
Int J App Pharm, Vol 17, Issue 5, 2025, 499-509Original Article
TRANSMUCOSAL BUCCAL PATCHES CARRYING BENIDIPINE HYDROCHLORIDE WITH A UNIDIRECTIONAL RELEASE OPTIMIZED BY CENTRAL COMPOSITE DESIGN
KUMARA SWAMY SAMANTHULA1*, CHANDRASHEKAR THALLURI1, SATYA OBBALAREDDY2, DANIEL KOTHAPALLY3, SRIKANTH PAREPALLI4
1Department of Pharmaceutics, Faculty of Pharmaceutical Science, Assam Down Town University, Guwahati-781026, Assam, India. 2Department of Health System Management Studies, JSS Academy of Higher Education and Research, Mysuru-570004, India. 3Department of Pharmaceutics, Chaitanya Deemed to be University, Hanamkonda, Warangal, Telangana-506001, India. 4Vaagdevi Pharmacy College, Bollikunta, Warangal-506005, Telangana, India
*Corresponding author: Kumara Swamy Samanthula; *Email: kumar4koty@gmail.com
Received: 23 Mar 2025, Revised and Accepted: 10 Jun 2025
ABSTRACT
Objective: This investigation aimed to develop buccal patches of benidipine hydrochloride (BH) that provide unidirectional release and adhere well to the mucous membranes.
Methods: The buccal patches of BH with a unidirectional drug release were made utilizing the solvent casting process using hydroxy propyl methyl cellulose, eudragit RL 100, and polyvinylpyrrolidone. The patches' water-resistant backing layer allowed for the controlled delivery of medication in one direction. Thickness, mass, surface pH, folding endurance, moisture absorption, in vitro release, ex-vivo permeation release, and accelerated stability experiments were all used to assess them.
Results: Based on the optimized formulation, the moisture absorption percentage is 40.50±1.39%, whereas the range for the original formulation was 32.18±1.69% to 44.95±1.52%. According to the in vitro drug release assays, formulation F9 was able to release 99.86±1.39% of the medication until 6 h. The study found progressive and constant drug penetration, with 69.53±1.86 compared to 54.88±1.39% in 6 h from the improved formulation.
Conclusion: A possible alternative drug delivery strategy for the systemic distribution of BH was suggested by the manufactured unidirectional buccal patches of BH, which released the maximal amount of medication during the prescribed mucoadhesion duration.
Keywords: Benidipine hydrochloride, Buccal patches, Ex-vivo mucoadhesion, Swine buccal mucosa
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijap.2025v17i5.54308 Journal homepage: https://innovareacademics.in/journals/index.php/ijap
INTRODUCTION
The oral cavity is conveniently accessible for self-medication and is widely accepted by patients. Over the past three decades, there has been a significant focus on the study of buccal drug delivery systems. Administering drugs through the buccal route provides unique benefits compared to swallowing for systemic effects. When administered orally via the buccal route, patients are more inclined to adhere to their medication regimen. Bypassing first-pass metabolism improves medication absorption. Rapid absorption is due to abundant blood capillaries and high blood flow rates. The medication is unaffected by stomach acid even in this hostile environment. Because of the increased blood flow on the mucosal surface, the action starts quickly. A prolonged residency time boosts absorption, which in turn enhances the therapeutic efficiency of the medicine. Because of its unique properties, the mucosal surface increases the duration of time that a dosage form spends at the absorption site [1, 2].
Research shows that stress and unhealthy behaviours lower the immune system, which in turn increases the risk of cardiovascular disease, infections, and malignancies. When it comes to managing high blood pressure, there are several types of medications recommended, such as beta-blockers, calcium channel blockers, angiotensin receptor blockers, ACE inhibitors, renin-antagonists, diuretics, alpha-adrenergic agonists, and other central-acting drugs [3]. Several medications for high blood pressure cannot be effectively given through the usual method of swallowing, considering our current knowledge of how the body absorbs and metabolism them.
Due to the significant presystemic hepatic clearance of many drugs after being taken orally, there is often a lack of correlation between membrane permeability, absorption, and bioavailability [4]. Most anti-hypertensive medications undergo significant first-pass metabolism, exhibit low water solubility, have short half-lives, and offer limited oral bioavailability. By using the transmucosal method of drug administration, first-pass metabolism is avoided, allowing for direct access to the systemic circulation via the internal jugular vein, which in turn enhances bioavailability. One possible method for administering hypertension medications over an extended period of time is by trans-buccal delivery systems [5, 6].
Benidipine hydrochloride (BH) is a synthetic calcium channel antagonist that belongs to the 1,4-dihydropyridine derivative group. It is similar to other compounds like nifedipine and nicardipine and is commonly used to manage hypertension and angina pectoris. BH demonstrates a gradual onset and prolonged duration of action. From a clinical perspective, this treatment is commonly utilized due to its long-lasting effects when taken orally just once daily. The drug's therapeutic effect gradually emerges and lasts for an extended period, aiding in enhancing patient adherence [7, 8]. However, due to its significant hepatic first-pass metabolism and high lipophilicity, this drug falls under Biopharmaceutical Classification System (BCS) class II and has low oral bioavailability. Enhancements in BH bioavailability have been attempted through various methods, including the use of solid dispersions and nanosuspension, but unfortunately, their impact has been limited. Fortunately, the buccal route of administration of Benidipine hydrochloride does not seem to circumvent its first-pass metabolism in any published studies. This study aimed to develop a variety of mucoadhesive buccal patches formulated with BH by combining hydrophilic and lipophilic polymers [9, 10].
MATERIALS AND METHODS
Materials
Benidipine hydrochloride was sourced from Ranbaxy Laboratories Bangalore. Hydroxy propyl methyl cellulose (HPMC E15) Eudragit RL 100 and Polyvinylpyrrolidone (PVP K30) were obtained from Loba Chemicals, Mumbai. The rest of the ingredients were purchased from commercial enterprises and were of analytical quality.
Experiment design (response surface methodology/Central composite design)
Design Expert® (Version 10.0.1, State-Ease Inc., India) was used for the design of the experiments and formulation optimization of the manufacturing of buccal patches designed for controlled release distribution. Response surface technique, in particular the three-level, two-factor Central composite design, was employed. The Central composite design determined the best design for assessing the quadratic response surface and polynomial model based on the formulation variables and prior research. This allowed for process optimization with the fewest feasible runs, which were 11 runs total, including 3 duplicated center points. The following is a three-factor, three-level model quadratic equation that was developed by a computer and describes a non-linear polynomial model.
Y = b0+b1X1+b2X2+b12X1X2+b11X12+b22X22 --------------- (1)
The observed responses associated with specific components at different levels are represented by the variable Y in the given polynomial equations. These reactions can be the result of a confluence of variables operating at different levels. X1, X2, and X3 are the references to the study's independent variables. Regression coefficients are denoted by b1, b2, and b12, whereas b0 represents the intercept of the polynomial equation.
The primary impacts of the separate components and their interconnections at each level are also reflected by the names X1X2, X1X3, and X2X3. Conversely, X12 and X22 denote the independent variables' quadratic models. The analysis of the research is affected by curvature due to these quadratic models [11].
Preparation of mucoadhesive buccal patches of BH
After carefully measuring the polymers, it was dissolved in distilled water using the magnetic stirring method for a period of 4-5 h. Following that, BH was added to the polymeric solution while stirring it constantly. The necessary amount of propylene glycol was added and left to stand for 1-2 h at room temperature. The drug-containing polymeric solution from each formulation was cautiously transferred to a Petri plate made of glass. A level surface was used to position the Petri dishes, and an inverted funnel was put on top to cover them. This allowed the solvent to evaporate slowly and uniformly at ambient temperature, yielding a flexible patch (table 1). To make the secondary backing polymeric layer, which served as a support for the unidirectional release of BH, 1.5–2.5 milliliters of propylene glycol and ethyl cellulose were dissolved in 20 milliliters of dichloromethane and ethanol (DCM: ETH) solvent mixture. This mixture was then added to the primary layer on a Petri dish and left to dry at room temperature. The dried patches were skillfully taken off, meticulously inspected for any flaws or air bubbles, and then meticulously cut into small patches.
Table 1: Composition of 32 factorial design formulations of BH mucoadhesive buccal patches
| Ingredients (mg) | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | F10 | F11 |
| Benidipine HCl | 76.88 | 76.88 | 76.88 | 76.88 | 76.88 | 76.88 | 76.88 | 76.88 | 76.88 | 76.88 | 76.88 |
| HPMC E15 | 1500 | 3000 | 2250 | 1500 | 2250 | 1500 | 2250 | 2250 | 3000 | 2250 | 3000 |
| Eudragit RL100 | 1500 | 2000 | 1500 | 2000 | 2000 | 1000 | 1500 | 1000 | 1000 | 1500 | 1500 |
| PVP K 30 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Propylene glycol (ml) | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| DCM: ETH (1:1) | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 |
| Ethyl Cellulose | 200 | 200 | 200 | 200 | 200 | 200 | 200 | 200 | 200 | 200 | 200 |
| Propylene glycol (ml) | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
Evaluation of buccal patches
Fourier transform infrared (FT-IR) study
The KBr disk approach was used in order to record the FTIR (Bruker-210329) spectra of the drug, polymers, and formulations. FTIR analysis was performed on four different drug mixtures: pure drug BH, and a physical combination of optimized formulation. A KBr disk was created by compressing a mixture of approximately two to three milligrams of sample with dried potassium bromide of the same weight. The spectra that were acquired by scanning the disk were reported across the range of 4000-400/cm [12, 13].
Thickness uniformity test
Flexible patches can deliver medications to mucosal membranes. They also provide a determined medicinal dosage, unlike lotions and ointments. From each batch of buccal patches, three patches were measured individually using a Vernier caliper, and the average thickness was computed [14, 15].
Weight uniformity test
This is an essential quality control test to ensure that the patch's weight is consistent to prevent under-or overdose. There ought to be a consistent weight in each batch. The analytical weighing device was used to weigh each batch of buccal patches separately, and then the mean weights were computed [16].
Surface pH of patch
In order to explore the potential for any in vivo adverse effects, the buccal patches' surface pH was measured. It aimed for a neutral surface pH since acids and bases might irritate the mucous membrane in the mouth. This was accomplished with the use of a composite glass electrode. One milliliter of pH 6.8 phosphate buffer was spread on the patch for two hours at room temperature to cause it to swell. After bringing the electrode to the surface of the patch and allowing it to equilibrate for one minute, the pH was determined in triplicate [17].
Folding endurance
The folding durability was measured by selecting three buccal patches at random from each batch. Folding the patches in the same spot over and over again eventually caused them to break. Buccal patches that could be manually folded up to 100 times were deemed to have adequate qualities. The value of the folding endurance was determined by the number of times the patches could be folded in the same spot without breaking.
Moisture absorption studies
Research on how well different polymers absorb water gives us an estimate of how adequately the formulation holds its shape after water has been absorbed. This research employed an agar plate model to simulate buccal mucosal secretion. Three buccal patches were weighed independently (W1) and then placed in 5% agar gel plates, core side up, for each formulation. The patches were then incubated at 37±1 ºC. Patches were taken from Petri dishes every 1 hour until 8 h, and excess surface water was gently blotted using blotting paper [18]. The enlarged patch was reweighed (W2) and the moisture absorption % was estimated in triplicate using the formula:
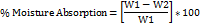
Where, W1 = initial weight of the patch
W2 = final weight of the enlarged patch
Content uniformity test
The BH content of the prepared buccal patches was assessed in every instance. The BH content was tested using three patches, each with an area of 2x2 cm2, from each formulation. A triple of each formulation was performed, and the BH content was determined by taking a single patch from each.
In vitro drug release studies
By employing a United States Pharmacopeia (USP) Type 5 rotary paddle over the disc dissolution test device, the BH release inquiry from the buccal patches was conducted. The dissolving media consisted of 500 ml of fluid (pH 6.8) at 37 °C±0.5 °C, agitating at a speed of 50 rpm. A cyanoacrylate adhesive backing layer attached a 2x2 cm2patch to a glass disc. To keep the patch on top of the glass slide disc, it was positioned at the very center of the dissolution container. 5 milliliters of the sample were taken away and replaced with the same amount of dissolution media at regular intervals of one hour. The samples were prepared by passing them through a 0.45 mm filter, diluting them with a new solution at a pH of 6.8, and then measuring their absorbance at 239 nm using spectrophotometry. The experiment was carried out three times with the goal of obtaining representative averages [19].
Ex-vivo residence time
The buccal mucosa of swine was swiftly transferred to the lab in a cooled normal saline solution after collection from the nearby slaughterhouse. Separating the buccal mucosa from the surrounding fat and tissues was done with great care using a tiny, sharp blade. The ex-vivo mucoadhesion duration investigations were conducted after applying a patch to newly separated swine buccal mucosa using a locally adapted USP disintegration device. A glass slab was affixed vertically to the equipment, and the buccal mucosa of swine was bonded to its surface. After 0.5 ml of a pH 6.8 phosphate buffer was applied to one surface of the buccal patch, it was allowed to come into touch with the mucosal membrane by gently pressing down with a fingertip for 20 seconds and assessed in triplicate [20].
Ex-vivo drug permeation study
The optimized formulation underwent an ex-vivo buccal drug permeation testing at 37 °C±0.5 °C using a Franz diffusion cell. Transferring the newly excised buccal mucosa to the donor and receptor compartments was the next step. 20 to 30 milliliters of phosphate buffer solution with a pH of 6.8 was added to the receiver chamber. One half an hour was given to the buccal mucosa to settle. To find out the quantity of BH permeated into the swine buccal mucosa, we took 2 ml aliquots from the receptor compartment and promptly replaced them with the identical volume of solution. The experiment was carried in triplicate [21, 22].
Stability studies
The optimized formulation underwent buccal patch stability testing in accordance with International Council for Harmonization (ICH) recommendations. Once the optimized formulations were chosen, stability experiments were conducted at accelerated circumstances of 40±2 °C and 75±5% relative humidity (RH). The formulations were then stored in desiccators and placed in an amber-colored screw-cap container, where they remained for three months. Periodically, the formulations were examined for their outward appearance, ability to absorb moisture, and in vitro drug release. The experiment was carried in triplicate [23].
RESULTS AND DISCUSSION
FTIR characterization
The incompatibility of the drug and polymers used to formulate buccal patches was investigated using FTIR analysis. BH is a dihydro-pyridine group derivative showing characteristic peaks at the wavelength regions 3016.53 (aromatic C-H stretch), 3342.12 (NH stretch), 2924.91 (CH3, CH2 stretch), 1620.16 (C=O stretch), 1523.24 (NO2 stretch), and 1487.37 (C=C stretch) cm‐1 are seen in the infrared spectra of pure drug, respectively. The fact that the BH peak did not vanish or move in the drug and excipients spectra shown in fig. 1 indicates that the two substances were compatible. The fact that all of the drug peaks are present in the optimal form and no additional new peaks have been seen indicates that there is no interaction between the drug and carrier material.
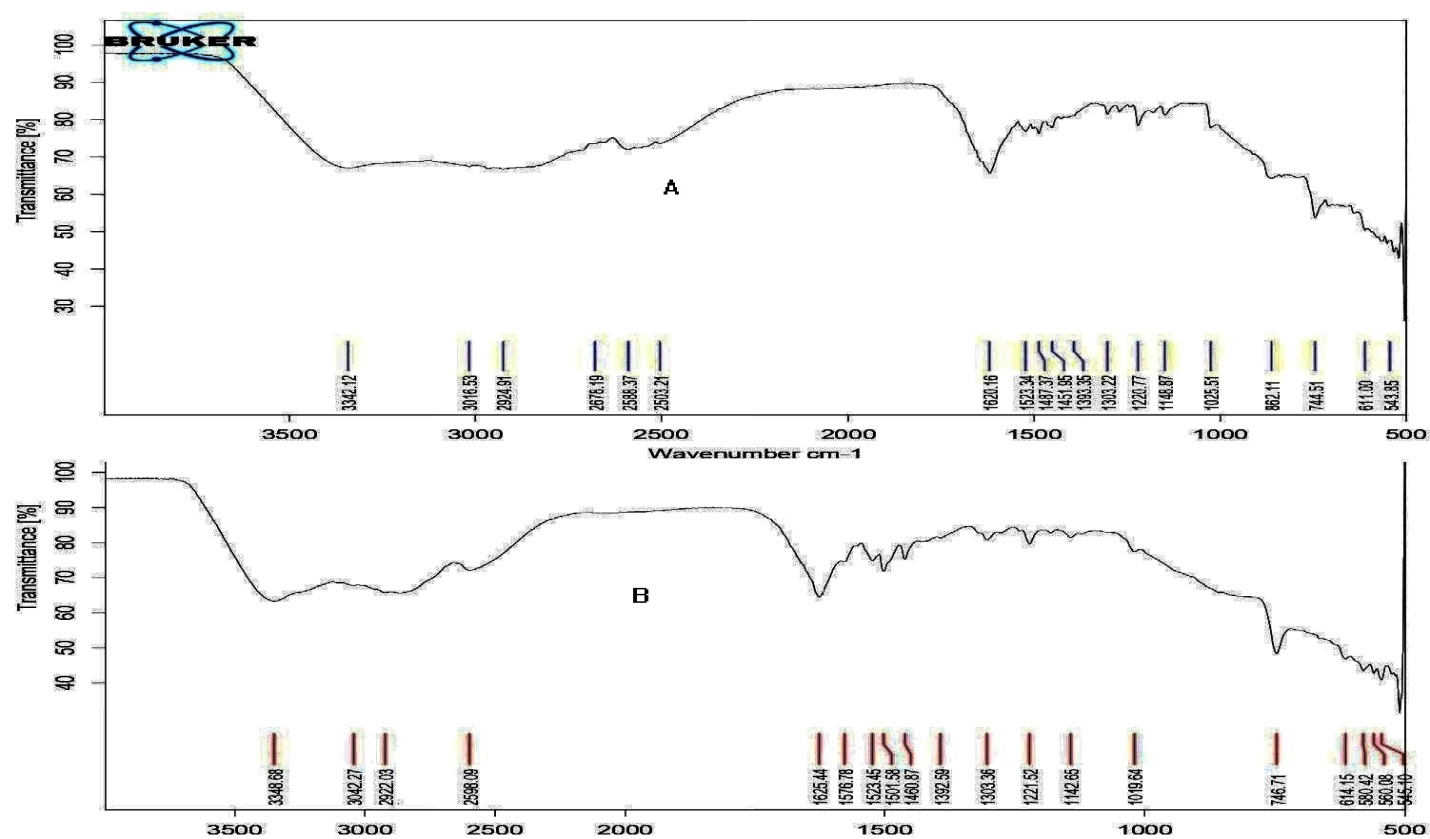
Fig. 1: FTIR spectrum of A) pure drug BH, B) Optimized formulation contains BH, HPMC E15 and Eudragit RL 100
Thickness uniformity test
After reviewing our prior work published [24], we decided to use a response surface methodology experiment to further optimise the batch formulation of HPMC E15 and Eudragit RL 100 in terms of weight uniformity, thickness, surface pH, and content uniformity. The goal was to develop drug-loaded patches that could be released into the buccal environment.
There was a clear correlation between the weight and thickness of the patches, and the thickness rose as the polymer concentration increased as well. The patches' thickness was found to be uniform, according to the results of the thickness variation test, which showed standard deviation (SD) values ranging from 0.32±0.016 to 0.39±0.013 mm (table 2).
Weight uniformity test
Tests were conducted to ensure that the weight of drug-loaded patches (2x2 cm2), and the results of the test showed (table 2) that the weight of the patches was uniform, as shown by the standard deviation values. It was observed that the weight of the patches somewhat increased with higher polymer concentration, ranging from 125.12±1.19 to 131.13±1.33 mg.
Surface pH of patch
All of the formulations' developed mucoadhesive buccal patches had surface pH values close to or equal to neutral. As a consequence, there should have been no signs of mucosal irritation, and the patient was able to comply with the treatment; the outcomes are detailed in table 2.
Content uniformity test
The medicine was evenly distributed throughout the polymer matrix, and each formulation had a respectable quantity of drug. All of the formulations had drug contents that ranged from 98.33±1.36 to 101.63±1.63. Table 2 displays all of the data, and the assay values were all within the boundaries.
Folding endurance
Throughout the process of being folded over 100 times, the material showed no signs of cracking, indicating an endurance range of 86±1.36 to 98±1.43. These designed buccal patches were guaranteed to be flexible by the matter of folding endurance research. The values were determined to be within the specified limits and demonstrated satisfactory film characteristics. The results are all in table 2, and there were no outliers among the assay values.
Ex-vivo residence time
Selected patches had an ex-vivo mucoadhesion residence period ranging from 5.33±1.36 to>6±0.09 h. Over the course of the research, not a single patch came loose from its mucosal membrane attachment, suggesting that the time allotted was enough for patch retention (table 2). These studies, which ensured consistency in production and performance, proved that the formulations were suitable for buccal delivery by measuring folding endurance and ex-vivo mucoadhesion residence time [25].
Table 2: Evaluation parameters for factorial design formulations of mucoadhesive buccal patches
| Formulation codes | Thickness uniformity* | Weight uniformity* | Surface pH* | Drug content* | Folding endurance* | Ex-vivo residence time* |
| F1 | 0.33±0.025 | 125.43±1.16 | 6.6±0.12 | 99.58±1.25 | 90±1.23 | 5.55±1.85 |
| F2 | 0.38±0.018 | 131.13±1.33 | 6.6±0.09 | 98.66±1.63 | 98±1.43 | >6±0.09 |
| F3 | 0.35±0.015 | 126.26±1.42 | 6.7±0.14 | 99.36±2.15 | 92±1.55 | 5.37±1.66 |
| F4 | 0.35±0.013 | 128.51±1.65 | 6.5±0.18 | 98.33±1.36 | 91±1.63 | 5.86±1.93 |
| F5 | 0.36±0.018 | 127.66±1.66 | 6.6±0.19 | 101.63±1.63 | 96±1.66 | >6±0.05 |
| F6 | 0.33±0.013 | 126.25±1.27 | 6.5±0.15 | 98.33±2.21 | 86±1.36 | 5.66±1.33 |
| F7 | 0.32±0.016 | 125.33±1.83 | 6.6±0.14 | 100.15±1.87 | 92±1.15 | 5.33±1.36 |
| F8 | 0.34±0.013 | 125.12±1.19 | 6.7±0.13 | 99.26±1.49 | 90±1.85 | 5.75±1.18 |
| F9 | 0.36±0.015 | 126.14±1.65 | 6.6±0.13 | 99.39±1.36 | 95±1.36 | 5.93±1.15 |
| F10 | 0.32±0.018 | 126.27±1.33 | 6.5±0.07 | 101.48±2.33 | 92±1.41 | 5.33±1.43 |
| F11 | 0.39±0.013 | 128.63±1.66 | 6.6±0.15 | 98.96±1.33 | 97±1.28 | >6±0.08 |
*Experiment was performed in triplicates (n=3) and each value is expressed as mean±SD.
Moisture absorption studies
In order to determine how well the formulation held up when exposed to moisture, study investigators conducted absorption experiments. Fig. 2 displays the results of the research conducted on moisture absorption. The range of moisture percentages absorbed by different formulations using HPMC E15 and eudragit RL100 varied from around 32.18±1.69% to 44.95±1.52% and the optimized formulation shows 40.50±1.39%. According to Govindasamy et al. [26], the swelling behaviour of a material could suggest numerous facts regards its relative water intake capacity of polymers and to what extent the formulation retains its integrity after it absorbs water. The developed buccal patches exhibited swelling and erosion when positioned on the agar gel plate, suggesting that the drug discharge mechanism begins with the polymer swelling and continues with the diffusion of the drug from the swelled matrix. This suggests that these formulations' ability to absorb moisture is enhanced when the concentration of eudragit RL100 polymer increases.
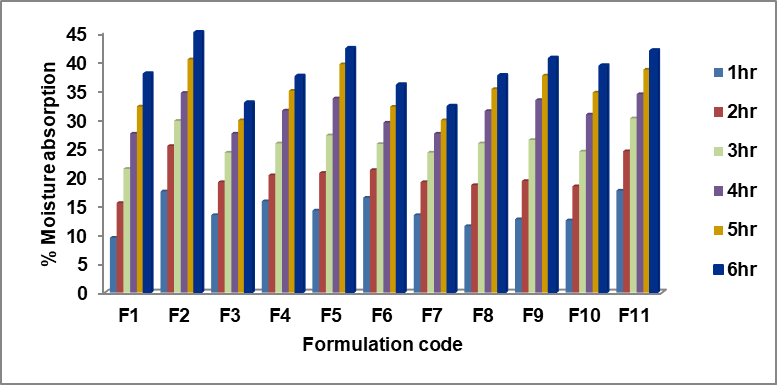
Fig. 2: % Moisture absorption of formulations F1 – F11 (n=3)
In vitro drug release studies
By combining the rate-controlling polymer eudragit RL 100 with the HPMC E15, in vitro release testing illustrated that the resulting patches exhibited an acceptable release rate. Formulations F1 and F6 had cumulative release percentages of 100.23±1.85 and 99.85±2.38 percent, respectively, after the 4 h in the low-level batch of both polymer combinations. However, the buccal patch matrix could not maintain its integrity, leading to the burst action and the subsequent release of the medication. It is evident that the formulations in F1 and F6 do not have enough potency to controllably slow down the drug's release over 6 h.
Using the intermediate-level batches of both polymer combinations, formulations F3, F4, F7, F8, F9, and F10 exhibited drug release rates of 99.98±2.63%, 100.25±2.45%, 99.25±2.19%, 100.86±2.33%, 99.86±1.39%, and 100.33±2.78% at the end of 6 h, respectively. This proves that each of these formulations is enough to slow the drug's action for 6 h. After careful consideration, the optimal formulation was determined to be F9, which highlights a controlled release over a 6-hour duration (fig. 3). It might be associated with the fact that diffusion is enabled in the HPMC polymer due to its enlargement. Haju and Samnthula et al. [27, 28] brought attention to the fact that the release pattern improved with increasing concentrations of gelling HPMC polymer and decreasing concentrations of eudragit RL 100 polymer, suggesting that the swellable HPMC polymer played a crucial role in BH release from the buccal patch.
Formulations F2, F5, and F11 with the high-level batches of both polymer combinations proved drug dissolution rates of 86.95±2.75%, 93.37±2.66%, and 90.66±2.31% after 6h, respectively. The transmucosal buccal patches are made of the positively charged polymer eudragit RL-100, which increases the drug's bioavailability by promoting contact between the patch and the mucous layer. A delay in medication release occurred because the patches' water-swollen gel-like form, caused by an increase in polymer concentration, significantly reduced the dissolving medium's ability to penetrate the patches.
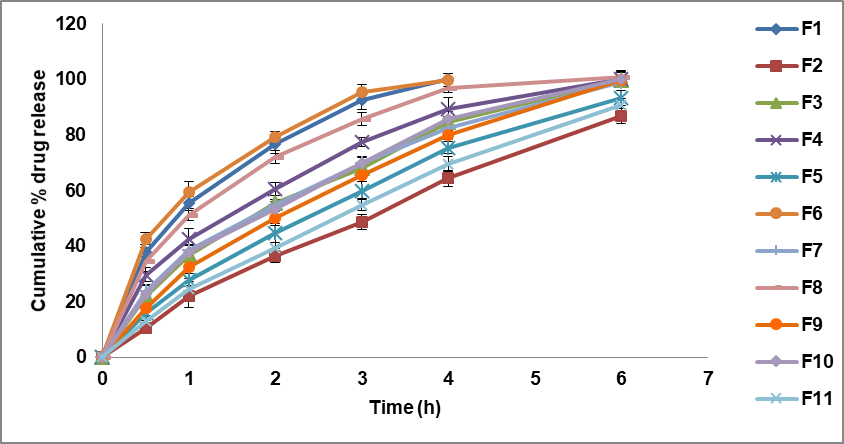
Fig. 3: In vitro dissolution profile of BH buccal patches (Data presented as mean±SD, n=3)
Ex-vivo drug permeation study
The chosen optimized formulation F9 was then subjected to ex-vivo permeation experiments via the buccal mucosa of swine, alongside the formulation F9 with polyethylene glycol 6000 grade as an enhancer, and a control group that did not receive an enhancer. The findings revealed that the drug penetration was gradual and consistent, and the test drug diffusion was 69.53±1.86 compared to 54.88±1.39% in 6 h from the optimized formulation, as shown in fig. 4. Adhikari et al. [29] found that all of the patches that were tested may have a connection to the fact that the drug penetration process varies among porcine buccal mucosa, as shown by their characteristics.
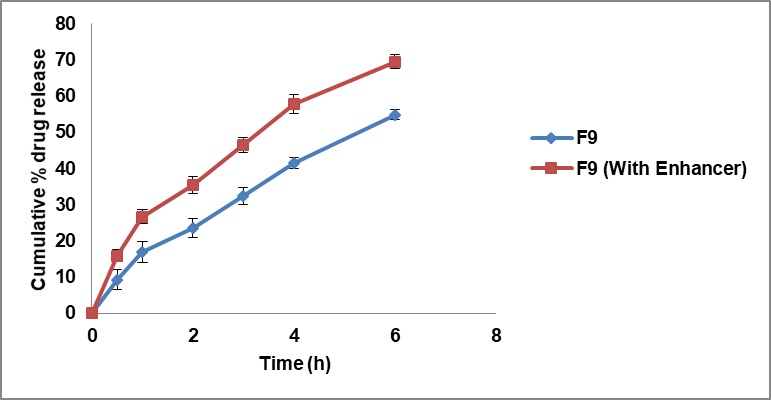
Fig. 4: Ex-vivo permeation profiles of BH buccal patches (Data presented as mean±SD, n=3)
Stability studies
Based on the results of the accelerated stability testing, the drug content of the BH buccal patches was unique from 99.25±1.24 to 99.74±1.86 percent. Probably between 53.85±1.48 and 55.63±1.66 percent of the moisture was absorbed by the patches. Tested patches maintained consistent drug content, water absorption, and in vitro drug release during and after the accelerated stability testing (table 3). According to El Sharawy et al. [30], suggests that when stored correctly, the buccal patches, as prepared, will not undergo any degradation.
Table 3: Stability studies of BH mucoadhesive buccal patches
| Formulation code | No. of months | Drug content* | Moisture absorption* | In vitro drug release* |
| F9 | 1 | 99.74±1.86 | 55.63±1.66 | 99.33±1.23 |
| 3 | 99.25±1.24 | 53.85±1.48 | 98.96±2.15 | |
| 6 | 99.66±1.33 | 54.16±1.17 | 99.18±1.81 |
*Data presented as mean±SD, n=3.
Application of factorial design for optimization of buccal patches
Time requires for 50 percentage drug release (t50) Y1
The "T50 percentage drug release" refers to the time it takes for 50% of the drug to be released from fabricated buccal trans mucosal dosage form. This parameter is often used to characterize the release profile of a drug from a pharmaceutical formulation, particularly for extended-release formulations.
The time requires for 50 percentage drug release (t50) of all formulations of buccal patches release (F1-F11) were carefully recorded, and all values range of 0.663±0.17 to 2.671±1.37 h. Based on the observations, it became evident that the chosen amount of both polymers (combination of HPMC E15 with eudragit RL 100) had a reliable impact on the t50 percentage drug release, denoted as Y1. To analyze this relationship, a linear regression analysis was employed. The mathematical representation of the linear regression coefficient equation is provided below.
t50 percentage drug release (Y1) =+28.14-1.35 X1-1.39 X2-9.27X12+1.381X12+1.575X22
The analysis of variance (ANOVA) analysis yielded a regression coefficient value (R2) of 0.965, indicating a robust goodness of fit. The calculated p-value of 0.028 is below the typical threshold of p<0.05, signaling the significant influence of the current model on t50 percentage drug release through the utilization of polymer thickeners.
Examining the polynomial equation, both X1 (HPMC E15) and X2 (Eudragit RL 100) exhibited closely aligned coefficient values of 1.35 and 1.39, respectively. Notably, when considered individually, X1 and X2 demonstrated negative coefficient signs, impacting t50 percentage drug release. However, their combined effect, denoted as X12, displayed a significant impact on the same parameter, with a value approximately nine times greater than when considered alone. This suggests that elevating the concentration of both polymer thickeners results in a decreased time required for drug release percentage.
In the context of buccal patches, HPMC E15 predominantly acts as a matrix-forming agent. It facilitates the creation of a cohesive matrix that houses the active pharmaceutical ingredient (BH), while also offering controlled release properties. Its viscosity and swelling characteristics can be adjusted to regulate the rate of drug release from the patch, enabling sustained or controlled release of the drug over time. Eudragit RL 100, an acrylic polymer, is recognized for its film-forming attributes. Additionally, it contributes to controlled drug release. By adjusting the thickness of the eudragit RL 100 layer or incorporating it within the matrix, the release kinetics of the drug from the patch can be further managed. The results were depicted in fig. 5 and 6.
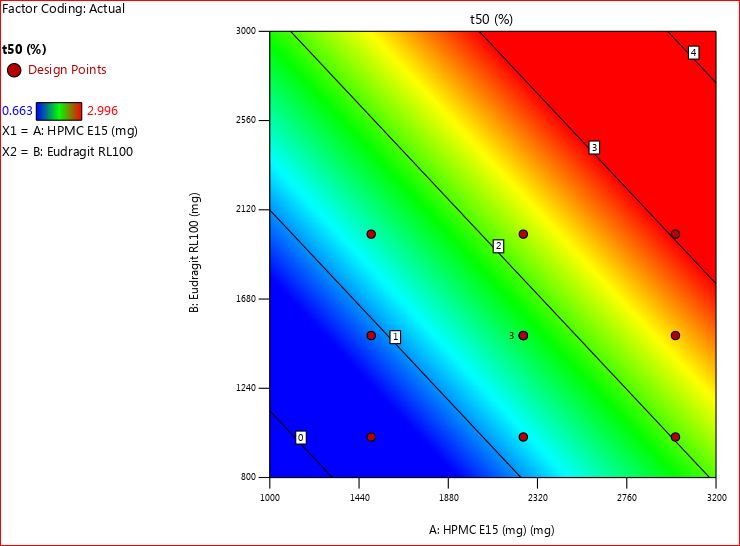
Fig. 5: 2D contour plot for t50 percentage drug release (Y1)
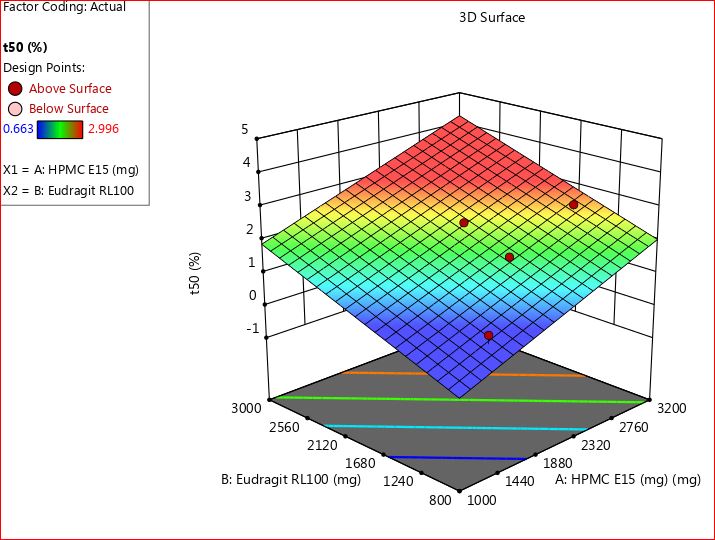
Fig. 6: 3D RSM plot for t50 percentage drug release (Y1)
Time requires for 90 percentage drug release (t90) Y2
Determining the t90 percentage is vital as it signifies the duration required for 90% of the drug to be released. This metric holds particular importance for medications with narrow therapeutic windows or those where achieving precise plasma concentrations is crucial for efficacy and safety. Moreover, it is essential for controlled-release formulations as it guarantees the sustained release of the drug over the intended timeframe, ensuring therapeutic levels within the body are maintained for extended periods.
The time required for 90% drug release (t90) across all formulations of trans mucosal buccal patches (F1-F11) was meticulously documented, with recorded values ranging from 2.981±0.17 to 6.232±1.37 h. Through careful observation, it became apparent that the selected dosage of both polymers (the combination of HPMC E15 with eudragit RL 100) significantly influenced the t90 percentage drug release, represented as Y2.
The relationship was analyzed using linear regression, and the mathematical representation of the equation for the regression coefficient is provided below.
t90 percentage drug release (Y2) =+25.38 – 4.28 X1 – 5.17 X2-10.08 X12+2.17X12+3.74 X22
The ANOVA data showed a regression coefficient value (R2) of 0.9627, indicating a robust goodness of fit, suggesting a strong relationship. The calculated p-value of 0.032 is below the conventional threshold of p<0.05, signifying statistical significance. This indicates that the model significantly impacted the t90 percentage drug release by utilizing the selective biodegradable polymer.
Analyzing the polynomial equation, both X1 (HPMC E15) and X2 (Eudragit RL 100) displayed closely aligned coefficient values of 4.28 and 5.17, respectively. Interestingly, when examined separately, X1 and X2 exhibited negative coefficient signs, affecting t90 percentage drug release. However, their joint effect, represented as X12, demonstrated a substantial impact on the same parameter, approximately two times greater than their individual effects. This implies that increasing the concentration of both polymer thickeners leads to a reduction in the time required for drug release percentage.
In buccal patches, HPMC E15 mainly acts as a substance that forms a sturdy structure where the drug is held. It helps in making a strong matrix that contains the active ingredient (BH) and allows the drug to be released in a controlled manner. Its thickness and how much it swells can be changed to control how fast the drug is released from the patch, giving a steady release over time.
Eudragit RL 100, on the other hand, is a type of acrylic polymer that forms a thin layer on the patch. It also helps in controlling how the drug is released. By adjusting how thick this layer is or by adding it to the matrix, we can further control how quickly the drug is released from the patch. Fig. 7 and 8 presented the findings. Shiledar and Kumria et al. [31, 32] made similar observations; they discovered that the initial drug release was concentration dependent and that drug release reduced as the polymer amount increased.
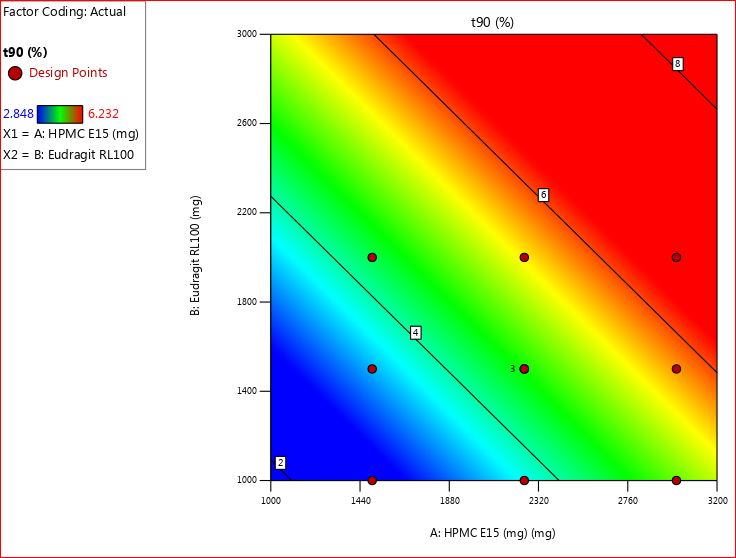
Fig. 7: 2D contour plot for t90 percentage drug release (Y2)
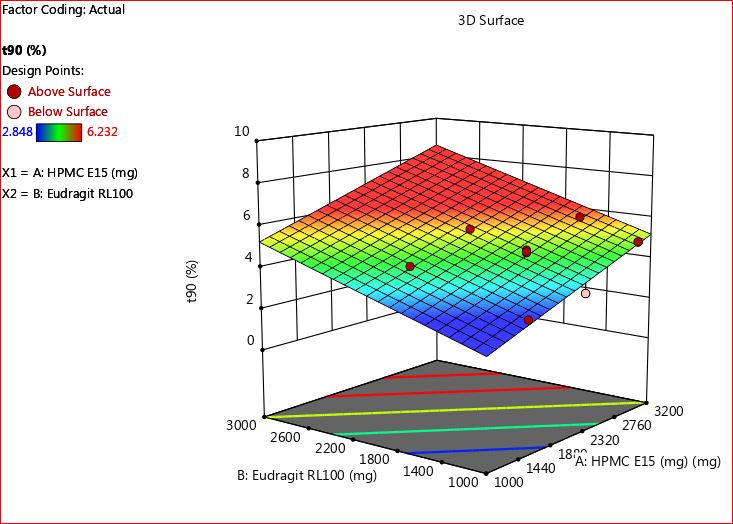
Fig. 8: 3D RSM plot for t90 percentage drug release (Y2)
Selection of optimized batch as function of desirability of all response variables
To choose the best and most optimized formulation, we relied on the desirability response, a vital function within the Design Expert software. This method was pivotal in pinpointing the most dependable formulation. By utilizing the desirability graph in the software, we merged different responses from dependent variables, simplifying the process of crafting an ideal formulation with the desired physicochemical properties.
For optimization purposes, the desirability scale ranged from 0 to 1. It consolidated the values of individual response variables and was utilized to assess each formulation from F1 to F11. A desirability value close to zero signaled unfavorable and unacceptable conditions for the responses, making a formulation undesirable. Conversely, as the value approached one, the formulation became highly preferable and desirable.
The desirability of all formulations was compared graphically using software in light of the results. This approach provided potential solutions for optimizing the best desirable and controlled release batch within the specified time. The information was illustrated through desirability and overlay plots, showcased in fig. 9 and 10. Notably, from fig. 9, it was evident that F9 stood out as the most desirable formulation, highlighted by a blue circle due to its highest desirability value of 1.00.
To analyze the release profiles of the fabricated matrix-extended formulations from batches F1 to F11, we utilized the DD solver model software. This software proved to be a powerful tool in decoding the complex release patterns. Through the application of various mathematical models, the study yielded insightful results, as depicted in fig. 11 to 14.
In the analysis using the zero-order model, the F9 formulation showed a substantial correlation with an R2 value of 0.9101, indicating a strong fit with the observed data. The Akaike Information Criterion (AIC) value was calculated as 47.58, while the Model Selection Criterion (MSC) value reached 1.72.
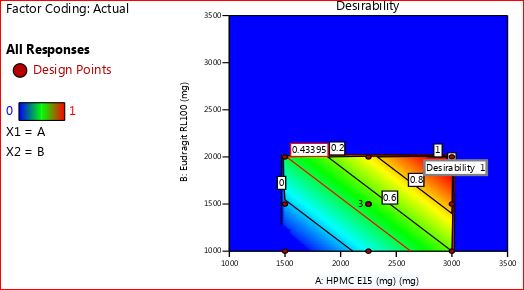
Fig. 9: Desirability plot for all response variables
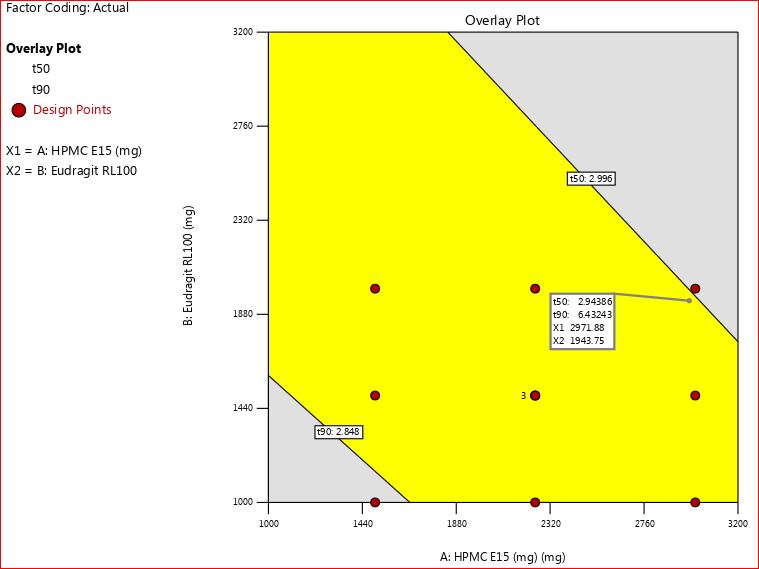
Fig. 10: Overlay plot for all response variables
In the context of first-order studies, the F9 formulation exhibited a higher R2 value of 0.9839, confirming its excellent fit with the model. The AIC value was recorded as 35.55, and the MSC value stood at 3.44.
Turning to the Higuchi model, formulation F9 demonstrated an impressive alignment with an R2 value of 0.9743. The AIC value was determined as 38.80, while the MSC value reached 2.98.
Expanding the investigation to the Korsmeyer-Peppas equation, formulation F9 displayed a remarkable agreement with an R2 value of 0.9981. The calculated AIC value was 22.69, and the MSC value was 5.28. Notably, the exponent release (n) value stood at 0.649, suggesting a non-Fickian diffusion-type mechanism governing the release. The results were shown in table 4.
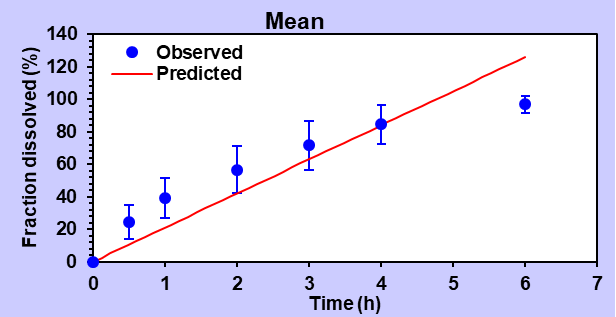
Fig. 11: Zero-order release
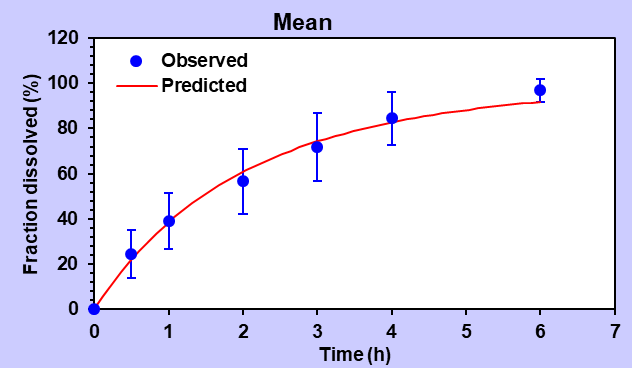
Fig. 12: First-order release
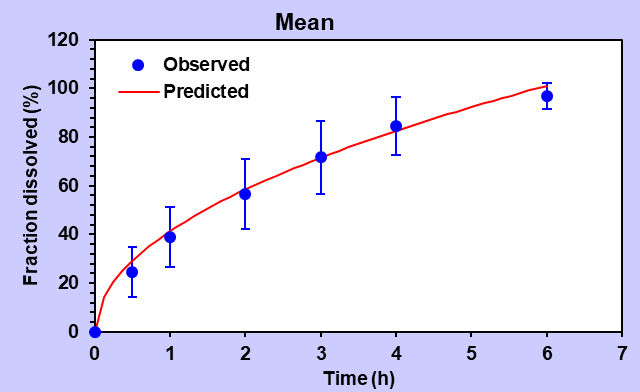
Fig. 13: Higuchi release order
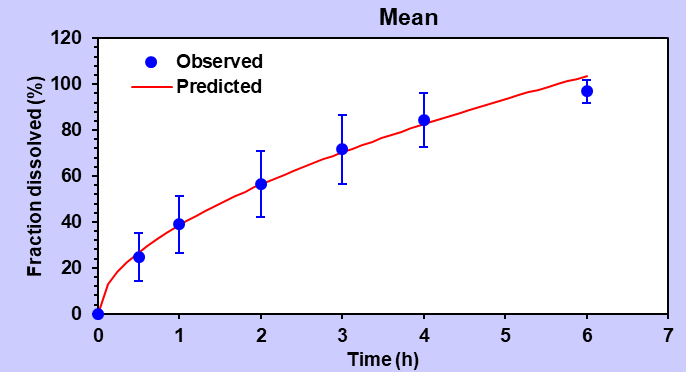
Fig. 14: Korsmeyer-peppas release order
Table 4: Summary data of various release order kinetic mechanisms for optimized formulation (F9)
| Type of release order | R2 | R2adjusted | Akaike information criterion (AIC) | Model selection criterion (MSC) | Exponent release value, n (Sphere) |
| Zero order | 0.9101 | 0.9101 | 47.58 | 1.72 | 0.649 Non-Fickian diffusion-type mechanism |
| First order | 0.9839 | 0.9839 | 35.55 | 3.44 | |
| Higuchi Model | 0.9743 | 0.9743 | 38.80 | 2.98 | |
| Korsmeyer-Peppas | 0.9981 | 0.9977 | 22.69 | 5.28 |
CONCLUSION
The goal of developing BH unidirectional transmucosal buccal patches was to increase bioavailability, decrease metabolite-dependent adverse medication effects, and circumvent hepatic first-pass metabolism. At the molecular level, FTIR shows that the medication and polymers in the patch formulation are compatible. The results of the in vitro drug release tests showed that formulation F9 could release 99.86±1.39% of the medicine up to 6 h after administration. In 6 h, the optimized formulation had a medication penetration rate of 69.53±1.86%, compared to 54.88±1.39% in the original formulation, according to the research. The results of the ANOVA revealed a robust goodness of fit, indicating a significant association, with a regression coefficient value (R2) of 0.9627. The statistical significance is shown by the fact that the computed p-value of 0.032 is lower than the commonly used p<0.05 threshold. This proves that the model's use of the selective biodegradable polymer had a substantial effect on the t90 percentage of medication release. The developed unidirectional buccal patches of BH released the greatest quantity of medicine within the stipulated mucoadhesion period, suggesting an alternate drug delivery approach for the systemic distribution of BH.
ACKNOWLEDGMENT
The authors express their sincere gratitude to the Faculty of Pharmaceutical Science, Assam Down Town University, for generously providing access to state-of-the-art facilities, infrastructure, and significant technical assistance.
FUNDING
The authors are thankful to the management of Assam Down Town University, and this research was supported by Assam Down Town University's Seed Money Grant (AdtU/DRA-II/2023-24/153).
ABBREVIATIONS
BH-Benidipine hydrochloride, HPMC E15-Hydroxy Propyl Methyl Cellulose, PVP K30-Polyvinylpyrrolidone, BCS-Biopharmaceutical Classification System, FT-IR-Fourier transform infrared, USP-United States Pharmacopeia, ICH-International Council for Harmonization, ANOVA-Analysis of Variance, AIC-Akaike Information Criterion, MSC-Model selection criterion.
AUTHORS CONTRIBUTIONS
The authors confirm their contribution to the article: study conception, design, planning, securing funding and management: KSS; data collection: KSS, CT; analysis and interpretation of results: KSS, CT, DK; draft manuscript preparation: KSS, SO, SP.
CONFLICT OF INTERESTS
The authors declare that there is no conflict of interest
REFERENCES
Rao S, Song Y, Peddie F, Evans AM. A novel tri-layered buccal mucoadhesive patch for drug delivery: assessment of nicotine delivery. J Pharm Pharmacol. 2011 Jun;63(6):794-9. doi: 10.1111/j.2042-7158.2011.01283.x, PMID 21585377.
Chen J, Pan H, Duan H, Deng W, Zhang F, Yang X. Self-assembled liposome from core sheath chitosan-based fibres for buccal delivery of carvedilol: formulation characterization and in vitro and ex vivo buccal absorption. J Pharm Pharmacol. 2020 Mar;72(3):343-55. doi: 10.1111/jphp.13210, PMID 31863466.
Bakris G, Ali W, Parati G. ACC/AHA versus ESC/ESH on hypertension guidelines JACC guideline comparison. J Am Coll Cardiol. 2019 Jun;73(23):3018-26. doi: 10.1016/j.jacc.2019.03.507, PMID 31196460.
Arpa MD, Yagcılar AP, Biltekin SN. Novel benzydamine hydrochloride and chlorhexidine gluconate-loaded bioadhesive films for local treatment of buccal infections. J Drug Deliv Sci Technol. 2023 Jun 1;84:104497. doi: 10.1016/j.jddst.2023.104497.
Penjuri SC, Damineni S, Ravouru N. Design and development of diltiazem hydrochloride transmucosal drug delivery system. Ceska Slov Farm. 2013 Feb 1;62(1):19-27. PMID 23578264.
Mane PP, Bushetti SS, Keshavshetti GG. Development and in vitro evaluation of mucoadhesive buccal films of nebivolol. Indian J Pharm Sci. 2014 Mar;76(2):166-9. PMID 24843191, PMCID PMC4023287.
Patel S, Patel AP. Formulation and evaluation of benidipine nanosuspension. Res J Pharm Technol. 2021;14(8):4111-6. doi: 10.52711/0974-360X.2021.00712.
Suzuki H, Miyamoto N, Masada T, Hayakawa E, Ito K. Solid dispersions of benidipine hydrochloride. I. Preparations using different solvent systems and dissolution properties. Chem Pharm Bull. 1996 Feb 15;44(2):364-71. doi: 10.1248/cpb.44.364.
Yao K, Nagashima K, Miki H. Pharmacological pharmacokinetic and clinical properties of benidipine hydrochloride a novel long-acting calcium channel blocker. J Pharmacol Sci. 2006;100(4):243-61. doi: 10.1254/jphs.dtj05001x, PMID 16565579.
Gajbhiye K, Hakam N, Rathod G, Tawar M. Formulation and evaluation of transdermal patches of benidipine hydrochloride. Asian J Pharm Technol. 2021;11(3):207-12. doi: 10.52711/2231-5713.2021.00034.
Farooqui PA, Gude RA. Formulation development and optimisation of fast dissolving buccal films loaded glimepiride solid dispersion with enhanced dissolution profile using central composite design. Int J Pharm Pharm Sci. 2023 Jun 1;15(6):35-54. doi: 10.22159/ijpps.2023v15i6.47992.
Prusty A, Mishra AK, Gupta BK. Effect of polymer in release profile of extended-release matrix tablet of an anti-hypertensive drug and to study impact of agglomerative phase of comminution (APOC) method on drug release. Int J Pharm Sci Res. 2018 Oct 1;9(10):4158-65. doi: 10.13040/IJPSR.0975-8232.9(10).4158-65.
Guo JH, Cooklock KM. The effects of backing materials and multilayered systems on the characteristics of bioadhesive buccal patches. J Pharm Pharmacol. 1996 Mar;48(3):255-7. doi: 10.1111/j.2042-7158.1996.tb05912.x, PMID 8737049.
Bhagurkar AM, Darji M, Lakhani P, Thipsay P, Bandari S, Repka MA. Effects of formulation composition on the characteristics of mucoadhesive films prepared by hot-melt extrusion technology. J Pharm Pharmacol. 2019 Mar;71(3):293-305. doi: 10.1111/jphp.13046, PMID 30485903.
Chen G, Bunt C, Wen J. Mucoadhesive polymers-based film as a carrier system for sublingual delivery of glutathione. J Pharm Pharmacol. 2015 Jan;67(1):26-34. doi: 10.1111/jphp.12313, PMID 25303221.
Nair AB, Kumria R, Harsha S, Attimarad M, Al Dhubiab BE, Alhaider IA. In vitro techniques to evaluate buccal films. J Control Release. 2013 Feb 28;166(1):10-21. doi: 10.1016/j.jconrel.2012.11.019, PMID 23219961.
Navamanisubramanian R, Nerella R, Duraipandian C, Seetharaman S. Quality by design approach for optimization of repaglinide buccal tablets using box-behnken design. Future J Pharm Sci. 2018 Dec 1;4(2):265-72. doi: 10.1016/j.fjps.2018.10.002.
Semalty A, Semalty M, Nautiyal U. Formulation and evaluation of mucoadhesive buccal films of enalapril maleate. Indian J Pharm Sci. 2010 Sep;72(5):571-5. doi: 10.4103/0250-474X.78522, PMID 21694987.
Buddhadev SS, C Garala K, SS, Rahamathulla M, Ahmed MM, Farhana SA. Quality by design aided self nano emulsifying drug delivery systems development for the oral delivery of benidipine: improvement of biopharmaceutical performance. Drug Deliv. 2024 Dec 31;31(1):2288801. doi: 10.1080/10717544.2023.2288801, PMID 38073402.
Reddy KR, Nagabhushanam MV. The role of needle in formulation of pH sensitive swellable microbeads prepared with hydrophilic polymers for atorvastatin and their characterization studies. Int J App Pharm. 2017;9(3):20-30. doi: 10.22159/ijap.2017v9i3.16952.
Rani NI, Dev DH. Formulation and evaluation of fast disintegrating tablet of propranolol hydrochloride using modified tamarind seed gum as a natural superdisintegrant. Asian J Pharm Clin Res. 2022;15(9):185-92. doi: 10.22159/ajpcr.2022.v15i9.45284.
Mathew RO, Varkey JO. Formulation and in vitro evaluation of self nano emulsifying drug delivery system of quercetin for enhancement of oral bioavailability. Int J Curr Pharm Sci. 2022 Jan 15;14(1):60-9. doi: 10.22159/ijcpr.2022v14i1.44113.
Peddapalli H, Chinnala KM, Banala N. Design and in vitro characterization of mucoadhesive buccal patches of duloxetine hydrochloride. Int J Pharm Pharm Sci. 2017;9(2):52-9.
Samanthula KS, Satla SR, Bairi AG. Development in vitro and ex vivo evaluation of mucoadhesive buccal patches of candesartan cilexetil. Res J Pharm Technol. 2019;12(6):3038-44. doi: 10.5958/0974-360X.2019.00514.6.
Gawai NI, Zaheer ZA. Formualtion and development of mucoadhesive sustained release buccal tablets and patches of 5-fluorouracil using different polymers. Asian J Pharm Clin Res. 2018;11(5):174-85. doi: 10.22159/ajpcr.2018.v11i5.20241.
Govindasamy P, Kesavan BR, Narasimha JK. Formulation of unidirectional release buccal patches of carbamazepine and study of permeation through porcine buccal mucosa. Asian Pac J Trop Biomed. 2013 Dec 1;3(12):995-1002. doi: 10.1016/S2221-1691(13)60192-6, PMID 24093793.
Haju SS, Yadav S. Formulation and evaluation of cilnidipine mucoadhesive buccal film by solvent casting technique for the treatment of hypertension. Int J Pharm Pharm Sci. 2021 Sep 1;13(9):34-43. doi: 10.22159/ijpps.2021v13i9.42641.
Samanthula KS, Satla SR, Bairi AG. Bioadhesive polymers permeation enhancers and types of dosage forms for buccal drug delivery. J Drug Delivery Ther. 2021;11(1):138-45. doi: 10.22270/jddt.v11i1.4495.
Adhikari SN, Nayak BS, Nayak AK, Mohanty B. Formulation and evaluation of buccal patches for delivery of atenolol. AAPS PharmSciTech. 2010 Sep;11(3):1038-44. doi: 10.1208/s12249-010-9459-z, PMID 20533098.
El Sharawy AM, Shukr MH, Elshafeey AH. Formulation and optimization of duloxetine hydrochloride buccal films: in vitro and in vivo evaluation. Drug Deliv. 2017 Jan 1;24(1):1762-9. doi: 10.1080/10717544.2017.1402216, PMID 29172829.
Shiledar RR, Tagalpallewar AA, Kokare CR. Formulation and in vitro evaluation of xanthan gum-based bilayered mucoadhesive buccal patches of zolmitriptan. Carbohydr Polym. 2014;101:1234-42. doi: 10.1016/j.carbpol.2013.10.072, PMID 24299896.
Kumria R, Al Dhubiab BE, Shah J, Nair AB. Formulation and evaluation of chitosan-based buccal bioadhesive films of zolmitriptan. J Pharm Innov. 2018 Jun;13(2):133-43. doi: 10.1007/s12247-018-9312-6.