
Int J Curr Pharm Res, Vol 17, Issue 6, 1-8Review Article
MODIFIED RELEASE MULTI-UNIT SYSTEM: A REVIEW
JYOTI G. RATHOD1*, SHASHIKANT N. DHOLE2, NILESH S. KULKARNI3
Department of Pharmaceutics, PES's Modern College of Pharmacy (For Ladies), Moshi affiliated to Savitribai Phule Pune University, Pune, Mumbai, India
*Corresponding author: Jyoti G. Rathod; *Email: jyotirathod61@gmail.com
Received: 15 Aug 2025, Revised and Accepted: 04 Oct 2025
ABSTRACT
Modified release multiunit dosage forms (MUFs) have emerged as a promising approach to achieve controlled and site-specific drug delivery with improved therapeutic outcomes. Pelletization, particularly via extrusion–spheronization, offers numerous formulation advantages, including uniform size, excellent flowability, and enhanced drug release control. This review provides a comprehensive overview of pelletization techniques with an emphasis on extrusion–spheronization, detailing its critical process parameters, formulation variables, and evaluation methods. Additionally, the classification of modified release systems and the comparative advantages of MUFs over single-unit systems are discussed. The integration of these technologies supports the development of patient-centric and robust drug delivery platforms with greater safety, efficacy, and compliance.
Keywords: Modified release, Multiunit dosage form, Pelletization, Extrusion–spheronization, Drug delivery systems, Controlled release, Sustained release, Multiparticulates, Formulation development, Pharmaceutical pellets
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijcpr.2025v17i6.7062 Journal homepage: https://innovareacademics.in/journals/index.php/ijcpr
INTRODUCTION
Modified Release (MR) drug delivery systems are innovative pharmaceutical formulations engineered to release the active pharmaceutical ingredient (API) in a controlled, sustained, or delayed manner, rather than immediately upon administration [1]. The primary objective of these systems is to deliver drugs at a predetermined rate, for a specified duration, or at a particular site within the gastrointestinal (GI) tract, depending on the desired therapeutic outcome. Unlike conventional immediate-release dosage forms, which dissolve and absorb rapidly and often necessitate frequent dosing, MR systems are designed to optimize drug release kinetics, ensuring consistent plasma drug concentrations over an extended period [2].
Modified release systems encompass a broad range of delivery mechanisms, including extended-release, sustained-release, delayed-release, and pulsatile-release formulations. Each of these systems is developed with the intention to enhance therapeutic efficacy, reduce side effects, and improve patient convenience. This approach has become increasingly important in modern therapeutics, particularly in the management of chronic diseases, where prolonged drug exposure and steady plasma levels are critical for maintaining consistent pharmacological effects [3].
Differences from conventional immediate-release systems
The fundamental distinction between modified release and conventional drug delivery systems lies in how and when the drug is released and absorbed in the body. In conventional or immediate-release formulations, the drug disintegrates and dissolves rapidly after administration, leading to quick absorption and often resulting in a sharp spike in plasma drug concentration [4-6]. This abrupt rise is typically followed by a rapid decline, potentially necessitating multiple doses throughout the day to maintain therapeutic levels. Such fluctuations can lead to periods of sub-therapeutic exposure, or conversely, to peak-related side effects [7].
In contrast, MR formulations are engineered to release the drug gradually or at specific intervals, thus smoothing out the peaks and troughs seen in plasma concentration profiles. By maintaining the drug concentration within the therapeutic window for a longer duration, MR systems help to improve drug efficacy while minimizing the risk of adverse effects. This not only optimizes the pharmacodynamic profile of the drug but also enhances its overall clinical utility [8].
Therapeutic advantages of modified release drug delivery
Modified release drug delivery systems offer a wide range of clinical and patient-oriented advantages, making them particularly beneficial in the treatment of chronic and long-term conditions. One of the most significant benefits is the reduction in dosing frequency, which directly contributes to improved patient compliance. This is especially valuable for geriatric and pediatric patients, or individuals managing multiple medications (polypharmacy), where simplifying the dosing regimen is crucial for adherence [8, 9].
Additionally, MR systems enable more consistent plasma drug concentrations, which not only improves therapeutic efficacy but also reduces the incidence of dose-dependent side effects. For drugs with a narrow therapeutic index, this controlled release is particularly critical in avoiding toxic peaks or ineffective troughs. Moreover, by delivering the drug at targeted sites within the GI tract, MR systems can increase site-specific bioavailability, improve local therapeutic action, and minimize systemic exposure.
Another noteworthy advantage is the protection of the drug substance from degradation in the harsh gastric environment, as seen with enteric-coated formulations. This contributes to better chemical stability, enhanced shelf-life, and overall product performance. Additionally, controlled drug release can lead to cost savings for both healthcare systems and patients by reducing the frequency of administration and potentially lowering the total amount of drug required over time [10, 11].
Multiunit dosage forms (MUFs)
Multiunit dosage forms (MUFs) are advanced drug delivery systems comprising numerous small, discrete units such as pellets, beads, microspheres, or mini-tablets, each functioning as an individual drug carrier. These subunits can be encapsulated within a capsule or compressed into a tablet, and they collectively deliver the drug in a controlled fashion. Unlike monolithic or single-unit systems, MUFs distribute the drug widely throughout the gastrointestinal tract upon administration, resulting in more consistent absorption and reduced inter-and intra-subject variability [12, 13].
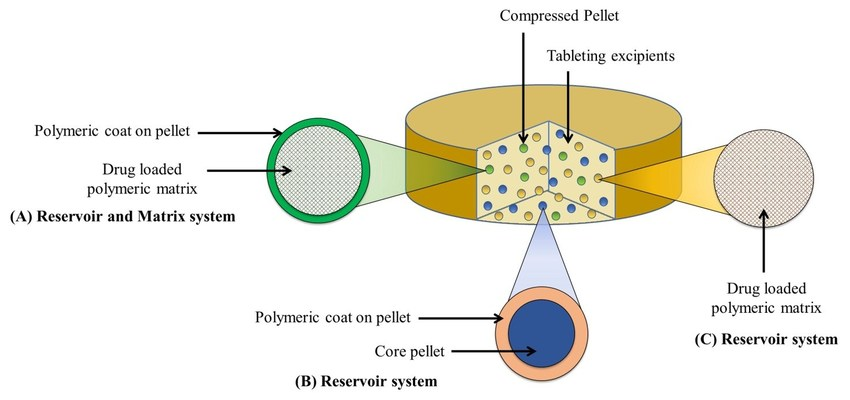
Fig. 1: Multi-unit pellet system [14]
Advantages over single-unit systems
Multiunit systems provide several critical advantages over traditional single-unit dosage forms. Firstly, the risk of dose dumpinga sudden and uncontrolled release of the drug-is significantly reduced due to the presence of multiple subunits. If a few units fail or are damaged, they represent only a fraction of the total dose. Secondly, the uniform distribution throughout the GI tract ensures that drug absorption is less affected by gastric emptying rates or localized irritation. This contributes to reduced variability in bioavailability, leading to more predictable pharmacokinetics [15].
Additionally, MUFs can be tailored for immediate, sustained, or delayed drug release, allowing for flexible and precise control over therapeutic outcomes. Their suitability for coating with polymers also facilitates site-specific delivery, making them highly advantageous for drugs that need to be released at specific pH levels or intestinal regions. Moreover, MUFs offer better gastric tolerance and reduce the risk of local irritation since the drug is not concentrated in a single unit [16-18].
Table 1: Advantages of multiunit dosage forms over single-unit systems
| Parameter | Single-unit dosage forms | Multiunit dosage forms (MUFs) |
| Risk of Dose Dumping | High | Low |
| GI Distribution | Limited to one location | Uniform throughout GI tract |
| Bioavailability Variability | High | Reduced |
| Customizable Drug Release | Limited | Flexible (IR, SR, DR, pulsatile) |
| Gastric Irritation | Higher | Lower |
| Suitability for Coating | Difficult | Easy to coat with functional polymers |
| Reproducibility and Flexibility | Less | More |
Classification of modified-release multiunit systems
Modified release multiunit dosage forms (MR-MUFs) have become a cornerstone in advanced drug delivery due to their ability to fine-tune drug release patterns based on therapeutic demands. These systems are composed of multiple discrete subunits, such as pellets, beads, or microspheres, each engineered to achieve a controlled release profile. Depending on the clinical objective, MR-MUFs can be designed for delayed release, extended release, pulsatile release, or site-specific delivery. These diverse systems employ various pharmaceutical technologies, including functional coatings, matrix-forming agents, and osmotic systems, to deliver the drug in a predetermined manner [19-22].
Delayed release multiunit systems
Delayed-release systems are formulated to withhold the release of the active pharmaceutical ingredient (API) for a predefined period after administration. This is typically achieved by coating the multiunit pellets with enteric polymers that resist dissolution in the acidic environment of the stomach but disintegrate in the higher pH of the small intestine. Such systems are especially beneficial for drugs that are unstable in gastric fluids or cause irritation to the gastric mucosa. By delaying drug release until the dosage form reaches the intestine, these systems enhance drug stability and patient tolerability. Enteric-coated pellets, for example, are widely used for medications like omeprazole and erythromycin to prevent premature degradation or irritation.
Extended-release multiunit systems
Extended release (ER) multiunit systems are designed to release the drug over a prolonged period, thereby maintaining steady plasma drug concentrations and minimizing the need for frequent dosing. This approach is particularly valuable in managing chronic conditions that require sustained therapeutic levels, such as hypertension, diabetes, and asthma. In these systems, the pellets or beads are either imgded in matrix-forming polymers or coated with rate-controlling membranes, which regulate the diffusion or erosion of the drug over time. Extended release systems reduce peak-trough fluctuations and enhance overall treatment compliance, particularly in patients who require long-term therapy. Drugs like theophylline and metoprolol are commonly formulated using extended release multiunit systems.
Pulsatile release multiunit systems
Pulsatile release systems are engineered to release drugs in one or more pulses after a specified lag time, rather than continuously. These systems are ideal for conditions where drug release needs to coincide with the body's biological rhythms, such as in chronotherapy. For instance, symptoms of diseases like asthma, arthritis, or cardiovascular conditions often exhibit circadian variation, and pulsatile systems allow drug release to be synchronized with these patterns. The mechanism typically involves time-dependent coatings, enzyme-degradable polymers, or pressure-sensitive materials that rupture or degrade at the desired interval. The result is a “burst” release of the drug at the right time and site, providing improved therapeutic outcomes with minimized side effects [23].
Site-specific release multiunit systems
Site-specific release systems are designed to deliver the drug at a particular location within the gastrointestinal tract, such as the colon, jejunum, or ileum. These systems are crucial for treating local diseases, such as inflammatory bowel disease, or for enhancing the bioavailability of drugs that are absorbed in specific regions. The release is often governed by pH-sensitive coatings, enzymatic triggers, or time-controlled mechanisms that ensure the drug is protected until it reaches the targeted area. Colon-targeted delivery, for instance, uses coatings that remain intact in the stomach and small intestine but dissolve in the neutral to slightly alkaline environment of the colon. This approach not only maximizes therapeutic efficiency but also minimizes systemic exposure and potential side effects [24].
Technologies used in modified-release multiunit systems
To achieve these specialized release patterns, a variety of formulation and process technologies are employed. Polymeric coatings, such as methacrylic acid copolymers (e. g., Eudragit), are commonly used for both delayed and extended release systems. Matrix technologies, using hydrophilic or lipophilic materials, control drug release by diffusion or erosion. Osmotic systems use semipermeable membranes and osmotic pressure to push the drug out in a controlled manner. Furthermore, enzyme-responsive polymers are increasingly utilized in site-specific and pulsatile systems to ensure release in response to gastrointestinal enzymes or microbial activity [25].
Table 2: MR-MUF types and characteristics
| Type | Purpose | Mechanism | Technology used | Applications |
| Delayed release | Protect drug from stomach or target intestine | pH-sensitive polymer coatings | Enteric coatings (Eudragit L/S) | Acid-labile drugs, gastric irritation |
| Extended release | Maintain prolonged drug levels | Diffusion, erosion | Matrix systems, coated pellets | Chronic diseases, sustained therapy |
| Pulsatile release | Timed bursts of drug release | Time-based or pressure-triggered mechanisms | Multi-layer coatings, enzyme-sensitive layers | Chronotherapy, hormone therapy |
| Site-specific release | Deliver drug at a defined GI location | pH or enzyme-triggered | Colon-targeting coatings, osmotic systems | IBD, colonic infections, targeted absorption |
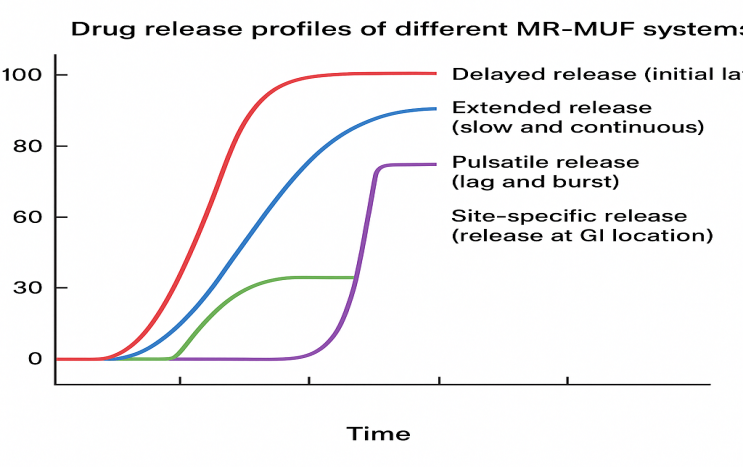
Fig. 2: Drug release profiles of different MR-MUF systems
Pelletization techniques in the development of modified-release multiunit systems
Pelletization plays a pivotal role in the pharmaceutical industry, particularly in the design and development of multiunit dosage forms (MUFs) intended for modified release drug delivery. This process involves converting fine powders or granular drug blends into small, dense, and generally spherical particles known as pellets. These discrete, multiparticulate units serve as versatile drug carriers that can be filled into capsules, compressed into tablets, or coated with functional polymers to achieve tailored drug release profiles, including delayed, extended, or site-specific delivery [26].
Pellets offer several formulation advantages, including improved flow properties, enhanced mechanical integrity, uniform packing, and reproducible drug release, owing to their narrow size distribution and spherical geometry. They are especially beneficial in minimizing the risk of dose dumping, improving gastrointestinal transit behavior, and enabling precise application of functional coatings. A variety of pelletization techniques have been developed, each suited for different formulation challenges and drug properties. The following sections detail the most prominent pelletization approaches currently employed in pharmaceutical manufacturing [27].

Fig. 3: Important aspects during pelletized drug delivery development
Extrusion–spheronization
Among the various pelletization methods, extrusion–spheronization remains the most extensively used and well-established technique in industrial-scale production. It involves two primary unit operations: first, a wet mass is prepared by blending the active pharmaceutical ingredient (API) with excipients and a granulating fluid to form a cohesive mixture. This mass is then extruded through a die to form cylindrical extrudates of uniform diameter. In the second stage, these extrudates are broken and rounded into spherical pellets within a high-speed spheronizer, where a rotating friction plate facilitates the shaping process.
This method is highly effective for achieving high drug loading, and it is compatible with a wide range of hydrophilic and hydrophobic drugs. The resulting pellets exhibit excellent sphericity, mechanical robustness, and narrow particle size distribution, making them ideal for further coating with polymers to modulate drug release. Due to its high reproducibility, scalability, and ability to accommodate diverse formulation variables, extrusion–spheronization is considered a gold standard for developing sustained and controlled release multiunit systems [28].
Powder layering
Powder layering is a conventional and widely used technique for fabricating drug-loaded pellets, particularly suitable for heat-sensitive APIs. In this method, inert starter cores-commonly sugar spheres or microcrystalline cellulose pellets—are coated with fine drug powder using a binder solution or polymeric dispersion as an adhesive. This process is typically carried out in pan coaters or fluidized bed processors, where alternating cycles of binder spraying and powder application allow for the gradual build-up of drug layers.
Powder layering is advantageous due to its simplicity and its ability to achieve rapid drug deposition without exposure to solvents or high temperatures. It is especially useful for developing immediate-release or modified-release formulations, depending on the polymer used for coating. However, the success of this method relies heavily on the uniform distribution of binder and precise control over process parameters, as inconsistencies may result in uneven coating and dose variation [29].
Solution or suspension layering
Solution/suspension layering represents a more refined wet coating approach, where the drug is either dissolved or suspended in a liquid medium and sprayed onto inert cores using fluidized bed coating equipment. Upon spraying, the solvent or dispersion medium evaporates, depositing the drug as a uniform film over the pellet surface. This method is preferred for low-dose drugs, taste-masking applications, and modified release systems, offering a high degree of control over the drug loading and coating thickness. By tailoring the polymeric composition and layering parameters, it is possible to design pellets with desired release kinetics, such as sustained or delayed profiles. Although this technique ensures excellent content uniformity and coating precision, it requires careful solvent management and efficient drying mechanisms to prevent agglomeration, which may compromise pellet integrity.
Hot-melt extrusion (HME)
Hot-melt extrusion (HME) is a solvent-free and thermally driven pelletization method that has gained significant attention for its ability to enhance the solubility and bioavailability of poorly water-soluble drugs. In this process, a blend of the drug and thermoplastic polymers or waxes is fed into a twin-screw extruder, where the mixture is subjected to high shear and temperature. The molten mass is then extruded and shaped into uniform pellets or granules upon cooling. This method promotes the formation of amorphous solid dispersions, significantly improving drug dissolution profiles. Moreover, HME enables matrix-based controlled release, making it suitable for sustained-release applications. However, it poses challenges for thermally labile APIs, and the formulation must be optimized to prevent thermal degradation during processing. Despite these limitations, HME is valued for its continuous processing, solvent-free nature, and compatibility with novel polymeric carriers.
Freeze pelletization
Freeze pelletization is an emerging technique particularly suited for drugs that are thermolabile or sensitive to moisture, such as peptides, proteins, and biological agents. In this method, molten formulations containing the drug and lipid-based or waxy excipients are dropped into a cryogenic medium (e. g., liquid nitrogen), where they rapidly solidify into spherical pellets. This quick freezing preserves the physical and chemical integrity of sensitive compounds by avoiding exposure to high processing temperatures or drying steps.
The technique yields well-shaped, stable pellets with good mechanical properties and is adaptable to modified release applications by incorporating suitable lipophilic or hydrophilic excipients. However, the method requires specialized cryogenic equipment, and its scalability for commercial manufacturing remains a challenge, thus currently limiting its widespread industrial use [30].
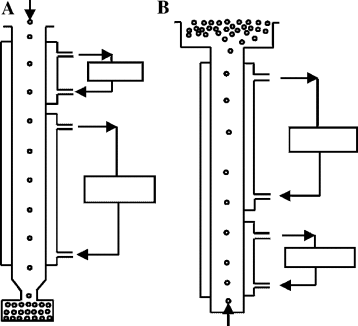
Fig. 4: Freeze pelletization I and II
Spray drying
Spray drying is a rapid and continuous technique used to convert a liquid drug-excipient mixture into dry, spherical particles by spraying it into a chamber filled with hot drying air. As the atomized droplets come into contact with the heated air, the solvent evaporates almost instantaneously, forming free-flowing pellets or microspheres. This technique is particularly suitable for heat-stable, water-soluble drugs and is widely employed in the preparation of porous or hollow pellets for various release profiles [31].
Spray drying allows for the development of immediate, extended, or fast-dissolving drug delivery systems, depending on the excipients used and the processing conditions. It also supports enhanced drug solubility by generating amorphous solid forms. While the process offers excellent scalability and high throughput, it may not be appropriate for drugs sensitive to thermal degradation, and it requires careful formulation to maintain product stability.
Table 3: Comparative overview of pelletization techniques
| Technique | Mechanism | Advantages | Limitations | Suitable applications |
| Extrusion–Spheronization | Wet mass extruded and spheronized into pellets | High drug loading, excellent sphericity, scalable | Sensitive to moisture, multi-step process | Sustained and delayed-release pellets |
| Powder Layering | Dry powder layered onto inert cores | No heat/solvent exposure, fast deposition | Requires precise process control | IR and MR pellets, heat-sensitive drugs |
| Solution/Suspension Layering | Drug solution/suspension sprayed on cores | Precise coating control, uniform drug distribution | Solvent handling, risk of agglomeration | Low-dose, taste-masked, sustained/delayed release |
| Hot-Melt Extrusion (HME) | Molten mixture extruded and shaped | Solvent-free, improved bioavailability of poorly soluble drugs | Limited to heat-stable APIs | Matrix-based sustained-release, solubility enhancement |
| Freeze Pelletization | Droplets solidified in cryogenic liquid | Suitable for thermolabile drugs, no drying required | Requires cryogenic setup, limited scalability | Biologics, protein-based formulations |
| Spray Drying | Atomized liquid dried in hot air stream | Fast, continuous, porous particle formation | Not ideal for thermosensitive APIs | Fast-dissolving or extended-release microspheres |
Extrusion–spheronization: process parameters and formulation variables
Extrusion–spheronization is a multi-step pharmaceutical technique designed to produce spherical pellets with high mechanical strength, uniform size, and excellent flow properties, making them ideal candidates for modified release multiparticulate dosage forms. The performance and quality of pellets derived from this technique are influenced by a complex interplay between process parameters and formulation components. Optimization of both is essential for achieving desirable product characteristics, particularly for extended-or site-specific drug delivery.
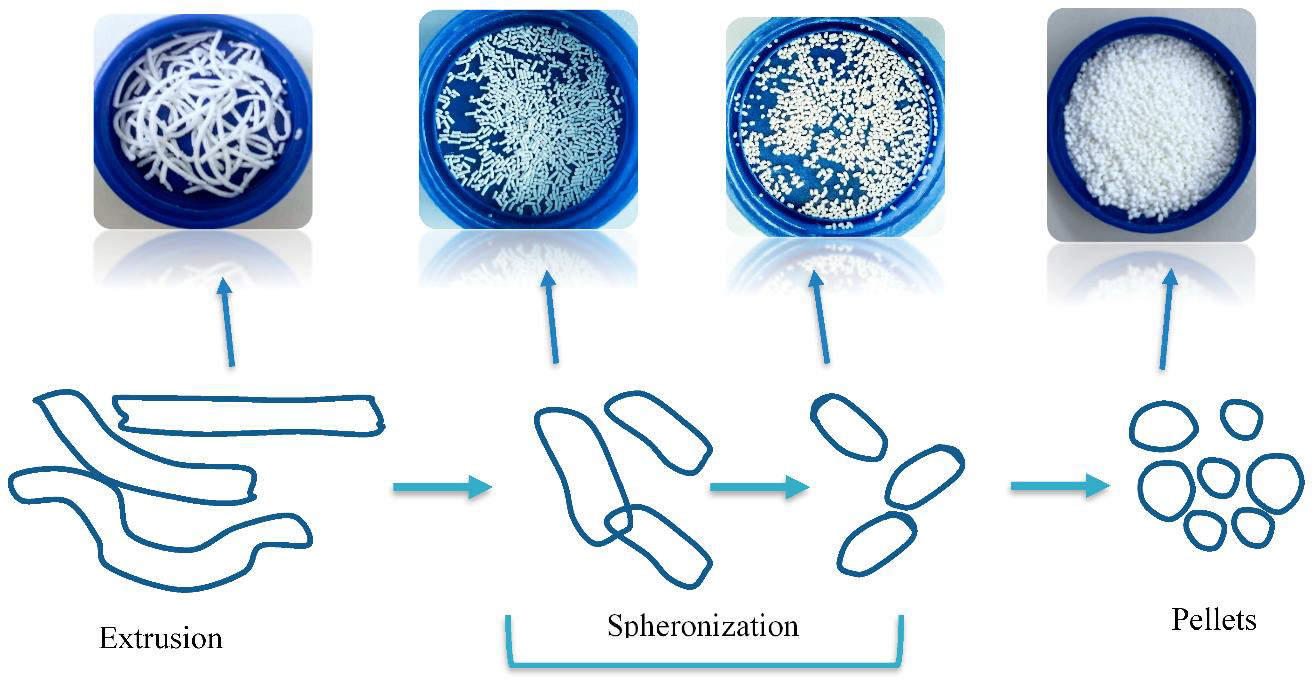
Fig. 5: Extrusion spheronization technique [32]
Process parameters influencing pelletization
Moisture content of wet mass
The moisture content plays a critical role in determining the plasticity and cohesiveness of the extrudable mass. Insufficient moisture results in poor extrudability and weak pellets, whereas excess water leads to agglomeration and loss of sphericity during spheronization. Optimal moisture levels ensure smooth extrusion, minimal friction, and uniform pellet formation [33].
Extruder screen size and type
The die or screen sizes of the extruder controls the diameter of the extrudates, which ultimately determines the final pellet size. A smaller screen size produces finer pellets, while larger screens are used for coarse or macro-pellets. The type of extruder axial or radial also affects extrudate shape and flow dynamics during the process.
Extrusion speed and force
The speed at which the wet mass is pushed through the extruder affects the uniformity and density of the extrudates. High-speed extrusion may generate heat, altering the plasticity of the mass, while slower speeds may lead to inconsistencies in strand formation. Optimizing extrusion force is essential to avoid mechanical stress on sensitive APIs.
Spheronization time and plate speed
The rotation speed and duration of spheronization significantly impact pellet roundness, smoothness, and sphericity index. Shorter spheronization times may lead to elongated pellets, while excessive processing can result in erosion of the surface. Plate speed is typically adjusted between 500–1500 rpm depending on the formulation [34].
Drying conditions
After spheronization, pellets must be dried under controlled conditions to remove residual moisture. The drying temperature and duration influence pellet hardness, drug crystallinity, and release behavior. Improper drying may cause cracks or compromise coating adhesion in subsequent processing steps.
Table 4: Critical process and formulation parameters in extrusion–spheronization
| Parameter | Function/Impact | Considerations |
| Moisture content | Affects plasticity and pellet formation | Must be optimized to prevent agglomeration or weak pellets |
| Screen size | Determines extrudate and pellet diameter | Selected based on desired pellet size |
| Extrusion speed | Influences extrudate density and heat generation | Requires balance between throughput and quality |
| Spheronization time/speed | Controls sphericity and surface finish | Typically 1–5 min at 800–1200 rpm |
| Drying conditions | Affects pellet strength and residual moisture | Should prevent cracks and maintain drug stability |
| Microcrystalline cellulose (MCC) | Provides structure, cohesion, and plasticity | Ideal matrix former for most APIs |
| Binder (e. g., PVP, HPMC) | Enhances cohesiveness and pellet integrity | Varies by drug and formulation type |
| Drug loading | Determines pellet drug content and release behavior | High load may reduce sphericity |
| Granulating fluid | Ensures proper wet mass consistency | Water, ethanol, or aqueous blends commonly used |
| Lubricants/disintegrants | Modify extrusion properties and drug release profile | Optional, formulation-dependent |
Evaluation and characterization of pellets produced by extrusion–spheronization
The success of any pellet-based drug delivery system depends significantly on the physical, mechanical, and functional properties of the pellets. Therefore, thorough evaluation and characterization are essential to ensure that the pellets are robust, uniform, and compatible with further processing, such as coating or compression into tablets. Parameters like size distribution, sphericity, flowability, surface morphology, mechanical strength, and in vitro drug release profile directly affect the performance and bioavailability of modified release formulations.
Pellet size and size distribution
Pellet size typically ranges from 500 to 1500 µm, depending on the formulation requirements and final dosage form. Sieving analysis using a standard sieve set or laser diffraction techniques is commonly employed to determine the size distribution. A narrow particle size distribution ensures uniform coating, consistent drug release, and better flow characteristics. Non-uniform sizes may lead to variable coating thickness and unpredictable therapeutic outcomes [35].
Sphericity and shape analysis
Pellets produced by extrusion–spheronization should exhibit high sphericity, as this improves flowability, minimizes surface area variation, and ensures even coating application. Sphericity is quantitatively evaluated using image analysis systems or scanning electron microscopy (SEM). Ideally, a sphericity index (aspect ratio close to 1) indicates near-perfect spherical shape. Poorly shaped pellets may exhibit inconsistent release and processing challenges [36].
Flow properties
Good flowability is essential for accurate filling into capsules or tablet dies. Flow behavior is assessed using parameters such as Angle of repose, Carr’s index and Hausner ratio.
Lower compressibility index and Hausner ratio indicate better flow, which facilitates uniform die filling and prevents segregation during manufacturing. MCC-based pellets typically show excellent flow characteristics due to their smooth surface and uniform shape [37].
Mechanical strength and friability
Pellets should possess sufficient mechanical strength to withstand subsequent processing, such as fluidized bed coating or tablet compression, without fracturing. Friability tests using a Roche friabilator, and crushing strength measurements help determine the robustness of the pellets. Excessively friable pellets may generate fines, leading to inconsistent dosing and coating defects.
Surface morpholo0gy
The surface characteristics of pellets influence coating adhesion, drug release, and particle–particle interaction. SEM imaging provides insights into the texture, porosity, and surface uniformity. Smooth surfaces are preferred for functional polymer coatings, while rough or porous pellets may result in uneven layering or excessive coating material consumption [38].
Drug content uniformity
Uniform drug distribution is essential for dose accuracy in each unit. Assay methods such as UV-Vis spectrophotometry or HPLC are used to determine the drug content across different pellet samples. Acceptable relative standard deviation (RSD) ensures homogeneity and consistent therapeutic performance, especially in high drug-loading formulations [39].
In vitro drug release studies
A critical evaluation parameter, dissolution testing simulates in vivo drug release under physiological conditions. Using USP Apparatus I (basket) or II (paddle), pellets are tested in different pH conditions to mimic GI tract transitions (e. g., pH 1.2 for stomach, pH 6.8 for intestine). The release profile helps classify the system as immediate, delayed, or sustained release. Data from these studies guide polymer selection and coating thickness optimization [40].
Table 5: Evaluation parameters for pellets
| Parameter | Test method | Significance |
| Size and Size distribution | Sieving or Laser Diffraction | Ensures uniform coating and consistent drug release |
| Sphericity | Image Analysis/SEM | Determines surface uniformity and coating suitability |
| Flow properties | Angle of Repose, Carr’s Index, Hausner Ratio | Affects capsule filling and uniformity |
| Mechanical strength/friability | Roche Friabilator/Crushing Test | Prevents pellet breakage during handling and coating |
| Surface morphology | Scanning Electron Microscopy (SEM) | Evaluates coating adhesion potential |
| Drug content uniformity | HPLC/UV-Vis Spectrophotometry | Ensures dosage accuracy and batch consistency |
| In vitro drug release | USP Dissolution Apparatus I/II | Confirms desired modified release behavior |
CONCLUSION
Extrusion–spheronization stands out as a highly versatile and industry-preferred technique for the development of modified release multiunit systems (MUFs). This process enables the production of spherical, uniform, and mechanically robust pellets with excellent flow characteristics and controlled drug release capabilities. Its adaptability to a wide range of active pharmaceutical ingredients irrespective of solubility profile and excipients, makes it a valuable platform for designing both immediate and sustained release formulations.
Through precise control of formulation variables (such as binder type, filler composition, and drug loading) and process parameters (including moisture content, extrusion force, and spheronization speed), extrusion–spheronization allows for consistent scale-up, high reproducibility, and enhanced product performance. Moreover, the pellets produced are particularly amenable to functional coating technologies, allowing for the design of advanced release profiles tailored to therapeutic requirements.
This review highlights the central role of extrusion–spheronization in the advancement of oral drug delivery systems. As regulatory expectations for robustness, reproducibility, and patient compliance grow, this technique offers a scalable and scientifically sound approach for developing next-generation pharmaceutical dosage forms. Future research should emphasize formulation optimization using QbD principles, the integration of innovative excipients and polymers, and modeling tools for predictive performance, further extending the utility and precision of this technology in modern drug delivery science.
FUNDING
Nil
AUTHORS CONTRIBUTIONS
All authors have contributed equally
CONFLICT OF INTERESTS
Declared none
REFERENCES
Rahman MA, Ahuja A, Baboota S, Bhavna, Bali V, Saigal N. Recent advances in pelletization technique for oral drug delivery: a review. Curr Drug Deliv. 2009;6(1):122-9. doi: 10.2174/156720109787048339, PMID 19418964.
Vaphare AM, Banerjee SK, Gadhave MV, Gaikwad DD. Pelletization techniques: a review. Asian J Pharm Res Dev. 2014;2(3):103-14.
Deb R, Ahmed AB. Pellets and pelletization techniques: a critical review. Int Res J Pharm. 2016;4(4):90-5. doi: 10.7897/2230-8407.04414.
Punia S, Bala R, Rana AC. Pelletization techniques: a literature review. Int Res J Pharm. 2012;3(3):30-4.
Begum AR, Ganesh NS, Chandy V. A study on different pellet formation techniques and its evaluation parameters a review. Int J Curr Pharm Sci. 2019;11(2):7-13. doi: 10.22159/ijcpr.2019v11i2.33020.
Kammili L, Senthil V, Rathi V. Pelletization technology: a quick review. Int J Pharm Sci Res. 2011;2(6):1337-55. doi: 10.13040/IJPSR.0975‑8232.2(6).1337‑55.
Sharma A, Chaurasia S. Multiparticulate drug delivery system: pelletization through extrusion and spheronization. Int Res J Pharm. 2013;4(2):137-40.
Trivedi NR, Rajan MG, Johnson JR, Shukla AJ. Pharmaceutical approaches to preparing pelletized dosage forms using the extrusion spheronization process. Crit Rev Ther Drug Carrier Syst. 2007;24(1):1-40. doi: 10.1615/critrevtherdrugcarriersyst.v24.i1.10, PMID 17430098.
Sarkar S, Heng PW, Liew CV. Insights into the functionality of pelletization aid in pelletization by extrusion spheronization. Pharm Dev Technol. 2013;18(1):61-72. doi: 10.3109/10837450.2011.621210, PMID 21981607.
Koo OM, Heng PW. The influence of microcrystalline cellulose grade on shape and shape distributions of pellets produced by extrusion spheronization. Chem Pharm Bull (Tokyo). 2001;49(11):1383-7. doi: 10.1248/cpb.49.1383, PMID 11724226.
Petrovick GF, Pein M, Thommes M, Breitkreutz J. Spheronization of solid lipid extrudates: a novel approach on controlling critical process parameters. Eur J Pharm Biopharm. 2015;92:15-21. doi: 10.1016/j.ejpb.2015.02.004, PMID 25681745.
Dukic Ott A, Thommes M, Remon JP, Kleinebudde P, Vervaet C. Production of pellets via extrusion spheronisation without the incorporation of microcrystalline cellulose: a critical review. Eur J Pharm Biopharm. 2009;71(1):38-46. doi: 10.1016/j.ejpb.2008.08.005, PMID 18771727.
Dukic Ott A, De Beer T, Remon JP, Baeyens W, Foreman P, Vervaet C. In vitro and in vivo evaluation of enteric coated starch based pellets prepared via extrusion/spheronisation. Eur J Pharm Biopharm. 2008;70(1):302-12. doi: 10.1016/j.ejpb.2008.04.019, PMID 18579353.
Anisha Gulhane, Bhoyar BB. A review on multi-unit particulate system: advanced technology for novel drug delivery system. Int J Pharm Sci. 2025;3(6):780-9. doi: 10.5281/zenodo.15597547.
Sinha VR, Agrawal MK, Kumria R. Influence of formulation and excipient variables on the pellet properties prepared by extrusion spheronization. Curr Drug Deliv. 2005;2(1):1-8. doi: 10.2174/1567201052772898, PMID 16305403.
Koo OM, Heng PW. The influence of MCC grade on extrusion spheronization outcomes. Chem Pharm Bull PMC. 2001;49(11):1383-7. doi: 10.1248/cpb.49.1383.
Khan A, Malviya R, Sharma PK. Multi-unit drug delivery system: a brief review of pelletization technique. World Appl Sci J. 2014;31(3):2137-40. doi: 10.5829/idosi.wasj.2014.31.12.8498.
Petrovick GF, Thommes M. Quality aspects in the development of pelletized dosage forms. Eur J Pharm Biopharm. 2022;182:113-27. doi: 10.1016/j.ejpb.2022.05.012.
Rahman MA, Ahuja A, Baboota S, Bhavna, Bali V, Saigal N. Recent advances in pelletization technique for oral drug delivery: a review. Curr Drug Deliv. 2009;6(1):122-9. doi: 10.2174/156720109787048339, PMID 19418964.
Muley S, Nandgude T, Poddar S. Extrusion spheronization a promising pelletization technique: in-depth review. Asian J Pharm Sci. 2016;11(6):684-99. doi: 10.1016/j.ajps.2016.08.001.
Shah RD, Kabadi M, Pope DG, Augsburger LL. Physicomechanical characterization of the extrusion spheronization process. I. Instrumentation of the extruder. Pharm Res. 1994;11(3):355-60. doi: 10.1023/a:1018996500749, PMID 8008698.
Sinha VR, Agrawal MK, Kumria R, Bhinge JR. Influence of operational variables on properties of piroxicam pellets prepared by extrusion spheronization: a technical note. AAPS PharmSciTech. 2007;8(1):E137–E41. doi: 10.1208/pt0801020, PMID 17408219.
Koo OM, Heng PW. The influence of microcrystalline cellulose grade on shape and shape distributions of pellets produced by extrusion spheronization. Chem Pharm Bull (Tokyo). 2001;49(11):1383-7. doi: 10.1248/cpb.49.1383, PMID 11724226.
Arshad MS, Zafar S, Yousef B, Alyassin Y, Ali R, AlAsiri A. A review of emerging technologies enabling improved solid oral dosage form manufacturing and processing. Adv Drug Deliv Rev. 2021;175:113840. doi: 10.1016/j.addr.2021.113840.
PaLkowski L, Karolak M, Kubiak B, Blaszczynski J, Slowinski R, Thommes M. Optimization of pellets manufacturing process using rough set theory. Eur J Pharm Sci. 2018;124:295-303. doi: 10.1016/j.ejps.2018.08.027, PMID 30157461.
Abdul S, Chandewar AV, Jaiswal SB. A flexible technology for modified-release drugs: multiple-unit pellet system (MUPS). J Control Release. 2010;147(1):2-16. doi: 10.1016/j.jconrel.2010.05.014, PMID 20493217.
Wang J, Kan S, Chen T, Liu J. Application of quality by design (QbD) to formulation and processing of naproxen pellets by extrusion spheronization. Pharm Dev Technol. 2015;20(2):246-56. doi: 10.3109/10837450.2014.908300, PMID 25069591.
De Barros JM, Lechner T, Charalampopoulos D, Khutoryanskiy VV, Edwards AD. Enteric-coated spheres produced by extrusion/spheronization provide effective gastric protection and efficient release of live therapeutic bacteria. Int J Pharm. 2015;493(1-2):483-94. doi: 10.1016/j.ijpharm.2015.06.051, PMID 26188314.
Dukic Ott A, Thommes M, Remon JP, Kleinebudde P, Vervaet C. Production of pellets via extrusion spheronisation without the incorporation of microcrystalline cellulose: a critical review. Eur J Pharm Biopharm. 2009;71(1):38-46. doi: 10.1016/j.ejpb.2008.08.005, PMID 18771727.
Khadam VK, Chawra HS, Singh RP. Extrusion spheronization, pelletization technique and Wurster coating (bottom spray): a review. Future J Pharm Health Sci. 2023;3(3):263-76. doi: 10.26452/fjphs.v3i3.474.
McConnell EL, Basit AW. Modified release oral drug delivery. In: Aulton ME, Taylor KM, editors. Aulton’s pharmaceutics: the design and manufacture of medicines. 5th ed. Amsterdam: Elsevier; 2018. p. 564-79.
Al Hashimi N, Begg N, Alany RG, Hassanin H, Elshaer A. Oral modified release multiple unit particulate systems: compressed pellets, microparticles and nanoparticles. Pharmaceutics. 2018 Oct 4;10(4):176. doi: 10.3390/pharmaceutics10040176, PMID 30287798, PMCID PMC6321440.
Foppoli A, Cerea M, Palugan L, Zema L, Melocchi A, Maroni A. Evaluation of powder layering vs. spray coating techniques in the manufacturing of a swellable/erodible pulsatile delivery system. Drug Dev Ind Pharm. 2020;46(8):1230-7. doi: 10.1080/03639045.2020.1788060, PMID 32597251.
Agrawal S, Fernandes J, Shaikh F, Patel V. Quality aspects in the development of pelletized dosage forms. Heliyon. 2022;8(2):e08956. doi: 10.1016/j.heliyon.2022.e08956, PMID 35243077.
Sosnik A, Augustine R. Challenges in oral drug delivery of antiretrovirals and the innovative strategies to overcome them. Adv Drug Deliv Rev. 2016;103:105-20. doi: 10.1016/j.addr.2015.12.022, PMID 26772138.
Roy P, Shahiwala A. Multiparticulate formulation approach to pulsatile drug delivery: current perspectives. J Control Release. 2009;134(2):74-80. doi: 10.1016/j.jconrel.2008.11.011, PMID 19105973.
Waghmare SM, More NN, Jagtap SR, Mandhare TA, Soni GK, Kale AY. Techniques and evaluation tests for colon cancer treatment using pellets: a review. Curr Res Pharm Sci. 2024;13(4):157-66. doi: 10.24092/CRPS.2023.130401.
Arora S, Rathore C. Formulation and in vitro evaluation of polymer-based extended release pellet tablets of diltiazem. Int J Life Sci Pharma Res. 2023;13(6):299–309. doi: 10.22376/ijlpr.2023.13.6.
Rahman MA, Ahuja A, Baboota S, Bhavna, Bali V, Saigal N. Recent advances in pelletization technique for oral drug delivery: a review. Curr Drug Deliv. 2009;6(1):122-9. doi: 10.2174/156720109787048339, PMID 19418964.
Kan S, Lu J, Liu J, Wang J, Zhao Y. A Quality by design (QbD) case study on enteric-coated pellets: screening of critical variables and establishment of design space at laboratory scale. Asian J Pharm Sci. 2014;9(5):268-78. doi: 10.1016/j.ajps.2014.07.005.