
Int J App Pharm, Vol 17, Issue 3, 2025, 93-100Review Article
A SYSTEMATIC REVIEW OF EUCALYPTOL AS AN INNOVATIVE THERAPEUTIC AGENT FOR RESPIRATORY DISEASES
I NYOMAN GEDE TRI SUTRISNA1,2, NI NYOMAN AYU DEWI3, DYAH KANYA WATI4, NI MADE LINAWATI5, I MADE AGUS GELGEL WIRASUTA6*
1Doctoral Study Program, Faculty of Medicine, Udayana University, Denpasar-80232, Indonesia. 2Universitas Pendidikan Nasional, Denpasar-80225, Indonesia. 3Department of Biochemistry, Faculty of Medicine, Udayana University, Denpasar-80232, Indonesia. 4Pediatric Emergency and Intensive Care, Prof. Ngoerah Hospital, Denpasar-80119, Indonesia. 5Histology Department, Faculty of Medicine, Udayana University, Denpasar-80232, Indonesia. 6*Pharmacy Department, Faculty of Mathematics and Natural Science, Udayana University, Badung-80361, Indonesia
*Corresponding author: I Made Agus Gelgel Wirasuta; *Email: gelgel.wirasuta@unud.ac.id
Received: 23 Jan 2025, Revised and Accepted: 25 Mar 2025
ABSTRACT
Eucalyptol (1,8-cineole), a natural monoterpenoid found in eucalyptus essential oil, has long been used in traditional medicine for its anti-inflammatory, mucolytic, antimicrobial, and mild bronchodilatory properties. Recent pharmacological studies have further validated its therapeutic potential, making it a promising candidate for managing chronic respiratory diseases. This study systematically reviews the available evidence on eucalyptol’s effects in treating these conditions. The review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines to ensure a clear and reproducible process for selecting articles, extracting data, and analyzing results. Literature was collected from reliable databases such as PubMed, ScienceDirect, and Scopus. The bibliometric analysis incorporated in this study further strengthens its novelty, providing a quantitative overview of research trends and highlighting key themes and emerging areas of interest. A total of 35 studies met the inclusion criteria, evaluating the pharmacological effects of eucalyptol on respiratory diseases. Eucalyptol has demonstrated anti-inflammatory effects through cytokine modulation, enhances mucus clearance, and reduces airway resistance. Several studies showed improvement in lung function and symptom relief in patients. Eucalyptol holds significant potential as an adjunctive therapy for chronic respiratory diseases due to its anti-inflammatory, mucolytic, antimicrobial, and mild bronchodilatory properties. In addition to improving lung function and patient quality of life, eucalyptol also reduces the risk of side effects associated with long-term therapy. Despite these benefits, challenges remain, including low bioavailability, volatility, and the need for optimized pharmaceutical formulations. Future research should focus on nanoemulsions, inhalable drug delivery systems, and controlled-release technologies to enhance its therapeutic efficacy. Large-scale clinical trials targeting vulnerable populations, including pediatric and geriatric patients, are essential to establish eucalyptol’s clinical utility.
Keywords: Eucalyptol, Respiratory diseases, Systematic review, Pharmaceutical formulation
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijap.2025v17i3.53775 Journal homepage: https://innovareacademics.in/journals/index.php/ijap
INTRODUCTION
Eucalyptol (1,8-cineole) is a primary component of eucalyptus essential oil and has long been used in traditional medicine to address various respiratory disorders. Modern studies have revealed that eucalyptol exhibits a wide range of pharmacological effects, including anti-inflammatory, mucolytic, and antimicrobial activities, making it a potential therapeutic candidate in the management of chronic respiratory diseases [1–3]. Eucalyptol has been shown to inhibit the production of pro-inflammatory mediators such as TNF-α and IL-1β, which play a crucial role in the inflammatory processes underlying various lung diseases [4, 5]. Eucalyptol inhibits inflammation by blocking ERK-dependent NF-κB activation, reducing nuclear NF-κB expression and lowering pro-inflammatory cytokines like TNF-α [6]. Additionally, eucalyptol’s mucolytic effects aid in thinning mucus and enhancing sputum clearance, which is critical in reducing airway obstruction in diseases like chronic bronchitis and COPD [7].
Respiratory diseases, such as asthma, chronic bronchitis, and Chronic Obstructive Pulmonary Disease (COPD), are leading causes of global morbidity and mortality, affecting over 500 million people worldwide. These conditions significantly impact the quality of life and contribute to the healthcare cost burden [8]. Research on four common respiratory diseases in the Asia-Pacific region estimated that the average annual cost per patient is approximately $4,191, with total expenses reaching $22 million for the study sample. These costs stem primarly from medical treatments, hospital visits, and reduced work productivity, highlighting the urgent need for more cost-effective therapeutic options [9]. Current therapies for these respiratory conditionstypically include a combination of inhaled corticosteroids, bronchodilators, and anti-inflammatory drugs. While effective in symptom management, long-term use of these therapies often leads to serious side effects, including osteoporosis, hypertension, and an increased risk of infections [10, 11]. In this context, there is an urgent need to identify safe and effective therapeutic agents as alternatives or adjuncts to conventional treatments. This highlights the urgent need for safer and more effective therapeutic agents as alternatives or adjuncts to conventional treatments. Eucalyptol’s potential as an adjunct therapy for respiratory diseases has been evaluated in numerous clinical and preclinical studies, with results showing improved lung function, reduced exacerbation rates, and enhanced quality of life for patients [12]. Despite these promising findings, there remain several unanswered questions regarding the specific mechanisms of action, long-term safety, and potential interactions between eucalyptol and conventional therapies. Beyond pharmacological approaches, patient education and counseling have also been shown to improve medication adherence, disease management, and health outcomes [13].
This article aims to systematically review the existing body of literature on eucalyptol as an innovative therapeutic agent for managing respiratory diseases, with the overarching goal of establishing a comprehensive scientific foundation that supports the development of safer and more effective treatment options. By examining the pharmacological properties and therapeutic potential of eucalyptol, this review seeks to highlight its capacity to address unmet needs in the treatment of respiratory conditions, particularly chronic disorders. Furthermore, it explores eucalyptol’s role as a promising alternative in the management of chronic respiratory diseases, thereby contributing to the diversification of therapeutic approaches and enhancing patient outcomes in a field where innovation and safety are critical priorities.
MATERIALS AND METHODS
Data collection
This systematic review was conducted following the guidelines of the PRISMA to ensure transparency and high quality in the screening and selection process of relevant articles [14]. In addition to the systematic review, a comprehensive bibliometric analysis was performed to identify and analyze research trends in the field of eucalyptol and its use in the therapy of respiratory diseases. This approach aimed to map future research directions and provide insights into emerging research trends and collaborations in this area.
Eligibility criteria
Studies were included if they investigated eucalyptol in the context of respiratory diseases, focusing on its effects or mechanisms of action, either in clinical or preclinical settings. Only original research articles were considered, while book chapters, review articles, and bibliographies were excluded. The studies had to focus on the therapeutic effects of eucalyptol, including improvements in respiratory function, symptom relief, or other relevant clinical outcomes. Studies written in English were included, provided eucalyptol was a central focus or discussed as a primary therapeutic agent in respiratory disease treatment. Studies could be included with or without a comparison group, where applicable, to assess the effects of eucalyptol.
Search strategy
A comprehensive search strategy was developed across three databases: PubMed, ScienceDirect, and Scopus. Keywords and Boolean operators (AND, OR) were used to combine terms in various permutations to ensure the identification of all relevant articles. The following search string was applied: ["Eucalyptus globulus" OR "Eucalyptol"] AND ["Respiratory disease" OR "Respiratory tract infection"]. The search was not restricted by publication date to maximize the scope of included studies, ensuring comprehensive coverage of the literature.
Selection process
The search results were screened by two independent reviewers in two phases. First, the titles and abstracts of all retrieved studies were reviewed, and studies that clearly did not meet the inclusion criteria were excluded. In the second phase, the remaining articles were assessed in full for eligibility based on the criteria outlined above. Disagreements between reviewers were resolved through discussion, and consensus was reached before final selection.
Data collection
Data were collected using the reference management software Zotero. Relevant articles that met the eligibility criteria were exported in. ris format for further analysis. Each article’s publication details, study design, outcomes, and findings were extracted for subsequent synthesis.
Data analysis process
VOSviewer software was used to visualize and evaluate research trends through bibliometric mapping. This process involved identifying and mapping keywords that appeared at least twice across the reviewed literature. Irrelevant or less significant terms were systematically excluded to ensure a focused analysis. The mapping helped identify clusters of research topics and the relationships between them, providing an overview of the main themes and trends related to the role of eucalyptol in respiratory disease therapy.
This structured methodological approach, adhering to PRISMA guidelines, ensures comprehensive, transparent, and accurate insights into the scientific landscape of eucalyptol in respiratory disease therapy.
RESULTS AND DISCUSSION
Literature search
The literature search process identified 486 relevant articles from three major databases: PubMed (n=9), ScienceDirect (n=412), and Scopus (n=65). The search process is depicted in the PRISMA flow diagram (fig. 1), which involves the stages of identification, screening, eligibility assessment, and final selection of articles for review.
After removing 10 duplicate articles, 476 unique articles were analyzed further. From this total, 261 articles were excluded based on the following criteria: book chapters (n=62), review articles (n=124), non-English articles (n=2), conference proceedings (n=4), and others (n=24). During the eligibility assessment phase, 215 articles remained and were re-screened to ensure relevance to the topic. A further 180 articles were excluded because the keywords were not the primary focus of the study. Ultimately, 35 articles met the inclusion criteria and were included in the systematic review, as shown in fig. 1.
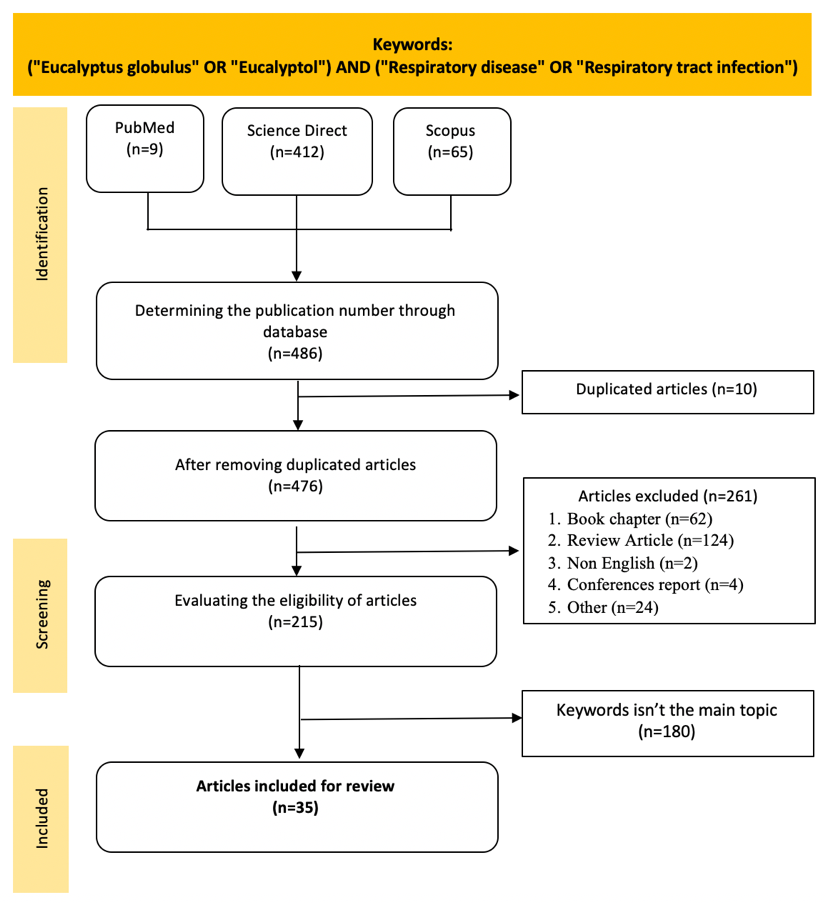
Fig. 1: Screening literature flowchart based on PRISMA method
Pharmacological potential of eucalyptus in respiratory disease therapy
Eucalyptus has long been utilized in traditional medicine to treat respiratory disorders owing to its bioactive compounds, such as eucalyptol and cineole, which possess antibacterial, anti-inflammatory, mucolytic, and bronchodilatory activities. Recent research has uncovered the mechanisms and therapeutic potential of these compounds, particularly in the treatment of respiratory tract diseases.
Recent studies indicate that eucalyptus is effective as a complementary agent in alleviating symptoms of respiratory infections such as cough, bronchitis, and asthma. Its active compounds work on the respiratory tract and the immune system, providing protective and restorative effects for patients with both acute and chronic respiratory disorders. Table 1 summarizes the plant parts, active compounds, analytical methods, and pharmacological mechanisms, offering a comprehensive overview of eucalyptus's role in respiratory therapy.
Clinical evidence supports eucalyptol’s role in improving lung function and reducing airway inflammation. A randomized controlled trial (ISRCTN07600011) in 242 COPD patients showed that 200 mg of eucalyptol, taken thrice daily for six months, significantly reduced exacerbation frequency, severity, and duration while improving lung function, dyspnea, and quality of life [7]. Similarly, a multicenter trial in 247 asthma patients found that eucalyptol treatment led to significant improvements in lung function (p = 0.0398), asthma symptoms (p = 0.0325), and quality of life (p = 0.0475) compared to placebo, highlighting its potential as an adjunct therapy for respiratory diseases [15]. While clinical trials have used a standard oral dose of 200 mg three times daily, preclinical studies have tested a wider range of doses (e. g., 50–500 mg/kg in animal models). This discrepancy highlights the challenge of dose translation from preclinical to human studies, where factors such as metabolism and bioavailability may impact efficacy. Future research should focus on optimizing dosing strategies to ensure therapeutic consistency between preclinical and clinical findings.
Advancements in pharmaceutical technology have enabled the development of various eucalyptol-based formulations, such as aerosols, capsules, essential oils, and nanoemulsions, which enhance the delivery efficiency of active compounds via inhalation, topical application, and oral administration. Specifically, nanoemulsion technology improves the stability and penetration of these compounds, resulting in more effective therapeutic effects for infectious and inflammatory conditions. Table 2 presents various eucalyptus-based formulations and their pharmacological effects, providing a comprehensive view of eucalyptus-based therapies for respiratory disorders.
Bibliometric analysis: computational mapping in eucalyptol research
This study generates a bibliometric map based on keyword co-occurrence to identify the main clusters in eucalyptol research. This visualization provides valuable insights for researchers to recognize dominant themes and highlight underexplored areas in the field. From the 35 selected articles, keyword co-occurrence analysis was used to identify research trends and thematic clusters. The frequency of each keyword is represented by the size of the nodes on the bibliometric map (fig. 2). The largest node, "eucalyptol," reflects the most frequently discussed topic in the literature, while smaller nodes, such as "trachea" and "aerosol," indicate more specific areas of research focus.
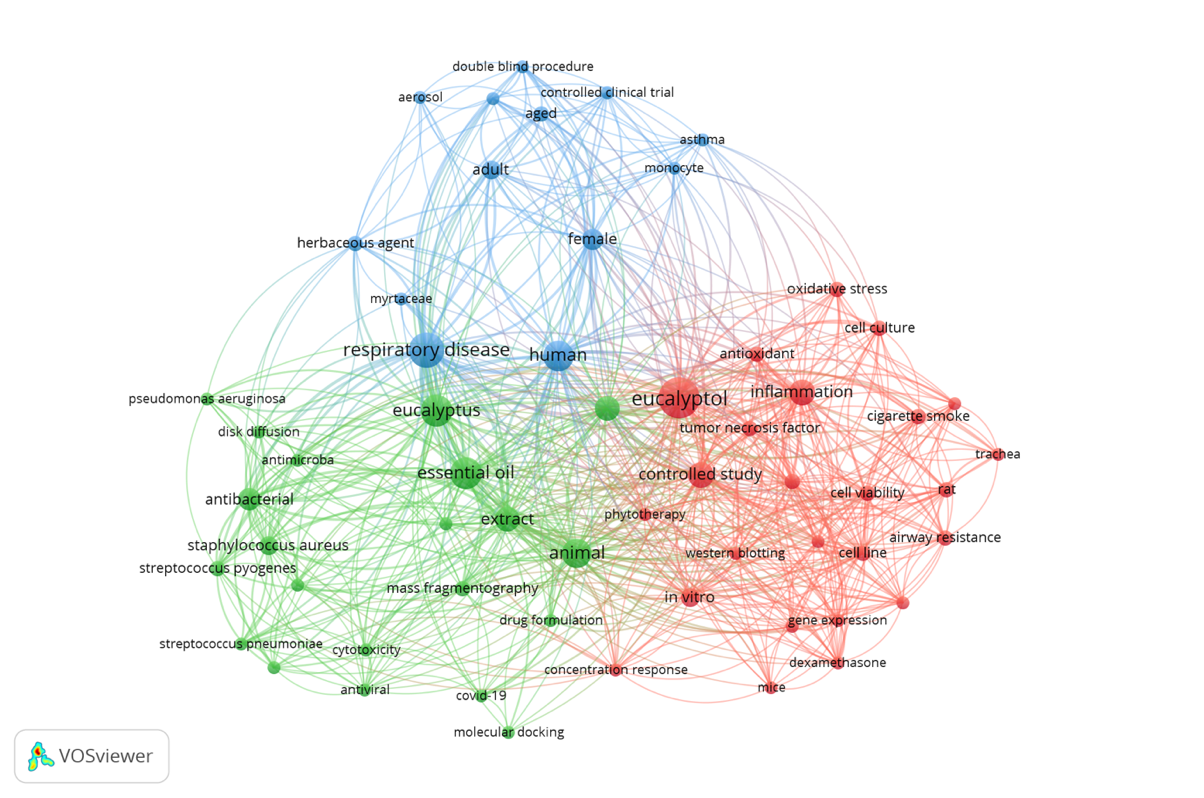
Fig. 2: Bibliometric map of keyword Co-occurrence network in eucalyptol research
The bibliometric mapping revealed three main thematic clusters that cover various aspects of eucalyptol research. The first cluster highlights the antimicrobial effects of eucalyptol, particularly its ability to combat respiratory tract pathogens. This positions eucalyptol as an effective antimicrobial agent for respiratory infections. Studies in this cluster emphasize eucalyptol's ability to inhibit bacterial growth, making it a promising candidate for respiratory disease treatments. The second cluster focuses on eucalyptol’s anti-inflammatory and antioxidant properties, which help reduce inflammation and protect tissues from oxidative stress-induced damage. Research within this cluster indicates that eucalyptol plays a protective role against tissues by reducing the damaging effects of free radicals and inflammation, reinforcing its potential as an antioxidant agent. The third cluster examines the clinical applications of eucalyptol in respiratory therapy, particularly its potential as a bronchodilator and its ability to enhance the efficacy of treatment when used as an adjunct therapy. Research in this cluster explores the practical applications of eucalyptol in the management of respiratory diseases, offering deeper insights into its use in complementary therapy.
These three clusters provide a comprehensive understanding of the role and potential of eucalyptol in health, particularly in respiratory treatment. Each cluster contributes unique insights into the pharmacological effects and clinical applications of eucalyptol, allowing researchers to identify key trends and opportunities for further exploration. This analysis underscores the need for further investigation into eucalyptol’s benefits as an adjunct component in respiratory therapy to maximize its potential.
Table 1: Summary of eucalyptus species' plant part, compound, site of action, pharmacological mechanism, dosage form, delivery method, formulation, description, and comparative drugs
| S. No. | Type/Cultivar | Part of plant | Compound | Site of action | Mechanism of pharmacology | Dosage form | Delivery method | Formulation | Reference |
| 1 | Eucalyptus camaldulensis | Leaves | Eucalyptol | Respiratory tract | Antibacterial (Pseudomonas aeruginosa) | Aerosol | Inhalation | Eucalyptol in 40% olive oil solution | [16] |
| 2 | Eucalyptus citriodora | Leaves | Eucalyptol | Alveolar macrophages | Immunomodulatory | Aerosol | Inhalation | Eucalyptol 10 mg/ml | [17] |
| 3 | Eucalyptus citriodora, E. globulus, Mentha piperita, Origanumsyriacum, Rosmarinusofficinalis | Multiple | Essential Oils | Upper Respiratory Tract | Provides rapid symptom relief in upper respiratory tract infections through anti-inflammatory and analgesic effects | Fluids for in vitro research | In vitro Delivery (Laboratory) | Eucalyptol, dissolved in culture medium at a concentration of 100 µM | [18] |
| 4 | Eucalyptus deglupta | Leaves | Eucalyptol | Respiratory tract | Antimicrobial | Liquid extracts and essential oils | Inhalation, Oral (Green Pharmacy) | Essential oil from Eucalyptus tereticornis leaves with the main content of eucalyptol, coumarin, α-pinene, β-pinene, p-cymene, limonene, γ-terpinene, α-terpineol, and aromadendrene | [19] |
| 5 | Eucalyptus globulus | Leaves | Cineole | Respiratory tract | Mucolytic, anti-inflammatory, bronchodilator; reduces inflammation, enhances mucus clearance, improves lung function | E. globulus leaf extract | Antibacterial | Methanol extract of eucalyptus leaves; MIC for Staphylococcus aureus (64 mg/l), Streptococcus pyogenes (32 mg/l), S. pneumoniae (16 mg/l), and Haemophilus influenzae (16 mg/l) | [20] |
| 6 | Eucalyptus globulus | Leaves | Eucalyptol | Respiratory Tract | Inhibits Wnt/β-catenin signaling pathway by dephosphorylating GSK-3, reducing inflammatory gene expression | Inhaler | Inhalation | Eucalyptus inhaler (contains 28 mg eucalyptol, 3 mg thymol, 10 mg menthol) | [21] |
| 7 | Eucalyptus globulus | Leaves | Eucalyptol | Mice lungs | Reduces airway hyperresponsiveness | Capsule | Oral | Cineole 200 mg per capsule | [15] |
| 8 | Eucalyptus globulus | Leaves | Eucalyptol | Nasal polyps | Reduces phosphorylation of eNOS, anti-inflammatory | Enteric-coated capsules | Oral (Enteric Capsule) | Eucalyptol, limonene, and pinene | [3] |
| 9 | Eucalyptus globulus | Leaves | Eucalyptol | Respiratory tract | Antioxidant, anti-inflammatory; inhibits superoxide anion (O2−) production, partially inhibits SOD, reduces H2O2 and TNF-α | Oral Solution | Oral (Through the Mouth) | Eucalyptol (99% purity) was diluted in 1% Tween-80 solution. | [22] |
| 10 | Eucalyptus globulus | Leaves | Eucalyptol | Grass carp hepatocytes | Antioxidant, anti-inflammatory | Aerosol | Inhalation | Eucalyptol (50 mg/kg) | [23] |
| 11 | Eucalyptus spp. | Leaves | Eucalyptol | Human bronchial tissue | Anti-inflammatory (asthma treatment) | Capsule | Oral | Cineole 200 mg per capsule | [24] |
| 12 | Eucalyptus spp. | Leaves | Eucalyptol | Mouse lungs | Lung repair, antioxidant, anti-inflammatory | Pure Essential Oils | Topical | Essential oils of some Eucalyptus species (main components: eucalyptol and citronellal) | [25] |
| 13 | Eucalyptus globulus | Leaves | Eucalyptol | Nervous system, microglia | Reduces inflammation and nerve damage by inhibiting GSDMD-mediated pyroptosis, lowering IL-1β, TNF-α, TLR4/NF-κB expression, and microglia activation | Pure Essential Oils | Topical | Eucalyptus globulus essential oil (79% cineole) combined with antibiotics | [26] |
| 14 | Eucalyptus globulus | Leaves | Eucalyptol | Respiratory tract | Antiviral, molecular docking against COVID-19 | Aerosol | Inhalation | Eucalyptol (50 mg/kg) | [27] |
| 15 | Eucalyptus globulus | Leaves | Eucalyptol | Respiratory tract | Antimicrobial, anti-inflammatory | Aerosol | Inhalation | Eucalyptol (1 mg/ml) | [28] |
| 16 | Eucalyptus globulus | Leaves | Eucalyptol | Respiratory tract | Antimicrobial, anti-inflammatory for COVID-19 | Nano-Emulsion solution in nebulizer | Inhalation | Eucalyptol nano-emulsion (4% v/v) with Tween 80 as surfactant | [29] |
| 17 | Eucalyptus spp. | Leaves | Eucalyptol | Mouse macrophages | Antioxidant, immunomodulatory | Solution (culture medium) | In vitro (cell culture) | Eucalyptol (5 μM) | [30] |
| 18 | Eucalyptus spp. | Leaves | Eucalyptol | Olfactory system | Enhances olfactory sensitivity through repeated smell training that triggers increased aroma identification and discrimination | Smell exercise | Olfactory | Eucalyptol, phenyl ethyl alcohol, citronellal, and eugenol | [31] |
| 19 | Eucalyptus globulus | Leaves | Eucalyptol | Respiratory tract | Anti-inflammatory, antioxidant | Solution (culture medium) | In vitro (cell culture) | Eucalyptol (10 ⁻ ¹ ⁰ –10 ⁻⁵ M) | [32] |
| 20 | Eucalyptus globulus | Leaves | Eucalyptol | Respiratory tract | Antibacterial (S. aureus, S. pyogenes) | Spray | Inhalation | Essential oils: E. citriodora (10%), E. globulus (20%), M. piperita (20%), Origanumsyriacum (30%), R. officinalis (20%) | [33] |
| 21 | Eucalyptus globulus | Leaves | Eucalyptol | Respiratory tract | Cross-protection against influenza virus | Suspension in Tween-80 0.5% | Oral | Eucalyptol (30, 60, and 120 mg/kg) | [34] |
| 22 | Eucalyptus griffthsii | Leaves | Eucalyptol | Respiratory tract | Antioxidant, immunomodulatory | Plant tissue isolation method | Topical | Eucalyptol (100 µM) | [35] |
| 23 | Eucalyptus globulus | Leaves | Eudesmol, α-pinene, β-pinene, γ-terpinene, α-terpineol | Nervous system, microglia | Reduces inflammation and nerve damage by inhibiting GSDMD-mediated pyroptosis, lowering IL-1β, TNF-α, TLR4/NF-κB expression, and microglia activation | Pure Essential Oils | Topical | E. globulus essential oil with eudesmol content | [36] |
| 24 | Eucalyptus spp. | Leaves | Not specified | Digestive and respiratory tracts | Antimicrobial, used in traditional medicine for digestive and respiratory diseases | Computer Model | In Silico (Molecular Interactions) | Eucalyptol, citronellol, alpha-terpineol, d-limonene | [27] |
| 25 | Eucalyptus globulus | Leaves | Eucalyptol | Respiratory tract | Antimicrobial, anti-inflammatory | Essential oils | Inhalation | Essential oils from Eucalyptus species | [25] |
| 26 | Eucalyptus globulus | Leaves | Eucalyptol | Respiratory tract | Antiviral, cross-protection against influenza virus | Solution (oral) | Oral | Eucalyptol (200 mg per capsule) | [24] |
| 27 | Eucalyptus globulus | Leaves | Eucalyptol | Respiratory tract | Antimicrobial, anti-inflammatory | Aerosol | Inhalation | Eucalyptol (1 mg/ml) | [28] |
| 28 | Eucalyptus globulus | Leaves | Eucalyptol | Respiratory tract | Reduces bronchial inflammation and hyper responsiveness | Inhalation | Inhalation | Eucalyptol nano-emulsion in nebulizer | [23] |
| 29 | Eucalyptus radiata | Leaves | Eucalyptol | Airway epithelium | Mucolytic, bronchodilator | Pure Essential Oils | Topical | Eucalyptol-rich essential oils | [24] |
| 30 | Eucalyptus globulus | Leaves | Eucalyptol | Respiratory tract | Adjuvant to flu vaccine | Oral solution | Oral | Eucalyptol (99%) | [34] |
| 31 | Eucalyptus globulus | Leaves | Eucalyptol | Respiratory tract | Antimicrobial, anti-inflammatory | Inhaler | Inhalation | Eucalyptol (20 mg per inhaler) | [21] |
| 32 | Eucalyptus globulus | Leaves | Eucalyptol | Respiratory tract | Antimicrobial and anti-inflammatory effect for asthma patients | Capsule | Oral | Eucalyptol (200 mg per capsule) | [24] |
| 33 | Eucalyptus globulus | Leaves | Eucalyptol | Respiratory tract | Bronchodilator and anti-inflammatory | Nebulizer | Inhalation | Eucalyptol-rich essential oils | [28] |
Identification of research gaps through cluster mapping
This bibliometric analysis reveals key gaps in eucalyptol research through the mapping of three major clusters: biomedical research related to inflammation and oxidative stress, antimicrobial studies and formulation development, and clinical studies in respiratory diseases. The analysis highlights the need for more in-depth clinical data in humans to confirm preclinical findings, particularly in the context of respiratory inflammation. Furthermore, there is a lack of research focusing on the bioavailability and stability of eucalyptol in innovative pharmaceutical formulations, which is crucial for enhancing its therapeutic effectiveness.
Another gap identified is the scarcity of data regarding the effectiveness of eucalyptol in vulnerable populations, such as pediatric and geriatric patients, as well as the limited evaluation of its long-term safety. This underscores the urgent need for comprehensive clinical trials, the development of more stable formulations, and studies involving specific populations to ensure that eucalyptol can be optimized as an effective therapeutic agent.
Table 3 presents a summary of these research gaps and offers recommendations for future studies, including innovations in pharmaceutical formulations and broader clinical evaluations, to maximize eucalyptol's potential in the treatment of various respiratory diseases.
Table 3: Research gaps in eucalyptol studies based on keyword mapping
| Research cluster | Dominant areas in research | Identified gaps | Further research recommendations | Ongoing research efforts |
| Cluster 1: Biomedical Research. Inflammation and Oxidative Stress | Focus on the anti-inflammatory effects of eucalyptol, especially through in vitro studies, animal testing (rats/mice), and molecular mechanisms such as IL-6 and TNF-α expression. |
|
|
Some clinical trials are investigating eucalyptol’s effects in respiratory conditions (e. g., COPD, asthma), but large-scale human studies remain limited. |
| Cluster 2: Antimicrobials and Drug Formulation Development | Studies on the antibacterial and antiviral effects of eucalyptol against respiratory pathogens, as well as the use of disk diffusion methods, molecular docking, and the development of essential oil-based formulations. |
|
|
Nanoparticle-based eucalyptol formulations are being explored to enhance bioavailability. Some preliminary antiviral studies are expanding beyond SARS-CoV-2. However, clinical validation remains scarce. |
| Cluster 3: Clinical Research in Respiratory Diseases | Clinical trials have investigated the effectiveness of eucalyptol in respiratory diseases (asthma, COPD) in adult patients, often via aerosol or inhalation formulations. |
|
|
Some moderate-scale trials have included geriatric patients, but pediatric studies remain lacking. Long-term safety data is still insufficient. |
Future research directions based on these findings
This research not only identifies gaps in eucalyptol studies but also provides strategic directions for future research to enhance the understanding and clinical application of eucalyptol in respiratory disease therapy. The bibliometric findings highlight several key areas for further development.
Large-scale clinical trials involving diverse populations, such as pediatric, geriatric, and patients with comorbidities, are needed to confirm the safety profile and efficacy of eucalyptol in managing conditions like asthma and COPD. Additionally, the development of innovative pharmaceutical formulations, such as nanoparticles or slow-release systems, could improve the bioavailability and stability of eucalyptol, thereby maximizing its therapeutic effects. Further research into the pharmacological interactions between eucalyptol and other respiratory medications is also crucial to ensure safe and effective therapeutic combinations, particularly in relation to pharmacokinetics and pharmacodynamics.
Exploration of the molecular pathways influenced by eucalyptol at the cellular and genetic levels will provide deeper insights into its anti-inflammatory and antioxidant mechanisms, which are relevant for the treatment of chronic inflammatory conditions. Studies on the antimicrobial activity of eucalyptol against acute respiratory pathogens such as influenza, RSV, and pneumonia-causing bacteria are also important to confirm its potential as an adjunctive antimicrobial agent in respiratory infections.
Lastly, long-term safety testing is essential to assess potential side effects, particularly in vulnerable populations, to establish safe dosages for prolonged use. Focusing on these areas is expected to address the existing research gaps and propel eucalyptol toward becoming a more effective, safe, and widely applicable therapy for respiratory diseases.
CONCLUSION
Eucalyptol exhibits significant pharmacological potential as an adjunct therapy for asthma, chronic bronchitis, and COPD, with documented effects on lung function, airway responsiveness, and symptom relief. Studies indicate that eucalyptol contributes to improved lung function, reduced airway resistance, and modulation of inflammatory cytokines (TNF-α, IL-1β, and IL-6), leading to better respiratory outcomes. Additionally, its mucolytic and antimicrobial properties support its role in alleviating symptoms and reducing the severity of exacerbations in chronic respiratory diseases. These benefits position eucalyptol as a valuable complementary therapy that may help reduce corticosteroid dependence and associated adverse effects. However, bioavailability limitations, volatility during inhalation, and formulation challenges must be addressed to maximize therapeutic effectiveness. Future research should prioritize nanoemulsion-based formulations, targeted inhalation therapies, and long-term safety studies. Large-scale clinical trials focusing on pediatric, geriatric patients and those with respiratory comorbidities are crucial to validating eucalyptol’s efficacy in real-world applications. Addressing these gaps will facilitate the integration of eucalyptol into mainstream respiratory disease management, offering a safer and more effective alternative to long-term corticosteroid therapy.
ACKNOWLEDGEMENT
The authors express their gratitude to the rector of Udayana University, Indonesia for providing access to their facilities during the course of this study.
ETHICS STATEMENT
This research did not involve human participants, animal subjects, or any material that requires ethical approval.
FUNDING
This study received no external funding.
AUTHORS CONTRIBUTIONS
Literature Review: I N. G. T. S., Ni N. A. D., D. K. W.; Data Curation: I N. G. T. S., Ni N. A. D., D. K. W.; Conceptualization: I N. G. T. S., Ni M. L., I M. A. G. W.;Visualization: I N. G. T. S., Ni M. L.; Writing-Original Draft: I N. G. T. S., Ni N. A. D., D. K. W., Ni M. L., I M. A. G. W.; Evaluation: D. K. W., Ni M. L., I M. A. G. W.; Supervision: Ni M. L., I M. A. G. W.
CONFLICT OF INTERESTS
The authors have no conflicts of interest regarding this investigation.
REFERENCES
Hoch CC, Petry J, Griesbaum L, Weiser T, Werner K, Ploch M. 1,8-cineole (eucalyptol): a versatile phytochemical with therapeutic applications across multiple diseases. Biomed Pharmacother. 2023 Nov;167:115467. doi: 10.1016/j.biopha.2023.115467, PMID 37696087.
Huoran W, Jianzhong L, Arnold R, Zhihe W. A review of the benefits of eucalypt essential oils for the human health and environment improvement. Agric For. 2023;2(2):38. doi: 10.57237/j.jaf.2023.02.001.
Qiu XY, Yan LS, Kang JY, YU GU C, Chi Yan Cheng B, Wang YW. Eucalyptol limonene and pinene enteric capsules attenuate airway inflammation and obstruction in lipopolysaccharide induced chronic bronchitis rat model via TLR4 signaling inhibition. Int Immunopharmacol. 2024 Mar 10;129:111571. doi: 10.1016/j.intimp.2024.111571, PMID 38309095.
Juergens UR, Dethlefsen U, Steinkamp G, Gillissen A, Repges R, Vetter H. Anti-inflammatory activity of 1.8-cineol (eucalyptol) in bronchial asthma: a double-blind placebo-controlled trial. Respir Med. 2003;97(3):250-6. doi: 10.1053/rmed.2003.1432, PMID 12645832.
Juergens UR. Anti-inflammatory properties of the monoterpene 1.8-cineole: current evidence for co-medication in inflammatory airway diseases. Drug Res (Stuttg). 2014 Dec;64(12):638-46. doi: 10.1055/s-0034-1372609, PMID 24831245.
Kim KY, Lee HS, Seol GH. Eucalyptol suppresses matrix metalloproteinase-9 expression through an extracellular signal regulated kinase-dependent nuclear factor kappa B pathway to exert anti-inflammatory effects in an acute lung inflammation model. J Pharm Pharmacol. 2015 Aug 1;67(8):1066-74. doi: 10.1111/jphp.12407, PMID 25773735.
Worth H, Schacher C, Dethlefsen U. Concomitant therapy with cineole (Eucalyptole) reduces exacerbations in COPD: a placebo controlled double blind trial. Respir Res. 2009;10(1):69. doi: 10.1186/1465-9921-10-69, PMID 19624838.
Wasnik RN, Cope M, Cowan A, Pakhale S. Assessing drug deposition efficacies environmental impact and affordability for inhalers among chronic respiratory diseases: a systematic review; 2024. doi: 10.1101/2024.06.05.24308532.
Wang DY, Ghoshal AG, Bin Abdul Muttalif AR, Lin HC, Thanaviratananich S, Bagga S, Faruqi R, Sajjan S, Brnabic AJM, Dehle FC, Cho SH. Quality of life and economic burden of respiratory disease in Asia-Pacific Asia-pacific burden of respiratory diseases study. Value in Health Regional Issues. 2016 May 1;9:72–7. doi: 10.1016/j.vhri.2015.11.004.
Lugogo N, Chipps BE, Panettieri RA, Trudo F, Ambrose CS. Long term use of maintenance systemic corticosteroids is associated with multiple adverse conditions in a large real-world cohort of us adults with severe asthma. J Asthma Allergy. 2022 Dec 7;15:1753-61. doi: 10.2147/JAA.S375005, PMID 36514709.
Omar S, Bangwal R, Sharma S, Mathur P. Effects of patient counseling on drug dose regime and medication compliance in asthma patients: a prospective study. Asian J Pharm Clin Res. 2021 Jun 1;14(7):86-9. doi: 10.22159/ajpcr.2021.v14i7.41766.
Juergens LJ, Worth H, Juergens UR. New perspectives for mucolytic anti-inflammatory and adjunctive therapy with 1,8-cineole in COPD and asthma: review on the new therapeutic approach. Adv Ther. 2020;37(5):1737-53. doi: 10.1007/s12325-020-01279-0, PMID 32200535.
Unnati P, Anushreya S, Keerthana G. Impact of patient counseling on health knowledge and medication adherence in asthma and chronic obstructive pulmonary disease patients. Asian J Pharm Clin Res. 2020;13(5):183-6. doi: 10.22159/ajpcr.2020.v13i5.37324.
Page MJ, MC Kenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021 Mar 29;372:n71. doi: 10.1136/bmj.n71, PMID 33782057.
Worth H, Dethlefsen U. Patients with asthma benefit from concomitant therapy with cineole: a placebo controlled double-blind trial. J Asthma. 2012;49(8):849-53. doi: 10.3109/02770903.2012.717657, PMID 22978309.
Jori A, Bianchetti A, Prestini PE, Gerattini S. Effect of eucalyptol (1,8-cineole) on the metabolism of other drugs in rats and in man. Eur J Pharmacol. 1970 Mar 1;9(3):362-6. doi: 10.1016/0014-2999(70)90236-0, PMID 5440307.
Kennedy Feitosa E, Cattani Cavalieri I, Barroso MV, Romana Souza B, Brito Gitirana L, Valenca SS. Eucalyptol promotes lung repair in mice following cigarette smoke-induced emphysema. Phytomedicine. 2019 Mar 1;55:70-9. doi: 10.1016/j.phymed.2018.08.012, PMID 30668445.
Bruchhage KL, Koennecke M, Drenckhan M, Plotze Martin K, Pries R, Wollenberg B. 1,8-cineol inhibits the Wnt/β-catenin signaling pathway through GSK-3 dephosphorylation in nasal polyps of chronic rhinosinusitis patients. Eur J Pharmacol. 2018 Sep 15;835:140-6. doi: 10.1016/j.ejphar.2018.07.060, PMID 30081034.
Castro MA, Girotti JR, Dumrauf B, Rodenak Kladniew B, Zaro MJ, Otero CM. In vitro evaluation of antiatherogenic potential of origanum paniculatum lippia alba clinopodium nepeta and eucalyptus globulus essential oils. J Herb Med. 2023 Dec 1;42:100785. doi: 10.1016/j.hermed.2023.100785.
Salari MH, Amine G, Shirazi MH, Hafezi R, Mohammadypour M. Antibacterial effects of eucalyptus globulus leaf extract on pathogenic bacteria isolated from specimens of patients with respiratory tract disorders. Clin Microbiol Infect. 2006;12(2):194-6. doi: 10.1111/j.1469-0691.2005.01284.x, PMID 16441463.
Nahaei MR, Kalejahi M, Rahbarfam P, Dizaj SM, Lotfipour F. Evaluation the antibacterial effects of two commercial products of eucalyptus globulus against common microbial causes of respiratory tract infections. Pharm Sci. 2016;22(4):285-90. doi: 10.15171/PS.2016.44.
Caldas GF, Limeira MM, Araujo AV, Albuquerque GS, Silva Neto JD, Silva TG. Repeated doses and reproductive toxicity studies of the monoterpene 1,8-cineole (eucalyptol) in wistar rats. Food Chem Toxicol. 2016;97:297-306. doi: 10.1016/j.fct.2016.09.020, PMID 27644596.
Rui Y, Han X, Jiang A, HU J, LI M, Liu B. Eucalyptol prevents bleomycin-induced pulmonary fibrosis and M2 macrophage polarization. Eur J Pharmacol. 2022 Sep 15;931:175184. doi: 10.1016/j.ejphar.2022.175184, PMID 35964659.
Worth H, Dethlefsen U. Patients with asthma benefit from concomitant therapy with cineole: a placebo-controlled double blind trial. J Asthma. 2012 Oct;49(8):849-53. doi: 10.3109/02770903.2012.717657, PMID 22978309.
Cimanga K, Kambu K, Tona L, Apers S, De Bruyne T, Hermans N. Correlation between chemical composition and antibacterial activity of essential oils of some aromatic medicinal plants growing in the democratic republic of congo. J Ethnopharmacol. 2002 Feb 1;79(2):213-20. doi: 10.1016/S0378-8741(01)00384-1, PMID 11801384.
Pereira V, Dias C, Vasconcelos MC, Rosa E, Saavedra MJ. Antibacterial activity and synergistic effects between Eucalyptus globulus leaf residues (essential oils and extracts) and antibiotics against several isolates of respiratory tract infections (Pseudomonas aeruginosa). Ind Crops Prod. 2014 Jan 1;52:1-7. doi: 10.1016/j.indcrop.2013.09.032.
Panikar S, Shoba G, Arun M, Sahayarayan JJ, Usha Raja Nanthini A, Chinnathambi A, Alharbi SA, Nasif O, Kim HJ. Essential oils as an effective alternative for the treatment of COVID-19: molecular interaction analysis of protease (Mpro) with pharmacokinetics and toxicological properties. Journal of Infection and Public Health. 2021 May 1;14(5):601–10. doi: 10.1016/j.jiph.2020.12.037.
Kennedy Feitosa E, Oliveira Melo P, Evangelista Costa E, Serra DS, Cavalcante FS, DA Ponte EL. Eucalyptol reduces airway hyperresponsiveness in rats following cigarette smoke exposed. Pulm Pharmacol Ther. 2020 Apr;61:101887. doi: 10.1016/j.pupt.2020.101887, PMID 31923458.
Tulbah AS, Bader A, Ong HX, Traini D. In vitro evaluation of nebulized eucalyptol nano emulsion formulation as a potential COVID-19 treatment. Saudi Pharm J. 2022 Dec 1;30(12):1691-9. doi: 10.1016/j.jsps.2022.09.014, PMID 36164456.
Yang N, Guo J, WU H, Gao M, XU S. Eucalyptol ameliorates chlorpyrifos induced necroptosis in grass carp liver cells by down-regulating ROS/NF-κB pathway. Pestic Biochem Physiol. 2024 Jan 1;198:105726. doi: 10.1016/j.pestbp.2023.105726, PMID 38225081.
Konstantinidis I, Tsakiropoulou E, Bekiaridou P, Kazantzidou C, Constantinidis J. Use of olfactory training in post-traumatic and postinfectious olfactory dysfunction. Laryngoscope. 2013;123(12):E85-90. doi: 10.1002/lary.24390, PMID 24114690.
Juergens LJ, Tuleta I, Stoeber M, Racke K, Juergens UR. Regulation of monocyte redox balance by 1,8-cineole (eucalyptol) controls oxidative stress and pro-inflammatory responses in vitro: a new option to increase the antioxidant effects of combined respiratory therapy with budesonide and formoterol? Synergy. 2018 Dec;7:1-9. doi: 10.1016/j.synres.2018.05.001.
Ben Arye E, Dudai N, Eini A, Torem M, Schiff E, Rakover Y. Treatment of upper respiratory tract infections in primary care: a randomized study using aromatic herbs. Evid Based Complement Alternat Med. 2011;2011:690346. doi: 10.1155/2011/690346, PMID 21052500.
LI K, Zhou R, Wang Jia W, LI Z, LI J, Zhang P. Zanthoxylum bungeanum essential oil induces apoptosis of HaCaT human keratinocytes. J Ethnopharmacol. 2016 Jun 20;186:351-61. doi: 10.1016/j.jep.2016.03.054, PMID 27041402.
Koennecke M, Benecke F, Masche A, Linke R, Bruchhage KL, Pries R. Increased phosphorylation of eNOS in nasal polyps of chronic rhinosinusitis patients can be diminished by 1,8-cineol. Nitric Oxide. 2018 Aug 1;78:89-94. doi: 10.1016/j.niox.2018.06.002, PMID 29885366.
Wang D, Shi Z, Liu C, Wang Q, Liu H, He J. E. globulus leaf EO exhibits anti-inflammatory effects by regulating GSDMD-mediated pyroptosis, thereby alleviating neurological impairment and neuroinflammation in experimental stroke mice. J Ethnopharmacol. 2024 Jan 30;319(3):117367. doi: 10.1016/j.jep.2023.117367.