
Int J Curr Pharm Res, Vol 17, Issue 3, 24-28Original Article
COMPARATIVE STUDY OF EFFICACY AND SAFETY OF ESCITALOPRAM WITH ESCITALOPRAM AND CLONAZEPAM IN PATIENTS SUFFERING FROM GENERALIZED ANXIETY DISORDER–A PROSPECTIVE RANDOMIZED CONTROL STUDY
D. KUSHBU1*, N. M. RIYAZ1, O. ANWAR BASHA2, SHAIK AQUIB AHMED1
1Department of Pharmacology, Government Medical College, Ananthapuram, Andhra Pradesh, India. 2Department of Psychaitry, Government Medical College, Kadapa, India
*Corresponding author: D. Kushbu; *Email: drkush786@gmail.com
Received: 20 Jan 2025, Revised and Accepted: 10 Mar 2025
ABSTRACT
Objective: Anxiety Disorders (AD) are one of the common psychiatric illnesses that adversely affect an individual’s quality of life, functioning and productivity. Generalized Anxiety Disorder (GAD) is a mental and behavioural disorder characterized by excessive, uncontrollable and often irrational worry about events or activities. This study was conducted to assess the efficacy and safety of Escitalopram and Clonazepam combination in comparison with Escitalopram alone.
Methods: Institutional ethical committee approval and informed consent obtained prior to the study. Total of 159 participants were included, among which 78 in group A and 81 in group B. Group A received Escitalopram and group B received Escitalopram and Clonazepam combination. Participants were followed up at 2nd, 4th, 6th and 8th w and reduction in anxiety was assessed using generalized anxiety disorder-7 (GAD-7) questionnaire.
Results: The mean±standard deviation (SD) values of GAD-7 scale at baseline and at the end of 8th week in Group A were 15.68±2.85 and 7.01±1.42 respectively and in group B were 16.63±2.86 and 3.54±0.88 respectively; with a p-value of<0.0001. The decrease in GAD-7 scores is significant in both groups, but more reduction is observed in group B. Mild adverse drug reactions were noted in both the groups. There was no significant difference in socio-demographics data between the groups like age, gender, education and marital status.
Conclusion: Results of our study show that Escitalopram and Clonazepam combination is more beneficial for Generalized Anxiety disorder patients. Combining a benzodiazepine to a SSRI improves efficacy and tolerability; thus increasing compliance among patients.
Keywords: Generalized anxiety disorder, Escitalopram, Clonazepam, SSRIs, BZDs, GAD-7
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijcpr.2025v17i3.55046 Journal homepage: https://innovareacademics.in/journals/index.php/ijcpr
INTRODUCTION
Anxiety Disorders (AD) are one of the common psychiatric illnesses that adversely affect an individual’s quality of life, functioning and productivity and, thus, loss of national income. Anxiety disorders are characterised by physical symptoms and are frequently associated with psychiatric illnesses. Anxiety disorders are broadly classified into three types such as Generalized Anxiety Disorder (GAD), panic disorder and social anxiety disorder.
GAD is a mental and behavioural disorder characterized by excessive, uncontrollable and often irrational worry about events or activities. Worry often interferes with daily functioning, and individuals are often overly concerned about everyday matters such as health, finances, death, family, relationship concerns, or work difficulties. Symptoms may include excessive worry, restlessness, trouble sleeping, exhaustion, irritability, sweating, and trembling [1]. Generalized anxiety disorder is diagnosed when an individual is worrying constantly and unable to control the worry; affecting the daily activities of the individual on most of the days, for a span of at least 6 mo.
As per World Health Organization (WHO), world mental health report, 2022 [2]; in 2019, 13% (970 million) of global population were living with mental disorders, among whom 4% (301 million) with anxiety disorder and 3.8% (280 million) with depressive disorder. In both males and females, depression and anxiety were two common mental disorders with 3% and 5% prevalence respectively. Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2019 [3] estimated a substantial increase in prevalence estimates of depression and anxiety as a result of COVID-19 pandemic, which were 27.6% and 25.6%, respectively.
National Mental Health Survey (NMHS) 2015-16 [4], a multicentred survey in 12 states across six regions (North [Punjab and Uttar Pradesh]; South [Tamil Nadu and Kerala]; East [Jharkhand and West Bengal]; West [Rajasthan and Gujarat]; Central [Madhya Pradesh and Chhattisgarh]; and North-East [Assam and Manipur]); was done to assess the prevalence, pattern and outcomes of mental disorders by National Institute of Mental Health and Neuro Sciences (NIMHANS). According to the study, the GAD had a lifetime as well as current weighted prevalence of 0.57 (95% CI); where females (0.8%), 40-49 age group (0.8%) and urban metro residents (1.3%) had higher rates than their counterparts.
Management of GAD includes psychotherapy, pharmacotherapy or both. Recommended first-line pharmacological treatments are selective Serotonin Reuptake Inhibitors (SSRI), selective Norepinephrine Reuptake Inhibitors (SNRI) and Pregabalin; while second-line are Tricyclic Anti-depressants (TCA), Mono Amino Oxidase inhibitors (MAOI) and Benzodiazepines (BZD). First-line SSRI therapy is associated with high rate of discontinuation due to late onset of efficacy; which can be taken care by use of BZD. Not many studies available assessing the combination of Escitalopram and Clonazepam.
Hence, this study was conducted to assess the efficacy and tolerability of combination of Escitalopram and Clonazepam with Escitalopram alone in the management of GAD. This study shall fill the gap and aid clinicians to plan for effective treatment for General Anxiety Disorder.
MATERIALS AND METHODS
This was a prospective, randomized and controlled study. Prior Institutional Ethics Committee approval obtained with approval number; 7-12-23, dt. 30/12/2023. Study conducted for a period of 6 mo from January 2024 to June 2024. The study population included patients attending Psychiatry outpatient department (OPD), Government General Hospital (GGH), Ananthapuram. The study subjects; diagnosed with Generalized Anxiety Disorder, satisfying the inclusion and exclusion criteria and who were willing to give written informed consent were included in the study. Simple random sampling method was used for randomization.
Sample size was calculated based on below formula [5]
![]()
Zα/2 = 1.96 (from Z table) at type I error of 5%
Zβ = 0.84 (From Z table) at 80% power
P1 = Proportion of patients with remission after 8 w treatment with Escitalopram = 61.3% = 0.613 (as reported in a study done by Wang Get al.) [6]
P2 = Assumption of 20% increase in remission after 8 w treatment with combination of Escitalopram and Clonazepam = 81.3% = 0.813
P = Pooled prevalence = [p1+p2]/2
= 0.713
Sample size amounted to = 80 participants per group
So, Final sample considered for the study = 80 participants per group
Statistical analysis
Data entered into Microsoft Excel and analysis done using IBM Statistical Package for the Social Sciences (SPSS) version 25.0 (IBM Corp., Armonk, NY, USA). Categorical variables were presented in number and percentage and continuous data were presented in mean and standard deviation (SD) or median and interquartile ranges depending on the normality of the data after testing normality of the data by using Shapiro-Wilk test. Proportions were compared using Chi-Square test with or without Yate’s correction or Fisher’s exact test. Student t-test was used for normally distributed continuous data and the Mann-Whitney U test was used for variables violating normal distribution. For all the comparisons P value of less than 0.05 was considered statistically significant.
Procedure
After receiving approval from Institutional Ethics Committee, written informed consent was taken from potential participants and were screened for selection criteria. A total of 166 patients were included and divided into two groups; group A of 78 patients (3 dropouts) received Escitalopram 10 mg and group B of 81 patients (4 dropouts) received Escitalopram10 mg and Clonazepam 0.5 mg combination. Anxiety reduction assessment was conducted at multiple points – 0 d, 2nd w, 4th w, 6th w and 8th w. Impact of the treatment on anxiety was assessed by using Generalized Anxiety Disorder-7 (GAD-7) [7] questionnaire and assessed as per the score obtained.
Efficacy and safety parameters
Patients were monitored for the following
Reduction in anxiety: assessed by using the GAD-7 questionnaire.
GAD-7 score
0-4: minimal anxiety
5-9: mild anxiety
10-14: moderate anxiety
15-21: severe anxiety
Adverse drug reactions (ADRs)
ADRs due to the study drugs were monitored, assessed and managed by the healthcare team. Participants were educated on recognizing and reporting any adverse effects.
Methods of collection of data
Details of collection of data explained and Informed consent obtained from patient. Efficacy and Safety of study drugs was assessed by symptomatic relief using a questionnaire called Generalised Anxiety Disorder-7 (GAD-7) score.
Inclusion criteria were patients in the age group of 18-59 y, diagnosed with generalized anxiety disorder as per DSM-V criteria and who were willing to give written informed consent.
Exclusion criteria were patients with age<18 y and>59 y, H/o hypersensitivity and ADRs to Clonazepam and Escitalopram, pregnant and lactating mothers, sever hepatic or renal impairment, uncontrolled hypertension, recent myocardial infarction, severe cardiac decompensation, recent cerebrovascular accident, seizures, glaucoma, Parkinson’s disease, bipolar disorder and depression.
RESULTS
The socio-demographic characteristics of the study participants is shown in table 1. Both the study groups were comparable with respect to age, gender, marital status and educational qualification; no statistically significant difference observed between the two groups.
Table 1: Sociodemographic characteristics of study population
| Characteristic | Number of participants n (%) |
| Age | |
| 18-30 | 56 (35.22%) |
| 31-45 | 72 (45.28%) |
| 46-60 | 31 (19.49%) |
| Gender | |
| males | 107 (67.30%) |
| females | 52 (32.70%) |
| Marital Status | |
| single | 28 (17.61%) |
| married | 98 (61.63%) |
| divorcee | 33 (20.75%) |
| Education | |
| <10th class | 56 (35.22%) |
| 10-graduate | 80 (50.31%) |
| post-graduate | 23 (14.46%) |
Data is mentioned in number and percentages in brackets
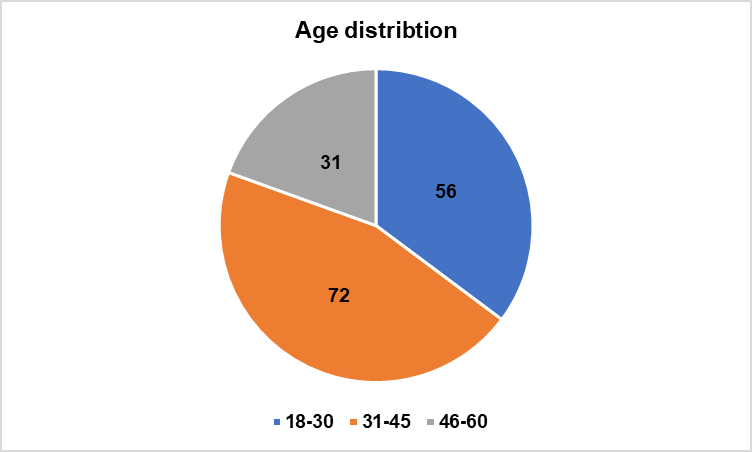
Fig. 1: Age distribution of participants among the two study groups
Table 2: Gender distribution of study participants between the two study groups
| Study group | Males N(%) | Females n(%) |
| Escitalopram (group A) | 48 (61.5%) | 30 (38.5%) |
| Escitalopram+Clonazepam (group B) | 55 (67.9%) | 26 (32.1%) |
In study population; among group A, 61.5% were males and 38.5% were females. Among group B, 67.9% were males and 32.1% were females.
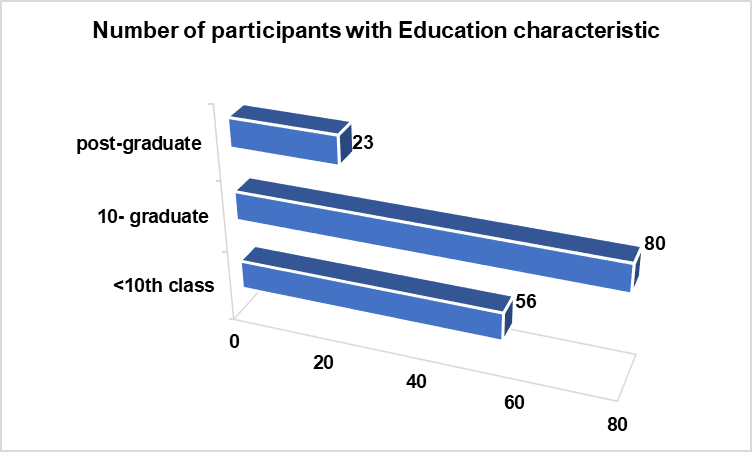
Fig. 2: Number of participants with their education characteristic among the two study groups
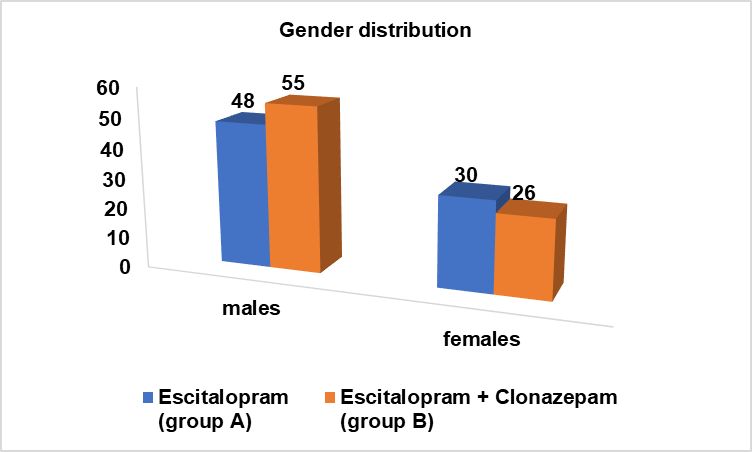
Fig. 3: Number of males and females in the study groups
Table 3: Comparing the GAD-7 score before and after drug usage
| Drug | Before mean±SD | After mean±SD | P value |
| Escitalopram | 15.68 ±2.85 | 7.01±1.42 | <0.0001 |
| Escitalopram+Clonazepam | 16.63±2.86 | 3.54±0.88 | <0.0001 |
Data is mentioned in mean±SD
The comparison of GAD-7 score among the two groups showed that Escitalopram and Clonazepam combination was more effective in reduction of anxiety when compared to Escitalopram alone. The mean±SD before and after the treatment were 15.68±2.85 and 7.01±1.42 respectively for Escitalopram group and 16.63±2.86 and 3.54±0.88 respectively for Escitalopram and Clonazepam combination group. The decrease in the GAD-7 scores were significant (p=<0.0001) in both the groups, but more reduction was observed in Escitalopram and Clonazepam group.
Comparison of the GAD-7 score among males and females showed that there was significant reduction in anxiety within the two study groups and the difference was not significant between the two groups. However, the reduction of anxiety scores was more in group B.
Table 4: Comparing the GAD-7 score by gender before and after drug usage
| Study group | Males | Females | ||||
| Before | After | P value | Before | After | P value | |
| Escitalopram (group A) | 15.81±3.41 | 7.39±1.39 | <0.0001 | 16.16±1.66 | 6.8±1.39 | <0.0001 |
| Escitalopram+Clonazepam (group B) | 17.03±2.96 | 3.67±0.86 | <0.0001 | 16.57±2.65 | 3.61±0.94 | <0.0001 |
Data is mentioned in mean±SD
Table 5: Adverse drug reactions (ADRs) seen in patients
| Escitalopram | % |
| nausea | 10 |
| diarrhoea | 15 |
| insomnia | 24 |
| anorexia | 20 |
| Headache | 8 |
| Escitalopram+Clonazepam | % |
| nausea | 12 |
| headache | 14 |
| dizziness | 8 |
| drowsiness | 6 |
| fatigue | 7 |
Both the treatment groups showed a better safety profile with mild adverse effects like diarrhoea, insomnia, headache in group A and headache, dizziness, drowsiness in group B. There was no statistically significant difference between the two groups with respect to tolerability.
DISCUSSION
Generalized anxiety disorder is characterized by excessive anxiety, worry about a variety of events or activities (e.g., work or school performance), restlessness and trouble focussing; that occurs on most days for at least 6 mo. Most guidelines recommend SSRIs, SNRIs and Pregabalin for the first line pharmacological treatment based on efficacy and tolerability. The second line drugs are TCAs, MAOIs or BZDs. There is the high rate of discontinuation with initial SSRIs therapy as their effect takes 4-8 w, so BZDs are added for initial rapid symptom relief and improves adherence to medication. There are very few studies assessing the role of BZDs as an adjunctive to SSRIs in the treatment of GAD.
This study was performed to compare the efficacy and safety of Escitalopram with Escitalopram and Clonazepam combination in the management of generalized anxiety disorder.
Escitalopram and Clonazepam combination group showed a significant improvement in reduction of GAD-7 scores at the end of 8 weeks. The mean±SD values of GAD-7 scores before and after the treatment were 15.94±2.85 and 7.16±1.41 respectively for Escitalopram group and 16.88±2.86 and 3.65±0.88 respectively for Escitalopram and Clonazepam combination group. The incidence of adverse events were low in Escitalopram and Clonazepam combination group with nausea and headache predominance; and in Escitalopram group, insomnia and anorexia predominates.
The results of our study are consistent with the other studies; As per a multicentre naturalistic study by Wang SM et al. [8]; Clonazepam and other BZDs (Alprazolam and Lorazepam) when used as an adjunctive to SSRIs and SNRIs in the management of GAD, showed a significant improvement in all the three efficacy parameters; Clinical Global Impression-Stress, Clinical Global Impression – Anxiety and Clinical Global Impression – Sleep. Among the 75 participants receiving Clonazepam; 24 (32%) participants received Escitalopram. The improvement in efficacy parameters was significant at 6 w follow-up in comparison to baseline values; CGI-Anxiety scores at baseline and 6 w follow-up were 4.89±0.94 and 2.63±0.75 respectively with mean change in score of 2.27±1.15, which was statistically significant (p<0.001). Among the three BZD groups, the total incidence of adverse events was significantly lower for Clonazepam (26.7%) group than for the alprazolam (48.4%) and lorazepam groups (43.9%) (p<0.05) and all the events were mild and no serious events reported.
Davidson JR et al. [9] designed a study to evaluate the efficacy and tolerability of escitalopram in the treatment of generalized anxiety disorder (GAD). Outpatients (18 y or older) who met DSM-IV criteria for GAD, with baseline Hamilton Rating Scale for Anxiety (HAMA) scores>or = 18, were randomly assigned to double-blind treatment with escitalopram (10 mg/day for the first 4 w) or placebo for 8 w. The primary efficacy variable was the mean change from baseline in total HAMA score at Week 8. The escitalopram group (N = 158) showed a statistically significant and clinically relevant, greater improvement at endpoint compared with placebo (N = 157) in HAMA total score and HAMA psychic anxiety subscale score at Week 1 and at each study visit thereafter. Response rates at Week 8 were 68% for escitalopram and 41% for placebo (P<.01). Low adverse events rates were reported in Escitalopram group and the discontinuation rates were comparable to placebo (8.9% vs 5.1%, p=0.27).
Waugh J, et al. [10] worked on “Escitalopram: a review of its use in the management of major depressive and anxiety disorders” and found that significantly greater reductions in Hamilton Rating Scale for Anxiety (HAM-A) scores, primary efficacy parameter; were observed in 124 patients, meeting the DSM-IV criteria for generalised anxiety disorder (GAD), who received escitalopram 10 mg/day (titrating to 20 mg/day after 4 w if required) than in 128 patients meeting the same criteria who received placebo for 8 w. Secondary efficacy parameters, Hamilton rating scale-anxiety (HAM-A) psychic anxiety subscale, clinical global impression-severity scale (CGI-S), hospital anxiety and depression (HAD) anxiety subscale and a patient-rated QOL scale; also showed significant improvement by 4 w. Escitalopram was well tolerated with low discontinuation rates, similar to placebo.
According to a meta-analysis done by Gomez AF et al. [11]; the combined use of Antidepressants and BZDs during initial medication treatment could yield the optimal risk-benefit ratio of both medications. BZDs could reach their maximal efficacy rapidly, but the effects level off after about 4 w of regular treatment and Antidepressants reach their maximal efficacy within 4-8 w. This type of combined use could minimize the adverse risks of BZDs, while potentially improving Antidepressant treatment compliance by decreasing initial unpleasant adverse effects.
Our study shows that the combination of Escitalopram and Clonazepam is safer in comparison to Escitalopram alone in terms of adverse effects and hence less chances of non-compliance. Consistent with our study is a meta-analysis done by T A Furukawa et al. where the combination of Benzodiazepines and anti-depressants is assessed in major depression patients with co-existing anxiety, showing that the combination is more compliant by the patients. The analysis aggregated nine studies with a total of 679 patients; the combination therapy group was 37% (95%CI: 19-51%) less likely to dropout than the antidepressant alone group. The intention-to-treat analysis showed that the former were 63% (18-127%) to 38% (15-66%) more likely to show response (defined as 50%or greater reduction in the depression scale from baseline) up to 4 w.
CONCLUSION
This was a randomized controlled study conducted to compare the efficacy and safety of Escitalopram alone with Escitalopram and Clonazepam combination in patients suffering from Generalized Anxiety Disorder.
Prior institutional ethics committee approval and written informed consent were obtained. Patients were randomized into two groups; group a received Escitalopram alone and group B received Escitalopram and Clonazepam combination.
Patients were assessed with Generalized Anxiety Disorder-7 score (GAD-7 score). At the end of the study, the combination group B showed more reduction of GAD-7 scores that was statistically significant (p<0.05). The incidence of adverse effects was less in the combination group B.
The results of this study shows that a combination of BZD and SSRIs is effective in the management of generalized anxiety disorder improves compliance and tolerability.
ACKNOWLEDGEMENT
We express our sincere gratitude to Dr. S. Sharon Sonia, Prof and HOD, Dept. of Pharmacology, GMC, Ananthapuram; for guiding and helping us in the completion of this study.
We also extend our heartfelt thanks to all the participants of this study; without whose co-operation this study couldn’t have completed.
FUNDING
Nil
AUTHORS CONTRIBUTIONS
Dr. D. Kushbu, the principal investigator, designed the study; Dr. Aquib Ahmed collected the data; Dr. N. M. Riyaz and Dr. O. Anwar Basha analysed the results. All authors reviewed and approved the final manuscript.
CONFLICT OF INTERESTS
Declared none
REFERENCE
Munir S, Takov V. Generalized anxiety disorder. In: Treasure Island, (FL): StatPearls Publishing; 2022 Oct 17. PMID 28722900.
World Health Organization. World mental health report: transforming mental health for all. In: Geneva: World Health Organization; 2022. Available from: https://www.who.int/publications/i/item/9789240049338. [Last accessed on 25 Jan 2025].
COVID. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet. 2021 Nov 6;398(10312):1700-12. doi: 10.1016/S0140-6736(21)02143-7. PMID 34634250, PMCID PMC8500697.
Gururaj G, Varghese M, Benegal V, Rao GN, Pathak K, Singh LK. National Mental Health Survey of India. Government of India. Bengaluru: National Institute of Mental Health and Neuro Sciences. NIMHANS Publication No. 128. Supported by Ministry of Health and Family Welfare; 2015-16. p. 62.
Charan J, Biswas T. How to calculate sample size for different study designs in medical research? Indian J Psychol Med. 2013;35(2):121-6. doi: 10.4103/0253-7176.116232, PMID 24049221.
Wang G, You X, Wang X, Xu X, Bai L, Xie J. Safety and effectiveness of escitalopram in an 8-week open study in Chinese patients with depression and anxiety. Neuropsychiatr Dis Treat. 2018 Aug 14;14:2087-97. doi: 10.2147/NDT.S164673. PMID 30147321.
Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006 May 22;166(10):1092-7. doi: 10.1001/archinte.166.10.1092, PMID 16717171.
Wang SM, Kim JB, Sakong JK, Suh HS, Oh KS, Woo JM. The efficacy and safety of clonazepam in patients with anxiety disorder taking newer antidepressants: a multicenter naturalistic study. Clin Psychopharmacol Neurosci. 2016 May 31;14(2):177-83. doi: 10.9758/cpn.2016.14.2.177, PMID 27121429, PMCID PMC4857865.
Davidson JR, Bose A, Korotzer A, Zheng H. Escitalopram in the treatment of generalized anxiety disorder: double-blind, placebo-controlled, flexible-dose study. Depress Anxiety. 2004;19(4):234-40. doi: 10.1002/da.10146, PMID 15274172.
Waugh J, Goa KL. Escitalopram: a review of its use in the management of major depressive and anxiety disorders. CNS Drugs. 2003;17(5):343-62. doi: 10.2165/00023210-200317050-00004, PMID 12665392.
Gomez AF, Barthel AL, Hofmann SG. Comparing the efficacy of benzodiazepines and serotonergic anti-depressants for adults with generalized anxiety disorder: a meta-analytic review. Expert Opin Pharmacother. 2018 Jun;19(8):883-94. doi: 10.1080/14656566.2018.1472767, PMID 29806492, PMCID PMC6097846.