
Int J App Pharm, Vol 17, Issue 5, 2025, 292-301Original Article
IDENTIFICATION AND CHARACTERIZATION OF NEW IMPURITIES IN DEXAMETHASONE OPHTHALMIC SUSPENSION
BHUPINDER KUMAR1, RAJU MUDHULKAR2*, SREEDHAR GUNDEKARI3, DAMA VENUGOPALA RAO4, LAKSHMIPRIYA ANAMALAGUNDAM5
1,4,5Dr. Reddy’s Laboratories Ltd, IPDO, Hyderabad-500090, India. 2,3Department of Chemistry, Koneru Lakshmaiah Education Foundation, Bowrampet, Hyderabad-500043, Telangana, India
*Corresponding author: Raju Mudhulkar; *Email: m.raju@klh.edu.in
Received: 10 Feb 2025, Revised and Accepted: 05 Jun 2025
ABSTRACT
Objective: This study was intended to identify two unknown impurities (Imp-A and Imp-B) observed at trace level in the six-month stability samples of dexamethasone ophthalmic suspension subjected to accelerated conditions (40 °C/75% relative humidity) using chromatographic and spectroscopic techniques.
Methods: During a six-month stability study of dexamethasone ophthalmic suspension, two unknown impurities were detected at level of exceeding the specification (0.20%) in chromatographic analysis. Impurities were isolated, enriched by preparative HPLC and characterized by using sophisticated spectroscopic techniques, including nuclear magnetic resonance (NMR), high-resolution mass spectrometry (HR-MS) and infrared (IR). Efficient and selective separation of these impurities was achieved using ultra-high performance liquid chromatography (UHPLC) on a Waters Acquity Bio H-Class System with XBridge C18 column (150 mm × 4.6 mm, particle size 3.5 µm) column at 40 °C. The mobile phase consisted of 0.02M ammonium formate and acetonitrile with detection at 240 nm using a photodiode array (PDA) detector. The results demonstrated that this mass-compatible UPLC method is suitable for the identifying and quantifying Imp-A and Imp-B in the dexamethasone ophthalmic suspension samples.
Results: Two impurities were formed in dexamethasone ophthalmic suspension samples under accelerated stability conditions, which were identified by spectroscopic techniques like LC-HRMS, NMR (1D and 2D) and IR. Major structural modifications of Imp-A and Imp-B with respect to dexamethasone were found on carbon C17. Mono-isotopic mass of 379.1921 [M+H]+and empirical formula of C21H28FO5 for Imp-A, and mono-isotopic mass of 407.1838 [M+H]+and empirical formula of C22H28FO6 for Imp-B observed during LC-HRMS study found in line with theoretical mono-isotopic mass and empirical formula for both impurities. The chemical shifts and splitting patterns observed in the 1D and 2D NMR spectra indicated the presence of a steroidal ring system in both impurities, consistent with the core structure of dexamethasone, suggesting that Imp-A and Imp-B are structurally related to the parent compound. The 2D 1H-13C HSQC spectra of Imp-A and Imp-B revealed correlations between hydrogen and carbon atoms, supporting their structural relationship with dexamethasone. Additionally, 1H-13C HMBC spectra confirmed the presence of a conjugated steroidal ring system in both impurities. Based on the combined LC-HRMS and NMR data, the structures of Imp-A and Imp-B were characterized and found to be related to dexamethasone.
Conclusion: The LC-HRMS method developed for the identification of unknown impurities of dexamethasone was found to be highly sensitive and selective. This method is capable of detecting trace level of impurities in dexamethasone and can used for the identifying unknown impurities of dexamethasone drug product. Combination of different spectroscopic techniques provides a pivotal tool for elucidating the structure impurities at trace level.
Keywords: Dexamethasone ophthalmic suspension, High-resolution mass spectrometry, Impurity isolation, Nuclear magnetic resonance, Ultra-performance liquid chromatography method
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijap.2025v17i5.53923 Journal homepage: https://innovareacademics.in/journals/index.php/ijap
INTRODUCTION
The identification, structure elucidation, and quantification of pharmaceutical impurities and degradation products are critical analytical activities throughout the research, development, and production stages of drug formulations [1, 2]. Impurity profiling, encompassing both synthesis-related impurities and degradation products, is essential for confirming the stability of a formulation [3]. This is typically achieved through preliminary stability studies and forced degradation or stress testing of the bulk drug material. The identification, qualification, and quantification of impurities are indispensable for ensuring the safety, efficacy, and overall quality of pharmaceutical substances and their dosage forms [1-3].
Dexamethasone, a potent synthetic corticosteroid with well-established anti-inflammatory and immunosuppressive properties, is extensively used in treating inflammation, allergic conditions, and adrenal cortex insufficiency [4]. It has also been shown to reduce neointimal hyperplasia in arteries, making it a valuable agent in drug-eluting stents for preventing restenosis [5-8]. A prominent example is the Dexamet™ stent (Abbott Vascular Devices Ltd.), which utilizes a phosphorylcholine (PC) polymer and dexamethasone coating to enhance therapeutic efficacy. TobraDex® (Alcon Laboratories Inc., Fort Worth, TX), a widely prescribed combination of Tobramycin (0.3%) and dexamethasone (0.1%), is FDA-approved for treating steroid-responsive inflammatory ocular conditions where the risk of bacterial infection exists. This combination product is often used empirically for inflamed red eyes, except in cases of suspected viral infection, and is the most frequently prescribed steroid/antibiotic ophthalmic formulation [9-12].
Given that dexamethasone treatment often requires long-term administration, ensuring the product's quality throughout its shelf life is of paramount importance for delivering safe and effective therapy. According to ICH Q3B (R2), for structurally unknown or unidentified impurities, there is an identification threshold based on total daily intake [13]. For most pharmaceutical products, this threshold is 0.1%. If impurity levels exceed this threshold during stability studies, structural identification of the impurity is mandatory to assess its impact on safety.
Hyphenated liquid chromatography methods, particularly those coupled with mass spectrometry (LC-MS), are widely employed for separating and identifying impurities in both drug substances and formulations [14-24]. High-resolution mass spectrometry, particularly with a quadrupole time-of-flight (Q-TOF) detector, is becoming increasingly popular due to its ability to provide highly accurate mass data, facilitating precise structural elucidation and reaction mechanism prediction. Electrospray ionization in positive mode is favoured in impurity analysis due to its ability to detect thermally labile, non-volatile, and polar compounds, making it suitable for a broad range of pharmaceutical impurities [18].
A comprehensive literature review reveals that while several dexamethasone impurities and degradation pathways have been discussed and reported [14, 18, 19, 25]. Two novel impurities (Impurity-A and Impurity-B) were Identified in the present study under long-term and accelerated stability conditions (40 °C/75% relative humidity) using high-performance liquid chromatography. These findings highlight the importance of further investigation into the stability and safety profiles of dexamethasone formulations. Such studies could provide insights into potential shelf-life extensions, offering both therapeutic and commercial benefits.
MATERIALS AND METHODS
Chemicals and reagents
The Tobramycin (0.3%) and Dexamethasone (0.1%) ophthalmic suspension utilized in this study was sourced from Dr. Reddy’s Laboratories Limited, India. Acetonitrile and methanol, both UPLC/MS grade, were obtained from Biosolve, while ammonium formate and acetic acid (Extra pure grade) were procured from Finar. Potassium bromide (KBr) was acquired from Sigma-Aldrich, and deuterated dimethylsulfoxide (DMSO-d6) was procured from Eurisotop. All analytical solutions were prepared using in-house Milli-Q water (Merck Milli-Q Integral 10 system).
Chromatographic conditions
A UPLC method was developed for the analysis of the unknown impurities of Tobramycin and Dexamethasone ophthalmic suspension. Chromatographic analysis was performed on Waters ACQUITY UPLC H-Class Bio System (Waters, Milford, MA, USA). Data acquisition and processing were performed using Waters Unifi software (Version 1.9.4.053). Chromatographic separation was achieved on XBridge C18 column (150 mm × 4.6 mm and 3.5 µm particle size). Two such columns were connected in series using a Merck Chromolith column coupler to replicate the related substances method by HPLC. The mobile phase A consisted of 20 mmol ammonium formate aqueous solution (pH 3.00±0.05 with dilute acetic acid). Mobile phase B consists of a mixture of 300 ml of 20 mmol ammonium formate buffer (pH 6.00±0.05 with dilute acetic acid), 200 ml of methanol and 500 ml acetonitrile. A stock solution of suspension (4 ml/10 ml v/v) was prepared by dissolving an appropriate amount in diluent dexamethasone composed of 0.02 M ammonium formate (pH 3.0) and methanol in 4:6 (v/v) ratio. The solution was degassed and filtered, through 0.22 mm filters under vacuum prior to introduction into the system. The linear gradient programme was optimized by the percentage of mobile phases as follows: Ttime/mobile phase-A: B (%): T0/55:45, T5/55:45, T15/45:55, T40/45:55, T60/20:80, T75/20:80, T80/0:100, T90/0:100, T91/55:45, T100/55:45. The flow rate was set at 0.6 ml min-1. Injection volume was 50 µl** and the column temperature was maintained at 40 °C. The analytes were detected in photo diode array detector at 240 nm with the sample temperature maintained at 5±1 °C.
High-resolution mass spectrometer conditions
The aforementioned UPLC system was coupled with a VION IMS Q-ToF mass spectrometer and used for HRMS analyses. Electrospray ionization (ESI) was performed in positive ionization mode. The capillary voltage was set to 3.0 V, and the source temperature was maintained at 140 °C. The desolvation temperature and gas flow were maintained at 400 °C and 800 l/h, respectively. Mass spectrometric data were acquired over m/z range of 50-2000.
Preparative HPLC conditions
Preparative chromatography was conducted using a Waters preparative HPLC equipped with MassLynx software, 2545 quaternary pump module, 2489 dual UV detector, and 2767 sample manager with auto-fraction collector. The separation of impurity products was performed using Waters C18 column (5 µm, 250 × 19 mm) at a flow rate of 2 ml/min. The mobile phase consisted of a mixture of ammonium formate (pH 6.0, adjusted with dilute acetic acid) and methanol. An optimized gradient elution program was employed to collect various fractions, which were then concentrated using liquid-liquid extraction. The purified fractions were lyophilized using a Lyofreeze lyophilizer and used for NMR analyses.
Nuclear magnetic resonance spectroscopy
The structures of the impurities were elucidated by NMR spectroscopy. 1H, 13C, and DEPT NMR spectra of dexamethasone and its impurities were recorded in DMSO-d6 using a Bruker Avance 600 MHz NMR instrument equipped with cryogenic probe. The probe temperature was regulated at 25 ℃. The 1H NMR spectra referenced to the singlet of tetramethylsilane (TMS) at 0 ppm, while 13C NMR spectra were referenced to the septet of DMSO-d6 at 39.5 ppm.
Infrared spectroscopy
Infrared spectra were recorded using a Perkin Elmer (model-Spectrum Two) equipped with Lab Solutions software to identify the functional groups present in the compounds. Potassium bromide was used as a medium to form sample pellets.
Sample preparation
A diluent consisting of 0.02 M ammonium formate (pH 3.0 adjusted with dilute acetic acid) and methanol (4:6 v/v) was used for sample preparation. 4 ml of tobramycin and dexamethasone ophthalmic suspension sample was dissolved in 6 ml of diluent and subjected to UPLC-HRMS analysis. A blank solution was injected prior to sample injection to ensure that no interference occurred during data processing.
RESULTS AND DISCUSSION
Method development and optimization
Multiple batches of dexamethasone ophthalmic suspension were analyzed using the LC-HRMS method. In brief, gradient elution was employed to achieve the separation of processed and degradation impurities [15]. Optimal resolution was obtained using an ACQUITY UPLC with XBridge C18 column (150 mm × 4.6 mm and particle size 3.5 µm) during method development. A series of experiments were conducted with gradient elution using mobile phases A and B, varying the column temperature between 20 and 50 °C. Successful separation of dexamethasone and its impurities was achieved with the column temperature was set to 40 °C. The optimized gradient program was as follows: Ttime/mobile phase-A: B (%): T0/55:45, T5/55:45, T15/45:55, T40/45:55, T60/20:80, T75/20:80, T80/0:100, T90/0:100, T91/55:45, T100/55:45. Under these conditions, the impurities A and B were observed at levels 0.39% and 0.22%, respectively. The structures of dexamethasone and its impurities are presented in fig. 1. The UPLC retention behaviour of the impurities is shown in fig. 2, with retention times of 27.00 min for Dexamethasone, 29.79 and 32.66 min for Imp-A and Imp-B, respectively. Dexamethasone ophthalmic suspension (0.1% w/v) is approved for topical administration at a frequency of 4 to 6 times per day [9-12]. According to FDA and ICH guidelines, topical drug products with concentrations exceeding 0.1% and less than 1% must have impurities identified if present at levels greater than 0.10% [13].
Structure elucidation of impurities
The structural identification of impurities was carried out using the UPLC coupled with HRMS method. The protonated mono-isotopic mass of the impurities were determined by analyzing the samples in ESI positive ion mode. The mass and NMR and IR spectroscopic data of pure dexamethasone is provided for comparative purposes. Impurities were isolated by preparative HPLC and characterized using LC-HRMS, NMR and IR [14, 16].
Structural elucidation of Imp-A
The MS/MS fragmentation spectrum of Imp-A is shown in fig. 3. The HR-MS spectrum in positive ion mode revealed an intense molecular ion peak at m/z = 379.1921 [M+H]+, corresponds to the theoretical value (m/z= 379.1843 [M+H]+), confirming the empirical formula of the Imp-A as C21H27FO5 with a mass error of 1.6 ppm (table 1). The fragmentation patterns indicated the loss of an HF molecule, producing a fragment at m/z 359.1870, followed by the loss of a water molecules, resulting in fragments at m/z 341.1768, 323.1693. These data suggest the presence of a fluorine atom at C9 and position of hydroxyl (-OH) groups at C11 and C17 (fig. 3). A subsequent loss of formic acid, yielding m/z 295.1740 suggests the presence of both-COOH and-OH groups at C17. However, the –COOH group was absent at C17 of dexamethasone [18].
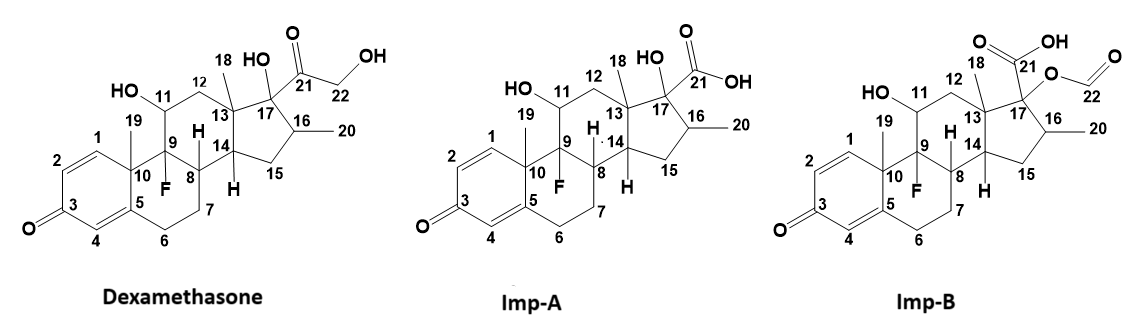
Fig. 1: Structures of dexamethasone, Imp-A and Imp-B
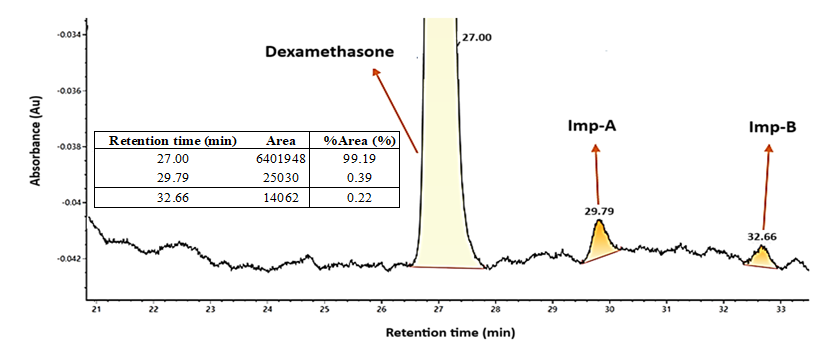
Fig. 2: UPLC chromatogram of dexamethasone and its impurities
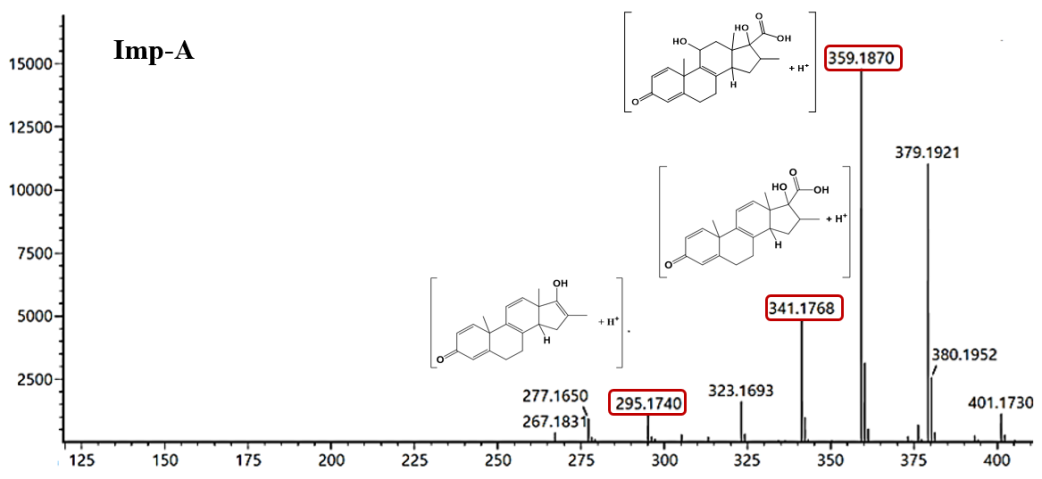
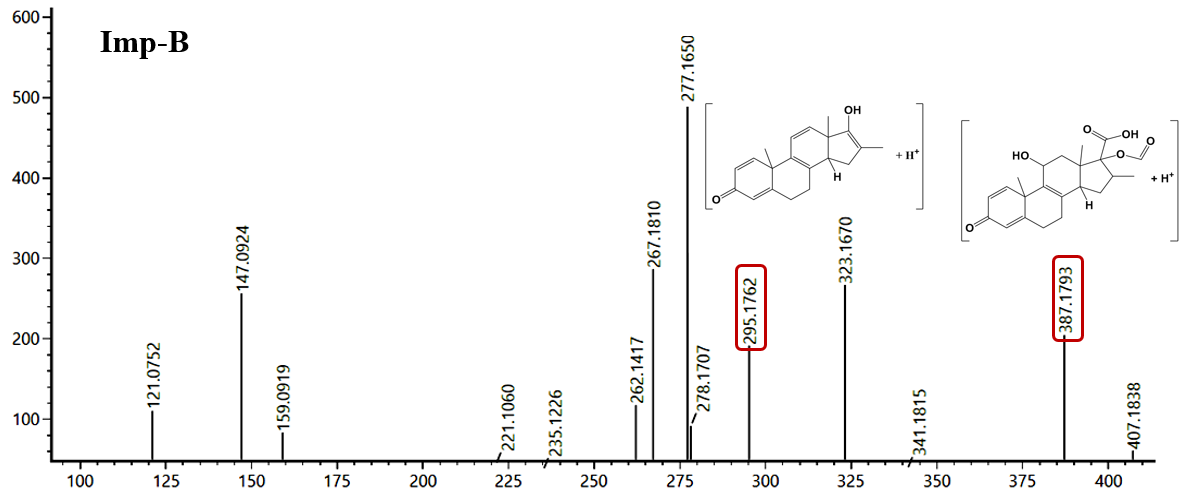
Fig. 3: Representative HRMS/MS chromatogram of Imp-A and Imp-B
Table 1: Accurate mass, elemental composition and error (ppm) for Imp-A and Imp-B
| Name | Theoretical mass (Da) | Theoretical elemental composition | Theoretical mass [M+H]+ | Observed mass [M+H]+ | Observed elemental composition | Error (ppm) |
| Imp-A | 378.1843 | C21H27FO5 | 379.1915 | 379.1921 | C21H28FO5 | 1.6 |
| Imp-B | 406.1792 | C22H27FO6 | 407.1864 | 407.1838 | C22H28FO6 | -6.4 |
The IR spectrum of the Imp-A displayed a stretching band at 3534.77 cm-1, corresponds to the presence of OH group. Strong stretching bands at 1689.06 cm-1 and 1655.52 cm-1are consistent with carbonyl (C=O) groups, possibly originating from both the ring structure and the acid group, respectively. Additional strong stretching bands observed at 2946.23 cm-1 and 1596.21 cm-1 corresponds to SP3 C-H, and C=C conjugation within the ring structure, respectively. For comparative studies, overlay spectrum of Imp-A and Imp-B with dexamethasone is illustrated in fig. 4.
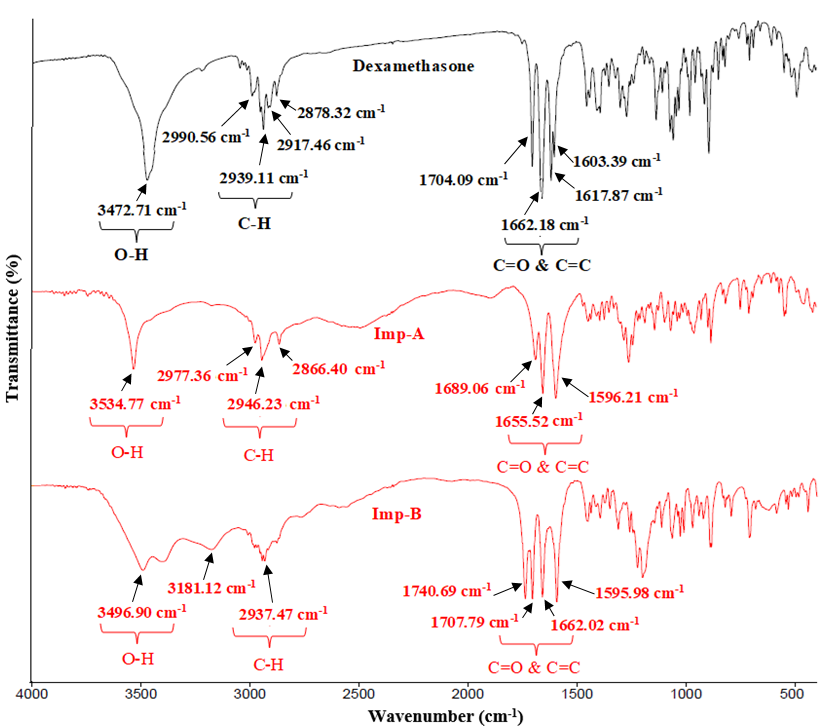
Fig. 4: Overlay of the IR spectrum of dexamethasone, Imp-A and Imp-B
The NMR (1H, 13C and DEPT-135) spectroscopic results of the Imp-A (fig. 5 and table 2) provided necessary information about its molecular structure. The 1H NMR spectrum of Imp-A (fig. 5a) revealed a relatively complex pattern, with three signals of equal intensity in the downfield region (δ 6-7.3 ppm). A doublet at δ 7.291 ppm was assigned to the proton at C1, while a doublet at δ 6.212 ppm was attributed to the proton at C2 and a singlet at δ 5.997 ppm was assigned to the proton at C4. Additionally, large singlets at δ 1.489 ppm and δ 0.998 ppm were assigned to methyl groups at C19 and C18, respectively and while a doublet at δ 0.850 was assigned to methyl group at C20. The positions and splitting patterns observed in the spectrum suggests the presence of steroid ring, a common structural feature in dexamethasone [14, 26].
The 13C NMR spectrum (fig. 5b) of the Imp-A displays 21 distinct carbon resonances. The most downfield peaks at δ 185.29 ppm and 174.79 ppm, corresponds to the carbonyl carbon at C3 and carboxylic acid at C21, respectively. Out of 21 observed peaks, seven peaks disappeared in DEPT-135 technique. The positive signals observed in DEPT-135 spectra at chemical shift of (δ) 152.91 ppm, 128.96 ppm, 124.13 ppm, 70.89 ppm, 42.44 ppm, 35.18 ppm, 33.73 ppm, 22.90 ppm, 16.90 ppm and 15.39 ppm remain unchanged (fig. 5c). The key features in the DEPT-135 data include inverted signals at δ 35.54 ppm, 32.16 ppm 30.30 ppm and 27.26 ppm, which originate from CH2 groups. The disappearance of the signals at185.29 ppm, 174.79 ppm, 167.15 ppm, 101.38 ppm, 85.49 ppm, 48.02 ppm and 47.27 ppm peaks indicate quaternary carbons. Signals at δ 152.91 ppm, 128.96 ppm, 124.13 ppm, 70.89 ppm, 42.44 ppm, 35.18 ppm, 33.73 ppm originate from CH groups, while the remaining signals at δ 16.90 ppm, 22.90 ppm, and 15.39 ppm arise from CH3. Four doublet signals at 101.38 ppm (1JCF=174.3), 48.02 ppm (2JCF=22.78), 70.89 ppm (2JCF =37.24) and δ 33.73 ppm (2JCF = 19.2) indicate the presence of fluorine atom at C9.
2D 1H-13C HSQC (Heteronuclear Single Quantum Coherence) spectrum of Imp-A (fig. 6a and 6b) revealed the correlations from H1, H2, H4, H6, H7, H8, H11, H12, H14, H15, H16, H18, H19 and H20 with their respective carbon atoms (C1, C2, C4, C6, C7, C8, C11, C12, C14, C15, C16, C18, C19 and C20) confirming the presence of dexamethasone structure in the impurity. Furthermore, 1H-13C HMBC NMR spectrum for Imp-A (fig. 7a and 7b) revealed that the H1 proton is coupled to C3, C5 and C19, supporting the presence of a conjugated steroidal ring system. Additionally, the HMBC spectrum showed OH11’ correlating with C9 and OH17’ correlating with C13, confirming the presence of hydroxyl groups on C11 and C17. Other notable correlations observed in Imp-A include H6 to C4, H7 to C5 and C9, H19 to C9, H1 to C9 and C18, H20 to C15 and C17. The combined NMR, MS and IR data provide conclusive evidence for the structural assignments of Imp-A, as illustrated in fig. 1.
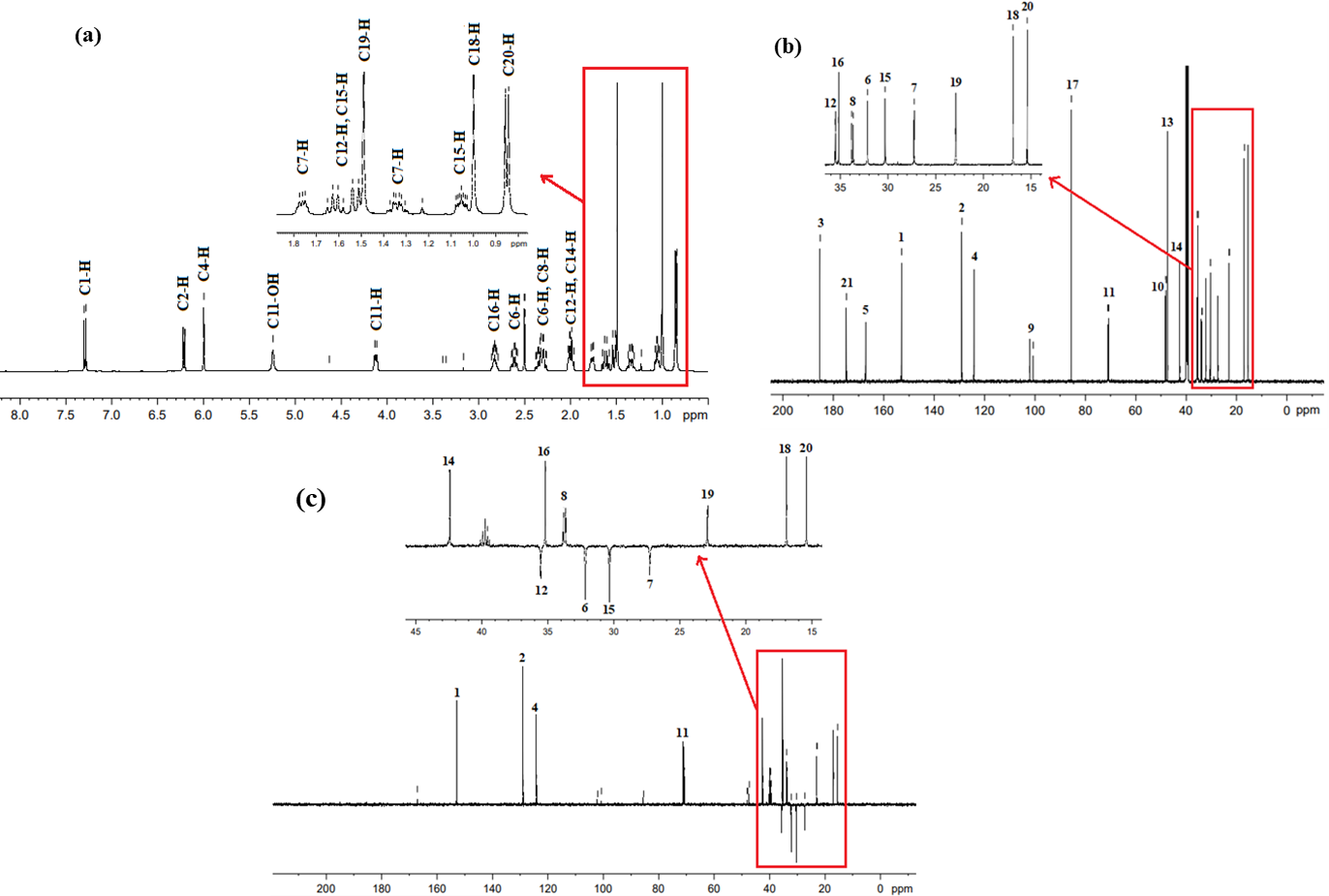
Fig. 5: (a) 1H NMR, (b) 13C NMR, and (c) DEPT-135 spectra of Imp-A recorded in DMSO-d6 at room temperature
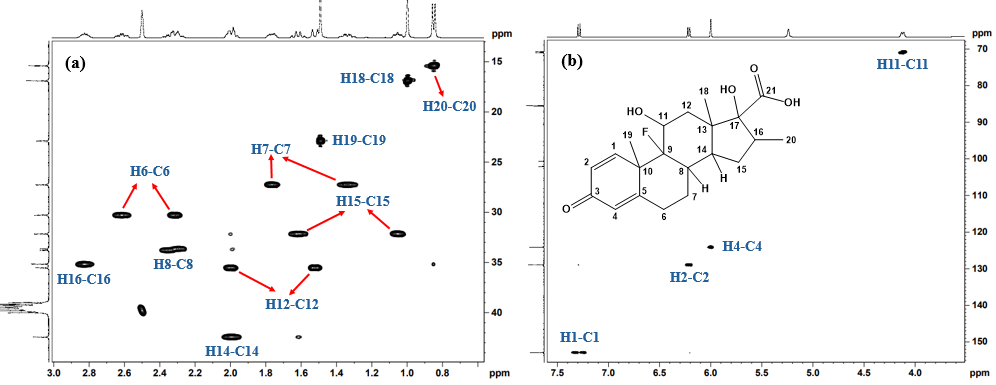
Fig. 6: 1H-13C HSQC NMR spectra of Imp-A in DMSO-d6 at room temperature, showing the chemical shift range (a) 13C: 10-50 ppm; 1H: 0.6-3.0 ppm, (b) 13C: 70-155 ppm; 1H: 3.5-7.5 ppm
Structural elucidation of Imp-B
The MS/MS fragmentation spectrum of Imp-B is presented in the fig. 3. The HRMS/MS spectrum in the positive ion mode revealed a prominent molecular ion peak at m/z = 407.1838 [M+H]+, which corresponds to the theoretical m/z value of 407.1792 [M+H]+, confirming the empirical formula of Imp-B as C22H27FO6 with error-6.4 ppm (table 1). The fragmentation pattern shows the loss of HF molecule, yielding a fragment at m/z 387.1793, followed by the loss of formic acid to produce m/z 341.1815 and subsequent loss of a water molecule, forming the fragments at m/z 323.1670. These observations suggest the presence of a fluorine atom at C9, formyl at C17 (-OCHO) and a hydroxyl group at C11 (fig. 3). The other possibility of forming fragment m/z 341.1815 by losing formic acid from m/z 387.1793 apparently shows the presence of carboxyl (–COOH) group on C17 along with formyl (–HCHO) group, while dexamethasone contains hydroxyl (-OH) and hydroxymethyl carbonyl (-COCH₂OH) groups at C17 [18].
The IR spectrum of the Imp-B showed a stretching band at 3496.90 cm-1, indicative of an OH group. Strong bands at 1740.69 cm-1, 1707.79 cm-1, and 1662.02 cm-1are consistent of carbonyl (C=O) groups, likely originating from aldehyde, cyclic, and carboxylic acid functional groups, respectively. Additionally, strong stretching band at 2937.47 cm-1 and 1595.98 cm-1 corresponds to SP3 C-H and C=C conjugation within the ring [14]. For comparative studies, overlay spectrum of Imp-A and Imp-B with dexamethasone is illustrated in fig. 4.
The NMR (1H, 13C and DEPT-135) spectroscopic results of the Imp-B (fig. 8 and table 2) provided necessary information about its molecular structure. The 1H NMR spectrum of the Imp-B (fig. 8a) provides preliminary chemical shift assignments. Three signals of equal intensity appear in the downfield region (δ 6-7.3 ppm). A doublet at δ 7.279 ppm is assigned to the proton at C1, while a doublet at δ 6.223 ppm attributed to the proton at C2, and a singlet at δ 6.011 ppm corresponds to the proton at C4. Large singlets at δ 1.490 ppm and δ 0.996 ppm are assigned to methyl groups at C19 and C18, respectively, while a doublet at δ 0.844 ppm is assigned to methyl group at C20. These positions and splitting patterns suggest the presence of a steroid ring, a common structural motif in dexamethasone [14, 26].
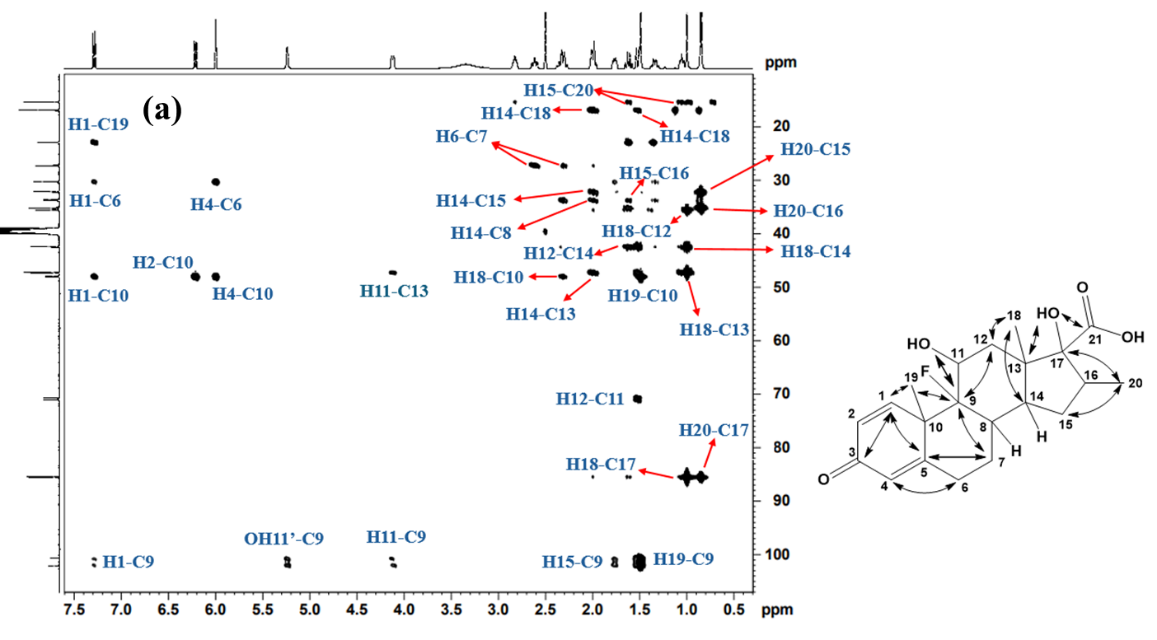
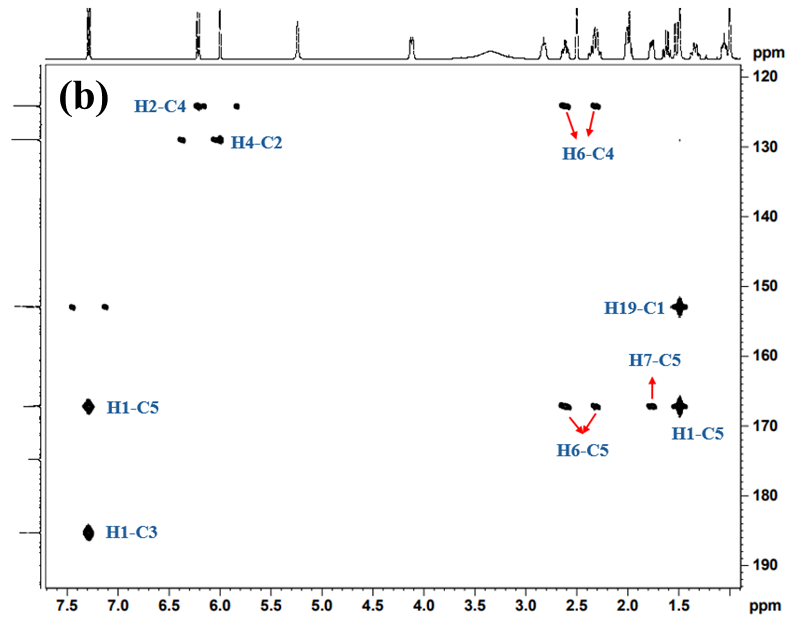
Fig. 7: 1H-13C HMBC NMR Spectrum of Imp-A recorded in DMSO-d6 at room temperature showing chemical shift range (a) 13C: 10-110 ppm; 1H: 0.4-7.5 ppm, (b) 13C: 120-190 ppm; 1H: 0.9-7.5 ppm
The 13C NMR (fig. 8b) of the Imp-B shows 22 distinct carbon resonances. The most downfield peaks at δ 185.27, 169.76 ppm, and 160.87 ppm corresponds to the carbonyl carbons at C3, C21 (carboxylic acid) and C22 (carbonyl), respectively. Out of 22 peaks, seven peaks disappeared in DEPT-135 spectrum, while peaks at δ 160.87, 152.67, 129.04, 124.20, 70.57, 42.88, 35.46, 35.38, 35.42, 33.66, 32.96, 30.18, 22.90, 27.18, 16.83, and 16.37 ppm remain unchanged (fig. 8c). Key features of the DEPT-135 data include inverted signals at δ 35.46 ppm, 32.96 ppm, 30.18 ppm, and 27.18 ppm corresponding to CH2 group, and the disappearance of the signals at δ 185.27 ppm, 169.76 ppm peaks indicate the presence of carbonyl carbons. Additionally, the absence of the signals at δ 166.86 ppm, 101.13 ppm, 92.09 ppm, 47.88 ppm, and 47.65 ppm suggest quaternary carbons. The remaining signals originate from CH groups, while peaks at δ 35.46, 32.96, and 30.18 ppm are assigned to CH₃ groups. Four doublet signals at 101.13 ppm with 1JCF=174.3, 47.88 ppm with 2JCF=22.8, 70.57 ppm with 2JCF =37.3andδ 33.66 ppm with 2JCF = 10.5 confirm the presence of the fluorine atom at C9.
The 1H-13C HSQC spectrum of Imp-B (fig. 9a and 9b) revealed correlationsbetweenH1, H2, H4, H6, H7, H8, H11, H12, H14, H15, H16, H18, H19 and H20 with their respective carbon atoms C1, C2, C4, C6, C7, C8, C11, C12, C14, C15, C16, C18, C19 and C20, confirming the presence of dexamethasone structure in the impurity. Furthermore, the 1H-13C HMBC NMR spectrum for Imp-B (fig. 10a and 10b) revealed that the H1 proton is coupled to C3, C5, and C19, while H2 is coupled to C4, C10, supporting the presence of steroidal ring. HMBC correlations between OH11’ and C9 confirm the presence of hydroxyl group on C11. Additional HMBC correlations observed in Imp-B include H6 to C4, H7 to C5 and C9, H12 to C9 and C18, H20 to C15 and H17. These findings further support the proposed structure of Imp-B. The combined NMR, MS and IR data provide conclusive evidence for the structural assignments of Imp-B, as illustrated in fig. 1.
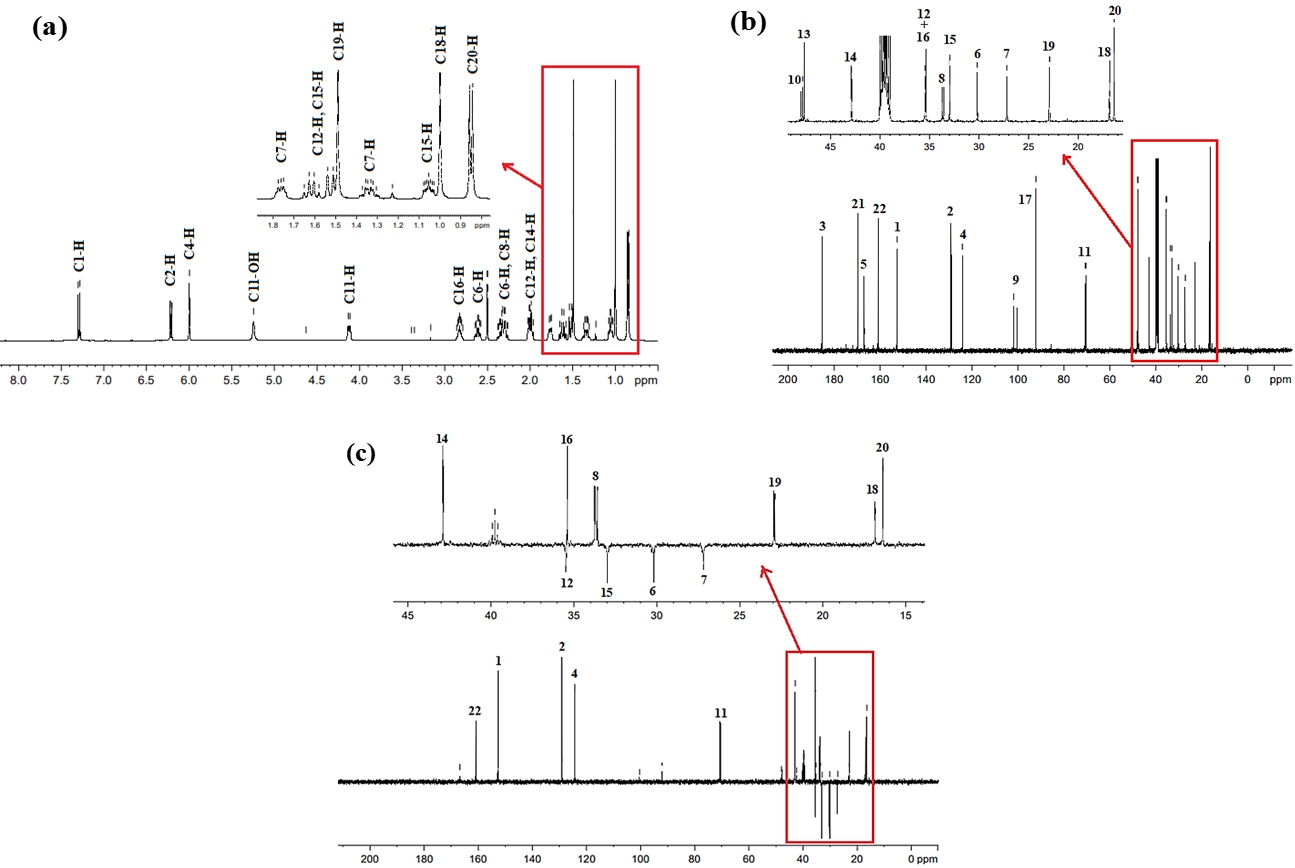
Fig. 8: (a) 1H NMR, (b) 13C NMR, and (c) DEPT-135 spectra of Imp-B recorded in DMSO-d6 at room temperature
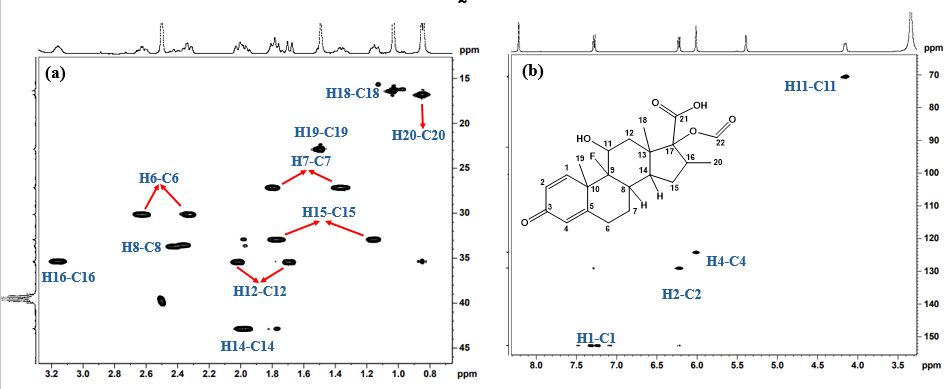
Fig. 9: 1H-13C HSQC NMR Spectra of Imp-B recorded in DMSO-d6 at room temperature, showing chemical shift range(a)13C: 13-46 ppm; 1H: 0.6-3.2 ppm, (b) 13C: 65-155 ppm; 1H: 3.2-8.4 ppm
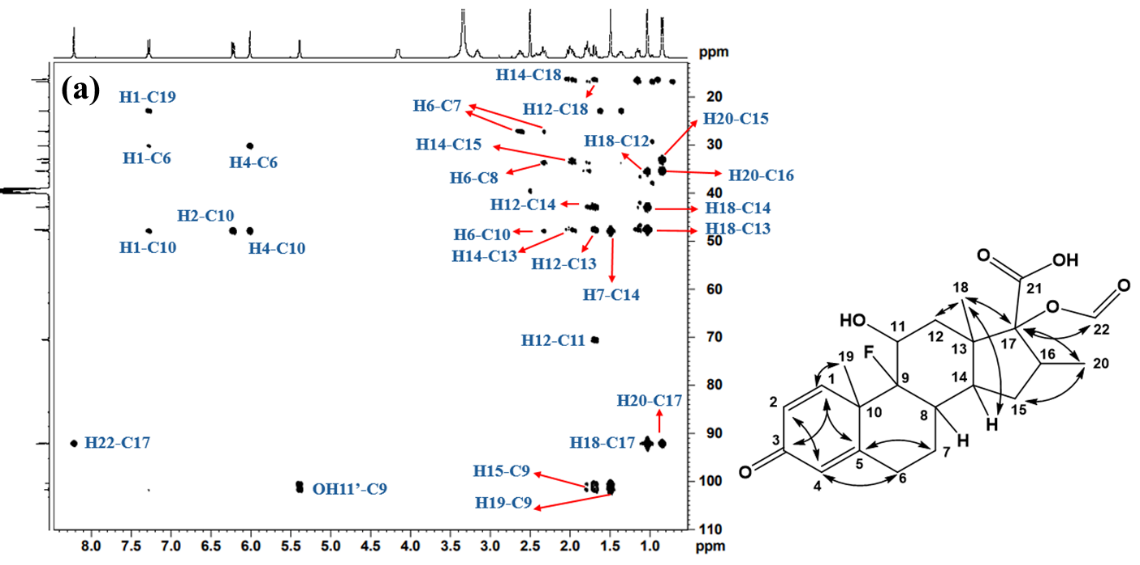
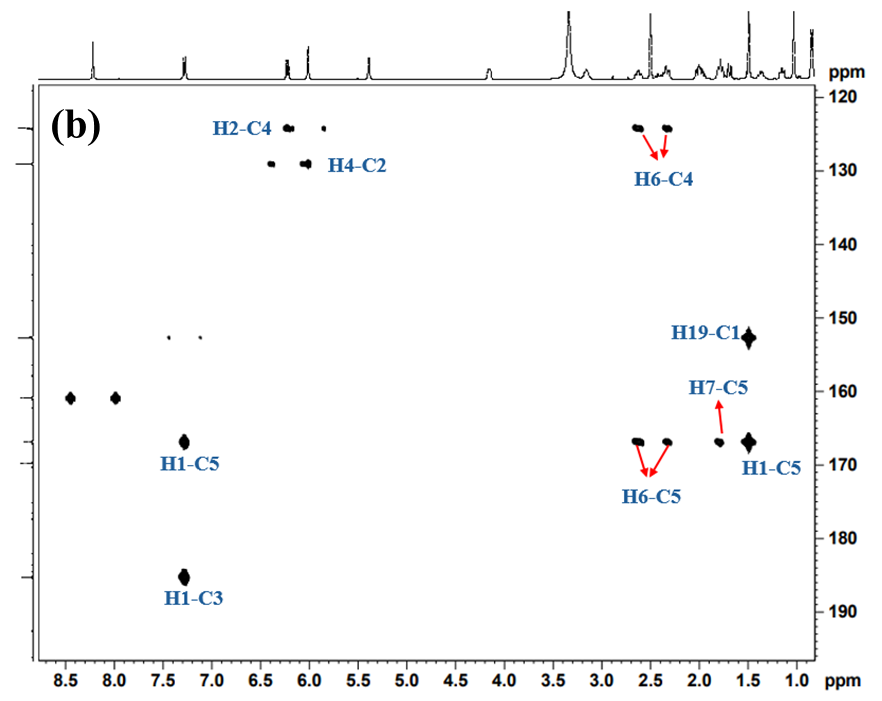
Fig. 10: 1H-13C HMBC NMR spectrum of Imp-B recorded in DMSO-d6 at room temperature showing chemical shift range (a) 13C: 10-110 ppm; 1H: 0.5-8.5 ppm, (b) 13C: 120-195 ppm; 1H 0.8-8.7 ppm
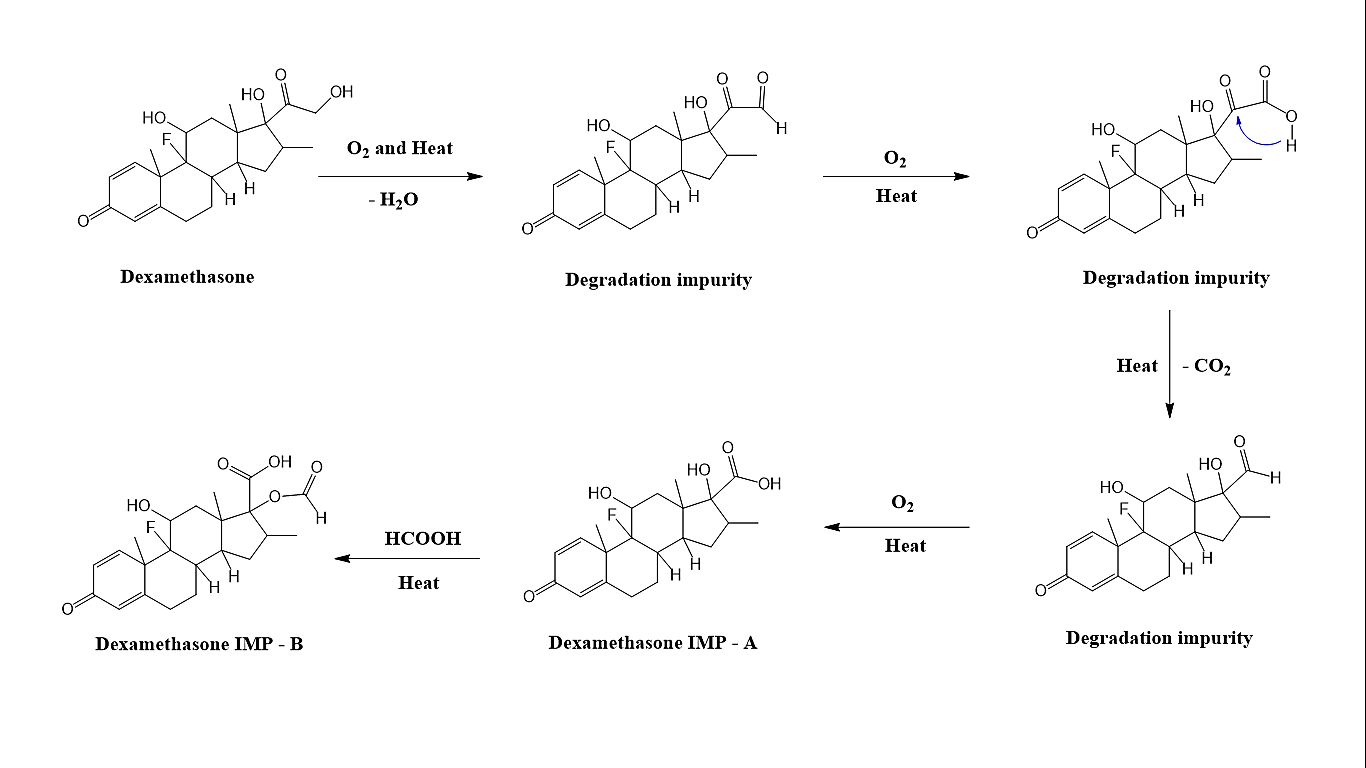
Fig. 11: Proposed pathway for impurities formation under accelerated conditions
Force degradation studies of analytes
Forced degradation studies were conducted to evaluate the stability of dexamethasone sample under thermal (50 °C) and oxidation (10% H2O2) conditions. For oxidative degradation, 1 ml of 10% hydrogen peroxide (H₂O₂) was added to 1 ml of the dexamethasone sample solution and incubated at 60 °C for 30 min. Thermal degradation was examined by exposing the dexamethasone sample solution to 60 °C for twelve hours. After exposing sample solution to thermal and oxidation conditions, 50 µl** of sample solution was injected into the LC-HRMS system for chromatographic purity and mass analysis. Under these stress conditions, impurity levels at 240 nm were found to be 0.45% (RRT 1.1) and ~0.56% (RRT 1.2), whereas no impurities were detected in the initial dexamethasone sample. HRMS analysis confirmed these impurities as Imp-A and Imp-B. These results demonstrate that thermal and oxidative conditions induce the formation of these impurities in the dexamethasone sample. All the experiments were performed in triplicate. We have presented the proposed pathway for impurity formation under accelerated conditions (fig. 11).
Table 2: 1H, 13C NMR and DEPT-135 signal assignments for dexamethasone, Imp-A, Imp-B
| Position | Dexamethasone API | Imp-A | Imp-B | DEP-135 | |||
| 1H (δ, ppm) | 13C (δ, ppm) | 1H (δ, ppm) | 13C (δ, ppm) | 1H (δ, ppm) | 13C (δ, ppm) | ||
| 1 | 7.287 (d, J=10.0) | 152.78 | 7.291 (d, J= 10) | 152.91 | 7.279 (d, J=10.2) | 152.67 | -CH |
| 2 | 6.218 (dd, J=1.8, 10.0) | 128.98 | 6.212 (dd, J=1.7, 10.0) | 128.96 | 6.223 (dd, J=1.7, 10.2) | 129.04 | -CH |
| 3 | - | 185.26 | - | 185.29 | - | 185.27 | -C |
| 4 | 6.004 (s) | 124.11 | 5.997 (s) | 124.13 | 6.011 (s) | 124.20 | -CH |
| 5 | - | 167.04 | - | 167.15 | - | 166.86 | -C |
| 6 | 2.334 (m), 2.614 (m) | 30.26 | 2.330 (m), 2.612 m) | 30.30 | 2.326 (m), 2.634 (m) | 30.18 | -CH2 |
| 7 | 1.344 (m), 1.764 (m) | 27.25 | 1.339 (m), 1.764 (m) | 27.26 | 1.362 (m), 1.780 (m) | 27.18 | -CH2 |
| 8 | 2.300 (m) | 33.62 (d), 2JCF = 19.4 |
2.294 (m) | 33.73 (d, 2JCF =19.2) |
2.339 (m) | 33.66 (d,2JCF =10.5) |
-CH |
| 9 | - | 101.21 (d), 1JCF = 175.3 |
- | 101.38 (d, 1JCF =174.3) |
- | 101.13 (d,1JCF=174.3) |
-C |
| 10 | - | 47.93 (d), 2JCF =23.0 |
- | 48.02 (d, 2JCF =22.78) |
- | 47.88 (d, 2JCF=22.8) |
-C |
| 11 | 4.136 (m) | 70.69 (d), 2JCF = 36.83 |
4.124 (m) | 70.89 (d, 2JCF =37.24) |
4.16 (m) | 70.57 (d, 2JC=37.3) |
-CH |
| 11 (OH) | 5.280 (m) | - | 5.242 (m) | - | 5.389 (m) | - | - |
| 12 | 1.419 (m), 2.112 (m) | 35.80 | 1.525 (m), 1.996 (m) | 35.54 | 1.686 (m), 1.997 (m) | 35.46 | -CH2 |
| 13 | - | 47.43 | - | 47.27 | - | 47.65 | -C |
| 14 | 2.112 (m) | 43.25 | 2.001 | 42.44 | 1.996 (m) | 42.88 | -CH |
| 15 | 1.064 (m), 1.619 (m) | 31.99 | 1.053 (m), 1.616 (m) | 32.16 | 1.062 (m), 1.616 (m) | 32.96 | -CH2 |
| 16 | 2.942 (m) | 34.87 | 2.826 (m) | 35.18 | 3.158 (m) | 35.38 | -CH |
| 17 | - | 90.13 | - | 85.49 | - | 92.09 | -C |
| 17 (OH) | 4.951 | - | 4.630 | - | - | - | - |
| 18 | 0.861 (s) | 16.64 | 0.998 (s) | 16.90 | 0.996 (s) | 16.83 | -CH3 |
| 19 | 1.481 (s) | 22.89 (d, J=5.5) | 1.489 (s) | 22.90 (d, J=5.4) | 1.490 (s) | 22.90 (d, J=5.5) | -CH3 |
| 20 | 0.779 (d, J=7.2) | 15.28 | 0.850 (d, J=7.2) | 15.39 | 0.844 (d, J=7.0) | 16.37 | -CH3 |
| 21 | - | 211.13 | - | - | - | - | -C |
| 21 | - | - | 12.387 | 174.79 | 12.790 | 169.76 | -C |
| 22 | - | - | - | - | 8.217 (s) | 160.87 | -CHO |
| 22 | 4.082 (dd, J=5.9, 19.2 4.462 (dd, J=5.9, 19.2) |
66.25 | - | - | - | - | - |
| 22 (OH) | 4.680 (t, J=5.9) | - | - | - | - | - | - |
*s=singlet, d=doublet, t=triplet, dd=doublet of doublet, m=multiplet; Coupling constant J in Hz; DEPT=distortionless enhancement by polarization transfer.
CONCLUSION
In conclusion, two previously unidentified impurities, designated as Imp-A and Imp-B, were detected and isolated during the stability studies of dexamethasone ophthalmic suspension. These impurities were characterized using UPLC-HRMS. Based on the acquired m/z data, fragmentation patterns, and insights into the formulation process, it is believed that the impurities originated from degradation. The molecular structures of Imp-A and Imp-B were further elucidated through ¹H NMR, ¹³C NMR, IR and mass spectrometry.
ACKNOWLEDGEMENT
The authors would like to acknowledge the management of Dr. Reddy’s Laboratories Ltd, IPDO, Hyderabad, for providing laboratory and analytical instrumentation facilities. The manuscript has been assigned the registration number PUB00715-24.
FUNDING
Nil
AUTHORS CONTRIBUTIONS
All authors have approved the final version of the manuscript. BK: Designing the study and Data collection); RM: Data analysis, Writing-Original draft preparation, Writing-Revised and Editing, Supervision; SG: Editing; DVR: Formal analysis, HPLC facility and Resources; LA: NMR facility.
CONFLICT OF INTERESTS
All authors declare that there is no conflict of interest regarding the publication of this article.
REFERENCES
Gorog S. Critical review of reports on impurity and degradation product profiling in the last decade. TrAC Trends Anal Chem. 2018 Apr;101:2-16. doi: 10.1016/j.trac.2017.09.012.
Dispas A, Avohou HT, Lebrun P, Hubert P, Hubert C. Quality by design approach for the analysis of impurities in pharmaceutical drug products and drug substances. TrAC Trends Anal Chem. 2018 Apr 1;101:24-33. doi: 10.1016/j.trac.2017.10.028.
Himabindu G, Reddy YS, Prasad AV, Ramadas C, Sharma HK. New stability indicating RP-HPLC methods for the determination of related substances and assay of trametinib acetic acid: a mass balance approach. Anal Sci. 2024 Nov;40(11):2005-15. doi: 10.1007/s44211-024-00633-4, PMID 39080194.
Brunton LL, Knollmann BC. Goodman and gilmans: the pharmacological basis of therapeutics. 14th ed; 2022.
Schepers A, Pires NM, Eefting D, De Vries MR, Van Bockel JH, Quax PH. Short-term dexamethasone treatment inhibits vein graft thickening in hypercholesterolemic ApoE3Leiden transgenic mice. J Vasc Surg. 2006 Apr;43(4):809-15. doi: 10.1016/j.jvs.2005.11.019, PMID 16616241.
Muller DW, Golomb G, Gordon D, Levy RJ. Site-specific dexamethasone delivery for the prevention of neointimal thickening after vascular stent implantation. Coron Artery Dis. 1994 May;5(5):435-42. doi: 10.1097/00019501-199405000-00010, PMID 7921375.
Strecker EP, Gabelmann A, Boos I, Lucas C, Xu Z, Haberstroh J. Effect on intimal hyperplasia of dexamethasone released from coated metal stents compared with non-coated stents in canine femoral arteries. Cardiovasc Intervent Radiol. 1998 Nov-Dec;21(6):487-96. doi: 10.1007/s002709900309, PMID 9853167.
Hoffmann R, Langenberg R, Radke P, Franke A, Blindt R, Ortlepp J. Evaluation of a high-dose dexamethasone-eluting stent. Am J Cardiol. 2004 Jul 15;94(2):193-5. doi: 10.1016/j.amjcard.2004.03.061, PMID 15246899.
Owji N, Khalili MR, Khademi B, Shirvani M, Sadati MS. Comparison of the effectiveness of onion extract topical steroid and petrolatum emollient in cosmetic appearance of upper blepharoplasty scar. J Curr Ophthalmol. 2020 Dec 12;32(4):408-13. doi: 10.4103/JOCO.JOCO_39_20, PMID 33553845.
Rhee SS, Mah FS. Comparison of tobramycin 0.3%/dexamethasone 0.1% and tobramycin 0.3%/loteprednol 0.5% in the management of blepharo-keratoconjunctivitis. Adv Ther. 2007 Jan-Feb;24(1):60-7. doi: 10.1007/BF02849993, PMID 17526462.
Notivol R, Amin D, Whitling A, Wells D, Kennedy M, Cockrum PC. Prophylactic effectiveness of tobramycin dexamethasone eye drops compared with tobramycin/vehicle eye drops in controlling post-surgical inflammation in cataract patients: prospective randomised double masked two arm parallel group placebo-controlled multicentre study. Clin Drug Investig. 2004;24(9):523-33. doi: 10.2165/00044011-200424090-00003, PMID 17523714.
Notivol R, Bertin D, Amin D, Whitling A, Kennedy M, Cockrum PC. Comparison of topical tobramycin dexamethasone with dexamethasone neomycin polymyxin and neomycin polymyxin gramicidin for control of inflammation after cataract surgery: results of a multicenter prospective three-arm randomized double masked controlled parallel group study. Clin Ther. 2004;26(8):1274-85. doi: 10.1016/s0149-2918(04)80113-9, PMID 15476908.
Impurities in new drug substances International Conference on harmonization (ICH). 2006;Q3A:R2. Available from: https://database.ich.org/sites/default/files/Q3A%28R2%29%20Guideline.pdf.
Ummiti K, Vakkala S, Panuganti V, Annarapu MR. Isolation identification and characterization of 17-oxo dexamethasone an oxidative degradation impurity of dexamethasone using flash chromatography and NMR/HRMS/IR. J Liq Chromatogr Relat Technol. 2014 May;37(17):2403-19. doi: 10.1080/10826076.2013.836712.
Chen Q, Zielinski D, Chen J, Koski A, Werst D, Nowak S. A validated stability indicating HPLC method for the determination of dexamethasone related substances on dexamethasone coated drug eluting stents. J Pharm Biomed Anal. 2008 Nov 4;48(3):732-8. doi: 10.1016/j.jpba.2008.07.010, PMID 18722070.
Surukonti SR, Manabolu Surya SB, Katari NK, Yerla RR. Investigating betrixaban maleate drug degradation profiles isolation and characterization of unknown degradation products by mass-triggered preparative HPLC HRMS and NMR. J Pharm Biomed Anal. 2023 Oct 25;235:115643. doi: 10.1016/j.jpba.2023.115643, PMID 37633165.
Ketha NV, Kolli D, Subbappa PK. Structural elucidation of novel degradation impurity and development validation of a single HPLC method for all putative impurities of clobetasol propionate in a foam drug product. J Chromatogr Sci. 2024 May 31;62(5):444-53. doi: 10.1093/chromsci/bmad016, PMID 36857571.
Karatt TK, Nalakath J, Perwad Z, Albert PH, Abdul Khader KK, Syed Ali Padusha M. Mass spectrometric method for distinguishing isomers of dexamethasone via fragment mass ratio: an HRMS approach. J Mass Spectrom. 2018 Nov;53(11):1046-58. doi: 10.1002/jms.4279, PMID 30098588.
Arvaniti OS, Ioannidi AΑ, Politi A, Miserli K, Konstantinou I, Mantzavinos D. Dexamethasone degradation in aqueous medium by a thermally activated persulfate system: kinetics and transformation products. J Water Process Eng. 2022 Oct;49:103134. doi: 10.1016/j.jwpe.2022.103134.
Sudha D, Malarkodi R, Gokulakrishnan A, Liyakath Ali AR. LC-MS/MS and GC-MS profiling and the antioxidant activity of Carissa carandas Linn. Int J Pharm Pharm Sci. 2024 Jun;16(6):39-45. doi: 10.22159/ijpps.2024v16i6.50818.
Patole S, Gosar A, Shaikh T. Impurities characterization in pharmaceuticals: a review article. Int J Pharm Pharm Res. 2019 Jul;15(4):46-64.
Rathore MK, Mohan Reddy TR. Tandem mass spectrometric method for the trace level determination of 2-aminopyridine: a potential genotoxic impurity in tenoxicam API. Int J Pharm Pharm Sci. 2024 Apr;16(4):50-6. doi: 10.22159/ijpps.2024v16i4.49902.
Rajagopaludu P, Saritha N, ND, Maddi S. Method development and validation of erythromycin and olaparib in human plasma by liquid chromatography tandem mass spectrometry. Asian J Pharm Clin Res. 2022;15(4):28-33. doi: 10.22159/ajpcr.2022.v15i4.44044.
Bodke SS, Bhangale CJ, Bhandare SN. Stability indicating UPLC method for estimation of benazepril and hydrochlorothiazide in bulk and combined dosage form. Int J Pharm Pharm Sci. 2024 Jan;16(1):22-9. doi: 10.22159/ijpps.2024v16i1.49457.
Matter B, Ghaffari A, Bourne D, Wang Y, Choi S, Kompella UB. Dexamethasone degradation in aqueous medium and implications for correction of in vitro release from sustained release delivery systems. AAPS PharmSciTech. 2019 Oct 23;20(8):320. doi: 10.1208/s12249-019-1508-7, PMID 31646399.
Dembitsky VM. Biological activity and structural diversity of steroids containing aromatic rings, phosphate groups or halogen atoms. Molecules. 2023 Jul 20;28(14):5549. doi: 10.3390/molecules28145549, PMID 37513423.