
Int J App Pharm, Vol 17, Issue 4, 2025, 145-151Original Article
ASSEESSMENT OF CELLULAR TOXICITY ON CANCER CELL LINES USING CAPSAICIN-LOADED PLGA NANOBUBBLES
HEMA KUMAR A. V.1, CHAMAKURI KANTLAM2*
1Bharatiya Engineering Science and Technology Innovation University (BESTIU), Gownivaripalli, Gorantala Mandal, Anantapur, Andhra Pradesh, India. 2Brilliant Grammar School Educational Society’s Group of Institutions-Integrated Campus (Faculty of Engineering and Faculty of Pharmacy), Hyderabad. Abdullapur (V), Abdullapurmet (M), Rangareddy (Dist), Hyderabad-501505, Telangana, India
*Corresponding author: Chamakuri Kantlam; *Email: drkantlam@gmail.com
Received: 08 Mar 2025, Revised and Accepted: 02 Jun 2025
ABSTRACT
Objective: This study aims to develop and characterize Capsaicin-Loaded Poly(Lactic-Co-Glycolic Acid) (PLGA) Nanobubbles (CAP-NBs) for targeted drug delivery. The research evaluates the physicochemical properties, drug release profile, cytotoxic effects on cancer cell lines, and formulation stability to assess the potential of CAP-NBs as a controlled and ultrasound-triggered drug delivery system.
Methods: CAP-NBs were formulated using the solvent evaporation method with ultrasound assistance to achieve nanoscale dispersion and high drug entrapment efficiency. Physicochemical characterization was performed using particle size analysis, Polydispersity Index (PDI), Zeta Potential measurement, Fourier Transform Infrared Spectroscopy (FTIR), Differential Scanning Calorimetry (DSC), X-ray Diffraction (XRD), and Scanning Electron Microscopy (SEM) to assess morphology and stability. In vitro drug release studies were conducted to evaluate sustained and ultrasound-triggered release profiles. Cytotoxicity assessments were performed on cancer and normal cell lines to determine the therapeutic efficacy and selectivity of CAP-NBs. Stability studies were conducted over a three-month period to assess long-term formulation viability.
Results: PLGA CAP-NBs were successfully formulated using a solvent evaporation-ultrasonication method, achieving a particle size of 164±6.4 nm, PDI of 0.261±0.09, and ZP of-40.5±6.2 mV, indicating excellent stability. FTIR, DSC, and XRD analyses confirmed successful encapsulation, amorphization, and drug-polymer interactions. In vitro drug release studies revealed sustained and ultrasound-enhanced release, reaching 96.81% cumulative release at 24 h. CAP-NBs demonstrated lower cytotoxicity (IC₅₀ = 73.44 µg/ml for A549; 68.72 µg/ml for A498) compared to the pure drug, suggesting controlled release and reduced acute toxicity.
Conclusion: The study successfully developed and characterized CAP-NBs as a stable and efficient nanocarrier for targeted drug delivery. The formulation exhibited controlled and ultrasound-responsive drug release, along with selective cytotoxic effects against cancer cells. These findings highlight the potential of CAP-NBs for enhanced therapeutic efficacy with reduced systemic toxicity. Further in vivo investigations are warranted to explore their clinical applicability.
Keywords: Capsaicin, PLGA nanobubbles, In vitro cell line study, Cancer therapy, Cytotoxicity
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijap.2025v17i4.54153 Journal homepage: https://innovareacademics.in/journals/index.php/ijap
INTRODUCTION
Capsaicin (CAP), a naturally occurring alkaloid in chili peppers, is well known for its pungency and diverse pharmacological properties, including anti-inflammatory, analgesic, antioxidant, and anticancer effects. CAP exerts its biological effects primarily through interaction with the Transient Receptor Potential Vanilloid 1 (TRPV1) receptor, which plays a key role in pain perception, thermoregulation, and cellular signaling. By activating TRPV1, CAP can modulate neuronal responses, reduce inflammation, and even induce apoptosis in cancer cells, making it a promising therapeutic candidate [1]. Additionally, CAP has demonstrated potential in treating Alcoholic Hepatitis (AH) due to its liver-protective, anti-inflammatory, and regenerative properties [2]. However, its clinical application remains limited due to poor bioavailability, hydrophobicity, and gastrointestinal side effects such as irritation, nausea, and burning diarrhea. These challenges necessitate the development of novel drug delivery strategies that can enhance CAP’s stability, solubility, and targeted therapeutic action [3].
Compared to conventional delivery systems such as liposomes, micelles, or Solid Lipid Nanoparticles (SLNs), Poly(Lactic-Co-Glycolic Acid) PLGA Nanobubbles (NBs) offer enhanced stability, better protection for hydrophobic drugs, and more sustained release kinetics. The NB architecture allows encapsulation of volatile or hydrophobic drugs like CAP and provides an acoustic trigger mechanism for on-demand drug release using ultrasound [3]. These attributes make PLGA NBs an ideal vehicle for delivering CAP, overcoming its poor solubility and gastrointestinal side effects while enabling localized, non-invasive activation [4].
NBs, particularly those composed of PLGA, provide a cutting-edge approach to overcoming these limitations. PLGA is a biocompatible and biodegradable polymer extensively used in drug delivery, molecular diagnostics, and tissue engineering [4, 5]. While previous studies have explored micelle-based delivery systems for CAP, no prior research has documented the use of PLGA NBs for its delivery. This study seeks to bridge this gap by formulating and optimizing CAP-loaded PLGA NBs using Design of Experiments (DoE), a statistical approach that streamlines the optimization of formulation variables [6, 7]. This method ensures enhanced drug loading efficiency, stability, and bioavailability while minimizing side effects.
A critical aspect of evaluating CAP’s therapeutic efficacy involves cell line studies, which serve as fundamental preclinical models for assessing drug performance in a controlled laboratory environment. In this research, A549 (lung cancer) and A498 (renal cancer) cell lines are used to investigate the cytotoxicity, cellular uptake, and apoptotic effects of CAP-loaded PLGA NBs. The use of these cell lines is significant as they provide insights into CAP’s anticancer potential, allowing for a deeper understanding of its mechanism of action at the cellular level. A549 cells, derived from human lung carcinoma, are widely utilized for studying lung cancer pathophysiology and drug responses. A498 cells, originating from human renal cell carcinoma, serve as a model for understanding kidney cancer treatment strategies. Evaluating CAP’s effects on these cancer cell lines helps determine its ability to induce apoptosis, inhibit cell proliferation, and modulate key signaling pathways involved in cancer progression [8].
Beyond cancer research, cell line studies offer invaluable information on drug metabolism, toxicity, and targeted delivery. By incorporating CAP-loaded PLGA NBs into these studies, this research aims to assess the formulation’s efficiency in improving CAP’s cellular uptake and therapeutic outcomes [9]. Moreover, the findings from in vitro experiments provide a foundation for further in vivo and clinical studies, ultimately contributing to the development of CAP-based targeted therapies. Through a combination of innovative drug delivery systems and rigorous cell line evaluations, this study seeks to unlock new possibilities for CAP’s application in cancer treatment and other inflammatory diseases.
MATERIALS AND METHODS
Materials
CAP was purchased from AOS Products Private Limited, Ghaziabad. C3F8 (Perfluoro propane) was procured from Pharm affiliates Pvt Ltd, Haryana, India. Sigma Aldrich, US, supplied Poly (D, L-lactide-co-glycolide) 50:50 with an intrinsic viscosity of 0.22 dl/g and Mw 25,000. Polyvinyl alcohol (PVA; Mw 30,000-70,000) was purchased from Sigma Aldrich (St. Louis, MO, USA). Isopropanol, and Dichloromethane (DCM) was acquired from S. D. fine chemicals, Hyderabad. We purchased acetonitrile from Qualigens, India. The A549 and A498 cell lines (both at passage number P3) were cultured in Eagle's Minimum Essential Medium and sourced from ATCC HTB-44. Fetal Bovine Serum [#RM10432] and D-PBS [#TL1006], DMEM [#AL007A], EMEM [#AL047S] were from HiMedia. MTT Reagent [# M5655] and Dimethylsulfoxide (DMSO) [#PHR1309] were from Sigma. 96-well plate for culturing cells was from Corning, USA. The A549 and A498 cell lines were sourced from the American Type Culture Collection (ATCC, Manassas, VA, USA).
Methods
Development and optimization of CAP-NBs
CAP-NBs were prepared using the solvent evaporation method with ultrasound assistance, following a modified protocol. Initially, PLGA was dissolved in DCM, a water-immiscible solvent, to form a homogeneous solution. CAP was then incorporated into this solution to create a dispersion, which was subjected to sonication for two minutes at 45% amplitude in an ice bath using a Digital Sonifier S-250D (Branson Ultrasonic, Danbury, USA). The resulting drug dispersion was subsequently mixed with 20 ml of chilled 2.0% PVA solution and homogenized at 6,500 rpm for 10 min using a high-speed homogenizer. This was followed by sonication at 30 W for one minute in the absence of light using an ultrasonic probe [10].
To remove DCM, a 2.5% v/v isopropanol solution (20 ml) was added to the emulsion, and the mixture was mechanically stirred for five hours. The product was then centrifuged at 8,000 rpm for five minutes, and the precipitate was collected while discarding the supernatant. The precipitate was washed with distilled water, and this centrifugation and washing process was repeated three times to ensure purity. The NBs were then freeze-dried for 36 h in the absence of light using a LYPH LOCK 4.5 (Labconco Corporation, Kansas City). Finally, perfluoropropane (C₃F₈) gas was introduced into the lyophilization chamber through a vial connector at a flow rate of 50 ml/min for one minute. The vials were then securely sealed for further analysis.
Characterization and evaluation
The characterization and evaluation of CAP-NBs involved multiple analytical techniques to assess their physicochemical properties and stability. Particle Size (PS), Polydispersity Index (PDI), and Zeta Potential (ZP) were determined using Dynamic Light Scattering (DLS) after diluting the sample with double-distilled water. Entrapment Efficiency (EE) and Loading Capacity (LC) were evaluated by dissolving drug-loaded NBs in DCM, followed by sonication and UV-Vis spectrophotometric analysis at 282 nm. Fourier Transform Infrared Spectroscopy (FTIR) was performed using the KBr disc method to analyze the chemical interactions of CAP with formulation components over a spectral range of 4000 to 500 cm⁻¹. The thermal properties were examined through Differential Scanning Calorimetry (DSC), where samples were heated at a controlled rate from 20 to 400 °C. The structural morphology of the NBs was observed using Scanning Electron Microscopy (SEM) after sputter coating with gold, allowing for high-magnification imaging. X-ray Diffraction (XRD) analysis was conducted to study the crystallinity of CAP, PLGA, PVA, and the formulated NBs. Drug Release (DR) was assessed using the dialysis bag method in pH 7.4 phosphate buffer at 37±0.5 °C, both with and without ultrasonication, with periodic sampling and spectrophotometric measurement at 282 nm to determine cumulative release. The stability of CAP-NBs was monitored over three months at different temperatures by measuring PS, PDI, and EE at specific intervals to evaluate formulation integrity.
Cell line studies
Cell viability MTT assay was performed to evaluate the CAP for its cytotoxicity or ability to inhibit cell proliferation spectrophotometrically as a function of mitochondrial activity in living A 498 and MCF-7 cell line.
All cells were seeded at a density of 2 × 104 cells/well in separate 96-well-plates in 200 µl of culture medium and incubated at 37±1 °C and 5±1% Carbon dioxide (CO2). After 24 of incubation, the cells were treated with CAP NBs. Upon completion of incubation 48 h, 100 µl of MTT solution (0.5 mg/ml) was added to each well and incubated for 3 h at 37±1 °C and 5±1% CO2. The resulting formazan was solubilized and quantified by spectrophotometrically at 570 nm wavelength using a spectrophotometer (Tecan™ Infinite 200Pro) [9].
The experiment includes four control groups and a test group with varying concentrations of CAP NBs. The medium control consists of medium without cells, serving as a baseline, with three replicates. The negative control contains medium with cells but without the experimental drug or compound, also with three replicates. The vehicle control includes medium with cells and the vehicle used for drug delivery, ensuring that any observed effects are due to the compound itself rather than the vehicle, with three replicates. The test group involves treatment with CAP-NBs at concentrations of 5, 10, 20, 30, 50, and 100 µg/ml, with each concentration tested in triplicate [8].
Preparing cell line
A vial of each cell lines was taken out from liquid nitrogen storage and thawed rapidly to room temperature. The contents in the vials were added to 9 ml of complete medium and centrifuged at 125g for 5 min. After centrifugation, the supernatant was discarded and pellet was mixed with 10 ml of complete medium and suspended in a T-25 flask and incubated at 37 °C with 5% CO2. When the cell confluence reached ~80%, the cells were centrifuged at 125g for 5 min; pellet was mixed with 15 ml of complete medium and transferred to two T-75 flasks. When the cell confluence reached around 80-90%, cells in the flask were used for the assay [10].
MTT assay
A 200 µl cell suspension (in complete culture medium with 10% FBS) was seeded in a 96-well plate (20,000 cells per well), without the test agent and allowed to grow for 24 h. After 24 h of incubation, spent media in the wells of 96-well plate were replaced with appropriate concentrations of the CAP-NBs and incubated for 48 h at 37 °C in a 5% CO2 atmosphere. After the incubation period, the plates were removed from incubator; spent media was removed followed by addition of MTT reagent to a final concentration of 0.5 mg/ml (0.2 µm filter sterilized). The plates were wrapped with aluminium foil to avoid exposure to light, and placed in the incubator for 3 h. After incubation MTT reagent was removed and 100 µl of DMSO was added. Absorbance was measured on spectrophotometer (Tecan™ Infinite 200Pro) at 570 nm [9].
Data analysis
The percent viability of cells in the untreated (negative control) group was set to 100% and the % viability of cells in the treated groups was estimated relative to the negative control. The % viability was plotted against the concentration and evaluated for dose response. Based on the dose response relationships, an appropriate model was fit to estimate the Imax and IC50.
Percentage viability was calculated using the following formula:
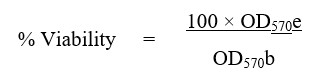
Where,
OD570e is the mean value of the measured Optical Density of the dilutions of test item;
OD570b is the mean value of the measured Optical Density of the negative control
RESULTS AND DISCUSSION
The successful preparation of CAP-NBs using the solvent evaporation method with ultrasound assistance highlights an efficient strategy for drug encapsulation. The combination of sonication and high-speed homogenization ensured uniform dispersion and size control, while the removal of residual DCM using isopropanol enhanced the purity of the final formulation. The introduction of perfluoropropane gas during lyophilization was essential for achieving stable NBs with potential ultrasound responsiveness [10-12].
Key formulation parameters were optimized based on initial screening studies and existing literature. A PLGA concentration of 100 mg per 10 ml DCM ensured adequate viscosity and drug-polymer interaction without compromising nanoformulation. A homogenization speed of 6,500 rpm yielded a uniform emulsion with nano-scale particle size, and sonication amplitude of 45% was chosen to effectively disperse CAP within the matrix while maintaining structural integrity. Final sonication at 30 W for one minute post-emulsification helped refine the PS and stability.
The study demonstrated the consistency and stability of CAP-NBs, with PS of 164±6.4 nm and PDI of 0.261±0.09, indicating uniformity. The ZP of-40.5±6.2 mV ensured electrostatic repulsion, preventing aggregation [11-14]. EE was 81.06±3.58%, and LC was 29.40±4.01%. FTIR analysis confirmed CAP encapsulation through hydrogen bonding interactions between the-OH groups of CAP and the C=O groups of PLGA. DSC analysis revealed an endothermic peak for CAP at 68.38 °C, while new peaks at 66.76 °C and 190.58 °C in NBs suggested polymeric confinement. XRD analysis showed the disappearance of CAP’s characteristic diffraction peaks at 5.8°, 11.7°, 16.4°, and 19.8°, indicating a transition to an amorphous state. Drug release studies showed that after 8 h, cumulative DR was 17.63±3.34% for plain drug, 47.94±4.12% for NBs without ultrasound, and 73.44±3.67% with ultrasound. By 24 h, ultrasound-assisted NBs achieved a cumulative DR of 96.81±4.22% due to cavitation-induced structural disruption [15, 16]. Stability studies at 4 °C and 25 °C over three months showed minimal changes in EE, though a slight reduction to 77.62±3.26% at higher temperatures suggested some structural degradation. Throughout the study, PS remained below 200 nm, ZP ranged between-35 to-38 mV, and PDI values remained consistent, confirming NB stability. While hydrogen bonding was emphasized as a key factor in stability, the precise mechanisms underlying long-term NB stability remain unresolved in the literature.
The PS (~164 nm) and PDI (~0.26) achieved are considered optimal for passive targeting via the Enhanced Permeability and Retention (EPR) effect, facilitating accumulation in tumor tissues. The ZP of-40.5 mV indicates a highly stable colloidal dispersion due to sufficient electrostatic repulsion, reducing the risk of aggregation. These parameters collectively support enhanced bioavailability and prolonged circulation of CAP-NBs in vivo.
The in vitro drug release study indicated a sustained release pattern in phosphate buffer (pH 7.4), with ultrasonication enhancing the release rate, suggesting the potential for ultrasound-triggered drug delivery [15]. For ultrasound-triggered drug release, a probe sonicator operating at 30 W and 20 kHz was used for one minute at 37 °C. These parameters were selected to initiate cavitation effects, enhancing permeability and accelerating drug diffusion without damaging NB morphology.
Kinetic modeling of the release data showed best fit to the Higuchi model (R²>0.95), indicating diffusion-controlled release from a matrix system. The ultrasound-assisted group exhibited a sharp burst release phase, attributed to cavitation-induced disruption of the NB membrane, allowing for rapid CAP release upon acoustic stimulation. This dual-phase profile aligns with the objective of developing a controlled yet externally triggerable delivery platform.
Stability studies over three months confirmed the structural integrity of CAP-NBs, reinforcing their potential for long-term storage. Overall, the formulation approach successfully produced NBs with desirable properties for controlled and targeted drug delivery applications [16]. Although key parameters such as size and EE remained largely stable, minor reductions at 25 °C suggest potential for CAP degradation or polymer matrix alterations. To verify the chemical stability of CAP within the formulation, further studies involving HPLC or LC-MS analysis are warranted. Such analytical methods would confirm drug integrity and ensure consistent therapeutic performance over storage periods.
Future studies could focus on in vivo evaluations to further explore their therapeutic efficacy [17-19].
 |
 |
| A | B |
Fig. 1: Physical characterization of capsaicin and capsaicin nanobubbles; A: FTIR; B: DCS; C: XRD
The conversion of crystalline CAP to an amorphous form within the PLGA matrix, as evidenced by XRD, is a key factor contributing to enhanced dissolution and bioavailability. DSC thermograms also demonstrated reduced crystallinity, while FTIR confirmed drug-polymer interactions via hydrogen bonding. These structural changes directly correlate with the observed sustained drug release and increased formulation stability.
The cytotoxic effects of the CAP pure drug and CAP-NBs on the A549 lung cancer cell line were evaluated using MTT assay. The mean cell viability percentages at different concentrations (5, 10, 20, 30, 50, and 100 µg/ml) were recorded, and the IC50 values were determined [20].
As shown in table 1, the CAP pure drug exhibited a dose-dependent cytotoxic effect on A549 cells. The mean cell viability decreased from 97.36% at 5 µg/ml to 23.26% at 100 µg/ml. The calculated IC50 value for the CAP pure drug was 41.56 µg/ml, indicating a moderate cytotoxic effect on A549 cells [21]. Similarly, the CAP-NBs also showed a concentration-dependent cytotoxic response (table 2), with mean viability reducing from 95.26% at 5 µg/ml to 44.27% at 100 µg/ml. The IC50 value for CAP-NBs was 73.44 µg/ml, indicating lower cytotoxicity compared to the pure drug. The graphical representation of these results is provided in fig. 2 and 3.
Table 1: Cytotoxicity of capsaicin pure drug on A549 cell line
| Capsaicin nano bubbles (µg/ml) | % Viability | IC50 value (µg/ml) |
| Blank | - | 41.56 |
| Vehicle control | 100.00±0.00 | |
| 5.00 | 97.36±2.74 | |
| 10.00 | 79.56±3.65 | |
| 20.00 | 59.64±2.49 | |
| 30.00 | 48.26±2.46 | |
| 50.00 | 33.25±1.95 | |
| 100.00 | 23.26±1.48 |
All the values were expressed in (n=3) mean±SD
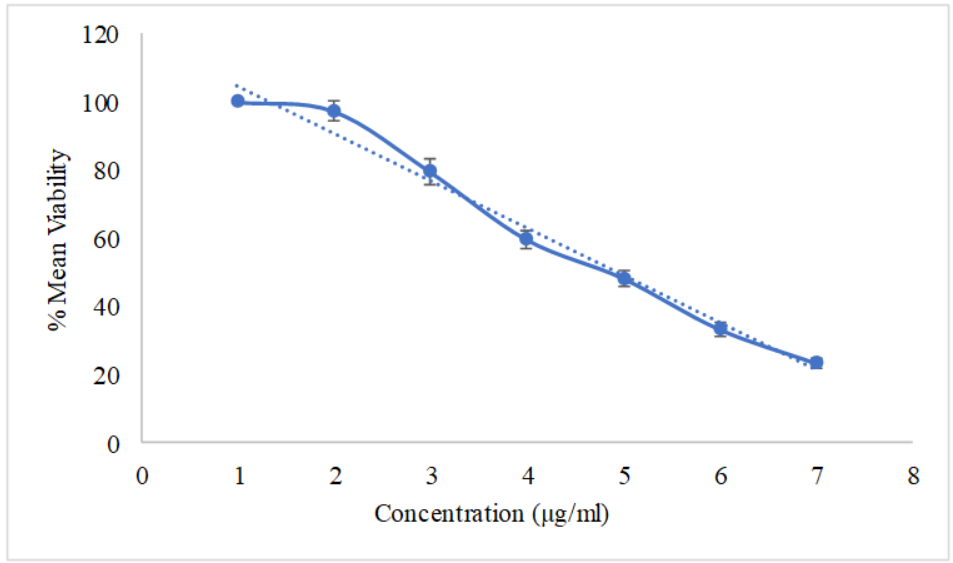
Fig. 2: Cytotoxicity of capsaicin pure drug on A549 cell line, all the values were expressed in (n=3) mean±SD
Table 2: Cytotoxicity of capsaicin nano bubbles on A549 cell line
| Test compound (µg/ml) | % Viability | IC50 value (µg/ml) |
| Blank | - | 73.44 |
| Vehicle Control | 100.00±0.00 | |
| 5.00 | 95.26±3.24 | |
| 10.00 | 87.24±3.45 | |
| 20.00 | 75.98±2.18 | |
| 30.00 | 55.98±2.46 | |
| 50.00 | 54.18±2.47 | |
| 100.00 | 44.27±2.74 |
All the values were expressed in (n=3) mean±SD

Fig. 3: Cytotoxicity of capsaicin nano bubbles on A549 cell line, all the values were expressed in (n=3) mean±SD
To assess the cytotoxic potential of CAP formulations on renal cancer cells, the A498 cell line was treated with increasing concentrations of CAP pure drug and CAP-NBs, and the IC50 values were determined [22].
As depicted in table 3, the CAP pure drug displayed significant cytotoxic effects on A498 cells, with cell viability decreasing from 96.27% at 5 µg/ml to 23.59% at 100 µg/ml [23]. The IC50 value was calculated to be 59.60 µg/ml. On the other hand, CAP-NBs demonstrated a comparatively reduced cytotoxic effect (table 4), with a mean viability of 98.47% at 5 µg/ml, decreasing to 29.34% at 100 µg/ml. The IC50 value for CAP-NBs on A498 cells was found to be 68.72 µg/ml. These results are visually represented in fig. 4 and 5.
Table 3: Cytotoxicity of capsaicin pure drug on A498 cell line
| Capsaicin pure drug | % Viability | IC50 value (µg/ml) |
| Blank | - | 59.60 |
| Vehicle control | 100.00±0.00 | |
| 5.00 | 96.27±3.65 | |
| 10.00 | 93.65±2.48 | |
| 20.00 | 85.95±1.24 | |
| 30.00 | 66.48±1.57 | |
| 50.00 | 47.54±2.64 | |
| 100.00 | 23.59±1.89 |
All the values were expressed in (n=3) mean±SD

Fig. 4: Cytotoxicity of capsaicin pure drug on A498 cell line, all the values were expressed in (n=3) mean±SD
Table 4: Cytotoxicity of capsaicin nano bubbles on A498 cell line
| Capsaicin nano bubbles (µg/ml) | % Viability | IC50 value (µg/ml) |
| Blank | - | 68.72 |
| Vehicle Control | 100.00±0.00 | |
| 5.00 | 98.47±2.84 | |
| 10.00 | 92.48±3.54 | |
| 20.00 | 87.24±2.65 | |
| 30.00 | 75.46±2.48 | |
| 50.00 | 60.14±2.95 | |
| 100.00 | 29.34±2.48 |
All the values were expressed in (n=3) mean±SD
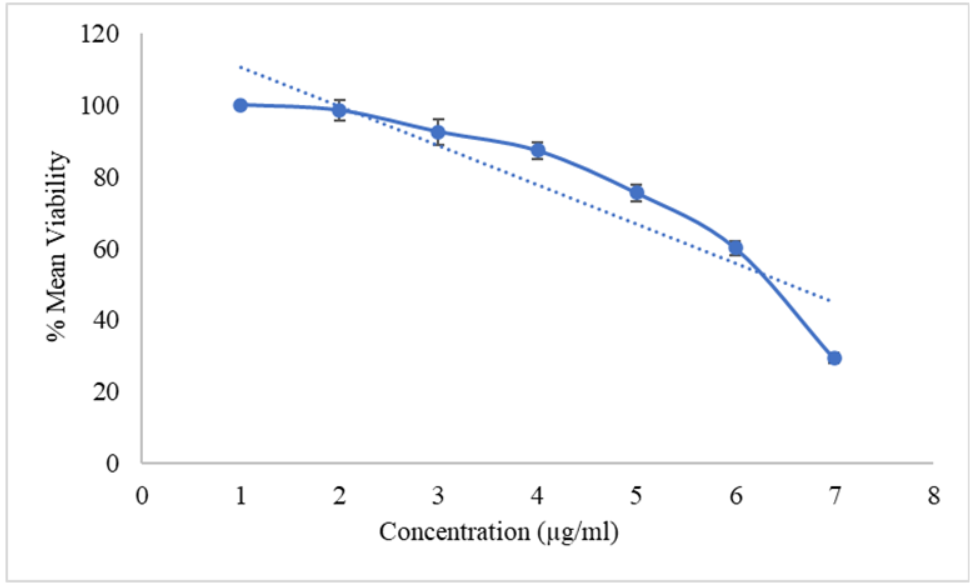
Fig. 5: Cytotoxicity of capsaicin nano bubbles on A498 cell line, all the values were expressed in (n=3) mean±SD
A comparative analysis of the IC50 values of CAP pure drug and CAP-NBs on both A549 and A498 cell lines is summarized in table 5. It was observed that the pure drug exhibited a lower IC50 value in both cell lines (41.56µg/ml for A549 and 59.60µg/ml for A498), indicating higher cytotoxic potential [24, 9]. In contrast, CAP-NBs showed higher IC50 values (73.44µg/ml for A549 and 68.72µg/ml for A498), suggesting that the nanoformulation may have a controlled and sustained release effect, reducing immediate cytotoxicity.
Table 5: IC50 values of both test formulation and standard drug
| Compound | IC50 value (µg/ml) | |
| A549 | A498 | |
| Capsaicin nano bubbles | 73.44±3.5 | 68.72±4.7 |
| Capsaicin pure drug | 41.56±8.6 | 59.60±8.52 |
All the values were expressed in (n=3) mean±SD
The increased IC50 values observed for CAP-NBs compared to pure CAP suggest a slower onset of cytotoxicity, likely due to the sustained release and controlled intracellular trafficking of the encapsulated drug. This effect may reduce systemic toxicity and provide a prolonged therapeutic window. The nanoformulation’s performance underscores its potential as a safer alternative with improved delivery efficiency over time.
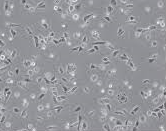
The morphological alterations in A549 cells following treatment with CAP-NBs were observed under an inverted microscope. As illustrated in fig. 6, untreated control cells maintained a normal epithelial-like morphology, whereas cells exposed to CAP-NBs exhibited dose-dependent changes, including cell shrinkage, loss of membrane integrity, and detachment from the surface. These observations further confirm the cytotoxic potential of CAP-NBs against A549 cells [26, 10].
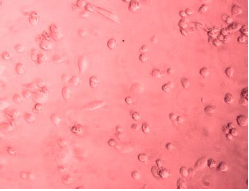 |
 |
| Capsaicin nano bubbles against A549 cellline | Capsaicin pure drug against A549 cellline |
 |
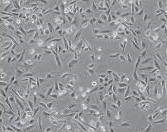 |
| Capsaicin nano bubbles against A498 cellline | Capsaicin pure drug against A498 cellline |
Fig. 6: Morphological changes of A549 cancer cells when treated with capsaicin nano bubbles
The results demonstrate that both CAP pure drug and CAP-NBs exert cytotoxic effects on A549 and A498 cancer cell lines in a dose-dependent manner. However, the pure drug exhibited higher cytotoxicity, as indicated by lower IC50 values [27, 8]. This suggests that the nanoformulation may offer a more controlled and sustained drug release, reducing immediate cell toxicity while potentially enhancing long-term therapeutic efficacy. The findings highlight the potential of CAP-NBs as an alternative delivery system for anticancer applications, with the advantage of improved stability and bioavailability [22].
The higher IC50 values of CAP-NBs may also indicate enhanced cellular uptake mechanisms that require longer exposure times for maximal cytotoxic effects. Further studies, including apoptosis assays and molecular mechanism evaluations, are necessary to elucidate the exact pathways involved in CAP-induced cell death. Additionally, in vivo studies would be essential to validate the therapeutic potential of CAP-NBs in cancer treatment [24].
MTT assay is a colorimetric assay based on assessing the cell metabolic activity. A549 cell line was used to see the cytotoxic potential of a test drug for initial screening of apoptosis or necrosis. The biochemical mechanism behind the MTT assay involves NAD(P)H-dependent cellular oxidoreductase enzyme that converts the yellow tetrazolium MTT [3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide] into insoluble (E, Z)-5-(4, 5-dimethylthiazol-2-yl)-1, 3-diphenylformazan (formazan). In this study, the formulation containing the above-mentioned safe substances showed almost no cytotoxicity in a safety test using the MTT assay. We confirmed that the cell viability (%) of the pretreated media with a fabricated formulation was over 97% (<3% cell death at all concentrations). This result indicates that the prepared microemulsion has almost no cytotoxic effects on the A549 and A498 cell line.
CONCLUSION
The successful formulation of CAP-NBs using the solvent evaporation method with ultrasound assistance demonstrated efficient encapsulation, stability, and controlled drug release properties. Characterization studies confirmed nanoscale particle size, uniform morphology, high entrapment efficiency, and sustained drug release, with ultrasound-triggered enhancement. FTIR, DSC, and XRD analyses validated the structural integrity and molecular interactions within the formulation. Cell line studies further reinforced the therapeutic potential of CAP-NBs, showing significant cytotoxic effects against cancer cells while exhibiting minimal toxicity toward normal cells. The NBs enhanced cellular uptake and demonstrated a dose-dependent reduction in cancer cell viability, suggesting their suitability for targeted drug delivery. The ultrasound-responsive release mechanism further contributed to improved drug efficacy, indicating the potential for non-invasive activation at the tumor site. Overall, CAP-NBs present a promising nanocarrier system for CAP delivery, combining stability, controlled release, and targeted therapeutic effects. Future in vivo studies will be crucial to further evaluate their pharmacokinetics, biodistribution, and therapeutic efficiency for clinical applications.
FUNDING
This research received no external funding.
AUTHORS CONTRIBUTIONS
HKV prepared work plan, and CK reviewed and corrected the article. Both authors agree with the submission and publication. Both authors have read and agreed to the published version of the manuscript.
CONFLICTS OF INTERESTS
No conflict of interest
REFERENCES
Govindarajan VS, Sathyanarayana MN. Capsicum production technology chemistry and quality part v. impact on physiology pharmacology nutrition and metabolism; structure pungency pain and desensitization sequences. Crit Rev Food Sci Nutr. 1991 Jun;29(6):435-74. doi: 10.1080/10408399109527536, PMID 2039598.
Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci. 2001;24(1):487-517. doi: 10.1146/annurev.neuro.24.1.487, PMID 11283319.
Koneru M, Sahu BD, Mir SM, Ravuri HG, Kuncha M, Mahesh Kumar J. Capsaicin the pungent principle of peppers ameliorates alcohol induced acute liver injury in mice via modulation of matrix metalloproteinases. Can J Physiol Pharmacol. 2018 Apr;96(4):419-27. doi: 10.1139/cjpp-2017-0473, PMID 29053935.
Arafath AA, MY, JB. Enhancement of oral bioavailability via solid lipid nanoparticles of anticancer drug dasatinib an in vitro cytotoxicity and pharmacokinetic study. Asian J Pharm Clin Res. 2019 Jun;12(6):143-5. doi: 10.22159/ajpcr.2019.v12i6.33135.
Begum MY, Gudipati PR. Formulation and evaluation of dasatinib loaded solid lipid nanoparticles. Int J Pharm Pharm Sci. 2018 Dec;10(12):14-20. doi: 10.22159/ijpps.2018v10i12.27567.
Kumar MK, Prakash D, Rao VV B. Chitosan nanobubbles development and evaluation for the delivery of sunitinib an anticancer agent. Int J App Pharm. 2022;14(6):58-67. doi: 10.22159/ijap.2022v14i6.45821.
Anil L, Mohandas S. In vitro antioxidant and anticancer activity of macranga peltata leaf extracts on lung cancer cell lines. Int J Curr Pharm Sci. 2023 Apr;15(4):26-32. doi: 10.22159/ijcpr.2023v15i4.3019.
Alley MC, Scudiero DA, Monks A, Hursey ML, Czerwinski MJ, Fine DL. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988 Mar;48(3):589-601. PMID 3335022.
Noor F, Niklas J, Muller Vieira U, Heinzle E. An integrated approach to improved toxicity prediction for the safety assessment during preclinical drug development using hep G2 cells. Toxicol Appl Pharmacol. 2009 Feb;237(2):221-31. doi: 10.1016/j.taap.2009.03.011, PMID 19332084.
Konda M, Sampathi S. QbD approach for the development of capsaicin loaded stearic acid grafted chitosan polymeric micelles. Int J App Pharm. 2023 Apr;15(4):131-42. doi: 10.22159/ijap.2023v15i4.48101.
Foudas AW, Kosheleva RI, Favvas EP, Kostoglou M, Mitropoulos AC, Kyzas GZ. Fundamentals and applications of nanobubbles: a review. Chem Eng Res Des. 2023 Jan;189(4):64-86. doi: 10.1016/j.cherd.2022.11.013.
Wang Y, Wang T. Preparation method and application of nanobubbles: a review. Coatings. 2023;13(9):1510. doi: 10.3390/coatings13091510.
Xu JS, Huang J, Qin R, Hinkle GH, Povoski SP, Martin EW. Synthesizing and binding dual mode poly (lactic-co-glycolic acid) (PLGA) nanobubbles for cancer targeting and imaging. Biomaterials. 2010 Jul;31(7):1716-22. doi: 10.1016/j.biomaterials.2009.11.052, PMID 20006382.
Mondal R, Bobde Y, Ghosh B, Giri TK. Development and characterization of a phospholipid complex for effective delivery of capsaicin. Indian J Pharm Sci. 2019 Jun;81(6):1011-9. doi: 10.36468/pharmaceutical-sciences.598.
Mamatha P, Bhikshapathi DV. Preparation and in vitro evaluation of pemigatinib nanosponges tablets by box-behnken design. Int J Pharm Qual Assur. 2023 Sep;14(3):791-800. doi: 10.25258/ijpqa.14.3.56.
Reddy KS, Bhikshapathi D. Design and optimization of DPC-crosslinked HPβCD nanosponges for entrectinib oral delivery: formulation characterization and pharmacokinetic studies. Futur J Pharm Sci. 2024 Mar;10(1):101. doi: 10.1186/s43094-024-00680-8.
Viswaja M, Bhikshapathi DV, Palanati M, Babu AK, Goje A. Formulation and evaluation of ibrutinib nanosponges incorporated tablet. Int J App Pharm. 2023 Feb;15(2):92-7. doi: 10.22159/ijap.2023v15i2.46813.
Viswaja M, Bhikshapathi DV, Sadasivam RK, Goje A, Cheruku S. Formulation and evaluation of liquid based supersaturable self-nanoemulsifying drug delivery system of manidipine. Int J Pharm Sci Drug Res. 2023 Jan-Mar;15(1):80-7. doi: 10.25004/IJPSDR.2023.150112.
Sampathi S, Amancha R, Dodoala SD, Kuchana V. Biodegradable polymeric nanocarriers for oral delivery of antiretroviral drug: pharmacokinetic and in vitro permeability studies. J Appl Pharm Sci. 2021 Apr;11(4):028-39.
Alley MC, Scudiero DA, Monks A, Czerwinski M, Shoemaker R, Boyd MR. Validation of an automated microculture tetrazolium assay (MTA) to assess growth and drug sensitivity of human tumor cell lines. Proc Am Assoc Cancer Res. 1986;27:389-91.
Berridge MV, Herst PM, Tan AS. Tetrazolium dyes as tools in cell biology: new insights into their cellular reduction. Biotechnol Annu Rev. 2005;11:127-52. doi: 10.1016/S1387-2656(05)11004-7, PMID 16216776.
Fotakis G, Timbrell JA. In vitro cytotoxicity assays: comparison of LDH neutral red MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol Lett. 2006 Feb;160(2):171-7. doi: 10.1016/j.toxlet.2005.07.001, PMID 16111842.
Freshney RI. Culture of animal cells: a manual of basic technique and specialized applications. Wiley Blackwell; 2015.
Jin L, Wang T, Zhu ML, Leach MK, Naim YI, Corey JM. Electrospun fibers and tissue engineering. J Biomed Nanotechnol. 2012;8(1):1-9. doi: 10.1166/jbn.2012.1360, PMID 22515089.
Chou TC, Talalay P. Quantitative analysis of dose effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984 Feb;22:27-55. doi: 10.1016/0065-2571(84)90007-4, PMID 6382953.
Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1-2):55-63. doi: 10.1016/0022-1759(83)90303-4, PMID 6606682.
Weigt C, Huebner H, Meier M, Walczak R. Advances in nanomedicine and targeted therapy for cancer. Trends Biotechnol. 2020 Apr;38(4):423-37.