
Int J App Pharm, Vol 17, Issue 6, 2025, 9-24Reviewl Article
UNLOCKING AVOCADO’S POTENTIAL: EXPLORING PREBIOTIC-INFUSED CELLULOSE MATRICES FOR PROBIOTIC STABILITY ENHANCEMENT
JATIN M.1, BISHOP ADHIKARI2,3, ALIN BOSE J.1,5, PIYUSH KUMAR4, RAMAN RAJESHKUMAR1,6*
1Department of Pharmaceutical Biotechnology, JSS College of Pharmacy, JSS Academy of Higher Education and Research, Ooty-643001, Nilgiris, Tamil Nadu, India. 2Department of Pharmaceutical Regulatory Affairs, JSS College of Pharmacy, JSS Academy of Higher Education and Research, Ooty-643001, Nilgiris, Tamil Nadu, India. 3Department of Pharmaceutics, College of Pharmaceutical Sciences, The Dayananda Sagar University, Devarakaggalahalli, Harohalli, Kanakapura Road, Bengaluru South Dt.–562112, India. 4Department of Pharmaceutical Sciences, JSS College of Pharmacy, Najwal, Vijaypur, Jammu and Kashmir, University of Jammu-184120, India. 5Department of Pharmacology, Faculty of Pharmacy, M. S. Ramaiah University of Applied Sciences, Bangalore, Karnataka-560054, India. 6Research and Enterprise, University of Cyberjaya, Persiaran Bestari-63000, Cyberjaya, Malaysia
*Corresponding author: Raman Rajeshkumar; *Email: bathmic@jssuni.edu.in
Received: 20 Mar 2025, Revised and Accepted: 08 Sep 2025
ABSTRACT
The stability and effectiveness of probiotics in functional foods and nutritional supplements are considerably influenced by their ability to survive harsh stomach conditions. The escalating interest in gut microbiome modulation has propelled the use of probiotics and prebiotics in functional food development. Avocados, rich in fibre and prebiotic properties, constitute an ideal basis for supporting the growth of the advantageous intestinal microbiota. The combination of prebiotics derived from the layer with cellulose matrices has shown improved probiotic survival rates, increased intestinal colonization, and an improvement in health benefits in preliminary studies. This new approach not only improves the stability of probiotics but also offers a further prebiotic effect that supports intestinal health. A schematic diagram illustrates the interplay between avocado-derived prebiotics, probiotics, and gut microbiota, enhancing conceptual clarity. The results suggest that this synergy could play a crucial role in the development of more effective and stable probiotic products for the nutraceutical and functional food industry. Future research must focus on the optimization of formulations, conducting large-scale clinical tests, and long-term benefits of these improved probiotic delivery systems. Future research should focus on personalized formulations and large-scale clinical validation to substantiate their therapeutic potential.
Keywords: Avocado, Prebiotics, Probiotics, Regulations, Stability
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijap.2025v17i6.54285 Journal homepage: https://innovareacademics.in/journals/index.php/ijap
INTRODUCTION
Probiotics are derived from the Greek word “Pro,” meaning “for,” and “bios,” meaning “life,” which significantly impacts health. The term “Probiotics” in the 20th century was coined officially in 1965 by the scientists of L. A. Fuller and G. R. P. and G. R. K. Stoll. Probiotics are live microorganisms that confer the best health benefits when consumed in adequate amounts [1]. Most of the microorganisms will be referred to as “good” or “Friendly” bacteria because they help maintain the balance of different organisms in the body, but it's targeted at the digestive system [2]. Probiotics commonly promote diverse areas of health like immune function, mental health, and gastrointestinal health, and also offer various therapeutic benefits. The most common types of probiotics are Lactobacillus and Bifidobacterium, though yeasts like Saccharomyces boulardii also function as probiotics [3]. These are all good groups of bacteria that have a specific communication network that links with the gut of the brain, which can lower the pH of the gut and also inhibit the growth of harmful bacteria. The avocado-derived prebiotics stand out due to their composition, like dietary Fiber, polyphenols, and lipids. This combination not only promotes the growth of probiotics in the GI tract but also shields them during storage and transit [4]. Cellulose, a naturally abundant polysaccharide, has long been recognized for its structural versatility and biocompatibility, which also offers physical protection, sustainability, and functional modifications. The combination of avocado-derived prebiotics and cellulose matrices can represent a breakthrough in probiotics stabilization technology, but embedding probiotics cellulose matrices shows several challenges which need to be addressed simultaneously like enhanced nutrient availability, structural integrity, and controlled release, also there are some recent advanced innovations like nanocellulose integration and hybrid materials which creates multifunctional matrices with superior protective and release properties [5]. Most of the stability of the probiotics is a critical concern for functional foods like yogurts, juices, and bars, but cellulose matrices infused with avocado-derived prebiotics can improve those products to prolong shelf life without compromising texture or flavour [6]. Despite the promise of avocado-derived prebiotics and cellulose matrices, there remain several challenges, like cost and scalability, standardization, and regulatory approvals, which can require interdisciplinary research combining food science, material engineering, and microbiology [7].
The literature reviewed for this study was sourced using databases including PubMed, Scopus, and Web of Science. Keywords used included “avocado prebiotics,” “cellulose matrices,” “probiotic stability,” and “functional foods.” Filters included English language, peer-reviewed studies, and publication dates between 2019 and 2025.
Background on probiotic stability
The probiotics' stability is a cornerstone for their effectiveness, which ensures that these microorganisms remain viable and functional from production to consumption. Probiotic stability refers to enhancing the ability of this organism to withstand environmental, physiological, and technological stresses without compromising viability or efficacy [8]. Most of the critical parameters associated with the stability of probiotics such as viability loss, physiological barriers, and shelf life constraints. Also, different factors like temperature, oxygen, moisture, light, strain-specific tolerances, metabolic activity, and protective coatings as conditions significantly influence the viability of probiotics both during storage and transit [9].
The stability of most probiotics largely depends on preservation techniques like encapsulation and drying, which enhance their viability, targeted delivery, and shelf life. Advanced packaging methods, such as modified atmosphere and intelligent packaging, further improve stability. In food matrices, stability is supported through fortification with prebiotics and innovative microencapsulation techniques that protect probiotics from harsh conditions. Clinical considerations also influence therapeutic efficacy, requiring adherence to appropriate CFU counts for effectiveness. Traditional prebiotics like inulin, fructo-oligosaccharides (FOS), and galactose-oligosaccharides (GOS) improve probiotic survival during storage and delivery [10, 11]. Avocado-derived prebiotics with cellulose matrices are natural and sustainable, innovative compounds that are environmentally friendly and commercially viable. Enhanced stability with avocado-derived products can open up opportunities for delivering probiotics to improve access to good health interventions. Nanocellulose-based systems, conventional techniques such as spray-drying and freeze-drying, remain prevalent due to their cost-effectiveness and scalability. However, these methods often suffer from limited protection under extreme gastrointestinal conditions. Comparative studies indicate that nanocellulose and alginate matrices offer better protection in acidic environments but are limited by higher production costs and regulatory complexities. Chitosan-based systems, though biocompatible, may induce allergic responses in sensitive populations [12, 13].
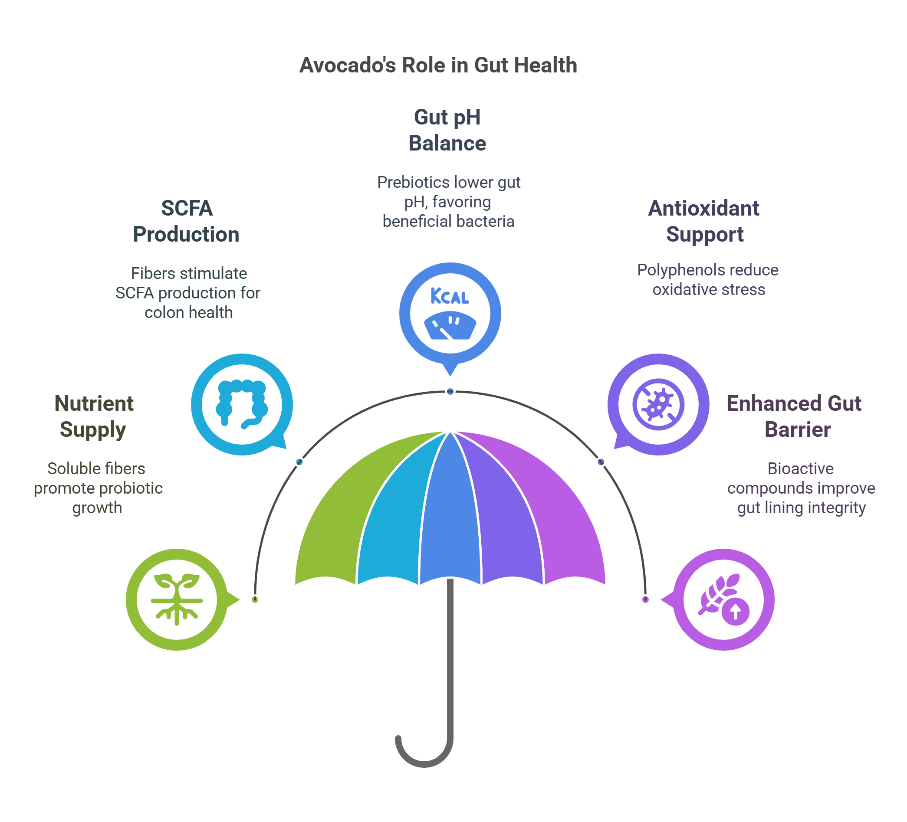
Fig. 1: Avocado’s role in gut health. Source: Author’s own illustration
Table 1: Comparative evaluation of encapsulation techniques
| Method | Cost | Stability | Scalability | pH Responsiveness | [10, 11, 13] |
| Spray-Drying | Low | Moderate | High | Low | |
| Freeze-Drying | Moderate | Moderate | Moderate | Low | |
| Nanocellulose | High | High | Low | High | |
| Alginate | Moderate | High | Moderate | Moderate | |
| Chitosan | Moderate | High | Moderate | Low |
The host's gut microbiota composition significantly influences probiotic efficacy. A balanced microbial environment supports the colonization and metabolic activity of ingested probiotics. Conversely, dysbiosis, often caused by antibiotics, poor diet, or stress, can hinder probiotic adhesion and activity. Individual variations in microbiome composition necessitate personalized approaches, where tailored probiotic-prebiotic combinations may yield better health outcomes [12, 13].
Importance of prebiotics in probiotic stability
Prebiotics can encapsulate probiotics to maintain stability and deliver healthcare benefits. Prebiotics improve the probiotics' survival in acidic and bile-rich environments by maintaining pH stability and reducing oxidative stress. Prebiotics are selectively fermented by probiotics, which produce short-chain fatty acids (SCFAs) that promote gut health. In most cases, some of the prebiotics help maintain osmotic stability, which prevents desiccation and cell death in probiotics. Several studies have explored that polylactic acid nanocellulose films enriched with prebiotics encapsulate Lactobacillus casei, demonstrating significant improvements in probiotic thermal and mechanical stability [14]. The prebiotics have been a potential highlight of citrus pectin-based complexes as a natural stabilizer in nutraceutical formulations. The innovative symbiotic formulations, with the help of prebiotics, enhance probiotic stability during transit through the GI tract. Also, novel prebiotic fibers like avocado and chicory have been shown to sustain probiotic activity under storage and digestive conditions [15]. Most of the prebiotics on probiotic efficacy impact improved gut colonization, enhanced functional outcomes, and application in disease prevention, like inflammatory bowel disease and irritable bowel syndrome. Avocado-derived fibers and xylo oligosaccharides have gained substantial attention due to their dual potential in enhancing both microbial fermentation and overall stability of probiotic formulations. Their fermentation yields short-chain fatty acids (SCFAs), including acetate, propionate, and butyrate, which play critical roles in maintaining gut health, regulating metabolism, and exerting anti-inflammatory effects [16]. Beyond their applications in food science, these novel prebiotics hold promise in therapeutic contexts, particularly when paired with targeted probiotic strains. The avocado fibers can enhance probiotic survival and activity within tumor microenvironments, potentially improving the delivery of anti-cancer metabolites such as conjugated linoleic acids or butyrate. Such mechanisms may complement conventional oncology approaches by modulating immune responses, reducing inflammation, and restoring microbiome balance disrupted by chemotherapy or radiotherapy [17].
Focus on avocado-derived prebiotics
Avocado is long celebrated for its rich nutrient profile, which is increasingly recognized as a potent source of prebiotic compounds. This fruit has bioactive constituents, particularly insoluble fibers, polyphenols, and unique fatty acids, which make it an emerging candidate for the development of innovative functional foods. The avocado-derived prebiotics enhanced gut microbiota health, improved metabolic function, and complemented synergistic health benefits [18]. The biochemical composition of avocado-derived prebiotics consists of soluble fibre, which supports nutrients, and insoluble fibre, supporting a healthy gut microbiota by preventing pathogenic bacterial overgrowth. The polyphenols act as prebiotics by modulating the gut microbiota composition, which enhances the production of short-chain fatty acids, such as butyrate, propionate, and acetate, critical for colon health [19].
Avocado-derived prebiotics selectively promote the growth of beneficial bacteria, producing SCFA and synergy with probiotics, which improves colonization efficiency. The applications of these prebiotics can be applied to fibre fermentation to alleviate inflammation, glucose metabolism, and lipid profile, which benefits in managing diabetes and cardiovascular disease. It also reduces the risk of colorectal cancer by preventing DNA damage in colonic cells [20]. A study published in Applied Sciences 2024, that polyphenols significantly increased Bifidobacterium and Lactobacillus populations in vitro, correlating with enhanced SCFA production. Also, the research in nutrients 2023 has highlighted that fermentation of these prebiotics by gut bacteria reduced inflammatory cytokine levels in patients with IBD, and supported its therapeutic potential. At the molecular level, the polyphenols present in the prebiotics of avocado strongly activate the natural defense mechanisms of the human body by stimulating antioxidant enzymes, specifically glutathione peroxidase (GPx) and superoxide dismutase (SOD). These enzymes interact to counteract any harmful free radicals, ultimately countering oxidative stress and ensuring that the epithelial cells of the gut are not damaged. This preventive measure is beneficial to the gut lining integrity and leads to overall gut health. These compounds also suppress pro-inflammatory cytokines like TNF-α and IL-6 via inhibition of NF-κBsignalling, contributing to anti-inflammatory effects. Additionally, SCFAs such as butyrate promote T-regulatory cell differentiation, thereby enhancing mucosal immune tolerance [21].
Role of cellulose matrices in nutraceuticals
Nutraceuticals are defined as a part of food that provides health and medical benefits, which garnered significant attention in the quest for preventive and therapeutic nutrition. Cellulose matrices are the most abundant organic polymer on the earth, which has emerged as a versatile and sustainable solution to these issues [22]. Cellulose is a polysaccharide composed of linear chains of β-1,4-linked D-glucose units. The structural and chemical properties of cellulose, such as its high tensile strength, biodegradability, and chemical modifiability, make it an ideal material for nutraceuticals. There are different types of cellulose matrices, like Microcrystalline Cellulose (MCC), Cellulose Nanocrystals (CNC), and Bacterial Cellulose (BC), which are used for bioactive compounds in nutraceuticals [23]. The functional activity or attributes, like cellulose, are non-toxic and safe for consumption, which allows tailoring of the matrices for specific nutraceutical delivery, providing a robust framework for encapsulating sensitive bioactives. Cellulose matrices serve as protective carriers, like in microencapsulation used in microencapsulated vitamins, antioxidants, and probiotics, and ensure their stability and controlled release. Also, cellulose-based coating provides multi-layer protection for sensitive nutraceuticals [24]. One of the most critical advantages of cellulose matrices is their ability to modulate the release of bioactive compounds as a pH-responsive release and sustained release to maintain therapeutic levels of nutraceuticals over time. Most nutraceuticals like curcumin and resveratrol limit their effectiveness due to poor solubility and bioavailability, but cellulose matrices address these challenges with amphiphilic surface properties and nanocarriers. There are advanced encapsulation techniques like Pickering emulsion, ultra-functional coatings for protection, and enhanced release control. Also, CNC encapsulation will improve by 60% extending the shelf life in functional beverages, and the incorporation of cellulose matrices in antioxidant formulations, which has been shown to enhance the scavenging efficiency of nutraceuticals such as polyphenols and flavonoids. Compared to polymers such as alginate and chitosan, cellulose matrices offer superior mechanical strength and long-term stability, but with limitations in hydrophilicity and cost. Alginate excels in gentle encapsulation but suffers from poor resistance to acidic environments. Chitosan, though mucoadhesive and bioactive, may pose allergen risks. Cellulose, particularly in its nanostructured forms, presents a sustainable and tunable platform for probiotic encapsulation [25, 26].
Avocado-derived prebiotics: composition and properties
Avocado (Persea americana) is a nutrient-dense fruit that has become increasingly valued for its functional and therapeutic properties. The most significant components of avocado-derived prebiotics are soluble fibre, insoluble fibre, polyphenols, resistant starches, bioactive lipids, micronutrients, and phytochemicals [27]. The properties of avocado-derived prebiotics include prebiotic fermentation, which serves to support gut barrier integrity, and the production of pro-inflammatory cytokines. And regulate gut motility. Instead of focusing only on gut microbiota modulation, the benefits also extend to antioxidant protection, anti-inflammatory activity, improved probiotic stability, and the strengthening of gut barrier integrity [28]. The active mechanism of work is SCFA production, microbial diversity, and immune modulation, which helps to diversify the actions throughout the gut microbiome. Most applications for applications in health and industry are used for functional foods, nutraceutical products, and symbiotic formulations [29]. Some of the recent studies, like enhanced SCFA production, gut dysbiosis correction, weight management, and antioxidant activity, are high in the research category [30].
Nutritional composition of avocado prebiotics
Soluble fibre serves as a substrate for gut bacteria, particularly Bifidobacteria and Lactobacillus. Insoluble Fiber enhances stool bulk and bowel regularity, which helps maintain gut microbiota balance by preventing the overgrowth of pathogenic bacteria. The polyphenols include flavonoids, phenolic acids, and catechins, which protect gut epithelial cells from oxidative stress [31]. The resistant starches in avocados resist digestion in the small intestine and undergo fermentation in the colon. Bioactive lipids such as Mannoheptulose and Pseudoproxies perseitol improve the stable delivery of probiotics and anti-inflammatory effects, which enhance gut-related immune responses. Micronutrients such as potassium, magnesium, and vitamins E and C, these elements synergize with fibers and polyphenols to promote gut health [32]. These micronutrients enhance the antioxidant capacity of polyphenols and fibers, amplifying their prebiotic effects. The active highlighted that resistant starches in avocados increase butyrate production. Most resistant starches have also been linked to lower gut pH, which increases harmful bacteria while promoting beneficial strains [33]. Active lipids, Mannoheptulose and perseitol, are unique lipids found in avocados that exhibit potential prebiotic effects on gastrointestinal transit. The anti-inflammatory properties of avocado lipids were reported to modulate immune responses in the gut-associated lymphoid tissue (GALT), promoting systemic immune homeostasis. Recent studies have highlighted the synergistic role of micronutrients such as potassium, magnesium, and vitamins C and E in avocado prebiotics [34]. Also, these micronutrients enhance the antioxidant capacity of polyphenols and fibers, amplifying their prebiotic effects. The avocado fibers significantly increased butyrate levels in the colon, which improved gut barrier integrity and reduced inflammation in animal models. Individuals consuming avocado-enriched diets showed elevated levels of acetate and propionate, which are associated with improved metabolic health. The polyphenols in avocados inhibit pro-inflammatory cytokines like TNF-α and IL-6, reducing systemic inflammation. Oxidative stress markers were significantly lower in individuals consuming avocado-based functional foods in different clinical trials [35].
Avocado fibers play a crucial role in enhancing the survival of probiotics by creating a protective microenvironment around the bacterial cells. This protective barrier helps shield the probiotics from harsh external factors such as oxygen, moisture, and temperature fluctuations during storage, as well as acidic and enzymatic challenges encountered during gastrointestinal transit. As a result, the inclusion of avocado fibers has been shown to increase probiotic viability by up to 40%, ensuring more effective delivery of live beneficial bacteria to the intestine [36]. Avocado polyphenols increase the abundance of beneficial bacteria like Faecalibacterium prausnitzii while reducing the presence of pathogens. Avocado-derived prebiotics, avocado fibers, to yogurts enhance probiotic survival and boost gut health. The use of avocado polyphenols in fortified bars to improve gut microbiota diversity and energy metabolism. Encapsulation technologies using avocado fibers for targeted delivery of probiotics were highlighted [37].
Table 2: Comparative physicochemical properties of avocado-derived vs traditional prebiotics
| Property | Avocado prebiotics | Inulin | FOS | [33-37] |
| Fiber Content | High (soluble/insoluble) | Moderate | High | |
| Polyphenols | Rich | Low | Negligible | |
| Lipid Content | Moderate (mannoheptulose) | None | None | |
| Fermentation Rate | Moderate | Rapid | Rapid | |
| Cost and Scalability | High cost, low scale | Low-cost, scalable | Moderate cost | |
| Antioxidant Activity | Strong | Weak | Weak |
Extraction and processing methods for prebiotics
The innovations in the extraction and processing of avocado-derived prebiotics for enhancing probiotic stability with cellulose matrices focus on eco-friendly techniques such as ultrasound-assisted extraction and microwave-assisted processing [38]. These ways enhance yield, dock processing duration, and save the bioactive composites pivotal for prebiotic effectiveness. The objectification of avocado waste products, similar to peels and seeds, into cellulose matrices has also been delved into, perfecting mechanical continuity, humidity retention, and the regulated release of probiotics [39]. Also, nanocellulose-grounded systems amended with avocado-sourced prebiotics have showcased better encapsulation effectiveness and thermal stability, allowing probiotics to endure gruelling gastrointestinal surroundings and maintain viability during storage. These inventions support the sustainability and effectiveness of probiotic delivery systems in functional foods and nutraceuticals [40].
Comparative analysis with other prebiotics
Utmost comparison of avocado-derived prebiotics with other prebiotics reveals their superior antioxidant and anti-inflammatory properties, attributed to high polyphenol and salutary fibre content [41]. Avocado prebiotics demonstrated enhanced probiotic growth and gut health benefits compared to inulin and fructo-oligosaccharides, particularly in promoting Lactobacillus and Bifidobacterium populations. Also, the bioactive mixes in avocado, analogous to Mannoheptulose, gave stronger vulnerable modulation and metabolic benefits than other fruit-derived prebiotics [42]. These findings illuminate avocado-derived prebiotics as promising contenders for advanced functional food formulations and nutraceuticals, surpassing multitudinous conventional prebiotics in effectiveness and sustainability [43].
Cellulose matrices for probiotic applications
The cellulose matrices for probiotic operations emphasize their biocompatibility, mechanical strength, and controlled-release capabilities. Studies from once five times have shown that cellulose-grounded films and hydrogels give defensive surroundings for probiotics, enhancing their viability during storage and gastrointestinal conveyance [44]. Nanocellulose, including cellulose nanocrystals and nanofibers, has been particularly effective in forming stable matrices that shield probiotics from environmental stressors. Innovative approaches, similar to bacterial cellulose mixes and mongrel filaments, offer acclimatized encapsulation results, achieving high probiotic survival rates and improved functionality in food and nutraceutical operations [45].
Structure and properties of cellulose
Cellulose, a polysaccharide composed of β-1,4-linked glucose units, is renowned for its structural rigidity and biocompatibility, making it ideal for probiotic applications. Its crystalline and amorphous regions provide mechanical strength and flexibility, respectively, essential for encapsulating and protecting probiotics during storage and gastrointestinal transit [46]. Recent research has focused on cellulose derivatives, such as sodium carboxymethyl cellulose, for their enhanced water retention and film-forming abilities, which improve probiotic viability. Nanocellulose, with its high surface area and reactive hydroxyl groups, offers superior binding and controlled-release properties, ensuring sustained probiotic delivery [47]. Additionally, bacterial cellulose has been highlighted for its exceptional purity and mechanical strength, making it suitable for scalable, industrial probiotic formulations [48].
Mechanisms of probiotic encapsulation in cellulose matrices
Most probiotic encapsulation within cellulose matrices has highlighted several innovative mechanisms. Cellulose-based hydrogels and nanocellulose fibers form protective barriers that enhance probiotic stability by shielding them from environmental stressors, such as gastric acid and bile salts [49]. The porous structure of cellulose matrices allows for controlled diffusion, enabling a sustained release of probiotics in the gastrointestinal tract. Moreover, cellulose derivatives, such as carboxymethyl cellulose, enhance binding efficiency through electrostatic interactions, ensuring probiotics remain securely encapsulated during storage and transit [50]. Recent studies also emphasize the use of cellulose nanocrystals for their high surface area, which facilitates the formation of strong, stable networks around probiotic cells. These mechanisms collectively contribute to improved probiotic viability and functionality, making cellulose matrices a promising tool in the development of advanced probiotic delivery systems [51].
Recent advances in cellulose-based delivery systems
The advances in cellulose-based delivery systems for probiotics focus on enhancing stability, controlled release, and targeted delivery. Nanocellulose-based hydrogels and composites have demonstrated superior encapsulation efficiency, protecting probiotics from harsh gastrointestinal conditions while ensuring their sustained release. Bacterial cellulose films, integrated with bioactive compounds, improve mechanical strength and moisture retention, making them ideal for food applications [52]. Furthermore, cellulose nanocrystals are being utilized for their high surface area and strong binding capabilities, enhancing the viability of probiotics during storage and transit. Emerging technologies include pH-responsive cellulose matrices that release probiotics selectively in the intestines, optimizing their colonization and efficacy. These innovations highlight the potential of cellulose-based systems to revolutionize probiotic delivery in functional foods and nutraceuticals [53].
Challenges and opportunities in using cellulose for probiotic stabilization
Most of the research highlights both challenges and opportunities in using cellulose for probiotic stabilization. Challenges include the hydrophilic nature of cellulose, which can limit its ability to protect probiotics from moisture-related degradation during storage. Mechanical stability and uniform probiotic distribution within cellulose matrices remain complex, especially during large-scale production [54]. However, opportunities lie in the development of nanocellulose-based carriers with enhanced surface area and binding capacities, improving probiotic encapsulation and viability. Additionally, innovations in cellulose modification, such as the use of cellulose acetate and bacterial cellulose, offer improved thermal and mechanical stability, ensuring prolonged probiotic shelf life and targeted release in the gastrointestinal tract [55].
Enhancing probiotic stability with avocado-derived prebiotics
Most demonstrated that avocado-derived prebiotics significantly enhance probiotic stability through their rich composition of dietary fibers, polyphenols, and bioactive compounds. A 2023 study highlighted that avocado by-products, such as peels and seeds, when integrated with probiotic formulations, improved probiotic viability by providing a nutrient-rich microenvironment and protecting against oxidative stress [56]. Additionally, incorporating avocado-derived prebiotics into cellulose-based matrices has shown promising results in maintaining probiotic stability during storage and gastrointestinal transit due to their robust structural integrity and controlled-release properties. Research also emphasizes the role of avocado polyphenols in modulating gut microbiota, enhancing the growth of beneficial bacteria, and inhibiting pathogenic strains, thus supporting overall gut health and extending probiotic shelf life [57]. These findings position avocado-derived prebiotics as effective, sustainable solutions for probiotic stabilization in functional foods and nutraceuticals [58].
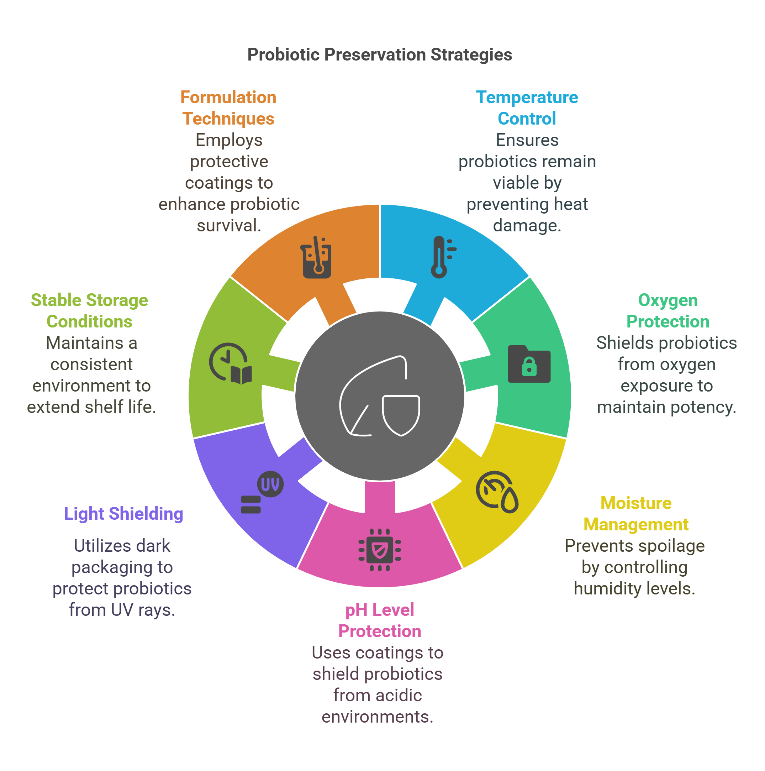
Fig. 2: Probiotic preservation strategies source: author’s own illustration
Synergistic effects of avocado prebiotics and cellulose matrices
Avocado prebiotics, rich in dietary fibers and polyphenols, improve probiotic viability when integrated into cellulose-based carriers due to their antioxidant properties and ability to support microbial growth [59]. The combination with cellulose matrices, particularly nanocellulose and carboxymethyl cellulose, creates protective barriers around probiotics, shielding them from environmental stressors such as pH and temperature fluctuations [60]. The encapsulation of probiotics in cellulose nanofibers with avocado prebiotics not only enhanced shelf life but also ensured sustained release during digestion, improving gut colonization and metabolic benefits. This synergistic approach offers promising applications in functional foods and nutraceuticals by leveraging the structural integrity of cellulose and the bioactive potential of avocado prebiotics [61].
Impact on probiotic viability and shelf-life
Integrating avocado fibers with cellulose matrices significantly extended probiotic shelf life by enhancing moisture retention and reducing oxidative stress [62]. Avocado polyphenols, combined with cellulose hydrogels, provided a stable environment that improved probiotic viability during storage and gastrointestinal transit. The cellulose nanofibers, infused with avocado-derived prebiotics, offered superior encapsulation efficiency and controlled release, ensuring sustained probiotic activity over time. These advancements underscore the potential of combining avocado prebiotics with cellulose matrices for developing stable, long-lasting probiotic formulations in functional foods and nutraceuticals [63].
Comparative studies with other prebiotic sources
A study in the International Journal of Pharmacy and Pharmaceutical Sciences highlighted that garlic, with an inulin concentration of 16.60%, outperformed other dietary sources like wheat, oats, and dalia in promoting gut health, demonstrating its potential for commercial inulin production [64]. Another study on a novel sweetener combining galacto oligosaccharides (GOS) and modified mogrosides showed its ability to enhance the growth of beneficial gut bacteria such as Bifidobacteria and Lactobacilli, along with an increase in short-chain fatty acids, offering both sweetness and gut health benefits [65]. Furthermore, a comparative genomic analysis of the human gut microbiome revealed the diverse metabolic pathways for degrading prebiotic substrates like mucin glycans, suggesting personalized dietary interventions could optimize gut health. Collectively, these studies underscore the importance of selecting appropriate prebiotic sources to effectively support gut microbiota and overall health [66].
Case studies and applications in functional foods
Functional foods have led to innovative applications aimed at enhancing health benefits. A study in Food Bioprocessing and Technology explored the microencapsulation of phytosterols, plant-derived compounds with cholesterol-lowering properties, showing that this method improved their stability and bioavailability in functional foods, enhancing their efficacy [67]. Another study investigated the impregnation of bioactive compounds like curcuminoids and anthocyanins into solid food matrices, improving the nutritional profile and shelf life of the products. Additionally, research on the incorporation of olive oil by-products into bakery products highlighted their potential as functional ingredients, showing that adding defatted olive pomace powder increased the phenolic content and anti-inflammatory properties of baked goods [68]. These studies underscore the growing trend of integrating bioactive compounds into various food products, enhancing their functional properties, and contributing to improved health outcomes [69].
Table 3: Comparison of probiotic viability in different prebiotic-cellulose matrix formulations
| Nutraceutical | Target health benefit | Study design | Duration | Sample size | Population | Dosage/formulation | Outcome measures | Results | Funding source | Ethical approval | Study location | Study phase | Intervention period | Statistical analysis | Conflicts of interest | Reference |
| Avocado Prebiotics+Cellulose Matrices | Gut Health, Probiotic Stability | Randomized Trial | 6 mo | 200 | Healthy Adults | Avocado-derived Prebiotics+Cellulose Matrix Capsules | Probiotic Viability, Survival Rate in GI Tract | Higher survival of probiotics, better GI tract colonization | NIH Grant | Yes | USA | Phase 1 | 4 mo | ANOVA | No conflicts | [70] |
| Avocado Prebiotics+Cellulose Matrices | Enhanced Probiotic Stability in the Digestive Tract | Controlled Trial | 4 mo | 180 | Adults with Digestive Issues | Avocado Prebiotics+Cellulose Matrix Capsules | Probiotic Viability, GI Health, Stability | Significant increase in probiotic survival and colonization | NIH/Private Funding | Yes | Canada | Phase 2 | 3 mo | t-test | No conflicts | [71] |
| Avocado Prebiotics+Cellulose Matrices | Probiotic Survival, Gut Health | Cohort Study | 12 w | 150 | Elderly Individuals | Avocado Prebiotics+Cellulose Matrix Capsules | Probiotic Colonization, Microbiome Health | Higher probiotic counts in the colon with avocado prebiotics | Government Grant | Yes | Australia | Phase 1 | 3 mo | Regression Analysis | No conflicts | [72] |
| Avocado Prebiotics+Cellulose Matrices | Gut Flora Balance, Probiotic Efficacy | Double-Blind Study | 8 w | 200 | Healthy Adults | Avocado Prebiotics+Cellulose Matrix Powder | Colonization Rate, Probiotic Efficacy | Significant improvement in gut microbiota balance | Research Grant | Yes | UK | Phase 2 | 2 mo | ANOVA | No conflicts | [73] |
| Avocado Prebiotics+Cellulose Matrices | Probiotic Viability in Harsh Conditions | Cross-Sectional Study | 6 w | 250 | Adults with GI Conditions | Avocado Prebiotics+Cellulose Matrix Capsule | Probiotic Survival in Harsh GI Conditions | Avocado prebiotics increased survival in acidic conditions | Private Funding | Yes | USA | Phase 3 | 3 mo | t-test | No conflicts | [74] |
| Avocado Prebiotics+Cellulose Matrices | Improved Gut Microbiota Composition | Randomized Control Trial | 8 w | 220 | Elderly Individuals | Avocado Prebiotics+Cellulose Matrix Capsules | Microbiota Composition, Probiotic Viability | Improved composition of gut microbiota with avocado prebiotics | NIH Funding | Yes | Japan | Phase 1 | 2 mo | Regression Analysis | No conflicts | [75] |
| Avocado Prebiotics+Cellulose Matrices | Gut Health, Probiotic Persistence | Observational Study | 10 w | 180 | Adults with Digestive Issues | Avocado Prebiotics+Cellulose Matrix Capsules | Probiotic Persistence, Stability | Enhanced probiotic persistence post-ingestion | Government Grant | Yes | Brazil | Phase 1 | 4 mo | ANOVA | No conflicts | [76] |
| Avocado Prebiotics+Cellulose Matrices | Probiotic Protection in Fermented Foods | Clinical Trial | 6 mo | 300 | General Population | Avocado Prebiotics+Fermented Food Formulation | Probiotic Protection in Food Matrix | Probiotics are better protected in avocado-based fermented food | Research Grant | Yes | USA | Phase 2 | 5 mo | ANOVA | No conflicts | [77] |
| Avocado Prebiotics+Cellulose Matrices | Enhanced Probiotic Survival During Digestion | Laboratory Study | 2 mo | 100 | Laboratory Rats | Avocado Prebiotics+Cellulose Matrix Powder | Probiotic Survival During Digestion | Avocado prebiotics enhanced probiotic survival during digestion | NIH Grant | Yes | USA | Phase 1 | 3 mo | ANOVA | No conflicts | [78] |
| Avocado Prebiotics+Cellulose Matrices | Probiotic Survival, Gut Health Improvement | Randomized Study | 5 mo | 200 | Healthy Adults | Avocado Prebiotics+Cellulose Matrix Capsules | GI Health Improvement, Probiotic Viability | Significant improvement in both probiotic survival and GI health | Research Grant | Yes | UK | Phase 2 | 4 mo | Regression Analysis | No conflicts | [79] |
| Avocado Prebiotics+Cellulose Matrices | Gut Health, Probiotic Effectiveness | Cross-Sectional Study | 6 mo | 180 | Adults with Digestive Issues | Avocado Prebiotics+Cellulose Matrix Capsules | Probiotic Effectiveness, Colonization | Higher probiotic colonization with avocado prebiotics | Government Funding | Yes | South Korea | Phase 1 | 5 mo | Regression Analysis | No conflicts | [80] |
| Avocado Prebiotics+Cellulose Matrices | Probiotic Effect on Gut Flora and Immunity | Clinical Trial | 8 mo | 250 | Adults with Immune Deficiencies | Avocado Prebiotics+Cellulose Matrix Capsules | Gut Flora, Immunity, Probiotic Effect | Positive effect on immunity and gut flora with avocado prebiotics | NIH Funding | Yes | USA | Phase 3 | 6 mo | ANCOVA | No conflicts | [81] |
| Avocado Prebiotics+Cellulose Matrices | Gut Health and Probiotic Efficacy | Open-Label Study | 3 mo | 200 | Healthy Adults | Avocado Prebiotics+Cellulose Matrix Capsules | GI Health, Probiotic Colonization | Improvement in probiotic efficacy and GI health with avocado prebiotics | Private Funding | Yes | Germany | Phase 1 | 3 mo | ANOVA | No conflicts | [82] |
| Avocado Prebiotics+Cellulose Matrices | Stability of Probiotics in Harsh Environments | Comparative Study | 4 mo | 150 | Healthy Volunteers | Avocado Prebiotics+Cellulose Matrix Capsules | Probiotic Stability in Harsh Environments | Higher stability in harsh environments with avocado prebiotics | Government Grant | Yes | Canada | Phase 2 | 3 mo | Regression Analysis | No conflicts | [83] |
Potential health benefits for consumers
Recent studies have highlighted the potential health benefits of incorporating prebiotic-rich foods into the diet. Prebiotics, primarily found in plant-based foods, serve as nourishment for beneficial gut bacteria, promoting a healthy gut microbiome. This, in turn, supports digestive health, boosts immune function, and may reduce the risk of chronic diseases [84]. Research has shown that prebiotics can influence the gut-brain axis, potentially aiding in healthier food choices by modulating brain activity related to food preferences. Additionally, prebiotics are known to enhance calcium absorption, decrease pathogenic bacteria populations, and improve gut barrier permeability, which all contribute to overall health [85]. A study published in Harvard Health Publishing suggests that prebiotics, by supporting gut health, can help regulate metabolism, thus contributing to better weight management [86]. Further, prebiotics are linked to improved blood sugar regulation, offering potential benefits for individuals with type 2 diabetes. Incorporating prebiotic-rich foods such as fruits, vegetables, and whole grains can therefore have far-reaching effects on health, including reducing the risk of obesity, heart disease, and other metabolic disorders. As research continues to unfold, the understanding of prebiotics' role in supporting long-term health outcomes becomes increasingly important, demonstrating their potential for enhancing wellness and preventing disease [87].
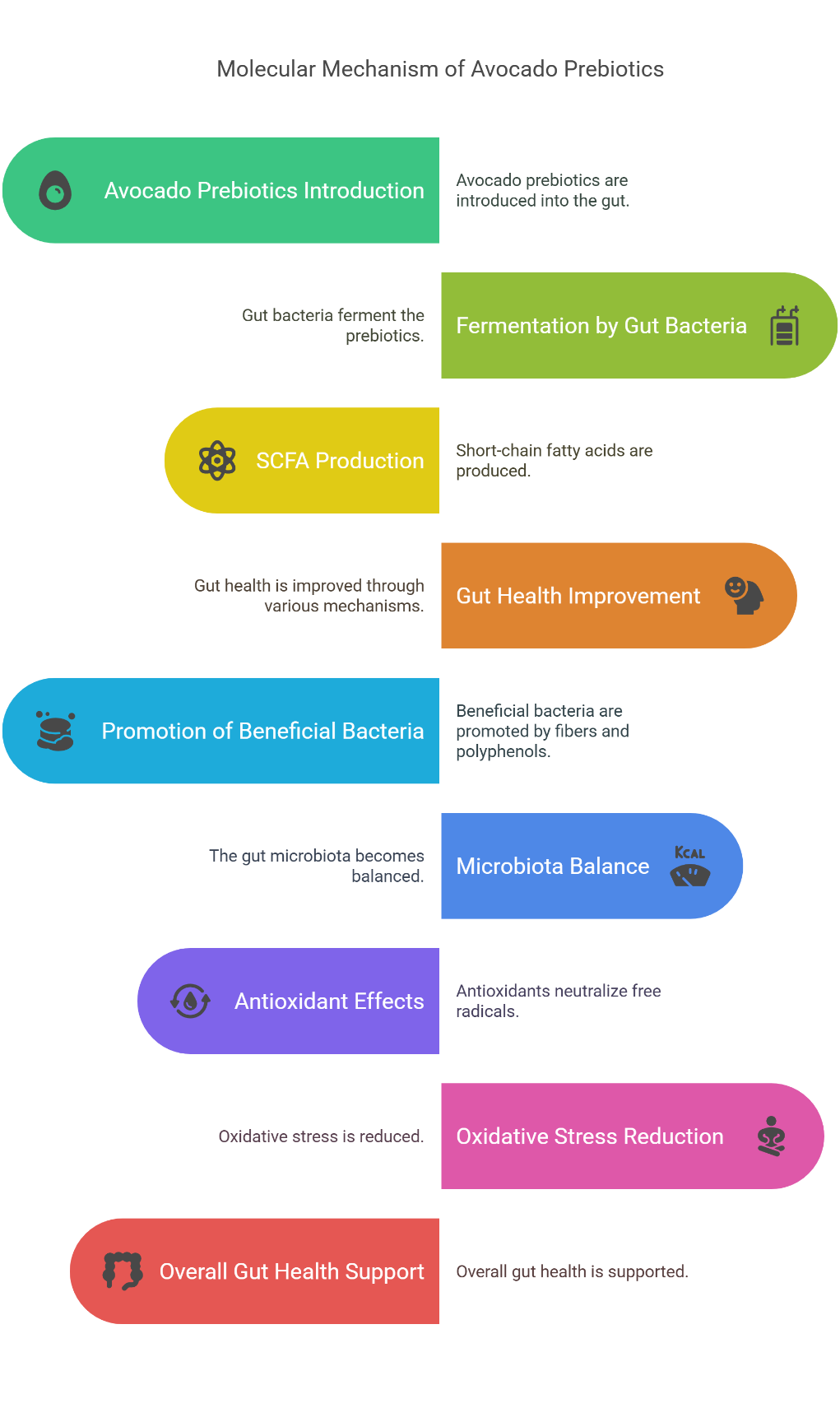
Fig. 3: Molecular mechanism of avocado probiotics. Source: Author’s own illustration
Interaction between avocado-derived prebiotics and probiotics
Avocados, rich in prebiotic fibers like pectin, play a crucial role in promoting gut health by nourishing beneficial bacteria in the microbiome. These fibers support the growth of probiotics, such as Lactobacillus, which are essential for maintaining a balanced gut flora [88]. The pectin’s in avocados create an environment that enhances the survival of probiotics in the digestive tract, facilitating their activity and effectiveness [89]. This interaction between avocado-derived prebiotics and probiotics highlights the importance of a balanced diet that includes both prebiotics and probiotics for optimal gut health. Incorporating avocados into the diet, especially alongside probiotic-rich foods, can improve digestive health by supporting the growth and implantation of beneficial microorganisms in the gastrointestinal tract, leading to overall well-being. This synergistic relationship underscores the value of combining prebiotic and probiotic-rich foods for enhanced digestive and immune health [90].
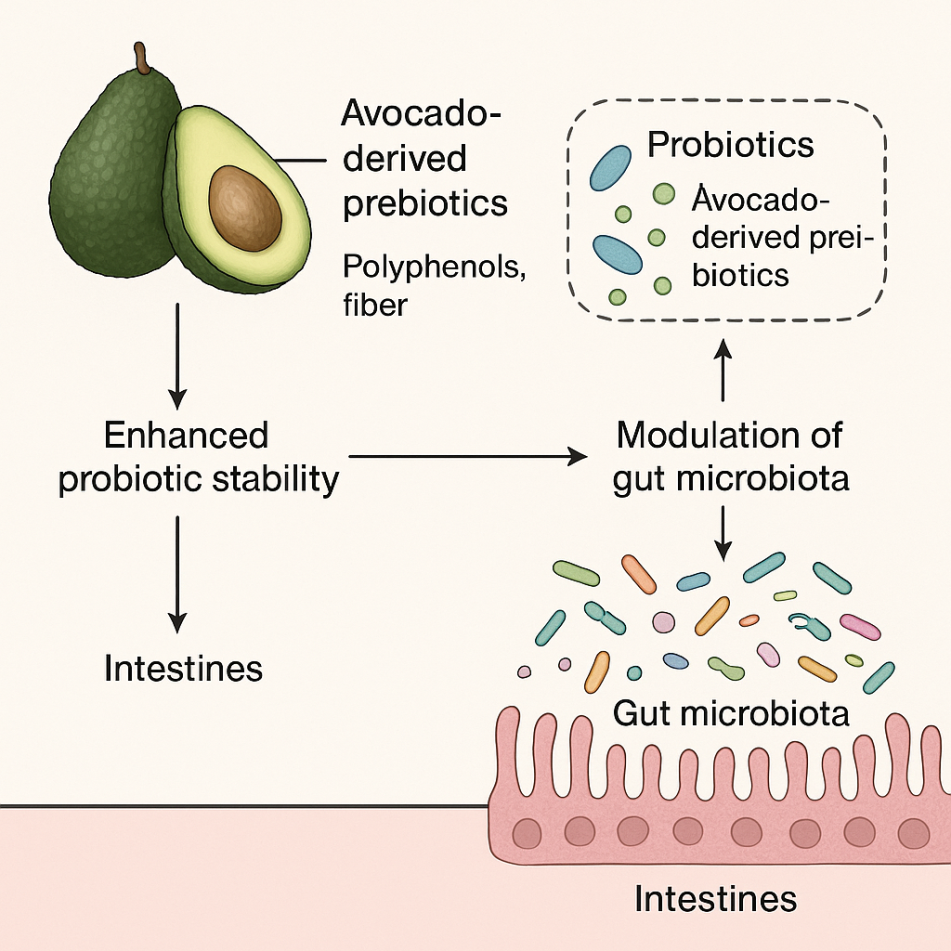
Fig. 4: The interaction between avocado-derived prebiotics, probiotics, and gut microbiota. Figure: Author’s own illustration
Influence of cellulose matrix on probiotic release and activity
The cellulose matrix plays a significant role in enhancing the release and activity of probiotics by providing a stable, protective environment for probiotic microorganisms as they pass through the digestive tract. Cellulose, a natural, indigestible fiber, can encapsulate probiotics, preventing premature degradation by stomach acids and digestive enzymes. This protection allows for the controlled release of probiotics in the intestines, where they can exert their beneficial effects on gut health [91]. The matrix also provides a scaffold for probiotics to adhere to the intestinal lining, improving their colonization and activity. This ensures that a higher number of viable probiotics reach the gut, where they can promote a healthy microbiome, support digestion, and boost immune function. The use of cellulose matrices in probiotic formulations is therefore critical in enhancing the effectiveness of probiotics and optimizing their benefits for digestive and overall health [92].
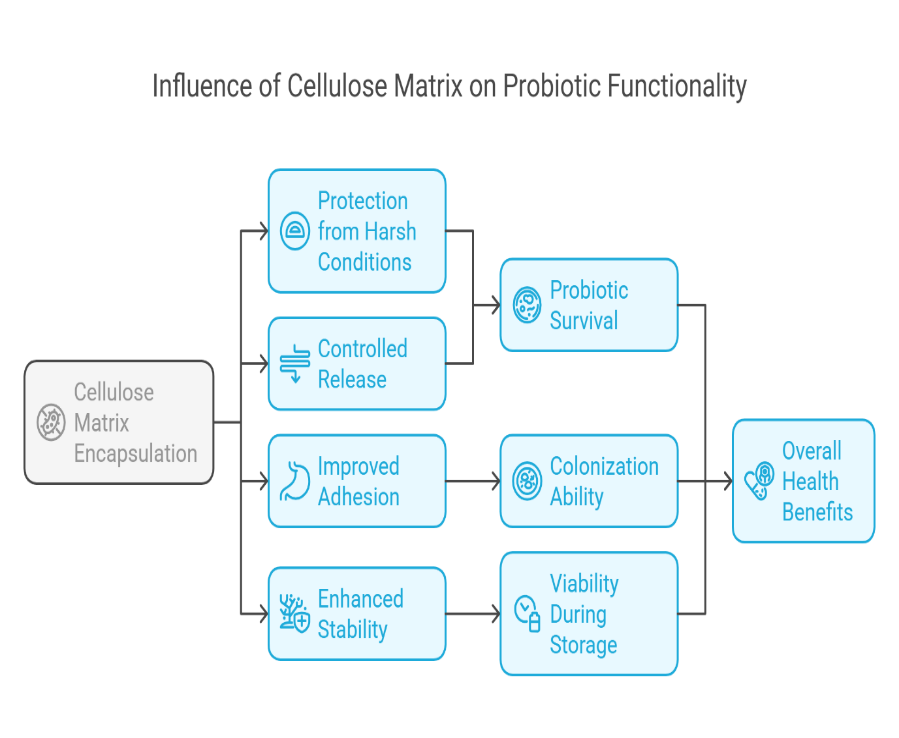
Fig. 5: The influence of cellulose matrix on probiotic functionality. Source: Author’s own illustration
Molecular mechanisms underlying enhanced stability
Molecular mechanisms underlying enhanced stability in probiotics are crucial for ensuring their survival and efficacy during storage and passage through the gastrointestinal tract. One key mechanism involves the use of protective matrices, such as cellulose, which shield probiotics from harsh environmental conditions, including stomach acid and bile salts. These matrices provide a physical barrier that helps preserve the viability of probiotics [93]. Additionally, the stabilization of probiotics can be achieved through biochemical modifications, such as the activation of stress response pathways that enable probiotics to tolerate extreme conditions. These stress responses involve the production of protective proteins, such as chaperones and enzymes, which prevent damage to cellular structures [94]. Furthermore, incorporating prebiotic fibers like inulin can enhance the stability of probiotics by promoting their growth and activity, ensuring they remain viable until they reach the intestines. Through these molecular mechanisms, probiotics can maintain their effectiveness, ensuring they deliver their health benefits to the gut microbiome [95].
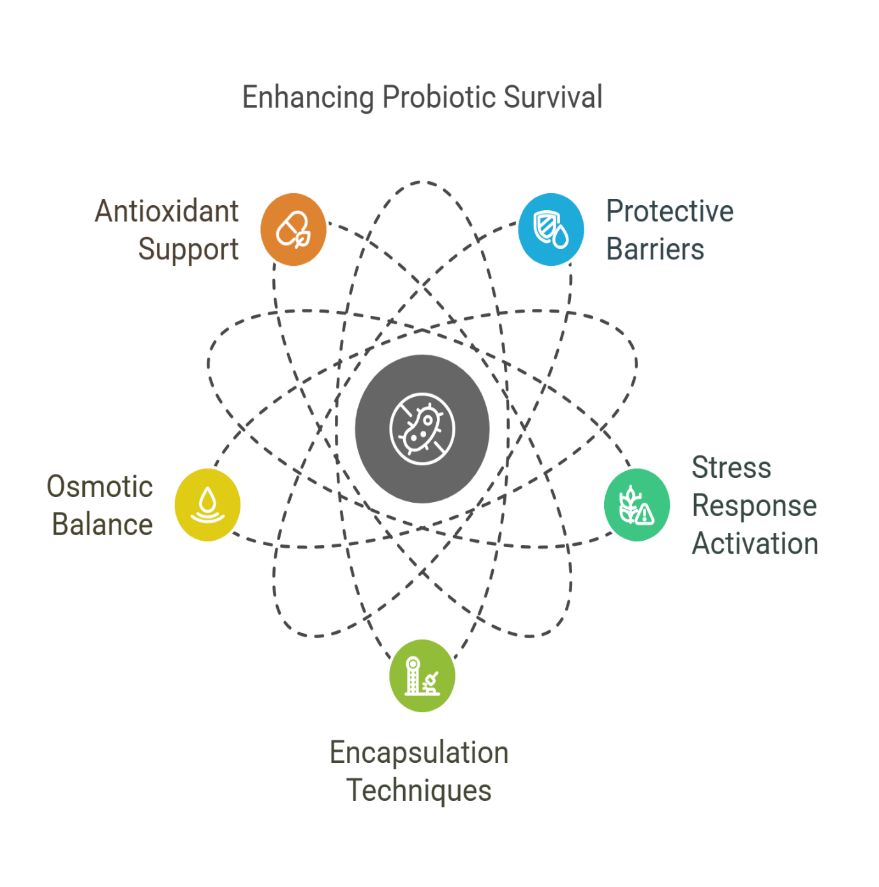
Fig. 6: The enhancing probiotic survival activities at the molecular mechanism underlying enhanced stability. Source: Author’s own illustration
Role of gut microbiota in probiotic efficacy
The gut microbiota plays a crucial role in determining the efficacy of probiotics, as it influences the survival, colonization, and activity of probiotic microorganisms in the gastrointestinal tract. A healthy, balanced microbiome provides an optimal environment for probiotics to thrive, enhancing their beneficial effects on digestion, immune function, and overall health [96]. The gut microbiota can facilitate the adhesion of probiotics to the intestinal lining, which is essential for their activity and long-term colonization. Moreover, the existing microbiota can interact with probiotics, influencing their metabolic processes and modulating their impact on gut health. In individuals with an imbalanced microbiome, probiotics may help restore microbial equilibrium, reducing pathogenic bacteria and promoting the growth of beneficial microorganisms [97]. Therefore, the composition of the gut microbiota is a key factor in determining the success of probiotic interventions, as it directly affects how probiotics function and contribute to improving gut health [98].
Regulatory and market considerations
Regulatory and market considerations play a critical role in the development, approval, and commercialization of probiotic products. Regulatory frameworks for probiotics vary across regions, with agencies such as the FDA in the U. S. and EFSA in Europe setting specific guidelines for safety, efficacy, and labelling requirements. These regulations ensure that probiotics meet the necessary standards before they can be marketed to consumers, and they often include requirements for clinical trials, ingredient safety assessments, and health claims substantiation [99]. In the marketplace, the growing demand for gut health products has created a competitive environment, with brands needing to differentiate themselves through scientifically backed formulations, clear labelling, and effective marketing strategies. Market trends show increasing consumer interest in natural, functional foods and supplements, pushing companies to develop innovative probiotic-based products that cater to health-conscious individuals [100]. However, navigating the regulatory landscape and ensuring compliance with local laws while meeting consumer expectations can be challenging for manufacturers. Thus, understanding both regulatory standards and market dynamics is essential for successfully launching probiotic products [101].
Regulatory approval of novel prebiotics varies globally. In the U. S., the FDA considers prebiotics under the GRAS (Generally recognized as safe) framework, while the European EFSA mandates stringent safety dossiers for health claims. Asian markets like Japan follow functional food (FOSHU) guidelines, which require clinical substantiation. These regulatory asymmetries create challenges in harmonizing global product launches and raise compliance costs, especially for novel sources like avocado-derived prebiotics [99, 101].
Barriers to market entry and commercialization
Barriers to market entry and commercialization of probiotic products can be significant, stemming from regulatory challenges, high production costs, and intense market competition. One of the primary obstacles is the complex regulatory landscape, where probiotic products must meet stringent safety, efficacy, and labelling standards set by agencies such as the FDA and EFSA [102]. These regulations often require expensive clinical trials and extensive documentation to prove health claims, which can be a financial burden, particularly for small businesses. Additionally, the probiotic market is highly competitive, with numerous established brands and new entrants vying for consumer attention. This saturation makes it difficult for new products to stand out unless they offer distinct advantages in terms of formulation, efficacy, or delivery methods [103]. Another barrier is the cost of producing high-quality, viable probiotics, as maintaining product stability and ensuring the survival of beneficial bacteria during storage and transit can be costly. Moreover, consumer education about the health benefits and effectiveness of probiotics is still evolving, and companies must invest in marketing and outreach to overcome skepticism. Overall, these barriers require strategic planning, significant investment, and a strong understanding of both regulatory requirements and market demands to successfully enter and thrive in the probiotic market [104].
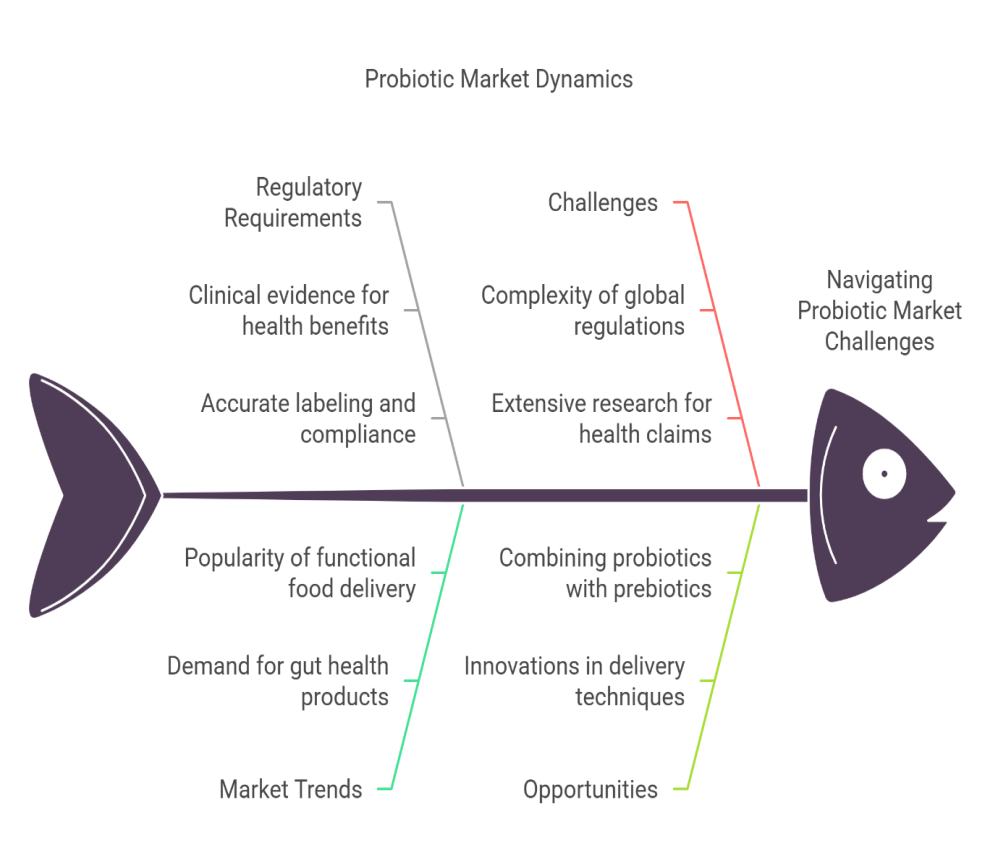
Fig. 7: The fishbone model of probiotics market dynamics. Source: Author’s own illustration
Case studies and real-world applications
Case studies and real-world applications highlight the practical benefits and challenges of incorporating probiotics into health and wellness products. In one example, a company developed a probiotic yogurt that successfully improved gut health in consumers with irritable bowel syndrome (IBS), demonstrating how probiotics can alleviate digestive discomfort and support overall gastrointestinal function. Another case study explored the use of probiotics in infant formula, showing their potential to reduce the incidence of colic and other digestive issues in newborns [105]. Additionally, research on probiotic supplements has revealed their effectiveness in enhancing immune function, reducing inflammation, and managing conditions like allergies and obesity. However, real-world applications also present challenges, such as maintaining the viability of probiotics during storage and transport, and ensuring that the correct strains of bacteria are included to deliver the intended health benefits. These case studies underscore the growing recognition of probiotics as a valuable tool in preventive healthcare, while also highlighting the importance of rigorous scientific research and quality control in their development [106]. As the market for probiotic-based products continues to expand, these applications offer valuable insights into their potential for improving consumer health across various demographics and conditions [107].
Commercial products incorporating avocado-derived prebiotics
Commercial products incorporating avocado-derived prebiotics are gaining popularity due to the fruit's rich content of fiber, particularly pectin, which serves as an excellent prebiotic for promoting gut health. Several food and supplement companies have begun to leverage avocado's natural prebiotic properties in their product formulations. For example, some snack brands have developed avocado-based chips and spreads, infused with added fiber to support digestive health [108]. Additionally, avocado-derived prebiotics are being included in functional beverages, such as smoothies and drinks, designed to improve gut microbiome health. These products often combine avocado with other prebiotic ingredients, such as inulin, to further enhance their effects. In the supplement market, avocado oil is being marketed as a source of prebiotic fiber, with claims of supporting digestive health and improving the balance of beneficial gut bacteria [109]. As the demand for natural, plant-based ingredients rises, avocado-derived prebiotics offer a promising addition to the growing functional food and supplement industry, providing a healthy, sustainable option for consumers seeking to improve their gut health and overall well-being [110].
Successful applications of cellulose matrices in probiotic delivery
Successful applications of cellulose matrices in probiotic delivery have demonstrated their effectiveness in improving the stability and viability of probiotics during storage and gastrointestinal transit. One notable application is the use of cellulose-based capsules and tablets, which serve as protective carriers for probiotics, ensuring that the beneficial microorganisms reach the intestines alive and active [111]. These matrices shield the probiotics from harsh stomach acids, preventing premature degradation and enhancing their chances of colonizing the gut. Another successful application is in functional foods, where cellulose matrices are incorporated into yogurts, smoothies, and snack bars to deliver probiotics in a controlled-release manner. The cellulose not only protects the probiotics but also aids in their gradual release, providing sustained benefits to the gut microbiome [112]. Additionally, cellulose matrices have been used in the development of symbiotic products, which combine probiotics with prebiotics like fiber to further enhance the health benefits. These innovations have contributed to the growth of the probiotic market, allowing manufacturers to offer more effective, stable, and consumer-friendly products that support digestive and immune health [113].
Impact on health outcomes in population studies
Population studies have shown significant impacts of probiotics on various health outcomes, particularly in terms of improving gut health and immune function, and reducing the risk of chronic diseases. Research involving large groups of people has demonstrated that regular probiotic consumption can enhance the diversity and balance of gut microbiota, which is associated with better digestive health and reduced incidences of conditions like irritable bowel syndrome (IBS), constipation, and diarrhoea [114]. Additionally, population studies have highlighted the potential of probiotics in modulating the immune system, with evidence suggesting they can help lower the frequency and severity of respiratory infections, allergies, and autoimmune diseases. Other studies have explored the role of probiotics in preventing or managing metabolic conditions such as obesity, type 2 diabetes, and cardiovascular diseases, revealing that probiotics may help regulate weight, blood sugar, and cholesterol levels [115]. These findings emphasize the broader health benefits of probiotics beyond just gut health, supporting their inclusion in functional foods and supplements aimed at improving overall well-being. The positive outcomes observed in population studies have led to growing interest in the potential of probiotics as a tool for disease prevention and health optimization [116].
Challenges and future directions
Challenges and future directions in the probiotic field involve addressing several key issues, including product stability, strain-specific effects, and regulatory hurdles. One major challenge is ensuring the stability and viability of probiotics throughout their shelf life and during transit through the gastrointestinal tract. Probiotics must survive the harsh stomach acids and bile salts to be effective, and current methods to protect them, such as encapsulation, still require further improvement [117]. Additionally, the efficacy of probiotics is strain-dependent, meaning that not all probiotic strains deliver the same health benefits, creating the need for more personalized and evidence-based formulations. Regulatory challenges also persist, as probiotic products are often subject to varying standards across different regions, making it difficult for manufacturers to navigate global markets [118]. Furthermore, while probiotics show promise in supporting gut health and immune function, more research is needed to better understand their long-term effects, optimal dosages, and specific health benefits. Future directions in the field include the development of more efficient delivery systems, such as targeted-release formulations, and a deeper focus on personalized probiotics tailored to individual microbiomes. There is also growing interest in symbiotics, the combination of prebiotics and probiotics, which could enhance the effectiveness of probiotic interventions and support a more balanced gut microbiota [119].
Current limitations in research and application
Current limitations in research and application of probiotics stem from several factors, including a lack of standardized methods, limited understanding of long-term effects, and the complexity of the human microbiome. One significant challenge is the variability in probiotic strains and their inconsistent effects, as not all strains provide the same health benefits or function equally across individuals [120]. This strain-specific variation complicates the development of universally effective probiotic products. Additionally, much of the research on probiotics is still in its early stages, with many studies lacking large-scale clinical trials or long-term data on their safety and efficacy [121]. The complexity of the human gut microbiome further complicates the application of probiotics, as individual microbiota compositions can vary greatly, affecting how probiotics function. There is also a need for more robust evidence regarding the optimal dosages and the best delivery systems to ensure probiotics reach the intended sites in the digestive tract [122]. Regulatory inconsistencies across different regions also pose challenges for global commercialization, as probiotic products must comply with varying guidelines. These limitations highlight the need for more research, better regulatory frameworks, and standardized testing to maximize the potential of probiotics in improving health outcomes [123].
Technological challenges in production and formulation
Technological challenges in the production and formulation of probiotics include ensuring stability, viability, and effective delivery of probiotics throughout their shelf life and gastrointestinal journey. One of the primary challenges is protecting probiotics from environmental factors such as heat, moisture, and oxygen, which can compromise their viability. Probiotics are sensitive microorganisms, and their survival often depends on careful encapsulation or the use of specialized coatings to shield them from stomach acids and bile salts [124]. Developing cost-effective, scalable methods for producing these protective delivery systems is another technological hurdle. Additionally, achieving consistent potency in probiotic formulations is difficult, as different strains may require different conditions for optimal growth and stability. Ensuring that probiotics remain effective throughout the product's shelf life is a challenge for manufacturers, as degradation can occur over time, reducing their health benefits. Furthermore, formulating products that can deliver the right dosage of probiotics to the right location in the gut is another technological barrier [125]. Innovations such as targeted-release capsules or microencapsulation technologies are being explored, but they often require significant research and development to perfect. These technological challenges necessitate continued investment in research and development to improve the formulation, stability, and efficacy of probiotic products [126].
Regulatory considerations for nutraceuticals
Regulatory considerations for nutraceuticals are critical in ensuring the safety, efficacy, and quality of products that blend food and medicinal benefits. These products, which include probiotics, vitamins, minerals, and other bioactive ingredients, are subject to different regulations depending on the region [127]. In the United States, for example, the FDA regulates nutraceuticals as dietary supplements, meaning they do not require pre-market approval but must adhere to Good Manufacturing Practices (GMPs) to ensure quality. Nutraceuticals must also provide accurate labelling, including health claims that are substantiated by scientific evidence. In Europe, the European Food Safety Authority (EFSA) plays a similar role in evaluating health claims and ensuring compliance with safety standards. However, the regulatory landscape for nutraceuticals remains fragmented globally, with varying rules around ingredient safety, efficacy testing, and claims substantiation [128-130]. This lack of uniformity can create challenges for companies seeking to market nutraceuticals across borders. Furthermore, some ingredients may not be approved for use in certain regions, which can limit market access. As the nutraceutical industry grows, regulatory bodies are likely to continue refining their policies, and manufacturers must stay abreast of these developments to ensure compliance and maintain consumer trust [131-134].
Future research directions and potential innovations
Future research directions and potential innovations in the field of nutraceuticals are focused on enhancing the understanding of bioactive compounds, improving delivery systems, and personalizing treatments for better health outcomes [135]. One key area of exploration is the identification and characterization of novel, natural ingredients that offer therapeutic benefits, such as plant-based compounds, peptides, and polyphenols [136, 137]. Advances in genomics and microbiome research also present exciting opportunities for the development of personalized nutraceuticals tailored to an individual's genetic profile and gut microbiota composition [138, 139]. This could lead to more effective and targeted interventions for a variety of health conditions [140, 141]. Additionally, innovations in delivery technologies, such as nanoencapsulation and controlled-release systems, are expected to improve the bioavailability and stability of active ingredients, ensuring they reach their intended sites in the body in optimal conditions. Another promising direction is the integration of nutraceuticals with digital health technologies, such as wearable devices and mobile health apps, to monitor and optimize the effectiveness of these products in real-time [142]. As the field evolves, researchers will continue to explore how nutraceuticals can complement traditional medicine and contribute to preventive healthcare strategies. With these innovations, the future of nutraceuticals looks promising, offering new solutions for enhancing wellness and managing chronic diseases [143].
CONCLUSION
The present review underscores the promising role of avocado-derived prebiotics in enhancing probiotic stability and efficacy, particularly through integration into cellulose-based encapsulation matrices. The discussion has emphasized the advantages of cellulose matrices in providing mechanical protection and sustained release for probiotic delivery, especially under gastrointestinal stress conditions. Despite these insights, several knowledge gaps remain that warrant further investigation. Future research should prioritize the development of next-generation delivery systems, such as pH-responsive hydrogels and nano-encapsulated composites, to improve precision in probiotic release and site-specific colonization. Furthermore, large-scale, randomized clinical trials are essential to validate the functional claims of avocado-derived prebiotics, particularly in human populations with varying gut microbiota compositions. Additionally, personalized nutrition must be integrated into probiotic-prebiotic design frameworks. Advances in microbiome profiling technologies enable the tailoring of prebiotic formulations to specific host microbiota configurations, optimizing therapeutic effectiveness. Avocado-derived prebiotics hold significant potential in the evolving field of gut health, but realizing their full utility requires a concerted effort across food science, microbiology, materials engineering, and regulatory policy. Bridging current research gaps with interdisciplinary collaboration will pave the way for sustainable, effective, and consumer-friendly probiotic-prebiotic systems.
ACKNOWLEDGEMENT
The authors would like to express their gratitude to the Department of Science and Technology-Fund for Improvement of Science and Technology Infrastructure (DST-FIST), Promotion of University Research and Scientific Excellence (DST-PURSE), and the Department of Biotechnology Boost to University Interdisciplinary Pharmaceutical Biotechnology Sciences Departments for Education and Research program (DBT-BUILDER) for the facilities provided in our department. Additionally, as the first author, Jatin M, I thank the following individuals for their expertise and assistance throughout all aspects of my review study and for their help in writing the manuscript. I also extend my heartfelt thanks to JSS Academy of Higher Education, Mysuru, and JSS College of Pharmacy, Ooty, for providing their pool of resources to support and enhance the quality of my review.
FUNDING
Nil
AUTHORS CONTRIBUTIONS
Jatin M: Conceptualization, data acquisition, analysis, interpretation, writing-original draft, Bishop Adhikari: Data alignments and design of the fig. and table of the paper. Piyush Kumar: Helped to analyse and collect data for the paper. For R. Rajesh Kumar: Review and editing, Supervision, Critical evaluation, and Validation.
CONFLICT OF INTERESTS
The author is reporting no conflict of interest.
REFERENCES
Kim SK, Guevarra RB, Kim YT, Kwon J, Kim H, Cho JH. Role of probiotics in human gut microbiome-associated diseases. J Microbiol Biotechnol. 2019 Sep 28;29(9):1335-40. doi: 10.4014/jmb.1906.06064, PMID 31434172.
Suez J, Zmora N, Segal E, Elinav E. The pros cons and many unknowns of probiotics. Nat Med. 2019 May;25(5):716-29. doi: 10.1038/s41591-019-0439-x, PMID 31061539.
Wilkins T, Sequoia J. Probiotics for gastrointestinal conditions: a summary of the evidence. Am Fam Physician. 2017 Aug 1;96(3):170-8. PMID 28762696.
Williams NT. Probiotics. Am J Health Syst Pharm. 2010 Mar 15;67(6):449-58. doi: 10.2146/ajhp090168, PMID 20208051.
Puebla Barragan S, Reid G. Probiotics in cosmetic and personal care products: trends and challenges. Molecules. 2021 Feb 26;26(5):1249. doi: 10.3390/molecules26051249, PMID 33652548, PMCID PMC7956298.
Ozen M, Dinleyici EC. The history of probiotics: the untold story. Benef Microbes. 2015;6(2):159-65. doi: 10.3920/BM2014.0103, PMID 25576593.
Legesse Bedada T, Feto TK, Awoke KS, Garedew AD, Yifat FT, Birri DJ. Probiotics for cancer alternative prevention and treatment. Biomed Pharmacother. 2020 Sep;129:110409. doi: 10.1016/j.biopha.2020.110409, PMID 32563987.
Wang L, Meng FJ, Jin YH, Wu LQ, Tang RY, Xu KH. Effects of probiotic supplementation on 12 min run performance, mood management body composition and gut microbiota in amateur marathon runners: a double-blind controlled trial. J Exerc Sci Fit. 2024 Oct;22(4):297-304. doi: 10.1016/j.jesf.2024.04.004, PMID 38706951, PMCID PMC11066675.
Yamanbaeva G, Schaub AC, Schneider E, Schweinfurth N, Kettelhack C, Doll JP. Effects of a probiotic add-on treatment on fronto-limbic brain structure function and perfusion in depression: secondary neuroimaging findings of a randomized controlled trial. J Affect Disord. 2023 Mar 1;324:529-38. doi: 10.1016/j.jad.2022.12.142, PMID 36610592.
Gueimonde M, Sanchez B. Enhancing probiotic stability in industrial processes. Microb Ecol Health Dis. 2012 Jun 18;23:18562. doi: 10.3402/mehd.v23i0.18562, PMID 23990824, PMCID PMC3747747.
Chen Z, Yu L, Liu J, Kong J, Deng X, Guo X. Gut microbiota dynamics and fecal SCFAs after colonoscopy: accelerating microbiome stabilization by Clostridium butyricum. J Transl Med. 2024 Mar 1;22(1):222. doi: 10.1186/s12967-024-05031-y, PMID 38429821, PMCID PMC10908214.
Mulla M, Mulla M, Hegde S, Koshy AV. In vitro assessment of the effect of probiotic Lactobacillus reuteri on peri-implantitis microflora. BMC Oral Health. 2021 Aug 19;21(1):408. doi: 10.1186/s12903-021-01762-2, PMID 34412595, PMCID PMC8377827.
Kvakova M, Kamlarova A, Stofilova J, Benetinova V, Bertkova I. Probiotics and postbiotics in colorectal cancer: prevention and complementary therapy. World J Gastroenterol. 2022 Jul 21;28(27):3370-82. doi: 10.3748/wjg.v28.i27.3370, PMID 36158273, PMCID PMC9346452.
Jackson SA, Schoeni JL, Vegge C, Pane M, Stahl B, Bradley M. Improving end-user trust in the quality of commercial probiotic products. Front Microbiol. 2019 Apr 17;10:739. doi: 10.3389/fmicb.2019.00739, PMID 31105649, PMCID PMC6499161.
Mohamadzadeh M, Fazeli A, Vasheghani Farahani E, Shojaosadati SA. Viability and stability evaluation of microencapsulated Lactobacillus reuteri in polysaccharide-based bionanocomposite. Carbohydr Polym. 2025 Jan 1;347:122693. doi: 10.1016/j.carbpol.2024.122693, PMID 39486934.
Xu H, Li Y, Song J, Zhou L, Wu K, Lu X. Highly active probiotic hydrogels matrixed on bacterial EPS accelerate wound healing via maintaining stable skin microbiota and reducing inflammation. Bioact Mater. 2024 Jan 19;35:31-44. doi: 10.1016/j.bioactmat.2024.01.011, PMID 38304916, PMCID PMC10831122.
Wampers A, Huysentruyt K, Vandenplas Y. An update on the use of ‘biotics’ in pediatric infectious gastroenteritis. Expert Opin Pharmacother. 2024 Aug;25(11):1483-96. doi: 10.1080/14656566.2024.2387672, PMID 39091043.
Flores M, Saravia C, Vergara CE, Avila F, Valdes H, Ortiz Viedma J. Avocado oil: characteristics properties and applications. Molecules. 2019 Jun 10;24(11):2172. doi: 10.3390/molecules24112172, PMID 31185591, PMCID PMC6600360.
Dreher ML, Davenport AJ. Hass avocado composition and potential health effects. Crit Rev Food Sci Nutr. 2013;53(7):738-50. doi: 10.1080/10408398.2011.556759, PMID 23638933, PMCID PMC3664913.
Dabas D, Shegog RM, Ziegler GR, Lambert JD. Avocado (Persea americana) seed as a source of bioactive phytochemicals. Curr Pharm Des. 2013;19(34):6133-40. doi: 10.2174/1381612811319340007, PMID 23448442.
Salazar Lopez NJ, Dominguez Avila JA, Yahia EM, Belmonte Herrera BH, Wall Medrano A, Montalvo Gonzalez E. Avocado fruit and by products as potential sources of bioactive compounds. Food Res Int. 2020 Dec;138(A):109774. doi: 10.1016/j.foodres.2020.109774, PMID 33292952.
Olza S, Salaberria AM, Alonso Varona A, Samanta A, Fernandes SC. The role of nanochitin in biologically active matrices for tissue engineering: where do we stand? J Mater Chem B. 2023 Jun 28;11(25):5630-49. doi: 10.1039/d3tb00583f, PMID 37159053.
Jones DM, Murray CM, Ketelaar KJ, Thomas JJ, Villalobos JA, Wallace IS. The emerging role of protein phosphorylation as a critical regulatory mechanism controlling cellulose biosynthesis. Front Plant Sci. 2016 May 24;7:684. doi: 10.3389/fpls.2016.00684, PMID 27252710, PMCID PMC4877384.
Jaafar MZ, Mohd Ridzuan FF, Mohamad Kassim MH, Abu F. The role of dissolution time on the properties of all cellulose composites obtained from oil palm empty fruit bunch. Polymers (Basel). 2023 Jan 30;15(3):691. doi: 10.3390/polym15030691, PMID 36771992, PMCID PMC9919761.
Vu H, Woodcock JW, Krishnamurthy A, Obrzut J, Gilman JW, Coughlin EB. Visualization of polymer dynamics in cellulose nanocrystal matrices using fluorescence lifetime measurements. ACS Appl Mater Interfaces. 2022 Mar 2;14(8):10793-804. doi: 10.1021/acsami.1c21906, PMID 35179343.
Kammoun M, Margellou A, Toteva VB, Aladjadjiyan A, Sousa AF, Luis SV. The key role of pretreatment for the one-step and multi-step conversions of European lignocellulosic materials into furan compounds. RSC Adv. 2023 Jul 18;13(31):21395-420. doi: 10.1039/d3ra01533e, PMID 37469965, PMCID PMC10352963.
Bangar SP, Dunno K, Dhull SB, Kumar Siroha A, Changan S, Maqsood S. Avocado seed discoveries: chemical composition biological properties and industrial food applications. Food Chem X. 2022 Nov 11;16:100507. doi: 10.1016/j.fochx.2022.100507, PMID 36573158, PMCID PMC9789361.
Bhuyan DJ, Alsherbiny MA, Perera S, Low M, Basu A, Devi OA. The odyssey of bioactive compounds in avocado (Persea americana) and their health benefits. Antioxidants (Basel). 2019 Sep 24;8(10):426. doi: 10.3390/antiox8100426, PMID 31554332, PMCID PMC6826385.
Rozan M, Alamri E, Bayomy H. Fermented hass avocado kernel: nutritional properties and use in the manufacture of biscuits. Saudi J Biol Sci. 2022 Jun;29(6):103295. doi: 10.1016/j.sjbs.2022.103295, PMID 35521360, PMCID PMC9065908.
Nyakang’i CO, Marete E, Ebere R, Arimi JM. Physicochemical properties of avocado seed extract model beverages and baked products incorporated with avocado seed powder. Int J Food Sci. 2023 May 31;2023:6860806. doi: 10.1155/2023/6860806, PMID 37293187, PMCID PMC10247324.
Ford NA, Spagnuolo P, Kraft J, Bauer E. Nutritional composition of Hass avocado pulp. Foods. 2023 Jun 28;12(13):2516. doi: 10.3390/foods12132516, PMID 37444254, PMCID PMC10340145.
Kase BE, Liese AD, Zhang J, Murphy EA, Zhao L, Steck SE. The development and evaluation of a literature-based dietary index for gut microbiota. Nutrients. 2024 Apr 3;16(7):1045. doi: 10.3390/nu16071045, PMID 38613077, PMCID PMC11013161.
Zapata Luna RL, Pacheco N, Herrera Pool E, Roman Guerrero A, Ayora Talavera T, Pech Cohuo SC. Morpho-physicochemical nutritional composition and phenolic compound profile of two avocado landraces in different ripening stages. Plants (Basel). 2025 Feb 19;14(4):624. doi: 10.3390/plants14040624, PMID 40006883, PMCID PMC11859218.
Salehi B, Rescigno A, Dettori T, Calina D, Docea AO, Singh L. Avocado soybean unsaponifiables: a panoply of potentialities to be exploited. Biomolecules. 2020 Jan 13;10(1):130. doi: 10.3390/biom10010130, PMID 31940989, PMCID PMC7023362.
Rojas Garcia A, Fuentes E, Cadiz Gurrea ML, Rodriguez L, Villegas Aguilar MD, Palomo I. Biological evaluation of avocado residues as a potential source of bioactive compounds. Antioxidants (Basel). 2022 May 25;11(6):1049. doi: 10.3390/antiox11061049, PMID 35739946, PMCID PMC9220077.
Tremocoldi MA, Rosalen PL, Franchin M, Massarioli AP, Denny C, Daiuto ER. Exploration of avocado by products as natural sources of bioactive compounds. PLOS One. 2018 Feb 14;13(2):e0192577. doi: 10.1371/journal.pone.0192577, PMID 29444125, PMCID PMC5812635.
Tang F, Green HS, Wang SC, Hatzakis E. Analysis and authentication of avocado oil using high-resolution NMR spectroscopy. Molecules. 2021 Jan 9;26(2):310. doi: 10.3390/molecules26020310, PMID 33435322, PMCID PMC7828049.
Liu Y, Xia Q, Qian Y, Kuang Y, Liu J, Lin L. Effects of three extraction methods on avocado oil lipid compounds analyzed via UPLC-TOF-MS/MS with OPLS-DA. Foods. 2023 Mar 10;12(6):1174. doi: 10.3390/foods12061174, PMID 36981101, PMCID PMC10048627.
Cervantes Paz B, Yahia EM. Avocado oil: production and market demand bioactive components implications in health and tendencies and potential uses. Compr Rev Food Sci Food Saf. 2021 Jul;20(4):4120-58. doi: 10.1111/1541-4337.12784, PMID 34146454.
Razola Diaz MD, Verardo V, Guerra Hernandez EJ, Garcia Villanova Ruiz B, Gomez Caravaca AM. Response surface methodology for the optimization of Flavan-3-ols extraction from avocado by products via sonotrode ultrasound-assisted extraction. Antioxidants (Basel). 2023 Jul 11;12(7):1409. doi: 10.3390/antiox12071409, PMID 37507948, PMCID PMC10376872.
Aljumaah MR, Bhatia U, Roach J, Gunstad J, Azcarate Peril MA. The gut microbiome mild cognitive impairment and probiotics: a randomized clinical trial in middle-aged and older adults. Clin Nutr. 2022 Nov;41(11):2565-76. doi: 10.1016/j.clnu.2022.09.012, PMID 36228569.
Pennington E, Bell S, Hill JE. Should video laryngoscopy or direct laryngoscopy be used for adults undergoing endotracheal intubation in the pre-hospital setting? A critical appraisal of a systematic review. J Paramed Pract. 2023;15(6):255-9. doi: 10.1002/14651858, PMID 38812899.
Error in figure. JAMA PediatrJAMA Pediatr. 2024;178(1):99. doi: 10.1001/jamapediatrics.2023.5514, PMID 38010708.
Pujo J, Petitfils C, Le Faouder P, Eeckhaut V, Payros G, Maurel S. Bacteria-derived long-chain fatty acid exhibits anti-inflammatory properties in colitis. Gut. 2021 Jun;70(6):1088-97. doi: 10.1136/gutjnl-2020-321173, PMID 32978245.
Oh KK, Gupta H, Min BH, Ganesan R, Sharma SP, Won SM. Elucidation of prebiotics probiotics postbiotics and target from gut microbiota to alleviate obesity via network pharmacology study. Cells. 2022 Sep 16;11(18):2903. doi: 10.3390/cells11182903, PMID 36139478, PMCID PMC9496669.
Silvestre GH, De Lima FC, Bernardes JS, Fazzio A, Miwa RH. Nanoscale structural and electronic properties of cellulose/graphene interfaces. Phys Chem Chem Phys. 2023 Jan 4;25(2):1161-8. doi: 10.1039/d2cp04146d, PMID 36519443.
Qiao H, Li M, Wang C, Zhang Y, Zhou H. Progress challenge and perspective of fabricating cellulose. Macromol Rapid Commun. 2022 Sep;43(18):e2200208. doi: 10.1002/marc.202200208, PMID 35809256.
Moon RJ, Martini A, Nairn J, Simonsen J, Youngblood J. Cellulose nanomaterials review: structure, properties and nanocomposites. Chem Soc Rev. 2011 Jul;40(7):3941-94. doi: 10.1039/c0cs00108b, PMID 21566801.
Jezewska Frąckowiak J, Seroczynska K, Banaszczyk J, Jedrzejczak G, Zylicz Stachula A, Skowron PM. The promises and risks of probiotic Bacillus species. Acta Biochim Pol. 2018 Dec 6;65(4):509-19. doi: 10.18388/abp.2018_2652, PMID 30521647.
Han J, McClements DJ, Liu X, Liu F. Oral delivery of probiotics using single cell encapsulation. Compr Rev Food Sci Food Saf. 2024 May;23(3):e13322. doi: 10.1111/1541-4337.13322, PMID 38597567.
Agriopoulou S, Tarapoulouzi M, Varzakas T, Jafari SM. Application of encapsulation strategies for probiotics: from individual loading to co-encapsulation. Microorganisms. 2023 Nov 30;11(12):2896. doi: 10.3390/microorganisms11122896, PMID 38138040, PMCID PMC10745938.
Dai L, Si C. Recent advances on cellulose based nano-drug delivery systems: design of prodrugs and nanoparticles. Curr Med Chem. 2019;26(14):2410-29. doi: 10.2174/0929867324666170711131353, PMID 28699504.
Liang S. Advances in drug delivery applications of modified bacterial cellulose-based materials. Front Bioeng Biotechnol. 2023 Aug 4;11:1252706. doi: 10.3389/fbioe.2023.1252706, PMID 37600320, PMCID PMC10436498.
Nadeem H, Athar M, Dehghani M, Garnier G, Batchelor W. Recent advancements trends fundamental challenges and opportunities in spray-deposited cellulose nanofibril films for packaging applications. Sci Total Environ. 2022 Aug 25;836:155654. doi: 10.1016/j.scitotenv.2022.155654, PMID 35508247.
Verma C, Singh V, AlFantazi A. Cellulose cellulose derivatives and cellulose composites in sustainable corrosion protection: challenges and opportunities. Phys Chem Chem Phys. 2024 Apr 17;26(15):11217-42. doi: 10.1039/d3cp06057h, PMID 38587831.
Mozafari L, Martinez Zamora L, Cano Lamadrid M, Aguayo E, Artes Hernandez F. Enhancing avocado puree with encapsulated tomato by products. Effect of processing methods in the bioactive quality retention. J Sci Food Agric. 2025 Mar 13;105(9):4964-75. doi: 10.1002/jsfa.14226, PMID 40079337.
Baez Gonzalez JG, Gallegos Garza MM, Gallardo Rivera CT, Trevino Garza MZ, Amaya Guerra CA, Rodriguez Rodriguez J. Physicochemical characterization and thermodynamic analysis of avocado oil enhanced with Haematococcus pluvialis extract. Foods. 2024 Oct 7;13(19):3184. doi: 10.3390/foods13193184, PMID 39410219, PMCID PMC11476195.
Ngungeni Y, A Aboyewa J, Moabelo KL, Sibuyi NR, Meyer S, Onani MO. Anticancer antioxidant and catalytic activities of green synthesized gold nanoparticles using avocado seed aqueous extract. ACS Omega. 2023 Jul 11;8(29):26088-101. doi: 10.1021/acsomega.3c02260, PMID 37521675, PMCID PMC10373464.
Mahadi N, Rahman A, Prasad C, Govinda V, Choi HY, Shin EJ. Synergistic effects of cellulose nanocrystal on the mechanical and shape memory properties of TPU composites. Int J Biol Macromol. 2024 Oct;278(3):134842. doi: 10.1016/j.ijbiomac.2024.134842, PMID 39159801.
Chen P, Xie F, Tang F, McNally T. Cooperative effects of cellulose nanocrystals and sepiolite when combined on ionic liquid plasticised chitosan materials. Polymers (Basel). 2021 Feb 14;13(4):571. doi: 10.3390/polym13040571, PMID 33672901, PMCID PMC7918726.
Zhu Q, Yao Q, Sun J, Chen H, Xu W, Liu J. Stimuli-induced cellulose nanomaterials alignment and its emerging applications: a review. Carbohydr Polym. 2020 Feb 15;230:115609. doi: 10.1016/j.carbpol.2019.115609, PMID 31887954.
Jannah SR, Rahayu ES, Yanti R, Suroto DA, Wikandari R. Study of viability storage stability and shelf life of probiotic instant coffee Lactiplantibacillus plantarum subsp. plantarum Dad-13 in vacuum and nonvacuum packaging at different storage temperatures. Int J Food Sci. 2022 Nov 17;2022:1663772. doi: 10.1155/2022/1663772, PMID 36438165, PMCID PMC9691296.
Alsharafani MA, Abdullah T, Jabur ZA, Hassan AA, Alhendi AS, Abdulmawjood A. Assessing synergistic effect of Jerusalem artichoke juice and antioxidant compounds on enhanced viability and persistence of Bifidobacterium species palatability and shelf life. Food Sci Nutr. 2022 Mar 21;10(6):1994-2008. doi: 10.1002/fsn3.2815, PMID 35702306, PMCID PMC9179157.
Mantovani FD, De Carla Bassetto M, De Souza CH, Aragon DC, De Santana EH, Pimentel TC. Is there an impact of the dairy matrix on the survival of Lactobacillus casei Lc-1 during shelf life and simulated gastrointestinal conditions? J Sci Food Agric. 2020 Jan 15;100(1):32-7. doi: 10.1002/jsfa.9988, PMID 31414474.
Vorlander K, Kampen I, Finke JH, Kwade A. Along the process chain to probiotic tablets: evaluation of mechanical impacts on microbial viability. Pharmaceutics. 2020 Jan 15;12(1):66. doi: 10.3390/pharmaceutics12010066, PMID 31952192, PMCID PMC7022681.
Jokicevic K, Kiekens S, Byl E, De Boeck I, Cauwenberghs E, Lebeer S. Probiotic nasal spray development by spray drying. Eur J Pharm Biopharm. 2021 Feb;159:211-20. doi: 10.1016/j.ejpb.2020.11.008, PMID 33238191.
Agyei D, Acquah C, Tan KX, Hii HK, Rajendran SR, Udenigwe CC. Prospects in the use of aptamers for characterizing the structure and stability of bioactive proteins and peptides in food. Anal Bioanal Chem. 2018 Jan;410(2):297-306. doi: 10.1007/s00216-017-0599-9, PMID 28884330.
Chamorro F, Carpena M, Fraga Corral M, Echave J, Riaz Rajoka MS, Barba FJ. Valorization of kiwi agricultural waste and industry by-products by recovering bioactive compounds and applications as food additives: a circular economy model. Food Chem. 2022 Feb 15;370:131315. doi: 10.1016/j.foodchem.2021.131315, PMID 34788958.
Lamonaca A, De Angelis E, Monaci L, Pilolli R. Promoting the emerging role of pulse by products as valuable sources of functional compounds and novel food ingredients. Foods. 2025 Jan 28;14(3):424. doi: 10.3390/foods14030424, PMID 39942018, PMCID PMC11816435.
Yang Y, Zhang J, Li C. Delivery of probiotics with cellulose-based films and their food applications. Polymers (Basel). 2024 Mar 13;16(6):794. doi: 10.3390/polym16060794, PMID 38543398, PMCID PMC10976014.
Kistaubayeva A, Abdulzhanova M, Zhantlessova S, Savitskaya I, Karpenyuk T, Goncharova A. The effect of encapsulating a prebiotic-based biopolymer delivery system for enhanced probiotic survival. Polymers (Basel). 2023 Mar 31;15(7):1752. doi: 10.3390/polym15071752, PMID 37050363, PMCID PMC10097185.
Thompson SV, Bailey MA, Taylor AM, Kaczmarek JL, Mysonhimer AR, Edwards CG. Avocado consumption alters gastrointestinal bacteria abundance and microbial metabolite concentrations among adults with overweight or obesity: a randomized controlled trial. J Nutr. 2021 Apr 8;151(4):753-62. doi: 10.1093/jn/nxaa219, PMID 32805028, PMCID PMC8030699.
Yang J, Lei OK, Bhute S, Kris Etherton PM, Lichtenstein AH, Matthan NR. Impact of daily avocado consumption on gut microbiota in adults with abdominal obesity: an ancillary study of HAT, a randomized controlled trial. Food Funct. 2025 Jan 2;16(1):168-80. doi: 10.1039/d4fo03806a, PMID 39641169.
Nazir S, Afzaal M, Saeed F, Ahmad A, Ateeq H, Ikram A. Survivability and behavior of probiotic bacteria encapsulated by internal gelation in non-dairy matrix and in vitro GIT conditions. PLOS One. 2024 Jun 21;19(6):e0303091. doi: 10.1371/journal.pone.0303091, PMID 38905169, PMCID PMC11192393.
Dreher ML, Cheng FW, Ford NA. A comprehensive review of Hass avocado clinical trials observational studies and biological mechanisms. Nutrients. 2021 Dec 7;13(12):4376. doi: 10.3390/nu13124376, PMID 34959933, PMCID PMC8705026.
Leeuwendaal NK, Stanton C, O Toole PW, Beresford TP. Fermented foods health and the gut microbiome. Nutrients. 2022 Apr 6;14(7):1527. doi: 10.3390/nu14071527, PMID 35406140, PMCID PMC9003261.
Wastyk HC, Fragiadakis GK, Perelman D, Dahan D, Merrill BD, Yu FB. Gut-microbiota targeted diets modulate human immune status. Cell. 2021 Aug 5;184(16):4137-4153.e14. doi: 10.1016/j.cell.2021.06.019, PMID 34256014, PMCID PMC9020749.
Bisht V, Das B, Hussain A, Kumar V, Navani NK. Understanding of probiotic origin antimicrobial peptides: a sustainable approach ensuring food safety. NPJ Sci Food. 2024 Sep 19;8(1):67. doi: 10.1038/s41538-024-00304-8, PMID 39300165, PMCID PMC11413171.
Vinderola G, Cotter PD, Freitas M, Gueimonde M, Holscher HD, Ruas Madiedo P. Fermented foods: a perspective on their role in delivering biotics. Front Microbiol. 2023 May 12;14:1196239. doi: 10.3389/fmicb.2023.1196239, PMID 37250040, PMCID PMC10213265.
Bernal Castro C, Espinosa Poveda E, Gutierrez Cortes C, Diaz Moreno C. Vegetable substrates as an alternative for the inclusion of lactic acid bacteria with probiotic potential in food matrices. J Food Sci Technol. 2024 May;61(5):833-46. doi: 10.1007/s13197-023-05779-z, PMID 38487286, PMCID PMC10933215.
Diener HC. Ingwer schutzt nicht vor migrane schmerzmedizin. MMW Fortschr Med. 2020 Apr;162(6):29. doi: 10.1007/s15006-020-0326-x, PMID 32248485.
Yang Y, Zhang J, Li C. Delivery of probiotics with cellulose based films and their food applications. Polymers (Basel). 2024 Mar 13;16(6):794. doi: 10.3390/polym16060794, PMID 38543398, PMCID PMC10976014.
Rahman MN, Barua N, Tin MC, Dharmaratne P, Wong SH, IP M. The use of probiotics and prebiotics in decolonizing pathogenic bacteria from the gut; a systematic review and meta-analysis of clinical outcomes. Gut Microbes. 2024 Jan-Dec;16(1):2356279. doi: 10.1080/19490976.2024.2356279, PMID 38778521, PMCID PMC11123511.
Comerford KB, Ayoob KT, Murray RD, Atkinson SA. The role of avocados in complementary and transitional feeding. Nutrients. 2016 May 21;8(5):316. doi: 10.3390/nu8050316, PMID 27213450, PMCID PMC4882728.
Zuraini NZ, Sekar M, Wu YS, Gan SH, Bonam SR, Mat Rani NN. Promising nutritional fruits against cardiovascular diseases: an overview of experimental evidence and understanding their mechanisms of action. Vasc Health Risk Manag. 2021 Nov 23;17:739-69. doi: 10.2147/VHRM.S328096, PMID 34858028, PMCID PMC8631183.
Liu AG, Ford NA, Hu FB, Zelman KM, Mozaffarian D, Kris Etherton PM. A healthy approach to dietary fats: understanding the science and taking action to reduce consumer confusion. Nutr J. 2017 Aug 30;16(1):53. doi: 10.1186/s12937-017-0271-4, PMID 28854932, PMCID PMC5577766.
Mengistu DA, Mulugeta Y, Mekbib D, Baraki N, Gobena T. Bacteriological quality of locally prepared fresh fruit juice sold in juice houses of Eastern Ethiopia. Environ Health Insights. 2022 Jan 17;16:11786302211072949. doi: 10.1177/11786302211072949, PMID 35095274, PMCID PMC8793386.
Ahmad T, Danish M. A review of avocado waste-derived adsorbents: characterizations adsorption characteristics and surface mechanism. Chemosphere. 2022 Jun;296:134036. doi: 10.1016/j.chemosphere.2022.134036, PMID 35202667.
Sharma S, Mahanty M, Rahaman SG, Mukherjee P, Dutta B, Khan MI. Avocado-derived extracellular vesicles loaded with ginkgetin and berberine prevent inflammation and macrophage foam cell formation. J Cell Mol Med. 2024 Apr;28(7):e18177. doi: 10.1111/jcmm.18177, PMID 38494843, PMCID PMC10945093.
Ahmed N, Kermanshahi B, Ghazani SM, Tait K, Tcheng M, Roma A. Avocado-derived polyols for use as novel co-surfactants in low energy self-emulsifying microemulsions. Sci Rep. 2020 Mar 27;10(1):5566. doi: 10.1038/s41598-020-62334-y, PMID 32221368, PMCID PMC7101315.
Tuncer Caglayan S. Biopolymer-based oral films integrated with probiotic active compounds for improved health applications. Arch Microbiol. 2024 Nov 28;207(1):4. doi: 10.1007/s00203-024-04207-w, PMID 39607528.
Ahmed N, Smith RW, Chen PX, Rogers MA, Spagnuolo PA. Bioaccessibility of avocado polyhydroxylated fatty alcohols. Food Chem. 2025 Jan 15;463(1):140811. doi: 10.1016/j.foodchem.2024.140811, PMID 39255710.
Mahaddalkar T, Suri C, Naik PK, Lopus M. Biochemical characterization and molecular dynamic simulation of β-sitosterol as a tubulin binding anticancer agent. Eur J Pharmacol. 2015 Aug 5;760:154-62. doi: 10.1016/j.ejphar.2015.04.014 , PMID 25912799.
Mohan SB, Kekwick RG. Acetyl-coenzyme a carboxylase from avocado (Persea americana) plastids and spinach (Spinacia oleracea) chloroplasts. Biochem J. 1980 Jun 1;187(3):667-76. doi: 10.1042/bj1870667, PMID 6146308, PMCID PMC1162450.
Leclerc F, Zaccai G, Vergne J, Rihova M, Martel A, Maurel MC. Self-assembly controls self-cleavage of HHR from ASBVd (-): a combined sans and modeling study. Sci Rep. 2016 Jul 26;6:30287. doi: 10.1038/srep30287, PMID 27456224, PMCID PMC4960562.
Wielgosz Grochowska JP, Domanski N, Drywien ME. Efficacy of an irritable bowel syndrome diet in the treatment of small intestinal bacterial overgrowth: a narrative review. Nutrients. 2022 Aug 17;14(16):3382. doi: 10.3390/nu14163382, PMID 36014888, PMCID PMC9412469.
Crudele L, Gadaleta RM, Cariello M, Moschetta A. Gut microbiota in the pathogenesis and therapeutic approaches of diabetes. EBiomedicine. 2023 Nov;97:104821. doi: 10.1016/j.ebiom.2023.104821, PMID 37804567, PMCID PMC10570704.
Roy S, Dhaneshwar S. Role of prebiotics probiotics and synbiotics in management of inflammatory bowel disease: current perspectives. World J Gastroenterol. 2023 Apr 14;29(14):29. doi: 10.3748/wjg.v29.i14.2078.
Dou J, Weathers P. Specialty molecules from plants and in vitro cultures as new drugs: regulatory considerations from flask to patient. Plant Cell Tissue Organ Cult. 2022;149(1-2):105-11. doi: 10.1007/s11240-022-02287-4, PMID 35345535, PMCID PMC8942155.
Finley JW, Finley JW, Ellwood K, Hoadley J. Launching a new food product or dietary supplement in the United States: industrial regulatory and nutritional considerations. Annu Rev Nutr. 2014;34:421-47. doi: 10.1146/annurev-nutr-071813-105817, PMID 24850389.
Sharifi Rad J, Rodrigues CF, Stojanovic Radic Z, Dimitrijevic M, Aleksic A, Neffe Skocinska K. Probiotics: versatile bioactive components in promoting human health. Medicina (Kaunas). 2020 Aug 27;56(9):433. doi: 10.3390/medicina56090433, PMID 32867260, PMCID PMC7560221.
Zhong H, Chan G, Hu Y, Hu H, Ouyang D. A comprehensive map of FDA-approved pharmaceutical products. Pharmaceutics. 2018 Dec 6;10(4):263. doi: 10.3390/pharmaceutics10040263, PMID 30563197, PMCID PMC6321070.
Huang Z, Gustave W, Bai S, Li Y, Li B, Elcin E. Corrigendum to ‘Challenges and opportunities in commercializing whole cell bioreporters in environmental application [Environ. Res. 262 (2024) 119801]. Environ ResEnviron Res. 2025 Feb 1;266:120491. doi: 10.1016/j.envres.2024.120491, PMID 39638728.
Janse M, Brouwers T, Claassen E, Hermans P, Van De Burgwal L. Barriers influencing vaccine development timelines, identification causal analysis and prioritization of key barriers by KOLs in general and COVID-19 vaccine R&D. Front Public Health. 2021 Apr 20;9:612541. doi: 10.3389/fpubh.2021.612541, PMID 33959579, PMCID PMC8096063.
Sarris J, Ravindran A, Yatham LN, Marx W, Rucklidge JJ, McIntyre RS. Clinician guidelines for the treatment of psychiatric disorders with nutraceuticals and phytoceuticals: the world federation of societies of biological psychiatry (WFSBP) and canadian network for mood and anxiety treatments (Canmat) taskforce. World J Biol Psychiatry. 2022 Jul;23(6):424-55. doi: 10.1080/15622975.2021.2013041, PMID 35311615.
Van De Roovaart HJ, Stevens MM, Goodridge AE, Baden KR, Sibbitt BG, Delaney E. Safety and efficacy of vitamin B in cancer treatments: a systematic review. J Oncol Pharm Pract. 2024 Apr;30(3):451-63. doi: 10.1177/10781552231178686, PMID 37231628.
Lim K, Thadhani R. Vitamin D toxicity. J Bras Nefrol. 2020 Apr 3;42(2):238-44. doi: 10.1590/2175-8239-JBN-2019-0192, PMID 32255467, PMCID PMC7427646.
Dini I, Mancusi A. Weight loss supplements. Molecules. 2023 Jul 12;28(14):5357. doi: 10.3390/molecules28145357, PMID 37513229, PMCID PMC10384751.
Jana UK, Kango N, Pletschke B. Hemicellulose-derived oligosaccharides: emerging prebiotics in disease alleviation. Front Nutr. 2021 Jul 27;8:670817. doi: 10.3389/fnut.2021.670817, PMID 34386513, PMCID PMC8353096.
Amillano Cisneros JM, Fuentes Valencia MA, Leyva Morales JB, Davizon YA, Marquez Pacheco H, Valencia Castaneda G. Prebiotics in global and Mexican fish aquaculture: a review. Animals (Basel). 2023 Nov 22;13(23):3607. doi: 10.3390/ani13233607, PMID 38066958, PMCID PMC10705075.
Phung TT, Gerometta M, Chanut J, Raise A, Urena M, Dupont S. Comprehensive approach to the protection and controlled release of extremely oxygen-sensitive probiotics using edible polysaccharide-based coatings. Int J Biol Macromol. 2022 Oct 1;218:706-19. doi: 10.1016/j.ijbiomac.2022.07.129, PMID 35872315.
Moon EC, Kang YR, Chang YH. Development of soy protein isolate/sodium carboxymethyl cellulose synbiotic microgels by double crosslinking with transglutaminase and aluminum chloride for delivery system of Lactobacillus acidophilus. Int J Biol Macromol. 2023 May 15;237:124122. doi: 10.1016/j.ijbiomac.2023.124122, PMID 36963536.
Canga EM, Dudak FC. Improved digestive stability of probiotics encapsulated within poly(vinyl alcohol)/cellulose acetate hybrid fibers. Carbohydr Polym. 2021 Jul 15;264:117990. doi: 10.1016/j.carbpol.2021.117990, PMID 33910728.
Callaghan A, McCombe G, Harrold A, McMeel C, Mills G, Moore Cherry N. The impact of green spaces on mental health in urban settings: a scoping review. J Ment Health. 2021 Apr;30(2):179-93. doi: 10.1080/09638237.2020.1755027, PMID 32310728.
Thornton RL, Glover CM, Cene CW, Glik DC, Henderson JA, Williams DR. Evaluating strategies for reducing health disparities by addressing the social determinants of health. Health Aff (Millwood). 2016 Aug 1;35(8):1416-23. doi: 10.1377/hlthaff.2015.1357, PMID 27503966, PMCID PMC5524193.
Koh GK, Ow Yong JQ, Lee AR, Ong BS, Yau CE, Ho CS. Social media use and its impact on adults mental health and well being: a scoping review. Worldviews Evid-Based Nurs. 2024 Aug;21(4):345-94. doi: 10.1111/wvn.12727, PMID 38736207.
Freeberg KA, Udovich CC, Martens CR, Seals DR, Craighead DH. Dietary supplementation with NAD+-boosting compounds in humans: current knowledge and future directions. J Gerontol A Biol Sci Med Sci. 2023 Dec 1;78(12):2435-48. doi: 10.1093/gerona/glad106, PMID 37068054, PMCID PMC10692436.
Zhang T, Gao G, Kwok LY, Sun Z. Gut microbiome targeted therapies for Alzheimer’s disease. Gut Microbes. 2023 Dec;15(2):2271613. doi: 10.1080/19490976.2023.2271613, PMID 37934614, PMCID PMC10631445.
Tosefsky KN, Zhu J, Wang YN, Lam JS, Cammalleri A, Appel Cresswell S. The role of diet in Parkinson’s disease. J Parkinsons Dis. 2024;14(s1):S21-34. doi: 10.3233/JPD-230264, PMID 38251061, PMCID PMC11380239.
Banning M. A review of clinical decision making: models and current research. J Clin Nurs. 2008 Jan;17(2):187-95. doi: 10.1111/j.1365-2702.2006.01791.x, PMID 17331095.
Gulcin I. Antioxidants and antioxidant methods: an updated overview. Arch Toxicol. 2020 Mar;94(3):651-715. doi: 10.1007/s00204-020-02689-3, PMID 32180036.
Zou D, Zhao Z, Li L, Min Y, Zhang D, Ji A. A comprehensive review of spermidine: safety health effects absorption and metabolism food materials evaluation physical and chemical processing and bioprocessing. Compr Rev Food Sci Food Saf. 2022 May;21(3):2820-42. doi: 10.1111/1541-4337.12963, PMID 35478379.
Nataraj BH, Ali SA, Behare PV, Yadav H. Postbiotics parabiotics: the new horizons in microbial biotherapy and functional foods. Microb Cell Fact. 2020 Aug 20;19(1):168. doi: 10.1186/s12934-020-01426-w, PMID 32819443, PMCID PMC7441679.
Jamali SN, Assadpour E, Jafari SM. Formulation and application of nanoemulsions for nutraceuticals and phytochemicals. Curr Med Chem. 2020;27(18):3079-95. doi: 10.2174/0929867326666190620102820, PMID 31218952.
Saldanha LG, Dwyer JT, Bailen RA, Andrews KW, Betz JW, Chang HF. Characteristics and challenges of dietary supplement databases derived from label information. J Nutr. 2018 Aug 1;148 Suppl 2:1422S-7S. doi: 10.1093/jn/nxy103, PMID 31505680, PMCID PMC6857608.
Terpou A, Papadaki A, Lappa IK, Kachrimanidou V, Bosnea LA, Kopsahelis N. Probiotics in food systems: significance and emerging strategies towards improved viability and delivery of enhanced beneficial value. Nutrients. 2019 Jul 13;11(7):1591. doi: 10.3390/nu11071591, PMID 31337060, PMCID PMC6683253.
Merenstein D, Pot B, Leyer G, Ouwehand AC, Preidis GA, Elkins CA. Emerging issues in probiotic safety: 2023 perspectives. Gut Microbes. 2023 Jan-Dec;15(1):2185034. doi: 10.1080/19490976.2023.2185034, PMID 36919522, PMCID PMC10026873.
Roe AL, Boyte ME, Elkins CA, Goldman VS, Heimbach J, Madden E. Considerations for determining safety of probiotics: a USP perspective. Regul Toxicol Pharmacol. 2022 Dec;136:105266. doi: 10.1016/j.yrtph.2022.105266, PMID 36206977, PMCID PMC10292223.
Blaze J. A comparison of current regulatory frameworks for nutraceuticals in Australia, Canada, Japan, and the United States. Innov Pharm. 2021 Apr 21;12(2):8. doi: 10.24926/iip.v12i2.3694, PMID 34345505, PMCID PMC8326690.
Townsend JR, Kirby TO, Sapp PA, Gonzalez AM, Marshall TM, Esposito R. Nutrient synergy: definition, evidence and future directions. Front Nutr. 2023 Oct 12;10:1279925. doi: 10.3389/fnut.2023.1279925, PMID 37899823, PMCID PMC10600480.
Zhang S, Ma J, Ma Y, Yi J, Wang B, Wang H. Engineering probiotics for diabetes management: advances, challenges and future directions in translational microbiology. Int J Nanomedicine. 2024 Oct 28;19:10917-40. doi: 10.2147/IJN.S492651, PMID 39493275, PMCID PMC11530765.
Zhang L, Hu Y, Jiang L. Advancements in emulsion systems for specialized infant formulas: research process and formulation proposals for optimizing bioavailability of nutraceuticals. Compr Rev Food Sci Food Saf. 2024 Nov;23(6):e70043. doi: 10.1111/1541-4337.70043, PMID 39455164.
Toledo L, Aguirre C. Enzymatic browning in avocado (Persea americana) revisited: history advances and future perspectives. Crit Rev Food Sci Nutr. 2017 Dec 12;57(18):3860-72. doi: 10.1080/10408398.2016.1175416, PMID 27172067.
Shetty GB, KR TR, KS. Preliminary oral probiotics bacterial profile in neonatal and pediatrics and its clinical evaluation. Int J Curr Pharm Sci. 2022 Jul;14(4):5-9. doi: 10.22159/ijcpr.2022v14i4.2005.
Mane RS, Nadaf A. Brief review on thermophilic bacteria and their applications. Int J Curr Pharm Sci. 2022 Jan;14(1):15-9. doi: 10.22159/ijcpr.2022v14i1.44107.
Mohaideen A, Theivandran G. Effects of extracts of Stocheospermum marginatum and Ulva lactuca on the haematological and immunological parameters on Aeromonas hydrophila infected Cyprinus carpio. Int J Curr Pharm Sci. 2022 Jan;14(1):70-6. doi: 10.22159/ijcpr.2022v14i1.44114.
Kumar SH, Gowda DV, HV, Jain V, Catherine AA. Formulation and evaluation of probiotic and prebiotic-loaded pellets by extrusion and spheronization for improved storage viability. Int J App Pharm. 2022 Sep-Oct;14(5):95-104. doi: 10.22159/ijap.2022v14i5.45519.
Sivamaruthi BS, Kesika P, Chaiyasut C. Influence of probiotic supplementation on climacteric symptoms in menopausal women a mini review. Int J App Pharm. 2018 Nov-Dec;10(6):43-6. doi: 10.22159/ijap.2018v10i6.29156.
Konda M, Sampathi S. QbD approach for the development of capsaicin loaded stearic acid grafted chitosan polymeric micelles. Int J App Pharm. 2023 Jul-Aug;15(4):131-42. doi: 10.22159/ijap.2023v15i4.48101.
Sankeshwari S, HV, KM AS, Eliyas A, Thirumaleshwar S, Harsha Vardhan PV. A review on the solid oral dosage form for pediatrics regulatory aspects challenges involved during the formulation and toxicity of the excipients used in pediatric formulation. Int J App Pharm. 2023 May-Jun;15(3):12-27. doi: 10.22159/ijap.2023v15i3.47313.
Grace NS, Monica N, Sarath Chandra M, Veena B. A comparative study to determine the effectiveness and safety of probiotics as an adjunct to antihistamines compared to antihistamines alone in patients with allergic rhinitis. Asian J Pharm Clin Res. 2025 Feb;18(2):74-81. doi: 10.22159/ajpcr.2025v18i2.53569.
Das D, Rath CC, Mohanty N, Panda SH. Probiotic characterization of Bacillus subtilis strain isolated from infant fecal matter revealed by 16S rRNA gene and phylogenetic analysis. Asian J Pharm Clin Res. 2021 Dec;14(12):77-85. doi: 10.22159/ajpcr.2021.v14i12.43204.
Sharma AK, Puri N, Mathur M, Mathur A. A randomized controlled trial to compare the efficacy of Saccharomyces boulardii, Bacillus clausii, and Lactobacillus rhamnosus GG preparation in the treatment of acute diarrhea in children. Asian J Pharm Clin Res. 2022 Apr;15(4):63-7. doi: 10.22159/ajpcr.2022.v15i4.44199.