
Int J App Pharm, Vol 17, Issue 4, 2025, 201-209Original Article
COMPARATIVE PHYSICOCHEMICAL EVALUATION OF DIFFERENT POLYMERS AS A MATRIX FOR THE FORMULATION OF SUSTAINED RELEASE TABLET USING FACTORIAL DESIGN
ALAA BUR1, ABDULLAH H. MAAD2,3*, YUSRA AHMED1, MALAZ YOUSEF1, ZUHEIR OSMAN1
1Department of Pharmaceutics, Faculty of Pharmacy, University of Khartoum, Khartoum, Sudan. 2Department of Pharmaceutics, College of Pharmacy, University of Al-Ameed, Karbala, Iraq. 3Department of Pharmacy Practice, College of Clinical Pharmacy, Hodeidah University, Al-Hodeidah, Yemen
*Corresponding author: Abdullah H. Maad; *Email: dr.ph.abdullah.maad@gmail.com
Received: 11 Mar 2025, Revised and Accepted: 24 May 2025
ABSTRACT
Objective: This research aims to evaluate and compare the physicochemical properties of various naturally occurring biocompatible polymers used as matrix materials in sustained-release tablet formulations. Metronidazole serves as the model drug in these formulations.
Methods: This study contributes to ongoing efforts to investigate the potential of natural polymers in developing drug delivery systems. Nine batches of Metronidazole matrix tablets were formulated using the wet granulation technique, incorporating varying concentrations of guar gum and Hydroxypropyl Methylcellulose (HPMC) polymers, as well as their combination in a 4:1 ratio. A 3² full factorial statistical design was employed to assess the impact of both polymer type and concentration on the physical properties of the granules and tablets, including drug release percentage, half-life of drug release(T₅₀%), and dissolution efficiency after 8 h.
Results: The designed factors showed a significant influence (p<0.05) on most of the responses studied, except for the polymer type, which exhibited no significant effect on the dissolution efficiency after 8 h. HPMC-based granules exhibited the best flow properties, with an angle of repose of 32.0° in F8 (HPMC 55%), the lowest Carr’s Index of 11.8, and the best Hausner ratio of 1.12, indicating superior flowability compared to Guar-based formulations. HPMC-based tablets showed the highest hardness, with F8 (HPMC 55%) achieving 7.5 kg/cm². The highest swelling index was observed in the blended formulation F9 (Guar/HPMC 55%), reaching 2.93 at 7 h, compared to 2.62 in F1 (Guar 45%) and 1.86 in F2 (HPMC 45%). F1 (Guar 45%) exhibited the highest cumulative drug release, with 89.4% released at 24 h, compared to 85.4% in F9 (Guar/HPMC 55%). The combination of Guar gum and HPMC (F9, 55%) resulted in significant release retardation compared to individual polymers. The combination of Guar gum and HPMC (F9, 55%) resulted in the longest half-life (T₅₀%) of drug release, at 6.93 h, compared to 3.75 h in F1 (Guar 45%) and 5.27 h in F2 (HPMC 45%). Dissolution efficiency was highest in F5 (HPMC 50%), with 78.8% after 8 h, compared to 69.7% in F9 (Guar/HPMC 55%), showing that the combination of Guar gum and HPMC results in a slower release, reflected by the lower dissolution efficiency.
Conclusion: The blend of guar gum and HPMC in a 4:1 ratio provides a more effective release-retardation profile compared to guar gum alone. The formulation (F9), comprising a blend of guar gum and HPMC polymer at a higher concentration of 55%, was identified as the optimal formulation. It demonstrated favorable granule flow properties and exhibited the most effective sustained release profile.
Keywords: Sustained release matrix tablet, Metronidazole, Guar gum, Hydroxypropyl methylcellulose (HPMC)
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijap.2025v17i4.54169 Journal homepage: https://innovareacademics.in/journals/index.php/ijap
INTRODUCTION
Modified-release drug delivery systems have advantages over conventional release, including low dosing frequency, fewer side effects, patient compliance, etc. These systems can be categorized into delayed release, receptor targeting, site-specific targeting, and sustained release [1]. Sustained-release systems utilize specific polymers to release drugs over an extended period gradually. Polymers, which are large molecules with various functional groups, are classified into natural (e. g., guar gum), synthetic (e. g., polyesters), and semisynthetic (e. g., cellulose derivatives) types [2]. Natural polymers are the most preferred among all polymer types, primarily because they include low toxicity, renewability, flexibility for modification, biodegradability, and cost-effectiveness [3]. Guar gum is a naturally occurring polymer extracted from the endosperm of guar plant seeds, characterized by its high molecular weight polysaccharides. Hydrophilic and susceptible to enzymatic degradation, it exhibits super hemocompatibility and interacts efficiently with living cells. These properties make it highly suitable as a biomaterial for applications requiring prolonged systemic circulation and targeted effects [4]. One of the semi-synthetic polymers is Hydroxypropyl methylcellulose (HPMC), or hypromellose [5]. It is commonly used in the formulation of sustained-release drug systems because it has swelling properties when absorbed water and forms a gel on the tablet's surface, controlling the drug release [6]. HPMC comes in various grades with different molecular weights and viscosities, making it suitable for multiple roles, including emulsification, thickening, adhesion, and gel formation [7]. Methocel K15M, a high-viscosity grade of HPMC with a viscosity of approximately 15,000 cP at 2% concentration and a gelation temperature range of 70 °C to 90 °C, was employed to achieve controlled drug release. Comparatively, other grades of HPMC, such as E3 and E5, have lower viscosities (around 3,000 cP and 5,000 cP, respectively) and different gelation temperatures, influencing their applications in drug formulations. The higher viscosity of Methocel K15M contributes to a more controlled and consistent drug release, while the lower viscosity grades may facilitate faster release profiles. These differences highlight the importance of selecting the appropriate HPMC grade based on desired drug release characteristics [5]. Hydrophilic polymers, including guar gum and HPMC, have an application in the formulation of oral controlled-release tablets. This is attributed to their capacity for polymer chain relaxation, leading to volume expansion or swelling upon contact with the dissolution medium or biological fluid. Consequently, this prompts drug molecules to initiate diffusion out of the system, with the rate governed by the nature and composition of the polymer, along with formulation technology [8, 9]. Guar gum and HPMC are widely used in sustained-release formulations due to their hydrophilic gel-forming properties, biocompatibility, and ability to modulate drug-release kinetics [10]. Their combined use has been reported to enhance the regulation of drug release by optimizing matrix swelling and erosion mechanisms [11]. Metronidazole is a crystalline powder that is white to pale yellow, odorless, and has a bitter taste. It is an antibiotic used to treat infections like trichomoniasis, amoebiasis, and giardiasis. It is classified as a Class I drug according to the Biopharmaceutics Classification System (BCS). Metronidazole is rapidly absorbed into the bloodstream, with a bioavailability of greater than 90%, and an average elimination half-life of about 8.5 h [12]. In this study, the aim was to evaluate and compare the physicochemical properties of various polymer types as matrix materials for formulating sustained-release tablets, with Metronidazole serving as the model drug. Metronidazole was selected as the model drug due to its moderate water solubility and short half-life (6–8 h), which necessitates frequent dosing to maintain therapeutic levels. Developing a sustained-release formulation can enhance patient compliance by reducing dosing frequency. Additionally, metronidazole is susceptible to degradation in acidic conditions, making controlled-release formulations an effective strategy to improve its stability in gastrointestinal transit [13]. This study hypothesizes that increasing polymer concentration will result in a significant reduction in metronidazole release rate due to enhanced gel barrier formation. Additionally, it is expected that formulations containing both guar gum and HPMC will exhibit superior release retardation compared to those containing a single polymer, due to a synergistic swelling and matrix-forming effect. The study aims to determine whether polymer type significantly impacts drug release kinetics.
MATERIALS AND METHODS
Materials
Metronidazole was obtained as a gift from (Blue Nile Pharmaceutical Co. Ltd., Sudan). Microcrystalline Cellulose PH101 (MCC) was purchased from (Shin Poong Pharm. Co. Ltd, Korea) while Guar gum was kindly gifted by (Gitaf Gums Production and Marketing Company, Sudan). Hydroxypropyl methylcellulose (Methocel K15M) was sourced from (Zaheamg Neo Dankong Pharmaceutical Co., Ltd, China). Magnesium stearate and talc were from (Techno Pharmchem, India). Hydrochloric acid (HCl) and potassium phosphate monobasic were all procured from Merck (Darmstadt, Germany).
Preparation of metronidazole matrix tablets
Metronidazole matrix tablets (200 mg dose) were formulated using the wet granulation method. The drug, polymers, and Microcrystalline Cellulose (MCC) were precisely weighed and mixed using geometrical dilution (as outlined in table 1). A fixed amount of water (20 ml per 100 g of powder mixture) was gradually added to create a wet mass, which was mixed until a cohesive mass with sufficient compressibility was achieved. The wet mass was then passed through sieve no. 12 (1200 μm) to form granules. These granules were dried for 3 h at 60 °C in a controlled humidity environment (relative humidity maintained at 40%) to ensure uniform moisture content and passed through a sieve with a 1400 μm mesh. The dried granules were blended with talc and magnesium stearate for 3 min before being compressed into tablets using a 10 mm diameter flat-faced punch tableting machine (Erweka, Germany).
Table 1: Composition of the formulations developed using the 32full factorial design
| Ingredients | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 |
| Metronidazole (%) | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 |
| Guar gum (%) | 45 | - | 36 | 50 | - | 40 | 55 | - | 44 |
| HPMC K15M (%) | - | 45 | 9 | - | 50 | 10 | - | 55 | 11 |
| Microcrystalline cellulose (%) | 13 | 13 | 13 | 8 | 8 | 8 | 3 | 3 | 3 |
| Magnesium stearate (%) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Talc (%) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
The total weight of the tablets is 500 mg.
Evaluation of the prepared granules
The granules obtained were analyzed for their micrometric characteristics, which included assessing the Angle of Repose, Compressibility Index, and Hausner’s Ratio [14]. Micrometric properties were evaluated in triplicate. Results are reported as mean±standard deviation (SD).
Angle of repose
25g of granule samples were allowed to flow through a funnel orifice onto a horizontally placed paper. The angle of repose was then calculated using the equation: θ = tan⁻¹ (
 ), where 'H' represents the pile height and 'R' denotes the base radius of the granule pile.
), where 'H' represents the pile height and 'R' denotes the base radius of the granule pile.
Compressibility index and Hausner's ratio
The compressibility index (CI) and the Hausner's Ratio (HR) were calculated from the bulk density and the tapped density, as per the following equations:
Compressibility Index (CI) = 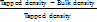 ×100
×100
Hausner's Ratio (HR) = 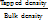
Evaluation of the matrix tablets
The matrix tablet formulations were evaluated using hardness, friability, drug content, weight variation, and drug release characteristics, by the specifications outlined in the British Pharmacopoeia (BP) [15].
Weight variation test
The weight variation test of the tablets was performed using an electronic balance.
Hardness test
The hardness of the tablets was determined using a Monsanto hardness tester.
Friability test
The friability of tablets was determined using a Roche friabilator (Campbell Electronics, India) for 4 min at a speed of 25 rpm.
Determination of drug content
Drug content was analyzed by powdering ten tablets, taking powder equivalent to 200 mg Metronidazole, and then dissolving it in a 100 ml volumetric flask containing 0.1M Hydrochloric Acid (HCl). The resulting solution was filtered via (Whitman filter paper) and subsequently diluted to achieve a theoretical concentration of 5μg/ml concentration. The absorbance of this solution was measured at λ = 277 nm by UV/Visible spectrophotometer (Shimadzu 1601, Kyoto, Japan), with 0.1M HCl solution serving as a blank. The drug content of tablets was determined using the equations derived from the Metronidazole calibration curves.
Conducting of the metronidazole calibration curves
Two standard solutions of Metronidazole were prepared, one in 0.1N HCl buffer and the other in phosphate buffer. These solutions underwent scanning between 200 and 400 nm, with their respective buffers as blanks. The maximum absorbance was observed at approximately 277 nm for Metronidazole in 0.1N HCl buffer and at 320 nm for Metronidazole in phosphate buffer. Calibration curves for Metronidazole in each medium were constructed over a working concentration range of 2–10 μg/ml at their respective maximum absorption wavelengths.
Swelling behavior of the sustained-release matrix tablets
Pre-weighted tablets (W1) were inserted in a type I dissolution apparatus containing 900 ml of 0.1N HCl at 37±0.5 °C with continuous stirring. At various time intervals (t), the tablets were withdrawn, wiped gently with tissue paper, and re-weighed (W2). The swelling index (S. I) was calculated using the following equation provided by reference[16]:
Swelling Index (S. I) = [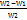
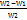 ] X 100
] X 100
In vitro drug release study
An In vitro release study was performed using a United States Pharmacopeia (USP) XXIII dissolution rate test apparatus (Apparatus 1), which has a rotating speed of 50 rpm, and a temperature of 37±1 °C. The test took 2 h in 0.1M HCl (900 ml), followed by 24 h in pH 6.8 phosphate buffer (900 ml) as per the protocol outlined in reference [17]. The dissolution study was conducted in pH 6.8 phosphate buffer, simulating intestinal conditions, as metronidazole is predominantly absorbed in the small intestine. Additionally, pH 6.8 provides a physiologically relevant medium for evaluating extended-release formulations, ensuring realistic in vitro-in vivo correlation. Sink conditions were maintained throughout the drug release studies. The volume of the dissolution medium (900 ml) was sufficient to keep the concentration of metronidazole below its solubility limit, preventing precipitation and ensuring a constant driving force for dissolution. The absorbance of the solution was measured at 277 nm during the initial 2 h, and for the subsequent 24 h, it was measured at 320 nm. These measurements were employed to determine the quantity of drugs released over time.
Dissolution Efficiency (DE)
Dissolution efficiency (DE, %) quantifies the Area Under the Curve (AUC) up to a specified time limit (t), presented as a percentage of the area of a rectangle representing 100% dissolution within the same timeframe. The AUC is computed using the trapezoidal method [18].
AUC=Σ {(y1+y2) × (t2-t1)} ×0.5
The DE% was calculated by the equation
DE (%) =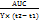 ×100
×100
Where Y represents the percentage dissolved at t2, t1 is the time at which the first sample was withdrawn, and t2 is the time for all active ingredients to dissolve. DE could be defined for every sampling time. In this study, DE was calculated for the drug released after the first 8 h, setting t1 at 0 h and t2 at 8 h.
Kinetics and mechanism of drug release
To determine the mechanism of drug release, in vitro drug release data were analyzed by various mathematical models; including the zero-order, the first-order, Higuchi, Korsmeyer–Peppas, and Hixson-Crowell release models. For a value of n equal to 0.45, the reported diffusion mechanism was Fickian diffusion. When the value of n falls between 0.45 and 0.89, the diffusion mechanism is classified as Anomalous (non-Fickian) diffusion. If n equals 0.89, the mechanism is considered Case-II transport. For values of n greater than 0.89, the mechanism is termed super Case-II transport. The correlation coefficient (R2) was calculated for each model to evaluate their fit to the experimental data. The model with the highest (R2) value was selected as the best-fit model, providing insights into the drug release mechanism. Additionally, the diffusion coefficient (n) and the release rate constant (k) parameters were predicted using the model.
Half-life of drug released
The time required to release 50% of the drug (T50%) was estimated by fitting the release data to the best-fit model of the Krosmeyer–Peppas equation.
Analysis of data
A 32 full factorial design was executed with Design Expert V8.0.6 software to identify the independent variable that significantly influenced the selected responses (table 2). Analysis of Variance (one-way ANOVA) was employed for detecting significant differences, with a threshold set at p<0.05.
Table 2: Factors and levels evaluated in the 32 full factorial design
| Factors | Levels |
| Polymer type (X1) | (-1) Level coded for Guar gum polymer type. (0) Level coded for HPMC K15M polymer type. (1) Level coded for a combination of guar gum and HPMC polymer type. |
| Polymer concentration (X2) | (-1) Level coded for 45% polymer concentration level. (0) Level coded for 50% polymer concentration level. (1) Level coded for 55% polymer concentration level. |
RESULTS
Evaluation of micromeritics properties of the granules
The properties of granules among the 9 different formulations obtained from the design were recorded as presented in table 3. Guar-based formulations (F1, F4, and F7) showed poorer flow, with F7 (Guar 55%) having the highest angle of repose (36.4°), Hausner ratio (1.22), and Carr’s Index (18.1%). In contrast, HPMC-based formulations (F2, F5, and F8) exhibited superior flow properties, with the lowest angle of repose (32.0°), Hausner ratio (1.12), and Carr’s Index (11.8%) at 55% concentration (F8). Blended Guar/HPMC formulations (F3, F6, and F9) exhibited intermediate values, suggesting a balance between both polymers.
Table 3: Micromeritics properties of the prepared metronidazole granules
| Run | Polymer type (X1) | Polymer concentration (X2) | Angle of repose* (°) | Hausner’s ratio* | Carr’s index* (%) |
| F1 | Guar | 45% | 34.5±0.1 | 1.20±0.01 | 17.5±0.1 |
| F2 | HPMC | 45% | 34.2±0.3 | 1.18±0.10 | 14.4±0.3 |
| F3 | Guar/HPMC | 45% | 33.0±0.1 | 1.19±0.01 | 16.9±0.2 |
| F4 | Guar | 50% | 34.7±0.1 | 1.21±0.01 | 17.6±0.1 |
| F5 | HPMC | 50% | 33.2±0.3 | 1.16±0.03 | 13.7±0.4 |
| F6 | Guar/HPMC | 50% | 34.0±0.1 | 1.20±0.11 | 17.2±0.1 |
| F7 | Guar | 55% | 36.4±0.1 | 1.22±0.12 | 18.1±0.0 |
| F8 | HPMC | 55% | 32.0±0.3 | 1.12±0.01 | 11.8±0.1 |
| F9 | Guar/HPMC | 55% | 35.0±0.2 | 1.21±0.01 | 17.5±0.2 |
*All values are expressed as mean±SD, n=3.
Evaluation of tablets' physical properties
The properties of tablets among the 9 different formulations obtained from the design were recorded as presented in table 4. Weight variation across all formulations is relatively consistent, ranging from 500±2 mg (F1, F2, and F3) to 507±5 mg (F6) with only minimal deviations, indicating uniformity in tablet weight. Hardness tends to increase with higher polymer concentrations, with F8 (HPMC 55%) showing the highest hardness (7.5 kg/cm²), suggesting that higher HPMC concentrations improve tablet strength. On the other hand, Guar-based tablets (F1, F4, and F7) generally show lower hardness, especially at 55% concentration (F7, 5.0 kg/cm²), indicating a decrease in tablet strength with higher Guar content. The friability values ranged between 0.60% and 0.97% with all formulations complying with the pharmacopeial limit of not more than 1%. The lowest friability was observed in F9 (0.60±0.01), while the highest was recorded in F7 (0.97±0.02%) though still within acceptable limits. The drug content is consistent across all formulations, with all values ranging from 97% to 102%, suggesting efficient drug incorporation and uniformity in content.
Table 4: Physical properties of the prepared metronidazole tablets
| Formulations | Polymer type (X1) | Polymer concentration (X2) | Weight Variationa (mg) |
Hardnessb (Kg/cm2) | Friabilityc (%) | Drug contentd (%) |
| F1 | Guar | 45% | 500.12±8.0 | 5.8±0.1 | 0.9±0.02 | 100.01±3.0 |
| F2 | HPMC | 45% | 500.18±9.0 | 6.5±0.3 | 0.8±0.04 | 102.04±2.0 |
| F3 | Guar/HPMC | 45% | 505.08±2.4 | 6.3±0.2 | 0.7±0.02 | 99.03±4.1 |
| F4 | Guar | 50% | 505.04±5.2 | 5.5±0.1 | 0.9±0.01 | 98.02±1.3 |
| F5 | HPMC | 50% | 505.02±5.1 | 6.9±0.2 | 0.8±0.03 | 97.14± 1.4 |
| F6 | Guar/HPMC | 50% | 507.22±5.2 | 6.9±0.1 | 0.8±0.02 | 97.12±1.2 |
| F7 | Guar | 55% | 505.04±6.0 | 5.0±0.2 | 0.9±0.02 | 97.01±1.1 |
| F8 | HPMC | 55% | 504.06±2.4 | 7.5±0.2 | 0.8±0.02 | 97.02±1.3 |
| F9 | Guar/HPMC | 55% | 503.03±7.0 | 7.1±0.1 | 0.6±0.01 | 97.04±2.1 |
a: mean±% deviation, n=20. b: mean±SD, n=5. c: Tablets equivalent to 6.5g. d: mean±SD, n=10.
Evaluation of the swelling behavior of the tablets
The swelling behavior of the Metronidazole matrix tablets across the different formulations is summarized in table 5 and fig. 1. The swelling index of the tablets generally increases with time, reaching the highest value at 7 h. Notably, Formulation F9 (Guar/HPMC 55%) exhibits the highest swelling index. Formulation F3 (Guar/HPMC 45%) also shows substantial swelling, with values showing a slight decrease from 2.63 at 10 min to 2.59 at 7 h. Formulation F2 (HPMC 45%) shows lower swelling indexes with the initial 10-minute value at 1.2 and only reaching 1.86 at 7 h. Guar-based formulations (F1, F4, and F7) tend to show moderate swelling with F1 (Guar 45%) showing steady swelling increasing from 1.84 at 10 min to 2.62 at 7 h, while F7 (Guar 55%) follows a similar pattern but with slightly lower values.
Table 5: Swelling indexes of the different metronidazole tablet formulations*
| Time | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 |
| 10 min. | 1.84±0.01 | 1.2±0.24 | 2.63±0.32 | 1.47±0.06 | 0.99±0.37 | 1.47±0.25 | 2.53±0.15 | 0.79±0.07 | 1.42±0.11 |
| 30 min. | 1.84±0.11 | 0.80±0.15 | 2.25±0.25 | 1.18±0.32 | 1.01±0.01 | 1.39±0.24 | 1.85±0.02 | 0.93±0.03 | 1.23±0.01 |
| 1 h. | 1.22±0.07 | 1.15±0.01 | 2.18±0.08 | 1.36±0.15 | 1.35±0.11 | 1.49±0.15 | 1.95±0.01 | 1.29±0.15 | 1.58±0.05 |
| 2 h. | 1.37±0.15 | 1.61±0.11 | 2.34±0.07 | 1.72±0.01 | 1.72±0.32 | 1.82±0.07 | 2.18±0.11 | 1.66±0.24 | 1.72±0.07 |
| 3 h. | 1.63±0.25 | 1.64±0.07 | 2.54±0.15 | 2.14±0.11 | 1.84±0.07 | 2.15±0.22 | 2.23±0.15 | 2.02±0.01 | 2.61±0.18 |
| 4 h. | 1.79±0.11 | 2.04±0.13 | 2.70±0.01 | 2.28±0.25 | 2.14±0.15 | 2.43±0.11 | 2.56±0.07 | 2.23±0.09 | 2.73±0.15 |
| 5 h. | 2.13±0.05 | 2.58±0.07 | 2.83±0.24 | 2.66±0.07 | 2.13±0.24 | 2.66±0.01 | 2.78±0.25 | 2.38±0.11 | 3.00±0.32 |
| 6 h. | 2.26±0.08 | 1.88±0.05 | 2.60±0.11 | 2.92±0.04 | 2.08±0.04 | 2.88±0.21 | 2.81±0.16 | 2.43±0.15 | 3.03±0.25 |
| 7 h. | 2.62±0.15 | 1.86±0.01 | 2.59±0.05 | 3.00±0.24 | 2.04±0.25 | 3.01±0.03 | 2.87±0.07 | 2.43±0.32 | 3.13±0.18 |
*All values are expressed as mean±SD, n=3.
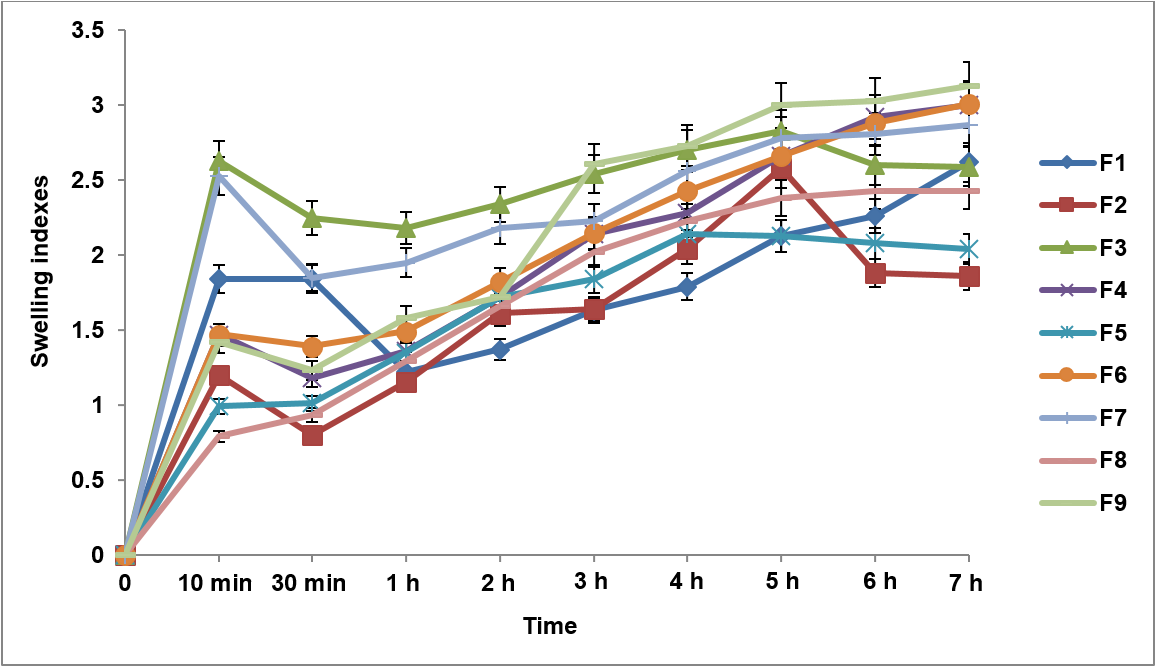
Fig. 1: Swelling indexes of the different metronidazole tablet formulations, error bars indicate the standard deviation of replicates, n=3
Table 6: The analysis of variance (ANOVA) of the swelling indexes of the nine formulations
| Factors | Swelling indexes | |
| Effect | P-value* | |
| Polymer type | 5.163 | 0.007 |
| Polymer concentration | 3.660 | 0.014 |
| Interaction | 0.220 | 0.551 |
*P ≤ 0.05 is the cutoff point for significant influence.
Evaluation of the in vitro dissolution profile of the matrix tablets
The in vitro dissolution profile of the Metronidazole matrix tablets, as shown in table 7 and fig. 2, provides insights into the drug release behavior over 24 h for all nine formulations (F1–F9). The in vitro dissolution profiles of the Metronidazole matrix tablets showed that Formulation F1 (Guar 45%) exhibited the highest drug release at 24 h with 89.4% of the drug released, while Formulation F9 (Guar/HPMC 55%) demonstrated the lowest release at 85.4%. F1 also had the highest release in the first hour (30.7%), while F9 had the lowest initial release (20.3%). Formulations F2 (HPMC 45%), F5 (HPMC 50%), and F8 (HPMC 55%) showed slower drug release with F8 reaching 85.1% at 24 h. Blended Guar/HPMC formulations (F3, F6, and F9) exhibited controlled release profiles with F3 releasing 88.1%, F6 reaching 87.1%, and F9 having the slowest release at 85.4% over 24 h.
Table 7: Cumulative drug release percentage of all formulations over 24 H*
| Time (H) | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 |
| 1 | 30.7±1.2 | 29.0±0.1 | 24.3±2.5 | 29.4±1.0 | 29.2±1.1 | 20.4±2.2 | 22.0±1.5 | 21.2±1.0 | 20.3±1.8 |
| 2 | 39.1±0.3 | 38.4±1.5 | 34.5±0.1 | 38.5±1.1 | 35.9±1.9 | 32.4±1.4 | 36.0±1.9 | 33.9±0.1 | 31.1±1.2 |
| 3 | 48.3±1.1 | 45.4±1.6 | 41.7±1.5 | 46.4±1.2 | 42.8±1.9 | 40.9±0.1 | 44.4±1.5 | 38.6±1.4 | 39.1±1.1 |
| 4 | 55.3±1.5 | 48.8±1.4 | 42.9±1.1 | 48.3±0.1 | 45.5±1.1 | 43.8±1.5 | 48.2±1.3 | 42.5±1.1 | 41.3±1.5 |
| 5 | 57.4±0.8 | 50.8±1.0 | 44.6±0.4 | 50.7±1.5 | 47.3±1.4 | 45.5±1.9 | 52.0±0.1 | 44.5±1.5 | 42.8±0.6 |
| 6 | 58.9±1.2 | 53.4±0.6 | 47.0±1.9 | 55.5±1.5 | 50.0±0.4 | 48.6±1.1 | 55.5±0.4 | 48.0±0.1 | 45.9±0.8 |
| 7 | 61.8±0.1 | 56.2±1.5 | 50.1±0.3 | 56.7±1.3 | 52.2±1.5 | 50.4±0.6 | 58.9±1.9 | 51.7±1.9 | 48.6±1.5 |
| 8 | 63.9±1.3 | 60.2±0.4 | 51.9±1.5 | 58.6±1.3 | 54.2±0.6 | 52.9±1.9 | 60.8±0.3 | 53.1±0.6 | 53.0±0.3 |
| 9 | 67.2±1.9 | 62.2±1.7 | 54.0±0.6 | 64.4±0.4 | 56.5±1.2 | 55.2±1.3 | 62.4±0.6 | 54.8±1.9 | 56.7±1.3 |
| 10 | 68.5±0.6 | 65.0±1.1 | 56.9±0.1 | 65.5±0.3 | 59.1±1.9 | 57.7±0.4 | 65.8±0.1 | 56.4±0.8 | 58.8±1.9 |
| 11 | 69.1±0.8 | 66.7±0.6 | 59.1±1.3 | 69.5±0.1 | 62.0±0.1 | 60.2±1.2 | 67.3±0.8 | 58.0±0.4 | 60.3±0.1 |
| 12 | 72.2±1.4 | 69.3±1.7 | 62.8±0.1 | 70.8±0.6 | 64.4±1.3 | 62.0±0.3 | 69.6±0.1 | 59.0±0.1 | 61.6±0.4 |
| 24 | 89.4±0.7 | 87.9±0.2 | 88.1±0.4 | 88.5±0.6 | 86.6±0.1 | 87.1±0.8 | 85.9±0.3 | 85.1±0.6 | 85.4±0.1 |
*All values are expressed as mean±SD, n=3.
Table 8: The analysis of variance (ANOVA) of the percentage of drug released from the nine formulations
| Factors | Percentage drug released | |
| Effect | P-value* | |
| Polymer type | 2.000 | 0.033 |
| Polymer concentration | -7.103 | 0.003 |
| Interaction | 0.850 | 0.205 |
*P ≤ 0.05 is the cutoff point for significant influence.
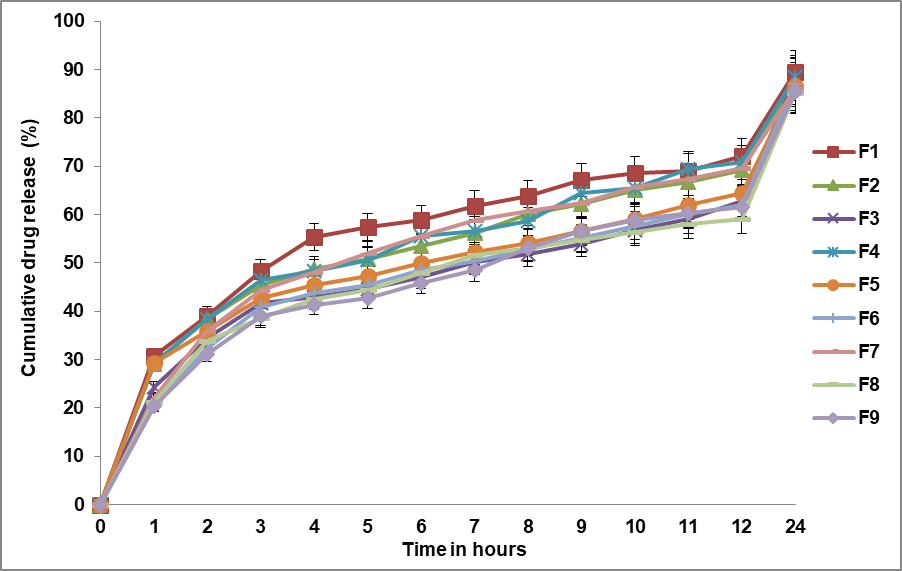
Fig. 2: Cumulative drug release profiles of all formulations over 24 H, Error bars indicate the standard deviation of replicates, n=3
Evaluation of the dissolution efficiency of the tablets after 8 h
The Dissolution efficiency after 8 h of drug release for all nine different formulations is presented in fig. 3. The dissolution efficiency of the Metronidazole matrix tablets after 8 h varied among the formulations with F5 (HPMC 50%) showing the highest dissolution efficiency at 78.8%, while F9 (Guar/HPMC 55%) exhibited the lowest at 69.7%.
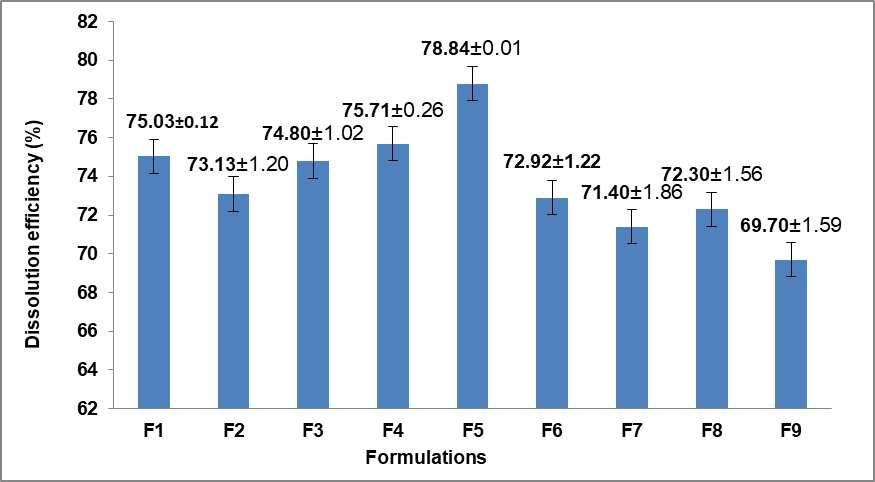
Fig. 3: Dissolution efficiency (%) after 8 H of drug release for all formulations, error bars indicate the standard deviation of replicates, n=3
Table 9: The analysis of variance (ANOVA) of the dissolution efficiency after 8 H of the nine formulations
| Factors | Dissolution efficiency after 8 h | |
| Effect | P-value* | |
| Polymer type | 1.449 | 0.175 |
| Polymer concentration | -3.192 | 0.042 |
| Interaction | 1.779 | 0.157 |
*P ≤ 0.05 is the cutoff point for significant influence.
Kinetics of drug release and the half-life of drug released (T50%)
The results of drug release kinetics and half-life (T50%) of the nine different formulations obtained from 32 full factorial design were shown in table 10 and fig. 4, respectively. The drug release kinetics of the Metronidazole matrix tablets were analyzed using the Korsmeyer-Peppas model with correlation coefficients (R²) ranging from 0.963 to 0.992, indicating a strong fit to the model. The release exponent (n) values varied from 0.305 (F5) to 0.427 (F7). The release rate constant (K) was highest for F1 (32.1), while F9 (17.6) had the lowest. The half-life (T50%) values ranged from 3.75 h (F1, the shortest) to 6.93 h (F9, the longest), confirming that F1 released the drug most rapidly while F9 had the most prolonged release.
Table 10: Parameters from korsmeyer-peppas model fitting and half-life (T50%)
| Formulations | Correlation coefficients (R2)a | Release exponent (n)b | Release rate (K)c | Half-life (T50%)d (hour) |
| F1 | 0.979 | 0.336 | 32.1 | 3.75 |
| F2 | 0.992 | 0.335 | 29.9 | 4.63 |
| F3 | 0.974 | 0.343 | 26.4 | 6.73 |
| F4 | 0.988 | 0.339 | 30.1 | 4.49 |
| F5 | 0.992 | 0.305 | 29.3 | 5.76 |
| F6 | 0.963 | 0.404 | 23.3 | 6.63 |
| F7 | 0.965 | 0.427 | 25.2 | 4.99 |
| F8 | 0.966 | 0.382 | 23.9 | 6.88 |
| F9 | 0.979 | 0.421 | 17.6 | 6.93 |
a: correlation coefficient. b: diffusion coefficient. c: Release rate constant. d: The time required to release 50% of the drug (T50%)
Table 11: The analysis of variance (ANOVA) of the half-life (T50%) of drug released of the nine formulations
| Factors | Half-life (T50%) | |
| Effect | P-value* | |
| Polymer type | -2.353 | 0.005 |
| Polymer concentration | -1.123 | 0.044 |
| Interaction | 2.579 | 0.015 |
*P ≤ 0.05 is the cutoff point for significant influence.
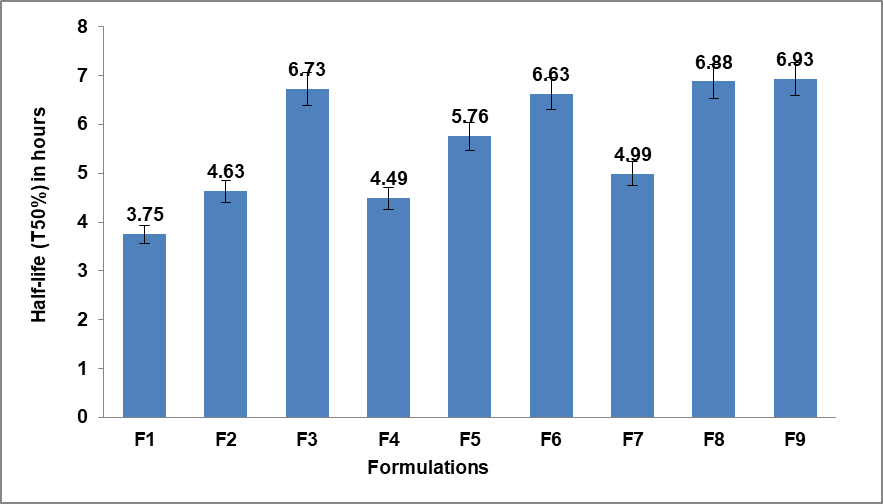
Fig. 4: The half-life (T50%) of the drug released from the nine formulations, Error bars indicate the standard deviation of replicates, n=3
DISCUSSION
The micromeritic properties of granules including the angle of repose, Hausner’s ratio, and Carr index are influenced by the type and concentration of polymers used, as shown in table 3. For Guar gum formulations (F1, F4, and F7), higher concentrations lead to increased angles of repose, Hausner’s ratios, and Carr indices, indicating reduced flowability and greater compressibility. Conversely, HPMC formulations (F2, F5, and F8) maintain stable and low angles of repose, consistently low Hausner’s ratios, and decreased Carr indices at higher concentrations, reflecting improved flowability and reduced compressibility, these findings align with a previous study [19]. Mixed formulations of Guar and HPMC (F3, F6, and F9) demonstrate angles of repose that are intermediate with improved Hausner’s ratios and Carr indices compared to pure Guar, highlighting a balanced enhancement in flow properties due to the synergistic effects of both polymers. HPMC improves the micromeritic properties of granules more effectively than Guar gum. When these two polymers are combined, the properties are further optimized, making them suitable for various pharmaceutical applications. This highlights the critical role of polymer type and concentration in achieving the desired granule characteristics [20]. From the results presented in table 4, the evaluation of the physical properties of the prepared tablets demonstrated that all formulations met the British Pharmacopoeia (BP) requirements, confirming their pharmaceutical quality and suitability for oral administration [15]. The uniformity of dosage units, a critical factor for ensuring consistent therapeutic outcomes, was maintained across all formulations as evidenced by the acceptable weight variation and drug content ranging between 85% and 115% of the label claim. Tablet hardness values between 5 and 7 kg/cm² reflect a good balance between mechanical strength and patient compliance, ensuring the tablets can withstand handling and packaging without compromising disintegration. Moreover, the friability results, below 1%, further support the physical integrity and durability of the tablets during transportation and use [15, 21]. The swelling behavior of the formulated sustained-release matrix tablets was assessed by measuring the swelling index over time. As summarized in table 5, following the initial 30 min, all formulations except for F5 and F8, which contained higher concentrations of HPMC at 50% and 55% respectively demonstrated an initial decrease followed by an increase in the swelling index. This behavior was associated with the onset of matrix erosion, indicating a combination of swelling and erosion in the matrix, making the mechanism complex [6]. Over time, the swelling index continued to increase, reflecting an accelerated rate of hydration and proportional weight gain by the tablet, particularly up to 7 h. Overall, the cumulative percent drug release decreased with an increase in gum concentration and swelling index. This phenomenon was attributed to the gradual erosion of the gelled layer in tablets containing higher guar gum content. The formation of a thick gel structure delayed the release of the drug from the tablet matrix [22]. Among all the formulations, F9, which contained guar gum/HPMC combination in a concentration of 55%, had the highest swelling index value. Guar gum forms a highly viscous gel layer, while HPMC contributes to controlled hydration and polymer relaxation, leading to prolonged hydration and gel formation [23]. This outcome was statistically validated (table 6), as both the polymer type and polymer concentration factors demonstrated a significant influence (p<0.05) on the swelling index response. Tablets that contain a combination of polymers demonstrated a higher swelling index compared to those with only HPMC, while the formulations with guar gum exhibited the lowest swelling index. Furthermore, an increase in polymer concentration increased the swelling index. These results align with the findings of Sriamornsak et al. (2007), who also observed that the swelling behavior of matrix tablets was significantly influenced by both the type of polymer and its concentration [24]. As depicted in table 7, an initial burst release (30.7% in the first hour) was observed in the formulation F1, which had a lower concentration of guar gum-based polymer. This occurrence could be linked to the reduced polymer content, leading to a loss of tablet integrity. Consequently, drug dissolution from the tablet surface contributed to a relatively rapid release rate during this initial phase. This result is consistent with the findings of El Nabarawi et al. (2017), who observed a similar burst release in gastroretentive floating matrix tablets with lower polymer concentrations [25]. Statistical analysis of the factors, table 8, indicated a positive and significant effect (p<0.05) of polymer type on the drug release response. As a result, formulations containing Guar gum/HPMC (e. g., F3, F6, and F9) demonstrated the most effective retardation of drug release. Following these, formulations containing HPMC exhibited an intermediate release, while those with guar gum alone showed relatively higher release rates. This was attributed to the faster hydration and lower gel viscosity of guar gum, which promoted more rapid matrix erosion and drug diffusion. In contrast, HPMC formed a more viscous gel layer, slowing the drug release. These findings were supported by a previous study, which discussed the impact of polymer type on drug release behavior [26]. The retardation in release associated with the combined polymer formulations might be attributed to the addition of guar gum to non-ionic cellulose like HPMC. This addition was suggested to increase viscosity and influence the properties of the gel layer around the tablets, resulting in a slower penetration of the dissolution medium into the core of the matrix [27]. Furthermore, there was a significant negative influence (p<0.05) of the polymer concentration factor on the drug release. As the concentration of the polymer increased, the release of the drug retarded due to the heightened density of the swollen hydrogels network, resulting in an increased diffusion path length required for the drug to release [28]. The interaction effect of the two factors also displayed significance, supporting the superior retardation of drug release in formulations containing combined polymers at higher concentration levels. The analysis of variance (ANOVA) of the dissolution efficiency of the nine formulations (table 9) indicated no significant effect observed for either polymer type or the interaction of the factors (p>0.05) and a negative significant effect of polymer concentration (p<0.05) on the dissolution efficiency after 8 h. This result suggested that, if the polymer type factor (X1) was maintained at any constant and the polymer concentration (X2) was increased, the dissolution efficiency value would decrease. That could be attributed to the fact that an increase in polymer concentration leads to the swelling of the hydrogel network resulting in a slower release rate and consequently reducing the dissolution efficiency of the drug [18, 29]. In our study, we investigated the drug release mechanisms from various formulations (F1–F9) using the Korsmeyer-Peppas model. The observed diffusion exponent (n) values ranged from 0.305 to 0.427 (table 10), indicating that the initial drug release predominantly follows a Fickian diffusion mechanism [30]. This aligns with our cumulative drug release data, where formulations with lower polymer concentrations (e. g., F1) exhibited higher initial release rates. Our swelling index results indicate a complex interaction between diffusion and erosion. Formulations with higher concentrations of HPMC and guar gum (e. g., F9) exhibited greater swelling indices over time, suggesting effective gel formation. Notably, while initial swelling supports diffusion, the subsequent erosion of the gel layer significantly influences the drug release profile, particularly in later stages. For instance, F9, which had the highest swelling index (3.13 at 7 h) also showed a controlled release pattern. This suggests that the erosion of the gel layer, which occurs concurrently with swelling, plays a critical role in modifying the drug release dynamics. In summary, our findings highlight that while diffusion is the primary mechanism governing initial drug release, erosion becomes increasingly significant as the gel layer evolves. This dual mechanism complicates the interpretation of the release kinetics, reinforcing the need to consider both factors when analyzing drug release from polymeric matrices. The time required to release 50% of the drug (T50%) was determined by fitting the release data to the Krosmeyer–Peppas equation that showed high values of R2, as summarized in table 8. Among the different runs, the range for the release time of Metronidazole varied from 3.75 to 6.93 h. Statistical analysis of T50%response revealed that; both polymer type (X1) and polymer concentration (X2) factors exerted a negative significant influence (p<0.05) on the T50%, as shown in table 11. This indicates that, at a constant polymer concentration, changing the polymer type from guar gum to HPMC to their combination led to a decrease in the half-life of drug release. That finding could be because of the higher hydration power of HPMC compared to guar gum, resulting in the formation of a more viscous gel layer due to the swelling of HPMC matrix tablets. This, in turn, caused a lengthening of the diffusion pathway required for the drug to be released and consequently retarded drug release [31]. Similarly, at a constant polymer type, increasing the polymer concentration from its lower to higher level resulted in a decrease in the half-life of the drug released.
CONCLUSION
This study demonstrated that polymer type and concentration significantly influenced the physicochemical properties and drug release profile of metronidazole tablets. HPMC-based granules (F8, 55%) exhibited the best flow properties with an angle of repose of 32.0°, Carr’s Index of 11.8, and Hausner ratio of 1.12. Tablet hardness was highest in HPMC-based F8 (7.5 kg/cm²), indicating superior compressibility. The highest swelling index was observed in F9 (Guar/HPMC 55%) at 2.93 after 7 h, suggesting synergistic hydration from the polymer blend. Cumulative drug release was highest in F1 (Guar 45%) with 89.4% at 24 h, while F9 (Guar/HPMC 55%) showed the lowest release (85.4%), confirming release retardation from the polymer blend. Dissolution efficiency (DE%) values ranged from 69.7% to 78.8%, with drug release half-lives (T50%) extending from 3.75 to 6.93 h, confirming the sustained-release effect. Formulation F9, which contains a combination of guar gum and HPMC in a 55% concentration, emerged as the best formulation with acceptable granule flow properties and an optimal sustained release profile. Utilizing natural guar gum in conjunction with HPMC, in a 4:1 ratio, resulted in a superior release-retarding profile compared to using Guar gum alone. Further studies should explore the impact of polymer molecular weight and cross-linking density on drug release behavior. Higher molecular weight polymers may enhance gel strength and prolong drug dissolution, while cross-linked hydrogels could offer more controlled swelling and erosion dynamics. Investigating the in vivo performance of these formulations will be essential to validate their clinical applicability.
FUNDING
This research received no external funding.
AUTHORS CONTRIBUTIONS
Alaa Bur contributed to conceptualization, data collection, formal analysis, and investigation. Abdullah H Maad contributed to methodology development and manuscript drafting. Yusra Ahmed contributed to resource gathering, data validation, and visualization. Malaz Yousef participated in data collection and investigation. Zuheir Osman supervised the study, provided critical review and editing, and approved the final version of the manuscript.
CONFLICTS OF INTERESTS
The authors declare no conflicts of interest.
REFERENCES
Alam S, Bishal A, Bandyopadhyay B. Formulation and evaluation of metformin hydrochloride sustained release matrix tablets. Int J Curr Pharm Sci. 2021;13(5):82-8. doi: 10.22159/ijcpr.2021v13i5.1899.
Vroman I, Tighzert L. Biodegradable polymers. Materials. 2009;2(2):307-44. doi: 10.3390/ma2020307.
Ngwuluka NC, Ochekpe NA, Aruoma OI. Naturapolyceutics: the science of utilizing natural polymers for drug delivery. Polymers. 2014;6(5):1312-32. doi: 10.3390/polym6051312.
Majee SB, Mishra T, Gupti S. Gastroretentive effervescent floating tablets (GREFT) of drugs acting on cardiovascular diseases. Int J Pharm Pharm Sci. 2024;16(7):21-7. doi: 10.22159/ijpps.2024v16i7.51296.
Al Kamarany MA, Maad AH, Mohajab AA, Isra’a Al-masrafi I. Impact monitoring of low and high dexamethasone doses on COVID-19 outcomes in Hodeidah Yemen: a pharmacoepidemiological study. Asian J Pharm Clin Res. 2025;18(1):52-5. doi: 10.22159/ajpcr.2025v18i1.53226.
Siepmann J, Peppas NA. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv Drug Deliv Rev. 2001;48(2-3):139-57. doi: 10.1016/s0169-409x(01)00112-0, PMID 11369079.
Guarve K, Kriplani P. HPMC-A marvel polymer for pharmaceutical industry patent review. Recent Adv Drug Deliv Formul. 2021;15(1):46-58. doi: 10.2174/1872211314666210604120619, PMID 34086557.
Khudhur ZO, Maad AH, Ghanimi H, Abdolmaleki A. Fullerene nanoparticle as new therapeutic agent for the nervous system disorders. Nanomed J. 2024;11(4):342-59. doi: 10.22038/nmj.2024.78043.1903.
Bhatt P, Patel D, Patel A, Patel A, Nagarsheth A. Oral controlled release systems: current strategies and challenges misra a shahiwala a editors. Novel drug delivery technologies: innovative strategies for drug re-positioning. Singapore: Springer Singapore; 2019. p. 73-120. doi: 10.1007/978-981-13-3642-3_4.
Naeem A, Yu C, Wang X. Highly swellable cytocompatible and biodegradeable guar gum based hydrogel system for controlled release of bioactive components of liquorice (Glycyrrhiza glabra L.): synthesis and evaluation. Int J Biol Macromol. 2024;273(Pt1):132825. doi: 10.1016/j.ijbiomac.2024.132825.
Verma D, Sharma SK. Recent advances in guar gum based drug delivery systems and their administrative routes. Int J Biol Macromol. 2021 Jun 30;181:653-71. doi: 10.1016/j.ijbiomac.2021.03.087, PMID 33766594.
Azhar M, Mishra A. Review of nanoemulgel for treatment of fungal infections. Int J Pharm Pharm Sci. 2024;16(9):8-17. doi: 10.22159/ijpps.2024v16i9.51528.
Medina Lopez JR, Gomez Vargas YJ, Mendez Hernandez HR, Reyes Ramirez FD, Ruiz Segura JC, Hurtado M. Estimation of in vitro and in vivo performance of metronidazole oral dosage forms. Int J App Pharm. 2023;15(4):291-5. doi: 10.22159/ijap.2023v15i4.48137.
Bayor MT, Tuffour E, Lambon PS. Evaluation of starch from new sweet potato genotypes for use as a pharmaceutical diluent binder or disintegrant. J App Pharm Sci. 2013;3(8 Suppl 1):S17-S23. doi: 10.7324/JAPS.2013.38.S4.
Stationery Office British Pharmacopoeia. London, UK: The Stationery Office; 2008.
Gangurde HH, Chordiya MA, Tamizharasi S, Senthilkumaran K, Sivakumar T. Formulation and evaluation of sustained release bioadhesive tablets of ofloxacin using 32 factorial design. Int J Pharm Investig. 2011;1(3):148-56. doi: 10.4103/2230-973X.85964, PMID 23071937.
Singh J, Garg R, Gupta GD. Enhancement of solubility of lamotrigine by solid dispersion and development of orally disintegrating tablets using 32 full factorial design. J Pharm (Cairo). 2015;2015:828453. doi: 10.1155/2015/828453, PMID 26634173.
Kassaye L, Genete G. Evaluation and comparison of in-vitro dissolution profiles for different brands of amoxicillin capsules. Afr Health Sci. 2013;13(2):369-75. doi: 10.4314/ahs.v13i2.25, PMID 24235938.
Shah RB, Tawakkul MA, Khan MA. Comparative evaluation of flow for pharmaceutical powders and granules. AAPS PharmSciTech. 2008;9(1):250-8. doi: 10.1208/s12249-008-9046-8, PMID 18446489.
Rao YM, Veni JK, Jayasagar G. Formulation and evaluation of diclofenac sodium using hydrophilic matrices. Drug Dev Ind Pharm. 2001;27(8):759-66. doi: 10.1081/DDC-100107239, PMID 11699827.
Ahmed Y, Maad AH, Hassan HA, Abdallah DB, Yousef M, Kadhum AA. Evaluation of carboxymethyl millet starch and pregelatinized millet starch as pharmaceutical excipients using factorial experimental designs. Int J App Pharm. 2025;17(2):321-8. doi: 10.22159/ijap.2025v17i2.52161.
Naga Durga DH, Lohithasu D, Ramana Murthy KV. Development and evaluation of diclofenac sodium controlled release dosage forms using natural hydrophilic and hydrophobic polymers and its comparative studies. Indian J Pharm Educ Res. 2017;51(1):116-27. doi: 10.5530/ijper.51.1.16.
Dahma Z, Torrado Salmeron C, Alvarez Alvarez C, Guarnizo Herrero V, Martinez Alonso B, Torrado G. Topical meloxicam hydroxypropyl guar hydrogels based on low substituted hydroxypropyl cellulose solid dispersions. Gels. 2024;10(3):207. doi: 10.3390/gels10030207, PMID 38534625.
Sriamornsak P, Thirawong N, Korkerd K. Swelling erosion and release behavior of alginate based matrix tablets. Eur J Pharm Biopharm. 2007;66(3):435-50. doi: 10.1016/j.ejpb.2006.12.003, PMID 17267187.
El Nabarawi MA, Teaima MH, Abd El Monem RA, El Nabarawy NA, Gaber DA. Formulation release characteristics and bioavailability study of gastroretentive floating matrix tablet and floating raft system of mebeverine HCl. Drug Des Devel Ther. 2017 Apr 3;11:1081-93. doi: 10.2147/DDDT.S131936, PMID 28435220.
Berradi A, Aziz F, Achaby ME, Ouazzani N, Mandi L. A comprehensive review of polysaccharide based hydrogels as promising biomaterials. Polymers. 2023;15(13):2908. doi: 10.3390/polym15132908, PMID 37447553.
Shah U, Patel B, Patel M. Formulation and evaluation of controlled release matrix tablet of diltiazem HCl by using HPMC and guar gum as polymeric matrix material. Ars Pharm. 2012;53(4):16-20.
Maad AH, Al Gamli AH, Shamarekh KS, Refat M, Shayoub ME. Antiproliferative and apoptotic effects of Solenostemma Argel leaf extracts on colon cancer cell line HCT-116. Biomed Pharmacol J. 2024;17(3):1987-96. doi: 10.13005/bpj/3001.
Yavari N, Azizian S. Mixed diffusion and relaxation kinetics model for hydrogels swelling. J Mol Liq. 2022 Oct 1;363:119861. doi: 10.1016/j.molliq.2022.119861.
Ritger PL, Peppas NA. A simple equation for description of solute release II. fickian and anomalous release from swellable devices. J Control Release. 1987;5(1):37-42. doi: 10.1016/0168-3659(87)90035-6.
Colombo P, Bettini R, Santi P, Peppas NA. Swellable matrices for controlled drug delivery: gel layer behaviour mechanisms and optimal performance. Pharm Sci Technol Today. 2000;3(6):198-204. doi: 10.1016/S1461-5347(00)00269-8, PMID 10840390.