fibrosis. Asian J Pharm Sci. 2018;13(1):91-100. doi: 10.1016/j.ajps.2017.08.005, PMID 32104382.
Wang Q, WU P, Ren W, Xin K, Yang Y, Xie C. Comparative studies of salinomycin loaded nanoparticles prepared by nanoprecipitation and single emulsion method. Nanoscale Res Lett. 2014;9(1):351. doi: 10.1186/1556-276X-9-351, PMID 25147486.
Rudokas M, Najlah M, Alhnan MA, Elhissi A. Liposome delivery systems for inhalation: a critical review highlighting formulation issues and anticancer applications. Med Princ Pract. 2016;25 Suppl 2:60-72. doi: 10.1159/000445116, PMID 26938856.
Weber S, Zimmer A, Pardeike J. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (nlc) for pulmonary application: a review of the state of the art. Eur J Pharm Biopharm. 2014;86(1):7-22. doi: 10.1016/j.ejpb.2013.08.013, PMID 24007657.
Lopez KL, Ravasio A, Gonzalez Aramundiz JV, Zacconi FC. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) prepared by microwave and ultrasound-assisted synthesis: promising green strategies for the nanoworld. Pharmaceutics. 2023;15(5):1333. doi: 10.3390/pharmaceutics15051333, PMID 37242575.
Shi C, Guo K, Zhang L, Guo Y, Feng Y, Cvijic S. In vitro and In vivo evaluation of inhalable ciprofloxacin sustained release formulations. Pharmaceutics. 2023;15(9):2287. doi: 10.3390/pharmaceutics15092287, PMID 37765256.
Loira Pastoriza C, Todoroff J, Vanbever R. Delivery strategies for sustained drug release in the lungs. Adv Drug Deliv Rev. 2014 Aug;75:81-91. doi: 10.1016/j.addr.2014.05.017, PMID 24915637.
MC Clements DJ. Edible nanoemulsions: fabrication properties and functional performance. Soft Matter. 2011;7(6):2297-316. doi: 10.1039/C0SM00549E.
Shafiq S, Shakeel F, Talegaonkar S, Ahmad FJ, Khar RK, Ali M. Development and bioavailability assessment of ramipril nanoemulsion formulation. Eur J Pharm Biopharm. 2007;66(2):227-43. doi: 10.1016/j.ejpb.2006.10.014, PMID 17127045.
Bali V, Ali M, Ali J. Novel nanoemulsion for minimizing variations in bioavailability of ezetimibe. J Drug Target. 2010;18(7):506-19. doi: 10.3109/10611860903548362, PMID 20067438.
Wang X, Jiang Y, Wang YW, Huang MT, HO CT, Huang Q. Enhancing anti-inflammation activity of curcumin through O/W nanoemulsions. Food Chem. 2008;108(2):419-24. doi: 10.1016/j.foodchem.2007.10.086, PMID 26059118.
Patravale VB, Mandawgade SD. Novel cosmetic delivery systems: an application update. Int J Cosmet Sci. 2008;30(1):19-33. doi: 10.1111/j.1468-2494.2008.00416.x, PMID 18377627.
Beloqui A, Solinis MA, Delgado A, Evora C, Del Pozo Rodriguez A, Rodriguez-Gascon A. Biodistribution of nanostructured lipid carriers (NLCS) after intravenous administration to rats: influence of technological factors. Eur J Pharm Biopharm. 2013;84(2):309-14. doi: 10.1016/j.ejpb.2013.01.029, PMID 23461861.
Khurana S, Jain NK, Bedi PM. Development and characterization of a novel controlled release drug delivery system based on nanostructured lipid carriers gel for meloxicam. Life Sci. 2013;93(21):763-72. doi: 10.1016/j.lfs.2013.09.027, PMID 24113071.
Fathi HA, Allam A, Elsabahy M, Fetih G, El-Badry M. Nanostructured lipid carriers for improved oral delivery and prolonged antihyperlipidemic effect of simvastatin. Colloids Surf B Biointerfaces. 2018 Feb 1;162:236-45. doi: 10.1016/j.colsurfb.2017.11.064, PMID 29197789.
Salvi VR, Pawar P. Nanostructured lipid carriers (NLC) system: a novel drug targeting carrier. J Drug Deliv Sci Technol. 2019 Jun;51:255-67. doi: 10.1016/j.jddst.2019.02.017.
Graczyk H, Bryan LC, Lewinski N, Suarez G, Coullerez G, Bowen P. Physicochemical characterization of nebulized superparamagnetic iron oxide nanoparticles (SPIONs). J Aerosol Med Pulm Drug Deliv. 2015;28(1):43-51. doi: 10.1089/jamp.2013.1117, PMID 24801912.
Verma NK, Crosbie Staunton K, Satti A, Gallagher S, Ryan KB, Doody T. Magnetic core shell nanoparticles for drug delivery by nebulization. J Nanobiotechnology. 2013;11:1. doi: 10.1186/1477-3155-11-1, PMID 23343139.
Han X, LI Y, Liu W, Chen X, Song Z, Wang X. The applications of magnetic particle imaging: from cell to body. Diagnostics. 2020;10(10):800. doi: 10.3390/diagnostics10100800, PMID 33050139.
Nishimoto K, Mimura A, Aoki M. Application of magnetic particle imaging to pulmonary imaging using nebulized magnetic nanoparticles. Open J Med Imaging. 2015;5:49-55.
Ali A, Zafar H, Zia M, Ul Haq I, Phull AR, Ali JS. Synthesis characterization applications and challenges of iron oxide nanoparticles. Nanotechnol Sci Appl. 2016 Aug 19;9:49-67. doi: 10.2147/NSA.S99986, PMID 27578966.
Verma NK, Crosbie Staunton K, Satti A, Gallagher S, Ryan KB, Doody T. Magnetic core-shell nanoparticles for drug delivery by nebulization. J Nanobiotechnology. 2013;11:1. doi: 10.1186/1477-3155-11-1, PMID 23343139.
Yang Z, Wang D, Zhang C, Liu H, Hao M, Kan S. The applications of gold nanoparticles in the diagnosis and treatment of gastrointestinal cancer. Front Oncol. 2021;11:819329. doi: 10.3389/fonc.2021.819329, PMID 35127533.
Nguyen KT, Zhao Y. Engineered hybrid nanoparticles for on demand diagnostics and therapeutics. Acc Chem Res. 2015;48(12):3016-25. doi: 10.1021/acs.accounts.5b00316, PMID 26605438.
Hadinoto K, Sundaresan A, Cheow WS. Lipid polymer hybrid nanoparticles as a new generation therapeutic delivery platform: a review. Eur J Pharm Biopharm. 2013;85(3 Pt A):427-43. doi: 10.1016/j.ejpb.2013.07.002, PMID 23872180.
Sgorla D, Bunhak EJ, Cavalcanti OA, Fonte P, Sarmento B. Exploitation of lipid-polymeric matrices at nanoscale for drug delivery applications. Expert Opin Drug Deliv. 2016;13(9):1301-9. doi: 10.1080/17425247.2016.1182492, PMID 27110648.
Clawson C, Ton L, Aryal S, FU V, Esener S, Zhang L. Synthesis and characterization of lipid polymer hybrid nanoparticles with pH-triggered poly(ethylene glycol) shedding. Langmuir. 2011;27(17):10556-61. doi: 10.1021/la202123e, PMID 21806013.
Zhang L, Feng Q, Wang J, Zhang S, Ding B, Wei Y. Microfluidic synthesis of hybrid nanoparticles with controlled lipid layers: understanding flexibility-regulated cell nanoparticle interaction. ACS Nano. 2015;9(10):9912-21. doi: 10.1021/acsnano.5b05792, PMID 26448362.
Zhang L, Zhang L. Lipid polymer hybrid nanoparticles: synthesis characterization and applications. Nano Life. 2010;1:163-73. doi: 10.1142/S179398441000016X.
Ragelle H, Colombo S, Pourcelle V, Vanvarenberg K, Vandermeulen G, Bouzin C. Intracellular siRNA delivery dynamics of integrin targeted pegylated chitosan-poly(ethylene imine) hybrid nanoparticles: a mechanistic insight. J Control Release. 2015 Aug 10;211:1-9. doi: 10.1016/j.jconrel.2015.05.274, PMID 25989603.
Hoppentocht M, Hagedoorn P, Frijlink HW, DE Boer AH. Technological and practical challenges of dry powder inhalers and formulations. Adv Drug Deliv Rev. 2014 Aug;75:18-31. doi: 10.1016/j.addr.2014.04.004, PMID 24735675.
Kulkarni A, Jagnade V, Hole K. Effect of formulation excipients on aerosolisation performance of budesonide. Indo Am J Pharm Res. 2017;7(11):909-19.
Sahab Uddin M, Hossain M, Al Mamun A. Pharmacopoeial standards and specifications for pharmaceutical aerosols: in process and finished products quality control tests. Equal Contributors Advances in Research. 2016;6(3):1-12. doi: 10.9734/AIR/2016/22442.
Agu RU, Ugwoke MI. In vitro and in vivo testing methods for respiratory drug delivery. Expert Opin Drug Deliv. 2011;8(1):57-69. doi: 10.1517/17425247.2011.543896, PMID 21174605.
HO KK, Kellaway IW, Tredree R. Particle size analysis of nebulised aerosols using Fraunhofer laser diffraction and inertial compaction methods. J Pharm Pharmacol. 2011;38(S12):26P. doi: 10.1111/j.2042-7158.1986.tb14255.x.
Radivojev S, Zellnitz S, Paudel A, Frohlich E. Searching for physiologically relevant in vitro dissolution techniques for orally inhaled drugs. Int J Pharm. 2019 Feb 10;556:45-56. doi: 10.1016/j.ijpharm.2018.11.072, PMID 30529665.
Eedara BB, Tucker IG, Das SC. In vitro dissolution testing of respirable size anti-tubercular drug particles using a small volume dissolution apparatus. Int J Pharm. 2019 Mar 25;559:235-44. doi: 10.1016/j.ijpharm.2019.01.035, PMID 30684598.
Nokhodchi A, Chavan S, Ghafourian T. In vitro dissolution and permeability testing of inhalation products: challenges and advances. Pharmaceutics. 2023;15(3):983. doi: 10.3390/pharmaceutics15030983, PMID 36986844.
May S, Jensen B, Wolkenhauer M, Schneider M, Lehr CM. Dissolution techniques for in vitro testing of dry powders for inhalation. Pharm Res. 2012;29(8):2157-66. doi: 10.1007/s11095-012-0744-2, PMID 22528980.
Davies NM, Feddah MR. A novel method for assessing dissolution of aerosol inhaler products. Int J Pharm. 2003;255(1-2):175-87. doi: 10.1016/s0378-5173(03)00091-7, PMID 12672613.
Salama RO, Traini D, Chan HK, Young PM. Preparation and characterisation of controlled release co-spray dried drug polymer microparticles for inhalation 2: evaluation of in vitro release profiling methodologies for controlled release respiratory aerosols. Eur J Pharm Biopharm. 2008;70(1):145-52. doi: 10.1016/j.ejpb.2008.04.009, PMID 18534832.
Marques MR, Loebenberg R, Almukainzi M. Simulated biological fluids with possible application in dissolution testing. Dissolut Technol. 2011;18:15-28.
Moss OR. Simulants of lung interstitial fluid. Health Phys. 1979;36(3):447-8. PMID 489300.
Hassoun M, Royall PG, Parry M, Harvey RD, Forbes B. Design and development of a biorelevant simulated human lung fluid. J Drug Deliv Sci Technol. 2018 Oct;47:485-91. doi: 10.1016/j.jddst.2018.08.006, PMID 30283501.