
Int J App Pharm, Vol 17, Issue 6, 2025, 423-435Original Article
DESIGN AND STATISTICAL OPTIMIZATION OF MUSKMELON PECTIN-BASED TELMISARTAN ORAL FAST DISSOLVING FILMS THROUGH QUALITY BY DESIGN
THAKUR REKHABAI, SANTOSH KUMAR RADA*
Department of Pharmaceutics, GITAM School of Pharmacy, GITAM (Deemed to be University), Rushikonda, Visakhapatnam-530045, Andhra Pradesh, India
*Corresponding author: Santosh Kumar Rada; *Email: srada@gitam.edu
Received: 02 May 2025, Revised and Accepted: 27 Aug 2025
ABSTRACT
Objective: This study aimed to design and statistically optimize oral fast-dissolving films containing telmisartan based on muskmelon pectin using a quality-by-design approach.
Methods: Cucumis melo pectin was extracted by acid hydrolysis. The physicochemical properties of muskmelon pectin were evaluated using FTIR, NMR, DSC, and SEM. The fast-dissolving films were made by solvent casting, and they were optimized using a 23-factorial design. A two-way factorial design was used because it effectively assesses the impact of three film-forming polymers, namely muskmelon pectin, apple pectin, and citrus pectin, at two concentrations (0 mg and 350 mg), on important formulation responses such as percentage drug dissolution and dissolution efficiency within 10 min. With a few experimental runs, this strategy enables the investigation of both the independent and combined effects of factors. The Critical Quality Attributes (CQA) of each of the eight formulations were analyzed, including dissolution efficiency (DE%), disintegration time (DT), tensile strength (TS), thickness, and the percentage of drug dissolved in 10 min (PD%). The optimized formulation was evaluated for in vivo bioavailability in Wistar rats. The accelerated stability tests were performed according to ICH guidelines.
Results: The extracted muskmelon pectin was found to be amorphous and water-soluble, with a high degree of esterification, and exhibited satisfactory swelling index, viscosity, and pH values. Instrumental analyses using FT-IR, NMR, SEM, XRD, and DSC confirmed the material as pectin. The optimized formulation (F2) demonstrated favorable critical quality attributes, including drug dissolution efficiency at 10 min (DE₁₀min), tensile strength (TS), and percentage drug dissolved at 10 min (PD₁₀min), with drug release reaching approximately 95.61%. In vivo pharmacokinetic studies showed that TMF2 had significantly higher maximum plasma concentration (Cmax) and area under the curve (AUC), and relative bioavailability of 175.85%, showing a 75.85% enhancement over pure telmisartan, indicating the developed formulation significantly boosts drug absorption and may offer superior therapeutic effectiveness. Additionally, stability studies over six months revealed no significant changes in drug content, mechanical strength, or dissolution profiles, confirming the formulation’s stability and suitability for therapeutic use.
Conclusion: Telmisartan OFDFs were successfully designed and optimized using muskmelon pectin as a new film-forming agent through 23 factorial designs, which exhibited 95.61% of the drug dissolution in 10 min and dissolution efficiency in 10 min alone, showing its better and efficient drug delivery.
Keywords: Telmisartan, Oral fast dissolving films, Quality by design, Dissolution efficiency, solvent evaporation, Factorial designs
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijap.2025v17i6.54850 Journal homepage: https://innovareacademics.in/journals/index.php/ijap
INTRODUCTION
Efficient drug delivery methods are necessary to guarantee the active pharmaceutical ingredient's quick availability at the site of action to maximize therapeutic results and reduce side effects, out of all the different delivery methods, oral fast-dissolving dosage forms, such oral fast-dissolving films (OFDF), have drawn a lot of interest because of their patient-friendly features. FDFs provide significant advantages for young, elderly, and dysphagic individuals who frequently have trouble swallowing traditional tablets or capsules since they dissolve rapidly in the oral cavity without the need for water [1]. To ensure a quick beginning of action and better patient compliance, the US Food and Drug Administration (USFDA) states that oral fast-disintegrating tablets (OFDT) or films must dissolve in a matter of seconds. Choosing a suitable film-forming agent that offers sufficient mechanical strength, quick disintegration, and effective drug release is crucial to the effective formulation of FDFs. Natural polymers' biodegradability, biocompatibility, and multifunctional qualities have led to an increase in their exploration for such uses. Of these, pectin a polysaccharide derived from plant cell walls, stands out as a viable option because of its capacity to gel, swell, and form films. Muskmelon pectin possesses a high degree of esterification 61.55% and high solubility 95%, promoting rapid film disintegration and improved flexibility, compared to citrus and apple pectins (Xiaobin Ma et al. 2020). Its sustainable origin and unique characteristics make it a promising alternative for fast-dissolving film formulation. Moreover, the functional properties of natural pectin can be greatly improved by chemical or physical modification, which qualifies it for innovative drug delivery applications [2].
Telmisartan, classified as a BCS Class II drug, has low aqueous solubility (~0.078 mg/ml), leading to poor and variable oral bioavailability (~42%). This limits its therapeutic efficiency, causing delayed onset and inconsistent blood pressure control, which may affect patient adherence. Enhancing solubility through advanced systems like fast-dissolving films could improve clinical outcomes and compliance [3]. This study investigates the use of pectin is a novel natural biopolymer derived from muskmelon, as a new film-forming agent that may enhance the dissolution and bioavailability of the medication when combined with other ingredients to create fast-dissolving films. Muskmelon pectin has a distinct advantage in creating thin, quickly dissolving films that can efficiently deliver medications that are poorly soluble in water because of its superior hydration, swelling, and biocompatibility properties [4]. The limitations caused by the poor solubility of telmisartan may be overcome by muskmelon pectin-based OFDFs, which facilitate more rapid dispersion and its dissolution in the oral cavity. Quality by Design (QbD) was used to systematically formulate and optimize the telmisartan fast-dissolving films. The goal of the QbD framework is to comprehend how formulation and process variables affect critical quality attributes (CQAs) like dissolution efficiency, mechanical strength, disintegration time, and drug content uniformity. The primary determinants of film performance were found and optimized through factorial design studies and risk assessment in order to produce a stable, scalable formulation with improved therapeutic potential. In order to improve the drug's bioavailability through the mouth and patient compliance, this study intends to create telmisartan fast-dissolving films using musk melon pectin, a novel film-forming agent. This will also address an urgent need in the efficient treatment of hypertension [5].
MATERIALS AND METHODS
Material
Telmisartan pure drug, was acquired as a gift sample from Aurobindo Pharma Pvt Ltd in Hyderabad, India. Each of the other chemicals and solvents used was of analytical and pharmaceutical grade.
Methods
Pectin extraction from muskmelon rind
The muskmelon (Cucumis melo L.) rind was collected from a nearby fruit juice shop. The rind was then thinly sliced and allowed to air-dry until the moisture had come off of it completely. The rinds were dried and then ground into a fine powder. Using an analytical balance, 5 g of rind powder was meticulously weighed and added to 150 ml of purified water. The liquid's pH was lowered to 2 by adding powdered anhydrous citric acid. For an hour, the mix was heated at 80 °C while being swirled occasionally. After passing the filtrate using a muslin cloth and adding an equivalent amount of ethanol, it was incubated in an incubator chamber set at about 4 °C for three hours in order to allow pectin to separate. After that, the precipitate was collected and subjected to several ethanol purification procedures. The precipitant was cleaned, then dried in a hot air oven. The dried pectin was stored in a desiccator after being filtered using a #120 sieve to create uniformly sized particles [6].
The following formula was used to determine the pectin yield:
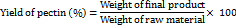
Assays
Determining the esterification degree (DE)
A 0.2 g sample of dried pectin was dissolved in 20 ml of distilled water after being moistened with ethanol. Until the pectin was completely dissolved, the sample was kept at 45 °C in a mechanical shaking water bath. The sample was mixed with three drops of phenolphthalein. The solution was titrated using sodium hydroxide at 0.1 N. As soon as some pink color started to show, the result was noted as the preliminary titration volume. The quantity of 0.1 N sodium hydroxide solution used for the first titration was used to determine the number of free carboxy groups. Then, to neutralize the polygalacturonic acid, 10 ml of 0.1 N sodium hydroxide were added. To de-esterify the pectin, the sample was sealed with a stopper, shaken vigorously, and then left to stand at room temperature for two hours [7]. The neutralized sodium hydroxide was mixed with 10 ml of 0.1 N hydrochloric acid, and the mixture was shaken until the pink hue vanished. After adding three drops of phenolphthalein, the sample was titrated once more using 0.1 N sodium hydroxide. After some pink coloration appeared, the titration volume was noted as the final titration volume. The amount of 0.1 N sodium hydroxide solution used for the final titration was used to determine the number of esterified carboxy groups [8].
The DE was calculated from the following formula
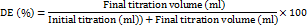
The equivalent weight was evaluated by the following equation:
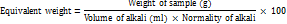
Determination of galactolacturonic acid
10 mg of the dried pectin sample was weighed, and 10 ml of 0.05 N NaOH was added to dissolve it. After allowing the solution to settle, 9.8 ml of purified water were added. After adding 1 ml of the carbazole reagent to 2 ml of this solution, a white precipitate was seen to form. After that, 12 ml of sulfuric acid were added while being continuously stirred. To develop color, the solution was left to stand for ten minutes. At 525 nm, the colorimetric measurement was made. In place of the carbazole reagent, 1 ml of 99% ethanol was added to the blank. The calibration graph plotted with galacturonic acid standard solutions was used to calculate the concentration of galacturonic acid [9].
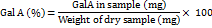
Determination of the methoxyl content
25 ml of 0.25 N NaOH was added to the neutral solution that was produced by the equivalent weight determination step. After thoroughly mixing the solution, it was left to stand for half an hour, neutralized the excess NaOH with 0.25N HCl, and titrated the released methanol with 0.25N NaOH. The following formula is used for the determination of methoxyl content [10].
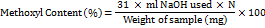
Physical-chemical properties of pectin
Organoleptic examination of isolated pectin
The isolated compound was evaluated for organoleptic characteristics like color, odor, texture, touch, taste, and texture.
Solubility behavior
To test the solubility of various solvents, one portion of dry pectin powder was shaken with ethanol, methanol, ether, and water [11].
Pectin pH
A digital pH meter was used to measure the pH of a 1% w/v solution containing the extracted pectin.
Viscosity
The Brookfield viscometer was filled with a 1 percent w/v solution containing muskmelon pectin, and a spindle was chosen based on the sample's predicted viscosity [12].
Swelling index
In a 25 ml stoppered measuring cylinder, transferred 25g of muskmelon pectin. Water was poured into the cylinder until the 20 ml mark was reached. Throughout the day, agitate gently at intervals and let it stand. Calculate the volume that the swollen [13].
Bulkiness and bulk density
50 g of precisely weighed material was placed in a graduated measuring cylinder. After setting the cylinder on a bulk density apparatus, the volume of the powder was determined. After that, the powder was tapped in a bulk density device until its volume was constant. Bulk volume, or total volume, was calculated [14].
Angle of repose
Using the provided formula, the prepared particles' angle of repose was determined.
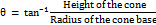
Powder flow property
The angle of repose was used to measure the flow characteristics. The formula and the readings were used to determine the angle of repose.
Hausner's ratio
The following formula can be used to determine Hausner's ratio.
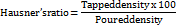
Powder compressibility
Compressibility was another name for this characteristic. Five grams of finely ground pectin were put into a measuring cylinder, and bulk density equipment was used to perform calculations [15].
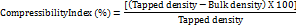
Characterization of muskmelon pectin
FTIR
Using an FTIR spectrophotometer (M/s Bruker), the extracted muskmelon pectin's FT-IR spectra were captured in KBr disc over the 4000-400 cm-1 range. The findings are displayed in fig. 1.
SEM
The extracted muskmelon pectin's surface morphology was investigated using a SEM JSM 6100 (M/s JOEL, Tokyo, Japan). Images showing the surface arrangement were obtained by scanning the extract powder at an acceleration voltage of 10 kV. The SEM image of the extracted muskmelon pectin is shown in fig. 2 [16].
Thermal evaluation
The purpose of the DSC studies was to examine the extracted muskmelon pectin's thermodynamic compatibility. About three to five percent of the extract was put in an aluminum pan and heated at a temperature of 10 °C per minute to 300 °C in an atmosphere of nitrogen at a flow rate of 20 ml per minute, as illustrated in fig. 3
XRD
The extracted musk melon pectin was characterized in the solid-state using X-ray diffraction studies. Using Cu Kα radiation and a voltage of 40 kV, the X-ray diffractometer recorded the samples' diffraction pattern. The findings are shown in fig. 4 [17].
NMR
The pectin was dissolved in D2O, moved to an NMR tube, recorded at 400MHz frequency to obtain the ¹H NMR spectrum, and then looked for peaks that indicate the presence of sugar ring protons and methyl esters. The findings are displayed in fig. 5.
Formulation and development of ODFs
Telmisartan OFDFs are prepared with 23 factorial techniques. 23 factorial design was employed to optimize the formulation variables, with the quantities of A-muskmelon pectin, B-apple pectin, and C-citrus pectin serving as the primary determinants. The inclusion of muskmelon, apple, and citrus pectins in the formulation was aimed at comparing their film-forming capacities, mechanical strength, and disintegration behavior, as each has been reported in literature to possess suitable properties for oral fast-dissolving films. Preliminary trials with varying concentrations indicated that 350 mg of each pectin produced optimal films in terms of uniformity, flexibility, and rapid disintegration. Therefore, 350 mg was selected as the standard polymer level for comparative evaluation. Prior research done before establishing the experimental design served as the basis for these decisions. Utilizing the Design Expert® software, a total of eight runs were performed by preparing several batches of telmisartan by the factorial design. The current study looked at two important response variables: PD10% in 10 min, or the percentage of the drug dissolved in ten minutes, and DE10% in 10 min, or the efficacy of dissolution within ten minutes. ANOVA was used to analyze the experimental results, fitting the response variables within the factorial design framework. Table 1 contains details about the formulation of the design matrix [18].
Table 1: The details about the formulation of the 23 design matrix
| S. No. | Ingredients (mg) | TF1 | TF2 | TF3 | TF4 | TF5 | TF6 | TF7 | TF8 |
| 1 | Telmisartan Drug | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 |
| 2 | Muskmelon pectin | 0 | 350 | 0 | 350 | 0 | 350 | 0 | 350 |
| 3 | Apple pectin | 0 | 0 | 350 | 350 | 0 | 0 | 350 | 350 |
| 4 | Citrus pectin | 0 | 0 | 0 | 0 | 350 | 350 | 350 | 350 |
| 5 | Glycerol | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| 6 | Sodium saccharin | 60 | 60 | 60 | 60 | 60 | 60 | 60 | 60 |
| 7 | Citric acid | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| 8 | Menthol | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| 9 | Distilled Water (ml) | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
Preparation of telmisartan OFDFs
The first step in the formulation process was to disperse 350 mg of the pectin in 5 ml of purified water, and this was then agitated on the magnetic stirrer at ambient temperature for 30 min at 2000 RPM (Solution A). A thorough mixing of the polymeric solutions was done. After that, citric acid and sodium saccharin were added and agitated for fifteen minutes. One ml of methanol was used to dissolve the drug, menthol, and it was then sonicated for half an hour. Following another 15 min of stirring, glycerol and polymeric solution were incorporated into the drug solution. The resultant solution was transferred to a Petri plate and dried at 40 degrees Celsius in a hot air oven. The films were divided into 2 x 2 cm² parts and kept in a desiccator in aluminum bags [19].
Evaluation of telmisartan OFDFs
Morphological study: Each formulation's three separate 2x2 cm² OFDFs were examined visually for surface quality, color, transparency, and homogeneity.
Weight variation: Each formulation's three 2x2 cm2 OFDFs were weighed by employing a digital weighing balance.
Thickness: A screw gauge with a range of 0–10 mm and a minimum count of 0.01 mm was used to measure the its thickness of the three samples, taken from each formulation. The standard deviation and mean were determined by analyzing the gathered data [20].
In vitro disintegration time
The USP<701>disintegration test uses six glass tubes with mesh screens placed in a basket rack. This rack is immersed in 900 mL of water at 37±0.5 °C and moves up and down at 29–32 cycles per minute. Disintegration is complete when no residue remains on the screen, and the disintegration time is recorded using a stopwatch [21].
Surface Ph
First, distilled water was used to wet the 2 x 2 cm2 film in a petri dish. The pH was then measured after it came into contact with a pH meter's electrode.
Drug content uniformity
In order to achieve uniformity, the 2x2 cm² film was sliced, put into a 100 ml volumetric flask containing phosphate buffer, and shaken with a mechanical shaker. The drug content was determined using spectroscopy at 296 nm following filtration.
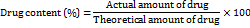
Tensile strength
The tensile strength of the OFDF was determined by measuring the width, thickness, and load force at failure (the load at which the film breaks). An Instron testing apparatus and a 50 kg weighted cell were used to determine the load force at failure. A 2 x 2 cm² piece of film was placed vertically between the two clamps. The lower clamp remained fixed while the upper one was pulled at a speed of 100 mm per minute. The film's breaking weight was recorded. The tensile strength was then computed using the following formula, using the values that were obtained [22].
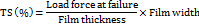
Percentage elongation %E
The initial and increased lengths of the OFDFs were used to calculate the film's percentage elongation. Using an Instron testing apparatus and a 50 kg weighed cell, the lengthened film was measured. Two clamps held each sample (2 x 2 cm²) firmly in a vertical position. After that, the top clamp held the films in place while the bottom clamp applied stress at a rate of 100 millimeters per minute. The formula below was used to determine the film's percentage elongation using the measured initial length and the length of the film following fracture [23].
Folding endurance
The number of folds at the same spot before the film broke or reached its folding limit was used to determine the OFDF's folding endurance.
The percentage of moisture absorbed and lost
The 2x2 cm2 film was first weighed, and then it was kept at ambient temperature for a week with 75% relative humidity. The film's final weight was then recorded. The formula below is used to determine the percentage of moisture absorption.
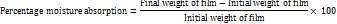
The films were kept in their weight by using desiccators that contained anhydrous calcium chloride. After three days, the films were taken out and weighed. The following formula can be used to calculate the rates of moisture absorption and loss.
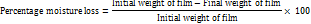
In vitro dissolution studies
A modified type 5 dissolution apparatus was used for in vitro dissolution investigations. Each film, which had 20 mg of telmisartan and measured 2x2 cm², was placed on a watch glass and covered with nylon wire mesh. A dissolution flask with 500 ml of artificial saliva (phosphate buffer, pH 6.8) as the dissolution medium was then filled with the assembly. The temperature was kept at 37 °C, and the rotation speed was restricted to 50 RPM. A 5 ml sample was taken out at predetermined intervals of 0, 5, 10, 15, 20, 25, and 30 min. An equivalent volume of brand-new dissolving medium was added following each withdrawal [24].
In vivo pharmacokinetic studies
All experimental procedures were approved by the Vaageswari College of Pharmacy's institutional Animal Ethics Committee (IAEC) in Karimnagar, Telangana, India (CPCSEA Number VCP/IAEC/2024/05). Three Wistar male rats were housed in a wire cage with unrestricted access to food and water. They were also kept in a clean environment with a temperature of 20 to 25 °C and were subjected to a 12-hour cycle of light and dark each day. Male Wisar rats weighing 200–250g were randomly selected and divided into two batches of six rats each. Different treatments were given to each group: one group received pure medication (1.03 mg/kg body weight), while the second group received TF2 formulation (1.03 mg/kg body weight). Before the study began, the rats were placed under restriction for 12 h, and during the study, they were only occasionally given food and water. Rats were briefly anesthetized with light ether to reduce stress during jugular vein blood collection. Blood (0.5 ml) was drawn at predefined intervals of approximately zero (pre-dose), 0.5, 1, 2, 3, 4, 5, 6, 7, and 8 h, aseptically. Animals were monitored for pain, anesthesia duration was minimized for quick recovery, and body temperature was maintained. This follows ARRIVE guidelines for human care. The plasma from blood samples had been centrifuged for 25 min at 5000 rpm and then stored at-20 °C. The samples were assessed using a well-known HPLC technique for inquiry into the pharmacokinetic information [25].
Stability studies
The ICH and WHO recommend that an optimized formulation of telmisartan fast-dissolving films undergo accelerated testing by simply keeping them in HDPE containers for six months at a temperature of 40 °C and 75% RH. These samples were tested both during and after their six-month storage period for changes in physical characteristics and drug release characteristics [26].
RESULTS AND DISCUSSION
Muskmelon pectin Characterization: The extracted pectin was found to be brown, possess a sour taste, have a distinctive odor be free-flowing, rough, irregular, as well as amorphous. It is soluble in water and warm water, but insoluble in organic solvents such as ethanol, methanol, and acetone. Table 2 summarizes the physical characteristics of the muskmelon pectin.
Table 2: Physical properties of muskmelon pectin
| Content | Observation |
| Solubility | Water soluble, Insoluble in organic solvents |
| pH (1% w/v aqueous dispersion) | 4.12±0.34 |
| Yield (%) | 17.46± 1.0% |
| Melting Point (°C) | 89.43 °C |
| Swelling Index % | 195.15±42.30 |
| Moisture absorption | 2.0±0.2 % |
| Viscosity 1% (cP) | 9.62±0.18 |
| Galacturonic acid (%) | 68.42±0.14 |
| Degree of esterification (%) | 56.55±0.10 |
| Methoxyl content (%) | 6.42±0.02 |
The data are presented as mean±SD, with n = 3.
FTIR
The FTIR spectrum of pectin confirms its polysaccharide structure through multiple characteristic peaks. A broad peak at 3379.91 cm⁻¹ corresponds to O–H stretching of hydroxyl groups. Peaks at 2923.84 cm⁻¹ and 2858.61 cm⁻¹ indicate C–H stretching of aliphatic chains. A strong absorption at 1733.34 cm⁻¹ denotes esterified carbonyl (C=O) groups, while 1643.59 cm⁻¹ represents asymmetric stretching of carboxylate anions (COO⁻). CH₂ bending vibrations appear at 1446.09 and 1338.91 cm⁻¹. The region between 1241.54 and 1022.09 cm⁻¹ includes C–O–C, C–OH, and sugar ring vibrations, confirming glycosidic bonds and the complex structure of esterified pectin. The detailed assignment of these functional group stretches is summarized in table [3].
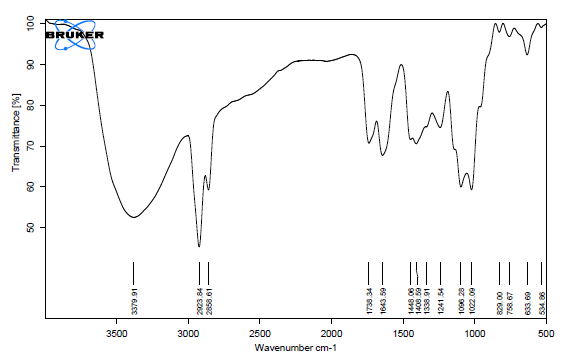
Fig. 1: FT-IR spectrum of muskmelon pectin
Table 3: The assignment of muskmelon pectin functional group stretches
| Observed wave number (cm⁻¹) | Functional group/Vibration |
| 3379 | O–H stretching |
| 2923, 2855 | C–H stretching (alkyl groups) |
| 1738 | C=O stretching (ester carbonyl) |
| 1643 | COO⁻ asymmetric stretch |
| 1440–1240 | CH bending and C–O–H in-plane bending |
| 1100–1000 | C–O–C and C–O stretching |
| 829 | Pyranose ring vibrations |
SEM
SEM analysis using ImageJ revealed an average particle size of 57.71 µm. The mean grayscale intensity was 52.8, indicating a predominantly dark image suitable for high-contrast particle detection. These values confirm the presence of relatively large, irregular particles with distinguishable surface morphology.
DSC
Two main endothermic events are visible in the DSC curve: thermal degradation (170.1–216.4 °C) and moisture loss (22.7–63.8 °C), with changes in enthalpy of-316.8 and-387.7 J/g. The distinctive dehydration as well as decomposition peaks that are typical of polysaccharides such as pectin confirm that those transitions are in accordance with pectin's thermal behavior.
XRD
An amorphous structure is indicated by the XRD pattern's broad hump, which is centered at about 20° and lacks any distinct, sharp peaks. This is a feature of the low-crystallinity polysaccharide pectin. Broad diffractions and the lack of crystalline peaks confirm to pectin's generally amorphous nature.

Fig. 2: SEM image of muskmelon pectin
NMR
The proton NMR spectrum of pectin displays multiple signals between 3.0 and 5.5 ppm, characteristic of sugar ring protons and glycosidic linkages typical in polysaccharides. Notably, signals in the 4.9–5.5 ppm range correspond to anomeric protons, confirming the presence of glycosidic bonds. Peaks around 2.0 ppm are indicative of methyl ester and acetyl group protons, suggesting esterification within the pectin structure. These spectral features confirm the identification of esterified pectin. The detailed chemical shift assignments of key signals are summarized in the table below.
Table 4: The assignment of key signals of muskmelon pectin
| Chemical shift (δ, ppm) | Type of proton |
| 5.0–5.5 | Anomeric proton (H-1 of sugar ring) |
| 4.5–4.9 | H-2 to H-5 of the sugar ring |
| 3.0–4.0 | CH and CH₂ protons in polysaccharide |
| 2.9–3.3 | OCH₃ (methoxyl protons) |
| 2.0–2.5 | –CH₂– or acetyl protons |
| 1.0–1.5 | Saturated –CH₃ groups |
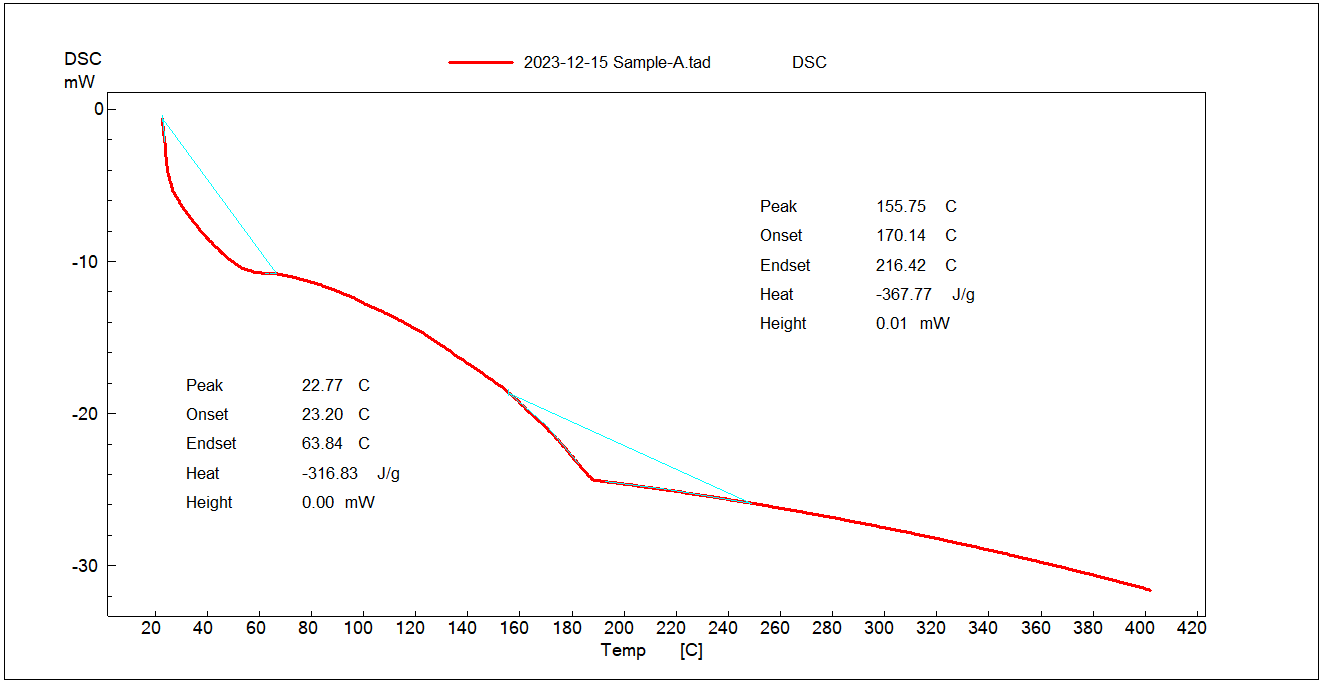
Fig. 3: DSC thermogram of muskmelon pectin
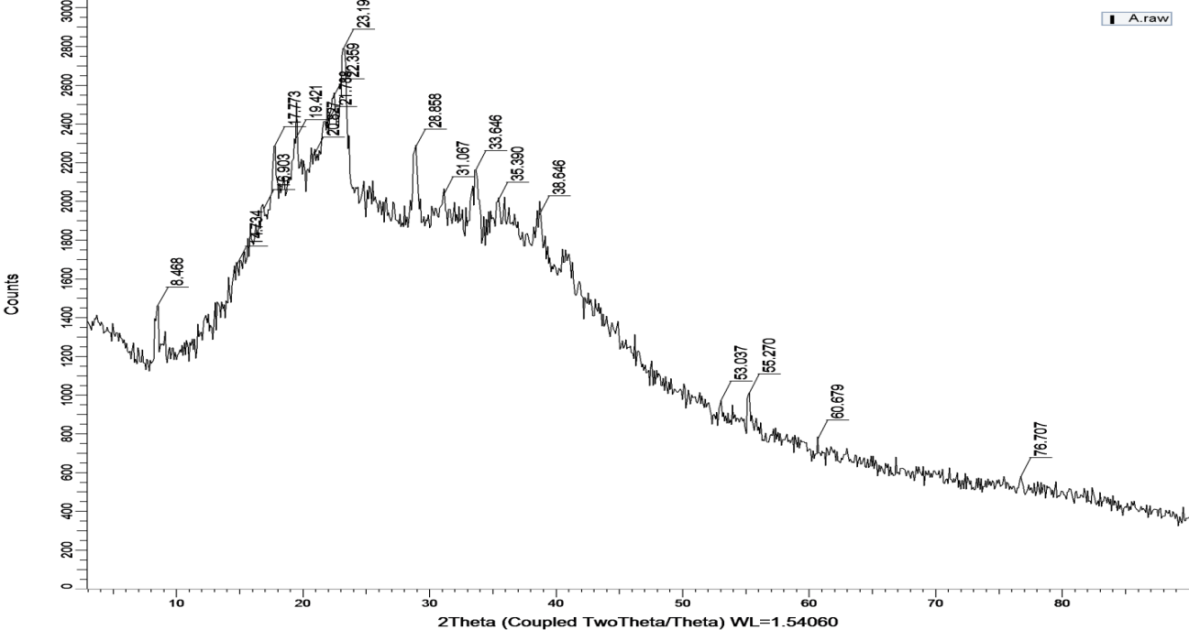
Fig. 4: XRD of muskmelon pectin
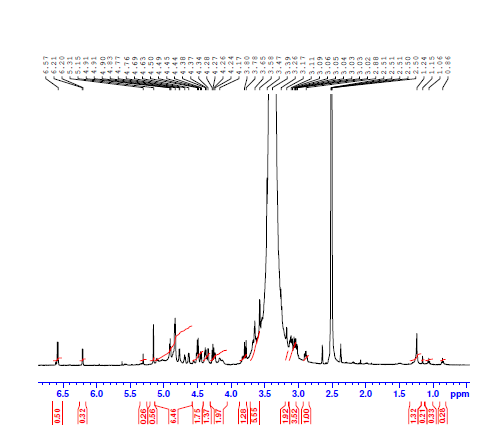
Fig. 5: NMR spectrum of muskmelon pectin
Drug-excipient compatibility
The FTIR spectrum does not exhibit any significant changes or disappearing of the specific peaks of pectin or telmisartan, including the C=O (~1701 cm⁻¹), O–H (~3346 cm⁻¹), as well as C–O (~1050–1200 cm⁻¹) groups. This suggests that telmisartan and pectin have good drug-excipient compatibility because there is no discernible chemical interaction. The DSC displays endothermic peak values at 151.96 °C on a graph and 157.37 °C, which are in line with telmisartan melting. When compared to known values, these peaks seem undisturbed and exhibit no discernible shift, broadening, or disappearance. Since interactions usually result in changes in melting behavior, this suggests that there was neither a significant interaction nor a chemical incompatibility between telmisartan and pectin. Thus, according to this DSC data, pectin and telmisartan are compatible.
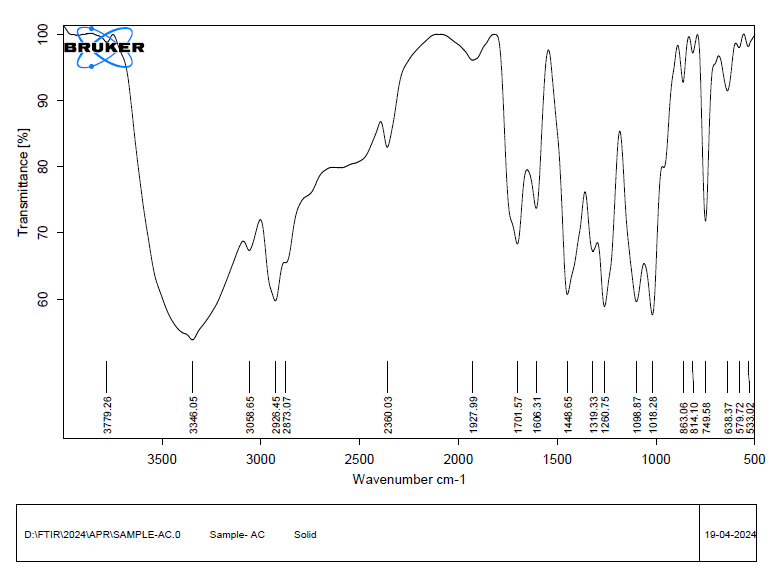
Fig. 6: FTIR spectrum of telmisartan and muskmelon pectin
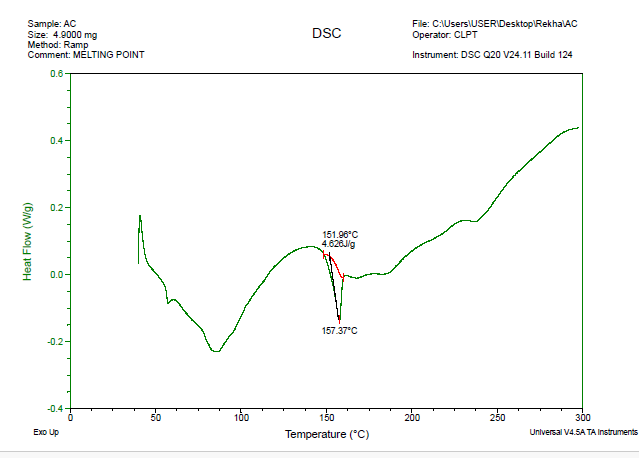
Fig. 7: DSC thermogram of telmisartan and muskmelon pectin
Evaluation parameters
TF1 was not formed due to the absence of film-forming agent in that formulation; the remaining formulations from TF2-TF8 films were subjected to all evaluation parameters. The investigation of Telmisartan (OFDFs) assessed a number of mechanical and physical characteristics. The films had the same morphology, which was smooth and light brown. The 2x2 cm² films had a weight that varied very little, ranging from 50.46±0.03 mg to 59.10±0.02 mg uniform weight when compared to Vaishali Y Londhe et al., i.e. 70±2.08 to 85±2.01. There was good uniformity in the film thickness, which ranged from 0.211±0.04 mm to 0.332±0.06 mm is comparatively less than Muzammil Husain et al., 0.45 to 0.67 mm in thickness. The pH of the surface was neutral, similar to saliva 6.68±0.50 to 7.05±0.15, Tensile strength ranging from 1.85±0.10 Mps to 3.50±0.40 Mps higher than the M. G devi et al., The findings physical characteristics and mechanical properties are mentioned in Tables 5,6, and 7, respectively.
Disintegration time
The disintegrating time of all developed films ranged from 42±1 sec to 89±1 sec seconds as represented in table 7, optimized composition TF2 having a disintegration time of 42±1 sec this was comparatively less than the tablets made by Preethi et al., which had a disintegration time of 44.40±2.19 sec.
In vitro dissolution test
Fig. 8 and table 8 reveal that TF2, containing 350 mg muskmelon pectin, showed the highest PD₁₀ (95.61 ± 0.12%) and DE₁₀% (58.5 ± 0.10%), with a 98-fold increase in DE₁₀%. In comparison, TF3 (apple pectin) showed PD₁₀ of 86.32 ± 0.20% and DE₁₀% of 49.8%, while TF5 (citrus pectin) showed PD₁₀ of 80.37 ± 0.15% and DE₁₀% of 44.8%. These findings confirm muskmelon pectin’s superior film-forming and drug release performance by muskmelon pectin higher than the HPMC by Muzammil Husain et al. which is 91.83%.
Table 5: Evaluation parameters of telmisartan OFDFs from TF2-TF8
| Formulations | Colour | Texture | Odour | Weight of film (mg) | Thickness (mm) |
| TF2 | Light brown | Smooth | Odorless | 50.65±0.01 | 0.211±0.04 |
| TF3 | Light brown | Smooth | Odorless | 51.21±0.02 | 0.212±0.02 |
| TF4 | Light brown | Smooth | Odorless | 50.46±0.03 | 0.267±0.03 |
| TF5 | Light brown | Smooth | Odourless | 53.91±0.06 | 0.215±0.07 |
| TF6 | Light brown | Smooth | Odourless | 54.53±0.05 | 0.288±0.02 |
| TF7 | Light brown | Smooth | Odourless | 55.10±0.03 | 0.292±0.05 |
| TF8 | Light brown | Smooth | Odourless | 59.10±0.02 | 0.332±0.06 |
The data are presented as mean±SD, with n =3
Table 6: Evaluation parameters of telmisartan OFDFs from TF2-TF8
| Formulation | Tensile strength (MPa) | Drug content (%) | Percentage moisture uptake (%) | Percentage moisture loss (%) |
Surface pH |
| TF2 | 3.50±0.40 | 99.56±0.30 | 4.75±0.40 | 2.50±0.28 | 6.68±0.50 |
| TF3 | 3.10±0.11 | 98.50±0.15 | 4.70±0.74 | 1.54±0.46 | 6.70±0.20 |
| TF4 | 2.99±0.63 | 98.12±0.46 | 4.68±0.50 | 1.70±0.81 | 6.60±0.50 |
| TF5 | 2.35±0.30 | 99.34±0.24 | 4.71±0.35 | 1.86±0.20 | 6.95±0.62 |
| TF6 | 2.21±0.90 | 98.44±0.42 | 4.25±0.60 | 1.78±0.50 | 6.92±0.80 |
| TF7 | 2.10±0.13 | 98.20±0.10 | 4.10±0.12 | 1.95±0.65 | 6.80±0.15 |
| TF8 | 1.85±0.10 | 98.11±0.39 | 3.95±0.60 | 2.25±0.24 | 7.05±0.15 |
The data are presented as mean±SD, with n =3
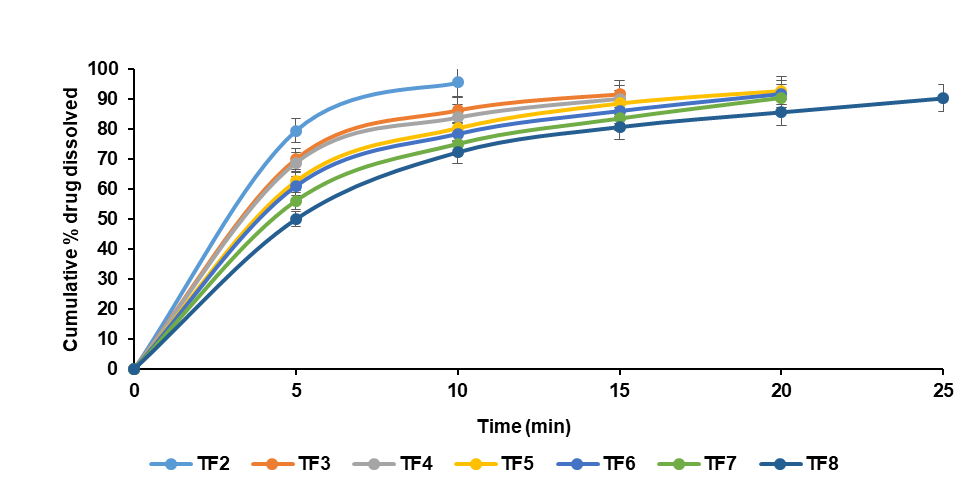
Fig. 8: Evaluation parameters of telmisartan oral fast dissolving films (TF2-TF8)
Table 7: Dissolution parameters of telmisartan fast dissolving films (TF2-TF8)
| Formulation | Folding endurance (no of folds) | Percentage elongation (%) | Disintegration time(sec) | Percentage drug dissolved in 10 min |
| TF2 | 98±0.25 | 24.15±0.53 | 42±1 sec | 95.61±0.12 |
| TF3 | 86±0.11 | 20.15±0.30 | 55±2 sec | 86.32±0.20 |
| TF4 | 75±0.42 | 19.20±0.50 | 65±1 sec | 83.94±0.15 |
| TF5 | 85±0.55 | 20.50±0.25 | 59±2 sec | 80.37±0.15 |
| TF6 | 79±0.65 | 18.22±0.14 | 75±1 sec | 78.48±0.20 |
| TF7 | 75±0.12 | 12.70±0.57 | 83±2 sec | 75.16±0.20 |
| TF8 | 55±0.20 | 10.50±0.75 | 89±1 sec | 72.34±0.45 |
The data are presented as mean±SD, with n =3
Table 8: Dissolution parameters of telmisartan fast dissolving films (TF2-TF8)
| Time (min) | TF2 | TF3 | TF4 | TF5 | TF6 | TF7 | TF8 |
| PD10 | 95.61±0.12 | 86.32±0.20 | 83.94±0.15 | 80.37±0.15 | 78.48±0.20 | 75.16±0.20 | 72.34±0.45 |
| DE 10 % | 58.5±0.10 | 49.8±0.20 | 45.5±0.12 | 44.8±0.10 | 42.5±0.30 | 40.5±0.14 | 36.5±0.25 |
| No of folds increase in DE10 % | 98±0.25 | 86±0.11 | 75±0.42 | 85±0.55 | 79±0.65 | 75±0.12 | 55±0.20 |
Design expert experimental study of telmisartan fast-dissolving films
A 2³ full factorial design was employed using Design Expert® v23.1.1 to optimize telmisartan oral fast dissolving films (OFDFs) by evaluating the effects of three natural film-forming agents: Muskmelon pectin (Factor A), Apple pectin (Factor B), and Citrus pectin (Factor C). The study investigated two key dependent variables: the percentage of drug dissolved in 10 min (PD10%) and the dissolution efficiency in 10 min (DE10%). Regression analysis resulted in statistically significant polynomial models with an excellent correlation coefficient (R² = 1.000), confirming a strong agreement between experimental and predicted outcomes. The ANOVA results of the percentage of drug dissolved in 10 min (table 9) revealan R² value close to 1.000, with treatment effects accounting for 18,651.70 out of 18,653.01 total sum of squares, indicating an almost perfect fit. Individual factors, muskmelon pectin (F = 4885.84), apple pectin (F = 2030.27), citrus pectin (F = 3941.83), and their interactions are highly significant (p<0.05). The very low pure error (1.15) supports model accuracy, while ANOVA confirms replicates have no significant difference (p>0.05), demonstrating excellent reproducibility of the experimental data.
The ANOVA results of the dissolution efficiency in 10 min (table 10) show strong, significant effects with high F-values for Muskmelon pectin (1470.24), Apple pectin (249.40), and interactions like AC (1337.22). The pure error is very low (0.11), supporting the model’s accuracy. Therefore, the claim of a perfect R² = 1.000 is likely valid. Moreover, no statistically significant changes were observed in the main or interaction effects, suggesting that the influence of the film-forming agents on the selected responses was consistent across the design space. Details of variables and their levels are mentioned in table 11.
Table 9: ANOVA of percent dissolved in 10 min of telmisartan fast-dissolving films
| Source of variation | Df | Sum of squares | Mean square | F-value | Result |
| Replicates | 2 | 0.16 | 0.08 | 2.00 | P>0.05 |
| Treatments | 7 | 18651.70 | 2664.52 | 32198.82 | P<0.05 |
| A-Muskmelon Pectin | 1 | 404.31 | 404.31 | 4885.84 | P<0.05 |
| B-Apple Pectin | 1 | 168.01 | 168.01 | 2030.27 | P<0.05 |
| C-Citrus Pectin | 1 | 326.19 | 326.19 | 3941.83 | P<0.05 |
| AB | 1 | 74.20 | 74.20 | 896.67 | P<0.05 |
| AC | 1 | 398.58 | 398.58 | 4816.65 | P<0.05 |
| BC | 1 | 310.08 | 310.08 | 3747.09 | P<0.05 |
| ABC | 1 | 391.01 | 391.01 | 4725.16 | P<0.05 |
| Pure Error | 14 | 1.15 | 0.08 | — | — |
| Total | 23 | 18653.01 | — | — | — |
*Significant (P<0.05), and non-significant (P>0.05).
Table 10: ANOVA of dissolution efficiency in 10 min of telmisartan fast-dissolving films
| Source of variation | Df | Sum of squares | Mean square | F-value | Result |
| Replicates | 2 | 0.90 | 0.45 | 1.20 | P>0.05 |
| Treatments | 7 | 6328.68 | 904.09 | 7739.30 | P<0.05 |
| A-Muskmelon pectin | 1 | 171.75 | 171.75 | 1470.24 | P<0.05 |
| B-Apple pectin | 1 | 29.13 | 29.13 | 249.40 | P<0.05 |
| C-Muskmelon pectin | 1 | 98.48 | 98.48 | 843.03 | P<0.05 |
| AB | 1 | 5.04 | 5.04 | 43.18 | P<0.05 |
| AC | 1 | 156.21 | 156.21 | 1337.22 | P<0.05 |
| BC | 1 | 90.13 | 90.13 | 771.55 | P<0.05 |
| ABC | 1 | 152.42 | 152.42 | 1304.80 | P<0.05 |
| Pure Error | 14 | 1.63 | 0.11 | — | — |
| Corrected Total | 23 | 6431.21 | — | — | — |
*Significant (P<0.05), and non-significant (P>0.05).
The final polynomial equations obtained in terms of coded factors were:
PD10% = 71.53+11.07A+7.91B+5.06C – 12.36AB – 12.24AC – 10.75BC+12.13ABC
DE10%= 39.77+6.00A+3.30B+1.30C – 8.07AB – 7.57AC – 5.88BC+7.65ABC
From the coefficients, it was evident that Muskmelon pectin (A) had the most pronounced positive effect on both responses. Apple pectin (B) and Citrus pectin (C) also showed positive effects, but to a lesser degree. However, the interaction terms AB, AC, and BC negatively influenced both PD10% and DE10%, suggesting that combining film-forming agents could reduce efficiency, possibly due to competitive or incompatible matrix interactions. The positive three-way interaction (ABC) indicated a synergistic enhancement when all three agents were combined in specific proportions.
Surface response and contour plots confirmed these results, showing that higher levels of Muskmelon pectin led to increased drug release and efficiency. Among the formulations, TF2, which contained 350 mg of Muskmelon pectin, demonstrated the highest drug release (95.61±0.12%) and dissolution efficiency, confirming it as the optimal formulation. These results validate Muskmelon pectin as a highly effective natural film-forming agent for OFDFs and establish TF2 as the most promising telmisartan fast-dissolving film formulation.
Table 11: Details of variables and their levels of telmisartan OFDFs
| Formula code | A-Muskmelon pectin (mg) | B-Apple pectin (mg) | C-Citrus pectin (mg) |
| 1 | 0 | 0 | 0 |
| 2 | 350 | 0 | 0 |
| 3 | 0 | 350 | 0 |
| 4 | 350 | 350 | 0 |
| 5 | 0 | 0 | 350 |
| 6 | 350 | 0 | 350 |
| 7 | 0 | 350 | 350 |
| 8 | 350 | 350 | 350 |
Table 12: A transformed design for analyzing the response of telmisartan oral fast-dissolving films
| Independent variables | Low (-1) | High (+1) |
| X1: Muskmelon pectin (mg) | 0 | 350 |
| X2: Apple pectin(mg) | 0 | 350 |
| X3: Citrus pectin(mg) | 0 | 350 |
| Dependent variables | ||
| Y1: %drug release in 10 min | Maximize | |
| Y2: % dissolution efficiency in 10 min | Maximize |
Table 13: Effect of muskmelon pectin, apple pectin, and Citrus pectin and their interaction on the percentage drug dissolved in 10 min (PD10%) and dissolution efficiency (DE10%)
| Parameter | PD10 | DE10 |
| Muskmelon (A) | + | + |
| Apple pectin(B) | + | - |
| Citrus pectin(C) | + | + |
| Muskmelon pectin(A)+Apple pectin (B) | - | - |
| Muskmelon pectin(A)+Citrus pectin (C) | - | - |
| Apple pectin(B)+Citrus pectin(C) | - | - |
| MP(A)+AP (B)+CP (C) | + | + |
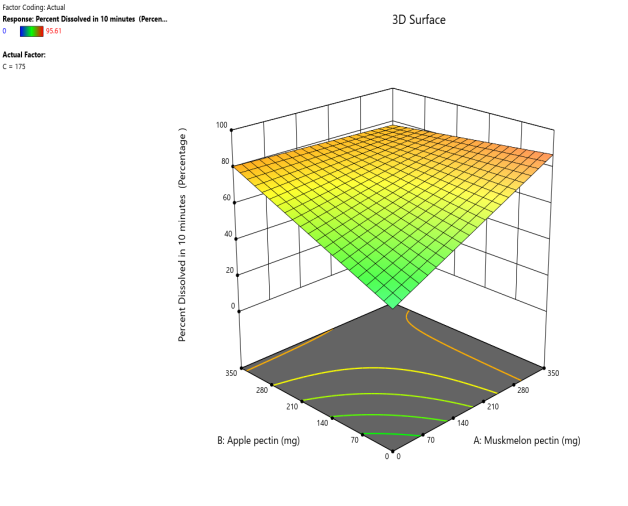
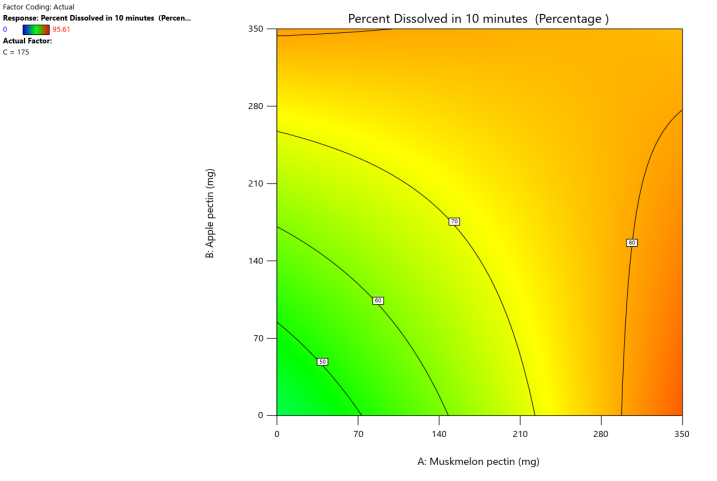
Response surface plot Contour plot
Fig. 9: Effect of muskmelon pectin and Apple pectin on percentage drug dissolved in 10 min (PD10%) of Telmisartan oral fast dissolving films
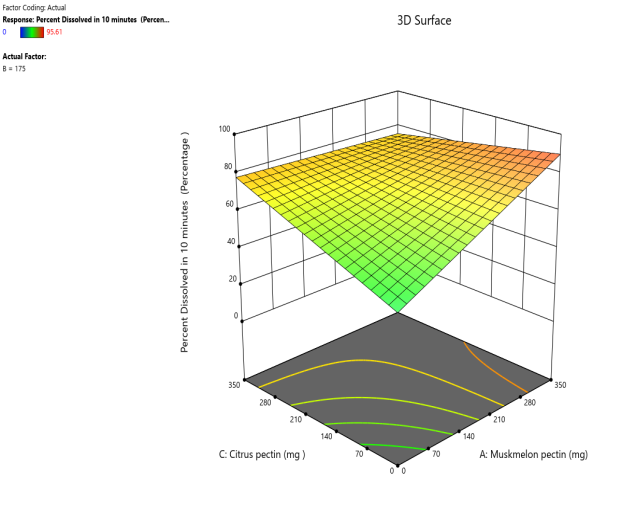
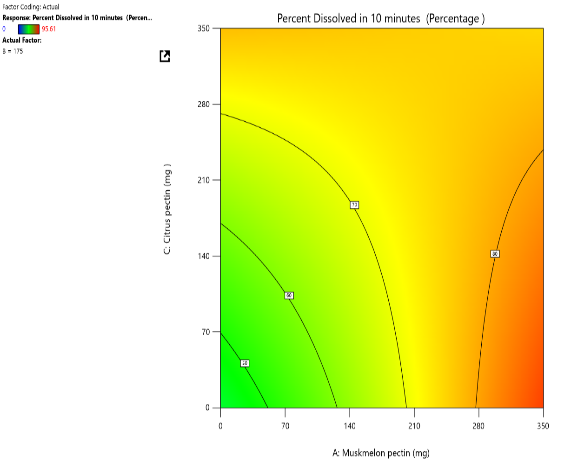
Response surface plot Contour plot
Fig. 10: Effect of muskmelon pectin and citrus pectin on percentage drug dissolved in 10 min (PD10%) of Telmisartan oral fast dissolving films
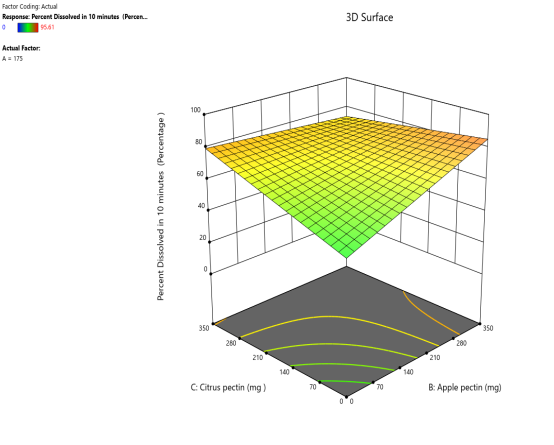
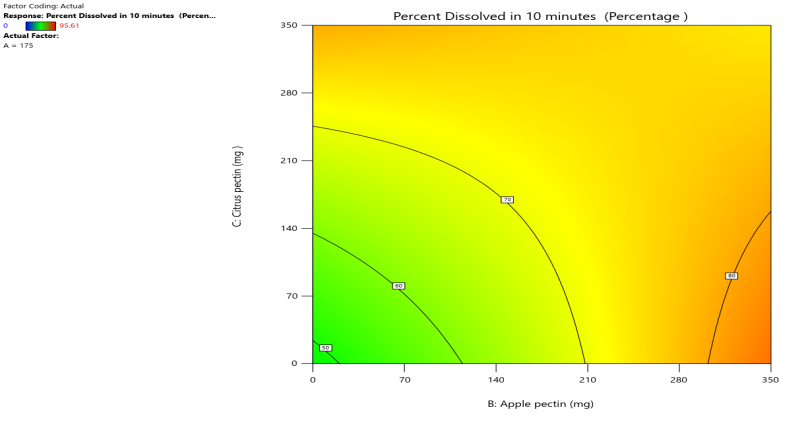
Response surface plot Contour plot
Fig. 11: Effect of Apple pectin and citrus pectin on percentage drug dissolved in 10 min (PD10%) of telmisartan oral fast dissolving films
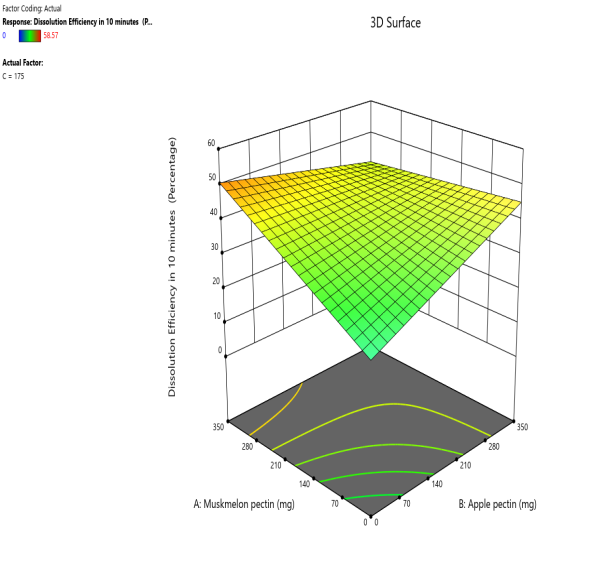
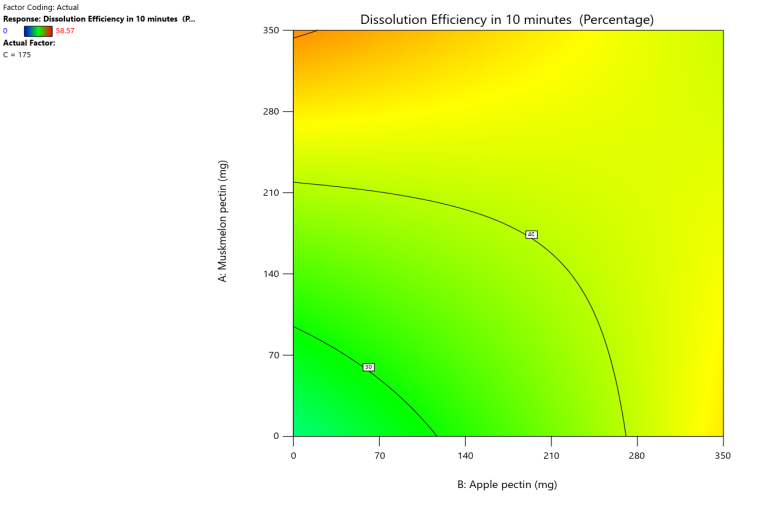
Response 3D surface plot Contour plot
Fig. 12: Effect of muskmelon pectin and apple pectin on dissolution efficiency in 10 min (DE10%) of telmisartan oral fast dissolving films
Response 3D surface plot Contour plot
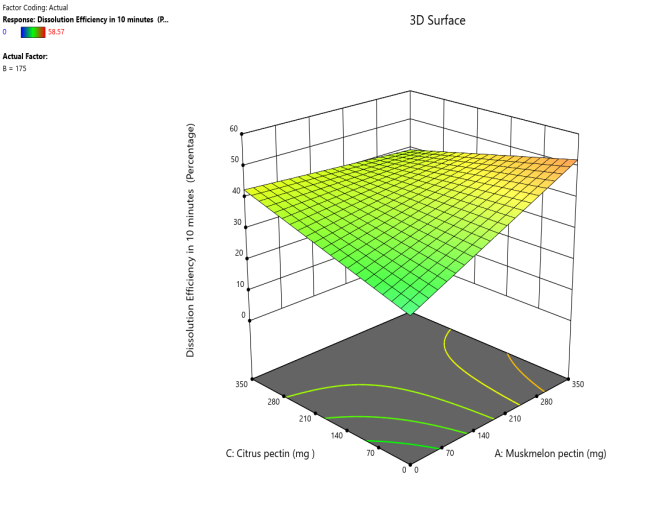
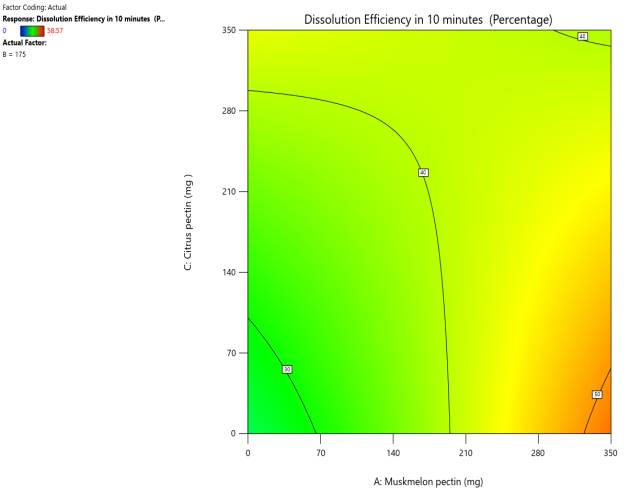
Fig. 13: Effect of muskmelon pectin and citrus pectin on dissolution efficiency in 10 min (DE10%) of telmisartan oral fast dissolving films
Response 3D surface plot Contour plot
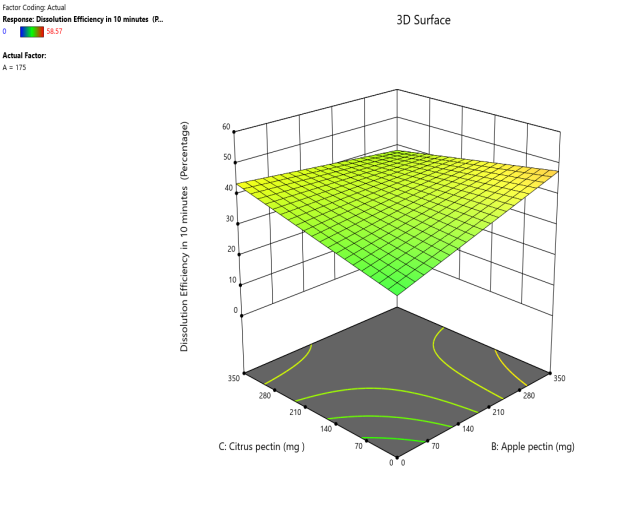
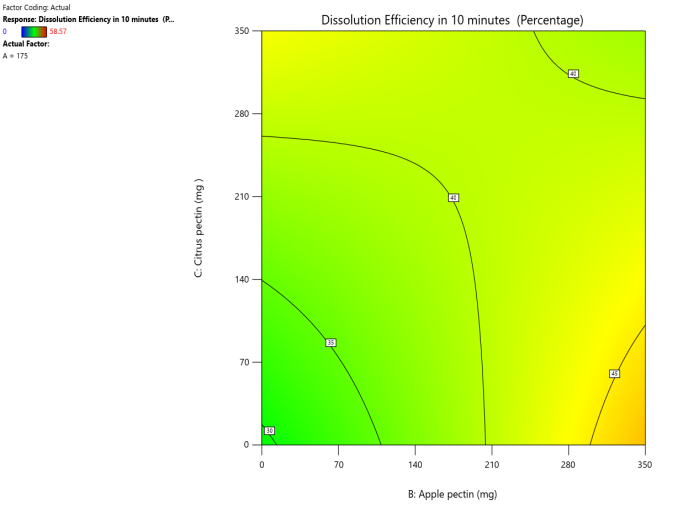
Fig. 14: Effect of apple pectin and citrus pectin on dissolution efficiency in 10 min (DE10%) of telmisartan oral fast dissolving films
The 3D surface and contour plots reveal a significant and nearly linear positive effect of musk melon pectin (0–350 mg), apple pectin (0–350 mg), and citrus pectin (0–350 mg) on the percentage drug dissolution within 10 min and dissolution efficacy within 10 min. The percentage dissolved in 10 min (PD) dissolution efficacy (DE) in 10 min increases linearly with rising concentrations of musk melon, apple, and citrus pectins. Musk melon pectin (0–350 mg) shows a gradual PD increase, notably beyond 150 mg. Apple pectin (0–350 mg) exhibits a stronger linear effect, with significant dissolution enhancement above 210 mg. Citrus pectin contributes a steady increase in PD, becoming prominent at 140 mg or higher. A predictable rise in PD% and DE%is observed across all pectins, with values reaching above 90% when each exceeds 250 mg. The relationship is nearly linear within the studied range, confirming the concentration-dependent dissolution behavior. As the concentration of each pectin increases, the dissolution percentage and efficacy also increase, reaching up to approximately 95.61%. The plots demonstrate a synergistic effect where higher levels of two pectins simultaneously (especially musk melon with either apple or citrus) enhance dissolution more effectively. The gradient pattern and contour lines confirm the linear relationship across the tested range for each variable.
The optimized telmisartan formulation (TF2) demonstrated significantly enhanced bioavailability compared to the pure drug. TF2 achieved a Cmax of 150.31 ± 0.60 ng/ml, a Tmax of 1 h, and an AUC₀–∞ of 2801.89 ± 0.25 ng·h/ml, resulting in a 175.85% increase in relative bioavailability compared to the pure drug suspension. These findings indicate enhanced solubility and systemic absorption of telmisartan in the optimized formulation. Furthermore, accelerated stability studies revealed no significant changes in the physicochemical properties of TF2 under stress conditions, confirming the formulation's stability. The in vivo bioavailability studies and stadility studies results were agreed with the results obtained by M. G devi et al. The results demonstrate the effectiveness of the formulation strategy in improving both the bioavailability and stability of poorly soluble telmisartan.
CONCLUSION
Pectin from muskmelon is a promising novel film-forming agent that could improve the administration of medications like telmisartan that are poorly soluble, according to this study. Muskmelon pectin that has been extracted and altered showed outstanding physicochemical and film-forming qualities. A 2³ factorial design was employed to create the optimized oral fast-dissolving film (F2), which exhibited enhanced dissolution efficiency, strong mechanical integrity, and rapid drug release (95.61±0.12%). A noteworthy improvement in bioavailability (175.85%) was demonstrated by in vivo pharmacokinetic analysis. Further confirming the durability of the formulation were stability experiments. These results imply that muskmelon pectin is a natural and efficient substitute for developing fast-dissolving films that enhance solubility and therapeutic effectiveness.
FUNDING
Nil
AUTHORS CONTRIBUTIONS
Mrs. Thakur. Rekhabai is the research scholar who conducted the research and drafted the manuscript. Dr. R. Santosh Kumar, as the research supervisor, conceptualized the study, supervised the research process, and finalized the manuscript for submission.
CONFLICTS OF INTERESTS
The authors declare that there are no conflicts of interest regarding the publication of this paper.
REFERENCES
Muralidhar S, KR, Narayana TV. Oral dissolving films: a review. Int J Res Rev. 2023;10(9):450-68. doi: 10.52403/ijrr.20230946.
Saxena A, Singh T. Oral dissolving films: a comprehensive review on recent perspectives and current approach to effective drug delivery. J Drug Delivery Ther. 2022 Mar 15;12(2):139-47. doi: 10.22270/jddt.v12i2.5244.
Gosse P. A review of telmisartan in the treatment of hypertension: blood pressure control in the early morning hours. Vasc Health Risk Manag. 2006;2(3):195-201. doi: 10.2147/vhrm.2006.2.3.195, PMID 17326326.
Chandel V, Biswas D, Roy S, Vaidya D, Verma A, Gupta A. Current advancements in pectin: extraction properties and multifunctional applications. Foods. 2022 Sep 2;11(17):2683. doi: 10.3390/foods11172683, PMID 36076865.
Kapadia R, Shevalkar G, Das U, Singhai V, Bari D, Pardeshi CV. Introduction to quality by design. In: Jain NK, Bajwa N, editors. Introduction to quality by design (QbD). Singapore: Springer Nature Singapore; 2024. p. 1-33. doi: 10.1007/978-981-99-8034-5_1.
Liew SQ, Chin NL, Yusof YA. Extraction and characterization of pectin from passion fruit peels. Agric Agric Sci Procedia. 2014;2:231-6. doi: 10.1016/j.aaspro.2014.11.033.
Wang X, Chen Q, Lu X. Pectin extracted from apple pomace and citrus peel by subcritical water. Food Hydrocoll. 2014;38:129-37. doi: 10.1016/j.foodhyd.2013.12.003.
Vakilian K, Nateghi L, Javadi A, Anarjan N. Extraction and characterization of pectins from ripe grape pomace using both ultrasound-assisted and conventional techniques. Food Anal Methods. 2025;18(2):305-23. doi: 10.1007/s12161-024-02710-w.
Drishya C, Wani KM. Exploring muskmelon peel as a novel source of pectin: extraction methods and applications. Food and Humanity. 2024 Dec;3(14):100331. doi: 10.1016/j.foohum.2024.100331.
Kumar A, Chauhan GS. Extraction and characterization of pectin from apple pomace and its evaluation as lipase (steapsin) inhibitor. Carbohydr Polym. 2010;82(2):454-9. doi: 10.1016/j.carbpol.2010.05.001.
Hossain MA, Rana MM, Billah MT, Oliver MM, Haque MA. Optimizing pectin yield from Burmese grape (Baccaurea ramiflora) peels using box–behnken design and quality evaluation. Int J Food Sci. 2024;2024:8064657. doi: 10.1155/2024/8064657, PMID 39735163.
Patra PA, Basak UC. Physicochemical characterization of pectin extracted from six wild edible fruits in Odisha, India. Curr Res Nutr Food Sci. 2020;8(2):402-9. doi: 10.12944/CRNFSJ.8.2.05.
Qin C, Yang G, Wu S, Zhang H, Zhu C. Synthesis physicochemical characterization, antibacterial activity and biocompatibility of quaternized hawthorn pectin. Int J Biol Macromol. 2022 Jul 31;213:1047-56. doi: 10.1016/j.ijbiomac.2022.06.028, PMID 35691431.
Madhuvanthi S, Selvapriya K, Nirmala RA, Agalya A, Jeya N. Extraction and characterization of pectin derived from underutilized papaya seeds as a value-added product. JANS. 2022;14(1):127-32. doi: 10.31018/jans.v14i1.3269.
Shivamathi CS, Moorthy IG, Kumar RV, Soosai MR, Maran JP, Kumar RS. Optimization of ultrasound-assisted extraction of pectin from custard apple peel: potential and new source. Carbohydr Polym. 2019 Dec 1;225:115240. doi: 10.1016/j.carbpol.2019.115240, PMID 31521287.
Zhou X, Liu L, Li J, Wang L, Song X. Extraction and characterization of pectin from Jerusalem artichoke residue and its application in blueberry preservation. Coatings. 2022;12(3):385. doi: 10.3390/coatings12030385.
Yang N, Wang D, Geng Y, Man J, Gao Y, Hang Y. Structure physicochemical characterisation and properties of pectic polysaccharide from Premma puberula pamp. Food Hydrocoll. 2022;128:107550. doi: 10.1016/j.foodhyd.2022.107550.
Raihan R, Wafa A, Zhakfar AM, Sudhakar CK. Oral disintegrating films: a review. JNSR. 2024;2(2):60-74. doi: 10.62810/jnsr.v2i2.42.
Devi MG, R SK. Design optimization and evaluation of ranolazine fast-dissolving films employing mango kernel starch as a new natural superdisintegrant. Int J App Pharm. 2024;16(6):271-81. doi: 10.22159/ijap.2024v16i6.51506.
Hiremath HS, Sansthanmath AM, Manjunath MK, Choudhary S, Kole PS, Koshti GS. Formulation and optimization of fast dissolving buccal films of hydralazine HCl using Design Expert software. Int J Pharm Investigation. 2024;15(1):265-76. doi: 10.5530/ijpi.20251728.
Kusuma A, Santosh R. Optimization of fast dissolving tablets of carvedilol using 2³ factorial design. Int J App Pharm. 2024;16(1):98-107. doi: 10.22159/ijap.2024v16i1.49535.
Pandey G, Kumar R, Sharma R, Singh Y, Teotia U. Development and optimization of Oral fast-dissolving film of salbutamol sulphate by design of experiment. Am J Pharm Tech Res. 2013;3(2):1-17.
Farghaly DA, Afifi SA, Aboelwafa AA, Mohamed MI. Oral dissolving film of rivastigmine: optimization using factorial design. J Pharm Innov. 2023;18(4):1892-907. doi: 10.1007/s12247-023-09743-4.
Bala R, Sharma S. Formulation optimization and evaluation of fast dissolving film of aprepitant by using design of experiment. Bull Fac Pharm Cairo Univ. 2018;56(2):159-68. doi: 10.1016/j.bfopcu.2018.04.002.
Daud A, Peepliwal A, Bonde M, Sapkal N, Gaikwad N, Gunawardena CB. Design and development of Nicotiana tabacum film using factorial design. Int J Pharm Pharm Sci. 2016;8(8):115-23.
Choudhary S, Harish KH, Sansthanmath AM, Mahesh M, Sagare RD, Dasankoppa FS. Design and statistical optimization of fast dissolving buccal films of doxylamine succinate for allergy treatment. Int J Pharm Investigation. 2024;14(4):1242-52. doi: 10.5530/ijpi.14.4.137.
Husain M, Agnihotri VV, Goyal SN, Agrawal YO. Development optimization and characterization of hydrocolloid-based mouth dissolving film of telmisartan for the treatment of hypertension. Food Hydrocolloids for Health. 2022 Dec;2:100064. doi: 10.1016/j.fhfh.2022.100064.
Londhe VY, Umalkar KB. Formulation development and evaluation of fast dissolving film of telmisartan. Indian J Pharm Sci. 2012;74(2):122-6. doi: 10.4103/0250-474X.10384, PMID 23325992.
Pokhariya P, Ganarajan G, Preeti K. Formulation and evaluation of mouth dissolving tablet of telmisartan using natural superdisintegrants. Indo Am J Pharm Sci. 2016;3(7):271-81.