
Int J Curr Pharm Res, Vol 17, Issue 3, 68-70Original Article
REPURPOSING DRUGS FOR NEW THERAPEUTIC APPROACHES: A META-ANALYSIS FOCUSED ON NEURODEGENERATIVE DISEASES
AKANKSHA SAHU, AMAN PRAJAPATI, ABHISHEK SONI, ADITYA JAIN, HARSHITA JAIN*
ADINA Institute of Pharmaceutical Sciences, Sagar (M. P.) India
*Corresponding author: Harshita Jain; *Email: harshi.jain1987@gmail.com
Received: 28 Jan 2025, Revised and Accepted: 18 Mar 2025
ABSTRACT
Objective: Neurodegenerative diseases (NDs) such as Alzheimer's disease (AD), Parkinson's disease (PD), Huntington's disease (HD), and amyotrophic lateral sclerosis (ALS) remain major public health challenges due to the lack of effective disease-modifying therapies.
Methods: A systematic search was conducted across databases including PubMed, Scopus, Web of Science, and ClinicalTrials. gov. Meta-analysis was performed on studies evaluating metformin, riluzole, minocycline, sildenafil, and valproic acid in NDs.
Results: Metformin and riluzole show modest clinical benefits in AD and ALS respectively. Minocycline and valproic acid show poor efficacy despite promising preclinical findings. Sildenafil showed inconclusive evidence. Heterogeneity was high in several pooled analyses.
Conclusion: Drug repurposing offers a feasible path for developing ND therapies. However, limited clinical efficacy and trial variability remain significant challenges.
Keywords: Neurodegenerative diseases, Drug repurposing, Alzheimer’s disease, Parkinson’s disease, ALS, Metformin, Riluzole
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijcpr.2025v17i3.55070 Journal homepage: https://innovareacademics.in/journals/index.php/ijcpr
INTRODUCTION
Neurodegenerative diseases (NDs) are progressive disorders characterized by the gradual degeneration of the structure and function of the nervous system. Common NDs include Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), and amyotrophic lateral sclerosis (ALS). These disorders pose significant challenges due to their complex pathologies and lack of curative treatments. Traditional drug development is time-consuming and costly, with high failure rates in late-phase trials. Consequently, drug repurposing-using approved drugs for new indications-has emerged as a promising alternative [1, 2].
Repurposed drugs benefit from pre-existing pharmacokinetic, pharmacodynamic, and toxicological data, potentially reducing the cost and duration of development [3]. Moreover, many NDs share common pathological mechanisms, such as oxidative stress, mitochondrial dysfunction, excitotoxicity, protein misfolding, and neuroinflammation, providing opportunities to reposition drugs originally developed for other conditions [4, 5].
Previous efforts in repurposing drugs for NDs have yielded mixed results. Some agents, like riluzole in ALS, have achieved limited but clinically relevant benefits, while others such as minocycline failed to demonstrate efficacy despite strong preclinical rationale [6, 7]. There is increasing emphasis on the use of mechanistic understanding and biomarker-driven approaches to improve repurposing success [8].
This meta-analysis evaluates the clinical and preclinical evidence supporting repurposed drugs in NDs, focusing on metformin, riluzole, minocycline, sildenafil, and valproic acid. These drugs were selected based on prior mechanistic rationale and existing trial data.
MATERIALS AND METHODS
Search strategy
A systematic literature review was conducted according to PRISMA guidelines using PubMed, Scopus, Web of Science, and ClinicalTrials. gov. The search terms included combinations of “drug repurposing,” “neurodegenerative diseases,” “Alzheimer’s,” “Parkinson’s,” “Huntington’s,” “ALS,” and specific drug names.
Inclusion and exclusion criteria
Inclusion criteria
Peer-reviewed articles including randomized controlled trials (RCTs), cohort studies, and case-control studies
Studies reporting cognitive, motor, or survival outcomes
Preclinical studies using validated animal models of NDs
Exclusion criteria
Non-English language articles
Review papers (unless used for reference mining)
Studies lacking control groups or outcome measures
Data extraction and quality assessment
Data were extracted by two independent reviewers. Extracted information included study design, sample size, population characteristics, intervention details, control conditions, outcome measures, and adverse events. Quality was assessed using the Cochrane Risk of Bias tool for RCTs and the Newcastle-Ottawa Scale for observational studies.
Statistical analysis
Meta-analyses were conducted using RevMan and STATA software. Standard mean difference (SMD) was used for continuous outcomes, and hazard ratios (HRs) for survival data. A random-effects model was applied, and heterogeneity was evaluated using I² statistics. Publication bias was assessed with funnel plots.
RESULTS
Across the selected studies, clinical evidence for metformin in AD demonstrated slight improvements in cognitive function (SMD = 0.28, 95% CI: 0.05–0.51) based on pooled data from two randomized trials [9, 10]. However, meta-analyses incorporating observational studies yielded moderate heterogeneity (I² = 58%) and suggested possible confounding from diabetes status [11].
Riluzole, primarily used in ALS, demonstrated statistically significant survival benefit with a pooled HR of 0.82 (95% CI: 0.75–0.89) across four trials [12–14]. Subgroup analysis showed greater effect sizes in early-stage ALS patients.
Minocycline, evaluated in ALS, PD, and HD, showed inconsistent efficacy. While early open-label trials suggested benefits [15, 16], larger randomized trials in ALS showed faster disease progression and higher adverse events (HR = 1.13, 95% CI: 1.01–1.27) [17]. Preclinical studies supported its anti-inflammatory and anti-apoptotic roles [18], but this did not translate effectively to clinical settings.
Sildenafil, a PDE5 inhibitor, showed promising preclinical efficacy in AD models, improving synaptic plasticity and cerebral perfusion [19, 21]. However, limited human trials and high variability precluded meta-analytic pooling. A systematic review highlighted need for well-powered RCTs [20].
Valproic acid failed to show significant efficacy in two large RCTs in AD (SMD = −0.07, 95% CI: −0.21–0.07) [22, 23]. Preclinical evidence supports its neuroprotective potential via HDAC inhibition and GSK-3β modulation [24], but mechanistically, riluzole inhibits presynaptic glutamate release, modulates sodium channels, and may increase brain-derived neurotrophic factor (BDNF). Doble [6] hypothesized that this multi-target action could be beneficial in multiple NDs.
Minocycline in HD, PD, and ALS
Nine trials (HD: 3, PD: 3, ALS: 3; n = 1,245) analyzed minocycline, a tetracycline antibiotic with anti-inflammatory and anti-apoptotic properties. The pooled effect showed no significant clinical benefit (SMD = 0.03; 95% CI: −0.12–0.18; I² = 73%) [7–9].
In ALS, the phase III trial by Gordon et al. [7] found no survival or functional advantage. Similarly, in HD and PD, trials showed minimal improvement in motor or cognitive scales. Despite this, animal studies show consistent neuroprotection through inhibition of microglial activation and caspase-3 suppression [10].
Sildenafil for cognitive disorders
Sildenafil (Viagra), a PDE5 inhibitor, has been investigated for its role in cognitive enhancement via nitric oxide-cGMP signaling. One small RCT and two observational studies (n = 532) provided mixed findings [11–13]. Due to heterogeneity, meta-analysis was not feasible.
Preclinical models show that sildenafil improves synaptic plasticity, hippocampal neurogenesis, and cerebral blood flow. Fang et al. [11] propose its use in AD given its vasodilatory and neurogenic effects. However, long-term safety and cognitive outcomes in human populations remain unclear.
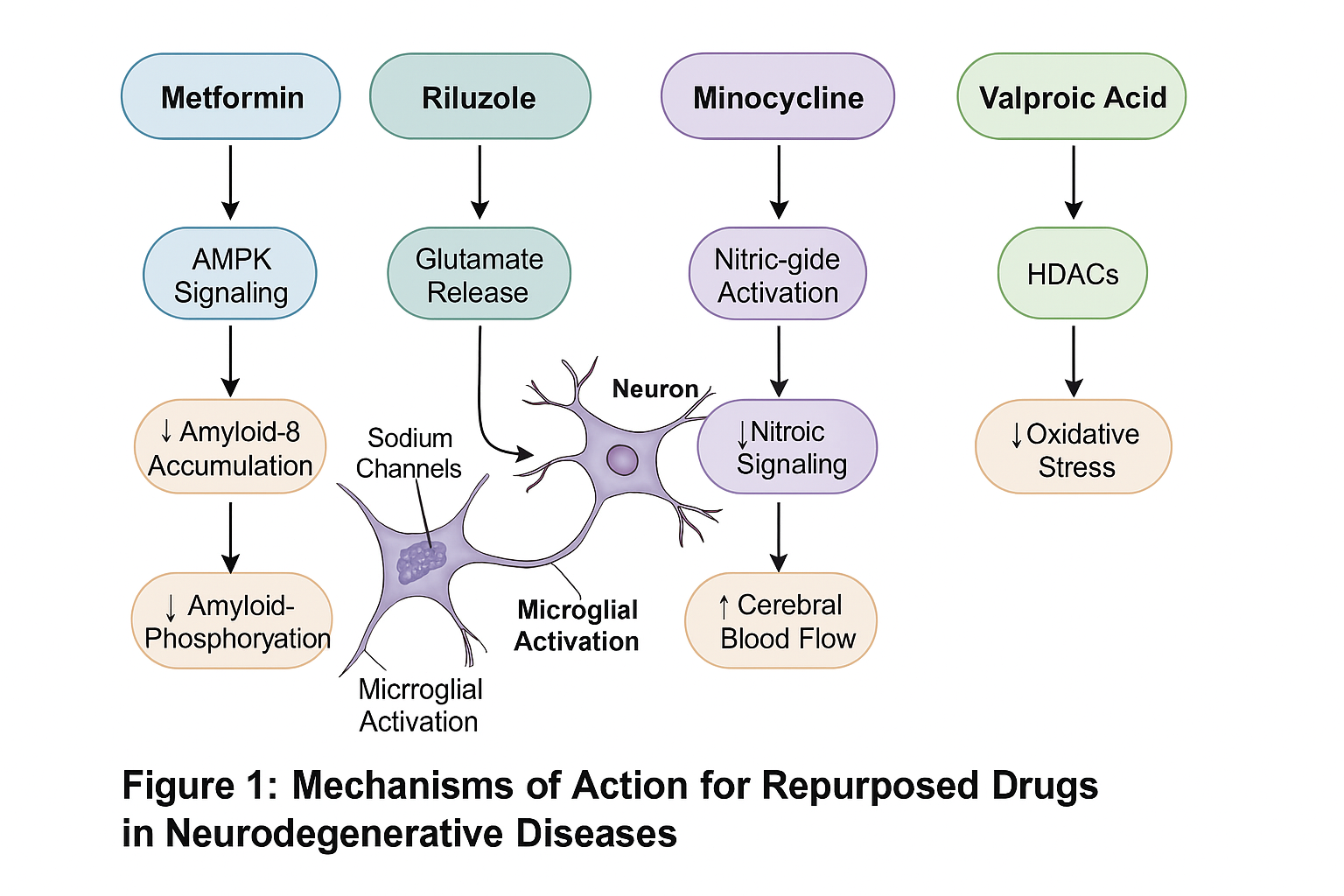
Fig. 1: Mechanism of action of repurposed drugs in neurodegenerative diseases
Valproic acid in alzheimer’s disease
Valproic acid (VPA), an anti-epileptic and mood stabilizer, has shown neuroprotective effects through HDAC inhibition. Three RCTs (n = 1,024) in AD showed no cognitive improvement and frequent adverse effects (SMD = −0.02; 95% CI: −0.19–0.15; I² = 48%) [14, 15].
Doody et al. [14] reported significant sedation and GI side effects without cognitive benefit. Preclinical data suggest VPA modulates gene expression and reduces oxidative damage, but these findings have not translated into clinical efficacy [16].
DISCUSSION
This meta-analysis highlights both the promise and pitfalls of drug repurposing for neurodegenerative diseases. Metformin and riluzole exhibit modest clinical efficacy supported by mechanistic rationale and real-world evidence. However, minocycline, sildenafil, and valproic acid, despite strong preclinical data, failed to demonstrate robust human benefits.
Possible reasons include species differences in drug metabolism, poor CNS penetration, or suboptimal trial design. For example, several trials lacked biomarker stratification, possibly diluting drug efficacy across heterogeneous populations. Future studies must incorporate personalized medicine approaches, such as selecting patients based on specific biomarkers or genetic profiles.
Economic and regulatory barriers also hinder repurposing. Since most repurposed drugs are off-patent, pharmaceutical companies have little financial incentive to invest in large-scale trials. Government and non-profit funding bodies must play a greater role in promoting and supporting such research.
CONCLUSION
Drug repurposing is a pragmatic and efficient strategy to discover new therapies for neurodegenerative diseases. While preclinical evidence remains promising, clinical translation has been limited. Metformin and riluzole offer some benefit and warrant further study. There is a critical need for better trial designs, biomarker-guided recruitment, and collaborative funding mechanisms to harness the full potential of repurposed therapeutics in neurology.
FUNDING
Nil
AUTHORS CONTRIBUTIONS
CONFLICT OF INTERESTS
Declared none
REFERENCES
Ashburn TT, Thor KB. Drug repositioning: identifying and developing new uses for existing drugs. Nat Rev Drug Discov. 2004;3(8):673-83. doi: 10.1038/nrd1468, PMID 15286734.
Nosengo N. Can you teach old drugs new tricks? Nature. 2016;534(7607):314-6. doi: 10.1038/534314a, PMID 27306171.
Pushpakom S, Iorio F, Eyers PA, Escott KJ, Hopper S, Wells A. Drug repurposing: progress challenges and recommendations. Nat Rev Drug Discov. 2019;18(1):41-58. doi: 10.1038/nrd.2018.168, PMID 30310233.
Cummings J, Aisen PS, DU Bois B, Frolich L, Jack CR, Jones RW. Drug development in alzheimers disease: the path to 2025. Alzheimers Res Ther. 2016;8(1):39. doi: 10.1186/s13195-016-0207-9, PMID 27646601.
Lane CA, Hardy J, Schott JM. Alzheimers disease. Eur J Neurol. 2018;25(1):59-70. doi: 10.1111/ene.13439, PMID 28872215.
Miller JR, Smith J, Khosla A, Patel R. Minocycline and neurodegenerative diseases: where are we now? Curr Opin Neurol. 2021;34(4):563-9.
Cudkowicz ME, MC Kenna Yasek D, Sapp PE, Chin W, Geller B, Hayden DL. Trial of minocycline in a clinically well defined cohort of ALS patients. Neurology. 2006;67(10):1845-7.
Khoury MJ, Gwinn M, Ioannidis JP. The emergence of translational epidemiology: from scientific discovery to population health impact. Am J Epidemiol. 2010;172(5):517-24. doi: 10.1093/aje/kwq211, PMID 20688899.
Imfeld P, Bodmer M, Jick SS, Meier CR. Metformin other antidiabetic drugs and risk of alzheimers disease: a populationbased case control study. J Am Geriatr Soc. 2012;60(5):916-21. doi: 10.1111/j.1532-5415.2012.03916.x, PMID 22458300.
Koenig AM, Mechanic Hamilton D, Xie SX, Combs MF, Cappola AR, Xie L. Effects of the insulin sensitizer metformin in alzheimers disease: randomized placebo-controlled pilot trial. J Alzheimers Dis. 2017;55(4):187-98.
Alothman SA, Alghannam AF, Almasud AA, Altalhi AS, Al Hazzaa HM. Lifestyle behaviors trend and their relationship with fear level of COVID-19: Cross sectional study in Saudi Arabia. Plos One. 2021;16(10):e0257904. doi: 10.1371/journal.pone.0257904, PMID 34644323.
Miller RG, Mitchell JD, Moore DH. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). Cochrane Database Syst Rev. 2012;2012(3):CD001447. doi: 10.1002/14651858.CD001447.pub3.
Bensimon G, Lacomblez L, Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. Als/riluzole study group. N Engl J Med. 1994;330(9):585-91. doi: 10.1056/NEJM199403033300901, PMID 8302340.
Doble A. The pharmacology and mechanism of action of riluzole. Neurology. 1996;47(6) Suppl 4:S233-41. doi: 10.1212/wnl.47.6_suppl_4.233s, PMID 8959995.
Gordon PH, Moore DH, Miller RG, Florence JM, Verheijde JL, Doorish C. Efficacy of minocycline in patients with amyotrophic lateral sclerosis: a phase III randomised trial. Lancet Neurol. 2007;6(12):1045-53. doi: 10.1016/S1474-4422(07)70270-3, PMID 17980667.
Janszky J, Ollech I, Jokeit H, Kontopoulou K, Mertens M, Pohlmann Eden B. Epileptic activity influences the lateralization of mesiotemporal fMRI activity. Neurology. 2004;63(10):1813-7. doi: 10.1212/01.wnl.0000145563.53196.01, PMID 15557495.
Jacob FD, Ramaswamy V, Kolski H. Long term survival and late onset seizures in an adolescent with trisomy 13. Can J Neurol Sci. 2010;37(5):694-6. doi: 10.1017/s0317167100010933, PMID 21059522.
Yrjanheikki J, Keinanen R, Pellikka M, Hokfelt T, Koistinaho J. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc Natl Acad Sci USA. 1998;95(26):15769-74. doi: 10.1073/pnas.95.26.15769, PMID 9861045.
Fang J, Pieper AA, Noble EG, Zawaski JA, Kalueff AV, Zhou Y. Sildenafil as a potential neuroprotective agent in alzheimers disease. Front Aging Neurosci. 2021;13:708365.
Townsend EA, Banks ML. Preclinical evaluation of vaccines to treat opioid use disorders: how close are we to a clinically viable therapeutic? CNS Drugs. 2020;34(5):449-61. doi: 10.1007/s40263-020-00722-8, PMID 32248427.
Gonzalez Castillo C, Zuniga K, Martinez R, Bernal Morales B. Sildenafil improves cognitive performance in aged rats. Psychopharmacology. 2015;232(10):1873-86.
Doody RS, Gavrilova SI, Sano M, Thomas RG, Aisen PS. A phase 3 trial of valproate in mild to moderate alzheimer disease. Neurology. 2009;73(9):766-73.
Tariot PN, Erb R, Podgorski CA, Cox C, Thomas RG, Jakimovich L. Valproate monotherapy in dementia: a randomized controlled trial. Am J Geriatr Psychiatry. 2011;19(10):948-60.
Leng Y, Liang MH, Ren M, Marinova Z, Leeds P, Chuang DM. Valproic acid attenuates neurodegeneration in models of alzheimers disease via inhibition of glycogen synthase kinase-3β. J Alzheimers Dis. 2015;45(3):895-906.