
Int J App Pharm, Vol 17, Issue 4, 2025, 337-343Original Article
IN VIVO PHARMACOKINETICS STUDY OF DICLOFENAC SODIUM LOADED BASELLA ALBA MUCILAGE BASED CARRIER FOR ENHANCEMENT OF ORAL BIOAVAILABILITY
MOUMITA CHOWDHURY1, PINTU KUMAR DE2*
1Guru Nanak Institute of Pharmaceutical Science and Technology, 157/F Nilgunj Road, Panihati, Sodepur, Kolkata-114, West Bengal, India. 2Department of Pharmaceutical Technology, JIS University 81, Nilgunj Road, Agarpara, Kolkata-700109, West Bengal, India
*Corresponding author: Pintu Kumar De; *Email: pintu.de@jisuniversity.ac.in
Received: 31 Jan 2025, Revised and Accepted: 07 May 2025
ABSTRACT
Objective: The present study aims to evaluate the in vivo bioavailability and pharmacokinetics of diclofenac sodium entrapped in a natural polysaccharide, Basella alba mucilage-based carrier.
Methods: In this study, mucilage from Basella alba was chemically modified to improve its physicochemical properties and evaluated for its extent of modification by elemental analysis. The Diclofenac sodium-loaded sustained-release hydrogel bead developed from tailored Basella alba mucilage was evaluated for particle size, drug entrapment and Scanning electron microscopy. The resulting beads were used to assess the in vivo pharmacokinetics and bioavailability study. The pharmacokinetics of diclofenac sodium and diclofenac sodium loaded formulation were studied and compared with a marketed formulation of Diclofenac sodium (Subsyde®-CR DRCM capsule) at an equivalent dose in three groups of Wistar albino rats containing six rats in each group. Blood samples were collected via retro-orbital puncture for a period of 24 h. The diclofenac sodium concentration in a plasma sample of rats was detected by HPLC and the pharmacokinetic parameters were analyzed by noncompartmental modeling using PK solver.
Results: The elemental analysis and degree of substitution confirmed the chemical modification of Basella alba mucilage. The Basella alba mucilage-based beads showed uniform surface with drug entrapment efficiency of 71.56%±0.85. The pharmacokinetic parameters of raw drug and test formulation showed significant difference (p<0.01), whereas test and marketed formulation appeared similar without any significant difference. The results showed higher mean residence time, increased tmax, and decreased Cmax of drug-loaded beads and marketed formulation than the pure drug. The relative bioavailability of Diclofenac sodium increased from 54.50% to 96.88% by entrapping it in Basella alba mucilage-based bead.
Conclusion: Thus, the present work unfolded the potential of Basella alba mucilage as a bioavailability enhancer, advocating its use in developing novel pharmaceutical formulations.
Keywords: Pharmacokinetics, Diclofenac sodium, Basella alba, Bioavailability, Marketed formulation
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijap.2025v17i4.53926 Journal homepage: https://innovareacademics.in/journals/index.php/ijap
INTRODUCTION
The oral route of drug administration is considered the most common and acceptable route of drug delivery as compared to other routes despite its poor bioavailability. Diclofenac sodium is broadly recognized as a Nonsteroidal Anti-Inflammatory Drug (NSAID) with potent analgesic and anti-inflammatory activity [1]. It is completely absorbed orally but is affected by first-pass metabolism, resulting in half of the drug reaching systemic circulation [2]. However, it exhibits a short half-life with rapid elimination, alleviating the need for extended-release formulation [3]. Apart from this, the unfavorable characteristics of the drug, like low bioavailability, poor pharmacokinetics, and untimely degradation, demand new frontiers in the arena of NSAIDs. However, only a few limited research activities have been done to improve the oral bioavailability of drugs to achieve better therapeutic response, especially for drugs irritating the gastric mucosa.
In a recent study carried out by Monika et al. (2024), bioavailability was enhanced by designing nanocapsules from pomegranate peel extract using a pH-sensitive polymer, Eudragit L-100, to sustain the release of the drug [4]. Zhang et al. (2024) have utilized a metal-organic framework to encapsulate Diclofenac sodium to achieve controlled release for 48 h and enhanced bioavailability [5]. Bilosomes of Diclofenac sodium were developed to enhance the therapeutic activity of the drug by Zafar et al. (2021). In vivo bioavailability study showed a 2.15-fold higher peak plasma concentration than the pure drug and a higher t1/2 value, revealing a higher residence time [6].
However, the nanoformulations prepared from the synthetic reagents need to be replaced with the natural polymer to overcome their setbacks. Therefore, natural polysaccharides have drawn much attention in this context. Natural polysaccharides, being hydrophilic and hemo-compatible, are selected as efficient approaches for developing micro and nano drug carriers to sustain the release of drugs, targeting a specific area, thereby improving the bioavailability of the drug, providing a challenge to overcome their stability issues by various modifications [7].
Tan et al. (2021) have isolated sago pulp from sago waste and modified it to its carboxymethyl derivative to develop hydrogels by crosslinking modified sago pulp and chitosan for sustained release and improved bioavailability of diclofenac sodium [8]. In another work by Upadhyay et al. (2019), a pharmacokinetics study of locust bean gum and sodium alginate microbeads showed a 3.7-fold increase in t1/2 and a 2.9-fold increase in mean residence time compared to the pure drug, therefore advocating the use of natural polysaccharides in enhancing bioavailability [9]. In an in vivo pharmacokinetics study by Niu et al. (2019), alginate hydrogel beads from carboxymethylated chitosan Zinc oxide nanoparticles in rats indicated improved bioavailability as compared to the conventional formulation [10]. Thus, chemically modified natural polysaccharides can be used to prolong the release of the drug, improving the bioavailability of the formulation.
Thus, to unveil the new functionalities of natural polysaccharides and to replace the synthetic analogs in a newer direction, the current research used modified Basella alba mucilage to prepare hydrogel beads loaded with Diclofenac Sodium (DS) and inspected the pharmacokinetics study in vivo to reveal the novel functionalities of Basella alba mucilage. The in vivo pharmacokinetics study of pure drugs, drug-loaded formulation and marketed products were studied in Wistar albino rats and the pharmacokinetic parameters were analyzed.
MATERIALS AND METHODS
Materials
Diclofenac sodium was procured from Micro Labs, Hyderabad. Basella alba was purchased from a grocery shop in Panihati, cleaned, and air dried to prepare herbarium, which was further identified by Botanical garden, Botanical Survey of India, Shibpur and was given the identification no. as MC0105. The analytical grade reagents were used as received and the water used was freshly prepared double distilled water.
Methods
Extraction of Basella alba mucilage and its carboxymethylation
At first, the Basella alba fruits and stem were subjected to aqueous extraction followed by precipitation with acetone to get the isolated mucilage. The mucilage was chemically modified by the Carboxymethylation process, where, initially, sodium hydroxide treatment, activated hydroxyl group followed by treatment with monochloroacetic acid at 15 °C temperature, followed by increasing temperature to introduce carboxymethyl group, thereby tailored mucilage was obtained following the method given by Chowdhury et al. [11].
Physicochemical characterization of the Basella alba mucilage and its carboxymethyl derivative
Percentage yield
The percentage yield of the Basella alba mucilage after extraction was calculated using the formula:
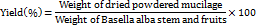
The percentage yield of the Carboxymethylated mucilage after modification was calculated using the formula:

Swelling index
The swelling index was determined by adding mucilage powder in distilled water overnight using the given formula [12].
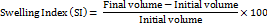
Water holding capacity
The water-holding capacity of the mucilage was determined by mixing it with water and then removing the supernatant by centrifuge and following the given formula [13].

Viscosity determination
The viscosity was determined by Brookfield viscometer (TokiSangyo viscometer, model no. TV-10, Japan) using spindle no. S1.
Estimation of the degree of substitution (o-carboxymethyl substitution)
The degree of substitution is found by first converting the modified mucilage to acid form by adding concentrated Hydrochloric acid and then adding a base (Sodium hydroxide) and back titration with Hydrochloric acid following the method given by Chowdhury et al. [11].
Elemental analysis
The elemental analysis of Basella alba mucilage and its carboxymethyl derivative was done with an elemental analyzer to detect the changes (Model Vario EL III, Elementer, Hanau, Germany). The percentage of Carbon (C), Hydrogen (H), and Oxygen (O) was estimated to confirm the carboxymethylation of the native mucilage.
Preparation of diclofenac sodium-loaded Basella alba mucilage-based hydrogel beads
The tailored Basella alba mucilage was able to form a hydrogel bead in the presence of Al3+ ions rather than divalent ions; therefore, aluminum chloride was taken as a cross-linking agent to prepare the hydrogel beads. Diclofenac sodium was the drug of choice and sodium carboxymethyl cellulose was used to improve the mechanical strength of the beads. The hydrogel beads were prepared by the ionotropic gelation method. The same method was followed to prepare blank Basella alba mucilage-based beads.
Physicochemical characterization of the hydrogel beads
Particle size of beads
Digital slide calipers were used to determine the particle size of 50 beads randomly collected from the sample.
Drug entrapment efficiency
The entrapment efficiency was calculated using the formula [14]:
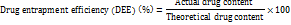
Scanning electron microscopy
Diclofenac sodium loaded beads and blank beads were coated with gold palladium film, mounted, and observed under a Scanning electron microscope and photos were taken at 17kV acceleration voltage.
Experimental rats
Eighteen healthy male albino rats weighing 200-250 g were obtained from M/S Chakraborty Enterprise, Kolkata. They were maintained in standard cages of polycarbonate and fur-marked for identification and a thorough veterinary examination was done. The rats were placed in cages (six rats) consisting of husk bedding at 20 to 24 °C temperature and relative humidity of 50 to 60%, following 12 h of dark and light cycles. Rats were acclimatized before the experiment for 10 days and were given a normal laboratory diet with free access to water. The protocol of the experiment followed schedule Y requirements of the Drug and Cosmetics Act OECD Principles of Good Laboratory Practices and it was reviewed and approved by the Institutional Animal Ethics Committee, TAAB Biostudy Services, Kolkata (Registration no.1938/P. O./Rc/S/17/CPCSEA). Throughout the experimental procedures, strict maintenance of ethical guidelines was followed as per the competent authority.
Pharmacokinetics study design
The pharmacokinetics study was carried out in three groups of albino rats, six in each group. The animals were not given food overnight for 12 h before dosing and had free access to water only.
Group I: Administered Pure Drug (Diclofenac sodium at a dose of 50 mg/kg body weight) orally [15].
Group II: Administered Test formulation (Diclofenac sodium loaded Basella alba mucilage beads equivalent to 50 mg Diclofenac sodium/kg body weight) orally
Group III: Administered Marketed Formulation (Subsyde®-CR DRCM capsule, a marketed controlled release formulation, manufactured by Raptakos Brett and C. Ltd. (Each capsule contains Diclofenac sodium IP 100 mg) at a dose equivalent to 50 mg Diclofenac sodium/kg body weight) orally.
Blood collection
0.5 ml of fasting blood sample at zero hours was withdrawn from the rats of all groups early in the morning. All the animals were administered the drug orally using an oral feeding syringe by oral route. 0.5 ml blood was withdrawn from each rat by a retro-orbital puncture at 0.5, 1,2,3,6,8,12, and 24 h. Therefore, a total of nine blood samples were collected for 24 h, out of which one sample was collected before drug administration and the rest samples after drug ingestion. The sample was collected in 0.5 ml heparinized centrifuge tubes and kept over ice. The tubes were centrifuged at 5000 rpm for ten minutes to allow the collection of plasma; the plasma collected was labeled appropriately with details like rat number, study date, and collection time and kept at-20 °C for further investigation. During the study period, the rats were monitored for any abnormal signs or symptoms.
Extraction of plasma
The frozen plasma sample was kept at room temperature for the thawing process. 50 μl of IS solution (1000ng/ml naproxen as mobile phase) was mixed with 150 μl plasma sample and transferred in polypropylene microcentrifuge tubes of capacity 1.5 ml. The plasma sample was deproteinized using the acetonitrile protein precipitation method. Protein precipitation was done by mixing 300 μl acetonitrile into the mixture. The sample was then vortexed for a few minutes and then centrifuged for 5 min at 6000 rpm. The supernatant obtained was filtered through a 0.45-micron filter using a micropipette. 20 μl of the filtered clean supernatant was then injected into the HPLC system [16].
Pharmacokinetic study of diclofenac sodium by HPLC
The concentration of Diclofenac sodium in a plasma sample of rats was detected using High-Performance Liquid Chromatography (HPLC). The HPLC system used was the Shimadzu-20AD model type (Model no: SPD-M20A 230V, Mfg by Shimadzu Corp.). The analysis of Diclofenac sodium from rat plasma was carried out using C18 column and Phosphate buffer of pH 3and Methanol in the ratio of 30:70 was taken as mobile phase, maintaining the flow rate at 1 ml/min at ambient temperature condition following pressure range of pump at 0 to 6000psi.
Analysis of pharmacokinetic parameters
The pharmacokinetic parameters of Diclofenac sodium were estimated, and its processing was done using the non-compartmental model of PK Solver, a menu-driven add-in program for Microsoft Excel (China Pharmaceutical University) [17]. The data were observed as mean standard deviation (mean±SD). The maximum plasma concentration (Cmax) and time taken to achieve maximum plasma concentration (tmax) were determined from the plasma concentration-time profile. The linear regression analysis of the log-linear plot of the plasma concentration-time profile gives an elimination rate constant (Ke). The linear trapezoidal rule is used to calculate the total area under the plasma concentration-time curve (AUC0-t). The AUC from 0 to infinity (AUC0-∞) was determined from AUC+C24/Ke where C24 is the plasma concentration measured at 24 h. The half-life of Diclofenac sodium (t ½) was calculated as ln2/Ke. The apparent clearance (CL) was estimated from dose/AUC0-∞, and the volume of distribution (Vd) was obtained from CL/Ke. mean residence time (MRT) indicates the average time the drug remains in the body [18].
Statistical analysis
All the experimental results were expressed as Mean± Standard Deviation (SD). The significant difference between the group-administered raw drug (Group I) with the group-administered test (Group II) and marketed formulation (Group III) was statistically analyzed using one-way ANOVA (Analysis of Variance) and graphs were plotted using Graph Pad Prism 9 software. The p-value (p<0.01) was considered the significant level.
RESULTS AND DISCUSSION
The percentage yield of the mucilage was observed to be 24% w/w, which suggests that the fruits and stem of Basella alba are a good source of mucilage. The observed value of the swelling index and water-holding capacity, as per table 1 indicate that the mucilage can be used to modify the release of drug from dosage form. A similar observation of physicochemical properties was confirmed when Chatchawal et al. (2010) extracted mucilage from the stem of Basella alba and studied its physical and biological properties [19]. Carboxymethylation decreased the viscosity of the Basella alba mucilage as per table 1. The degree of substitution value in table 1 shows a good extent of carboxymethyl substitution in the mucilage as the average number of substituted groups in each sugar unit is 0.58.
Table 1: Physicochemical characterization of Basella alba mucilage and Carboxymethylated Basella alba mucilage
| S. No. | Parameter | Result | |
| Basella alba mucilage | Carboxymethylated Basella alba mucilage | ||
| Color | Brownish | Light Brown | |
| Percentage yield(%w/w)* | 24±1.2 | 99.5±1.7 | |
| Swelling index (%)* | 16.2±1.3 | 17.8±0.9 | |
| Water holding capacity (g water/g dry sample wt)* | 2.22±1.1 | 2.34±1.6 | |
| Viscosity (cP) (0.1% w/v solution)* | 62.4±2.08 | 26.8±1.15 | |
| Degree of substitution (O-carboxymethyl)* | - | 0.58±0.03 | |
Data were presented as mean ±standard deviation, where n is 3
Elemental analysis
The observed value of elemental analysis represented in table 2 shows a significantly increased oxygen content and decreased carbon and hydrogen content in the carboxymethylated mucilage compared to the Basella alba mucilage. The increase in oxygen content can be due to the incorporation of –CH2COONa in the three replaceable –OH groups. The reduction in carbon content may be due to the distortion of the polymer chain due to continuous stirring during the carboxymethylation process. During the carboxymethylation process, water was generated, which may be the reason for the decreased hydrogen content in the modified mucilage. Therefore, the results of elemental analysis show that Basella alba mucilage has been successfully converted to its carboxymethyl derivative.
Table 2: Result of elemental analysis
| Sample | C% | H% | O% |
| Basella alba mucilage | 38.47 | 6.85 | 50.76 |
| Carboxymethylated Basella alba mucilage | 30.85 | 5.48 | 60.35 |
The drug-loaded Basella alba mucilage-based hydrogel beads were found to have an average diameter of 1365.23±0.14 μm with an average Drug entrapment efficiency of 71.56%±0.85 by studying the randomly collected 50 beads from the sample. The photographs from SEM shown in fig. 1 show the spherical shape of blank and Diclofenac sodium-loaded beads. The surface of the blank bead (fig. 1a) shows some distortion on the surface, whereas the surface of drug-loaded hydrogel beads is uniform with no distortion or cracks, as shown in fig. 1b.
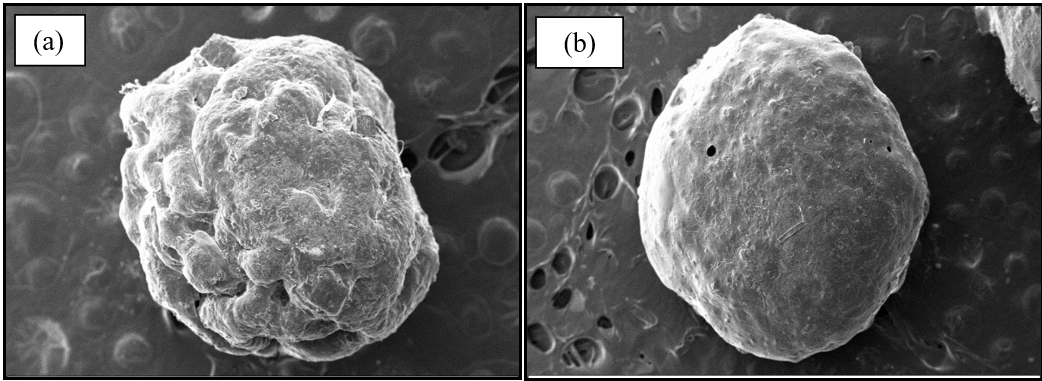
Fig. 1: Scanning electron microscopy photographs (a) Blank Basella alba mucilage-based hydrogel bead (magnification-121X) (b) Diclofenac sodium loaded Basella alba mucilage-based hydrogel beads (magnification-94X)
Pharmacokinetics study
The non-compartmental model permits the analysis of pharmacokinetic data without any specific model; therefore, this model-independent method is mostly utilized to study the pharmacokinetic parameters. The plasma concentration-time curves of Diclofenac sodium after administering a dose of 50 mg/kg body weight orally in rats are depicted in fig. 2, 3and 4 for raw drug, Diclofenac sodium loaded formulation, and marketed formulation (Subsyde®-CR capsule) respectively for 24 h. The test and marketed formulation were administered to the rats at a dose equivalent to 50 mg Diclofenac sodium/kg body weight.
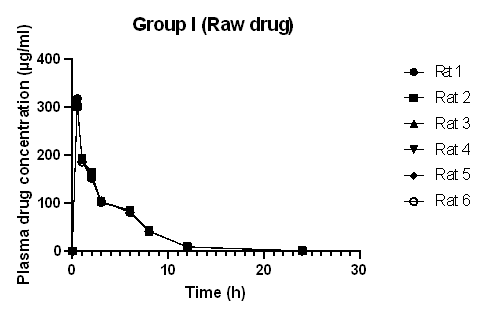
Fig. 2: Plasma concentration-time curve of diclofenac sodium administered as a raw drug (Group I) (Graph plotted from data expressed as mean±standard deviation, where n =6)
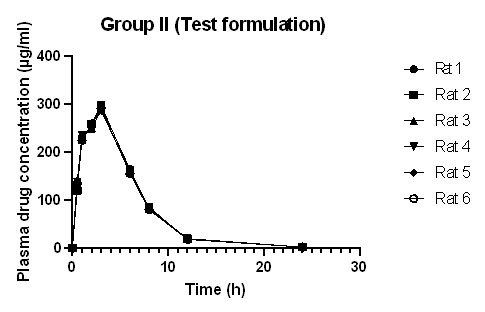
Fig. 3: Plasma concentration-time curve of diclofenac sodium loaded bead (Group II) (Graph plotted from data expressed as mean±standard deviation, where n =6)
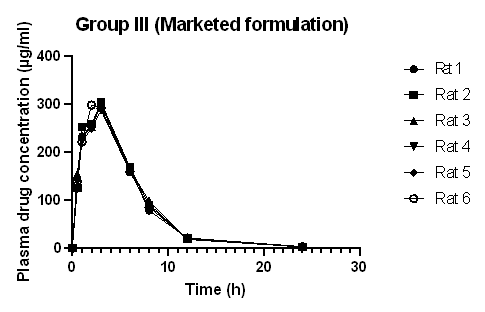
Fig. 4: Plasma concentration-time curve of diclofenac sodium marketed formulation Subsyde®-CR capsule (Group III) (Graph plotted from data expressed as mean±standard deviation, where n =6)
The plasma drug concentration increased and decreased rapidly after oral administration of Diclofenac sodium. After 8 h, the concentration of the drug in plasma decreased to 41.62 μg/ml in rats administered drug only (Group I), 83.08 μg/ml in rats administered Diclofenac sodium-loaded Basella alba mucilage-based beads (Group II), and 88.29 μg/ml in rats administered marketed product (Group III). This shows that the absorption of the drug and elimination of Diclofenac sodium was rapid in rats administered raw drugs, whereas the absorption and elimination of Diclofenac sodium from test formulation and marketed formulation appeared to be slower. Diclofenac sodium reached the maximum plasma concentration in 0.5 h when administered in raw form, but when the same dose was administered in the form of a test and marketed formulation to rats, the time taken to reach the highest concentration was 3h and 2.8 h, respectively.


Fig. 5a, b: Mean plasma concentration-time curve of Diclofenac sodium administered as a raw, test, and marketed formulation (Graph plotted from data as mean±standard deviation (n =6). *Indicates significance at p<0.01 from Diclofenac sodium administered group (Gr I), ns: non-significant)
The mean plasma concentration-time profile of Diclofenac sodium in rats of groups I, II, and III were compared in fig. 5 (a,b). The graph indicates a significant difference between the plasma concentration-time profile of group I administered raw drug and group II administered test formulation (p<0.01), whereas there is no significant difference between the plasma concentration-time profile of group II administered test formulation and group III administered marketed formulation.
Table 3: Pharmacokinetic parameters of diclofenac sodium in plasma of Group I, II, III rats
| S. No. | Pharmaco-kinetic parameters* |
Unit | Gr I (Diclofenac sodium) | Gr II (DFS loaded bead) |
Gr III (Subsyde®-CR capsule) |
| Cmax | µg/ml | 311.19±5.814 | 291.71±4.273 | 298.315±5.891 | |
| tmax | h | 0.5±0.184 | 3±0.211 | 2.83±0.405 | |
| AUC 0-t | µg. h/ml | 1065.10±11.185 | 1888.71±16.406 | 1946.58±39.927 | |
| AUC 0-∞ | µg. h/ml | 1067.40±11.77 | 1897.31±16.58 | 1958.36±40.77 | |
| ke | h-1 | 0.26±0.012 | 0.24±0.008 | 0.23±0.005 | |
| t1/2 | h | 2.66±0.112 | 2.88±0.018 | 3.03±0.105 | |
| MRT | h | 4.09±0.072 | 4.86±0.036 | 4.96±0.137 | |
| CL/F | (mg/kg)/(μg/ml)/h | 0.047±0.002 | 0.026±0.002 | 0.026±0.001 | |
| Vd/F | (mg/kg)/(μg/ml) | 0.18±0.011 | 0.11±0.001 | 0.112±0.004 | |
| Relative Bioavailability | % | 54.50 | 96.88 | - |
*All the data are presented as mean±Standard deviation, where n is 6
Table 3 depicts the mean pharmacokinetic parameters of Diclofenac sodium in the plasma of all rats of groups I, II, and III. The mean tmax for the drug-loaded bead was 3 h and 2.83 h for the marketed formulation, which is higher as compared to the tmax of the raw drug. The t½ of Diclofenac sodium, Diclofenac sodium loaded bead, and the marketed formulation was found to be 2.66, 2.88, and 3.03 h, respectively. This is in agreement with the report stated in the literature that the half-life of Diclofenac sodium in plasma ranges from 1 to 3 h [16, 20]. The increased value of mean tmax and the decreased value of mean Cmax of the drug-loaded beads and marketed formulation compared to the raw drug indicated the sustained release effect of the formulation. This result is comparable with the result of the in vivo pharmacokinetic analysis and oral bioavailability study of Berberine nanoparticles developed from chitosan and sodium alginate, studied by Kohli et al. [21]. The pharmacokinetic parameters of the test and marketed formulation appeared similar without any significant difference. The mean residence time of Diclofenac sodium was higher in the test and marketed formulation than in the raw drug. In most species, Diclofenac sodium binds with protein (more than 99%); as a result, a low volume of distribution and clearance value is obtained [22]. The relative bioavailability of Diclofenac sodium increased from 54.50 to 96.88% by formulating it into a Basella alba mucilage-based bead. The relative bioavailability of the loaded bead was also similar to that of the marketed capsule. This result is in agreement with the study carried out by Zafar et al., where Diclofenac sodium-loaded lipid nanoparticle showed improved relative bioavailability as compared to pure drug [6]. The absolute bioavailability of Diclofenac sodium was low due to the low aqueous solubility of the drug, and the increased relative bioavailability is because of the sustained release of the drug from the beads, which gives a longer absorption and distribution period for the Diclofenac sodium loaded formulation, than that of the raw Diclofenac sodium drug.
Thus, the study explores a novel aspect of Basella alba mucilage as bioavailability enhancer. Since polysaccharides used to improve in vivo bioavailability are very few and limited to chitosan and alginate in literature, therefore the findings of the study highlights the uniqueness of Basella alba mucilage and advocate its novelty in this context.
CONCLUSION
The present study revealed the flexibility of Basella alba mucilage to be carboxymethylated to entrap Diclofenac sodium within the hydrogel beads. The modification improved the physicochemical properties of the mucilage and the substitution was confirmed by elemental analysis. The functionalized Basella alba mucilage-based beads were found to be stable and had potential to entrap and modify the release of the drug efficiently, thereby acting as a cost-effective and potent substitute for the existing synthetic polymers. The efficiency of the tailored polysaccharide was studied by an in vivo pharmacokinetics study in Wistar albino rats, wherein the drug concentration in plasma was measured using HPLC, and the pharmacokinetics parameters were estimated. The results of in vivo studies and relative bioavailability studies showed higher mean residence time, increased tmax, and decreased Cmax of drug-loaded beads and the marketed formulation (Subsyde®-CR capsule) compared to the raw drug. The pharmacokinetic parameters of the Diclofenac sodium-loaded Basella alba mucilage-based beads and marketed formulation appeared similar without any significant difference, confirming the sustained release property of the formulation. Thus, the increment of relative bioavailability of Diclofenac sodium from 54.50% to 96.88% suggests that Basella alba mucilage can be used as a potential drug carrier to improve bioavailability. Therefore, future researches should focus more on the exploration, scalability and commercialization of underutilized natural polysaccharides to design novel dosage forms.
ACKNOWLEDGMENT
The authors sincerely thank Guru Nanak Institute of Pharmaceutical Science and Technology and JIS University for providing facilities to complete the research.
FUNDING
Nil
AUTHORS CONTRIBUTIONS
Moumita Chowdhury have carried out the research work and drafted the manuscript. Pintu Kumar De have reviewed the manuscript.
CONFLICT OF INTERESTS
The authors declared no conflict of interest.
REFERENCES
Nurwaini S, Anggraini AF, Sukmawati A, Imanto T, Utami W. Formulation and evaluation of sodium diclofenac slow-release tablet using a combination of mangrove fruit starch (Bruguiera gymnorrhiza) and sodium carboxy methyl cellulose (CMC) as matrix. Int J App Pharm. 2024;16(06):72-7. doi: 10.22159/ijap.2024.v16s6.TY2040.
Gagliardi A, Chiarella E, Voci S, Ambrosio N, Celano M, Cristina Salvatici M. Difucosin: diclofenac sodium salt loaded fucoidan-sericin nanoparticles for the management of chronic inflammatory diseases. Int J Pharm. 2024 Apr 25;655:124034. doi: 10.1016/j.ijpharm.2024.124034, PMID 38531433.
Khadra I, Obeid MA, Dunn C, Watts S, Halbert G, Ford S. Characterisation and optimisation of diclofenac sodium orodispersible thin film formulation. Int J Pharm. 2019;561:43-6. doi: 10.1016/j.ijpharm.2019.01.064, PMID 30772459.
Monika P, Chandraprabha MN, Krishna RH, Maanya V, Likhitha C, Pooja N. Development of targeted nanocapsules using pomegranate peel extract with enhanced bioavailability and anti-inflammatory activity for ulcerative colitis: in vitro studies. Hybrid Adv. 2024;7:100334. doi: 10.1016/j.hybadv.2024.100334.
Zhang Y, Yang D, Shuai B, Ding H, Yang J, Wang J. Diclofenac sodium nanomedicine results in pain-relief and differential expression of the RNA transcriptome in the spinal cord of SNI rats. Int J Pharm. 2024 Jun 25;659:124276. doi: 10.1016/j.ijpharm.2024.124276, PMID 38821436.
Zafar A, Alruwaili NK, Imam SS, Yasir M, Alsaidan OA, Alquraini A. Development and optimization of nanolipid-based formulation of diclofenac sodium: in vitro characterization and preclinical evaluation. Pharmaceutics. 2022;14(3):507. doi: 10.3390/pharmaceutics14030507, PMID 35335883.
Ngwuluka NC, Ochekpe NA, Aruoma OI. Naturapolyceutics: the science of utilizing natural polymers for drug delivery. Polymers. 2014;6(5):1312-32. doi: 10.3390/polym6051312.
Tan LS, Tan HL, Deekonda K, Wong YY, Muniyandy S, Hashim K. Fabrication of radiation cross-linked diclofenac sodium loaded carboxymethyl sago pulp/chitosan hydrogel for enteric and sustained drug delivery. Carbohydr Polym Technol Appl. 2021;2:100084. doi: 10.1016/j.carpta.2021.100084.
Upadhyay M, Adena SK, Vardhan H, Yadav SK, Mishra B. Locust bean gum and sodium alginate based interpenetrating polymeric network microbeads encapsulating capecitabine: improved pharmacokinetics, cytotoxicity & in vivo antitumor activity. Mater Sci Eng C Mater Biol Appl. 2019 Nov;104:109958. doi: 10.1016/j.msec.2019.109958. PMID 31500043.
Niu B, Jia J, Wang H, Chen S, Cao W, Yan J. In vitro and in vivo release of diclofenac sodium-loaded sodium alginate/carboxymethyl chitosan-ZnO hydrogel beads. Int J Biol Macromol. 2019 Dec 1;141:1191-8. doi: 10.1016/j.ijbiomac.2019.09.059, PMID 31518622.
Chowdhury M, Kumar De P. Tailored Basella Alba mucilage-based bipolymeric hydrogel beads for controlled release of diclofenac sodium. Int J App Pharm. 2023;15(5):106-16. doi: 10.22159/Ijap.2023v15i5.48803.
Fegade TD, Patil VR, Deshmukh TA. Evaluation of Vateria indica Modified Gum as a Release Retardant Matrix in the tablet dosage form. Int J Pharm Pharm Sci. 2023;15(4):28-32. doi: 10.22159/Ijpps.2023v15i4.47329.
Haju SS, Yadav S. Formulation and evaluation of cilnidipine mucoadhesive buccal film by solvent casting technique for the treatment of hypertension. Int J Pharm Pharm Sci. 2021;13(9):34-43. doi: 10.22159/Ijpps.2021v13i9.42641.
Jeevana JB, Ramya K. Development and in vitro evaluation of phytosomes of ellagic acid. Asian J Pharm Clin Res. 2023;16(3):105-9. doi: 10.22159/ajpcr.2023.V16i3.47129.
McLean MK, Khan SA. Toxicology of frequently encountered nonsteroidal anti-inflammatory drugs in dogs and cats: an update. Vet Clin North Am Small Anim Pract, 2018;48(6):969-84. doi: 10.1016/j.cvsm.2018.06.003, PMID 30149968.
Yuan J, Ma H, Cen N, Zhou A, Tao H. A pharmacokinetic study of diclofenac sodium in rats. Biomed Rep. 2017;7(2):179-82. doi: 10.3892/br.2017.942, PMID 28781777.
Zhang Y, Huo M, Zhou J, Xie S. PK Solver: an add-in program for pharmacokinetic and pharmacodynamic data analysis in microsoft excel. Comput Methods Programs Biomed. 2010;99(3):306-14. doi: 10.1016/j.cmpb.2010.01.007, PMID 20176408.
Peris Ribera JE, Torres Molina F, Garcia Carbonell MC, Aristorena JC, Pla Delfina JM. Pharmacokinetics and bioavailability of diclofenac in the rat. J Pharmacokinet Biopharm. 1991;19(6):647-65. doi: 10.1007/BF01080872, PMID 1815046.
Chatchawal C, Nualkaew N, Preeprame S, Porasuphatana S, Priprame A. Physical and biological properties of mucilage from Basella alba L. Stem and Its gel formulation. Isan J Pharm Sci. 2010;6(3):104-12. doi: 10.14456/ijps.2010.26.
Davies NM, Anderson KE. Clinical pharmacokinetics of diclofenac. Therapeutic insights and pitfalls. Clin Pharmacokinet. 1997;33(3):184-213. doi: 10.2165/00003088-199733030-00003, PMID 9314611.
Kohli K, Mujtaba A, Malik R, Amin S, Alam MS, Ali A. Development of natural polysaccharide-based nanoparticles of berberine to enhance oral bioavailability: formulation, optimization, ex vivo, and in vivo assessment. Polymers. 2021;13(21):3833. doi: 10.3390/polym13213833, PMID 34771389.
Uns Q, Asma H, Khawla R. Compartmental and non-compartmental pharmacokinetic analysis of extended-release diclofenac sodium Tablet. 2016;19(1):161-5.