
Int J Curr Pharm Res, Vol 17, Issue 5, 46-49Original Article
EVALUATION OF ANTI-ANXIETY ACTIVITY OF FICUSTHONNINGII ROOT IN MICE
MANISHA*, AJEET PAL SINGH, AMAR PAL SINGH
Department of Pharmacology, St. Soldier Institute of Pharmacy, Lidhran Campus, Behind NIT (R. E. C.), Jalandhar –Amritsar bypass, NH-1, Jalandhar-144011, Punjab, India
*Corresponding author: Manisha; *Email: manisha.bahti94@gmail.com
Received: 05 Jun 2025, Revised and Accepted: 25 Jul 2025
ABSTRACT
Objective: To evaluate Anxiolytic activity of ethanolic extracts of Ficus Thonningii in male and female mice by light-dark model, open field test, Elevated plus maze models.
Methods: Healthy, adult mice of either sex weighing 25-40 g, maintained under standard laboratory conditions, at temperature 25±2 °C. The behavioral effects of an acute or sub-acute (14 d course) will be orally administered. Ficus Thonningii (200 and 400 mg/kg) ethanolic extract will be evaluated in male and female mice in various animal models. The animals were housed in plastic cages in groups of ten per cage, at room temperature, about 21+1 ~ C, and with free access to water and food. They were kept on an artificial 12 h/12 h d/night cycle.
Results: In the present study, we have undertaken the anxiolytic activity of ethanolic and aqueous extracts of Ficusthonningii in male and female mice, at the dose (200 and 400 mg/kg) mg/kg with oral administration respectively.
Conclusion: In conclusion, Ficusthonningii plant extract showed significant anxiolytic activity by using Elevated plus maze, open field test, light/dark exploration parameters. Probably due to GABA facilitatory action of phytoconstituents such as flavonoids, tannic acid, marmesinin, phenols, saponin etc.
Keywords: Ficusthonningii, Anxiolytic activity, Open field test (OFT), Light dark model (LDM), animals
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijcpr.2025v17i5.7024 Journal homepage: https://innovareacademics.in/journals/index.php/ijcpr
INTRODUCTION
A drug or other intervention that reduces anxiety is called an anxiolytic. Anxiolytic agents have the opposite meaning, causing anxiety; anxiolytic agents can refer to all of these different types of psychoactive drugs or interventions available. Research shows many recreational drugs are anxiolytic, but some substances, including alcohol, initiate the process with an anxiolytic effect initially. Anxiolytic agents have been used to treat anxiety disorder and associated physical and psychological conditions. Light therapy and other interventions have had anxiolytic outcomes [1].
Anxiety is a class of resonant emotional stimulation episodes that include feelings of worry or fear; in contrast to patients who have panic, patients with concern usually have an imprecise realization of the cause of danger. Research shows anxiety is the most frequent behavioral issue, and research related to the contributors of kids' anxiety shows that their physical development, social, family, and affective contributors are significant predictors of the child's anxiety level [2]. Anxiety is a frequently occurring psychological illness that comes with mood changes, irrational, violent behavior, low quality of life, bodily dysfunction, cognitive impairment. The most frequent phobias are agoraphobia and simple phobia, which have the most frequent prevalence. The lifetime prevalence of obsessive-compulsive disorder (OCD) and panic disorder (PD) is 2% while the prevalence of generalized anxiety disorder (GAD) is 3%–30% and social phobia (SP) is 2%–16%. Mood states of anxiety are shown to be governed by monoamines (dopamine, nor adrenaline, and serotonin), neuropeptides (galanin, neuropeptide Y, arginine vasopressin, tachycardia, and substance P), neurosteroids, and cytokines [3]. The two medications most frequently used for the treatment of anxiety are SSRIs and benzodiazepines. Synthetic medications used for the treatment of anxiety also have several adverse side effects. Natural substances have been shown to have the same ability to treat illness while possessing fewer side effects than synthetic drugs [4]. Since prehistoric times, mankind has relied on plants as a source of food, shelter and medicine. From the beginning of time, humans have utilized plants for food, shelter, and medicine. Before allopathic medicine arose, illnesses were treated with remedies from plants. Plants are still commonly used in both ethnomedicine and ethnoveterinary practice, beyond their commercial use in manufacturing, cosmetics, and pharmaceutical purposes. Also, the development of traditional medicines has benefited as well. Ficusthonningii is one of the species of Ficus. It is native to Africa. Latest evolutionary research shows that Ficusthonningiimay be the classification for many different species. One tree in the Moraceae plant family is Ficusthonningii It is a fruiting tree that has been utilized for disease treatment for a long time. Ficusthonningiiprefers light, deep, and well-drained soils and is found in upland woodlands in tropical and subtropical Africa [5-7]. It is a historically important plant species that has both medicinal and nutritional uses. The usually yellow fruits, 10-20 mm in diameter, change to pink when they are ripe. The well-known ornamental tree Ficusthonningii is also employed as an added-value species within agroforestry systems. Its bark is used to fabricate bark cloth, and leaves are used for fodder. Like many woody trees, Ficusthonningii is used extensively by ethnomedical practitioners to treat a wide range of maladies. Whereas every plant part has medicinal value, the latex-rich leaves are preferred as latex has historically been associated with efficacy. For mice treated with Alprazolam (Standard), open arms entries and total time in open arms were all significantly (p<0.001) greater than the baseline levels. For all Ficusthonningii root dosages (200 and 400 mg/kg), total time spent and entry into the open arms increased significantly (P<0.05) in a dose-dependent fashion [8]. The number of squares crossed and rearings were significantly (p<0.05, 0.01) increased by the root of Ficusthonningii. Ficusthonningii root significantly (p<0.05) increased the number of social interactions if we consider the social interaction test. In comparing to the vehicle treated control group (for the alprazolam and the extract (Ficusthonningii root 200 and 400 mg/kg p. o.)) there was a significant increase in rearing and squares crossed in the 5 min intervals. The best anti-anxiety action was seen primarily at the dose of 400 mg/kg. The mice in the various levels of treatment groups visited fewer squares over the two-hour time span in the open field test. Relative to control, it was shown that mice visited less squares overtime while they were exposed to the ethanolic extract of Ficusthonningii root and ethanolic soluble fraction, suggesting the possiblility of anxiolytic actions [9]. Elevated Plus Maze (EPM), Light Dark Model (LDM), and Open Field Test (OFT) are used to measure anxiolytic activity. Total locomotor time and the amount of time spent in open arms were both enhanced in EPM after treatment with ethanolic and aqueous extracts. Treatment with these extracts increased the amount of time spent in the light compartment in the light-dark experiment. The amount of time spent in the central compartment and motility both increased in the open field test treatment with extract. Root extract has demonstrated notable anxiolytic efficacy at 200 mg/kg and 400 mg/kg [10].
MATERIALS AND METHODS
Procurement of the extract
Ficusthonninhii root ethanolic extracts were collected from the dealer of ShreedhaPhyto extracts Jaipur – 302019. The same group also provided a certification of the Plant’s identify and quality.
Experimental animals
24 adult either sex mice weighing between 25-30g were obtained from the animal house of Pharmacology Department. ST. Soldier institute of pharmacy, Jalandhar-Amritsar Bypass NH-I, Behind NIT Jalandhar, Punjab India – 144011. Healthy, adult mice of either sex weighing (25-40 g), maintained under standard laboratory conditions; at temperature 25±2 °C and a 12 h light-12 h dark period will be employed for the experimentation. Food and water will be provided ad libitum.
Acute oral toxicity study
Acute toxicity study for the ethanolic extract of “FICUS THONNINGII” will be done according to the OECD guidelines No: 423 and low, medium and high dose will be selected for treatment [11].
Methods
The overnight fasted mice will be divided into 04 groups, each group consisting of 06 animals. The EERFT will be given in various doses (0, 200, 400 and 600 mg/kg) by oral route with a gavage. After administration of the extract, the animal will be observed continuously for the first 2 h and at 24 h to detect changes in behavioural responses and also for tremors, convulsion, salivation, diarrhoea, lethargy, sleep, and coma and also will be monitored up to 14 d for the toxic symptoms and mortality.
Table 1: Treatment schedule
| Groups (where N=4) | Treatment (For 14 d) |
| Group I | Naive animal, received standard pellet diet and tap water ad libitum daily. |
| Group II | Standard group received 0.25 mg/kg Alprazolam orally daily. |
| Group III | Test group-I received 200 mg/kg Ethanolic extract of FicusThonningiiroot orally daily |
| Group IV | Test group –II received 400 mg/kg Ethanolic extract of FicusThonningiiroot orally daily |
Total No. of animals required
No. of the animal in each group (n) = 06
No. of groups (N) = 04
Total no. of animals required = 24
Parameters for antianxiety in mice
The behavioral effects of an acute or sub acute (14 h course) will be orally administered. Ficus Thonningii (200 and 400 mg/kg) ethanolic extract will be evaluated in male and female mice in various animal models. The animals were housed in plastic cages in groups of ten per cage, at room temperature about 21+1 ~ C, and with free access to water and food. They were kept on an artificial 12 h/12 h day/night cycle.
Body weight analysis
During the same time of study, weekly body weight analysis will be recorded.
Statistical analysis
All the results will be expressed as standard error of mean (S. E. M.). Data will be analyzed using one-way ANOVA (Graph pad prism version 5.00 software) followed by suitable post testDunnett’s t-test. p<0.05 will be considered as statistically significant.
RESULTS
All the parameters were performed with suitable time interval to prevent unwanted stress in animals. Ficusthonningii includes a variety of physiologically active chemicals with physiological effects that may account for its healing potential in a variety of diseases.
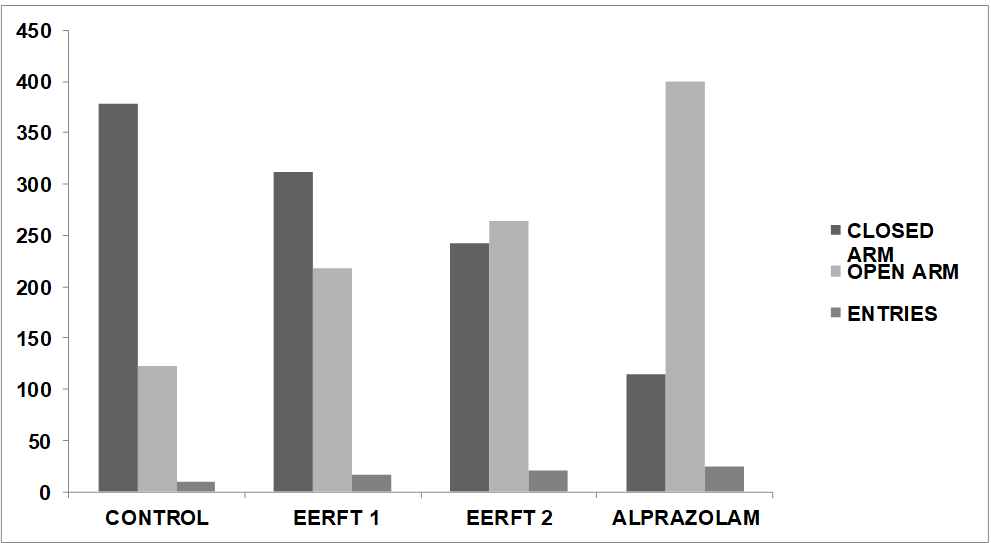
Fig. 1: Graphical representation of effects of different doses of Ficus thonningii root ethanolic extracton anxiolytic effect as compared to standard (Alprazolam)
Table 2: Phytochemical constituents of crude leaf/roots extracts of Ficusthonningii
| Phytoconstituent | Aqueous extract | Ethanolic extract |
| Anthraquinones | + | + |
| Flavonoids | + | ++ |
| Alkaloids | - | + |
| Terpenoids | - | ++ |
| steroids | - | ++ |
| Saponins | ++ | + |
(-) = absent, (+) = low, (++) = high, (+++) = very high
Elevated plus-maze test
The vehicle-treated mice (Normal control) had spent more time in closed arm and showed less entries in open arm compared to closed arm of the maze during 10 min.
Group 1: In this group animals were treated with normal saline solution in which mice spent 122.8±2.34 sec in open arm and 378.6±1.53 sec in closed arm and no. of crossings were 10±2.01.
Group 2: In this group animals were treated with low dose of Ethanolic extract (200 mg/kg) solution in which mice spent 218.1±1.99 sec in open arm and 312.1±1.22 sec in closed arm and no. of crossings were 17±1.59
Group 3: In this group animals were treated with high dose of Ethanolic extract (400 mg/kg) solution in which mice spent 264.6±2.31 sec in open arm and 242.1±0.56 sec in closed arm and no. of crossings were 20±0.22.
Group 4: In this group animals were treated with Alprazolam (0.25 mg/kg) solution in which mice spent 400.6±0.22 sec in open arm and 114.6±0.21 sec in closed arm and no. of crossings were 24±0.21.
Animals treated with Alprazolam (Standard) showed significant (p<0.001) increase in the percentage of open arms entries as well as time spent in open arm. All the doses of Ficusthonningiiroot (200 and 400 mg/kg) showed a significant increase in the time spent and number of entries into open arms (P<0.05) in dose dependent manner.
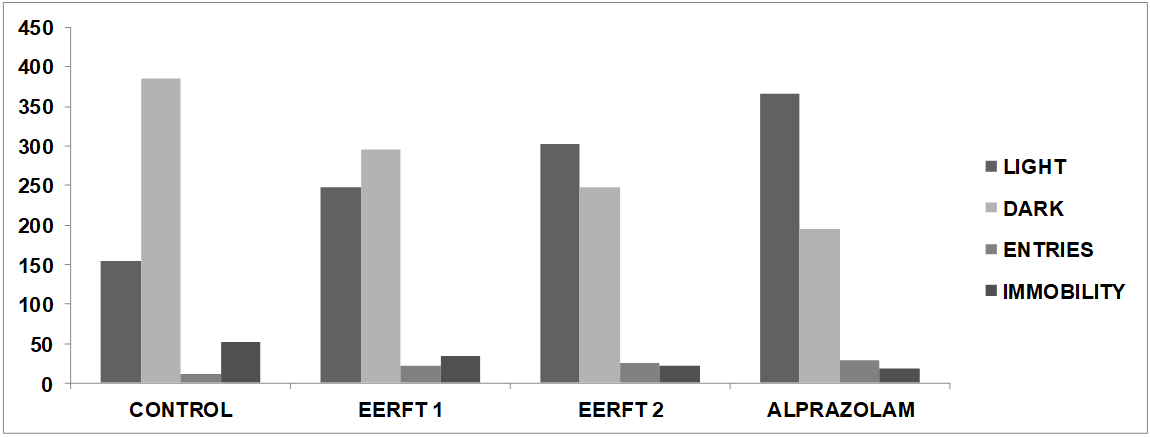
Fig. 2: Graphical representation of effects of different doses of Ficusthonningi iroot ethanolic extracton anxiolytic effect as compared to standard (Alprazolam)
Light dark model
The vehicle-treated mice (Normal control) had spent more time in dark, less number of entries and showed less mobility compared to test and standard drug treated group animals during 10 min.
Group 1: In this group animals were treated with Normal saline solution in which mice spent 154.8±1.02 sec in light chamber and 384.6±0.24 sec in dark chamber and no. of crossings were 12±0.12. Immobility period was 52±1.65 sec.
Group 2: In this group animals were treated with low dose of Ethanolic extract (200 mg/kg) solution in which mice spent 248.3±1.06 sec in light chamber and 294.5±0.55 sec in dark chamber and no. of crossings were 22±1.64. Immobility period was 34.1±0.29 sec.
Group 3: In this group animals were treated with high dose of Ethanolic extract (400 mg/kg) solution in which mice spent 302.6±0.26 sec in light chamber and 248.1±2.01 sec in dark chamber and no. of crossings were 25±1.01. Immobility period was 22.3±0.11 sec.
Group 4: In this group animals were treated with Alprazolam (0.25 mg/kg) solution in which mice spent 366.3±2.56 sec in light chamber and 194±0.25 sec in dark chamber and no. of crossings were 28±0.21. Immobility period was 18.5±0.16 sec.
In the light/dark exploration test, Ficusthonningiiroot significantly (p<0.05, 0.01) increased the latency of entry into the dark box, time spent in the light box, and number of rearing and assisted rearing. Treatment with Alprazolam significantly increased the time spent (P<0.001) in light box as well as the number of crossings between the light and dark boxes, but duration of immobility was significantly reduced. Ficusthonningiiroot treated mice also exhibited dose dependent significant increase in the time spent in light box and the number of crossings between light and dark boxes. The duration of immobility was also significantly reduced as compared to the vehicle treated group.
Open field test
The vehicle-treated mice (Normal control) had spent more time in center area, less number of grooming and showed less crossing compared to test and standard drug treated group animals during 10 min.
Group 1: In this group animal was treated with normal saline solution in which mice spent 17.8±1.53 sec time at centre, 26.3±2.34 sec. in grooming and 50±2.01 line of crossings.
Group 2: In this group animal was treated with low dose of Ethanolic extract (200 mg/kg) solution in which mice spent 32.5±1.22 sec time at centre, 9.6± 1.99 sec. in grooming and 25±1.59 line of crossings.
Group 3: In this group animal was treated with high dose of Ethanolic extract (400 mg/kg) solution in which mice spent 36.5± 0.56 sec time at centre, 7.6± 2.31 sec. in grooming and 22± 0.22line of crossings.
Group 4: In this group animal was treated with Alprazolam (0.25 mg/kg) solution in which mice spent 42.6±0.21 sec time at centre, 3.2± 0.22 sec. in grooming and 10± 0.21 line of crossings. Ficusthonningiiroot significantly (p<0.05, 0.01) increased the number of sectional crossings and rearing. Considering the social interaction test, Ficusthonningiiroot significantly (p<0.05) increased the number of interactions. Peak anti-anxiety effects were mostly observed at the dose of 400 mg/kg), Alprazolam and extract (Ficusthonningiiroot 200 and 400 mg/kg p. o.) treated mice showed significant increase in the number of rearing and number of squares crossed during 5-min interval as compared to vehicle treated control group. During Open field test, as time passed on, the number of squares visited by the mice of the different test groups decreased over time for an interval of 2h. It was seen that the ethanolic extract of Ficusthonningiiroot and its ethanolic soluble fraction showed the lower number of squares visited by the mice over time, compared to the control, indicating plant extract might have potential of anxiolytic effect.
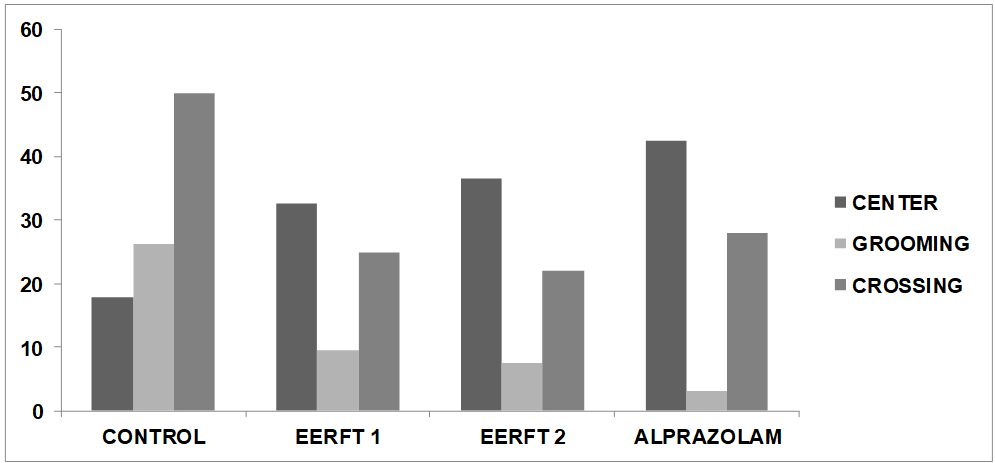
Fig. 3: Graphical representation of effects of different doses of Ficusthonningiiroot ethanolic extracton anxiolytic effect as compared to standard (Alprazolam)
CONCLUSION
The goal of the current study was to assess the anxiolytic impact of ethanolic extracts of the root portion of Ficusthonningii in mice using an elevated plus maze, an open field test, and a light-dark paradigm. The results were compared with those of a standard medication, alprazolam. Significant anxiolytic efficacy has been demonstrated by Ficusthonningiiethanolic extract in all LDM, OFT, and EPM models. Ficusthonningii extract given at two doses (200 mg/kg and 400 mg/kg) in a light-dark paradigm shown a reduction in the amount of time spent in the dark box and a notable increase in the amount of time spent in the light box, as well as the number of crossings between the light and dark boxes. Ficusthonningii extract given at two doses (200 mg/kg and 400 mg/kg) demonstrated a markedly higher number of rearings, fewer crossings, and a shorter grooming time in an open field test. As demonstrated by the common medication Alprazolam, Ficusthonningii exhibited anxiolytic effects. The outcomes were similar in all light-dark, open field, and elevated plus maze models. Ficusthonningii's ethanolic extract has the ability to lengthen its duration of action. As a result, we may deduce that it may also have anxiolytic effects. It can also enhance the amount of time spent in the open arm, entry in the open arm (in the elevated plus model), and light field (in the light dark field).
ACKNOWLEDGMENT
It’s our privilege to express the profound sense of gratitude and cordial thanks to our respected chairman Mr. Anil Chopra and Vice Chairperson Ms. Sangeeta Chopra, St. Soldier Educational Society, Jalandhar for providing the necessary facilities to complete this research work.
FUNDING
Nil
AUTHORS CONTRIBUTIONS
All authors have contributed equally
CONFLICT OF INTERESTS
Declared none
REFERENCES
Youngstedt SD, Kripke DF. Does bright light have an anxiolytic effect? an open trial. BMC Psychiatry. 2007 Dec 30:7:62. doi: 10.1186/1471-244X-7-62, PMID 17971237.
Khabbaz Mafinezhad M, Hosseini S, Hazar N. Stress anxiety and depression frequency among medical students of shahid sadoughi university of medical sciences in 2019. Health Res Dev. 2024 Mar 10;2(1):1-15. doi: 10.61186/jhrd.2.1.1.
Edition F. Diagnostic and statistical manual of mental disorders. Am Psychiatric Association. 2013;21(21):591-643.
Craske MG, Stein MB. Anxiety. Lancet. 2016;388(10063):3048-59. doi: 10.1016/S0140-6736(16)30381-6, PMID 27349358.
Ajayi AO. Antimicrobial nature and use of some medicinal plants in Nigeria. Afr J Biotechnol. 2008;7:595-9.
Meneses C, Valdes Gonzalez M, Garrido Suarez BB, Garrido G. Systematic review on the anxiolytic and hypnotic effects of flower extracts in in vivo pre-clinical studies published from 2010 to 2020. Phytother Res. 2023 May;37(5):2144-67. doi: 10.1002/ptr.7830, PMID 37039741.
Schmidt E, Lotter M, Mc Cleland W. Trees and shrubs of Mpumalanga and Kruger national park. Jacana media. 3rd ed. 1-1216; 2002.
Dangarembizi R, Erlwanger KH, Moyo D, Chivandi E. Phytochemistry pharmacology and ethnomedicinal uses of Ficus thonningii (Blume Moraceae): a review. Afr J Tradit Complement Altern Med. 2013 Jan 25;10(2):203-12. doi: 10.4314/ajtcam.v10i2.4, PMID 24146443.
WA. Anti-anxiety effects in mice following acute administration of Ficus thonningii (wild fig). Insights Depress Anxiety. 2018;2(1):40-7. doi: 10.29328/journal.ida.1001009.
Gupta V, Wankhede S, Deshmukh V, Juvekar A. Anxiolytic effect of Couroupita guianensis aubl flower extracts in mice. Int J Pharm Bio Sci. 2013 Aug 28;4(2):420-6.
The Organization of Economic Co-Operation and Development (OECD). The OECD guideline for testing of chemicals: 423 acute oral toxicity. Paris: OECD; 2001. p. 1-14.