
Int J Curr Pharm Res, Vol 17, Issue 4, 113-116Original Article
THE STUDY OF COLISTIN SUSCEPTIBILITY AMONG CARBAPENEM-RESISTANT GRAM-NEGATIVE BACILLI BY BROTH MICRODILUTION METHOD USING READY-TO-USE COLISTIN MIC PLATE KIT
K. SNEHITHA, S. SWAPNA*, P. RATNAKUMARI
Department of Microbiology, Andhra Medical College, Visakhapatnam, India
*Corresponding author: S. Swapna; *Email: swapna.sasapu@gmail.com
Received: 15 Apr 2025, Revised and Accepted: 10 Jun 2025
ABSTRACT
Objective: Carbapenem-resistant Gram-negative bacteria (CR-GNB) pose a major threat to the treatment of infectious diseases. There is a recent increase of Colistin use as a last resort antibiotic for carbapenem-resistant and extensively drug-resistant GNB infections. As per EUCAST (2023) and CLSI(2023) guidelines, gradient diffusion or disc diffusion are inappropriate for colistin susceptibility testing and colistin MIC should be performed by broth microdilution method.
The aim of the present study is to isolate Carbapenem-resistant Gram-negative bacilli (CR-GNB) and to detect colistin susceptibility in CR-GNB using commercial broth microdilution plate kit.
Methods: This is a cross-sectional descriptive study conducted at the Department of Microbiology, Andhra Medical College, Visakhapatnam, for a period of 6 mo from October 2023 to March 2024. A total of 58 CR-GNB isolated from 4905 samples were taken for colistin susceptibility testing by the HiMIC plate kit Broth microdilution technique.
Results: This study examined 4905 clinical samples comprising of Urines, Blood, and Exudates. Out of which 1269 were GNB, 41 were Gram-positive cocci and 3595 were sterile. Of these, 1269 GNB, 58 were identified as CR-GNB, according to EUCAST (2023) guidelines. The most common CR-GNB organism isolated was Klebsiella spp. (47%), followed by Escherichia coli (41%), Acinetobacter spp. (7%), and Pseudomonas aeruginosa (5%). All 58 isolates were tested for colistin susceptibility, with 4 isolates showing resistance and 54 being in the sensitive range according to EUCAST guidelines. Of the colistin-resistant isolates, 3 were Klebsiella spp. and 1 was E. coli with MICs of 16 mcg/ml, 16 mcg/ml, 8 mcg/ml, and 4 mcg/ml, respectively.
Conclusion: This colistin plate kit is a high-throughput, less time-consuming, ready-to-use method for colistin susceptibility testing in routine microbiology laboratories, which guides clinicians in lowering mortality and morbidity of CR-GNB infections by appropriate use of colistin.
Keywords: Carbapenem resistance, EUCAST, Colistin, Broth micro dilution, Ready-to-use
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijcpr.2025v17i4.7037 Journal homepage: https://innovareacademics.in/journals/index.php/ijcpr
INTRODUCTION
Carbapenem-resistant Gram-negative bacteria (CR-GNB) are increasingly being identified worldwide and pose a major threat to the treatment of infectious diseases [1]. The number of deaths is 2-fold higher among patients with bacteraemia caused by CR-GNB than Carbapenem Susceptibility variants. Given the paucity of new drugs, the focus of management shifted to older drugs such like colistin as a last resort treatment option for CR-GNB [2]. There is a recent increase of colistin use for carbapenem-resistant and extensively drug-resistant Gram negative bacterial infections [3].
Routine methods for colistin antimicrobial susceptibility testing like gradient diffusion and disk diffusion are inappropriate because, colistin molecules are large cationic molecules that diffuse poorly in diffusion-based assays [2]. As per EUCAST 2023 [4] and CLSI 2023 [5] guidelines, Colistin MIC should be performed by broth microdilution method. Colistin susceptibility testing is needed in routine clinical laboratories to allow appropriate therapeutic decision-making.
Hence, this study was doneto evaluate the scope of Broth microdilution by commercial plate kit in detecting the Colistin resistance in CR-GNB isolates.
MATERIALS AND METHODS
This cross-sectional descriptive study was conducted at Department of Microbiology, Andhra Medical College, Visakhapatnam for a period of 6 mo from October 2023 to March 2024. A total of 4905 clinical samples such as Pus, Body fluids, Blood and Urine were processed during the study period.
Study procedure
All clinical samples were collected aseptically and were inoculated onto Nutrient agar, Blood agar, and MacConkey agar and were incubated at 35 ̊-37 °C for 18-24 h. Gram-negative bacilli were identified by colony morphology, Gram staining, motility, and the standard biochemical tests. The Gram-negative bacilli that were intrinsically resistant to colistin were excluded from the study.
Antimicrobial susceptibility testing
AST was performed by Kirby-Bauer disk diffusion method and interpreted using EUCAST [4] guidelines 2023. The following antibiotics were tested on Mueller Hinton agar: Amikacin, Gentamicin, Ciprofloxacin, Levofloxacin, Ceftazidime, Ceftazidime-clavulanic acid, Cotrimoxazole, Imipenem, Meropenem, Ertapenem, Fosfomycin, Nalidixic acid, Nitrofurantoin, Piperacillin and Tazobactam.
Detection of CR-GNB
According to EUCAST [4] guidelines 2023, Meropenem (10µg) and Ertapenem (10µg) disks were used to detect CR-GNB with disc diffusion screening cut-offs for Meropenem<28 mm, Ertapenem<25 mm.
The Carbapenem-resistant Gram-negative bacilli (CR-GNB) isolates were subjected to colistin susceptibility testing by broth microdilution method using a commercial HiMIC plate kit (Himedia Pvt. Ltd) as per the manufacturer’s instructions [6].
Colistin susceptibility testing
Carbapenem-resistant Gram-negative bacilli were taken for colistin susceptibility testing by broth microdilution technique using commercial HiMICTM plate kit [6]. Colistin HiMICTM Plate Kit (fig. 1) is a ready-to-use kit, designed to detect MIC of Colistin against test organisms by broth microdilution method, in the range of 0.25 mcg/ml to 16 mcg/ml.
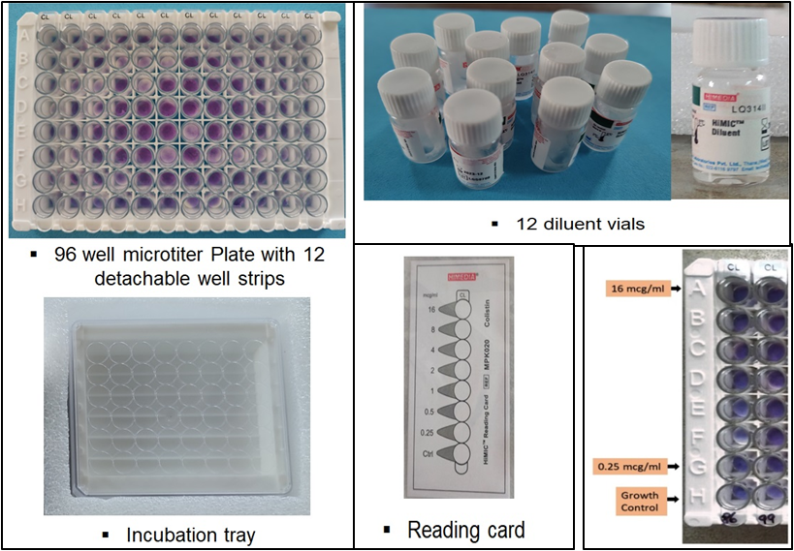
Fig. 1: HiMICTM plate kit contents

Fig. 2: Preparation of final inoculum
Colistin susceptibility test procedure
Only pure cultures were used for the test. Four to five similar colonies were transferred to 5 ml Tryptone Soya Broth and incubated at 35°-37 °C for 2-8 h. The inoculum turbidity was compared with that of 0.5 McFarland standards. Then the inoculum was diluted for further two dilutions to get final inoculum of 106cfu/ml (fig. 2). The final inoculum is used to test the colistin susceptibility as per manufacturer’s instructions.
A 200 µl** of final test inoculum was transferred into HiMICTMdiluent vial and vortexed well for proper mixing of the inoculum. The required number of detachable microtitre well strips were taken and labelled them. An amount of 220 µl** of inoculum mixture from the diluent vial was transferred into all the 8 wells and incubated at 35°-37 °C for 2-8 h. As per the instructions from the manufacturer, the wells were interpreted using the reading card given. The first purple well of the lowest concentration was interpreted as the Minimum Inhibitory Concentration (MIC) of the test inoculum.
Interpretation of results and quality control were performed as per EUCAST Guidelines version 13.0, 2023 (table 1-2).
Table 1: Interpretation: as per EUCAST guidelines version 13.0, 2023
| When testing | Incubation | Interpretative criteria (mcg/ml) |
| ≤S | ||
| Enterobacteriaceae. Acinetobacter spp. | 33 ℃ – 37 ℃ for 18 h | 2 |
| Pseudomonas spp. | 4 |
Table 2: Quality control (as per EUCAST guidelines)
| Organism | Target MIC value (mcg/ml) |
| Escherichia coli, ATCC 25922 | 0.5 – 1 |
| Escherichia coli, NCTC 13846 | 4 |
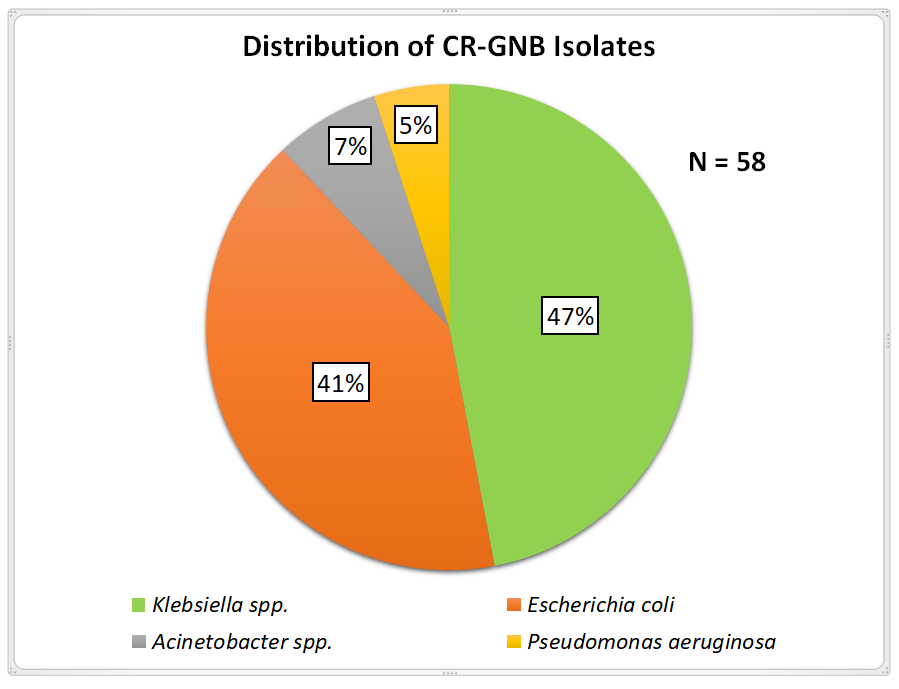
Fig. 3: Distribution of CR-GNB isolates
RESULTS
Out of Total 4905 clinical samples tested in this study, 3595 were culture Sterile and 1310 were culture Postivies. Among Culture Positives, 1269 were Gram Negative Bacilli, in which 58 were CR-GNB isolates (N=58) (fig. 3). Out of 58 CR-GNB isolates Klebsiella spp. were 27(47%), Escherichia coli 24(41%), Acinetobacter spp. 4(7%), Pseudomonas aeruginosa 3(5%), which were tested for Colistin Susceptibilty.
Interpretation of colistin susceptibility
Out of total 58 (n) CR-GNB isolates. 54 isolates were sensitive and 4 isolates were resistant to Colistin. Among 4 Resistant isolates 3 were Klebsiella pneumoniae and 1 was Escherichia coli. Minimum Inhibitory Concentration (MIC) for the three Klebsiella pneumoniae isolates were 16mcg/ml, 16mcg/ml, 8mcg/ml respectively, and for E. coli was 4mcg/ml (fig. 4 and table 3)
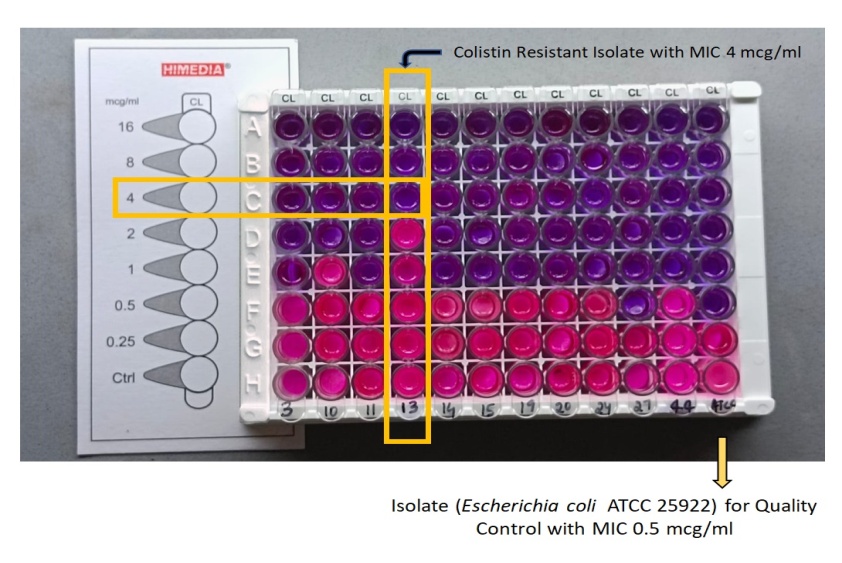
Fig. 4: MIC interpretation of colistin susceptibility
Table 3: MIC interpretation of colistin-resistant isolates
| Colistin-resistant isolates (4) | MIC (mcg/ml) |
| Klebsiella pneumoniae | 16 |
| Klebsiella pneumoniae | 16 |
| Klebsiella pneumoniae | 8 |
| Escherichia coli | 4 |
DISCUSSION
In our study, among 58 total CR-GNB isolates Klebsiella pneumoniae was the predominant organism (47%). Similar findings have been reported by other studies. In a study by Chew et al. [7]. 41% of CR-GNB were Klebsiella pneumoniae. In a study by Mohanty S et al. [8]. 63.4% of CR-GNB were Klebsiella pneumoniae. Dafopoulou K et al. [9] reported 67.2% of CR-GNB were Klebsiella pneumoniae.
In our study among 58 CR-GNB, 6.9% isolates were resistant to colistin by BMD. In a study by RahulRanjan et al. [2] among CR-GNB, 9% of isolates were resistant to colistin by BMD correlates with present study. However, in a study by Chew et al. [7] 32.89% of CR-GNB isolates were colistin resistant by BMD, this high colistin resistance rate reported as compared to our study could be due the inclusion of isolates from non-invasive infections where as our study included both invasive and non-invasive infections. Matuschek E et al. [10] reported 5.3% colistin-resistant isolates (table 4).
Table 4: Colistin resistance among CR-GNB isolates
| Study | Total CR-GNB isolates | Colistin-resistant isolates | Percentage |
| Rahul Ranjan et al.(2022) [2] | 100 | 9 | 9% |
| Chew et al. (2017) [7] | 76 | 25 | 32.89% |
| Matuschek E et al. (2018) [10] | 19 | 1 | 5.3% |
| Present study | 58 | 4 | 6.9% |
CONCLUSION
According to EUCAST4and CLSI5guidelines, it is essential to test colistin susceptibility using the broth microdilution method because false susceptibility by gradient diffusion approach could result in ineffective colistin therapy.
This colistin plate kit used in this study is a high-throughput, less time-consuming, ready-to-use practical method for colistin susceptibility testing in routine microbiology laboratories. This study may guide clinicians significantly in lowering mortality and morbidity of carbapenem-resistant infections by appropriate use of colistin.
ACKNOWLEDGEMENT
Nil
FUNDING
Nil
AUTHORS CONTRIBUTIONS
The first author of the study K SNEHITHA contributed conceptual design, literature search, collection of patient samples and data collection. The second author S SWAPNA contributed sample processing, Data analysis, statistical analysis and wrote the first draft of the manuscript. The third author P RATNA KUMARI guided the work and corrected the manuscript.
CONFLICT OF INTERESTS
The study declared no conflicts of interest.
REFERENCES
Nordmann P, Naas T, Poirel L. Global spread of carbapenemase producing Enterobacteriaceae. Emerg Infect Dis. 2011;17(10):1791-8. doi: 10.3201/eid1710.110655, PMID 22000347.
Ranjan R, Iyer RN, Jangam RR, Arora N. Evaluation of in vitro colistin susceptibility and clinical profile of carbapenem-resistant Enterobacteriaceae-related invasive infections. Indian J Med Microbiol. 2023 Jan-Feb;41:40-4. doi: 10.1016/j.ijmmb.2022.12.008, PMID 36870748.
Lomonaco S, Crawford MA, Lascols C, Timme RE, Anderson K, Hodge DR. Resistome of carbapenem and colistin resistant Klebsiella pneumoniae clinical isolates. PLOS One. 2018 Jun 8;13(6):e0198526. doi: 10.1371/journal.pone.0198526, PMID 29883490.
European committee on antimicrobial susceptibility testing (EUCAST). Break point tables for interpretation of MICs and zone diameters; 2023. Available from: https://www.eucast.org.
CLSI performance standards for antimicrobial susceptibility testing. 32nd ed. Vol. M100. Clinical Laboratory Standard Institute; 2023. Available from: www.clsi.org/standards/products/iso-documents/documents.
Colistin HiMIC Plate kitMPK020 (Package insert). Maharashtra: HiMedia Laboratories; 2023.
Chew KL, La MV, Lin RT, Teo JW. Colistin and polymyxin B susceptibility testing for carbapenem resistant and mcr-positive Enterobacteriaceae: comparison of sensititre microscan vitek 2 and etest with broth microdilution. J Clin Microbiol. 2017;55(9):2609-16. doi: 10.1128/JCM.00268-17, PMID 28592552.
Mohanty S, Gaind R. In vitro susceptibility of carbapenem resistant Enterobacteriaceae to colistin: a hope at present. Indian J Med Microbiol. 2016;34(4):558-60. doi: 10.4103/0255-0857.195381, PMID 27934845.
Dafopoulou K, Zarkotou O, Dimitroulia E, Hadjichristodoulou C, Gennimata V, Pournaras S. Comparative evaluation of colistin susceptibility testing methods among carbapenem nonsusceptible klebsiella pneumoniae and acinetobacter baumannii clinical isolates. Antimicrob Agents Chemother. 2015 Aug;59(8):4625-30. doi: 10.1128/AAC.00868-15, PMID 26014928.
Matuschek E, Ahman J, Webster C, Kahlmeter G. Antimicrobial susceptibility testing of colistin evaluation of seven commercial mic products against standard broth microdilution for Escherichia coli Klebsiella pneumoniae Pseudomonas aeruginosa and Acinetobacter spp. Clin Microbiol Infect. 2018 Aug;24(8):865-70. doi: 10.1016/j.cmi.2017.11.020, PMID 29221995.