
Int J Curr Pharm Res, Vol 17, Issue 4, 45-51Original Article
PHARMACOVIGILANCE STUDY OF ANTICANCER DRUGS IN A DISTRICT GOVERNMENT HOSPITAL OF CENTRAL INDIA: A PROSPECTIVE OBSERVATIONAL STUDY
ANERI PATEL*, ANJALI KUSHWAH, KAMAYANI GUPTA
Department of Pharmacology, Mahatma Gandhi Memorial Medical College, Indore, Madhya Pradesh, India
*Corresponding author: Aneri Patel; *Email: aneripatel2311.96@gmail.com
Received: 12 Apr 2025, Revised and Accepted: 05 Jun 2025
ABSTRACT
Objective: Anticancer drugs are responsible for most adverse drug reactions (ADRs) in cancer patients worldwide. ADR reporting of anticancer drugs is sporadic in India, especially in rural areas, due to the lack of awareness and knowledge about the Pharmacovigilance Programme of India (PvPI). This study assessed the causality and severity pattern of ADRs with anticancer drugs in cancer patients.
Methods: An observational, prospective, and single-center study was conducted at the oncology unit of the District Government Hospital, Jhabua, of Madhya Pradesh. Patients of either sex, aged>18 y, reporting ADRs with anticancer drugs from April 1, 2024, to July 31, 2024, were included. The analysis focused on demographic data of the patients, suspected drugs, causality assessment using the World Health Organization-Uppsala Monitoring Centre (WHO-UMC) scale, and severity assessment.
Results: The age group of 41-60 y (61%) and females (68%) reported the highest number of ADRs. The maximum number of ADRs was reported with the use of platinum compounds (cisplatin, carboplatin, and oxaliplatin) (34%), followed by taxanes (paclitaxel and docetaxel) (28%). The gastrointestinal system (39.38%) was the most commonly involved, and nausea (20.33%) was the most common ADR among all. The majority of ADRs were certain (76%) and mild (48.36%).
Conclusion: ADR reporting, monitoring, and timely management are important to increase the outcome of anticancer treatment. It’s important to increase awareness regarding PvPI among healthcare professionals and patients.
Keywords: Anticancer drugs, Pharmacovigilance, Adverse drug reactions, World Health Organization-Uppsala Monitoring Centre scale
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijcpr.2025v17i4.54590 Journal homepage: https://innovareacademics.in/journals/index.php/ijcpr
INTRODUCTION
Cancer is one of the most significant public health challenges worldwide in the 21stcentury. It is the second leading cause of death globally and is responsible for nearly one in six deaths (16.8%) [1]. The 2022 cancer statistics report showed that the newly diagnosed cases were 20 million and the mortality rate was 10 million [2]. In India, the crude incidence rate is 100.4 per one lakh, and one in nine people is likely to develop cancer in his/her lifetime. In India, lung cancer (10.6%) and oral cavity cancer (8.4%) are common among men, and breast cancer (28.8%) and cervical cancer (10.6%) are common among females [3].
The anticancer drugs that are used as single agents or in combination to treat patients are also responsible for many adverse drug reactions (ADRs). The World Health Organization (WHO) defined adverse drug reaction as “a response that is noxious and unintended and which occurs at doses normally used in humans for the prophylaxis, diagnosis, or therapy of disease or the modification of physiological function” [4]. The prevalence of adverse drug reactions (ADRs) due to anticancer drugs in India is 10%-12% [5]. Adverse drug reaction reporting of anticancer drugs is sporadic in India, especially in rural areas, due to the lack of awareness and knowledge about the Pharmacovigilance Programme of India (PvPI) [6].
Hence, this study was carried out to assess the causality and severity pattern of adverse drug reactions (ADRs) with anticancer agents in patients diagnosed with cancer and to increase the awareness of the Pharmacovigilance Programme of India (PvPI) in healthcare professionals and patients of the rural areas.
MATERIALS AND METHODS
Aim and objective
This study was done to assess the causality and severity pattern of adverse drug reactions (ADRs) with anticancer agents in cancer patients.
Study design
This study was a cross-sectional, prospective, observational, non-interventional, and single-center study.
Study site
After receiving the required approval from the authorities (MGMMC/5072-82/2024 dated 29/03/2024), the study was carried out at the oncology unit of the District Government Hospital in Jhabua, Madhya Pradesh.
Sample size
The patients admitted and registered throughout the study period served as the basis for designing the sample size. Between April 2024 and July 2024, a total of 153 patients were enrolled in the study.
Inclusion criteria
Newly diagnosed cancer and/or known cases of carcinoma of either sex and over the age of 18 y that required treatment with anticancer drugs either as a single-drug regimen or in a combination of two or more drugs after surgery and/or radiotherapy, who reported adverse drug reactions (ADRs), were included in the study.
Exclusion criteria
The patients are only on radiotherapy, surgery, or other modalities of management for cancer; the pregnant or lactating women and the patients who were not willing to give written informed consent were excluded from the study.
Tools utilized for data collection and analysis
The data was collected from the patients who had experienced adverse drug reactions (ADRs) by filling out the suspected adverse drug reaction monitoring form (version 1.4), and the World Health Organization-Uppsala Monitoring Centre (WHO-UMC) causality assessment scale [7] was applied.
Data analysis
The data were entered in Microsoft Excel (Windows 10; Version 2016). The collected data of demographic and clinical variables were analyzed using descriptive statistics such as frequencies and percentages and were represented in tables and figures. The causality and severity assessment of adverse drug reactions (ADRs) was done.
RESULTS
Out of 153 patients, the predominance of adverse drug reactions (ADRs) was seen more in females (n=104, 68%) than in males (n=49, 32%) fig. 1.
The adverse drug reactions (ADRs) were highest in the age group 41-60 y (n=94, 61%), followed by 21-40 y (n=32, 21%), 61-80 y (n=25, 17 %,) and only two patients (1%) were above 80 y of age (fig. 2).
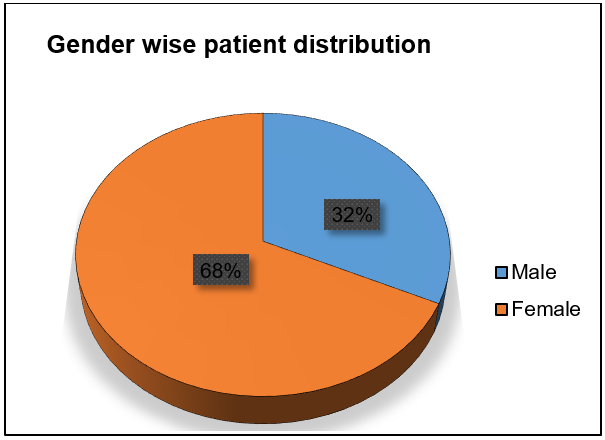
Fig. 1: Gender wise distribution of patients
Table 1: Demographics of the study population
| Demographic details | Number of patients (n=153) (%) |
| Educational status | |
| Educated | 119 (78%) |
| Uneducated | 34 (22%) |
| Marital status | |
| Married | 138 (90%) |
| Unmarried | 15 (10%) |
| Case type | |
| Old | 80 (52%) |
| New | 73 (48%) |
| Comorbidities | |
| Hypertension | 32 (21%) |
| Diabetes mellitus | 20 (13%) |
| Hypertension and diabetes mellitus | 34 (22%) |
| Others | 30 (20%) |
| No comorbidities | 37 (24%) |
| Social habits | |
| Smoking | 10 (7%) |
| Alcohol | 19 (12%) |
| Substance abuse | 25 (16%) |
| Smoking and alcohol both | 26 (17%) |
| Smoking, alcohol and substance abuse | 35 (23%) |
| No social habits | 38 (25%) |
| Solid malignancies | |
| Breast | 44 (29%) |
| Cervix | 21 (14%) |
| Tongue | 17 (11%) |
| Buccal mucosa | 29 (19%) |
| Uterus | 3 (2%) |
| Ovary | 3 (2%) |
| Gall bladder | 3 (2%) |
| Shoulder | 1 (0.65%) |
| Rectum | 3 (2%) |
| Pancreas | 1 (0.65%) |
| Glottis | 5 (3%) |
| Neck | 3 (2%) |
| Others | 15 (9%) |
| Haematological malignancy | |
| Leukaemia | 5 (3%) |
| Family history | |
| Positive for | |
| Breast | 5 (3%) |
| Uterus (Fibroids) | 3 (2%) |
| Cervix | 3 (2%) |
| Gallbladder | 1 (0.65%) |
| Tongue | 3 (2%) |
| Negative family history | 138 (90%) |
| Chemotherapy regimen | |
| Single regimen | 42 (27%) |
| Double regimen | 93 (61%) |
| Triple regimen | 18 (12%) |
All the cytotoxic drugs were administered through the intravenous route. The highest number of adverse drug reactions (ADRs) were seen with the platinum compounds (carboplatin 20%, cisplatin 13%, and oxaliplatin 1%), followed by taxanes (paclitaxel 21% and docetaxel 7%) (table 2).
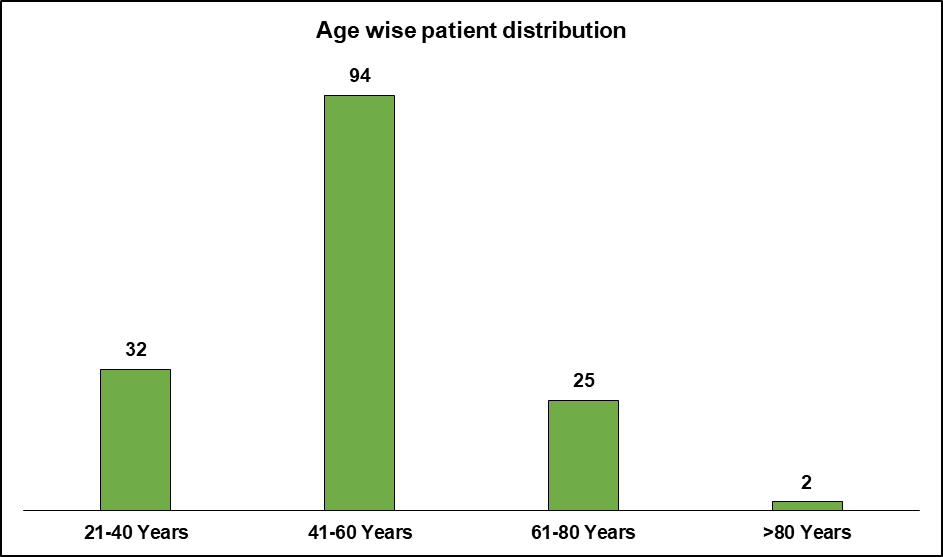
Fig. 2: Age-wise distribution of patients
In this study, the prevalence of adverse drug reactions (ADRs) was seen more with educated (n=119, 78%), married (n=138, 90%), old cases (n=80, 52%), patients with no comorbidities (n=37, 24%), and patients with no social habits (n=38, 25%). The majority of the adverse drug reactions (ADRs) were seen in patients with breast cancer (n=44, 29%), followed by buccal mucosa cancer (n=29, 19%) and cervical cancer (n=21, 14%). Among that, the majority of adverse drug reactions (ADRs) were seen with a double chemotherapy regimen (n=93, 61%) (table 1). Paclitaxel plus carboplatin was the commonly prescribed double chemotherapy regimen.
Table 2: Functional classification of cytotoxic drugs
| Functional classification | Cytotoxic drugs | Number for cytotoxic drugs (n=282) (%) |
| Platinum Compounds | Cisplatin | 38 (13%) |
| Carboplatin | 55 (20%) | |
| Oxaliplatin | 3 (1%) | |
| Alkylating Agents | Cyclophosphamide | 23 (8%) |
| Antimetabolites | Gemcitabine | 1 (0.35%) |
| 5-Fluorouracil | 20 (7%) | |
| Capecitabine | 3 (1%) | |
| Taxanes | Paclitaxel | 60 (21%) |
| Docetaxel | 20 (7%) | |
| Antibiotics | Doxorubicin | 7 (3%) |
| Dactinomycin | 1 (0.35%) | |
| Tyrosine Protein Kinase Inhibitor | Imatinib | 7 (3%) |
| Topoisomerase-2 Inhibitor | Etoposide | 1 (0.35%) |
| SERM | Tamoxifen | 18 (6%) |
| Anthracycline | Epirubicin | 25 (9%) |
Only 19 (12%) patients had experienced a single adverse drug reaction, and the rest, 134 (88%), had experienced more than one adverse drug reaction.
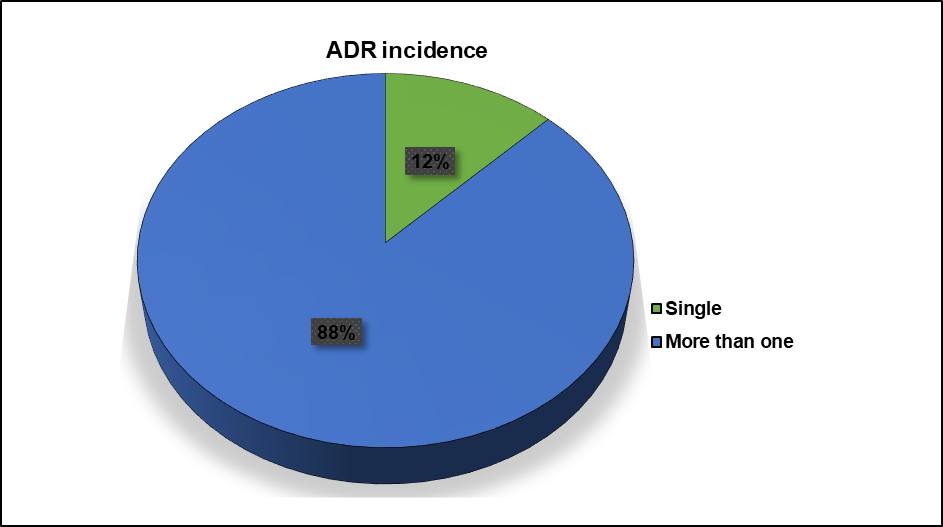
Fig. 3: Incidence of adverse drug reactions (ADRs) among the patients
The gastrointestinal system (n=102) was the most commonly involved system, followed by the integumentary (n=38) and musculoskeletal (n=20) systems (fig. 4). Nausea (n=85, 20.33%) was the most commonly experienced adverse drug reaction, followed by vomiting (n=83, 19.85%) and urticaria (n=61, 14.59%) (table 3). During the chemotherapy, the most commonly prescribed adjuvant drugs were ranitidine (100%) and vitamins (100%).
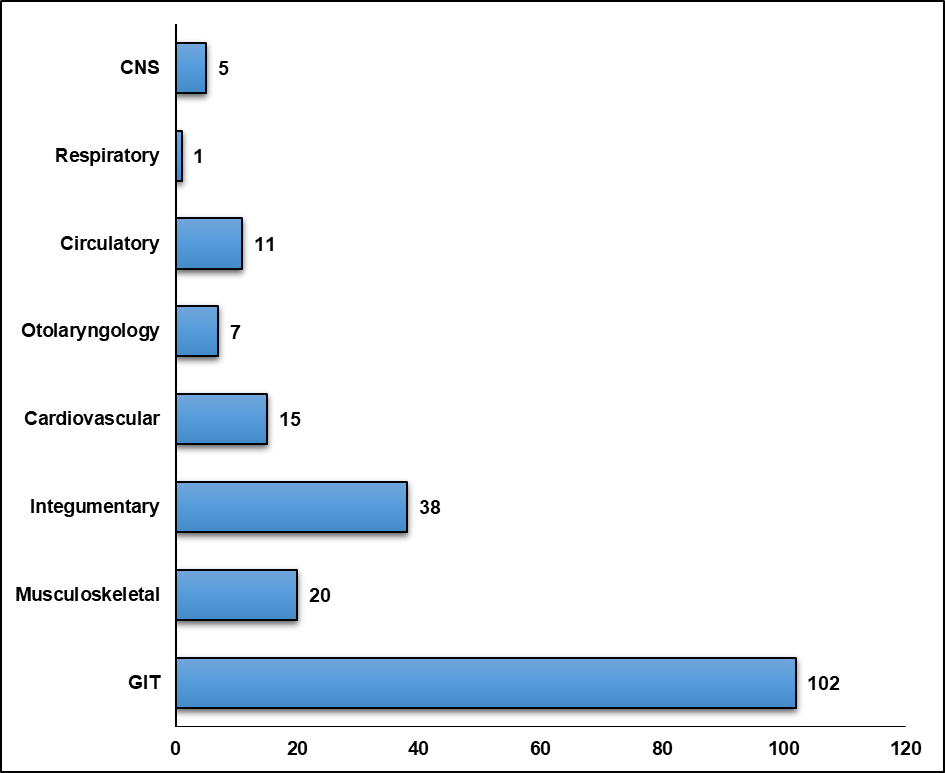
Fig. 4: System-wise distribution of adverse drug reactions (ADRs)
Table 3: Commonly encountered adverse drug reactions (ADRs) with anticancer drugs
| Adverse drug reactions | Frequency (n=418)(%) |
| Gastrointestinal system | |
| Nausea | 85 (20.33%) |
| Vomiting | 83 (19.85%) |
| Gastroesophageal reflux disease (GERD) | 29 (6.93%) |
| Constipation | 18 (4.30%) |
| Diarrhoea | 3 (0.71%) |
| Hepatotoxicity | 1 (0.23%) |
| Musculoskeletal system | |
| Pedal oedema | 15 (3.58%) |
| Muscle cramps | 10 (2.39%) |
| Integumentary system | |
| Urticaria | 61 (14.59%) |
| Alopecia | 49 (11.72%) |
| Skin necrosis | 7 (1.67%) |
| Cardiovascular system | |
| Bradycardia | 14 (3.34%) |
| Otorhinolaryngial system | |
| Sore throat | 1 (0.23%) |
| Hearing loss | 6 (1.43%) |
| Stomatitis | 3 (0.71%) |
| Circulatory system | |
| Anaemia | 10 (2.39%) |
| Deep vein thrombosis (DVT) | 3 (0.71%) |
| Respiratory system | |
| Cough | 1 (0.23%) |
| Central nervous system | |
| Headache | 5 (1.19%) |
| Sweating | 2 (0.47%) |
| Fever | 3 (0.71%) |
| Hot flushes | 9 (2.15%) |
The outcome of the reported adverse drug reactions (ADRs) showed that 14% (n=21) of patients were “recovered”, 6% (n=9) had “not recovered”, and 80% (n=123) were recovering (fig. 5). There was no dose modification or drug discontinuation after the reported adverse drug reactions (ADRs).
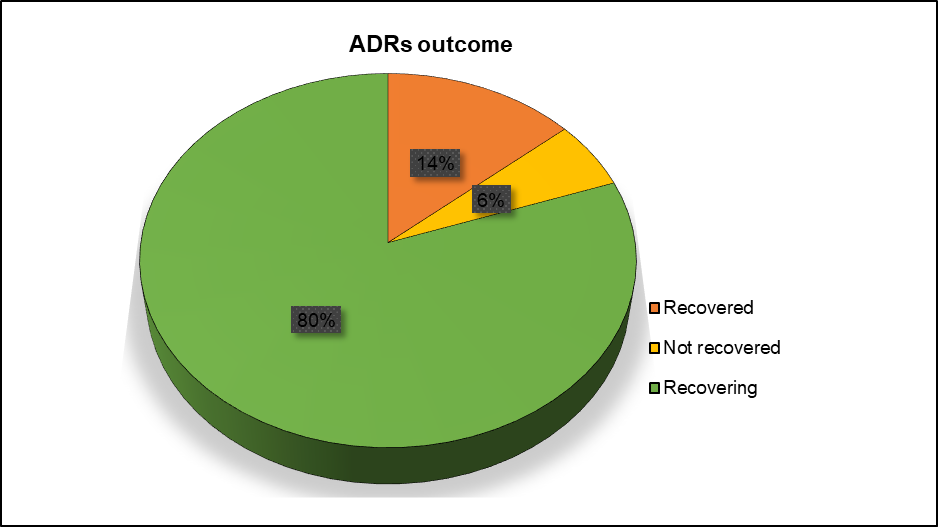
Fig. 5: Outcome of the adverse drug reactions (ADRs) in the patients
According to the World Health Organisation-Uppsala Monitoring Centre (WHO-UMC) scale of causality assessment, 117 (76%) of the reported adverse drug reactions (ADRs) were certain and 36 (24%) were probable (fig. 6).
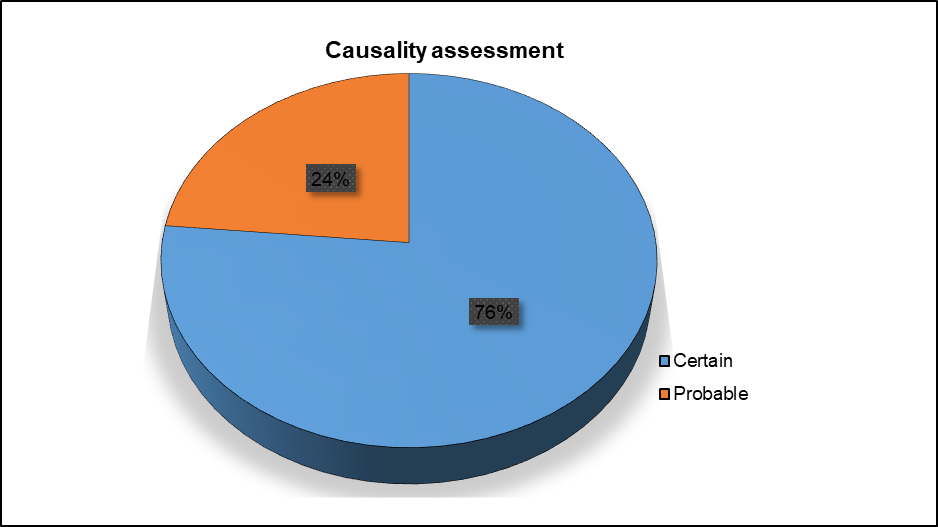
Fig. 6: Causality assessment of adverse drug reactions (ADRs) using the World Health Organization-Uppsala monitoring centre (WHO-UMC) scale, According to the severity assessment, 74 (49%) of the adverse drug reactions (ADRs) were mild (fig. 7)
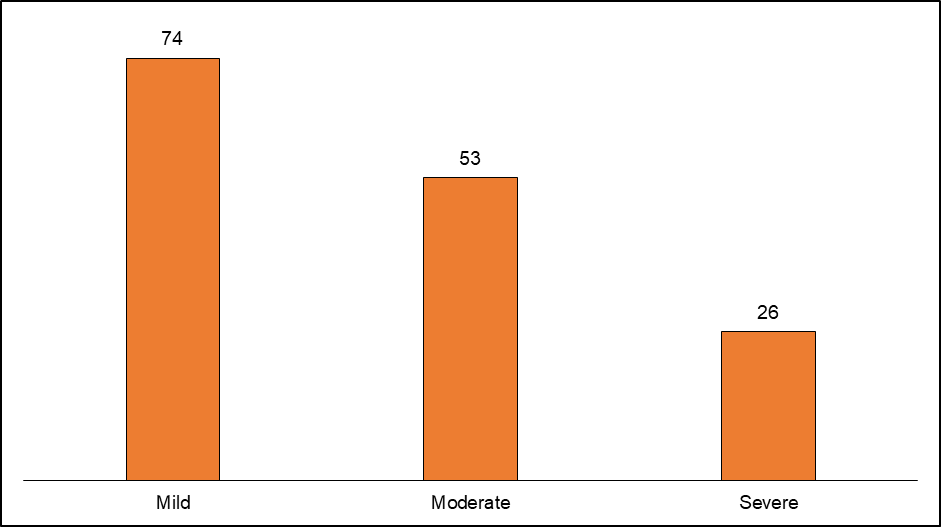
Fig. 7: Severity assessment of adverse drug reactions (ADRs)
DISCUSSION
The adverse drug reactions (ADRs) reported due to the use of anticancer drugs in a district government hospital, Jhabua of Madhya Pradesh, were collected in the suspected adverse drug reaction monitoring form (version 1.4) and analyzed.
In this study, the maximum number of adverse drug reactions (ADRs) was reported in females, showing similar results to Raj Kumar et al. [8] and contrasting results with Prasad A et al. and Giri K et al. [9, 10], respectively. The changes in the hormones in every stage of life causing alteration in the pharmacokinetics of the drug lead to an increased incidence of adverse drug reactions (ADRs) [11]. In this study, the highest number of adverse drug reactions (ADRs) were seen in the middle-age group (41-60 y), showing similar results to Raj Kumar et al. [8], as the accumulation of drugs increases with the reducing capacity of metabolism and excretion with age, leading to an increased incidence of adverse drug reactions (ADRs).
In this study, the adverse drug reactions (ADRs) were seen more with educated patients, married patients, old or previously diagnosed cases of cancer, patients having no comorbidities, and patients with no social habits. Breast cancer is the most common in this study, showing similar results to Mandal A et al. [12] and contrasting results with the study done in eastern India by Prasad A et al. [9], where bronchogenic carcinoma was the most common.
In this study, the majority of adverse drug reactions (ADRs) were seen with the double chemotherapy regimen and with the use of platinum compounds (cisplatin, carboplatin, and oxaliplatin) (34%), followed by taxanes (paclitaxel and docetaxel) (28%), showing similar results with Mathur R et al. [13] and contrasting results with Poddar S et al. [14], where antimetabolites and alkylating agents were the most common drugs causing adverse drug reactions (ADRs).
The majority of the patients in this study had experienced more than one adverse drug reaction. The gastrointestinal system was the most commonly involved, followed by the integumentary, having contrasting results with the study done by Swagata Dutta et al. [15], where the hematological system was majorly affected. Nausea was the most commonly experienced adverse drug reaction in this study, showing similar results with Sunny S et al. [16] and contrasting results with Swagata Dutta et al. [15], where alopecia was the most common adverse drug reaction. Chemotherapy-induced nausea and vomiting is due to the activation of the chemoreceptor trigger zone [9]. The most commonly prescribed adjuvant drugs were ranitidine and vitamins with chemotherapy.
The outcome of reported adverse drug reactions (ADRs) showed that the majority of the patients were recovering. The majority of the adverse drug reactions (ADRs) were certain as per the World Health Organization-Uppsala Monitoring Centre (WHO-UMC) causality assessment scale, contrasting with Chopra D et al. [17], where most of the results were probable. In this study, the majority of the adverse drug reactions (ADRs) were mild as per the severity assessment. This finding is also in concordance with other studies done by Mathur R et al. [13] and Swagata Dutta et al. [15].
The major limitation of the study was that it included only the spontaneously reported adverse drug reactions (ADRs). It is based predominantly on clinical evidence, particularly on the basis of patient-reported symptoms. There was a possibility of selective underreporting of adverse events by patients due to a lack of awareness.
CONCLUSION
Anticancer drugs have the potential to damage the rapidly dividing cells in the body and thereby can cause adverse drug reactions (ADRs). Hence, regular and sustained monitoring and timely reporting can minimize the occurrence of adverse drug reactions (ADRs). It can also increase patient compliance, reduce mortality due to adverse drug reactions (ADRs), and also reduce economic burden. An awareness regarding the Pharmacovigilance Programme of India (PvPI) should be created among all healthcare professionals and patients to encourage spontaneous reporting and to reduce the burden of adverse drug reactions (ADRs) with effective pharmacovigilance.
ACKNOWLEDGMENT
The authors would like to thank the authorities of the District Government Hospital, Jhabua, Madhya Pradesh, India, for granting us the necessary permission for the study. We express our sincere gratitude to all the healthcare professionals of the oncology unit at Jhabua for their valuable guidance and timely support.
FUNDING
Nil
AUTHORS CONTRIBUTIONS
Conceptualization: Dr. Aneri Patel; Methodology: Dr. Anjali Kushwah, Dr. Kamayani Gupta; Formal analysis and data collection: Dr. Aneri Patel, Dr. Kamayani Gupta; Writing and original draft preparation: Dr. Aneri Patel, Dr. Anjali Kushwah; Final review: Dr. Aneri Patel, Dr. Anjali Kushwah, Dr. Kamayani Gupta
CONFLICTS OF INTERESTS
There are no conflicts of interest
REFERENCES
Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024 May-Jun;74(3):229-63. doi: 10.3322/caac.21834, PMID 38572751.
Jokhadze N, Das A, Dizon DS. Global cancer statistics: a healthy population relies on population health. CA Cancer J Clin. 2024 May-Jun;74(3):224-6. doi: 10.3322/caac.21838, PMID 38572764.
Sathishkumar K, Chaturvedi M, Das P, Stephen S, Mathur P. Cancer incidence estimates for 2022 and projection for 2025: result from national cancer registry programme India. Indian J Med Res. 2022 Oct-Nov;156(4&5):598-607. doi: 10.4103/ijmr.ijmr_1821_22, PMID 36510887, PMCID PMC10231735.
Geneva Switzerland world health organization meeting on international drug monitoring: the role of national centres; 1971 and world health organization. International drug monitoring: the role of national centres report of a WHO meeting. World Health Organization; 1972. Available from: https://iris.who.int/handle/10665/40968.
Pirmohamed M, James S, Meakin S, Green C, Scott AK, Walley TJ. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ. 2004 Jul 3;329(7456):15-9. doi: 10.1136/bmj.329.7456.15, PMID 15231615, PMCID PMC443443.
Shukla S, Sharma P, Gupta P, Pandey S, Agrawal R, Rathour D. Current scenario and future prospects of adverse drug reactions (ADRs) monitoring and reporting mechanisms in the rural areas of India. Curr Drug Saf. 2024;19(2):172-90. doi: 10.2174/1574886318666230428144120, PMID 37132145.
The use of the WHO-UMC system for standardised case causality assessment. Available from: https://www.who.int/docs/default-source/medicines/pharmacovigilance/whocausality-assessment.pdf. [Last accessed on 25 Apr 2025].
Kumar R SR, Jindal A SR, Garg P, Kaur A, Kumar S, Tilak Raj R. Pharmacovigilance study of anticancer drugs in a Tertiary Care Teaching Hospital in North India: a retrospective study. Cureus. 2023 Sep 10;15(9):e44984. doi: 10.7759/cureus.44984, PMID 37822427, PMCID PMC10562879.
Prasad A, Datta PP, Bhattacharya J, Pattanayak C, Chauhan AS, Panda P. Pattern of adverse drug reactions due to cancer chemotherapy in a Tertiary Care Teaching Hospital in Eastern India. J Pharmacovigilance. 2013 Feb 8;1(2):107.
Giri K, Palandurkar K, Giri R, Agrawal U. A retrospective pharmacovigilance analysis at tertiary care hospital: an observational study. Asian J Pharm Clin Res. 2022 Aug 7;15(8):51-6. doi: 10.22159/ajpcr.2022.v15i8.45019.
Soldin OP, Chung SH, Mattison DR. Sex differences in drug disposition. J Biomed Biotechnol. 2011 Feb 23;2011:187103. doi: 10.1155/2011/187103, PMID 21403873, PMCID PMC3051160.
Mandal A, Gangopadhyay T, Mandal S. Drug utilization study in a radiotherapy unit of a Tertiary Care Teaching Hospital in Rural West Bengal, India. Asian J Pharm Clin Res. 2022 Nov 7;15(11):126-30. doi: 10.22159/ajpcr.2022.v15i11.45709.
Mathur DR, Vohra DA, Jain DS, Malhotra DH. Assessment of pattern of adverse drug reactions from anti-cancer drugs in a Tertiary Care Hospital: a cross-sectional study. IJMSCRR. 2022 Aug 31;5(4):263-8.
Poddar S, Sultana R, Sultana R, Akbor MM, Azad MA, Hasnat A. Pattern of adverse drug reactions due to cancer chemotherapy in Tertiary Care Teaching Hospital in Bangladesh. Dhaka Univ J Pharm Sci. 2010 Jun 25;8(1):11-6. doi: 10.3329/dujps.v8i1.5330.
Datta S, Zosangpuii C, Ningthoujam G, Paonam SD, Leisangthem TD, Nameirakpam MD. A retrospective study on adverse drug reactions of anticancer drugs in a Tertiary Care Hospital in Northeast India. J Clin Diagn Res. 2021;15(11): FC01-FC05. doi: 10.7860/JCDR/2021/51095.15687.
Sunny S, Thampi A, JJ, Shetty N, Babasahib SK, Chacko C. Assessment of adverse effects of most commonly prescribed anticancer drugs in a Tertiary Care Teaching Hospital. IJOPP. 2018;10(4):270-5. doi: 10.5530/ijopp.10.4.55.
Chopra D, Rehan HS, Sharma V, Mishra R. Chemotherapy induced adverse drug reactions in oncology patients: a prospective observational survey. Indian J Med Paediatr Oncol. 2016 Jan-Mar;37(1):42-6. doi: 10.4103/0971-5851.177015, PMID 27051157, PMCID PMC4795375.