
Int J App Pharm, Vol 17, Issue 3, 2025, 398-412Original Article
FACTORIAL DESIGN, OPTIMIZATION OF SWEETSOP STARCH AS A NEW NATURAL SUPERDISINTEGRANT IN THE FORMULATION OF CARVEDILOL FAST DISSOLVING TABLETS FOR BIOAVAILABILITY ENHANCEMENT
RAMA CHAKRADHAR DEVANI, SANTOSH KUMAR RADA*
Department of Pharmaceutics, GITAM School of Pharmacy, GITAM (Deemed to be University), Rushikonda, Visakhapatnam-530045, Andhra Pradesh, India
*Corresponding author: Santosh Kumar Rada; *Email: srada@gitam.edu
Received: 14 Nov 2024, Revised and Accepted: 10 Mar 2025
ABSTRACT
Objective: Carvedilol is a BCS class-II nonselective cardiac beta blocker with low bioavailability (35%) due to its poor solubility. To enhance the solubility and bioavailability, it can be formulated in Fast Dissolving Tablets (FDTs) employing a new natural superdisintegrant, Sweetsop starch, through factorial design.
Methods: Sweet Sop Starch (SSS) was extracted from the pulp of Annona squamosal L. The micromeritics characteristics of SSS were assessed, and the resulting product was utilized as a new superdisintegrant in the direct compression method of formulating Carvedilol (CRV) FDTs. The SSS was evaluated employing Fourier-transform infrared spectroscopy (FTIR), powdered x-ray diffraction, differential scanning calorimetry, and scanning electron microscopy. SSS, Potato starch (PS), and Sodium Starch Glycolate (SSG) were employed as superdisintegrants, and factorial design was used to investigate their disintegration property, Wetting Time (WT), and in vitro dissolution. The hardness, friability, homogeneity of Drug Content (DC), Water Absorption Ratio (R), in vivo pharmacokinetics, and stability parameters of formulated carvedilol FDTs were assessed.
Results: Micromeritic characteristics revealed that the produced SSS was fine, free-flowing, and crystalline. FTIR and DSC investigations indicated that there were no drug-excipient interactions. From the prepared formulations (F1 to F8), the one with a 5% SSS containing formulation CF2, demonstrated 98.44±1% drug release within ten minutes with 45±0.11 sec WT and had 32±01 sec DT. The optimized formula attained peak plasma concentration extremely quickly and showed 176.11 % relative bioavailability.
Conclusion: The formula containing 5% SSS showed good mechanical and physical characteristics, increased drug dissolution, and promoted quick disintegration with enhanced relative bioavailability in the management of hypertension and patient acceptance.
Keywords: Superdisintegrant, Sweetsop starch, In vitro dissolution, Bioavailability
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijap.2025v17i3.53266 Journal homepage: https://innovareacademics.in/journals/index.php/ijap
INTRODUCTION
The most common mode of administration is oral due to its features such as self-administration, improved patient compliance and higher therapeutic efficacy [1]. FDTs are a desirable alternative for patients due to their many advantages. The fact that they dissolve fast in the mouth without the need for water makes them an excellent choice for patients who have trouble swallowing or who cannot take tablets or capsules, especially for bedridden, elderly, and pediatric patients [2].
The optimal usage of superdisintegrants remains difficult since they must be able to dissolve in the oral cavity as quickly as feasible [3]. Much research has been conducted on natural superdisintegrants like Starch derivatives (Corn and potato starch), Alginates, Guar gum, Chitosan, and Plantago ovata [4]. Natural superdisintegrants are non-toxic, reduce the risk of adverse reactions, are biocompatible, and are cost-effective, making them suitable for pharmaceutical applications [5].
A literature survey indicated that sweet sop starch has not previously been employed as a superdisintegrant. In this study, we extracted starch from the pulp of sweet sop fruit [6], which requires fewer processing steps and is less expensive, and we utilized it as a supedisintegrant to improve the solubility and dissolution of carvedilol. A comparison will be made using existing marked superdisintegrants and newly synthesized superdisintegrants (SSS).
Carvedilol is a BCS class II antihypertensive drug having low solubility used in the treatment of hypertension. To increase the solubility, absorption, as well as therapeutic efficacy, FDTs were produced using a new superdisintegrant employing a 23 factorial design [7]. Newly developed superdisintegrant efficiency is compared with traditional disintegrants such as PS and SSG [7] CRV is a BCS classification II drug having low solubility used in the treatment of hypertension. To increase the solubility, dissolving rate, absorption, as well as therapeutic efficacy, FDTs were produced using a new superdisintegrant [8].
The current study intends to formulate and optimize FDTs of CRV. 23 Factorial design was employed to understand the impact of each independent superdisintegrant and the interaction of each other at the level of 0 and 5 %. Here, SSS (A), PS(B), along SSG(C) as independent variables, and DT, WT, and % percent dissolved in ten minutes were the independent variables [9, 10].
METHODS AND MATERIALS
Materials
The following ingredients were bought from Molychem in Mumbai: Carvedilol, PS, SSG, Microcrystalline cellulose (MCC), mannitol, magnesium stearate, and talc from Yarrow Chem Mumbai, Maharashtra. In the lab, SSS was isolated from the pulp of Annona squamosa L.
Method
Isolation of sweetsop starch
The sweetsop fruits (Annona squamosa L. Voucher Number 0194; the botanical specimen was confirmed by plant taxonomist (IAAT: 337) were cleaned, the outer layer, the seeds removed, and the pulp extracted. After chopping the pulp into cubes of 5 to 6 cm, it was quickly washed in a sodium sulphite solution and dried at 50 °C. The dried chips were ground in a ball mill for two hours, and the dry chips were mixed with five times their weight in distilled water and kept aside for 2 h. After passing the mixture through a muslin cloth, the obtained starch milk was centrifuged at 5000 rpm for 0.5 h, and then decantation of supernatant was done. The resultant starch sediment, which included a thin coating of brown mucus, was mixed with a 0.3% sodium hydroxide (w/v) solution and repeatedly rinsed and centrifuged until we got a pure white starch. The starch was dried and kept in a sealed container.
Analysis of sweetsop starch characteristics
SSS was assessed: for the following properties
Solubility
The SSS solubility has been measured with aqueous and non-aqueous solvents including petroleum ether, acetone, dichloromethane, alcohol, and chloroform [11].
Viscosity
A 4 %w/v aqueous dispersion of SSS has been analyzed using a viscometer (Ostwald).
Swelling index (SI)
1 g of SSS was taken into 2 graded test tubes one was added with light paraffin and the second one was added with distilled water. The dispersions were kept aside for twelve hrs. Sediment volumes of the tubes are noted. The SI of the material has been computed utilizing the subsequent formula:
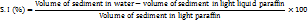
pH
Using a digital pH meter, 1.0% w/v suspensions of SSS were analyzed in triplicate, and the findings were noted.
Density
The density (g/cc) of an SSS dispersion in DW has been analyzed with the liquid displacement method. Benzene was used as the displacing liquid.
Particle size (PS)
This analysis was performed by sifting through standard sieves.
Moisture absorption
When handling, storing, and combining SSS into different formulations, especially in culinary and medicinal applications, moisture absorption is an important feature to consider. The final product's effectiveness and shelf life can be strongly impacted by the moisture content. To preserve the quality and functionality of SSS in various applications, it is imperative to maintain the ideal moisture level. The moisture absorption has been evaluated using a desiccator at 25 C, retaining a relative humidity of around 84%.
Bulk density (BD)
BD was determined using 50 times tapping in a graduated cylinder, and the results were calculated accordingly.
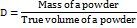
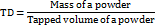
Measurement of powder flow
It was determined using the set funnel method. The below formula calculates the angle of repose.
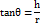
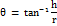
θ = angle of repose; h = height of pile; r = radius of pile
Compressibility index (CI)
The CI was found via measurement of the beginning volume (V0) and concluding volume (V) after subjecting a sample of SSS to one hundred tapings in a tube. It was then noted using the following eq.
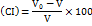
Estimation of ash content
The isolated starch has been heated to a temperature that evaporates the organic chemical and its derivatives, resulting in elements of minerals along with inorganic compound residues. The percentage of ash must be less than one percent.
% ASH = ((Ashed wt.)-(Crucible wt.)) x 100/((Crucible and sample wt.)-(Crucible wt.))
Estimation of loss on drying (LOD)
A single g of powdered SSS was measured and placed in a preheated assessing jar with a cover. It was then dried in an oven at 105ºC until a consistent weight
Identification test: iodine test
The SSS solution was treated with a solution of potassium iodide with iodine in water) then it turns out into a bright blue-black color that indicates the existence of starch.
Analysis of the amount of amylose
The amount of amylose in isolated starch has been determined using Juliano's (1971) technique, with slight changes. Pure amylose (40 mg) had been added to a test tube holding 1 ml of 95% ethanol along with 9 ml of sodium hydroxide 1N. The sample in the tube had been heated in boiling water for over 10 min, generating a gel, then cooled. The resultant gel (1, 2, 3, 4, and 5 ml) is transferred to five 100 ml-volumetric flasks and treated using 0.2, 0.4, 0.6, 0.8, as well as 1 ml of acetic acid 1N, respectively. Each volumetric flask with a capacity of 100 ml was then filled with two milliliters of iodine solution with enough distilled water. The accomplished mixture is agitated to get homogenous and then kept aside for 20 min. Then analyzed utilizing a UV-VIS spectrophotometer at 620 nm. The amount of amylose in the sample was calculated by interpolating the sample's absorbance value with a linear calibration curve, employing Eq [12].
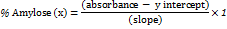
Drug-excipient compatibility
Fourier transform infrared spectroscopy (FTIR)
The IR spectrum for SSS has been obtained from samples made with an FT-IR device (Tokyo, Japan) (BRUKER) in potassium bromide. Then it was produced in KBr disks at a pressure of six to eight tons using a hydrostatic press. FTIR analysis was employed to evaluate the compatibility between SSS and CRV. CRV's FTIR and their 1:1 mixture with SSS and CRV were recorded [13].
Differential scanning calorimetry (DSC)
DSC measurements of CRV, CRV with SSS (1:1) are performed using a Perkin Elmer thermal analyzer that had a heated temperature range between 50-300 °C with a rate of heating of 10 °C per minute [14].
X-ray diffractometry (XRD)
The diffraction pattern of SSS has been obtained using an XRD (Analytical Spectra’s Pvt. Ltd., Singapore). It took place at the ambient temp (30ºC) using a diffractometer; target, Cu (λ1.54 A), filters, Ni; voltage, 40 ⱪV; power 30mA; scanned rate 4 °/min; and recorded between 30-150 ° with the full scale 200 [15].
Morphology by scanning electron microscope (SEM)
The morphology for SSS was observed using a scanning electron microscope. Scanning electron micrographs of isolated starch sample sputtered using gold to a layer thickness of approximately thirty nm (Sputter Coater Type E 5100, Biorad GmbH, Munich, Germany) were obtained at 5000X magnification using a DSM 940 equipment (Carl Zeiss, Oberkochen, Germany). The accelerating voltage ranged from 0.3 to 40 kV. The granule size was calculated using the validated scale bar on the SEM image [15].
Preparation of carvedilol fast-dissolving tablets
Direct compression has been used in the preparation of the tablets. Table 1 displays the ingredients of the various fast-dissolving Carvedilol tablet formulations. Before combining, each ingredient was run through a #100 mesh filter to guarantee uniformity in the particle size. Carvedilol was mixed with a mortar and pestle after SSS, SSG, PS, mannitol, and MCC were carefully measured out and blended. Lastly, the powder has talc and mg stearate added to it. The tablets were finally prepared using a rotary press with eight stations [16].
Table 1: Formulae of carvedilol FDT
| Formula (Mg/tablet) | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 |
| Carvedilol | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 |
| SSS | --- | 5 | --- | 5 | --- | 5 | --- | 5 |
| PS | --- | --- | 5 | 5 | --- | --- | 5 | 5 |
| SSG | --- | --- | --- | --- | 5 | 5 | 5 | 5 |
| Mannitol | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 |
| MCC | 33.5 | 28.5 | 28.5 | 23.5 | 28.5 | 23.5 | 23.5 | 18.5 |
| Magnesium Stearate | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Talc | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Total weight | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
The optimization technique
To optimize the formulation variables 23 factorial design was used, total of 8 runs were generated using the Design Expert® software. Table 2 illustrates detailed information on the investigated response variables and the design matrix. These choices of concentrations were based on preliminary studies conducted before setting up the experimental design. SSS, PS, and SSG as superdisintegrants are the independent variables, and WT, DT, and PD in 10 min are response variables (dependent variables) [17].
Table 2: Formulation composition constraints of carvedilol FDTs
| Parameter | Low (-1) | High (+1) | Constraints |
| A: SSS | 0 | 5 | |
| B: PS | 0 | 5 | |
| C: SSG | 0 | 5 | |
| Dependent variables | |||
| Y1: WT (Sec) | Minimize | ||
| Y2: DT (Sec) | Minimize | ||
| Y3: PD in 10 min (%) | Maximize |
Evaluation of carvedilol FDTs
Hardness
This was assessed using a Monsanto hardness tester, and the force was measured in kilograms (kg). Hardness values were determined for three tablets from each formulation.
In analysis, the Monsanto tester measures force in kilograms per centimeter and needs force to be delivered to the tablet diametrically using an integrated spring was used.
Uniformity of weight
Twenty pills were the subject of a weight variance investigation, which comprised calculating the actual weight difference between the tablets and their average weight.
Friability
A Roche friabilator was used to evaluate the tablets' friability. The tablets were turned 100 times around at a speed of 25 revolutions per minute for 4 min. The pills were weighed again after the penalties were removed, and the % of weight reduction was computed [18].

Drug content
Ten tablets were ground into powder for the content uniformity test. A volume of powder equal to ten milligrams of CRV was then extracted and filtered into a pH 6.8 buffer. Spectrophotometric absorbance measurement, following appropriate dilution with buffer was used to quantify the CRV concentration.
Wetting time (WT)
A petri dish of the same diameter was filled with five circular tissue sheets, each measuring 0.1 m in diameter. Ten ml of aqueous medium, including amaranth (water-soluble dye), was poured onto the plate. The FDTs were then carefully kept on a tissue sheet. The time elapsed until water fully covered the top of the tab is referred to as the WT [19].
Water absorption ratio (R)
6 ml of water and a piece of tissue paper folded twice were put on a tiny plate. After placing the tablet on tissue paper, it saturated fully and it was then reweighed. The R is calculated using the equation below.
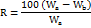
Where, Wa = Weight of tablet after water absorption, Wb= Weight of tablet before water absorption.
In vitro DT
Using a pH 6.8 buffer and USP disintegration equipment, the DT of FDTs was determined. The temperature was 37±0.2 °C and the buffer volume was 900 ml. The time taken for complete DT of six tablets was noted, and the mean disintegration time was noted [20].
In vitro dissolution
The in vitro dissolving rate investigation of CRV-FDTs requires the use of dissolution test equipment (Electrolab TDT-08L). The dissolving medium was the 900 ml pH 6.8 buffer with dissolution test equipment (Electrolab TDT-08L). At prearranged intervals, 5 ml of the sample was obtained and replaced with buffer, purified, and analyzed with a T360 UV/Visible double-beam spectrophotometer at 242 nm. The cumulative percent has been then noted. Each test was conducted in thrice (n = 3) [21].
Stability studies
The ICH and WHO recommend that an optimum composition of fast-dissolving carvedilol tablets be subjected to expedited testing, which can be achieved by simply storing the tablets in HDPE containers for six months at a temperature of 40 °C and 75° RH. The physical changes and dissolution properties had been evaluated both during as well as after their 6-month storage [22].
In vivo pharmacokinetic studies
The Institutional Animal Ethics Committee (IAEC) approved the experimental protocol (approval number: AKRGCP/Pheuc/2021-3). 3 male wistar rats 200–250g (Procured from Jeevan Life Sciences, Malkajgiri, Hyderabad) were housed in cages. They also experienced a daily cycle of 12 h of light and 12 h of darkness. Randomly selected rats were divided into two batches, each containing six rats. One group was given a pure drug (52.08 µg/kg body weight), and another group was given the F2 formulation (52.08 µg/Kg body weight). All groups received different treatments. The rats were given a 12 h restriction before the study began, and during that time, food and water were only periodically available to them. The dose was given to the wiser rats using a catheter. After the rats were given the drug, they were given moderate ether Anesthesia. At predefined intervals, blood was extracted. The blood plasma samples are centrifuged at 5000 rpm and kept at-20 °C. The samples were assessed with methanol and acetonitrile (80:20 %v/v) as mobile phase with a flow rate of 1 ml/min using a PDA detector analyzed with HPLC (Shimadzu LC) [23].
RESULTS AND DISCUSSION
The SSS that was produced was a fine, crystalline powder. The SI of 74% indicated water caused the sweetsop starch to expand significantly. It was discovered that the sweetsop starch bulk density and tapped density were, respectively, 0.545 and 0.505 g/cc. Ash content of SSS was found to be 0.92±0.14%, amylose content was 24.44±0.04 (%) and loss on drying was 10.27±0.07%. Moisture absorption was found to be 4.4±0.6%, and the angle of repose was 26.38±2.34, these results indicated that isolated SSS having good SI and flow properties are essential for FDTs. Due to its crystalline nature and physical properties resembling those of a superdisintegrant (table 3), it was suggested that sweetsop starch could serve as a new natural superdisintegrant in the manufacturing of FDTs.
Table 3: The sweetsop starch's physical and micromeritic characteristics
| Parameter | Observation |
| Solubility | In soluble in all tested organic and aqueous solvents. |
| Iodine test | Bluish violet color-presence of starch. |
| pH (1% aqueous dispersion) | 4.88±0.02 |
| Viscosity (4%w/v aq. dispersion) | 317±0.005cps |
| LOD | 10.27±0.07% |
| Content of Ash | 0.92±0.14% |
| SI | 74±0.04% |
| Moisture absorption | 6.4±0.6% |
| Content of Amylose | 24.44±0.04 (%) |
| CI | 14.11±2% |
| BD | 0.545±0.063g/cc |
| Angle of repose | 26.38 ±2.34 |
| TD | 0.505±0.03g/cc |
| Particle size | 5.2±3.2µm |
*n=3, Mean±SD
Moisture absorption and storage conditions
Sweetsop starch's moisture absorption properties are critical to its functionality and stability when used in tablet formulations. In real-world storage conditions, temperature and relative humidity significantly influence moisture uptake, which can affect the tablet's disintegration efficiency and shelf life. Unlike controlled laboratory environments, variations in humidity can lead to moisture-induced degradation of active ingredients or reduced tablet hardness.
A comparison between SSS and traditional disintegrants, including synthetic and natural types such as SSG, and potato starch, provides valuable insights given in table 4. Synthetic disintegrants like SSG are generally designed to minimize moisture absorption, thereby maintaining disintegration efficiency even under humid conditions. Disintegrants like SSS and PS tend to have higher moisture uptake than SSG, but can be modified chemically or physically to enhance their performance and stability.
Table 4: Quantitative comparisons of disintegration efficiency and moisture uptake
| Disintegrants | Disintegration time (s) | Moisture uptake (%) |
| SSS | 32.04±1.2 | 6.4±0.6 |
| SSG | 30.04±0.8 | 5.8±0.5 |
| PS | 321.48±2.2 | 8.3±0.7 |
*n=3, Mean±SD
Drug–exipient compatibility studies
The FTIR spectra of SSS are shown in fig. 1 The IR band beginning at 1337 cm-1 demonstrated C-H stretching within the propyl group, whereas the band in 1417 cm-1 was attributed to demonstrate the bending vibration for CH2. The 1640 cm-1 IR band illustrates the bend vibration of H-O-H. The region at 2928 cm-1 resulted from the symmetry stretching of H-C-H. The IR peak at 2359 cm-1 has been attributed to symmetry and asymmetric stretching on the methyl group produced via hydroxypropylation. The natural starch structure's hydroxy group is represented by the broad band around 3343 cm-1, while the propylated starch's hydroxy group is represented by a medium-sharp peak at 3896 cm-1. A high peak at 1012 cm-1 indicates the ether bond. (C–O) stretching. The spectrum of carvedilol with SSS (fig. 3) shows a strong absorbance band around 3059.72 cm-1 due to N-H stretching. The wavelength at 2955.80 cm-1 is likely due to O-H connection stretching. C-H stretching is responsible for 2868 cm-1, C-O stretching for 1265 cm-1, C=C aromatic for 1599 cm-1, C-N stretching for 1322.74 cm-1, N-H stretching of the linkage for 1520 cm-1, and C=CH2 bending for 862 cm-1. The identical peaks are also identified at the FTIR spectra of CRV (fig. 2): (-NH) 3037.43, (-OH) 2927.53, (-CH) 2868.83, (-CO) 1246.61, (-C=C) 1599.75, (-C-N) 1228.97, and (C=CH2) 670.86. The FTIR fig. showed that there were no interactions among them. Therefore, sweetsop starch can be utilized as a superdisintegrant in formulating FDTs-containing CRV.
SSS's crystalline nature is indicated by the existence of well-defined diffractions at 14.9, 19.3, and 22.8, and it also has a doublet extending from 16.8 to 17.8. The observed patterns correspond to a crystal polymorph structure depicted in fig. 4.
SSS granules were in size from 2-5μm and had a polygonal form. Fig. 5 shows that the particle shape of starch ranged from irregular to spherical.
The DSC thermogram of CRV-SSS starch also shows a prominent endothermic peak at 115. 58 °C. The DSC of pure CRV exhibits a noticeable endothermic curve at its melting point of 120.37 °C. The peaks in this region clearly show that SSS did not interact with the CRV, as it falls between 115 and 120 °C, which is the drug's melting point. Fig. 6 and 7 display the DSC of pure CRV along with CRV-SSS.
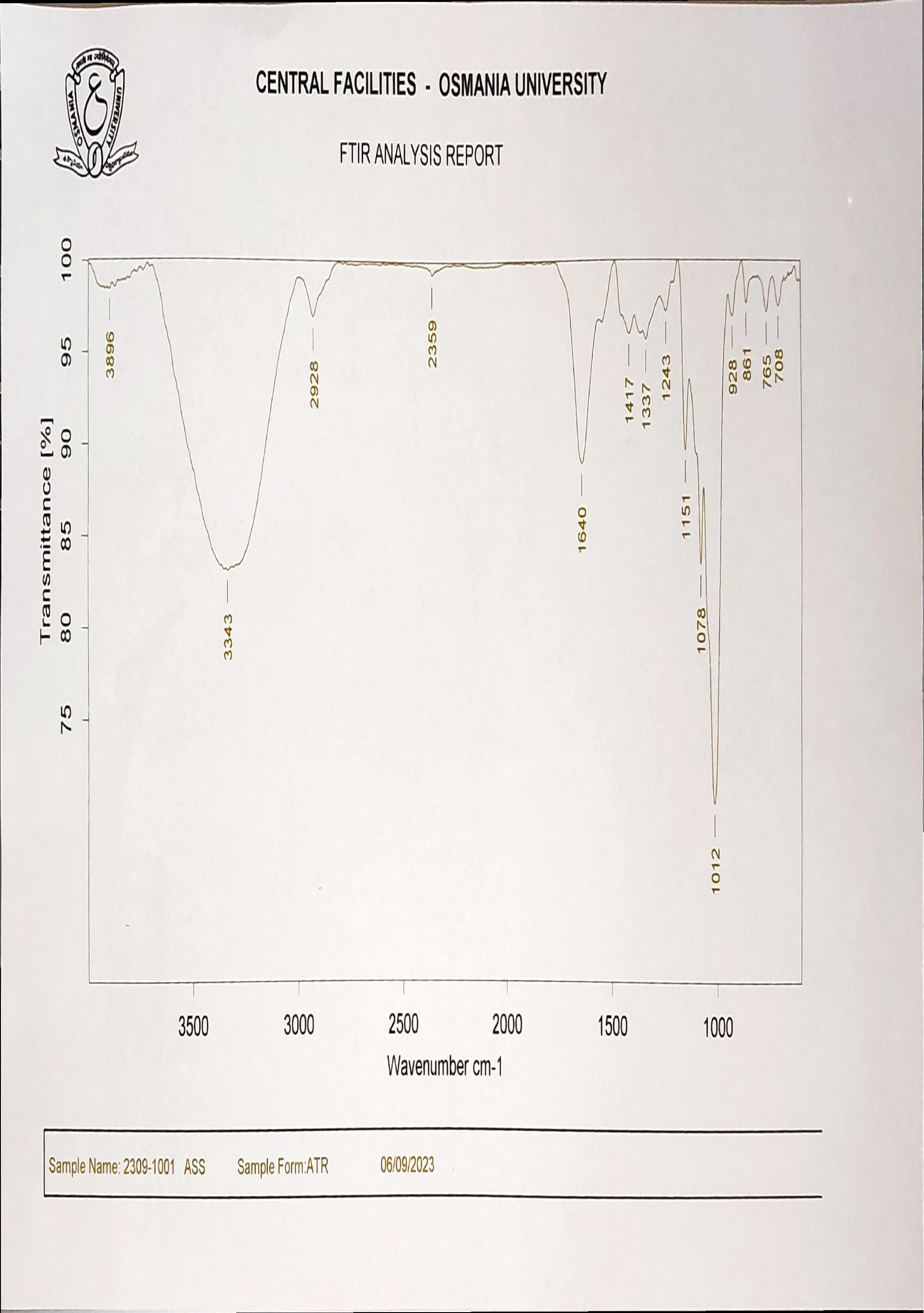
Fig. 1: IR spectrum of SSS
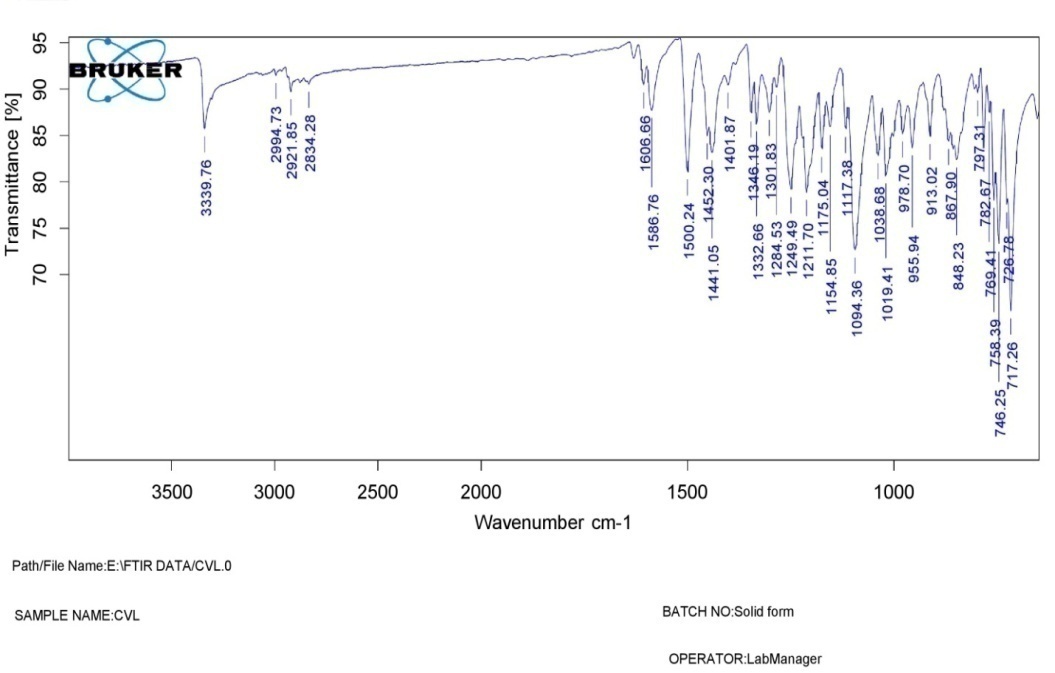
Fig. 2: Carvedilol IR spectrum

Fig. 3: IR spectra of CRV with sweetsop starch

Fig. 4: XRD of SSS

Fig. 5: SEM image of sweetsop starch (1000x)

Fig. 6: DSC of CRV
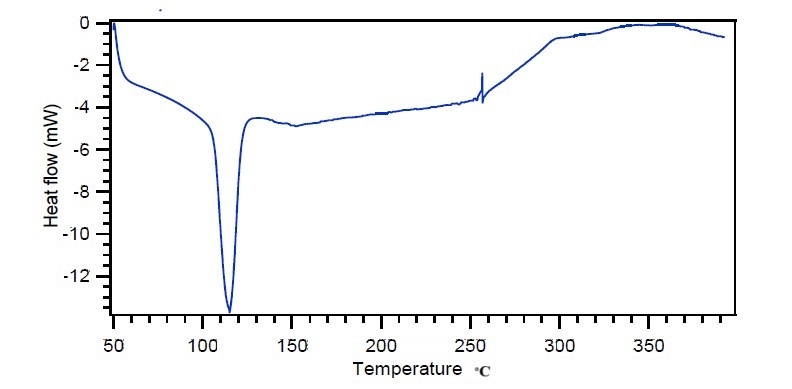
Fig. 7: DSC of CRV with SSS
Evaluation test
Hardness
The tablets ranged in hardness from 3.7 to 3.9 kg/cm2. It suggests a high level of adaptability and the capacity to manage stress that arises from different sources. The tablets' hardness was higher than that of the tablets made in compliance with S. Jaya, et al., it has a hardness of 3.5 kg/cm2 [24].
Friability
It was found that the weight loss was less than 0.17%, demonstrating the tablets' strong mechanical resilience. The friability findings (<0.17%), were best to withstand the mechanical shocks compared to the tab manufactured by Raha Khalid Dhahir et al., which has a friability of 0.8 % [25].
Drug content uniformity
The medication content of all manufactured FDT was 12.45 ±0.46 mg of CRV, under the recommended DC.
Water absorption ratio and WT
The definition of constraints and criteria for FDT was found to be met by the in vitro wetting time of the carvedilol FDT prepared with 5% of sweetsop starch as a superdisintegrant (fig. 4). Table 5 displays a wetting time for the formulations. WT of the best formulation was found to be 45±0.11 sec. The WT was shorter than the tablets made by Sharma V et al., it has a WT of 70±8 sec [26]. The water absorption ratio of 81±0.02% was observed in the optimized formulation F2 of Carvedilol FDT.
Table 5: Post-compression parameters of carvedilol FDT
| S. No. | Hardness Kg/cm2 | Friability (%)±SD | Drug content (mg/tab)±SD | DT (s)±SD | WT (sec)±SD | R (%)±SD |
| F1 | 3.7±0.001 | 0.11±0.011 | 12.12±.0.12 | 450±01 | 487±0.12 | 24.12±0.01 |
| F2 | 3.9±0.001 | 0.12±0.021 | 12.45±0.46 | 32±01 | 45±0.11 | 81±0.02 |
| F3 | 3.8±0.002 | 0.15±0.014 | 12.11±0.15 | 321±01 | 471±0.11 | 53±0.01 |
| F4 | 3.9±0.020 | 0.12±0.021 | 12.21±0.14 | 46±02 | 51±0.12 | 81±0.02 |
| F5 | 3.7±0.021 | 0.13±0.014 | 12.33±0.54 | 30±02 | 53±0.21 | 69±0.31 |
| F6 | 3.8±0.011 | 0.14±0.031 | 12.41±0.77 | 34±01 | 49±0.20 | 78±0.31 |
| F7 | 3.9±0.021 | 0.16±0.041 | 12.34±0.98 | 46±02 | 48±0.12 | 77±0.41 |
| F8 | 3.8±0.022 | 0.17±0.022 | 12.22±0.41 | 29±01 | 32±0.14 | 91±0.21 |
(Mean ±SD, n=3)
| F1 |  |
 |
| Time = 0 | Time = 478 Sec | |
| F2 | 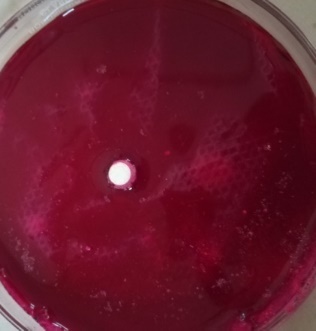 |
 |
| Time = 0 | Time = 45 Sec | |
| F3 | 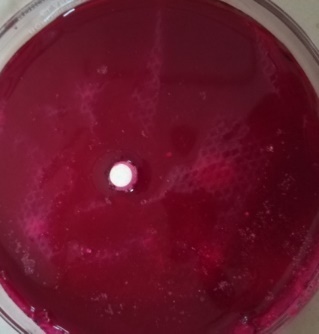 |
 |
| Time = 0 | Time = 471 Sec | |
| F4 |  |
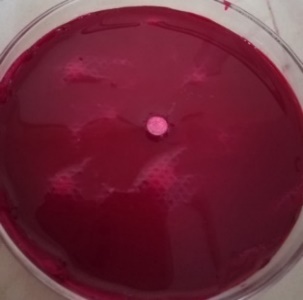 |
| Time = 0 | Time = 51 Sec | |
| F5 | 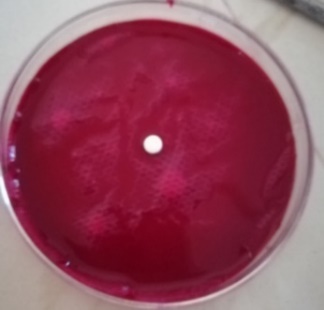 |
 |
| Time = 0 | Time = 53 Sec | |
| F6 |  |
 |
| Time = 0 | Time = 49 Sec | |
| F7 |  |
 |
| Time = 0 | Time = 48 Sec | |
| F8 |  |
 |
| Time = 0 | Time = 32 Sec |
Fig. 8: WT of carvedilol FDT made with sweetsop starch
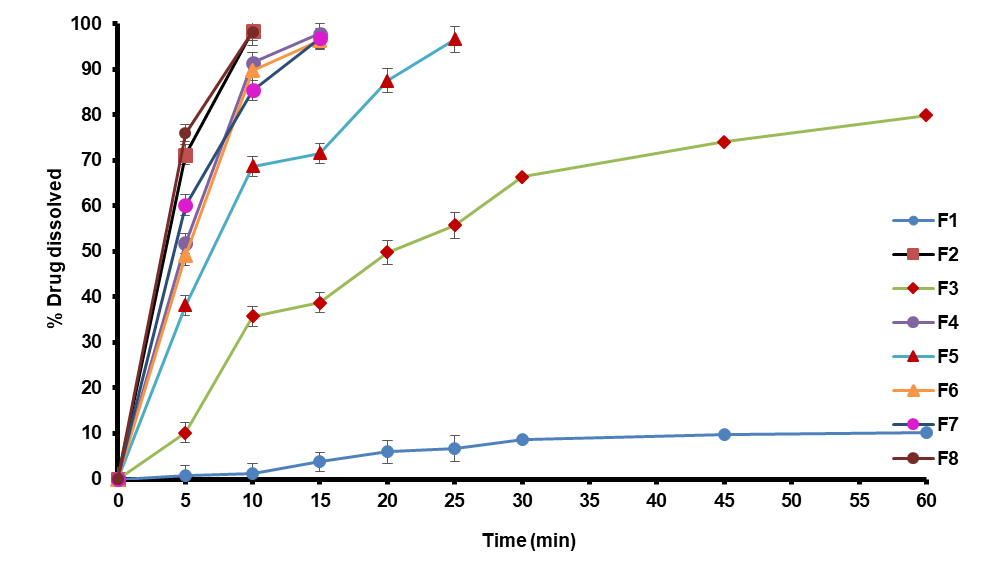
Fig. 9: Carvedilol FDTs dissolution profiles (F1-F8)
In vitro disintegration time
Table 5 indicates that the duration required for all formulated tablets to disintegrate ranges from 29±01 to 450±01 sec. The optimal formulation containing SSS had a DT of 32±02 sec, which was shorter than the FDTs prepared by Nani Parfati et al., which had a disintegration time of 42±02 sec [27].
In vitro dissolution studies
Fig. 5 displays the profile of the fast-dissolving Carvedilol tablets from in vitro dissolution experiments using sweetsop starch. Fig. 9 provided the dissolution data for every formulation, ranging from F-1 to F-8. Based on the findings, it is recommended that the novel superdisintegrant sweetsop starch be employed in the formulation of Carvedilol FDT. The optimized formulation, which contains SSS alone showed a 98.44±1 drug release in 10 min, which is much higher than the 97.08% release of tablets in 50 min that was made by CK Sahoo, et al. [28].
Factorial design
WT, DT, and PD in 10 min are examples of response variables (dependent variables) that have been analyzed using the polynomial regression technique, with the independent variables, such as superdisintegrants like sweetsop starch (A), potato starch (B), and sodium starch glycolate (C). Equations 1, 2, and 3 provide the polynomial Form for WT, DT, and PD in ten minutes, respectively.
WT=154.50+110.25A+4B+109C-1.25AB-105.25AC+1.5BC+4.25ABC (R2=1.000) …. 1
PD10= 71.21+23.49A+6.71B+14.52C-6.11AB-14.78AC-0.2037BC+4.30ABC ……… 2
(R2=1.000))
DT =125.00+89.75A+14.50B+87.25C-16.75AB-83.50AC-14.25BC+19ABC (R2=1.000) …….. 3
The meaning of the R2 indicates which suit is correct. An inference may be drawn using polynomial calculations by evaluating the variable's magnitude and statistical significance (positive or negative).
Using Design Expert 7.11 software, Surface Response Curves (SRC) and Contour Plots (CP) were created after the equation was generated. The proportions of each ingredient and their resulting interdependencies with WT, DT, and percent drug dissolution in ten minutes are compared in this equation. The accompanying table 6 shows the relationship between the superdisintegrants Sweet sop starch (A), potato starch(B), and sodium starch glycolate (C), as well as how these interactions affect the PD in 10 min, wetting time, and DT.
Table 6: Superdisintegrants' interactions and their impact on WT, DT, and percent dissolved in 10 min
| Superdisintegrants' interactions | Effect on | ||
| Wetting time | Disintegration time | % Dissolved in 10 min | |
| Sweetsop starch X Potato starch (AB) | - | - | - |
| Sweetsop starch X Sodium starch glycolate (AC) | - | - | - |
| Potato starch X Sodium starch glycolate (BC) | - | - | + |
| Sweetsop starch(A) | + | + | + |
| Potato starch(B) | + | + | + |
| SSG (C) | + | + | + |
| Sweetsop starch X Potato starch X Sodium starch glycolate (ABC) | + | + | + |
'-' signifies a detrimental impact, whereas '+' signifies a beneficial impact.
CP and SRP were made based on the interplay of effects on the WT, DT, and % dissolved in 10 min. Fig. 10 to 18 display the SRP and CP of the effect of various independent variables' responses on DT, WT, and % dissolved in 10 min. Contour plots of WT, DT, and PD in 10 min are linear. RS P and CP demonstrate the impact of superdisintegrants concentration on WT, DT, and PD in 10 min. Plots indicate that the superdisintegrant percentage ranges from 4 to 5%, which lowers the tablet's DT, WT, and enhanced PD in 10 min. The concentration of superdisintegrant is inversely related to WT and DT, directly proportional to PD in 10 min.
Table 7 displays the correlation between the superdisintegrant concentration and the WT, DT, and PD in 10 min.
Optimized formula
With a 5% concentration of SSS, formulation F2 demonstrated a shorter disintegration time, a higher percentage dissolved in 10 min, and a wetting time. Formulation F2, which is similar to formulation F8. Formulation F2, has a single superdisintegrant, SSS, in a concentration range of 5%. F2 is therefore seen as an optimized formulation that was more cost-effective. These results suggest that a single superdisintegrant formulation with an optimal concentration of sweetsop starch can effectively enhance the dissolution profile of CRV FDTs. The ability of SSS to achieve a high percentage of CRV dissolved within ten minutes is particularly noteworthy and underscores its potential for developing FDTs. From the response surface plot and contour plot, it was concluded that the WT and DT plots are linear with an inverse relation to the superdisintegrant concentration. PD in 10 min of RSP and CP indicates the superdisintegrant concentration is directly proportional and the best concentration was identified to be 4-5%.
Pharmacokinetic data analysis
For pharmacokinetic studies, the plasma concentration versus time profile obtained is shown in fig. 19. And table 6. Pharmacokinetic parameters, including Tmax, Cmax, AUC, Bioavailability (BA), rate of elimination (Ke), constant, and rate of absorption constant (Ka) were calculated from the plasma profile by using MS Excel. The carvedilol plasma concentration after the oral administration in pure form i. e. A and in fast-dissolving tablets i. e. B. From the plot, it can be concluded that after administration of FDTs of CRV, plasma concentration is higher initially within a short period and attains its Cmax concentration within 1 hour. A faster disintegration time associated with SSS may reduce the Tmax from 3 h to 1, and improved relative bioavailability through optimized formulation can lead to a higher AUC and Cmax. All pharmacokinetic parameters of CRV pure drug and optimized formula are given in table 8.
Table 7: Relation between concentration of superdisintegrant on WT, DT, PD in 10 min
| The interactions among ABC | Relation Founded | Recommended ratio | ||
| Disintegration time | Wetting time | On percent dissolved in 10 min | ||
| SSS X PS(AB) | Linear | Linear | Linear | 4-5% |
| SSSXSSG (AC) | Linear | Linear | Linear | 4-5% |
| PSXSSG (BC) | Linear | Linear | Linear | 4-5% |
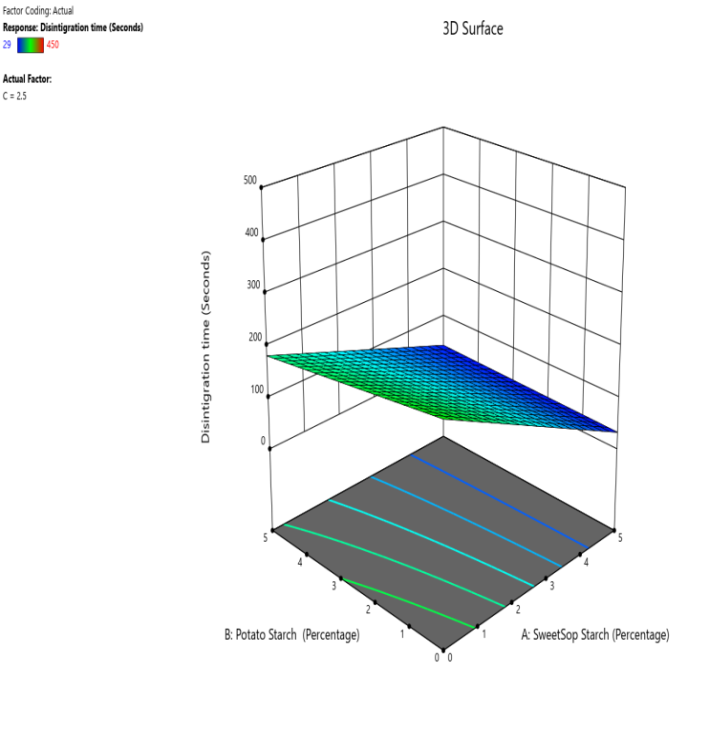
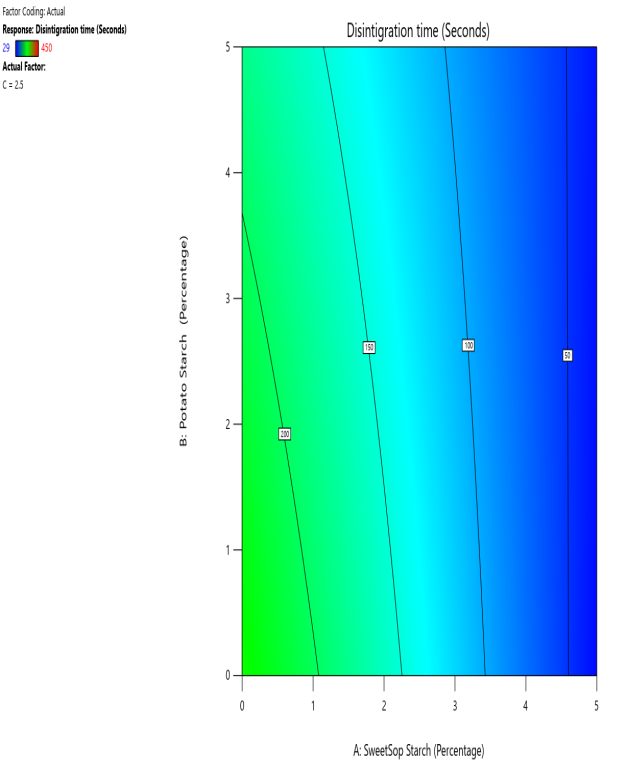
Fig. 10: The contour and response (3D surface graph) demonstrate the effect of SSS and potato starch on the DT
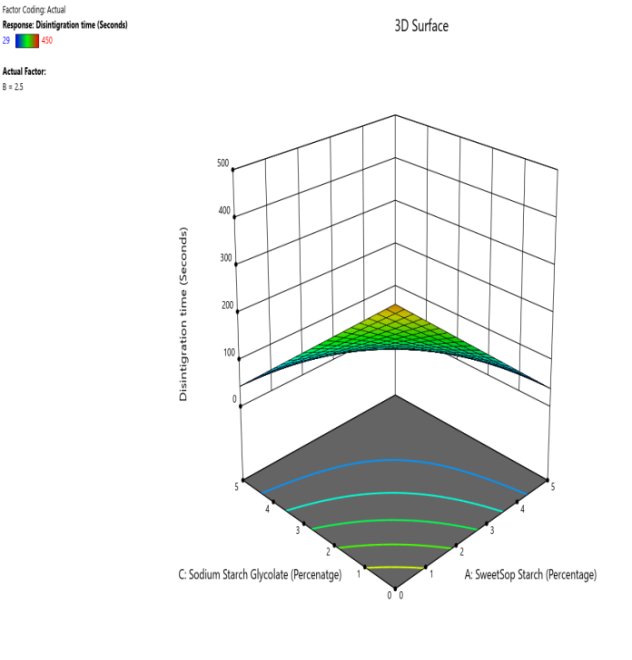

Fig. 11: The contour and response (3D surface graph) demonstrate the effect of SSS and SSG on the DT
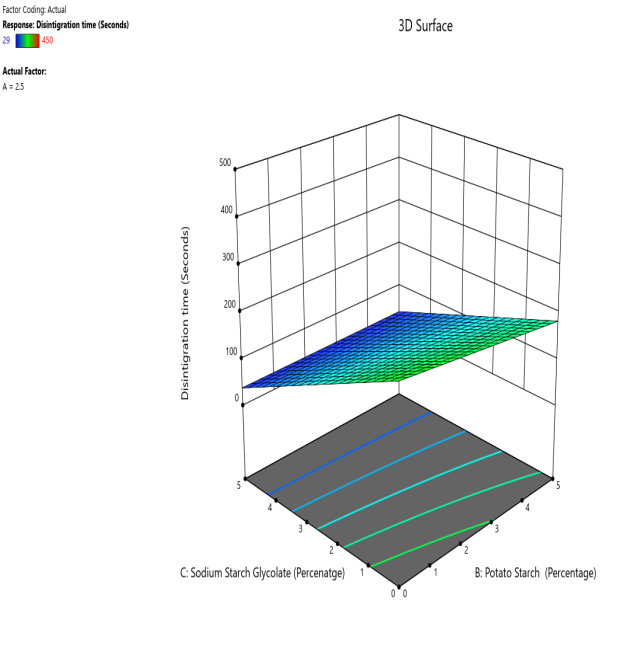
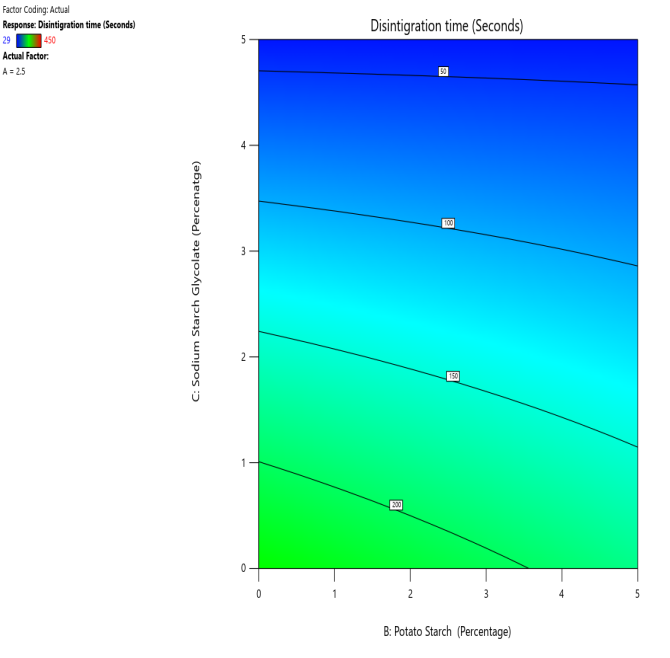
Fig. 12: The contour and response (3D surface graph) demonstrate the effect of potato starch and SSG on the DT

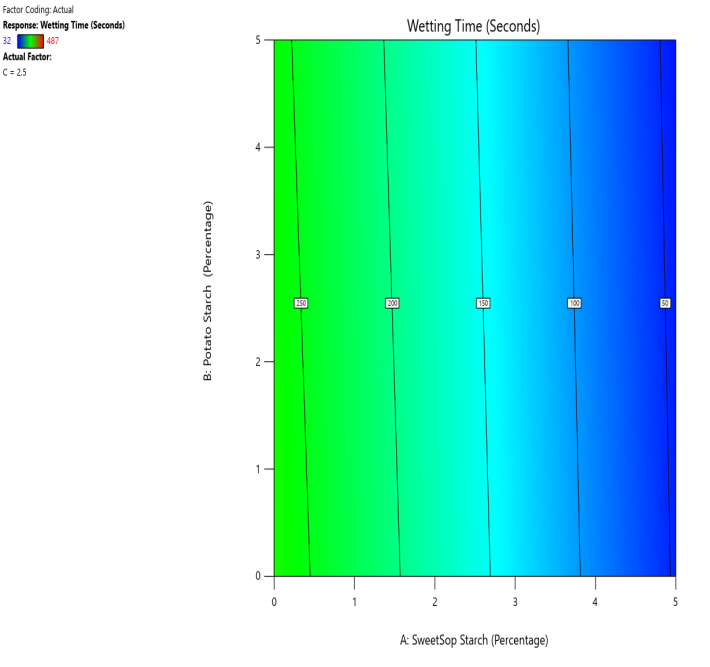
Fig. 13: The contour and response (3D surface graph) demonstrate the effect of SSS and potato starch on the WT
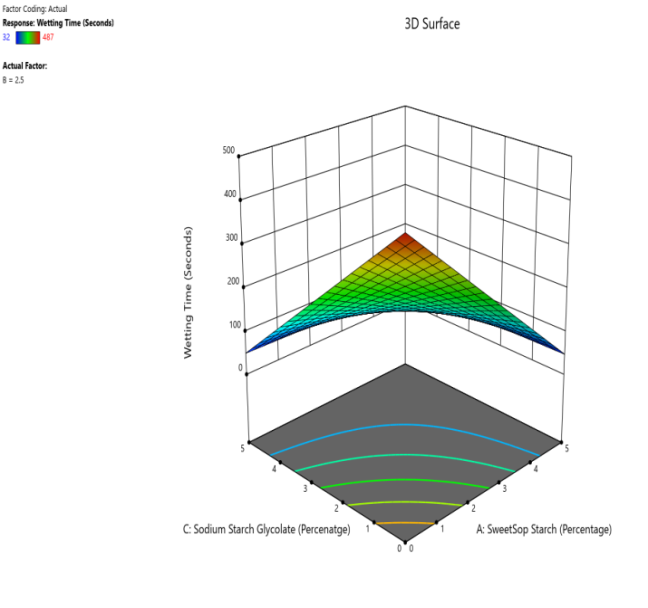

Fig. 14: The contour and response (3D surface graph) demonstrate the effect of SSS and SSG on the WT
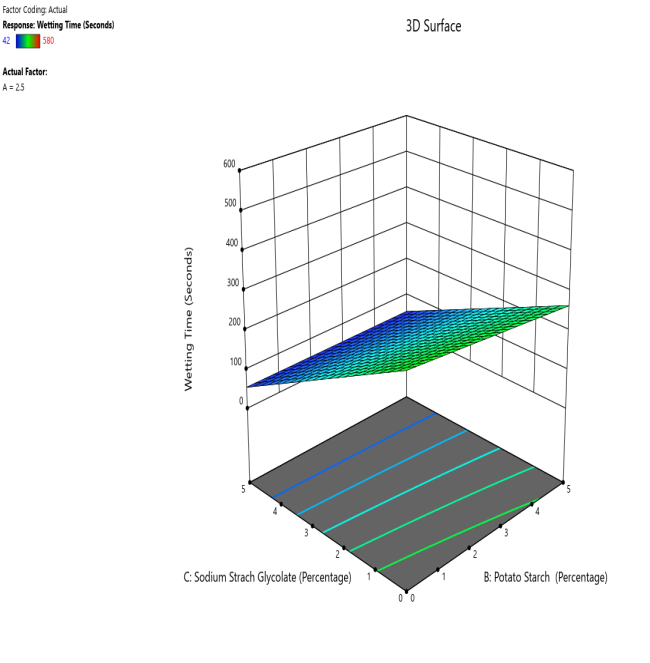
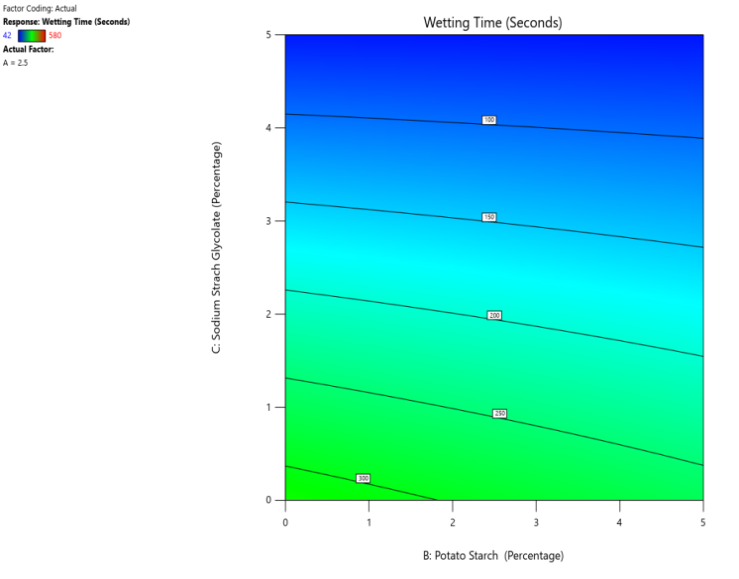
Fig. 15: The contour and response (3D surface graph) demonstrate the effect of potato starch and SSG on the WT

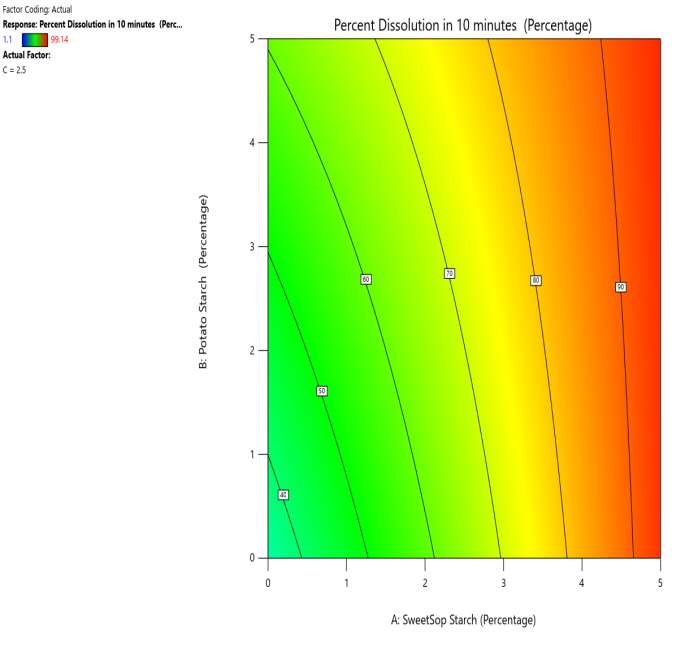
Fig. 16: The contour and response (3D surface graph) demonstrate the effect of SSS and potato starch on the PD in 10 min


Fig. 17: The contour and response (3D surface graph) demonstrate the effect of SSS and SSG on the PD in 10 min
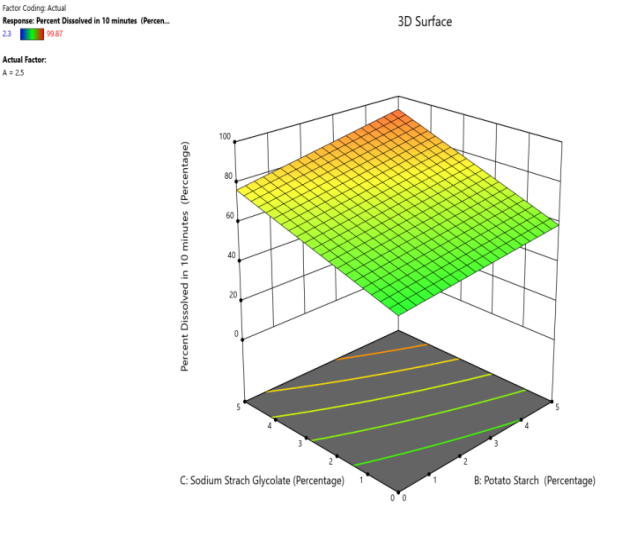

Fig. 18: The contour and response (3D surface graph) demonstrate the effect of potato starch and SSG on the PD in 10 min)

Fig. 19: Plasma concentration profile of CRV pure drug and optimized formulation F2
Table 8: Pharmacokinetic parameters of CRV pure drug, optimized formula F2 Employing SSS
| Pharmacokinetic parameter | Pure CRV | Optimized CRVFDTs employing SSS |
| Ka (h-1) | 0.924±0.55 | 1.408±0.5 |
| Kel (h-1) | 0.101±0.25 | 0.108±0.30 |
| AUC0-∞ (ng. h/ml) | 2752.8±0.35 | 4848.12±0.42 |
| Cmax (ng/ml) | 290.12±0.30 | 730.47±0.50 |
| Tmax (h) | 3 | 1 |
| RBA (%) | ---- | 176.11 |
(Mean ±SD, n=3)
Stability studies
For 6 mo, the FDT formulations' physical characteristics, WT, water absorption ratio, DT, hardness, and in vitro drug dissolution were observed. These outcomes show that there was no significant alteration in the characteristics of before and after stability, and the results are shown in fig. 20. Table 9 has proven equivalent to the tablets made by Anusha K et al. [29].
Table 9: Summary of stability studies of optimized formulation
Formulation (F2) |
DC (mg/tab) n±SD |
WT (Sec) n±SD | R (%) n±SD | DT (sec) n±SD | In vitro drug dissolution (%) | Hardness (kg/cm2) n±SD |
| Before stability | 12.45±0.46 | 45.02±0.11 | 81.08±0.02 | 32.14±0.1 | 98.44±1.21 | 3.9±0.001 |
| After stability | 12.45±0.5 | 45.05±0.21 | 81.24±0.20 | 32.04±0.5 | 98.12± 1.53 | 3.9±0.01 |
(Mean ±SD, n=3)

Fig. 20: In vitro dissolution profiles of the optimized formulation in the stability studies
CONCLUSION
The new superdisintegrant, sweetsop starch, had been extracted from the pulp of the sweetsop fruit. It was fine, crystalline, and free-flowing with superdisintegrant characteristics, SSG, and SSS, as novel super disintegrants, were used to make carvedilol FDTs using a direct compression approach with a 23factorial design. The optimal formulation of CRV (CF2) FDTs with 5% SSS had an acceptable DT, maximum dissolution, and enhanced relative bioavailability without affecting the stability of the formulations. Hence, sweetsop starch was recommended to be utilized as a new natural superdisintegrant to enhance the in vitro dissolution, and relative bioavailability of selected poorly soluble drugs.
FUNDING
Nil
ABBREVIATIONS
FDTs-Fast Dissolving Tablets, SSS-Sweet Sop Starch, CRV-Carvedilol, FTIR-Fourier-Transform Infrared Spectroscopy, PS-Potato Starch, SSG-Sodium Starch Glycolate, WT-Wetting Time, R-Water Absorption Ratio, DC-Drug Content, MCC-Microcrystalline cellulose, SI-Swelling Index, PS-Particle Size, BD-Bulk Density, TD-Tapped Density, CI-Compressibility Index, LOD-Loss on Drying, DSC-Differential Scanning Calorimetry, XRD-X-ray Diffractometry, SEM-Scanning Electron Microscope, DT-Disintegration Time, RSP-Response Surface Plot, CP-Contour Plot PD10-Percent Dissolved in ten minutes.
AUTHORS CONTRIBUTIONS
The authors report that this publication is based on the Ph. D. thesis (Chakradhar D), who conducted the preliminary research, collected the data, carried out the work, and produced the entire manuscript. (Santosh Kumar R) was the supervisor, and he revised the text and validated the data for this study.
CONFLICTS OF INTERESTS
All authors have none to declare
REFERENCES
Sahithi M, Santosh Kumar R. Optimization of statistically designed aceclofenac fast dissolving tablets employing starch glutamate as a novel superdisintegrant. Int J Appl Pharm. 2020;12(1):77-88. doi: 10.22159/ijap.2020v12i1.34987.
Ashish P, Harsoliya MS, Pathan JK, Shruti S. A review: formulation of mouth dissolving tablet. Int J Pharm Clin Sci. 2011;1(1):1-8.
Raj, Behin Sundara, IS R, Punitha, Suraj Dube. Formulation and characterization of fast disintegrating tablets of amlodipine using super disintegrants. J App Pharm Sci. 2012;2(8):118-23. doi: 10.7324/JAPS.2012.2819.
Masih A, Kumar A, Singh S, Tiwari AK. Fast dissolving tablets: a review. Int J Curr Pharm Sci. 2017;9(2):8. doi: 10.22159/ijcpr.2017v9i2.17382.
Kumari N, Sharma R. An immediate release tablet of carvedilol with natural super disintegrants fenugreek seed mucilage and synthetic super disintegrants. Asian J Pharm Technol. 2020;10(3):156-64. doi: 10.5958/2231-5713.2020.00027.6.
Prakash Rao AH, Rada SK, Kandukuri S. Optimization of starch crotonate as a novel superdisintegrant in the formulation of fast dissolving tablets through 2³ factorial design. Int J App Pharm. 2021;7:247-56. doi: 10.22159/ijap.2021v13i4.41335.
Pinho LA, Temer AC, Ribeiro C, Sa-Barreto LL, Cunha Filho MS. The popularization of orodispersible tablets in the pharmaceutical market. Infarma. 2018;30(2):77-84. doi: 10.14450/2318-9312.v30.e2.a2018.pp77-84.
Aboud HM, El Komy MH, Ali AA, El Menshawe SF, Abd Elbary A. Development optimization and evaluation of carvedilol loaded solid lipid nanoparticles for intranasal drug delivery. AAPS Pharm Sci Tech. 2016;17(6):1353-65. doi: 10.1208/s12249-015-0440-8, PMID 26743643.
Lionberger RA, Lee SL, Lee L, Raw A, YU LX. Quality by design: concepts for ANDAs. AAPS J. 2008;10(2):268-76. doi: 10.1208/s12248-008-9026-7, PMID 18465252.
Borse LB, Bendale AR, Borse SL, Naphade VD, Jadhav AG. Formulation and evaluation of mouth dissolving tablet rivaroxaban and its validation. Biosci Biotechnol Res Asia. 2022;19(4):943-54. doi: 10.13005/bbra/3043.
DE Los Santos Santos MA, Balois Morales R, Bello Lara JE, Leon Fernandez AE, Jimenez Zurita JO, Bautista Rosales PU. Phytochemical compounds in soursop fruit starches (Annona muricata L.). Rev Bio Cienc. 2023;10. https://doi.org/10.15741/revbio.10.e1502.
Swarnalatha N, Maravajhala V. Formulation in vitro and in vivo evaluation of taste masked oral disintegrating tablets of fexofenadine hydrochloride using semisynthetic and natural superdisintegrants. Int J App Pharm. 2021;13(5):99-108. doi: 10.22159/ijap.2021v13i5.41558.
Singh R, Saxena S, Jain S. Formulation and evaluation of mouth dissolving tablet containing non-steroidal anti-inflammatory drug. Res J Pharm Technol. 2021;14(1):432-6. doi: 10.5958/0974-360X.2021.00078.0.
Kumar I, Chaudhary D, Thakur B, Pandit V. Formulation and evaluation of piroxicam fast dissolving tablets using direct compression and sublimation method. J Drug Delivery Ther. 2020;10(3-s):17-25. doi: 10.22270/jddt.v10i3-s.4063.
Kumari PV, Rao YS. Formulation and evaluation of orodispersible tablets of donepezil hydrochloride. Int J Curr Pharm Sci. 2020;12(4):45-51. doi: 10.22159/ijcpr.2020v12i4.39049.
Gugulothu D, Choudhary SK. Design and in vitro evaluation of floating drug delivery system of glipizide using a combination of natural mucilages and synthetic polymers. Int J Pharm Pharm Sci. 2021;13(7):40-8. doi: 10.22159/ijpps.2021v13i7.41644.
Nirmala D, Vidyavathi M. Preparation and evaluation of fast dissolving tablets of pitavastatin by 3² full factorial design. Int J Appl Pharm. 2020;12(1):108-14. doi: 10.22159/ijap.2020v12i1.35678.
Anusha K, Rada SK. Oral disintegrating tablets: best approach for faster therapeutic action of poorly soluble drugs. Egypt Pharm J. 2021;20(2):105-14. doi: 10.4103/epj.epj_63_20.
Anusha K, Santosh KR. Optimization of starch hyaluronate as a new super disintegrant in the formulation of fast dissolving tablets of nisoldipine. Eur Chem Bull. 2023;12(3):1606-32.
Nagar P, Singh K, Chauhan I, Verma M, Yasir M, Khan A. Orally disintegrating tablets: formulation preparation techniques and evaluation. J Appl Pharm Sci. 2011;4(1):35-45.
Aboud HM, El Komy MH, Ali AA, El Menshawe SF, Abd Elbary A. Development optimization and evaluation of carvedilol loaded solid lipid nanoparticles for intranasal drug delivery. AAPS PharmSciTech. 2016;17(6):1353-65. doi: 10.1208/s12249-015-0440-8, PMID 26743643.
Mazzo DJ. The ICH stability guideline. In: Mazzo DJ, editor. International stability testing. CRC Press; 2020. p. 1-13. doi: 10.1201/9781003076087-1.
Eisa AM, El Megrab NA, El Nahas HM. Formulation and evaluation of fast dissolving tablets of haloperidol solid dispersion. Saudi Pharm J. 2022;30(11):1589-602. doi: 10.1016/j.jsps.2022.09.002, PMID 36465849.
Jaya S, Amala V. Formulation and in vitro evaluation of oral disintegrating tablets of amlodipine besylate. Int J App Pharm. 2019;11(1):49. doi: 10.22159/ijap.2019v11i1.28457.
Dhahir RK, Al Kotaji M. Formulation of orally disintegrating tablets of cinnarizine by using the direct compression method. Int J Appl Pharm. 2019;11(1):117-23. doi: 10.22159/ijap.2019v11i1.29599.
Sharma V, Arora V. Comparison of various natural superdisintegrants in the formulation of fast dissolving carvedilol tablet. Int J Pharm Sci Res. 2012;3(10):3947. doi: 10.13040/IJPSR.0975-8232.3(10).3947-54.
Parfati N, Rani KC, Charles N, Geovany V. Preparation and evaluation of atenolol-β-cyclodextrin orally disintegrating tablets using co-process crospovidone sodium starch glycolate. Int J App Pharm. 2018;10(5):190. doi: 10.22159/ijap.2018v10i5.27982.
Sahoo CK, Mohanty D, Bhaskar J, Ramana DV. Formulation and evaluation of fast dissolving tablets of carvedilol using sodium starch glycolate. Int J Pharm Sci Rev Res. 2018;51(1):35-40.
Kusuma A, Santosh Kumar R. Optimization of fast dissolving tablets of carvedilol using 2³ factorial design. Int J App Pharm. 2024;16(1):98-107. doi: 10.22159/ijap.2024v16i1.49535.