
Int J App Pharm, Vol 17, Issue 5, 2025, 85-93Review Article
NOVEL APPROACHES IN ETHOSOMAL DRUG DELIVERY: APPLICATION IN DERMATOLOGY AND BEYOND
SIMRAN NEGI1 , SHALU VERMA1*
, SHALU VERMA1* , PRAYAG RAJ1
, PRAYAG RAJ1 , ALKA SINGH2
, ALKA SINGH2
1Department of Pharmaceutics, Uttaranchal Institute of Pharmaceutical Sciences, Uttaranchal University, Prem Nagar, Dehradun-248007, India. 2School of Pharmaceutical Sciences and Technology, Sardar Bhagwan Singh University, Balawala, Dehradun, Uttarakhand-248007, India.
*Corresponding author: Shalu Verma; *Email: vermashalu339@gmail.com
Received: 31 Mar 2025, Revised and Accepted: 30 Jul 2025
ABSTRACT
Skin is a complex and dynamic structure with multiple layers, and it is the body's largest organ. It is the protective barrier between the external and internal environment; it comprises three layers (epidermis, dermis, and hypodermis). The skin performs various vital functions in the body. A conventional drug delivery system (CDDS) is the method of administering drugs into the body through oral, parenteral, and topical administration. This delivery system has several drawbacks, including low bioavailability, uncontrolled release, and poor solubility. A novel carrier and TDDS (Transdermal Drug Delivery) systems were introduced to overcome these drawbacks. It is an advanced drug delivery system designed to overcome the drawbacks of conventional methods and improve therapeutic outcomes. Some examples of novel and transdermal carriers are liposomes, niosomes, ethosomes, and microspheres. Ethosomes are preferred over liposomes and niosomes due to their high ethanol content, which enhances skin penetration and allows deeper drug delivery. TDDS delivers drugs into the deeper layer of the skin and provides a therapeutic effect by avoiding first-pass metabolism. They are generally used to treat various diseases. Ethosomes are highly advanced lipid-based nano-carriers designed to improve transdermal and dermal drug delivery systems. The main aim of writing this review paper is to summarize the focus on the key aspects, such as types of ethosomes, the mechanism of action of ethosomes, the method of preparation, composition, application, characterization, the patent, marketed formulation, and factors of ethosomes.
Keywords: Ethosomes, Skin, Applications, Transdermal, Bioavailability
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijap.2025v17i5.54400 Journal homepage: https://innovareacademics.in/journals/index.php/ijap
INTRODUCTION
Skin is the body's largest organ, which regulates the body's temperature and protects against germs. Skin comprises minerals, fat, protein, water, and other essential nutrients [1]. The skin is composed of three layers: the Epidermis, the top layer of skin, which helps in forming new skin; it also serves as a protective barrier and provides skin color. The middle layer, the Dermis layer, helps in hair growth. It has collagen and elastin, helps in feelings and sensations, produces sweat, and supplies blood to the body. The bottom layer of skin is known as the Hypodermis layer. This layer helps regulate body temperature, helps to connect nerves and blood vessels, and has connective tissues [2]. The skin's main job is to act as a protective agent and prevent harmful substances from entering the body. Various barriers are present in the skin, which make it difficult for medication to reach the site of action. The stratum corneum is the most significant barrier. It is a foremost barrier that decreases drug permeability and decreases the therapeutic effect of bioactive components of the drug [3]. Medication in the form of creams and gels was used in the layer of skin to get therapeutic results, but now novel drugs are being used to achieve better results. One of these is ethosomes. The significant drawbacks of the conventional drug delivery system (CDDS) were an uncontrolled release of drugs, unregulated doses of drugs, and they had low bioavailability, lower efficacy, and sometimes toxicity was observed. Transdermal drug delivery system (TDDS), or a novel drug delivery system (NDDS), was introduced to overcome these drawbacks. As this drug delivery system is highly advanced, it avoids first-pass metabolism, reduces toxicity, directly reaches the systemic circulation, enhances permeation of the drug, they are highly stable, and has several benefits over conventional drug delivery systems [4].
TDDS is a procedure of directly delivering drugs into the bloodstream. TDDS encounters the barriers property of stratum corneum (Horny layer). It aims to achieve systemic medication by directly or topically applying medication to interact with the skin layer and avoiding first-pass metabolism. Liposomes, niosomes, and ethosomes are used in the vesicular or TDDS [5]. Firstly, if we talk about the liposomes, they are tiny, spherical-shaped vesicles made up of cholesterol and natural phospholipids. They are biodegradable and biocompatible and are used in making medical applications. However, they are less stable and exposed to oxidation, which is a more stable evaluation of the niosome. Niosomes are synthetic vesicles, and they are made up of non-ionic surfactant and cholesterol. They are very much identical to liposomes, but compared to liposomes, niosomes are more stable, less expensive, and pocket-friendly. Niosomes are further used to deliver drugs, especially in a TDDS [6]. Ethosomes are an advanced formulation of niosomes. Ethosomes are known as ethanolic vesicles as they contain more ethanol in the vesicular structure, as shown in fig. 1 and table 1 [7]. In ethosomes, ethanol breaks the gaps of the intensely packed lipid present in the outermost layer of skin that is known as stratum corneum; ethanol makes it simple for the ethosomes to deeply penetrate, as they are highly flexible and permit them to squeeze through the small gaps in the skin barrier, as shown in fig. 3. Once the drug reaches the skin's deepest layer, it ensures effective delivery. This unique combination of ethanol and flexible vesicles makes ethosomes very efficient for TDDS [8]. Ethosomes have a size range from 10 nm to a specific micron. The size of ethosomes is dependent on the concentration of ethanol and phospholipid [9]. Ethosomes are highly advanced TDDS systems that are produced to improve the absorption of medicines through the different layers of skin. These are very tiny and soft vesicles that consist of ethanol, phospholipid, water, and many more essential components and excipients [10, 11]. Ethosomes are very flexible, carrying hydrophilic and lipophilic drugs. Ethosomes are painless, meaning they do not require a needle, making them more comfortable for patients. They are commonly used to cure hormonal imbalance, skin disorders, pain, and nowadays they are widely used in the formulation of cosmetics [12]. Ethosomes are very stable, and we can store medicine for a longer duration of time. Generally, ethosomes are promising a new revolution, by making treatment more effective and safer. Ethosomes are very stable, and we can store medicine longer [13, 14].
To ensure a thorough review of ethosomes and their role in transdermal drug delivery systems (TDDS), a systematic literature search was conducted using major scientific databases, including PubMed, Scopus, Web of Science, and Google Scholar. The search strategy incorporated Boolean operators (AND, OR) to refine results with relevant keywords such as transdermal drug delivery, ethosomes, vesicular systems, skin permeability, and ethanol-enhanced penetration. Articles published between 2010 and 2024 were prioritized to include the latest advancements in ethosomal formulations. Selection criteria focused on peer-reviewed studies detailing the formulation, mechanism, advantages, and clinical applications of ethosomes, while excluding papers that were solely based on animal models with limited translational relevance or those lacking experimental validation.
Types of ethosmes systems
Ethosomes are an advanced novel carrier of TDDS that can be classified based on their composition and application [15]. As shown in fig. 2, ethosomes are classified into three types: classical ethosomes, binary ethosomes, and trans ethosomes.
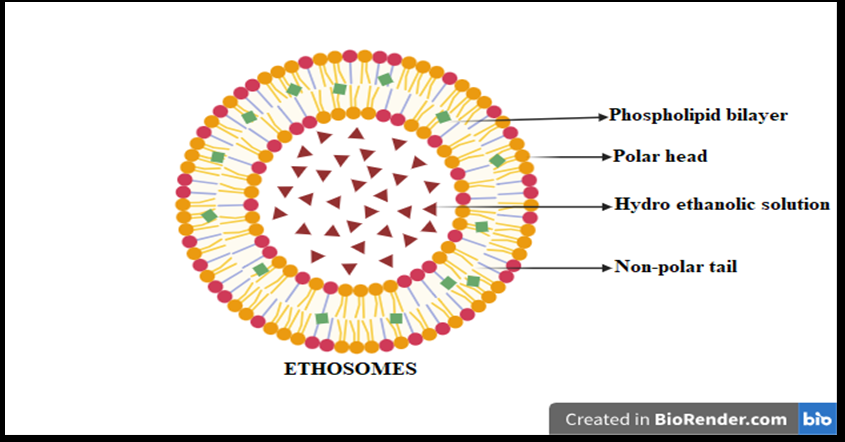
Fig. 1: Systemic structure of ethosome
Table 1: Ethosomes vs. liposomes vs. niosomes
| Parameter | Ethosomes | Liposomes | Niosomes | References |
| Permeation efficiency | High—due to ethanol-enhanced skin penetration | Moderate—relies on phospholipid bilayer | Moderate—depends on non-ionic surfactants | [16] |
| Stability | Improved stability due to ethanol, but sensitive to high temperatures | Moderate—prone to oxidation and degradation | Higher stability than liposomes, but susceptible to surfactant phase transitions | [17] |
| Clinical efficacy | Enhanced drug absorption and bioavailability | Effective for localized delivery, but limited deep penetration | Suitable for controlled drug release, but may have lower permeability than ethosomes | [18] |
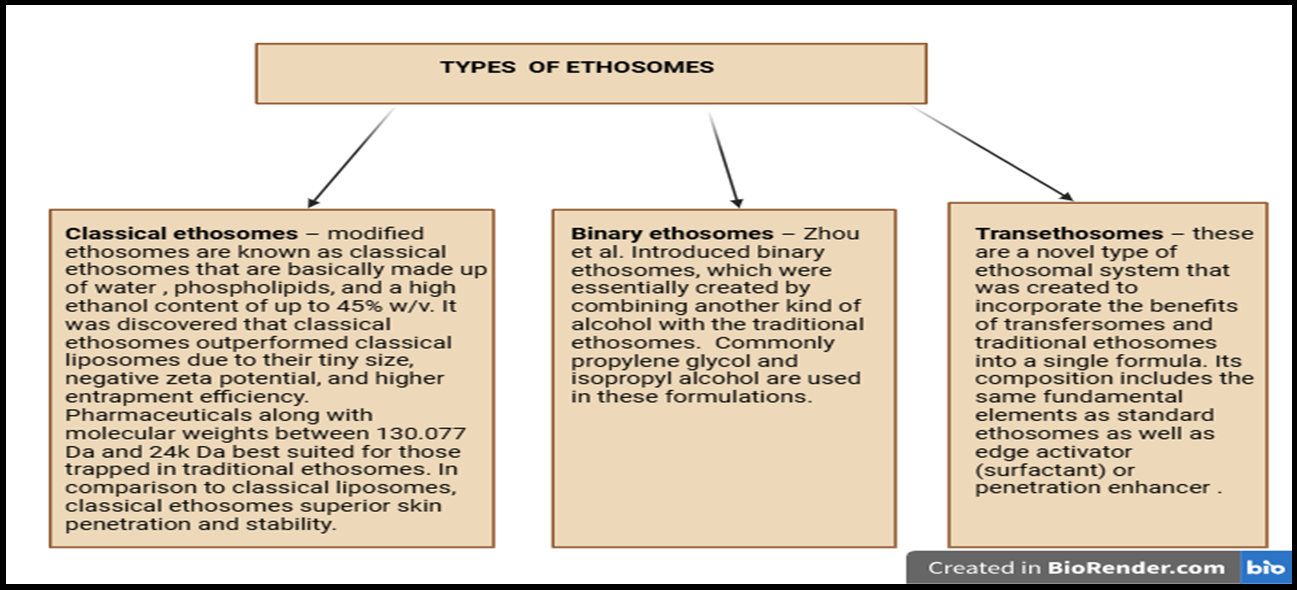
Fig. 2: Types of ethosomes
Mechanism of action
Ethosomes utilize a high ethanol concentration to enhance transdermal drug delivery. Ethanol disrupts the stratum corneum's lipid organization by reducing lipid packing density, weakening van der Waals forces, and breaking hydrogen bonds between ceramides, fatty acids, and cholesterol. This increase in membrane fluidity lowers the phase transition temperature and makes the lipid bilayer more permeable. The altered lipid structure allows ethosomes to penetrate deeper into the skin and efficiently deliver hydrophilic and lipophilic drugs, significantly improving therapeutic efficacy as shown in fig. 3 [19, 20].
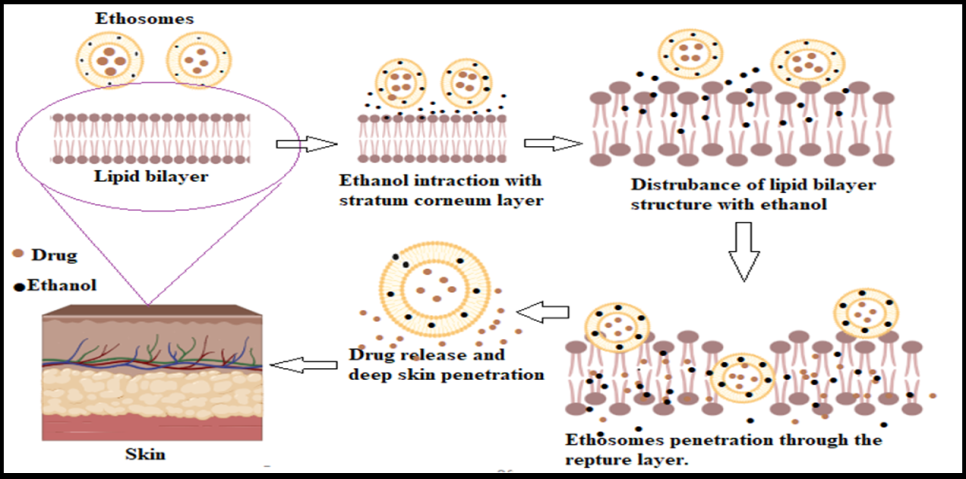
Fig. 3: Mechanism of penetration of the ethosomal drug delivery system
Table 2: Different excipients used in ethosomes
| Excipients of ethosomes | Uses | References |
| Phospholipids | Essential components of the vesicle bilayer aid ethosome formation. Examples: Phosphatidylcholine, Dipalmitoylphosphatidylcholine (DPPC), Soy phosphatidylcholine (SPC). | [20] |
| Ethanol | Enhances skin penetration by fluidizing the lipid bilayer, facilitating better drug delivery—a standard concentration of 20% to 40%. | [21, 22] |
| Water | Solvent disperses lipids and other components, such as distilled and purified water. | [23] |
| Cholesterol | Modulates lipid bilayer fluidity and improves ethosome stability—typical usage: 10% to 20% w/w. | [24] |
| Glycerol | Functions as a humectant, stabilizing the formulation and improving texture. | [25, 26] |
| Sodium Chloride | Adjusts osmolarity and ensures isotonicity of formulations, such as sodium chloride (NaCl). | [22] |
| Carrier | Used to enhance drug encapsulation or modify the release profile. Examples: Poloxamers, Cyclodextrins (β-cyclodextrin). | [22] |
| pH Adjuster | Maintains the desired pH, influencing formulation stability and drug release, such as citric acid and sodium hydroxide. | [22] |
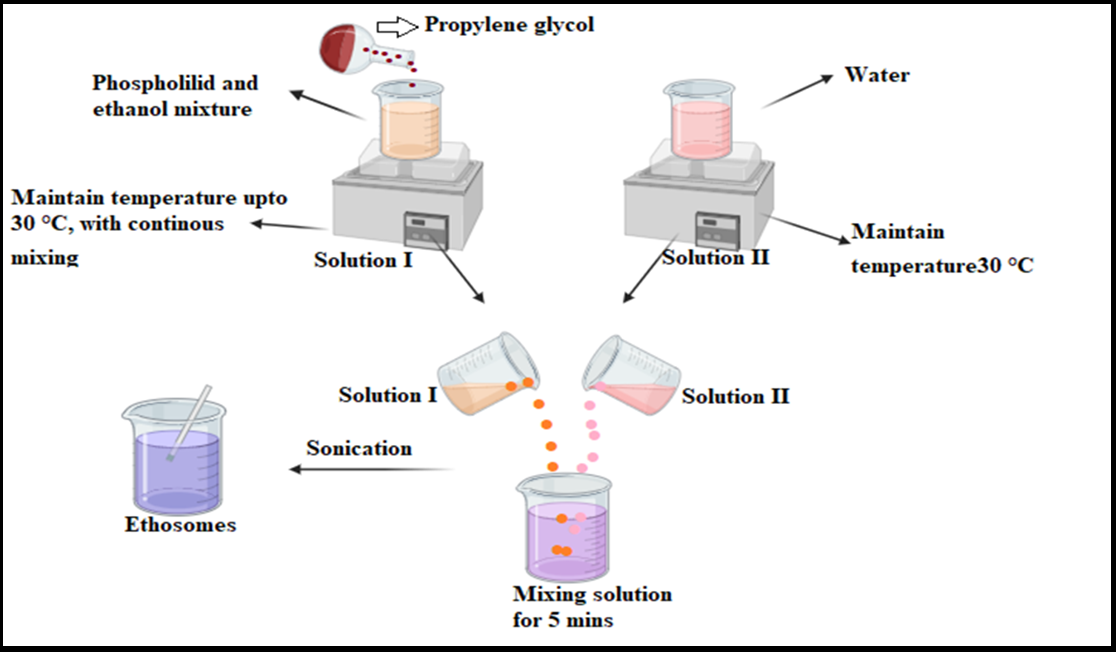
Fig. 4: Systematic representation of the cold method
Ethosome compositions
Ethosomes are very tiny, soft vesicles that consist of ethanol, phospholipid, water, and many other essential components and excipients. Table 2 highlights the excipients used in the formulation of ethosomes.
Method of preparation of ethosomes
Cold method
Phospholipids and lipid components are mixed with ethanol at a controlled temperature while continuously stirring. Polyol or propylene glycol is added to stabilize the mixture maintained in a water bath. Water is heated and gradually incorporated into the ethanol-lipid blend with continuous stirring until a uniform ethosomal dispersion is obtained. Vesicle size can be further reduced through sonication, as shown in fig. 4 [27]. This method is moderately scalable, requires controlled mixing conditions, and is particularly suitable for heat-sensitive drugs, ensuring drug stability throughout the process.
Hot method
This process involves heating the phospholipid at 40 °C in a water bath to form a colloidal solution. Meanwhile, ethanol and propylene glycol are heated to 40 °C in a separate vessel. Once both solutions reach the same temperature, they are combined to form ethosomes, ensuring efficient drug encapsulation depending on its hydrophilic or lipophilic nature. Vesicle size reduction can be achieved using sonication, as shown in fig. 5 [27]. This highly scalable method has low production costs and higher encapsulation efficiency, making it ideal for lipophilic drugs. However, due to thermal exposure, it may not be suitable for heat-sensitive compounds.
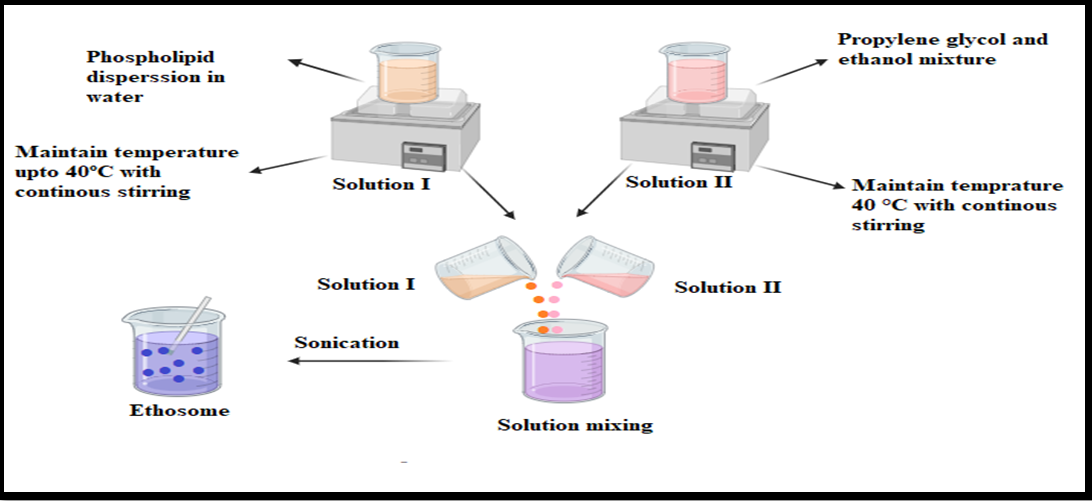
Fig. 5: Systematic representation of the hot method
Thin film hydration technique
Lipids and an organic solvent are dissolved in a round-bottom flask, followed by evaporation using a rotary evaporator above the lipid transition temperature. This process creates a thin lipid film along the flask’s inner walls, then hydrated with an ethanolic mixture. The final ethosomal suspension is obtained through sonication, improving uniformity, as shown in fig. 6 [28]. This highly scalable method allows for precise control over vesicle size, making it suitable for hydrophilic and lipophilic drugs. However, it requires specialized equipment like rotary evaporators, which may increase production costs.
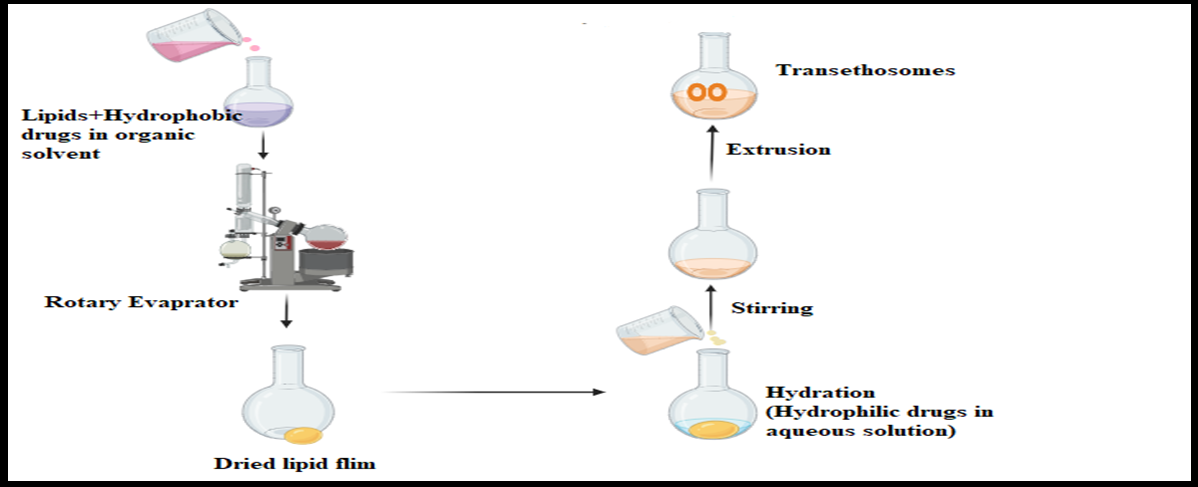
Fig. 6: Systematic representation of the thin film hydration method
Classic method
When the drugs and phospholipids are mixed with ethanol and then heated in a water bath heated to 30 °C. In a covered vessel, double-distilled water is provided to the solution that contains lipids in a stream while continuously stirring at 700 rpm. A hand extruder is utilized to homogenize the obtained solution of vesicles by passing it through a polycarbonate membrane 3 times [28].
Ethosomal-based formulation for TDDS
Ethosome-based formulations are a recent approach in transdermal drug delivery, and their unique combination of excipients improves skin penetration. These formulations are highly effective for hydrophilic and lipophilic drugs; they penetrate the skin and give therapeutic responses. Some drugs based on ethosome formulations are mentioned below in table 3.
Application of ethosomes
Ethosomes are primarily used in skin care products to enhance skin color, provide moisture, prevent ageing, and reduce acne marks and pigmentation. However, they are often used to improve effectiveness in antiviral and antifungal medicines such as clotrimazole and acyclovir. They are also exploring the potential of ethosomes in cancer treatment and how to deliver a vaccine without needing a needle. These specialties make ethosomes valuable in cosmetic and medicinal fields [40].
Antifungal
Clotrimazole and ketoconazole are active antifungal agents, and an ethosomal system has been used to encapsulate antifungal drugs. For example, Wang et al., through their study, it was revealed that the use of ethosomes as an antifungal agent enhances skin permeation and improves antifungal activity against fungal infections like Candida albicans with the help of hexyl-amino levulinate [41].
Table 3: Ethosomes-based formulations
| Drugs | Application | Method | Excipients | Key finding | Reference |
| Minoxidil | Alopecia treatment. | Hot method. | Phospholipid 90G, Ethanol. | When phospholipid and drug were mixed in a 1:4 ratio, 75% of the drug was successfully encapsulated, leading to better release than other mixtures. | [29] |
| Clotrimazole. | Antifungal. | Mechanical dispersion method. | Phosphatidylcholine, Soya lecithin, Carbopol 934. | The best formulation achieved 52.6% to 60% drug entrapment efficiency, with RE5* being the most effective. | [30] |
| Acyclovir | Antiviral | Hot method. | Palmitoyl chloride, Phosphatidyl choline, Cholesterol. | Encapsulation efficiency was 87.75%, compared to 39.13% for ACV ethosomes. After 24 h, ACV-C16 ethosomes achieved 5.3 times higher skin absorption than ACV-C16 hydroalcoholic solution and 3.43 times higher than ACV ethosomes. | [31] |
| Silver sulfadiazine | Antibacterials also reduce the bacterial burden and healing time in burn injuries. | Cold method | Soya lecithin, Propylene glycol, Ethanol, Cholesterol. |
Optimized ethosomal suspension resulted in high SSD encapsulation efficiency (92.03±0.79%) and significantly reduced microbial colonies. It also enhanced wound healing, achieving 96.83% contraction compared to 59.41% in the untreated group. | [32] |
| Curcumin | Skin cancer | Ethanol injection method | Lecithin, Ethanol, Cholesterol, Glycerol, Water. |
The optimized formulation achieved 92.24±0.20% entrapment efficiency with a vesicle size of 247±5.25 nm, making it highly effective. | [33] |
| Thymoquinone | Brest cancer | Conventional mechanical Dispersion method. |
Soya lecithin, ethanol, cholesterol, and chloroform. | Researchers discovered an optimized formulation using specific polymers in controlled quantities, achieving 99% drug encapsulation. The formulation has an average vesicle size of 20±1 nm and a zeta potential of-63±2 mV. | [34] |
| Ketoconazole | Antifungal | Thin film hydration method. | Phosphatidylcholine, Tween80, Ethanol, Methanol, Fluorescein, Carbopol940, | Optimized vesicles were round, with an average size of 151.34±8.73 nm and a zeta potential value of+34.82±2.64 mV. Entrapment efficiency was approximately 95% of the drug. | [35] |
| Rosmarinic Acid | Anti-aging treatment | Ethanol injection method | Soya lecithin, propylene glycol, ethanol, cholesterol | The resulting vesicle size is (453.10-796.80 nm) and good efficiency of entrapment (46.73-65.99%), and the negative zeta potential is(-45.40±-86.90mv). | [36] |
| Anthralin | Psoriatic | The thin-film hydration method | Phosphatidylcholine, cholesterol, chloroform, ethanol, methanol, diethyl ether. | The efficiency of the drug to encapsulate ≥97.2% and ≥77% was obtained for ethosomes, and the size of the particle, 116-199 nm and 146-381 nm, was recorded. | [37] |
| Cryptotanshinone | Acne | Ethanol injection technique | Soybean phosphatidylcholine, Oleic acid, Carbomer 974, Polyethylene glycol 400 (PEG-400), and Tyrosinase. | This acne activity was observed in rabbits, and the resulting ethosomes have an average vesicle size of 69.1±1.9 nm. Drug encapsulation efficiency is 0.445±0.007 mg/ml 40.31±0.67%, and the optimized gels were 2.5 and 2.1 times that of conventional gels. | [38] |
| Carvedilol | Antihypertensive | The ethanol injection Method | Phospholipone 100 H, Cholesterol, Transcutol P. | The ethosomes' vesicular size ranges between 201.55 and 398.55 nm. The drug's efficiency of entrapment is 30.00-90.66%, and the capacity of loading is 7.64-43.04%, with a zeta potential of-30.30 to-44.90 mV. | [39] |
Topical drug delivery
Topical formulation of ethosomes that are used for the treatment of eczema and dermatitis. Ethosomal preparation allows drugs (corticosteroids) to penetrate the deeper layer of skin for therapeutic responses. For example, Goindi et al. Their study or review includes the fact that ethosomes are successful and highly effective carriers in tropical drug delivery systems that increase skin penetration and improve drug stability. Their analysis reveals that ethosomes can be used for systemic and localized disease treatment, specifically those requiring long-term therapy [42].
Cosmetic application
Cosmetic ethosomal formulations are used in moisturizing and anti-aging creams because they contain APIs like vitamin C. They deliver the drug deep into the skin, which helps reduce pigmentation and wrinkles [43]. For example, Akhtar et al., [44]. Their study concluded that the development of stable tocopherol succinate-loaded ethosomes is significant in cosmetic and TDDS. Their study highlights the capability of ethosomes to improve the permeation of cosmetic ingredients like antioxidants, retinol, and sunscreen agents, which is challenging to deliver effectively using traditional formulations. Their study also suggests that ethosomes have better efficacy, stability, and skin penetration of these ingredients, improving their performance in cosmetic treatments.
Anticancer
Ethosomal preparation has been used to deliver anticancer agents like doxorubicin to the tumour, and it also improves targeting and decreases systemic toxicity [51]. For example, Shinde et al. concluded that ethosomes are a good carrier for the therapy of skin cancer by offering innovative formulations like gel and patches. They provide an effective, stable, and safe alternative to conventional therapy [52].
Anti-inflammatory and antioxidant
Potent drugs like curcumin contain anti-inflammatory and antioxidant compounds, and they can be encapsulated in an ethosomal preparation to enhance transdermal absorption and also help in the cure of inflammatory skin conditions [45]. For example, Pathan et al., [46]. In this research, he revealed that ethosomes are carriers for loxoprofen, a non-steroidal anti-inflammatory drug, to improve the delivery of transdermal preparation. As a result, the ethosome system significantly improved the drug permeation through skin and anti-inflammatory effects. He also mentioned that it has potential as an effective transdermal delivery system.
Vaccination
Ethosomes can deliver transdermal vaccines, providing a painless alternative to traditional injections [47]. For example, Panwar et al. [48]. Demonstrated that ethosomes enhance the transdermal delivery of bioactive substances, including vaccines, by improving skin permeability and bypassing first-pass metabolism. These features make ethosomes a promising, innovative solution for vaccine delivery, reducing patient discomfort while ensuring adequate immunization [48].
Recent advancements
Recent advancements in ethosomes have mainly focused on improving their stability and efficacy and expanding their application in drug delivery. Various formulations have been developed for commercial use, some of which are mentioned below in table 4, and several patents related to ethosomes, concentrating on their preparation, composition, and application, are discussed below in table 5. Ethosomal formulations have been evaluated in several clinical studies to assess their efficacy and safety. One notable example is ethosomal minoxidil for alopecia, which demonstrated enhanced transdermal penetration and improved hair regrowth compared to conventional formulations. Studies have also reported that ethosomes improve drug bioavailability while maintaining low systemic toxicity, making them viable candidates for skin-targeted drug delivery. However, ethanol content may cause mild irritation in some patients, emphasizing the need for optimized formulations with stabilizers to mitigate potential side effects [49]. Regulatory agencies such as the FDA (Food and Drug Administration) and EMA (European Medicines Agency) oversee transdermal ethosomal formulations under guidelines for topical and nanocarrier-based drug delivery systems [50]. These frameworks emphasize safety, efficacy, and quality control, requiring comprehensive data on drug permeation, stability, and skin compatibility before market approval. Ethosomes incorporated into transdermal patches must comply with Good Manufacturing Practices (GMP) and demonstrate sustained drug release while minimizing skin irritation risks [51].
Table 4: Marketed formulation of ethosomes
| Formulation type | Product name | Active ingredient | Uses | Manufacturing country | Reference |
| Topical gel | Etoderm | Diclofenac sodium | Pain relief conditions like muscle pain, arthritis, and inflammation | India | [52] |
| Ointment or Topical cream | Dermovate (clobetasol propionate) | Clobetasol Propionate |
Inflammatory skin condition | UK | [53] |
| Tropical cream | Eli Quin | Ketoconazole | Anti-fungal | India | [54] |
| Topical gel | Minoxidil ethosomal gel | Minoxidil | Hair growth | USA | [55] |
| Transdermal patch | Klonopin | Clonazepam | Anxiety, panic attacks, and seizures | USA | [56] |
| Topical gel | Curaheal | Curcumin | Antioxidant, anti-inflammatory | India | [57] |
Table 5: Patents on ethosomes-based formulations [58]
| Patent number | Title | Year |
| US 20220304903A1 | The method of preparing bioactive substance is encapsulated ethosomes, ethosome composition, and cosmetic composition, including ethosome composition. | 2022 |
| WO2021201310A1 | The composition of the ethosomes includes vitamins, Dexpanthenol encapsulated therein, and the preparation method. | 2020 |
| AU2019206649B2 | Encapsulated cannabinoid formulations for transdermal delivery | 2019 |
| TR201818665A2 | Rosmarinic acid-loaded liposomes and ethosomes are suitable for use in cosmetics. | 2018 |
| JP7181880B2 | A core/shell structural platform for immunotherapy | 2018 |
| KR101810160B1 | Generating method for ethosomes with bioactive compounds, ethosomes, and cosmetic composition including ethosomes. | 2017 |
| US11452679B2 | Method of preparing bioactive substance-encapsulated ethosome, ethosome composition, and cosmetic composition including ethosome composition. | 2017 |
| US20220304903A1 | Method of preparing bioactive substance-encapsulated ethosome, ethosome composition, and cosmetic composition including ethosome composition. | 2017 |
Challenges and future prospects
Ethosomes are very small based on their lipid structure, which allows efficient drug delivery through the skin. They can potentially treat various diseases and skin conditions; however, several challenges must be addressed to enhance their future applications. One primary concern is stability, as ethosomes lose structural integrity over time, affecting drug delivery efficiency. Recent studies have explored stability enhancement strategies, including lyophilization, which removes water content to prevent degradation. Additionally, cryoprotectants such as trehalose and sucrose help stabilize ethosomes during freezing and drying, preventing vesicle rupture and improving formulation robustness. Polymer coatings, like chitosan or PEG, further enhance mechanical stability, drug retention, and prolonged release, reducing premature degradation.
Recent advancements in ethosomal drug delivery are exploring combinatorial approaches and hybrid systems to enhance therapeutic efficacy. Microneedle-assisted ethosomes improve penetration by creating microchannels, facilitating better drug absorption, while iontophoresis, using electrical currents, increases drug flux across the skin barrier, optimizing transdermal delivery. Additionally, Transethosomes, a hybrid formulation incorporating penetration enhancers alongside ethanol, have shown superior skin permeation, drug retention, and bioavailability compared to traditional ethosomes. These emerging technologies highlight innovative directions in ethosomal research, offering enhanced stability, efficiency, and broader applications for future pharmaceutical formulations.
CONCLUSION
Through this review paper, we briefly describe ethosomes and the discovery of ethosomes, which introduced a new era for successfully delivering drugs through the skin. Ethosomes are lipid-based nanocarriers with a high ethanol concentration, as ethanol helps increase the drug's permeability through the skin by dissolving the skin barrier layer (stratum corneum). Ethosomes have many positive aspects, such as being safe to use, easily prepared, stable, and having no side effects. They also improve drug bioavailability and have better patient compliance. Twosomes indicate the capability to deliver a large-scale therapeutic agent for treating conditions like acne, anti-fungal, anti-diabetic, anti-cancer, etc. However, more research is required to determine the cost of the formulation, enhance stability, and increase capability; skin irritation remains an area for further study.
ACKNOWLEDGEMENT
The authors thank Mr. Jitender Joshi, president, and Prof. (Dr.) Dharam Buddhi, Vice-Chancellor of Uttaranchal University, for their research-related encouragement.
FUNDING
Nil
AUTHORS CONTRIBUTIONS
Shalu Verma: Investigation, Conceptualization, drafting, Supervision. Alka Singh: Review, editing, and visualization. Simran Negi: Writing review and editing. Prayag Raj: writing and analysis.
CONFLICT OF INTERESTS
Declared none
REFERENCES
Haftek M, Abdayem R, Guyonnet Debersac P. Skin minerals: key roles of inorganic elements in skin physiological functions. Int J Mol Sci. 2022;23(11):6267. doi: 10.3390/ijms23116267, PMID 35682946.
Gilaberte Y, Prieto Torres L, Pastushenko I, Juarranz A. Anatomy and function of the skin. In: Nanoscience in dermatology. Amsterdam: Elsevier; 2016 Jan 1. p. 1-14. doi: 10.1016/B978-0-12-802926-8.00001-X.
Bouwstra JA, Ponec M. The skin barrier in healthy and diseased state. Biochim Biophys Acta. 2006 Dec 1;1758(12):2080-95. doi: 10.1016/j.bbamem.2006.06.021, PMID 16945325.
Kumar V, Praveen N, Kewlani P, Arvind, Singh A, Gautam AK. Transdermal drug delivery systems. In: Santra TS, Shinde AU, editors. Advanced drug delivery. Singapore: Springer Nature Singapore; 2023. p. 333-62. doi: 10.1007/978-981-99-6564-9_13.
Alenzi AM, Albalawi SA, Alghamdi SG, Albalawi RF, Albalawi HS, Qushawy M. Review on different vesicular drug delivery systems (VDDSs) and their applications. Recent Pat Nanotechnol. 2023;17(1):18-32. doi: 10.2174/1872210516666220228150624, PMID 35227188.
Uchegbu IF, Florence AT. Non-ionic surfactant vesicles (niosomes): physical and pharmaceutical chemistry. Adv Colloid Interface Sci. 1995 Jun 27;58(1):1-55. doi: 10.1016/0001-8686(95)00242-I.
Das SK, Chakraborty S, Roy C, Rajabalaya R, Mohaimin AW, Khanam J. Ethosomes as novel vesicular carrier: an overview of the principle preparation and its applications. Curr Drug Deliv. 2018 Jan 16;15(6):795-817. doi: 10.2174/1567201815666180116091604, PMID 29336262.
Mbah CC, Builders PF, Attama AA. Nanovesicular carriers as alternative drug delivery systems: ethosomes in focus. Expert Opin Drug Deliv. 2014;11(1):45-59. doi: 10.1517/17425247.2013.860130, PMID 24294974.
Touitou E, Dayan N, Bergelson L, Godin B, Eliaz M. Ethosomes, novel vesicular carriers for enhanced delivery: characterization and skin penetration properties. J Control Release. 2000 Apr 3;65(3):403-18. doi: 10.1016/s0168-3659(99)00222-9, PMID 10699298.
Mbah CC, Builders PF, Attama AA. Nanovesicular carriers as alternative drug delivery systems: ethosomes in focus. Expert Opin Drug Deliv. 2014;11(1):45-59. doi: 10.1517/17425247.2013.860130, PMID 24294974.
Mohanty D, Mounika A, Bakshi V, Akiful Haque M, Keshari Sahoo C. Ethosomes: a novel approach for transdermal drug delivery. IJCTR. 2018;11(8):219-26. doi: 10.20902/IJCTR.2018.110826.
Verma P, Pathak K. Therapeutic and cosmeceutical potential of ethosomes: an overview. J Adv Pharm Technol Res. 2010;1(3):274-82. doi: 10.4103/0110-5558.72415.
Colaco HA, Mestre B, Pela DCO T, Doutora P, Beco AC, Reis P, Auxiliar P, Pela C, Manuela De Jesus M, Gaspar G, Auxiliar I. Development of ethosomes as skin carriers for Sambucus nigra l. extracts. Universidade de Lisboa Faculdade de Farmacia; 2021.
Chauhan AS, Chand P, Parashar T. Lipid-based nanoparticles: strategy for targeted cancer therapy. BIO Integr. 2025;6(1). doi: 10.15212/bioi-2024-0107.
Mohammed MI, Makky AM, Abdellatif MM. Formulation and characterization of ethosomes bearing vancomycin hydrochloride for transdermal delivery. Int J Pharm Pharm Sci. 2014 Nov 1;6(11):190-4.
Alfawaz Altamimi AS, Arockia Babu M, Afzal M, Bishoyi AK, Roopashree R, Saini S. Exosomes derived from natural killer cells: transforming immunotherapy for aggressive breast cancer. Med Oncol. 2025;42(4):114. doi: 10.1007/s12032-025-02647-y, PMID 40100465.
Musielak E, Krajka Kuzniak V. Liposomes and ethosomes: comparative potential in enhancing skin permeability for therapeutic and cosmetic applications. Cosmetics. 2024;11(6):191. doi: 10.3390/cosmetics11060191.
Fadaei MS, Fadaei MR, Kheirieh AE, Rahmanian Devin P, Dabbaghi MM, Nazari Tavallaei K. Niosome as a promising tool for increasing the effectiveness of anti-inflammatory compounds. Excli J. 2024 Feb 7;23:212-63. doi: 10.17179/excli2023-6868, PMID 38487088.
Satyam G, Shivani S, Garima G. Ethosomes: a novel tool for drug delivery through the skin. J Pharm Res. 2010;3(4):688-91.
Opatha SA, Titapiwatanakun V, Chutoprapat R. Transfersomes: a promising nanoencapsulation technique for transdermal drug delivery. Pharmaceutics. 2020;12(9):855. doi: 10.3390/pharmaceutics12090855, PMID 32916782.
Gupta R, Badhe Y, Rai B, Mitragotri S. Molecular mechanism of the skin permeation enhancing effect of ethanol: a molecular dynamics study. RSC Adv. 2020 Mar 24;10(21):12234-48. doi: 10.1039/d0ra01692f, PMID 35497613.
Ghosh A, Sirisolla JD. Ethosomes: design, composition and characterization in different disease management. YMER. 2024;23(9):1173-84.
Paiva Santos AC, Silva AL, Guerra C, Peixoto D, Pereira Silva M, Zeinali M. Ethosomes as nanocarriers for the development of skin delivery formulations. Pharm Res. 2021 May 25;38(6):947-70. doi: 10.1007/s11095-021-03053-5, PMID 34036520.
Ruwizhi N, Aderibigbe BA. The efficacy of cholesterol-based carriers in drug delivery. Molecules 2020;25(18):4330.
Mast R. Glycerine in creams lotions and hair care products. In: Jungermann E, Sonntag NO, editors. Glycerine. Boca Raton: CRC Press; 2018. p. 345-79. doi: 10.1201/9780203753071-13.
Richard C, Souloumiac E, Jestin J, Blanzat M, Cassel S. Influence of dermal formulation additives on the physicochemical characteristics of catanionic vesicles. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2018 Dec 5;558:373-83. doi: 10.1016/j.colsurfa.2018.09.007.
Chauhan N, Vasava P, Khan SL, Siddiqui FA, Islam F, Chopra H. Ethosomes: a novel drug carrier. Ann Med Surg (Lond). 2022 Oct 1;82:104595. doi: 10.1016/j.amsu.2022.104595, PMID 36124209.
Neeraj Kumar, Anubhav Dubey, Ashish Mishra Pallavi Tiwari. Ethosomes: a novel approach in transdermal drug delivery system. International Journal of Pharmacy & Life Sciences. 2020;11(5):6598-607.
Dhurke R, Pravalika G, Chandhana P, Chiranjitha I. Minoxidil ethosomes for treatment of alopecia. International Journal of Recent Scientific Research. 2020;11(1):37112-7. doi: 10.24327/ijrsr.2020.1101.5056.
Parmar P, Mishra A, Pathak A. Preparation and evaluation of ethosomal gel of clotrimazole for fungal infection by mechanical dispersion method. Current Research in Pharmaceutical Sciences. 2016 Jul 9;6(2):45-9.
Zhou Y, Wei YH, Zhang GQ, Wu XA. Synergistic penetration of ethosomes and lipophilic prodrug on the transdermal delivery of acyclovir. Arch Pharm Res. 2010;33(4):567-74. doi: 10.1007/s12272-010-0411-2, PMID 20422366.
Seyedehhamideh Razavi, Alireza Partoazar, Nasrin Takzaree. Silver sulfadiazine nanoethogel for burn healing: characterization and investigation of its in vivo effects. Nanomedicine. 2018 Jun;13(11):1319–31. doi: 10.2217/nnm-2017-0385.
Peram MR, Jalalpure S, Kumbar V, Patil S, Joshi S, Bhat K. Factorial design-based curcumin ethosomal nanocarriers for the skin cancer delivery: in vitro evaluation. J Liposome Res. 2019;29(3):291-311. doi: 10.1080/08982104.2018.1556292, PMID 30526186.
Nasri S, Ebrahimi Hosseinzadeh B, Rahaie M, Hatamian Zarmi A, Sahraeian R. Thymoquinone-loaded ethosome with breast cancer potential: optimization in vitro and biological assessment. J Nanostructure Chem. 2020;10(1):19-31. doi: 10.1007/s40097-019-00325-w.
Ahmed TA, Alzahrani MM, Sirwi A, Alhakamy NA. The antifungal and ocular permeation of ketoconazole from ophthalmic formulations containing trans-ethosomes nanoparticles. Pharmaceutics. 2021;13(2):151. doi: 10.3390/pharmaceutics13020151, PMID 33498849.
Abd El Alim SH, Kassem AA, Basha M, Salama A. Comparative study of liposomes ethosomes and transfersomes as carriers for enhancing the transdermal delivery of diflunisal: in vitro and in vivo evaluation. Int J Pharm. 2019;563:293-303. doi: 10.1016/j.ijpharm.2019.04.001, PMID 30951860.
Fathalla D, Youssef EM, Soliman GM. Liposomal and ethosomal gels for the topical delivery of anthralin: preparation, comparative evaluation and clinical assessment in psoriatic patients. Pharmaceutics. 2020;12(5):446. doi: 10.3390/pharmaceutics12050446, PMID 32403379.
Yu Z, LV H, Han G, Ma K. Ethosomes loaded with cryptotanshinone for acne treatment through topical gel formulation. PLOS One. 2016;11(7):e0159967. doi: 10.1371/journal.pone.0159967, PMID 27441661.
Tarek M Ibrahim, Marwa H Abdallah, Nagia A El Megrab, Hanan M El Nahas. Transdermal ethosomal gel nanocarriers; a promising strategy for enhancement of the anti-hypertensive effect of carvedilol. Journal of Liposome Research. 2019 Sep;29(3):215-28. doi: 10.1080/08982104.2018.1529793.
Paiva Santos AC, Silva AL, Guerra C, Peixoto D, Pereira Silva M, Zeinali M. Ethosomes as nanocarriers for the development of skin delivery formulations. Pharm Res. 2021 May 25;38(6):947-70. doi: 10.1007/s11095-021-03053-5, PMID 34036520.
Wang Y, Song J, Zhang F, Zeng K, Zhu X. Antifungal photodynamic activity of hexyl-aminolevulinate ethosomes against Candida albicans biofilm. Front Microbiol. 2020 Sep 11;11:2052. doi: 10.3389/fmicb.2020.02052, PMID 33042036.
Goindi S, Dhatt B, Kaur A. Ethosomes-based topical delivery system of antihistaminic drug for treatment of skin allergies. J Microencapsul. 2014 Nov 1;31(7):716-24. doi: 10.3109/02652048.2014.918667, PMID 24963956.
Verma P, Pathak K. Therapeutic and cosmeceutical potential of ethosomes: an overview. J Adv Pharm Technol Res. 2010;1(3):274-82. doi: 10.4103/0110-5558.72415, PMID 22247858.
Akhtar N, Akhtar N. Development of stable tocopherol succinate-loaded ethosomes to enhance transdermal permeation: in vitro and in vivo characterizations. J Cosmet Dermatol. 2022 Oct 1;21(10):4942-55. doi: 10.1111/jocd.14907, PMID 35274433.
Omidian H, Wilson RL, Chowdhury SD. Enhancing therapeutic efficacy of curcumin: advances in delivery systems and clinical applications. Gels. 2023 Aug 1;9(8):596. doi: 10.3390/gels9080596, PMID 37623051.
Pathan IB, Jaware BP, Shelke S, Ambekar W. Curcumin-loaded ethosomes for transdermal application: formulation optimization in vitro and in vivo study. J Drug Deliv Sci Technol. 2018 Apr 1;44:49-57. doi: 10.1016/j.jddst.2017.11.005.
Rezapour E, Kariminezhad H, Mostafazadeh A, Ghasemi R, Pourbagher R, Neyshaburi EZ. Enhancement of transdermal vaccination using electrothermal active interfaces. J Drug Deliv Sci Technol. 2024 Jun 1;96:105639. doi: 10.1016/j.jddst.2024.105639.
Pawar S, Shivarkar R, Dhole S. Ethosome: a novel carrier used in transdermal and topical drug delivery. International Journal of Pharmaceutical Research and Applications. 2024 May-Jun;9(3):2442-52. doi: 10.35629/4494-090324422452.
Abdallah HM, El Megrab NA, Balata GF, Eissa NG. Niosomal and ethosomal gels: a comparative in vitro and ex vivo evaluation for repurposing of spironolactone. J Drug Deliv Sci Technol. 2022 Aug 1;74:103583. doi: 10.1016/j.jddst.2022.103583.
Claudia Paiva Santos A, Gama M, Peixoto D, Sousa Oliveira I, Ferreira Faria I, Zeinali M. Nanocarrier based dermopharmaceutical formulations for the topical management of atopic dermatitis. Int J Pharm. 2022 Apr 25;618:121656. doi: 10.1016/j.ijpharm.2022.121656, PMID 35278601.
Ferreira L, Mascarenhas Melo F, Rabaca S, Mathur A, Sharma A, Giram PS. Cyclodextrin-based dermatological formulations: dermopharmaceutical and cosmetic applications. Colloids Surf B Biointerfaces. 2023 Jan 1;221:113012. doi: 10.1016/j.colsurfb.2022.113012, PMID 36395617.
Diclofenac topical: uses side effects, interactions, pictures, warnings and dosing-webmd. Available from: https://www.webmd.com/drugs/2/drug-22221-1065/diclofenac-sodiumtopical/diclofenac-1-topical/details. [Last accessed on 22 Mar 2025].
Buy dermovate cream and ointment online. Next-day delivery UK. Available from: https://www.cloudpharmacy.co.uk/online-doctor/eczema-psoriasis/dermovate-cream-ointment. [Last accessed on 22 Mar 2025].
Ketoconazole Bp 2.0% W/w Cream (kt-sal Cream). Available from: https://www.indiamart.vol.20gat₹130/pieceinsurat.com./proddetail/ketoconazole-bp-2-0-w-w-cream-kt-sal-cream-2851103006148.html?srsltid=afmbooqoojb5qnuvomz-gmbwcwy2oastkiof1ooaciijrpgbi1pzdkth. [Last accessed on 22 Mar 2025].
Kimbonguila A, Matos L, Petit J, Scher J, Nzikou JM. Effect of physical treatment on the physicochemical, rheological and functional properties of yam meal of the cultivar ngumvu from Dioscorea alata L. of Congo. Int J Recent Sci Res. 2019;10(11):30693-5.
Clonazepam (Klonopin): uses side effects, interactions pictures, warnings and dosing-WebMD. Available from: https://www.webmd.Com/drugs/2/drug-920-6006/klonopin-oral/clonazepam-oral/details. [Last accessed on 22 Mar 2025].
Anuradha BR, Bai YD, Sailaja S, Sudhakar J, Priyanka M, Deepika V. Evaluation of anti-inflammatory effects of curcumin gel as an adjunct to scaling and root planing: a clinical study. J Int Oral Health. 2015 Jul;7(7):90-3. PMID 26229378.
Google Patents. Available from: https://patents.google.com. [Last accessed on 5 May 2025].