
Int J Curr Pharm Res, Vol 17, Issue 5, 56-61Original Article
EVALUATION OF SCREENING METHODS FOR BEHAVIORAL AND MUSCLE COORDINATION ACTIVITY OF ETHANOLIC EXTRACT OF CHAMAEMELUM NOBILE FLOWERS IN MICE
TAMANNA THAKUR*, AJEET PAL SINGH, AMAR PAL SINGH
Department of Pharmacology, St. Soldier Institute of Pharmacy, Lidhran Campus, Behind NIT (R. E. C.), Jalandhar –Amritsar by pass, NH-1, Jalandhar-144011, Punjab, India
*Corresponding author: Tamanna Thakur; *Email: tamannathr666@gmail.com
Received: 05 Jun 2025, Revised and Accepted: 29 Jul 2025
ABSTRACT
Objective: To evaluate muscle relaxants Activity of ethanolic extract of Chamaemelum in albino mice by using Open field test, Locomotion activity test, Rota Rod Method, and Inclined Plane Model.
Methods: A total of 24 Swiss albino mice aged 6–8 w of either sex, weighing about 20-35g and maintained under standard laboratory conditions with controlled temperature (25±2 ˚C), humidity (40±10 %), and 12 h light and dark cycles each.
Various CNS models using mice were used to evaluate behavioral and muscular coordination. Diazepam was a standard drug used for behavioral and muscular coordination activities. The ethanolic extract of Chamaemelum Nobile flowers with low and high doses (250 and 500 mg/kg) was administered orally in Swiss mice for 14 d and assessed Open field test, Locomotion activity test, Rota Rod Method, and Inclined Plane Model (IPM). The effects of diazepam (DZP; 4 mg/kg) through the i. p route were also assessed. The biochemical estimations after the 14th day evaluated plasma corticosterone levels.
Result: In the present study, Chamaemelum Nobile (250 mg/kg and 500 mg/kg) Ethanolic extract was evaluated in albino Swiss mice in Open field test, Locomotion Activity Test, Rota Rod Method, and Inclined Plane Model. The effects of diazepam (DZP; 4 mg/kg) were also being assessed.
Conclusion: In conclusion, study show that the ethanolic extract of Chamaemelum Nobile flowers is effective in inducing significant protection against behavioral and muscle coordination, as demonstrated by various CNS models for control.
Keywords: Chamaemelum nobile, Behavioral and muscle coordination, Open field test, Locomotion activity test, Rota rod method, Inclined plane Model and Plasma corticosterone levels
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijcpr.2025v17i5.7033 Journal homepage: https://innovareacademics.in/journals/index.php/ijcpr
INTRODUCTION
A muscle relaxant is a drug that reduces skeletal muscle tone and function, commonly used to relieve muscle spasms, pain, and hyperreflexia [1]. It generally refers to two main therapeutic categories: neuromuscular blockers and spasmolytics [2]. Neuromuscular blockers work by disrupting transmission at the neuromuscular end plate without affecting the central nervous system (CNS), and are commonly used to induce temporary paralysis during surgery, intensive care, and emergency medicine [3]. Spasmolytics like carisoprodol, cyclobenzaprine, metaxalone, and methocarbamol are frequently prescribed to manage conditions such as low back or neck pain, fibromyalgia, tension headaches, and myofascial pain syndrome [4]. Muscle relaxants are also considered beneficial in painful conditions based on the theory that pain triggers spasms and spasms, in turn, cause pain; however, substantial evidence challenges this concept [5]. Due to their enhancement of CNS inhibition, most spasmolytic agents commonly cause side effects such as sedation and drowsiness, and prolonged use may lead to dependence. Many of these drugs also carry a risk of abuse, leading to strict prescription regulations. Common spasmolytics include methocarbamol, carisoprodol, chlorzoxazone, cyclobenzaprine, gabapentin, metaxalone, and orphenadrine [6]. Chamaemelum nobile is commonly used to treat skin and mucous membrane inflammations, as well as various bacterial infections affecting the skin, oral cavity, gums, and respiratory tract. In the form of an aqueous extract, it is often used as a mild sedative to soothe nerves, reduce anxiety, and manage conditions like hysteria, nightmares, insomnia, and other sleep disturbances [7]. Various chamomile preparations have been developed, with herbal tea being the most popular, consumed in over a million cups daily. This research explores the traditional use of chamomile, assessing its therapeutic and preventive properties while emphasizing recent discoveries supporting its potential as a health-promoting therapeutic agent. For the past four decades, benzodiazepines have been widely used to treat different types of anxiety [8]. The essential oil of German chamomile primarily contains the terpenoids α-bisabolol and its oxide, along with azulenes such as chamazulene and acetylene derivatives. Chamazulene and bisabolol are highly unstable and are best preserved in alcoholic tinctures. In contrast, Roman chamomile essential oil has lower levels of chamazulene and is mainly composed of esters of angelic and tiglic acids, along with compounds like farnesene and α-pinene. Roman chamomile also contains up to 0.6% sesquiterpene lactones of the germacranolide type, primarily nobilin and 3-epirubicin. The major bioactive components across both types include α-bisabolol, bisabolol oxides A and B, chamazulene (or azulene), farnesene, spiro-ether sesquiterpene lactones, glycosides, hydroxycoumarins, flavonoids (such as apigenin, luteolin, patulin, and quercetin), coumarins (herniarin and umbelliferone), terpenoids, and mucilage [9] flowers also contain several important phenolic compounds, mainly the flavonoids apigenin, quercetin, and patulin in the form of glucosides and various acetylated derivatives. Among these, apigenin stands out as the most promising flavonoid, found in minimal amounts as free apigenin but primarily existing as various glycoside forms [10].
MATERIALS AND METHODS
Procurement of animals
A total of 24 Swiss albino mice aged 6–8 w of either sex, weighing about 20-35g, were procured from the AHF, Lovely Professional University in Phagwara, Punjab. The animals were acclimatized for seven days to the housing conditions of the Central Animal House Facility of St. Soldier Institute of Pharmacy, Jalandhar, before experiments. Animals were housed and maintained under standard laboratory conditions with controlled temperature (25±2˚C), humidity (40±10 %), and 12 h light and dark cycles each. The animals were fed with a standard rodent pellet diet and water ad libitum. The experiments were carried out between 09:00 to 17:00 h. The laboratory animals were maintained as per CPCSEA guidelines. The experimental protocol were approved by the Institutional Animal Ethics Committee (IAEC) and was carried out as per the guidelines of the Committee for Control and Supervision of Experimental Animals (CPCSEA), Ministry of Environment and Forests, Government of India (Reg. No. 2011/PO/Re/S/18/CPCSEA and date of registration is 1/5/2018) for the use and care of experimental animals. Adequate measures were taken to minimize pain or discomfort during the animal’s experimental procedure. The research protocol is duly approved by IAEC/CPCSEA (IAEC/SSIP/2024/PR-049).
Plant material
Chamaemelum Nobile flower extract was Procured/obtained from M/s Kshipra Biotech Private Limited, Indore, Madhya Pradesh. They also provided a certification of the plant's identity and quality (Certificate of Analysis).
Drugs and chemicals
Diazepam 4 mg/kg and Normal saline (normal saline 0.9% NaCl (10 ml/kg, or 1 ml/100 g body weight orally) were administered according to the weight of animals. The extracts were suspended in distilled water and subjected to muscle relaxant activity using the Rotarod apparatus, locomotor activity, open field test, and Inclined plane model. The extracts were administered orally (p. o.), in doses of 250 mg and 500 mg/kg.
Photochemical characterization
The different extracts were subjected to general phytochemical analysis for the presence of carbohydrates, proteins, amino acids, tannins, phenolics, flavonoids, alkaloids, anthraquinones, glycosides, saponins, and steroidal nucleus using the standard methods.
Table 1: Grouping of animals
| Groups | Treatment | Day | No. of animal |
| Group-I | Naïve animal received a standard pellet diet and tap water adlibitum daily. Control group, normal saline 0.9% NaCl (10 ml/kg, or 1 ml/100 g body weight orally). | 1-14 d | 06 |
| Group-II | Standard group received 4 mg/kg of Diazepam (i. p) | 7th d and 14th d | 06 |
| Group-III | Test Group I received CNEFE 250 mg/kg (orally) daily up to 14 d | 1-14 d | 06 |
| Group-IV | Test Group II received CNEFE 500 mg/kg (orally) daily up to 14 d | 1-14 d | 06 |
Experimental design
The duration of the study period is 14 d. Total 24 animals were used in study, with six animals were in each of the four groups.
Group I consisted of naïve animals that received a standard pellet diet and water ad libitum, along with administered normal saline (0.9% NaCl) at a dose of 10 ml/kg (or 1 ml/100 g body weight) orally for 14 d.
Group II, the standard group, was given 4 mg/kg of Diazepam intraperitoneal route (i. p.) day scheduled on the 7th and 14th d.
Group III, the test group for low dose, received CNEFE (Chamaemelum nobile ethanolic flowers extracts) at a dose of 250 mg/kg orally for 14 d.
Group IV, the test group for higher dose, received CNEFE (Chamaemelum nobile ethanolic flowers extracts) at a dose of 500 mg/kg orally for 14 d.
At the end of the treatment period, behavioral and motor activity assessments were performed. Motor activity and behaviour were evaluated using the open field test and the locomotion activity test. Muscle coordination was assessed using the inclined plane method and the rotarod method. On the 15th d, blood (0.3 ml) was withdrawn from the tail vein of all groups of mice. Blood samples were centrifuged at 2500 rpm for 10 min using a refrigerated centrifuge to separate the plasma, which was used for the estimation of corticosterone levels.
Behavioral/Skeletal muscle activity animal model
The behavioral effects of an acute or sub-acute (14 d course) will be orally administered. Chamaemelum Nobile flowers extract (250 and 500 mg/kg) Ethanolic extract will be evaluated in Swiss mice by the Open field test, Locomotion Activity test, Rota rod Method, and Inclined Plane Model (IPM). The effects of diazepam (DZP; 4 mg/kg) will also be assessed.
Open field test in mice
In this model, a group of 6 animals is treated with the test Compounds or the vehicle orally 30 min before testing the Mice are observed in a Square open field area (68×68×45 cm) equipped with 2 rows of 8 photocells, Sensitive to infrared light, placed 40 and 125 mm above the floor and the measurements are made in the dark Ventilated Sound attenuating box and Interruption of photocell beam can be collected by a microcomputer and Interruption of light beams as a movement of Mice in a cage. After 30 min, each animal was placed in the center of the box and the number of lines crossed was counted for each mice.
Locomotor activity
The spontaneous locomotor activity was assessed with the help of an actophotometer. Each animal was observed for 5 min in a Square field arena (30 cm×30 cm×30 cm) equipped with six photocells in the outer wall. Interruptions of photocell beams (locomotor activity) were recorded using a six-digit counter. To see the locomotor activity, the Actophotometer was turned on and each mouse was placed in a divided in the activity Cage for 5 min. The basal activity Score for all the animals was noted. Control normal Saline, Standard Diazepam, and two different doses of aqueous extract of the flower were given orally, and after 1 h of re-testing. The locomotor activity can be easily measured using an Actophotometer, which operates on photoelectric cells that are connected in a circuit with a counter. When the beam of light falls on the photocell, it is cut off by the animal, and the count is recorded.
Inclined plane model
A simple behavior model in mice to detect compounds with skeletal muscle relaxant effects. The plane consists of two rectangular plywood boards connected at one end by a hinge. One board is the base, the other is the movable inclined plane, which is set at 65°. The inclined plane method is used to assess skeletal muscle relaxation. Mice with a body weight between 20 to 30 g are used. The plane consists of two rectangular plywood boards connected at one end by a hinge. One board is the base, the other is the movable inclined plane, a rubber mat with ridges 0.2 cm in height is fixed to the inclined plane, which is set at 65 degrees. The test compound or the standard is administered to groups of 24 mice either i. p. or s. c. or orally. 30, 60, and 90 min thereafter, the mice are placed at the upper part of the inclined plane and are given 30 s to hang on or to fall off.
Rota rod method
Rotarod is a horizontal metal rod coated with rubber, 3 cm in diameter, placed at a rotation of 25 rpm. The metal rod is about 50 cm above the surface to prevent the animal from jumping off the roller. The albino mice were placed on the revolving rod for a period of 10 min. Only those animals that remained in the revolving rod for at least 1 min were included in the study. The initial basal reading of the number of rotations covered by each animal before falling from the rotarod during this period was recorded. The test and standard compound were administered 1 hour before placing the rats on the rotarod. The number of animals falling from the rotarod during this period was counted. The difference in fall of time from the rotating rod between the control and treated mice was taken as an index of muscle relaxation.
Biochemical estimation
Collection of blood samples
On the 15th day, blood (0.3 ml) was withdrawn from the tail vein of all groups of mice. Blood samples were centrifuged at 2500 rpm for 10 min using a refrigerated centrifuge (Paramount Scientific Works, Ambala Cantt, India) to separate the plasma, which was used for the estimation of corticosterone levels.
Estimation of plasma corticosterone levels
The quantitative estimation of corticosterone levels in the blood plasma was performed by the method of Bartos and Perez (1979). To 1.0 ml of sample in ethanol, 0.50 ml of 0.10 % solution of p-nitroso-N, N-dimethylaniline in ethanol was added, and the tubes were immersed in ice water for 5 min, and then 0.50 ml of 0.10 N sodium hydroxide was added. The tubes were plugged with cotton wool and were left to stand at 0 °C for 5 h, protected against light. To the above solution, 2.0 ml of buffer for pH 9.8, 5.0 ml of 0.10 % solution of phenol in ethanol, and 0.50 ml of 1.0 % aqueous solution of potassium ferricyanide were added. The tubes were kept in a water bath at 20±2 °C for 10 min. The solution was read at 650 nm using a UV-visible spectrophotometer (UV 3200 UV-VIS Spectrophotometer, Somajiguda, Hyderabad).
Statistical analysis
All the results were expressed as Mean±SEM. The data of all the groups were analyzed by one-way ANOVA followed by Tukey’s test using software Graph Pad Prism In Stat (Graph Pad Software Inc., USA). A value of p<0.05 was considered to be significant.
RESULTS
Effect of Chamaemelum nobile flowers extract on body weight (g) of mice
In both test groups, mice of 6 no. in each group were treated with Chamaemelum Nobile extract (250 and 500 mg/kg/p. o), respectively showed a significant (p<0.05) increase in body weight as compared to the control group. In treatment with DZP (4 mg/kg i. p), the body weight significantly increased as compared to the normal group.

Fig. 1: Graph showing the effect of Chamaemelum nobile extract on body weight (g) of mice
Effect of Chamaemelum Nobile flowers extract on feed intake (g) of mice
The mice of the Chamaemelum Nobile extract (250 and 500 mg/kg/p. o) treated group showed a significant (p<0.05) increase in feed intake as compared to the control group. In treatment with DZP (4 mg/kg i. p), the feed intake significantly (p<0.05) increased as compared to the control group.
Effect of Chamaemelum Nobile flowers extract on water intake (ml) of mice
The mice of the Chamaemelum Nobile extract (250 and 500 mg/kg/p. o) treated group showed a significant (p<0.05) increase in body water intake as compared to the control group. In treatment with DZP (4 mg/kg i. p), the water intake significantly (p<0.05) increased as compared to the test group.

Fig. 2: Graph showing the effect of Chamaemelum Nobile extract on food intake of mice
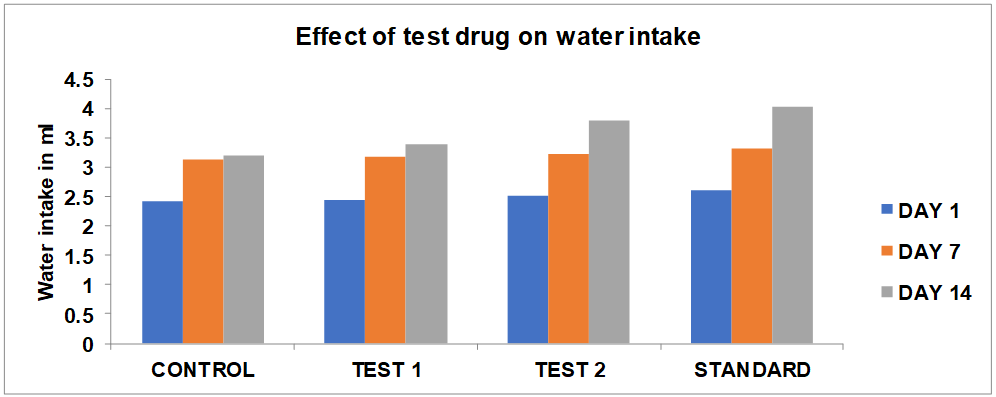
Fig. 3: Graph showing effect of Chamaemelum Nobile extract on water intake (ml) of mice
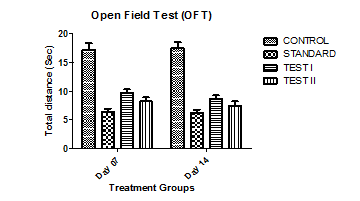
Fig. 4: Effect of ethanolic flower extracts of Chamaemelum Nobile on total distance (number of square crossings) when the control group is compared with the standard group and drug-treated groups on the initial and final day of study
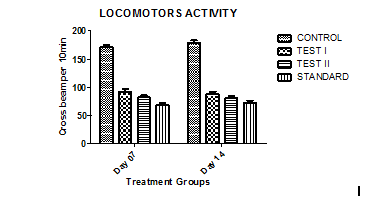
Fig. 5: Showing the Effect of Chamaemelum Nobile extracts on locomotor activity of mice
Values are expressed as mean±SEM (One-way ANOVA followed by Tukey’s test). The mice of the Chamaemelum Nobile extract (250 and 500 mg/kg/p. o.) treated group showed no significant (p<0.05) difference in locomotor activity as compared to the control group. Treatment with DZP (4 mg/kg p. o.) also showed significant change as compared to the control group.
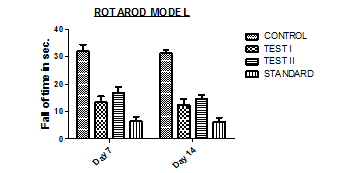
Fig. 6: Effects of different doses of Chamaemelum Nobile flowers ethanolic extract on skeletal muscle relaxant as compared to standard (Diazepam)
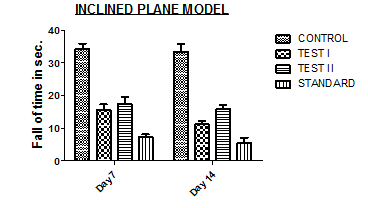
Fig. 7: Graphical representation of effects of different doses of Chamaemelum Nobile flowers ethanolic extract on skeletal muscle relaxant as compared to standard (Diazepam)
Treatment with Diazepam significantly increased the fall time (P<0.05) in the Inclined Plane Model. Ethanolic extract of Chamaemelum Nobile flowers treated mice also exhibited dose-dependent, significantly increased fall time. The duration of fall of time was also significantly reduced as compared to the vehicle-treated group. However, there is little significant difference between Chamaemelum Nobile flower extracts-treated animals and Diazepam-treated animals. The above observation suggests that Chamaemelum Nobile has Skeletal Muscle Relaxant activity.
Plasma corticosterone levels
Groups 1 to 4 were bled on day 15, and then corticosterone levels were combined to obtain the average levels in tail blood. For treatment of Groups, see their respective experimental design.
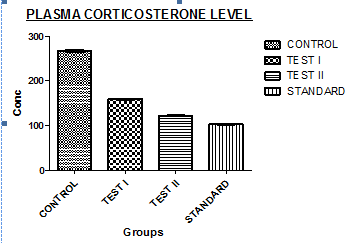
Fig. 8: Shows the plasma corticosterone level
Graph showing the corticosterone levels in Post Rota rod/Inclined plane test experiments
Group 1: In this mice were treated with Normal saline, and the corticosterone level was measured, which is 267.34± 7.76ng/ml.
Group 2: In this mice were treated with a low dose of the Ethanolic extract, and the corticosterone level was measured, which is 159.66±4.42ng/ml.
Group 3: In this mice were treated with a High dose of the Ethanolic extract, and the corticosterone level was measured, which was 122.48±2.34ng/ml.
Group 4: In this mice were treated with Standard (Diazepam), and corticosterone level was measured, which was 102.22±1.36ng/ml.
It is known that stress enhances the activity of the hypothalamic-pituitary-adrenal (HPA) axis and results in increased secretion of corticosteroids from the adrenal cortex. Cortisol and corticosterone are thus often used as biomarkers for stress and depressive disorders. Although corticosterone is considered the main glucocorticoid involved in the regulation of stress responses in rodents, researchers often choose to detect cortisol for stress indicators in consideration of convenience and kit availability.
DISCUSSION
The presence of medicinal substances in the higher plants is well established. Plants have provided a source of inspiration for novel drug compounds, as plant-derived medicines have made significant contributions towards human health. Phytomedicine can be used for the treatment of diseases, as is done in the case of the Unani and Ayurvedic systems of medicine, or it can be the base for the development of a medicine, a natural blueprint for the development of a drug.
Progress in unraveling the neurochemical mechanism is, as in so many areas of psychopharmacology, limited by the lack of good animal models of the clinical condition. There is no known animal condition corresponding to the inherited condition of depression in humans, but various procedures have been described that produce in animals behavioral states typical of human depression.
After the selection of Chamaemelum Nobile flowers, acute oral toxicity was detected with Ethanolic extracts (CNFEE) having doses (5, 50, 300, 1000 mg/kg) via the oral route, showing no change in behavioral responses, and observation shows no acute oral toxicity. Hence, depending upon it, the Dose was 250 mg/kg and 500 mg/kg for our experimental work.
Successive isolation of botanical compounds from plant material is largely dependent on the type of solvent used in the extraction procedure. The traditional healers use primarily water as the solvent, but we found in this study that the plant extracts by ethanol provided more consistent activity compared to those extracted by water. The results of the skeletal muscle activity of the plant Chamaemelum Nobile against the investigated RRA and IPM parameters are shown in the table. The higher dose extract produced a greater effect compared to the lower dose extract in both parameters. This might have resulted from the higher quantities of active constituents in the Ethanolic extract showing some degree of skeletal muscle activity. Further trials using solvents of various polarities will explore the effects of solvent composition on extract efficacy.
Preliminary phytochemical analysis of Chamaemelum Nobile revealed the presence of phenol compounds, Proteins, tannins, glycosides, Carbohydrates, Starch, Vitamins, and Minerals, etc. It is not surprising that there are differences in the pharmacological effects of plant species, due to the phytochemical properties and differences among species.
It is possible that the active chemical constituents were not soluble in ethanol or water. The drying process may have caused conformational changes to occur in some of the chemical constituents found in these plants. The active compound(s) may be present in insufficient quantities in the crude extracts to show activity with the dose levels employed. Lack of activity can thus only be proven by using large doses.
Alternatively, if the active principle is present in high enough quantities, there could be other constituents exerting antagonistic effects or negating the positive effects of the bioactive agents with no significant activity. Extracts may be active against other biological activities that were not tested.
Chamaemelum Nobile (250 mg/kg and 500 mg/kg) Ethanolic extract was evaluated inalbino Swiss mice in OFT, LA, RRA and IPM. The effects of diazepam (DZP; 4 mg/kg) were also being assessed.
The higher dose of the Ethanolic extract of Chamaemelum Nobile showed the most remarkable activity. This plant can be further subjected to isolation of the therapeutic Antidepressant compound, and further pharmacological evaluation, exhibiting significant.
CONCLUSION
The ethanolic extract of Chamaemelum Nobile exhibited significant muscle relaxant activity in a dose-dependent manner. Maximum muscle relaxation was observed with 500 mg/kg of Chamaemelum Nobile. The Rotarod test showed a highly significant reduction in the time spent by the animals on the revolving rod when compared to the controls. The results from the Actophotometer test and Rotarod test showed that the extract significantly reduced the motor coordination of the tested animals. The effect of flower extracts of Chamaemelum Nobileon the total number of square crossings observed increased when compared to control groups.
ACKNOWLEDGMENT
It’s our privilege to express the profound sense of gratitude and cordial thanks to our respected chairman Mr. Anil Chopra and Vice Chairperson, Ms. Sangeeta Chopra, St. Soldier Educational Society, Jalandhar for providing the necessary facilities to complete this research work.
FUNDING
Nil
AUTHORS CONTRIBUTIONS
All authors have contributed equally
CONFLICT OF INTERESTS
Declared none
REFERENCES
Shadman AS, Priyambada, Supriyana. Comparative study of muscle relaxant activity of midazolam with diazepam in male albino mice. Int J Pharm Sci Rev Res. 2019;58(2):9-14. doi: 10.9790/0853-1709050914.
Sharmila R. Muscle relaxants in treating temporomandibular joint disorder an update. J Pharm Sci Res. 2015;7(8):611-4.
Jabeen A, Ramya B, Soujanya J, Bhattacharya B. Evaluation of anxiolytic muscle relaxant and locomotor activity of Cuminum cyminum. J Med Plants Stud. 2017;5(2):259-62.
Ge HY, Fernandez De Las Penas C, Yue SW. Myofascial trigger points: spontaneous electrical activity and its consequences for pain induction and propagation. Chin Med. 2011;6:13. doi: 10.1186/1749-8546-6-13, PMID 21439050.
Beebe FA, Barkin RL, Barkin S. A clinical and pharmacologic review of skeletal muscle relaxants for musculoskeletal conditions. Am J Ther. 2005;12(2):151-71. doi: 10.1097/01.mjt.0000134786.50087.d8, PMID 15767833.
Chou R, Peterson K, Helfand M. Comparative efficacy and safety of skeletal muscle relaxants for spasticity and musculoskeletal conditions: a systematic review. J Pain Symptom Manage. 2004;28(2):140-75. doi: 10.1016/j.jpainsymman.2004.05.002, PMID 15276195.
Srivastava JK, Shankar E, Gupta S. Chamomile: a herbal medicine of the past with bright future. Mol Med Rep. 2010;3(6):895-901. doi: 10.3892/mmr.2010.377, PMID 21132119.
Kavya Sree K, Vijusha M, Rajani A, Hemamalini K, Ratna Sundari EG. Screening of behavioural muscle co-ordination and anxiolytic activities of methanolic extract of tabebuia rosea (Bertol.). Asian J Pharm Clin Res. 2013;6 Suppl 5:187-90. doi: 10.13040/IJPSR.0975-8232.5(2).526-31.
Lemberkovics E, Kery A, Marczal G, Simandi B, Szoke E. Phytochemical evaluation of essential oils medicinal plants and their preparations. Acta Pharm Hung. 1998;68(3):141-9. PMID 9703700.
Babenko NA, Shakhova EG. Effects of chamomilla recutita flavonoids on age related liver sphingolipid turnover in rats. Exp Gerontol. 2006;41(1):32-9. doi: 10.1016/j.exger.2005.08.008, PMID 16183236.