
Int J App Pharm, Vol 17, Issue 4, 2025, 408-419Original Article
DESIGN OF EXPERIMENTS-BASED OPTIMIZATION OF ORAL THIN FILM FORMULATION OF ESCITALOPRAM OXALATE FOR ENHANCED PATIENT COMPLIANCE
SUMANTH BHUKYA1*, JAYAPAL REDDY GANGADI2, POLI REDDY PAPAGATLA3
1,2Faculty of Pharmaceutical Sciences, Motherhood University, Roorkee, Haridwar, Uttarakhand, India. 3Department of Pharmacology, Nalanda College of Pharmacy, Nalgonda, Telangana, India
*Corresponding author: Sumanth Bhukya; *Email: bsumanthpharma@gmail.com
Received: 08 Feb 2025, Revised and Accepted: 19 May 2025
ABSTRACT
Objective: This study aimed to develop and optimize an Oral Thin Film (OTF) formulation of escitalopram oxalate to enhance patient compliance, improve drug acceptability, and facilitate rapid systemic absorption.
Methods: A Central Composite Design (CCD) under Response Surface Methodology (RSM) was employed to optimize the formulation. Polymer (300–500 mg) and plasticizer (100–300 mg) concentrations were selected as independent variables, and their effects on critical response parameters—tensile strength, folding endurance, disintegration time, and dissolution rate-were evaluated. The optimized OTF was characterized for thickness, disintegration, and dissolution behaviour.
Results: The optimal polymer and plasticizer concentrations were identified as 400 mg and 220 mg, respectively, yielding desirable film properties. The OTF thickness ranged from 0.48 to 0.57 mm, with formulations between 0.50 and 0.55 mm exhibiting the most favorable dissolution and disintegration profiles. The optimized OTF exhibited a tensile strength of 15.3 N/mm², folding endurance of 159 folds, and a disintegration time of 73 sec. Dissolution studies demonstrated 98% drug release within 10 min, confirming rapid dissolution for a fast onset of action.
Conclusion: The study successfully developed an optimized OTF formulation of escitalopram oxalate, supporting the global shift toward patient-centric drug delivery. The formulation enhances therapeutic outcomes by ensuring rapid systemic absorption and improved patient adherence.
Keywords: Escitalopram, Oral thin films, Central composite design, Dissolution, Patient-centric
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijap.2025v17i4.53907 Journal homepage: https://innovareacademics.in/journals/index.php/ijap
INTRODUCTION
The evolution of drug delivery systems has been increasingly driven by the need to address patient-specific challenges while ensuring therapeutic efficacy. Oral Thin Films (OTFs) represent a paradigm shift in this domain, offering unique advantages such as rapid disintegration, ease of administration, and the potential for mucosal absorption, thereby circumventing gastrointestinal degradation and first-pass metabolism [1, 2]. These attributes are particularly transformative for vulnerable populations, including paediatric, geriatric, and psychiatric patients, who frequently face dysphagia-related barriers to conventional solid dosage forms [3, 4]. Within this framework, escitalopram oxalate—a first-line Selective Serotonin Reuptake Inhibitor (SSRI) for major depressive disorder and generalized anxiety disorder—emerges as a compelling candidate for OTF innovation [5, 6].
Fig. 1: Structure of escitalopram
Escitalopram (fig. 1), the pharmacologically active S-enantiomer of citalopram, exhibits high selectivity for serotonin transporters, enhancing serotonergic neurotransmission at low therapeutic doses (5–20 mg) [7, 8]. Despite its therapeutic advantages, conventional escitalopram formulations, including tablets and oral solutions, present several pharmacokinetic limitations, such as delayed onset of action, hepatic first-pass metabolism, and variable gastrointestinal absorption, which may impact bioavailability and therapeutic response [9, 10]. Furthermore, swallowing difficulties in psychiatric populations exacerbate the issue of non-adherence to conventional dosage forms, necessitating alternative drug delivery approaches [11]. However, its formulation into OTFs is challenged by intrinsic physicochemical properties: bitter taste, moderate aqueous solubility, and dose-sensitive mechanical stability. These hurdles necessitate a scientifically rigorous approach to balance patient acceptability with optimal drug release kinetics [12–14].
Existing studies on OTFs for SSRIs have primarily focused on fluoxetine and paroxetine, with limited exploration of escitalopram-based formulations [15, 16]. Current research gaps lie in the systematic optimization of OTFs for escitalopram, particularly through advanced design-of-experiments (DoE) methodologies that address multifactorial dependencies between formulation variables and critical quality attributes. A key gap in the literature is the lack of comprehensive studies that quantitatively correlate excipient composition with dissolution efficiency and mechanical robustness in escitalopram OTFs.
The urgency for patient-centric solutions is underscored by the rising prevalence of psychiatric disorders in populations with dysphagia, such as the elderly, where conventional tablets or capsules compromise adherence and therapeutic outcomes [17, 18]. OTFs not only eliminate the need for water but also offer discrete administration, a critical psychological advantage in mental health management [19]. Moreover, OTFs enable rapid mucosal absorption of escitalopram, potentially bypassing hepatic metabolism and reducing the time to peak plasma concentration, which is crucial for patients experiencing acute depressive episodes [20, 21]. While existing studies have explored OTFs for other SSRIs, escitalopram’s low dose requirement and solubility limitations demand a tailored formulation strategy that integrates taste-masking, solubilization, and mechanical robustness without compromising disintegration efficiency.
This study introduces a novel, DoE-driven framework to optimize escitalopram OTFs, employing Central Composite Design (CCD) under Response Surface Methodology (RSM) to systematically correlate polymer and plasticizer concentrations with tensile strength, folding endurance, disintegration time, and dissolution rate. Unlike prior OTF research, this approach rigorously quantifies the interplay between excipient ratios and performance metrics, ensuring reproducibility and scalability. By resolving the dichotomy between rapid drug release and mechanical integrity, this work advances the translational potential of OTFs for low-dose, high-bioavailability therapeutics. Furthermore, it pioneers the integration of escitalopram’s unique physicochemical profile with OTF technology, addressing both patient-centric needs (taste masking, ease of use) and pharmacokinetic optimization (enhanced mucosal absorption).
Aligned with global initiatives toward precision drug delivery, this research establishes a methodological blueprint for SSRIs and beyond, emphasizing the synergy between advanced statistical modeling and formulation science. The outcomes hold significant implications for psychiatric pharmacotherapy, offering a clinically viable alternative to conventional dosage forms while setting a precedent for patient adherence-driven innovation.
MATERIALS AND METHODS
Escitalopram oxalate procured from Athos Chemicals Limited, Gujarat, India. Pullulan, β-Cyclodextrin were procured fron Shreeji Pharma International, Vadodara, India. Polyethylene glycol (PEG) 400 and other chemicals were obtained from Sd Fine Chemicals Ltd, Hyderabad, India.
UV–VIS spectrophotometric assay method for escitalopram
The quantification of escitalopram oxalate in oral thin films (OTFs), both in terms of drug loading and release, was performed using a validated UV–VIS spectrophotometric method. The maximum absorbance wavelength for escitalopram oxalate was identified as 238 nm using a Shimadzu Double Beam UV–VIS Spectrophotometer (UV-2700i). For content uniformity studies, 50 mg of escitalopram oxalate was accurately weighed, dissolved in ethanol, and diluted to a final volume of 100 ml in a volumetric flask. For dissolution studies, water was employed as the diluent. A standard calibration curve was constructed by preparing serial dilutions of the stock solution to achieve concentrations ranging from 500 µg/ml to 10 µg/ml. The samples were analysed at 238 nm with ethanol as the blank. Each experiment was performed in triplicate and repeated over three consecutive days to ensure reproducibility. The absorbance-concentration relationship was plotted, and the regression coefficient was calculated from the linear equation, confirming the method's reliability and suitability for precise quantification [16, 17].
Preparation of oral thin films of escitalopram
Escitalopram formulated into oral thin film by solvent casting method. A film of about 2 cm2 area must have 10 mg of escitalopram was prepared. Accurate weight of pullulan was dissolved in beaker (50 ml capacity) containing 10 ml of distilled water. It was allowed to stir for few minutes using magnetic stirrer (MS-500, REMI, India) until it dissolved and then sucralose (10 mg), citric acid (50 mg) was added and stirred to dissolve. On its dissolution, accurately 250 mg of escitalopram was added with stirring. When the solution becomes homogenous, an accurate quantity of PEG 400 and watermelon flavour were added to the above solution and stirred. The obtained solution of each batch was allowed to stand for half an hour to remove air-bubble, if any. The smooth homogeneous solution of each batch was poured gently in transparent glass petri-plates of uniform size (10 × 10 × 0.5 cm3) and allowed to dry at ambient temperature (28±1 °C) until the preparation became a dry film. Films were further stored in a desiccator at 25 °C with 40% relative humidity for 24 h before characterization to maintain consistency in mechanical and physicochemical properties. The developed each dry film was then carefully removed from plates using spatula, segmented into pieces (2 × 2 cm2) and stored in aluminium sachets at 2–8 °C until further studies [18].
Design of experiments
A Central Composite Design (CCD) under the response surface methodology framework was utilized to optimize the formulation of escitalopram oxalate oral thin films. The experimental design involved two independent variables: polymer concentration (A), varied between 300 mg (-1) and 500 mg (+1), and plasticizer concentration (B), varied between 100 mg (-1) and 300 mg (+1) were given in table 1. The design included 13 randomized experimental runs to minimize potential biases and ensure robust data collection. Three critical response parameters: tensile strength (R1), folding endurance (R2), and disintegration time (R3), were evaluated to achieve the optimization of thin film [18, 19]. The study was conducted using Design-Expert software (version 13.0.5.0), which facilitated the creation of the design, data analysis, and interpretation of results. The absence of blocks in the design further simplified the experimental approach. This methodology allowed for the systematic evaluation of factor-response relationships and the identification of optimal formulation conditions to achieve the desired film characteristics. The Experimental Results of response variable for each optimization trial were enumerated in table 2.
Table 1: Factors and response variables for the optimization of escitalopram oxalate oral thin films
| Factor | Name | Units | Coded low | Coded high | Mean | Std. Dev. |
| A | Polymer concentration | mg | -1 ↔ 300.00 | +1 ↔ 500.00 | 400.00 | 81.65 |
| B | Plasticizer concentration | mg | -1 ↔ 100.00 | +1 ↔ 300.00 | 200.00 | 81.65 |
| Response variables, Tensile strength (R1), Folding endurance (R2), Disintegration time (R3) |
Characterization of oral thin films (OTFs)
Weight uniformity
The weight uniformity of the OTFs was evaluated by individually weighing three films from each batch (dimensions: 2 × 2 cm²) using a calibrated digital single-pan analytical balance. The mean weight was calculated to ensure consistency across the films [18].
Film thickness uniformity
The thickness of each OTF (2 × 2 cm²) was measured at five predetermined points: four corners and the centre, using a calibrated digital vernier calliper. Measurements were performed in triplicate, and the mean thickness values were calculated. Thickness data was further utilized for determining the mechanical properties, including ultimate tensile strength, folding endurance, and elongation at break [19].
Folding endurance
The folding endurance of the OTFs was assessed manually in triplicate by repeatedly folding each film (2 × 2 cm²) at the same point until rupture occurred. The number of folds before rupture was recorded and the mean value was reported as the folding endurance.
Surface pH
To determine the surface pH, an OTF (2 × 2 cm²) was placed in a closed Petri dish containing 5 ml of distilled water at room temperature. After the film was moistened, the surface pH was measured using a digital pH meter (Equiptronics, EQ-611, Mumbai, India). The pH probe was placed in direct contact with the wetted film surface, and the pH was recorded. This test ensured that the films would not cause irritation to the tongue or mouth during use [20].
Ultimate tensile strength (UTS) and elongation at break (EL)
The ultimate tensile strength (UTS) of the oral thin films (OTFs) was determined as the maximum force required to break the film, calculated by dividing the force at the breaking point by the total cross-sectional area of the film. The elongation at break (EL), expressed as a percentage, represents the distance the film stretched from its original length between grips before breaking.
Both parameters were measured in triplicate for each batch using a texture analyzer (CT-3 Texture Analyzer, Brookfield) equipped with a 25 kg load cell. Testing was performed under standard laboratory conditions following the ASTM International test method for thin plastic sheets (D 882–02). OTF samples (2 × 2 cm²) were vertically clamped with a 1 cm gap between the upper and lower clamps. While the lower clamp remained stationary, the upper clamp moved upward at a speed of 0.55 mm/s to pull the film apart. Data collection and calculations were performed using Texture Pro CT software [20].
Fourier transform infrared (FTIR) study
Fourier Transform Infrared (FTIR) spectroscopy was performed to analyse the functional groups and potential interactions in Escitalopram, Pullulan, and the optimized Escitalopram oral thin films. The spectra were recorded using a PerkinElmer FTIR spectrometer in the wavenumber range of 4000–400 cm⁻¹. Samples were prepared by the KBr pellet method, ensuring uniform dispersion of each sample in potassium bromide. A resolution of 4 cm⁻¹ and 16 scans per sample were used to achieve high-quality spectra. The obtained spectra were analysed to identify characteristic peaks and evaluate any chemical interactions or changes in the optimized formulation compared to pure components [20].
SEM analysis
Scanning Electron Microscopy (SEM) was employed to examine the surface morphology of the optimized Escitalopram oral thin films. The analysis was performed using a JEOL JSM-IT500 SEM instrument. Thin film samples were mounted on aluminium stubs using double-sided carbon adhesive tape and coated with a thin layer of gold using a sputter coater to ensure conductivity. The images were captured at an accelerating voltage of 15 kV with varying magnifications to observe surface characteristics, including uniformity, texture, and potential defects in the film structure. The obtained micrographs were analysed to evaluate the film's quality and homogeneity [20].
In vitro disintegration
The in vitro disintegration time of oral thin films (OTFs) was assessed using phosphate buffer (pH 6.8), maintained at 37±0.5 °C to simulate the physiological conditions of human saliva. Each OTF (2 × 2 cm²) was tested in triplicate to determine the mean disintegration time. The films were placed in a glass petri plate (3.5-inch internal diameter, 1-inch height) containing 10 ml of phosphate buffer (pH 6.8) maintained at 37±0.5 °C, replicating physiological temperature. The time taken for complete disintegration of the films was recorded. This method ensures reliable and reproducible assessment of disintegration performance under conditions that closely mimic the oral environment.
In vitro dissolution study
The in vitro dissolution study was conducted in triplicate using the USP dissolution apparatus type II, with 200 ml of phosphate buffer (pH 6.8) as the dissolution medium, maintained at 37±0.5 °C to simulate physiological conditions. The rotation speed of the paddle was set at 50 rpm. Aliquots of 5 ml were withdrawn at predetermined intervals, and the withdrawn volume was replenished with an equivalent volume of fresh medium to maintain sink conditions. Each sample was filtered through a 0.45 µm Millipore filter to remove any particulate matter. The drug release was quantified using a UV–VIS spectrophotometer at the appropriate wavelength for escitalopram. The cumulative amount of drug released over time was calculated by referencing a standard calibration curve of escitalopram in phosphate buffer (pH 6.8).
RESULTS AND DISCUSSION
Experimental optimization of escitalopram oral thin films
Tensile strength (R1)
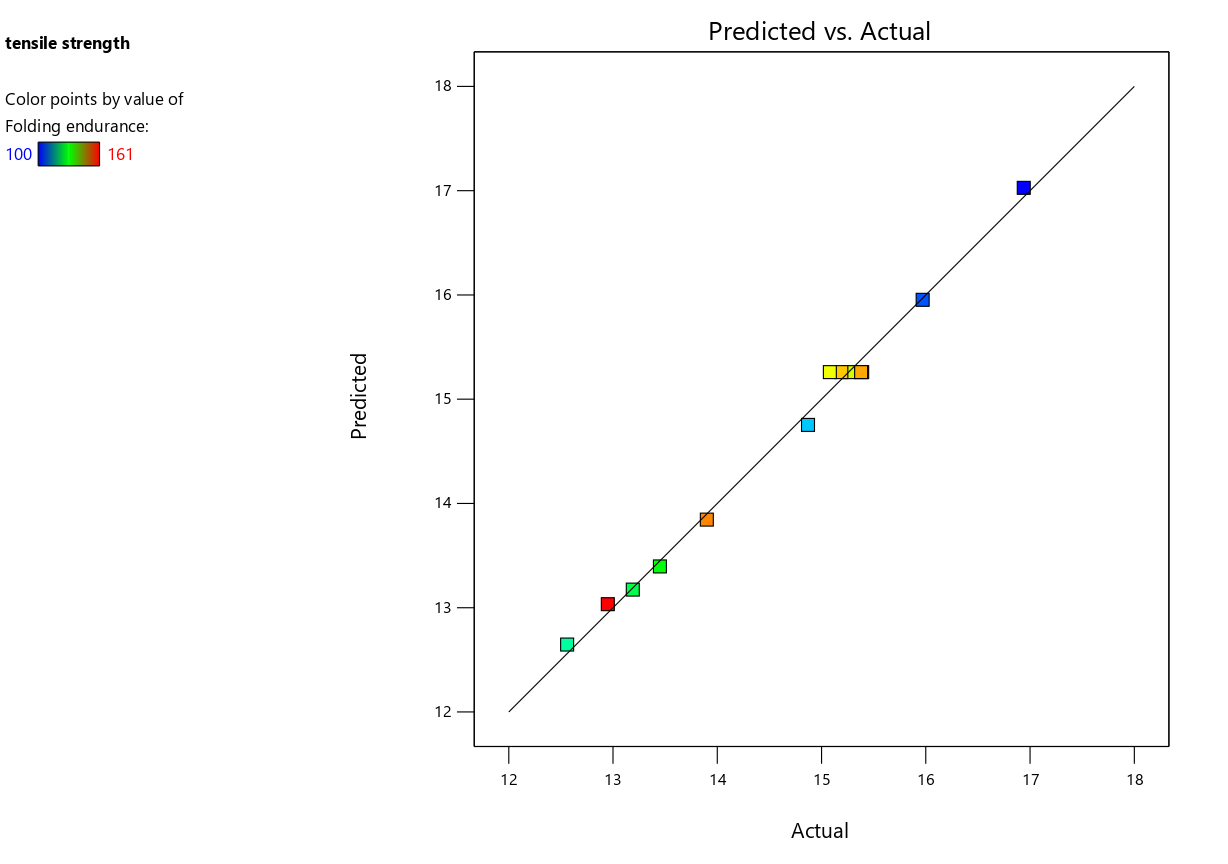
The quadratic model emerged as the best fit for predicting the tensile strength of Escitalopram OTFs. The sequential p-value for the quadratic model was highly significant (<0.0001), indicating a strong relationship between the response and the independent variables. The adjusted R² (0.9907) and predicted R² (0.9800) values were both very close to 1, demonstrating the model’s high accuracy and ability to predict tensile strength reliably. Furthermore, the lack of fit p-value for the quadratic model was 0.5478, which is not significant, confirming that the model fits the data well without substantial unexplained variation.
The ANOVA results (table 3) for the quadratic model confirmed its statistical significance, explaining the majority of the variability in tensile strength, with a sum of squares of 20.24 out of a total of 20.35. The model showed a highly significant F-value of 256.04 (p-value<0.0001), and the residual sum of squares was minimal (0.1107), indicating a good fit. Among the individual factors affecting tensile strength, polymer concentration (A) was found to be the most significant, contributing the largest sum of squares (7.73) and exhibiting an F-value of 488.70 (p-value<0.0001). This suggests that polymer concentration had the strongest impact on the tensile strength of the Escitalopram OTFs. Plasticizer concentration (B) also significantly influenced tensile strength, with a sum of squares of 2.96 and an F-value of 187.17 (p-value<0.0001), although its effect was slightly less pronounced than that of polymer concentration. The interaction between polymer and plasticizer concentrations (AB) exhibited a statistically significant impact on tensile strength, as evidenced by a sum of squares of 5.83, an F-value of 368.94, and a p-value<0.0001, confirming a strong synergistic effect between these variables. Furthermore, the quadratic terms for polymer concentration (A²) and plasticizer concentration (B²) were also significant, with F-values of 53.33 (p = 0.0002) and 205.36 (p<0.0001), respectively, indicating pronounced nonlinear contributions to the mechanical properties of the films.
Table 2: Experimental design and observed responses for escitalopram oral thin films
| Run | Factor 1 | Factor 2 | Response 1 | Response 2 | Response 3 |
| A: Polymer concentration | B: Plasticizer concentration | Tensile strength | Folding endurance | Disintegration time | |
| mg | mg | ||||
| 1 | 300 | 300 | 13.9 | 153 | 84 |
| 2 | 400 | 341.421 | 12.95 | 161 | 87 |
| 3 | 400 | 200 | 15.26 | 143 | 71 |
| 4 | 500 | 100 | 16.94 | 100 | 102 |
| 5 | 500 | 300 | 13.45 | 130 | 95 |
| 6 | 400 | 200 | 15.08 | 145 | 73 |
| 7 | 258.579 | 200 | 13.19 | 126 | 78 |
| 8 | 400 | 200 | 15.38 | 151 | 70 |
| 9 | 541.421 | 200 | 15.97 | 105 | 105 |
| 10 | 400 | 200 | 15.39 | 155 | 72 |
| 11 | 400 | 58.5786 | 14.87 | 112 | 79 |
| 12 | 300 | 100 | 12.56 | 121 | 70 |
| 13 | 400 | 200 | 15.19 | 149 | 74 |
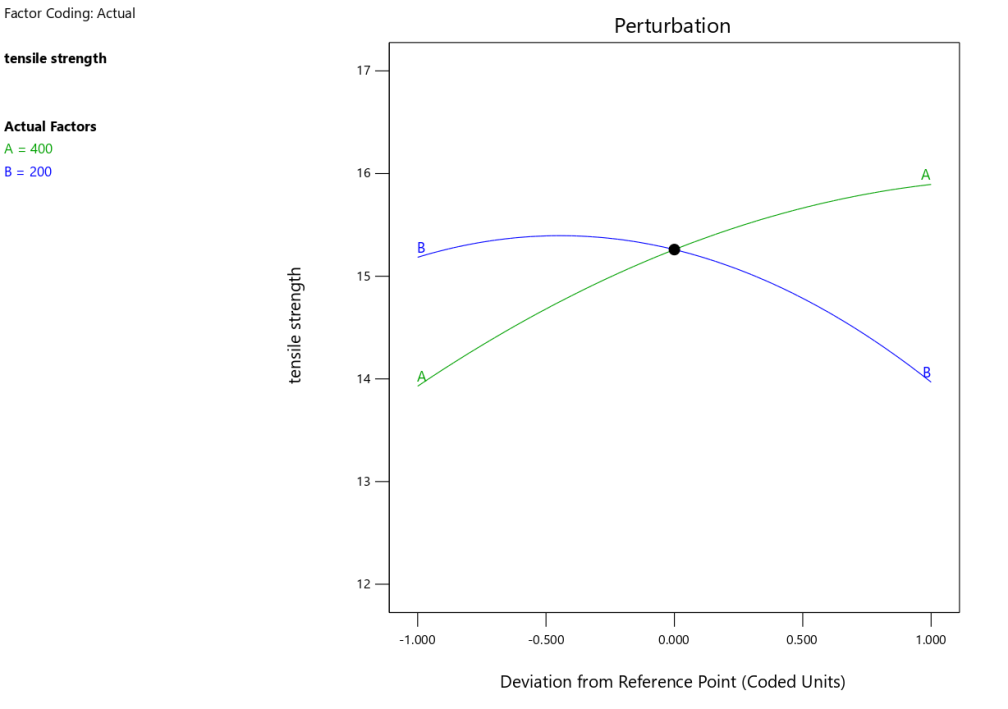
The strong agreement between the predicted and actual tensile strength values was also confirmed by the predicted vs. actual plot (fig. 2a). The perturbation plot (fig. 2b) provides insight into the effect of varying each factor while keeping the others constant. From this plot, it is evident that polymer concentration (A) has a more significant effect on tensile strength compared to plasticizer concentration (B), as the curve for polymer concentration exhibits a steeper slope. In contrast, the curve for plasticizer concentration is relatively flat, suggesting that the tensile strength is less sensitive to changes in plasticizer concentration.
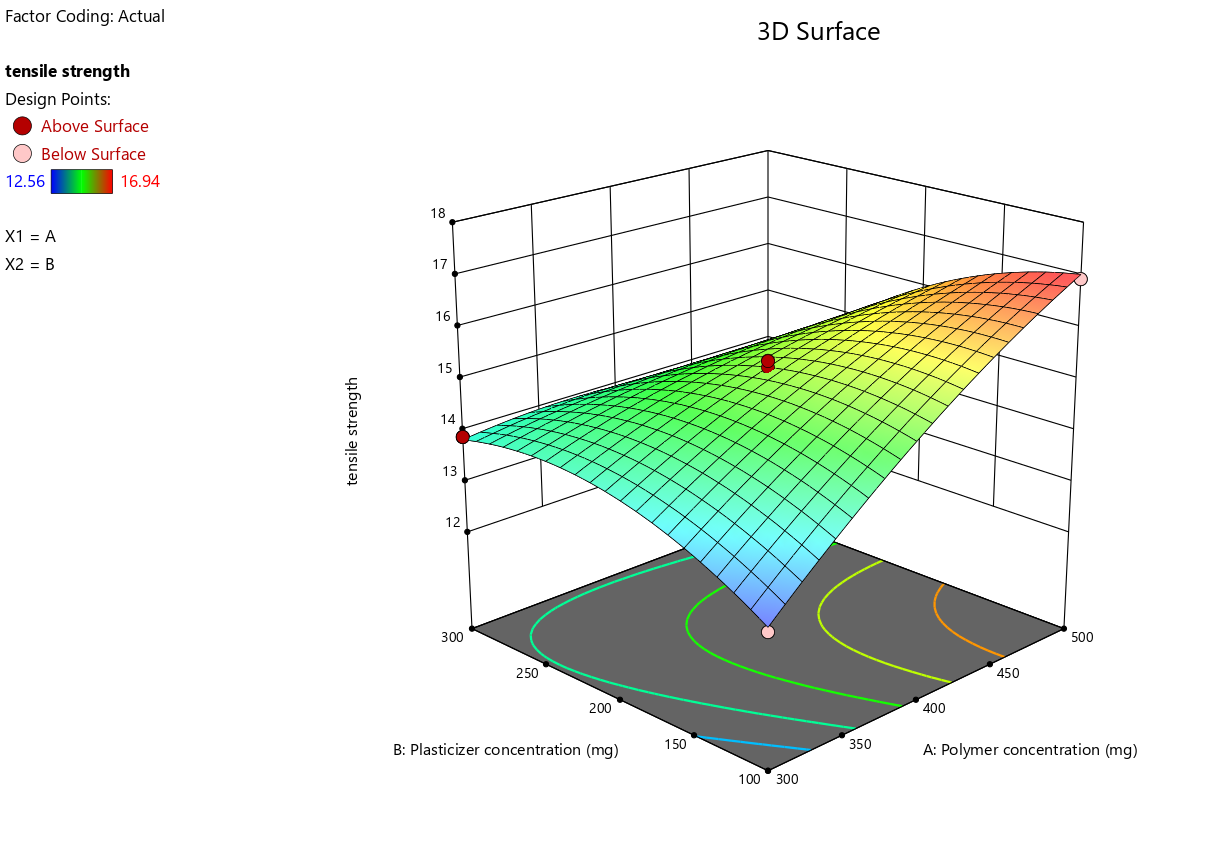
The three-dimensional (3D) response surface plots (fig. 2c) further elucidate the interaction effects between polymer and plasticizer concentrations on tensile strength. These plots highlight a clear interaction between the two factors, with tensile strength showing a peak in the response surface at specific levels of polymer and plasticizer concentrations. The curvature observed in the plots indicates a nonlinear relationship, emphasizing that both factors must be optimized simultaneously to achieve the desired tensile strength.
Folding endurance (R2)
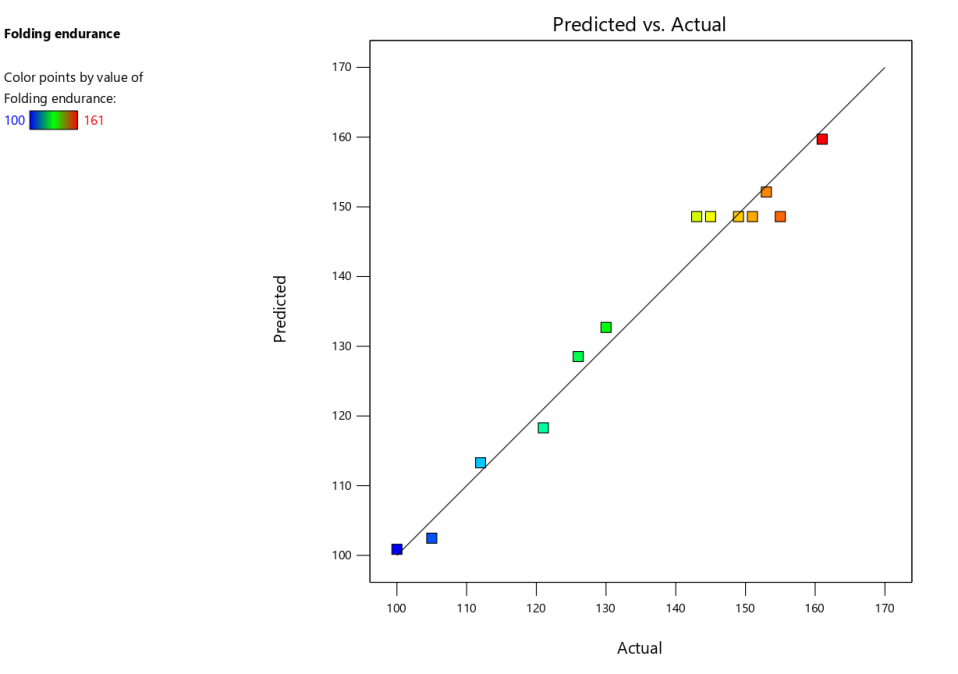
The optimization of Escitalopram Oral Thin Films (OTFs) was further evaluated through the folding endurance response, with the quadratic model identified as the most appropriate fit based on the ANOVA results. The quadratic model showed a highly significant F-value of 54.98 (p-value<0.0001), indicating that this model explained most of the variability in folding endurance. The adjusted R² was 0.9574, and the predicted R² was 0.9252, both suggesting a strong model fit and predictive accuracy. The residual sum of squares was 123.42, demonstrating a good fit between the model and experimental data.
The analysis of individual factors revealed that polymer concentration (A) had a significant effect on folding endurance, contributing 678.93 to the sum of squares with an F-value of 38.51 (p-value = 0.0004). This shows that polymer concentration had a substantial impact on the folding endurance of the Escitalopram OTFs. Similarly, plasticizer concentration (B) was the most influential factor, with a sum of squares of 2154.85 and a highly significant F-value of 122.21 (p-value<0.0001), indicating that plasticizer concentration played a critical role in enhancing the flexibility of the films. However, the interaction term (AB) was found to be statistically insignificant, with a p-value of 0.8186, indicating that the combined influence of polymer and plasticizer concentrations on folding endurance was negligible. This suggests that variations in polymer and plasticizer levels primarily exerted independent effects on the mechanical flexibility of the films, with no appreciable synergistic or antagonistic interaction between them. Consequently, the overall folding endurance was predominantly governed by the individual contributions of these formulation components rather than their combined interplay.
The quadratic terms for both polymer concentration (A²) and plasticizer concentration (B²) were also significant. The A² term, with a sum of squares of 1905.41 and an F-value of 108.07 (p-value<0.0001), indicated that polymer concentration had a nonlinear effect on folding endurance. Similarly, the B² term contributed 254.63 to the sum of squares with an F-value of 14.44 (p-value = 0.0067), confirming that plasticizer concentration also exhibited a nonlinear effect on folding endurance.
The Lack of Fit was not significant (p-value = 0.7188), which indicates that the model's predictions were robust and did not suffer from poor fit due to unexplained variability in the data.
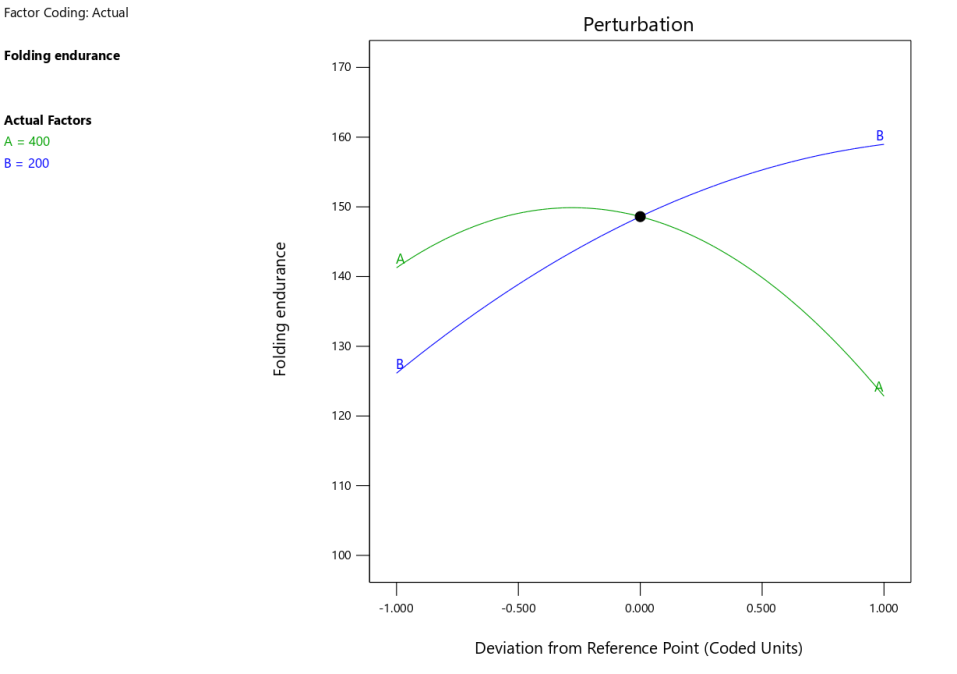
The predicted vs. actual plot (fig. 3a) confirmed the model’s reliability in predicting folding endurance. The data points aligned closely with the regression line, indicating good predictive capability. The perturbation plot (fig. 3b) further illustrated how folding endurance was influenced by changes in individual factors. The steep slopes observed for polymer and plasticizer concentrations highlighted their significant contributions to the response.
Table 3: ANOVA summary for the response variables of escitalopram oral thin films
| Source | Tensile strength | Folding endurance | Disintegration time | |||||||||
| Sum of squares | Mean square | F-value | p-value | Sum of squares | Mean square | F-value | p-value | Sum of squares | Mean square | F-value | p-value | |
| Model | 20.24 | 4.05 | 256.04 | <0.0001 | 4847.35 | 969.47 | 54.98 | <0.0001 | 1787.51 | 357.50 | 159.14 | <0.0001 |
| A-Polymer concentration | 7.73 | 7.73 | 488.70 | <0.0001 | 678.93 | 678.93 | 38.51 | 0.0004 | 823.85 | 823.85 | 366.73 | <0.0001 |
| B-Plasticizer concentration | 2.96 | 2.96 | 187.17 | <0.0001 | 2154.85 | 2154.85 | 122.21 | <0.0001 | 41.92 | 41.92 | 18.66 | 0.0035 |
| AB | 5.83 | 5.83 | 368.94 | <0.0001 | 1.0000 | 1.0000 | 0.0567 | 0.8186 | 110.25 | 110.25 | 49.08 | 0.0002 |
| A² | 0.8431 | 0.8431 | 53.33 | 0.0002 | 1905.41 | 1905.41 | 108.07 | <0.0001 | 678.37 | 678.37 | 301.97 | <0.0001 |
| B² | 3.25 | 3.25 | 205.36 | <0.0001 | 254.63 | 254.63 | 14.44 | 0.0067 | 220.11 | 220.11 | 97.98 | <0.0001 |
| Residual | 0.1107 | 0.0158 | 123.42 | 17.63 | 15.73 | 2.25 | ||||||
| Lack of Fit | 0.0421 | 0.0140 | 0.8174 | 0.5478 | 32.22 | 10.74 | 0.4711 | 0.7188 | 5.73 | 1.91 | 0.7634 | 0.5708 |
| Pure Error | 0.0686 | 0.0172 | 91.20 | 22.80 | 10.00 | 2.50 | ||||||
| Cor total | 20.35 | 4970.77 | 1803.23 | |||||||||
Table 4: Results summary of characterization experiments of escitalopram oral thin films
| Formulation | Thickness (mm) | Weight variation (mg) | Surface pH | Folding endurance | Tensile strength (MPa) | Elongation break (%) | Disintegration time (Sec) |
| F1 | 0.048±0.02 | 38.4±2.65 | 6.88±0.06 | 153±3.6 | 13.90±0.75 | 2.056 | 84±0.84 |
| F2 | 0.049±0.02 | 37.5±3.68 | 6.92±0.15 | 161±2.9 | 12.95±0.97 | 2.124 | 87±0.92 |
| F3 | 0.052±0.01 | 38.9±2.15 | 6.89±0.10 | 143±1.7 | 15.26±1.06 | 1.836 | 71±0.63 |
| F4 | 0.053±0.01 | 39.6±3.26 | 6.83±0.10 | 100±3.6 | 16.94±0.42 | 1.532 | 102±1.03 |
| F5 | 0.049±0.01 | 36.8±4.21 | 6.81±0.02 | 130±1.4 | 13.45±0.94 | 2.018 | 95±0.71 |
| F6 | 0.056±0.01 | 37.2±2.96 | 6.87±0.09 | 145±2.1 | 15.08±0.87 | 1.817 | 73±0.62 |
| F7 | 0.053±0.02 | 38.1±1.27 | 6.85±0.09 | 126±1.6 | 13.19±0.61 | 1.942 | 78±0.85 |
| F8 | 0.057±0.01 | 37.9±3.52 | 6.83±0.05 | 151±1.8 | 15.38±0.76 | 1.846 | 70±0.48 |
| F9 | 0.057±0.02 | 38.3±2.27 | 6.88±0.04 | 105±4.2 | 15.97±0.84 | 1.783 | 105±0.82 |
| F10 | 0.055±0.03 | 37.6±3.91 | 6.89±0.05 | 155±1.9 | 15.39±0.77 | 1.832 | 72±0.59 |
| F11 | 0.050±0.02 | 38.1±4.19 | 6.82±0.10 | 112±2.3 | 14.87±0.82 | 0.987 | 79±0.65 |
| F12 | 0.054±0.03 | 37.8±2.83 | 6.94±0.02 | 121±2.7 | 12.56±0.69 | 1.451 | 70±0.91 |
| F13 | 0.056±0.03 | 38.8±2.54 | 6.95±0.04 | 149±2.1 | 15.19±0.78 | 1.825 | 74±0.55 |
| (n=3; Average ±SEM) |
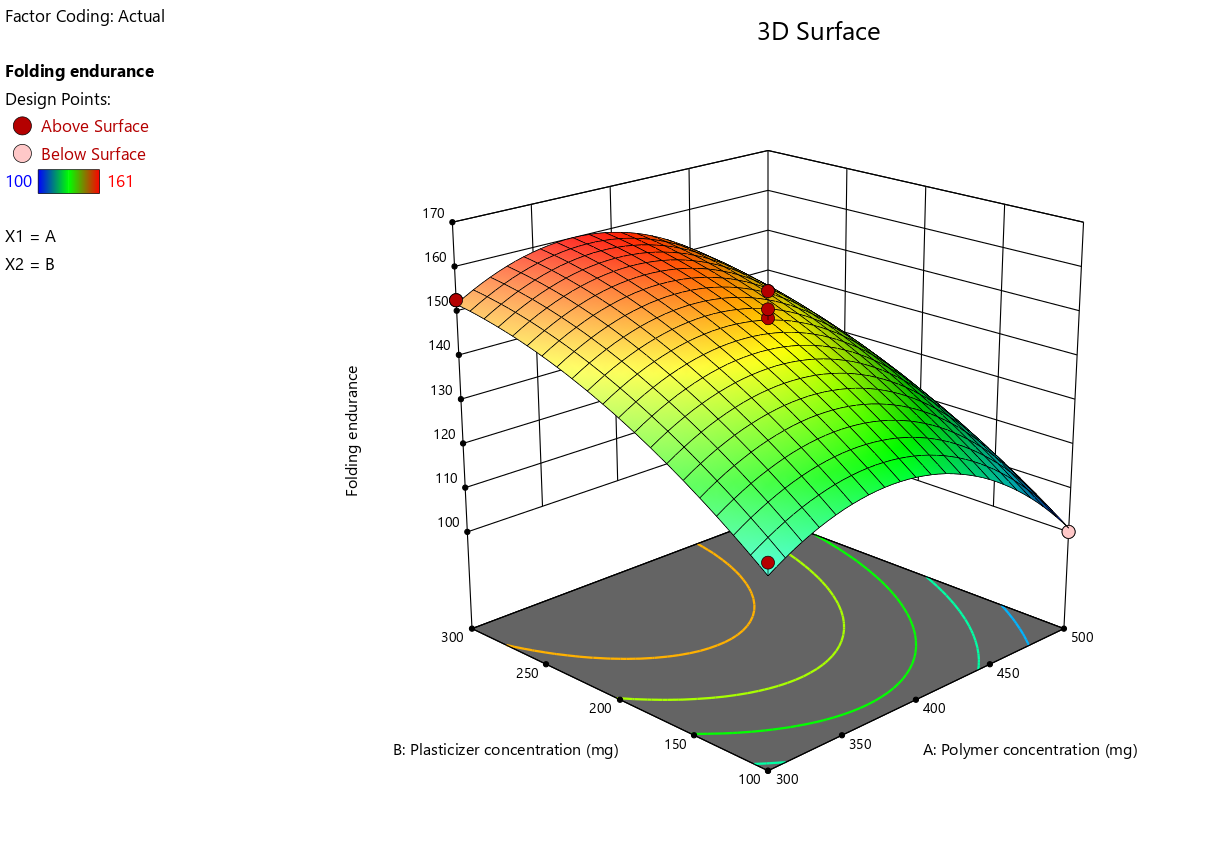
The 3D-response surface plots (fig. 3c) provided valuable insights into the combined effects of polymer and plasticizer concentrations. These plots visually demonstrated the optimal regions for maximizing folding endurance, with higher plasticizer and polymer concentrations generally leading to improved flexibility of the films. These findings align with the statistical analysis and suggest that a careful balance of these factors is essential for achieving the desired properties in Escitalopram OTFs.
Disintegration time (R3)
The optimization of Escitalopram Oral Thin Films (OTFs) was further assessed with respect to the disintegration time response. The results indicated that the quadratic model was the most appropriate fit, as it showed a highly significant F-value of 159.14 (p-value<0.0001), suggesting that this model adequately explained the variability in disintegration time. The adjusted R² value of 0.9851 and the predicted R² value of 0.9688 both confirmed the model’s high explanatory and predictive power. The residual sum of squares was 15.73, which was minimal, indicating a good fit of the model to the experimental data.
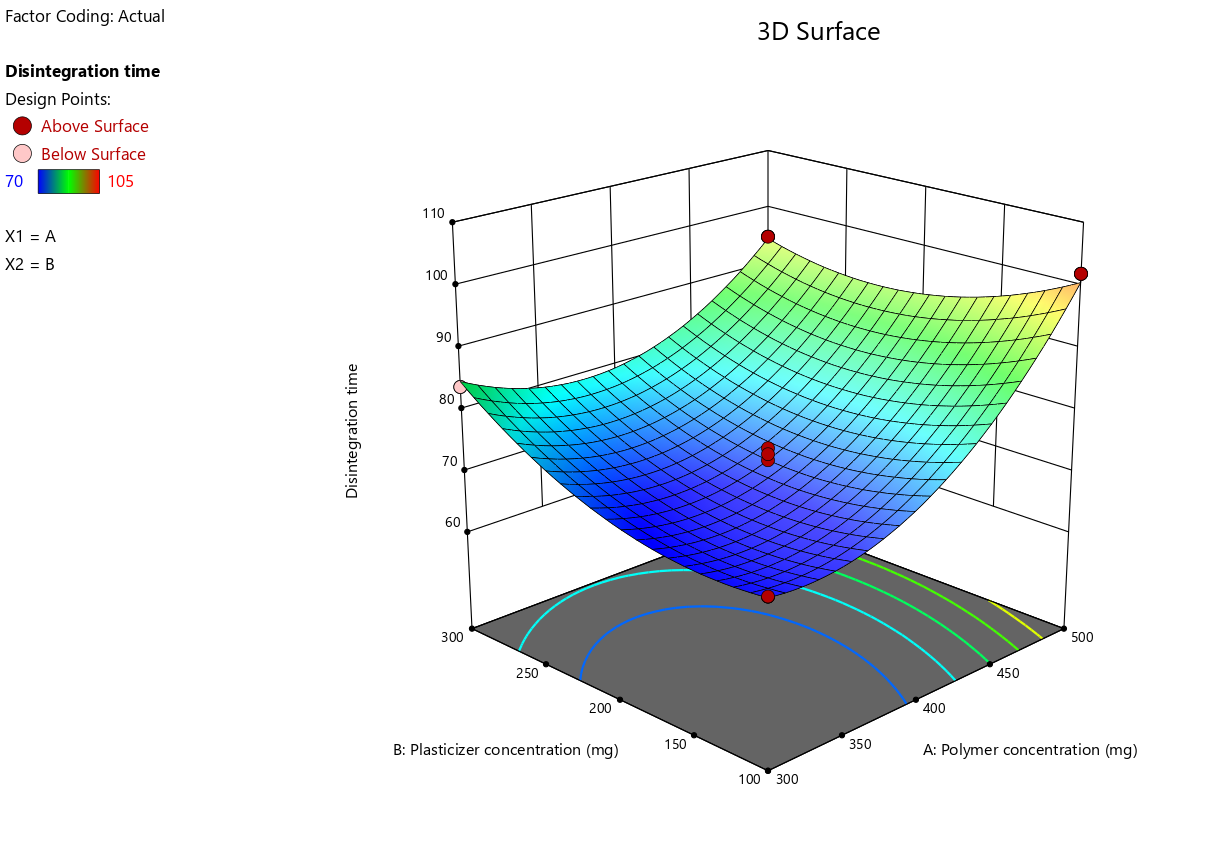
The individual factors influencing disintegration time were polymer concentration (A) and plasticizer concentration (B). Polymer concentration (A) had the most significant effect on disintegration time, contributing 823.85 to the sum of squares with an F-value of 366.73 (p-value<0.0001). This indicates that increasing polymer concentration led to a significant delay in disintegration time. On the other hand, plasticizer concentration (B) had a smaller, though still significant, impact on disintegration time, contributing 41.92 to the sum of squares with an F-value of 18.66 (p-value = 0.0035). The interaction between polymer and plasticizer concentrations (AB) was also found to be significant, with a sum of squares of 110.25 and an F-value of 49.08 (p-value = 0.0002), suggesting that the combination of these factors could synergistically influence the disintegration behaviour of the films.
 |
 |
| 2a | 2b |
 |
|
| 2c |
Fig. 2: Statistical plots of ANOVA of tensile strength (R1)
 |
 |
| 3a | 3b |
 |
|
| 3c |
Fig. 3: Statistical plots of ANOVA of folding endurance (R2)
The quadratic terms for both polymer concentration (A²) and plasticizer concentration (B²) were statistically significant. The A² term contributed 678.37 to the sum of squares, with an F-value of 301.97 (p-value<0.0001), indicating that the effect of polymer concentration on disintegration time is nonlinear. Similarly, the B² term contributed 220.11 to the sum of squares, with an F-value of 97.98 (p-value<0.0001), suggesting a nonlinear influence of plasticizer concentration on disintegration time. The Lack of Fit was found to be not significant (p-value = 0.5708), indicating that the model provided a good fit to the data without significant unexplained variability.
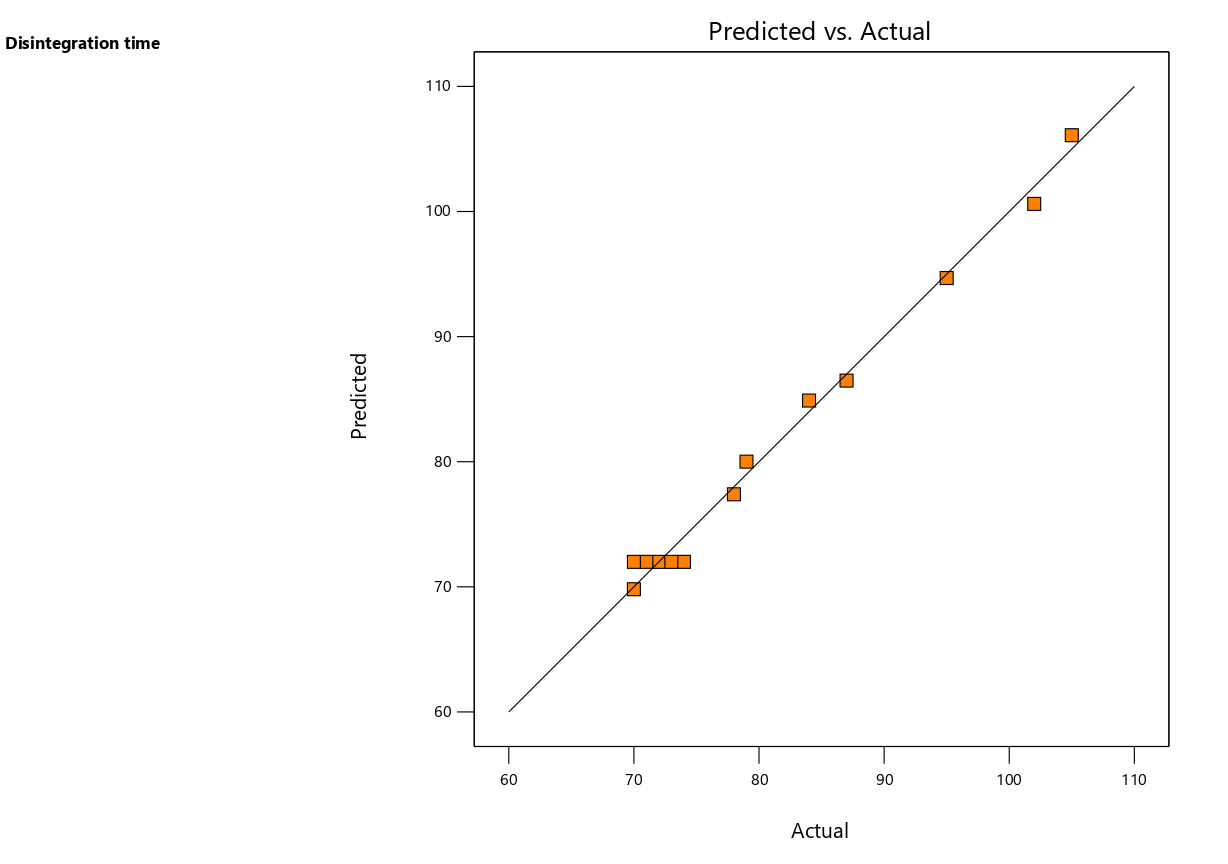
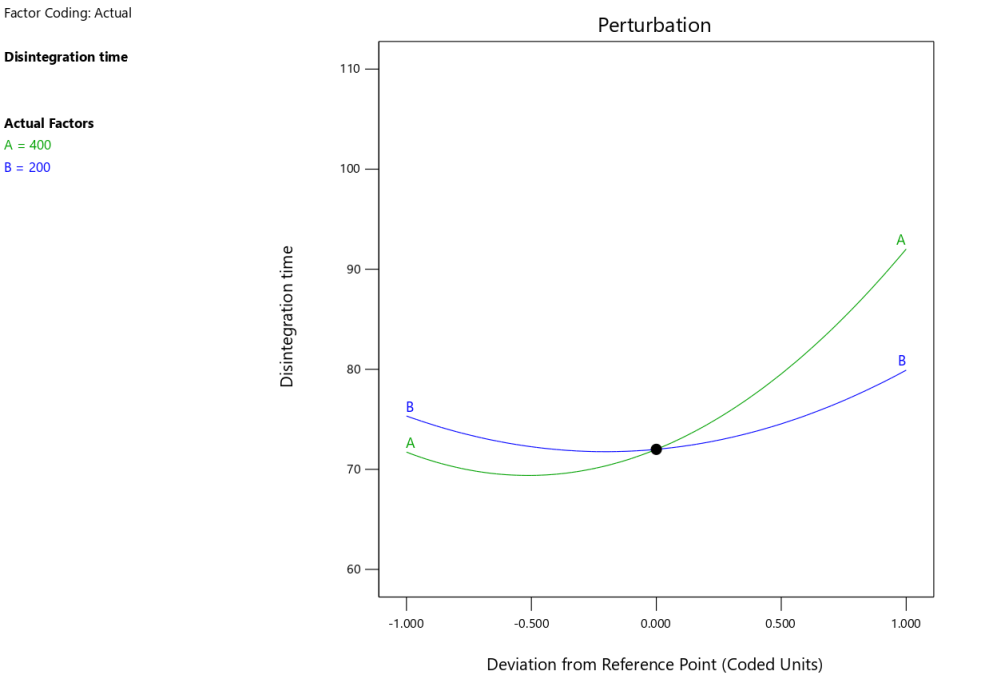
The predicted vs. actual plot (fig. 4a) confirmed the robustness of the quadratic model, as the data points aligned well with the regression line, indicating reliable predictions of disintegration time. The perturbation plot (fig. 4b) showed that both polymer and plasticizer concentrations had significant effects on disintegration time, with a steeper slope for polymer concentration, which aligned with its greater impact in the statistical analysis. The 3D-response surface plots (fig. 4c) further illustrated how variations in polymer and plasticizer concentrations influenced disintegration time. These plots revealed that higher polymer concentrations led to longer disintegration times, while plasticizer concentration had a more moderate impact, further confirming the findings from the ANOVA.
Overall, the optimization of Escitalopram OTFs through the quadratic model revealed important insights into the roles of polymer and plasticizer concentrations in determining the physical properties of the films. These results are valuable for the formulation of OTFs with controlled disintegration behaviour, optimizing both the performance and the user experience of the dosage form.
Final optimization
The MODR (Method Operable Design Region) is graphically represented in fig. 5, illustrating the optimal ranges of polymer (400 mg) and plasticizer concentrations (220 mg) for achieving the desired responses-tensile strength, folding endurance, and disintegration time-in Escitalopram oral thin films. Final formulation details with all ingredients for the optimized formulation were enumerated in table 5.
 |
 |
| 4a | 4b |
 |
|
| 4c |
Fig. 4: Statistical plots of ANOVA of disintegration time (R3)
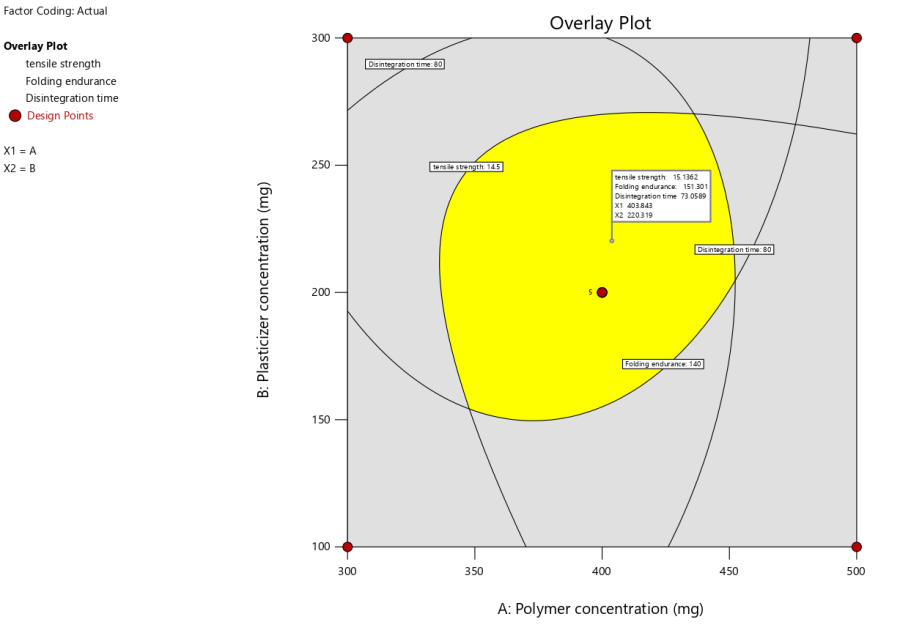
Fig. 5: Overlay plot for optimization of polymer and plasticizer concentrations
Table 5: Final optimized formulation and the response variable results for escitalopram oral thin films
| Ingredient | Quantity | Unit formula |
| Escitalopram | 250 mg | 10 mg |
| Pullulan | 400 mg | 16 mg |
| PEG 400 | 220 mg | 9.2 mg |
| Sucralose | 10 mg | 0.4 mg |
| Citric Acid | 50 mg | 2 mg |
| Watermelon Flavour (C73423) | 2 mg | 0.08 mg |
| Response variable | Result | |
| Tensile Strength | 15.3 N | |
| Folding Endurance | 159 | |
| Disintegration time | 73 Sec |
Characterization of escitalopram thin films
The characterization results of the Escitalopram oral thin films (OTFs) for different formulations (F1 to F13) are summarized in terms of thickness, weight variation, surface pH, folding endurance, tensile strength, Elongation break (%) and disintegration time in table 4. Each of these parameters provides important insights into the quality and performance of the films, which can inform the selection of the optimal formulation for therapeutic use.
Thickness (mm)
The thickness of the films varied from 0.48 mm to 0.57 mm across different formulations, with F1 (0.48±0.02 mm) exhibiting the lowest thickness and F8, F9, and F13 showing the highest (0.57±0.01 mm). Thin films are preferred for faster dissolution and disintegration, which are desirable for oral administration. Formulations with thicknesses in the range of 0.50 mm to 0.55 mm, such as F1, F2, F5, and F12, appear to strike a good balance between handling strength and performance.
Weight variation (mg)
The weight variation of the films ranged from 368±4.21 mg (F5) to 396±3.26 mg (F4). This parameter is critical for ensuring consistent dosage in each film. Formulations with minimal weight variation, such as F5 (368±4.21 mg), show good consistency in film preparation, which is important for dose accuracy and reproducibility.
Surface pH
The surface pH of the films ranged from 6.81±0.02 (F5) to 6.95±0.04 (F13), indicating that all formulations have a near-neutral pH, which is important to avoid irritation upon contact with the oral mucosa. The pH values in the range of 6.8-7.0 are ideal, as they are close to the physiological pH of the mouth (approximately 6.8-7.4), ensuring minimal risk of irritation.
Folding endurance
Folding endurance values varied from 100±3.6 (F4) to 161±2.9 (F2). Folding endurance is a critical factor for assessing the mechanical strength and flexibility of the films. Formulations with higher folding endurance values, such as F2 (161±2.9), suggest superior mechanical properties, which would be beneficial for the handling and shelf-life of the films. Lower values, such as those in F4 (100±3.6), may indicate weaker films, which could lead to issues during storage and use.
Tensile strength (MPa) and elongation break
Tensile strength values ranged from 12.56±0.69 MPa (F12) to 16.94±0.42 MPa (F4). The tensile strength of a film indicates its resistance to breaking under tension. Higher tensile strength values, such as F4 (16.94±0.42 MPa), indicate stronger films that are less likely to tear during handling or administration. On the other hand, lower tensile strength values, like F12 (12.56±0.69 MPa), suggest films that may be more prone to breaking, compromising their integrity.
The elongation break (%) reflects the flexibility and mechanical robustness of the films, varying from 0.987% (F11) to 2.124% (F2). Formulations with higher elongation break values (e. g., F2, F5) exhibit better flexibility, while those with lower values (e. g., F4, F11) are more brittle. A trade-off is observed between tensile strength and elongation, where higher tensile strength (e. g., F4: 16.94 MPa) corresponds to lower flexibility. Plasticizer concentration plays a crucial role, with optimal levels enhancing flexibility and folding endurance, as seen in F2 and F5. These formulations demonstrate a balanced mechanical profile, making them ideal for fast-dissolving films, while others like F11 may require further optimization to improve flexibility and usability.
FTIR analysis of OTFs
FTIR spectra of Escitalopram, pure Pullulan, and the optimized Escitalopram-pullulan OTF (fig. 6) were analyzed to assess molecular interactions and excipient compatibility. The spectrum of pure Escitalopram exhibited characteristic peaks corresponding to its functional groups, including O–H stretching (3500–3200 cm⁻¹), aromatic C=C stretching (1600–1500 cm⁻¹), and C–N stretching (1200–1000 cm⁻¹). Pullulan displayed broad O–H stretching (3400 cm⁻¹), C–H stretching (2900 cm⁻¹), and C–O–C stretching (1000–1200 cm⁻¹), confirming its polysaccharide structure.
In the FTIR spectrum of the optimized OTF, the characteristic peaks of both Escitalopram and Pullulan were retained, but notable shifts and broadening were observed in the O–H stretching region, indicating hydrogen bonding interactions between the hydroxyl groups of Pullulan and the functional groups of Escitalopram. These interactions suggest enhanced drug dispersion within the polymer matrix, contributing to improved film uniformity and drug stability. The absence of new peaks or significant structural changes confirms the absence of chemical degradation or covalent interactions, ensuring the integrity of the formulation. Such hydrogen bonding interactions may also influence drug release kinetics by modulating the hydration and disintegration properties of the film, ultimately supporting the observed rapid dissolution behavior.
SEM analysis
The SEM image in fig. 7 depicts a rough, fibrous surface with an irregular texture, characteristic of an amorphous or semi-crystalline matrix typically observed in thin films formulated for rapid drug release. This rough surface increases the available surface area, potentially enhancing the rate of disintegration and dissolution. The pronounced surface irregularities may also promote faster hydration and erosion of the film, facilitating rapid disintegration upon contact with saliva.
Disintegration time (sec)
The disintegration time ranged from 70±0.48 sec (F8) to 105±0.82 sec (F9). Disintegration time is a crucial factor in ensuring that the film dissolves rapidly in the mouth for quick drug absorption. Formulations with shorter disintegration times, such as F8 (70±0.48 sec) and F12 (70±0.91 sec), are preferable for fast release of the active ingredient, ensuring quicker therapeutic effects. Longer disintegration times, such as F9 (105±0.82 sec), may delay onset of action.
Formulation F8 emerges as the optimized formulation after evaluating all key parameters. With a thickness of 0.57 mm, F8 is on the higher end of the range but still falls within the optimal limits, ensuring mechanical stability and ease of handling. The weight of F8 (379±3.52 mg) is well within acceptable variation limits, which guarantees accurate dosing. The surface pH of 6.83±0.05 is ideal for oral administration, minimizing potential irritation. In terms of folding endurance, F8 exhibits a value of 151±1.8, demonstrating a good balance between flexibility and strength, making it robust for both handling and storage. The tensile strength of 15.38±0.76 MPa indicates that the film has good mechanical strength without compromising its ability to disintegrate. Most notably, F8 exhibits the shortest disintegration time of 70±0.48 seconds, which is critical for ensuring a rapid onset of action in oral delivery. Therefore, Formulation F8 showcases the best overall performance in terms of physical properties, mechanical strength, and rapid disintegration, making it the most promising candidate for further development.
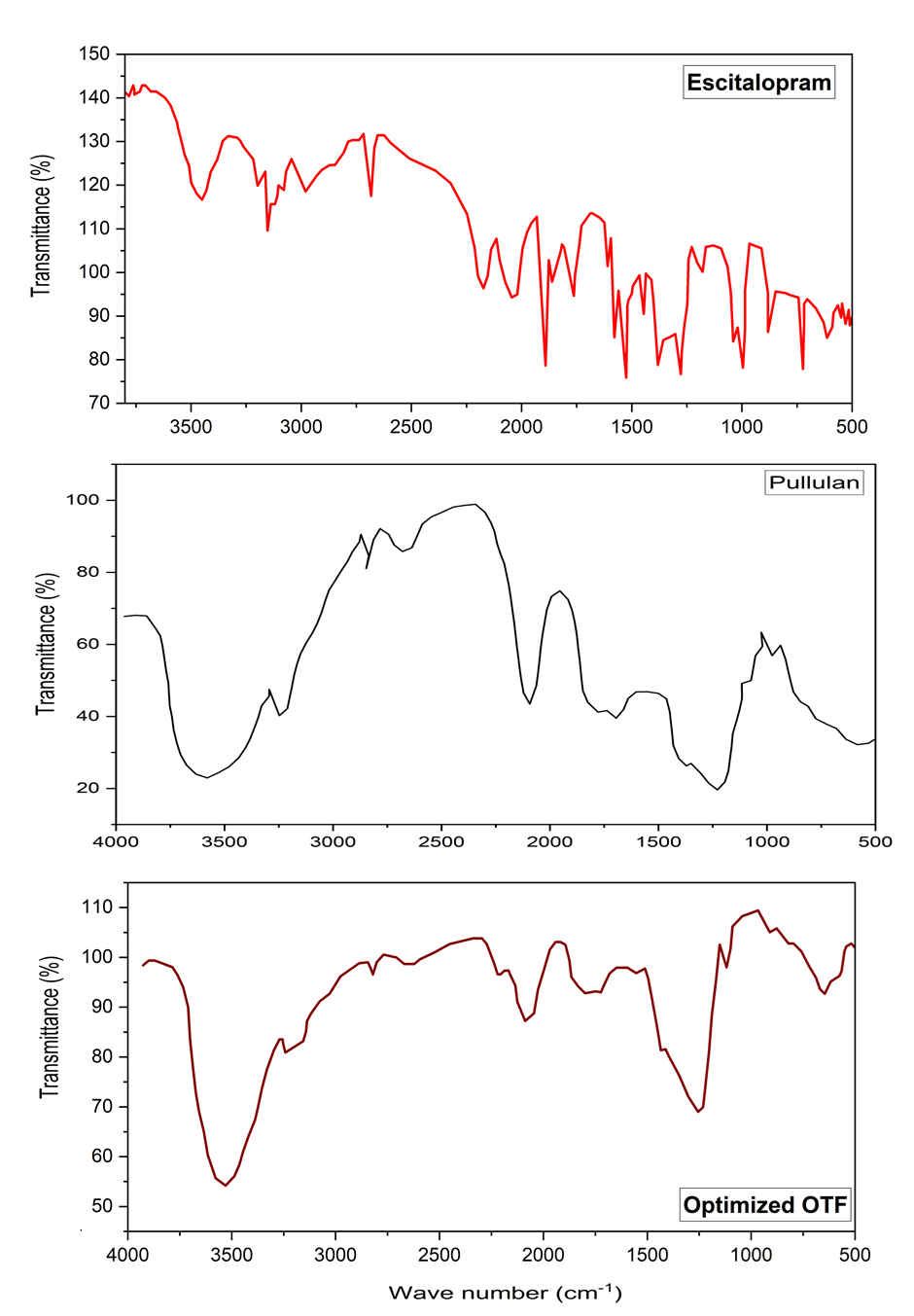
Fig. 6: FTIR spectra of escitalopram, pullulan and optimized OTF
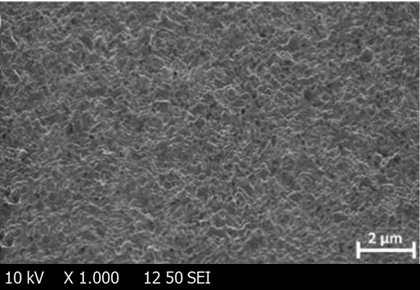
Fig. 7: SEM image of escitalopram oral thin film (optimized)
Table 6: Dissolution of optimized OTFs
| Time (min) | Escitalopram (Nexito forte) | Escitalopram (Oral thin film) |
| 0 | 0 | 0 |
| 3 | 3.5±1.51 | 85.7±3.95 |
| 5 | 10.2±1.88 | 93.1±1.62 |
| 10 | 21.3±2.05 | 98.6±1.81 |
| 30 | 63.7±3.08 | 98.7±1.25 |
| 60 | 90.4±1.89 | 98.7±1.97 |
| 90 | 93.3±2.09 | 98.9±1.34 |
| 120 | 98.2±1.55 | 98.9±1.22 |
(n=3; Average ±SEM)
In vitro drug release study
The dissolution profile of the optimized Escitalopram oral thin film (OTF) was compared to the commercially available dispersible tablet (Nexito Forte), and the results are summarized in table 6 and fig. 8. Dissolution testing was conducted in 200 ml of phosphate buffer (pH 6.8) at 50 rpm and 37±0.5 °C. The OTF exhibited a significantly faster drug release, with 85.7% release at 3 min, 93.1% at 5 min, and 98.6% at 10 min. In contrast, the tablet formulation released only 3.5% at 3 min and 21.3% at 10 min, reaching 63.7% dissolution at 30 min. Both formulations approached complete release by 90 min, with the OTF achieving 98.9% and the tablet 93.3%. To substantiate the observed differences, a paired t-test was conducted, confirming a statistically significant improvement in the dissolution rate of the OTF compared to the tablet (p<0.05 at all-time points). Furthermore, one-way ANOVA revealed a significant formulation effect (p<0.0001), demonstrating that the OTF formulation achieved a superior dissolution profile. The rapid dissolution of the OTF suggests enhanced bioavailability due to faster absorption, which can be advantageous for patients requiring immediate therapeutic effects or those with dysphagia. The significantly faster release profile of the OTF aligns with its potential for improved pharmacokinetic performance compared to traditional solid oral dosage forms.
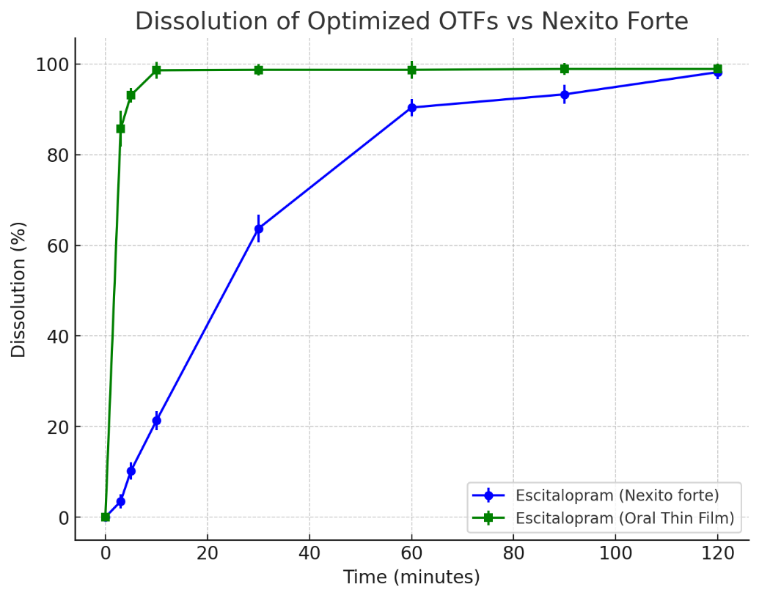
Fig. 8: Line graph of in vitro dissolution of escitalopram oral thin films
DISCUSSION
The optimization of Escitalopram Oral Thin Films (OTFs) using a quadratic model revealed that polymer and plasticizer concentrations critically influenced key physicochemical properties, including tensile strength, folding endurance, and disintegration time. These findings align with previous studies demonstrating that higher polymer concentrations enhance mechanical strength but may prolong disintegration, while plasticizers improve flexibility at the cost of reduced tensile strength [21, 22]. Our results corroborate these trends, with polymer concentration exhibiting the strongest effect on tensile strength and disintegration, whereas plasticizer concentration primarily enhanced film flexibility. The interaction between these factors further demonstrated synergistic effects, necessitating a balanced optimization approach. The final optimized formulation (400 mg polymer and 220 mg plasticizer) successfully achieved an equilibrium between mechanical robustness and rapid disintegration, consistent with prior reports on OTFs [23].
Characterization studies confirmed uniformity across batches, with thickness (0.48–0.57 mm), minimal weight variation, near-neutral pH (6.81–6.95), and optimal mechanical properties, in agreement with pharmacopeial standards for oral films [24]. FTIR analysis confirmed the absence of drug-excipient interactions, ensuring formulation stability-a critical factor observed in similar OTF studies [25]. SEM analysis suggested an amorphous structure, which is known to facilitate rapid disintegration, as reported by [26]. Among the formulations, F8 exhibited the shortest disintegration time (70±0.48 sec), outperforming F9 (105±0.82 sec), reinforcing the impact of polymer-plasticizer balance on disintegration kinetics, as previously noted by Pries et al. [27].
The in vitro drug release study demonstrated a significant enhancement in dissolution rate for the optimized OTF compared to a commercial dispersible tablet. Specifically, the OTF achieved 85.7% drug release within 3 min and 98.6% within 10 min, whereas the tablet released only 21.3% in the same period. These results are consistent with studies highlighting the superiority of OTFs in enhancing dissolution rates due to their large surface area and amorphous nature [28]. The rapid drug release observed in our study suggests potential bioavailability advantages, particularly for psychiatric medications requiring fast onset, as emphasized by Hirani et al. [29].
The systematic optimization of Escitalopram OTFs underscores the critical role of excipient selection in tailoring mechanical and dissolution properties. The superior dissolution profile of the optimized OTF (F8) over conventional tablets suggests promising clinical benefits, particularly for patients with dysphagia or those requiring rapid therapeutic onset. These findings are in line with recent advancements in OTF technology, where fast-dissolving films have demonstrated improved patient compliance and pharmacokinetic performance [30]. However, further in vivo pharmacokinetic studies are warranted to validate the bioavailability and therapeutic efficacy of this formulation compared to existing oral dosage forms.
CONCLUSION
The optimization of Escitalopram OTFs was successfully achieved through a quadratic modelling approach, providing valuable insights into the influence of polymer and plasticizer concentrations on the physical and mechanical properties of the films. The optimized formulation exhibited favourable characteristics, including rapid dissolution, controlled disintegration time, high tensile strength, and good folding endurance, making it a promising alternative to conventional dosage forms. These findings contribute to the development of high-quality Escitalopram OTFs with the potential for enhanced therapeutic efficacy and improved patient compliance. To further establish the clinical utility of this formulation, future studies should focus on pharmacokinetic evaluations to assess drug absorption, bioequivalence trials comparing the optimized OTF with existing oral formulations, and in vivo studies to validate its therapeutic performance. These investigations will be essential in translating the formulation into clinical applications and ensuring regulatory approval.
ACKNOWLEDGEMENT
Authors are thankful to the Faculty of Pharmaceutical Sciences of Motherhood university, Roorkee, India, for providing necessary facilities in the successful completion of the current research.
FUNDING
Nil
AUTHORS CONTRIBUTIONS
Sumanth Bhukya contributed to the conceptualization, investigation, and data curation. Jayapal Reddy Gangadi was responsible for data interpretation, manuscript preparation, and supervision. Poli Reddy Papagatla performed the results analysis.
ABBREVIATIONS
ANOVA – Analysis of Variance, ASTM – American Society for Testing and Materials, CCD – Central Composite Design, DoE – Design of Experiments, EL – Elongation at Break, FTIR – Fourier Transform Infrared (Spectroscopy), KBr – Potassium Bromide, MODR – Method Operable Design Region, OTFs – Oral Thin Films, PEG – Polyethylene Glycol, RSD – Relative Standard Deviation, RSM – Response Surface Methodology, SEM – Scanning Electron Microscopy/Standard Error of the mean (context-dependent), SSRI – Selective Serotonin Reuptake Inhibitor, USP – United States Pharmacopeia, UTS – Ultimate Tensile Strength, UV-VIS – Ultraviolet-Visible (Spectrophotometry)
CONFLICT OF INTERESTS
Authors disclose no conflict of interest with current work.
REFERENCES
Karki S, Kim H, Na SJ, Shin D, Jo K, Lee J. Thin films as an emerging platform for drug delivery. Asian J Pharm Sci. 2016;11(5):559-74. doi: 10.1016/j.ajps.2016.05.004.
Jacob S, Boddu SH, Bhandare R, Ahmad SS, Nair AB. Orodispersible films: current innovations and emerging trends. Pharmaceutics. 2023;15(12):2753. doi: 10.3390/pharmaceutics15122753, PMID 38140094.
Morath B, Sauer S, Zaradzki M, Wagner AH. Orodispersible films-recent developments and new applications in drug delivery and therapy. Biochem Pharmacol. 2022;200:115036. doi: 10.1016/j.bcp.2022.115036, PMID 35427572.
Sevinc Ozakar R, Ozakar E. Current overview of oral thin films. Turk J Pharm Sci. 2021;18(1):111-21. doi: 10.4274/tjps.galenos.2020.76390, PMID 33634686.
Landy K, Rosani A, Estevez R. Escitalopram. StatPearls; 2023 Nov 10. Available from: https://www.ncbi.nlm.nih.gov/books/NBK557734/.
Goodman WK, Bose A, Wang Q. Treatment of generalized anxiety disorder with escitalopram: pooled results from double-blind, placebo-controlled trials. J Affect Disord. 2005;87(2-3):161-7. doi: 10.1016/j.jad.2004.11.011, PMID 15982747.
Bræstrup C, Sanchez C. Escitalopram: a unique mechanism of action. Int J Psychiatry Clin Pract. 2004;8 Suppl 1:11-3. doi: 10.1080/13651500410005496, PMID 24930683.
Waugh J, Goa KL. Escitalopram: a review of its use in the management of major depressive and anxiety disorders. CNS Drugs. 2003;17(5):343-62. doi: 10.2165/00023210-200317050-00004, PMID 12665392.
Gupta AK, Kumar A. Practical approaches for taste masking of bitter drug: a review. Int J Drug Deliv Technol. 2010;2(2):71-6. doi: 10.25258/ijddt.v2i2.8848.
Song D, Won Y, Kim H, Kim H, Choi S. Pharm CL. Bitter taste masked oral thin film formulation of sildenafil citrate; 2011 Dec 6. WO2013085224A1. Available from: https://patents.google.com/patent/WO2013085224A1/en. [Last accessed on 03 Jun 2025].
Sohi H, Sultana Y, Khar RK. Taste masking technologies in oral pharmaceuticals: recent developments and approaches. Drug Dev Ind Pharm. 2004;30(5):429-48. doi: 10.1081/ddc-120037477, PMID 15244079.
Khayyat YM, Abdul Wahab RA, Natto NK, Al Wafi AA, Al Zahrani AA. Impact of anxiety and depression on the swallowing process among patients with neurological disorders and head and neck neoplasia: systemic review. Egypt J Neurol Psychiatry Neurosurg. 2023;59(1):64. doi: 10.1186/s41983-023-00674-y.
Bushuven S, Niebel I, Huber J, Diesener P. Emotional and psychological effects of dysphagia: validation of the Jugendwerk Dysphagia Emotion and Family Assessment (JDEFA). Dysphagia. 2022;37(2):375-91. doi: 10.1007/s00455-021-10289-1, PMID 33817751.
Gupta MS, Kumar TP, Gowda DV. Orodispersible thin film: a new patient-centered innovation. J Drug Deliv Sci Technol. 2020;59:101843. doi: 10.1016/j.jddst.2020.101843.
Rao N. The clinical pharmacokinetics of escitalopram. Clin Pharmacokinet. 2007;46(4):281-90. doi: 10.2165/00003088-200746040-00002, PMID 17375980.
El-Ansary AL, Salama NN, FM. AA, Hassib HB, Mohamed MA. Validated spectrophotometric methods for determination of escitalopram through study of charge transfer and ion pair complexation. J Chem Acta. 2013;2:119-28.
Babu CV, Murthy Kolapalli VR. Design and optimization of escitalopram oxalate oral dissolving films by response surface methodology. Int J App Pharm. 2024 May 7;16(3):262-71. doi: 10.22159/ijap.2024v16i3.49662.
Prajapati VD, Chaudhari AM, Gandhi AK, Maheriya P. Pullulan based oral thin film formulation of zolmitriptan: development and optimization using factorial design. Int J Biol Macromol. 2018;107(B):2075-85. doi: 10.1016/j.ijbiomac.2017.10.082, PMID 29074082.
Gupta A, Kumar J, Verma S, Singh H. Application of quality by design approach for the optimization of orodispersible film formulation. Asian J Pharm Clin Res. 2018 Jul 27;11(14):8-11. doi: 10.22159/ajpcr.2018.v11s2.28508.
Kamali H, Farzadnia P, Movaffagh J, Abbaspour M. Optimization of curcumin nanofibers as fast dissolving oral films prepared by emulsion electrospinning via central composite design. J Drug Deliv Sci Technol. 2022;75:103714. doi: 10.1016/j.jddst.2022.103714.
Patil PH, Belgamwar VS, Patil PR, Surana SJ. Solubility enhancement of lamotrigine using inclusion complexation and preparation of fast-dissolving sublingual films. Carbohydr Polym. 2016;137:250-9. doi: 10.1016/j.carbpol.2015.10.073.
Chen J, Pan H, Yang Y, Xiong S, Duan H, Yang X. Self-assembled liposome from multi-layered fibrous mucoadhesive membrane for buccal delivery of drugs having high first-pass metabolism. Int J Pharm. 2018;547(1-2):303-14. doi: 10.1016/j.ijpharm.2018.05.062, PMID 29803794.
Liu Y, Liu J, Zhang X, Zhang R, Huang Y, Wu C. In situ gelling gelrite/alginate formulations as vehicles for ophthalmic drug delivery. AAPS PharmSciTech. 2010;11(2):610-20. doi: 10.1208/s12249-010-9413-0.
Rockville MD. United States Pharmacopeia and National Formulary (USP-NF): United States Pharmacopeial Convention. United States Pharmacopeia 46-National Formulary 41; 2023.
Kulkarni AS, Deokule HA, Mane MS, Ghadge DM. Exploration of different polymers for use in the formulation of oral fast-dissolving films. J Drug Deliv Sci Technol. 2021;63:102454. doi: 10.1016/j.jddst.2021.102454.
El-Setouhy DA, El-Malak NS, El-Gazayerly ON. Formulation of a novel tianeptine sodium orodispersible film. Saudi Pharm J. 2015;23(1):19-30. doi: 10.1016/j.jsps.2014.04.003.
Kang MH, Park MJ, Yoo HJ, hyuk KY, Lee SG, Kim SR. RIPL peptide (IPLVVPLRRRRRRRRC)-conjugated liposomes for enhanced intracellular drug delivery to hepsin-expressing cancer cells. Eur J Pharm Biopharm. 2014;87(3):489-99. doi: 10.1016/j.ejpb.2014.03.016, PMID 24704199.
Dixit RP, Puthli SP. Oral strip technology: overview and future potential. J Control Release. 2009;139(2):94-107. doi: 10.1016/j.jconrel.2009.06.014, PMID 19559740.
Hirani JJ, Rathod D, Vadalia K. Orally disintegrating tablets: a review. Trop J Pharm Res. 2009;8(2):161-72. doi: 10.4314/tjpr.v8i2.44525.
Ohlfest JR, Zellmer DM, Panyam J, Swaminathan SK, Oh S, Waldron NN. Immunotoxin targeting CD133+ breast carcinoma cells. Drug Deliv and Transl Res. 2013;3(2):195-204. doi: 10.1007/s13346-012-0066-2.