
Int J App Pharm, Vol 17, Issue 4, 2025, 91-116Review Article
BEYOND NEEDLES: INNOVATIONS IN TRANSDERMAL ANTIBIOTIC DELIVERY SYSTEMS
RAWANDM DAGHMASDa*, SHEREEN M. ASSAFb
aFaculty of Pharmacy, Middle East University, Amman, Jordan. bJordan University of Science and Technology, Irbid, Jordan
*Corresponding author: Rawandm Daghmasd; *Email: r.daghmash@meu.edu
Received: 09 Mar 2025, Revised and Accepted: 20 May 2025
ABSTRACT
As a substitution to traditional needle injections, other non-invasive treatments have lately evolved. Transdermal Drug Delivery Systems (TDDS) are the most appealing of these methods due to their low rejection rate, exceptional simplicity of application, and superior efficiency and endurance among patients. Because this strategy primarily includes local administration, it can could minimize local drug concentration accumulation and broad-spectrum drug distribution to tissues not specifically targeted by the medication. Furthermore, the physicochemical features of the skin translate to several hurdles and limitations in transdermal distribution, necessitating various studies to address these bottlenecks. In this study, we explain the many types of accessible TDDS approaches for delivering antibiotics, as well as a critical evaluation of each method's individual merits and limitations, characterization methodologies, and potential. Advancements in research on these alternative technologies are mentioned in this review.
Keywords: Transdermal antibiotics, Transdermal drug delivery system advantages, Limitations, Microneedles, Characterization
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijap.2025v17i4.54157 Journal homepage: https://innovareacademics.in/journals/index.php/ijap
INTRODUCTION
Skin is the outer lipid the body's layer, having a thickness about 2–3 mm [1, 2]. Aside from serving as a barrier, it also aids in the absorption of numerous medicinal and non-therapeutic substances [3]. The epidermis and the underlying dermis are two skin layers that play a vital role in percutaneous absorption. The hypodermis, or subcutaneous adipose tissue layer, serves as the primary food store, as well as offers a physical protection and thermal insulation. Stratum lies under the dermis and connects it to the underlying structures [4-7]. The cutaneous appendages, which cover just around 0.1 percent of the skin's surface, are made up of hair shafts and sebum glands, as well as sweat glands (eccrine and apocrine) [8, 9]. They provide crucial physiological activities and may also play a role in chemical transport through the skin [4-7]. Fig. 1 representing the anatomy of human skin.
The dermis is a complex structure that contains diverse cell types, including fibroblasts, endothelial cells, nerve cells, mast cells, endothelial cells, nerve cells, and blood cells [4-7]. In humans, this layer, which is primarily made of connective tissue, with a thickness ranges from 3–5 mm and contains important skin structures such as blood capillaries, a lymphatic network, and a network of sensory nerves [10, 11]. When a molecule is applied topically, it can permeate into the dermis layer and enter the central blood circulation [12]. The dermal vasculature provides sink conditions for a penetrant while also acting as a driving factor for percutaneous absorption. The epidermis is a multilayered membrane that has two compartments: the outermost Stratum Corneum (SC) layer and the viable epidermis [10, 12]. The basal epidermal layer produces epidermal cells (or keratinocytes), which migrate to the skin's surface. The epidermis is composed of melanocytes, Langerhans cells, migratory macrophages, and lymphocytes [10, 12-14]. The major obstacle to percutaneous absorption is located in the skin's outer layer, also known as the horny layer [10, 12]. The SC layer is roughly 10–20 µm thick and has a key plays an important function in preventing the movement of water and xenobiotics. The SC cells, known as corneocytes, are regularly supplied by the gradual upward migration of cells generated by the viable epidermis's bottom layer. Corneocytes are hexagonal anucleate cells made of insoluble keratins surrounded by crosslinked proteins and immersed in an intercellular lipid domain. Because the living skin layers and blood vessels are highly permeable and the peripheral circulation is relatively fast, and the rate-limiting step is the SC diffusion. Since SC cells are thought to be dead and have no metabolic action, diffusion across the stratum corneum is passive guided by physical and chemical rules. The composition and structure of SC can explain its barrier properties. The SC structure may be represented as a model of bricks (corneocytes) and mortar (intercellular lipids) [5]. The intercellular lipid domain is the sole continuous area in the stratum corneum [15, 16]. Ceramids, free fatty acids, cholesterol, and cholesterol esters are the primary components of intercellular lipids [17]. The structure of the lipid domains is thought to be critical for skin barrier function. The lack of phospholipids, which separates SC from other biological membranes, is a notable property of SC [4, 17]. The skin's extraordinary barrier characteristics are due to the SC's particular composition, which prevents the passage of highly polar, ionic, and macromolecules substances. Highly lipophilic compounds that quickly partition into the SC, on the other hand, do not easily diffuse into the hydrophilic epidermis and dermis, and their clearance is slowed [6].
Hair follicles, for example, are skin appendages supported by the dermis and may be responsible for passive medicine absorption via the transdermal route (fig. 1) [18]. Because substances like therapeutic agents may move into the systemic circulation immediately after passing the skin, this method has attracted pharmaceutical experts to conduct drug delivery studies for the previous twenty years [19]. Because of the avoidance of dosage variations, first-pass metabolized in the liver, and enhanced bioavailability, the transdermal route is regarded a preferable option than oral route of drug administration [20]. Due to being non-invasive and the simplicity of distribution of the drug, this enhances patient compliance [21]. Various issues should be addressed when designing transdermal drug delivery systems, such as the epidermal barrier, which only permits lipophilic substances with a molecular weights below 500 kDa (kilodaltons) to pass through [22]. The rate of drug entry over this barrier is extremely sluggish. However, efficient delivery of hydrophilic medicines via the skin remains a difficult challenge [23]. Also, skin is capable of absorption and excretion, as well as selectively transmitting chemical compounds required for bodily hemostasis.
Transdermal delivery is divided into two types: active and passive. Active approaches disturb the stratum corneum, while passive methods modulate drug-vehicle interactions to promote skin diffusion. Transdermal drug delivery technologies, including electroporation, phonophoresis, and laser-assisted procedures, enhance medication absorption and bioavailability by overcoming the skin's natural barrier [24, 25]. Studies had showed that Niosomes have the ability to improve drug solubility, bioavailability, and release, making them helpful in pharmaceutical applications [26].
Since the extensive antibiotics use began in the 1940s, concomitant Antimicrobial Resistance (AMR) became a source of concern, due to the significant morbidity and mortality [27]. AMR has grown into a major hazard to worldwide global health, and controlling it is a top focus for public health officials [28].
gut microbiome is considered as an important AMR preventative factor by enhancing host colonization resistance [29, 30]. Alteration in taxonomic content have been linked to increased vulnerability to a variety of diseases, comprising diabetes, obesity, liver illness, colon cancer, and Inflammatory Bowel (IBD) [31-33].
Antibiotics has a major influence on the makeup of the gut microbiome, resulting in a significant decrease in the microbiota's taxonomic abundance and multiplicity diversity [34, 35]. The extensive use of antibiotics can lead to prolonged chronic infections such as vancomycin-resistant enterococci (VRE) and Clostridium difficile, and bowel colonization is acknowledged to lead bloodstream infections in vulnerable people [36-39]. Due to antibiotic treatment, dysbiosis in the gut microbiome has been linked to a variety of disorders in neonates and preterm newborns, including allergies, asthma, atopy, obesity, and abnormalities with brain development [40, 41].
Gut identified as a key pool for Antimicrobial Resistance Genes (ARGs), that pass in feces and so, they contribute to the antibiotic resistome of other environments [42-44]. Geographic variations in antibiotic resistomes of fecal material support the link among antibiotic usage and the evolution of resistance through the gut. ARG profiles from various nations correspond with antibiotic usage in particular locations, both in terms of antibiotic type and quantity of antibiotic utilized. The longer the duration of use of antibiotics clinically, like cephalosporins and tetracycline the more ARGs [42, 45].
The detected dysbiosis and fast accumulation of ARGs emerge from an antibiotic challenge to gut microbes, leading in enhanced buildup and transmission of these genes [46]. The mutations are more noticeable with increased concentrations of antibiotic exposure in the stomach, which is largely determined by dosage, length of therapy, and, importantly, mode of administration of the antimicrobial drug. Decreasing the quantity of antibiotic that enters the stomach is likely to reduce AMR advancement. As a result, medication delivery mode, particularly patient-convenient delivery methods that limit unnecessary antibiotic exposure to the gut microbiota, should be addressed as a major tactic, which is a smaller segment of larger antimicrobial stewardship program. The field of AMR is at a crossroads. This rising issue quickly turns into a disaster, and significant antimicrobial stewardship efforts are required to protect world health. The manner in which antibiotics are administered might be a crucial component of any such program, and medication delivery mechanisms play a vital role in the battle against AMR. Numerous studies have investigated transdermal administration techniques for antibiotics, which could enhance therapeutic results and possibly slow the emergence of AMR: such as Microemulsion-Based Bacteriophage Transdermal administration: Using a microemulsion technology, an ex vivo and in vivo investigation examined the transdermal administration of a T4 bacteriophage specific to Escherichia coli. According to the results, this method might be a useful substitute for conventional antibiotic therapy in the treatment of bacterial infections [47]. Dissolving Microneedle Arrays for Gentamicin Delivery: Studies on the transdermal administration of gentamicin using dissolving microneedle arrays showed promise in the treatment of neonatal sepsis. This technique might offer a less invasive way to give antibiotics, which could increase compliance and decrease misuse [47]. Vancomycin Hydrochloride Nano-Ethosomes and Iontophoresis: A study investigated the use of iontophoresis and nano-ethosomes in conjunction to improve transdermal delivery of vancomycin hydrochloride. Better medication penetration and bioavailability were shown by the in vitro and in vivo studies, indicating a potential substitute for traditional delivery methods [47]. Vancomycin Hydrochloride Polymeric Microarray Patches: The creation and assessment of polymeric microarray patches for transdermal administration of vancomycin hydrochloride revealed encouraging pharmacokinetic characteristics. This strategy might make dosing more constant and possibly lower the chance that resistance will arise as a result of inadequate dosage [47]. Although these trials demonstrate novel transdermal delivery strategies for antibiotics, more clinical investigations are required to prove a link between TDDS and a decrease in the emergence of AMR. More conclusive proof that transdermal antibiotic distribution is an important part of AMR prevention would come from extensive clinical trials evaluating the long-term effects of this approach on resistance trends [47].
The claim that TDDS can prevent antibiotic resistance by reducing disruptions to the gut flora is now being investigated. There are currently few direct comparative clinical studies examining microbiome changes in people treated with TDDS versus oral antibiotics. However, indirect data suggests that the method of antibiotic delivery may impact gut microbiota makeup. For example, a study published in Nature found that neonates who were directly treated with antibiotics had lower amounts of good gut bacteria, such as Bifid bacterium, which led to worse immunological responses to immunizations later in infancy. This effect was not detected in newborns whose mothers were given antibiotics during birth, implying that direct antibiotic exposure had a greater influence on the infant gut flora [48]. While this study does not directly compare TDDS to oral antibiotic delivery, it does highlight the importance of antibiotic exposure pathways for gut microbiota integrity. Transdermal administration skips the gastrointestinal route, which may reduce direct interactions with gut flora. As a result, it is likely that TDDS could attenuate gut microbiome disruptions caused by oral antibiotics, potentially lowering the development of antibiotic resistance connected to microbiome imbalances. Additional research is required to support this notion. Comparative clinical research comparing microbiota changes in TDDS patients against those receiving oral antibiotics would give more conclusive data about the influence of transdermal delivery on gut microbiome composition and its involvement in antibiotic resistance prevention [48].
This review focuses on antibiotic transdermal delivery techniques that manage to prevent AMR. These precautions include avoiding the stomach and adopting customized delivery routes, as well as reducing avoidable antimicrobial challenge that might cause development of AMR.
This review will cover the advantages of TDDS, challenges faced while using TDDS, transdermal delivery of antibiotics, new technologies used for transdermal antibiotics delivery, and characterization methods.
Search criteria
A thorough literature search was conducted using predetermined keywords to find all pertinent articles published up to 2025 in Web of Science (http://www.webofknowledge.com), ResearchGate (https://www.researchgate.net), PubMed (https://pubmed.ncbi.nlm.nih.gov), and Google Scholar (https://scholar.google.com). Both phrase and text words were used. Among the most popular terms were “Transdermal antibiotics, Transdermal drug delivery system advantages, Limitations, Microneedles, and lastly Characterization of Transdermal drug delivery systems ".
Transdermal drug delivery system (TDDS) advantages
TDD provides offers huge advantages including: bypassing the gastrointestinal tract, and thus, eliminating the effect of the first pass metabolism, no interference with the PH, enzymes, and the bacteria of the intestine, so this will enhance the bioavailability [50-52], and this had been supported by the study which investigated the transdermal administration of vancomycin hydrochloride utilizing nano-ethosomes and iontophoresis. The study found a maximal transdermal flow of 550 µg/cm²/h, demonstrating efficient systemic administration. In vivo tests revealed equivalent bacterial count decreases between intramuscular and transdermal injection, implying similar treatment efficacy [53]. Also, a study of a cationic nanoemulsion gel for transdermal administration of rifampicin indicated considerable improvements in pharmacokinetic characteristics. The study found a 4.34-fold rise in Maximum Plasma Concentration (Cmax) and a 4.74-fold increase in Area Under The Curve (AUC) when compared to oral delivery, showing significantly increased bioavailability [54]. Furthermore, a study looked into the usage of transfersomal nanoparticles for transdermal delivery of clindamycin phosphate. The data revealed a much larger cumulative amount of medication penetration and flux than typical gel formulations, implying improved transdermal absorption [55].
A more uniformly distributed plasma levels of molecules, and a decrease frequency of dosing due to prolongation the duration of action, so, this will lead to a reduction in side effects, as well as, maintaining a more stable plasma concentrations, so this will improve therapy, decreasing in first-pass drug metabolism. Furthermore, a reduction in the side effect, which might be due to the continual usage of medication, such as, liver damage or renal failure [56]. Using TDDS enhances the compliance of the patients by eliminating of the bad taste of the antibiotics. TDDS are often less expensive on a monthly basis when compared to conventional methods, as patches aim to administer medications for 1 to 7 d. Another advantage of TDDS is that with programmable systems, multiple dosage dosing, when needed or at different rate drug administration is conceivable, adding extra benefits to traditional patch dosage forms [57, 58].
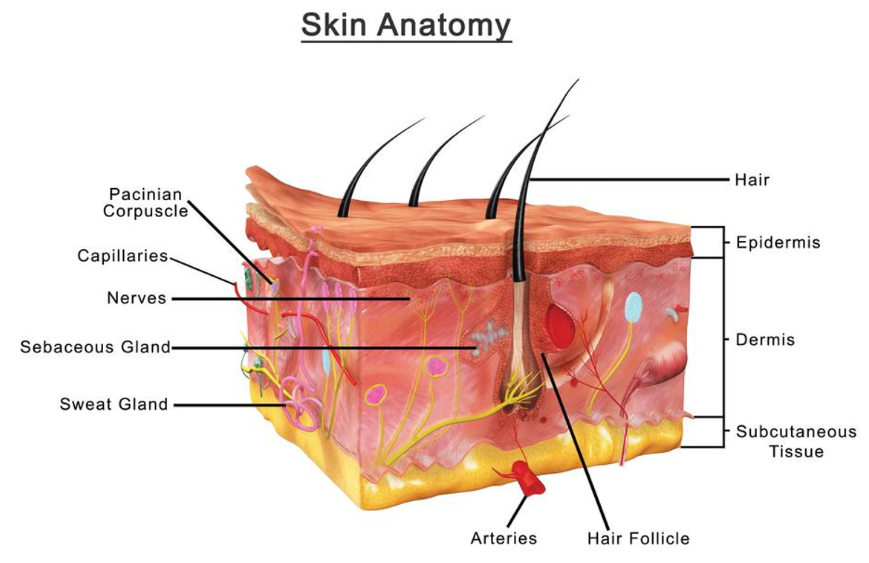
Fig. 1: A graph representing the anatomy of human skin[49]
Challenges faced while using transdermal drug delivery system
Skin permeation
Because of the specific environment of the epidermal skin layer, only a limited number of medicines may be given for systemic therapy meaningful rates. The whole section of the transdermal medication industry is made up of about 15 medicines. Aside from high potency, the physicochemical properties of drugs that are frequently cited as beneficial for percutaneous administration they comprise of modest hydrophobicity and low molecular weight [59]. However, a significant proportion of pharmacological substances do not fit these standards. This is more accurate for macromolecules such as insulin, human growth hormone, or cyclosporine, which pose significant drug delivery challenges. Overcoming poor skin miscibility to xenobiotics can be accomplish through a number of methods, and this is an active area of research. Their efficacy and applicability will differ based on the physicochemical nature of the component. So, skin permeability can be solved by either chemical method, physical methods, and a prodrug approach [59].
Overcoming the skin permeation barrier: challenges and solutions
Due to the unique properties of the epidermal layer, the choice of medicines that can be given systemically are restricted to a few. Potency and physicochemical properties are important features to study when the drug is chosen for percutaneous administration. Some of the physicochemical properties include adequate lipophilicity and small size (low molecular weight). Many drugs do not comply with these needs. The techniques of how to overcome bad skin permeability are an area for research. According to the physicochemical properties of the xenobiotics, the efficacy and applicability are determined [59].
Chemical methods
Permeation enhancers improve drug penetration over epidermis by two mechanisms: either by boosting drug partitioning into the stratum corneum's barrier region, or, they increase drug diffusivity in this region, or by both mechanisms [60]. It is known that stratum corneum is made of up multilamellar layers, which compose of intercellular continuous lipid ‘mortar' and keratin 'bricks' [61-63]. Medication nature plays a major role in permeation and of which these two mechanisms might influence the percutaneous transport (rate limiting). As a result, it is expected that the extent of permeability enhancement acquired with a particular permeation enhancer will differ amongst lipophilic and hydrophilic medicines. Many mechanisms are involved in enhancing skin permeation by increasing the humidity of stratum corneum, extracting intercellular lipids, enhancing drug thermodynamic action, increasing stratum corneum hydration, altering proteinaceous corneocyte composition [64, 65]. Permeation enhancers are traditionally classified into categories depend on the chemical structure more than their method of activity. This is owing in part to the lack of ability in in distinguishing whether they have a main or multiple method of activity. Furthermore, chemicals from the same class might exert their impact via several methods. More than 300 chemicals have been found to be skin permeability enhancers.
Enhancers are divided into categories: Alcohols such as, ethanol, pentanol, lauryl alcohol, benzyl alcohol, glycerol, and propylene glycols. Amines such as, diethanolamine and triethanolamine. Esters such as, ethyl acetate, isopropyl palmitate, and isopropyl myristate. Fatty acids such as, valeric acid, linoleic acid, oleic acid, and lauric acid. Amides such as, pyrrolidone derivatives, 1-dodecylazacycloheptane-2-one[Azone®]urea, dimethylacetamide, and dimethylformamide. Surfactants such as, sodium laureate, cetyltrimethylammonium bromide, Brij®, Tween® and sodium cholate. Hydrocarbons such as, alkanes and squalene. Terpenes such as, D-limonene, carvone and anise oil. Phospholipids such as, lecithine. Sulfoxides such as, dimethyl sulfoxide.
SC hydration level cannot be overstated. The diffusional resistance to xenobiotics is much lower in case of completely hydrated stratum corneum than in a dehydrated one. Skin discomfort is the most prevalent disadvantages. Skin irritation is caused by the same processes that cause increased drug transport, by breaking lipid bilayers of the stratum corneum [66]. One approach to addressing this challenge is to discover permeation enhancer mixes with synergistic effects [67].
Karande et al. 2004 effectively tested 5000 binary chemical mixes using a screening technique [68]. The majority of transdermal products exploit the occlusion effect, which may be categorized as enhancers functioning through increasing the fluidity of the stratum corneum.
Enhancers have traditionally been employed to augment small-molecule delivery, with relatively little effectiveness in increasing the penetration of macromolecules. Overall, while chemical treatments are successful, they cannot compete with physical augmentation procedures that produce a larger degree of skin permeabilization [68].
Physical methods
Hypodermic needles are the earliest and the most prevalent methods of physically bypassing the epidermal barrier. Throughout many circumstances, it is the only practical means of delivering substances that are poorly absorbable or unstable. This method of administration leads in the rapid distribution of huge volumes of medicine [69]. This method has many drawbacks being painful, and need to be administered with the help of professionals. Also it would be problematic to be used with patients suffering from phobia. So, several methods are being developed to be used instead of using needles (physical skin enhancement methods) like jet injections, laser, thermal ablation, dermabrasion, iontophoresis, MNs (MNs), ultrasound, electroporation, and combinations of the of the previous methods. These approaches are considered as convenient. Also affords a more controlled drug delivery pattern [69].
Prodrugs
The prodrug administration comprises various domains of concern, including skin permeability, chemical and enzymatic stability, and potential of skin irritation. Those factors must be addressed in order to build a good prodrug, the major rationale for using this strategy is to change the physicochemical characteristics main drug and thus entry of the main drug is greater than parent molecule. A chemical alteration of the component of interest provides an excellent chance to change its capacity to pass the stratum corneum when the diffusional barrier (lipid region) and the parent medication is moderately hydrophilic, increasing its lipophilicity can result in increased percutaneous flow. On the other hand, is paid for by increasing the size of the permeant. By producing more hydrophilic prodrugs, it was feasible to enhance the flow of very hydrophobic substances (which undergo viable tissue-controlled diffusion as opposed to stratum corneum-controlled diffusion) [70].
Skin irritation
The decrease in systemic adverse effects is a significant benefit of topical and transdermal medication administration. On the other hand, medication distribution via these channels introduces the possibility of other certain adverse reactions such as skin irritation at the site of action. Irritating contact dermatitis (ICD), an inflammatory response generated by repetitive or direct contact of the skin with mild irritants, and allergic contact dermatitis (ACD), a delayed, T-cell-mediated inflammatory reaction to a particular allergen, are two types of skin irritation responses. ICD reactions include erythema, scaling, and burns causing necrosis necrotic burns, whereas ACD reactions include erythema, edema, and, on rare occasions, vesiculation. Furthermore, the beginning of ACD responses is very diverse and depends on the irritant that triggered the response as well as the individual who exhibits the allergic response physiological pH changes is the major cause of skin irritation due to, hydration, delipidization, and disruption of lipid packing in stratum corneum. Also, bacteria proliferation, physiological and immunological reactions, as well drug (active pharmaceutical ingredient (API)) or vehicles or excipients used or the formulation used may influence skin irritation. As well, duration of TDDS application [71].
During the device design phase, the risk of generating skin irritation could be reduced by managing the device itself and formulation components. Additional methods for reducing adverse skin reactions was described by using a corticosteroid before applying the transdermal antibiotic, inclusion of corticosteroids in the formulation, and structural changes to chemical permeation enhancers, to name a few. Later, we'll go through the factors that have been discovered to cause skin irritation, and also the as well as techniques for preventing or treating these responses [72].
The role of drug features in skin irritation
The API was identified as the primary reason for ACD in individuals [79]. Therapeutic agents cause moderate-to-severe irritability in animal trials are frequently deemed out for usage in topical preparations. Predicting a drug's irritation potential without first studying it in an animal or cell model is challenging. It has been observed, for example, that the dissociation constant (pKa) of an API might change the dermal pH, causing cutaneous discomfort. Berner et al.1988 demonstrated that benzoic acid analogues that has a pKa values of 4 or less induced skin irritation in individuals after 24 h of exposure [73, 74]. Additionally, medicines with pKa values greater than 8 have been reported to cause dermal irritation. A number of studies found an increase in irritation with increasing pKa. According to this research, medicines with pKa values between 4 and 8 should cause the least amount of skin irritation. Furthermore, rather than omitting potentially irritating medications, by reducing their concentration in a topical or transdermal formulas may minimize the ensuing irritation to harmless and acceptable levels [75, 76].
The role of vehicle and devices in skin irritation
Skin's surface pH of the has been found between 5.4 to 5.9, which is critical for maintaining the defensive role of the skin against microbes and illnesses [77]. The skin is capable of producing buffers that prevent major pH shifts. However, external stimuli such as bathing and rubbing on solutions, medications, and cosmetics to the skin's surface can change its pH and hence aggravate or provoke dermal irritation. Alkaline solutions with a pH of 9 or higher, for example, have been known to induce skin irritation. Also, this investigation, aqueous solutions with pH levels of 5 and 7 did not irritate the skin [78].
Other investigation done by, Ananthapadmanabhan et al. 2003 discovered that applying a pH 10 solution to the skin, as opposed to a pH 4 or 6.5 solution, raised the transition temperature of stratum corneum lipids [79]. Enlargement and inflammation of the stratum corneum and the loss of the primary function of the skin as a barrier is known to give a rise in water loss through the epidermis. As a result, it is critical for the skin products to have a pH that is very near to the skins pH. In order to minimize skin irritation. Some universal medicinal solvents are skin irritants and so should not be utilized in topical treatments. Before in vivo testing is done, the solvents should be substituted with solvents that have tolerable irritation limits and good safety. To a certain extent, topical solvents approved safe for usage comprise of isopropyl alcohol, propylene glycol, isopropyl myristate, and polyethylene glycols (up to 60 percent are available commercially) [80].
A wide range of transdermal and local topical methods need extended skin occlusion in dosage forms of a cream, lotion or patches, etc., medicinal formulations. Non permeable skin occlusions for water have shown to raise the pH and warmth of the skin's surface while also retaining moisture and perspiration to produce a humid atmosphere. These circumstances encourage bacterial and yeast proliferation on the skin's surface. Sweat has been proven in studies to cause occlusion-induced irritation [81]. ICD responses are prevalent following skin occlusion, and their frequency has been observed to rise with occlusion time [71, 82].
Furthermore, occlusion may enhance the irritability of the topically applied API. Van der Valk and Maibach, for example, demonstrated that postexposure occlusion improved the irritation response to frequent temporary exposure sodium lauryl sulfate in ten healthy participants [83]. Occlusion has also been shown to cause functional impairment to the skin barrier, as measured by trans epidermal water loss [84].
Choosing the matrix-type patch vs a reservoir-type patch for the therapeutic agent administration might influence the likelihood of skin irritation. Each system's constituent have been observed to cause skin responses when used. Skin responses to the adhesive, hydroxypropyl cellulose, and ethanol in the original TDS have been recorded in the case of transdermal distribution of estradiol. Also, methacrylate reactions in the transdermal nicotine patch have been described [85]. Because most patch reactions are ACD reactions, predicting the irritation potential of particular patch components is challenging. Moreover, investigations comparing discomfort produced by matrix vs reservoir-type patches are inconsistent. Nevertheless, according to one source, matrix patches that are loaded with drug induce fewer cutaneous responses than reservoir release patches [86]. In contrast, comparable skin irritation was found after applying a fentanyl matrix or reservoir patch to the skin of healthy human individuals [87].
Formulation impact on skin irritation
The formulation used to distribute a medicine has been demonstrated to alter the appearance and severity of skin irritation. For example, hydrogels have been shown to minimize skin irritation by water absorption from the surface of skin [71]. Research that compared the safety and effectiveness of a lotion comprising benzoyl peroxide encapsulated in polymeric microspheres to a lotion containing free benzoyl peroxide found that controlled release of benzoyl peroxide from the microspheres decreased skin irritation in people while maintaining effectiveness. Depending on these findings, researchers hypothesized that controlled-release devices might be effective in minimizing discomfort caused by medicines used locally on the skin. medications [88]. Liposomes have also demonstrated to help with skin irritation. Liposomes' proposed methods for reducing skin irritation include increasing the moisture of the skin, hydration of the epidermis and prolonged drug release, and thus, prevent reaching dangerous drug concentrations in the skin [89]. When delivered to patients, tretinoin encapsulated in liposomes revealed less skin irritation than the equipotent gel formulation [90]. Employing a hydrogel or a cream containing a medicine encapsulated in liposomes or microspheres will reduce dermal irritation possibility of formulas used topically on the skin.
The addition of a soothing agent to the dermal products to topical formulations has been demonstrated to avoid or minimize skin irritation by reinforcing the skin's protective barrier. Zhai et al., 1998 for example, demonstrated that rubbing the skin with a cream having paraffin wax in cetyl alcohol before applying creams containing prior to treatment with sodium lauryl sulfate or ammonium hydroxide avoided irritation generated by these recognized irritants [91]. Furthermore, Wigger-Albeti et al. 1997 demonstrated that putting petrolatum to the skin prior to the irritating agent inhibited the irritating action of recognized irritants sodium hydroxide, toluene, and lactic acid on human skin. Petrolatum was shown to be less efficient in protecting against toluene-induced irritation [92].
Chemical permeation enhancers
The stratum corneum is an impenetrable wall external substances such as medicines. As a result, permeation-enhancing compounds are frequently used to help medications pass through the stratum corneum. Fatty acids, organic solvents (such as acetone and ethanol), alcohols, esters, and surfactants, and several others are permeation-enhancing substances. It is often assumed that higher efficacy of challenging to reduce the irritation induced these substances since the same processes that generate penetration also cause irritation. While powerful enhancers can temporarily compromise the rigidity of the stratum corneum barrier, their impact is not fully restricted to the stratum corneum, and interactions with living epidermal cells that can produce cytotoxicity and irritation. Investigations have been conducted with some success with the goal of minimizing the irritation capability of recognized permeation enhancers without diminishing their strength. Mixing permeation enhancers (synergistic combinations) and manipulating their chemical structures are two published ways for minimizing skin irritation caused by permeation enhancers. Ben-Shabat, Baruch, and Sintov created propylene glycol mono-and di-ester derivatives of saturated and unsaturated fatty acids to advance permeation without causing excessive irritation [93]. None of the studied derivatives improved penetration more than the corresponding fatty acid, while propylene glycol conjugates of oleic and linoleic acid reduced skin irritation while maintaining similar enhancing potential as compared to the free fatty acid. Sintov and Ben-Shabat have also reported on the creation of fatty acid–drug conjugates to improve penetration while maintaining safety [94]. Finally, Karande and Mitragotri analyzed several permeation enhancer combinations, their safety, and their synergistic enhancement in improving drug permeability [95]. One example offered was a cyclodextrin–enhancer combination that lowered the enhancer's adverse effects while retaining its permeability ability. In conclusion, it appears that a mix of permeability enhancers, solvents, and medicines may be capable of increasing potency without causing excessive irritation.
Anti-irritants and corticosteroids in reducing skin irritation
Steroids applied to the skin before irritant formulations have been shown to lessen skin responses. In humans, applying 0.1 percent triamcinoloneacetonide cream to the skin have demonstrated to reduce the occurrence and/or extreme skin responses related to with TDS exposure. This was found by comparing cumulative irritation scores obtained in individuals who received steroid pretreatment vs those who did not get pretreatment [96]. Finally, it has been demonstrated that including anti-irritants into irritating formulations reduces unfavorable skin responses [97]. Glycerol [98, 99], triamcinolone acetonide [99], lobetasol, and diphenhydramine are some anti-irritants that have been found to reduce adverse effects induced by irritants [100]. These solutions were demonstrated to minimize skin irritation caused by nonanoic acid, sodium lauryl sulfate, and captopril gel.
Transdermal drug delivery of antibiotics
Active transdermal drug delivery
Exterior stimulation, like electrical, physical stimulation, or mechanical, are known to improve skin permeability for molecules [101]. Active transdermal delivery is TDDS enhanced by using suitable equipment that is known to deliver molecules in a faster way. Additionally, this technique of improved TDDS has the potential to expedite the therapeutic effectiveness of administered medications (fig. 2) [102-104].
Microneedle (MN)
The MNs are considered to be a novel DDS, were drugs can be directly administered to blood circulation [105]. Needles in micro size are used to pierce the skin, reaching the epidermis, in order to deliver drugs. MNs are small and thin in order to be painless and to be able to transport medicines directly to the blood circulation region for active absorption, reducing pain [106]. Many attempts has been done by scientist in order to optimize MNs in order to obtain an effective insertion throw the skin [106].
MNs fabrication has been extensively studied, taking into account the purpose, drug type, dosage, and usage targets [107]. Till now, laser-mediated methods and photolithography have been used to create the metal MNs, typically achieve penetration depths of 700–1,500 µm. These MNs provide strong mechanical properties but can cause discomfort if penetration is too deep. A microneedle's 3D shape is created by cutting or ablating a flat metal/polymer exterior with a laser [108, 109].
Photolithography is a process of intricately constructing MNs that has the benefit of being able to produce needles of diverse forms and substances. This approach is mostly employed in creatinga dissolving/hydrogel MNs or silicon MNs by etching photoresist to create an inverse mold based on the microneedle structure. Since photolithography enables fine control over MN dimensions and forms, resulting in highly homogeneous arrays. Such precision can improve mechanical strength and ensure uniform skin penetration. However, the materials commonly employed, such as silicon, can be brittle, potentially restricting mechanical robustness. Drug loading capacity in photolithography-fabricated MNs is frequently limited by the material characteristics and fabrication technique. Silicon MNs produced through photolithography often penetrate the skin at depths ranging from 500–1,000 µm, making them effective for transdermal delivery while minimizing deep tissue discomfort [110].
Also, advanced 3D printing processes, including as two-photon polymerization and microstereolithography, have transformed MN manufacture, allowing for the creation of sophisticated and personalized shapes. According to studies, 3D-printed MNs have higher mechanical strength and better drug delivery effectiveness. For example, morphology-customized MNs improved not only mechanical strength but also drug loading capacity and release behavior, thanks to their highly customizable topologies. Furthermore, magnetic field-aided 3D printing (MF-3DP) has been used to create MNs with increased mechanical properties, surpassing limitations of existing production methods.3D-printed polymer MNs generally penetrate between 300–800 µm, with some custom designs reaching up to 1,200 µm, allowing for tunable drug release based on depth [111, 112]. Microstereolithography are widely investigated to produce MNs, the penetration depth of MNs varies based on fabrication method and material properties, typically ranging between 500 µm and 1,500 µm [113].
There are many types of MNs such as solid MNs which it role to pierce the skin to produce pores to make drug absorption much easier, coated MNs enhance the delivery of drugs which they are deposited at the surface of MNs, dissolving MNs which are constructed of medication formulations that dissolve once it gets in to contact with the interstitial fluid naturally administered melting needles that incorporate drug storage in hollow needles followed by delivery, and microneedle patches paired with various patch [114, 115].
The penetration depth of MNs is an important determinant in both efficacy and patient satisfaction. MNs penetrate the stratum corneum, reaching the epidermis or upper dermis at depths of 500 µm to 1,500 µm. Shorter MNs (≤500 µm) typically generate mild discomfort and do not stimulate deeper pain receptors. However, when MN duration increases, pain perception rises significantly. One study found that increasing MN length from 480 µm to 1,450 µm led to a sevenfold increase in pain ratings. As a result, while greater penetration can improve drug delivery to specific skin layers, it may also cause increased discomfort and systemic consequences. Balancing penetration depth is critical for improving treatment effects while reducing unpleasant feelings [116].

Fig. 2: Difference between various transdermal delivery systems in depth of dermal penetration [117]
Hydrogel-forming MNs
Hydrogel MNs arrays are fabricated using a process cold crosslinking of polymers in an aqueous [118].
These kinds of MNs are mainly made up of baseplate where micron-scale needles are organized. Drugs are mainly kept in a tank linked to the top of the base plate. The release mechanism is by releasing the drug from the reservoir to the blood circulation [119]. Several researches [119-122] demonstrated that hydrogel-forming MN arrays can efficiently distribute a range of compounds, including hydrophilic, hydrophobic, high molecular, and high dosage chemicals [119-122]. Hydrogel MNs have many advantages of being biodegradable and biocompatible, so no accumulation of ruminants in the skin. They are also not confined to the delivery of medications inside the needles [121].
In order to overcome the problems associated with oral therapies for tuberculosis (TB), such as liver damage, hydrogel-forming microneedles (MNs) were created. Four TB medications—isoniazid, pyrazinamide, ethambutol, and rifampicin-were manufactured in MN arrays using various tablet forms, including lyophilized, directly compressed, and poly (ethylene glycol) tablets. Polymers (S-97, PVA, and PVP) were used to make hydrogel films, which were then cross-linked at various temperatures. According to in vitro research, the characteristics of the medication affected its ability to permeate the hydrogel sheets. The two drugs with the highest penetration rates were ethambutol (46.99 mg) and rifampicin (3.64 mg). This method showed how hydrogel-forming MN arrays might be used to effectively distribute TB drugs transdermally [123].
Infection is a major cause of death for infants and young children in less developed nations. The WHO suggests oral amoxicillin and injectable gentamicin as treatments; however, gentamicin must be administered by medical personnel, which can be difficult in rural regions. This was addressed by the development of a novel transdermal delivery system for gentamicin that uses polymeric microneedle (MN) arrays. The MN arrays were created using two polymers, PVP and sodium hyaluronate, along with 30% gentamicin. These arrays, which have 19 × 19 needles and a height of 500 μm, are robust and have a 378 μm skin penetration capacity, making them a simpler and safer option for outpatient therapy [124].
The synthesis of dissolving MNs starting First with the selection of the polymers starts, which are then blended with gentamicin and dissolved in deionized water. Mixing and sonification are done at 37 °C for 1–2 h. The resulting preparation was then dispensed in 100 mg into the MN mold at positive pressure (3–4 bar) for 15 min, until the mold was filled properly. Further, MNs array were dried at 19 °C for 24 h. As they are dry, they are evaluated through a Leica EZ4D digital light microscope [124].
A study of gentamicin-loaded dissolving Microneedle (MN) arrays gives a thorough assessment of their potential for transdermal medication administration. However, no direct comparison of in vitro release data between this unique technology and conventional administration procedures is provided, making it difficult to completely evaluate the MN system's therapeutic potential. In vitro permeation investigations revealed that dissolving MNs supplied 14.85% (4.45 mg) of the gentamicin load over a 6-hour period. Furthermore, approximately 75% of the gentamicin contained in the MN array was successfully administered within 24 h. This suggests a prolonged release profile, with the medicine delivered constantly from both the needle tips and the baseplate of the MN array. In vivo studies in animal models demonstrated that the Cmax of gentamicin reached with MN administration ranged from 2.21±1.46 μg/ml to 5.34±4.23 μg/ml, depending on the dose administered. The time required to achieve these peak concentrations (Tmax) ranged between 1 and 6 h, showing a dose-dependent absorption profile. Notably, the Area Under the Curve (AUC) values for MN delivery were larger than for Intramuscular (IM) injection, indicating that the MN approach provides more prolonged drug release. While the study sheds light on the pharmacokinetics of gentamicin administered by dissolving MNs, it does not provide a direct comparison of in vitro release patterns between the MN system and traditional administration methods, such as intramuscular injection. Comparative data would be useful in completely clarifying the therapeutic potential and efficiency of the MN delivery method relative to established procedures [124].

Fig. 3: A graph representing hydrogel films synthesis and crosslinking [123]
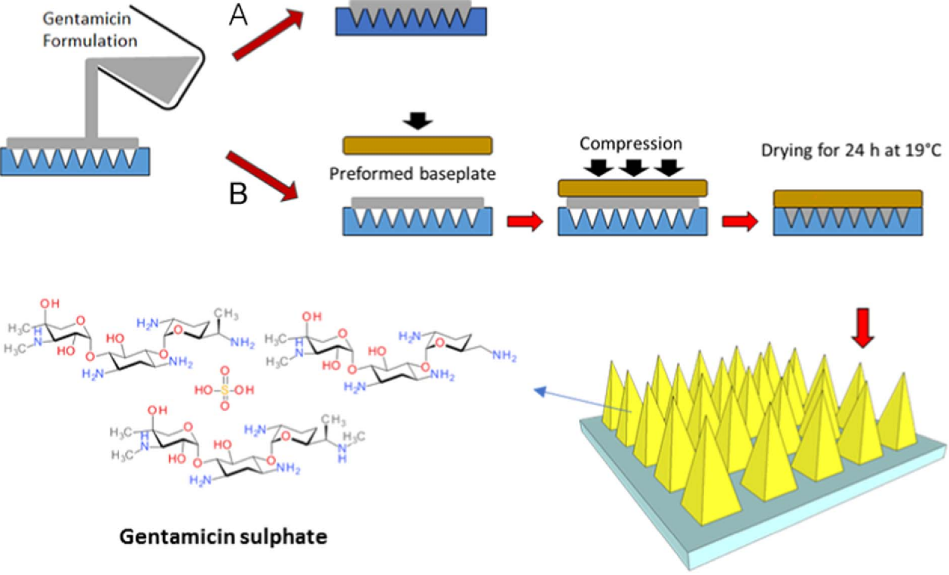
Fig. 4: The synthesis of MNs (A) one-step and (B) two-step [124]
In another study, a composite pharmaceutical system consisting of dry reservoirs of Cefazolin (CFZ) and hydrogel-forming microneedles (MNs) had been developed. Gantrez® S-97 and Carbopol® 974P NF crosslinked with PEG 10,000 were used to create the hydrogel system. According to swelling kinetic experiments, the hydrogel system that was created could achieve 4000% swelling in PBS with a pH of 7.4. Additionally, CFZ was able to achieve ≈100% cumulative penetration across the swollen hydrogel sheet, according to the results of a solute diffusion analysis. Optical coherence tomography demonstrated that the hydrogel system could penetrate the stratum corneum when formed into MNs, allowing the hydrogel-forming MNs to be inserted intradermally into the skin of an ex vivo neonatal pig. Furthermore, two distinct dry reservoirs filled with CFZ were created and characterized using Lyophilized (LYO) wafers and Directly Compressed Tablets (DCT). In less than a minute, these dry reservoir systems dissolved in phosphate buffer saline pH 7.4 demonstrating rapid dissolution. Full-thickness ex vivo neonatal pig skin was used in in vitro permeation experiments. According to HPLC analysis, the dry reservoir combination of DCT and hydrogel-forming MNs could transfer up to 80 µg of CFZ into the epidermis in just two hours after application. Furthermore, at 24 h, CFZ up to 1.8 mg may be delivered into and across the skin using a DCT reservoir in conjunction with hydrogel-forming MNs [125].
The current work focuses on employing Smart Film technology to improve solubility when delivering the hydrophobic antibiotic rifampicin. Unlike earlier methods, a one-step procedure can be used to create smart drug reservoirs (Smart Reservoirs) for hydrophobic substances. HF-MNs and three distinct rifampicin Smart Films (SFs) concentrations were generated in this investigation by fabricating SFs using a blend of polymers, specifically 15% w/w Poly Methyl Vinyl Ether-alt-Maleic Acid (PMVE/MA) and 5% w/w PEG with a molecular weight of 10,000 Da. This combination was chosen to achieve the desired mechanical strength and swelling properties necessary for effective skin penetration and drug delivery. In order to enhance the structural integrity of the HF-MNs, the polymer mixture underwent a crosslinking process. This involved thermal treatment at 80 °C for 24 h, facilitating the formation of a robust hydrogel network capable of efficient drug delivery. The physicochemical and mechanical characteristics, morphology, Raman surface mapping, interaction with the cellulose matrix, and maintenance of the loaded drug in the amorphous state of both HF-MNs and SFs were then thoroughly characterized. Their transdermal penetration effectiveness and medication loading were also investigated. The resultant SFs demonstrated that the majority of the medication was in the amorphous state and that the API was intact inside the cellulose matrix. The release kinetics of rifampicin from Smart Reservoirs were studied over a 24 h period. The study found that rifampicin had a consistent and regulated release profile, with 500±22 μg permeating the skin and 80±7 μg remaining inside the layers. This persistent discharge indicates that the system has the potential to provide long-term therapeutic benefits. The HF-MNs and SFs combination effectively delivers rifampicin transdermally. The formulation's success depends on the polymer ratios, crosslinking circumstances, and drug release kinetics [126].
Antibiotic delivery methods that avoid the human gut have been proposed as a potential solution to this issue. An alternate antibiotic delivery method, the antibiotic Hydrogel-Forming Microarray Patch (HF-MAP) system, has been created in this work. With>600% swelling in PBS over a 24 h period, the Poly(Vinyl Alcohol)/Poly(Vinyl Pyrrolidone) (PVA/PVP) microarray demonstrated exceptional swelling properties. It was demonstrated that the HF-MAP's points could pierce a skin model that was thicker than the stratum corneum. The drug reservoir for the antibiotic tetracycline hydrochloride was mechanically strong and dissolved entirely in an aqueous media in a matter of minutes. Using a Sprague Dawley rat model, in vivo animal studies demonstrated that administering antibiotics via HF-MAP produced a sustained release profile with a transdermal bioavailability of 19.1% and an oral bioavailability of 33.5% when compared to animals receiving oral gavage and Intravenous (IV) injection. At 24 h, the HF-MAP group's maximum drug plasma concentration was 7.40±4.74 μg/ml, while the oral (5.86±1.48 μg/ml) and intravenous (8.86±4.19 μg/ml) groups' drug plasma concentrations peaked shortly after drug administration and had dropped to below the limit of detection. The outcomes showed that HF-MAP can deliver antibiotics over an extended period of time [127].
Hollow MNs
Hollow MNs(HMNs) have the same fundamental construction as traditional hypodermic needles, namely the hollow core contains the drug inside, but they are much shorter their length is less than 1000m [128, 129]. Due to application of HMNs, the drug can be diffuse passively or actively, either by osmoses pressure generated from the needle lumen [129].
Mainly, the role of HMNs is to perforate the skin to enable the drug to be released from the needle core [130]. When using HMNs, the medication flow rate can be purposefully regulated using standard apparatuses that control flow as syringes and micropumps [124]. solutions are employed, a fluid medication formula may be given directly into the skin without the need to dry the pharmaceuticals, preventing thermolabile chemicals from being damaged [124]. One advantage of employing HMs is that the time and quantity of medicine administered may be controlled, allowing for a wide range of drug concentration-time profiles [131]. Furthermore, the pressure in an HM may be altered, resulting in a change in flow rate [128]. This characteristic enables the administration of either a quick bolus injection or a gradual infusion [128].
A hollow MN array was manufactured through a Three-Dimensional (3D) printing technique. The design of the MN composed of an emptytank. The hollow MN array were also fabricated through conventional methods, such as Stereolithography (SLA) technology using class-I resin feature. Software was used to improve the location, direction, support, and position of the job to print. Printing consisted of fourfactors: The volume of the resin needed to print the hollow MNs, the resolution of each sheet, and the dimensions of supports touching edges, the positioning of the item to be printed in regards to the platform, and the temperature of the resin tank, which was 29.8 °C.
The resin, which is unpolymerized and is attached to the hollow MNs is rinsed softly with 99.9% IPA, to avoid damage to the printed object. The hollow MN was exposed to UV curing for 60 min. at 60 °C. to increase durability of the printed MN [132].
The HMNs array were found to be suitable for the delivery of high molecular weight antibiotics as in the case of rifampicin which has a molecular weight of 822.94 g/mol, and transdermal delivery is important for such drug, since its oral delivery presents problems as sensitivity to stomach acids, low bioavailability and the risk of toxicity to the liver. The synthesized hollow MNs were optimized by the addition of holes to the sub-apical parts at the side of the MN tip. These holes increased the durability and integrity. The quality of hollow MNs were assessed through optical and electronic microscopy. Moreover, the consistency of the array system and the ability to penetrate the skin are evaluated. Rifampicin delivery through hollow MN array systems was examined using ex vivo permeation studies and pig skin to evaluate bioavailability. The results showed that rifmbicin had good bioavailability when delivered using hollow MNs array [132].
Degradable MNs
Biodegradable polymers are used to produce this kind of MNs, such as polylactic acid, polyglycolic acid, Poly(Lactide-Co-Glycolide) (PLGA), and chitosan. The medication are mainly enclosed in side these polymers (acts like a matrix), then the release will start when the MNs interacts with the interstitial fluid. Because MNs are biodegradable, there will be no MN residues [133, 134].
Due to having benefits like accurate loading, complete dermal elimination, and attachment of the drug tank, biodegradable MNs have gained prominence over other MNs. Natural biopolymers have been utilized as a material in medicinal applications for millennia and have grown in relevance in medication delivery. The section that follows discusses the different polysaccharide and polypeptide-based biomaterials utilized to make biodegradable MNs. Furthermore, the biocompatibility and biodegradability of these biomaterials, as well as their use in transdermal medication administration [135].
Antifungal agents used orally or topically face many problems like low bioavailability, emergence of resistance and low sustainability. Transdermal delivery through polymeric MNs can provide a solution to these complications. One of the polymers used for the synthesis of MNs is Chitosan-Polyethylenimine (CP-Polymer), which is a ecofriendly and a biocompatible substance, and further, it has an antimicrobial activity, that is insusceptible to drug resistance and provides continuous drug release [136].
Production and characterization of CP-copolymer: The production method of CP-copolymer was done in reference to a technique that was formerly described in literature. After passing through the night, mixing until it dissolves and adjusting the pH to 7.0 by adding 1 M of NaOH. Afterwards, CDI was mixed well with the previously prepared solutions in a ratio of 1:2 (CDI: amines in chitosan), mixing is done for an hour. Consequently PEI was added using a dropper and under constant mixing and with a ratio of 2:1 (PEI: amines in chitosan) by molarity. The solution is allowed to stand overnight to complete the polymerization reaction. Then dialysis takes place on the produced solution against water for an extra 48 h to eliminate excess free PEI. Lastly, CP powder that was achieved by lyophilization and was characterized by 1H NMR spectroscopy. Afterwards, the zeta potential was calculated using a Malvern Nano-ZS Zeta Sizer machine. The pH was identified through a pH meter [136].
Synthesis of drug-loaded MN patches: MN patches were produced through a micromolding procedure. Poly(dimethylsiloxane) (PDMS) mold was initially made by adding the mixture of PDMS and curing agent (weight ratio of 10:1) into a stainless-steel master assembly, that has 10×10 arrays of sharp-pointed pyramidal MNs with a base width of 300 μm, height of 1000 μm, and a space between them of 700 μm. Then, the gas was removed from the PDMS mold and dehydrated using an oven at a temperature of 70 °C for 1 h. Afterwards, dissolving CP powders was done in an aqueous solution with acetic acid (1%, v/v). To control pH at 6, dialysis then was performed on the CP solution (20 mg/ml) (MWCO: 10 kDa) against DI water to eliminate excess acetic acid. The next step was dehydrating CP solution in an oven at 37 °C tilla of the concentration of the CP solution is up to a maximum of 5 wt% and the solution becomes sticky. At this point the CP solution was ready for CP-MN patch synthesis. Drug loading starts with fluconazole (F) or amphotericin B (AB) which were dissolved in DMSO (40 mg/ml). An aliquot of 125 μl of the drug solution was taken and mixed well with 875 μL CP solution along the night. Afterwards, concentrated CP and CP+AB solution were added into PDMS mold and centrifuged at 4000 rpm, 10 min, and was let to stand all night. In the final steps of the MN patches synthesis, the HA solution was added into the moulds using centrifugation to allow support of the substrate of the MN patch, and the MN patch was left to dry overnight, and it was removed with caution from the molds. The CP/AB hydrogel after soaking into CP/AB MNs in buffer solution, was nearly 7 [136].
The mechanical durability of was checked through an Instron 5543 Tensile Meter. A constant force was added to the outermost of MNs, which were positioned uphill on a flat stainless-steel probe, till a dislocation of 400 μm was attained at a continuous speed of 0.5 mm/min.
The efficacy of the treatment using MN batches in vivo was tested through mice models with fungal infection and Mpatches loaded with Amphotericin B, which is an anti-fungal agent. The results of the in vivo study have proved that this approach has high potential in the treatment of fungal infections, especially that the MN patches were able to attain good bioavailability. The results were also attributed to the use of an anti-fungal polymer, which added to the effect of amphotericin B [136].
The DH/VEGF@Gelma-MNs are prepared in this work using high-quality biocompatible gelatin methacrylate (Gelma) and polyvinyl alcohol (PVA), loading with Doxycycline Hydrochloride (DH) and Vascular Endothelial Growth Factors (VEGF). Gelma-MNs have conical features that are in alignment, good mechanical and swelling qualities, and the ability to enter the skin. DH/VEGF@Gelma-MNs exhibit biocompatibility and antibacterial activity to prevent Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus) from growing. Additionally, DH/VEGF@Gelma-MNs strongly encourage human umbilical vein endothelial cells' (HUVECs') migration and tube formation. Compared to DH/VEGF@Gelma-plain patch (DH/VEGF@Gelma-PPs) flat patches and DH/VEGF solution injection, DH/VEGF@Gelma-PNs treatment speeds up diabetic wound closure in vivo. According to histological analysis, DH/VEGF@Gelma-MNs covering exhibits angiogenesis, increased collagen deposition and remodeling, follicle and sebaceous gland development, and intact wound re-epithelialization. In conclusion, DH/VEGF@Gelma-MNs present an interesting approach based on a synergistic MN therapeutic platform by releasing DH and VEGF to improve diabetic wound healing, which has antibacterial and angiogenic effects [137].
Another study aimed to create dissolving microneedle patches (CP) based on sorbitol and hyaluronic acid, microfiber-coated microneedle patches (MP), microfibers loaded with polyvinyl pyrollidone, and microfibers based on Eudragit S-100. Scanning electron microscopy and differential scanning calorimetry, X-ray diffraction, were used to analyze the formulations' morphology and phase, respectively. Antimicrobial assays, in vitro drug release, substrate liquefaction tests, and in vivo antibiofilm investigations were carried out. The surface of MF was uniform, and the network was interconnected. Microstructures with homogeneous surfaces and sharp tips were found by morphological study of CP. Clarithromycin was added as an amorphous solid to MF and CP. The liquefaction test revealed that hyaluronic acid was responsive to the hyaluronate lyase enzyme. The alkaline pH (7.4) responsive drug release from fiber-based formulations (MF, MB, and MP) was approximately 79%, 78%, and 81%, respectively, within 2 h. Around 82% of the medication was released by CP in just two hours. MP's inhibitory zone against Staphylococcus aureus (S. aureus) was approximately 13% greater than those of MB and CP. In comparison to MB and CP, MP was found to be effective for managing microbial biofilms because it was able to eradicate S. aureus from infected wounds reasonably quickly and cause skin regeneration after application [138].
Zinc Oxide Nanoparticles (ZnO NPs) have been used to prepare antibacterial agents and have been shown to have low toxicity and antibacterial qualities. ZnO NPs have a few drawbacks, though, including poor dispersion in various mediums, which lessens their antibacterial activity. With organic cations and organic/inorganic anions, ionic liquids (ILs) are a type of low melting point salts that have good biocompatibility and the ability to both improve ZnO NP dispersion and have antibacterial qualities. A new transdermal drug delivery technology called microneedles (MNs) can efficiently create a transport channel in the epidermis and deliver the medication to a specific depth without causing discomfort, harm to the skin, or excessive stimulation. Dissolving Microneedles (DMNs) have advanced quickly due to a number of benefits. When compared to solitary ZnO NPs and a single IL, this study confirms that ZnO NPs dispersed in imidazolidinyl IL had superior and enhanced antibacterial activities. Consequently, the ZnO NPs/IL dispersion exhibited strong antibacterial properties. Then, to create DMNs, ZnO NPs/IL dispersions with complementary antibacterial qualities were employed as antibacterial agents. According to in vitro antibacterial studies, DMNs likewise possessed strong antibacterial qualities. Moreover, DMNs were used to treat infections in wounds. In order to cause microbial death and hasten wound healing, antibacterial DMNs were introduced into the infected site, dissolved, and then released [139].
Solid MNs
The "poke-and-patch" method is used for solid MNs. Solid MNs are mainly built of various metals such as silicon, titanium, stainless steel, and titanium. These MNs are used to produce micropores, after that the drug applied, so the drug entrance resistance is minimal [106, 140-142]. In one investigation, super-short solid silicon MNs (70–80 µm) outperformed sharp-tipped MNs in terms of skin permeability. In addition, they can be kept attached to the skin for a longer durations of time than sharp MNs [143]. Narayanan and colleagues created solid silicon MNs and gold-coated them to boost mechanical strength and biocompatibility. Solid MNs are considered as a promising approach for enhancing drug bioavailability in TDDS [144].
Human papillomavirus causes a skin infection called warts. The current therapy is based on mechanical obliteration of the wart, like cryotherapy and electrocautery. These therapies cause severe pain and mostly, the treatment either fails or the warts reoccur. This research compared between cryotherapy and modern bleomycin MB patch as therapy for warts, using 42 patients who suffer from warts. The efficacy of the two treatments was measured by the guidelines of the Physicians Global Assessment (PGA) and the Patients Global Assessment (PaGA). The results of the study showed no significance difference between cryotherapy and Bleomycin MN patch in the effectiveness in eliminating the wart, however the Bleomycin MN patch was extremely less painful when compared with cryotherapy. Furthermore, the MN patch treatment increased compliance in patients who were deterred by the pain of the cryotherapy [129].
The medical research community is searching for substitute antibiotics due to the global trend of growing antibiotic resistance to Gram-negative bacteria.
When used against gram-negative bacteria, antibiotics like polymyxin B (PMB) have high effectiveness and low resistance rates. However, the therapeutic constraints of topical PMB treatment, such as its short residence period, make its application in treating skin infections challenging. In order to administer PMB to the skin, we provide Porous Silicon Microneedle (pSi MN) patches. The fabrication of pSi MN patches involves first producing the projections using dry Si etching techniques, then electrochemically etching to produce porous layers surrounding the MNs. Gram-negative Escherichia coli's inhibition zone is measured to assess the antibacterial activity of pSi MNs. Next, chemical mapping employing matrix-assisted laser desorption/ionization confirms that PMB is effectively transported into the epidermal layer of ex vivo skin models. Imaging using mass spectrometry (MALDI-MSI). The findings show that pSi MN patches are a useful and effective method for transdermal PMB delivery that preserves bioactivity and antibacterial efficiency, hence increasing the potential of existing transdermal drug delivery methods [145].
Photomechanical waves
Photodynamic waves enter subcutaneously (SC), permitting the medicine to flow via the temporarily created channel [146, 147]. The wave causes limited ablation, which is done through exposing the incident wave to a low radiation dose of around 5–7 J/cm2 in order to expand the depth to 50–400 m for effective transmission. This restricted ablation had a longer rise and duration than previous direct ablation procedures, necessitating the regulation of photodynamic wave characteristics to assure product delivery to the appropriate depth in the skin. Within minutes, the wave produced via laser pulse enhanced skin permeation, permitting the diffusion of macromolecules throw the skin [147].
For the treatment of acne, multiple antibiotics and photosensitizers are used. Treatment with antibiotic has its drawbacks like adverse reactions, drug resistance, and low efficacy due to small skin diffusion. A study was performed on a novel therapy in which erythromycin and branched polyethyleneimin-hematoporphyrin (bPEI-HPP) conjugates were filled into liposomes (cationic photosensitizer-erythromycin loaded liposomes) [148].
The formulation of bPEI-HPP conjugates starts with adding 1 g of HPP, bPEI and 100 mg of HPP, after dissolving each discretely with 20 ml DMSO for four hours. Then DCC and NHS were added as catalysts and a coupling reagent to HPP solution with molar ratio of bPEI to catalyst = 1:1.5. After 4 h the HPP is added by a dropper to the bPEI solution and was left all night under constant stirring. Through a dialysis membrane the bPEI-HPP conjugate (MWCO: 1000) was dialyzed against distilled water for 2 d. Removal of the solution of the membrane comes next, followed by filtration. Afterwards, lyophilization is performed. The geometry of the bPEI-HPP conjugate was measured by1 H NMR, spectrometer and Fourier Transform Infrared Spectrometry (FT-IR) [148].
CP-Ls prepared in the interior side of a round-bottom flask, a lipid film was formed through evaporating 10 ml of chloroform solution that contains a decent quantity of DPPC (7.5 mg), cholesterol (2.5 mg), erythromycin (1 mg) and bPEI-HPP (10 mg; CP-L 1 and 20 mg; CP-L 2) to attain a specific molar ratio in the liposome blend. Ammonium sulphate solution (10 ml, 1 mmol) is added to lipid film. Then, heating and sonification are applied to the solution [148].
Penetration studies are performed using Franz cell diffusion system and fluorescence microscopy. The effectiveness in the penetration of the CP-L was found to be higher than that of the bPEI-HPP, the penetration efficiency of CP-Ls was greater than that of bPEI-HPP, unloaded cationic photosensitizer and free HPP since CP-Ls, since CP-Ls contained of a phospholipid that share great similarities between the cell membrane lipid composition. To evaluate the efficacy of antibacterial in vitro, the bacteria, Propionibacterium acnes (P. acnes) was used.
CP-l 2 had caused losing viability rate of the bacteria by 95% from the Colony Forming Unit (CFU) assay, and was 2.4-times greater than erythromycin-loaded liposomes (39%) and 1.9-fold higher than bPEI-HPP-loaded liposomes (50%). As a result, it was recommended that polycationic photosensitizer and antibiotic-loaded liposomes together can be a novel therapy for the treatment of acne [148].
Passive transdermal drug delivery
With the purpose of improving the effectiveness of TDDS drug molecules must attain the following criteria, having a low MW (less than 1 kDa), low skin irritation probability, have both hydrophilic and lipophilic affinity, and short half-life [149]. Skin permeation might be influenced by several factors like, the species used, site and age of skin, moisture content, temperature, exposure duration, application area, methods used for pretreatment, and penetrant physical characteristics [149].
Multiple trials have been performed to enhance the efficiency of TDDs by either using chemical enhancers to improve the spread ability of the medicine through the dermal layers, or by increasing the solubility of the drug molecules, so this can be achieved by using microemulsions, super-strong formulations, and vesicles [57, 150]. Penetration enhancers can be either used with other penetration enhancer or alone used to obtain superior permeability throw the skin. Eutectic combinations and nanoparticle composite self-assembled vesicles are examples of synergistic systems.
Vesicles
Vesicles are water-filled colloidal carriers made up of amphiphilic bilayer structure. To accomplish transdermal absorption, vesicles can transport both hydrophilic and lipophilic and molecules. Vesicles can be employed to produce extended release of drug molecule in case of TDDS. Thus, vesicles can also be used to modulate the absorption rate via its multilayered structure. Vesicles can be classified in to liposomes, ethosomes, Solid Lipid Nanoparticles (SLN), and nanostructured lipid carriers (NLCs) based on the features of the component molecules [151].
Liposome
Liposomes are soft spherical vesicles bilayer structure (can either single or multiple layer). Their primary constituents are generally phospholipids, and cholesterol. Phospholipid mostly made up two parts of polar head and two hydrophobic hydrocarbon chains. The polar head has groups that have a positive or a negative charge. The lengths and extent of unsaturation of hydrocarbon chain molecules vary. Liposomes arise spontaneously when a dried lipid film is reconstituted in an aqueous solution. Liposomes may be both hydrophilic and hydrophobic due to their unique configuration that permits encapsulation of both hydrophilic and hydrophobic compounds. Nevertheless, other researchers have discovered that liposomes can only stay on the skin's surface and cannot penetrate through the epidermis, this will lead to reducing the quantity of medicine taken into the bloodstream. This characteristic improves medication retention on the skin, extending their action at the injury site, and allowing for long-term sustained release. Liposomes are thus the ideal approach for the local therapy of skin disorders [152].
Transfersomes are highly flexible liposomes (deformable liposomes, or elastic). Elasticity is the most important criteria, which is due to the presence of surfactants having only one chain. The surfactants increase the fluidity of the phospholipid bilayer vesicles become extremely malleable, transforming them into first-generation transfersomes. Second-generation transfersomes have evolved throughout time, with a minimum of a single primary bilayer building block (usually fluid-phase phosphatidylcholine lipids) and at least two or more polar lipophilic molecules. Third-generation transfersomes are composed of amphiphilic surfactants that can posses phospholipids. The capability of deformation has aided in the construction of transfersomes that are able of piercing skin pores 5 to 10 times smaller than their size, allowing for the administration of skin-piercing medicines with MWs of up to 1000 kDa. Furthermore, TDDS utilizing transfersomes enables the delivery of macromolecular medicines like as peptides or proteins [153].
A research aimed to apply and formulate Ethosomal Gels (EGs) to be used in TDDs thatgained significantattention, since they have good solubility in water and known for being biocompatible. Another goal of this research is to characterize ethosomes of antileprotic medicine, which is Dapsone (DAP) along with an antibiotic Cloxacillin Sodium (CLXS). This approach increases the drug delivery at the site of action, more than that of the conventional method through gel formula of DAPS, and reduces the related issues of oral administration [154].
Ethosomes were formulated using cold method. Ethosomes were formulated through modified cold technique. The preparation starts with dissolving Phospholipid (3-5% w/v) and cholesterol (0.5-1% v/v) in ethanol (30-40% v/v) at room temperature and with forceful stirring. Through the process of stirring propylene glycol (5-10% v/v) at a water bath with temperature of the drug is dissolved in distilled water to form a solution of (1% w/v solution), that is heated up to 30±1 °C. Gradual addition of the solution on ethanolic lipid solution with constant stirring on a rate of 900 rpm. The resultant mixture was in the form of a dispersion of vesicles that were permitted to cool at room temperature for 45 min [154].
Characterization was done through four aspects; particle size, entrapment Efficiency (EE), zeta potential and permeation studies. Particle size (Vesicular size) was examined by the SEM and the results ranged between 127±9.01 to 215±7.23 nm, which is reliant on soya lecithin and ethanol concentrations.
The mean (as an entrapment) efficiency of preparations was in-between 52.31% and 73.51% and 49.07% to 71.91% for DAP and CLXS respectively. The vesicular charge was affected by the profound ethanol concentration in ethosomes which moved the vesicular charge from positive to negative. It was detected that F1 and F2 preparations have zeta potential of-25.08±1.03 mV and-50.11±1.97 mV respectively and do not accumulate quickly. The drug release of ethosomes ranged from 84.68% to 96.58% and 64.89% to 84.21% for DAP and CLXS respectively. Ethosomal gel was formulated using optimum ethosomes, in which the physical and chemical properties and the release characteristics were investigated. The results of the study showed that G5 ethosomes allowed the antileprotic agent to have higher efficacy and stability, with decreased adverse reactions and lower toxicity linked with the antileprotic agent [154].
Investigating flexible nanoliposomes for topical daptomycin administration and recording penetration rates and bacteriostatic efficacy against skin infections were the goals of this investigation. The daptomycin-laden flexible nanoliposomes (DAP-FL) were optimized using response surface methods, and the investigation index was the quantity of drug loaded into the particles. Lecithin to sodium cholate (17:1 (w/w)) and lipid to medication (14:1 (w/w)) were the ideal lipid ratios. The ultrasonic therapy lasted 20 min, and the hydration temperature was fixed at 37 °C. The resulting DAP-FL exhibited a narrow size distribution (polydispersity index of 0.15), and a modest mean particle size (55.4 nm). The average drug loading percentage was 5.61%±0.14%, and the average entrapment efficiency was 87.85%±2.15%. The percentage and amount of cumulative daptomycin penetration from DAP-FL within 12 h were determined using skin mounted between the donor and receptor compartments of a modified Franz diffusion cell. These results directly demonstrated quick and effective antibacterial activity against Staphylococcus aureus, at 96.28%±0.70% and (132.23±17.73) μg/cm2 *5 = 661.15±88.65 μg/cm2. Daptomycin was found in the mouse's multilayer skin tissues and underlying structures in the dorsal skin after local application of DAP-FL. Significant inhibition of bacterial growth and injury-induced biofilms was observed at effective therapeutic concentrations that were sustained for several hours. These findings show that daptomycin can more effectively penetrate the skin, where it has a potent antibacterial effect and activity against biofilms, when the DAP-FL is present. There may be a new way to use daptomycin in clinical settings with this innovative formulation [155].
Another work investigates the in vivo action of Chrysomycin A (CA) and develops a transdermal liposomal formulation of CA for the concurrent treatment of cutaneous Methicillin-Resistant Staphylococcus Aureus (MRSA) infection and cutaneous melanoma. With an IC50 value of less than 0.1 μm in B16-F10 cells, the produced liposomes (TD-LP-CA) have a potent anticancer effect. They also inhibit MRSA proliferation with a Minimum Inhibitory Concentration (MIC) of 1 μm and eliminate established MRSA biofilms at 10× MIC in vitro. More significantly, TD-LP-CA exhibits superior subcutaneous tumor penetration following skin application in vivo and improved Stratum Corneum (SC) penetration, reaching more than 500 μm beneath the skin's surface as a result of alteration with the TD peptide. After topical dermal application, TD-LP-CA exhibits a moderate inhibitory impact on subcutaneous melanoma with a 75% tumor suppression rate, as well as a good therapeutic effect against intradermal MRSA infection in mice. Because they can penetrate the skin barrier, the liposomes made here may be a potential vehicle for transcutaneous CA transfer in the treatment of superficial conditions such infections and skin cancers [156].
Ethosomes
Phospholipids, alcohols, and water make up ethosomes. Ethosomes contain greater alcohol contents than liposomes. It aids in drug molecule penetration, with phospholipids also playing a role [157]. As water particles around the lipid head group are exchanged by alcohol, ethosomes become more flexible and fluid. Ethosomes have the distinctive size of tiny size, a stable structure, and a great capability of delaying the release of the drug. Hence, ethosomes can transport pharmaceuticals profound permeation or straight through the skin when compared to conventional liposomes. These formulations have also been shown to significantly increase medication release into the circulation as well as drug transdermal effectiveness. Ethosomes are a form of multiphase dispersion system with increased stability and retention time [158].
A study aimed to search and inspect the efficiency of the use of ethosomes in the transport of the drug vancomycin hydrochloride. The ethosomes carrying vancomycin were prepared with cold method. These ethosomes contain of 1–3 % w/v Soy Phospholipids (SPC), 30 – 45 % v/v of ethanol and water up to 100% v/v. The synthesis starts with dissolving 250 mg of vancomycin hydrochloride and SPC in ethanol and the blend is heated up to 30 °C. Then, distilled water is added gradually, with continuous stirring at 700 rpm in a locked container. The temperature was kept at 30 °C throughout the procedure. Stirring was sustained for an additional 5 min. The resulting ethosome suspension was relocated to a 15 ml centrifugation tube and centrifuged at 13,000 rpm for 1 hour at 4 °C through a cold centrifugation system (Sigma 3K 30, Germany), then re-suspending occurred in PBS. The formula was then kept at 4 °C [159].
In vitro characterization is done to test the vesicles size, geometry entrapment, efficiency, zeta potential, penetration, assay against methicillin resistant MRSA and stability. The study concluded that the vesicles are unilamellar spherical in shape. The vesicles size, entrapment, efficiency, zeta potential, penetration all rely on the concentration of membrane constituents. The preparation which has 1% phospholipid, 45% ethanol and 10% propylene glycol exhibited the lowest average particle size (71.60 nm±17.6) and highest transdermal flux (414.7 µg/cm2. h) crosswise the skin of shaved rat, which was 72.67 times drug solution. The results from the microbiological assay have proposed that the MIC is influenced by exterior charge of ethosomes. The improved preparation could be kept at 4 °C for 90 d and reserved 89.82%±1.00 of primary drug content. The ethosomes can have future potential as a carrier for vancomycin hydrochloride in TDD [159].
Preparing and characterizing ethosomes of the antileprotic medication Dapsone (DAP) and the antibiotic Cloxacillin Sodium (CLXS) may help these medications reach their intended site more effectively than commercially available gel preparations of DAP and also address issues with oral administration of CLXS. After being made using the cold approach, ethersomes were examined for zeta potential, particle size, Entrapment Efficiency (EE), and penetration. Using Scanning Electron Microscopy (SEM), the size of the vesicles was observed to vary according on the amounts of ethanol and soy lecithin, ranging from 127±9.01 to 215±7.23 nm. For DAP and CLXS, the average percentage of drug entrapment efficiency varied from 52.31% to 73.51% and 49.07% to 71.91%, respectively. Ethamomes' high ethanol content has caused the vesicular charge to change from positive to negative. It was found that the F1 and F2 formulations did not aggregate quickly and had zeta potentials of-25.08±1.03 mV and-50.11±1.97 mV, respectively. For DAP and CLXS, the ethosome drug release ranged from 84.68% to 96.58% and 64.89% to 84.21%, respectively. Ethosomal gel was made using an optimized ethosome, and its physicochemical properties and release were examined. Last but not least, G5 showed a stronger (p<0.05) antileprotic impact to increase the medications' efficacy, stability, and decrease their toxicity and adverse effects [160].
Paolino et al. (2005) used human skin samples to assess the penetration of ethosomes containing ammonium glycyrrhizinate. The results showed a considerable increase in drug penetration compared to standard formulations, highlighting ethosomes' potential to improve transdermal medication delivery [161] Lodzki et al. (2003) used a mouse model to examine transdermal cannabidiol administration via ethosomal carriers. The study discovered that cannabidiol-loaded ethosomes resulted in considerable drug levels in the bloodstream and anti-inflammatory effects, demonstrating the efficiency of ethosomes in enabling systemic drug absorption via the skin. These findings give solid evidence of ethosomes' increased skin permeability, highlighting their potential as excellent carriers for transdermal medication delivery [162].
Solid lipid nanoparticles (SLN)
The first generation of lipid-based nanocarriers, known as solid lipid nanoparticles (SLNs), consist of biocompatible lipid nucleus and an amphiphilic surfactant in the outer surface with a size ranges from 50 to 1,000 nm. They are solid at body temperature and stabilized by emulsifiers [163, 164]. They offer many benefits, including biocompatibility, biodegradability, convenience of large-scale synthesis through the use of the high pressure homogenization process, and protection of the medicine from harsh environmental conditions [165]. SLNs have a few drawbacks as well. Their flawless crystalline structure results in limited drug loading efficiency [165], and the crystallization process during storage conditions may cause drug expulsion. Initial burst release [166], which typically happens with these formulations, is another disadvantage. Drug molecules in SLNs arrange themselves between fatty acid chains or glycerides, and there is a propensity for previously dissolved drug to be expelled during storage times and polymorphic changes in solid lipid structures [166]. Fig. 5 illustrates drug orientation in SLNs.
In this study, a transdermal patch was created utilizing cephalexin as a model antibiotic. Solid lipid nanoparticles (SLNs) based on α-tocopherol succinate were loaded with cephalexin. The SLNs were formulated with a drug-to-lipid ratio of 20%, resulting in nanoparticles with a diameter of 180±7 nm and a drug loading efficiency of 7.9%. These SLNs demonstrated enhanced skin penetration and exhibited significant antibacterial activity against Staphylococcus aureus.The transdermal patches were fabricated by dispersing the cephalexin-loaded SLNs into a poly-isobutylene adhesive solution, followed by solvent casting. The optimal patch formulation contained 90% adhesive solution, 7% cephalexin, and 3% cephalexin-loaded SLNs. This formulation not only inhibited S. aureus growth more effectively than a patch containing 90% adhesive solution and 10% cephalexin but also promoted approximately 25.5% greater proliferation of human fibroblast skin cells in vitro compared to media without the patch.However, the reported drug loading efficiency of 7.9% for the cephalexin SLNs is relatively low. Enhancing this efficiency could lead to improved therapeutic outcomes. Strategies to achieve higher drug loading include optimizing the surfactant system and refining the lipid composition. Selecting appropriate surfactants can stabilize the lipid core more effectively, while modifying the lipid matrix can enhance drug incorporation and retention within the nanoparticles. Implementing these adjustments may result in SLNs with superior drug loading capacities, thereby enhancing the efficacy of the transdermal patch [167].
Nanostructured lipid carriers (NLCs)
The unstructured matrix of NLCs, a second generation of lipid-based nanocarriers made from a combination of liquid and solid lipids, results from the various ingredients' moieties [169]. NLCs were created to get around the drawbacks of SLNs. Because of their irregular crystal structure, NLCs offer a larger drug loading capacity. They can also prevent drug expulsion by preventing lipid crystallization throughout the manufacturing and storage processes. The formulation of NLCs contains liquid lipids, which reduces the amount of drug ejection during formulation and storage. In contrast to SLNs, NLCs can exhibit more controlled release profiles and improve drug solubility in lipid matrices [170]. NLCs have a lower melting point than SLNs despite being solid at body temperature. This is because of their unstructured nature and defective crystalline characteristics, which allow for greater space for drug breakdown and payload in the liquid portion of the NLCs. In this sense, NLCs have a greater loading capacity than SLNs. Another benefit of NLCs is their ability to separate nanoparticles from the rest of the medium and prepare dosage forms for parenteral administration. Previous studies have also confirmed that NLCs are less susceptible to gelation than SLNs during the preparation and storage period [169, 170]. Fig. 5 illustrates drug orientation in NLCs.

Fig. 5: Drug location within the lipid matrix of the solid lipid nanoparticle (SLN) [168]
Fig. 6: Drug location within the lipid matrix of the nanostructured lipid carriers (NLCs) [168]
In order to treat bacterial resistance strains transdermally, the current study set out to create and evaluate a transdermal chitosan-based polymeric hydrogel's antibacterial activity. Ipenem-cilastatin (IMI-CIL) loaded NLCs were created and optimized using the Box–Behnken design model and the micro-emulsion method. Compritol 888 ATO was used as the lipid core, while Tween 80 was used as a stabilizer. A thorough assessment of the improved nanoformulation was conducted, taking into account factors including molecular interaction, entrapment efficiency, surface morphology, zeta potential, particle diameter, and crystallinity. The optimized nanoparticles showed a polydispersity index (PDI) of 0.286, a zeta potential of-19.8 mV, and an average particle size of 129 nm. No signs of molecular interaction were found despite thorough examinations of crystallinity and FTIR. The hydrogel and the NLCs loaded with IMI-CIL both demonstrated long-lasting in vitro drug release. Furthermore, a noteworthy 15.77-fold increase in drug permeability was shown by the hydrogel enhanced with permeation enhancers. Additionally, as compared to IMI-CIL solution, which showed little activity against non-resistant bacterial species and no activity against resistant isolates, the IMI-CIL loaded NLCs showed much higher antibacterial activity. In conclusion, the hydrogel formulation of NLCs loaded with IMI-CIL plays a crucial part in providing prolonged drug release and enhancing the antibacterial activity of carbapenem antibiotics. This shows promise as a possible remedy for bacterial illnesses that are resistant to medications [171].
Polymeric nanoparticles
Nanoparticles (NPs) are nanocarriers with diameters ranging from 1 to 1000 nm that may be divided into numerous categories based on their composition. Drug delivery as NPs results in controlled and targeted release trend varies in the drug's in vivo dynamics, and an extension of the drug's residence duration in the blood, all of which contribute to increased drug bioavailability and decreased toxicity and adverse reactions. Polymerization and crosslinking are often utilized to create NPs, and biodegradable polymeric substances as in gelatin and Polylactic Acid (PLA) are frequently used [172-174]. Polymeric NPs are receiving interest in TDDS because they can overcome the constraints of existing lipid-based systems, such as protecting unstable pharmaceuticals from degradation and denaturation and attaining constant drug release to lessen adverse effects. A rise in the concentration gradient boosts permeation. Polymeric NPs are characterized as nanospheres, nanocapsules, and polymer micelles based on their production technique and structure. Polylactic acid, PLGA, polycaprolactone, polyacrylic acid, and natural poly esters are examples of commonly used polymers (including chitosan, gelatin, and alginate). Under certain circumstances, like the existence of an artificial membrane that replicates the physiological lipid bilayer membrane, these polymer chains can be formed by covalent attachment of two or more single polymeric units. Despite the fact that these polymers may create a complicated configuration of the membranes due to the high MW of the composing polymer chains; as a result, polymeric NPs with extreme mechanical durability and non-deformability will not be able to enter through the holes with diameters less or equal to their size. However, because these NPs are resistant to degradation, medications can be retained for an extended length of time before being released from the NPs and diffusing further through the skin [174-178].
Nanofibrous composites
Nanofibers are nanometer-sized diameters fibers. Nanofibers may be made from a variety of polymers, resulting in a wide range of physical qualities,application and possibilities. Collagen, keratin, cellulose, gelatin, silk fibroin, and polysaccharides such as chitosan and alginate are examples of natural polymers [179-182]. PLA, PLGA, Polyurethane (PU), polycaprolactone (PCL), poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV), and poly(ethylene-co-vinylacetate) are examples on synthetic polymers (PEVA) [179-181, 183]. Covalent bonding hold polymer chains together [184]. The diameters of nanofibers are determined by the kind of polymer in manufacturing procedure [185]. When compared to microfibers, all polymer nanofibers are distinguished by their large surface area-to-volume ratio, elevated permeability due to high number of pores, durability, and functionalization flexibility [181, 186]. Drawing, electrospinning, self-assembly, template synthesis, and thermal-induced phase separation are all ways for creating nanofibers. Because of its simple setup, capacity to constant bulk production of nanofibers from a variety of polymers, and ability to make ultrathin fibers with adjustable diameters, compositions, and orientations, electrospinning is the most often used technology to create nanofibers[186]. Due to Because of this flexibility, the form and arrangement of the fibers may be controlled, allowing variable configurations (i. e. hollow, flat, and ribbon shaped) to be manufactured depending on the intended application reasons. Researchers at the University of Minnesota have created nanofibers as thin as 36 nm using a new melt processing approach suitable for industrial mass manufacturing [187].
Lately, a category of nanomaterials, which is called nanofiber, has gained a lot of interest, due to its large range of biomedical applications.
A nanofiber within nanostructured carriers, usually, has a dimension of lower than 100 nm in length. Nevertheless, nanofibers with the length of less than 1000 nanometers are synthesized using an extra fine synthesis procedure called electrospinning. As the width of polymer fibers contracts from micrometers (e. g., 10–100 µm) to sub micro meters or nanometers (e. g., 10–100 nm), the outcome is greatly larger specific surface area. This inherent property allows polymeric nanofibers to have futuristic applications [188]. Electrospun micro and nanofibers have multiple possible biomedical applications such as scaffolds for the growth of neural cells [189, 190], implants [191], stents and guidance structures [192]. Nanofiber have significant applications in TDD such as wound dressing and carriers, and for cosmetic usage [192, 193].
The fabrication of nanofibers can start from either natural polymers such as chitosan, fibronectin, gelatin, collagen, and silk or ethyl cellulose or from synthetic polymers (e. g. PLA, PGA, PLGA, tyrosine-derived polycarbonates, PCL, PU, PVP, PVA), or numerous blends of the above. For the delivery of Hydrophilic and lipophilic drugs, fiber mats were found very suitable [194, 195]. Multiple factors affect drug release from the fiber mats such as drug to polymer ratio, fiber diameter, morphology, and/or porosity. The functionalization of the nanofibers (e. g., surface graft polymerization) is an additional methodology that can be employed to control drug release [188]. A numerous number of techniques have been established to synthesize nanofibers. For instance template-based synthesis, which synthesizes or extrudes nanoscale fibers of few hundred nm is commercially accessible templates of nanoporous membranes [196]. However, phase separation process yields nanofibrous films straight after freeze drying the polymeric mixture, but this procedure is extremely tiresome and wastes time. Fibers manufactured through a technique in the range between 50 to 500 nm and of smaller length [197]. The self-assembly technique, includes a practice wherein the atomic and molecular masses position themselves via connecting into stable and architecturally identified units at meso or nanoscale. However, this method has a drawback of being time consuming. This method yields nanofibers in the range of 100 nm [198]. Melt blown process uses a melt blowing fiber spinning technology. Polymer blend is sucked out by a small hole parallel to applying high speed streams of heated air. The fibers produced ranges between 150-1000 nm [199]. Electrospinning has the least disadvantages of all methods. The fibers manufactured by this procedure range from nanometers to micrometers. Additionally it has advantages of being cost effective and a high scale process that is able to synthesize large volumes of nanofibers from various starting material [200].
An additional method is nanospider. It is originated from the electrospinning and commercial method, but modifications are added to enable large production scale of polymeric nanofibers that come in diameter of 50–300 nm into nonwovens [201]. Modifications are added to polymeric melts or solutions the micro/nanofibrous nonwovens made by melt electrospinning technique to offer benefits in biomedical application as in tissue culturing, wound healing dressing, transdermal patches, and enzyme immobilization, and targeted drug delivery [202]. Airbrush Spray is a cost effective, fast and simply flexible method to fabricate nonwoven micro/nanofibrous membranes [203]. It employs electrospinning practices, electrical charge is added to the polymer melt or solution as it squeezed out from the syringe, pipette or nozzle and the fibers gathered on an aluminum paper due to the attractive forces produces because of oppositely charged polymers [185].
In a study done by N Nematpour et al. (2020), nanofibrous transdermal patch was synthesized by electrospinning technique as shown in fig. 6. This patch contains Dextran, Polycaprolactone (PCL) and Graphene oxide (GO) and the antibiotic, tetracycline, and intended to be used as wound dressing. GO was manufactured by hummer technique with slight modifications. To make GO solution, a quantity enough to make a concentration of 1 mg/ml was dissolved in distilled water. Afterwards, the solution was sonicated using BANDELIN bath sonicator, to ensure complete shredding [204].
The drug was placed then on the graphene oxide nano sheets by introducing 80 mg of tetracycline to prepared GO solution and then sonicated for 1 hour followed by 18 h of stirring. 1 ml from the prepared solution was withdrawn to determine the quantity of drug loading. After that, the solution was added with 50% w/w dextran to create drug@GO/dextran nanofibers as shown in fig. 7. To begin the fabrication of the dermal patch, the outer PCL layer was created using an electrospinning apparatus. Following the initial protective pure PCL layer, an interfacial thin layer of dextran/PCL (1:1) was applied to the first layer to serve as an interface between the hydrophobic exterior PCL layer and the hydrophobic inside the medicine that constitute of dextran. Lastly the preceding layers were electrospined with the third layer of drug@GO/dextran solution [204].
FTIR and SEM were used to assess the physiochemical characteristics for the nanofiber. The swelling index, mechanical properties, and release profile were also investigated. Using 50 percent w/w dextran loaded via TC@GO, a homogenous nanofibrous framework with a diameter of 50 nm was formed. A supplementary hydrophobic PCL layer kept moisture out of the patches inside, which featured TC-loaded GO fixed on dextran nanofiber. Furthermore, the subsequent layer increases the mechanical characteristics of the composite. A controlled drug release of TC was also seen in a drug release investigation with nano fiber drug carrier for up to three days. Finally, the nano dressing demonstrated high antibacterial action against E. coli and S. aureus, indicating that it might be employed as a dermal patch for the therapeutic protocol of persistent infected lesions [204].

Fig. 7: Diagrammatic representation of nanofibrous transdermal patch [205]
Wound healing, after getting exposed to injuries, necessitates a speedy transport of the treatment to the site of injury, with the least possible unwanted related reactions. This study explored the inclusion of the antibiotic ciprofloxacin in a nanofiber transdermal patch along with hydrophilic biodegradable PVA and sodium alginate (NaAlg) electrospun composite. The aim of the transdermal patch is to provide local transport of the treating agent to the site of action. SEM was used to confirm loading of ciprofloxacin into the composite nanofiber patch. Electro Spinning Instrument ESPIN-NANO was used to manufacture electrospun nanofibers membrane or patches. The PVA nanofiber was electrospun from 8 wt% PVA solution at applied voltage 15 kV. A magnetic stirrer was use to mix the polymer solution 3–4 h to dissolve PVA entirely in deionized water. The electro spinning of NaAlg solution is troublesome, that needs to manage viscosity and spinability. The addition of other polymers was required to solve these issues. Thus, the PVA was added in the Na-Alg to improve the composite nanofibers. The PVA and NaAlg solutions were prepared each independently of 8% and 2% (w/w). Later on, the PVA and NaAlg solution in the ratio 5.5:1 was blended through magnetic stirring machine for 8–10 h. Afterwards, the solution was electrospun at applied voltage 15 kV, flow rate 0.5 ml/min, collector speed 2000 rpm and tip to collector distance 15 cm. Ciprofloxacin was loaded into the electrospun nanofiber patch by active loading. Primarily, ciprofloxacin solution was made and mixed with PVA and PVA/NaAlg solution according to the method mentioned before stirred well for 3 h. Ciprofloxacin solubility is greater in PVA solution than PVA/PVA-NaAlg i. e. 35 mg/ml and 32 mg/ml respectively. Both the solutions were electrospun under enhanced spinning conditions to give ciprofloxacin loaded nano-fibers. These were allowed to dry at room temperature under vacuum [206].
Male rabbits were used to perform in vivo studies, where nanofiber patches, both loaded and unloaded with the ciprofloxacin were used to compare the drug release. Sustained and controlled release of the drug was noticed from the transdermal patch, and how the release trends occur were investigated in this study. The nanofiber transdermal patch is being controlled by the rules of the Higuchi and Korsmeyer–Peppas model for drug release. The result of this study have shown that the nanofiber patchesthat constitute of the drug, is faster in treating the injury than the nanofiber patch that does not contain the drug. Another result was seen, is that the hydroxyproline formed in wound site with time displays that its content are highest in the drug loaded PAV-NaAlg patch [206].
Wound dressings aim to increase the speed of healing and decrease infection. An innovative nanofiber dressing was invented that has numerous functions. These wound dressing have three components, which are; chloramphenicol (CAM), beta-glucan (βG) and chitosan (CHI), of which βG and CHI arethe polymers forming the nanofiber. The morphological properties of the polymers forming the nanofibers were studied and so both βG and CHI, only βG, only CHI and only copolymers, polyethylene oxide (PEO) and hydroxypropylmethylcellulose (HPMC) were manufactured. 1% CAM was added to the four nanofiber formulations [207].
Synthesis of eight different polymer solutions was done. The entire dry composition of all-polymer solutions was maintained at 2.5% (w/w). The polymer solutions were prepared with the exact similar solvents. A final concentration of 60% (w/w) ethanol, 3% (w/w) acetic acid and 34.5% (w/w) water. Next, dissolution and hydration of the polymer and active ingredients is performed: CHI was dissolved in a blend of the suitable quantity of SBG®, water and acetic acid and heated on a magnetic blender all night at 50 °C. Because is considered soluble in ethanol upon heating, it was initially dissolved in ethanol, and then under continuous stirring at 70 °C for no less than 80 min. The 5% PEO solution and the βG and/or CHI-encompassing solution were added to an HPMC-dispersion in ethanol. The quantity of HPMC and ethanol was optimized to reach a final ethanol concentration of 60% (w/w) and the PEO amount required for each formula. All solutions were stirred all night long at 50 °C and permitted to stand for 24 h at room temperature before performing electrospinning on the solutions. Then, a solution of CAM and ethanol (10 mg/ml) was prepared with the polymer solution on the final day before electrospinning. Concentration of CAM is 1% [207]. Nanofibers that include CAM had a pattern of burst drug release, accompanied with a great absorption ability. Nanofibers with all active ingredients (βG, CHI and CAM) displayed anti-inflammatory action that is dose dependent, along with antibiotic action of activity. This allows multifunctional al nanofibers to have a great future as wound healing dressing [207].
The goal of this study was to introduce antibiotic-loaded nanofiber substrates that could be used for topical skin distribution, reduce antibiotic consumption, and extend storage period. Using FTIR and Raman spectroscopy, the identification of Amoxicillin (AMX) had been done, a model medication with low solubility, in our nanofibers. After electrospinning, AMX-loaded Pullulan (Pull) nanofibers demonstrated the ability to retain the antibacterial qualities of the AMX medication for at least eight months of storage. By sandwiching the Pull layer between two hydrophobic electrospun layers (such PLGA biopolymer), the release trend can be adjusted from burst release in mono-layer AMX: Pull nanofibers to sustained release. For effective topical administration of antibiotics in wound healing and skin therapies, the AMX-loaded Pull construct can be regarded as a new nanobrous solid dispersion of a drug that is poorly water soluble [208].
Nanofiber scaffolds
Nanofibrous scaffolds are synthetic extracellular matrices that mimic the tissue environment development. Because of their high surface/volume ratio, they stimulate cell adhesion, proliferation, and differentiation more effectively than other types of scaffolds [205].
In a study, that investigated the nanofiber composite that constitute of polyvinyl alcohol/Gum tragacanth/graphene oxide and are used for antibiotic delivery, it concluded that that wound healing is faster in case drug loaded PVA-NaAlg transdermal patch as shown in (fig. 6). A blend of two synthetic and natural polymers (polyvinyl alcohol and gum tragacanth) were mixed with tetracycline comprising graphene oxide were utilized to generate electros pun nanofiber scaffolds that can be employed in TDD systems [209].
GO was produced by an altered Hummers Technique. The process begins with dissolving 2 g of graphite powder in 60 ml H2SO4. The resulting solution is cooled using an ice bath at 0 °C. Within 30 min, the quantity of 7.5 g of KMnO4was slowly added. The temperature of the blend was gradually raised to 40 ° C and was maintained for additional 5 h in order to oxidize the graphite. An amount of 75 ml of deionized water (DI) was added to the solution and then, adding 7.5 ml of H2O2 with continuous for 24 h. The resultant precipitate was re-suspended in deionized water and dialyzed for 48-72 h and after that it was lyophilized [209].
Preparing PVA/GT/GO/TCH suspensions and electrospinning as in fig. 8: aqueous solutions of 10 wt% of PVA and 0.5wt% GT were formed. Mixing of PVA and GT with a ratio of 60/40 (w/w) has resulted in a homogeneous aqueous solution which is PVA/GT. Then, GO suspensions was formed through dissolving 20 mg of its powder in DI water (final concentration = 1 mg/ml). The solution is the sonicated for 1 h. TCH solution was prepared by the addition of TCH into the GO solutions and were mixed for 24 h. Following that, the GO/TCH blends were added to the PVA/GT solutions and were mixed by stirring for 1 h. In the electrospinning procedure, the previously prepared solution was added to 5-mL plastic syringe and the nanofibers were gathered on aluminum sheet wrapped about a rotatory collector. The speed of spinning, electric potential and distance between nozzle and collector were kept constant at 0.7 ml/h, 16 kV and 12 cm respectively [209].
Nanofiber scaffolds’ chemical and physical features were studied through SEM, FTIR, XRD, and stretching tests. The SEM results showed that the dimension of the nanofiber was lower than 100 nm in diameter, while the stretching test showed that the durability can be enhanced by the adding graphene oxide. Cell viability of the nanofiber was examined through MTT assay and the results showed that the used nanofibers were viable against the cells used in MTT test. Diffusion agar tests were used to explore the antibiotic activity and the exhibited that nanofibers composite have greater antibiotic action than other used nanofiber composites [209].

Fig. 8: Diagrammatic representation of nanofibrous scaffolds [209]
One major obstacle to the healing of many skin injuries is wound infection. This emphasizes the necessity of developing fresh and enhanced antibacterial agents with unique and distinct modes of action. In order to create a novel wound dressing, Tanacetum polycephalum essential oil (EO), a naturally occurring antibacterial and anti-inflammatory agent, and Amoxicillin (AMX), an antibiotic, are electrospun into PVA/gelatin-based nanofiber mats both separately and together. To put it briefly, we created a PVA/gelatin layer loaded with amoxicillin as the first layer that comes into direct contact with the wound surface to protect it from external bacteria. On top of that layer, we created a PVA/gelatin/Tanacetum polycephalum essential oil layer that helps to clean the wound from infection and speeds up wound closure. Lastly, a third layer of PVA/gelatin is constructed on top of the middle layer to ensure the desired mechanical qualities. To create bead-free fibers, the electrospinning parameters were changed for every layer. Using SEM and FTIR, the morphology of the produced nanofiber scaffolds was described. Microscopic pictures showed that each layer of nanofiber with a consistent fiber size of 126.888 to 136.833 nm was made with smooth, bead-free microstructures. Although the diameter of the nanofibers was enhanced by EO and AMX, the physical structure of the nanofiber remained unchanged.
The hydrophilicity of nanofibers was demonstrated by the water contact angle test, which showed 47.35°. Nanofibers having a contact angle between 51.4° and 65.4° are remained hydrophilic even though EO and AMX had minimal effect on decreasing hydrophilicity. When compared to control and PVA/gelatin nanofiber, multilayer nanofibers loaded with EO and AMX destroyed 99.99% of g-positive and g-negative bacteria. Additionally, MTT results demonstrated the acceleration of cell growth in addition to verifying the non-toxicity of nanofibers. Because of their beneficial effects on angiogenesis, collagen deposition, granulation tissue formation, epithelialization, and wound closure, in vivo wound evaluation in mice models revealed that developed nanofibrous scaffolds would be a suitable choice for wound treatment [210].
In another study, they demonstrated the feasibility of creating a dual drug-loaded patch using nanoparticles (NPs) and ultrafine fibers using a single piece of equipment the electrospinning unit. Drugs can be locally released through the skin and tympanic membrane using such NP/fiber devices. In short, rhodamine (RHO)-loaded PLGA NPs were used to adorn dexamethasone (DEX)-loaded poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBHV) fiber meshes, with RHO serving as a second drug model. DEX-loaded PHBHV fibers (i. e., by electrospinning mode) and RHO-loaded PLGA NPs (i. e., by electrospray mode) were successfully prepared and easily assembled to form a TDDS patch by appropriately adjusting the electrospinning working parameters. FTIR and dynamometry were used to characterize the patch.
Following that, Human Dermal Fibroblasts (HDFs) were used to evaluate the patch in vitro. The fiber mesh stiffness was greatly decreased by the addition of DEX. According to in vitro experiments, HDFs remained alive for eight days after coming into contact with drug-loaded samples, and no notable cytotoxic effects were observed. Ultimately, a controlled release of DEX from the electrospun patch over a period of 4 w was achieved due to the fibers' beaded structure. This could potentially achieve the therapeutic goal of a TDDS's local, sustained, and prolonged anti-inflammatory action, which is required in chronic inflammatory conditions as well as other pathological conditions, like the treatment of sudden sensorineural hearing loss [211].
Topical gels
Topical gels are semisolid materials that have a liquid phase inside a 3D polymeric matrix of naturally derived or synthetic gums, which characterized by a great level of cross bonding [212].
Gels are a relatively recent family of dosage forms that are formed by entrapping a considerable volume of aqueous or hydroalcoholic liquid in a network of colloidal solid particles. When compared to ointments and creams, gel formulations often enable quicker medication release. These are superior in terms of use and patient acceptance [213].
Most topical gels are made with organic polymers, like carbomers, which give the products a visually attractive, clear, glittering look and allow them to be readily wiped off the skin with water. The type of base employed in the formulation of a topical dermatological treatment has a significant impact on its efficacy. Bases with a high concentration of oleaginous ingredients have an emollient impact on dry, irritated skin. More crucially, bases composed of non-volatile oleaginous chemicals (for example, hydrocarbon bases) can establish an occlusive barrier on the skin, preventing moisture from escaping into the environment. As a consequence, moisture collects between the skin and the ointment layer, causing the SC to become hydrated. Increasing the moisture in SC results in the 'opening up' of intra-and inter-cellular channels and pathways, allowing medication molecules to pass more easily. Furthermore, the moisture layer acts as a dissolving medium for the medicine, which is otherwise scattered as tiny particles in the ointment base. Because only the dissolved medication given to the skin as a unique molecular entity may enter the SC, skin occlusion leads in increased transdermal drug absorption [214, 215].
Gels are semi-rigid structures in which the mobility of the dispersing medium is constrained by the overlapping of a 3D network of the dispersed phase particles. The semi-solid state is created by the higher viscosity caused by the interlacing and subsequent internal function. Gelling agents combine or entangle to generate a 3D colloidal network when dispersed in a suitable solvent. This network structure is also important for a gel's deformation resistance and hence its viscoelastic qualities. Certain gels' elasticity may be related to the existence of a double helix structure, which is akin to a water absorption capacity, as well as the rheological characteristic of each polymer tested [216, 217].
Extended release gel
In research performed by Ramirex et al. (2020) gelscontaining three polymers and loaded with broad spectrum antibiotic piperacillin/Tazobactam for transdermal delivery were prepared. The topical route of administration was chosen since it has poor oral abruption and short half-life. Piperacillin/Tazobactam is given by injection, which is an inconvenient route of administration [218].
Three concentrations of the drug were prepared in each polymer type, which are 1%, 5% and 10%. Comparison between the three concentrations in the three different polymers was performed.
The characterization for the physical features was performed for four tests: 1) Drug release and was done through a dissolution machine. 2) Skin permeation, which was done in rats and franz cell method 3) stability studies were done on skin pH and on the following temperatures; 25 °C, 37 °C, 45 °C.
Chemical stability was also compared between the different formulations through simulating the skins wound conditions. Oxidation reduction reactions on four conditions: 0.01 mol/1HCl, 0.01 mol/1 NaOH, 0.1/mol H2O2 and 1M K1 [218].
Three separate gels from three bases were made. The bases were methylcellulose, carbomer homopolymer type B and petroleum. To compare and characterize the gels physically, six concentrations were made, which are; 0.5, 1.0, 2.0, 3.0, 4.0, 5.0 % (w/v). The concentration chosen for the bases was 5 % (w/v).
Multiple amounts of carbomer were mixed well with purified water through an automated mixer (120 rpm), for 3 h, at ambient temperature. Then, the gel bases were added to suitable amounts of triethanolamine, along with adjusting pH to 7.0. Afterwards, the gel bases were left for about 12vhours to permit the release of air captured bubbles, and also to give ethanolamine enough time for ethanolamine crosslinking. The gels pH was kept at 7.0 instead of 5 (Skin’s pH) due to stability and quality issue, such as over-neutralization occurs at pH 5, which cause reduced viscosity and clarity of the gels.
Petroleum gels was prepared as a fine paste was prepared of mineral oil and proper amounts of the antibiotic (Piperacillin/Tazobactam).
Different types of cellulose are available and in order to make a decision about the most appropriate type of cellulose and to determine the most suitable concentrations in regards to maintain good stability and viscosity, five concentrations (05, 1.0,2.0,3.0,4.0,5.0) were prepared with methylcellulose, hydroxypropyl methylcellulose, hydroxypropyl cellulose. A viscometer was used to determine the best cellulose type, in which it was found to be methylcellulose.
Piperacillin/Tazobactam was mixed with the gel base as 0.02 gm of the drug and 1.98 g of the base when a concentration of 1% is required, while 0.1 g of the drug and 1.8 g of the gel are needed when the concentration 5% is required.
The formula that contained 10% of the drug with methycellulose was the most stable physically at all temperatures and was most resistant to chemical degradation. Release of the drug substance was the highest using the polymer methycellulose, which have reached up to 40.9%, while it was 27.59% and 3.97% for carbomer and petroleum, respectively. Furthermore, permeation studies have displayed that also the formula that contains 10% of the drug and methylcellulose (12.7%), had the highest permeation, while carbomer had the lowest permeation rate with 6.08% and petroleum had an intermediate drug release rate of 6.97%. Methlcellulose 5% gel containing 10% Piperacillin/Tazobactam is the most promising antibacterial gel for local and transdermal delivery [218].
Hydrogel dressing
Three dimensions (3D) hydrogel system of hydrophilic polymers, absorb water and increase in size more than 10% due to their geometrical architecture, extensive crosslinking and the presence of hydrophilic functional groups for instance; NH2,-COOH,-OH,-CONH2,-CONH-, and-SO3H. This geometry is maintained during this physical process. Since the hydrogel have distinguished ability to uphold water, this provides them with elasticity.
The change in the size of a hydrogel comes after undergoing a gel-sol phase transition, as a reaction to either physical or chemical stimuli. The physical stimuli factors vary like magnetic and electric field, changes in temperature, light power and pressure. While for the chemical factors, they include alterations in chemical composition or pH. These modifications in the structure can be revocable mostly; and thus, hydrogels have the ability of regaining their primary conformation and thus they have flexibility and elasticity. There are factors that control the reaction of hydrogels to the stimuli, which includes by the type of the monomer, charge density, hanging chains, and the degree of cross linking. The extent of reaction is directly proportional to the strength of the applied stimulus [219].
Hydrogels dressing technology was first established in 1950s. It consisted of 90% water in order to maintain, moisture fluid movement in the wound and ensures conditions suitable for granulation, epithelialization, and autolytic debridement. They vary in their viscosity; however, they are regularly clear and lustrous. The moisture provided by the hydrogel gives a cooling effect which reduces pain, and facilitates changing the dressing, since they do not stick to the wound, due to the lack of interfacial tension among the hydrogel exterior and the wound fluids [220-223].
The reduced efficacy in skin wound infections, in phototherapy is due to low permeation of light and laser induced non-specific thermal diffusion. However, microwave thermal therapy (MWTT) has great benefits, since it permeates well into the skin. The heat produced is faster and helps in delivering the treatment agent to the site of action. This study presented an all-in-one MW-therapeutic hydrogel for treating bacterial infections through TDD. To form a polymer, microwave-responsive ionic liquid (IL), i. e., vinylbenzyl trimethylammonium chloride (VBTMACl), and a transdermal-enhancer IL, i. e., [2-(methacryloyloxy) ethyl] trimethylammonium chloride (ChMACl), are copolymerized with acrylic acid (AA) to form the hydrogel VACPHs. PVP is combined with VACPHs to enhance their durability and stability. Levofloxacin (LEVO) was chosen for loading into the hydrogel in this experiment. It was found VACPHs-LEVO displays strong antibacterial action. It is suggested that microwave heating increased the penetration of the antibiotic [224].
In a study done by amoğlu, and Arıca (2003), they have presented a synthetic hydrogel that depends on poly(hydroxyethyl methacrylate), (pHEMA), and chitosan. This hydrogel has high response to changing pH. The hydrogel was synthesized through incorporating 2-hydroxyethyl-methacrylate monomer (HEMA) and chitosan by starting photo-polymerization through UV and an initiator (i. e., α-α; ′-azo-isobutyronitrile; AIBN). A number of models of HEMA and chitosan hydrogels were synthesized and used as models to study the swelling and response to different pH. The equilibrium water absorption was found maximum at 60 min. The pHEMA/chitosan membrane thickness and density were 600 μm and 1.26 g cm−3, respectively. Drug release investigations were done using amoxicillin. After the hydrogel was filled with 100 mg of amoxicillin, the release was 80% during ten hours at pH 7.4. The pHEMA/chitosan membrane can be a used in the future for the transdermal delivery of antibiotics and other biomedical applications [225].
Nanoparticulates carrying antibiotics were also presented by Thakkar, et al. (2016) and it is intended for the treatment of burns. The goal of the nanoparticulates is to enhance drug delivery to avoid burn infection. Enhancing epithelisation also speeds healing. The nanoparticulates presented with this study are made of cubosomes, with Silver Sulfadiazine (SSD) and Aloe vera for local therapy of infected burns [226].
Fabrication of Cubosome dispersals was performed using emulsification procedure. The researchers prepared various concentrations of a lipid phase Glyceryl Monooleate (GMO) and Poloxamer 407. The optimized preparation was chosen and included aloe vera gel and carbopol 934. The end product was called cubogels [226].
Fabrication of cubosomal dispersals was performed through an aqueous emulsification of monoglyceride/surfactant. The constituents of multiple formulations are presented in table 1. Poloxamer 407 was utilized as surfactant within concentrations of 0-2% w/w and the lipid based self-assembling monoglyceride (GMO) have also been employed in concentrations ranging between 0-30% w/w. GMO was liquefied using water bath at 70 °C. Poloxamer 407 was dissolved into distilled water at 70 °C independently. The resulting solution with the poloxamer 407, was added drop by drop to the liquefied blend of GMO at the same temperature under powered mixing at 1500 rpm for 2 h. The dispersion was permitted to cool at room temperature. Afterwards, ultra-sonication was applied on the dispersionat 5 amplitude for 15 min. Storage of Cubosomes is at room temperature [227-229].
Characterization of cubogels was done through in vitro release, rheological features, pH, bioadhesion, Transmission Electron Microscopy and in vivo Wound Healing Study. The study results have displayed those multiple concentrations of GMO had substantial influence on the size of the molecule and drug release. In vitro drug release studies have presented that batch CG3 (15% GMO and 1% P407) demonstrated controllable drug release within 12 h (i. e. 98.25%), which provides superior bio adhesion and burn healing ability. Also, in vivo study in rats discovered that an optimum formula of cubogel comprising of SSD and aloe vera has faster curative speed than cubogel with SSD alone, and other wound healing preparations available commercially. Thus, it can be employed in the future as a therapy for deep second degree burns [226].
Another study had created topical patches loaded with bacitracin zinc as a novel therapeutic agent for possible wound healing. For synthesis, a free radical polymerization method was refined. Acrylic acid and PEG-8000 were chemically cross-linked in an aqueous solution with the aid of tween 80 as a surfactant and Carbopol 934 as a permeation enhancer. As initiators and cross-linkers, ammonium persulfate and N,N'-Methylenebisacrylamide (MBA) were employed. Patches were assessed for swelling dynamics, sol-gel analysis, and in vitro drug release in different mediums using FTIR, DSC, TGA, and SEM. The permeation investigation was conducted using a Franz diffusion cell. The drug-loaded patches' effects on irritation and wound healing were also investigated. The development of a cross-linked hydrogel network was validated by the characterization investigations. Formulations with the highest concentrations of Polyethylene glycol-8000 and the lowest quantities of N,N'-Methylenebisacrylamide showed the greatest swelling and drug release. Since more swelling, drug release, and drug penetration through the skin were noted at pH 7.4, the pH-sensitive behavior of the patches was further validated. The Draize scale revealed no erythema or irritation on the manufactured patches. In comparison to commercially available formulations, manufactured patches also showed faster wound healing. Thus, through improved drug deposition, such a polymeric network may be a potential solution for accelerating wound healing and small skin damage [230].
A study had used an ex vivo pig dermal explant model to investigate the antibacterial effectiveness of antibiotic-loaded glutathione-conjugated PEG hydrogels (GSH-PEG) against acute bacterial skin and skin structure infections. GSH-PEG hydrogels loaded with vancomycin or meropenem at three distinct dosage levels were loaded over the course of one hour. The Franz cell diffusion method, in vitro immersed circumstances, and ex vivo using a pig dermis model were used to track drug release. Porcine dermis explants inoculated with isolates of Pseudomonas aeruginosa or Staphylococcus aureus treated with GSH-PEG hydrogels loaded with meropenem or vancomycin, respectively, were used to evaluate antibacterial activity ex vivo. Tissue vitality and integrity under the experimental conditions, was determined by histological evaluation of the explants. Vancomycin and meropenem hydrogels showed a dosage-dependent release; the in vitro Franz cell diffusion data closely matched the ex vivo vancomycin release, but not the high dose meropenem release. At 48 h, high dose hydrogels loaded with vancomycin had a>3 log10 clearance against every S. aureus isolate. When tested against susceptible, intermediate, and resistant P. aeruginosa isolates, high dosage meropenem-loaded hydrogels reduced CFU/ml by 6.5, 4, and 2 log10, respectively. Our results show that GSH-PEG hydrogels may be used to deliver antibiotics locally and flexibly to treat bacterial skin infections [231].
Three synergistic antibiotics that are often used in clinical practice can be carried by a multifunctional hydrogel that has been designed. The drug encapsulation efficiencies of this hydrogel were found to be 88% for polymyxin B, 94% for neomycin, and 97% for bacitracin. These three antibiotics had distinct release characteristics, according to drug release data. Following the discharge of its antibiotics, the hydrogel absorbed liquid until it became saturated, which happened within 48 h, according to a swelling test. Additionally, this hydrogel demonstrated outstanding antibacterial properties against Pseudomonas aeruginosa and Escherichia coli, as well as biocompatibility; as a result, it can shield a lesion from microbial invasion. By employing ultrasound imaging to check for colonization whenever the alginate hydrogel is applied to a wound, it may be possible to avoid the establishment of wound biofilms in the early stages of infection. When we compared the hydrogel to commercially available wound dressings, we found that the latter lacked the previously described beneficial characteristics. In conclusion, our multipurpose hydrogel is an appropriate medium for ultrasound imaging to identify the development of colonies in wounds and is capable of delivering three different kinds of antibiotics at once. In the future, the hydrogel is anticipated to play a significant role in preventing wound infections [232].
In order to break bacterial biofilms and reduce the bacterial load while preserving host cell viability and scaffold integrity, this study intends to evaluate the efficacy of human-derived collagen hydrogel to deliver continuous antibiotic release. After being processed to produce a gellable liquid, human collagen extracted from flexor tendons was mixed with different quantities of either gentamicin (50–500 mg/l) or clindamycin (10–100 mg/l). Utilizing liquid chromatography-mass spectrometry, the elution kinetics of antibiotics from the hydrogel were examined. To evaluate the effectiveness of bacterial inhibition, the gel was applied topically to MRSA and Clostridium perfringens in well-established Kirby-Bauer and Crystal Violet models.
To evaluate cytotoxicity, 2D mammalian cell monolayers were topically treated, and cell death was measured. In order to measure cell mortality and mobility, bacteria-enhanced in vitro scratch experiments were treated with hydrogel imgded with antibiotics and photographed throughout time. Both MRSA and C. perfringens biofilms were successfully inhibited by collagen hydrogel implanted with antibiotics (cHG+abx), which showed continuous antibiotic release for up to 48 h while staying bioactive for up to 72 h. In an in vitro scratch model, it was discovered that administering cHG+abx with antibiotic doses up to 100X minimum inhibitory concentration was non-toxic and promoted mammalian cell motility. A promising drug delivery method that enables safe, accurate bacterial targeting for efficient bacterial suppression in a pro-regenerative scaffold is collagen hydrogel [233].
Ionic liquids
Ionic liquids (IL) comprise of a group of salts that are either organic cations or organic/inorganic anions, upon mixing with the ratio of 1:1 it forms true ionic liquid (at ambient temperature) [234].
ILs have many applications. They are used as strong solvents and electrolytes, electrical battries, they have also been utilized as sealing agents, since they have extremely low vapour pressure [235-237].
IL has been suggested to be used in biofilms utilized in treatment of infected skin wounds. The obstacles available for drug absorption is presented by the SC, in which it have hindered the development of successful wound dressings. IL can be used in increasing the delivery of the treatment agents todeep layers of the skin [238].
The production of the biofilms starts with purifying and washing of Trihexyltetradecyl phosphonium (tetraalkylphosphonium) chloride, or CYPHOS 101and with 1 M sodium bicarbonate and water. Followed by extraction with hexanes till the UV-Vis absorption is further than 300 nm have vanished and the pH of 2 ml of water after shaking with an equivalent amount of the (IL) remains the same. CYPHOS 101 was dried at 80 °C under vacuum for 24 h. Then, purification of Geranic acid was performed from the available in the markets, technical grade (Sigma-Aldrich) by repeated (5–7×) recrystallization from a solution of 70 wt% geranic acid/30 wt% acetone at −70 °C. Purity of products was evaluated through 1H NMR spectroscopy and conductivity measurements.
The characterization of the physiochemical features and the deactivation factors on the biofilm forming microbes; Salmonella enterica and Pseudomonas aeruginosa were studied. Skin models were also created to study the cell toxicity, dermal sensitivity against IL biofilms, and delivery of treatment to the site of action. Choline-geranate was studied as an IL component of the biofilm and was found to have versatile and exceptional antimicrobial action, negligible skin cells toxicity, and excellent permeation augmentation for the treatment. Choline-geranate showed similar or even superior effects against bleach treatment and against biofilms of S. enterica and P. aeruginosa, respectively. Moreover, choline-geranate improved delivering cefadroxil by more than sixteen times and was deprived of causing skin irritation. Biofilm-infected wound models were created to prove the effectiveness of choline-geranate IL biofilms against microbial infections. For the success mentioned formerly of IL biofilms, they can be used in the future for dermal delivery of antibiotics [238].
Methods for characterization of tdds
It is a key element to characterize and assess the efficiency and efficacy of novel TDD treatment methods. And thus, multiple techniques are being employed for this purpose. The best methods used for this are, diffusion cells, tape stripping, and microscopic and spectroscopic examination [113, 239]. These methods replace visual inspection for the treatment absorption from the skin and they depend on imaging the treatment or the drug on each skin layer [240].
Diffusion cell method
The diffusion test procedure is considered one of the most effective in drug permeation characterization. This technology identifies, both the effect of the drug on the skin like irritation and toxicity and also drug permeation using franz cells [241, 242]. The diffusion cells are composed of a hollow cavity for drug application, a membrane which allows treatment diffusion, and an acceptor chamber, to which the drug is expected to permeate. Diffusion cells are classified into static and flow through cells. In the static cells, the Franz diffusion cell, the donor, the membrane, and the acceptor chambers can be positioned either vertically or horizontally. Some Franz cells have an opening rofm the above and taking samples for evaluation are performed under atmospheric pressure. Nevertheless, mostly, the cells have a closed above ending and thus, measuring samples are taken under amplified pressure. This results in overestimation of the measured samples. To solve this obstacle, hand-sampler” Franz diffusion cells have been invented in which it they are automated and reduces man made errors [242].
Tape stripping
Is a simple and non-invasive process where the SC absorption is measured through applying the treatment on the skin and allowing penetration to the subcutaneous (SC) layer and the evaluating the permeation level is performed through an adhesive tape to the SC skin, after the treatment is applied topically and allowed to stand for a specified time duration. After stripping the tape, the composition on the tape is examined to evaluate penetration [102, 242-244]. It is important to mention that the tape should be placed on the same location of the skin and a rolling machine should be moved over the tape. Additionally, the speed of tape removal is vital. The gentler the tape removal, the more SC skin is adhered to the tape.
Examination of the components left on the tape can be done by High-Performance Liquid Chromatography (HPLC), which provides quantifiable measurements. Spectroscopic techniques which is affords semi-quantitative data.
Spectroscopy can also measure atomic absorption. The most common technique is attenuated total reflectance-Fourier transform infrared spectroscopy (ATR-FTIR). This involves detecting spectroscopic data after exposing the sample to irradiation, then, variations in oscillations and bonding angles amongst atoms because of the absorption or scattering of infrared rays. The changes in radiation after transition of the sample can be calculated through plotting the transmitted radiation as a function of wavelength/wavenumber. The analysis produces a spectrum in which analysis is done the qualitative and quantitative data. Thus, the depth of permeation can be identified through the wavelength of the infrared radiation, the refractive index of the ATR crystal, and the calculated material and angle of reflection. Tape stripping can be used in combination with ATR-FTIR spectroscopy, which enables to measure multiple xenobiotics compounds in specific dermal layers. Though, it is considered a difficult technique where peaks that represents both the drug and the skin can overlap [244].
Microscopic and spectroscopic methods
It is an important method for detecting data on the longitudinal drug permeation into the skin based on the concept that multiple dermal layers different light depending on drug absorption. Two main microscopic techniques can be utilized in skin permeation studies; confocal laser scanning microscopy (CLSM) and two-photon fluorescence microscopy [244-247].
CLSM is a very common, non-invasive procedure in which it is used for detecting fluorescent compounds in the skin. It can be employed to assess dermal constructions, with the least effect of destruction on the skin samples. It has been excessively used to make assessments of the efficacy of permeation enhancers on the skin. In addition, it is used for the in vivo and in vitro studies. Moreover, CLSM can diagnose dermal dysfunction, detect skin cancers and identify keratinization and pigmentation conditions. CLSM can be applied to probe the mechanism underlying the promotion of transdermal transport by nanoparticle formulations. Fluorescent markers as in fluorescein, Nile red, and 5-bromodeoxyuridine can be utilized for encapsulating nanostructured preparations, The CLSM technology is used to evaluate the permeation of these fluorescent markers through Transdermal tissue [247].
Moreover, two-photon microscope (2-PFM) has recently emerged as a vital method for imaging skin cells [246]. This method uses a Ti-sapphire laser as the excitation source. In single-photon fluorescence. A fluorescent photon is created upon exposer to a high-energy photon that stimulates the fluorophore and enhances electron energy level. The combination energy transfer of two low-energy photons is adequate to promote the same electron to higher energy level. The differences between 2-PFM and CLSM, includes that the 2-PFM works with an adaptable Ti-sapphire high-frequency pulsed laser, which produces red and near-infrared rays in the wavelength range of 650–1100 nm [248, 249]. The other major is the lack of a pinhole in front of the detector. The most important benefit of 2-PFM is that the overall energy is transferred to the sample is minor to that in other systems. Furthermore, the two-photon excitation phenomenon includes fluorescence excitation of the teste model in tiny sizes, therefore, plummeting the probability of photobleaching and photodamage. Dermal Specimens can be examined without cryofixation or sectioning. UV-absorbing fluorophores imaging is considered to have lower scattering and lower absorption that allows deep tissue imaging. The disadvantage of the 2-PFM method is that it requires laser high-cost expensive lasers and intricate cooling systems. In addition to having less lateral resolution than other techniques; nevertheless, the resolution difference is insignificant [246].
CONCLUSION
Transdermal drug delivery systems (TDDS) are substitute approaches of drug delivery which have the benefits of having low rejection rate, ease of administration, and superior efficiency and acceptance amongst patients. Because of the prevention of dosage variations, first-pass effect of the liver, and improved bioavailability, the transdermal route is regarded a preferable option than the oral route of drug administration. Due to being non-invasive and the simple in distribution of the drug, this enhances patient compliance. Especially, it can be an alternative to needle injections, especially in patient with needle phobia. Multiple factors need to be focused on upon designing TDDs, such as the epidermal barrier, which only permits lipophilic substances of molecular weights lower than 500 kDa to pass through the dermal membranes. Therefore, the rate of drug delivery through this barrier is extremely slow. Also, the skin is responsible for both absorption and excretion, in addition to the selective transport of chemical compounds required for bodily hemostasis. Antimicrobial resistance has become a global health problem and recently research has been conducted to investigate the relation between AMR and TDDs. Some challenges are faced during the use of TDDS like the skin permeation and the dermal barrier. Transdermal antibiotics can be either delivered actively by microneedles, or by photomechanical wave, or can be delivered passively by forming vesicles which are filled with water and the amphiphilic bilayer composes the colloidal carriers. These carriers can transport both lipophilic and hydrophilic substances. Depending on the features and the elements of the vesicles, they are categorizes into liposomes, transfersomes, or ethosomes. Or by forming PNs, Nano fibrous composites or scaffolds, or by forming topical gels or hydrogels. The characterization of the different methods of transdermal delivery is very important for identifying the benefits and draw backs of these delivery systems.
Future prospections
Due to the lake of studies in the transdermal delivery of antibiotics, many studies can be conducted in the future covering this area because it’s a promising area for research especially for antibiotics.
ACKNOWLEDGMENT
The author is grateful to the Middle East University, Amman, Jordan for the financial support granted to cover the publication fee of this article.
AUTHORS CONTRIBUTIONS
Rawand M. Daghmash is the principle investigator contributed in writing, revising, and formatting the manuscript. Dr. Shereen M. Assaf contributed in revising the manuscript.
CONFLICT OF INTERESTS
Declared none
REFERENCES
Jeong JH, Lee CY, Chung DK. Probiotic lactic acid bacteria and skin health. Crit Rev Food Sci Nutr. 2016;56(14):2331-7. doi: 10.1080/10408398.2013.834874, PMID 26287529.
Ghanem AM. A review on recent advances in transdermal drug delivery systems of tamsulosin. Int J App Pharm. 2024;16(2):28-33. doi: 10.22159/ijap.2024v16i2.49950.
Alonso C, Carrer V, Espinosa S, Zanuy M, Cordoba M, Vidal B. Prediction of the skin permeability of topical drugs using in silico and in vitro models. Eur J Pharm Sci. 2019 Aug 1;136:104945. doi: 10.1016/j.ejps.2019.05.023, PMID 31163216.
Schaefer H, Redelmeier TE. Skin barrier. Karger Publishers; 1996.
Barry BW. Dermatological formulations; 1983. p. 127-233.
Lu GW, Flynn GL. Cutaneous and transdermal delivery processes and systems of delivery. In: Modern Pharmaceutics. Vol. 2. CRC Press; 2016. p. 61-118.
Smith EW, Maibach HI. Percutaneous penetration enhancers. CRC Press; 1995.
Das PS, Saha P. Design and characterisation of transdermal patches of phenformin hydrochloride. Int J Curr Pharm Sci. 2017;9(6):90-3. doi: 10.22159/ijcpr.2017v9i6.23437.
Anwar N, Jan SU, Gul R. Formulation and evaluation of glibenclamide gel for transdermal drug delivery. Int J Curr Pharm Sci. 2020;12(5):35-9. doi: 10.22159/ijcpr.2020v12i5.39762.
Schaefer H. Skin permeability. Springer Science+Business Media; 2013.
Wysocki AB. Skin anatomy physiology and pathophysiology. Nurs Clin North Am. 1999;34(4):777-97. doi: 10.1016/S0029-6465(22)02423-9, PMID 10523436.
Schaefer H, Redelmeier TE, Lademann J. Skin penetration. In: Johansen JD, Frosch PJ, Lepoittevin JP, editors. Contact dermatitis. Springer; 2011. p. 215-27. doi: 10.1007/978-3-642-03827-3_12.
Fenner J, Clark RS. Anatomy physiology histology and immunohistochemistry of human skin. In: Skin Tissue Engineering and Regenerative Medicine; 2016. p. 1-17. doi: 10.1016/B978-0-12-801654-1.00001-2.
Hadgraft J. Skin the final frontier. Int J Pharm. 2001;224(1-2):1-18. doi: 10.1016/s0378-5173(01)00731-1, PMID 11512545.
Downing DT, Stewart ME, Wertz PW, Colton SW, Abraham W, Strauss JS. Skin lipids: an update. J Invest Dermatol. 1987;88(3) Suppl:2s-6s. doi: 10.1111/1523-1747.ep12468850, PMID 2950180.
Bouwstra J, Pilgram G, Gooris G, Koerten H, Ponec M. New aspects of the skin barrier organization. Skin Pharmacol Appl Skin Physiol. 2001;14 Suppl 1:52-62. doi: 10.1159/000056391, PMID 11509908.
Goldsmith LA. Biochemistry and physiology of the skin. Oxford University Press; 1991.
Reddy G. Review on transdermal drug delivery system. International Journal of Pharmaceutics and Drug Analysis. 2021;9(4):236-40. doi: 10.47957/ijpda.v9i4.482.
Antunes AF, Pereira P, Reis C, Rijo P, Reis C. Nanosystems for skin delivery: from drugs to cosmetics. Curr Drug Metab. 2017;18(5):412-25. doi: 10.2174/1389200218666170306103101, PMID 28266273.
Li J, Xu W, Liang Y, Wang H. The application of skin metabolomics in the context of transdermal drug delivery. Pharmacol Rep. 2017;69(2):252-9. doi: 10.1016/j.pharep.2016.10.011, PMID 28126641.
Manconi M, Caddeo C, Sinico C, Valenti D, Mostallino MC, Lampis S. Penetration enhancer containing vesicles: composition dependence of structural features and skin penetration ability. Eur J Pharm Biopharm. 2012;82(2):352-9. doi: 10.1016/j.ejpb.2012.06.015, PMID 22922162.
Bouwstra JA, Honeywell Nguyen PL. Skin structure and mode of action of vesicles. Adv Drug Deliv Rev. 2002;54 Suppl 1:S41-55. doi: 10.1016/s0169-409x(02)00114-x, PMID 12460715.
Jepps OG, Dancik Y, Anissimov YG, Roberts MS. Modeling the human skin barrier towards a better understanding of dermal absorption. Adv Drug Deliv Rev. 2013;65(2):152-68. doi: 10.1016/j.addr.2012.04.003, PMID 22525516.
Alkilani AZ, McCrudden MT, Donnelly RF. Transdermal drug delivery: innovative pharmaceutical developments based on disruption of the barrier properties of the stratum corneum. Pharmaceutics. 2015;7(4):438-70. doi: 10.3390/pharmaceutics7040438, PMID 26506371.
Yusuf NA, Abdassah M, Mauludin R, Chaerunisaa AY. Glibenclamide transethosome patch for transdermal delivery: formulation and evaluations. Int J App Pharm. 2023;15(5):303-9. doi: 10.22159/ijap.2023v15i5.48455.
Kazi KM, Mandal AS, Biswas N, Guha A, Chatterjee S, Behera M. Niosome: a future of targeted drug delivery systems. J Adv Pharm Technol Res. 2010;1(4):374-80. doi: 10.4103/0110-5558.76435, PMID 22247876.
Negut I, Grumezescu V, Grumezescu AM. Treatment strategies for infected wounds. Molecules. 2018;23(9):2392. doi: 10.3390/molecules23092392, PMID 30231567.
O Neill J. Review on antimicrobial resistance: tackling a global health crisis; 2015.
Pamer EG. Resurrecting the intestinal microbiota to combat antibiotic resistant pathogens. Science. 2016;352(6285):535-8. doi: 10.1126/science.aad9382, PMID 27126035.
Lawley TD, Walker AW. Intestinal colonization resistance. Immunology. 2013;138(1):1-11. doi: 10.1111/j.1365-2567.2012.03616.x, PMID 23240815.
Marchesi JR, Adams DH, Fava F, Hermes GD, Hirschfield GM, Hold G. The gut microbiota and host health: a new clinical frontier. Gut. 2016;65(2):330-9. doi: 10.1136/gutjnl-2015-309990, PMID 26338727.
Becattini S, Taur Y, Pamer EG. Antibiotic induced changes in the intestinal microbiota and disease. Trends Mol Med. 2016;22(6):458-78. doi: 10.1016/j.molmed.2016.04.003, PMID 27178527.
Francino MP. Antibiotics and the human gut microbiome: dysbioses and accumulation of resistances. Front Microbiol. 2016 Jan 12;6:1543. doi: 10.3389/fmicb.2015.01543, PMID 26793178.
Dethlefsen L, Huse S, Sogin ML, Relman DA. The pervasive effects of an antibiotic on the human gut microbiota as revealed by deep 16S rRNA sequencing. PLOS Biol. 2008;6(11):e280. doi: 10.1371/journal.pbio.0060280, PMID 19018661.
Dethlefsen L, Relman DA. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci USA. 2011;108 Suppl 1:4554-61. doi: 10.1073/pnas.1000087107, PMID 20847294.
Buffie CG, Jarchum I, Equinda M, Lipuma L, Gobourne A, Viale A. Profound alterations of intestinal microbiota following a single dose of clindamycin results in sustained susceptibility to clostridium difficile induced colitis. Infect Immun. 2012;80(1):62-73. doi: 10.1128/IAI.05496-11, PMID 22006564.
Ubeda C, Taur Y, Jenq RR, Equinda MJ, Son T, Samstein M. Vancomycin resistant enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans. J Clin Invest. 2010;120(12):4332-41. doi: 10.1172/JCI43918, PMID 21099116.
Madan JC, Salari RC, Saxena D, Davidson L, O Toole GA, Moore JH. Gut microbial colonisation in premature neonates predicts neonatal sepsis. Arch Dis Child Fetal Neonatal Ed. 2012;97(6):F456-62. doi: 10.1136/fetalneonatal-2011-301373, PMID 22562869.
Mai V, Torrazza RM, Ukhanova M, Wang X, Sun Y, Li N. Distortions in development of intestinal microbiota associated with late onset sepsis in preterm infants. PLOS One. 2013;8(1):e52876. doi: 10.1371/journal.pone.0052876, PMID 23341915.
Lu J, Claud EC. Connection between gut microbiome and brain development in preterm infants. Dev Psychobiol. 2019;61(5):739-51. doi: 10.1002/dev.21806, PMID 30460694.
Milliken S, Allen RM, Lamont RF. The role of antimicrobial treatment during pregnancy on the neonatal gut microbiome and the development of atopy asthma allergy and obesity in childhood. Expert Opin Drug Saf. 2019;18(3):173-85. doi: 10.1080/14740338.2019.1579795, PMID 30739516.
Forslund K, Sunagawa S, Kultima JR, Mende DR, Arumugam M, Typas A. Country specific antibiotic use practices impact the human gut resistome. Genome Res. 2013;23(7):1163-9. doi: 10.1101/gr.155465.113, PMID 23568836.
Rolain JM. Food and human gut as reservoirs of transferable antibiotic resistance encoding genes. Front Microbiol. 2013 Jun 24;4:173. doi: 10.3389/fmicb.2013.00173, PMID 23805136.
Bengtsson Palme J, Angelin M, Huss M, Kjellqvist S, Kristiansson E, Palmgren H. The human gut microbiome as a transporter of antibiotic resistance genes between continents. Antimicrob Agents Chemother. 2015;59(10):6551-60. doi: 10.1128/AAC.00933-15, PMID 26259788.
Hu Y, Yang X, Qin J, Lu N, Cheng G, Wu N. Metagenome wide analysis of antibiotic resistance genes in a large cohort of human gut microbiota. Nat Commun. 2013;4(1):2151. doi: 10.1038/ncomms3151, PMID 23877117.
Zhang L, Huang Y, Zhou Y, Buckley T, Wang HH. Antibiotic administration routes significantly influence the levels of antibiotic resistance in gut microbiota. Antimicrob Agents Chemother. 2013;57(8):3659-66. doi: 10.1128/AAC.00670-13, PMID 23689712.
Nainu F, Permana AD, Djide NJ, Anjani QK, Utami RN, Rumata NR. Pharmaceutical approaches on antimicrobial resistance: prospects and challenges. Antibiotics (Basel). 2021;10(8):981. doi: 10.3390/antibiotics10080981, PMID 34439031.
Saso A, Kampmann B. Vaccine responses in newborns. Semin Immunopathology. 2017 Nov;39(6):627-42. doi: 10.1007/s00281-017-0654-9, PMID 29124321.
Sullivan JV, Myers S. Skin structure and function wound healing and scarring. In: plastic surgery principles and practice. Elsevier; 2022. p. 1-14. doi: 10.1016/B978-0-323-65381-7.00001-0.
Akhtar N, Singh V, Yusuf M, Khan RA. Non-invasive drug delivery technology: development and current status of transdermal drug delivery devices techniques and biomedical applications. Biomed Tech (Berl). 2020;65(3):243-72. doi: 10.1515/bmt-2019-0019, PMID 31926064.
Pires LR, Vinayakumar KB, Turos M, Miguel V, Gaspar J. A perspective on microneedle based drug delivery and diagnostics in paediatrics. J Pers Med. 2019;9(4):49. doi: 10.3390/jpm9040049, PMID 31731656.
Ruby PK, Pathak SM, Aggarwal D. Critical attributes of transdermal drug delivery system (TDDS) a generic product development review. Drug Dev Ind Pharm. 2014;40(11):1421-8. doi: 10.3109/03639045.2013.879720, PMID 24467407.
Mohammed MI, Makky AM, Teaima MH, Abdellatif MM, Hamzawy MA, Khalil MA. Transdermal delivery of vancomycin hydrochloride using combination of nano-ethosomes and iontophoresis: in vitro and in vivo study. Drug Deliv. 2016;23(5):1558-64. doi: 10.3109/10717544.2015.1013200, PMID 25726990.
Hussain A, Altamimi MA, Alshehri S, Imam SS, Shakeel F, Singh SK. Novel approach for transdermal delivery of rifampicin to induce synergistic antimycobacterial effects against cutaneous and systemic tuberculosis using a cationic nanoemulsion gel. Int J Nanomedicine. 2020 Feb 14;15:1073-94. doi: 10.2147/IJN.S236277, PMID 32103956.
Abdellatif AA, Tawfeek HM. Transfersomal nanoparticles for enhanced transdermal delivery of clindamycin. AAPS PharmSciTech. 2016;17(5):1067-74. doi: 10.1208/s12249-015-0441-7, PMID 26511937.
Tanwar H, Sachdeva R. Transdermal drug delivery system: a review. Int J Pharm Sci. 2016;7(6):2274. doi: 10.13040/IJPSR.0975-8232.7(6).2274-90.
Hadgraft J, Lane ME. Passive transdermal drug delivery systems. American Journal of Drug Delivery. 2006;4(3):153-60. doi: 10.2165/00137696-200604030-00003.
Das A, Ahmed AB. Natural permeation enhancer for transdermal drug delivery system and permeation evaluation: a review. Asian J Pharm Clin Res. 2017;10(9):5-9. doi: 10.22159/ajpcr.2017.v10i9.19389.
Bos JD, Meinardi MM. The 500 dalton rule for the skin penetration of chemical compounds and drugs. Exp Dermatol. 2000;9(3):165-9. doi: 10.1034/j.1600-0625.2000.009003165.x, PMID 10839713.
Thong HY, Zhai H, Maibach HI. Percutaneous penetration enhancers: an overview. Skin Pharmacol Physiol. 2007;20(6):272-82. doi: 10.1159/000107575, PMID 17717423.
Michaels AS, Chandrasekaran SK, Shaw JE. Drug permeation through human skin: theory and in vitro experimental measurement. AIChE Journal. 1975;21(5):985-96. doi: 10.1002/aic.690210522.
Albery WJ, Hadgraft JJ. Percutaneous absorption: in vivo experiments. J Pharm Pharmacol. 1979;31(3):140-7. doi: 10.1111/j.2042-7158.1979.tb13456.x, PMID 34686.
Tojo K. Random brick model for drug transport across stratum corneum. J Pharm Sci. 1987;76(12):889-91. doi: 10.1002/jps.2600761209, PMID 3440932.
Goffin V, Henry F, Pierard Franchimont C, Pierard GE. Penetration enhancers assessed by corneoxenometry. Skin Pharmacol Appl Skin Physiol. 2000;13(5):280-4. doi: 10.1159/000029934, PMID 10940818.
Barry BW. Lipid protein partitioning theory of skin penetration enhancement. Journal of Controlled Release. 1991;15(3):237-48. doi: 10.1016/0168-3659(91)90115-T.
Karande P, Jain A, Ergun K, Kispersky V, Mitragotri S. Design principles of chemical penetration enhancers for transdermal drug delivery. Proc Natl Acad Sci U S A. 2005;102(13):4688-93. doi: 10.1073/pnas.0501176102, PMID 15774584.
Kogan A, Garti N. Microemulsions as transdermal drug delivery vehicles. Adv Colloid Interface Sci. 2006 Nov 16;123-126:369-85. doi: 10.1016/j.cis.2006.05.014, PMID 16843424.
Karande P, Jain A, Mitragotri S. Discovery of transdermal penetration enhancers by high throughput screening. Nat Biotechnol. 2004;22(2):192-7. doi: 10.1038/nbt928, PMID 14704682.
Hamilton JG. Needle phobia: a neglected diagnosis. J Fam Pract. 1995;41(2):169-75. PMID 7636457.
Sloan KB. Prodrugs: topical and ocular drug delivery. CRC Press; 1st edition. Informa Health Care; 1992:53:336.
Hurkmans JF, Bodde HE, Van Driel LM, Van Doorne H, Junginger HE. Skin irritation caused by transdermal drug delivery systems during long term (5 d) application. Br J Dermatol. 1985;112(4):461-7. doi: 10.1111/j.1365-2133.1985.tb02321.x, PMID 3994921.
Paudel KS, Milewski M, Swadley CL, Brogden NK, Ghosh P, Stinchcomb AL. Challenges and opportunities in dermal/transdermal delivery. Ther Deliv. 2010;1(1):109-31. doi: 10.4155/tde.10.16, PMID 21132122.
Berner B, Wilson DR, Guy RH, Mazzenga GC, Clarke FH, Maibach HI. The relationship of pKa and acute skin irritation in man. Pharm Res. 1988;5(10):660-3. doi: 10.1023/a:1015931105660, PMID 3244620.
Berner B, Wilson DR, Steffens RJ, Mazzenga GC, Hinz R, Guy RH. The relationship between pKa and skin irritation for a series of basic penetrants in man. Fundam Appl Toxicol. 1990;15(4):760-6. doi: 10.1016/0272-0590(90)90192-m, PMID 2086317.
Andersen PH, Nangia A, Bjerring P, Maibach HI. Chemical and pharmacologic skin irritation in man. A reflectance spectroscopic study. Contact Dermatitis. 1991;25(5):283-9. doi: 10.1111/j.1600-0536.1991.tb01875.x, PMID 1809531.
Mangia A, Andersen PH, Berner B, Maibach HI. High dissociation constants (pKa) of basic permeants are associated with in vivo skin irritation in man. Contact Dermatitis. 1996;34(4):237-42. doi: 10.1111/j.1600-0536.1996.tb02192.x.
Schmid Wendtner MH, Korting HC. The pH of the skin surface and its impact on the barrier function. Skin Pharmacol Physiol. 2006;19(6):296-302. doi: 10.1159/000094670, PMID 16864974.
Antoine JL, Contreras JL, Van Neste DJ. pH influence of surfactant induced skin irritation a non-invasive multiparametric study with sodium laurylsulfate. Derm Beruf Umwelt. 1989;37(3):96-100. PMID 2743874.
Ananthapadmanabhan KP, Lips A, Vincent C, Meyer F, Caso S, Johnson A. pH-induced alterations in stratum corneum properties. Int J Cosmet Sci. 2003;25(3):103-12. doi: 10.1046/j.1467-2494.2003.00176.x, PMID 18494892.
Williams A. Pharmaceutical solvents as vehicles for topical dosage forms. In: Augustijns P, Brewster ME, editors. Solvent systems and their selection in pharmaceutics and biopharmaceutics. Springer; 2007. p. 403-26. doi: 10.1007/978-0-387-69154-1_13.
ReferencesMatsumura H, Oka K, Umekage K, Akita H, Kawai J, Kitazawa Y. Effect of occlusion on human skin. Contact Dermatitis. 1995;33(4):231-5. doi: 10.1111/j.1600-0536.1995.tb00472.x, PMID 8654072.
Homick JL, Kohl RL, Reschke MF, Degioanni J, Cintron Trevino NM. Transdermal scopolamine in the prevention of motion sickness: evaluation of the time course of efficacy. Aviat Space Environ Med. 1983;54(11):994-1000. PMID 6651736.
Van Der Valk PG, Maibach HI. Post application occlusion substantially increases the irritant response of the skin to repeated short term sodium lauryl sulfate (SLS) exposure. Contact Dermatitis. 1989;21(5):335-8. doi: 10.1111/j.1600-0536.1989.tb04754.x, PMID 2620512.
Tsai TF, Maibach HI. How irritant is water? An overview. Contact Dermatitis. 1999;41(6):311-4. doi: 10.1111/j.1600-0536.1999.tb06990.x, PMID 10617210.
Dwyer CM, Forsyth AJ. Allergic contact dermatitis from methacrylates in a nicotine transdermal patch. Contact Dermatitis. 1994;30(5):309-10. doi: 10.1111/j.1600-0536.1994.tb00612.x, PMID 8088155.
Ross D, Rees M, Godfree V, Cooper A, Hart D, Kingsland C. Randomised crossover comparison of skin irritation with two transdermal oestradiol patches. BMJ. 1997;315(7103):288. doi: 10.1136/bmj.315.7103.288, PMID 9274549.
Marier JF, Lor M, Potvin D, Dimarco M, Morelli G, Saedder EA. Pharmacokinetics tolerability and performance of a novel matrix transdermal delivery system of fentanyl relative to the commercially available reservoir formulation in healthy subjects. J Clin Pharmacol. 2006;46(6):642-53. doi: 10.1177/0091270006286901, PMID 16707411.
Wester RC, Patel R, Nacht S, Leyden J, Melendres J, Maibach H. Controlled release of benzoyl peroxide from a porous microsphere polymeric system can reduce topical irritancy. J Am Acad Dermatol. 1991;24(5 Pt 1):720-6. doi: 10.1016/0190-9622(91)70109-f, PMID 1869643.
Sudhakar K, Fuloria S, Subramaniyan V, Sathasivam KV, Azad AK, Swain SS. Ultraflexible liposome nanocargo as a dermal and transdermal drug delivery system. Nanomaterials (Basel). 2021;11(10):2557. doi: 10.3390/nano11102557, PMID 34685005.
Schafer Korting M, Korting HC, Ponce Poschl EJ. Liposomal tretinoin for uncomplicated acne vulgaris. Clin Investig. 1994;72(12):1086-91. doi: 10.1007/BF00577761, PMID 7711421.
Zhai H, Willard P, Maibach HI. Evaluating skin protective materials against contact irritants and allergens an in vivo screening human model. Contact Dermatitis. 1998;38(3):155-8. doi: 10.1111/j.1600-0536.1998.tb05683.x, PMID 9536408.
Wigger Alberti W, Hinnen U, Elsner PJ. Predictive testing of metalworking fluids: a comparison of 2 cumulative human irritation models and correlation with epidemiological data. Contact Dermatitis. 1997;36(1):14-20. doi: 10.1111/j.1600-0536.1997.tb00916.x, PMID 9034682.
Ben Shabat S, Baruch N, Sintov AC. Conjugates of unsaturated fatty acids with propylene glycol as potentially less irritant skin penetration enhancers. Drug Dev Ind Pharm. 2007;33(11):1169-75. doi: 10.1080/03639040701199258, PMID 18058312.
Sintov A, Ben Shabat SJ. Design of fatty acid conjugates for dermal delivery and topical therapeutics. Crit Rev Ther Drug Carrier Syst. 2006;23(1):67-87. doi: 10.1615/critrevtherdrugcarriersyst.v23.i1.20, PMID 16749899.
Karande P, Mitragotri SB. Enhancement of transdermal drug delivery via synergistic action of chemicals. Biochim Biophys Acta. 2009;1788(11):2362-73. doi: 10.1016/j.bbamem.2009.08.015, PMID 19733150.
Wilson DE, Kaidbey K, Boike SC, Jorkasky DK. Use of topical corticosteroid pretreatment to reduce the incidence and severity of skin reactions associated with testosterone transdermal therapy. Clin Ther. 1998;20(2):299-306. doi: 10.1016/s0149-2918(98)80093-3, PMID 9589821.
Amkraut AA, Jordan WP, Taskovich LJ. Effect of coadministration of corticosteroids on the development of contact sensitization. J Am Acad Dermatol. 1996;35(1):27-31. doi: 10.1016/S0190-9622(96)90491-0, PMID 8682959.
Andersen F, Hedegaard K, Petersen TK, Bindslev Jensen C, Fullerton A, Andersen KE. Anti-irritants I: dose response in acute irritation. Contact Dermatitis. 2006;55(3):148-54. doi: 10.1111/j.1600-0536.2006.00752.x, PMID 16918613.
Andersen F, Hedegaard K, Petersen TK, Bindslev Jensen C, Fullerton A, Andersen KE. Comparison of the effect of glycerol and triamcinolone acetonide on cumulative skin irritation in a randomized trial. J Am Acad Dermatol. 2007;56(2):228-35. doi: 10.1016/j.jaad.2006.08.063, PMID 17156893.
Huang YB, Tsai YH, Chang JS, Liu JC, Tsai MJ, Wu PC. Effect of antioxidants and anti-irritants on the stability skin irritation and penetration capacity of captopril gel. Int J Pharm. 2002;241(2):345-51. doi: 10.1016/s0378-5173(02)00265-x, PMID 12100862.
Benson HA, Grice JE, Mohammed Y, Namjoshi S, Roberts MS. Topical and transdermal drug delivery: from simple potions to smart technologies. Curr Drug Deliv. 2019;16(5):444-60. doi: 10.2174/1567201816666190201143457, PMID 30714524.
Ramadon D, McCrudden MT, Courtenay AJ, Donnelly RF. Enhancement strategies for transdermal drug delivery systems: current trends and applications. Drug Deliv Transl Res. 2022;12(4):758-91. doi: 10.1007/s13346-021-00909-6, PMID 33474709.
Bird D, Ravindra NM. Transdermal drug delivery and patches an overview. Med Devices & Sens. 2020;3(6):e10069. doi: 10.1002/mds3.10069.
Dhiman S, Singh TG, Rehni AK. Transdermal patches: a recent approach to new drug delivery system. International Journal of Pharmacy and Pharmaceutical Sciences. 2011;3(5):26-34.
Agrawal S. Microneedles: an advancement to transdermal drug delivery system approach. Journal of Applied Pharmaceutical Science. 2020;10(3):149-59. doi: 10.7324/JAPS.2020.103019.
Zhao Z, Chen Y, Shi Y. Microneedles: a potential strategy in transdermal delivery and application in the management of psoriasis. RSC Adv. 2020;10(24):14040-9. doi: 10.1039/d0ra00735h, PMID 35498446.
Jung JH, Jin SG. Microneedle for transdermal drug delivery: current trends and fabrication. J Pharm Investig. 2021;51(5):503-17. doi: 10.1007/s40005-021-00512-4, PMID 33686358.
Shakya AK, Ingrole RS, Joshi G, Uddin MJ, Anvari S, Davis CM. Microneedles coated with peanut allergen enable desensitization of peanut sensitized mice. J Control Release. 2019 Nov 28;314:38-47. doi: 10.1016/j.jconrel.2019.09.022, PMID 31626861.
Lim J, Tahk D, Yu J, Min DH, Jeon NL. Design rules for a tunable merged-tip microneedle. Microsyst Nanoeng. 2018;4(1):29. doi: 10.1038/s41378-018-0028-z, PMID 31057917.
Dardano P, Calio A, Di Palma V, Bevilacqua MF, Di Matteo A, De Stefano L. A photolithographic approach to polymeric microneedles array fabrication. Materials (Basel). 2015;8(12):8661-73. doi: 10.3390/ma8125484, PMID 28793736.
Han D, Morde RS, Mariani S, La Mattina AA, Vignali E, Yang C. 4D printing of a bioinspired microneedle array with backward facing barbs for enhanced tissue adhesion. Adv Funct Materials. 2020;30(11):1909197. doi: 10.1002/adfm.201909197.
Balmert SC, Carey CD, Falo GD, Sethi SK, Erdos G, Korkmaz E. Dissolving undercut microneedle arrays for multicomponent cutaneous vaccination. J Control Release. 2020 Jan 10;317:336-46. doi: 10.1016/j.jconrel.2019.11.023, PMID 31756393.
Jeong WY, Kwon M, Choi HE, Kim KS. Recent advances in transdermal drug delivery systems: a review. Biomater Res. 2021;25(1):24. doi: 10.1186/s40824-021-00226-6, PMID 34321111.
Kim HM, Lim YY, An JH, Kim MN, Kim BJ. Transdermal drug delivery using disk microneedle rollers in a hairless rat model. Int J Dermatol. 2012;51(7):859-63. doi: 10.1111/j.1365-4632.2011.05343.x, PMID 22715835.
Li K, Yoo KH, Byun HJ, Lim YY, Kim MN, Hong HK. The microneedle roller is an effective device for enhancing transdermal drug delivery. Int J Dermatol. 2012;51(9):1137-9. doi: 10.1111/j.1365-4632.2010.04703.x, PMID 22233171.
Bal SM, Caussin J, Pavel S, Bouwstra JA. In vivo assessment of safety of microneedle arrays in human skin. Eur J Pharm Sci. 2008;35(3):193-202. doi: 10.1016/j.ejps.2008.06.016, PMID 18657610.
Jamaledin R, Yiu CK, Zare EN, Niu LN, Vecchione R, Chen G. Advances in antimicrobial microneedle patches for combating infections. Adv Mater. 2020;32(33):e2002129. doi: 10.1002/adma.202002129, PMID 32602146.
Donnelly RF, Singh TR, Garland MJ, Migalska K, Majithiya R, McCrudden CM. Hydrogel forming microneedle arrays for enhanced transdermal drug delivery. Adv Funct Mater. 2012;22(23):4879-90. doi: 10.1002/adfm.201200864, PMID 23606824.
Donnelly RF, Singh TR, Morrow DI, Woolfson AD. Microneedle mediated transdermal and intradermal drug delivery. Chichester: John Wiley & Sons; 2012. doi: 10.1002/9781119959687.
Donnelly RF, McCrudden MT, Zaid Alkilani A, Larraneta E, McAlister E, Courtenay AJ. Hydrogel forming microneedles prepared from super swelling polymers combined with lyophilised wafers for transdermal drug delivery. PLOS One. 2014;9(10):e111547. doi: 10.1371/journal.pone.0111547, PMID 25360806.
Migdadi EM, Courtenay AJ, Tekko IA, McCrudden MT, Kearney MC, McAlister E. Hydrogel forming microneedles enhance transdermal delivery of metformin hydrochloride. J Control Release. 2018 Sep 10;285:142-51. doi: 10.1016/j.jconrel.2018.07.009, PMID 29990526.
Kearney MC, McKenna PE, Quinn HL, Courtenay AJ, Larraneta E, Donnelly RF. Design and development of liquid drug reservoirs for microneedle delivery of poorly soluble drug molecules. Pharmaceutics. 2019;11(11):605. doi: 10.3390/pharmaceutics11110605, PMID 31766145.
Anjani QK, Permana AD, Carcamo Martinez A, Dominguez Robles J, Tekko IA, Larraneta E. Versatility of hydrogel forming microneedles in in vitro transdermal delivery of tuberculosis drugs. Eur J Pharm Biopharm. 2021 Jan;158:294-312. doi: 10.1016/j.ejpb.2020.12.003, PMID 33309844.
Gonzalez Vazquez P, Larraneta E, McCrudden MT, Jarrahian C, Rein Weston A, Quintanar Solares M. Transdermal delivery of gentamicin using dissolving microneedle arrays for potential treatment of neonatal sepsis. J Control Release. 2017 Nov 10;265:30-40. doi: 10.1016/j.jconrel.2017.07.032, PMID 28754611.
Sabri AH, Anjani QK, Utomo E, Ripolin A, Donnelly RF. Development and characterization of a dry reservoir hydrogel forming microneedles composite for minimally invasive delivery of cefazolin. Int J Pharm. 2022 Apr 5;617:121593. doi: 10.1016/j.ijpharm.2022.121593, PMID 35182702.
Abraham AM, Anjani QK, Adhami M, Hutton AR, Larraneta E, Donnelly RF. Novel smart reservoirs for hydrogel forming microneedles to improve the transdermal delivery of rifampicin. J Mater Chem B. 2024;12(18):4375-88. doi: 10.1039/d4tb00110a, PMID 38477350.
Zhao L, Vora LK, Kelly SA, Li L, Larraneta E, McCarthy HO. Hydrogel forming microarray patch mediated transdermal delivery of tetracycline hydrochloride. J Control Release. 2023 Apr;356:196-204. doi: 10.1016/j.jconrel.2023.02.031, PMID 36868520.
Chandran R, Tohit ER, Stanslas J, Tuan Mahmood TM. Recent advances and challenges in microneedle mediated transdermal protein and peptide drug delivery. In: Biomaterials and bionanotechnology. Elsevier; 2019. p. 495-525. doi: 10.1016/B978-0-12-814427-5.00014-7.
Ryu HR, Jeong HR, Seon Woo HS, Kim JS, Lee SK, Kim HJ. Efficacy of a bleomycin microneedle patch for the treatment of warts. Drug Deliv Transl Res. 2018;8(1):273-80. doi: 10.1007/s13346-017-0458-4, PMID 29204924.
Sanjay ST, Zhou W, Dou M, Tavakoli H, Ma L, Xu F. Recent advances of controlled drug delivery using microfluidic platforms. Adv Drug Deliv Rev. 2018 Mar 15;128:3-28. doi: 10.1016/j.addr.2017.09.013, PMID 28919029.
Bhatnagar S, Dave K, Venuganti VV. Microneedles in the clinic. J Control Release. 2017 Aug 28;260:164-82. doi: 10.1016/j.jconrel.2017.05.029, PMID 28549948.
Yadav V, Sharma PK, Murty US, Mohan NH, Thomas R, Dwivedy SK. 3D printed hollow microneedles array using stereolithography for efficient transdermal delivery of rifampicin. Int J Pharm. 2021 Aug 10;605:120815. doi: 10.1016/j.ijpharm.2021.120815, PMID 34153441.
Hong X, Wei L, Wu F, Wu Z, Chen L, Liu Z. Dissolving and biodegradable microneedle technologies for transdermal sustained delivery of drug and vaccine. Drug Des Devel Ther. 2013 Sep 4;7:945-52. doi: 10.2147/DDDT.S44401, PMID 24039404.
Park JH, Allen MG, Prausnitz MR. Biodegradable polymer microneedles: fabrication mechanics and transdermal drug delivery. J Control Release. 2005;104(1):51-66. doi: 10.1016/j.jconrel.2005.02.002, PMID 15866334.
Marin E, Briceno MI, Caballero George CJ. Critical evaluation of biodegradable polymers used in nanodrugs. Int J Nanomedicine. 2013;8:3071-90. doi: 10.2147/IJN.S47186, PMID 23990720.
Zan P, Than A, Duong PK, Song J, Xu C, Chen P. Antimicrobial microneedle patch for treating deep cutaneous fungal infection. Advanced Therapeutics. 2019;2(10):1900064. doi: 10.1002/adtp.201900064.
Tan Y, Wang Y, Zeng N, Zhang Q, Wu M, Wu Y. Degradable microneedle patch loaded with doxycycline hydrochloride and vascular endothelial growth factors for promoting diabetic wound healing. Advanced Therapeutics. 2024;7(2):2300264. doi: 10.1002/adtp.202300264.
Zafar S, Arshad MS, Rana SJ, Patel M, Yousef B, Ahmad Z. Engineering of clarithromycin loaded stimulus responsive dissolving microneedle patches for the treatment of biofilms. Int J Pharm. 2023 Jun 10;640:123003. doi: 10.1016/j.ijpharm.2023.123003, PMID 37146953.
Li X, Du W, Xu W, Ling G, Zhang P. Dissolving microneedles based on ZnO nanoparticles and an ionic liquid as synergistic antibacterial agents. J Mater Chem B. 2023;11(19):4354-64. doi: 10.1039/d3tb00127j, PMID 37159019.
He X, Sun J, Zhuang J, Xu H, Liu Y, Wu D. Microneedle system for transdermal drug and vaccine delivery: devices safety and prospects. Dose Response. 2019;17(4):1559325819878585. doi: 10.1177/1559325819878585, PMID 31662709.
Xie L, Zeng H, Sun J, Qian W. Engineering microneedles for therapy and diagnosis: a survey. Micromachines (Basel). 2020;11(3):271. doi: 10.3390/mi11030271, PMID 32150866.
Lee KJ, Jeong SS, Roh DH, Kim DY, Choi HK, Lee EH. A practical guide to the development of microneedle systems in clinical trials or on the market. Int J Pharm. 2020 Jan 5;573:118778. doi: 10.1016/j.ijpharm.2019.118778, PMID 31678394.
Wei Ze L, Mei Rong H, Jian Ping Z, Yong Qiang Z, Bao Hua H, Ting L. Super short solid silicon microneedles for transdermal drug delivery applications. International Journal of Pharmaceutics. 2010;389(1-2):122-9. doi: 10.1016/j.ijpharm.2010.01.024.
Pradeep Narayanan S, Raghavan SJ. Fabrication and characterization of gold coated solid silicon microneedles with improved biocompatibility. Int J Adv Manuf Technol. 2019;104(9-12):3327-33. doi: 10.1007/s00170-018-2596-3.
Tabassum N, Rudd D, Yan L, Voelcker NH, Alba M. Porous silicon microneedle patches for delivery of polymyxin based antimicrobials. Nano Select. 2024;5(7-8):2300116. doi: 10.1002/nano.202300116.
Dermol Cerne J, Pirc E, Miklavcic D. Mechanistic view of skin electroporation models and dosimetry for successful applications: an expert review. Expert Opin Drug Deliv. 2020;17(5):689-704. doi: 10.1080/17425247.2020.1745772, PMID 32192364.
Lin CH, Aljuffali IA, Fang JY. Lasers as an approach for promoting drug delivery via skin. Expert Opin Drug Deliv. 2014;11(4):599-614. doi: 10.1517/17425247.2014.885501, PMID 24490743.
Park H, Park H, Na K. Dual propionibacterium acnes therapy using skin penetration enhanced liposomes loaded with a photosensitizer and an antibiotic. J Porphyrins Phthalocyanines. 2015;19(8):956-66. doi: 10.1142/S1088424615500686.
Stanekzai A, Sudhakar C, Zhakfar A, Karan VS. Recent approaches in transdermal drug delivery system. Rese Jour of Pharm and Technol. 2019;12(9):4550-8. doi: 10.5958/0974-360X.2019.00783.2.
Gandhi K. Transdermal drug delivery a review. International Journal of Research in Pharmaceutical Sciences. 2012;3(3).
Babaie S, Bakhshayesh AR, Ha JW, Hamishehkar H, Kim KH. Invasome: a novel nanocarrier for transdermal drug delivery. Nanomaterials (Basel). 2020;10(2):341. doi: 10.3390/nano10020341, PMID 32079276.
Palac Z, Engesland A, Flaten GE, Skalko Basnet N, Filipovic Grcic J, Vanic Z. Liposomes for (trans) dermal drug delivery: the skin-PVPA as a novel in vitro stratum corneum model in formulation development. J Liposome Res. 2014;24(4):313-22. doi: 10.3109/08982104.2014.899368, PMID 24646434.
Cevc GJ. Transfersomes liposomes and other lipid suspensions on the skin: permeation enhancement vesicle penetration and transdermal drug delivery. Crit Rev Ther Drug Carrier Syst. 1996;13(3-4):257-388. doi: 10.1615/critrevtherdrugcarriersyst.v13.i3-4.30, PMID 9016383.
Tiwari R. Development characterization and transdermal delivery of dapsone and an antibiotic entrapped in ethanolic liposomal gel for the treatment of lepromatous leprosy. The Open Nanomedicine Journal. 2018 Apr;5:1. doi: 10.2174/1875933501805010001.
Li C, Zhang X, Huang X, Wang X, Liao G, Chen Z. Preparation and characterization of flexible nanoliposomes loaded with daptomycin a novel antibiotic for topical skin therapy. Int J Nanomedicine. 2013 Mar 24;8:1285-92. doi: 10.2147/IJN.S41695, PMID 23569376.
Cai Y, Chu Y, Gong Y, Hong Y, Song F, Wang H. Enhanced transdermal peptide modified flexible liposomes for efficient percutaneous delivery of chrysomycin a to treat subcutaneous melanoma and intradermal MRSA infection. Adv Healthc Mater. 2023;12(26):e2300881. doi: 10.1002/adhm.202300881, PMID 37267625.
Zhang Z, Liu Y, Chen Y, Li L, Lan P, He D. Transdermal delivery of 5-aminolevulinic acid by nanoethosome gels for photodynamic therapy of hypertrophic scars. ACS Appl Mater Interfaces. 2019;11(4):3704-14. doi: 10.1021/acsami.8b17498, PMID 30589527.
Shah PK, Majumdar SJ. Patent review on ethosomes: novel vesicular carrier for enhanced transdermal drug delivery system. Journal of Advanced Pharmacy Education & Research. 2014;4(4):380-7.
Mohammed MI. Formulation and characterization of ethosomes bearing vancomycin hydrochloride for transdermal delivery. Int J Pharm Pharm Sci. 2014;6(11):190-4.
Tiwari R, Tiwari G, Wal P, Wal A, Maurya P. Development characterization and transdermal delivery of dapsone and an antibiotic entrapped in ethanolic liposomal gel for the treatment of lapromatous leprosy. Open Nanomed J. 2018;5(1):1-15. doi: 10.2174/1875933501805010001.
ReferencesPaolino D, Lucania G, Mardente D, Alhaique F, Fresta M. Ethosomes for skin delivery of ammonium glycyrrhizinate: in vitro percutaneous permeation through human skin and in vivo anti-inflammatory activity on human volunteers. J Control Release. 2005;106(1-2):99-110. doi: 10.1016/j.jconrel.2005.04.007, PMID 15935505.
Lodzki M, Godin B, Rakou L, Mechoulam R, Gallily R, Touitou E. Cannabidiol transdermal delivery and anti-inflammatory effect in a murine model. J Control Release. 2003;93(3):377-87. doi: 10.1016/j.jconrel.2003.09.001, PMID 14644587.
Muller RH, Mader K, Gohla S. Solid lipid nanoparticles (SLN) for controlled drug delivery a review of the state of the art. Eur J Pharm Biopharm. 2000;50(1):161-77. doi: 10.1016/s0939-6411(00)00087-4, PMID 10840199.
Doktorovova S, Kovacevic AB, Garcia ML, Souto EB. Preclinical safety of solid lipid nanoparticles and nanostructured lipid carriers: current evidence from in vitro and in vivo evaluation. Eur J Pharm Biopharm. 2016;108:235-52. doi: 10.1016/j.ejpb.2016.08.001, PMID 27519829.
Yoon G, Park JW, Yoon IS. Solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs): recent advances in drug delivery. J Pharm Investig. 2013;43(5):353-62. doi: 10.1007/s40005-013-0087-y.
Makwana V, Jain R, Patel K, Nivsarkar M, Joshi A. Solid lipid nanoparticles (SLN) of efavirenz as lymph targeting drug delivery system: elucidation of mechanism of uptake using chylomicron flow blocking approach. Int J Pharm. 2015;495(1):439-46. doi: 10.1016/j.ijpharm.2015.09.014, PMID 26367780.
Nasrollahzadeh M, Ganji F, Taghizadeh SM, Vasheghani Farahani E, Mohiti Asli M. Drug in adhesive transdermal patch containing antibiotic loaded solid lipid nanoparticles. J Biosci Bioeng. 2022;134(5):471-6. doi: 10.1016/j.jbiosc.2022.08.003, PMID 36151004.
Ghasemiyeh P, Mohammadi Samani S. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: applications advantages and disadvantages. Res Pharm Sci. 2018;13(4):288-303. doi: 10.4103/1735-5362.235156, PMID 30065762.
Beloqui A, Solinis MA, Rodriguez Gascon A, Almeida AJ, Preat V. Nanostructured lipid carriers: promising drug delivery systems for future clinics. Nanomedicine. 2016;12(1):143-61. doi: 10.1016/j.nano.2015.09.004, PMID 26410277.
Shidhaye SS, Vaidya R, Sutar S, Patwardhan A, Kadam VJ. Solid lipid nanoparticles and nanostructured lipid carriers innovative generations of solid lipid carriers. Curr Drug Deliv. 2008;5(4):324-31. doi: 10.2174/156720108785915087, PMID 18855604.
Tariq A, Kazim T, Khan MF, Usman M, Khan A. Reshaping antibiotic delivery: chitosan based polymeric hydrogel for transdermal treatment of drug resistant bacteria. Polym Adv Technol. 2024;35(3):e6331. doi: 10.1002/pat.6331.
Dhal S, Gavara RR, Pal K, Banerjee I, Mishra M, Giri S. Facile transdermal delivery of upconversion nanoparticle by iontophoresis responsive magneto upconversion oleogel. Nano Ex. 2020;1(1):010012. doi: 10.1088/2632-959X/ab81e1.
Lee SY, Kang MS, Jeong WY, Han DW, Kim KS. Hyaluronic acid based theranostic nanomedicines for targeted cancer therapy. Cancers (Basel). 2020;12(4):940. doi: 10.3390/cancers12040940, PMID 32290285.
Jijie R, Barras A, Boukherroub R, Szunerits S. Nanomaterials for transdermal drug delivery: beyond the state of the art of liposomal structures. J Mater Chem B. 2017;5(44):8653-75. doi: 10.1039/c7tb02529g, PMID 32264260.
Jeong WY, Kim S, Lee SY, Lee H, Han DW, Yang SY. Transdermal delivery of minoxidil using HA-PLGA nanoparticles for the treatment in alopecia. Biomater Res. 2019;23(1):16. doi: 10.1186/s40824-019-0164-z, PMID 31695925.
Escobar Chavez JJ, Diaz Torres R, Rodriguez Cruz IM, Domnguez Delgado, Sampere Morales, Angeles Anguiano. Nanocarriers for transdermal drug delivery. RRTD. 2012;1(1):3. doi: 10.2147/RRTD.S32621.
Patra JK, Gitishree Das ON, Fernandes Fraceto L, Ramos Campos EV, Del Pilar Rodriguez Torres M, Acosta Torres LS, Diaz Torres LA. Nano based drug delivery systems: recent developments and prospects. Journal of Nanobiotechnology. 2018;16(71)2-33. doi: 10.1186/s12951-018-0392-8.
Buhecha MD, Lansley AB, Somavarapu S, Pannala AS. Development and characterization of PLA nanoparticles for pulmonary drug delivery: co-encapsulation of theophylline and budesonide a hydrophilic and lipophilic drug. Journal of Drug Delivery Science and Technology. 2019;53:101128. doi: 10.1016/j.jddst.2019.101128.
Mader P, Fliessbach A, Dubois D, Gunst L, Fried P, Niggli U. Soil fertility and biodiversity in organic farming. Science. 2002;296(5573):1694-7. doi: 10.1126/science.1071148, PMID 12040197.
Vargason AM, Anselmo AC, Mitragotri SJ. The evolution of commercial drug delivery technologies. Nat Biomed Eng. 2021;5(9):951-67. doi: 10.1038/s41551-021-00698-w, PMID 33795852.
Chan, BK J Eng SJ Leong. Scaffolding in tissue engineering: general approaches and tissue specific considerations. Eur Spine J. 2008;17(4):467-79. doi: 10.1007/s00586-008-0745-3.
Khajavi R, M Abbasipour, A Bahador. Electrospun biodegradable nanofibers scaffolds bone tissue eng. J Appl Polym Sci. 2016;133(3):42883. doi: 10.1002/app.42883.
Sivan M, Madheswaran D, Valtera J, Kostakova EK, Lukas D. Alternating current electrospinning: the impacts of various high voltage signal shapes and frequencies on the spinnability and productivity of polycaprolactone nanofibers. Materials & Design. 2022;213:110308. doi: 10.1016/j.matdes.2021.110308.
Teraoka I. Polymer solutions: an introduction to physical properties. Chichester: John Wiley & Sons; 2002. doi: 10.1002/0471224510.
Reneker DH, Chun IJ. Nanometre diameter fibres of polymer produced by electrospinning. Nanotechnology. 1996;7(3):216. doi: 10.1088/0957-4484/7/3/009.
Li D, Babel A, Jenekhe S, Xia Y. Nanofibers of conjugated polymers prepared by electrospinning with a two capillary spinneret. Advanced Materials. 2004;16(22):2062-6. doi: 10.1002/adma.200400606.
Soltani I, Macosko CW. Influence of rheology and surface properties on morphology of nanofibers derived from islands in the sea meltblown nonwovens. Polymer. 2018 Jun 6;145:21-30. doi: 10.1016/j.polymer.2018.04.051.
Zhang Y, Lim CT, Ramakrishna S, Huang ZM. Recent development of polymer nanofibers for biomedical and biotechnological applications. J Mater Sci Mater Med. 2005;16(10):933-46. doi: 10.1007/s10856-005-4428-x, PMID 16167102.
Bockelmann J, Klinkhammer K, Von Holst A, Seiler N, Faissner A, Brook GA. Functionalization of electrospun poly(ε-caprolactone) fibers with the extracellular matrix derived peptide GRGDS improves guidance of schwann cell migration and axonal growth. Tissue Eng Part A. 2011;17(3-4):475-86. doi: 10.1089/ten.TEA.2010.0369, PMID 20819000.
Gerardo Nava J, Fuhrmann T, Klinkhammer K, Seiler N, Mey J, Klee D. Human neural cell interactions with orientated electrospun nanofibers in vitro. Nanomedicine (Lond). 2009;4(1):11-30. doi: 10.2217/17435889.4.1.11, PMID 19093893.
Clements IP, Kim YT, English AW, Lu X, Chung A, Bellamkonda RV. Thin film enhanced nerve guidance channels for peripheral nerve repair. Biomaterials. 2009;30(23-24):3834-46. doi: 10.1016/j.biomaterials.2009.04.022, PMID 19446873.
Kim YT, Haftel VK, Kumar S, Bellamkonda RV. The role of aligned polymer fiber based constructs in the bridging of long peripheral nerve gaps. Biomaterials. 2008;29(21):3117-27. doi: 10.1016/j.biomaterials.2008.03.042, PMID 18448163.
Lietz M, Dreesmann L, Hoss M, Oberhoffner S, Schlosshauer B. Neuro tissue engineering of glial nerve guides and the impact of different cell types. Biomaterials. 2006;27(8):1425-36. doi: 10.1016/j.biomaterials.2005.08.007, PMID 16169587.
Xu J, Jiao Y, Shao X, Zhou C. Controlled dual release of hydrophobic and hydrophilic drugs from electrospun poly(l-lactic acid) fiber mats loaded with chitosan microspheres. Materials Letters. 2011;65(17-18):2800-3. doi: 10.1016/j.matlet.2011.06.018.
Song B, Wu C, Jiang Chang. Controllable delivery hydrophilic hydrophobic drugs electrospun poly (lactic‐co‐glycolic acid)/mesoporous silica nanoparticles composite mats. J Biomed Mater Res B Appl Biomater. 2012 Nov;100(8):2178-86. doi: 10.1002/jbm.b.32785.
Martin CR. Membrane based synthesis of nanomaterials. Chem Mater. 1996;8(8):1739-46. doi: 10.1021/cm960166s.
Ma PX, R Zhang. Synthetic nano‐scale fibrous extracellular matrix. J Biomed Mater Res. 1999;46(1):60-72. doi: 10.1002/(sici)1097-4636(199907)46:1<60::aid-jbm7>3.0.co;2-h.
Hartgerink JD, Beniash E, Stupp SI. Self assembly and mineralization of peptide amphiphile nanofibers. Science. 2001;294(5547):1684-8. doi: 10.1126/science.1063187, PMID 11721046.
Hassan MA, Yeom BY, Wilkie A, Pourdeyhimi B, Khan SA. Fabrication of nanofiber meltblown membranes and their filtration properties. Journal of Membrane Science. 2013 Jan 15;427:336-44. doi: 10.1016/j.memsci.2012.09.050.
Heunis TD, Dicks LM. Nanofibers offer alternative ways to the treatment of skin infections. J Biomed Biotechnol. 2010;2010:510682. doi: 10.1155/2010/510682, PMID 20798871.
Rozek Z. Potential applications of nanofiber textile covered by carbon coatings. Journal of Achievements of Materials and Manufacturing Engineering. 2008;27(1):35-8.
KO J, Bhullar SK, Mohtaram NK, Willerth SM, Jun MB. Using mathematical modeling to control topographical properties of poly (ε-caprolactone) melt electrospun scaffolds. J Micromech Microeng. 2014;24(6):65009. doi: 10.1088/0960-1317/24/6/065009.
Tutak W, Sarkar S, Lin Gibson S, Farooque TM, Jyotsnendu G, Wang D. The support of bone marrow stromal cell differentiation by airbrushed nanofiber scaffolds. Biomaterials. 2013;34(10):2389-98. doi: 10.1016/j.biomaterials.2012.12.020, PMID 23312903.
Nematpour N, Farhadian N, Ebrahimi KS, Arkan E, Seyedi F, Khaledian S. Sustained release nanofibrous composite patch for transdermal antibiotic delivery. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2020 Feb 5;586:124267. doi: 10.1016/j.colsurfa.2019.124267.
Gupta KC, Haider A, Choi YR, Kang IK. Nanofibrous scaffolds in biomedical applications. Biomater Res. 2014;18(1):5. doi: 10.1186/2055-7124-18-5, PMID 26331056.
Kataria K, Gupta A, Rath G, Mathur RB, Dhakate SR. In vivo wound healing performance of drug loaded electrospun composite nanofibers transdermal patch. Int J Pharm. 2014;469(1):102-10. doi: 10.1016/j.ijpharm.2014.04.047, PMID 24751731.
Schulte Werning LV, Murugaiah A, Singh B, Johannessen M, Engstad RE, Skalko Basnet N. Multifunctional nanofibrous dressing with antimicrobial and anti-inflammatory properties prepared by needle-free electrospinning. Pharmaceutics. 2021;13(9):1527. doi: 10.3390/pharmaceutics13091527, PMID 34575602.
Ajalloueian F, Asgari S, Guerra PR, Chamorro CI, Ilchenco O, Piqueras S. Amoxicillin loaded multilayer pullulan based nanofibers maintain long term antibacterial properties with tunable release profile for topical skin delivery applications. Int J Biol Macromol. 2022 Aug 31;215:413-23. doi: 10.1016/j.ijbiomac.2022.06.054, PMID 35700845.
Abdoli M, Sadrjavadi K, Arkan E, Zangeneh MM, Moradi S, Zangeneh A. Polyvinyl alcohol/Gum tragacanth/graphene oxide composite nanofiber for antibiotic delivery. Journal of Drug Delivery Science and Technology. 2020 Dec;60:102044. doi: 10.1016/j.jddst.2020.102044.
Soleiman Dehkordi E, Reisi Vanani V, Hosseini S, Lorigooini Z, Zvareh VA, Farzan M. Multilayer PVA/gelatin nanofibrous scaffolds incorporated with tanacetum polycephalum essential oil and amoxicillin for skin tissue engineering application. Int J Biol Macromol. 2024;262(1):129931. doi: 10.1016/j.ijbiomac.2024.129931, PMID 38331079.
Azimi B, Ricci C, Macchi T, Gunday C, Munafo S, Maleki H. A straightforward method to produce multi-nanodrug delivery systems for transdermal/tympanic patches using electrospinning and electrospray. Polymers. 2023;15(17):3494. doi: 10.3390/polym15173494, PMID 37688120.
Patil PB, Datir SK, Saudagar RB. A review on topical gels as drug delivery system. J Drug Delivery Ther. 2019;9(3-s):989-94. doi: 10.22270/jddt.v9i3-s.2930.
Fung L, Banker G, Chodes C. Modern pharmaceutics. New York: Marcel Dekker; 1990.
Ansel HC, Popovich NG, Allen LV. Pharmaceutical dosage forms and drug delivery systems. Lippincott Williams & Wilkins; 1995.
Kaur LP. Topical gels: a review. World Journal of Pharmacy and Pharmaceutical Research. 2010;3(1):17-24.
Pena LJ. Intoduction to dosage forms and formulations. Sci Gel Dosage Forms Theor Formulation Process. 1990;42:381-8.
Osborne DW, Amann AH. Topical drug delivery formulations. Informa Health Care; 1989.
Jaglal Y. Formulation of pH-responsive lipid polymer hybrid nanoparticles for co-delivery and enhanced antibacterial activity of 18β-glycyrrhetinic acid and vancomycin against MRSA; 2020.
Otto W, Drahoslav LJ. Hydrophilic gels in biologic use. Nature 1960;185:117-8. doi: 10.1038/185117a0.
Tsuruta T. Biomedical applications of polymeric materials. CRC press; 1993.
Peppas NA. Hydrogels in pharmaceutical formulations. European Journal of Pharmaceutics and Biopharmaceutics. 2000;50(1):27-46. doi: 10.1016/S0939-6411(00)00090-4.
Bhattarai N, J Gunn, M Zhang. Chitosan based hydrogels control localized drug deliv. Advanced Drug Delivery Reviews. 2010;62(1):83-99. doi: 10.1016/j.addr.2009.07.019.
Hoffman AS. Hydrogels for biomedical applications. Advanced Drug Delivery Reviews. 2012 Dec;64:18-23. doi: 10.1016/j.addr.2012.09.010.
Gao Y, Hao Y, Zhang W, Wei Y, Shu Y, Wang J. Microwave triggered ionic liquid based hydrogel dressing with excellent hyperthermia and transdermal drug delivery performance. Chemical Engineering Journal. 2022 Feb 1;429:131590. doi: 10.1016/j.cej.2021.131590.
Bayramoglu G, Arıca MY. A novel pH sensitive porous membrane carrier for various biomedical applications based on pHEMA/chitosan: preparation and its drug release characteristics. In: Macromolecular symposia. Wiley Online Library; 2003.
Thakkar V, Korat V, Baldaniya L, Gohel M, Gandhi T, Patel N. Development and characterization of novel hydrogel containing antimicrobial drug for treatment of burns. Int J Pharm Investig. 2016;6(3):158-68. doi: 10.4103/2230-973X.187343, PMID 27606259.
Kwon TK, Hong SK, Kim JC. In vitro skin permeation of cubosomes containing triclosan. Journal of Industrial and Engineering Chemistry. 2012;18(1):563-7. doi: 10.1016/j.jiec.2011.11.031.
Esposito E, Eblovi N, Rasi S, Drechsler M, Di Gregorio GM, Menegatti E. Lipid based supramolecular systems for topical application: a preformulatory study. AAPS PharmSci. 2003;5(4):E30. doi: 10.1208/ps050430, PMID 15198518.
Esposito E, Cortesi R, Drechsler M, Paccamiccio L, Mariani P, Contado C. Cubosome dispersions as delivery systems for percutaneous administration of indomethacin. Pharm Res. 2005;22(12):2163-73. doi: 10.1007/s11095-005-8176-x, PMID 16267633.
Afzal S, Barkat K, Ashraf MU, Khalid I, Mehmood Y, Shah NH. Formulation and characterization of polymeric cross-linked hydrogel patches for topical delivery of antibiotic for healing wound infections. Polymers. 2023;15(7):1652. doi: 10.3390/polym15071652, PMID 37050266.
Andrianopoulou A, Sokolowski K, Wenzler E, Bulman ZP, Gemeinhart RA. Assessment of antibiotic release and antibacterial efficacy from pendant glutathione hydrogels using ex vivo porcine skin. J Control Release. 2024;365:936-49. doi: 10.1016/j.jconrel.2023.12.008, PMID 38070603.
Yeh YW, Huang CC, Kuo WS, Liao TL, Tsai TL, Wu PC. Multifunctional hydrogel dressing that carries three antibiotics simultaneously and enables real time ultrasound bacterial colony detection. ACS Omega. 2023;8(11):10278-87. doi: 10.1021/acsomega.2c07806, PMID 36969425.
Jarman E, Burgess J, Sharma A, Hayashigatani K, Singh A, Fox P. Human derived collagen hydrogel as an antibiotic vehicle for topical treatment of bacterial biofilms. PLOS One. 2024;19(5):e0303039. doi: 10.1371/journal.pone.0303039, PMID 38701045.
Welton TJ. Room temperature ionic liquids solvents for synthesis and catalysis. Chem Rev. 1999;99(8):2071-84. doi: 10.1021/cr980032t, PMID 11849019.
Sheldon RJ. Catalytic reactions in ionic liquids. Chem Commun (Camb). 2001;(23):2399-407. doi: 10.1039/b107270f, PMID 12239988.
Endres F. Air and water stable ionic liquids in physical chemistry. Physical Chemistry Chemical Physics. 2006;8(18):2101-16. doi: 10.1039/b600519p.
Freemantle M. An introduction to ionic liquids. Royal Society of Chemistry; 2010. p. 1-25.
Zakrewsky M, Lovejoy KS, Kern TL, Miller TE, Le V, Nagy A. Ionic liquids as a class of materials for transdermal delivery and pathogen neutralization. Proc Natl Acad Sci U S A. 2014;111(37):13313-8. doi: 10.1073/pnas.1403995111, PMID 25157174.
Sheth NS, RB Mistry. Formulation and evaluation of transdermal patches and to study permeation enhancement effect of eugenol. Journal of Applied Pharmaceutical Science. 2011;1(3):96-101.
Salamanca CH, Barrera Ocampo A, Lasso JC, Camacho N, Yarce CJ. Franz diffusion cell approach for pre-formulation characterisation of ketoprofen semi-solid dosage forms. Pharmaceutics. 2018;10(3):148. doi: 10.3390/pharmaceutics10030148, PMID 30189634.
Lee JD, Kim JY, Jang HJ, Lee BM, Kim KB. Percutaneous permeability of 1-phenoxy-2-propanol a preservative in cosmetics. Regul Toxicol Pharmacol. 2019 Apr;103:56-62. doi: 10.1016/j.yrtph.2019.01.002, PMID 30611821.
Rajitha P, Shammika P, Aiswarya S, Gopikrishnan A, Jayakumar R, Sabitha M. Chaulmoogra oil based methotrexate loaded topical nanoemulsion for the treatment of psoriasis. Journal of Drug Delivery Science and Technology. 2019 Feb;49:463-76. doi: 10.1016/j.jddst.2018.12.020.
Escobar Chavez JJ, Merino Sanjuan V, Lopez Cervantes M, Urban Morlan Z, Pinon Segundo E, Quintanar Guerrero D. The tape stripping technique as a method for drug quantification in skin. J Pharm Pharm Sci. 2008;11(1):104-30. doi: 10.18433/j3201z, PMID 18445368.
Russell, LM, RH Guy. Novel imaging method quantify stratum corneum dermatopharmacokinetic s studies: proof of concept with acyclovir formulations. Pharm Res. 2012;29(12):3362-72. doi: 10.1007/s11095-012-0831-4.
Gu Y, Yang M, Tang X, Wang T, Yang D, Zhai G. Lipid nanoparticles loading triptolide for transdermal delivery: mechanisms of penetration enhancement and transport properties. J Nanobiotechnology. 2018;16(1):68. doi: 10.1186/s12951-018-0389-3, PMID 30217198.
Ahn J, Kim KH, Choe K, Lim JH, Lee SK, Kim YS. Quantitative two-photon microscopy imaging analysis of human skin to evaluate enhanced transdermal delivery by hybrid type multi-lamellar nanostructure. Biomed Opt Express. 2018;9(8):3974-82. doi: 10.1364/BOE.9.003974, PMID 30338168.
Berekmeri A, Tiganescu A, Alase AA, Vital E, Stacey M, Wittmann M. Non-invasive approaches for the diagnosis of autoimmune/autoinflammatory skin diseases a focus on psoriasis and lupus erythematosus. Front Immunol. 2019 Aug 21;10:1931. doi: 10.3389/fimmu.2019.01931, PMID 31497014.
Ashtikar M, Matthaus C, Schmitt M, Krafft C, Fahr A, Popp J. Non-invasive depth profile imaging of the stratum corneum using confocal raman microscopy: first insights into the method. Eur J Pharm Sci. 2013;50(5):601-8. doi: 10.1016/j.ejps.2013.05.030, PMID 23764946.
Zhang LW, NA Monteiro Riviere. Use confocal microsc nanopart drug deliv skin. J Biomed Opt. 2012 Jun;18(6):61214. doi 10.1117/1.JBO.18.6.061214.