
Int J App Pharm, Vol 17, Issue 6, 2025, 174-183Original Article
PREPARATION AND OPTIMIZATION OF GEPIRONE HCL SR MATRIX TABLETS USING HPMC K100M AND POLYOX WSR N80
DINESH DAS, ANJAN KUMAR*, CH. NIRANJAN PATRA
Roland Institute of Pharmaceutical Sciences, Biju Patnaik University of Technology, Odisha, India
*Corresponding author: Anjan Kumar; *Email: akd009@gmail.com
Received: 19 Apr 2025, Revised and Accepted: 13 Aug 2025
ABSTRACT
Objective: In adults, Gepirone Hydrochloride (HCl) is most commonly employed as a first-line treatment for Major Depressive Disorder (MDD). The elimination half-life of Gepirone HCl is approximately 5 h necessitates the development of sustained release formulation. In the current research, two polymers HPMC K100M and Polyox WSR N80 were used in combination by adopting a central composite design with an objective to minimize burst release and sustain drug release for 24 h.
Methods: The SR matrix tablets of Gepirone HCl were prepared by wet granulation method. Optimization of Gepirone HCl SR matrix tablets was achieved through central composite design (CCD). The selected CQAs were concentration of polymer HPMC K100M in the range of 20 to 40 mg per tablet and concentration of Polyox WSR N80 in the range of 30 to 50 mg per tablet. Three responses selected for optimization vis-à-vis Q 1 (Cumulative percent drug release from SR matrix tablet in 1 h), t 50 (time taken for release of 50% of API) and Q 18 (Cumulative percent drug release from SR matrix tablet at 18 h).
Results: Fourier Transform Infra Red (FT-IR) and Differential Scanning Calorimetry (DSC) study for the physical mixture of Gepirone HCl with HPMC K100M and Polyox WSR N80 in 1:1 ratio demonstrated compatibility. The granules exhibited improved flowability and compressibility. The optimized formulation (run 7) passed all the quality control tests for tablets. Scanning electron microscopy for tablet of run 7 exhibited a homogenous and porous gel network. In vitro dissolution study showed that the integration of two polymers HPMC K100M and Polyox WSR N80 were effective in minimizing the initial burst release, and sustained release of Gepirone HCl over 24 h.
Conclusion: Hence, the development of a once-daily formulation aligns with modern patient-centric approaches in pharmaceutical design, catering to convenience and improving quality of life for patients requiring long-term therapy.
Keywords: Swelling, Erosion, Central composite design, Once-daily formulation
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijap.2025v17i6.54651 Journal homepage: https://innovareacademics.in/journals/index.php/ijap
INTRODUCTION
In adults, Gepirone HCl is most commonly employed as a first-line treatment for major depressive disorder (MDD) in adults [1]. It acts as a selective serotonin receptor agonist, specifically targeting the 5-HT1A receptors. The recommended starting dose for adults is 18.2 mg taken orally once daily with food [2]. Depending on the response and tolerance of the patient, the dosage may be increased gradually from 18.2 to 72.6 mg per day. Gepirone HCl is supplied as an extended-release tablet and is available in the following strengths: 18.2 mg, 36.3 mg, 54.5 mg, and 72.6 mg. Gepirone HCl is absorbed with a peak plasma concentration (Cmax) reached within approximately 4 to 5 h after administration [3]. The bioavailability of oral gepirone ranges from 14 to 17% [4]. The elimination half-life of gepirone HCl is approximately 5 h. The partition coefficient (logP) of gepirone HCl is 1.55 [5]. The dissociation constants (pKa) of gepirone HCl are 7.51 and 2.23, indicating its basic nature. Its short half-life necessitates the development of an extended-release formulation.
Matrix tablets offer excellent control over the drug release rate. By adjusting the composition of the matrix using different polymers, formulators can fine-tune how quickly the drug is released [6]. Matrix tablets tend to have better physical and chemical stability. The matrix can protect the active pharmaceutical ingredient (API) from environmental factors like moisture and light. These tablets are versatile and can accommodate a wide range of drugs with different solubility profiles. Whether dealing with a water-soluble drug or a poorly soluble one, the matrix system can be adapted accordingly [7]. HPMC K100M based matrix tablets when exposed to gastrointestinal fluids, HPMC hydrates rapidly and forms a robust, viscous gel layer [8-10]. This gel acts as a barrier, primarily controlling drug release through diffusion [11]. It also contributes to good compressibility and helps maintain mechanical strength. Polyox WSR N80, because of very high molecular weights tends to form a visco-elastic sticky gel. Its slower erosion and dissolution properties help extend the drug release profile. Polyox can improve the pliability and overall stability of the matrix, which can be especially beneficial for drugs that require very prolonged release [12, 13]. Incorporating Polyox WSR N80, a high molecular weight polyethylene oxide derivative, into HPMC K100M based systems has been shown to enhance matrix robustness. Polyox contributes to a more coherent, interpenetrating polymer network within the gel, which not only improves the mechanical strength of the matrix but also helps maintain its structural integrity during dissolution. This combination of Polyox and HPMC is reported for many drugs like ranitidine HCl [14], metformin [15], repaglinide [15] and levofloxacin [16]. Hence in the current research, two polymers HPMC K100M and Polyox WSR N80 were used in combination as per the central composite design with an objective of sustained drug release for 24 h with low initial burst release.
MATERIALS AND METHODS
Material
Gepirone HCl was acquired as a complimentary sample from MSN Laboratories, Hyderabad. HPMC K100M was received as a gift sample from Colorcon, India. Polyox WSR N80 was procured from Chempoint, India.
Method
Drug excipient compatibility study
Fourier transform infra-red (FT-IR) study
FT-IR spectroscopic analysis was performed for pure drug Gepirone HCl and its physical mixtures with HPMC K100M and Polyox WSR N80 alone and also in combination in 1:1 ratio using IR Affinity, Shimadzu, Japan.
Differential scanning calorimetry (DSC) study
Thermal analysis was performed for the pure drug Gepirone HCl and its physical mixtures with HPMC K100M and Polyox WSR N80 alone and also in combination in a 1:1 ratio using DSC-60, Shimadzu, Japan. The DSC study was performed with a rate of rise in temperature of 10 °C per min up to 250 °C under a nitrogen atmosphere.
Preparation of gepirone HCl SR matrix tablets
The sustained release (SR) matrix tablets of Gepirone HCl were prepared by wet granulation method. In the first step, drug and excipients were passed through sieve number 60. The drug, polymers (HPMC K100M and Polyox WSR N80), and other excipients were dry mixed for 10 min in mortar and pestle. During dry mixing, Avicel PH101 was also added as diluents. The starch paste 10 % W/V binder solution (15 ml) was added to the dry mix and wet mixing was continued till the formation of a damp mass. The wet damp mass was passed through sieve no 16 to obtain granules. The wet granules were transferred to a fluidized bed dryer (FBD) and dried under a fluidized hot air stream at a temperature of 70 C for 15 min. The dried granules were again passed through sieve number 20. The lubricants talc (2%) and aerosil (1%) were mixed with dried and sieved granules for 5 min. The lubricated granules were compressed into circular, flat tablets of 6 mm diameter using Mini Press II, Karnavati, India to get tablets in the hardness of tablets in the range of 5 to Kg/cm2. The amount of starch powder incorporated from the paste to the damp mass was 15 mg per tablet. The batch size for each formulation was 100 tablets (table 1).
Table 1: Composition of sustain release (SR) matrix tablets of gepirone HCl as per central composite design (CCD)
| Run | Factor 1 HPMCK100 M | Factor 2 polyox WSR N80 | Response 1 (Q1) | Response 2 (t50) | Response 3 (Q18) |
| 1 | 0 | 0 | 11.31±0.61 | 13.51±0.57 | 88.68±2.6 |
| 2 | 1 | -1 | 18.72±0.81 | 11.63±0.62 | 91.53±3.7 |
| 3 | 0 | 0 | 11.62±0.62 | 13.21±0.47 | 93.56±2.6 |
| 4 | 0 | 1.41421 | 22.17±1.02 | 10.25±0.34 | 100.13±4.1 |
| 5 | 0 | 0 | 5.53±0.26 | 14.64±0.74 | 78.47±2.9 |
| 6 | -1 | 1 | 26.32±1.61 | 9.53±0.05 | 100.34±1.7 |
| 7 | -1.41421 | 0 | 11.17±0.57 | 11.25±0.07 | 85.61±3.5 |
| 8 | -1 | -1 | 35.54±2.67 | 8.12±0.03 | 100.98±2.6 |
| 9 | 0 | -1.41421 | 4.72±0.04 | 15.24±0.98 | 74.24±1.5 |
| 10 | 0 | 0 | 11.51 ±.54 | 13.48±0.74 | 87.51±2.6 |
| 11 | 1 | 1 | 12.36±0.62 | 14.24±0.68 | 82.35±3.1 |
| 12 | 0 | 0 | 11.87±0.84 | 13.7±0.59 | 87.56±2.8 |
| 13 | 1.41421 | 0 | 11.79±0.06 | 13.18 ±.97 | 88.52±2.9 |
| Factors | Low (-1) | Medium (0) | High (+1) | ||
| Concentration of HPMCK100M (mg) | 20 | 30 | 40 | ||
| Concentration of Polyox WSR N80 (mg) | 30 | 40 | 50 |
Mean±SD, n = 6
Optimization of Gepirone HCl SR matrix tablets was achieved through central composite design (CCD) utilizing Stat-Ease's Design Expert software (version 13.0) from Minneapolis, USA. Based on the recommended design, thirteen formulations were created and evaluated for important quality characteristics. Using the response surface approach, contour plots, and three-dimensional plots were produced. To identify the relevant model factor, an ANOVA was employed. Critical quality attributes (CQAs) were optimized using upper and lower bounds. The selected CQAs for the above research were concentration of polymer HPMC K100M in the range of 20 to 40 mg per tablet and concentration of Polyox WSR N80 in the range of 30 to 50 mg per tablet. The design space was defined using an overlay plot. Three responses selected for optimization of research were vis-à-vis Q1 (Cumulative percent drug release from SR matrix tablet in 1 h), t50 (time taken for release of 50% of API) and Q18 (Cumulative percent drug release from SR matrix tablet at 18 h).
Micromeritic properties of granules
The pure drug Gepirone HCl powders and granules of all the formulations were subjected for evaluation of micromeritic properties. The following tests, such as angle of repose, Carr’s index, Hausner’s ratio, granular friability index [17], and moisture content [18] were determined as per standard procedure [19].
Quality control tests for tablets
The following quality control tests, such as drug content, hardness, friability, thickness, and diameter of gepirone HCl tablets were determined as per standard procedure [20].
In vitro dissolution study
An In vitro dissolution test was performed for the optimized formulation for 24 h. In this study, the 0.1 N HCl was used as dissolution medium for 1st 2 h followed by phosphate buffer pH 6.8 for the remaining 22 h [21]. The dissolution was performed in a paddle-type apparatus with 100 rpm maintained at a temperature of 37±0.5 °C. The dissolution samples were diluted and analyzed spectrophotometrically at 235 nm. The drug dissolution data was put into zero order, first order, Higuchi and korsmeyer Pappa’s equation for analyzing drug release kinetics and mechanism of drug release.
Scanning electron microscopy (SEM)
The optimized formulation run 7 was removed from the dissolution medium after 4 h of dissolution testing. A small fragment of the SR tablet was subjected to coating with a thin layer of gold to enhance electrical conductivity. The scanning electron microscopic images were collected to evaluate gel formation and erosion resulting from the dissolution process.
Stability study
The stability study for the optimized tablet formulation was performed as per ICH guidelines [22] [Q1A(R2)] at three different conditions i. e. 40 °C±2 °C/75% RH±5% RH, 25°±2 °C/60±5% RH and 30°±2 °C/65±5% RH for 6 mo in a humidity controlled oven (90L, Stability Chamber, Thermolab, India). Samples were collected at 0, 1, 3, and 6 mo time intervals and analyzed for drug content, Q1, t50, and Q18.
RESULTS AND DISCUSSION
Drug excipient compatibility study
Fourier transform infrared (FT-IR) study
FT-IR study for pure drug gepirone HCl confirms the presence of the following functional groups by analyzing absorption bands (fig. 1). The broad peak at 3500 cm-1corresponds to the stretching vibrations of the N-H bond, indicative of primary or secondary amines in gepirone HCl. Peaks at 2900 cm-1 are due to the C-H stretching vibrations of aliphatic and aromatic hydrocarbons. A sharp peak at 1650 cm-1 indicates the presence of carbonyl groups. Medium to strong peaks associated with C-N stretching vibrations in the region of 1000 to 1350 cm-1, confirming the presence of amine groups. The PM of gepirone HCl with HPMC K100M and polyox WSR N80 alone and also in combination exhibited absorption bands in a similar region, suggesting the compatibility between gepirone HCl and polymers used in this study.
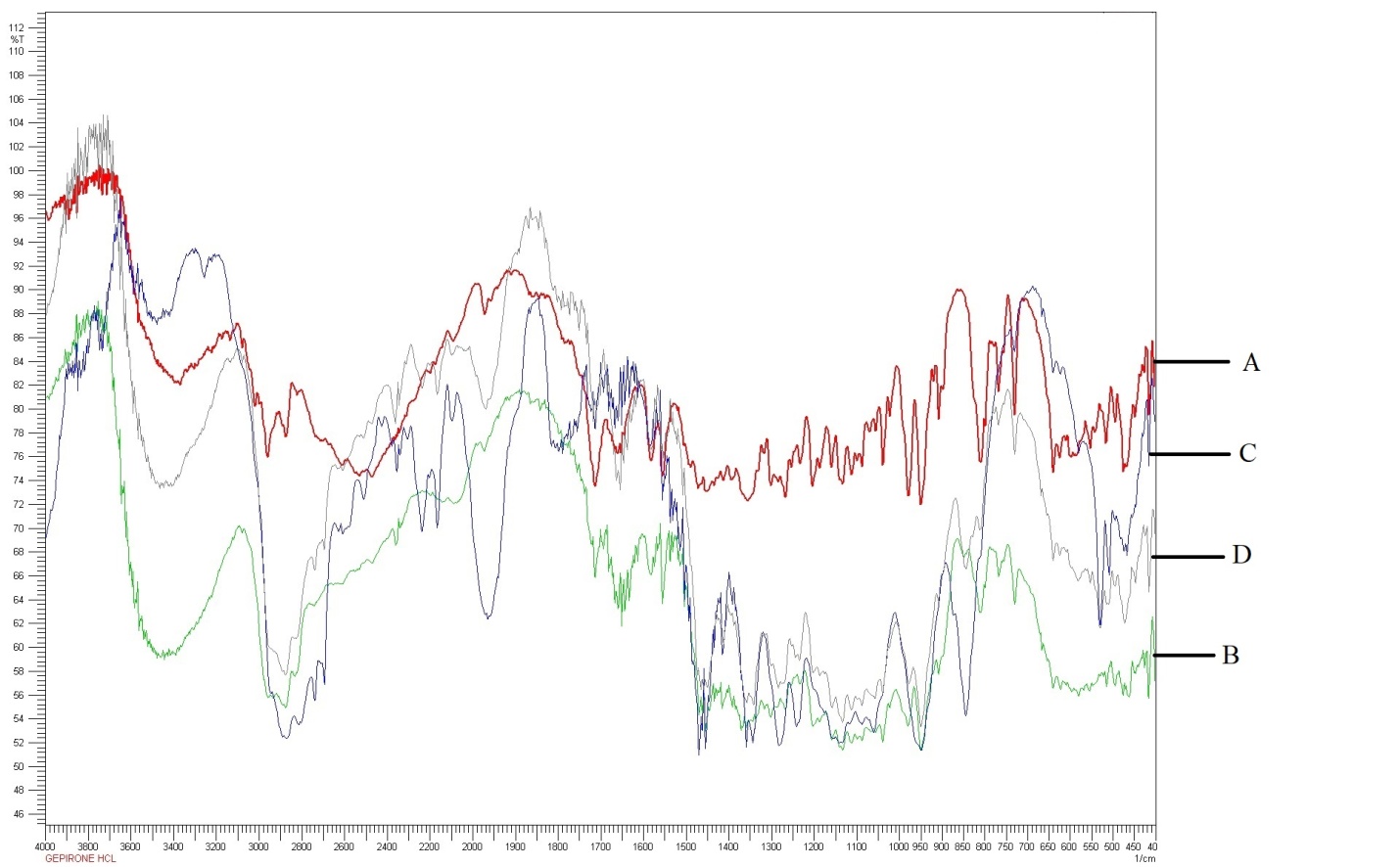
Fig. 1: FT-IR spectra of Gepirone HCl, PM of Gepirone HCl with HPMCK100M (1:1), Gepirone HCl with Polyox WSR N80 (1:1) and Gepirone HCl with HPMCK100M (1:1) and Polyox WSR N80 (1:1:1) for drug polymer compatibility
Differential scanning calorimetry (DSC) study
The thermogram for gepirone HCl exhibited a sharp melting peak at 213.2 °C with the onset and endset temperature of 210.4 °C and 216.1 °C respectively. This narrow melting temperature range with a sharp peak clearly indicates that gepirone HCl is a crystalline drug. The PMs of gepirone HCl with HPMC K100M (1:1), gepirone HCl with polyox WSR N80 (1:1) and the PM of gepirone HCl, HPMC K100M, and polyox WSR N80 (1:1:1) demonstrated peaks at 208.8 °C, 209.7 °C and 211.0 °C, respectively. The peaks for all three PMs were nearer to the peak of gepirone HCl suggesting no incompatibility between drug and polymers (fig. 2). All the PMs exhibited lower enthalpy in comparison to the enthalpy of gepirone HCl [23].
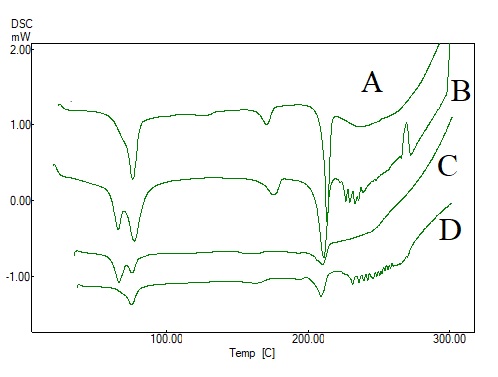
Fig. 2: DSC Thermogram of Gepirone HCl (A), PM of gepirone HCl with HPMCK100M (1:1) (B), Gepirone HCl with Polyox WSR N80 (1:1) (C) and Gepirone HCl with HPMCK100M and Polyox WSR N80 (1:1:1) for drug polymer compatibility
Preparation of gepirone HCl SR matrix tablets
The gepirone HCl SR matrix tablets were produced successfully with a yield more than 98 % suggesting proper selection of processing parameters like dry mixing time, wet mixing time, amount of binder, less adhesion of wet mass to sieves, drying time and temperature and optimum mixing of lubricants [24].
Characterization of gepirone HCl SR matrix tablets
Response surface analysis
Fig. 3 features a contour plot alongside a 3D representation of the Q1 response i. e. cumulative percent drug release at 1 h. The value of Q1 ranges from 4.72 % (run 9) to 35.54 % (run 8). The desirability of Q1 was targeted in the range of 6 to 12 % in 1 h of dissolution study. The following runs i. e. 1, 3, 7, 10, 12, and 13 were able to control the release of gepirone HCl in the percentage range of 6 to 12. A higher proportion of HPMC K100M i. e. in the medium or higher range is able to minimize the initial burst release effect and control the release of gepirone HCl in the desired range. When the concentration of both the polymers is in a higher range (run 5 and 9) the dissolution of gepirone HCl was much less i. e. less than the desired range (6 to 12 %). The runs having both polymers in a lower range exhibited burst release of more than 20% in 1st hour of the dissolution study (Run 4, 6, and 8). When the concentration polyox WSR N80 was in a higher range with the lower range of HPMC K100M were also unable to control the initial burst release within the desirable range (Run 2 and 6). The initial burst release was well controlled by an increased concentration of HPMC K100M similarly higher concentration of polyox WSR N80 also controlled initial burst release but it was slightly more than the desirable limit (run 11). Drug release from these hydrophilic matrices typically involves a combination of diffusion through the gel layer and erosion of the gel layer. The relative contribution of diffusion and erosion can be affected by the ratio of HPMC K100M and Polyox WSR N80. A higher HPMC K100M content might favor diffusion-controlled release due to the formation of a more robust gel, while a higher Polyox WSR N80 content could potentially lead to a greater contribution from erosion [25, 26].
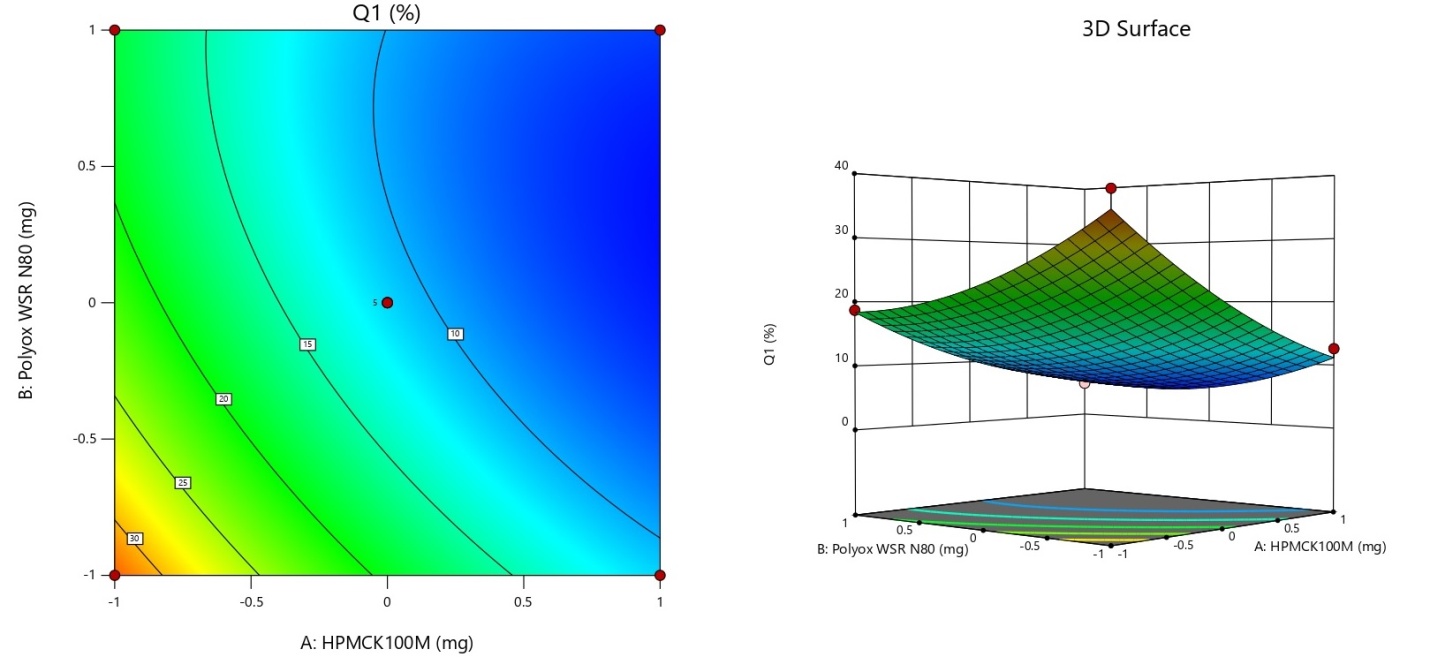
Fig. 3: Contour plots and 3 D-Response surface plot showing the influence of significant factors on Cumulative % drug release at 1 h (Q1)
Fig. 4 features a contour plot alongside a 3D representation of the t50 response i. e. time taken for dissolution of 50% gepirone HCl. The value of t50 ranges from 8.12 to 15.24 h. The desirability for dissolution of 50 % of the drug was fixed in the range of 10 to 13 h. It was observed that an increase in the concentration of both polymers resulted in delay in the time to reach the time for 50% of cumulative drug release. The desirability for t50 was attained only by 3 formulations i. e. Run 2, 4, 6, and 7.

Fig. 4: Contour plots and 3 D-Response surface plot showing the influence of significant factors on time taken for the release of 50% drug (t50)
Fig. 5 features a contour plot alongside a 3D representation of the Q18 response i.e. cumulative percent drug released in 18 h. The cumulative percent drug release ranges from 74.24 (run 9) to 100.98 (run 8). The desirability was fixed in the range of 70 to 90 cumulative percent drug dissolution or release in 18th h. The desirability of Q18 was attained by run 1, 5, 7, 9, 10, 11, 12 and 13.
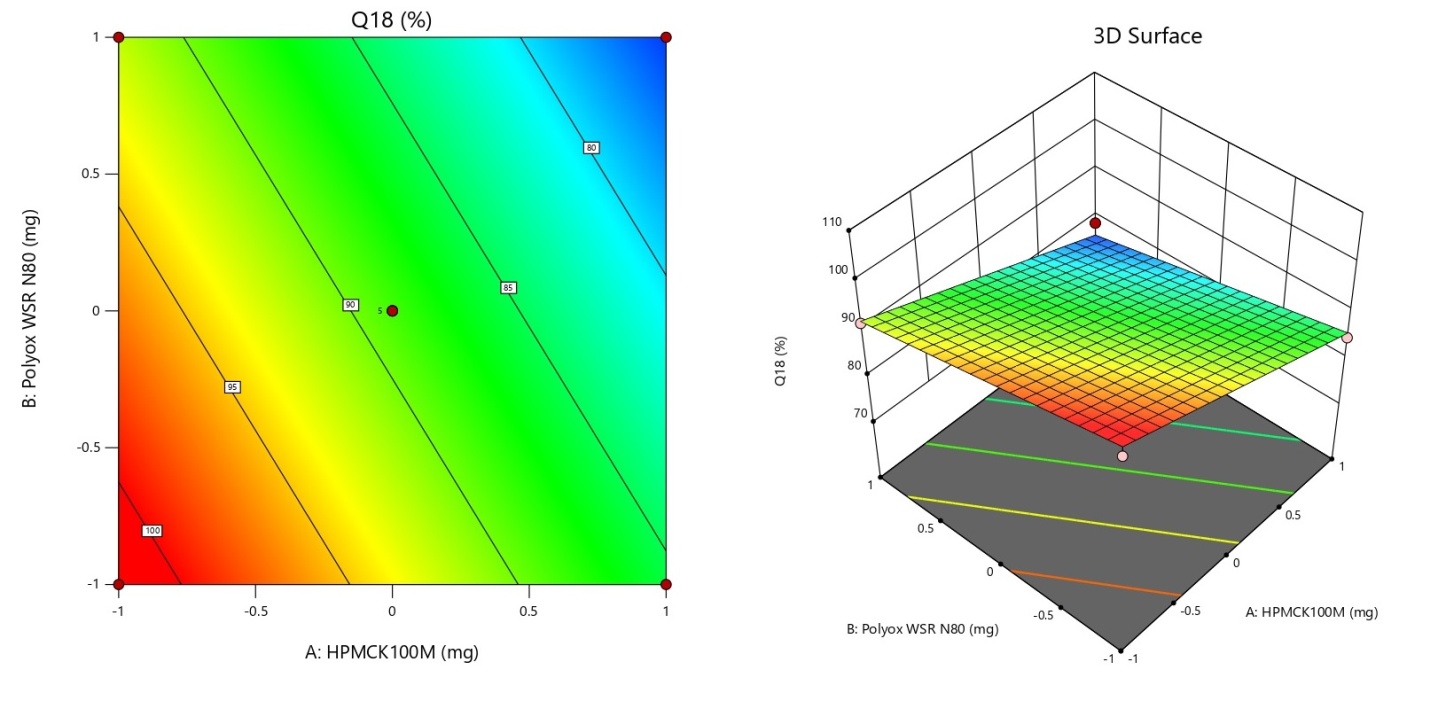
Fig. 5: Contour plots and 3D-Response surface plot showing the influence of significant factors on Cumulative % drug release at 18 h (Q18)
ANOVA of experimental design
For each response Q1, t50, and Q18 a regression equation based on coded factors was derived. This model enables the prediction of the relative influence of each factor. Below are the quadratic equations obtained from the regression analysis for each CQA.

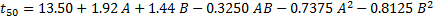
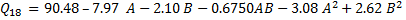
Table 2: summarizes the ANOVA results, highlighting the significance of various factors in our quadratic model. Analysis of the design matrix reveals that the model's F-value and p-value substantiate its significance for Q1, t50, and Q18. For the Q1 response, the analysis revealed that the factors A (HPMC K100M), B (Polyox WSR N80), their interaction (AB), along with their squared terms (A² and B²), were statistically significant (p<0.05, α = 0.05).
Table 2: Summary of analysis of variance (ANOVA) for different factors
| Source | Q1 | t50 | Q18 | |||
| F Value | P value | F Value | P value | F Value | P value | |
| Model | 54.20 | <0.0001 | 81.79 | <0.0001 | 43.51 | <0.0001 |
| A-HPMC K100M | 173.45 | <0.0001 | 223.26 | <0.0001 | 163.52 | <0.0001 |
| B-Polyox WSR N80 | 55.67 | <0.0001 | 126.37 | <0.0001 | 11.38 | 0.0119 |
| AB | 7.35 | 0.0302 | 3.20 | 0.1169 | 0.5867 | 0.4687 |
| A2 | 13.92 | 0.0073 | 28.64 | 0.0011 | 21.30 | 0.0024 |
| B2 | 24.85 | 0.0016 | 34.76 | 0.0006 | 15.33 | 0.0058 |
Similarly, for the t50 response, A, B, AB, A², and B² emerged as significant contributors. In contrast, when considering the Q18 response, the significant terms were A, B, A², and B². Value exceeding 0.1000 signify that the corresponding model terms are not statistically significant. This suggests a strong correlation between the observed and predicted values. Table 3 provides a summary of the CCD quadratic model employed during the optimization of the gepirone SR matrix tablets. For every critical response, the R² value is nearly identical to the adjusted R², demonstrating the model’s robust fit. Moreover, a high precision ratio for each response indicates an excellent signal-to-noise ratio.
Table 3: Summary of design of experiment with various parameters fitting to the quadratic model
| Responses | Q1 | t50 | Q18 |
| R2 | 0.9748 | 0.9832 | 0.9688 |
| Adjusted R2 | 0.9568 | 0.9712 | 0.9466 |
| Predicted R2 | 0.8209 | 0.8803 | 0.7901 |
| Adequate Precision | 23.1243 | 27.2525 | 22.0181 |
| mean | 14.94 | 12.55 | 88.77 |
| Standard Deviation | 1.79 | 0.3635 | 1.76 |
| P values | <0.0001 | <0.0001 | <0.0001 |
| Coefficient of Variation | 11.98 | 2.90 | 2.25 |
Q1: Cumulative Percent Drug Release at hour, t50: Time for 50 % of Cumulative percent drug release and Q18: Cumulative percent drug release at 18 h
Optimization and construction of overlay plot
For optimization, target specifications were set for responses Q1, t50, and Q18. Fig. 6 shows the overlay plot within the design space and illustrates the final optimized gepirone HCl SR matrix tablet. Table 4 presents a detailed summary of the optimization process, comparing both the predicted and the observed response values for the final formulation. Run 7 was selected as the optimum formulation because of its superiority in attaining the CQA i. e. Q1 (6 to 12 %), t50 (10 to 13 h), and Q18 (70 to 90%). The proportion of HPMC K100M and polyox WSR N80 played a significant role in attaining the desirable results. The optimal single-dose SR tablet (table 5), developed using CCD, comprises 18.2 mg of gepirone HCl, 40 mg of HPMC K100M, 30 mg of Polyox WSR N80, and additional excipients including starch paste (10% W/V) i. e. 15 mg per tablet, 3.8 mg of Avicel PH102, 2 mg of talc, and 1 mg of aerosol.

Fig. 6: Overlay contour plots depicting the design space and delineate the optimized formulation of Gepirone HCl SR matrix tablets
Table 4: Constrains for the process of optimization
| Name of factor | Lower limit | Upper limit | Optimized coded value | Optimized actual value |
| A. HPMC K100M | 20 | 40 | 1 | 40 |
| B-Polyox WSR N80 | 30 | 50 | -1 | 30 |
| Responses (CQA) | Desirable lower limit | Desirable upper limit | Predicted responses | Experimental responses |
| Q1 (%) | 6 | 12 | 11.17 | 10.93±0.56 |
| t50 (h) | 10 | 13 | 12.751 | 13.43±0.94 |
| Q18 (%) | 70 | 90 | 84.821 | 87.05±3.51 |
Mean±SD, n =6
Table 5: Composition of optimized run 7
| Ingredients | Quantity in mg/Tablet | Quantity in mg/100 Tablets |
| Gepirone HCl | 18.2 | 1800 |
| HPMC K100M | 40 | 4000 |
| Polyox WSR N80 | 30 | 3000 |
| Avicel PH 102 | 3.8 | 380 |
| Starch paste (10 %) (W/V) | 15 | 1500 |
| Talc | 2 | 200 |
| Aerosil | 1 | 100 |
| Total | 110 | 11000 |
Micromeritic properties of granules
The evaluation of micromeritic properties of pure drug powder Gepirone HCl suggests that it is a poorly flowable drug and it needs to be granulated for improvement of flowability and compressibility (table 6). All 13 formulations showed micromeritic properties desirable for proceeding to the next process, which is compression. The improvement in micromeritic properties suggests that the selection of binder, concentration of binder, sieve number, lubricating agent, etc were appropriate to achieve desirable flowability and compressibility [27, 28]. A granular friability index (GFI) of less than 1 % for all 13 formulations also suggests selection of right binder in right proportion [29].
Quality control tests for tablets
All 13 tablet formulations passed the quality control tests for tablets as the values were within the official specifications (table 7). The drug content for all formulations were above 97 % suggesting a uniform mixing of drug with excipients. Weight variation or deviation was within the allowed specification i. e.±7.5 %. Hardness for all formulations was above 5 Kg/cm2and percent loss in weight in the friability test was less than 1% suggesting optimum selection of binder. Hence, the prepared passed the quality control tests.
Table 6: Micromeritic properties of gepirone HCl and its granules
| Run | Angle of repose (θ) in degree | Carr’s index (%) | Hausner’s ratio | Granular friability index (%) | Moisture content (%) |
| Gepirone HCl | 41.58±1.74 | 23.61±1.82 | 1.65±0.02 | ** | 8±0.3 |
| 1 | 25.12±1.32 | 17.58±0.97 | 1.21±0.01 | 0.62±0.04 | 4.35±0.2 |
| 2 | 23.47±2.48 | 18.65±1.37 | 1.24±0.03 | 0.54±0.01 | 3.54±0.3 |
| 3 | 23.58±1.19 | 20.14±0.27 | 1.21±0.01 | 0.63±0.01 | 2.54±0.4 |
| 4 | 22.91±2.18 | 19.61±0.51 | 1.23±0.05 | 0.84±0.02 | 4.61±0.3 |
| 5 | 24.52±2.89 | 18.34±0.69 | 1.23±0.06 | 0.94±0.03 | 3.72±0.2 |
| 6 | 23.87±1.85 | 20.97±1.37 | 1.24±0.03 | 0.41±0.04 | 4.13±0.3 |
| 7 | 22.84±2.84 | 18.57±0.83 | 1.25±0.01 | 0.65±0.03 | 3.81±0.2 |
| 8 | 21.63±3.19 | 16.95±1.61 | 1.25±0.02 | 0.98±0.01 | 4.21±0.1 |
| 9 | 23.87±1.87 | 17.51±0.38 | 1.24±0.03 | 0.47±0.07 | 3.95±0.3 |
| 10 | 22.91±2.76 | 18.64±0.92 | 1.21±0.01 | 0.58±0.04 | 4.15±0.6 |
| 11 | 21.65±3.47 | 19.34±0.81 | 1.25±0.02 | 0.67±0.01 | 4.65±0.7 |
| 12 | 23.87±1.54 | 17.53±0.93 | 1.21±0.03 | 0.84±0.02 | 4.31±0.2 |
| 13 | 24.12±2.84 | 15.37±0.85 | 1.20±0.01 | 0.67±0.01 | 3.38±0.1 |
Mean±SD, n =6
Table 7: Quality control tests for SR tablets of gepirone HCL
| Run | Hardness* (Kg/cm2) | Thickness* (mm) | Friability** (%) | Drug content*** (%) | Weight variation**** (mg) |
| 1 | 5.2±0.4 | 2.33±0.3 | 0.8±0.11 | 98.56±3.25 | 100±4.52 |
| 2 | 5.4±0.3 | 2.33±0.2 | 0.8±0.05 | 99.74±2.26 | 100±3.91 |
| 3 | 5.5±0.3 | 2.33±0.3 | 0.6±0.04 | 98. 78±1.76 | 100±7.12 |
| 4 | 5.1±0.5 | 2.35±0.2 | 0.7±0.03 | 99.58±1.52 | 100±2.56 |
| 5 | 5.2±0.4 | 2.34±0.1 | 0.5±0.03 | 102.38±3.25 | 100±5.48 |
| 6 | 5.5±0.6 | 2.35±0.2 | 0.6±0.08 | 98.57±2.54 | 100±3.58 |
| 7 | 5.8±0.4 | 2. 34±0.3 | 0.8±0.12 | 99.85±2.97 | 100±2.87 |
| 8 | 5.7±0.5 | 2.33±0.2 | 0.3±0.09 | 98.45±2.69 | 100±3.54 |
| 9 | 5.5±0.4 | 2.32±0.3 | 0.6±0.05 | 99.38±3.54 | 100±6.57 |
| 10 | 5.3±0.2 | 2.33±0.2 | 0.3±0.05 | 98.62±2.56 | 100±5.94 |
| 11 | 5.6±0.4 | 2.34±0.1 | 0.8±0.04 | 97.58±3.74 | 100±4.81 |
| 12 | 5.5±0.3 | 2.34±0.3 | 0.9±0.02 | 99.87±1.89 | 100±3.49 |
| 13 | 5.8±0.3 | 2.34±0.2 | 0.6±0.02 | 97.19±3.58 | 100±7.14 |
Mean±SD, *n =6, **n =60, ***n = 10, and ****n = 20
In vitro dissolution study
In vitro dissolution study for all the formulations was performed and it was found that Run 4, 6, and 8 could not sustain the release of gepirone HCl for more than 18 h. The reason attributed can be because of the lower proportion of either HPMC K100M or polyox WSR N80. All other formulations exhibited gepirone HCl release for 24 h in sustained manner. By considering the initial burst release and time for 50 % of release of gepirone HCl run 7 was selected as the optimized formulation. The dissolution profile of run 7 is presented in table 8 and fig. 7. The release mechanism of gepirone HCl from Run 7 can be attributed to the release of gepirone HCl by the complex interplay of diffusion and polymer matrix erosion. HPMC K100M, a high-viscosity hydrophilic polymer, forms a gel-like barrier upon contact with aqueous media. This gel structure acts as a rate-controlling layer, slowing drug diffusion and mitigating the initial burst effect. Meanwhile, Polyox WSR N80 complements this mechanism by enhancing matrix integrity and providing controlled erosion properties. Together, these polymers create a robust drug delivery matrix that harmonizes sustained release with protection against burst release [30].

Fig. 7: Dissolution profile for optimized formulation (run 7)
Table 8: Evaluation of run 7
| Hardness (Kg/cm2) | Drug content (%) | In vitro drug release | |
| Time (h) | Cu. % drug release | ||
| 5.8±0.4 | 99.85±2.97 | 1 | 11.17±0.57 |
| 2 | 18.25±1.04 | ||
| 4 | 27.35±1.37 | ||
| 6 | 34.54±2.14 | ||
| 8 | 40.23±2.58 | ||
| 10 | 49.56±2.74 | ||
| 12 | 57.65±3.19 | ||
| 14 | 66.58±2.84 | ||
| 16 | 75.84±3.87 | ||
| 18 | 85.61±4.82 | ||
| 20 | 93.54±3.57 | ||
| 22 | 100.67±3.64 | ||
| 24 | 100.25±3.91 | ||
Mean±SD, n = 6
The dissolution data for run 7 was put into different In vitro release kinetic equations (table 9). A higher correlation coefficient for zero order equation suggests that gepirone HCl release followed zero-order kinetics. A higher correlation coefficient for the Higuchi equation and peppa's release exponent (1.02) suggests that gepirone HCl is released not only by diffusing through the polymer but also by the polymer matrix itself dissolving or eroding at a significant rate [31, 32]. The combination of HPMC K100M and Polyox WSR N80 is particularly interesting. While HPMC forms a robust gel matrix, Polyox can modify the viscosity and the overall mechanical strength of the swollen layer [33]. Their synergistic effect results in a controlled matrix where water penetration gradually triggers polymer relaxation. As the gel erodes, the entrapped drug is released in a manner that reflects a super case-II mechanism. The exponent n ≈ 1.02 reflects that the drug is being released predominantly due to these structural changes rather than classic diffusion [13]. This behavior has been documented in systems using similar hydrophilic polymers, where the interplay between swelling and erosion becomes the rate-limiting step [30].
Scanning electron microscopy (SEM)
SEM image of fig. 8 reveals the formation of gel layer and matrix erosion. This can be attributed to the synergistic effect of using a combination of HPMC K100M and Polyox WSR N80. Fig. 8 validates this synergy by revealing a homogenous and porous gel network, which is vital for ensuring predictable and reproducible drug release [12].
Stability study
The stability study for selected run 7 for 6 mo indicates no significant change in drug content, Q1, t50, and Q18 at P<0.05 level. The data of the stability study suggest that gepirone HCl SR matrix tablets are stable (table 10).

Fig. 8: SEM image of tablet collected at 4th h of dissolution testing
Table 9: In vitro release kinetics for the optimized formulation (Run 7)
| Run | Correlation coefficient | Korsmeyer pappa’s plot | |||
| Zero order | First order | Higuchi Equation | Correlation | Slope | |
| Run 7 | 0.971 | 0.917 | 0.976 | 0.996 | 1.02 |
Table 10: Stability study for optimized formulation (Run 7)
| Months | 40 °C±2 °C/75% RH±5% RH | |||||
| Drug content (%) | Q1 (%) | t50 (h) | Q18 (%) | Tablet discoloration | Cracking | |
| 0 | 99.85±2.56 | 11.17±0.87 | 12.75±0.67 | 85.61±2.64 | * | ** |
| 1 | 98.13±1.26 | 11.09±0.31 | 12.89±0.47 | 86.54±3.96 | * | ** |
| 3 | 98.85±1.61 | 11.03±0.29 | 12.71±0.84 | 85.96±2.99 | * | ** |
| 6 | 98.25±1.29 | 11.11±0.36 | 12.65±0.04 | 86.17±3.47 | * | ** |
| P<0.05 | NS | NS | NS | NS | - | - |
| Months | 25°±2 °C C/60%±5% RH | |||||
| Drug content (%) | Q1 (%) | t50 (h) | Q18 (%) | Tablet discoloration | Cracking | |
| 0 | 99.85±2.56 | 11.17±0.87 | 12.75±0.67 | 85.61±2.64 | * | ** |
| 1 | 99.43±1.15 | 11.19±0.42 | 12.13±0.26 | 84.12±2.91 | * | ** |
| 3 | 98.45±3.7 | 11.93±0.33 | 12.14±0.73 | 84.37±3.74 | * | ** |
| 6 | 98.79±2.4 | 11.61±0.25 | 12.13±0.61 | 85.41±2.81 | * | ** |
| P<0.05 | NS | NS | NS | NS | - | - |
| Months | 30 °C2 °C C/65%±5% RH | |||||
| Drug Content (%) | Q1 (%) | t50 (h) | Q18 (%) | Tablet discoloration | cracking | |
| 0 | 99.85±2.56 | 11.17±0.87 | 12.75±0.67 | 85.61±2.64 | * | ** |
| 1 | 98.74±3.49 | 11.02±0.18 | 12.26±0.61 | 85.14±3.96 | * | ** |
| 3 | 99.16±2.57 | 10.93±0.27 | 12.23±0.27 | 84.08±1.84 | * | ** |
| 6 | 98.19±1.27 | 11.01±0.17 | 12.14±0.15 | 85.18±2.26 | * | ** |
| P<0.05 | NS | NS | NS | NS | - | - |
Mean±SD, n = 6, NS = Not significant, *No discoloration **No cracking
CONCLUSION
The need for a once-daily formulation of gepirone HCl is underscored by its pharmacokinetic profile and therapeutic indications. The successful preparation and optimization of gepirone HCl SR matrix tablets by wet granulation method and Central Composite Design (CCD), respectively, marks a significant achievement in controlled drug delivery systems. The selection of run 7 as the optimum formulation highlights the precision of CCD in optimizing the formulation parameters to achieve the desired release profile. The integration of two polymers HPMC K100M and Polyox WSR N80 proved effective in modulating the drug release dynamics. Specifically, the polymers synergistically contributed to minimizing the initial burst release, thereby ensuring a steady and sustained release of gepirone HCl over 24 h. Hence, the development of a once-daily formulation aligns with modern patient-centric approaches in pharmaceutical design, catering to convenience and improving the quality of life for patients requiring long-term therapy. This can be ascertained in future studies through in vivo studies.
FUNDING
This research received no external funding.
AUTHORS CONTRIBUTIONS
Dinesh Das: Performed the practical experimental work. Anjan Kumar: Guided the candidate while executing the work. Ch. Niranjan Patra: Assisted in writing the manuscript.
CONFLICT OF INTERESTS
The authors declared no conflict of interest in the manuscript.
REFERENCES
Kaur Gill A, Bansal Y, Bhandari R, Kaur S, Kaur J, Singh R. Gepirone hydrochloride: a novel antidepressant with 5-HT1A agonistic properties. Drugs Today (Barc). 2019;55(7):423-37. doi: 10.1358/dot.2019.55.7.2958474, PMID 31347611.
Holmberg M, From Fabre Kramer E. Pharmaceuticals. Pharmacy Times. 2024;90(1):36-7.
Timmer CJ, Sitsen JM. Single and multiple dose pharmacokinetics and tolerability of gepirone extended release. Clin Drug Investig. 2002;22(12):819-26. doi: 10.2165/00044011-200222120-00002.
Fabre LF, Timmer CJ. Effects of food on the bioavailability of gepirone from extended release tablets in humans: results of two open-label crossover studies. Curr Ther Res Clin Exp. 2003;64(8):580-98. doi: 10.1016/j.curtheres.2003.09.012, PMID 24944406.
Robinson DS, Sitsen JM, Gibertini M. A review of the efficacy and tolerability of immediate release and extended release formulations of gepirone. Clin Ther. 2003;25(6):1618-33. doi: 10.1016/s0149-2918(03)80159-5, PMID 12860488.
Teixeira MT, Sa Barreto LL, Taveira SF, Gratieri T, Gelfuso GM, Marreto RN. The influence of matrix technology on the subdivision of sustained-release matrix tablets. AAPS PharmSciTech. 2019;21(1):8. doi: 10.1208/s12249-019-1554-1, PMID 31797144.
Sarangi DK, Patro CS, Patra CN, Sahoo NK, Das NR, Kaur K. In vivo assessment, formulation characterization and enhancing pharmacotherapy of encapsulated mini tablets for immediate release sildenafil citrate and sustained release bosentan. Results Chem. 2024;9:101652. doi: 10.1016/j.rechem.2024.101652.
Patra CN, Kumar AB, Pandit HK, Singh SP, Devi MV. Design and evaluation of sustained-release bilayer tablets of propranolol hydrochloride. Acta Pharm. 2007;57(4):479-89. doi: 10.2478/v10007-007-0038-0, PMID 18165191.
Diwedi R, Alexandar A, Chandrasekar M. Preparation and in vitro evaluation of sustained release tablet formulations of metformin HCl. Asian J Pharm Clin Res. 2012;5(1):45-8.
Ram D, Pankhaniya H. Formulation evaluation and optimization of sustained-release drug delivery system of cisapride tablet. Int J Pharm Pharm Sci. 2021;13(9):56-62. doi: 10.22159/ijpps.2021v13i9.41799.
Patra CN, Padhy S, Sen T, Bhattacharya S, Swain S, Sruti J. Formulation development and evaluation of SR matrix tablets of stavudine. Ind J Pharm Educ Res. 2013;47(2):214-20.
Vanza JD, Patel RB, Dave RR, Patel MR. Polyethylene oxide and its controlled release properties in hydrophilic matrix tablets for oral administration. Pharm Dev Technol. 2020;25(10):1169-87. doi: 10.1080/10837450.2020.1808015, PMID 32772604.
Martin LM, Rajabi Siahboomi AR. Applications of polyethylene oxide (POLYOX) in hydrophilic matrices. Hydrophilic Matrix Tablets for Oral Controlled Release. 2014 Sep 23;16:123-41. doi: 10.1007/978-1-4939-1519-4_5.
Gharti K, Thapa P, Budhathoki U, Bhargava A. Formulation and in vitro evaluation of floating tablets of hydroxypropyl methylcellulose and polyethylene oxide using ranitidine hydrochloride as a model drug. J Young Pharm. 2012;4(4):201-8. doi: 10.4103/0975-1483.104363, PMID 23493037.
He W, Huang S, Zhou C, Cao L, Yao J, Zhou J. Bilayer matrix tablets for prolonged actions of metformin hydrochloride and repaglinide. AAPS PharmSciTech. 2015;16(2):344-53. doi: 10.1208/s12249-014-0229-1, PMID 25319054.
Petra D, Biljana J. Designing polyethylene oxide and hydroxypropyl methylcellulose matrix tablets with comparable dissolution properties. Afr J Pharm Pharmacol. 2020;14(4):87-98. doi: 10.5897/AJPP2020.5133.
Garg N, Pandey P, Kaushik D, Dureja H. Development of novel multifunction directly compressible co-processed excipient by melt granulation technique. Int J Pharm Investig. 2015;5(4):266-74. doi: 10.4103/2230-973X.167692, PMID 26682197.
Rantanen J, Antikainen O, Mannermaa JP, Yliruusi J. Use of the near infrared reflectance method for measurement of moisture content during granulation. Pharm Dev Technol. 2000;5(2):209-17. doi: 10.1081/pdt-100100536, PMID 10810751.
Sinko PJ. Martin’s physical pharmacy and pharmaceutical sciences. Lippincott Williams & Wilkins; 2023.
Aulton ME, Taylor K. Aulton's pharmaceutics: the design and manufacture of medicines. Elsevier Health Sciences; 2013.
Cohen JL, Hubert BB, Leeson LJ, Rhodes CT, Robinson JR, Roseman TJ. The development of USP dissolution and drug release standards. Pharm Res. 1990;7(10):983-7. doi: 10.1023/a:1015922629207, PMID 2281043.
Khagga B, Kaitha MV, Dammu R, Mogili S. ICH guidelines “Q” series (quality guidelines) a review. GSC Biol and Pharm Sci. 2019;6(3):89-106. doi: 10.30574/gscbps.2019.6.3.0034.
Ghanbari E, Picken SJ, Van Esch JH. Analysis of differential scanning calorimetry (DSC): determining the transition temperatures and enthalpy and heat capacity changes in multicomponent systems by analytical model fitting. J Therm Anal Calorim. 2023;148(22):12393-409. doi: 10.1007/s10973-023-12356-1.
Tahara K, Yamamoto K, Nishihata T. Overall mechanism behind matrix sustained release (SR) tablets prepared with hydroxypropyl methylcellulose 2910. J Control Release. 1995;35(1):59-66. doi: 10.1016/0168-3659(95)00021-Y.
Sujja Areevath J, Munday DL, Cox PJ, Khan KA. Relationship between swelling erosion and drug release in hydrophillic natural gum mini-matrix formulations. Eur J Pharm Sci. 1998;6(3):207-17. doi: 10.1016/s0928-0987(97)00072-9, PMID 9795062.
Sung KC, Nixon PR, Skoug JW, Ju TR, Gao P, Topp EM. Effect of formulation variables on drug and polymer release from HPMC-based matrix tablets. Int J Pharm. 1996;142(1):53-60. doi: 10.1016/0378-5173(96)04644-3.
Patra CN, Swain S, Mahanty S, Panigrahi KC. Design and characterization of aceclofenac and paracetamol spherical crystals and their tableting properties. Powder Technol. 2015;274:446-54. doi: 10.1016/j.powtec.2015.01.053.
Jammula S, Patra CHN, Swain S, Panigrahi KC, Nayak S, Dinda SC. Design and characterization of cefuroxime axetil biphasic floating minitablets. Drug Deliv. 2015;22(1):125-35. doi: 10.3109/10717544.2013.871603, PMID 24417642.
Prakash SS, Patra CN, Santanu C, Kumar PH, Patro VJ, Devi MV. Studies on flowability compressibility and in vitro release of Terminalia chebula fruit powder tablets. Iranian Journal of Pharmaceutical Research. 2011;10(3):393-401. doi: 10.22037/ijpr.2010.894.
Talevi A, Ruiz ME. Korsmeyer peppas peppas sahlin and brazel peppas: models of drug release. The ADME Encyclopedia: a Comprehensive Guide on Biopharmacy and Pharmacokinetics; 2022. p. 613-21. doi: 10.1007/978-3-030-84860-6.
Korsmeyer RW, Gurny R, Doelker E, Buri P, Peppas NA. Mechanisms of solute release from porous hydrophilic polymers. Int J Pharm. 1983;15(1):25-35. doi: 10.1016/0378-5173(83)90064-9.
Lee PI. Kinetics of drug release from hydrogel matrices. J Control Release. 1985;2:277-88. doi: 10.1016/0168-3659(85)90051-3.
Peppas NA, Duncan R, Wnek GE, Hoffman AS, Gao GH, Kim SW. Highly cited research articles in Journal of Controlled Release: commentaries and perspectives by authors. J Control Release. 2014;190:29-74. doi: 10.1016/S0168-3659(14)00482-9.