
Int J App Pharm, Vol 17, Issue 5, 2025, 378-390Original Article
ISOLATION AND EVALUATION OF NATURAL GUM EXUDATES OF LANNEA COROMANDELICA (HOUTT.) MERR. AS NATURAL POLYMERIC BINDER AND DRUG RELEASE REGULATING AGENT IN COMPARISON WITH SYNTHETIC AND SEMISYNTHETIC POLYMERS
V. ANUSHA1, M. S. UMASHANKAR1*
1Department of Pharmaceutics, SRM College of Pharmacy, SRM Institute of Science and Technology, SRM Nagar, Kattankulathur, Tamil Nadu, India
*Corresponding author: M. S. Umashankar; *Email: umashans@srmist.edu.in
Received: 28 Feb 2025, Revised and Accepted: 01 Jul 2025
ABSTRACT
Objective: The present research study aims to isolate the natural gum from Lannea coromandelica (Houtt.) Merr and to analyze and explore the polymeric properties specifically binding property and drug release regulating properties of Lannea gum by formulation of tablets using Ibuprofen as model drug.
Methods: The natural Lannea gum was compared with standard synthetic and semi-synthetic polymers like Povidone K30 and Hydroxypropyl methyl cellulose K4M (HPMC K4M) respectively as standard polymers with binding property and drug release regulating property. The process includes formulation of total nine batches, where Lannea gum was used at concentrations of 7, 14, 21 mg as a novel binder and drug release regulating agent with above mentioned as binders and drug release regulating agent along with Avicel PH 102 as diluent and disintegrant, Ibuprofen as model drug, magnesium stearate as lubricant and Aerosil as Glidant with the technique of wet granulation method. The compatibility of formulation blend was analyzed by FTIR studies which indicated the gum is compatible with formulation ingredients. The thermal, surface morphological, physical properties of Lannea gum were identified by DSC, SEM and XRD studies respectively. The micromeritic properties of granules like bulk/tapped density, compressibility index, Hausnner’s ratio, angle of repose indicated satisfactory flow properties.
Results: The formulated tablets were evaluated for parameters like weight variation, hardness, disintegration time, drug content and In vitro drug release studies. In conclusion it could be confirmed that at 60 min formulation f3 containing Lannea gum produced drug release of 85.27%, f6 containing Povidone K30 produced drug release of 97.47% and f9 comprising HPMC K4M exhibited 81.15% drug release.
Conclusion: The study concludes that Lannea gum unveiled superior binding properties than Povidone K30 and slightly low potential with concern to its binding and drug release regulating properties when compared to HPMC K4M. Hence, Lannea coromandelica gum, can be used as a potential polymer in formulation of tablets as a binder and drug release regulating agent.
Keywords: Polymer, Natural, Synthetic, Drug release, Compatibility
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijap.2025v17i5.53905 Journal homepage: https://innovareacademics.in/journals/index.php/ijap
INTRODUCTION
Polymers play a significant role in formulation of dosage forms with wide range of applications as diluents, binders, disintegrant, suspending agents, emulsifying agents, thickening agents, gelling agents, etc. Natural plant derived materials are extensively acceptable as pharmaceutical excipients because of their additional advantages like renewability, non-toxic nature, cost effectiveness, biocompatibility and biodegradable nature [1]. These natural plant derived gums also act as drug release controlling agents and release retardants which makes them suitable in formulation of sustained and extended release dosage forms. The synthetic polymers could produce toxicities and their production and synthesis leads to environmental pollution. The naturally obtained products are advantageous when compared to synthetic polymers when compared to cost, toxicity, renewability etc [2]. Natural gums can be modified easily to produce a polymer with desirable properties which helps in formulating different pharmaceutical dosage forms. The plant based polymers have been extensively studied for their advantages and application in formulation of various pharmaceutical dosage forms and produce different activity like controlled release agent, film coating agents, suspending agent, drug release regulating agent, emulsifying agent, lubricant, disintegrant, binding agent, thickening agent, stabilizing agent etc with efficient characteristics and stability [3].
Gums are defined as the substances produced as a result of pathological conditions of the plant which are specifically composed of mixture of polysaccharides which could be hydrophilic or hydrophobic high molecular weight substances. They exhibit colloidal properties, which are insoluble in organic solvents like alcohol and ether. Gums can be characterized by physicochemical analysis, phytochemical characterization, physicochemical analysis includes solubility profile, swelling index, loss on drying, pH determination, micromeritic properties characterization, total ash content, melting point determination, etc. physicochemical and preliminary characterization includes test for carbohydrates, alkaloids, glycosides, tannins, flavonoids, terpenoids, steroids, amino acids and proteins [4].
The Lannea coromandelica tree grows in dry, humid, tropical and subtropical regions which is distributed throughout the world and specifically considered as inherent to western hemisphere, southern Europe, Africa, temperate and tropical Asia, tropical and subtropical Australia, and Pacific Islands [5]. The Lannea gum exhibits pharmaceutical excipient properties along and also the bark exhibits pharmacological properties like applications in various conditions of skin eruptions, dysentery, heart diseases, toothache etc. The gum can be collected by making an injury on the bark of the tree along with process of pruning, upon injury of the epithelial cells of the bark, the Lannea tree produces gum exudates [6]. The gum is composed of polysaccharides which is constituted with d-galactose and l-arabinose in the 4:1 ratio. The botanical name is presented in fig. 1. The present research study focuses on comparison and evaluation of natural gum isolated from Lannea coromandelica as polymeric binder and drug release regulating agent in comparison with synthetic polymers like povidone K30 and semisynthetic polymers like HPMC K4M (Hydroxypropyl methyl cellulose).
Fig. 1: Botanical name of Lannea
MATERIALS AND METHODS
Materials
Ibuprofen was acquired as a gift sample from Hetero Laboratory Pvt. Ltd, Hyderabad. Avicel PH 102, Povidone K30 (Polyvinylpyrrolidone K30/PVP K30) was purchased from SD fine chemicals. HPMC K 4M was purchased from Research lab fine Chem industries. Talc and magnesium stearate were purchased from Loba Chemie Pvt. Ltd. Lannea coromandelica gum was extracted by collecting the Natural gum exudates. All the other chemicals and reagents used were of analytical grade.
Lannea coromandelica was selected as natural plant material for extraction of gum (common name-Gumpena gum) because of the following comprehensions Gumpena gum is reported to be comparatively effective at ample minor concentration in comparison to the other natural gum binders. It is employed as a food grade material with no conveyed toxicity. The study of certain literature indicates that gum has been reported to be effective tablet binder, and it was found to be more effectual at lesser concentrations when compared to other natural binders. The gum is however unexplored in various fields of pharmaceutical product development and little literature is available on pharmaceutical dosage form development utilizing Lannea gum, since the gum was unexplored, it has been choosed as natural plant derived gum material to be evaluated for binding and drug release monitoring properties to explore it [7]. Ibuprofen was selected as model drug based on the dose of its administration. It can be administered at moderate doses as single dose, hence the handling during manufacturing of tablets will be easier when compared to low dose potent, drugs. The other reason includes its cost effectiveness and ease of availability when compared to other drugs.
Plant exudate collection
Lannea coromandelica gum exudates were collected from the Hyderabad botanical garden, situated in district Rangareddy, Telangana, India. The gum exudates are represented in fig. 2.
For gum collection, the selected Lannea coromandelica trees were exposed to stress by creating injury on the trunk along with pruning of trunk region and exudates were collected after few days to stress exposure. The Collected gum exudates were further shade dried for duration of 48 to 96 h until the gum exudates are completely dried without any traces of moisture.
Authentication of sample
The sample was collected and the plant material was authenticated at Botanical survey of India, Deccan regional Centre, Hyderabad, Telangana.
Purification and isolation of gum exudates
Purification
The gum exudates were collected from incisions made on the bark of Lannea tree. The gum collected was shade dried for 48-96 h, the extraneous materials were removed followed by pulverization into fine powder using a mixer [8]. The produced crude gum powder was dispersed in distilled water at a concentration of 1 part of gum to 10 parts of water for duration of 24 h, utilizing a mechanical stirrer. After the time interval the dispersion was filtered using a muslin cloth to remove the fibrous materials which leads to separation of filtrate.
Isolation of gum
After the separation of fibrous residues to the produced dispersion of extract, aliquots of acetone were added which helps in precipitation of gum. Acetone was added at a three-fold volume to the volume of dispersion produced in above step to precipitate gum [9, 10]. Finally the gum was washed with acetone and collected as precipitate, which was further dried in hot air oven at temperature which is lower than 40 °C. The gum was pulverized and sieved using #100 followed by packed in air tight container and stored in desiccator for future use. The sequence of steps are visualized in fig. 3.

Fig. 2: Lannea coromandelica tree and gum exudates

Fig. 3: Isolation and processing of Lannea coromandelica gum
Preformulation studies of ibuprofen
Ibuprofen was analyzed for various Preformulation parameters like organoleptic evaluation, micromeritic evaluation, melting point, and functional group analysis by FTIR studies, solubility studies in water and different organic solvents, construction of calibration curve in pH 6.8 phosphate buffer [12].
Construction of calibration curve of ibuprofen
A 1 mg/ml stock solution of Ibuprofen was prepared in the, pH 6.8 phosphate buffer by dissolving accurately weighed quantity of 50 mg ibuprofen in little quantity of ethanol followed by making up the volume to 50 ml with pH 6.8 phosphate buffer in a volumetric flask. From this stock solution, 1 ml was taken and made up to 100 ml with pH 6.8 phosphate buffer to produce 10 µg/ml. It was analyzed by UV-Visible spectrophotometer by scanning between 200 and 400 nm to determine the λmax of API in respective mediums. Ibuprofen showed absorbance maxima at 223 nm in 6.8 pH phosphate buffer [12].
Lannea coromandelica gum characterization
The Lannea gum was evaluated for different physicochemical properties. The physicochemical evaluation of gum was performed by organoleptic evaluation, identification tests, melting point, pH, moisture content, micromeritic properties, scanning electron microscopy-SEM, differential scanning Calorimetry-DSC, X-ray diffraction studies –XRD, Fourier transform infrared spectroscopy – FTIR [13]. The gum was evaluated for various phytochemical evaluation tests like test for carbohydrates, reducing sugars, monosaccharaides, proteins, alkaloids, tannins, flavonoids which helps to ensure the purity of the gum.
Determination of pH
The pH was determined by using a pH meter, a 1% w/v solution of gum powder was prepared by dispersing gum in water [15].
FTIR studies
The Fourier transform infrared (FTIR) spectra can be used to identify the functional vibrations in a substance and shift in vibrational or stretching bands of key functional groups will help to analyze them and the FTIR spectra was recorded by scanning wave number range of 4,000 and 400 cm−1 [17].
X-ray diffractometry (XRD)
The crystalline nature of samples could be determined using powder XRD analysis. The X-ray powder diffraction patterns were analyzed using Cu as anode material, with generator Settings at 30 mA, 45 kV, with an Intended Wavelength of Type-K-α1. The samples were scanned for 2 θ range up to 40 °C [18].
Thermal analysis
The differential scanning Calorimetry (DSC) is a frequently used thermo analytical technique that generates data on melting endotherms and glass transitions. The differential scanning Calorimetry (DSC) was performed utilizing a differential scanning calorimeter. Samples of 3-4 mg were encapsulates and hermetically sealed in flat bottomed aluminum pan with crimped on lid. The pans were positioned on sample pan holder [19]. Samples were allowed to equilibrate for 1 min and then heated in an atmosphere of nitrogen over a temperature up to 300 °C with a heating rate of 10 °C/min. An empty aluminum pan is served as reference. Nitrogen was used as a purge gas, at the flow rate of 20 ml/min for all the studies [20, 21].
Preparation of Ibuprofen granules
Ibuprofen granules were prepared followed by formulation of tablets using the technique of wet granulation. The materials used for preparation of granules were presented in table 1. The ibuprofen was used as model drug, all the formulation components were passed through sieve 40# initially. The procedure includes transferring the accurately weighed ingredients API, Lannea gum, Avicel PH 102 followed by dry mixing for 5 min, then the granulating vehicle distilled water was added and blended thoroughly for 8-10 min to produce a wet mass [22]. The vehicle was added until a damp mass was produced. The above procedure was followed for other formulations also which are comprised of synthetic polymer Povidone K30 and semi-synthetic polymer HPMCK 4M. The end point was determined by pressing the wet mass using thumb finger. The wet mass was passed through sieve no # 16 followed by drying in hot air oven at temperature of 40 °C-50 °C for duration of 15-30 min (until granules are dried completely) [23]. The dried granules were passed through sieve #18.
Preparation of ibuprofen tablets
The dried ibuprofen granules were thoroughly blended for 1-2 min with magnesium stearate and Aerosil, then the tablets were prepared by using Rimek 10 station tablet punching machine with 9 mm punch [24]. The total tablet weight was fixed to 350 mg.
Table 1: Formulation of Ibuprofen tablets using different polymers
| S. No. | Ingredients | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 |
| 1. | Ibuprofen | 200 | 200 | 200 | 200 | 200 | 200 | 200 | 200 | 200 |
| 2. | Lannea gum | 7 | 14 | 21 | --- | --- | --- | --- | --- | --- |
| 3. | Povidone K30 | --- | --- | --- | 7 | 14 | 21 | --- | --- | --- |
| 4. | HPMC k 4M | --- | --- | --- | --- | --- | --- | 7 | 14 | 21 |
| 5. | Avicel PH 102 | 134.5 | 127.5 | 120.5 | 134.5 | 127.5 | 120.5 | 134.5 | 127.5 | 120.5 |
| 6. | Distilled water | q. s | q. s | q. s | q. s | q. s | q. s | q. s | q. s | q. s |
| 7. | Magnesium stearate | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 |
| 8. | Aerosil | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Total (mg) | 350 | 350 | 350 | 350 | 350 | 350 | 350 | 350 | 350 |
FTIR analysis of formulation ingredients
The powder blend of API along with formulation ingredients like Lannea gum, Povidone K30, HPMC K4M, Avicel PH 102 were analyzed for compatibility using FTIR spectral analysis. FTIR spectra were obtained by using Shimadzu analyzer. All the spectra were analyzed by using Agilent technologies FTIR spectral Analyzer. This helps to identify the compatibility of the ingredients used in the formulation [25].
Compatibility studies of formulation ingredients
The pure drug and the corresponding mixtures of API with individual excipients and the blend of excipients were mixed and packed in glass vials, stoppered and sealed. They were studied for compatibility interactions by FTIR studies [26]. The vials were exposed to respective temperature and humidity condition for duration of 1 mo and samples were observed at time intervals of 15 d and one month for compatibility by physical observational studies
Evaluation of granules
The formulated Ibuprofen granules were evaluated for pre-compression parameters which include the determination of bulk density, tapped density, compressibility index, angle of repose and Hausnner’s ration.
Bulk density
It is usually defined as the ratio of total granules weight taken to bulk volume of granules. The procedure includes transferring accurat5ely weighed 10 g of granules into 50 ml graduated measuring cylinder. The undisturbed bulk volume was noted as Vo. It is determined by following equation [27]. It is denoted in g/ml.
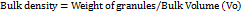
Tapped density
Tapped density is considered as the ratio of mass of granules taken to the granules tapped volume.
It is determined by transferring accurately weighed 10 g of formulated granules into a 50 ml graduated measuring cylinder, the cylinder was tapped using bulk density apparatus and the number of tapings were processed according to the procedure mentioned in Indian Pharmacopeia. After completing the procedure the tapped volume was noted as (VT) [27]. Tapped density was determined by the following equation.
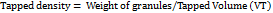
Compressibility index/Carr’s index (%)
It was determined by considering the following formula, which provides information about compressibility of granules or powdered materials.
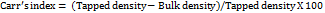
Hausnner’s ratio
It was calculated by the following equation
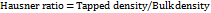
Angle of repose
The procedure involves fixed funnel method where, required quantity of granules were transferred into a funnel and the funnel height was adjusted to 2.5 cm with a fixed height. The sample was poured into the funnel by closing the funnel tip, the sample was allowed to flow from the funnel once it is filled completely [28]. As a pile of sample is produced the diameter and radius was calculated by determining average of three repeated trials. The angle of repose was determined using.
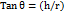
Angle of repose θ = tan-1(h/r)
Evaluation of tablets
Weight variation
Weight variation for tablets was calculated by taking twenty tablets at random and they were weighed individually and the average weight was calculated then it is compared with the individual weights of the tablet [28]. Weight variation was calculated using the formula.
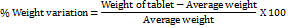
Hardness
The hardness of formulated tablets was determined using a Monsanto hardness tester by selecting 3 tablets from each formulation batch. The strength and resistance of the tablet to chipping, abrasion or breakage under conditions of storage, transportation and handling before usage depends on its hardness of the tablet formulated [29].
Friability
The Friability of prepared tablets was measured using Roche friabilator. Friability evaluates the ability of the tablet to with stand abrasion during packaging, handling and shipping. The required quantities of tablets were taken based on their weights and the procedure was followed according to USP [30]. The tablets initial weight is noted then the tablets were placed in the friabilator and allowed to rotate for 4 min with 25rpm and the loss in weight before rotation and after rotation indicates the friability losses and the acceptable range is between 0.5-1.0 percent.
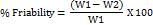
Where, W1= weight of tablets before test W2 = weight of tablets after test
Drug content
The procedure includes selection of ten tablets followed by determining their weights and they were powdered. An equivalent weight of 200 mg of ibuprofen was accurately weighed and transferred into a 100 ml volumetric flask. It was further dissolved by adding 1-2 ml of solvent and volume was made up with phosphate buffer pH 6.8. The solution was further filtered and diluted according to the requirement and was analyzed at 223 nm using UV-Visible spectrophotometer [31]. The drug content was determined from calibration curve of ibuprofen using phosphate buffer pH 6.8.
Disintegration time
The disintegration time was supported using tablet disintegration test apparatus. The procedure includes selection of six tablets and placing them individually in each tube of disintegration test apparatus. The assembly was immersed in beaker containing water which was maintained at a temperature of 37°±2 °C and time taken for tablet to disintegrate completely was noted as disintegration time [32].
In vitro drug release studies
The In vitro drug release was determined by performing dissolution studies using Dissolution Apparatus (Electrolab), with USP type II apparatus, the jar was filled with 900 ml phosphate buffer pH 6.8 as dissolution medium, which was maintained at temperature 37±0.5 °C at 50 rpm, followed by placing the tablets in the medium, 5 ml of sample was withdrawn at regular intervals according to the corresponding time points and equivalent volume of buffer was replaced into the jar [33]. The samples were analyzed spectrophotometrically at 223 nm and the percentage of drug release was calculated.
RESULTS AND DISCUSSION
Preformulation studies of ibuprofen
Ibuprofen was analyzed for organoleptic evaluation, micromeritic evaluation, and functional group analysis by FTIR studies, solubility studies construction of calibration curve in pH 6.8 phosphate buffer. The micromeritic studies indicated good flow properties. The results are presented in table 2 and calibration curve is presented in fig. 4.
Table 2: Preformulation evaluation of Ibuprofen
| S. No. | Test parameter | Result |
| 1. | Colour | White |
| 2. | Odour | Odourless |
| 3. | Physical state and nature | Crystalline solid |
| 4. | Solubility | Soluble in methanol, ethanol, acetone |
| 5. | Melting point | 76 ᵒC |
| 6. | Bulk density | 0.515 g/ml |
| 7. | Tapped density | 0.612 g/ml |
| 8. | Compressibility index | 15.84 % |
| 9. | Angle of repose | 32.24ᵒ |
| 10. | Hausnner’s ratio | 1.18 |
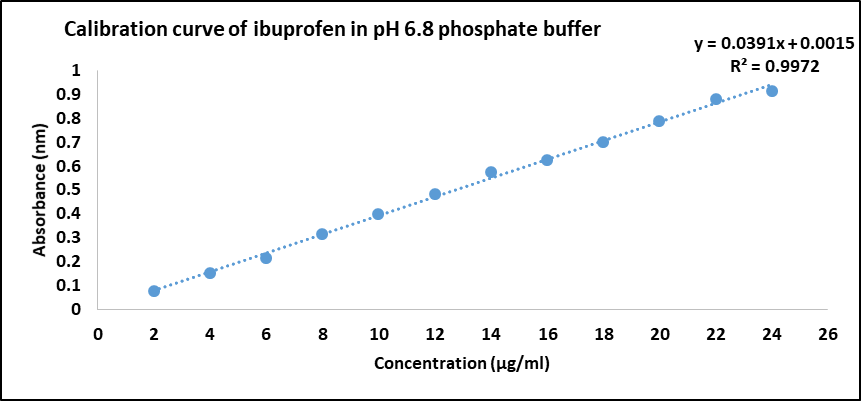
Fig. 4: Calibration curve of ibuprofen
Lannea coromandelica gum characterization
The Lannea gum was evaluated for different physicochemical properties. The physicochemical evaluation of gum was performed by organoleptic evaluation, melting point, pH, moisture content, micromeritic properties, swelling index, ash values, scanning electron microscopy-SEM, X-ray diffraction studies –XRD, Fourier transform infrared spectroscopy – FTIR, physicochemical parameters are presented in table 3 and phytochemical parameters are presented in table 4.
Table 3: Physicochemical characterization of Lannea coromandelica gum
| S. No. | Test parameter | Result |
| 1. | Bulk density | 0.612±0.18 |
| 2. | Tapped density | 0.681±0.11 |
| 3. | Compressibility index | 11.04±0.08 |
| 4. | Hausnner’s ratio | 1.12±0.19 |
| 5. | Angle of repose | 28.75±0.35 |
| 6. | Melting Point (DSC) | 73.4 C |
| 7. | pH | 6.6±0.27 |
(Bulk density and tapped density are expressed in g/ml, compressibility index in %)
Table 4: Phytochemical characterization of Lannea coromandelica gum
| S. No. | Phytochemical test | Observation | Inference |
| 1. | Test for carbohydrates 1. Molisch test |
+ | Indicates presence of carbohydrates |
| 2. | Test for proteins 1. Biuret test |
+ | Indicates presence of proteins |
| 3. | Test for Alkaloids 1. Mayer’s test 2. Dragendorff’s test |
- - |
Indicates absence of alkaloids |
| 4. | Test for Tannins 1. Ferric Chloride test |
- | Indicates absence of tannins |
| 5. | Test for flavonoids 1. Alkaline reagent test |
- | Indicates absence of flavonoids |
FTIR studies
The Fourier transform infrared (FTIR) spectra identified the functional groups in the Lannea gum and corresponding functional groups are presented in table. The spectra was recorded by scanning wave number in the FTIR range. The spectra is presented in fig. 5 and interpretation is visualized in table 5.
X-ray diffractometry (XRD)
The crystalline nature of samples could be determined using powder XRD analysis. The samples were scanned for 2 θ range up to 40 °C. The peaks were obtained at 12.8, 26.3, 27.4, 34.7, 31.1, 29.4, 27.9, 2 θ positions, the peak analysis indicated that Lannea gum was not crystalline in nature and it exhibited amorphous behavior in the peak analysis, the results are shown in fig. 6.
Thermal analysis
The differential scanning Calorimetry (DSC) was performed utilizing a differential scanning calorimeter. Samples of 3-4 mg were sealed in flat bottomed aluminum pan with a lid and were positioned on sample holder. They were heated in an atmosphere of nitrogen over a temperature up to 300 °C with a heating rate of 10 °C/min. The spectra is presented in fig. 7 which indicates the peak at temperature 73.40 °C.
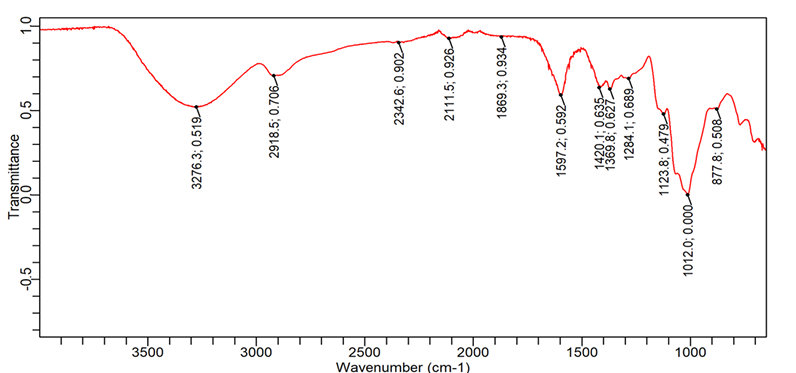
Fig. 5: FTIR spectra of Lannea gum
Table 5: FTIR analysis of Lannea gum
| S. No. | Observed peak | Functional group interpretation | Mode |
| 1. | 1420.11 | C-H | Bending |
| 2. | 2937.14 | C-H | Stretching |
| 3. | 1597.16 | C=C | Stretching |
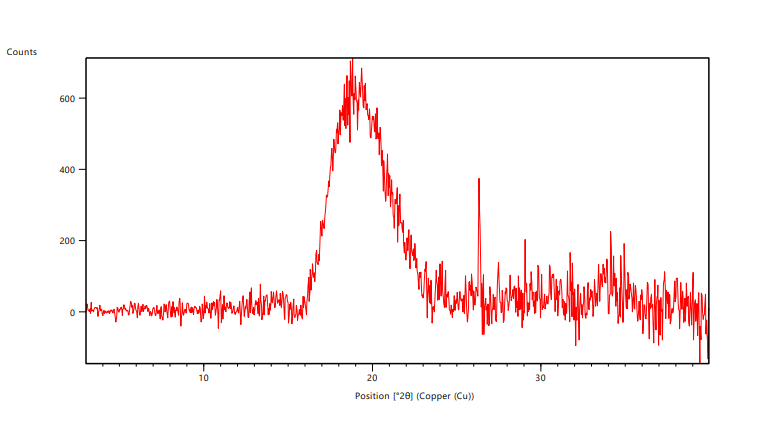
Fig. 6: XRD analysis of Lannea gum
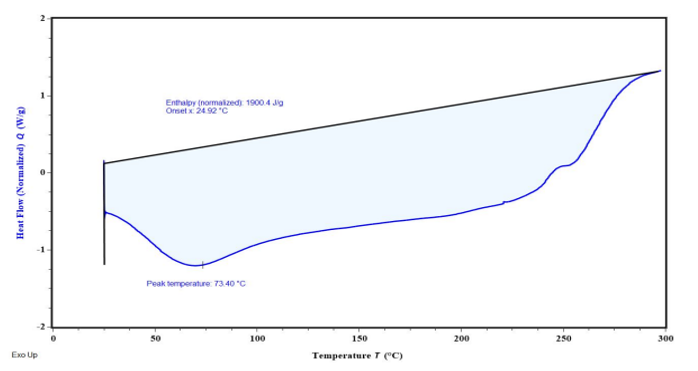
Fig. 7: DSC analysis of Lannea gum
FTIR analysis of formulation ingredients
The powder blend of API along with formulation ingredients like Lannea gum, Povidone K30, HPMC K4M, Avicel PH 102 were analyzed for compatibility using FTIR spectral analysis. The studies indicated the excipients were compatible with each other and also with the API. The corresponding spectra are visualized in fig. 8-11. The peaks observed for pure drug and in the physical mixtures with Lannea gum, Povidone K30, HPMC K4M and with excipients of blend along with Avicel PH 102 indicated that no chemical interaction was observed between ibuprofen and L. coromandelica gum along with formulation ingredients. The functional group interpretation is presented in table 6.
Compatibility studies of formulation ingredients
The sample vials were exposed to respective temperature and humidity condition for duration of 1 mo and samples were observed at time intervals of 15 d and one month for compatibility by physical observational studies. The results are presented in table 7.
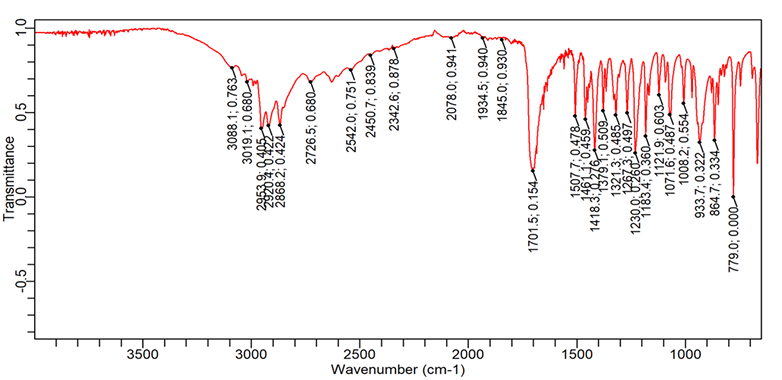
Fig. 8: FTIR spectra of ibuprofen
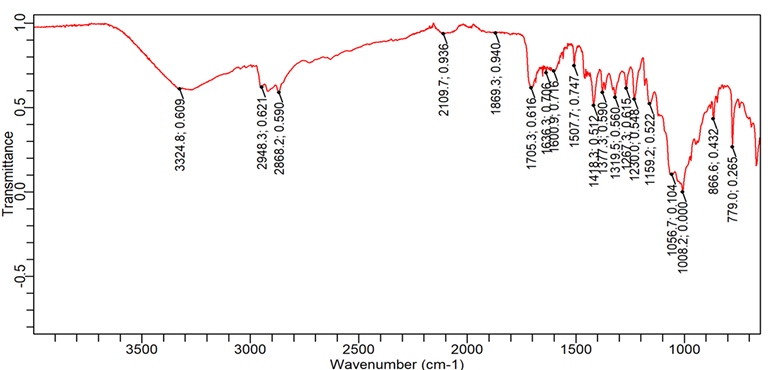
Fig. 9: FTIR spectra of Ibuprofen+Lannea gum+Avicel PH102
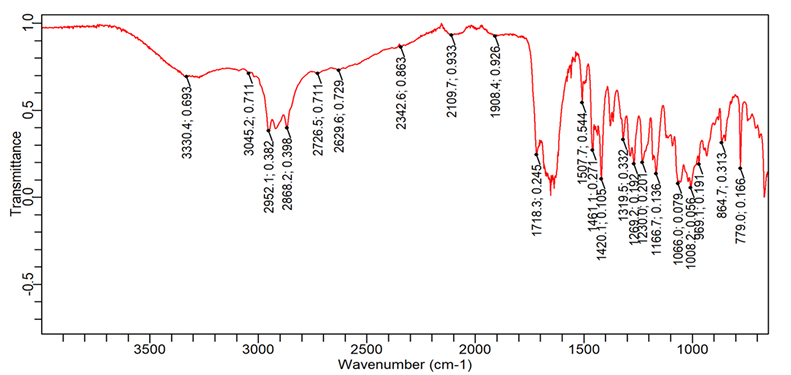
Fig. 10: FTIR spectra of Ibuprofen+Povidone K30+Avicel PH102
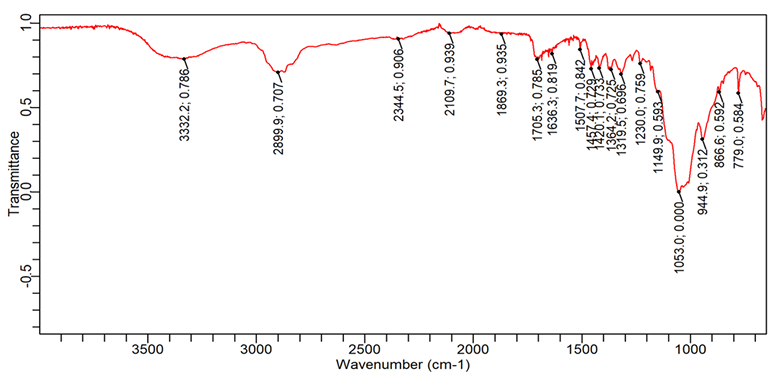
Fig. 11: FTIR spectra of Ibuprofen+HPMC K4M+Avicel PH102
Table 6: FTIR analysis of pure ibuprofen and blend of excipients
| S. No. | Functional group | Range | 1 | 2 | 3 | 4 |
| 1. | C=O (Stretching-acid) | 1760-1690 | 1701.5 | 1705.2 | 1718.3 | 1705.2 |
| 2. | O-H (Stretching) | 3300-2500 | 2953.9 | 2868.1 | 2726.5 | 2899.8 |
| 3. | C-H (bending-aromatic) | 900-600 | 779.0 | 779.0 | 779.01 | 779.01 |
| 4. | C=C (Stretching-aromatic) | 1620-1400 | 1461.1 | 1507.7 | 1420.1 | 1457.3 |
| 5. | C-H (Stretching-alkane) | 2850-3000 | 2868.1 | 2948.3 | 2952.0 | 2899.8 |
1= Pure Ibuprofen, 2= Ibuprofen+L. Gum+Avicel 102, 3= Ibuprofen+PVPK 30+Avicel 102, 4= Ibuprofen+HPMC K 4M+Avicel 102
Table 7: API and excipient compatibility study
| S. No. | Material | Initial observation | 40 °C/75%RH (15 d) | 40 °C/75%RH (one month) |
| 1. | API | White Crystalline solid | White Crystalline solid | White Crystalline solid |
| 2. | API+Lannea gum | White Colour blend | Complies, NC | Complies, NC |
| 3. | API+HPMC K4M | White Colour blend | Complies, NC | Complies, NC |
| 4. | API+Povidone K30 | White Colour blend | Complies, NC | Complies, NC |
| 5. | API+all excipients | White Colour blend | Complies, NC | Complies, NC |
NC: No change
Table 8: Evaluation of ibuprofen granules
| Formulation code | Bulk density (g/ml) | Tapped density (g/ml) | Carr’s index (%) | Hausnner’s ratio | Angle of repose (ᵒ) |
| F1 | 0.584±0.010 | 0.651±0.005 | 10.29±0.163 | 1.11±0.008 | 29.28±0.262 |
| F2 | 0.592±0.009 | 0.665±0.007 | 10.97±0.124 | 1.12±0.009 | 31.15±0.205 |
| F3 | 0.595±0.011 | 0.668±0.009 | 10.92±0.124 | 1.12±0.008 | 29.71±0.216 |
| F4 | 0.496±0.011 | 0.582±0.002 | 14.77±0.205 | 1.17±0.008 | 32.36±0.205 |
| F5 | 0.538±0.011 | 0.612±0.006 | 12.09±0.141 | 1.13±0.009 | 30.13±0.286 |
| F6 | 0.513±0.011 | 0.597±0.009 | 13.06±0.169 | 1.15±0.008 | 31.25±0.205 |
| F7 | 0.502±0.011 | 0.569±0.008 | 11.77±0.205 | 1.13±0.008 | 30.89±0.286 |
| F8 | 0.488±0.010 | 0.573±0.009 | 14.83±0.169 | 1.17±0.008 | 31.82±0.286 |
| F9 | 0.493±0.011 | 0.576±0.012 | 14.40±0.163 | 1.16±0.008 | 32.25±0.205 |
Data expressed as mean±standard deviation (SD), n=3
Evaluation of ibuprofen granules
The formulated ibuprofen granules were evaluated for various parameters like bulk density, tapped density, Carr’s index, Hausnner’s ration, angle of repose. The bulk density of all the formulated batches ranges from 0.488 g/ml to 0.595 g/ml. The angle of repose ranges from 29.71 to 32.36. All the formulated batches exhibited good flow properties and compressibility index, indicating their suitability for formulation of tablets. The results are presented in table 8 and fig. 12. One way Anova was employed to determine the significant difference among the formulations. The results of the one way Anova for Precompression parameters indicated that the all the formulations from f1-f9 are statistically significant with p<0.05.
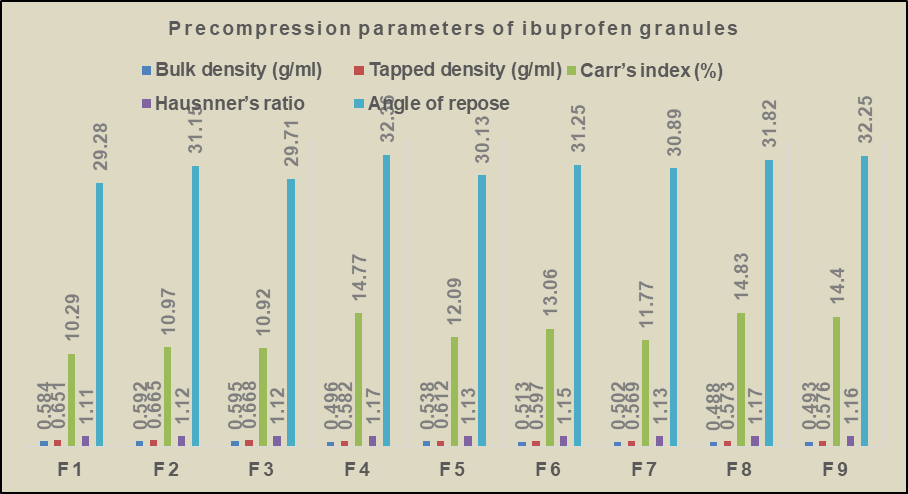
Fig. 12: Precompression parameters of Ibuprofen granules
Evaluation of Ibuprofen tablets
The characterization and evaluation of all the formulated batches indicated satisfactory and acceptable results. The weight variation of all the batches ranges from 349.25±1.47 to 350.86±1.11, which specifies that they are within the pharmacopeial limits. The hardness ranges between 5.6-5.8 and friability was in the range of 0.19%-0.42% within the limits indicating less than 1%. The disintegration time for all the formulated batches was less than 15 min. The drug content of all the formulated batches ranges between 97.22±0.37 to 100.51±0.25. The In vitro drug release studies were performed using drug dissolution analysis with pH 6.8 phosphate buffer as dissolution medium. The drug release at the end of 60 min from all the batches ranged from 81.15% to 97.47%. The results are presented in table 9 and comparison of disintegration times of various formulations is visualized in fig. 13.
When compared the batch F3 comprising Lannea gum at concentration of 21 mg (6 % of total tablet weight) exhibited drug release of 85.27% at end of 60 min whereas batch F6 containing Povidone K30 at concentration of 21 mg (6 % of tablet weight) shown 97.47% drug release at 60 min time point. The batch F9 containing 21 mg (6 % of tablet weight) of HPMC K4M presented a drug release of 81.15% at 60 min time point. The drug release studies data is presented in fig. 14-17. The Anova results for dissolution studies for formulations F3, F6 and F9 when considered and compared based on their composition, binding ability and drug releasing pattern as discussed above it revealed that F6 is statistically significant with F3 and F9.
The results of drug release study implies that the batches comprising Lannea gum specifically F3 has shown a lower drug release at the end of 60 min when compared to F6 comprising Povidone K30 at the end of 60 min indicating Lannea coromandelica gum possess extensive binding and drug release regulating ability in comparison with synthetic polymer Povidone k30 and it could be a superior polymeric excipient in formulation of tablets.
The results also infers that the batches comprising HPMC K4M precisely F9 has shown a lower drug release at the end of 60 min when compared to batches F3 and F6 comprising Lannea gum and Povidone k30 respectively at the end of 60 min. The study reveals that F3 with Lannea gum presented a nearby or slightly higher drug release (85.27%) than F9 containing HPMC K4M (81.15 %). It could be concluded that Lannea gum possess enhanced and superior polymeric properties, binding abilities and drug release regulating properties when compared to Povidone k30. The study also reveals that Lannea gum exhibits slightly low potential with concern to its binding ability, polymeric properties and drug release regulating properties when compared to HPMC K4M. The research study directs that Lannea gum can be used a natural polymer precisely as a binder and drug release regulating agent in formulation of solid dosage forms like tablets.
Table 9: Evaluation of Ibuprofen tablets comprising different polymers
| Formulation | Hardness (kg/cm2) | Weight variation (mg) | Friability (%) | Disintegration time (min) | Drug content (%) |
| F1 | 5.7±0.05 | 349.93±0.56 | 0.19 ±0.01 | 4.36±0.36 | 99.13±0.53 |
| F2 | 5.6±0.05 | 350.08±0.41 | 0.26± 0.01 | 8.23±0.15 | 100.51±0.09 |
| F3 | 5.8±0.19 | 349.88±1.42 | 0.31±0.01 | 11.47±0.57 | 99.95±0.08 |
| F4 | 5.7±0.08 | 349.95±0.50 | 0.28± 0.01 | 4.11±0.12 | 97.22±0.23 |
| F5 | 5.8±0.18 | 350.86±0.53 | 0.42± 0.03 | 7.6±0.34 | 99.18±0.42 |
| F6 | 5.6±0.15 | 350.59±1.59 | 0.32± 0.01 | 10.9±0.26 | 98.65±0.51 |
| F7 | 5.6±0.14 | 350.03±0.97 | 0.26± 0.03 | 5.1±0.22 | 98.47±0.02 |
| F8 | 5.8±0.15 | 349.74±0.28 | 0.29± 0.03 | 11.6±0.94 | 100.31±0.14 |
| F9 | 5.8±0.17 | 349.25±1.47 | 0.27± 0.01 | 11.9±0.22 | 99.73±0.09 |
Data expressed as mean±standard deviation (SD), n=3
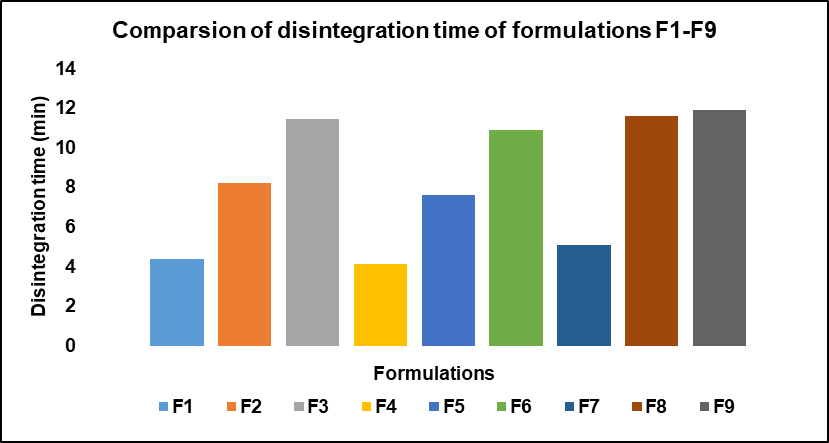
Fig. 13: Comparison of disintegration time of different formulations, disintegration times of different formulation from F1-F9, were compared and presented graphically
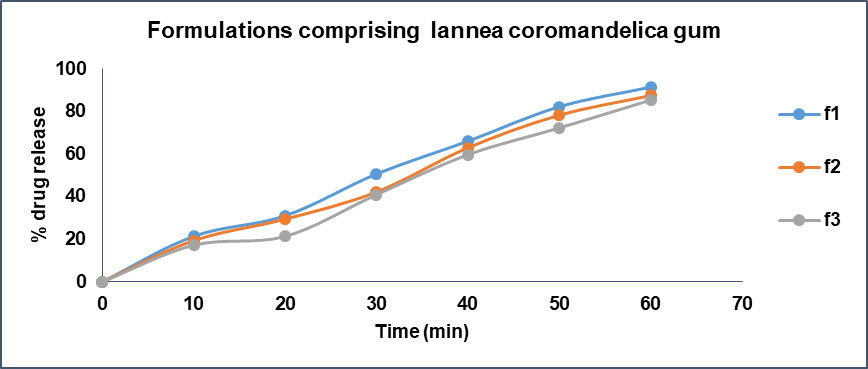
Fig. 14: Drug release profile of formulations comprising Lannea coromandelica gum
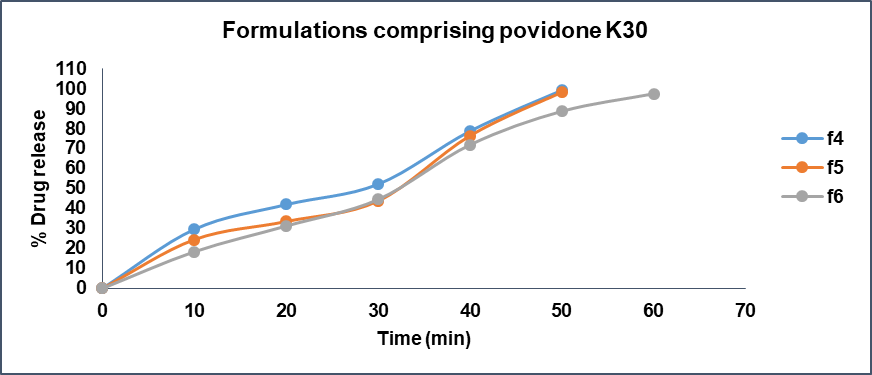
Fig. 15: Drug release profile of formulations comprising povidone K30
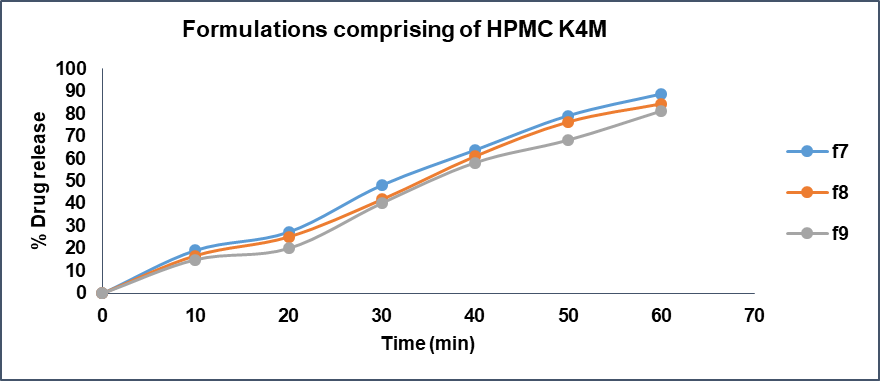
Fig. 16: Drug release profile of formulations comprising HPMC K4M
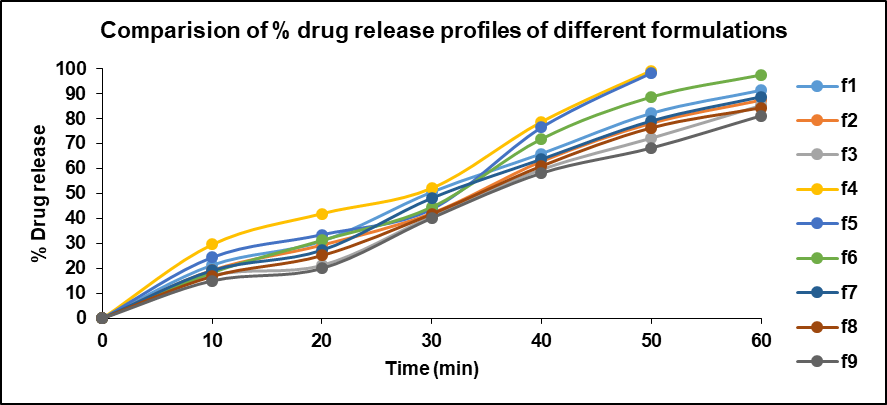
Fig. 17: Drug release profile of formulation batches F1-F9
Drug release kinetics
The formulations from F1-F9 were subjected to model dependent kinetic evaluation to analyze the best fit model and order of drug release which assists in further prediction about mechanism of drug release. The results are projected in table 10.
It indicates that all the formulations exhibited zero order release. The release mechanism was determined with Korsmeyer peppas, the n value being greater than 0.89 for all the formulations indicated that the mechanism is super case II transport. It suggests that higher the n value the mechanism of drug release is controlled by both diffusion and polymer relaxation and swelling, with relaxation playing a significant role producing a zero order drug release behavior.
Stability studies of batches F3 and F9 of Ibuprofen tablets
The formulation batches F3 comprising of Lannea gum and F9 composed of HPMC K4M exhibited satisfactory drug release behavior when compared to F6 composed of Povidone K30, F9 produced drug release of 81.15% in 60 min whereas F3 produced 85.27% release in 60 min, F6 exhibited 97.47% drug release in 6o min, by comparing this data the formulations F3 and F9 were subjected for further stability studies of one month. The stability studies were planned further for duration of 6 mo to evaluate the long term performance of tablets for different parameters like hardness, drug content, in vitro drug release, friability and tablets discoloration. The results are tabulated in table 11 and fig. 18.
Table 10: Model dependent release kinetics of different formulations
| Formulation | Zero order R2 | First order R2 | Higuchi R2 | Korsmeyer peppas R2 | n |
| F1 | 0.9901 | 0.9499 | 0.9090 | 0.9947 | 0.893 |
| F2 | 0.9916 | 0.9420 | 0.8877 | 0.9923 | 0.957 |
| F3 | 0.9878 | 0.9251 | 0.8515 | 0.9891 | 1.064 |
| F4 | 0.9755 | 0.9168 | 0.9040 | 0.9789 | 0.9 |
| F5 | 0.9650 | 0.8681 | 0.8297 | 0.9705 | 1.148 |
| F6 | 0.9880 | 0.9044 | 0.8634 | 0.9882 | 1.018 |
| F7 | 0.9918 | 0.9446 | 0.8912 | 0.9930 | 0.944 |
| F8 | 0.9911 | 0.9383 | 0.8704 | 0.9911 | 1.005 |
| F9 | 0.9875 | 0.9339 | 0.8509 | 0.9886 | 1.059 |
Table 11: Stability studies of formulations F3 and F9
| S. No. | Parameter | F3 (Initial) | F3 (after one month) at 40 °C/75% RH | F9 (Initial) | F9 (After one month) at 40 °C/75% RH |
| 1. | Drug content | 99.95±0.28 | 98.54 | 99.73±0.38 | 98.35 |
| 2. | In vitro % drug release at 60 min | 85.27 | 84.38 | 81.15 | 80.93 |
| 3. | Hardness | 5.8±0.19 | 5.8 | 5.8±0.17 | 5.7 |
4. 5. |
Friability Discoloration of tablets |
0.19 No discoloration |
0.23 No discoloration |
0.34 No discoloration |
0.36 No discoloration |
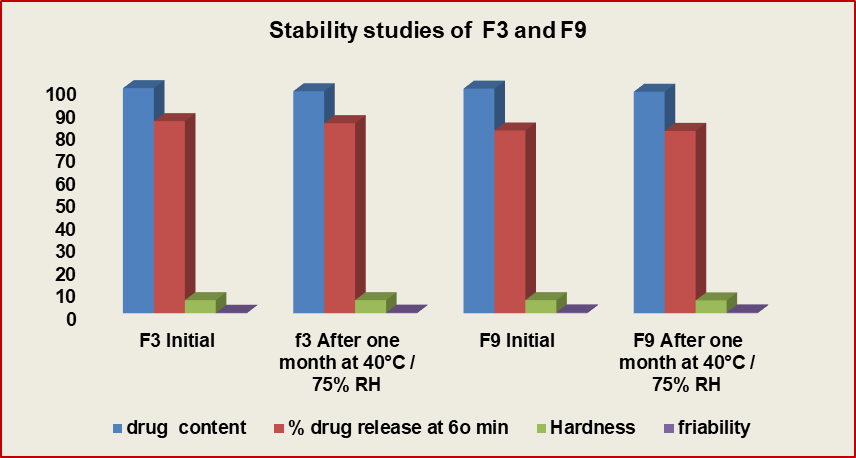
Fig. 18: Stability studies of Formulations F3 and F9, stability studies data of formulations F3 and F9 at 40 °C / 75% RH, after one month and three months duration
Lannea coromandelica typically exudes a water-soluble gum which is polysaccharide in nature, it is composed of constituents like galactose, arabinose, rhamnose, uronic acid. The gum is categorized under class of polysaccharides acknowledged as arabinogalactan [34]. This gum structure assists for hydrogen bonding and intermolecular interactions, impacting the binding ability of gum. Arabinogalactan dissolves totally in water forming a substance with sticky, viscous nature which helps in turn producing adhesive and binding properties. The Lannea gum interact with water producing viscous gel like structure, this viscosity supports in controlling the drug release from the formulations. The linkages between monosaccharide residues in Lannea gum plays critical role in determination of properties of the gum polysaccharide, including its binding and also drug release behavior.
The release profile of Lannea gum was compared with other natural polymers in literature and few studies are indicated below. Metformin HCL Controlled release matrix tablets were formulated using xanthan gum, Lannea gum and HPMC 100, the research study concluded that Lannea gum exhibited similar results and produced identical drug release behavior when compared to xanthan gum. Formulations comprising both the gums produced extended drug release for 24 h [35]. Lamivudine microspheres were formulated, utilizing Moi gum (Lannea gum) and guar gum. The study determined that Gum Lannea was found more effectual than guar gum in sustaining rate of drug release. The drug release studies also revealed that formulation containing mixture of both the gums retarded considerably lamivudine release beyond 10 h [36]. Vildagliptin once daily matrix tablets were developed to explore the potential of Lannea coromandelica gum and Terminalia catappa gum to slow down the drug release. Dissolution studies established that these gums can be employed as materials for creating the matrix in extended-release tablets. The study concluded that formulations containing the Lannea gum and Terminalia gums in their highest concentration exhibited similar drug release profiles by prolonging the release up to 24 h, indicating both the gums produced similar drug release profiles [37].
CONCLUSION
The present research study focuses on evaluation of polymeric properties of natural gum isolated from Lannea coromandelica specifically the binding properties and drug release governing properties by selecting ibuprofen as model drug. The natural gum was compared with synthetic polymer like Povidone K30 and semi-synthetic polymer HPMC K4M. The formulation batches were prepared by utilizing Povidone K30, HPMC K4 M, Avicel PH 102, magnesium stearate, talc as excipients. A total of nine batches were prepared from F1-F9, wet granulation procedure was utilized, where initial granules were prepared followed by compression of tablets using 9 mm punch. The granules were evaluated for Precompression parameters like bulk/tapped density, compressibility index, Hausnner’s ratio and angle of repose, the results indicated good flow property for all the batches. The formulated tablets were evaluated for post compression parameters like weight variation, hardness, friability, drug content, disintegration and drug release studies. The drug content for all the formulations was in the range 98.22% to 100.51%. The drug release from F6 comprising Povidone K30 at 21 mg concentration was found to be 97.47% whereas from F3 comprising Lannea gum at 21 mg concentration exhibited release of 85.27%, F9 comprising HPMC K4M exhibited 81.15% drug release at end of 60 min. It could be concluded that Lannea coromandelica has enhanced binding and drug release regulating potential properties when compared to synthetic polymer Povidone K30 and a marginally slightly low potential with concern to its binding ability, polymeric properties and drug release regulating properties when compared to HPMC K4M. Hence, Lannea coromandelica gum, a natural plant derived gum, can be used as a polymer in formulation of tablets as a binder and drug release regulating agent due to its binding ability and drug release governing ability.
FUNDING
Nil
AUTHORS CONTRIBUTIONS
V Anusha: Data collection, data processing, preparation of manuscript. Umashankar M. S: preparation of study protocol, data analysis, review of results.
CONFLICT OF INTERESTS
Declared none
REFERENCES
Malu Q, Caldeira GI, Catarino L, Indjai B, Da Silva IM, Lima B. Ethnomedicinal chemical and biological aspects of Lannea species a review. Plants (Basel). 2024;13(5):690. doi: 10.3390/plants13050690, PMID 38475536.
Swathi S, Lakshman K. Phytochemistry and pharmacological bio-activities of Lannea coromandelica: a review. Inn J Med Sci. 2022;10(5):1-6. doi: 10.22159/ijms.2022.v10i5.45853.
Gunjal JN. Patil MS, Chittam KP. Lannea coromandelica: an overview. Int J Pharm Biol Sci Arch. 2021;9(1):102-7. doi: 10.32553/ijpba.v9i1.181.
Lohithasu D, Ramana Murthy KV. Isolation and evaluation of binding property of Lannea coromandelica gum. Indian J Pharm Sci. 2016;78(2):224-30. doi: 10.4172/pharmaceutical-sciences.1000107.
Achika JI. A review of the phytochemistry and pharmacology of Lannea species. TJNPR. 2018;2(10):442-6. doi: 10.26538/tjnpr/v2i10.1.
Dash SK, Behera BC, Mohanty B. Isolation characterization and acute toxicity study of Terminalia elliptica (Saj) gum Buchanania lanzan (Chironji) gum and Albizia lebbeck (Siris) gum. Int J Health Sci. 2022;6 Suppl 8:3597-615.
Mate CJ, Mishra S. Exploring the potential of moi gum for diverse applications: a review. J Polym Environ. 2020;28(6):1579-91. doi: 10.1007/s10924-020-01709-8.
Vadivel K, Thangabalan B, Veera NK, Chetanajessygrace BD, Praveenkumar S, Babu M. Preliminary phytochemical evaluation of leaf extracts of Lannea coromandelica L. Int J Pharmacol Res. 2012;2(2):64-8.
Enauyatifard R, Azadbakht M, Fadakar Y. Assessment of ferula gummosa gum as a binding agent in tablet formulations. Acta Pol Pharm. 2012;69(2):291-8. PMID 22568044.
Odeku OA. Assessment of Albizia zygia gum as a binding agent in tablet formulations. Acta Pharm. 2005;55(3):263-76. PMID 16375837.
Alam B, Hossain S, Habib R, Rea J, Islam A. Antioxidant and analgesic activities of Lannea coromandelica Linn. bark extract. Int J Pharmacol. 2012;8(4):224-33. doi: 10.3923/ijp.2012.224.233.
Abdelfattah F, Taha N, Abdou A, Mursi N, Emara L. Prediction of in vivo performance of ibuprofen immediate release products using different dissolution models. J Appl Pharm Sci. 2022;12(8):193-201. doi: 10.7324/JAPS.2022.120820.
Sarojini S, Deepthi Kunam S, Manavalan R, Jayanthi B. Effect of natural almond gum as a binder in the formulation of diclofenac sodium tablets. Int J Pharm Sci Res. 2010;1(3):55-60.
Manna S, Kollabathula J. Formulation and evaluation of ibuprofen controlled release matrix tablets using its solid dispersion. Int J Appl Pharm. 2012;11(2):71-6. doi: 10.22159/ijap.2019v11i2.30503.
Yada K, Katakam P, Suddala S. Simultaneous UV-spectrophotometric estimation of ibuprofen and moxifloxacin in pH 6.8 phosphate buffer. Int J Pharm Anal Res. 2015;4(2):88-93.
Elijah I, Nep B, Conway R. Characterization of grewia gum a potential pharmaceutical excipient. J Excipients Food Chem. 2010;1(1):30-40.
Avbunudiogba JA, Okafo SE, Kabari F. Formulation and evaluation of controlled release salbutamol tablets using Cissus populnea Gum as matrix former. East Afr Scholars J Med Sci. 2020;3(3):109-15.
Gayathri R, Sundara Ganapathy R. Extraction and characterization of the gum isolated from Araucaria heterophylla. Int J Pharm Sci Res. 2018;9(3):1062-7.
Darekar AB, Kahane JU, Saudagar RB, Gondkar SB, Chavan MJ, Ashawat M. Characterization of grevillea robusta gum to establish it as a pharmaceutical excipient. World J Pharm Res. 2014;3(9):415-31.
Thakur N, Mittal P, Kaur R, Goswami M. Phytochemical screening of gum extracted from Curcuma amada. Int J Pharmacogn. 2015;2(8):419-25.
Babu SN, Gayathri R, Rajkumar P, Saravanan T, Lakshminarayanan B, Arathi. Isoaltion and characterisation of Araucaria heterophylla mucilage. Int J Phytopharmacol Res. 2012;3(6):6-8.
Das B, Dash S, Choudhury RC, Chakraborty J, Deb RS. Optimization and characterization of purified polysaccharide from Terminalia belarica gum as pharmaceutical excipient. Int J Pharm Res Allied Sci. 2014;3(1):21-9.
Deeksha, Malviya R, Sharma PK. Extraction and characterization of Aegle marmelos derived polymer as a pharmaceutical excipient. Polim Med. 2014;44(3):141-6. PMID 25696938.
Reddy RM, Manjunath K. Pharmaceutical application of natural gums mucilages and pectins. Int J Pharm Chem Sci. 2013;2(3):1233-9.
Prajapati VD, Jani GK, Moradiya NG, Randeria NP. Pharmaceutical applications of various natural gums mucilages and their modified forms. Carbohydr Polym. 2013;92(2):1685-99. doi: 10.1016/j.carbpol.2012.11.021, PMID 23399207.
Sharma DR, Sharma A, Kaundal A, Rai PK. Herbal gums and mucilage as excipients for pharmaceutical products. Res J Pharmacogn Phytochem. 2016;8(3):145-52. doi: 10.5958/0975-4385.2016.00026.1.
Pavithra T, Tamizh Mani T. Medicinal importance of odina wodier roxb. A Brief review study. World J Pharm Res. 2018;7(17):1354-65.
Jani GK, Shah DP, Prajapati VD, Jain V. Gums and mucilages: versatile excipient for pharmaceutical formulations. Asian J Pharm Sci. 2009;4(5):308-22.
Guru PR, Kar RK, Nayak AK, Mohapatra S. A comprehensive review on pharmaceutical uses of plant derived biopolysaccharides. Int J Biol Macromol. 2023;233(10):123454. doi: 10.1016/j.ijbiomac.2023.123454, PMID 36709807.
Oforikwakye K, Asantewaa Y, Kipo SL. Physicochemical and binding properties of cashew tree gum in metronidazole tablet formulations. Int J Pharm Pharm Sci. 2010;2(4):105-9.
Singh AK, Panner Selvam R, Sivakumar T. Isolation characterization and formulation properties of a new plant gum obtained from Mangifera indica. Int J Pharm Biomed Res. 2010;1(2):35.
Ghosh A, Deb J, Mishra S, Dhankhar N, Kumar S. Formulation and evaluation of oral controlled release preparation using potential agents obtained in nature. Int J Drug Form Res. 2011;2(5):448-62.
Sarangapani S, Rajappan M. Pharmacognostical and pharmaceutical characterization of delonix regia a novel matrix forming natural polymer. Int J Pharm. 2012;2(3):564-73.
Reddy AK, Jyothi MJ, Ashok Kumara CK. Lannea coromandelica the researchers tree. J Pharm Res. 2012;4(3):577-9.
Anil Kumar K, Ganga Rao B, Prabhakar T. Preparation and evaluation of controlled release matrix tablets of metformin hydrochloride by using Lannea coromandelica plant gum and other polymers. J Glob Trends Pharm Sci. 2018;9(4):5925-38.
Shankar NB, Udaya Kumar N, Balakrishna PK, Kumar RP. Preparation and in vitro evaluation of lamivudine entrapped MOI microspheres for oral administration. Res J Pharm Technol. 2008;1(4):437-40.
Mallikarjun PN, Nagoji KE. Formulation and characterization of extended release vildagliptin matrix tablets using natural gums. Am J PharmTech Res. 2023;13(5):112-8.