Int J App Pharm, Vol 17, Issue 4, 2025, 395-400Original Article
NANOPARTICLE PREPARATION OF SNAKEHEAD FISH EXTRACT (CHANNA STRIATA) BY IONIC GELATION METHOD USING CHITOSAN AS POLYMER
MOHAMAD ANDRIE, WINTARI TAURINA*
Departement of Pharmacy, Faculty of Medicine, Universitas Tanjungpura, Pontianak, West Kalimantan, Indonesia
*Corresponding author: Wintari Taurina; *Email: wintari.taurina@pharm.untan.ac.id
Received: 16 Apr 2024, Revised and Accepted: 23 Apr 2025
ABSTRACT
Objective: Snakehead fish extract has high protein content, including albumin, which plays a role in accelerating wound healing. However, snakehead fish extract has low stability and a large particle size. The aim of this study is to develop nanoparticles that can increase bioavailability, solubility, particle surface area, and improve the diffusion, penetration, and stability of the active compounds.
Methods: This study aims to formulate snakehead fish extract nanoparticles using the ionic gelation method. The polymers used were chitosan at concentrations of 0.02% (F1) and 0.01% (F2), with Na-TPP 0.01% serving as the cross-linking agent. Nanoparticles were formed through ionic gelation, stirring with a magnetic stirrer at 1500 rpm for 2 h followed by sonication for 2 h and converted into nanoparticle powder by the freeze-drying method. Both formulas of nanoparticles of snakehead fish extract were characterized by visual observation, %Transmittance, Particle size, polydispersity index. The optimum formula will be continued with Zeta potential testing, Scanning electron microscope and %Adsorption efficiency.
Results: The characteristics of nanoparticles differ between F1 and F2. In F1, the nanoparticles exhibited an average transmittance of 94.447%, a particle size of 3791.8 nm, and a polydispersity index of 0.785. Meanwhile, the nanoparticles in F2 had an average transmittance of 98.028%, a particle size of 683.6 nm, and a polydispersity index of 0.528. Statistical test results confirmed a significant difference between the two formulations (p<0.05). The concentration of chitosan affects the characteristics of the nanoparticles produced. A high concentration of chitosan causes the particles to become non-uniform and increases the potential for aggregation and precipitation. F2 was determined to be the optimum formula for the preparation of snakehead fish extract nanoparticles, achieving an average entrapment efficiency of 91.87%, a zeta potential of+10.46 nm, and a particle morphology, observed at 10.000x magnification, resembling wrinkled particles with the presence of aggregates.
Conclusion: Snakehead fish extract nanoparticles with a 0.01% chitosan concentration exhibited the best nanoparticle characteristics. Observations for 7 d showed that few floating particles, the solution looks a bit cloudy and gives off a distinctive fishy odor. The % transmittance averaged 98.028%, while the particle size measured 68.6 nm with a polydispersity index of 0.528. The zeta potential was recorded at+10.46 mV, and the entrapment efficiency reached 91.87%. At 10,000x magnification, the particle morphology appeared wrinkled, with a resemblance to particulate structures and the presence of aggregates in the nanoparticles.
Keywords: Albumin, Snakehead fish extract, Nanoparticles, Ionic gelation, Chitosan
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijap.2025v17i4.53876 Journal homepage: https://innovareacademics.in/journals/index.php/ijap
INTRODUCTION
Fish is one of the most widely utilized marine natural resources, as it serves as a valuable source of animal protein [1]. Snakehead fish is one of the types of fish that contains higher protein levels compared to others. Snakehead fish contains 25.5% protein and has albumin, omega-3, and omega-6 fatty acids, which are essential for wound healing [2, 3]. Albumin found in snakehead fish aids in the formation and healing of body tissues and serves as an alternative source of albumin [4, 5]. However, albumin has a large particle size of 30 micrometers (μm) and is sensitive to extreme changes in temperature and pH, which can cause protein denaturation and precipitation [6]. The effectiveness of albumin in wound healing may be compromised due to particle size and albumin instability, which can lead to a reduction in snakehead fish extract content. To overcome this challenge, albumin nanoparticles can be formulated from snakehead fish extract using the ionic gelation method, with chitosan serving as the polymer.
Nanoparticles are colloidal formations with sizes ranging from 10 to 1000 nm [7]. Nanoparticles have the advantage of increasing bioavailability, solubility, particle surface area, improving diffusion, penetration and stability of active ingredients. Ionic gelation is a method of forming nanoparticles that involves a cross-linking process between poly-electrons and their multivalent ion pairs. The formation of this cross-linking bond is expected to strengthen the mechanics of the particles formed [8]. The distinctive characteristics of nanoparticles allow them to surpass the limitations of traditional therapeutic and diagnostic agents [9]. Cells absorb nanoparticles more efficiently than larger macromolecules, making them promising candidates for transport and delivery systems [10]. Chitosan was chosen because of its advantages, extending contact duration, enhancing penetration, and can boost effectiveness. Chitosan is a natural biopolymer obtained from chitin, a renewable resource extracted from the exoskeletons of marine waste like shrimp and crab. It possesses several notable properties, including antibacterial activity, homeostatic action, adsorption capability, biodegradability, film-forming ability, biocompatibility, adjustable mechanical strength, and mucosal adhesion, making it highly valuable in biomedical engineering. Recent studies have developed nanoparticles composed of lysozyme, nafion, and chitosan, which exhibit strong antimicrobial activity against S. aureus and E. coli [11]. Snakehead fish extract nanoparticles were prepared using the ionic gelation method by incorporating negatively charged Sodium Tripolyphosphate (Na-TPP). In this process, the amino groups in chitosan interact with negatively charged polyanions. Conversion into nanoparticles is expected to increase the effectiveness and stability of snakehead fish extract.
This research aimed to formulate albumin nanoparticles from snakehead fish extract using the ionic gelation method with chitosan as a polymer. The study involved both physical and chemical characteristics powder of snakehead fish extract nanoparticles such as visual appearance, % transmittance, particle size, zeta potential, particle morphology, and % entrapment efficiency.
MATERIALS AND METHODS
Materials
Snakehead (Channa striata) was obtained from fisherman in Sungai Itik village, Kuburaya Regency, West Kalimantan, Indonesia, Chitosan (Chimultiguna), Natrium trypolyphosphate (Lux Chemical)
Instruments
Scanning Electron Microscopy (SEM) (SU3500, America), Particle Size Analyzer (PSA) and Zeta Potential Analyzer (ZPA) (SZ-HORIBA 100, Japan), Spectrophotometer UV-Vis (Shimadzu UV 2600i, Japan), Photometer (Microlab 300, Netherland).
Collection and extraction of snakehead fish extract
The Snakehead fish (Channa striata) was identified at the Biology Laboratory, Department of Biology, Faculty of Mathematics and Natural Sciences, Universitas Tanjungpura, Pontianak, West Kalimantan, under the identification number 0164/A/lB/FMIPA/UNTAN/2024. The Snakehead fish used have weights ranging from 500 to 1000 gs, obtained from fisherman in Sungai Itik Village, Kuburaya Regency, West Kalimantan, Indonesia. Snakehead fish were selected based on Indonesian National Standards number SNI 2729-2021. This includes a whole body with intact scales, free from parasites, without defects, and with a bright and shiny appearance. Afterward, we conduct a wet sorting process. The scales, fins, tail, and intestines are cleaned. After that, the snakehead fish is rinsed under running water. The snakehead fish is chopped into several pieces. The snakehead fish meat is steamed at 70 °C for 30 min. The meat wrapped with cloth and pressed with hydraulic press to obtain extract of snakehead fish.
Preparation nanoparticle of snakehead fish extract
Nanoparticles were initially prepared using different types of chitosan with molecular weight 5,5 kDa and degree of deacetylation>85% on a medium scale (60 ml) to identify the most optimal preparation method. Two formulas were created with varying concentrations of chitosan and Na-TPP in each. The volume ratio of the snakehead fish extract, chitosan, and Na-TPP was 1:2:1. The snakehead fish extract, being in liquid form, did not require dissolution. The Na-TPP solution was prepared by dissolving Na-TPP in distilled water pH 5, resulting in a transparent, clear solution. The chitosan solution was made by dissolving chitosan in acetate buffer at pH 4, yielding a transparent, clear solution.
Each formula was prepared by weighing 15 ml of the snakehead extract and mixing it into 30 ml of the solution. This mixture was then added drop by drop into 6 ml of chitosan in an acetate buffer solution at pH 4, while stirring with a magnetic stirrer at 1500 rpm for 30 min. Following this, 15 ml of Na-TPP solution in distilled water was measured and dripped into the combined water and chitosan mixture, continuing to stir at 1500 rpm for 1.5 h. Additionally, the nanoparticles of snakehead fish extract were processed into a powder form using a freeze-drying method. The fish is arranged in a container or tray in a thin layer and frozen in a freezer at approximately-40 °C until completely frozen, usually within 24 h. After freezing, the fish is transferred to a freeze dryer, where low temperature and pressure are applied to enable sublimation, the process in which ice directly turns into vapor without passing through the liquid phase. This process takes about 24 h. Once completed, the dried snakehead fish is stored in an airtight container or vacuum-sealed bag to prevent moisture absorption.
Observations of the powdered nanoparticles from the snakehead fish extract were conducted to examine the presence of floating particles, sedimentation, solution color, sediment, shape, and turbidity in order to identify the optimal formula for the next step.
Evaluation of physical characterization powder nanoparticles
The physical properties of nanoparticles in both formulas were evaluated through visual inspection, % transmittance, particle size, and polydispersity index. The size, distribution, and zeta potential of the nanoparticles were measured using a Particle Size Analyzer (PSA), while transmittance was assessed using UV/Vis spectrophotometry [14]. The most suitable formulation will undergo further testing, including zeta potential and SEM analysis. Nanoparticle morphology was examined using a Scanning Electron Microscope (SEM).
Evaluation of chemical characterization powder nanoparticles
Entrapment efficiency of the optimal formulation
The entrapment efficiency was assessed by measuring the concentration of free albumin in the optimal nanoparticle powder derived from snakehead fish extract. Photometry, utilizing bromocresol blue reagent, was employed to determine the entrapment efficiency. The nanoparticles of snakehead fish extract were centrifuged at 10,000 rpm for 30 min, and the supernatant was collected. A 10 µl** sample was transferred into a 1.5 ml tube, followed by the addition of 1,000 µl** of bromocresol blue reagent, and the mixture was vortexed until homogeneous. For the blank, 10 µl** of distilled water and 1,000 µl** of reagent were used. An albumin standard solution with a concentration of 200 mg/dl was prepared, and 10 µl** of this standard was mixed with 1,000 µl** of reagent. Each mixture was homogenized and incubated at room temperature (24-30 °C) for 10 min. Absorbance was measured using a Microlab 300 Photometer at a wavelength of 546 nm using the endpoint method. The drug Entrapment Efficiency (EE) was determined as follows [12, 13]
% Drug entrapment = 
RESULTS
Collection and extraction of snakehead fish
The Snakehead fish were collected from the Toampe ditch in RT 13/RW 005, Dusun Cempaka, Sungai Itik Village, Sungai Kakap District, Kuburaya Regency, West Kalimantan. The weight of the snakehead fish obtained was 707 g. This is based on the criteria for the weight of snakehead fish, with the highest albumin content ranging from 600 to 900 g per tail [15]. The processing stage of snakehead fish begins with sorting, which aims to separate snakehead fish that are still alive and selected with the criteria of the body and scales of the fish in good condition, no parasites, red gills, fresh fish and a distinctive aroma [16]. Furthermore, the weeding process involves separating the scales, right and left fins, dorsal and lower fins, and fish tails. The fish are then cut to expedite the extraction process and washed thoroughly to remove impurities. The processing continues with the steaming stage, where 114 g of snakehead fish are steamed at a temperature of 70 °C for 30 min. The goal of this step is to break apart the cells in the snakehead fish meat, allowing the nutrients to be optimally released during the pressing process. A steaming duration of 30 min is considered optimal for achieving the best yield, effectively removing albumin and oil from the snakehead fish meat [17]. Extending the steaming time can cause protein denaturation while a short time causes the cell lysis process to be less than optimal [18]. The yield of the snakehead fish extract is 25.36%.
Preparation of nanoparticle preparations snakehead fish extract
Nanoparticles were synthesized using the ionic gelation method, which relies on the electrostatic interaction between the chitosan amine group and the negatively charged polyanion group of Na-TPP. This interaction occurs when the chitosan amine group, protonated in an acidic environment, binds with the oxygen atom in Na-TPP. The protonated amine group of chitosan enables it to interact with negatively charged compounds, resulting in the coating of extract particles with a primer in the snakehead extract. The ionic interaction between chitosan and Na-TPP leads to nanoparticle formation, which is why this method is called ionic gelation. TPP was selected as a polyanionic crosslinker due to its non-toxic nature and gel-forming capability when combined with chitosan. The addition of Na-TPP helps stabilize the nanoparticles by linking the positive charge of chitosan and the negative charge of Na-TPP, with higher concentrations of Na-TPP contributing to larger particle sizes.
Table 1: Design of snakehead fish extract nanoparticle formula, chitosan, Na-TPP
| Formula | Chitosan concentration (%) | Na-TPP concentration (%) | Snakehead fish extract concentration (ml) |
| 1 | 0.02 | 0.01 | 15 ml |
| 2 | 0.01 | 0.01 | 15 ml |
Table 1 presents the formula for the snakehead fish extract nanoparticles, chitosan, and Na-TPP. The observation results revealed that the addition of Na-TPP during preparation resulted in a cloudy yellow solution. As Na-TPP was incorporated into the chitosan environment, a gradual cloudiness developed, indicating the presence of small particles in the suspension. This cloudy appearance (opalescence) is called the Tyndall effect. The Tyndall effect is a phenomenon of light scattering by colloidal particles. The results of observations of the F1 preparation from day 0 to day 7 were that many floating particles, and the solution looks a bit cloudy and gives off a distinctive fishy odor. The results of observations of the F2 preparation from day 0 to day 7 were that few floating particles, the solution looks a bit cloudy and gives off a distinctive fishy odor. The instability of nanoparticles in F1 and F2 occurred on day 3, which was marked by changes in turbidity behaviour and the presence of floating particles. The concentration of chitosan affects the resulting sediment, where at a concentration of 0.01, the resulting sediment is less. This is because the smaller the concentration of chitosan, the molecules will be evenly distributed, so that the chance of agglomeration and clumping between particles is reduced.
Characterization of nanoparticle
The physicochemical characterization of nanoparticles is crucial as their properties impact not only the absorption and release of active substances but also their interactions with biological components, such as proteins and tissue membranes, in the environments where they function.
Characterization of nanoparticles of snakehead fish extract was carried out in powder form with freeze dry method. Freeze dry is one of the drying methods that has the advantage of maintaining the quality of extracts from snakehead fish. This method can leave a sample air content of 1%, which meets the requirements of raw materials from nature in pharmaceutical preparations. The nanoparticles of snakehead fish extract were made in powder form to extend the shelf life; the suspension of snakehead fish extract nanoparticles was freeze-dried. The physicochemical analysis of nanoparticles, which serve as the primary drug carriers, involves evaluating their dimensions, size, surface characteristics, and encapsulation efficiency [19].
Table 2: Characterization results of chitosan-snakehead fish extract nanoparticle
| Formulation | Chitosan consentration (%) |
Na-TPP concentration (%) |
Chitosan: Na-TPP: snakehead fish extract | Nanoparticle characterization results | |||
| Replication | Particle size (Nm) | PI (Polidispersity Index) | % Transmittance | ||||
| 1 | 0.02 | 0.01 | 2:1:1 | 1 | 3890.2 | 0.997 | 94.543% |
| 2 | 3774.5 | 0.832 | 94.295% | ||||
| 3 | 3710.9 | 0.526 | 94.504% | ||||
| Average | 3791.8±90.090 | 0.785±0.2389 | 94.447±0.133 | ||||
| 2 | 0.01 | 0.01 | 2:1:1 | 1 | 714.3 | 0.597 | 97,992 |
| 2 | 607.7 | 0.428 | 98,152 | ||||
| 3 | 728.9 | 0.561 | 97,940 | ||||
| Average | 683.6±66.16 | 0.528±0.089 | 98.028±0.110 | ||||
Description: Na-TPP = Natrium Tripolifosfat; PI = Polydisperse Index, (Results are expressed as a mean±SD, n=3)
% Transmittance
Transmittance testing aims to determine the clarity of the nanoparticle [20]. Based on table 2, the transmittance of nanoparticles with 0.02% chitosan polymer produces a transmittance of 94.447%. Meanwhile, the transmittance value of nanoparticles with 0.01% chitosan polymer is 98.0128%. A transmittance value close to 100% suggests that the nanoparticles formed create clear and transparent dispersions with particle sizes in the nanometer range. Increasing chitosan concentration causes a decrease in % transmittance [21]. Turbidity in nanoparticles is due to colloidal formation due to the interaction between the negative charge of Na-TPP and the positive charge of chitosan. The smaller the chitosan concentration, the fewer the number of nanoparticles formed so that the light scattering is smaller and the more light is transmitted.
Particle size
Particle size plays a key role in the performance of nanoparticles. Achieving a narrow size distribution in nanoparticle formulation can be challenging if the suspension doesn't have a uniform droplet size distribution. As nanoparticles are taken up by cells through endocytosis, larger particle sizes can reduce absorption and may impact drug bioavailability. The extent of endocytosis varies depending on the type of target cell [22]. The results of the formulation of nanoparticles from snakehead fish extract in an aqueous phase with various concentrations of chitosan polymer are shown in table 2. Among the two nanoparticle formulas with variations of chitosan of 0.02% and 0.01% that meet the requirements for good nanoparticle size are in formula 2 with an average particle size diameter of 683.6 nm. The higher concentration of chitosan causes an excess amount of chitosan. This causes chitosan to tend to bind irregularly, then cross-link with Na-TPP, which forms a single particle with a large size. This can be stated that increasing the concentration of chitosan causes non-uniform particles to increase and has the potential for precipitation [23]. The concentration of Na-TPP added can affect the stability and strength of the nanochitosan matrix formed, resulting in stronger nanoparticles and making them difficult to break. The more cross-links formed between chitosan and Na-TPP, the stronger the amylose molecules will be. The strength of amylose causes the bioplastic structure to become strong and compact. The chitosan matrix will increase, causing the chitosan particles to become stronger and harder, and increasingly difficult to break into small pieces. Therefore, the number of chitosan particles produced is decreasing [24].
Polydispersity index
The polydispersity index test aims to determine the uniformity of the particles. Nanoparticles with a polydispersity index of 1 exhibit a broad size distribution and include large particles or clusters that may experience sedimentation. Table 2 shows the polydispersity index value of nanoparticles with 0.01% chitosan of 0.528 and 0.02% chitosan of 0.758. This polydispersity index value is below 0.7 which means it is the upper range where the distribution algorithm operates best and is quite homogeneous with a polydispersity index value close to 0 [25]. The 0.01% chitosan concentration produces nanoparticles with a smaller polydispersity index, this is because the chitosan molecules are homogeneously dispersed until the particles formed are uniform. A large concentration of chitosan is likely to cause aggregation [26].
Formula 2 was selected for further testing as it contains the lowest concentration of TPP and chitosan (0.01%) and has the fewest floating particles. Judging from the results of nanoparticle characterization such as transmittance, particle size, and polydispersity index, formula 2 is feasible to continue testing zeta potential and SEM morphology because the characterization results show a particle size that meets, a small polydispersity index, and a percentage transmittance value that is close to 100%.
Table 3: Normality test
| Tests of normality | Characteristics | Kolmogorov-Smirnova | Shapiro-Wilk | ||||
| Statistic | df | Sig. | Statistic | df | Sig. | ||
| Chitosan 0.02% | %Transmittance | .331 | 3 | . | .865 | 3 | .280 |
| Particle size (nm) | .242 | 3 | . | .973 | 3 | .683 | |
| Polydispersity index | .245 | 3 | . | .971 | 3 | .673 | |
| Chitosan 0.01% | %Transmittance | .294 | 3 | . | .920 | 3 | .454 |
| Particle size (nm) | .345 | 3 | . | .839 | 3 | .389 | |
| Polydispersity index | .308 | 3 | . | .901 | 3 | .253 | |
| a. Lilliefors significance correction | |||||||
Normal distributed test data sig shapiro wilk above 0.05.
The normality test is shown in table 3. The significant results of Shapiro Wilk in all test groups show sig values >0.05 so it can be concluded that the data characteristics of snakehead fish extract nanoparticle is normally distributed.
Table 4: Independent sample T-test
| Independent sampel t-test | |||||||||
| Levene’s test fir equality of variances | T-test for equality of means | ||||||||
| F | Sig | t | df | Sig (2-tailed) |
Mean difference |
Std. error difference | Difference | ||
| Lower | |||||||||
| Transmittance | Equal variances assumed | .285 | .622 | 47.883 | 4 | .000 | 3108.23333 | 64.91280 | 2928.00649 |
| Equal variances not assumed | 3.665 | .000 | 3108.23333 | 64.91280 | 2921.08797 | ||||
| Size | Equal variances assumed | .285 | .663 | 47.883 | 4 | .000 | 3108.23333 | 64.91280 | 2928.00649 |
| Equal variances not assumed | 47/833 | 3.66 | .000 | 3108.23333 | 64.91280 | 2928.00649 | |||
| Polydispersity index | Equal variances assumed | 2.464 | .192 | 1.741 | 4 | .157 | .25633 | .14724 | -.15248 |
| Equal variances not assumed | 1.741 | 2.544 | .196 | .25633 | .14724 | -2.6348 | |||
The results of the significant test are shown in table 4. The results show that in the test group of transmitter and particle size at equal variances assumed shows that the sig value (2-tailed)<0.05, which means that HO is rejected and H1 is accepted. This means that there is a significant difference between the results of the transmitter and the size test of 0.02% and 0.01% chitosan nanoparticles. The results show that in the test group of polydispersity index at equal variances assumed shows that the sig value (2-tailed)>0.05, which means that H0 is accepted and H1 is rejected. This means that there is no significant difference between the results of the polydispersity index test of 0.02% and 0.01% chitosan nanoparticles. Based on the results of this statistical test, it can be concluded that in the test group of transmitter and particle size, there is a significant difference between the formulation of snakehead fish nanoparticles with 0.02% and 0.01% chitosan polymers. The optimal formula that produces fish extract nanoparticles with good physical characteristics is F2 (chitosan 0.01). Based on the results of statistical tests, it can be concluded that in the transmittance and particle size test groups there are significant differences between the formulations of snakehead fish nanoparticles with 0.02% and 0.01% chitosan polymers. The optimal formula that produces snakehead fish extract nanoparticles with good physical characteristics is F2. F2 is determined as the optimum formula for making snakehead fish extract nanoparticles because the nanoparticles in F2 have an average transmittance of 98.028%, a particle size of 683.6 nm, and a polydispersity index of 0.528 compared to F1 with an average transmittance of 94.447%, a particle size of 3791.8 nm, and a polydispersity index of 0.785. The average transmittance, particle size, and polydispersity index of F2 are better than F1.
Zeta potential
After conducting preliminary tests such as transmittance, particle size test and polydispersity index, the optimal formula for chitosan (F2) was obtained so that further tests were carried out, namely zeta potential. Zeta potential describes the condition of the surface charge of nanoparticles which is sufficient to cause repulsive forces between the particles [27]. A good zeta potential value of>+30 indicates that the repulsion force is greater than the attractive force, thus increasing the stability of the dispersion system. The result of zeta potential of snakehead fish extract nanoparticle with polymer chitosan shows+10.46 mV (<+30), which means that the nanoparticles formed are unstable. The dispersion of slightly charged or neutral nanoparticles is unstable so that it has a tendency to clump, flocculation or agglomerate after long-term storage. The positive zeta potential value is due to the contribution of partial charges on the surface dominated by chitosan (positively charged) so that the potential difference between the electrical double layer and the medium is positive [28]. Particles with zeta potential values ≤ 30 mV can also be sterically stabilized. Increasing the zeta potential value is very important to improve the stability of nanoparticles in suspension. The zeta potential value can be stabilized by modifying the formulation composition by adjusting the polymer and crosslinker ratio between the chitosan and Na-TPP ratio. In addition, adding charged surfactants such as anionic and cationic surfactants also has an effect on increasing stability [29].
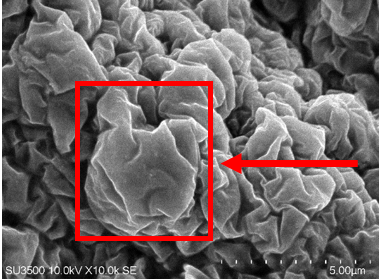
Particle morphology
SEM (Scanning Electron Microscopy) characterization is used to observe the morphology of a particle [24]. Optimal nanoparticle powder of snakehead fish extract was tested using SEM. morphology characterization was carried out with magnifications of 5000x and 10.000x. Based on fig. 1, the results of SEM morphology characterization at 5000x and 10.000x magnification show particles that resemble balls and wrinkled, and the presence of aggregates in these nanoparticles. These aggregates can be formed due to the effects of Brownian motion and Van der Waals forces. Nanometer-sized particles have a very large surface area, which causes the Van der Waals force to be more dominant than the gravitational force. As a result, particles will have a tendency to stick together and form a collection of small particles. In addition, aggregation causes the size and diameter of the resulting nanoparticles to be non-uniform. Nanoparticle aggregation can be mitigated by applying surface engineering techniques, incorporating charged groups, or utilizing steric stabilization. Ideally, nanoparticles should maintain a surplus of negative surface charge, especially in the fasting state, and create steric hindrance through surface functionalization with citrate, anionic surfactants, or high-molecular-weight polymeric chains such as polyethylene glycol (PEG) and polyvinylpyrrolidone (PVP) to inhibit clustering [30]. Good nanoparticle morphology is spherical so that it is easier to enter cells. This is because particle morphology affects the ability of nanoparticles to penetrate the target cell membrane [31, 32].
(a) |
(b) |
Fig. 1: Nanoparticles morphology using SEM magnification of 5000x (a) and magnification of 10000x (b)
% Entrapment efficiency
Targeted drug delivery is a strategy aimed at precisely and effectively delivering therapeutic agents to the intended site, while reducing exposure to unintended areas. Targeting drugs to reach the target tissue will increase the efficiency of the therapeutic effect produced by the active substance or drug, can also reduce the dose or amount of drugs that must be administered or consumed so as to reduce toxic effects or unwanted side effects of drugs [31]. A good nanoparticle delivery system has a high % absorption value. The absorption efficiency (EE) is measured to determine the ability of chitosan to protect the active substances that form nanoparticles [33]. The samples made for the entrapment efficiency test were optimal snakehead fish nanoparticle powder (Chitosan 0.01%) and snakehead fish extract (control). The absorption efficiency of snakehead fish extract nanoparticles with chitosan 0.01% (F2) is 91.87%, which means that the absorption efficiency is said to be good if the value is more than 80% [34]. The increasing % absorption efficiency value can cause the bond between the carrier system and the active substance to be stronger so that it can protect degradation from the external environment.
CONCLUSION
Snakehead fish extract nanoparticles with 0.01% chitosan concentration produced the best nanoparticle characteristics. Observations for 7 d showed that few floating particles, the solution was slightly cloudy, and had a distinctive smell of snakehead fish and the average of %transmittance was 98.028%, particle size was 683.6 nm, polydispersity index was 0.528, zeta potential was+10.46mV, % entrapment efficiency was 91.87% and has a particle morphology at 10.000 x magnification show particle like resemble, wrinkled and the presence of aggregates in these nanoparticles. In vivo validation or cytotoxicity studies are necessary to evaluate the efficacy and safety of these nanoparticles in biological systems to ensure their biocompatibility, minimize potential toxicity, and support their potential application in therapeutic or biomedical fields.
ACKNOWLEDGMENT
The authors thank to Dean of Medicine Faculty and Rector of Tanjungpura University Pontianak for the permission of all the facilities that have been given to the author.
AUTHORS CONTRIBUTIONS
Mohamad Andrie contributed in conceptualized and designed the study, gathered and analyzed the data, and drafted the initial manuscript. Involved in interpretation of the data and provided critical revisions to the content. Wintari Taurina assisted in data collection and contributed to data analysis, provided substantial input in the interpretation of the results, and reviewed and revised the manuscript for intellectual content. Each author has read and approved the final version of the manuscript.
CONFLICT OF INTERESTS
The authors declare no conflict of interest.
REFERENCES
Wulandari R, Nurmalasari, Wardi RY. Albumin levels of climbing perch (Anabas testudineus) in the cenning village river malangke district North Luwu cokroaminoto. J Chem Sci. 2020;2(1):21-3.
Arfiyanti, Ariyanti D. Additional food for pregnant women to prevent stunting in an effort to improve the quality of human resources for national defense. J Kim Saintek Pendidik. 2014;6(2):56-65. doi: 10.51544/kimia.v6i2.3494.
Andrie M, Sihombing D. Effectiveness of ointment containing snakehead fish extract (Channa striata) on the healing process of stage II open acute wounds in male wistar rats. Pharm Sci Res. 2017;4(2):4.
Ginta S. Effectiveness of the provision of snakehead fish nuggets and colored fruit extracts to blood protein (total protein albumin Hb) in PLHIV. J Phys: Conf Ser. 2020;1665(1):012026. doi: 10.1088/1742-6596/1665/1/012026.
Waicang R, Maria R, Herawati T. The effects of snakehead fish extract supplements on nephrotic syndrome patients. J Res Health. 2022;13(3):600-3.
Tungadi R, Wicita P. Formulation optimization and characterization of snakehead fish (Ophiocephalus striatus) powder nanoemulgel. Braz J Pharm Sci. 2020;56(17337):1-8. doi: 10.1590/S2175-97902019000417337.
Istianah. Folic acid encapsulation using chitosan nanoparticles with ionic gelation method. UIN Syarif Hidayatullah Jakarta; 2019.
Kadian R. Nanoparticles: a promising drug delivery approach. Asian J Pharm Clin Res. 2018;11(1):30-5. doi: 10.22159/ajpcr.2017.v11i1.22035.
GA, RG, VV G, AR. Targeting the target using nanoparticles-a review. Asian J Pharm Clin Res. 2017;10(12):6-10. doi: 10.22159/ajpcr.2017.v10i12.19734.
Abdassah M. Nanoparticles with ionic gelation. J Farmaka. 2017;15(1):45-52.
Yadav M, Kaushik B, Rao GK, Srivastava CM, Vaya D. Advances and challenges in the use of chitosan and its derivatives in biomedical fields: a review. Carbohydr Polym Technol Appl. 2023;5:5. doi: 10.1016/j.carpta.2023.100323.
Edityaningrum CA, Zulaechah AN, Putranti W, Arimurni DA. Formulation and characterization of carbamazepine chitosan nanoparticle. JFIKI. 2022;9(2):146-54. doi: 10.20473/jfiki.v9i22022.146-154.
Ambarwati R, Rustiani E. Formulation and evaluation of avocado seed extract nanoparticles (Persea americana mill) with plga polymer. Pharm J. 2022;7(4):305.
Asikin AN, Kusumaningrum I. Characteristics of snakehead fish protein extract based on fish weight size from mahakam river East Kalimantan. J Processor Fish. 2017;21(1):137. doi: 10.17844/jphpi.v21i1.21462.
Zdzisaw ES. Food quality and standards pertaining to fish. In: Food quality and standards. In: Encyclopedia of Life Support Systems (EOLSS) developed under the auspices of the UNESCO. Paris France: Eolss Publishers. 2009;2(10):134.
Indonesian National Standard. Fresh fish Jakarta: National Standardization Agency. SNI. 2021;2729:2021.
Mendoza SD. Physicochemical characteristics of skin lotion from snakehead fish (Channa striata) albumin extract. Nat Microbiol. 2020;3(1):641.
Salmatia S, Isamu KT, Sartinah A. Pengaruh proses perebusan dan pengukusan terhadap kandungan albumin dan proksimat ikan gabus (Channa striata). Fish Protech. 2020;3(1):67. doi: 10.33772/jfp.v3i1.11606.
Andrie M, Taurina W. Nanoencapsulation of ethanol extract of papaya leaf (Carica papaya linn.) using chitosan and testing its effectiveness as an anti-inflammatory. Int J App Pharm. 2024;16(2):264-71. doi: 10.22159/ijap.2024v16i2.49992.
Ariani LW, Purwanto UR. Nanoparticle formulation of hibiscus leaf extracts (Hibiscus rosa sinensis l.). Semarang: STIFAR; 2021.
Supraba W, Juliantoni Y, Ananto AD. The effect of stirring speeds on the entrapment efficiency in a nanoparticles formulation of java plum seed ethanol extract (Syzygium cumini). Acta Chim Asiana. 2021;4(1);197-103. doi: 10.29303/aca.v4i1.50.
Betala S, Mohan Varma M, Abbulu K. Formulation and evaluation of polymeric nanoparticles of an antihypertensive drug for gastroretention. J Drug Delivery Ther. 2018;8(6):82-6. doi: 10.22270/jddt.v8i6.2018.
Qonitannisa S, Fadli A, Sunarno. Synthesis of nanochitosan by ionic gelation method using acetic acid solvent with variation of chitosan concentration. J Mhs BiD Tek Dan Sains. 2020;7(2):1-4.
Guge SR, Lukum A, Kunusa WY. Nano chitosan production using ionic gelation method. Jambura J Chem. 2024;6(1):1-8.
Ngafif A. Optimization of sodium alginate and calcium chloride (CaCl2) as cross-linking agents of ethanol extract nanoparticles of katuk leaves (Sauropus androgynus (l.) merr). Berk Ilm Mhs Farm. 2020;7(2):13-23. doi: 10.48177/bimfi.v7i2.33.
Rahmatullah S, Permadi YW, Agmarina SN. Testing of nanoparticle character ionic gelation method of extract and tablet of African leaf (Vernonia amygdalina del.). J Wiyata. 2021;8(2):147-51.
Maharani P, Ikasari E, Purwanto U, Bagiana I. Optimization of Na-alginate and Ca-chloride in nanoparticles of purified fucoidan extract from brown seaweed (Sargassum polycystum). Pharm Med J. 2022;5(2):38-45. doi: 10.35799/Pmj.V5i2.45100.
Samudra AG, Ramadhani N, Lestari G, Nugroho BH. Formulation of chitosan nanoparticles from methanol extract of brown sea algae (Sargassum hystrix) using ionic gelation method. J Ilm Manuntung. 2021;7(1):92-9.
Kulkarni S, Nilesh. Characterization of self-microemulsifying dosage form: special emphasis on zeta potential measurement. Int J Pharm Biol Arch. 2019;10(3):172-9.
Shrestha S, Wang B, Dutta P. Nanoparticle processing: understanding and controlling aggregation. Adv Colloid Interface Sci. 2020;279:102162. doi: 10.1016/j.cis.2020.102162, PMID 32334131.
Tekade RK, Maheshwari R, Soni N, Tekade M, Chougule MB. Nanotechnology for the development of nanomedicine. In: Nanotechnology-based approaches for targeting and delivery of drugs and genes. Elsevier; 2017. p. 3-61. doi: 10.1016/B978-0-12-809717-5.00001-4.
Juliantoni Y, Hajrin W, Subaidah WA. Formulasi sediaan gel sari buah duwet (Syzygium cumini) dengan basis karbopol 940 sebagai gelling agent. SJP. 2020;1(2):30-3. doi: 10.29303/sjp.v1i2.14.
Putri GM, Atun S. Preparation and characterization of nanoparticles ethanol extract of temu kunci (Boesenbergia pandurata) in various variations of alginate composition. J Kim Basis. 2017;6(1):19-26.
Auliasari N, Hanifa HL, Permatasari A. Formulation and characterization of α-mangostin nanoparticle delivery system with chitosan alginate as polymer. Dissertation. 2023;3(1):222-8.