
Int J Curr Pharm Res, Vol 17, Issue 5, 103-108Original Article
“A STUDY OF EFFICACY AND SAFETY OF TABLET DEFLAZACORT IN PATIENTS WITH NASAL POLYPS ATTENDING A TERTIARY CARE HOSPITAL”
B. S. B. MALLIKA1, V. CH. V. SIVA KUMAR2, MANOGNA1, PILLA S. SURYA DURGA DEVI1*
1Department of Pharmacology, Rangaraya Medical College-Kakinada, Andhra Pradesh, India. 2Department of Ent, Government Medical College-Rajahmundry, Andhra Pradesh, India
*Corresponding author: Pilla S. Surya Durga Devi; *Email: sreesuryadurgadevi@gmail.com
Received: 06 Jun 2025, Revised and Accepted: 24 Jul 2025
ABSTRACT
Objective: The aim of this study is to assess the efficacy and safety of the tablet deflazacort in nasal polyps patients and also to assess the causality, severity, and preventability of identified adverse drug reactions.
Methods: Fifty patients with Nasal polyps were treated with Deflazacort. The efficacy of the drug was assessed by SNOT (Sino-nasal outcome test) SCORE at baseline (0), 1st, 2nd w. All the adverse drug reactions (ADRs) due to the study drug was reported in a Suspected Adverse Drug Reaction Reporting form in each follow-up.
Results: After the completion of treatment, symptom scores of all the patients were improved. Of all the patients, 32% showed complete recovery, 50% showed improvement of symptoms, and 18% showed no/little improvement of symptoms. On an average about 82% of patients showed improvement in symptoms.
Conclusion: The efficacy of Deflazacort in the treatment of Nasal polyps is proven in this study. Moreover, less Adverse drug reactions (ADRs) is also reported, which confers it as an optimal treatment choice for Diabetic patients who are requiring steroid therapy.
Keywords: Nasal polyps, Deflazacort, Steroid, Adverse drug reactions, Efficacy
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijcpr.2025v17i5.7065 Journal homepage: https://innovareacademics.in/journals/index.php/ijcpr
INTRODUCTION
Nasal polyps are benign lesions that arise from the mucosa of the nasal cavity or paranasal sinuses. They are the inflammatory outgrowths of paranasal mucosa caused by chronic mucosal inflammation that typically arises from the middle meatus and ethmoid region [1]. The prevalence of nasal polyps ranges from 1% to 4.3% [2]. Asthma is associated with 25% to 32.6% of patients with nasal polyps [3]. The exact etiology of nasal polyps is not understood. Factors considered are Rhinosinusitis, genetic predisposition, family history, atopy, food allergens, etc. Allergy is the most associated factor.
Nasal polyps typically affect middle-aged and elderly patients, the average age at diagnosis is 40 to 60 y and men are affected more than women [4]. Presentations of Nasal polyps are usually after 20 y. Clinically they present with symptoms of nasal obstruction/stiffness, partial or total anosmia, watery nasal discharge, don’t bleed on touch and sleep disturbances. Nasal polyps can be diagnosed with clinical examination alone. Anterior rhinoscopy reveals multiple, pale-colored edematous masses. Medical treatment (antihistamines, steroids) should be the first-line therapy. Surgery should not be proposed until Steroid therapy is unsuccessful after 6 mo of treatment [5].
Early polypoidal changes with edematous mucosa may revert to normal with antihistamine and control of allergy [6]. Steroids are used in those who cannot tolerate antihistaminic, asthma and polypoidal nasal mucosa. They significantly reduce polyp size, rhinorrhea, congestion and increase airflow. Steroids also prevent recurrence after Surgery. Adverse drug reactions associated with the use of oral steroids are glaucoma, weight gain, stomach upset, insomnia, mood changes (irritability, anxiety), dizziness, headache, water retention, glucose intolerance, osteoporosis, etc. Surgery is reserved for cases when polyps cause recurrent sinusitis, severe obstruction, and those who fail medical treatment. The recurrence of nasal polyps is a serious problem and is found in 60-70% of patients after endoscopic sinus surgery [7]. The anti-inflammatory action of corticosteroids is the main reason for its use in this condition. Prolonged use of steroids produces glucose intolerance due to increased hepatic production and decreased peripheral utilization of glucose.
Deflazacort is an oxazoline derivative of prednisolone with anti-inflammatory and immunosuppressive activity [8]. Deflazacort depresses osteoblast less compared to Prednisolone causing less decrease in Serum osteocalcin levels and also having less negative effect on growth rate in children. Deflazacort is safer than other steroids because of its pharmacological property of causing less calcium and hydroxyproline excretion, less metabolic effects on glucose balance, and less neuronal degeneration as compared with other steroids [9]. The anti-inflammatory potency of Deflazacort is about 10-20 times higher than prednisolone and 40 times higher than hydrocortisone and also duration of action of Deflazacort is longer than others [10]. Deflazacort is given orally at 6 mg tablet BD (twice daily) for 2 w. Because of its low liposolubility, a small part crosses the blood-brain barrier, hence less suppression on the hypothalamic-pituitary-adrenal (HPA) axis [11]. As there are limited studies with Deflazacort in Nasal polyps patients and its use prevents recurrence, the primary aim of this study is to assess the efficacy and safety of the tablet Deflazacort in patients with Nasal polyps.
MATERIALS AND METHODS
Study design
Prospective and Observational study.
Study setting
ENT (Ear, Nose and Throat) OPD (Outpatient Department) at a tertiary care teaching hospital.
Duration of study
4 mo (Sep 2024-Oct 2024, Mar 2025-Apr 2025).
Sample size
50 patients.
Selection criteria of subjects
Inclusion criteria
Patients diagnosed with Nasal polyps of age group between 20-60 y of either sex.
Patients willing to participate in the study.
Exclusion criteria
Patients below 20 y and above 60 y.
Known immunocompromised patients, those receiving any other immunosuppressives or immunomodulators.
Known active cases of Infection like tuberculosis, HIV etc.
Pregnant and Lactating women.
Patients who are unwilling to participate in the study.
Data collection procedure
After approval from the Institutional Ethics Committee (IEC/RMC/2024/113), informed written consent was taken from the patients willing to participate in the study. All the eligible patients who met the inclusion and exclusion criteria, with Nasal polyps were given treatment with tablet Deflazacort. Data regarding the patient was taken in case study form, which includes demographic details, Efficacy, Adverse drug reactions, etc. The efficacy of the drug was assessed by SNOT (Sino-nasal outcome test) SCORE [12] at baseline (0), 1st, 2nd w. All the adverse drug reactions (ADRs) due to drugs were reported in a Suspected Adverse Drug Reaction Reporting form [13] in each follow-up. Causality of adverse drug reaction is assessed by using the WHO Causality Assessment Scale [14]. Severity by Modified Hartwig and Seigel scale, [15] and Schumock and Thornton criteria were used to assess the preventability of ADRs [16].
Study parameters
1. Efficacy by SNOT SCORE (Annexure-1) [12].
2. Suspected adverse drug reaction reporting form (Annexure–2) [13]
3. Causality by WHO Causality Assessment Scale (Annexure-3) [14]
4. Severity by Modified Hartwig and Seigel scale (Annexure-4). [15]
5. Preventability by Schumock and Thornton scale(Annexure-5) [16]
Statistical analysis
After interpreting the patient data into MS Excel sheets, data was tabulated and analyzed. The results were expressed as numbers and percentages.
Confidentiality
We maintained confidentiality by using a unique ID system and patient initials in the ADR form for assessment. Patient identity will not be disclosed further also.
Ethical issues
An approval from the Institutional Ethical Committee (IEC/RMC/2024/113) was taken before initiating the study. Informed consent was taken from all the participants, and full confidentiality was maintained. The patient was assured that his/her replies were in no way going to prejudice the treatment given.
RESULTS
Demographic profile
In our study, fifty patients treated with Deflazacort are included under this analysis, where the maximum age is 60 y and minimum age is 20 y. Overall, a maximum of 34% of patients are reported in between the age group of 31-40 y and 58% are males in between 20-60 y of age. The age is divided into four groups: 20-30 y, 31-40 y, 41-50 y, 51-60 y. It is observed that 34% of study population belong to age group 31-40 y, 30% of study population belong to age group 41-50 y, 22% of study population belong to age group 20-30 y and 14% of study population belong to age group 51-60 y. This is shown in table 1, fig. 1. The maximum number of patients attending are Males (58%) and females include 42% of the study population. This is shown in table 2, fig. 2.
Table 1: Age-wise distribution of the study population
| Age group | Study population (n=50) | Percentage |
| 20-30 | 11 | 22% |
| 31-40 | 17 | 34% |
| 41-50 | 15 | 30% |
| 51-60 | 7 | 14% |
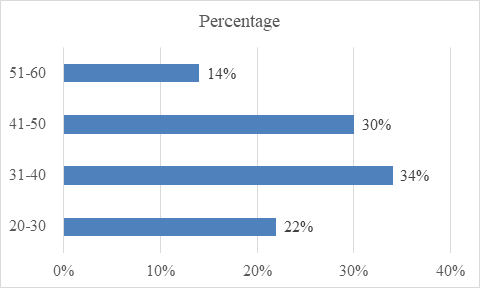
Fig. 1: Showing the age group of the study population
Table 2: Gender wise distribution of study population
| Gender | Number (n=50) | Percentage |
| Male | 29 | 58% |
| Female | 21 | 42% |
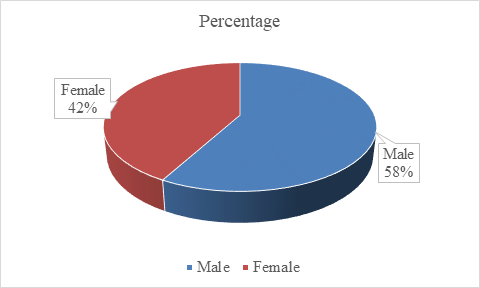
Fig. 2: Showing the gender distribution of study population
Allergy history
In the study population, most of the study population is associated with allergic history, such as allergic rhinitis, asthma, dust allergy; frequent attacks of sneezing, hay fever, etc. 68% of the study population had a history of allergy. Allergic history is absent in 32% of the study population. This is shown in table 3, fig. 3.
Family history
In the study population, most of them are not associated with the family history of nasal polyps. 14% of the study population had a family history of nasal polyps. Family history is absent in 86% of the study population. The details are shown in table 4, fig. 4.
Assessment of efficacy
In our study, Efficacy is assessed by SNOT scores, which are taken before start of drug (baseline, 0), after 1st w of treatment (1), end of 2nd w (2). In the study population, most of the patients showed improvement in the symptoms (scores). At baseline (0)[before start of drug] total score 1662, which is reduced to score of 1014 by End of 1st w (1) after treatment and from then to score of 584 by end of 2nd w (2) after treatment. The details are shown in table 5, fig. 5.
Table 3: Allergic history of study population
| Allergic history | Study population (n=50) | Percentage |
| Present | 34 | 68% |
| Absent | 16 | 32% |
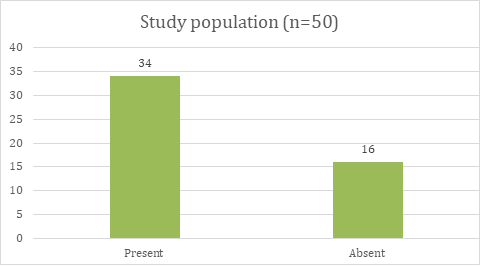
Fig. 3: Showing the allergic history of the study population
Table 4: Family history of nasal polyps of study population
| Family history of nasal polyps | Study population (n=50) | Percentage |
| Present | 7 | 14% |
| Absent | 43 | 86% |
Table 5: Assessment of efficacy by SNOT
| Assessment of efficacy by SNOT SCORE | Average of SNOT SCORE of 50 patients |
| Baseline(0) | 33.24 |
| End of 1st week(1) | 20.28 |
| End of 2nd week(2) | 11.68 |
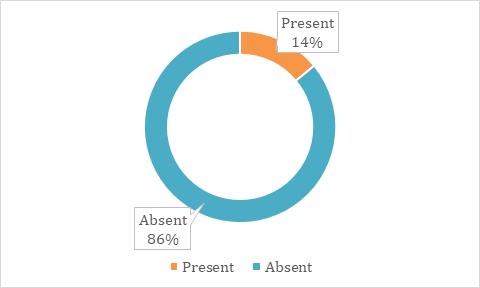
Fig. 4: Showing family history of nasal polyps in the study population

Fig. 5: Showing assessment of efficacy by SNOT SCORE
In our study, Efficacy is assessed by SNOT scores, which are taken before start of drug (baseline,0), after 1st w of treatment (1), end of 2nd w (2). Most of the patients showed improvement of symptoms (50%), about 32% showed complete recovery and about 18% showed no/little improvement of symptoms. This is shown in table 6, fig. 6.
Table 6: Assessment of efficacy of deflazacort
| Assessment of efficacy | Study population (n=50) | Percentage |
| Recovered | 16 | 32% |
| Symptoms improved | 25 | 50% |
| Not recovered | 9 | 18% |
Adverse drug reactions (ADRs)
Out of a study population of 50 patients, 20 patients reported ADRs out of which 6 patients (30%) reported headache, 5 patients (25%) reported Stomach upset (gastrointestinal symptoms), 4 patients (20%) reported Immunosuppression, 3 patients (15%) reported dizziness, 1 patients (5%) reported mood changes, 1 patient (5%) reported insomnia. The details are shown in table 7.
Causality, severity and preventability of ADRs
The results of Causality, Severity and Preventability scales of the Adverse Drug Reactions are shown here. It was explained that these ADRs are 100% possible by causality scale. 100% mildly severe by severity scale and 100% definitely preventable by preventability scale.
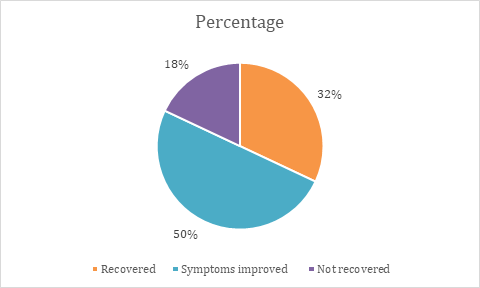
Fig. 6: Assessment of efficacy of deflazacort
Table 7: List of adverse drug reactions
| Name of the ADR | Number of patients | Percentage |
| Headache | 6 | 30% |
| Stomach upset | 5 | 25% |
| Immunosuppression | 4 | 20% |
| Dizziness | 3 | 15% |
| Mood changes (Irritable) | 1 | 5% |
| Insomnia | 1 | 5% |
| Total | 20 | 100% |
DISCUSSION
Demographic profile
In a study conducted by Adolfo Toledano Munaz [17] who has taken 165 patients as their study population showed that nasal polyps affect men (63%) and more frequently with a mean age of 46.5y, our study also showed the similar result.
Allergic history
In a study conducted by M Gelardi et al., [18], taking 455 patients as study population showed that nasal polyps are more common in allergic rhinitis patients than with non-allergic rhinitis patients. Our study also showed the same results. And the patients are enquired about their social history, there are drivers, daily wage workers, farmers, carpenters, housewives, miners, coal workers sweepers etc who are at risk of exposure to dust. They are given Deflazacort as treatment and conservative treatment to avoid exposure to dust (allergens) by use of a mask.
Family history
In our study, seven patients (14%) out of fifty patients had a family history of nasal polyps (parents, siblings or children). Those having a family history of nasal polyps should take preventive measures so as to avoid the disease such as use of a mask to avoid exposure to allergen (dust). In the study conducted by William A Greisner et al., ([19]) took 50 patients with nasal polyps and a control group of 30 patients without nasal polyps showed that 7 (14%) of 50 of the group with nasal polyps had family history of nasal polyps. This has also been proven in our study.
Assessment of efficacy
In our study, most of the patients showed improvement of symptoms (50%), about 32% showed complete recovery and about 18% showed no/little improvement of symptoms. On an average, about 82% of patients showed improvement in symptoms, hence Deflazacort is Efficacious in improvement in symptoms of Nasal polyps. In a study conducted by Cassano P, ([20]) treated nasal polyps with combination of oral Deflazacort with a topical Beclomethasone dipropionate in prevention of recurrence of nasal polyps after surgery. At 24 mo, recurrence was observed in 57% of patients, but most of the cases didn’t show any sign of further progression. No important side effects are observed during the study. Hence, Our study also showed the same results.
Adverse drug reactions (ADRs)
In a study conducted by Vishal R Tandon et al., [21] reported that overall incidence of adverse events in Deflazacort recipients to be lower than that recorded in patients with prednisone or methylprednisolone and are similar to that in betamethasone recipients. These ADRs can be prevented by prescribing related drugs as concomitant medication, eg. Tab. Pantop for Stomach upset, Tab. Amoxyclav for Immunosuppression etc.
When it comes to tolerability in our study, Deflazacort was well tolerated by the study population. In a Journal article from Coimbra University Hospitals, [22] retrospectively, all Patients attending drug allergic clinics for the last 10 y with immediate reactions to systemic corticosteroids are less reported but the challenge test with deflazacort was negative in all patients. It is concluded that Deflazacort was well tolerated in all cases.
According to WHO causality assessment scale, all Adverse Drug Reactions are possibly related to Deflazacort. The Reported ADRs are 100% mild severe in severity as per Modified Hartwig and Siegel Severity Scale. All ADRs have a known treatment, so all are preventable according to Schumock and Thornton’s Preventability Scale.
CONCLUSION
This represents a significant effort to study the efficacy of Deflazacort in the treatment of Nasal polyps. In our study, we found that about 82 % of patients enrolled in the study have shown improvement in symptoms from nasal polyps after oral administration of Deflazacort. Hence it is efficacious in treating Nasal polyps. The ADRs reported with Deflazacort, even if they affect the person, are generally tolerable and can be easily preventable. This proves that the drug, Deflazacort is efficacious in the treatment of Nasal polyps causing less ADRs and less diabetogenic (Diabetic-friendly drug).
ACKNOLEDGMENT
The authors would acknoledge the Dr NTR University of Health Sciences, Vijayawada, Andhra Pradesh as this study was conducted and accepted under Undergraduate Students Research Scholarships (UGSRS-2024) for their encouragement of young medical students towards research.
FUNDING
Nil
AUTHORS CONTRIBUTIONS
All authors have contributed equally.
CONFLICT OF INTERESTS
Declared none
REFERENCES
Georgy MS, Peters AT. Nasal polyps. Allergy Asthma Proc. 2012;33(3):22-3. doi: 10.2500/aap.2012.33.3537.
Bhat VS. Steroid therapy for nasal polyp: compliance due to cost and phobia in developing countries. JOENTR. 2018;10(6):312-6. doi: 10.15406/joentr.2018.10.00369.
Settipane GA. Epidemiology of nasal polyps. Allergy Asthma Proc. 1996;17(5):231-6. doi: 10.2500/108854196778662246.
Raciborski F, Arcimowicz M, Samolinski B, Pinkas W, Samel Kowalik P, Sliwczynski A. Recorded prevalence of nasal polyps increases with age. PDA. 2020;38(4):682-8. doi: 10.5114/ada.2020.99365.
Bonfils P. Medical treatment of paranasal sinus polyposis: a prospective study in 181 patients. Ann Otolaryngol Chir Cervicofac. 1998;115(4):202-14. PMID 9827187.
Textbook of diseases of ear nose and throat and head and neck surgery-PL Dhingra, Shruti Dhingra. 7th ed; 2021. p. 196.
De Conde AS, Mace JC, Levy JM, Rudmik L, Alt JA, Smith TL. Prevalence of polyp recurrence after endoscopic sinus surgery for chronic rhinosinusitis with nasal polyposis. Laryngoscope. 2017;127(3):550-5. doi: 10.1002/lary.26391, PMID 27859303.
Markham A, Bryson HM. Deflazacort. A review of its pharmacological properties and therapeutic efficacy. Drugs. 1995;50(2):317-33. doi: 10.2165/00003495-199550020-00008, PMID 8521761.
Gonzalez Perez O, Luquin S, Garcia Estrada J, Ramos Remus C. Deflazacort: a glucocorticoid with few metabolic adverse effects but important immunosuppressive activity. Adv Ther. 2007;24(5):1052-60. doi: 10.1007/BF02877711.
Parente L. Deflazacort: therapeutic index relative potency and equivalent doses versus other corticosteroids. BMC Pharmacol Toxicol. 2017;18(1):1. doi: 10.1186/s40360-016-0111-8.
Luzzani F, Glasser A. Differential binding in vitro to glucocorticoid receptors of deflazacort and prednisolone. Eur J Pharmacol. 1981;76(4):427-30. doi: 10.1016/0014-2999(81)90115-1.
https://melbentgroup.com.au/wpcontent/uploads/2016/10/meg-snot-22-scoring.pdf.
https://www.ipc.gov.in/images/adr_reporting_form_1.4_version.pdf.
https://who-umc.org/media/164200/who-umc-causality-assessment_new-logo.pdf.
Hartwig SC, Siegel J, Schneider PJ. Preventability and severity assessment in reporting adverse drug reactions. American Journal of Health System Pharmacy. 1992;49(9):2229-32. doi: 10.1093/ajhp/49.9.2229.
Schumock GT, Thornton JP. Focusing on the preventability of adverse drug reactions. Hosp Pharm. 1992;27(6):538. PMID 10118597.
Toledano Munoz A, Herraiz Puchol C, Navas Molinero C, Garcia Simal M, Navarro Cunchillos M, Galindo Campillo AN. Epidemiological study in patients with nasal polyposis [Epidemiological study in patients with nasal polyposis]. Acta Otorrinolaringol Esp. 2008;59(9):438-43. doi: 10.1016/S0001-6519(08)75115-X, PMID 19080774.
Gelardi M, Iannuzzi L, Tafuri S, Passalacqua G, Quaranta N. Allergic and non-allergic rhinitis: relationship with nasal polyposis asthma and family history. Acta Otorhinolaryngol Ital. 2014;34(1):36-41. PMID 24711681.
Greisner WA, Settipane GA. Hereditary factor for nasal polyps. Allergy Asthma Proc. 1996;17(5):283-6. doi: 10.2500/108854196778662192, PMID 8922148.
Cassano P, Marini F, Indraccolo AS, Curatoli FP. Corticosteroid therapy in the prevention of recurrent post-surgical nasal polyposis. Acta Otorhinolaryngol Ital. 1996;16(4):334-8.
Tandon VR, Singh P, Mahajan A, Khajuria V. Comparative adverse drug profile of deflazacort vs conventional corticosteroids in spontaneous reporting system of pharmacovigilance. JK Sci. 2014;16(1):16.
Sousa NG, Faria E, Carrapatoso I, Almeida E, Geraldes L, Chieira C. Deflazacort: a possible alternative in corticosteroid allergy. J Investig Allergol Clin Immunol. 2010;20(5):449-51.