
Int J Curr Pharm Res, Vol 17, Issue 5, 50-55Original Article
EVALUATION OF ANTIDEPRESSANT ACTIVITY OF AEGLEMARMELOS FRUIT EXTRACTS IN MICE
SAVITA*, AJEET PAL SINGH, AMAR PAL SINGH
Department of Pharmacology, St. Soldier Institute of Pharmacy, Lidhran Campus, Behind NIT (R. E. C.), Jalandhar –Amritsar by pass, NH-1, Jalandhar-144011, Punjab, India
*Corresponding author: Savita; *Email: jaswalsavita19@gmail.com
Received: 09 Jun 2025, Revised and Accepted: 27 Jul 2025
ABSTRACT
Objective: To evaluate Antidepressant Activity of Aqueous and Ethanolic extracts of Aeglemarmelos fruit in albino mice by using Forced swimming test (FST) and Tail suspension test (TST) parameters.
Methods: For the study, 25–40 g healthy adult Swiss albino mice of both sexes will be housed in a conventional laboratory environment with a temperature of 25±2 °C, and a 12 h light–12 h dark cycle. Food and water will be offered ad libitum. The two antidepressant Animal model are used for asses the activity. A) Forced swimming test: This model requires the mice to swim or float for five minutes while submerged 40 cm in 23±1 °C of water in a glass cylinder. b) Tail suspension test in mice: In this model the mice are suspended at the edge of shelf approximately 58 cm above the tabletop by adhesive tape that is placed approximately 1 cm from the tip of the tail, and the duration of immobility is recorded for a 5 min duration.
Results: In the present study, we have undertaken the antidepressant activity of ethanolic and aqueous extracts of Aeglemarmelos in Swiss albino mice, at the dose of 250 and 500 mg/kg, respectively, with oral administration respectively.
Conclusion: In conclusion both the extracts ethanolic and aqueous extracts of Aeglemarmelos have shown significant results as an antidepressant effect using forced swim test (FST) and Tail Suspension test (TST) parameters.
Keywords: Aeglemarmelos, Antidepressant, FST – forced swim test, TST – Tail suspension test
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijcpr.2025v17i5.7025 Journal homepage: https://innovareacademics.in/journals/index.php/ijcpr
INTRODUCTION
The rise of antidepressant medicines spearheaded the biochemical theory of depression, which largely would claim that the major lesion in the disease states was the derangement of central monoaminergic activity. Although the monoaminergic system is indisputably one of the main pieces to the puzzle with their roles in depression treatment, other systems in the brain and regulation of central nervous system activity must also be taken into consideration. Many herbs, plants, and metals have been suggested in ancient Indian texts for the treatment of depression and anxiety. This is because it has been noted that some medicinal herbs have anti-anxiety and anti-depressant properties. Only few of those herbs found in the herbal formulation have been used; hence, the need to explore and investigate more about natural antidepressants with little to no side-effects continues to intensify [1]. Bael, also known as Aeglemarmelos, is an Indian tree with vast medicinal value in the Rutaceae family. Bael is also known by several other common names, both on-site and off. Bael is well known to all people in India, and a lot of its healing properties were noted in the ancient Indian medical practices. All types of parts of the bael tree lead to so much potential as medicine that it is one of the most important medicinal plants in India, with the root, bark, leafs, flower, fruit, seed, and latex being used in various types of traditional systems of medicines. Bael is a medium-sized, armed, deciduous tree found in nature occurring in India, predominantly in the outer Himalayas, Shivaliks and dry forests. Bael is also cultivated for its fruits throughout the subcontinent, found in areas holding elevations from 250m-1200m in south India [2, 3]. Bael fruit exhibits many pharmacological actions; it has anti-dyspepsia, anti-dysentery, and anti-bacterial actions. As a dietary supplement, it has been used as a treatment for mental health issues, intermittent fever, hypoglycemia, antifungal properties, antimicrobial, analgesic, anti-inflammatory, antipyretic, antidyslipidemic activity, immunomodulatory, antiproliferative properties, wound-healing activity, antifertility, insecticidal properties, and many other conditions [4]. The bael plant is a "sink" for chemical pollutants because it absorbs toxic gasses from the atmosphere, rendering them neutral or inert. It is part of a collection of plant species called "Climate Purifiers", which produce the greatest percent of oxygen in sunlight of any plant. It is also a "fragrant" tree [5].
MATERIALS AND METHODS
Collection of data
A dose-dependent or time-dependent trial can be done using both aqueous and ethanolic extractions of Aeglemarmelos. In addition, the dosage amounts for each Aeglemarmelos extraction according to the literature review and proposed dosing schedule were: 250 and 500 mg/kg.
The data will be evidence gathered from a study using experimental animals. The study protocols were signed off by the Institutional Animal Ethics Committee (IAEC) and the study was guided by the Committee for the purpose of Control and Supervision of Experimental Animals (CPCSEA) guidelines of Ministry of Environment and Forests, Government of India (Reg. No. 2011/PO/Re/S/18/CPCSEA, registered 1/5/2018). Sufficient care was taken in mitigating any pain or distress to the animals used during the study. The research protocol is in accordance with IAEC/CPCSEA (IAEC/SSIP/2020/PR-015).
Chemical
All chemicals of analytical grade were procured from Sigma chemical, USA and SD. Fine Chem. Ltd., India.
Collection and preparation of plant material
The aquous and ethanolic extract of fruit part of the plant Aeglemarmelos were procured from Shreedha Phyto Extract, Jaipur. The same group also provided a certification of the plant's identity and quality (Certificate of Analysis).
Preparation and administration of crude extracts/standard drugs [6, 7]
Extraction is the fully established method for separating the active ingredients from the unrefined drugs. Their methods of extraction typically do not yield pure extracts but rather liquid, semi-solid or powder products that contain one or more of the medicinally active parts of plant or animal tissues plus some inactive or inert plant or animal components. They are meant for topical or taken or consumed orally. The extraction methods used on crude pharmaceuticals (plant parts) utilize standardized extraction techniques to obtain the therapeutically desirable components of the plant or animal drug from the water-soluble or insoluble, unwanted material by treating, or extracting, the crude with a menstrum (selective solvent); thereafter, the extract, after standardization to the easy normal, could become the medicine. These extracts consist of numerous metabolites with multiple terpenoids, flavonoids, lignans, alkaloids, glycosides etc.
Animals
Healthy, adult swiss albino mice of either sex weighing (25-40 g), maintained under standard laboratory conditions, at temperature 25±2 °C and a 12 h light-12 h dark period, was employed for the experimentation. Food and water were provided ad libitum.
Acute oral toxicity study [8]
After conducting an acute toxicity study with both ethanolic and aqueous extracts of 'Aeglemarmelos' with minimum protocol as per OECD recommendations No: 423, we selected low, medium, and high doses for therapy.
Method
Three groups of mice were formed from the overnight fasting mice. The EEAMF and AEAMF were administered orally via a gawage at various dose ranges (5, 50, 300, and 1000). After the animals were given the extract, they were closely monitored for the first two hours and the first twenty-four hours for any behavior changes, and were evaluated for tremors, convulsions, salivation, diarrhea, lethargy, sleep, and coma. Animals would be monitored for toxic symptoms and mortality for up to fourteen days. Two weeks after acute oral toxicity, the surviving mice were repaired and used again for experimental purposes.
Behavioral tests for depression in mice/mouse as a model for depression [9, 10]
One of the main advantages of using mice as models is their significant genetic, structural, and functional similarities to humans. Because more than 95% of the genomes of mice and humans are both identical, research into genetics and mice is more translatable to concerns of health in humans. Anxiety and depression Animals serve as experimental models in research related to brain and central nervous system functions. Animal model is very important to preclinical research in the neurobiology of psychiatric disease and also to find new treatment drugs as conduct screening paradigms using animal models. Since, more than 90% of mouse and human genes are homologues, rodents, with a focus on mice, are useful in this type of research. Animal models are very helpful when researchers cannot ethically or otherwise examine the effects of stress on humans. In addition to the ethical advantage to researchers, there is an also economical advantage, smaller body weight and doses, ease of handling and reproduction rates. These contributions would undoubtedly make research simpler and perhaps less expensive for the researcher. Animal models related to anxiety and depression are very helpful in determining new treatment modalities related to this type of disorders.
Acute or subacute (07 d period) behavioral effects will be given orally. Swiss mice will be tested using the forced swim test (FST) and tail suspension test (TST) with 250 and 500 mg/kg of Aegle marmelos ethanol and aqueous extract. We will also evaluate the effects of fluoxetine (FXT; 10 mg/kg).
Forced swimming test
The most common behavioral paradigm for assessing the effects of antidepressants in mice. Each mouse was placed in a 25×15×25 cm glass chamber with fresh water filled approximately 15 cm high and maintained at 26°±1 °C. In the test his chamber, every animal will swim vigorously for the first two minutes. The last three minutes of the five-minute test session will involve manually measuring the amount of immobility. Mice will be defined as immobile if they have stopped struggling and are floating in the water, using only enough movements to keep their head above the water. Following the swimming session, mice will be towel-dried and placed back in their housing.
Tail suspension test in mice
In this method, 30 min prior to testing, groups of 10 rats receive intraperitoneal injections of either the vehicle or the test drugs. Adhesive tape is placed around 1 centimeter from the tips of the mice's tails to suspend them over the edge of a shelf that is 58 cm above a tabletop for test purposes. The time required for recording duration of immobility is five minutes. A period of immobility is noted when the mice hang down passively and do not move for at least one minute; they are determined to be immobile.
Is a commonly employed method for evaluating novel knowledge of the effects of antidepressant drugs. Imipramine and fluoxetine have proven therapeutic efficacy to treat human depression, as they can reverse immobility, which is an indicator of helplessness. The measure of depression in this experimental model is based on immobility using the duration of immobility within a specified time. Extending the amount of time that the period of immobility lasts denotes mental depression, while shortening the time frame symbolizes a mental state without depression. The adhesive tape was placed at approximately one centimeter from the tips of the mice's tails, where they were suspended.
Immobility – the mouse hangs without engaging in any activity;
Swinging – keeping its body straight, the mouse continuously moves its paws in a vertical position and/or moves its body from side to side;
Curling – the mouse engages in active twisting movements.
Laboratory models employed to produced stress [11-15]
Chronic unpredictable mild stress procedure (CUMS)
The mice were subjected to chronic unpredictable mild stress with some modifications. Animals were subjected to stress paradigm once a day over a period of 3 w between 09:00 and 14:00 h.
Table 1: Chronic unpredictable mild stress procedure (CUMS)
| Weeks | Day-1 | Day-2 | Day-3 | Day-4 | Day-5 | Day-6 |
| Week-1 | I | E | F | O | T2 | X |
| Week-2 | I | O | X | T2 | E | T1 |
| Week-3 | O | F | T1 | X | T2 | I |
Note: I— Immobilization for 2 h; E— Exposure to empty water bottles for 1 hour; F— Exposure to foreign object for 24 h (e. g. piece of plastic); O— Overnight illumination; T2— Tail pinch (60s). X— Tilted cage at 45 degree for 7 h; T1— Tail pinch (30s).
Mice subjected to CUMS procedure were called as stressed mice. Unstressed mice were exposed to behavioral tests, and not subjected to CUMS procedure. Drugs were administered 30 min before CUMS procedure in case of stressed mice.
Procedures for tail suspension test stress
The tail suspension test (TST) is a behavioral test widely used for evaluating antidepressant-like activity of a drug (Steru et al., 1985). In the test, the mice were individually suspended 50 cm above the surface of a floor, using an adhesive tape placed 1 cm away from the tip of the tail. Each animal under test was both acoustically and visually isolated from other animals during test. The total period of immobility was recorded manually for 6 min. Animal was considered to be immobile when it didn’t show any body movement, hung passively and completely motionless.
Procedures for forced swimming stress
Animals were made to swim for different times in a rectangle plastic tank (45x35x18 cm) containing 15 cm of water, according to the stressed swimming test. The water temperature was kept at a constant approximate temperature of 23 °C. After swimming, the mice were dried with towels before collecting the blood sample from a tail vein.
Rapid effects of forced swimming stress/tail suspension test stress on dynamics and correlation of plasma corticosterone
Prior to being selected for blood extraction for hormone testing, mice were put through forced swimming of different durations in order to assess the acute effects of extreme stress on the dynamics and association of plasma corticosterone. Because the concentration of cortisol peaked at 3 min during forced swimming stress, corticosterone did not peak until 40 min in to the stress. Forced swimming test and tail suspension test showed a high correlation between both hormones. It seems cortisol and corticosterone reacted to extreme stressors in different patterns; specifically, cortisol reacted faster than corticosterone.
Biochemical estimation
Collection of blood samples
On 15th day, blood (0.3 ml) was withdrawn from tail vein from all groups of mice. Blood samples were centrifuged at 2500 rpm for 10 min using refrigerated centrifuge (Paramount Scientific Works, Ambalacantt, India) to separate the plasma, which was used for estimation of corticosterone levels.
Estimation of plasma corticosterone levels
This method was used to estimate tha amounts of corticosterone in the blood plasma quantitatively. The tubes containing the 1.0 ml samples in ethanol, 0.50 ml of a 0.10 N-sodium hydroxide, and 0.50 ml of a 0.10 percent solution of p-nitroso-N, N-dimethylaniline in ethanol was placed in an ice-water bath for five minutes. Next, tubes were capped with cotton-wool and kept in an opaque place at 0 °C for five hours. To this solution, added 2.0 ml of a pH 9.8 buffer, 5.0 ml of a 0.10 % phenol in ethanol solution, and 0.50 ml of a 1.0 % potassium ferricyanide aqueous solution. Finally, the tubes were placed in a water bath at 20±2 °C for ten minutes.
Groups 1 to 6 were tail bled on day 1 and then corticosterone levels were combined to obtain the average levels in tail blood. For treatment of Groups see their respective experimental design.
Experimental design
Group 1: control (solvent)
Group 2: mice treated with EEAMF (Low dose)
Group 3: mice treated with EEAMF (High dose)
Group 4: mice treated with AEAMF (Low dose)
Group 5: mice treated with AEAMF (High dose)
Group 6: mice treated with fluoxetine.
Body weight analysis
During the same time of study, body weight analysis will be recorded.
Statistical analysis
All the results will be expressed as standard error of mean (S. E. M.). Data will be analyzed using one-way ANOVA (Graphpad prism version 5.00 software) followed by suitable post test like Dunnett’s t-test. p<0.05 will be considered as statistically significant.
Total no. of animals required
No of the animal in each group = 06
No. of groups = 06
Total no. of animals required = 06×06 = 36
RESULTS
Acute oral toxicity study
The median lethal dose (LD50) of AEAMF and EEAMF was determined according to the OECD (425) guidelines, using four mice fasted overnight before receiving the different extracts of AEAMF and EEAMF at maximum dose levels of 1000 mg/kg orally (commencing doses of 5, 50 and 300 mg/kg). Upon the first dose of AEAMF or EEAMF, one mouse had food withheld for another 4 h.
Toxicity signs (dependent upon the damages to the mucous membranes, skin, fur, and eyes, and upon any circulatory, respiratory, somato-motor and behavioural patterns and signs) and mortality were measured for the first 24 h and thereafter for 14 days. The behavioural reactions were unchanged, and no signs of acute oral toxicity. The contrary four mice were given doses for the duration of two weeks accordingly to their assigned treatments, and then the LD50 was determined again.
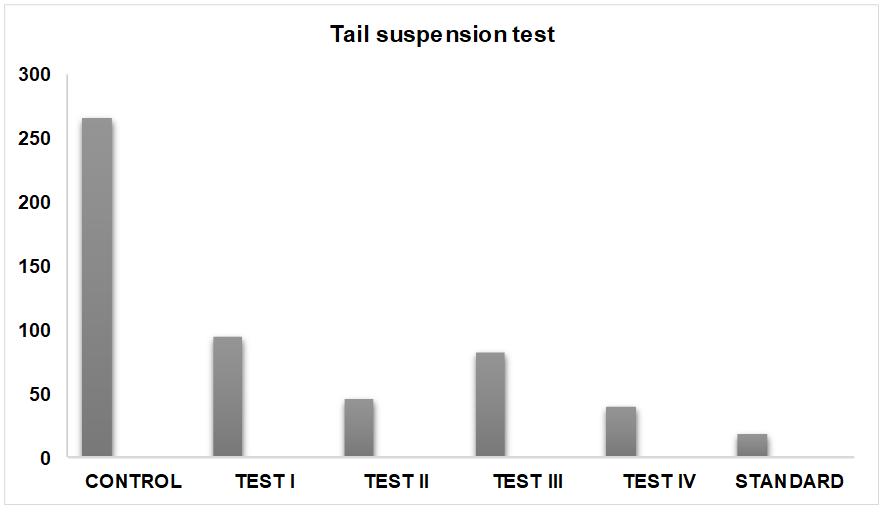
Fig. 1: Antidepressant effect of Aeglemarmelos fruit extracts tail suspension test
Evaluation of antidepressant effect of Aeglemarmelos fruit extracts in TST model
Group 1: In this group animals were treated with Normal saline solution in which mice Immobility period was 268±3.826 sec.
Group 2: In this group animals were treated with low dose of Aqueous extract (250 mg/kg) solution, in which mice Immobility period was 92.6±2.384 sec.
Group 3: In this group animals were treated with high dose of Aqueous extract (500 mg/kg) solution in which mice Immobility period was 45.4±1.845 sec.
Group 4: In this group animals were treated with low dose of Ethanolic extract (250 mg/kg) solution, in which mice Immobility period was 84.0±2.742 sec.
Group 5: In this group, animals were treated with high dose of Ethanolic extract (500 mg/kg) solution in which mice Immobility period was 36.6±2.664 sec.
Group 6: In this group, animals were treated with Fluoxetine (10 mg/kg) solution in which mice Immobility period was 18.6±1.628 sec.
Treatment with Fluoxetine significantly decreased the duration of immobility time (P<0.001) in TST. Ethanolic and aqueous extract of Aeglemarmelos treated mice also exhibited dose dependent significant decreased the duration of immobility time.
The duration of immobility was also significantly reduced as compared to the vehicle-treated group. But there is no significant difference between Aeglemarmelos extracts treated animals and Fluoxetine treated animal. The above observation suggests that Aeglemarmelos has antidepressant activity.
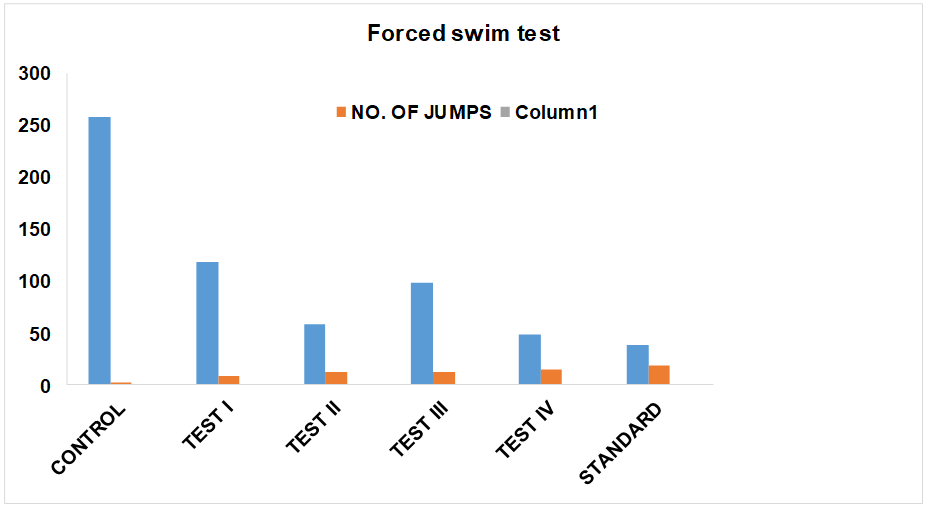
Fig. 2: Antidepressant effect of aeglemarmelos fruit extracts in forced swimming test
Evaluation of antidepressant effect of aeglemarmelos fruit extracts in FST model
Group 1: In this group animals were treated with Normal saline solution in which mice Immobility period was 246.4±2.966 sec. and numbers of jumps were 2.6± 0.384.
Group 2: In this group animals were treated with low dose of Aqueous extract (250 mg/kg) solution, in which mice Immobility period was 107±3.868 sec and number of jumps were 9.4±0.586.
Group 3: In this group animals were treated with high dose of Aqueous extract (500 mg/kg) solution, in which mice Immobility period was 48.4±2.368 sec and number of jumps were 12.8±0.386.
Group 4: In this group animals were treated with low dose of Ethanolic extract (250 mg/kg) solution, in which mice Immobility period was 98.8±3.644 sec and number of jumps were 12.2±0.782.
Group 5: In this group animals were treated with high dose of Ethanolic extract (500 mg/kg) solution in which mice Immobility period was 38.8±1.746 sec and number of jumps were 14.8±0.942.
Group 6: In this group animals were treated with Fluoxetine (10 mg/kg) solution, in which mice Immobility period was 34.8±1.726 sec and numbers of jumps were 18.8 ±0.826.
Treatment with Fluoxetine significantly decreased the duration of immobility time (P<0.001) in FST. Ethanolic and aqueous extract of Aeglemarmelos fruit treated mice also exhibited dose-dependent significant decreased the duration of immobility time. The duration of immobility was also significantly reduced as compared to the vehicle-treated group.
But there is no significant difference between Aeglemarmelos extracts-treated animals and Fluoxetine treated animal. The above observation suggests that Aeglemarmelos has antidepressant activity.
Plasma corticosterone levels
Estimation of plasma corticosterone levels
The technique was applied to quantitatively assess the levels of corticosterone in blood plasma. Following the addition of 0.50 ml of sodium hydroxide, 0.10 N, to a 1.0 ml sample in ethanol, and 0.50 ml of a 0.10 percent solution of p-nitroso-N, N-dimethylaniline in ethanol, the tubes were immersed in ice water for five minutes. The tubes were then stoppered with cotton-wool and were stored in a darkened space at 0 °C for five hours. 2.0 ml of pH 9.8 buffer, 5.0 ml of a 0.10 % phenol, in ethanol solution, and lastly, 0.50 ml of a 1.0 % aqueous solution of potassium ferricyanide were added to the solution mentioned above and the tubes were held at 20±2 °C, in a water bath, for ten minutes. The solution was read at 650 nm on a UV-visible spectrophotometer (UV 3200 UV-VIS Spectrophotometer, Somajiguda, Hyderabad). Groups 1 to 6 were tail bled on day 1 and then corticosterone levels were combined to obtain the average levels in tail blood. For treatment of Groups, see their respective experimental design.
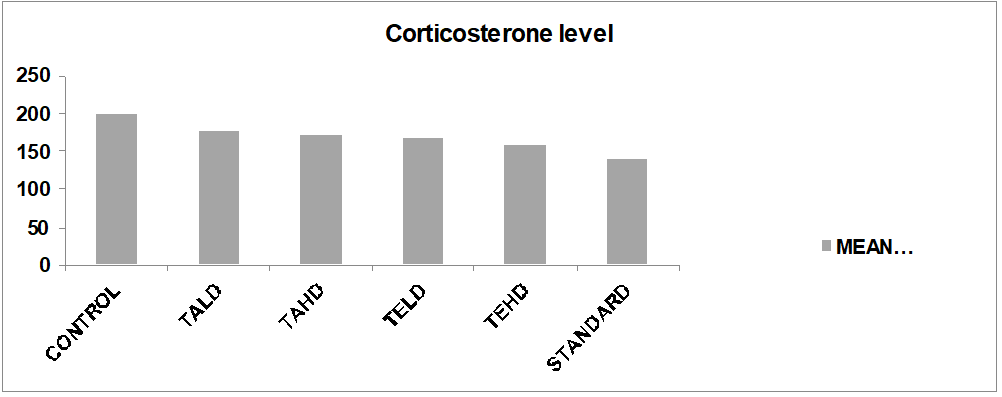
Fig. 3: Showing the corticosterone levels in post-tail suspension test experiments
Note:-TALD= Test Aqueous lower dose; TAHD= Test Aqueous higher dose; TELD= Test Ethanolic lower dose; TEHD= Test Ethanolic higher dose; STD= standard
Group 1: In this mice were treated with Normal saline and corticosterone level is measured, which is 198.88±0.58 ng/ml
Group 2: In this mice were treated with low dose of Aqueous extract and corticosterone level is measured which is 177.44±1.42 ng/ml
Group 3: In this mice were treated with High dose of Aqueous extract and corticosterone level is measured which is 171.96±2.24ng/ml
Group 4: In this mice were treated with low dose of Ethanolic extract and corticosterone level is measured which is 166.26±1.28 ng/ml
Group 5: In this mice were treated with High dose of Ethanolic extract and corticosterone level is measured which is 156.48±0.32 ng/ml
Group 6: In this mice were treated with Standard (Fluxetine) and corticosterone level is measured which is 142.62±0.38 ng/ml
It is recognized that stress can increase the activity of the hypothalamus-pituitary-adrenal (HPA) axis and induce the adrenal cortex to secrete increased amounts of corticosteroids. Because of that, cortisol and corticosterone are commonly referenced as biomarkers for depression and stress. Due to ease of use and the general availability of kits, researchers often choose to measure cortisol for stress indicators when corticosterone is considered to be the primary glucocorticoid involved in the regulation of the stress response in mice.
CONCLUSION
The objective of the present study is to evaluate the antidepressant effects of ethanol and aqueous extract of Aeglemarmelos fruit in mice using the TST and FST models, which will also be compared to treatment effects observed for the conventional medication fluoxetine. Nutritionally, Bael (Aeglemarmelos) fruit pulp powder provides a good pool of nutrients. The fruit pulp was high in vitamin C and carbs. Based on the aforementioned nutritional attributes, the portion of Bael in the current study could be considered a new source of nutritious food since it is rich in vitamin C, carbohydrates, and a caloric source that would provide adequate energy. Aeglemarmelos ethanol and aqueous extract exhibited significant enzeytic effectiveness in TST and FST models. Aeglemarmelos ethanol and aqueous extract were shown to significantly reduce immobility times in both TST (250 mg/kg and 500 mg/kg) respectively, and FST was significantly reduced again by Aeglemarmelos (250 mg/kg and 500 mg/kg). The effect seen by fluoxetine as a conventional medication that turned out to be classified as having some antidepressant intervention in Aeglemarmelos, effects of treatment were consistent with each other in TST and FST models. In conclusion, the fruit extract from Aeglemarmelos demonstrated effective antidepressant activity, most likely due to the GABA-facilitating effects of many of its phytoconstituents, such as flavonoids, tannic acid, marmesinin, phenols, saponin, and so on. Stress activates the hypothalamus-pituitary-adrenal (HPA) axis, causing the adrenal cortex to secrete more corticosteroids. Hence, cortisol and corticosterone are used as biomarkers of depression and stress, respectively. Due to ease of use and availability of kits, researchers tend to choose cortisol for detecting stress markers, although corticosterone is considered to be the main glucocorticoid involved in regulating stress responses in mice.
ACKNOWLEDGMENT
It’s our privilege to express the profound sense of gratitude and cordial thanks to our respected chairman Mr. Anil Chopra and Vice Chairperson Ms. Sangeeta Chopra, St. Soldier Educational Society, Jalandhar for providing the necessary facilities to complete this research work.
FUNDING
Nil
AUTHORS CONTRIBUTIONS
All authors have contributed equally
CONFLICT OF INTERESTS
Declared none
REFERENCES
Brigitta B. Pathophysiology of depression and mechanisms of treatment. Dialogues Clin Neurosci. 2002 Mar;4(1):7-20. doi: 10.31887/DCNS.2002.4.1/bbondy, PMID 22033824, PMCID PMC3181668.
Monika S, Thirumal M, Kumar PR. Phytochemical and biological review of Aegle marmelos Linn. Future Sci OA. 2023 Mar 1;9(3). doi: 10.2144/fsoa-2022-0068.
Singh R, Singh A, Babu N. Ethno-medicinal and Pharmacological activities of Aegle marmelos (Linn.) Corr: a review. J Pharm Innov J. 2019;8(6):176-81.
Lamia SS, Shimo MS, Rashed SS, Prima AA, Mony AT, Dash PR. Phytochemistry and pharmacological properties of Aegle marmelos l (Rutaceae): a review. Phytochemistry. 2018 May;3(3):45-54.
Choudhary Y, Saxena A, Kumar Y, Kumar S, Pratap V. Phytochemistry, pharmacological and traditional uses of Aegle marmelos. Pharmaceutical and Biosciences Journal. 2017 Oct 20:27-33.
Pandey MM, Rawat AK. Pharmacognosy and pharmacopoeial standards for Syzygium cumini. In: The genus Syzygium. CRC Press; 2017 Apr 7. p. 119-32.
Nain JY, Bhatt SH, Dhyani S, Joshi NU. Phytochemical screening of secondary metabolites of Datura stramonium. Int J Curr Pharm Res. 2013;5(2):151-3.
Gribaldo L, Gennari A, Blackburn K, Clemedson C, Deguercy A, Meneguz A. Acute toxicity. Altern Lab Anim. 2005 Jul;33(1Suppl 1):27-34. doi: 10.1177/026119290503301s07, PMID 16194139.
Vogel HG, editor. Drug discovery and evaluation: pharmacological assays. 2nd ed. Springer Science+Business Media; 2002 Jun 13. p. 17-72.
Taiwo AE, Leite FB, Lucena GM, Barros M, Silveira D, Silva MV. Anxiolytic and antidepressant-like effects of Melissa officinalis (lemon balm) extract in rats: influence of administration and gender. Indian J Pharmacol. 2012 Mar-Apr;44(2):189-92. doi: 10.4103/0253-7613.93846, PMID 22529473.
Kant GJ, Leu JR, Anderson SM, Mougey EH. Effects of chronic stress on plasma corticosterone, ACTH and prolactin. Physiol Behav. 1987 Jan 1;40(6):775-9. doi: 10.1016/0031-9384(87)90282-4, PMID 2823307.
Garrido P, de Blas M, Del Arco A, Segovia G, Mora F. Aging increases basal but not stress-induced levels of corticosterone in the brain of the awake rat. Neurobiol Aging. 2012 Feb 1;33(2):375-82. doi: 10.1016/j.neurobiolaging.2010.02.015, PMID 20416975.
Handa RJ, Burgess LH, Kerr JE, O’Keefe JA. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm Behav. 1994 Dec 1;28(4):464-76. doi: 10.1006/hbeh.1994.1044, PMID 7729815.
Bhattacharya S, Herrera Molina R, Sabanov V, Ahmed T, Iscru E, Stober F. Genetically induced retrograde amnesia of associative memories after neuroplastin ablation. Biol Psychiatry. 2017 Jan 15;81(2):124-35. doi: 10.1016/j.biopsych.2016.03.2107, PMID 27215477.
Petrella C, Giuli C, Agostini S, Bacquie V, Zinni M, Theodorou V. Maternal exposure to low levels of corticosterone during lactation protects against experimental inflammatory colitis-induced damage in adult rat offspring. PLOS One. 2014 Nov 18;9(11):e113389. doi: 10.1371/journal.pone.0113389, PMID 25405993.