
Int J Curr Pharm Res, Vol 17, Issue 3, 62-67Original Article
EVALUATION OF ANTIDEPRESSANT ACTIVITY OF CYMBOPOGONCITRATUS EXTRACTS IN MICE
SUMEET MAGOTRA*, AJEET PAL SINGH, AMAR PAL SINGH
Department of Pharmacology, St. Soldier Institute of Pharmacy, Lidhran Campus, Behind NIT (R. E. C.), Jalandhar–Amritsar by pass, NH-1, Jalandhar-144011, Punjab, India
*Corresponding author: Sumeet Magotra; *Email: sumeetmagotra@gmail.com
Received: 27 Jan 2025, Revised and Accepted: 16 Mar 2025
ABSTRACT
Objective: To evaluate Antidepressant Activity of Aqueous and Ethanolic extracts of Cymbopogoncitratus in albino mice by using Forced swimming test (FST) and Tail suspension test (TST) parameters.
Methods: Healthy, adult Swiss albino mice of either sex weighing (25-40 g), maintained under standard laboratory conditions at temperature 25±2 °C and a 12 h light-12 h dark period will be employed for the experimentation. Food and water will be provided ad libitum.
Antidepressant Animal model
A) Forced swimming test: In this model, the animal is placed in a glass cylinder (30 cm in diameter and 50-cm height) containing 40 cm of water at 23±1 °C for 5 min, forcing the mice to either swim or float.
b) Tail suspension test in mice: In this model, five animals are treated with test compounds or a vehicle via injection 30 min before being suspended by their tails. Immobility is recorded for 5 min, with the mouse considered immobile after hanging motionless for 1 min. A low-dose study found no effect of cymbopogoncitratus (lemongrass) oil on humans. However, higher doses enhance GABA-ergic transmission, like benzodiazepines, used for anxiety and sedation. To reduce anxiety, an adult would need 600–800 mg of pure oil, while most supplements contain lower doses, likely relying on the placebo effect. It may also synergize with other anxiolytics.
Results: In the present study, we have undertaken the antidepressant activity of ethanolic and aqueous extracts of Cymbopogoncitratus in Swiss albino mice at the dose of 250 and 500 mg/kg with oral administration, respectively.
Conclusion: In conclusion, both the extracts ethanolic and aqueous extracts of Cymbopogoncitratus have shown significant results as an antidepressant effect using forced swim test (FST) and Tail Suspension test (TST) parameters.
Keywords: Cymbopogoncitratus, Antidepressant, FST-forced swim test, TST-Tail Suspension test, Swiss albino mice
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijcpr.2025v17i3.55067 Journal homepage: https://innovareacademics.in/journals/index.php/ijcpr
INTRODUCTION
Disruptions of mind or cognition depression is characterized by disorders of mood. It can range from a very mild state that borders on normalcy to severe psychotic depression that is accompanied by delusions and hallucinations. Bipolar depression and unipolar depression are two of the various forms of depression. The development of antidepressant medications supported the biochemical theory of depression, which postulated that the primary lesion underlying the illness was a disruption in central monoaminergic activity. Although the monoaminergic system is undoubtedly one of the main pillars of these mechanisms in the treatment of depression, numerous interactions with other brain systems and the control of central nervous system function must also be considered. Numerous plants and metals have been recommended for the treatment of anxiety and depression in ancient Indian literature since it has been observed that a number of medicinal herbs offer anti-depressant and anti-anxiety properties. In the herbal formulation, only few of these herbs have been used [1, 2]. As a result, there is a growing need for research on natural antidepressant products with negligible or no adverse effects [1, 2]. Cohosh, Black, Kanna, Kougoed Rhodiola, Alpine, Golden Root, Rose Root, Russian, Golden Root, Rose Root, Saint John's Wort, Vervain, Blue, Wild Dagga, Wild Dagga, Lion's Tail, Lion's Ears, and Umunyane are among the herbs that have antidepressant properties. However, as no pharmacological activity related to depression has been studied thus far, we chose to investigate Cymbopogoncitratus's antidepressant properties in experimental mice [3].
The leaves of Cymbopogoncitratus are frequently used in herbal teas and supplements and have been utilized in traditional medicine. Both topical and oral use have been linked to a variety of effects, and current research backs up many of these claims. It is thought to have hypnotic, anticonvulsant, and anxiolytic effects in Brazilian traditional medicine. The plant's essential oil is employed as a carminative, depressive, analgesic, antipyretic, antibacterial, and antifungal agent in Indian traditional medicine, while the leaves are used as stimulants, sudorifics, antiperiodics, and anticatarrhals. In vitro, laboratory studies have demonstrated cytoprotective, antioxidant, and anti-inflammatory qualities in addition to antifungal capabilities (although the study indicated that Cymbopogonmartinii was more efficient). Lippiaalba, Cymbopogoncitratus, and Cymbopogonwinterianus are the sources of citronellol, an essential oil ingredient. By directly affecting the vascular smooth muscle and causing vasodilation, citronellol has been demonstrated to reduce blood pressure in rats. An infusion prepared from C. citratus was utilized as a low-cost treatment for HIV/AIDS patients' oral thrush in a small, randomized, controlled study [4, 5].
Along with myrcene, citronella, citronellol, and geraniol, lemon grass oil also includes 65–85% citral. The water and oil can be separated via cooling, condensation, and hydrosteam distillation. As a by-product of the distillation process, the hydrosol is utilized to make face cleansers, lotions, and creams, among other skincare items. Lemon grass oil and "negros oil," a blend of lemon grass oil and virgin coconut oil used in aromatherapy, are the primary constituents in these items [6, 7].
MATERIALS AND METHODS
Methods of collection of data
The methods for an extract of aqueous and ethanolic cymbopogoncitratus can be used by the dose dependent study and time-dependent study. Dose is fixed at 250 and 500 mg/Kg of each extract of cymbopogoncitratus on the basis of the literature review and dosage regimen is scheduled. The data will be collected based on laboratory animal experimentation. The experimental protocol was approved by the Institutional Animal Ethics Committee (IAEC) and was carried out as per the guidelines of Committee for the purpose of Control and Supervision of Experimental Animals (CPCSEA), Ministry of environment and Forests, Government of India (Reg. No. 2011/PO/Re/S/18/CPCSEA and date of registration is 1/5/2018) for the use and care of experimental animals. Adequate measures were taken to minimize pain or discomfort with animal’s experimental procedure. Research protocol is duly approved by IAEC/CPCSEA (IAEC/SSIP/2020/PR-016).
Chemical
All chemicals of analytical grade were procured from Sigma chemical, USA and S. D. Fine Chem. Ltd., India.
Collection and preparation of plant material
The aqueous and ethanolic extract of whole part of plant cymbopogoncitratus were procured from shreedha phyto extract, Jaipur. The same group also provided a certification of the plant's identity and quality (Certificate of Analysis).
Preparation and administration of crude extracts/standard drugs [8]
Extraction is the widely used method for separating the active ingredients from the unrefined medications. In typical extraction processes, selective solvents are used to separate the medicinally active parts of plant or animal tissues from the inactive or inert components. These plant-based products are comparatively impure liquids, semisolids, or powders that are solely meant to be applied topically or taken orally. Standardized extraction techniques are used for crude pharmaceuticals (medical plant parts) in order to extract the therapeutically desirable components and remove unwanted material by treating them with menstrum, a selective solvent. Following standardization, the resulting extract could be utilized as a medication. Numerous therapeutic plant metabolites, including alkaloids, glycosides, terpenoids, flavonoids, and lignans, are complexly mixed together in these extracts.
Animals
Healthy, adult swiss albino mice of either sex weighing (25-40 g), maintained under standard laboratory conditions at temperature 25±2 °C and a 12 h light-12 h dark period was employed for the experimentation. Food and water were provided ad libitum.
Acute oral toxicity study [9]
Low, medium, and high doses were chosen for therapy after an acute toxicity study of the ethanolic and aqueous extract of "Cymbopogoncitratus" was conducted in accordance with OECD recommendations No: 423.
Method
Four groups of three mice each were created from the overnight fasting mice. Using a gawage, the ECC and ACC were administered orally at different dosages (5, 50, 300, and 1000). Following the extract's administration, the animals were closely watched for the first two and twenty-four hours to look for behavioral changes, as well as for tremors, convulsions, salivation, diarrhea, lethargy, sleep, and coma. They were also watched for toxic symptoms and mortality for up to fourteen days. The survivor mice were restored and used again for experiments following 14 d of acute oral toxicity.
Behavioral tests for depression in mice/Mouse as a model for depression [10, 11]
One of the main benefits of utilizing mice as models is their striking genetic, anatomical, and physiological similarities to humans. Since over 95% of the mouse and human genomes are identical, mouse genetic research is particularly relevant to human disease. Depression and anxiety Animals are used as experimental models in studies pertaining to the brain and central nervous system. Preclinical research on the neurobiology of psychiatric diseases relies heavily on animal models, which are also used as screening techniques to find new treatment drugs. Since mice and humans share over 90% of their genes, rodents, particularly mice, have proven useful in studies. Additionally, animal models are especially useful when the effects of stress cannot be studied in humans for ethical and other reasons. Other benefits of using mice as a model include cost-effectiveness, a small dose relative to body weight, ease of handling, and a quicker breeding period, all of which will undoubtedly make the research easier for the researcher. Additionally, animal models of anxiety and depression are essential for developing new treatments for these conditions.
Acute or subacute (7 d period) behavioral effects will be given orally. Swiss mice will be tested using the Tail Suspension Test (TST) and the Forced Swim Test (FST) using ethanolic and aqueous extracts of Cymbopogoncitratus (250 and 500 mg/kg). We will also evaluate the effects of fluoxetine (FXT; 10 mg/kg).
Forced swimming test
The most popular behavioral paradigm for evaluating the effects of antidepressants in mice. Each mouse was made to swim in a 25×15×25 cm glass chamber filled with fresh water up to 15 cm high and kept at 26°±1 °C. During the first two minutes of the test, every animal will move enthusiastically. During the next four minutes of the six-minute testing session, the length of immobility will be manually recorded. Mice will be deemed immobile if they have stopped struggling and are still floating in the water, requiring only the motions required to maintain their head above the water. Mice will be towel-dried and put back in their habitat after their swimming session [12].
Tail suspension test in mice
In this approach, 30 min before testing, groups of 10 rats receive intraperitoneal injections of either the vehicle or the test drugs. Adhesive tape is positioned around 1 cm from the tip of the mice's tails to hang them on the edge of a shelf 58 cm above a tabletop for the test. Five minutes are allotted for recording the duration of immobility. When mice hang passively and without moving for at least a minute, they are said to be immobile.
A frequently used model for assessing novel antidepressant medications is the tail suspension test4. Drugs like imipramine and fluoxetine, which are therapeutically successful in treating human depression, can reverse immobility, which is a sign of helplessness. In this experimental model, the duration of immobility during a given time period is used as the measure of depression. The lengthening of the immobility period is a sign of mental depression. On the other hand, a decrease in the immobility period indicates a mental state free from depression. On the other hand, a decrease in the immobility period indicates a mental state free from depression. The adhesive tape was positioned about one centimeter from the tip of the mice's tail, and they were suspended on the edge of a table 50 centimeters above the floor. The duration of immobility was measured for five minutes.
(1) Immobility – the mouse hangs without engaging in any activity;
(2) Swinging – keeping its body straight, the mouse continuously moves its paws in a vertical position and/or moves its body from side to side;
(3) Curling – the mouse engages in active twisting movements [13].
Laboratory models employed to produced Stress [14-19]
Chronic unpredictable mild stress procedure (CUMS)
With some adjustments, the mice were exposed to mild, ongoing, and unexpected stress. Over the course of three weeks, the animals were exposed to the stress paradigm once daily from 9:00 to 14:00.
Table 1: The stressors were listed in the following order
| Weeks | Day-1 | Day-2 | Day-3 | Day-4 | Day-5 | Day-6 |
| Week-1 | I | E | F | O | T2 | X |
| Week-2 | I | O | X | T2 | E | T1 |
| Week-3 | O | F | T1 | X | T2 | I |
Note: I-Immobilization for 2 h, E-Exposure to empty water bottles for 1 h, F-Exposure to foreign object for 24 h (e. g. piece of plastic), O-Overnight illumination, T2-Tail pinch (60s), X-Tilted cage at 45° for 7 h, T1-Tail pinch (30s).
Mice subjected to CUMS procedure were called as stressed mice. Unstressed mice were exposed to behavioral tests and not subjected to CUMS procedure. Drugs were administered 30 min before CUMS procedure in case of stressed mice.
Procedures for tail suspension test stress
A common behavioral test for assessing a drug's antidepressant-like effects is the tail suspension test (TST) (Steru et al., 1985). Using sticky tape positioned 1 cm from the tip of the tail, each mouse was suspended 50 cm above the floor during the test. Throughout the test, every animal was visually and audibly separated from the others. For five minutes, the entire immobility duration was manually recorded. When an animal hung passively, showed no signs of movement, and remained absolutely still, it was deemed immobile.
Procedures for forced swimming stress
Animals were made to swim for varying lengths of time in a rectangle plastic tank (45x35x18 cm) with water that was 15 cm deep as part of the forced swimming test. A constant temperature of about 23 °C was maintained for the water. The mice were dried off with towels after swimming before a blood sample was taken from the tail vein.
Rapid effects of forced swimming stress/tail suspension test stress on dynamics and correlation of plasma corticosterone
Before being chosen to have their blood drawn for hormone tests, mice were subjected to forced swimming for varying lengths of time in order to investigate the quick impacts of extreme stress on the dynamics and correlation of plasma corticosterone. In contrast to the cortisol concentration, which peaked in 3 min during forced swimming stress, the corticosterone concentration did not peak until 40 min into the stress. Both the forced swimming test and the tail suspension test revealed a high association between the two hormones. According to the results, cortisol and corticosterone reacted to extreme stressors in distinct ways, with cortisol reacting more quickly than corticosterone.
Biochemical estimation
Collection of blood samples
On 15th day, blood (0.3 ml) was withdrawn from tail vein from all groups of mice. Blood samples were centrifuged at 2500 rpm for 10 min using refrigerated centrifuge (Paramount Scientific Works, Ambalacantt, India) to separate the plasma, which was used for estimation of corticosterone levels.
Estimation of plasma corticosterone levels
The quantitative estimation of corticosterone levels in the blood plasma was performed by the method of Bartos and Pesez, 1979. To 1.0 ml of sample in ethanol, 0.50 ml of 0.10 % solution of p-nitroso-N, N-dimethylaniline in ethanol was added and the tubes were immersed in ice water for 5 min, and then 0.50 ml of 0.10 N-sodium hydroxide was added. The tubes were plugged with cotton-wool, and were let to stand at 0 °C for 5 h, protected against light. To the above solution, 2.0 ml of buffer for pH 9.8, 5.0 ml of 0.10 % solution of phenol in ethanol and 0.50 ml of 1.0 % aqueous solution of potassium ferricyanide were added. The tubes were kept in a water bath at 20±2 °C for 10 min. The solution was read at 650 nm using a UV-visible spectrophotometer (UV 3200 UV-VIS Spectrophotometer, Somajiguda, Hyderabad).
Groups 1 to 6 were tail bled on day 1 and then corticosterone levels were combined to obtain the average levels in tail blood. For treatment of Groups see their respective experimental design.
Experimental design
Group 1: control (solvent)
Group 2: mice treated with ECC (Low dose)
Group 3: mice treated with ECC (High dose)
Group 4: mice treated with ACC (Low dose)
Group 5: mice treated with ACC (High dose)
Group 6: mice treated with fluoxetine.
Body weight analysis: During the same time of study, body weight analysis will be recorded.
Statistical analysis
All the results will be expressed as standard error of mean (S. E. M.). Data will be analyzed using one-way ANOVA (Graph pad prism version 5.00 software) followed by suitable post-test like Dunnett’s t-test. p<0.05 will be considered as statistically significant.
Total No. of animals required
No of the animal in each group = 06
No. of groups = 12
Total no. of animals required = 06 × 12 =72
First of all we will complete one parameter i. e. TST, then after rehabilitation/washing period. We will use same animals for second parameter i. e. FST by this way. We will minimize the number of experimental animals up to 50% i. e. 36.
RESULTS AND DISCUSSION
Acute oral toxicity study
The median lethal dose (LD50) of ACC and ECC was determined in accordance with the Organization for Economic Co-operation and Development (OECD, 425) guidelines using four mice which were fasted overnight before dosing with different extracts of ACC and ECC separately at maximum dose level up to 1000 mg/kg orally starting from dose of 5, 50, 300 mg/kg. One mouse was initially dosed and food was further withheld for 4 h. It was observed for the first 24 h and then for 14 d for signs of toxicity (changes in mucous membranes, skin, fur and eyes, circulatory, respiratory, somato-motor activity and behaviour pattern) and mortality. It has been observed that no change in behavioural responses and observation shows any acute oral toxicity. The remaining four mice were also dosed and observed for 2 w. Thereafter, the LD50 was estimated.
Evaluation of antidepressant effect of Cymbopogoncitratus leaves extracts in TST and FST models
Group 1: In this group animals were treated with Normal saline solution in which mice Immobility period was 265±3.808 sec.
Group 2: In this group animals were treated with low dose of Aqueous extract (250 mg/kg) solution in which mice Immobility period was 92.4±2.364 sec.
Group 3: In this group animals were treated with high dose of Aqueous extract (500 mg/kg) solution in which mice Immobility period was 44.4±1.865 sec.
Group 4: In this group animals were treated with low dose of Ethanolic extract (250 mg/kg) solution in which mice Immobility period was 81.0±2.744 sec.
Group 5: In this group animals were treated with high dose of Ethanolic extract (500 mg/kg) solution in which mice Immobility period was 39.8±2.686 sec.
Group 6: In this group, animals were treated with Fluoxetine (10 mg/kg) solution in which mice Immobility period was 17.2±1.646 sec.
Treatment with Fluoxetine significantly decreased the duration of immobility time (P<0.001) in TST. Ethanolic and aqueous extract of Cymbopogoncitratus treated mice also exhibited dose-dependent significant decreased the duration of immobility time. The duration of immobility was also significantly reduced as compared to the vehicle-treated group. But there is no significant difference between Cymbopogoncitratus extracts treated animals and Fluoxetine treated animal. The above observation suggests that Cymbopogoncitratus has antidepressant activity.
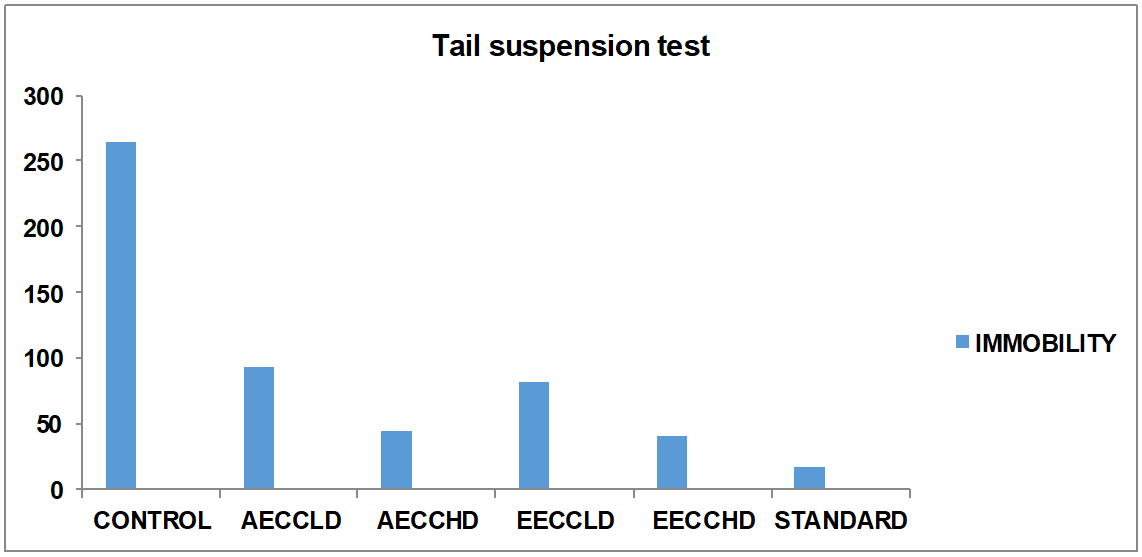
Fig. 1: Aqueous extract of cymbopogoncitratus leaves (AECC) and ethanolic extract of cymbopogoncitratus leaves (EECC)
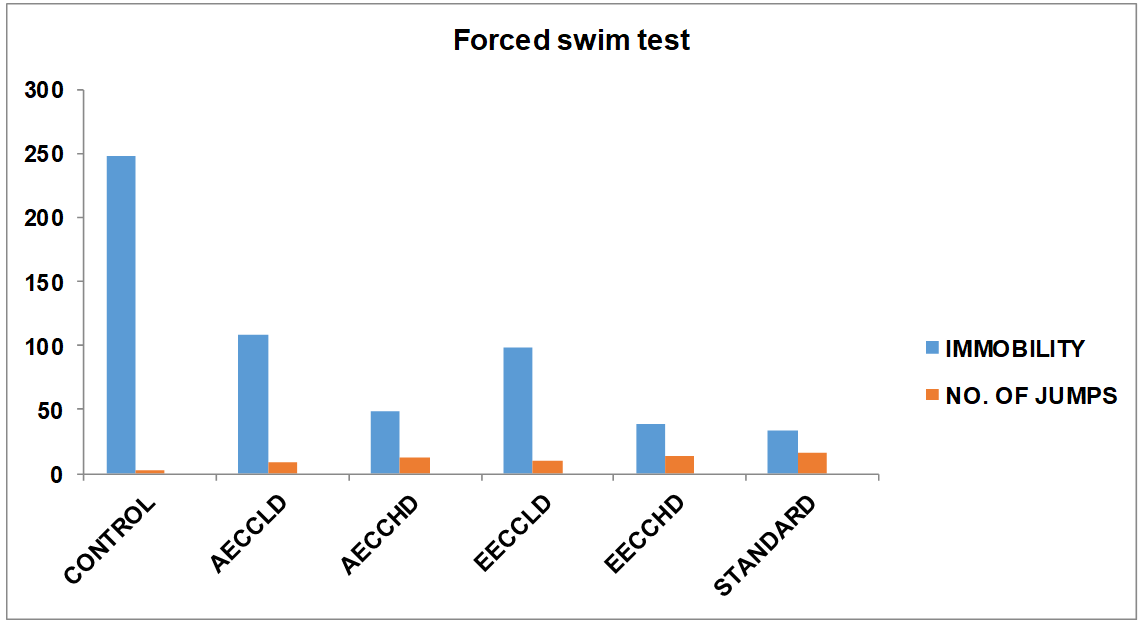
Fig. 2: Aqueous extract of cymbopogoncitratus leaves (AECC) and ethanolic extract of cymbopogoncitratus leaves (EECC)
Group 1: In this group animals were treated with Normal saline solution in which mice Immobility period was 248±2.984 sec. and numbers of jumps were 2.4±0.376.
Group 2: In this group animals were treated with low dose of Aqueous extract (250 mg/kg) solution in which mice Immobility period was 108±3.848 sec. and number of jumps were 9.2±0.506.
Group 3: In this group animals were treated with high dose of Aqueous extract (500 mg/kg) solution in which mice Immobility period was 48.4±2.388 sec. and number of jumps were 12.6±0.376.
Group 4: In this group animals were treated with low dose of Ethanolic extract (250 mg/kg) solution in which mice Immobility period was 98.8±3.656 sec. and number of jumps were 10.2±0.708.
Group 5: In this group animals were treated with high dose of Ethanolic extract (500 mg/kg) solution in which mice Immobility period was 38.8±1.716 sec. and number of jumps were 13.6±0.926.
Group 6: In this group animals were treated with Fluoxetine (10 mg/kg) solution in which mice Immobility period was 34±1.704 sec. and numbers of jumps were 16.8±0.814.
Treatment with Fluoxetine significantly decreased the duration of immobility time (P<0.001) in FST. Ethanolic and aqueous extract of Cymbopogoncitratus treated mice also exhibited dose dependent significant decreased duration of immobility time. The duration of immobility was also significantly reduced as compared to the vehicle-treated group.
But there is no significant difference between Cymbopogoncitratus extracts treated animals and Fluoxetine treated animal. The above observation suggests that Cymbopogoncitratus has antidepressant activity.
Plasma corticosterone levels
Groups 1 to 6 were tail bled on day 1 and then corticosterone levels were combined to obtain the average levels in tail blood. For treatment of Groups see their respective experimental design.
Evaluation of antidepressant activity of Cymbopogoncitratus extracts in mice
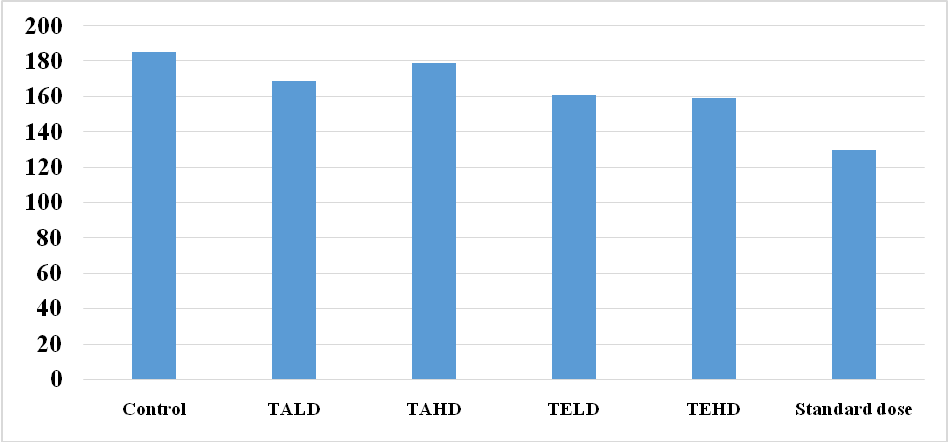
Fig. 3: Graph showing the corticosterone levels in post tail suspension test experiments, Note: TALD= Test Aqueous lower dose; TAHD= Test Aqueous higher dose; TELD= Test Ethanolic lower dose; TEHD= Test Ethanolic higher dose; STD= standard
Group 1: In this mice were treated with Normal saline and corticosterone level is measured, which is 11. 87±0.53 ng/ml.
Group 2: In this mice were treated with low dose of Aqueous extract and corticosterone level is measured which is 6.48±1.43 ng/ml.
Group 3: In this mice were treated with High dose of Aqueous extract and corticosterone level is measured, which is 5.99±2.26 ng/ml
Group 4: In this mice were treated with low dose of Ethanolic extract and corticosterone level is measured which is 5.16±1.27 ng/ml.
Group 5: In this mice were treated with High dose of Ethanolic extract and corticosterone level is measured which is 4.48±0.30 ng/ml.
Group 6: In this mice were treated with Standard (Fluxetine) and corticosterone level is measured which is 0.82±0.35 ng/ml.
It is known that stress enhances the activity of the hypothalamus-pituitary-adrenal (HPA) axis and results in increased secretion of corticosteroids from the adrenal cortex. Cortisol and corticosterone are thus often used as biomarkers for stress and depressive disorders. Although corticosterone is considered the main glucocorticoid involved in regulation of stress responses in rodents, researchers often choose to detect cortisol for stress indicators in consideration of convenience and kits availability.
CONCLUSION
Extracts from Cymbopogoncitratus exhibit a wide range of action against a panel of variables that cause the most prevalent psycosis disorders. The discovery of novel antidepressant chemicals with clinical efficacy is made possible by these promising extracts. Cymbopogoncitratusethanolic and aqueous extracts were examined separately for antidepressant efficacy using the FST and TST methods at dose levels of 250 and 500 mg/ml. Cymbopogoncitratus'sethanolic extract exhibited significantly higher activity than its aqueous counterpart. These outcomes were contrasted with those of fluoxetine, a common antidepressant. However, the extract's precise active ingredients that produced this effect were not identified. In conclusion, both extracts showed antidepressant active plant principles, despite the fact that active components were not separated.
ACKNOWLEDGMENT
It’s our privilege to express the profound sense of gratitude and cordial thanks to our respected chairman Mr. Anil Chopra and Vice Chairperson Ms. Sangeeta Chopra, St. Soldier Educational Society, Jalandhar for providing the necessary facilities to complete this research work.
FUNDING
Nil
AUTHORS CONTRIBUTIONS
All authors have contributed equally
CONFLICTS OF INTERESTS
There are no conflicts of interest
REFERENCES
Sekhon S, Gupta V. Mood disorders. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2025.
Bains N, Abdijadid S. Major depressive disorder. Treasure Island (FL): StatPearls Publishing; 2022.
LI Y, Pham V, Bui M, Song L, WU C, Walia A. Rhodiola rosea L: an herb with anti-stress anti-aging and immunostimulating properties for cancer chemoprevention. Curr Pharmacol Rep. 2017;3(6):384-95. doi: 10.1007/s40495-017-0106-1, PMID 30393593.
Kiani HS, Ali A, Zahra S, Hassan ZU, Kubra KT, Azam M. Phytochemical composition and pharmacological potential of lemongrass (Cymbopogon) and impact on gut microbiota. Applied Chem. 2022;2(4):229-46. doi: 10.3390/appliedchem2040016.
Kassahun T, Girma B, Joshi RK, Sisay B, Tesfaye K, Taye S. Ethnobotany traditional use phytochemistry and pharmacology of cymbopogoncitratus. Int J Herb Med. 2020;8(4):80-7.
Okpo SO, Edeh I. A comprehensive review on lemongrass (CymbopogonCitratus) oil extraction and its applications. EPRA Int J Researc H Dev (IJRD). 2023;8(4):258-73.
Ashaq B, Rasool K, Habib S, Bashir I, Nisar N, Mustafa S. Insights into chemistry extraction and industrial application of lemon grass essential oil a review of recent advances. Food Chem X. 2024 May 28;22:101521. doi: 10.1016/j.fochx.2024.101521, PMID 38952570.
Vishnoi NK. Advanced practical organic chemistry. 2nd ed. Delhi: Vikas Publishing House Private Limited; 2007. p. 54-5.
The organization of economic co-operation and development (OECD). The OECD guideline for testing of chemicals: 423 acute oral toxicity. Paris: OECD; 2001. p. 1-14.
Springer H, Vogel G, Editors. A textbook of drug discovery and evaluation (pharmacological assay). 2nd ed; 2008. p. 430.
Taiwo AE, Leite FB, Lucena GM, Barros M, Silveira D, Silva MV. Anxiolytic and antidepressant-like effects of Melissa officinalis (lemon balm) extract in rats: influence of administration and gender. Indian J Pharmacol. 2012 Mar-Apr;44(2):189-92. doi: 10.4103/0253-7613.93846, PMID 22529473.
Dunham NW, Miya TS. A note on a simple apparatus for detecting neurological deficit in rats and mice. J Am Pharm Assoc Am Pharm Assoc. 1957;46(3):208-9. doi: 10.1002/jps.3030460322, PMID 13502156.
Rodrigues AL, DA Silva GL, Mateussi AS, Fernandes ES, Miguel OG, Yunes RA. Involvement of monoaminergic system in the antidepressant like effect of the hydroalcoholic extract of siphocampylus verticillatus. Life Sci. 2002;70(12):1347-58. doi: 10.1016/s0024-3205(01)01498-9, PMID 11885577.