
Int J Curr Pharm Res, Vol 17, Issue 4, 60-65Original Article
ANTIDEPRESSANT AND ANXIOLYTIC ACTIVITY OF FICUS RELIGIOSA ADVENTITIOUS ROOT EXTRACTS IN MICE
GAURAV HASTIR*, AJEET PAL SINGH, AMAR PAL SINGH
Department of Pharmacology, St. Soldier Institute of Pharmacy, Lidhran Campus, Behind NIT (R. E. C.), Jalandhar –Amritsar by pass, NH-1, Jalandhar-144011, Punjab, India
*Corresponding author: Gaurav Hastir; *Email: gyhastir@gmail.com
Received: 20 Apr 2025, Revised and Accepted: 10 Jun 2025
ABSTRACT
Objective: To evaluate Antidepressant and anxiolytic activity of Aqueous and Ethanolic extracts of Ficus religiosa adventitious in albino mice by using Forced swimming test (FST) and Tail suspension test (TST) parameters.
Methods: Healthy, adult swiss albino mice of either sex weighing (25-40 g), maintained under standard laboratory conditions, at temperature 25±2 °C and a 12 h light-12 h dark period will be employed for the experimentation. Food and water will be provided ad libitum. The behavioral effects of an acute or sub-acute (10 d course) will be orally administered. “Ficus religiosa” (250 and 500 mg/kg) aqueous and ethanolic extract of adventitious root will be evaluated in male and female Swiss mice by elevated plus-maze (EPM). The effects of diazepam (DZP; 1 mg/kg) will also assess. The Tail Suspension test (TST) will be used to assess the effects of an ethanolic and aqueous extract of Ficusreligiosa (250 and 500 mg/kg) on male and female Swiss mice. Additionally, the effects of fluoxetine (10 mg/kg) will be evaluated.
Results: In the present study, we have undertaken the antidepressant activity and anxiolytic activity of ethanolic and aqueous extracts Ficus religiosa adventitious in Swiss albino mice, at the dose of 250 and 500 mg/kg with oral administration respectively.
Conclusion: In conclusion both the extracts ethanolic and aqueous extracts of Ficus religiosa adventitious have shown significant results as an antidepressant effect and anxiolytic activity using forced swim test (FST) and Tail Suspension test (TST) parameters.
Keywords: Ficus religiosa, Anxiolytic, Antidepressant, Corticosterone levels, TST-Tail suspension test, EPM – elevated plus maze
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijcpr.2025v17i4.7010 Journal homepage: https://innovareacademics.in/journals/index.php/ijcpr
INTRODUCTION
Depression is a mood disorder that can manifest itself in different ways. The centers for disease control and prevention (CDC) Trusted Source reported that between 2013 and 2016 about 8.1 percent of American adults aged 20 and over underwent depression in any given 2 w period. Depression can be defined as feelings of sadness, loss, or anger that interfere with everyday activities. Depression is a disorder of mood only and not a disorder of thought or cognition. The disorder can manifest in a very mild way, in a manner that is nearly normal lo severe psychotic depression, which is often include hallucinations and delusion [1].
Depression can exacerbate certain conditions, such as arthritis and asthma.
Heart disease
Cancer
Diabetes
Obesity
It is critical to understand that experiencing sadness occasionally is a natural aspect of life. Everyone experiences sad and distressing circumstances. However, you may be suffering from depression if you frequently feel depressed or hopeless. Depression is regarded as a severe illness that, if left untreated, might worsen. The symptoms of those who seek treatment frequently improve in a matter of weeks [2].
Anxiety is a normal and often adaptive emotion. But too much anxiety can turn into a health problem if it occurs often enough. Anxiety disorders are a variety of mental health disorders that features excessive anxiety, fear, nervousness, and worry. Many disorders, in addition to producing physical symptoms, alter the person's actions and emotional processing. Although pathological anxiety can have a significant effect on day-to-day functioning, low-level anxiety can be vague and disturbing.
There are 40 million individuals with anxiety disorders in the U. S., making it the most common category of mental disorders in the country, although only 36.9% of individuals with anxiety disorders receive treatment [3, 4].
An emotional and physiological reaction to known or unknown causes, anxiety can range from a normal reaction to extreme dysfunction (a sign of an anxiety disorder), hinder functioning, interfere with decision-making, and lower quality of life. One of the most common psychological diseases is anxiety, which manifests as erratic mood, hypervigilance, negative perception, and social phobia in the presence of potentially dangerous symptoms [5, 6].
Many herbs, such as Bacopamonnieri Linn., Casimiroaedulis, Citrus aurantium Linn. Emblicaofficinalis, Ginkgo biloba L., Hypericumperforatum Linn., Magnolia officinalis, Piper methysticum, Withaniasominifera Linn., Zingiberofficinale, and others, are used for their antidepressant and anxiolytic properties [7].
The most well-known species in the genus Ficus, Ficusreligiosa, usually referred to as peepal, has over 150 different names. In Indian culture, Ficusreligiosa has religious, mythical, and therapeutic significance. Numerous ancient sacred scriptures, including the Arthasastra, Puranas, Upanishads, Ramayana, Mahabharata, Bhagavadgita, and Buddhistic literature, among others, make reference to Ficusreligiosa [8].
The enormous perennial tree F. religiosa is examined for its medicinal properties in the bark, leaves, seeds, fruits, roots and latex. The therapeutic characteristics of all the components of the plant can be exploited and all parts are best used in conjunction with other medicinal herbs, however wood will not have any therapeutic properties because it is highly porous. Traditionally, F. religiosa has been employed for treating conditions such as diabetes, gonorrhea, amnesia, and wound healing. It has also been shown to have some activity as an anticancer, antibacterial, anti-convulsant, antiviral, antiprotozoal, anti-diarrheal, astringent, and anticholinergic agent [9, 10].
The enormous perennial tree F. religiosa is looked at in regard to the plant's bark, leaves, seeds, fruits, roots, and latex for possible medicinal uses. Each of the parts of the plant has medicinal properties that have been used in many ways, and best when used synergistically with other medicinal herbs, however the wood part has no therapeutic use with part of its porous nature. Traditional uses include treating of the plant for diabetes, gonorrhea, amnesia and wound healing. It has also shown to have potential in anti-cancer, anti-bacterial, anti-convulsant, anti-viral, anti-protozoal, anti-diarrheal, astringent, and anticholinergic activities [11-13].
MATERIALS AND METHODS
Source of data
All experiments were planned to generate data from the laboratory studies i. e.; experiments were performed as described in reference, experimental studies in journals and in textbooks available with college, SSIP library, Jalandhar, and various institutions.
Methods of collection of data
Study through research articles, research data based like Medline.
The data related to physicochemical details of the drug will be collected from drug information centre, various standard books, journals and other sources like literature data bases such as science direct etc.
The data was collected based on laboratory animal experimentation.
Effective animal experiment design, accurate data analysis, and the use of the fewest animals required to accomplish scientific goals-but not so few as to overlook biologically significant effects or necessitate needless repetition of experiments-are crucial for both ethical and financial reasons. Researchers are reminded that no experiment should ever be initiated without a clear understanding of how the resulting data are to be analyzed, and they are encouraged to seek advice from a statistician during the design phase. These recommendations are meant to assist biomedical researchers in carrying out their studies effectively and interpreting the outcomes so that they can derive all relevant information from the data that is produced. The various objectives of experiments (e. g., exploratory vs. confirmatory); the experimental unit; the need to document all experimental details (e. g., species, sex, age, microbiological status, strain and source of animals, and husbandry conditions); the use of formal experimental designs (e. g., completely randomized and randomized block); the assignment of experimental units to treatments through randomization; other aspects of the experiment (e. g., timing of measurements); the estimation of the experiment's size using power and sample size calculations; the screening of raw data for obvious errors; the use of the t-test or analysis of variance for parametric analysis; and the efficient design of graphical data are all covered.
Experimental animals
Rabbits and guinea pigs are, after mice and rats, the most commonly used mammals in research, but their popularity has waned since the 1980s. The guinea pig (Caviaporcellus) was one of the first animals used in medical research, but it ranks lower on the list than mice and rats because of its long gestation time (59-72 d), small litter size (2-5), difficulty accessing and obtaining vessels, and anesthetic challenges. Guinea pigs still have an important place in immunology, vaccination and infectious disease research, and are good models for hearing. The rabbit is primarily used in the making of polyclonal antibodies, product safety testing, and research in cardiology, orthopedics, and eye research. In the present study albino mice (25-30 gm) of both sexes were housed in different polypropylene cages with husk bedding, under standard light and dark cycle conditions, in the central animal house of St. Soldier Institute of Pharmacy, Jalandhar, Punjab. They were maintained on a standard laboratory pellet chow diet and given free access to water. Mice were acclimatized with the laboratory environment prior to the experimental trial. All experiments were conducted in a semi-soundproof laboratory space between 8 AM and 4 PM. The experimental protocol was approved by the Institutional Animal Ethics Committee (IAEC) and was carried out as per the guidelines of Committee for the purpose of Control and Supervision of Experimental Animals (CPCSEA), Ministry of environment and Forests, Government of India (Reg. No. 2011/PO/Re/S/18/CPCSEA and date of registration is 1/5/2018) for the use and care of experimental animals. Adequate measures were taken to minimize pain or discomfort with animal’s experimental procedure. Research protocol is duly approved by IAEC/CPCSEA (IAEC/SSIP/2019/PR-003).
Drugs and reagents/chemical
All the chemicals and biochemical reagents used in this study were of analytical grade and were freshly prepared before use. All chemicals of analytical grade were procured from Sigma chemical, USA and S. D. Fine Chem. Ltd., India.
Collection and identification of plant
The adventitious root of Ficusreligiosa was collected from the herbal garden, St. Soldier Institute of Pharmacy, Jalandhar. This plant was identified and Morphological authenticated by Dr. (Mrs) SunitaGarg, Head, Raw Materials Herbarium and Museum, Delhi (RHMD),National Institute of Science Communication And Information Resources (CSIR-NISCAIR), New Delhi, with reference no. NISCAIR/RHMD/Consult/2020/3676-77 dated 21-09-2020.
Preparation of plant material
Ficusreligiosa's adventitious root was thoroughly cleaned to get rid of any debris, then ground to a fine powder while preventing moisture contamination. After passing through a screen to achieve a consistent particle size, the powdered crude medication was dissolved in a number of solutions to ascertain its ideal solubility.
Preparation and administration of crude extracts/standard drugs
Extraction is the widely used method for separating the active ingredients from the unrefined medications. In typical extraction processes, selective solvents are used to separate the medicinally active parts of plant or animal tissues from the inactive or inert components. These plant-based products are comparatively impure liquids, semisolids, or powders that are solely meant to be applied topically or taken orally. Standardized extraction techniques are used for crude pharmaceuticals (medical plant parts) in order to extract the therapeutically desirable components and remove unwanted material by treating them with menstrum, a selective solvent. After standardization, the resulting extract could be utilized as a medication. Numerous therapeutic plant metabolites, including alkaloids, glycosides, terpenoids, flavonoids, and lignans, are complexly mixed together in these extracts.
Animals
Healthy, adult swiss albino mice of either sex weighing (25-40 g), maintained under standard laboratory conditions, at temperature 25±2 °C and a 12 h light-12 h dark period will be employed for the experimentation. Food and water will be provided ad libitum.
Acute oral toxicity study
The number of pharmaceutical compounds and chemicals employed in modern society has nearly reached infinite proportions. These days, they can be found in food items, medications, drinks, and other household and commercial manufactured goods. However, when used in large quantities that might cause an immediate hazardous effect, these chemicals or pharmacological substances may cause acute toxicity or chronic toxicity in the biological system over an extended period of time. Depending on the substance's nature, these effects could be minor or severe. The term "acute toxicity" refers to the undesirable consequence or effects that follow one or more doses of a chemical within a 24 h period, either immediately or shortly thereafter. Any effect that results in biochemical lesions and/or functional impairments in organs that could change how the organism functions overall or in specific organs is considered an undesirable (or bad) effect. However, studies of acute toxicity tend to determine the dose-dependent undesirable (or unfavorable) consequence or effects that may occur, including mortality and all other pertinent data for the assessment of acute toxicity. Nowadays, determining the lethal dosage (LD50), or the amount that kills 50% of test animals, is a key metric for assessing acute toxicity and serves as the first step in the general toxicity screening of chemical and pharmacological compounds.
Acute toxicity studies only provide data on a pharmacological agent's LD50, therapeutic index, and level of safety. Before pharmacological medicines are permitted to be sold on the market, a crucial process known as toxicity evaluation is typically conducted. On the other hand, several techniques have been created and implemented for assessing acute toxicity. But since the majority of these approaches have drawbacks, it is critical to create a better one that, if at all feasible, uses fewer animals. This study aims to present a novel approach to acute toxicity testing that, if used, should yield more precise and repeatable results with fewer animals. Low, medium, and high doses will be chosen for therapy once an acute toxicity study of the ethanolic and aqueous extract of Ficusreligiosa is completed in accordance with OECD recommendations No: 423.
Method
Acute toxicity studies only provide data on a pharmacological agent's LD50, therapeutic index, and level of safety. Before pharmacological medicines are permitted to be sold on the market, a crucial process known as toxicity evaluation is typically conducted. On the other hand, several techniques have been created and implemented for assessing acute toxicity. But since the majority of these approaches have drawbacks, it is critical to create a better one that, if at all feasible, uses fewer animals. This study aims to present a novel approach to acute toxicity testing that, if used, should yield more precise and repeatable results with fewer animals. Low, medium, and high doses will be chosen for therapy once an acute toxicity study of the ethanolic and aqueous extract of Ficusreligiosa is completed in accordance with OECD recommendations No: 423.
Mouse as a model for depression and anxiety
One of the main benefits of utilizing mice as models is their striking resemblance to human anatomy, physiology, and genetics. Since the mouse and human genomes share about 95% of their similarities, mouse genetic research is particularly relevant to human disease. Depression and anxiety Animals are used as experimental models in studies pertaining to the brain and central nervous system. Preclinical research on the neurobiology of psychiatric diseases relies heavily on animal models, which are also used as screening techniques to find new treatment drugs. Since humans and mice share over 90% of their genes, rodents, particularly mice, have proven useful in studies. Additionally, when it is not ethically or otherwise possible to study the effects of stress in humans, animal models are especially useful. The cost-effectiveness, small dose in relation to body weight, ease of handling, and faster breeding time are further benefits of using mice as a model. These factors will undoubtedly make the research easier for the researcher to manage. Animal models of anxiety and depression are also essential for developing new treatments for these conditions.
Experimental parameters
Antianxiety animal model
The behavioral effects of an acute or sub-acute (10 d course) will be orally administered. “Ficus religiosa” (250 and 500 mg/kg) aqueous and ethanolic extract of adventitious root will be evaluated in male and female Swiss mice by elevated plus-maze (EPM). The effects of diazepam (DZP; 1 mg/kg) will also assess.
Elevated plus maze (EPM)
A straightforward technique for evaluating mice's anxiety reactions is the Elevated Plus Maze. A shared central platform (10×10 cm) connected the four arms of this test, which included two opposing open arms (50×10 cm) and two opposing closed arms (50×10×40 cm). All of the equipment was raised 50 cm off the ground. Both open arms have a 1 cm Plexiglass border installed to prevent falls. The animals were put on the central platform facing an enclosed arm at the start of the test, and they had five minutes to freely roam the maze. The parameters listed below were scored: Time spent on the central platform and in the open and enclosed arms, as well as the quantity of entries in both arms. Mice's dislike of open areas served as the basis for the concept. The ratio of time spent on the open arms to time spent on the enclosed arms was used to evaluate the anxious behavior of the mice. The elevated plus maze is based on mice's natural aversion to darkness (in confined areas) and their innate fear of heights (in open areas).
Induction of experiment (Experimental protocol)
Group 1: anxiety control Naïve Animal (solvent)
Group 2: anxiety induced mice treated with AFR (Low dose)
Group 3: anxiety induced mice treated with AFR (High dose)
Group 4: anxiety induced mice treated with EFR (Low dose)
Group 5: anxiety induced mice treated with EFR (High dose)
Group 6: anxiety induced mice treated with diazepam (DZP; 1 mg/kg)
Antidepressant animal model
Acute or sub-acute (10 d course) behavioral effects will be given orally. The Tail Suspension test (TST) will be used to assess the effects of an ethanolic and aqueous extract of Ficusreligiosa (250 and 500 mg/kg) on male and female Swiss mice. Additionally, the effects of fluoxetine (10 mg/kg) will be evaluated.
Tail suspension test in mice
Thirty minutes before testing, groups of ten mice received intraperitoneal injections of either the vehicle or the test drugs. Adhesive tape was positioned around 1 cm from the tip of the mice's tails to hang them on the edge of a shelf 58 cm above a tabletop for the test. For eight minutes, the length of immobility was recorded. When mice hung passively and without moving for at least one minute, they were deemed immobile.
Induction of experimental experimental protocol
Group 1: Depression control Naïve animal (solvent)
Group 2: Depression induced mice treated with AFR (Low dose)
Group 3: Depression induced mice treated with AFR (High dose)
Group 4: Depression induced mice treated with EFR (Low dose)
Group 5: Depression induced mice treated with EFR (High dose)
Group 6: Depression induced mice treated with Fluoxetine (FXT; 10 mg/kg).
Biochemical estimation
Collection of blood samples
On 15th d, blood (0.3 ml) was withdrawn from tail vein from all groups of mice used in Tail Suspension Test. Blood samples were centrifuged at 2500 rpm for 10 min using refrigerated centrifuge (Paramount scientific works, Ambalacantt, India) to separate the plasma, which was used for estimation of corticosterone levels.
Estimation of plasma corticosterone levels
The Bartos and Pesez (1979) approach was used to quantitatively estimate the amounts of corticosterone in the blood plasma. After immersing the tubes in ice water for five minutes, 0.50 ml of 0.10 N sodium hydroxide was added to 1.0 ml of the sample in ethanol along with 0.50 ml of a 0.10 percent solution of p-nitroso-N,N-dimethylaniline in ethanol. After being sealed with cotton-wool, the tubes were left in a dark place at 0 °C for five hours. 2.0 ml of pH 9.8 buffer, 5.0 ml of a 0.10 % phenol in ethanol solution, and 0.50 ml of a 1.0 % potassium ferricyanide aqueous solution were added to the aforesaid solution. For ten minutes, the tubes were submerged in a water bath at 20±2 °C. A UV-visible spectrophotometer (UV 3200 UV-VIS Spectrophotometer, Somajiguda, Hyderabad) was used to read the solution at 650 nm.
Body weight analysis
During the same time of study, weekly body weight analysis will be recorded.
Statistical analysis
The data obtained will be analyzed using T-test or one way ANOVA (Graph pad prism version 5.00 software) followed by suitable post test.
Total No. of animals required
No. of animal in each group (n) = 6 or 7
No. of groups (N) = 12
Total no. of animals required =80
First of all we will complete one parameter i. e. Anxiolytic study, then after rehabilitation/washing period. We will use same animals for second parameter i. e. Antidepressant by this way, we can minimize the number of experimental animals up to 50% i. e. 40.
RESULTS AND DISCUSSION
Acute oral toxicity study
According to the Organization for Economic Co-operation and Development's (OECD, 425) guidelines, the median lethal dose (LD50) of AFR and EFR was calculated using five mice that had been fasted overnight before receiving oral doses of various extracts of AFR and EFR at maximum dose levels up to 1000 mg/kg, starting with doses of 5, 50, and 300 mg/kg. After the initial dosage, food was further withheld from one mouse for four hours. Signs of toxicity (alterations in mucous membranes, skin, fur, and eyes, as well as circulatory, respiratory, somato-motor, and behavioral patterns) and mortality were monitored for the first 24 h and subsequently for 14 d. No behavioral reactions have changed, and there is no evidence of acute oral toxicity. For two weeks, the other four mice were similarly given doses and monitored. The LD50 was then calculated.
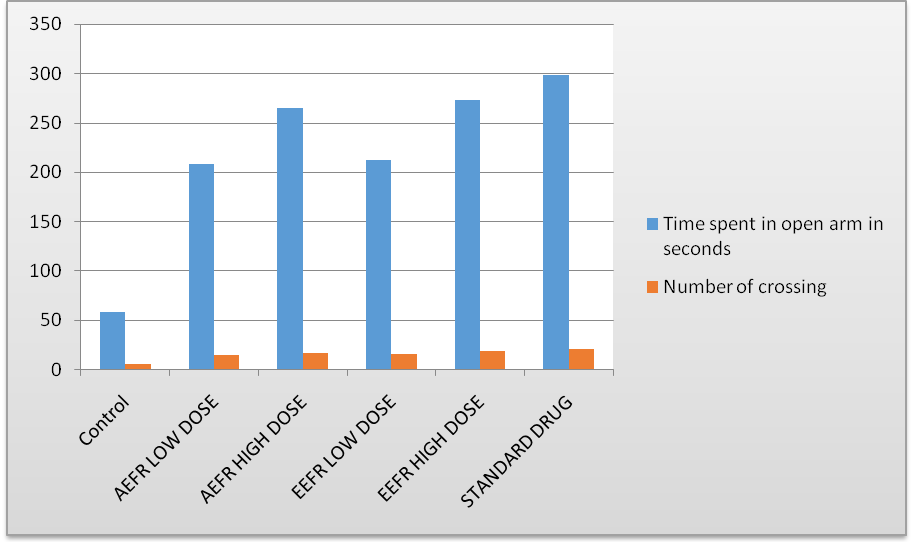
Fig. 1: Anxiolytic effect of Ficusreligiosa adventitious root extracts
Anti-depressant animal model
Table 1: Tail suspension test (TST)
| S. No. | Group and dose | Immobility (sec.) |
| 1. | Control 1% w/v sodium cmc, (0.5 mg/kg, p. o) | 256±4.808 |
| 2. | Aqueous extract (AEFRAR) | |
|
92.2±2.386* | |
|
44.4±1.866** | |
| 3. | Ethanolic extract (EEFRAR) | |
|
78.0±2.764* | |
|
36.8±2.696** | |
| 4. | Standard dose (Fluoxetine-10 mg/kg) | 16.4±1.628# |
*P<0.005, **P<0.01, #P<0.001, When compared with the control group. All values represent = Mean±SEM, n= 5/6 in each group
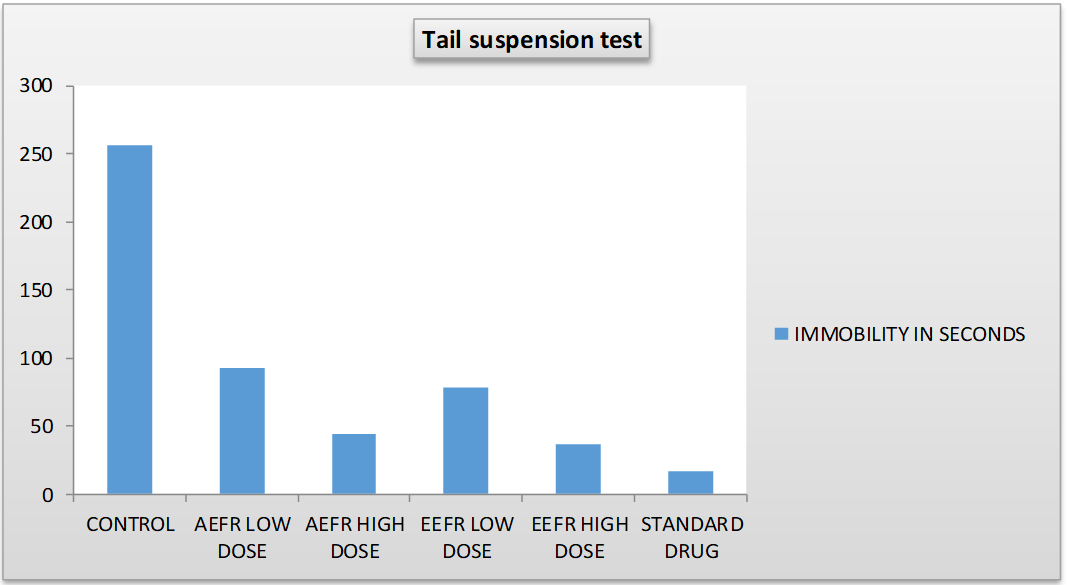
Fig. 2: Anti-depressant effect of ethanolic and aqueous extract of Ficusreligiosa adventitious root (aefrar)
Fig. 3: Showing the corticosterone levels in post tail suspension test experiments Note: TALD= Test Aqueous lower dose; TAHD= Test Aqueous higher dose; TELD= Test Ethanolic lower dose; TEHD= Test Ethanolic higher dose; STD= standard
Biochemical estimation
(a) Estimation of plasma corticosterone levels
Groups 1 to 6 were tail bled on day 1 and then corticosterone levels were combined to obtain the average levels in tail blood. For treatment of Groups see their respective Experimental design.
DISCUSSION
As in many other realms of psychopharmacology, the lack of solid animal models of the clinical state inhibits understanding of the neuro-chemical mechanism related to depression. While we have no knowledge of any animal condition that mimics hereditary human depression, a few methods have been described that cause animals to exhibit behaviors that resemble human depressed states to some degree.
Animal models are used in anxiety research, as proxy deployments to investigate the mechanisms of an emotional behavior, and as screening methods to find chemicals that could have therapeutic potential to treat anxiety disorders. The validation of these types of anxiety models as purely regulatory has resulted in clear issues with their translation to systems that are not the benzodiazepines/GABAA receptor complex. Most of the more than 30 animal models of anxiety that are currently used are behavioral based; although, some are physiological (e. g. hyperthermia) and some are endocrine (e. g. plasma corticosterone) based "acute stress" reactions. Interestingly, behavioral models can generally be subdivided into conditioned and unconditioned responses to stimuli that may induce anxiety in human subject.
In India herbal medicines have historically been the major form of medicine, based on the existence of various complex chemical compounds, these be secondary plant metabolites in one or more plant parts that give medicinal plants a therapeutical property. One specific medicinal plant, Ficusreligiosa (L.) also known as pepal, in the family Moraceae, has for centuries been used for diabetic remedies, antibacterial uses, antiulcer uses, as well as to treat skin ailments and gonorrhea. This plant, the Bo tree has the significance of providing cover to the Buddha as he unveiled the "Truths." Very high levels of total flavonoid, total phenolic, and percent inhibition of linoleic acid were determined in the fruit of F. religiosa. In many instances, the total phenolic contents, yields of extract, and antioxidant activity of the aqueous and aqueous organic solvent were better than the absolute organic solvents. The shaker method was the preferred technique, and it yielded higher total phenolic contents and antioxidant activity in general than the refluxing extraction method even if the latter produced bigger yield of extract. All previous studies show that Ficusreligiosa is having the activities related to CNS. It is having minerals, vitamins and amino acids as a chemical constituent which already have proved activity to cure the stress related disorders especially anxiety, depression and insomnia.
After selection of Ficusreligiosa, acute oral toxicity was detected with different extracts (EFR and AFR) having dose (5, 50, 300, 1000 mg/kg) via oral route, shows no change in behavioral responses and observation shows no acute oral toxicity. Hence depending upon it, Dose was selected 250 mg/kg and 500 mg/kg for our experimental work.
Anti-anxiety model
Ficusreligiosa (250 mg/kg and 500 mg/kg) aqueous and ethanolic extract were evaluated in swiss albino mice by elevated plus-maze (EPM). The effects of diazepam (DZP; 1 mg/kg) were also assessed.
In EPM (Elevated plus maze),when group-Control 1% w/v sodium CMC (0.5 mg/kg, p. o) drug administration have shown their time spent in open arms (58±2.852) and number of crossings (4.8±0.366) is insignificant. When aqueous extract of Ficusreligiosa (250 mg/kg) orally administered, have shown time spent in open arms (208.2±2.376) and number of crossings (14.2±0.384) is significant. When aqueous extract of Ficusreligiosa (500 mg/kg) orally administered, have shown time spent in open arms (264.4±1.654) and number of crossings (16.6±0.514) is significant.
When Ethanolic extract of Ficusreligiosa (250 mg/kg) orally administered, have shown time spent in open arms (212.2±2.778) and number of crossingss (15.0±0.446) is significant. When Ethanolic extract of Ficusreligiosa (500 mg/kg) orally administered, have shown time spent in open arms (272.8±2.598) and number of crossings (18.6±0.506) is significant. When standard dose of Diazepam (1 mg/kg) was orally administered, have shown time spent in open arms (298±1.814) and number of crossings (20.6±0.816) is more significant.
Antidepressant model
Ficusreligiosa (250 mg/kg and 500 mg/kg) Aqueous and ethanol extract was evaluated inswiss albino mice in Tail Suspension test (TST). The effects of fluoxetine (FXT; 10 mg/kg) were also be assessed.
In TST (Tail suspension test), Group-Control 1% w/v sodium CMC (0.5 mg/kg, p. o) drug administration have shown their immobility (256±4.808) insignificant. When aqueous extract of Ficusreligiosa (250 mg/kg) orally administered, have shown immobility (92.2±2.3846) is significant. When aqueous extract of Ficusreligiosa (500 mg/kg) orally administered, have shown immobility (44.4±1.866) is significant. When Ethanolic extract of Ficusreligiosa (250 mg/kg) orally administered, have shown immobility (78±2.764) is significant. When Ethanolic extract of Ficusreligiosa (500 mg/kg) orally administered, have shown immobility (36.8±2.696) is significant. When standard dose of fluoxetine (10 mg/kg) was orally administered, have shown immobility (16.4±1.628) is more significant.
Biochemical estimation
In Estimation of plasma corticosterone levels it is known that stress enhances the activity of the hypothalamus-pituitary-adrenal (HPA) axis and results in increased secretion of corticosteroids from the adrenal cortex. Cortisol and corticosterone are thus often used as biomarkers for stress and depressive disorders. Although corticosterone is considered the main glucocorticoid involved in regulation of stress responses in rodents, researchers often choose to detect cortisol for stress indicators in consideration of convenience and kits availability.
Group-Control 1% w/v sodium CMC (0.5 mg/kg, p. o) drug administration have shown their corticosterone level is (11.80±0.54) is insignificant. When aqueous extract of Ficusreligiosa (250 mg/kg) orally administered, have shown corticosterone level (6.46±1.44) is also insignificant. When aqueous extract of Ficusreligiosa (500 mg/kg) orally administered, have shown corticosterone level (5.96±2.28) is significant. When Ethanolic extract of Ficusreligiosa (250 mg/kg) orally administered, have shown corticosterone level (5.18±1.28) is significant. When Ethanolic extract of Ficusreligiosa (500 mg/kg) orally administered, have shown corticosterone level (4.46±0.30) is significant. When standard dose of fluoxetine (10 mg/kg) was orally administered, have shown corticosterone level (0.84±0.34) is more significant.
CONCLUSION
The behavioral effects of an acute or sub-acute (10 d course) will be orally administered. For evaluate anxiolytic and antidepressant effect of “Ficus religiosa” (250 or 500 mg/kg) aqueous and ethanolic extract of Ariel root will be evaluated in mice by elevated plus-maze (EPM). The effects of diazepam (DZP; 1 mg/kg) will also assess and Ficus religiosa (250 or 500 mg/kg) aqueous and ethanolic extract of Ariel root will be evaluated in mice in Tail Suspension test (TST). The effects of Fluoxetine (FXT; 10 mg/kg) will also be assessed respectively. 40 healthy albino mice of either sex were randomly divided into 6 groups of 6 or 7 each (n=6 or 7), weighing about 25-30 g were selected for the study.
On 15th day, blood (0.3 ml) was withdrawn from tail vein from all groups of mice. Blood samples were centrifuged at 2500 rpm for 10 min using refrigerated centrifuge (Paramount scientific works, Ambalacantt, India) to separate the plasma, which was used for estimation of corticosterone levels. They had showed the positive biochemical estimation results. Both the extracts have shown significant results as an antidepressant effect using TST parameter and Anxiolytic activity using EPM parameters respectively.
ACKNOWLEDGMENT
It’s our privilege to express the profound sense of gratitude and cordial thanks to our respected chairman Mr. Anil Chopra and Vice Chairperson Ms. Sangeeta Chopra, St. Soldier Educational Society, Jalandhar for providing the necessary facilities to complete this research work.
FUNDING
Nil
AUTHORS CONTRIBUTIONS
All authors have contributed equally
CONFLICT OF INTERESTS
Declared none
REFERENCES
Sekhon S, Gupta V. Mood disorder. In: Treasure Island, (FL): StatPearls Publishing; 2023 May 8. PMID 32644337.
https://my.clevelandclinic.org/Health/diseases/17843-Mood-Disorders.
Chand SP, Marwaha R. Anxiety. In: Treasure Island, (FL): StatPearls Publishing; 2023 Apr 24. PMID 29262212.
Munir S, Takov V. Generalized anxiety disorder. In: Treasure Island, (FL): StatPearls Publishing; 2022 Oct 17. PMID 28722900.
Department of Error. Lancet. 2021 Mar 6;397(10277):880. doi: 10.1016/S0140-6736(21)00473-6, PMID 33676627.
https://www.psychiatry.org/patients-families/anxiety-disorders/what-areanxiety-disorders.
Kaur R. A review article for herbal drugs as anxiolytic activity. Int J of Allied Med Sci and Clin Res. 2020 Aug 8;5(2):636-47.
Prasad PV, Subhaktha PK, Narayana A, Rao MM. Medico historical study of asvattha (sacred fig tree). Bull Indian Inst Hist Med (Hyderabad). 2006;36(1):1-20. PMID 18175640.
Gupta S, Porwal MC, Roy PS. Indigenous knowledge on some medicinal plants among the nicobari tribe of car nicobar Island. Indian J Tradit Know. 2004;3(3):287-93.
Nair R, Chanda SV. Antibacterial activities of some medicinal plants of the Western Region of India. Turk J Biol. 2007;31(4):231-6.
Choudhary GP. Evaluation of ethanolic extract of ficus religiosa bark on incision and excision wounds in rats. PlantaIndica. 2006;2(3):17-9.
Chandrasekar SB, Bhanumathy M, Pawar AT, Somasundaram T. Phytopharmacology of ficus religiosa. Pharmacogn Rev. 2010 Jul;4(8):195-9. doi: 10.4103/0973-7847.70918, PMID 22228961, PMCID PMC3249921.
Devanesan EB, Vijaya Anand AV, Kumar PS, Vinayagamoorthy P, Basavaraju P. Phytochemistry and pharmacology of ficus religiosa. Syst Rev Pharm. 2018 Jan 1;9(1):45-8. doi: 10.5530/srp.2018.1.9.