
Int J App Pharm, Vol 17, Issue 6, 2025, 344-353Original Article
MIXED POLYMERIC NANOMICELLES LOADED WITH MECLIZINE HYDROCHLORIDE: PREPARATION AND CHARACTERIZATION
HIND KHALID OMAR*, SHAIMAA NAZAR ABD ALHAMMID
Department of Pharmaceutics, College of Pharmacy, University of Baghdad, Baghdad, Iraq
*Corresponding author: Hind Khalid Omar; *Email: hind.ali2200@copharm.uobaghdad.edu.iq
Received: 06 Jul 2025, Revised and Accepted: 03 Sep 2025
ABSTRACT
Objective: The goal of this study was to make meclizine hydrochloride (MLZ) into nanomicelles using soluplus®® with TPGS or Tween 80 to make MLZ more soluble and easier to absorb when taken by mouth.
Methods: The maximum absorbance wavelength of MLZ was found, and calibration curves were made in both methanol and phosphate buffer pH 6.8 with 0.25% sodium lauryl sulfate (SLS). Nanomicelles were made by directly dissolving soluplus®® with either TPGS or Tween 80. The selected formulations were examined to determine particle size (PS), polydispersity index (PDI), zeta potential, drug content, dissolution rate, drug trapping efficiency, drug loading and in vitro drug release. Fourier-transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), and field emission scanning electron microscopy (FESEM) were used to study the best formulation, F17, further (soluplus®: Tween 80 = 50:25).
Results: The best formula was F17, which had a PZ of 57.75±1.0 nm, a PDI of 0.086±0.006, an EE% of 98.88±0.5%, a zeta potential of+3.90±0.18, a solubility factor of 7.56±0.12 mg/ml, drug content of 98.59%±0.28, drug loading of 24.86±0.11, and a 90 min release rate of 94%. The One-Way ANOVA test showed that there were statistically significant impacts on EE% (p<0.05), but not on PS (p>0.05). PDI did not exhibit any significant results (p>0.05). Stability tests of the best formula indicated that the PS and EE% stayed pretty consistent for at least three months.
Conclusion: Nanomicelles are effective carriers for enhancing the solubility of MLZ in the mouth, thereby increasing its availability to the body. Nanomicelles have a flawless dissolving profile, which makes them an attractive option for use in pharmaceuticals.
Keywords: Meclizine hydrochloride, TPGS, Soluplus®, Nanomicelles, Tween 80, Drug delivery
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijap.2025v17i6.55932 Journal homepage: https://innovareacademics.in/journals/index.php/ijap
INTRODUCTION
Micelles are colloidal structures that are amphiphilic and contain particles ranging from 5 to 100 nanometers in size. The molecules forming them have two halves, each with different affinities for water. These amphiphilic molecules join together at specific temperatures and concentrations. The critical micelle concentration (CMC) is the point where micelles begin to form and aggregation occurs. The critical micellar temperature (CMT) is the temperature at which micellar molecules start to stick together, similar to the critical micelle temperature. Below this temperature and concentration, only monomers are present, and no micelles form. The term "aggregation number" in relation to micelles refers to the number of monomer molecules that come together to form a micelle. Hydrophobic parts of the micelles are shielded from water, causing amphiphilic molecules to group and form micelles. This spontaneous process occurs because hydrogen bonds form in water, lowering the system's Gibbs free energy formula [1]. soluplus®® is an amphiphilic graft copolymer made of polyvinyl caprolactam, polyvinyl acetate, and polyethylene glycol. It is commonly used to enhance the solubility of poorly water-soluble drugs due to its self-assembling ability, biocompatibility, and low critical micelle concentration [2]. When combined with either D-α-tocopheryl polyethylene glycol 1000 succinate (TPGS) or Tween 80, Soluplus®® forms more stable nanomicelles that effectively encapsulate and transport drugs. TPGS, a water-soluble form of vitamin E, acts as a solubilizer and P-glycoprotein (P-gp) inhibitor, helping drugs pass through mucous membranes and reducing drug efflux, thereby boosting oral bioavailability [3]. Tween 80, a nonionic surfactant from polyethoxylated sorbitan and oleic acid, facilitates micelle formation and enhances drug movement across the gastrointestinal membrane through better dispersion and wetting. These polymers were chosen because they synergize to produce effective nanomicelles, are safe, and address solubility and permeability challenges typical of BCS class II drugs [4]. MLZ(MLZ) is a first-generation piperazine-based antihistamine with a half-life of six hours. It functions as an antagonist at H1 receptors and exhibits anticholinergic, CNS depressant, and local anesthetic effects. It is primarily used as an antiemetic and antivertigo agent to prevent and treat nausea, vomiting, and dizziness caused by motion sickness. Although the exact mechanism of how it alleviates nausea and dizziness is not fully understood, its central anticholinergic properties are partly responsible [5]. According to the biopharmaceutics classification system, MLZ is class II since it poorly dissolves in water, has a slow absorption rate, and yields only 30%–40% bioavailability when administered orally. It takes about an hour for meclizine to become effective, so taking it an hour before travel can help mitigate motion sickness. Its activity may be delayed due to inadequate solubility. MLZhas poor water solubility (0.261 mg/ml) and dissolves poorly at pH levels above 2.0. Its solubility and wettability are insufficient for use in oral or injectable formulations [6]. This study aimed to enhance the aqueous solubility and oral bioavailability of Meclizine hydrochloride, a poorly water-soluble BCS class II drug, by formulating it into polymeric nanomicelles using Soluplus®® in combination with either TPGS or Tween 80.
MATERIALS AND METHODS
Material
MLZ was purchased from Macline, China, with 98.5% purity. Solupus from BASF in Germany, with 98% purity. TPGs from Baoji Guokang Bio-Technology Co., Ltd., China, with 98% purity, and Tween 80 from Biotech Co. Methanol was obtained from HyperChem, China, with 98% purity.
Methods
Methods of MLZ preformulation
UV-wavelength (lambda max)
UV spectrophotometry (Shimadzu, Japan) is used to find the wavelength of maximum absorbance of Meclizine in the final solution. We do this by comparing it to methanol as a blank solvent in the range of 200 nm to 400 nm.
Preparation of the calibration curve of MLZ in methanol
A standard stock solution is prepared in methanol containing 50µg/ml of Meclizine Hydrochloride by dissolving 5 mg of the drug once in a 100 ml volumetric flask of methanol. The absorbance was measured using UV spectrophotometry at lambda max of MLZ in methanol [7].
Preparation of the calibration curve of MLZ in phosphate buffer (pH 6.8)
A standard stock solution is made by dissolving 5 mg of Meclizine Hydrochloride in a 100 ml volumetric flask of phosphate buffer with 0.25% SLS, which gives a concentration of 50µg/ml. SLS was added to make MLZ more soluble and to improve linearity. The absorbance was measured using UV spectrophotometry at lambda max of MLZ in phosphate buffer 6.8 [7].
Methods of the prepared nanomicelles characterization
Preparation of polymeric nanomicelles Polymeric nanomicelles
The direct dissolving approach is used to make polymeric nanomicelles Polymeric nanomicelles of MLZ. In this method, 25 mg of MLZ and different polymers are added to 10 ml of deionized distilled water. The drug-to-polymer ratios (1:1 to 1:4) were chosen based on preliminary solubility tests and previous research that showed these ratios worked well for increasing the solubility and encapsulation of drugs that don't dissolve well in water when mixed with Soluplus® and surfactants like TPGS and Tween 80 [7, 8], as shown in table 1.
The medicine is added to the hydrophobic core of the nanomicelles by agitating them at a high speed of 1500 rpm for 45 min at a temperature of 37.0±0.5 ᵒC. The solution is not filtered after agitation. There are 20 constructed formulas, from F1 to F20, as given in table 1 [8-10].
Table 1: Composition of MLZ polymeric nanomicelles formulation
| Formula code | Drug (mg) | Soluplus®(mg) | TPGs (mg) | TWEEN80 (mg) | Deionized water (ml) |
| F1 | 25 | 25 | 10 | ||
| F2 | 25 | 50 | 10 | ||
| F3 | 25 | 75 | 10 | ||
| F4 | 25 | 100 | 10 | ||
| F5 | 25 | 25 | 25 | 10 | |
| F6 | 25 | 25 | 50 | 10 | |
| F7 | 25 | 25 | 75 | 10 | |
| F8 | 25 | 25 | 100 | 10 | |
| F9 | 25 | 50 | 25 | 10 | |
| F10 | 25 | 50 | 50 | 10 | |
| F11 | 25 | 50 | 75 | 10 | |
| F12 | 25 | 50 | 100 | 10 | |
| F13 | 25 | 25 | 25 | 10 | |
| F14 | 25 | 25 | 50 | 10 | |
| F15 | 25 | 25 | 75 | 10 | |
| F16 | 25 | 25 | 100 | 10 | |
| F17 | 25 | 50 | 25 | 10 | |
| F18 | 25 | 50 | 50 | 10 | |
| F19 | 25 | 50 | 75 | 10 | |
| F20 | 25 | 50 | 100 | 10 |
Abbreviations: TPGS – D-α-Tocopheryl polyethylene glycol 1000 succinate; SLS – Sodium lauryl sulfate; MLZ – Meclizine hydrochloride; PBS – Phosphate buffer saline.
Particle size (PS) and polydisperse index (PDI)
The Malvern Zetasizer in the UK used dynamic light scattering (DLS) at 25 °C to find out the PS and PDI [8]. We examined undiluted samples, aware that there was a risk of repeated dispersion.
Entrapment efficiency (EE)
The indirect method is used to determine the entrapment efficiency (EE). The nanomicelle formulation was placed in a plastic conical (Amicon) tube with a molecular weight cutoff (MWCO) of 10 kDa. The tube was then spun in a centrifuge at 4000 rpm for 20 min at 25 °C. A UV spectrophotometer was used to measure the amount of unencapsulated drug. This amount was subtracted from the total drug amount (25 mg) to calculate the encapsulated drug. The methanol calibration curve is used to determine how much MLZ [11, 12] is present.
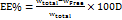 ……Eq1…. [12].
……Eq1…. [12].
Where:
Wtotal: total drug added to the formula
Wfree: amount of free (unentrapped) drug measured in the filtrate
Wencapsulated: entrapped drug (calculated by difference between Wtotal and Wfree)
Drug content
Drug content is measured by dissolving 1 ml of the Polymeric nanomicelles formula in 9 ml of methanol and then using a UV light spectrophotometer.
Drug content% ……Eq2…. [13].
……Eq2…. [13].
Where:
Wmeasured: the actual drug amount quantified from the formulation (after dilution)
Wtheoritical: the calculated amount of drug expected in the same volume
Drug loading (DL)
DL was experimentally evaluated using the indirect ultrafiltration method. 3 ml of the nanomicelle dispersion was placed into Amicon Ultra-4 centrifugal filter tubes (MWCO 10 kDa) and spun at 4000 rpm for 20 min at 25 °C. We collected the filtrate containing the free medicine and analyzed it using a spectrophotometer at 230 nm. To find out how much drug was trapped, we took the entire amount that was added and subtracted the free amount. Using Equation 3 [11, 12], the percentage of DL was then found.
DL= …Eq3. [12]
…Eq3. [12]
Where:
Wtotal: total drug added to the formula
Wfree: amount of free (unentrapped) drug measured in the filtrate
Wdrug: represents the weight of Meclizine hydrochloride (25 mg)
Wexcipients: is the combined weight of Soluplus®, TPGS or Tween 80 used in the nanomicelle formulation.
Measurement of zeta potential
The Malvern Zeta Sizer, which is based in the UK, was used to measure the zeta potential of the sample. We measured zeta potential three times and published the data as the mean±SD. A capillary zeta cell received a one-milliliter dose of the chosen MLZ Nano micelles formulation, and the zeta potential value was recorded [14].
Saturated solubility and solubility factor
Saturated solubility and solubility factor: We tested the saturation solubility of MLZ in phosphate buffer at 25 °C and a pH of 6.8. We used different types and amounts of polymers and shook the water bath for 48 h at 50 rpm. After that, the samples were spun in a centrifuge at 4000 rpm for 25 min with an Amicon filter 10 kDa to separate the undissolved MLZ. Using a UV spectrophotometer, the filtrate was collected and mixed with methanol to the right amount to measure how much MLZ had dissolved. After that, equation 3 [8, 15] was used to find the solubility factor (SF).
SF= …. Eq4… [15]
…. Eq4… [15]
In vitro release study
In vitro release study. The in vitro release of MLZ from micelle formulations, as well as pure MLZ powder, was examined using a dialysis membrane with a molecular weight cutoff of 8-14 kDa to retain the nanomicelles while allowing the free drug to diffuse. Sink conditions were confirmed by ensuring that the maximum drug concentration achieved during solubility studies was below one-third of the saturated solubility of Meclizine hydrochloride in the tested medium. Dialysis was conducted using 10 ml of sample inside the bag and 500 ml of phosphate buffer pH 6.8 with 0.25% SLS outside the bag as the receptor dissolution medium. Based on the solubility of Meclizine hydrochloride in pH 6.8 (≈ 0.05 mg/ml), a 500 ml receptor volume ensures sink conditions for a 25 mg of MLZ. The solubility of MLZ in pH 6.8 phosphate buffer was determined to be 0.434 mg/ml. Sink conditions are defined as a medium capacity at least three times the drug dose. For a 25 mg dose in 500 ml, the minimum solubility required is 0.15 mg/ml (3 × 25 mg ÷ 500 ml). The medium's drug capacity (500 ml × 0.434 mg/ml) equals 217 mg, which exceeds the 75 mg needed, confirming that sink conditions were maintained during the dissolution study. The USP dissolve equipment (RIGGTEK, Dissilio TX8, Germany) was set to 37 °C and stirred at 100 rpm. Every so often, 5 ml of the external medium was taken out and replaced with new dissolution medium. A UV spectrophotometer measures the absorbance of samples of the nanomicelles formula and pure MLZ that have been taken out. These are then plotted to get the dissolution curves [15].
Fourier transform infrared (FTIR) spectroscopy
FTIR is used to check the purity of drugs and see if they work well with other substances. We performed FTIR analysis using both the pure MLZ and the KBr pellet techniques to obtain the FTIR spectra. A sample of about 2 mg (in a 1:1 drug-to-polymer ratio) was mixed with 200 mg of dry KBr, crushed up very finely, and pressed into pellets under vacuum. The pellets were then examined in transmission mode throughout a wavenumber range from 4000 [17].
Field emission scanning electron microscope (FESEM)
FESEM was used to examine the morphology of the MLZ nano-micelles formulation (FESEM S-4160, Hitachi, Japan). Proper sample preparation is crucial for obtaining high-quality FESEM images. A drop of the nanomicelle dispersion was placed on a clean aluminum stub and allowed to dry in a dust-free chamber at room temperature for 24 h. Before imaging, the sample was coated with conductive platinum to enhance conductivity. The slow drying process helps prevent particle aggregation. This preparation involves carefully handling the nano-micelles to avoid contamination or damage, fixing them to preserve the formulation's structure, attaching the stub with conductive carbon tape, and coating it to reduce charging and improve image clarity. Finally, imaging is performed once the sample is prepared for FESEM analysis.
Powder X-ray diffraction (PXRD)
PXRD was employed to analyze the crystalline structure of the lyophilized polymeric nanomicelles powder. Using a Shimadzu (Japan) instrument (XRD-6000) with a Cu Kα filter, measurements were conducted at 40 kV voltage and 30 mA current. The scan covered a 2θ range from 2° to 80° [19].
Data statistical analysis
Statistical analysis was conducted using one-way ANOVA to evaluate how different formulation parameters-such as surfactant type and ratio, polymer combinations, and preparation methods-affect particle size, polydispersity index (PDI), drug content, entrapment efficiency (EE%), and in vitro release profiles. When significant differences (p<0.05) were identified, Tukey’s post hoc test was used to compare pairs of formulations. All analyses were performed with GraphPad Prism version [X], and the results are presented as mean±standard deviation (n = 3) [19].
RESULTS
Results of MLZ preformulation
UV-wavelength lambda max (λmax) and calibration curve
The λ max of Meclizine Hydrochloride was determined to be 229.5 nm in methanol and 230 nm in phosphate buffer (pH 6.8) (0.25% SLS) using UV spectroscopy, which is consistent with references [6], as shown in fig. 3 and fig. 4. The calibration curve for MLZ in phosphate buffer pH 6.8 containing 0.25% SLS was linear over the concentration range of 0–24 µg/ml, with a correlation coefficient (R²) of 0.9995. The linear regression equation was:
y = 0.0375x+0.0014.
The limits of detection (LOD) and quantification (LOQ) were calculated according to ICH guidelines using the equations:
LOD = 3.3 × (σ/S), LOQ = 10 × (σ/S),
Where σ = 0.0014 and S = 0.0375.
The LOD and LOQ were found to be 0.123 µg/ml and 0.373 µg/ml, respectively, as shown in fig. 1.
The calibration curve for Meclizine hydrochloride in phosphate buffer pH 6.8 containing 0.25% SLS was linear over the concentration range of 0–24 µg/ml with a correlation coefficient (R²) of 0.9995. The linear regression equation was:
y = 0.0375x+0.0014.
The limits of detection (LOD) and quantification (LOQ) were calculated according to ICH guidelines using the equations:
LOD = 3.3 × (σ/S), LOQ = 10 × (σ/S),
Where σ = 0.0014 and S = 0.0375.
The LOD and LOQ were found to be 0.123 µg/ml and 0.373 µg/ml, respectively.
As shown in fig. 2 [20].
Results of characterization of the prepared nanomicelles
Particle size (PS) and polydisperse index (PDI)
The particle size of the formulations (F1-F20) ranged from 57 nm to 71 nm. The impact of increasing Soluplus® concentration on the nanomicelle particle size is shown in F1 to F4, as listed in table 2. The effect of changing TPGS concentration while maintaining Soluplus® at 25 mg is illustrated in F5 to F8. Similarly, F9 to F12 demonstrate the effect of TPGS concentration variation with Soluplus® fixed at 50 mg, also shown in table 2, indicating a slight increase in particle size.
Similarly, for Tween 80 added at different concentrations in (F13-F20) with Soluplus® fixed at 25 mg (F13-F16) and 50 mg (F17-F20), the particle size (PS) slightly increased, as shown in table 2. Significant differences (P<0.05) were observed when comparing F1 and F17, indicating a significant change in PS. The PDI values ranged from 0.04 to 0.19, reflecting particle homogeneity.
Entrapment efficiency (EE)
Nanomiscelles made from Soluplus® alone exhibit low entrapment efficiency (54%-64%). When TPGS is added with a fixed Soluplus® concentration of 25 mg, the EE% improves to a good range of 60-76%. An excellent EE% of 75-99% is achieved when TPGS is added with a fixed Soluplus® amount of 50 mg, indicating significant differences (p<0.05). Soluplus®-tween 80 nanomicelles were better than soluplus®-TPGS in EE% (90-98%), P values<0.05.

Fig. 1: Calibration curve of MLZ in methanol
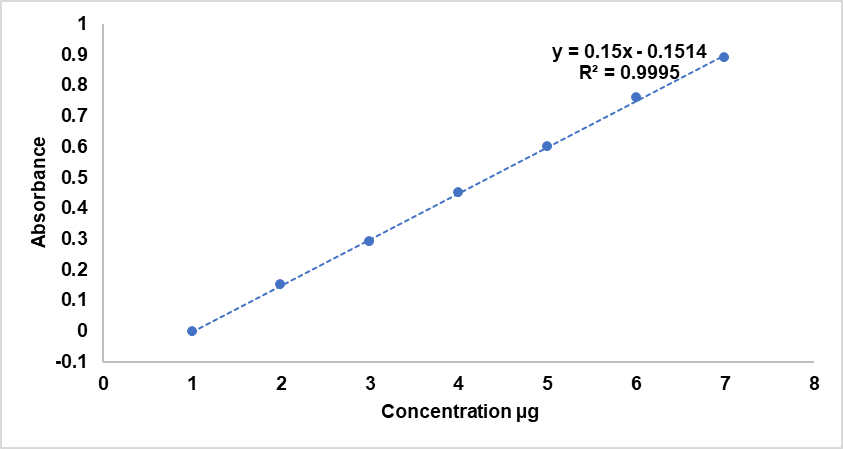
Fig. 2: Calibration curve of MLZ in phosphate buffer (pH 6.8) with 0.025 mg SLS

Fig. 3: Lambda max of MLZ in methanol

Fig. 4: Lambda max of MLZ in phosphate buffer 6.8 with 0.25% SLS
Table 2: Particle size (PS), PDI, and entrapment efficiency (EE %) of MLZ nanomiscelles formulas
| Formula code | Soluplus®(mg) | TPGs (mg) | TWEEN 80 (mg) | PS(nm) | PDI | EE% |
| F1 | 25 | 66.22±1.5 | 0.160±0.010 | 54.80±2.1 | ||
| F2 | 50 | 60.38±1.2 | 0.059±0.006 | 55.71±1.9 | ||
| F3 | 75 | 59.07±1.0 | 0.058±0.005 | 62.59±2.0 | ||
| F4 | 100 | 58.74±1.1 | 0.065±0.006 | 64.98±1.8 | ||
| F5 | 25 | 25 | 55.56±0.9 | 0.174±0.009 | 76.00±2.2 | |
| F6 | 25 | 50 | 62.69±1.3 | 0.079±0.008 | 75.29±1.7 | |
| F7 | 25 | 75 | 62.88±1.4 | 0.213±0.012 | 64.10±1.6 | |
| F8 | 25 | 100 | 66.52±1.6 | 0.280±0.014 | 60.47±1.5 | |
| F9 | 50 | 25 | 57.23±1.0 | 0.099±0.006 | 99.23±0.5 | |
| F10 | 50 | 50 | 58.80±1.2 | 0.160±0.010 | 98.95±0.4 | |
| F11 | 50 | 75 | 63.19±1.4 | 0.210±0.011 | 83.18±1.3 | |
| F12 | 50 | 100 | 67.78±1.5 | 0.244±0.013 | 75.29±1.4 | |
| F13 | 25 | 25 | 60.46±1.3 | 0.195±0.010 | 90.00±1.2 | |
| F14 | 25 | 50 | 61.10±1.2 | 0.105±0.007 | 81.57±1.1 | |
| F15 | 25 | 75 | 68.06±1.6 | 0.470±0.020 | 81.91±1.0 | |
| F16 | 25 | 100 | 71.20±1.8 | 0.226±0.012 | 67.69±1.3 | |
| F17 | 50 | 25 | 57.75±1.0 | 0.086±0.006 | 98.88±0.5 | |
| F18 | 50 | 50 | 59.84±1.2 | 0.139±0.008 | 97.99±0.6 | |
| F19 | 50 | 75 | 63.64±1.3 | 0.199±0.010 | 90.22±0.9 | |
| F20 | 50 | 100 | 66.08±1.5 | 0.140±0.007 | 81.10±1.0 |
Data are given as mean±SD, n = 3
Selection of the optimized formula of MLZ nano micelles
The formulas with the smallest particle size, PDI, and high EE%-specifically F9, F10, F17, and F18-were tested in triplicate. The best formulas were then chosen for further analysis, including drug loading, drug content, zeta potential, solubility factor, and in vitro release, to identify the most optimal formulation.
Drug loading and drug content
Drug content and the drug loading percentage (DL%) were also calculated for the formulations (F9, F10, F17, F18) as shown in table 3.
Zeta potential measurement
The zeta potential of the prepared formulations was measured for (F9, F10, F17, F18) as shown in table 3.
Saturated solubility and solubility factor
MLZ solubility is 0.43 mg/ml in Pb 6.8, and the solubility of the prepared Nanomicelles was calculated for (F9, F10, F17, F18) as shown in table 3.
In vitro release
Fig. 5 and fig. 6 represent the release of the formulas with the least particle size and PDI, and high EE% were (F9, F10, F17, F18).
The optimal formula characterization
Fourier transform infrared (FTIR) spectroscopy
The FTIR spectra of MLZ in fig. 7-a show the prominent characteristic peaks of pure MLZ. The FTIR spectra of Soluplus®® in fig. 7-b, the spectrum corresponding to Tween 80 in fig. 7-c, and fig. 7-d indicate the FTIR analysis of the chosen formula.
Table 3: Zeta potential, solubility factor (SF), drug loading (DL) and drug content of MLZ HCL nanomiscelles selected formulas
| Formula code | Soluplus® (mg) | TPGs (mg) | TWEEN 80 (mg) | Zeta potential | SF (mg/ml) | DL | Drug content |
| Pure MLZ, PB 6.8 | 0.434 mg/ml | ||||||
| F9 | 50 | 25 | +7.85±0.15 | 6.50±0.10 | 24.61±0.12 | 99.65±0.35 | |
| F10 | 50 | 50 | +5.68±0.20 | 7.13±0.08 | 24.83±0.09 | 97.24±0.41 | |
| F17 | 50 | 25 | +3.90±0.18 | 7.56±0.12 | 24.86±0.11 | 98.59±0.28 | |
| F18 | 50 | 50 | +9.70±0.14 | 7.73±0.09 | 24.52±0.14 | 98.23±0.33 |
Data are given as mean±SD, n = 3

Fig. 5: The release profile of F9 and F10 (SLP/TPGS) and pure drug powder in phosphate buffer (pH 6.8) with 0.025 SLS at 37 °C (mean±SD, n = 3)
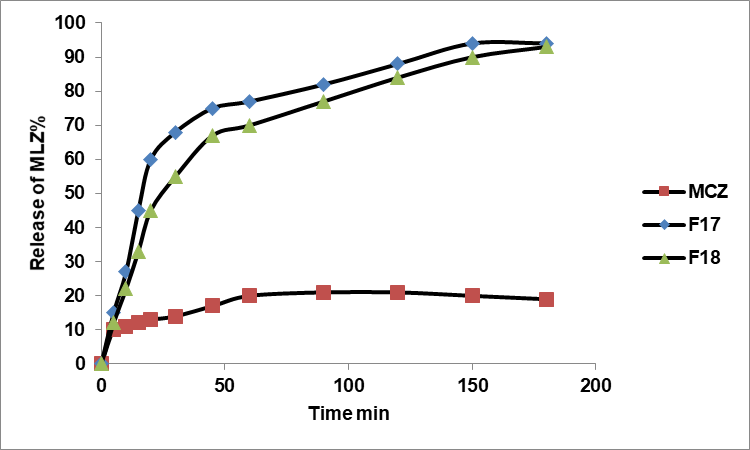
Fig. 6: The release profile of F9and F10 (SLP/Tween 80) and pure drug powder in phosphate buffer (pH 6.8) with 0.025 sls at 37 °C (mean±SD, n = 3)
Fig. 7: FTIR spectra of a. MLZ, b. Soluplus®, c. Tween 80, and d. the optimized nanomicrocapsule formulation F17 are presented. Notable peaks of MLZ-such as 3394.72 cm-1 (secondary amine N-H stretching), 3047.53 cm-1 (aromatic C–H stretching), several sharp bands between 1,600 cm-1 and 1,400 cm-1 (aromatic C=C stretching), 1276.88 cm-1 (C–N stretching), and 698.23 cm-1 (C–Cl stretching)-are significantly diminished or absent in the F17 spectrum. This indicates that MLZ was successfully encapsulated in the nanomicelles without undergoing chemical interaction

Fig. 8: 1. FESEM of MLZ showing is crystalline nature 2. FESEM of F17, illustrates spherical particles measuring 25.81 nm, 31.94 nm, 36.99 and 48.87 nm

Fig. 9: PXRD diffractogram of MLZ PXRD diffractogram of MLZ F17
Field emission scanning electron microscopy (FESEM)
Fig. 8 illustrates spherical particles measuring in the nanometer, range 25.81 nm to 48.87 nm.
Powder x‑ray diffraction (PXRD)
The PXRD pattern of pure MLZ (fig. 9-A) shows several sharp diffraction peaks at 2θ values around 8.9°, 15.8°, 18.9°, 21.6°, 24.3°, and 28.4°, indicating its crystalline nature. In contrast, the PXRD pattern of the nanomicelle-loaded film (fig. 9-B) displays a broad halo with no clear peaks. MLZ.
DISCUSSION
The PS of nanomicelles using soluplus® alone as the polymer for formulations (F1-F4), prepared at ratios of 1:1 to 1:4, ranged from 66.22 nm to 58.7 nm, as shown in table 2. Increasing the soluplus® concentration leads to more nanomicelles forming and a decrease in PS (P values<0.05). This occurs because higher soluplus® levels enhance micelle stabilization and reduce interfacial tension, preventing aggregation. Additionally, this promotes the formation of more numerous, smaller micelles with improved drug distribution [21].
The effect of changing TPGS concentration while keeping Soluplus® fixed at 25 mg is shown in (F5-F8) in table 2. It indicates an increase in PZ from 55.5 nm to 66.5 nm (P values<0.05). When Soluplus® is fixed at 50 mg (F9-F12), increasing TPGS from 25 mg to 100 mg raises PZ from 57.2 nm to 67.7 nm (P values<0.05), likely due to the bulkiness of TPGS molecules and the swelling properties of TPGS's hydrophilic part. Similarly, with Tween 80 added at different concentrations (F13-F16) while Soluplus® remains at 25 mg, the PS slightly increases from 60.4 nm to 71.2 nm, as shown in table 2. When Soluplus® is fixed at 50 mg (F17-F20), PS increases from 57.7 nm to 66.0 nm, attributed to the same reasons.
The limited variation in nanomicelle particle size, despite changing the ratios of Soluplus®, TPGS, and Tween 80, can be explained by several formulation and physicochemical factors. First, micelles formed from amphiphilic copolymers tend to reach a PSplateau beyond a certain polymer or surfactant concentration. This plateau is linked to the critical micelle concentration (CMC), where further increases in excipient levels do not significantly alter micelle size due to thermodynamic stabilization of the aggregates [23]. After micellization reaches equilibrium, extra copolymer or surfactant molecules usually stay in the bulk solution rather than incorporating into existing micelles [24].
Second, the opposing effects of the components might also contribute. Soluplus® forms relatively compact micelles because of its balanced hydrophilic-lipophilic structure, while TPGS and Tween 80 are bulkier surfactants that can cause slight micelle expansion through steric repulsion or interfacial swelling. When combined, these effects may cancel each other out, resulting in an overall neutral influence on PS [22].
The PS difference between formulation F1 (66.22±1.52 nm) and F17 (57.75±1.28 nm) was statistically tested with Tukey’s post hoc analysis. Results showed a significant difference (p<0.05), suggesting that the polymer/surfactant composition in F17 led to smaller and more uniform micelles. This size decrease likely results from improved micelle formation dynamics due to Tween 80. It is known that a PDI above 0.7 indicates an excessively broad PS distribution. The PDI values obtained-ranging from 0.04 to 0.19-demonstrate particle homogeneity, with 0.19 indicating a relatively narrow size distribution and high uniformity. This colloidal stability can be ascribed to the critical micelle concentration (CMC) of Soluplus® and the steric stabilization from its polyethylene glycol (PEG) chains. These PEG segments form a hydrated shell around the micelles, reducing aggregation and maintaining dispersion stability even after dilution.
Entrapment Efficiency (EE %) mainly depends on how effectively pharmaceuticals are encapsulated within the micellar core, which largely relies on their affinity and compatibility. When the drug has a greater affinity and compatibility with the micellar core, encapsulation efficiency increases, and vice versa [24]. Soluplus®-based nanomicelles showed an entrapment efficiency between 54% and 64% (P<0.05), as seen in table 2, indicating a moderate capacity for drug encapsulation. This points to effective interaction between the drug and polymer, though further optimization could improve drug retention in the micellar core [23]. The entrapment efficiency (EE) of Soluplus®-TPGs mixed nanomicelles is notably high, thanks to TPGs enhancing the stability and encapsulation of MLZ due to their surfactant and emulsifying properties [25]. As shown in (table 2), fixing soluplus® at 25 mg, the concentrations of TPGs (F5_F8) and Tween80 (F13_F16) significantly affected the EE% (P<0.05). When 25 mg of Soluplus® was used, EE% increased to 76% in F5 (Soluplus®: TPGs, 2:1) and to 90% in F13 (Soluplus®: Tween80, 2:1), both significantly better than the EE% in F1 (Soluplus®, 1) (P<0.05). The highest EE% was achieved when the soluplus® amount was doubled to 50 mg along with 25 mg of TPGs or Tween80: EE% reached 99.2% in F9 (Soluplus®: TPGs, 2:1) and 98.9% in F10 (Soluplus®: TPGs, 2:2). Similar increases were observed in F17 (Soluplus®: Tween80, 2:1) at 98.8% and in F18 (Soluplus®: Tween80, 2:1) at 97.9%, as shown in (table 2).
At higher surfactant ratios of TPGS or Tween 80, a decline in entrapment efficiency was observed. This could be due to saturation of nanomicelles, where excess surfactant cannot accommodate additional drug molecules, or it may facilitate drug leakage through the formation of smaller or mixed micelles with reduced core volume. Excess surfactant (TPGS or Tween 80) might not increase the number of micelles but instead generate mixed micelles or aggregates that poorly encapsulate the drug, leading to free drug leakage into the aqueous phase [26, 27].
Moreover, formulations with Soluplus®®-Tween 80 (F13–F20) consistently exhibited higher EE% than those with Soluplus®-TPGS (F1–F12). This difference is likely due to the hydrophilic–lipophilic balance (HLB) of the surfactants. Tween 80, with a higher HLB (~15.0) compared to TPGS (~13.2), promotes the formation of more hydrophilic micelles with greater solubilization capacity for hydrophobic drugs like Meclizine HCl. The higher HLB value also supports closer packing of micelles, which enhances drug encapsulation efficiency.
The drug content in the prepared nanomicelles (F9, F10, F17, F18) averaged 98% and the DL averaged 24.7, which is remarkably close to the theoretical drug content of 25 mg, demonstrating excellent drug incorporation within the micellar system, as shown in table 3. This high percentage indicates the polymer matrix's strong solubilizing ability and suggests minimal drug loss during formulation.
The positive zeta potential of (F9, F10, F17, F18), as shown in table 3, prevents particle aggregation because particles repel each other due to their surface charge. Generally, it is known that zeta potential indicates particle stability; thus, it is assumed that the prepared particles are relatively stable. This stability is likely due to the non-ionic nature of the polymers used in the formulation [28, 29]. MLZ solubility is 0.46 mg/ml in Pb 6.8, but its solubility in (F9, F10, F17, F18) shows a highly significant increase as shown in table 3.
The in vitro release of the formulations with the smallest particle size, lowest PDI, and highest EE%-specifically F9, F10, F17, and F18-is shown in fig. 5 and fig. 6. Fig. 5 presents the release profiles of F9 and F10, both containing Soluplus® and TPGS at ratios of 50:25 and 50:50, respectively. These formulations displayed rapid and enhanced drug release compared to the pure drug. F9 released about 94% of MLZ within 180 min, while F10 achieved approximately 86% in the same period. Conversely, the pure drug had limited release (~22%), reflecting its poor aqueous solubility. The improved release of F9 and F10 is likely due to their nano-sized particles and the solubilizing effects of Soluplus® and TPGS. Soluplus® enhances wettability and drug-polymer interactions, whereas TPGS can inhibit P-glycoprotein, improving membrane permeability. One-way ANOVA confirmed significant differences in release profiles among F9, F10, and pure MLZ (p<0.001). Tukey’s post-hoc test further showed that F9 and F10 differed significantly from molar concentration (p<0.001), with F9 also releasing faster than F10 (p<0.05), highlighting the influence of polymer ratio and micelle stability.
Fig. 6 shows the release profiles of formulations F17 and F18, both containing Soluplus® and Tween 80 at ratios of 50:25 and 50:50, respectively, compared to pure MLZ. F17 achieved over 94% drug release in 90 min, outperforming F18, which released about 85% in the same period. The MLZ powder showed poor dissolution (~22%). The release kinetics of F17 (~94% in 90 min) can be attributed to the moderate lipophilicity of Meclizine (log P = 2.8), which favors partitioning into the micellar core and allows rapid diffusion into the medium once micelles disassemble. The quick release is further aided by micelle instability below the CMC, leading to disintegration and drug release. The notable improvement in F17 likely results from an optimized polymer-to-drug ratio and Tween 80's surfactant effect, which has a higher HLB (15) compared to TPGS (~13), aiding in micelle disassembly and wettability. ANOVA tests showed significant differences (p<0.001). Tukey’s post-hoc analysis revealed F17 was significantly better than F18 and MLZ (p<0.001), and F18 outperformed MLZ (p<0.01), supporting the effectiveness of mixed polymeric micelles in enhancing MLZ solubility and release. Further Tukey’s test confirmed that F17 and F18 had significantly higher release rates than F9 and F10, respectively (p<0.05). The improved performance of Soluplus®®/Tween 80 formulations (F17, F18) is likely due to Tween 80’s higher HLB (~15) compared to TPGS (~13). This higher HLB promotes faster micelle disassembly upon dilution, boosting MLZ release. Additionally, Tween 80’s surfactant properties may more effectively reduce interfacial tension than TPGS, facilitating quicker drug diffusion through the dialysis membrane. The formula F17 is the optimal selected formula (PZ=57.75 nm, PDI=0.086, EE%=98.88%, Zeta potential=+3.9, Solubility factor=7.56 (mg/ml), Drug content =98.5% and 94% release rate in 90 min).
The FTIR spectra of MLZ in (fig. 7-a) display the main characteristic peaks of pure MLZ at 3394.72 cm-1 (secondary amine N-H stretching), 3047.53 cm-1 (aromatic C–H stretching), multiple sharp bands between 1,600 cm-1 and 1,400 cm-1 (aromatic C=C stretching), 1276.88 cm-1 (C–N stretching), and 698.23 cm-1 (C–Cl stretching). FTIR spectra reveal no significant shifts in the characteristic peaks of MLZ, suggesting no chemical interaction or degradation upon formulation. [8, 30, 31]. The FTIR spectra of Soluplus® in (fig. 7-b) show a distinct hydroxyl group peak at 3437 cm-1 (O-H stretching). Peaks at 1741 cm-1 and 1633 cm-1 correspond to the C=O stretching of the ester and tertiary amide groups, respectively [32, 33]. The spectrum corresponding to Tween 80 in (fig. 7-c) indicates a methyl group C-H stretching at 2960 cm-1, an ester group at 1740 cm-1, an alkene group at 1640 cm-1, and an ether group at 1100 cm-1 [34]. The FTIR analysis of the selected formula in (fig. 7-d) shows similar absorption peaks, demonstrating strong compatibility with polymers. This indicates no significant chemical interaction between excipients and the drug, confirming the drug's stability during formulation [35, 6].
FESEM of MLZ (fig. 8-1) shows irregular, crystalline structures with well-defined, rod-like and plate-shaped crystals, characteristic of a highly crystalline compound. Meanwhile, FESEM of F17 (fig. 8-2) revealed that the nanomicelles have a uniform spherical shape with particle sizes from 25 to 48 nm. These sizes are considerably smaller than those measured by DLS, which reported an average hydrodynamic diameter of 57.75 nm for the same sample. This difference arises from the distinct measurement principles and sample conditions: DLS measures the particle's hydrodynamic diameter in suspension, including the core, hydration shell, and any polymer or surfactant layers, while FESEM images dried samples under high vacuum, where the hydration layer is removed, and particles may shrink or collapse during solvent evaporation or preparation [8].
The PXRD pattern of pure MLZ(fig. 9-A) shows several sharp diffraction peaks while, the PXRD pattern of the nanomicelle-loaded film (fig. 9-B) displays a broad halo, confirming that Meclizine has become amorphous after incorporation. This decrease in crystallinity likely plays a key role in enhancing the drug's solubility and dissolution rate, as amorphous forms generally have higher free energy and increased molecular mobility. [37].
CONCLUSION
In this study, MLZ-loaded mixed micelles were formulated using Soluplus® combined with TPGS or Tween80 through a direct dissolution process. These micelles measured less than 100 nm on average, showing high uniformity and effective drug entrapment. Solubility assessments revealed enhanced solubility and better in vitro release of the MLZ. Soluplus®Future studies may focus on optimizing polymer-to-surfactant ratios to further improve stability and DL, assessing the physicochemical stability of the nanomicelles under ICH-recommended storage conditions, and evaluating their in vivo performance. In addition, extending this formulation approach to other lipophilic drugs or incorporating the nanomicelles into advanced dosage forms, such as sublingual films or soft capsules, could broaden the pharmaceutical applicability of this strategy.
FUNDING
Nil
AUTHORS CONTRIBUTIONS
Hind Khalid Omar carried out the experimental work, analyzed and interpreted the data, and wrote the manuscript. Dr. Shaimaa Nazar Abd AL-Hammid supervised the research, helped with the study design and data interpretation, and reviewed the final version of the manuscript.
CONFLICT OF INTERESTS
Declared none
REFERENCES
Bose A, Roy Burman D, Sikdar B, Patra P. Nanomicelles: types properties and applications in drug delivery. IET Nanobiotechnology. 2021 Feb;15(1):19-27. doi: 10.1049/nbt2.12018, PMID 34694727.
Attia MS, Elshahat A, Hamdy A, Fathi AM, Emad Eldin M, Ghazy FE. Soluplus® as a solubilizing excipient for poorly water-soluble drugs: recent advances in formulation strategies and pharmaceutical product features. J Drug Deliv Sci Technol. 2023 Nov;84:104519. doi: 10.1016/j.jddst.2023.104519.
Liu Y, Li L, Sun H, Zhao S, Ma C, Qu Z. TPGS-based nanocarriers for enhancing oral bioavailability: mechanisms and applications. J Control Release. 2023 Jan;350:120-32. doi: 10.1016/j.jconrel.2022.12.030.
Wang H, Sun X, Zhao J, Yang L, Li L, Han X. Surfactant-assisted nanocarriers in oral drug delivery: recent advances and challenges. J Control Release. 2023 Feb;351:90-105. doi: 10.1016/j.jconrel.2022.12.044.
Rao GS, Kumar A, Rani G, Reddy PR, Goud YP. QbD-based Eudragit-coated meclizine HCl immediate and extended release pellet formulations. J Drug Deliv Sci Technol. 2020 Dec;60:102081. doi: 10.1016/j.jddst.2020.102081.
Kalyani V, Rao NR, Prathyusha P. Liquisolid compact of meclizine hydrochloride. Int J Appl Pharm. 2025;17(3):88-94. doi: 10.22159/ijap.2025v17i3.51805.
Patel D, Sawant KK. Soluplus-based polymeric micelles of curcumin: formulation optimization, physicochemical characterization and cytotoxicity studies. Drug Dev Ind Pharm. 2021;47(2):276-87. doi: 10.1080/03639045.2020.1866026.
Sharma D, Maheshwari D, Philip G, Rana R. Formulation and optimization of polymeric micelles for oral delivery of poorly water-soluble drugs: a comparative study of TPGS and Tween 80. J Drug Deliv Sci Technol. 2020 Sep;59:101909. doi: 10.1016/j.jddst.2020.101909.
Sutrisno H, Handayani R, Wibowo D, Dewanti ID, Pratama E, Nugraheni R. Synthesis, characterization and optimization of biodegradable PCL-PEG-PCL triblock copolymeric micelles as nanocarriers for hydrophobic drug solubility enhancer. Int J Curr Pharm Res. 2019;11(4):125-32. doi: 10.22159/ijcpr.2019v11i4.34872.
Malekhosseini S, Rezaie A, Khaledian S, Abdoli M, Zangeneh MM, Hosseini A. Fabrication and characterization of hydrocortisone-loaded dextran-poly lactic-co-glycolic acid micelle. Heliyon. 2020;6(5):e03975. doi: 10.1016/j.heliyon.2020.e03975, PMID 32455174.
Xia HJ, Zhang ZH, Jin X, Hu Q, Chen XY, Jia XB. A novel drug phospholipid complex enriched with micelles: preparation and evaluation in vitro and in vivo. Int J Nanomedicine. 2013;S38584:545-54. doi: 10.2147/IJN.S39526.
Keshari P, Sonar Y, Mahajan H. Curcumin-loaded TPGS micelles for nose to brain drug delivery: in vitro and in vivo studies. Materials Technology. 2019;34(7):423-32. doi: 10.1080/10667857.2019.1575535.
Rao GS, Kumar A, Rani G, Reddy PR, Goud YP. QbD-based Eudragit-coated meclizine HCl immediate and extended release pellet formulations. J Drug Deliv Sci Technol. 2020 Dec;60:102081. doi: 10.1016/j.jddst.2020.102081.
Hamzah ML, Kassab HJ. Formulation and characterization of intranasal drug delivery of frovatriptan-loaded binary ethosomes gel for brain targeting. Nanotechnol Sci Appl. 2024 Jan 16;17:1-19. doi: 10.2147/NSA.S442951, PMID 38249545.
Abdulqader AA, Rajab NA. Preparation and characterization of posaconazole nano-micelles using d-α-tocopheryl polyethylene glycol 1000 succinate (TPGS). Iraqi J Pharm Sci. 2023;32(5):26-32. doi: 10.31351/vol32issSuppl.pp26-32.
Jassem NA, Abd Alhammid SN. Formulation and evaluation of canagliflozin self-nanomicellizing solid dispersion based on rebaudioside a for dissolution and solubility improvement. Iraqi J Pharm Sci. 2024;33 4 Suppl 1:43-56. doi: 10.31351/vol33iss(4SI)pp43-56.
Al Edhari GH, Al Gawhari FJ. Study the effect of formulation variables on preparation of nisoldipine-loaded nano bilosomes. IJPS. 2023;32Suppl:271-82. doi: 10.31351/vol32issSuppl.pp271-282.
Aldabbagh M, Baker F Abdallaha, Nibras Y Abdulla, Ibraheem J Ibraheem. Synthesis, characterization and anticancer activity of chitosan Schiff Base / PEG blend doped with gold and silver nanoparticles in treatment of breast cancer cell line MCF-7. IJPS. 2024;33(2):101-11. doi: 10.31351/vol33iss2pp101-111.
Abbas IK, Rajab NA, Hussein AA. Formulation and in vitro evaluation of darifenacin hydrobromide as buccal films. Iraqi J Pharm Sci. 2019;28(2):83-94. doi: 10.31351/vol28iss2pp83-94.
Kumar Y, Sharma R. UV spectrophotometric method development and validation of meclizine HCl in bulk and pharmaceutical dosage form. Asian J Pharm Clin Res. 2020;13(7):123-6. doi: 10.22159/ajpcr.2020.v13i7.37965.
Al Wiswasi N, Fatima J, Al Gawahri. Brimonidine soluplus nanomicelles: preparation and in vitro evaluation. Iraqi J Pharm Sci. 2025;34(1):246-55. doi: 10.31351/vol34iss1pp246-255.
Sulaiman HT. Soluplus and solutol HS-15 olmesartan medoxomil nanomicelle-based oral fast-dissolving film: in vitro and in vivo characterization. Farmacia. 2024;72(4):794-804. doi: 10.31925/farmacia.2024.4.7.
Ji S, Lin X, Yu E, Dian C, Yan X, Li L. Curcumin loaded mixed micelles: preparation characterization and in vitro antitumor activity. J Nanotechnol. 2018 Mar;2018(1):1-9. doi: 10.1155/2018/9103120.
Ahmed K, Kassab H. Evaluation of nanomicelles loaded with spironolactone. Iraqi J Pharm Sci. 2025;33(3):1130-41.
Ali R, Qamar W, Kalam MA, Binkhathlan Z. Soluplus-TPGS mixed micelles as a delivery system for brigatinib: characterization and in vitro evaluation. ACS Omega. 2024;9(40):41830-40. doi: 10.1021/acsomega.4c06264, PMID 39398132.
Poojar B, Ommurugan B, Adiga S, Thomas H, Sori RK, Maryam G. Methodology used in the study. Asian J Pharm Clin Res. 2017;10(7):1-5. doi: 10.22159/ajpcr.2017.v10i7.17364.
Guembe Michel N, Nguewa P, Gonzalez Gaitano G. Soluplus® based pharmaceutical formulations: recent advances in drug delivery and biomedical applications. Int J Mol Sci. 2025;26(4):1499. doi: 10.3390/ijms26041499, PMID 40003966.
Shakiba E, Khazaei S, Hajialyani M, Astinchap B, Fattahi A. Preparation and in vitro characterization of retinoic acid-loaded poly(ε-caprolactone)-poly(ethylene glycol)-poly(ε-caprolactone) micelles. Res Pharm Sci. 2017;12(6):465-78. doi: 10.4103/1735-5362.217427, PMID 29204175.
Malkawi A, Alrabadi N, Kennedy RA. Dual-acting zeta-potential changing micelles for optimal mucus diffusion and enhanced cellular uptake after oral delivery. Pharmaceutics. 2021;13(7):974. doi: 10.3390/pharmaceutics13070974, PMID 34199091.
Kumar A, Jain A, Yadav PK, Singhai AK. Development of oro-dispersible tablet of meclizine by using different super-disintegrating agents. J Drug Delivery Ther. 2022;12(4):7-14. doi: 10.22270/jddt.v12i4.5413.
Qazi F, Shoaib MH, Yousuf RI, Nasiri MI, Ahmed K, Ahmad M. Lipids bearing extruded spheronized pellets for extended release of poorly soluble antiemetic agent-meclizine HCl. Lipids Health Dis. 2017;16(1):75. doi: 10.1186/s12944-017-0466-x, PMID 28403892.
Alwan RM, Rajab NA. Nanosuspensions of selexipag: formulation characterization and in vitro evaluation. Iraqi J Pharm Sci. 2021;30(1):144-53. doi: 10.31351/vol30iss1pp144-153.
Nandi U, Ajiboye AL, Patel P, Douroumis D, Trivedi V. Preparation of solid dispersions of simvastatin and soluplus using a single-step organic solvent-free supercritical fluid process for the drug solubility and dissolution rate enhancement. Pharmaceuticals (Basel). 2021;14(9):846. doi: 10.3390/ph14090846, PMID 34577546.
Simoes A, Ramos A, Domingues F, Luis A. Pullulan tween 40 emulsified films containing geraniol: production and characterization as potential food packaging materials. Eur Food Res Technol. 2024;250(6):1721-32. doi: 10.1007/s00217-024-04514-y.
Blake D, Gazzara MR, Breuer I, Ferretti M, Lynch KW. Alternative 3′UTR expression induced by T cell activation is regulated in a temporal and signal-dependent manner. Sci Rep. 2024;14(1):10987. doi: 10.1038/s41598-024-61951-1, PMID 38745101.
Barot T, Nagula K, Patel M, Patel LD. Liquisolid compact of meclizine hydrochloride: development and optimization using factorial design. Int J App Pharm. 2025;17(2):259-67. doi: 10.22159/ijap.2025v17i2.53361.
Shen D, Jin T, Xiao Y, Zhu X, Hua Y. Preparation of pazopanib-fumarate disodium glycyrrhizinate nanocrystalline micelles by liquid-assisted ball milling. Eur J Pharm Sci. 2023;188:106530. doi: 10.1016/j.ejps.2023.106530, PMID 37459902.