
Int J Curr Pharm Res, Vol 17, Issue 6, 9-13Review Article
A COMPREHENSIVE REVIEW ON DOSAGE FORM FOR ANTICOAGULANT DRUG
MAHESHWARI M. NAVGHARE*, NILESH S. KULKARNI, VAISHNAVI K. DESHMUKH
Department of Pharmaceutics, PES Modern College of Pharmacy (for ladies), Moshi, Pune 412105, Maharashtra, India
*Corresponding author: Maheshwari M. Navghare; *Email: mahimnavghare8316@gmail.com
Received: 12 Aug 2025, Revised and Accepted: 02 Oct 2025
ABSTRACT
Thromboembolic disorders are a major global health issue, requiring effective therapies rooted in understanding thrombophilia and the coagulation cascade. Warfarin, though widely used, presents challenges like dietary restrictions, narrow therapeutic index, and constant monitoring. New generation oral anticoagulant like hirudin, bivalirudin, argatroban, apixaban, and rivaroxaban offer improved safety and pharmacokinetic profiles. To further optimize their clinical use, advanced drug delivery systems have been developed. Formulations like hydrogels, microspheres, micellar nanocomplexes, nanoparticles, and fast-dissolving oral films enhance bioavailability and therapeutic efficacy. These systems also provide targeted or sustained drug release and reduce systemic side effects. Hirudin-based hydrogels and microspheres maintain prolonged thrombin inhibition. Bivalirudin micelles and hydrogels offer localized anticoagulation with minimal bleeding. Rivaroxaban and apixaban in film and nanoparticle forms ensure rapid absorption and patient-friendly administration. Such innovations improve both clinical outcomes and patient compliance in anticoagulant therapy.
Keywords: NOAC (Non-vitamin k oral anticoagulant), Thrombin, Microbubbles, Acute coronary syndrome (ACS)
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijcpr.2025v17i6.7068 Journal homepage: https://innovareacademics.in/journals/index.php/ijcpr
INTRODUCTION
Understanding excessive blood clotting has evolved significantly since Virchow's 1856 theory, with inherited antithrombin (AT) deficiency first reported in 1965. Later, functional defects like AT Budapest and protein S (PS) and protein C (PC) deficiencies were identified. These conditions, collectively termed thrombophilias, involve inherited or acquired blood coagulation abnormalities. Genetic mutations, such as PS, AT, and PC deficiencies or factor V Leiden and prothrombin gene variants, play a major role. A recent Polish study identified novel mutations and confirmed the utility of genetic screening. AT deficiency, although rare, is the most severe and has well-characterized genetic subtypes. PC and PS deficiencies show variable expression and present diagnostic challenges. Functional and antigen assays, though helpful, can be misleading, especially in the presence of interfering conditions. Genetic testing, though complex (especially for PS due to pseudogenes), is vital for accurate diagnosis. Standardized guidelines for testing and further research are needed to improve diagnosis and care of inherited thrombophilias [1-6].
Orally administered anticoagulants serve long-term for the prevention and management of blood clots in veins and arteries. Warfarin was the standard for decades, but A new class of oral anticoagulants not dependent on vitamin K, including edoxaban, apixaban, rivaroxaban, and dabigatran, have been developed as newer therapeutic options. NOACs match warfarin in effectiveness but are easier to use, requiring no routine monitoring and offering fixed dosing [7]. They also have a lower risk of causing intracranial bleeding. In the U. S., NOACs are approved for VTE prevention post-surgery, VTE treatment, and Prophylaxis of stroke in individuals with atrial fibrillation. Rivaroxaban is also approved in Europe for preventing recurrent ischemia in ACS [8]. This article compares NOACs to warfarin, outlines approved dosages, summarizes clinical trials, discusses ongoing research, reviews real-world data, and highlights future opportunities and challenges [9].
Rivaroxaban and Dabigatran appeared first authorized as direct oral anticoagulants (DOACs) in Europe in 2008, followed by edoxaban in Japan and subsequently in Europe for apixaban in 2011. Currently four drugs are extensively utilized to prevention of Thromboembolic events in the venous circulation, particularly post-operative phase of significant orthopedic interventions surgeries. The aforementioned drugs are also approved for Prophylaxis of stroke in individuals with atrial fibrillation, with apixaban, rivaroxaban, and edoxaban additionally authorized to treat Acute thrombotic events in the deep veins and pulmonary arteries. In Prophylaxis of stroke in individuals with atrial fibrillation cases, edoxaban and rivaroxaban, having shorter half-lives, are taken on daily basis, while apixaban and dabigatran, with longer half-lives, require twice-daily dosing. However, these dosing schedules may not align perfectly with their pharmacokinetics. While all DOACs have proven effective and safe in trials, the logic behind their dosing regimens remains unclear. This study aimed to establish a comparative indicator based on factors like Extent of systemic absorption, Molar mass, Equilibrium constant for inhibitor binding, Binding affinity to plasma proteins, together with dose to evaluate along with compare the relative effectiveness and dosage intensity of individual agent [10].
Mechanism
Hemostasis through coagulation is a complex process involving numerous factors for clotting. The intrinsic pathway includes factors (I, IX, II, XI, XII and X), also known as fibrinogen, Christmas factor, prothrombin, plasma thromboplastin, Hageman factor and Stuart-Prower factor. Factor VII, also known as the stable factor, is a component of the extrinsic pathway, which also includes factors I, II, and X. In the common coagulation pathway factors I, II, V, VIII, and X are present in circulation as inactive precursors known as zymogens. Upon activation, they become serine proteases that sequentially activate other zymogens, eventually resulting in the generation of fibrin. While factors V, VIII, and XIII do not function as serine proteases, factors II, VII, IX, X, XI, and XII exhibit serine protease activity [11].
Intrinsic pathway
The longer pathway of secondary hemostasis is initiated by endothelial injury that reveals collagen, leading to the activation of factor XII into XIIa. This activated factor XIIa then converts factor XI into XIa, which subsequently activates factor IX into IXa, continuing the coagulation cascade. Factor IXa converts factor X into its active form, Xa. Each step increases the concentration of the activated factor, enhancing the response. Activated thrombin (factor IIa) further amplifies this pathway by providing feedback to several other factors. Factor XII is not essential for clotting, as its absence doesn’t impair hemostasis. Partial thromboplastin time (PTT) is used clinically to assess the intrinsic pathway [12].
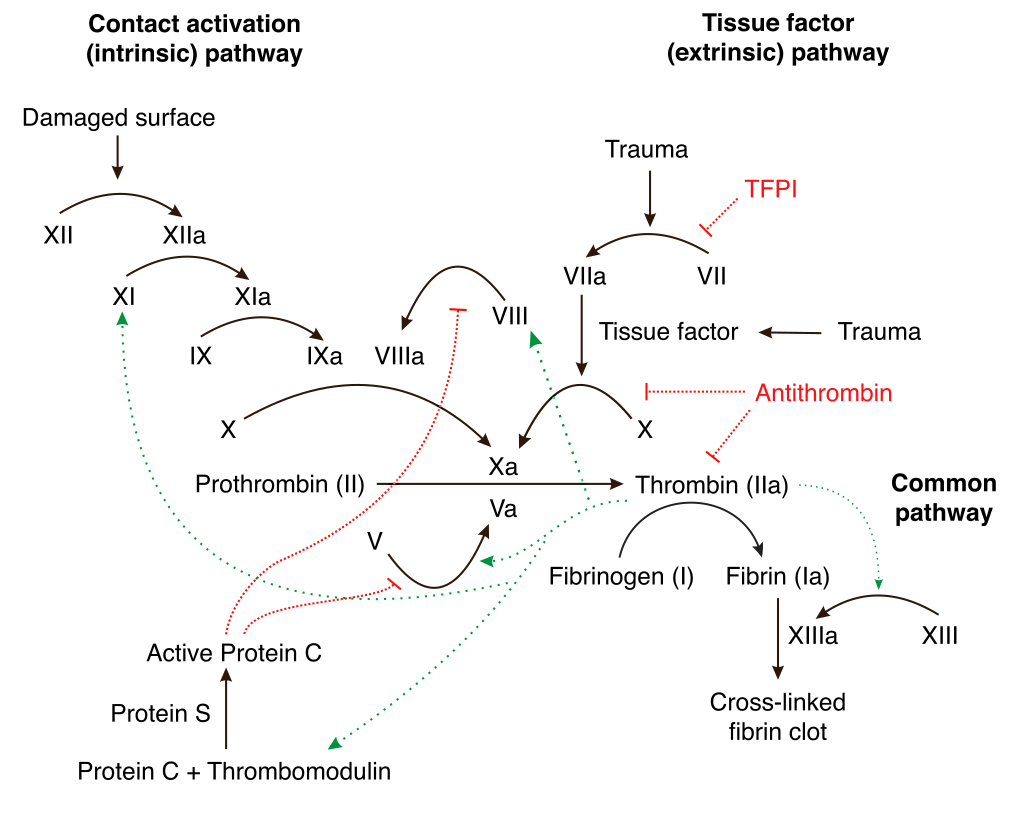
Fig. 1: Mechanism of coagulation pathway
Extrinsic pathway
This quicker pathway is assessed using prothrombin time (PT) and begins when damaged endothelial cells release tissue factor. Tissue factor activates factor VII to VIIa, which then converts factor X to Xasignaling the convergence point of both pathways.
Common pathway
The intrinsic and extrinsic pathways converge upon the conversion of factor X into its active form, Xa, a process mediated by two types of tenase complexes: the intrinsic complex (comprising factors VIII, IXa, phospholipids, and calcium) and the extrinsic complex (consisting of factor VII, tissue factor, and calcium). Once formed, Stuart-Prower factor, together with its cofactor factor V, converts (factor II) into its active form (IIa). Thrombin not only transforms fibrinogen (factor I) into fibrin but also enhances the functions of factors VIII, V, XIII, and X [12]. The resulting fibrin strands are cross-linked and stabilized by factor XIII, forming a mesh that strengthens the platelet plug [13-22].
Dosage form for anticoagulant drugs
Hirudin
Hirudin is a thrombin inhibitor composed of 65 amino acids, originally isolated from the saliva of leeches, is now made using recombinant technology. It binds strongly to thrombin, forming a nearly irreversible complex, and is mainly cleared by the kidneys. Used effectively in treating heparin-induced thrombocytopenia (HIT), it’s also beneficial during cardiopulmonary bypass and in hip surgery without raising bleeding risks. Though it slightly increases major bleeding in unstable angina or NSTEMI, it doesn’t raise life-threatening events. It’s approved for HIT and under consideration for broader cardiac use [23, 24].
Table 1: Table of Hirudin
| Drug (hirudin) | Material | Formulation | Method of preparation | Outcome |
| 1) | Hydrogenated phosphatidylcholine, polyethylene glycol 1500, Poloxamer 188, butanol and Perfluoropropane. | Microbubbles | Sonication-lyophilization method | Phospholipid-based gas-filled microbubbles demonstrates promise as a reliable and efficient system for hirudin delivery with high encapsulation efficiency and minimal impact on ultrasound imaging quality [25] |
| 2) | rHV2(recombinant hirudin variant-2), Pluronic®F127, thrombin | Hydrogel | Cold Method | PF127 gel effectively prolongs the antithrombotic activity and plasma level of rHV2, highlighting its potential as a promising drug delivery system [26] |
| 3) | Polyvinyl alcohol, (PLGA) Poly(lactic-co-glycolic) acid and Dichloromethane | Microsphere | Double emulsion solvent evaporation method | Microspheres composed of PLGA and F-127 encapsulating hirudin support motor function recovery and neural regeneration after CNS injury by maintaining prolonged thrombin inhibition [27] |
Bivalirudin
A synthetic analogue of hirudin is a bivalent thrombin inhibitor made of 12 amino acids. It binds reversibly to thrombin due to cleavage of its Arg-Pro bond, which makes it a lower-affinity inhibitor with a shorter half-life potentially making it safer than hirudin [28]. In a large Phase III trial, bivalirudin significantly reduced both ischemic and bleeding events compared to heparin during angioplasty in patients with unstable angina, leading to its approval in North America. It is cleared partially through the kidneys but also undergoes hepatic metabolism and proteolysis, making it safer for HIT patients with kidney issues [14].
Argatroban
Carboxylic acid compound that binds reversibly at the catalytic site of thrombin. It has been officially accepted for use in HIT and is being studied for arterial thrombosis treatment [15]. Argatroban is a selective, reversible thrombin inhibitor that effectively blocks both free and thrombin imgded in the thrombus, unlike heparin as well as hirudin, that show significantly diminished ability to inhibit clot-associated thrombin [48].
Rivaroxaban
Rivaroxaban, an oxazolidinone derivative, binds directly to factor Xa (FXa) with strong affinity and high Preferential binding, without the need for cofactors [16]. Rivaroxaban is an orally administered direct inhibitor of Factor (Xa) that targets both unbound and Thrombus-bound Factor (Xa), exhibiting rapid absorption with peak plasma concentrations reached within 2 to 4 h. It has high bioavailability (80–100%), moderate pharmacokinetic variability, and consistent profiles across populations. In younger adults, the elimination time spans 5 to 9 h, whereas in older individuals, it extends between 11 and 13 h. Its effects correlate with plasma levels, showing predictable pharmacodynamics. It doesn’t affect CYP enzymes or major drug transporters, has minimal drug interactions, and is approved for various thromboembolic disorders [17]. It comes in various dosages, with 20 mg typically prescribed for atrial fibrillation. Lower doses, such as 15 mg and 10 mg, are used to address and avert the occurrence of deep vein thrombosis and pulmonary embolism [18].
Table 2: Table of bivalirudin
| Drug (Bivalirudin) | Material | Formulation | Method of preparation | Outcome |
| 1) | N-acetyl-Lcysteine methyl ester, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine N-[maleimide (polyethylene glycol)-2000] (ammonium salt), Fibrinogen and thromboplastin | Micellar Nanocomplexes. | Thin-film hydration method | Clot-targeted bivalirudin micelles enhance anticoagulant efficacy and stability, offering a versatile platform for safer and more effective thrombosis therapy [29]. |
| 2) | Gelatin (Type A), methacrylic), Irgacure I-2959 (≥95%) sodium dodecyl sulfate (SDS) and anhydride (MA) | Hydrogel | Methacrylation of Gelatin | BV-coated GelMA hydrogel provides safe, localized anticoagulation for blood-contact devices without systemic bleeding risk [30]. |
| 3) | Allylamine, Bovine serum albumin (BSA), Glutaraldehyde aqueous solution (30%), Ethanol, and Phosphate-buffered saline tablet. | Film | Plasma Polymerization Deposition Method | Stable, substrate-independent polyallylamine coating strategy to enhance blood compatibility and functionalization of bioinert metal surfaces [31]. |
Table 3: Table of argatroban
| Drug (Argatroban) | Material | Formulation | Method of preparation | Outcome |
| 1) | Hydroxylesters, L-(1)-diethyl tartrate (DET), L-(1)-di-n-butyl tartrate (DnBT), L-(1)-di-i-propyl tartrate (DiPT), DL-diethyl malate (DEM), and triethyl citrate (TEC), Poly-DL-lactic acid (PLA), L-(1)-dimethyl tartrate (DMT), triethyl citrate (TEC) and Trifluoroethanol. | Film | Solvent casting method | The enhanced release of argatroban in DET-added PLA films is attributed to surface pore formation from increased water uptake [32]. |
| 2) | Polyacrylic Acid | Gel | Hydrogel-based drug-eluting balloon catheter preparation | The study concludes that locally delivered argatroban via hydrogel-coated balloon catheter effectively reduces restenosis, warranting further investigation for clinical application [33] |
Table 4: Table of rivaroxaban
| Drug (Rivaroxaban) | Material | Formulation | Method of preparation | Outcome |
| 1) | Macrogol stearate, Behenoyl polyoxyl-8 glycerides, Caprylo-caproyl polyoxyl-8 glycerides and Polyoxyl stearate. | Lipid Solid Dispersion | Spray drying method | The Box–Behnken experimental design facilitated the evaluation of formulation variables influencing rivaroxaban dissolution from lipid solid dispersions and enabled optimization to enhance drug release [34]. |
| 2) | Phospholipid (PL), Lipoid S PC, injection grade phosphatidylcholine, Chitosan (CS), Cholesterol, Tween 80 (T80), Dimethyl Sulfoxide (DMSO), Span 80 (S80), chloroform and Glacial acetic. | Liposomes | Conventional thin-film hydration method. | The formulation’s in vitro release profile showed an initial rapid release of approximately 25% within the first 2 h, followed by a sustained release that reached around 84% over 24 h [35]. |
| 3) | Poloxamer 188, PEG 4000, Sodium starch glycolate, Magnesium stearate, Talc and Povidone K30. | Fast disintegrating tablet | Direct compression process. | By forming compounds with Poloxamer 188, rivaroxaban's solubility and rate of dissolution can be improved. The rivaroxaban in the PEG 4000 tablet formulation was rapidly dissolved. Optimal formulations were selected based on P values, along with response surface and contour plots generated using Design Expert software [36]. |
| 4) | β-Cyclo-dextrin (βCD), Poloxamer 188 (PXM-188), Polyvinyl pyrolidine (PVP K-30) and soluplus (SOLO) | Inclusion Complex | Kneading and Solvent Evaporation Technique | The RIV: βCD (1:2) inclusion complex, prepared using kneading and solvent evaporation methods, enhanced the solubility and dissolution of RIV. Additionally, ternary complexes with Soloplus, formulated via solvent evaporation, demonstrated even greater improvements in RIV’s solubility and dissolution profile [37]. |
| 5) | Polyvinylpyrrolidone, Sodium lauryl sulfate (SLS) and Polyvinyl alcohol. | Microsphere | Spray-drying technique | The oral bioavailability was enhanced by about 2, 1.3, and 1.6 times relative to the drug powder, with corresponding AUC values of 2101.3 ± 314.8, 1325.2 ± 333.3, and 1664.0 ± 102.6 h•ng/ml, respectively [38]. |
| 6) | Propylene glycol, HPMC, Sodium starch glycolate and Aspartame. | Fast-Dissolving Oral Film | Solvent casting method | The in vitro release profile of the drug followed first-order kinetics, with Fickian diffusion as the release mechanism. These results indicate that rivaroxaban OTFs enable rapid drug release from the site of administration into systemic circulation [39]. |
| 7) | Carbon dioxide and Ethanol | Nanoparticles | Supercritical CO₂ Solubilization Method with Static Extraction and UV Quantification | The study revealed that using ethanol as a co-solvent greatly increased RXN solubility in supercritical CO₂ with the highest solubility at 30 MPa and 338 K, and models like Jouyban and Garlapati-Madras accurately predicted solubility behaviour [40]. |
Apixaban
Apixaban is a orally administered inhibitor of Factor Xa that targets both unbound and clot-associated forms. Apixaban directly and binds reversibly to the catalytic site of Factor Xa, competitively blocking its activity [41]. It's used to reduce Stroke susceptibility in individuals with non-valvular atrial fibrillation and treat otherwise prevent DVT/PE. It also provides thromboprophylaxis after Surgical replacement of the hip or knee joint. The drug has ~50% oral bioavailability, unaffected by food [19-22]. Apixaban is an oral anticoagulant with approximately 50% bioavailability and is primarily eliminated through feces, with about 25% excreted unchanged by the kidneys. Administered in an amount of 2.5 mg two times per day it has demonstrated both efficacy and safety in preventing venous thromboembolism (VTE) after planned orthopedic procedures, including hip or knee joint replacements [42].
Table 5: Table of Apixaban
| Drug (Apixaban) | Material | Formulation | Method of preparation | Outcome |
| 1) | PEG 600, Microcrystalline Cellulose, Sodium Starch Glycolate, Mannitol, Magnesium, Carboxy Methyl Ethyl Cellulose (CMEC), Hydroxy Propyl Methyl Cellulose, Hydroxy Propyl Methyl Cellulose Phthalate, Hydroxy Propyl Cellulose and Poly Vinyl Pyrrolidone (PVP). | Oridispersible Fast Dissolving Tablet | Hot melt extrusion method and Fusion method | The study developed Apixaban quick-release tablets using direct compression. Preformulation tests confirmed excipient compatibility, with Crospovidone-XL showing the best performance, achieving 98.14% drug release in 8 min and a 96.12% assay success rate [43]. |
| 2) | Hypromellose 2910 K4M And E15, Macrogol 6000, POLYOX Water-Soluble Resins (Polyethylene Oxide), Glycerin, Sucralose and Maltose, | Orodispersible film | Solvent Casting Mathod | Apixaban orodispersible films, made using hydroxypropyl methylcellulose and polyethylene glycol, showed high drug absorption in rats and met bioequivalence criteria in humans. These films offer a potential solution for NVAF treatment in patients with swallowing difficulties [44]. |
| 3) | Propylene Glycol, HPMC, Sodium Starch Glycolate And Aspartame. | Fast-Dissolving Oral Film | Solvent Casting Process | Apixaban oral thin films were successfully prepared using solvent casting, showing good stability, dissolution, and dose uniformity. The films demonstrated quick drug release, enhancing Apixaban's bioavailability [45]. |
| 4) | Glycerylmonostearate and Polyethylene glycol 200. | Solid Lipid Nanoparticles | High‐pressure homogenization | The F10-loaded Apixaban formulation using solid lipid nanoparticles (SLNs) notably enhanced both solubility and bioavailability, while its sustained-release design helps prevent hidden blood clotting events [46]. |
| 5) | Sodium lauryl, Lactose anhydrous, Crosscarmellose sodium, Sodium starch glycolate, crospovidone, Magnesium stearate and Microcrystalline cellulose. | Tablet | Direct Compression | The final 5 mg immediate-release apixaban tablet provides fast, reliable drug release and stable quality, supported by a robust analytical method offering potentially enhanced therapeutic efficacy [47]. |
CONCLUSION
Thrombophilia management relies on understanding coagulation. NOACs and advanced forms like nanoparticles and films boost safety, bioavailability, and patient compliance. Innovative dosage forms such as hydrogels, microspheres, nanoparticles, liposomes, and fast-dissolving oral films have significantly improved the delivery, safety, and efficacy of anticoagulant drugs. These advanced formulations offer targeted action, enhanced bioavailability, and better patient compliance, making them valuable tools in the effective management of thromboembolic disorders. As research progresses, the continued development and optimization of these formulations hold promise for more effective and personalized treatment strategies in the management of thromboembolic disorders.
FUNDING
Nil
AUTHORS CONTRIBUTIONS
All authors have contributed equally
CONFLICT OF INTERESTS
Authors declare that we have no conflict of interest
REFERENCES
Egeberg O. Inherited antithrombin deficiency causing thrombophilia. Thromb Diath Haemorrh. 1965 Jun;13(2):516-30. doi: 10.1160/TH15-02-0141, PMID 14347873.
Marder VJ, Aird WC, Bennett JS, Schulman S, White GC. Hemostasis and thrombosis: basic principles and clinical practice. 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2012.
Comp PC, Esmon CT. Recurrent venous thromboembolism in patients with a partial deficiency of protein S. N Engl J Med. 1984 Dec 13;311(24):1525-8. doi: 10.1056/NEJM198412133112401, PMID 6239102.
Mannucci PM, Franchini M. Classic thrombophilic gene variants. Thromb Haemost. 2015 Nov;114(5):885-9. doi: 10.1160/TH15-02-0141, PMID 26018405.
Muszbek L, Bereczky Z, Kovacs B, Komaromi I. Antithrombin deficiency and its laboratory diagnosis. Clin Chem Lab Med. 2010 Dec;48 Suppl 1:S67-78. doi: 10.1515/CCLM.2010.368, PMID 21062218.
Moll S. Thrombophilia: clinical practical aspects. J Thromb Thrombolysis. 2015 Apr;39(3):367-78. doi: 10.1007/s11239-015-1197-3, PMID 25724822.
Messerschmidt C, Friedman RJ. Clinical experience with novel oral anticoagulants for thromboprophylaxis after elective hip and knee arthroplasty. Arterioscler Thromb Vasc Biol. 2015 Apr;35(4):771-8. doi: 10.1161/ATVBAHA.114.303400, PMID 25767271.
Bacchus F, Schulman S. Clinical experience with the new oral anticoagulants for treatment of venous thromboembolism. Arterioscler Thromb Vasc Biol. 2015 Mar;35(3):513-9. doi: 10.1161/ATVBAHA.114.303396, PMID 25717178.
Carreras ET, Mega JL. Role of oral anticoagulants in patients after an acute coronary syndrome. Arterioscler Thromb Vasc Biol. 2015;35(3):520-4. doi: 10.1161/ATVBAHA.114.303401, PMID 25614282.
Ieko M, Naitoh S, Yoshida M, Takahashi N. Profiles of direct oral anticoagulants and clinical usage dosage and dose regimen differences. J Intensive Care. 2016;4:19. doi: 10.1186/s40560-016-0144-5, PMID 26966542.
Panova Noeva M, Eggebrecht L, Prochaska JH, Wild PS. Potential of multidimensional large scale biodatabases to elucidate coagulation and platelet pathways as an approach towards precision medicine in thrombotic disease. Hamostaseologie. 2019 Jun;39(2):152-63. doi: 10.1055/s-0038-1677520, PMID 30722070.
Grover SP, Mackman N. Intrinsic pathway of coagulation and thrombosis. Arterioscler Thromb Vasc Biol. 2019;39(3):331-8. doi: 10.1161/ATVBAHA.118.312130, PMID 30700128.
Chaudhry R, Usama SM, Babiker HM. Physiology coagulation pathways. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2018 Mar 1. PMID 29489185.
Maraganore JM, Bourdon P, Jablonski J, Ramachandran KL, Fenton JW II. Design and characterization of hirulogs: a novel class of bivalent peptide inhibitors of thrombin. Biochemistry. 1990 Jul 1;29(30):7095-101. doi: 10.1021/bi00482a021, PMID 2223763.
Fitzgerald D, Murphy N. Argatroban: a synthetic thrombin inhibitor of low relative molecular mass. Coron Artery Dis. 1996 Jun;7(6):455-8. doi: 10.1097/00019501-199606000-00008, PMID 8889361.
Perzborn E, Kubitza D, Misselwitz F. A novel oral direct factor Xa inhibitor in clinical development for the prevention and treatment of thromboembolic disorders. Hamostaseologie. 2007 Sep;27(4):282-9. doi: 10.1055/s-0037-1617095, PMID 17938768.
Mueck W, Stampfuss J, Kubitza D, Becka M. Clinical pharmacokinetic and pharmacodynamic profile of Rivaroxaban. Clin Pharmacokinet. 2014 Jan;53(1):1-16. doi: 10.1007/s40262-013-0100-7, PMID 23999929.
Alburyhi MM, Hamidaddin MA, Saif AA, Noman MA. Formulation and evaluation of rivaroxabanorodispersible tablets. World J Pharm Pharm Sci. 2024;13(2):2066-92.
Byon W, Garonzik S, Boyd RA, Frost CE. Apixaban: a clinical pharmacokinetic and pharmacodynamic review. Clin Pharmacokinet. 2019 Oct;58(10):1265-79. doi: 10.1007/s40262-019-00775-z, PMID 31089975.
Lassen MR, Raskob GE, Gallus A, Pineo G, Chen D, Hornick P. Apixaban versus enoxaparin for thromboprophylaxis after knee replacement (ADVANCE-2): a randomised double-blind trial. Lancet. 2010;375(9717):807-15. doi: 10.1016/S0140-6736(09)62125-5, PMID 20206776.
Lassen MR, Gallus A, Raskob GE, Pineo G, Chen D, Ramirez LM. Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N Engl J Med. 2010 Dec 23;363(26):2487-98. doi: 10.1056/NEJMoa1006885, PMID 21175312.
Lassen MR, Raskob GE, Gallus A, Pineo G, Chen D, Portman RJ. Apixaban or enoxaparin for thromboprophylaxis after knee replacement. N Engl J Med. 2009;361(6):594-604. doi: 10.1056/NEJMoa0810773, PMID 19657123.
Bao Z, Qi X, Zhu S, Zhang M, Liu X, Chen P. Recent progress on formulations of hirudin. Pharmaceutical Science Advances. 2025 May 21;3:100078. doi: 10.1016/j.pscia.2025.100078.
Wei D, Lyu J, Wang B, He Y, Bi L. Hirudin enhances perforator flap survival: clinical application report and mechanistic exploration. J Stomatol Oral Maxillofac Surg. 2024 Jun 1;125(3S):101868. doi: 10.1016/j.jormas.2024.101868, PMID 38588856.
Zhao YZ, Liang HD, Mei XG, Halliwell M. Preparation, characterization and in vivo observation of phospholipid-based gas-filled microbubbles containing hirudin. Ultrasound Med Biol. 2005;31(9):1237-43. doi: 10.1016/j.ultrasmedbio.2005.05.007, PMID 16176790.
Liu Y, Lu WL, Wang JC, Zhang X, Zhang H, Wang XQ. Controlled delivery of recombinant hirudin based on thermo-sensitive Pluronic F127 hydrogel for subcutaneous administration: in vitro and in vivo characterization. J Control Release. 2007;117(3):387-95. doi: 10.1016/j.jconrel.2006.11.024, PMID 17207884.
Sellers DL, Kim TH, Mount CW, Pun SH, Horner PJ. Poly(lactic-co-glycolic) acid microspheres encapsulated in Pluronic F-127 prolong hirudin delivery and improve functional recovery from a demyelination lesion. Biomaterials. 2014;35(31):8895-902. doi: 10.1016/j.biomaterials.2014.06.051, PMID 25064804.
Warkentin TE, Koster A. Bivalirudin: a review. Expert Opin Pharmacother. 2005 Jul;6(8):1349-71. doi: 10.1517/14656566.6.8.1349, PMID 16013985.
She ZG, Liu X, Kotamraju VR, Ruoslahti E. Clot-targeted micellar formulation improves anticoagulation efficacy of bivalirudin. ACS Nano. 2014;8(10):10139-49. doi: 10.1021/nn502947b, PMID 25270510.
Gao W, Shen H, Chang Y, Tang Q, Li T, Sun D. Bivalirudin hydrogel coatings of polyvinyl chloride on extracorporeal membrane oxygenation for anticoagulation. Front Cardiovasc Med. 2023 Dec 15;10:1301507. doi: 10.3389/fcvm.2023.1301507, PMID 38162136.
Lin S, Li X, Wang K, Shang T, Zhou L, Zhang L. An albumin biopassive polyallylamine film with improved blood compatibility for metal devices. Polymers (Basel). 2019 Apr 23;11(4):734. doi: 10.3390/polym11040734, PMID 31018520.
Mochizuki A, Niikawa T, Omura I, Yamashita S. Controlled release of argatroban from PLA film effect of hydroxylesters as additives on enhancement of drug release. J Appl Polym Sci. 2008 Jun 5;108(5):3353-60. doi: 10.1002/app.27970.
Imanishi T, Arita M, Hamada M, Tomobuchi Y, Hano T, Nishio I. Effects of locally administration of argatroban using a hydrogel coated balloon catheter on intimal thickening induced by balloon injury. Japan Circ J. 1997;61(3):256-62. doi: 10.1253/jcj.61.256, PMID 9152775.
Ganesh M. Design and optimization of Rivaroxaban lipid solid dispersion for dissolution enhancement using statistical experimental design. Asian J Pharm. 2016 Feb 26;10(1):59-64.
Elsayad MK, Mowafy HA, Zaky AA, Samy AM. Chitosan caged liposomes for improving oral bioavailability of rivaroxaban: in vitro and in vivo evaluation. Pharm Dev Technol. 2021 Mar 16;26(3):316-27. doi: 10.1080/10837450.2020.1870237, PMID 33356742.
Patra RK, Sahu SK, Mahapatra AK, Das R. Enhancement of solubility of Rivaroxaban and formulation of its fast-disintegrating tablets: using design of experiments. Res J Pharm Life Sci. 2023 May;4:40-55.
Khan WH, Asghar S, Khan IU, Irfan M, Alshammari A, Riaz Rajoka MS. Effect of hydrophilic polymers on the solubility and dissolution enhancement of Rivaroxaban/beta-cyclodextrin inclusion complexes. Heliyon. 2023 Sep;9(9):e19658. doi: 10.1016/j.heliyon.2023.e19658, PMID 37809727.
Choi MJ, Woo MR, Baek K, Kim JS, Kim JO, Choi YS. Novel Rivaroxaban-loaded microsphere systems with different surface microstructure for enhanced oral bioavailability. Drug Deliv Transl Res. 2024 Mar;14(3):655-64. doi: 10.1007/s13346-023-01420-w, PMID 37667087.
Kanna S, Nadendla RR, Satyanarayana J, Karthikeya V, Sonu MV, Bhargavi PN. Formulation and evaluation of fast-dissolving oral film of Rivaroxaban. J Young Pharm. 2023 Oct 1;15(4):687-95. doi: 10.5530/jyp.2023.15.94.
Askarizadeh M, Esfandiari N, Honarvar B, Ali Sajadian SA, Azdarpour A. Binary and ternary approach of solubility of Rivaroxaban for preparation of developed nano drug using supercritical fluid. Arab J Chem. 2024 Apr 1;17(4):105707. doi: 10.1016/j.arabjc.2024.105707.
Eikelboom JW, Weitz JI. New anticoagulants. Circulation. 2010 Apr 6;121(13):1523-32. doi: 10.1161/circulationaha.109.853119, PMID 20368532.
Lassen MR, Raskob GE, Gallus A, Pineo G, Chen D, Portman RJ. Apixaban or enoxaparin for thromboprophylaxis after knee replacement. N Engl J Med. 2009;361(6):594-604. doi: 10.1056/NEJMoa0810773, PMID 19657123.
Shireesha M, Reddy VP. Formulation and evaluation of apixaban orodispersible fast-dissolving tablets. Indo Am J Pharm Biosci. 2022 Dec 25;20(4):83-96.
Wang CC, Chen YL, Lu TC, Lee C, Chang YC, Chan YF. Design and evaluation of oral formulation for apixaban. Heliyon. 2023 Aug 1;9(8):e18422. doi: 10.1016/j.heliyon.2023.e18422, PMID 37534003.
Sagili SP, Deepika PP, Pavuluri E, Bai NJ, Priyadarshini KS, Kumar MS. Design and characterization of fast-dissolving oral film of apixaban. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2024;40:e20240022. doi: 10.62958/j.cjap.2024.022, PMID 39191637.
Mulay L, Hegde N, Kanugo A. Formulation optimization and characterization of solid lipid nanoparticles of apixaban. Recent Pat Nanotechnol. 2025 Jun;19(2):270-81. doi: 10.2174/0118722105284862240506045944, PMID 39099216.
Parveen S, Singh RP, Rathore G. Formulation evaluation and bioequivalence studies of apixaban tablet. AP. 2024;13(1):896-906. doi: 10.54085/ap.2024.13.1.96.
Mulay L, Hegde N, Kanugo A. Formulation optimization and characterization of solid lipid nanoparticles of apixaban. Recent Pat Nanotechnol. 2025 Jun;19(2):270-81. doi: 10.2174/0118722105284862240506045944, PMID 39099216.