
Int J App Pharm, Vol 17, Issue 5, 2025, 510-518Original Article
A NEW RELATED SUBSTANCES METHOD DEVELOPMENT AND VALIDATION OF METRONIDAZOLE AND PREDNISOLONE BY USING EFFECTIVE LIQUID CHROMATOGRAPHIC METHOD
M. MANORANJANI*1, D. ANITHA2, NANDURI GAYATRI DEVI3
*1Department of Chemistry, P B Siddhartha College of Arts and Science, Vijayawada, AP-520010, India. 2Chemistry Department (Basic Sciences and Humanities), Dhanekula Institute of Engineering and Technology, Ganguru, Vijayawada, India. 3Department of Chemistry, Ch. S. D. St. Theresa’s College for Women (A), Eluru-534003, Andhra Pradesh, India
*Corresponding author: M. Manoranjani; *Email: ranjani.20ranjani@gmail.com
Received: 31 Mar 2025, Revised and Accepted: 21 Jul 2025
ABSTRACT
Objective: The present study was aimed at developing and successively validating novel, simple, responsive and stable RP-UPLC (Reverse Phase Ultra Performance Liquid Chromatography) method for the estimation of active pharmaceutical ingredients of Metronidazole and Prednisolone and their related substances.
Methods: The method of chromatography was fine-tuned with the use of the solution containing the contaminant. A phenyl column with dimensions of 50x2.1 mm and a particle size of 1.7 μm was used in the chromatographic procedure. The mobile phase consisted of acetonirile and 0.1 percent ortho-phosphoric acid (OPA) in a 50:50 v/v ratio, and isocratic elution was employed. The experimental parameters called for a PDA (Photo Diode Array) detector operating at 271 nm and a flow rate of 0.2 ml/min.
Results: The resolution of Metronidazole, Prednisolone and their four related substances were greater than 2.0 for all pairs of components. The high correlation coefficient (R2>0.999) values indicated clear correlations between the investigated compound concentrations and their peak areas within the test ranges. The Repeatability and intermediate precision expressed by the Relative Standard Deviation (RSD) were less than 2. The accuracy evaluated by performing recovery studies via a spike method was 50-150%. The performance of the method was validated according to the present ICH (International Council for Harmonization) guidelines.
Conclusion: We used the created technique to measure Metronidazole and Prednisolone with their associated contaminants and it was determined to be appropriate to routine analysis.
Keywords: Metronidazole, Prednisolone, RP-UPLC, Development, Validation
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijap.2025v17i5.54959 Journal homepage: https://innovareacademics.in/journals/index.php/ijap
INTRODUCTION
The antiprotozoal [1] and antibiotic [2, 3] drug known as metronidazole and goes by many brand names, including Flagyl. It treats bacterial vaginosis, pelvic inflammatory disease [4, 5], endocarditis [6, 7] and bacterial vaginosis [8, 9]. Several parasitic diseases, including dracunculiasis [10], giardiasis [11], trichomoniasis [12] and amebiasis [13], respond well to it. When other antibiotics, such as vancomycin or fidaxomicin, are not available, it may be used to treat mild to severe Clostridioides difficile [14, 15] colitis [16]. We may take metronidazole by mouth, topically as a gel or lotion, or intravenously as a gradual infusion. Nausea, headaches, a metallic taste, and decreased appetite are common adverse effects. Seizures or drug allergies might happen from time to time. While some sources say it's not safe to take metronidazole in early pregnancy, others say it's fine for trichomoniasis dosages. In most cases, nursing may safely continue while using metronidazole.
As a steroid hormone known as a corticosteroid [17, 18], prednisolone is used to treat a variety of illnesses, including allergies, inflammation [19], autoimmune disorders [20, 21], cancer, electrolyte imbalances [22], and skin issues. Adrenocortical insufficiency, hypercalcemia [23], rheumatoid arthritis [24], inflammation of the eyes, asthma, dermatitis [25], multiple sclerosis [26], and phimosis [27] are among these disorders. It has several administration options, including oral, intravenous, topical, and ocular drops. Having a hydroxyl instead of a ketone at the 11th carbon is what distinguishes it from the similarly called prednisone. Symptoms such as nausea, dizziness, trouble sleeping [28], increased hunger, and exhaustion are common with short-term usage. Mental health issues are among the most serious side effects, and they may affect around 5% of the population. Weakness, yeast infections, easy bruising [29], bone loss, and other common adverse effects are reported with long-term usage. Using it for a short period in the third trimester is generally safe, but using it for a long period or in the first trimester might sometimes cause damage to the baby. Created from hydrocortisone, it is known as cortisol, and it is a glucocorticoid [30]. Fig. 1 shows the chemical structures of Metronidazole, Prednisolone and their related substances.
The development of a new and dependable UPLC technique for the detection of Metronidazole, Prednisolone, and their associated related substances has become more important due to the lack of existing methods in the literature.
MATERIALS AND METHODS
Chemicals
The following materials were procured from Merck India Ltd, Mumbai, India: acetonitrile, water, and ortho-phosphoric acid of HPLC (High Performance Liquid Chromatography) grade. The reference standards for Metronidazole and Prednisolone, as well as their related substances, were obtained from Glenmark Pharmaceuticals Pvt Ltd in Hyderabad.
Instrumentation
A Phenyl column (50x2.1 mm, 1.7 µ) and a detector of light diode array (model 2998) from Waters Acquity liquid chromatographic system were used in this study. The system was monitored using an Empower 2.0 data processing system.
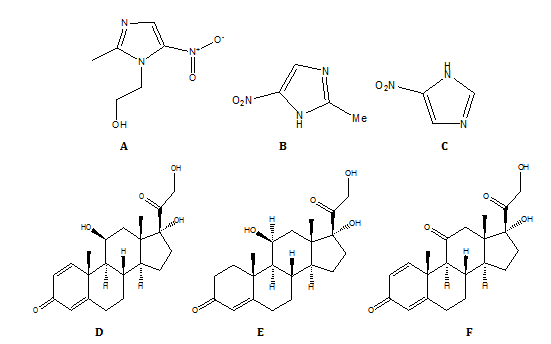
Fig. 1: Chemical structures of (A) Metronidazole (B) Metronidazole impurity-A (C) Metronidazole impurity-B (D) Prednisolone (E) Prednisolone impurity-A and (F) Prednisolone impurity-B
Preparation of buffer
In 1 L of HPLC-grade water, 1 ml of orthophosphoric acid is dissolved and then filtered through 0.22 µ filter paper.
Conditions
Using a mobile phase consisting of acetonitrile and 0.1% OPA in a 50:50 ratio, a Phenyl (50x2.1 mm, 1.7 μ) column with a flow rate of 0.2 ml/min, and the UPLC analysis was carried out using a reverse-phase UPLC system.
Diluents
The diluent utilised was mobile phase.
Preparation of standard stock solution
They measured 40 mg of Metronidazole and 4 mg of Prednisolone as working standards. The contents were transferred to a clean, dry 10 ml volumetric flask. Diluent was added, and the mixture was sonicated until completely dissolved. The flask was then filled to the mark with the same solvent.
Impurity preparation-impurity stock solution
They accurately weighed and transferred 5 mg each of Metronidazole impurity-A, Metronidazole impurity-B, Prednisolone impurity-A, and Prednisolone impurity-B working standards into a clean, dry 10 ml volumetric flask. Diluent was added, and the mixture was sonicated to ensure complete dissolution. The volume was then made up to the mark with the same solvent. Subsequently, 1 ml of this solution was transferred into another 10 ml volumetric flask and diluted to the desired concentration with diluent.
Preparation of the standard solution
A 10 ml volumetric flask was partially filled with diluent after pipetting 1 ml each of the standard stock solution and the impurity stock solution into it. The volume was then made up to the mark with diluent.
Preparation of sample stock solution
They weighed 57.8 mg of Metronidazole (equivalent to 40 mg of Metronidazole) and 18.8 mg of Prednisolone (equivalent to 4 mg of Prednisolone) and transferred them into a clean, dry 10 ml volumetric flask. Diluent was added, and the sample was dissolved using a sonicator. The flask was then filled up to the mark with the same solvent.
Preparation of the sample solution
A 10 ml volumetric flask was partially filled with diluent after pipetting 1 ml each of the sample stock solution and the impurity stock solution into it. The volume was then made up to the mark with diluent.
Validation procedure
The following analytical characteristics were evaluated in accordance with ICH Q2 (R1) guidelines: forced degradation, linearity, specificity, accuracy, precision, specificity, system appropriateness, and linearity [31, 32].
System suitability
To ensure the system is functioning properly, we have computed its appropriateness parameters. The USP plate count, USP tailing, and % RSD may all be measured and determined to be within the limit. (USP – United States Pharmacopeia)
Specificity
Specificity refers to the ability to test the analyte clearly, even when other factors, such as contaminants or excipients, are present in the sample solution or the norm solution. Analyses of both blank and spiked samples with prednisolone and metronidazole were conducted to determine its efficacy.
Accuracy
The process's accuracy may be defined as how near the actual value of the test findings it produces it is. We ran the recovery experiments with three different concentrations. The drug concentration, recovery rate, and standard deviation were measured with at least three injections at each stage [33].
Precision
The accuracy of the analytical procedure is defined by the consistency of the findings. In order to assess the precision [34] of the present system, it was subjected to repeated sampling analysis on a homogenous sample and examined for repeatability, intraday and inter-day variations, the accuracy of the current system was evaluated. The sample was analysed at various time intervals on the same-day as well as on different days.
Linearity
The analytical technique can only provide results within a certain bound, which is known as its linearity. The linearity spectra were evaluated using six different series of standard solutions, with peak area proportional to analyte concentration in the sample. The calibration curve was created and the regression equations were measured by plotting the peak area against the concentration of the standard solution. The intercept, slope, and coefficient of correlation were measured using the technique of least squares.
LOD and LOQ
The limit of detection (LOD) is the lowest detectable concentration of analyte in a sample, whereas the limit of quantification (LOQ) is the lowest concentration that can be estimated with a sufficient degree of accuracy and precision. Both LOD and LOQ were calculated independently using calibration curves. The limits of detection (LOD) were found to be 3.3s/n and 10 s/n, in accordance with the ICH recommendations; s/n stands for signal-to-noise ratio.
Robustness
The robustness [35] of an analytical technique is a measure of its dependability throughout frequent usage and its capacity to stay unaffected by tiny but purposeful changes in the system's method parameters. The examination of robustness was performed by infusing the standard solution into the HPLC system and modifying the chromatographic parameters, including the flow rate (±0.02 ml/min) and the organic phase composition (±10%). The separation factor, retention duration, and peak asymmetry were calculated by analysing the impact of the modified parameters.
Forced degradation
Stress degradation should be no interference between the peaks obtained for a chromatogram of preparations. Researchers carried out stress degradation investigations in accordance with ICH standards. Distancing the deterioration peaks is important, as is ensuring that the resolution between them is at least 2.0. Additionally, the major peaks' peak purity must pass. A deterioration rate of around 20% was achieved via the use of forced degradation tests subjected to different stress settings.
RESULTS AND DISCUSSION
The separation of active pharmaceutical components from their contaminants posed the greatest analytical problem during the development of a new approach. The chromatographic conditions were fine-tuned to ensure optimal performance.
Method optimization
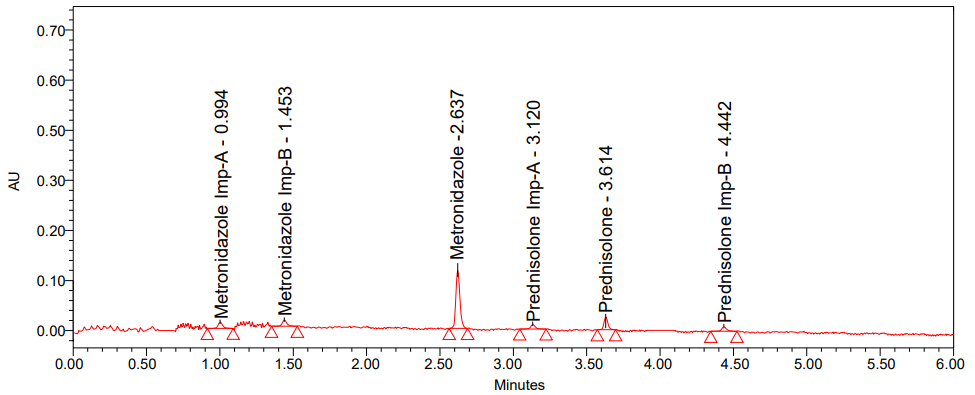
In order to find the best chromatographic conditions, we tried using isocratic and gradient modes with mobile phases that varied the ratio of phosphate buffer to acetonitrile. Nevertheless, in order to improve resolution and attain satisfactory retention periods, the mobile phase composition was adjusted at each session. Isocratic elution with acetonitrile and 0.1% OPA buffer was ultimately chosen because it yields a higher reaction from the active pharmaceutical component and any contaminants in it. A number of stationary phases, including C8, and C18 phenyl columns, were evaluated during the technique optimisation process [36]. Using symmetry C18 column the chromatogram (fig. 2) shows fifth peak was broad and less resolution between fifth and sixth peaks. From these trials, the peak shapes were relatively good with a column of phenyl 50x2.1 mm, 1.7 µ with a PDA (Photo Diode Array) detector. To achieve sufficient sensitivity, the mobile phase flow rate was set at 271 nm. Metronidazole and prednisolone had tailing factors of 1.05 and 1.13, respectively, and retention durations of about 2.634 and 3.617 min, according to the circumstances given above. For Metronidazole, the retention durations for impurity-A were 0.992 and 1.455 min, whereas for Prednisolone, they were 3.126 and 4.440 min, respectively. With 14802 and 8107 theoretical plates, respectively, for Metronidazole and Prednisolone, the column was able to provide accurate results; the percentage RSD for six duplicate injections was around 1.46% and 1.66%, indicating that the suggested method is quite exact. The developed procedure was verified according to ICH criteria.
Method validation
The ICH-approved optimized RP-UPLC technique was tested for system appropriateness, linearity, consistency, accuracy, and robustness.
System precision
Suitability parameters [37] of Metronidazole and Prednisolone, such as USP plate count, USP tailing and USP resolution, are acquired from the Empower software. Table 1 shows results of system precision and fig. 3 shows the chromatogram of standard.
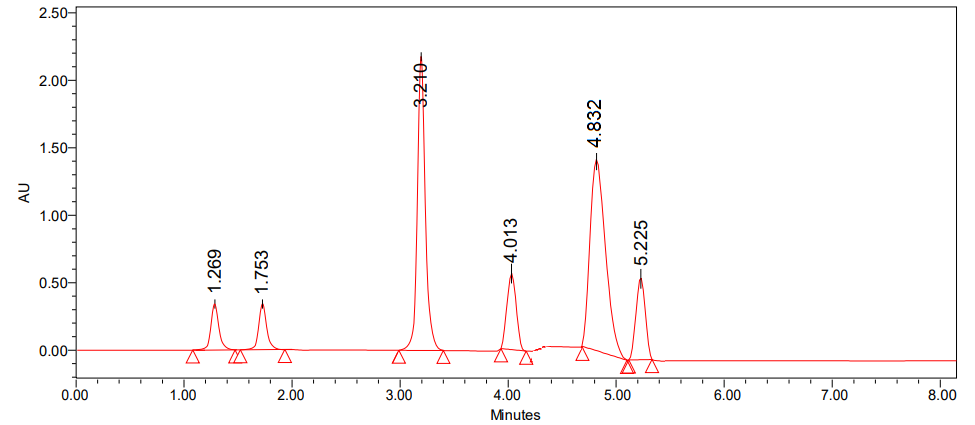
Fig. 2: Trial chromatogram
Table 1: Results of system precision
| Suitability parameter | Acceptance criteria | Metronidazole | Prednisolone | ||
| Mean | Std Dev | Mean | Std Dev | ||
| USP Plate count | NLT 2000 | 14796 | 30.956 | 8126 | 12.785 |
| USP Tailing | NMT 2.0 | 1.07 | 0.025 | 1.01 | 0.009 |
| USP Resolution | NMT 2.0 | - | - | 13.35 | 0.193 |
(n=6), NLT – Not Less Than, NMT – Not More Than, SD – Standard Deviation
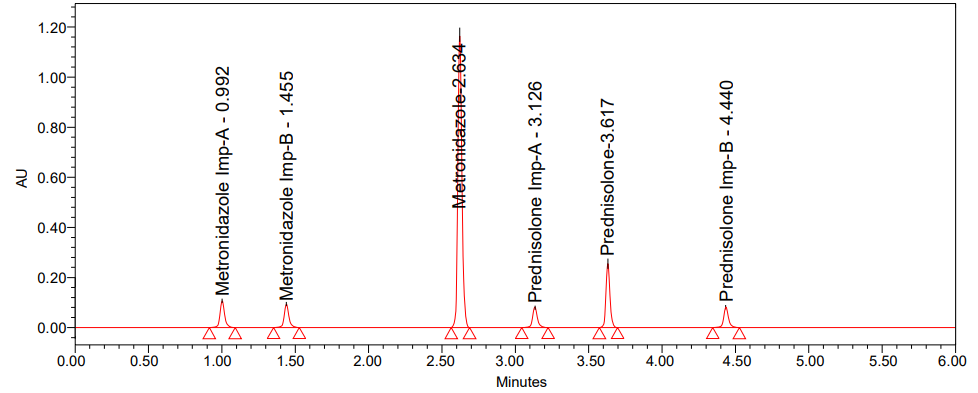
Fig. 3: Chromatogram of standard
Specificity
The interference was examined by separately analyzing the sample, standard, and placebo solutions according to the test technique. There was no interference of placebo with the primary peak, and the active components were well separated from blank and their excipients, as seen in the fig. below. Therefore, the approach is targeted. Fig. 4 shows the chromatogram of blank.
Fig. 4: Chromatogram of blank
Table 2: Linearity results of metronidazole, prednisolone and their related substances
A
| Linearity | Metronidazole | Imp-A | Imp-B | |||
| Conc. (µg/ml) | Area | Conc. (µg/ml) | Area | Conc. (µg/ml) | Area | |
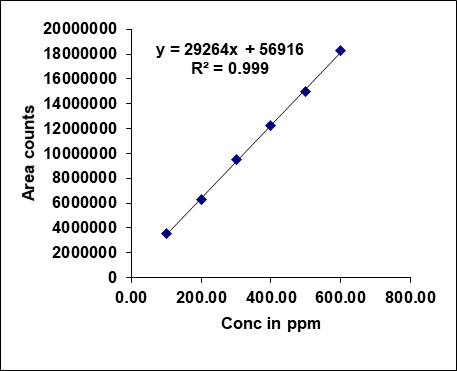
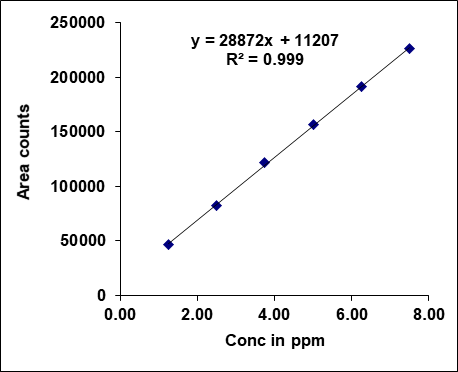
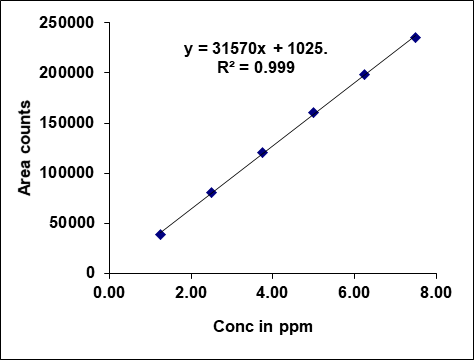
| Linearity-1 | 100.00 | 3568941 | 1.25 | 46387 | 1.25 | 38572 |
| Linearity-2 | 200.00 | 6244535 | 2.50 | 82387 | 2.50 | 80394 |
| Linearity-3 | 300.00 | 9545123 | 3.75 | 121691 | 3.75 | 120743 |
| Linearity-4 | 400.00 | 12248841 | 5.00 | 156383 | 5.00 | 160651 |
| Linearity-5 | 500.00 | 15004365 | 6.25 | 191934 | 6.25 | 198575 |
| Linearity-6 | 600.00 | 18256872 | 7.50 | 226351 | 7.50 | 235915 |
| Slope | 29263.68 | 28872.07 | 31569.51 | |||
| Intercept | 569159.87 | 11206.87 | 1025.07 | |||
| CC | 0.99956 | 0.99979 | 0.99977 | |||
B
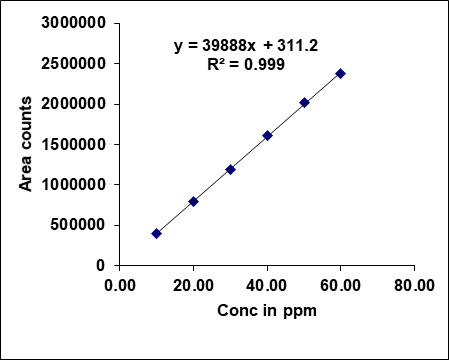
| Linearity | Prednisolone | Imp-A | Imp-B | |||
| Conc. (µg/ml) | Area | Conc. (µg/ml) | Area | Conc. (µg/ml) | Area | |
| Linearity-1 | 10.00 | 395853 | 1.25 | 7520 | 1.25 | 7416 |
| Linearity-2 | 20.00 | 798451 | 2.50 | 16189 | 2.50 | 14894 |
| Linearity-3 | 30.00 | 1187867 | 3.75 | 23257 | 3.75 | 21288 |
| Linearity-4 | 40.00 | 1607407 | 5.00 | 32136 | 5.00 | 28899 |
| Linearity-5 | 50.00 | 2013964 | 6.25 | 39731 | 6.25 | 35986 |
| Linearity-6 | 60.00 | 2374793 | 7.50 | 46841 | 7.50 | 42963 |
| Slope | 39887.94 | 6311.09 | 5682.79 | |||
| Intercept | 311.27 | 1.33 | 378.80 | |||
| CC | 0.99983 | 0.99948 | 0.99984 | |||
Linearity
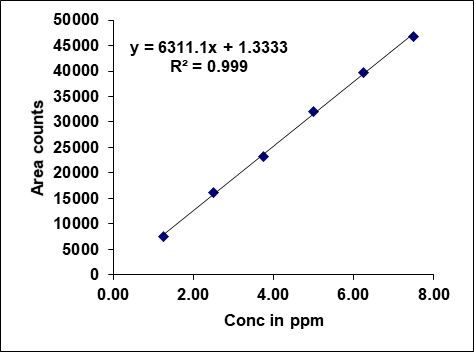
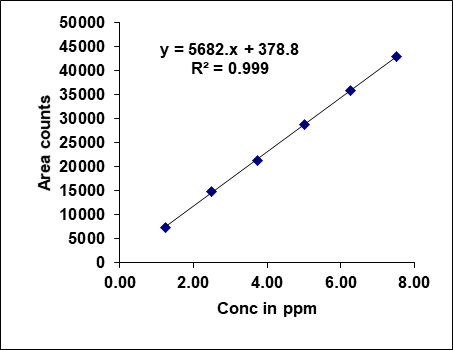
Metronidazole, prednisolone, and similar drugs have had their linearity peak area versus concentrations tested. The test solutions are made using impurity stock solutions at different concentration levels for the associated substance procedure. Metronidazole has a linearity range of 100-600µg/ml, Prednisolone from 10-60 µg/ml, and each impurity of Metronidazole and Prednisolone from 1.25-7.5 μg/ml. There is a linear relationship between the peak areas and the matching pitch concentrations under ideal chromatographic conditions. The correlation coefficients for all the components were under the limit (R2>0.999) [38]. Table 2 gives the results of linearity and fig. 5 gives the calibration plots of Metronidazole, Prednisolone and their related substances.
A B
C D
E F
Fig. 5: Calibration plots of (A) Metronidazole (B) Metronidazole imp-A (C) Metronidazole imp-B (D) Prednisolone (E) Prednisolone Imp-A (F) Prednisolone imp-B
Accuracy
In order to ensure accuracy, the sample solution containing the active pharmaceutical component was tested three times, with each sample containing known quantities of each impurity at concentration levels of 50%, 100%, and 150%, within the allowed range [39]. The % recoveries for all contaminants were checked and confirmed to be within the specified range. Table 3 gives the results of accuracy.
Table 3: Results of accuracy
| S. No. | % Level | Metronidazole | Prednisolone | ||||
| Conc. (µg/ml) | % Recovery | Std dev | Conc. (µg/ml) | % Recovery | Std dev | ||
| 1 | 50 | 200 | 100.4 | 0.153 | 20 | 99.3 | 0.235 |
| 2 | 100 | 400 | 100.2 | 0.147 | 40 | 100.0 | 0.211 |
| 3 | 150 | 600 | 99.5 | 0.089 | 60 | 99.9 | 0.172 |
(n=3)
Method precision
The degree to which series of measurements obtained from repeated homogeneous mixture samplings are closely related is the precision of an analytical procedure. A precise procedure involving related chemicals was carried out by injecting six separate 400 ppm and 40 ppm doses of Metronidazole and Prednisolone injections, each spiked with the respective imp-A and imp-B of the two drugs. Under the given experimental circumstances, the method proved to be accurate in determining the % RSD for each impurity. Intraday precision results were shown in table 4 and the method precision chromatogram was shown in fig. 6.
Table 4: Intraday precision results of metronidazole and prednisolone
| Sample number | % of related substances | |||||
| Metronidazole | Prednisolone | |||||
| Spiked impurities | Total impurities | % Purity (100-total imp) |
Spiked impurities | Total impurities | % Purity (100-total imp) |
|
| 1 | 5.02 | 0.55 | 99.45 | 3.06 | 0.41 | 99.59 |
| 2 | 5.04 | 0.57 | 99.43 | 3.07 | 0.42 | 99.58 |
| 3 | 5.09 | 0.56 | 99.44 | 3.09 | 0.41 | 99.59 |
| 4 | 5.03 | 0.57 | 99.43 | 3.01 | 0.41 | 99.59 |
| 5 | 5.01 | 0.55 | 99.45 | 3.03 | 0.42 | 99.58 |
| 6 | 5.05 | 0.56 | 99.44 | 3.04 | 0.43 | 99.57 |
| Average | 5.04 | 0.56 | 99.44 | 3.05 | 0.42 | 99.58 |
| Std Dev | 0.028 | 0.009 | 0.00894 | 0.029 | 0.008 | 0.00816 |
| % RSD | 0.56 | 1.60 | 0.01 | 0.95 | 1.96 | 0.01 |
(n=6)
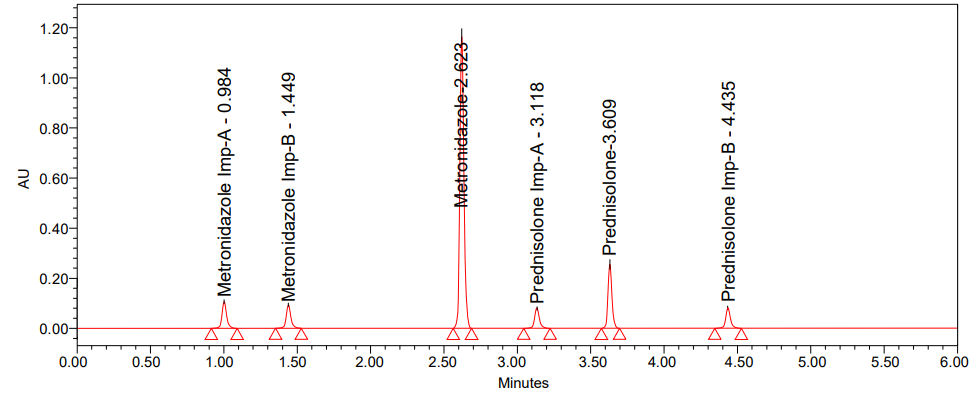
Fig. 6: Chromatogram of method precision
Table 5: Inter-day outcomes of accuracy of metronidazole and prednisolone
| Sample number | % Related substances | |||||
| Metronidazole | Prednisolone | |||||
| Spiked impurities | Total impurities | % Purity (100-total imp) |
Spiked impurities | Total impurities | % Purity (100-total imp) |
|
| 1 | 5.08 | 0.62 | 99.38 | 3.13 | 0.49 | 99.51 |
| 2 | 5.06 | 0.64 | 99.36 | 3.17 | 0.47 | 99.53 |
| 3 | 5.03 | 0.63 | 99.37 | 3.19 | 0.48 | 99.52 |
| 4 | 5.07 | 0.64 | 99.36 | 3.15 | 0.48 | 99.52 |
| 5 | 5.06 | 0.65 | 99.35 | 3.16 | 0.49 | 99.51 |
| 6 | 5.05 | 0.65 | 99.35 | 3.14 | 0.47 | 99.53 |
| Average | 5.06 | 0.64 | 99.36 | 3.16 | 0.48 | 99.52 |
| Std Dev | 0.017 | 0.011 | 0.011 | 0.022 | 0.009 | 0.009 |
| % RSD | 0.34 | 1.83 | 0.01 | 0.68 | 1.86 | 0.01 |
Mean±SD (n=6)
Intermediate precision
On different days, several analysts and tools were used to test six duplicates of the sample solution. The areas of the peaks were measured in order to get the mean percent RSD values. The results are shown in the table below. Inter-day precision results were shown in table 5.
LOD and LOQ
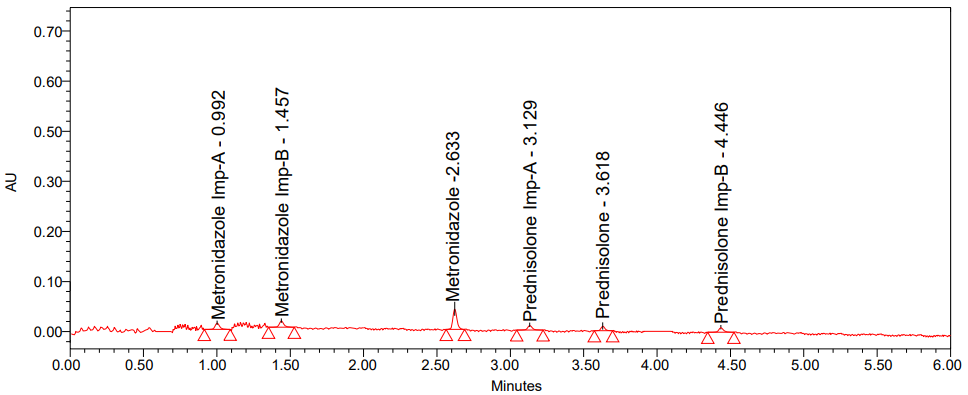
To determine the chemicals' LOD and LOQ, the lesser ones were injected steadily. Metronidazole and its related substances had LOD concentrations of 0.6, 0.15, and 0.15 with s/n values of 3, 3, and 3, respectively; Prednisolone and its related substances had LOD concentrations of 0.15, 0.15, and 0.15 with s/n values of 3, 3, and 3. Metronidazole and its related substances had LOQ concentrations of 2, 0.5, and 0.5 with s/n values of 10, 10, and 10 for Prednisolone and its related substances, respectively, and 0.5, 0.5, and 0.5 with s/n values of 10, 10, and 10 for Metronidazole. The ICH recommendations were used to verify this procedure [40, 41]. [The values of signal-to-noise ratio of LOD and LOQ were got from Empower software].
A
B
Fig. 6: Chromatogram of (A) LOD and (B) LOQ
Robustness
Flow rate, mobile phase in organic percentage, and other experimental parameters were chosen to assess the resilience of a previously constructed system that had been purposefully changed [42]. There was no discernible effect on the resolution of impurity-active pharmaceutical component separation, retention time, plate count, or tailing factor. This strategy proved to be quite reliable.
Table 6: Robustness data of metronidazole and prednisolone
| Parameter name | % RSD | |
| Metronidazole | Prednisolone | |
| Flow min (0.18 ml/min) | 0.75 | 0.58 |
| Flow plus (0.22 ml/min) | 0.16 | 0.24 |
| Organic min (-10%) | 0.38 | 0.69 |
| Organic plus (+10%) | 0.49 | 0.55 |
RSD-Relative standard deviation
Degradation studies
The medication samples of Metronidazole and Prednisolone were partially degraded by subjecting them to different forced degradation settings. Research on forced deterioration has shown that it works well for degradation products. Furthermore, the investigations provide information about the drug's instability under certain circumstances, which allows for the implementation of safeguards during formulation to prevent such instability [43].
Acid degradation
Acid degradation of Metronidazole and Prednisolone were studied in 1N HCl (Hydrochloric acid). 13.8% of Metronidazole and 14.8% of Prednisolone degradation was observed.
Alkali degradation
Alkali degradation of Metronidazole and Prednisolone were studied in 1N NaOH (Sodium Hydroxide). 13.3% of Metronidazole and 15.5% of Prednisolone degradation was observed.
Peroxide degradation
Peroxide degradation of Metronidazole and Prednisolone were studied in 10% hydrogen peroxide. 14.9% of Metronidazole and 15.9% of Prednisolone degradation was observed.
Reduction degradation
Reduction degradation of Metronidazole and Prednisolone were studied in 10% Sodium bi sulphite solution. 12.5% of Metronidazole and 14.3% of Prednisolone degradation was observed.
Thermal degradation
Thermal degradation of Metronidazole and Prednisolone were studied with analyzed standard solution. 12.1% of Metronidazole and 2.7% of Prednisolone degradation was observed.
Table 8: Forced degradation results of metronidazole and prednisolone
| Degradation condition | Metronidazole | Prednisolone | ||
| % Assay | % Deg | % Assay | % Deg | |
| Acid degradation | 86.2 | 13.8 | 85.2 | 14.8 |
| Alkali degradation | 86.7 | 13.3 | 84.5 | 15.5 |
| Peroxide degradation | 85.1 | 14.9 | 84.1 | 15.9 |
| Reduction degradation | 87.5 | 12.5 | 85.7 | 14.3 |
| Thermal degradation | 87.9 | 12.1 | 97.3 | 2.7 |
| Photo degradation | 96.3 | 3.7 | 98.4 | 1.6 |
| Hydrolysis degradation | 98.4 | 1.6 | 87.8 | 2.9 |
Photolytic degradation
The photo stability chamber was used for 6 h with 50 mg of Metronidazole and 10 mg of Prednisolone as working standards. By using these analyzed standards solution was prepared and injected. 3.7% of Metronidazole and 1.6% of Prednisolone degradation was observed
Hydrolysis degradation
Hydrolysis degradation of Metronidazole and Prednisolone was observed in HPLC-grade water. 1.6% of Metronidazole and 2.9% of Prednisolone degradation was observed.
CONCLUSION
We provide a straightforward, selective, verified, and well defined stability analysis that demonstrates the isocratic RP-UPLC technique was well-established for the quantitative assessment of Metronidazole and Prednisolone, together with associated chromatographic related substances. In RS (Related Substances) conditions, the suggested method is quick, easy, feasible, and inexpensive because all degradation products and associated active pharmaceutical ingredient-related substances are clearly separated, and the peaks are well-resolved and separate with an appropriate retention time. The method was better because of its wide range of linearity, use of readily available mobile phase and low retention time. All these factors make this method suitable for quantification of related substances in Metronidazole and Prednisolone in pharmaceutical dosage forms without interference and can be successfully used for routine analysis.
ACKNOWLEDGEMENT
P B Siddhartha College of Arts and Science administration has the author's gratitude for their support.
FUNDING
Nil
AUTHORS CONTRIBUTIONS
Gayathri Devi has collected the literature and information about the drugs, Anitha has carried out the research samples and Manoranjani prepared the manuscript.
CONFLICTS OF INTERESTS
Declared none
REFERENCES
Creek DJ, Barrett MP. Determination of antiprotozoal drug mechanisms by metabolomics approaches. Parasitology. 2014;141(1):83-92. doi: 10.1017/S0031182013000814, PMID 23734876.
Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399(10325):629-55. doi: 10.1016/S0140-6736(21)02724-0, PMID 35065702.
Sethi NJ, Safi S, Korang SK, Hrobjartsson A, Skoog M, Gluud C. Antibiotics for secondary prevention of coronary heart disease. Cochrane Database Syst Rev. 2021;2(2):CD003610. doi: 10.1002/14651858.CD003610.pub4, PMID 33704780.
Kreisel K, Torrone E, Bernstein K, Hong J, Gorwitz R. Prevalence of pelvic inflammatory disease in sexually experienced women of reproductive age United States, 2013-2014. MMWR Morb Mortal Wkly Rep. 2017;66(3):80-3. doi: 10.15585/mmwr.mm6603a3, PMID 28125569.
Curry A, Williams T, Penny ML. Pelvic inflammatory disease: diagnosis management and prevention. Am Fam Physician. 2019;100(6):357-64. PMID 31524362.
Hubers SA, DeSimone DC, Gersh BJ, Anavekar NS. Infective endocarditis: a contemporary review. Mayo Clin Proc. 2020;95(5):982-97. doi: 10.1016/j.mayocp.2019.12.008, PMID 32299668.
Delgado V, Ajmone Marsan N, De Waha S, Bonaros N, Brida M, Burri H. 2023 ESC guidelines for the management of endocarditis. Eur Heart J. 2023;44(39):3948-4042. doi: 10.1093/eurheartj/ehad193, PMID 37622656.
Chieng WK, Abdul Jalal MI, Bedi JS, Zainuddin AA, Mokhtar MH, Abu MA. Probiotics a promising therapy to reduce the recurrence of bacterial vaginosis in women? a systematic review and meta-analysis of randomized controlled trials. Front Nutr. 2022;9:938838. doi: 10.3389/fnut.2022.938838, PMID 36204368.
Ma S, Wang W, Su Y, Sun W, Ma L. Antibiotics therapy combined with probiotics administered intravaginally for the treatment of bacterial vaginosis: a systematic review and meta-analysis. Open Med (Wars). 2023;18(1):20230644. doi: 10.1515/med-2023-0644, PMID 37724125.
Hopkins DR, Weiss AJ, Torres Velez FJ, Sapp SG, Ijaz K. Dracunculiasis eradication: end stage challenges. Am J Trop Med Hyg. 2022;107(2):373-82. doi: 10.4269/ajtmh.22-0197, PMID 35895421.
Robertson LJ, Hanevik K, Escobedo AA, Morch K, Langeland N. Giardiasis why do the symptoms sometimes never stop? Trends Parasitol. 2010;26(2):75-82. doi: 10.1016/j.pt.2009.11.010, PMID 20056486.
Thorley N, Ross J. Intravaginal boric acid: is it an alternative therapeutic option for vaginal trichomoniasis? Sex Transm Infect. 2018;94(8):574-7. doi: 10.1136/sextrans-2017-053343, PMID 29223972.
Rawat A, Singh P, Jyoti A, Kaushik S, Srivastava VK. Averting transmission: a pivotal target to manage amoebiasis. Chem Biol Drug Des. 2020;96(2):731-44. doi: 10.1111/cbdd.13699, PMID 32356312.
Drewes JL, Chen J, Markham NO, Knippel RJ, Domingue JC, Tam AJ. Human colon cancer derived clostridioides difficile strains drive colonic tumorigenesis in mice. Cancer Discov. 2022;12(8):1873-85. doi: 10.1158/2159-8290.CD-21-1273, PMID 35678528.
Cymbal M, Chatterjee A, Baggott B, Auron M. Management of clostridioides difficile infection: diagnosis treatment and future perspectives. Am J Med. 2024;137(7):571-6. doi: 10.1016/j.amjmed.2024.03.024, PMID 38508330.
Huang Y, Zhang X, PengWang Y, Li Y, Yao J. Identification of hub genes and pathways in colitis associated colon cancer by integrated bioinformatic analysis. BMC Genom Data. 2022;23(1):48. doi: 10.1186/s12863-022-01065-7, PMID 35733095.
Chalitsios CV, Shaw DE, McKeever TM. Risk of osteoporosis and fragility fractures in asthma due to oral and inhaled corticosteroids: two population based nested case control studies. Thorax. 2021;76(1):21-8. doi: 10.1136/thoraxjnl-2020-215664, PMID 33087546.
Chalitsios CV, Shaw DE, McKeever TM. Corticosteroids and bone health in people with asthma: a systematic review and meta-analysis. Respir Med. 2021 May;181:106374. doi: 10.1016/j.rmed.2021.106374, PMID 33799052.
Kumar R, Clermont G, Vodovotz Y, Chow CC. The dynamics of acute inflammation. J Theor Biol. 2004;230(2):145-55. doi: 10.1016/j.jtbi.2004.04.044, PMID 15321710.
Sharma C, Bayry J. High risk of autoimmune diseases after COVID-19. Nat Rev Rheumatol. 2023;19(7):399-400. doi: 10.1038/s41584-023-00964-y, PMID 37046064.
Angum F, Khan T, Kaler J, Siddiqui L, Hussain A. The prevalence of autoimmune disorders in women: a narrative review. Cureus. 2020;12(5):e8094. doi: 10.7759/cureus.8094, PMID 32542149.
Alfarouk KO, Ahmed SB, Ahmed A, Elliott RL, Ibrahim ME, Ali HS. The interplay of dysregulated pH and electrolyte imbalance in cancer. Cancers. 2020;12(4):898. doi: 10.3390/cancers12040898, PMID 32272658.
Guise TA, Wysolmerski JJ. Cancer associated hypercalcemia. N Engl J Med. 2022;386(15):1443-51. doi: 10.1056/NEJMcp2113128, PMID 35417639.
Alwarith J, Kahleova H, Rembert E, Yonas W, Dort S, Calcagno M. Nutrition interventions in rheumatoid arthritis: the potential use of plant based diets. A review. Front Nutr. 2019 Sep;6:141. doi: 10.3389/fnut.2019.00141, PMID 31552259.
Devasenapathy N, Chu A, Wong M, Srivastava A, Ceccacci R, Lin C. Cancer risk with topical calcineurin inhibitors pimecrolimus and tacrolimus for atopic dermatitis: a systematic review and meta-analysis. Lancet Child Adolesc Health. 2023;7(1):13-25. doi: 10.1016/S2352-4642(22)00283-8, PMID 36370744.
Liu Z, Liao Q, Wen H, Zhang Y. Disease modifying therapies in relapsing-remitting multiple sclerosis: a systematic review and network meta-analysis. Autoimmun Rev. 2021;20(6):102826. doi: 10.1016/j.autrev.2021.102826, PMID 33878488.
Moreno G, Ramirez C, Corbalan J, Penaloza B, Morel Marambio M, Pantoja T. Topical corticosteroids for treating phimosis in boys. Cochrane Database Syst Rev. 2024;1(1):CD008973. doi: 10.1002/14651858.CD008973.pub3, PMID 38269441.
Pennington E, Bell S, Hill JE. Should video laryngoscopy or direct laryngoscopy be used for adults undergoing endotracheal intubation in the pre-hospital setting? A critical appraisal of a systematic review. J Paramed Pract. 2023;15(6):255-9. doi: 10.1002/14651858, PMID 38812899.
Liem EB, Hollensead SC, Joiner TV, Sessler DI. Women with red hair report a slightly increased rate of bruising but have normal coagulation tests. Anesth Analg. 2006;102(1):313-8. doi: 10.1213/01.ANE.0000180769.51576.CD, PMID 16368849.
Botia M, Escribano D, Martinez Subiela S, Tvarijonaviciute A, Tecles F, Lopez Arjona M. Different types of glucocorticoids to evaluate stress and welfare in animals and humans: general concepts and examples of combined use. Metabolites. 2023;13(1):106. doi: 10.3390/metabo13010106, PMID 36677031.
Senthil Rajan D, Muruganathan G, Shivkumar K, Ganesh T. Development and validation of HPLC method for simultaneous quantification of vasicine glycyrrhizin and piperine in poly herbal cough syrup. Int J Curr Pharm Res. 2020;12(2):15-9. doi: 10.22159/ijcpr.2020v12i2.37480.
Ravichandran V, Shalini S, Sundaram KM, Rajak H. Validation of analytical methods strategies and importance. Int J Pharm Pharm Sci. 2010;2(3):18-22.
Gadhvi MP, Bhandari A, Suhagia BN, Desai UH. Development and validation of RP-HPLC method for simultaneous estimation of atazanavir and ritonavir in their combined tablet dosage form. Res J Pharm Technol. 2013;6(2):200-3.
Mangukiya MA, Bagwe PV, Desai AA, Joshi SV. Development and validation of stability indicating HPLC method for determination of related substances and assay of monobenzone drug substance. J Indian Chem Soc. 2023;100(9):101060. doi: 10.1016/j.jics.2023.101060.
Vijayakumari M, Reddy Ch B. Stability indicating validated HPLC method for the determination of zanubrutinib in bulk and pharmaceutical dosage form. Asian J Pharm Clin Res. 2020;13:159-62.
Kumar SA, Debnath A, Rao JV, Sankar DG. Development and validation of a sensitive RP-HPLC method for simultaneous estimation of rosuvastatin and fenofibrate in tablet dosage form by using PDA detector in Gradient mode. Res J Pharm Technol. 2016;9(5):549-54. doi: 10.5958/0974-360X.2016.00104.9.
Raziq A, Jan SU. Relative comparison of stability and degradation of methylcobalamin tablets of different brands at different storage settings. Int J Appl Pharm. 2021;13(3):171-5. doi: 10.22159/ijap.2021v13i3.41263.
Kovac L, Casar Z, Trdan Lusin TT, Roskar R. Development of an analytical method for determination of related substances and degradation products of cabotegravir using analytical quality by design principles. ACS Omega. 2022;7(10):8896-905. doi: 10.1021/acsomega.1c07260, PMID 35309479.
Rajakumari R, Sreenivasa Rao S. Stress degradation studies and development of a validated RP-HPLC method for determination of tiagabine in presence of its degradation products. Int J Pharm Pharm Sci. 2016;8(1):230-6.
Nagulancha BR, Vandavasi KR. Stability indicating method development and validation for quantitative estimation of organic impurities of the antidiabetic drug glipizide in drug substance and pharmaceutical dosage form using HPLC. Biomed Chromatogr. 2023;37(11):e5727. doi: 10.1002/bmc.5727, PMID 37635093.
Gomathy S, Narenderan ST, Meyyanathan SN, Gowramma B. Development and validation of HPLC method for the simultaneous estimation of apigenin and luteolin in commercial formulation. J Crit Rev. 2020;7(19):4785-90. doi: 10.31838/jcr.07.19.560.
Athavia BA, Dedania ZR, Dedania RR, Swamy SM, Prajapati CB. Stability indicating HPLC method for determination of vilazodone hydrochloride. Int J Curr Pharm Sci. 2017;9(4):123-9. doi: 10.22159/ijcpr.2017v9i4.20975.
Pandit RK, Pandey V. Development and validation of an RP-HPLC chromatographic method for the determination of related substances in a poly pharmaceutical oral suspension with ion exchange resin based taste masking. Int J App Pharm. 2025;17(4):216-30. doi: 10.22159/ijap.2025v17i4.54312.