
Int J App Pharm, Vol 17, Issue 6, 2025, 25-37Reviewl Article
AN OVERVIEW OF BILAYER TABLET TECHNOLOGY: DESIGN, MANUFACTURING, AND THERAPEUTIC APPLICATIONS
VEERENDRA UMESH DHOKE1, SHUBHAM S. GUPTA2, BHAGYASHRI R. DHAKATE3, RAKSHA PUROHIT4, PRIYANKA A. MANDAL5, SHUBHAM KAMBLE6*GULSHAN GURUNANI7
1Department of Pharmaceutics, the Royal Gondwana College of Pharmacy Nagpur, Maharashtra, India. 2Department of Pharmaceutical Sciences, Gurunanak College of Pharmacy, Nagpur, Maharashtra, India. 3Department of Pharmaceutics, National College of Pharmacy, Nagpur, Maharashtra, India. 4Department of Pharmaceutical Sciences, Rashtrasant Tukadoji Maharaj Nagpur University Nagpur, Maharashtra, India. 5Department of Pharmaceutics, Siddhivinayak College of Pharmacy, Warora, Maharashtra, India. 6Department of Pharmaceutics the Royal Gondwana College of Pharmacy, Nagpur, Maharashtra, India. 7Pharmaceutical Chemistry, Gurunanak College of Pharmacy, Nagpur, Maharastra, India
*Corresponding author: Shubham Kamble; *Email: shubhkamble121@gmail.com
Received: 11 Apr 2025, Revised and Accepted: 26 Aug 2025
ABSTRACT
Bilayer tablets are innovative drug delivery systems designed to enhance therapeutic efficacy by enabling the simultaneous or sequential release of active pharmaceutical ingredients. They offer distinct advantages in managing diseases requiring combination therapy or controlled release. This system enables the incorporation of two different drugs or release profiles within a single unit, enhancing patient compliance and therapeutic outcomes. Bilayer tablets are particularly beneficial for drugs with narrow therapeutic windows, those requiring sustained release, or those with poor bioavailability. This review outlines the formulation strategies, types of bilayer tablets, manufacturing challenges, evaluation parameters, and current regulatory considerations.
Keywords: Bilayer tablets, Controlled release, Combination therapy, Formulation strategies, Regulatory considerations
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijap.2025v17i6.54542 Journal homepage: https://innovareacademics.in/journals/index.php/ijap
INTRODUCTION
Oral drug delivery remains the most preferred route for medication administration due to its convenience, non-invasiveness, high patient compliance, and broad formulation options. Among oral dosage forms, tablets are especially favored for their ease of handling, dosing accuracy, and stability profiles [1, 2]. The pharmaceutical industry has increasingly focused on the development of advanced oral dosage forms, such as controlled-release and sustained-release systems, to enhance therapeutic efficacy and minimize dosing frequency [2].
Bilayer tablets represent a significant advancement in oral drug delivery technology. These systems are designed to incorporate two different layers, each capable of delivering one or more active pharmaceutical ingredients (APIs) with distinct release profiles, typically one for immediate release and the other for sustained release [3, 4]. This design is particularly beneficial for combination therapy, especially in the management of chronic conditions such as hypertension, diabetes, and inflammation [5]. By physically separating APIs that are chemically or physically incompatible, bilayer tablets improve drug stability and minimize formulation challenges [6].
Furthermore, bilayer tablets offer greater flexibility in customizing drug release kinetics. They can be engineered to release drugs sequentially or simultaneously, depending on therapeutic needs. For example, a loading dose may be delivered via the immediate-release layer, while the maintenance dose is released slowly from the sustained-release layer [7]. Innovations such as floating bilayer tablets have further enhanced the utility of this dosage form by prolonging gastric residence time, which is especially valuable for drugs that are unstable or poorly soluble in intestinal fluids [8]. These systems are particularly effective in improving bioavailability and achieving long-acting therapeutic effects [9].
Advanced fabrication techniques now allow for multilayered matrix structures and coatings that further refine drug release patterns. Technologies such as gastro-retentive systems, osmotic pumps, and polymeric matrices have contributed to the evolution of bilayer tablets into precise and patient-friendly delivery tools [10].
A comprehensive literature search was conducted using PubMed, Scopus, and Google Scholar databases. Keywords included: “bilayer tablets,” “dual-release formulations,” “controlled release,” “multi-layered tablets,” “oral drug delivery systems,” and “tablet compression.” Filters were applied to include peer-reviewed articles published in English between 2018 and 2025, with special emphasis on studies from the last five years (2020–2024). Priority was given to review articles, original research, and regulatory reports relevant to bilayer tablet formulation, manufacturing technologies, and therapeutic applications.
Types of bilayer tablets
Bilayer tablets can be homogenous, which consists of uniform composition, and heterogeneous, which consists of well-defined layers of two or more compositions.
Homogenous type
All drug-layers are homogeneous in composition, and the two-layer tablet consists of the same drug in both layers with different drug-release characteristics. Usually, one layer is immediate-release, and the other is extended-release [11].
Heterogeneous type
Heterogeneous bilayer tablets are designed either for the sustained release of two drugs in combination or for separating incompatible substances within a single tablet [11].
To manufacture quality bilayer tablets under validated GMP conditions, we need a bilayer tablet press that can: Several advanced techniques have been developed for preparing bilayer tablets to achieve controlled and targeted drug release. Notable among these are Elan Drug Technology’s Dual Release Drug Delivery System, DUROS TROL Technology, EN SO TROL Technology, L-OROS Technology, and OROS Push Pull Technology [15]. These technologies enable precise modulation of drug release profiles to enhance therapeutic efficacy and patient compliance.
OROS push-pull technology
OROS Push-Pull Technology is a sophisticated drug delivery system designed to release medication in a controlled and consistent way over time. The tablet typically consists of two or three layers: one or more layers containing the active drug, and another called the “push layer.” The drug layer also includes ingredients that help suspend the drug evenly and draw in water. The push layer is made of special polymers that swell when they absorb fluid. As water enters the tablet through a semi-permeable outer membrane, the push layer expands and steadily pushes the drug out through a small, laser-drilled hole. This controlled mechanism helps deliver the medication at a consistent rate, improving its effectiveness and reducing the need for frequent dosing [16].
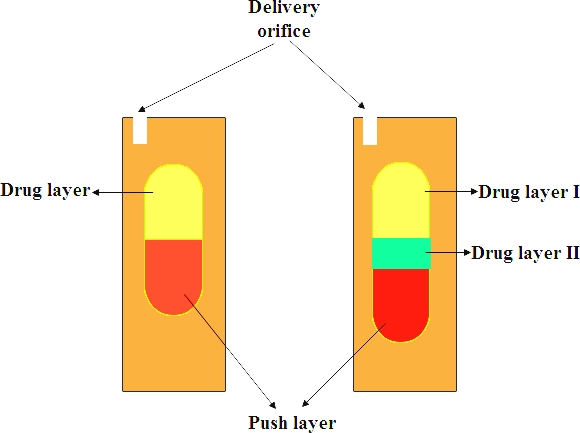
Fig. 1: Illustrates the OROS push-pull technology, showing the drug layers separated by a push layer. The delivery orifice releases the drug at controlled rates from both drug layers, offering both immediate and extended release characteristics.
L-OROS technology
It is a specialized drug delivery system designed to improve the effectiveness of medications that don’t dissolve well in water. It works by using a lipid-based formulation that holds the drug in a liquid form inside a soft gelatin capsule. Surrounding this core is a carefully engineered system that includes an osmotic push layer, a semi-permeable membrane, and a tiny hole called an egress orifice that controls how the drug is released. When the tablet comes into contact with fluid in the body, water enters through the membrane, activates the osmotic layer, and pushes the drug out in a slow, steady manner. This helps ensure that the drug is absorbed more reliably and over a longer period, improving both its effectiveness and the patient’s experience [17].
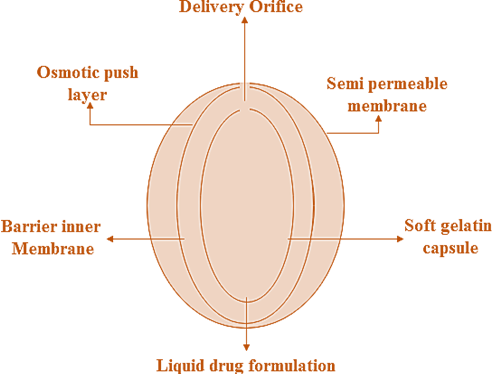
Fig. 2: Demonstrates the structure of L-OROS technology, highlighting the osmotic push layer, semi-permeable membrane, and the liquid drug formulation within a soft gelatin capsule. The system is designed to enable controlled drug release through the delivery orifice
EN-SO-TROL technology
EN-SO-TROL is an integrated drug delivery system designed to enhance the solubility and bioavailability of poorly soluble drugs. It employs a combination of solubility-enhancing agents and a semi-permeable membrane, which enables controlled drug release. The inclusion of a wicking agent in the system guarantees consistent drug delivery over time, thereby improving patient compliance and therapeutic outcomes. This technology is particularly effective in formulations that require precise dosing and extended therapeutic efficacy action [18].
DUROS technology
DUROS Technology is an advanced drug delivery system designed to provide a steady, long-term release of medication, sometimes lasting for months or even years. It works by using a tiny, implantable device made of titanium that safely stores the drug in liquid form. This protective design keeps the medication stable and shields it from the body's enzymes and external conditions. Once implanted, the device absorbs body fluids through a special membrane, which creates osmotic pressure. This pressure slowly moves a piston inside the device, pushing the drug out through a small opening at a constant rate. DUROS is especially useful for treating chronic conditions where patients need consistent medication over long periods, such as in cancer therapy, pain management, or hormone treatments [19].
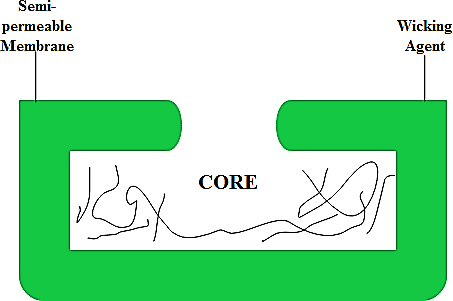
Fig. 3: Illustrates the EN-SO-TROL technology, showing the core surrounded by a semi-permeable membrane and a wicking agent. This system is designed to enhance drug delivery by utilizing the wicking agent to facilitate controlled release
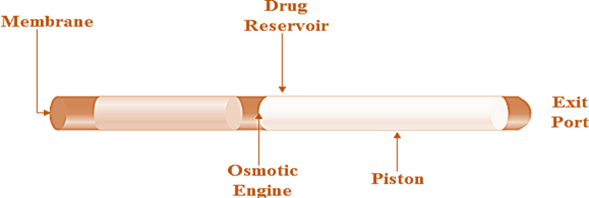
Fig. 4: DUROS technology shows the DUROS technology, depicting the osmotic engine, drug reservoir, piston, and exit port. This system is designed to provide controlled and sustained drug release over an extended period by utilizing osmotic pressure to move the drug through the exit port
Duredas™ technology (A dual-release drug delivery system, elan drug technologies)
A new bilayer tablet technology has been developed to provide either immediate (IR) and sustained release (SR) for two different drugs or for different rates of release of one drug all in one dosage form. It consists of the delivery system layered as classes of both an immediate-release granulate and a modified-release hydrophilic matrix complex in the same tablet during the tableting process. A combination of hydrophilic polymers are used, thus imparting modified-release properties [20].
Bilayer tablet production is primarily determined by the physical and/or chemical properties of active pharmaceutical ingredients (APIs) and excipients. The strength and failure of bilayer tablets depend on the material composition of the dosage form. Compression, in all its aspects-plasticity, brittleness, and viscoelasticity-must consider the deformation of plasticity and brittleness of materials under compression. Plasticity refers to a material’s ability to undergo permanent deformation without breaking, allowing it to retain its new shape once the stress is removed. In bilayer tablet manufacturing, plasticity helps the material flow into the die cavity and maintain uniformity in layer thickness, ensuring the tablet’s structural integrity [21]. However, excessive plasticity can lead to excessive deformation, which may affect the tablet's final shape. Brittleness, on the other hand, refers to a material's tendency to break or fracture under stress instead of deforming. Too much brittleness in bilayer tablets can lead to tablet breakage during compression or handling, causing issues like capping or delamination between layers [22]. Viscoelasticity, which combines both viscous (resistance to flow) and elastic (ability to return to original shape) properties, plays a crucial role in the manufacturing of bilayer tablets. During compression, the viscous component ensures that the material flows properly to fill the die cavity, enabling uniform tablet formation, while the elastic component allows some recovery after compression [23]. A material with an optimal viscoelastic profile can deform sufficiently under pressure without cracking or losing structural integrity, which is essential for achieving uniformity and mechanical strength in bilayer tablets. If plastic deformation remains below the bonding limit, it does not negatively affect the process; however, excessive plasticity can lead to undesirable deformation, while excessive elasticity can cause interfacial stress, leading to capping or delamination. Balancing these viscoelastic properties ensures that the material compresses efficiently, maintains strong interlayer adhesion, minimizes brittleness, and supports the structural integrity and therapeutic performance of the final dosage form. Compatibility of viscoelastic properties between both layers is particularly important to avoid interfacial failure and ensure a stable, high-quality bilayer tablet [24, 25]. However, since the breakdown of particles is more considerable in the middle layer of the die compared to the outer layer, it is essential to assess the material properties of a substance before manufacturing bilayer tablets. For a multilayer tablet formulation, volume reduction, mechanical durability, and cohesive strength among each layer are required. They must be highly compact (compressibility-the ability of a material to decrease in volume under pressure; compatibility-the ability of powdered material to convert into tablet form) [26]. To stack tablets, individual layers must be precisely controlled in weight by either optimizing particle size distribution, flow properties, and compression capabilities of the material, or by specifically designing the tablets [27].
Reproducibly making bilayer tablets relies on precise control of the compression force applied to the first layer, as this force significantly influences the binding and adhesion between the layers at their interface [28]. This ensures incredibly tight and uniform aeration between the layers. However, if the first layer is too elastic, excessive stress and strain can compromise the entire bilayer tablet structure and weaken layer adhesion due to contact breakage. Additionally, the compactability of the die itself is crucial to the entire process [29].
Compression improves both the tensile strength and the smoothness of the surfaces. A smoother surface of the first layer can help lower adhesion between molecular bonds, which might aid in layer delaminating [30]. A lightweight first layer, which moves very freely at a low compressive force, effectively ends up interacting with the second layer during final compression, when the bonding is to take place [31]. On the other hand bonding is extremely negatively affected with high compression force when it comes to the first layer. Even the distribution of lubricant reduces friction between the lubricant particles and mold [32]. For bilayer formulations, a lower amount of lubricant is needed, thus improving the contact and strength between the layers. On the flip side, bilayer tablets are more sensitive to lubricant levels than they are to brittle materials since the material flow affects the bilayer structure more [33].
It is critical to establish the right level of lubricant that will allow the first layer to release from the tablet press during product development [34]. Lubricant is mixed and blown onto the surface of the granule when it touches the die and punches in the processes of compression, hence diminishing friction and wear [35]. This also decreases intergranular bonding, influencing the tablet QA in ways such as breaking force and dissolution [36]. External lubrication has also been investigated in which the lubricant is applied to the dies and punches rather than internally mixed with the granules [36]. External lubrication sprays lubricant on the die and punches during every compression cycle (rather than being included in the bulk powder mixtures); it can raise crushing strength by as much as 40%. The technique has been verified to produce a magnesium stearate layer on the tablet surface via scanning electron microscopy. While this method is suitable for monolayer tablets, it will give an understanding of the effect of lubricant on the quality attributes of bilayer tablets [37].
In a typical bilayer tablet formulation, the two layers only sometimes weigh the same, giving rise to various tablet weight ratios (1:1, 1:2, 1:3, etc). It is incredibly tricky to keep the second layer's weight the same as the first in manufacturing [38]. The compaction of bilayer tablets is mainly influenced by environmental factors such as humidity and moisture. Strangely enough, little is known about moisture's effect on bilayer tablet strength [39]. Hygroscopic materials in tablets absorb and desorb water as a function of the relative humidity, which will alter the pore structure of these tablets [40]. Water can also pass through compacts from sodium starch glycolate, starches, microcrystalline cellulose, crospovidone, polyvinylpyrrolidone, and colloidal silicon dioxide. In the long run, the moisture-driven expansion delaminates the porous structures, as the interfacial contact between the layers may become ineffective [41, 42].
One of the significant challenges in bilayer tablet manufacturing is ensuring robust interfacial bonding between the layers [43]. The quality of bonding directly influences the tablet’s structural integrity and its performance during storage and use. Issues such as insufficient interlayer bonding can lead to separation, cracking, or delamination of the tablet. For successful bilayer tablet production, it is essential to achieve the right compression force to ensure proper bonding without compromising the properties of each layer. The use of excipients like microcrystalline cellulose (MCC) is commonly employed to enhance this interfacial adhesion and improve stability during environmental stress, such as humidity changes.
Moisture is another crucial factor affecting the stability of bilayer tablets. Since certain excipients, like microcrystalline cellulose (MCC), are hygroscopic, their interaction with moisture can lead to swelling, compromising the tablet's structure. Increased humidity and prolonged storage time can also reduce interfacial strength, causing delamination or altering the release profile of the active ingredients. To mitigate these issues, it is recommended that materials be preconditioned in the same conditions as the manufacturing environment (relative humidity) and that the tablets be packaged in blisters with moisture-resistant materials to prevent vapor ingress [24]. A detailed moisture control protocol ensures that tablets maintain their physical stability and quality parameters, such as tensile strength, interlayer adhesiveness, friability, and dissolution, under varying environmental conditions. In contrast, bilayer tablets utilizing microcrystalline cellulose (MCC) as widely used binder and disintegrant in the first layer (at the interface) demonstrate an increase in interfacial strength with the same changes in humidity and storage time [44].
Uniform dispersion of active pharmaceutical ingredients
Uniform dispersion of active pharmaceutical ingredients (APIs) in the individual layers of bilayer tablets is dependent on multiple factors such as the flowability of the material, size distribution of the particles, and compactibility of the bilayer mix [45]. Flowability of each layer is very important to achieve consistent weight and uniform distribution of API among the layers, thus, material flow properties are critical. Variations in layer weight and drug content due to poor flow can affect the performance and uniformity of the tablet [46].
Uniform particle size in each material layer
Each layer aims for a uniform particle size distribution to facilitate blending and limit segregation. This maintains a consistent pinout across the tablet's API dispersion [47].
Compressibility
The compressibility of bilayer mixture is an important factor in the final thickness of the cohesive tablet that is necessary to provide sufficient hardness and structural integrity. Inadequate compactibility can lead to capping, lamination or a defect in a tablet thereafter resulting in its quality [48].
Weight control methods
Different methods can be used to control the weight of both layers in bilayer presses that are instrumented. Normally, the first and second layers are individually weighted. Yet, one of the main challenges over weight control and homogeneity of the second layer is that often there are neither commercial available mechanisms to directly weigh the second layer on existing presses. In most instrumented bilayer presses, the compression force and the punch displacement are calculated automatically. With the latest improvements in compression machine design and accessory technologies, functions can now be product-specific like first-layer sampling, sealed feeders, pre-compression rolls (to create uniformity in the powder to give the same load to every single small sample), layer strain gauge sensitivity and upper punch maximum penetration. Aside from these theoretical physical benefits, such as loss-free compression and independent compaction, many other things contributed to the creation of high-end bilayer tablets. These consist of the sample's particle size distribution, angle of repose, photo microscopic examinations, densities, compressibility’s, and moisture sorption capacity. This type of medication delivery is most suitable for coronary vasodilators, as well as antihypertensive, antihistamine, analgesic, antipyretic, and antiallergenic medications. In certain bilayer tablets, both layers function as sustained-release components for specific antidiabetic medications [49].
Fabrication of the bilayer tablet
The outer layer releases the drug quickly and the inner layer releases drug slowly, acting as a second dose or giving it an extended release [52]. Bilayer tablets are formed by compressing 2 layers of each incompatible pharmaceuticals, to minimize the contact between 2 incompatible pharmaceuticals.
Compaction
Formulating the correct tablet is therefore dependent on some parameters, including good mechanical strength and good drug releasing profile. Nevertheless, obtaining these prerequisites can be difficult, specifically for the formulator if the drug has poor flow and compatibility properties, which may cause capping and/or lamination problems, particularly in bi-layer tablet preparations that employ the double compression principle. The properties of compressibility and consolidation of the material are the paramount parameters of the process of compaction.
Compression
A method that reduces the size of contents by removing empty spaces and allows the closeness of particles.
Consolidation
It is a characteristic of material in which mechanical strength is enhanced due to inter-particle interaction (bonding). However, the first layer compressive stress was proved to have a great effect on tablet crack.
Table 1: Key features and challenges of bilayer tablets in drug delivery
| Aspect | Description | Reference |
| 1. Control release of APIs | To control the release of one or two active pharmaceutical ingredients (APIs) at a time. | [15] |
| 2. Control release of therapeutic agents | To control the release of one to two therapeutic agents or excipients. | [15] |
| 3. Dissociation of incompatible APIs | To exploit the functional behavior of the extra instars for dissociating incompatible APIs and control their release from a layer (e. g., through osmotic properties). | [22] |
| 4. imgding incompatible APIs | To img two active pharmaceutical ingredients that are not compatible with one another while using the functional properties of the outer layer to regulate the release property from a single layer. | [22] |
| 5. Incorporation of API layer between passive layers | Incorporating the API layer between one or two passive layers translates to a larger available surface area for the API layer, resulting in swellable or erodible barriers for customized release profiles. | [23] |
| 6. Surface area modification | To change the entire surface area available for the API layer through the incorporation of 1 or 2 passive layers in the bilayer sheet, enabling the creation of swellable or erodible barriers for modified release. | [23] |
| 7. Fixed-dose combinations | To provide multiple active pharmaceutical ingredients in optimal fixed-dose combinations to prolong the product lifecycle and novel drug delivery systems, including chewable tablets, floating tablets, and buccal systems. | [23] |
| 8. Purpose of multilayered tablets | To regulate the release of APIs, separate incompatible APIs, and enhance API surface area via passive layers for controlled or customized release. | [50] |
| 9. Economical | Bilayer tablets are relatively economical compared to other oral dosage forms. | [51] |
| 10. Stability | Bilayer tablets have superior chemical and microbiological stability compared to all other oral dosage forms. | [51] |
| 11. Limitation (swallowing issues) | Kids and those who are unconscious may have trouble swallowing them. | [51] |
| 12. Compression difficulties | The characteristic amorphous form and lower density of some drugs can lead to difficulties compressing a tablet into a dense one. | [51] |
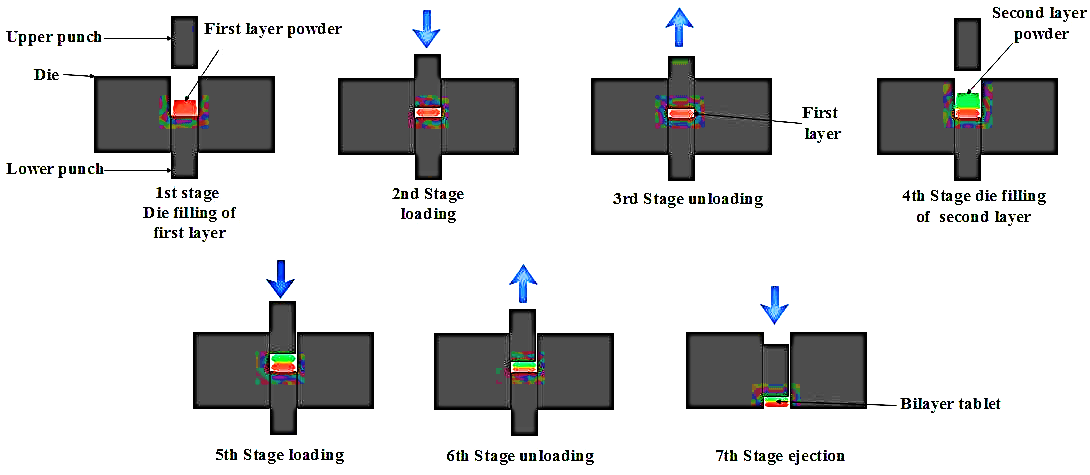
Fig. 5: Fabrication/Manufacturing of the bilayer tablet

Fig. 6: Single-sided tablet press showing powder filling and compression from one side to form a tablet [54] **https://www.adinathpharma.com/single-sided-rotary-tablet-press-machine.html
Types of bilayer tablet press
Single-sided tablet press
The most basic design is a single-sided press where the chambers of the double feeder are distinctly separated. Each chamber is fed by gravity or force with different powders, creating the two individual tablet layers. As the die moves beneath the feeder, it is first filled with the powder for the first layer, followed by the powder for the second layer. The complete tablet is then compressed in one or two steps [53].
The single-sided tablet press, while commonly used in bilayer tablet manufacturing, presents several limitations that can affect the quality and consistency of the final product:
Inadequate Individual Layer Weight Control: Single-sided presses often lack precise mechanisms to monitor and control the weight of each individual layer, leading to potential variability in dosing.
Short Dwell Time for First Layer: The design of single-sided presses can result in a very short dwell time for the first layer during compression. This insufficient dwell time may cause poor de-aeration, leading to issues like capping and inadequate hardness.
Lack of Distinct Visual Separation Between Layers: There is a tendency for slight mixing at the interface of the two layers in single-sided presses, which can result in a lack of clear visual separation between the layers. This can be problematic for quality control and product identification.
Difficulty in First-Layer Sampling: Sampling the first layer for in-process quality control is challenging in single-sided presses, making it difficult to perform timely weight recalibrations and ensure consistent tablet quality.
These limitations highlight the need for careful consideration when choosing tablet press equipment for bilayer tablet production, especially when precise layer control and product quality are critical [55].

Fig. 7: Double-sided tablet press showing simultaneous compression of two powder layers from both sides, enabling the formation of bilayer tablets with distinct layers for controlled drug release [56]**https://www.fluidpack.net/blogs/b4-double-sided-rotary-tablet-press-features-and-applications

Fig. 8: Bilayer tablet press with displacement monitoring – Enables real-time tracking of punch movement to ensure uniform tablet thickness and layer bonding [59] **https://shriramassociate.in/products/bilayer-tablet-press-machine?srsltid=AfmBOorLsFibriv7_5HI-4VpPvcwNkgmPgtK2uj1VCRHKOpB2y-i8rHG
Bilayer tablet press with displacement monitoring
The principle of displacement-based tablet weight control differs significantly from that based on compression force. In this method, the sensitivity of the control system when measuring displacement is not dependent on the tablet's weight but rather on the applied precompression force [57, 58]. This approach offers several advantages, including the ability to monitor and control the weight of each individual layer, thereby preventing capping and separation between the two layers. It also operates independently of the machine's stiffness and ensures adequate hardness even at maximum turret speed. Moreover, it minimizes the risk of cross-contamination between the layers and provides a clear visual separation, ultimately enhancing the overall yield [59].
Methods employed for bilayer tableting
Layered compression
With layered compression, each layer of the tablet is compressed individually before the layers are combined, giving precise control over each layer's properties and release profiles [60].
Granulation techniques
To improve the flowability of the powders and the uniformity of the tablets, various granulation techniques are used for each layer, such as wet granulation or dry granulation [61].
Excipients
Different excipients can be employed in each layer to tailor the drug release profile. For instance, hydrophilic polymers could serve in one layer for immediate release, while hydrophobic materials could be deployed in another for sustained release [62].
Coating techniques
Coating techniques in bilayer tablets serve to protect moisture-sensitive drugs, control drug release (e. g., enteric or sustained release), enhance mechanical strength, prevent layer separation, and improve taste and appearance. Common coating materials include HPMC, ethyl cellulose, and PVA, applied through methods like pan or spray coating [63].
Osmotic systems
In some bilayer tablets, osmotic principles are used for controlled release. Each layer may be compressed with a different set of compression parameters: adjusting the compression parameters (e. g., force and speed) for each layer can optimize the tablet's mechanical properties and performance [64].
Adhesives
Adhesives in bilayer tablets help bond the layers together to prevent delamination during compression, storage, or use. These are typically polymeric binders like HPMC or PVP, which enhance interfacial adhesion and can also influence drug release. Proper adhesive selection ensures structural integrity and consistent tablet performance [65].
Sequential release designs
The design can be customizable to elicit a specific sequential release of drugs in which one layer releases its drug first, followed by the second layer after a particular time [65].
Three-dimensional printing
Recent technological innovations have enabled 3D printing of bilayer tablets, providing novel customizations with drug release profiles and therapeutic aspects [66].
Applications of bilayer tablet
Controlled release
Bilayer tablets employ different materials and methods of compression to achieve and control the release of the drug over time. This can , however, be accomplished by engineering the first layer to consist of a fast-dissolving matrix to allow a rapid release of the drug, followed by materials that are able to dissolve at a more prolonged rate for the second layer thus giving the drug release over a longer time frame [67].
Immediate and extended release
Many therapeutic regimens have the need for patients to have not only immediate, but also sustained relief. This dual action can be effectively delivered by bilayer tablets. In pain management, for instance, one layer could deliver a fast-acting analgesic for immediate relief, and the other could contain a longer-lasting compound that maintains pain relief for several hours [68].
Combination therapies
Bilayer tablets are ideal for combination therapies needing two or more drugs to treat a particular condition. Physical and chemical interactions of incompatible drugs can be mitigated by compartmentalizing the active pharmaceutical ingredients (APIs) into separate layers. This is especially crucial wherein two drugs could become undesirable to be combined in one layer [69].
Targeted drug delivery
This feature enables selective delivery of the drug to different sites of the gastrointestinal tract. As an example, some drug products intended to be absorbed in the intestines could be coated in a bilayer tablet, formulated to resist disintegration in the stomach, to reach the optimal location of absorption. Bilayer tablets can also utilize pH-sensitive polymers for targeted drug delivery, ensuring that drugs are released in specific parts of the gastrointestinal tract. For example, a bilayer tablet could incorporate a pH-sensitive polymer like Eudragit in the outer layer, which dissolves in the higher pH of the intestine, while using a different polymer in the inner layer designed to release drugs in the acidic stomach environment. This strategy enables targeted drug release, improving treatment effectiveness while reducing side effects [70].
Enhancing patient compliance
Many of our patients are on complex dosing regimens with multiple medications taken at different times. Bilayer tablets eliminate this step by packaging multiple drugs or drug release profiles into a single dosage form. This can help ensure that the patient adheres to the treatment plan, which is important for managing chronic conditions like diabetes, hypertension, or psychiatric disorders [71].
Preventing drug degradation
Some of these drugs are susceptible to environmental factors, such as high humidity and light, that can cause degradation. Keeping these ingredients separated in distinct layers of a bilayer tablet allows formulators to stabilize unstable compounds, ensuring efficacy until absorption [72].
Reduction of side effects
Many medications can induce adverse effects when they reach high peak concentrations in the bloodstream. By controlling the release of drugs through bilayer formulations, manufacturers can reduce the likelihood of side effects. This is particularly beneficial in drugs with a narrow therapeutic index, where slight deviations from the effective dose can lead to toxicity [73].
Specific examples in diabetes treatment
In diabetic management, bilayer tablets are used to combine medications like metformin and glipizide. Metformin is designed for immediate release to control blood sugar spikes after meals, while glipizide is formulated for slow-release to manage blood sugar levels throughout the day. This combination not only enhances glucose control but also reduces the number of pills the patient has to take [74].
Applications of pain management
Bilayer pills, for instance, can allow for the quick release of an analgesic (a painkiller) like acetaminophen or ibuprofen in one layer and an opioid or non-opioid analgesic with a prolonged-release pharmacokinetic profile in the other layer. This dual-action medication first relieves pain quickly, followed by a long-lasting pain-controlling effect [75].
Formulation of cardiovascular drugs
Bilayer tablets can improve the way cardiovascular conditions like chronic heart failure or hypertension are treated. One layer may contain a fast-acting antihypertensive medicine, while the other layer may have a drug that maintains blood pressure control over time. This approach addresses both immediate and long-term management demands [76].
Therapeutic customization
A novel bilayer tablet formulation comprising a rapid-release layer of vitamin B6 and a sustained-release layer of melatonin was developed to achieve tailored drug delivery for improved therapeutic outcomes. The formulation strategy involved evaluating the effects of the viscosity and concentration of the sustained-release matrix material, the amount of diluent, and the particle size of melatonin on its release characteristics. Drug-release behavior was further assessed in various dissolution media, with in vitro tests confirming complete release of vitamin B6 within 10–15 min and approximately 90% cumulative release of melatonin over 8 h. Kinetic modeling indicated that melatonin release followed the Ritger–Peppas model, suggesting a non-Fickian diffusion mechanism involving both diffusion and erosion. Notably, the sustained-release layer primarily exhibited polymer swelling rather than erosion. Stability studies demonstrated the robustness of the bilayer tablets under stress conditions, including high temperature, humidity, and light exposure. These findings support the potential of this bilayer system as a customizable oral delivery platform for controlled melatonin release, although in vivo evaluation is warranted to confirm its therapeutic efficacy in humans [77].
Improved use of nutraceuticals
In nutraceuticals, bilayer systems are also advancing. These systems allow for the formulation of vitamins or dietary supplements so that one layer delivers a prolonged release of minerals or other elements required for general health, while the other layer offers quick nutritional advantages. For example, a bilayer tablet could release energy-boosting nutrients such as B-vitamins or caffeine in the immediate-release layer, while releasing minerals like calcium or magnesium in the sustained-release layer to support overall wellness throughout the day [78].
Table 2: Various advancements in the field of bilayer tablets
| Drug(s) | Dosage form | Rationale | Reference |
| Glipizide, Metformin Hydrochloride | Bilayer Tablets | To avoid interaction between incompatible drugs | [79] |
| Atenolol, Lovastatin | Bilayer Tablets | Synergistic effect in hypertension and biphasic release profile: Atenolol reduces heart rate and blood pressure, while Lovastatin lowers cholesterol levels. | [80] |
| Metformin Hydrochloride, Pioglitazone | Bilayer Tablets | Synergistic effect in diabetes mellitus: Metformin reduces insulin resistance, and Pioglitazone enhances insulin sensitivity. | [81] |
| Amlodipine, Atenolol | Bilayer Tablets | To improve the stability of drugs in combination: Amlodipine relaxes blood vessels, and Atenolol reduces the heart's workload. | [82] |
| Tramadol, Acetaminophen | Bilayer Tablets | Synergistic effect of drugs in pain: Tramadol inhibits the reuptake of serotonin and norepinephrine, while Acetaminophen inhibits COX enzymes to reduce pain. | [83] |
| Cefuroxime Axetil, Potassium Clavulanate | Bilayer Tablets | Synergistic effect against microbial infections, minimizing dose-dependent side effects: Cefuroxime Axetil inhibits bacterial cell wall synthesis, while Potassium Clavulanate protects against beta-lactamase enzymes. | [84] |
Atorvastatin Calcium |
Bilayer Buccal Tablets | To overcome bioavailability problems, reducing side effects and frequency of administration | [85] |
| Granisetron Hydrochloride | Bilayer Buccal Tablets | To overcome bioavailability problems, reducing side effects | [86] |
| Acetaminophen, Methocarbamol | Bilayer Tablets | Synergistic effect of drugs in back pain: Ibuprofen inhibits COX enzymes, while Methocarbamol relaxes muscles. | [87] |
| Salbutamol, Theophylline | Bilayer Tablets | Synergistic effect of drugs in asthma: Salbutamol relaxes bronchial muscles, and Theophylline improves lung function by inhibiting phosphodiesterase. | [88] |
| Metformin Hydrochloride | Bilayer Tablets | Synergistic effect in diabetes: Metformin reduces blood glucose levels by enhancing insulin sensitivity. | [89] |
| Losartan | Bilayer Tablets | Biphasic release profile: Losartan is used for hypertension and its extended release helps maintain steady blood pressure control. | [90] |
| Amlodipine Besilate, Metoprolol Succinate | Bilayer Tablets | Synergistic effect in hypertension: Amlodipine relaxes blood vessels, and Metoprolol reduces heart rate. | [91] |
| Misoprostol, Diclofenac | Bilayer Tablets | To minimize contact between drugs: Misoprostol protects the stomach lining, while Diclofenac reduces inflammation. | [92] |
| Diclofenac Sodium, Paracetamol | Bilayer Tablets | Synergistic effect in pain: Diclofenac inhibits COX-2, while Paracetamol inhibits COX-1 and COX-2 enzymes to reduce pain. | [93] |
| Valsartan, Clopidogrel Bisulfate | Bilayer Tablets | A bilayer tablet combining Valsartan and Clopidogrel Bisulfate offers dual therapeutic action for cardiovascular care. Valsartan, in the sustained-release layer, lowers blood pressure by blocking angiotensin II receptors, while Clopidogrel, in the immediate-release layer, prevents platelet aggregation by irreversibly inhibiting P2Y12 receptors. This design ensures rapid antiplatelet action with prolonged antihypertensive effect, improves patient compliance, and reduces drug interaction risks by separating the two drugs physically within the tablet. | [94] |
| Nifedipine | Gastro-Retentive Floating Bilayer Tablets | Treatment of hypertension and angina pectoris: Nifedipine relaxes blood vessels to reduce blood pressure. | [95] |
| Divalproex sodium | Bilayer Tablets | Divalproex sodium bilayer tablets combine an immediate-release layer for rapid symptom relief with a sustained-release layer for prolonged therapeutic effect. This dual-release mechanism ensures quick onset and extended control, enhancing treatment efficacy and patient compliance in epilepsy, bipolar disorder, and migraine prophylaxis. | [96] |
| Atenolol | Bilayer Buccal Tablets | To overcome bioavailability problems, reducing side effects and frequency of administration | [97] |
| Guaifenesin | Bilayer Tablets | Biphasic release profile: Guaifenesin helps loosen mucus in the chest, and its release is controlled for extended efficacy. | [98] |
| CefiximeTrihydrate, Dicloxacillin Sodium | Bilayer Tablets | Synergistic effect in bacterial infections: Cefixime inhibits bacterial cell wall synthesis, while Dicloxacillin works against beta-lactamase-producing bacteria. | [99] |
| Tramadol, Diclofenac | Bilayer Tablets | Bilayer tablets of Tramadol and Diclofenac combine rapid pain relief and sustained analgesia. Diclofenac in the immediate-release layer quickly reduces inflammation by inhibiting COX enzymes, while Tramadol in the sustained-release layer provides prolonged pain control through central opioid receptor activation and neurotransmitter modulation. This design enhances effectiveness and patient compliance. | [100] |
| Losartan Potassium | Bilayer Tablet | Treatment of hypertension: Losartan blocks the action of angiotensin II to help lower blood pressure. | [101] |
| Metformin Hydrochloride, Atorvastatin Calcium | Bilayer Tablets | To develop polytherapy for the treatment of NIDDM and hyperlipidemia: Metformin reduces insulin resistance, while Atorvastatin lowers cholesterol. | [102] |
| Montelukast, Levocetirizine | Bilayer Tablets | To improve the stability of drugs in combination: Montelukast reduces inflammation in the airways, and Levocetirizine is an antihistamine. | [103] |
| Atorvastatin, Propanalol | Bilayer Gastroretentive Matrix Tablet | Treatment of hypertension and hypercholesterolemia: Atorvastatin lowers cholesterol, and Atenolol reduces blood pressure. | [104] |
Evaluation of bilayer tablets
Overall appearance
The thickness of the tablet is now a crucial factor for duplicating its appearance and using filling equipment. The tablets' uniform thickness is a counting method in some filling equipment. A micrometre was used to measure the thickness of ten different tablets. Nice, Texture and general elegance of a tablet play a vital role in patient acceptance. Factors such as size, shape, colour, odor, flavour, particle morphology, structural integrity, and the consistency and legibility of any identification markings are all critical considerations [105].
Shape and size
A bilayer tablet's size and form are essential factors that must be carefully specified, monitored, and controlled during production. In addition to the tablet's mechanical properties and stability, these aspects significantly affect patient compliance, swallowing comfort, and overall customer preference. Bilayer tablet design can also impact the release profile of the active ingredients since varying sizes and shapes may alter the rates at which the active ingredients dissolve when in contact with gastric fluids. Specific ratios, for instance, might enhance the tablet's ability to stay afloat in a gastro-retentive formulation. At the same time, a rounded or streamlined form might facilitate more straightforward passage through the digestive system [106].
Measurement of thickness of bilayer tablet
The thickness of the tablet is a crucial factor in both duplicating appearances now and when filling equipment is used. The tablet's uniform thickness serves as a counting method in some filling equipment. A micrometer was used to measure the thickness of ten different tablets [107].
Tablet hardness
Tablet hardness influences their resistance to fracture or shipment while storing, transporting, and handling before use. The hardness of every other formulation's tablet was determined using a Monsanto hardness tester. The hardness was measured in kilograms per square meter [108].
Friability
Friability testing is used to assess the hardness of tablets and to assess a tablet's ability to tolerate abrasion during packaging, handling, and shipping. The Roche friability is commonly used to measure it [108].
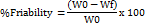
W0=Initial weight of tablets,
Wf=Final weight of tablets
Uniformity of weight
To ensure consistent tablet quality, weight uniformity testing is an essential part of the bilayer tablet formulation process. The statistical methods used for weight uniformity testing include descriptive statistics, where the mean, standard deviation, and range of tablet weights are calculated across multiple samples. This allows for the identification of any significant variations in weight. Additionally, Analysis of Variance (ANOVA) can be employed to determine whether there are significant differences in tablet weight across different production batches. For comparing two groups or batches, a t-test can be utilized to assess if any statistically significant weight differences exist. Furthermore, regression analysis may be applied to evaluate the relationship between tablet weight and other factors, such as compression force or the excipient composition. It is also crucial to define acceptance criteria based on established standards (e. g., USP or ICH guidelines) to determine acceptable weight variation limits for ensuring uniformity across batches [109].
Dissolution studies
Bilayer tablets are put through in vitro release experiments to simulating gastrointestinal fluid to see if they could deliver the necessary controlled medication. Because the average time for stomach emptying is around 2 h, drug release tests were conducted for two hours, use For two hours, use USP dissolving testing machine at 100rpm, 37.5 °C, and a pH 1.2 buffer (900 ml). These dissolving liquids were replaced by 900 ml of pH 6.8 buffer solution, and the study was maintained for just an additional 10 h. At various time intervals, 5 ml of a sample are removed and replace by 5 ml of medication dissolving media. A UV spectrophotometer in a multi-component model was used to evaluate the removed samples [110].
Stability studies
The stability of the bilayer tablets was assessed following the ICH Q1A(R2) and ICH Q1Eguidelines, which govern stability testing for new drug products. The tablets, packaged in suitable primary containers, were stored under accelerated conditions at 40 ± 2 °C and 75 ± 5% relative humidity for periods of 6 and 12 mo. Samples were collected at specified intervals, including a midpoint at 15 days, and evaluated for physical appearance, drug content, hardness, friability, and dissolution. ICH Q1A(R2), titled “Stability Testing of New Drug Substances and Products,” establishes the framework for designing stability studies, specifying conditions such as temperature, humidity, and testing schedules to ensure pharmaceutical quality, safety, and efficacy throughout the product’s shelf life. ICH Q1E, titled “Evaluation of Stability Data,” provides guidance on the statistical methods and models-like the Arrhenius equation and first-order kinetics-for analyzing stability results, determining shelf life, and predicting product expiration based on both accelerated and long-term stability data. Together, these guidelines represent the internationally recognized standard for the evaluation of pharmaceutical product stability [111].
CONCLUSION
Bilayer tablets represent a significant advancement in oral drug delivery systems, offering tailored therapeutic outcomes through the combination of immediate and sustained drug release. Their ability to house multiple active pharmaceutical ingredients with different release profiles enhances patient compliance, reduces dosing frequency, and allows for targeted drug delivery. Despite their promising benefits, bilayer tablets present notable manufacturing and formulation challenges, particularly in achieving robust interfacial bonding, maintaining layer uniformity, and ensuring stability under varying environmental conditions. Advanced technologies-such as displacement monitoring, osmotic systems, and novel coating techniques-are instrumental in overcoming these barriers. The therapeutic versatility of bilayer tablets is evident across a wide spectrum of diseases, including hypertension, diabetes, pain management, and microbial infections. As innovation in pharmaceutical engineering continues, bilayer tablet systems will play an increasingly critical role in achieving precise, effective, and patient-centric drug delivery.
ACKNOWLEDGEMENT
The authors would like to thank the Principal of The Royal Gondwana College of Pharmacy Nagpur for providing their support, facilities, and guidance in this research work.
FUNDING
Nil
AUTHORS CONTRIBUTIONS
S. K.:-Writing – Original Draft Preparation, Conceptualization, Literature Search, Writing – Review and Editing
CONFLICT OF INTERESTS
The authors declare no conflict of interest
REFERENCES
Maheshwari S, Singh A, Varshney AP, Sharma A. Advancing oral drug delivery: the science of fast dissolving tablets (FDTs). Intell Pharm. 2024;2(4):580-7. doi: 10.1016/j.ipha.2024.01.011.
Barik A, Dhar A. Introduction to different types of dosage forms and commonly used excipients. In: Nayak AK, Sen KK, editors. Dosage forms, formulation developments and regulations. Elsevier; 2024. p. 67-82. doi: 10.1016/B978-0-323-91817-6.00002-4.
Chaubey C, Bharkatiya M. Bimodal release bilayer tablet: a review. The Pharmaceutical and Chemical. 2024;11(4):10-7.
Udayasri G, Reddy SR. Review on bilayer tablets. IJRAR. 2023;10:2.
Nguyen NN, Pham DT, Nguyen DT, Trinh TT. Bilayer tablets with sustained-release metformin and immediate-release sitagliptin: preparation and in vitro/in vivo evaluation. J Pharm Investig. 2021;51(5):579-86. doi: 10.1007/s40005-021-00533-z.
Akhtar M, Jamshaid M, Zaman M, Mirza AZ. Bilayer tablets: a developing novel drug delivery system. J Drug Deliv Sci Technol. 2020;60:102079. doi: 10.1016/j.jddst.2020.102079.
Simao J, Chaudhary SA, Ribeiro AJ. Implementation of quality by design (QbD) for development of bilayer tablets. Eur J Pharm Sci. 2023;184:106412. doi: 10.1016/j.ejps.2023.106412, PMID 36828037.
Janczura M, Sip S, Cielecka Piontek J. The development of innovative dosage forms of the fixed-dose combination of active pharmaceutical ingredients. Pharmaceutics. 2022;14(4):834. doi: 10.3390/pharmaceutics14040834, PMID 35456668, PMCID PMC9025674.
Nguyen NN, Pham DT, Nguyen DT, Trinh TT. Bilayer tablets with sustained-release metformin and immediate-release sitagliptin: preparation and in vitro/in vivo evaluation. J Pharm Investig. 2021;51(5):579-86. doi: 10.1007/s40005-021-00533-z.
Hwang KM, Cho CH, Lee SH, Kim JY, Park ES. Preformulation and evaluation of multi-layer tablets. J Pharm Investig. 2024;54(2):161-74. doi: 10.1007/s40005-024-00673-y.
Inamdar I, Iftequar Ahmed S. Bilayer tablet overview: a revolutionary approach in sustained drug release & combination drug therapy. J Innov Pharm Biol Sci. 2024;10(2):8-19. doi: 10.56511/JIPBS.2023.10302.
Baghel US, Goyal M, Singh Y, Kushwah AS, Baghel A. Formulation and evaluation of bilayered matrix tablets of antidiabetic drugs. Int J Pharm Prof Res. 2025;16(1):51-67.
GEA Group. Bi-layer tableting: a challenge for tableting technology; 2024. Available from: https://www.gea.gea.com/en/customer-cases/bi-layer-tableting/. [Last accessed on 23 Mar 2025].
Siva Sai Kiran B, Sambasiva Rao P, Raveendra Babu G, Venkat Kumari M. Bilayer tablets a review. Int J Pharm Chem Biol Sci. 2015;5(3):510-6.
Blicharski T, Swiader K, Serefko A, Kulczycka Mamona S, Kolodziejczyk M, Szopa A. Challenges in technology of bilayer and multi-layer tablets: a mini-review. Curr Issues Pharm Med Sci. 2019;32(4):229-35. doi: 10.2478/cipms-2019-0039.
Soni N, Joshi D, Jain V, Pal P. A review on applications of bilayer tablet technology for drug combinations. J Drug Deliv Ther. 2022;12(1):222-7. doi: 10.22270/jddt.v12i1.5206.
Keraliya RA, Patel C, Patel P, Keraliya V, Soni TG, Patel RC. Osmotic drug delivery system as a part of modified release dosage form. ISRN Pharm. 2012;2012:528079. doi: 10.5402/2012/528079, PMID 22852100, PMCID PMC3407637.
Kale PS, Ghadge PS, Bobde MN, Shahi SR. Overview of bilayer tablet: a review. Int J Res Trends Innov. 2022;7(6):697.
Prasanna N, Srilatha, Subrahmanyam CVS. osmotic controlled release oral delivery system: an overview. Int J of Pharm Sci. 2024;2(3):530-58. doi: 10.5281/zenodo.10810393.
Bartere SN. Bilayer tablets a brief review. Int J Pharm Res Appl. 2023;8(3):556-62. doi: 10.35629/7781-0803556562.
Sonawane AY, Sonawane SS, Khapre J. Novel approach and current applications of bilayer tablet. Int J Pharm Sci. 2024;2(7):1491-504. doi: 10.5281/zenodo.12788774.
Suryawanshi SM, Kawtikwar PS, Uplenchwar PM, Suryawanshi RR. A review: brief overview on bilayered tablets and its introduction. IJPPR. 2023 Mar;26(4):407-20.
Meynard J, Amado Becker F, Tchoreloff P, Mazel V. Characterization of the viscoelasticity of pharmaceutical tablets using impulse excitation technique. Int J Pharm. 2022 Feb 5;613:121410. doi: 10.1016/j.ijpharm.2021.121410, PMID 34942329.
Blicharski T, Swiader K, Serefko A, Kulczycka Mamona S, Kolodziejczyk M, Szopa A. Challenges in technology of bilayer and multi-layer tablets: a mini-review. Curr Issues Pharm Med Sci. 2019;32(4):229-35. doi: 10.2478/cipms-2019-0039.
Prajapati M, Kanavaje AM, Naeem SN. A review of the emerging trend in bi-layer tablet technology. Indo Am J Pharm Sci. 2024;11(8):166-78.
Gaonkar V. Fundamentals and potentials of bilayer tablet technology. IJPPR. 2021 Mar;20(4):146-75.
Hwang KM, Cho CH, Lee SH, Kim JY, Park ES. Preformulation and evaluation of multi-layer tablets. J Pharm Investig. 2024;54(2):161-74. doi: 10.1007/s40005-024-00673-y.
Munu I, Nicusan AL, Crooks J, Pitt K, Windows Yule C, Ingram A. Using in line measurement and statistical analyses to predict tablet properties compressed using a styl’one compaction simulator: a high shear wet granulation study. Int J Pharm. 2025;669:125098. doi: 10.1016/j.ijpharm.2024.125098, PMID 39694157.
ACG Group. Navigating the complexities of bilayer tablet manufacturing. ACG World. Available from: https://www.acg-world.com/blogs/navigating-complexities-bilayer-tablet-manufacturing.
Kim SH, Kook JH, Seo DW, Kang MJ. The effect of compression pressure on the first layer surface roughness and delamination of metformin and evogliptin bilayer and trilayer tablets. Pharmaceuticals. 2023;16(11):1523. doi: 10.3390/ph16111523, PMID 38004389.
Chang SY, Sun CC. Insights into the effect of compaction pressure and material properties on interfacial bonding strength of bilayer tablets. Powder Technol. 2019;354:867-76. doi: 10.1016/j.powtec.2019.07.021.
Guo Y, Xiao P, Dong H, Guo X, Yin T, Zhang Y. Effect of compression process parameters on the physical properties and in vitro release mechanism of the press coated nifedipine matrix sustained release tablets. Powder Technol. 2023;429:118884. doi: 10.1016/j.powtec.2023.118884.
Chang SY, Sun CC. Interfacial bonding in formulated bilayer tablets. Eur J Pharm Biopharm. 2020;147:69-75. doi: 10.1016/j.ejpb.2019.12.009, PMID 31870828.
Pharmaceutical tablet lubrication. Pharmaceutical networking; 2019. Available from: https://www.pharmaceuticalnetworking.com./wpcontent/uploads/2019/06/pharmaceutical-tablet-lubrication-1.pdf.
Hackl E, Ermonlina I, Kabova E. Effect of lubricants on the properties of tablets compressed from varied-size granules. Br J Pharmacol. 2023;8(2):S1-2. doi: 10.5920/bjpharm.1395.
Veronica N, Heng PW, Liew CV. Magnesium stearate fatty acid composition lubrication performance and tablet properties. AAPS PharmSciTech. 2024;25(8):262. doi: 10.1208/s12249-024-02980-x, PMID 39500792.
Lura V, Klinken S, Breitkreutz J. A systematic investigation of external lubrication of mini-tablets on a rotary tablet press with focus on the tensile strength. Eur J Pharm Biopharm. 2024;198:114236. doi: 10.1016/j.ejpb.2024.114236, PMID 38423137.
Singh A, Das S, Gupta S, Ghosh S. The challenges of producing bilayer tablet: a review. J Drug Delivery Ther. 2021;11(4-S):171-5. doi: 10.22270/jddt.v11i4-S.4922.
Kondo H, Toyota H, Kamiya T, Yamashita K, Hakomori T, Imoto J. Effect of the external lubrication method for a rotary tablet press on the adhesion of the film coating layer. Chem Pharm Bull (Tokyo). 2017;65(9):848-53. doi: 10.1248/cpb.c17-00376, PMID 28867712.
Daou J. Moisture control and degradation management. Tablets Capsules Mag. 2022;22(1):8-13.
Manzoo A. Sodium starch glycolate as a superdisintegrant. J Contemp Pharm. 2021;5(1):33-9.
Soni N, Joshi D, Jain V, Pal P. A review on applications of bilayer tablet technology for drug combinations. J Drug Deliv Ther. 2022;12(1):222-7. doi: 10.22270/jddt.v12i1.5206.
Deebes M, Mahfouf M, Omar C. A new data-driven modelling framework for moisture content prediction in continuous pharmaceutical tablet manufacturing. In: Sheu SH, editor. Industrial engineering and applications Europe. Cham: Springer Nature Switzerland; 2024. p. 107-20. doi: 10.1007/978-3-031-58113-7_10.
Chang SY, Sun CC. Interfacial bonding in formulated bilayer tablets. Eur J Pharm Biopharm. 2020;147:69-75. doi: 10.1016/j.ejpb.2019.12.009, PMID 31870828.
Finsgar M. Mapping active pharmaceutical ingredients distributions in tablets using time of flight secondary ion mass spectrometry with multivariate curve resolution. Microchem J. 2024;207:112156. doi: 10.1016/j.microc.2024.112156.
Won DH, Park H, Ha ES, Kim HH, Jang SW, Kim MS. Optimization of bilayer tablet manufacturing process for fixed dose combination of sustained release high dose drug and immediate release low dose drug based on quality by design (QbD). Int J Pharm. 2021;605:120838. doi: 10.1016/j.ijpharm.2021.120838, PMID 34197909.
Desai PM, Truong T, Marathe S. Detailed accounts of segregation mechanisms and the evolution of pharmaceutical blend segregation analysis: a review. Int J Pharm. 2024;665:124739. doi: 10.1016/j.ijpharm.2024.124739, PMID 39321901.
Sahoo SK, Patra CN, Sahu RK. Influence of compressibility and compactibility on the mechanical properties of bilayer tablets: a review. J Drug Deliv Sci Technol. 2022;69:103131. doi: 10.1016/j.jddst.2022.103131.
Sonawane AY, Sonawane SS, Khapre J. Novel approach and current applications of bilayer tablet. Int J Pharm Sci. 2024;2(7):1491-504. doi: 10.5281/zenodo.12788774.
Kondapalli K, Korni RD, Bhavani H, Priya DL. Multi-layered tablets: a comprehensive review on technologies and research. GSC Biol Pharm Sci. 2024;29(2):178-88. doi: 10.30574/gscbps.2024.29.2.0406.
Gopinath C, HimaBindu V, Nischala M. An overview on bilayered tablet technology. J Glob Trends Pharm Sci. 2013;4(2):1077-85.
Ishitsubo N, Oguro S, Shimahashi H, Kawanishi M, Adachi T, Mitsuda K. Development physicochemical characteristics and pharmacokinetics of a new sustained release bilayer tablet formulation of tramadol with an immediate release component for twice daily administration. Eur J Drug Metab Pharmacokinet. 2024 Jan;49(1):87-100. doi: 10.1007/s13318-023-00865-1, PMID 38064122, PMCID PMC10781817.
Hake J, Pol V, Shinde R, Kundekar K, Deshmukh A, Kale P. Bilayer tablet technology: an innovative approach to multimodal drug delivery. Int Res J Mod Eng Technol Sci. 2025 Apr;7(4):4584-92. doi: 10.56726/IRJMETS72687.
TabPlus. Tablet Press Machine. Gujarat, India: TabPlus. Available from: https://tabplus.co.in/tablet-press-machine/. [Last accessed on 09 Sep 2025].
Nagpure N, Chaple DR, Kasliwal RH, Gholse Y, Zade S, Kumar R. Dual release tablets: a novel approach to optimized drug release. World J Pharm Res. 2024;14(2):173-97.
Sonawane AY, Sonawane SS, Khapre J. Novel approach and current applications of bilayer tablet. Int J Pharm Sci. 2024;2(7):1491-504. doi: 10.5281/zenodo.12788774.
Fluid pack. B4-Double-sided rotary tablet press features and applications; 2024. Available from: https://www.fluidpack.net/blogs/b4-double-sided-rotary-tablet-press-features-and-applications.
Pathloth N, Reddy MS, Vijetha KA. A review on bilayer tablets: dual release systems for controlled drug administration. Int J Pharm Res Appl. 2024;9(1):1798-803.
PTK-GB LTD. PR3000 bi-layer tablet press; 2025. Available from: https://www.ptkgb.com/production-tablet-presses/ptk-pr3000-bi-layer-tabletpress/.
Kim SH, Kook JH, Seo DW, Kang MJ. The effect of compression pressure on the first layer surface roughness and delamination of metformin and evogliptin bilayer and trilayer tablets. Pharmaceuticals (Basel). 2023;16(11):1523. doi: 10.3390/ph16111523, PMID 38004389.
Zarekar Shivani Gulab, Raykar Sonali Dattu Zende Gauri Sampat, Puranik Aboli K, Sanjay J Ingle. A review on the wet granulation technique and its modules. World J Bio Pharm Health Sci. 2024;20(2):113-24. doi: 10.30574/wjbphs.2024.20.2.0836.
Khan S, Sharma A, Singh S, Kumar M. Role of excipients in modulating drug release from bilayer tablets: a comprehensive review. J Drug Deliv Sci Technol. 2023;77:103841. doi: 10.1016/j.jddst.2023.103841.
Navabhatra A, Yingngam B. Coating techniques for biocompatible surfaces. In: Kulkarni S, Haghi AK, Yingngam B, Ogwu MC, editors. Medical applications for biocompatible surfaces and coatings. Royal Society of Chemistry; 2024. p. 76-118.
KK, GD. From challenges to advancement for bilayer tablet technology as drug delivery system. Int J Drug Deliv Technol. 2024;14(4):1676-82. doi: 10.25258/ijddt.14.4.64.
Pan P, Svirskis D, Waterhouse GI, Wu Z. Hydroxypropyl methylcellulose bioadhesive hydrogels for topical application and sustained drug release: the effect of polyvinylpyrrolidone on the physicomechanical properties of hydrogel. Pharmaceutics. 2023 Sep 15;15(9):2360. doi: 10.3390/pharmaceutics15092360, PMID 37765328.
Sadhu VR, Bopparaju P, Kantamneni P. Bilayer tablet technology: a novel approach. GSC Biol Pharm Sci. 2019;7(2):22-8. doi: 10.30574/gscbps.2019.7.2.0033.
Ishitsubo N, Oguro S, Shimahashi H, Kawanishi M, Adachi T, Mitsuda K. Development physicochemical characteristics and pharmacokinetics of a new sustained release bilayer tablet formulation of tramadol with an immediate-release component for twice daily administration. Eur J Drug Metab Pharmacokinet. 2024 Jan;49(1):87-100. doi: 10.1007/s13318-023-00865-1, PMID 38064122, PMCID PMC10781817.
Soni N, Joshi D, Jain V, Pal P. A review on applications of bilayer tablet technology for drug combinations. J Drug Deliv Ther. 2022;12(1):222-7. doi: 10.22270/jddt.v12i1.5206.
Parmar A, Jagwani A, Khanam T, Gehalot N, Jain V. Comprehensive review on formulation and manufacturing techniques of bilayer tablets. Int J Pharm Sci. 2024;2(8):3117-21. doi: 10.5281/zenodo.13316959.
Parmar A, Jagwani A, Khanam T, Gehalot N, Jain V. Comprehensive review on formulation and manufacturing techniques of bilayer tablets. Int J Pharm Sci. 2024;2(8):3117-21. doi: 10.5281/zenodo.13316959.
Parmar A, Jagwani A, Khanam T, Gehalot N, Jain V. Comprehensive review on formulation and manufacturing techniques of bilayer tablets. Int J Pharm Sci. 2024;2(8):3117-21. doi: 10.5281/zenodo.13316959.
Al Noumani H, Al Harrasi M, Jose J, Al Naamani Z, Panchatcharam SM. Medication adherence and patients characteristics in chronic diseases: a national multi-center study. Clin Nurs Res. 2022;31(3):426-34. doi: 10.1177/10547738211033754, PMID 34287084.
Yokoyama R, Kimura G, Huwyler J, Hosoya KI, Puchkov M. Impact of insoluble separation layer mechanical properties on disintegration and dissolution kinetics of multilayer tablets. Pharmaceutics. 2020 May 29;12(6):495. doi: 10.3390/pharmaceutics12060495, PMID 32485803.
Nikam V, Dighe S, Khapare J, Somwanshi S, Kotade K. Tablet in tablet technique for oral drug delivery. Neuro Quantology. 2022 Nov;20(18):1041-51. doi: 10.48047/NQ.2022.20.18.
Atif Khattak SUS, Shah PA, Rashid SA, Hashmat Ullah H Gul, Saima Mahmood, Mudassar Mazher, Rabia Baloch, Sidra Mumtaz. Metformin adorned bilayer tablets for type II diabetes management: formulation development and characterization. J Popul Ther Clin Pharmacol. 2024;31(4):1952-67. doi: 10.53555/jptcp.v31i4.6048.
Huang YT, McCarthy C, Jani M. Balancing the evidence: an update on analgesic use in rheumatic and musculoskeletal diseases. Front Drug Saf Regul. 2023;3:1117674. doi: 10.3389/fdsfr.2023.1117674.
Andrews GP, Li S, Almajaan A, Yu T, Martini L, Healy A. Fixed dose combination formulations: multilayered platforms designed for the management of cardiovascular disease. Mol Pharm. 2019;16(5):1827-38. doi: 10.1021/acs.molpharmaceut.8b01068, PMID 30702301.
Wang Y, Xu J, Gao N, Lv H, Sun M, Zhang P. Controlled release of bilayer tablet comprising vitamin B6 rapid release layer and melatonin sustained release layer. Pharmaceutical Science Advances. 2023;1(2):100008. doi: 10.1016/j.pscia.2023.100008.
Parameswaran S, Rajkumar R. Bilayer tablet formulation of beetroot and isoniazid: integration of herbal nutrition and anti-tubercular therapy. Biomed Pharmacol J. 2025;18(1):693-700. doi: 10.13005/bpj/3120.
Prabu SL. Formulation and evaluation of polymeric bilayer matrix tablet containing glipizide and metformin hydrochloride. BEBA. 2020;4(1):1-11. doi: 10.23880/beba-16000139.
Mounika P, Pandey A, Kishore MR, Sanjit. Bilayer tablets of lovastatin (IR) and atenolol (CR) formulation. World J Pharm Res. 2019;8(3):839-44. doi: 10.20959/wjpr20193-14266.
KV, PS N. Latest advancements on gastro floating bi-layer tablets of metformin and pioglitazone for the treatment of diabetes mellitus. Int J Drug Deliv Technol. 2024;14(4):1683-93. doi: 10.25258/ijddt.14.4.77.
Gandhi B, Bhagwat A, Matkar S, Matkar S, Wale T, Kokane O. Formulation and evaluation of bilayer tablets of atenolol and amlodipine for the treatment of hypertension. Res J Pharm Technol. 2025;18(5):2037-42. doi: 10.52711/0974-360X.2025.00291.
Shinkai M, Katsumata N, Kawai S, Kuyama S, Sasaki O, Yanagita Y. Phase III study of bilayer sustained release tramadol tablets in patients with cancer pain: a double blind parallel group non-inferiority study with immediate release tramadol capsules as an active comparator. Support Care Cancer. 2023 Dec 29;32(1):69. doi: 10.1007/s00520-023-08242-z, PMID 38157081, PMCID PMC10756890.
Jammula S, Patra ChN, Swain S, Panigrahi KC, Nayak S, Dinda SC. Design and characterization of cefuroxime axetil biphasic floating minitablets. Drug Deliv. 2015;22(1):125-35. doi: 10.3109/10717544.2013.871603, PMID 24417642.
Porwal A, Dwivedi H, Pathak K. Gastroretentive bilayer film for sustained release of atorvastatin calcium and immediate release of amlodipine besylate: pharmaceutical pharmacokinetic evaluation and IVIVC. Pharm Dev Technol. 2020;25(4):416-31. doi: 10.1080/10837450.2019.1705486, PMID 31852330.
Pawar N, Desai P, Jadhav K, Shah P, Shrotri SN. Formulation and evaluation of bilayer tablets of granisetron HCl and ibuprofen for the treatment of migraine. IJPSN. 2024 Oct 15;17(5):7596-604.
Kumar KA, Pramod K, Ranjith Kumar N, Rajavardhan Reddy M. Design and characterization of bilayered tablets of acetaminophen and methocarbamol. J Innov Dev Pharm Technol Sci. 2024;7(9):98-103.
Liu T, Wang J, Feng Y, Wang H, Xu Y, Yin T. Further enhancement of the sustained release properties and stability of direct compression gel matrix bilayer tablets by controlling the particle size of HPMC and drug microencapsulation. Powder Technol. 2024;448:120256. doi: 10.1016/j.powtec.2024.120256.
Liu T, Wang J, Feng Y, Wang H, Xu Y, Yin T. Further enhancement of the sustained release properties and stability of direct compression gel matrix bilayer tablets by controlling the particle size of HPMC and drug microencapsulation. Powder Technol. 2024;448:120256. doi: 10.1016/j.powtec.2024.120256.
Maddiboyina B, Hanumanaik M, Nakkala RK, Jhawat V, Rawat P, Alam A. Formulation and evaluation of gastro retentive floating bilayer tablet for the treatment of hypertension. Heliyon. 2020;6(11):e05459. doi: 10.1016/j.heliyon.2020.e05459, PMID 33241144.
Tuyen NT, Nghiem LQ, Tuan ND, Le PH. Development of a scalable process of film-coated bi-layer tablet containing sustained-release metoprolol succinate and immediate-release amlodipine besylate. Pharmaceutics. 2021;13(11):1797. doi: 10.3390/pharmaceutics13111797, PMID 34834212.
Shah SN, Ali H, Yasmin R, Perveen S, Zafar F, Israr F. Design and optimization studies of tablet in tablet formulation of diclofenac and misoprostol: application of response surface methodology and compressional behaviour strategy. bioRxiv. 2022 Nov 5. doi: 10.1101/2022.11.04.515246.
Gupta MM, Khoorban A, Ali A, Ramlogan O, Talukdar D. Comparative quality control study of different brands of diclofenac sodium tablet available in local and government pharmacies by in vitro testing. Cureus. 2020 Nov 5;12(11):e11348. doi: 10.7759/cureus.11348, PMID 33304683.
Fatima H, Mohammed S, Begum A. Effect of croscormellose sodium in sustained release layer of valsartan in bilayer tablet with clopidogrel as immediate drug release and valsartan as sustained drug release. J Drug Deliv Ther. 2023;13(12):35-50. doi: 10.22270/jddt.v13i12.6067.
Ughade S, Bawankar RD, Mundhada DR. Nifedipine gastroretentive drug delivery system: formulation characterization and evaluation. Indian J Pharm Sci. 2023;85(3):667. doi: 10.36468/pharmaceutical-sciences.1133.
Gunda DR, Kolli DT. Development and in vitro assessment of divalproex sodium bilayered tablets. J App Pharm Sci Res. 2024 Aug;7(2):46-52. doi: 10.31069/japsr.v7i2.08.
Elsayed MM, Aboelez MO, Mohamed MS, Mahmoud RA, El Shenawy AA, Mahmoud EA. Tailoring of rosuvastatin calcium and atenolol bilayer tablets for the management of hyperlipidemia associated with hypertension: a preclinical study. Pharmaceutics. 2022;14(8):1629. doi: 10.3390/pharmaceutics14081629, PMID 36015255.
Simao J, Chaudhary SA, Ribeiro AJ. Implementation of quality by design (QbD) for development of bilayer tablets. Eur J Pharm Sci. 2023;184:106412. doi: 10.1016/j.ejps.2023.106412, PMID 36828037.
Ferdousi FM, Safrin F, Ali MN, Al Juhan A, Akter P. Composition and drug release characteristics of bi-layered and multilayered tablets: a comprehensive review. J Drug Deliv Ther. 2025;15(1):169. doi: 10.22270/jddt.v15i1.6936.
Shah DD, Sorathia ZH. Tramadol/diclofenac fixed dose combination: a review of its use in severe acute pain. Pain Ther. 2020;9(1):113-28. doi: 10.1007/s40122-020-00155-7, PMID 32062853.
Ahmed SI, Zaheer Z, Khan FN, Hasan M. The Formulation and evaluation of gastro bilayer floating tablets of losartan potassium as immediate release layer and ramipril hydrochloride as sustained release floating layer. Int J Pharm Investig. 2020;10(3):294-9. doi: 10.5530/ijpi.2020.3.53.
Bhagwat SS, Patil PP, Aher SJ, Mohsina Patwekar FP. Formulation and characterization of bilayer tablet of anti-hyperlipidemic BCS Class II drug. Res Rev J Pharm Sci. 2023;14(1):1-18. doi: 10.37591/(rrjops).v14i1.1261.
Oh DJ, Shin SY, Lim CY, Chae YB, Yang JH, Hwang SJ. Development of a stable fixed dose combination of montelukast sodium and levocetirizine dihydrochloride using multi-layering API coating technology. Int J Pharm. 2025;675:125527. doi: 10.1016/j.ijpharm.2025.125527, PMID 40154822.
Roy SK, Naskar S, Kundu S, Koutsu K. Formulation and evaluation of sustained release bilayer tablets of propranolol hydrochloride. Int J Pharm Pharm Sci. 2014;7(4):264-9.
Kolhe RC, Chaudhari RY. Development and evaluation of antidiabetic polyherbal tablet using medicinal plants of traditional use. Int J Curr Pharm Sci. 2023 Mar;15(2):17-21. doi: 10.22159/ijcpr.2023v15i2.2095.
Alam S, Alam S, Bishal A, Bandyopadhyay B. Formulation and evaluation of metformin hydrochloride sustained release matrix tablets. Int J Curr Pharm Sci. 2021 Sep;13(5):82-8. doi: 10.22159/ijcpr.2021v13i5.1899.
Sarkar P, Das S, Majee SB. Solid dispersion tablets in improving oral bioavailability of poorly soluble drugs. Int J Curr Pharm Res. 2022 Mar;14(2):15-20. doi: 10.22159/ijcpr.2022v14i2.1961.
Kolhe RC, Chaudhari RY. Development and evaluation of antidiabetic polyherbal tablet using medicinal plants of traditional use. Int J Curr Pharm Res. 2023 Mar;15(2):17-21. doi: 10.22159/ijcpr.2023v15i2.2095.
International Council for Harmonisation (ICH). ICH Q4B. In: Uniformity of dosage units general chapter. European Medicines Agency; 2008. Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-q-4-b-annex-6-note-evaluation-and-recommendation-pharmacopoeial-texts-use-ich-regions-uniformity-dosage-unites-general-chapter-step-3_en.pdf.
Bassetto R, Amadio E, Ciampanelli F, Perin S, Ilari P, Gaballo P. Designing an effective dissolution test for bilayer tablets tailored for optimal melatonin release in sleep disorder management. Front Nutr. 2024 May 6;11:1394330. doi: 10.3389/fnut.2024.1394330, PMID 38769992, PMCID PMC11102985.
RAPS. ICH releases overhauled stability guideline for combination products. Regulatory focus; 2025 Apr. Available from: https://www.raps.org/news-and-articles/news-articles/2025/4/ich-releases-overhauled-stability-guideline-for-co?utm.