
Int J Curr Pharm Res, Vol 17, Issue 6, 14-19Original Article
PHARMACOLOGICAL EVALUATION OF ANTI-PARKINSON ACTIVITY OF DELPHINIUM DENUDATUM (JADWAR) FLOWERS ETHANOLIC EXTRACT IN MICE
AKASHDEEP*, AJEET PAL SINGH, AMAR PAL SINGH
Department of Pharmacology, St. Soldier Institute of Pharmacy, Lidhran Campus, Behind NIT (R. E. C.), Jalandhar –Amritsar bypass, NH-1, Jalandhar-144011, Punjab, India
*Corresponding author: Akashdeep; *Email: akashdeepkaur725@gmail.com
Received: 10 Aug 2025, Revised and Accepted: 01 Oct 2025
ABSTRACT
Objective: This study aimed to evaluate the neuroprotective effects of ethanolic extracts of Delphinium denudatum (Jadwar) flowers in an MPTP-induced mouse model of Parkinson’s disease (PD).
Methods: Parkinsonism was induced in Swiss albino mice using 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Mice were divided into five groups: control, negative control (MPTP), standard drug (Levodopa+Carbidopa), and two test groups receiving low (200 mg/kg) and high (400 mg/kg) doses of the ethanolic extract. The extract was administered orally for 7 d, followed by MPTP injections for another 7 d. Behavioral assessments were conducted using rota rod test, hanging test, forced swim test, open field test, actophotometer, elevated plus maze, and catalepsy bar test.
Results: The high-dose group (400 mg/kg) demonstrated significant improvement in motor coordination, grip strength, reduced immobility time, enhanced locomotor activity, and decreased cataleptic behavior compared to the MPTP group. These results were comparable to those observed in the standard treatment group, indicating a potential neuroprotective effect of the plant extract.
Conclusion: Delphinium denudatum ethanolic flower extract showed promising anti-parkinsonian activity by mitigating MPTP-induced motor and behavioral deficits in mice. This suggests its potential as a complementary therapeutic strategy for managing Parkinson’s disease, warranting further clinical exploration.
Keywords: Parkinson's disease (PD), 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), Delphiniumdenudatum (Jadwar) flowers ethanolic extract, Rota rod test, Hanging test, Swimming test, Open field test, Actophotometer test, Elevated plus maze test, Catalepsy bar test, Neuroprotective activity
© 2025 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/)
DOI: https://dx.doi.org/10.22159/ijcpr.2025v17i6.7055 Journal homepage: https://innovareacademics.in/journals/index.php/ijcpr
INTRODUCTION
Parkinson’s disease (PD), with a prevalence of 1% to 2% in the population aged over 60 years, is believed to be a multicentric second most common neurodegenerative disease in world surpassed in frequency only by Alzheimer’s disease [1], characterized by a slowly expanding degeneration of neurons particularly in the mesencephalon [2]. PD is associated with the oxidative stress and mitochondrial dysfunction and characterized clinically by combination of motor symptoms (rest tremor, bradykinesia, rigidity, postural instability, stooped posture and freezing of gait [FoG]) and non-motor symptoms (including psychiatric and cognitive disorders) [3]. In Parkinson’s disease (PD), also visual signs and symptoms as part of sensory dysfunction are a common complaint that leads to a significant decrease in the vision related quality of life [4] as the Cognitive impairment and depression are more common in patients with Parkinson disease (PD) whereas dementia commonly occurring in advanced stage of disease. Pathologically, PD is characterized by loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc) and the deposition of synuclein and cytoplasmic protein aggregates (Lewy bodies) inside degenerated neurons [5]. The Progressive loss of nigrostriatal dopamine (DA) neurons is the neuropathological hallmark of Parkinson's disease (PD) [6]. PD is not considered a hereditary condition; however, genetic risk factors should be considered as an etiology, along with environmental factors. The available treatment options for early Parkinson's disease have changed little in the past decade and include carbidopa/levodopa, dopamine agonists, and monoamine oxidase type B (MAO-B) inhibitors to reduce or delay the appearance of motor complications and along with this current therapy produce adverse events like “on-off phenomenon” [7] Delphinium denudatum (Jadwar) belongs to Family-Ranunculaceae, an annual or perennial erect and hardy ornamental herb grows in the western Himalayas from Kashmir to Uttarakhand at an altitude of 2400-3600 m especially on grassy slopes. The plant density in Himalayan region was most in Sirmaur followed by Solan and Shimla with only a few plants found in Bilaspur district. The roots are used in various medical formulations in Unani and Ayurveda to reduce the withdrawal symptoms in people on de-addiction therapy. Jadwar is Arabic word Persian Zadwar, which means the antidote. In Persian it is also called Mah-parwin (Moon and Pleiades), as it blossoms in the beginning of summer when the Pleiades rise. Indian Jadwar named as Narbisi/Nirbisi due to its antidotal properties. Nir means to oppose or to remove and Bisi means Bis or Vish (Poison). Some of its vernacular names are Antila, Balootularz, Jadwar, Mahpervin, Zadwar, Nirbisi, Nirbishi, Vishalakarani, Nilobikh, Nirvisha, Mahferfin, Apavisha, Avisha. It is one of the most important drugs used in the indigenous system of medicine in India, especially in Unani Medicine [8, 9]. Folk medicines are the tribal people’s attitudes and response towards the natures, they are the result of the nature interrelation towards the natural vegetation or the flora of the adjoin area, primitive man live on the mercy of the natures and they use the nature for the several purposes, well the folk knowledge transmit from the one generation to the another by them means of the talks and the other means. Traditional knowledge is the nature excavation for the different purposes. Delphinium denudatum (Jadwar) is a potent drug of Central Nervous System, an annual herb belongs to family Ranunculaceae occurring in the western Himalayas from Kashmir to Uttarakhand. The word Jadwar is Arabic form of Persian Zadwar means the great purifier or antidote. It is also called Nirbisi due to its antidotal properties [10, 11].
MATERIALS AND METHODS
Plant material
The flower extract of Delphinium denudatum was collected from Shivay herbal healthcare along with COA. Flower parts of Delphinium denudatumwere shade dried and powdered ethanolic Extract was prepared by soxhlet apparatus. Yield: 10 g/kg. Ethanolic extract of Delphinium denudatumwas administered in different doses (200 and 400 mg/kg, p. o) to mice.
Animal protocol
All the experiments were carried out using Adult male Swiss Albino Mice weighing 20–25 g, approximately 2 mo old procured from the Disease Free Small Animal House, Lovely Professional University, Phagwara. The animals had free access to food and water, and they were housed in a natural (12h each) light-dark cycle. Food given to mice consisted of wheat in the form of dahlia boiled in water. The animals were acclimatized for at least 5 d to the laboratory conditions before behavioral experiments. The experimental protocol was approved by the Institutional Animals Ethical Committee (IAEC) and the care of animals was taken as per guidelines of CPCSEA, Ministry of Forests and Environment, Government of India.
Drugs and chemicals
1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine hydrochloride (MPTP-HCL) was purchased from Scientific Sale Corporation, HSP. All other chemicals used in the present study were of analar quality. All drug solutions were freshly prepared before use.
MPTP-induced Parkinson’s disease model in mice
The 30 mice will be used for the evaluation of the Antiparkinson activity of ethanolic extract. The study will be conduct for 7 d. The animals will allow to acclimatizing for one week before the experiments. Animals will divide into 5 groups each group containing 6 mice. MPTP (1-methyl 4-phenyl 1,2,3,6-tetrahydropyridine) model will use for study. MPTP will be given to animals for 7 Consecutive days to create the symptoms of Parkinson's disease. Behaviour parameters will be conducted regularly.
Experimental protocol
Swiss mice weighing between-30-35 g will used for the study. The animals will keep in polypropylene cages and maintained under standardized conditions. Standard laboratory conditions: 12 h’ light and dark cycle, maintain temperature: 25±2 °C Relative humidity: 60±5%. Standard pellet diet and water ad libitum is given to animals.
Animals were divided into the 5 groups each group contain 6 mice.
Group I Taken as ‘Control group’ and receives regular food and drinking water for 7 d.
Group II Treated with MPTP for 7 d.
Group III Treated with L-Dopa+Carbidopa for 7 d.
Group IV Treated with Delphinium denudatum (Jadwar) Flowers extract 200 mg/kg and then received MPTP for 7 consecutive days.
Group IV is Treated with Delphinium denudatum (Jadwar) Flowers extract 400 mg/kg and then received MPTP for 7 consecutive days.
Extraction of Delphinium denudatum (Jadwar) flowers
Jadwar samples were taken from a plantation, and the flowers was separated from the flesh, sliced thinly, and dried in an oven at 40-50 °C. The dried flowers were crushed, sieved through a 30-mesh sieve, and 100 gs of the material that passed through was mixed with 300 ml of 70% ethanol. This mixture was stirred for 2 h, left to macerate for 24 h, filtered, and then macerated two more times. The resulting filtrate was concentrated using a rotary vacuum evaporator.
Experimental parameters
Behaviour parameters
Rotarod Test
Hanging/Grip Test
ForcedSwim Test
Open field Test
Actophotometer Test
Elevated plus maze Test
Catalepsy bar Test/wooden bar test
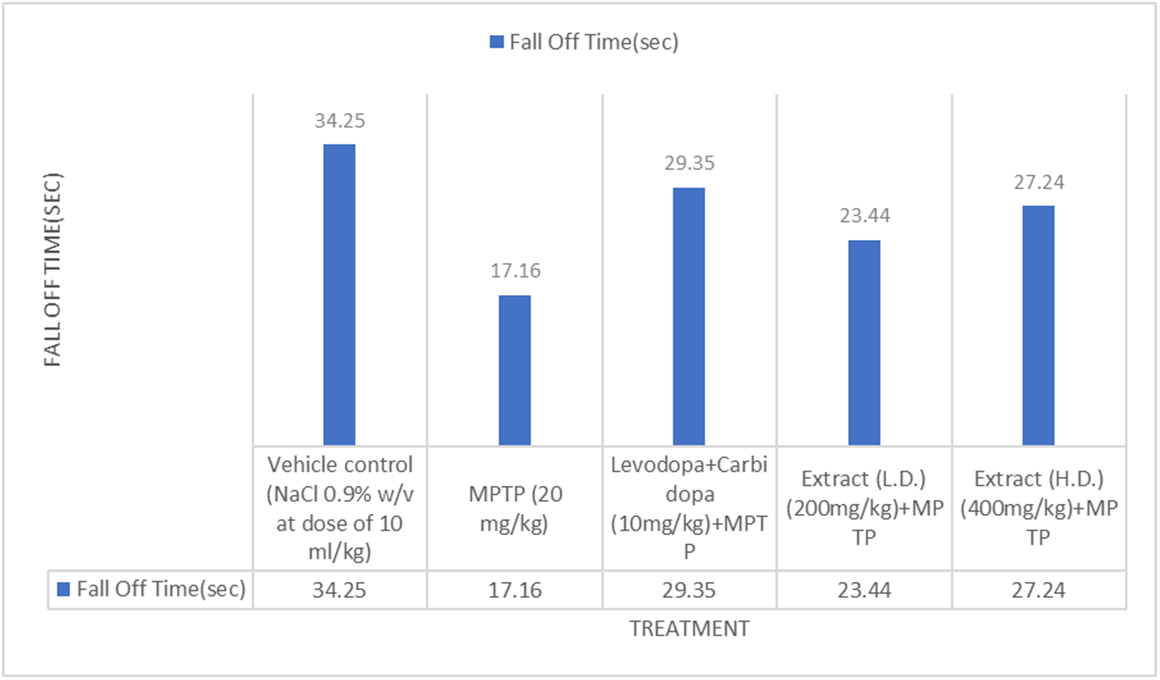
Fig. 1: Effect of ethanolic extracts of Delphinium denudatum on fall time in seconds in 0.5 mm Bar Test. All values are represented as mean±standard error of the mean; n = 6, a represents significant difference (P<0.05) compared to vehicle control and b,represents significant difference (P<0.05) compared to MPTP control. Results were analysed using analysis of variance Tukey’s post-hoc test. a =P<0.05 vs. VEH-VEHcontrol; b =P<0.05 vs. MPTP control
STATISTICAL ANALYSIS
The results were expressed as mean±standard deviation (S. D.). Statistical analysis was performed for all the result by using one-way ANOVA followed by Tukey’s test as post hoc analysis. A value of P<0.05 was considered to be statistically significant.
RESULTS
The results are discussed as
Bar test (0.5 mm)
Effect of Ethanolic extract of Delphinium denudatum (Jadwar)on bar test (0.5 mm)
Bar test (2 mm)
Effect of Ethanolic extract of Delphinium denudatum (Jadwar)on bar test (2 mm)
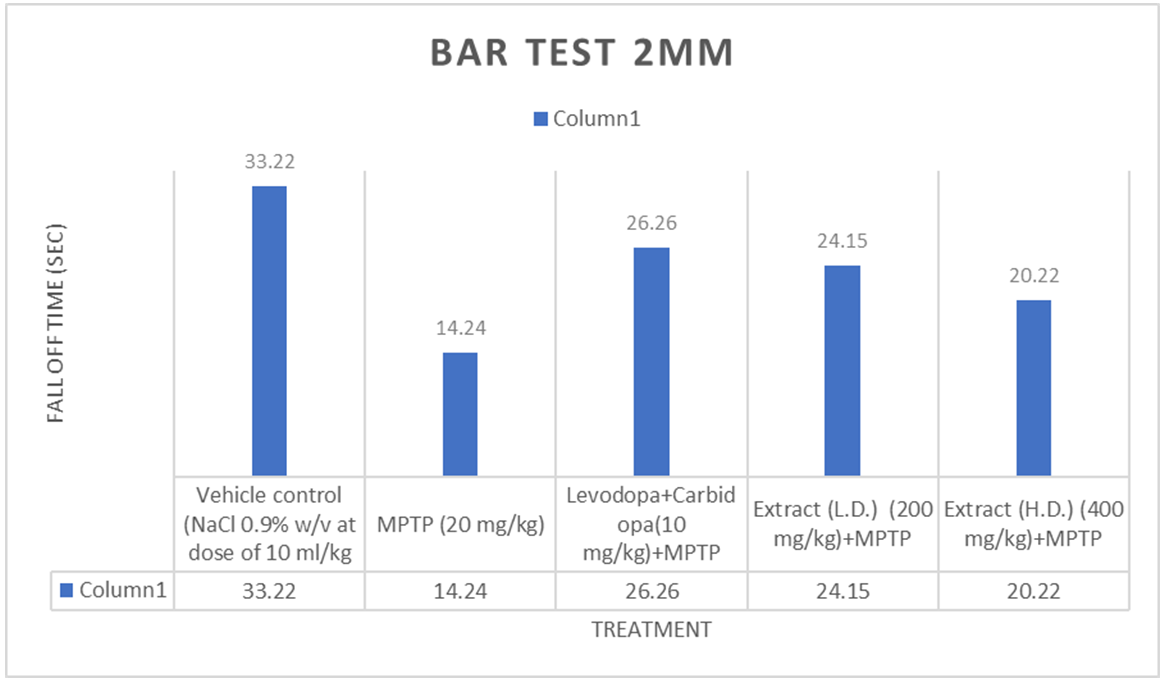
Fig. 2: Effect of ethanolic extracts of Delphiniumdenudatum on fall time in seconds in 2 mm Bar Test. All values are represented as mean±standard error of the mean; n = 6, a represents significant difference (P<0.05) compared to vehicle control and b,represents significant difference (P<0.05) compared to MPTP control. Results were analysed using analysis of variance Tukey’s post-hoc test. a =P<0.05 vs. VEH-VEH control; b =P<0.05 vs. MPTP control
Bar test (4 mm)
Effect of Ethanolic extract of Delphinium denudatum (Jadwar)on bar test (4 mm)
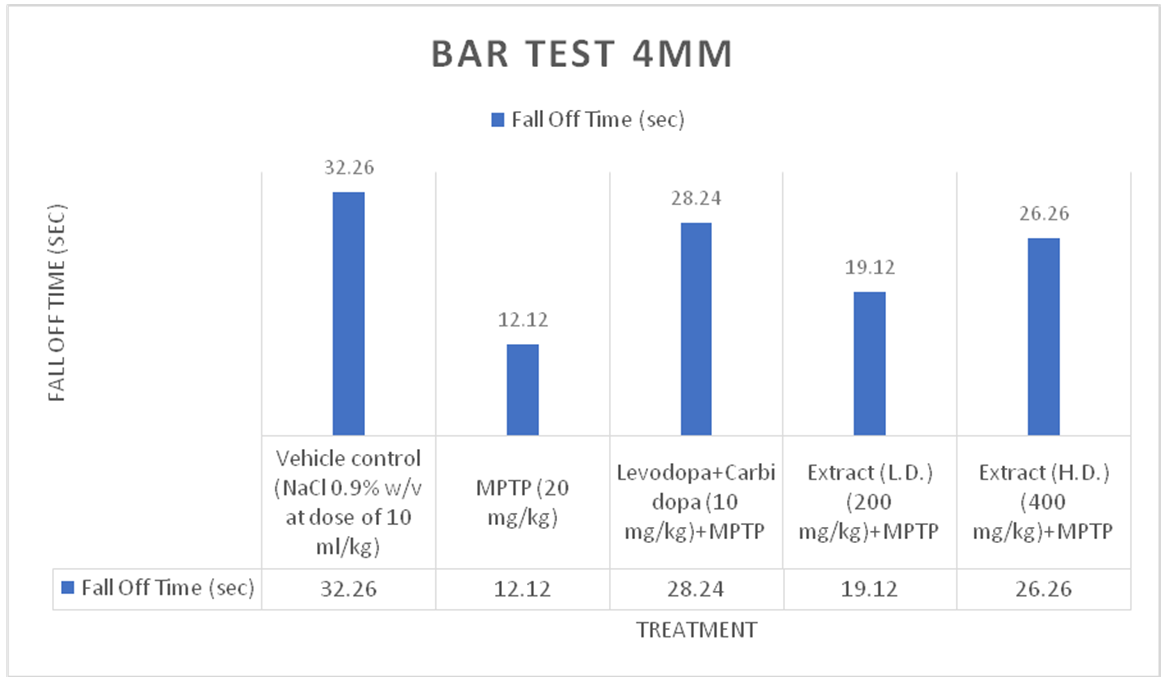
Fig. 3: Effect of ethanolic extracts of Delphiniumdenudatum on fall time in seconds in 4 mm Bar Test. All values are represented as mean±standard error of the mean; n = 6, a represents significant difference (P<0.05) compared to vehicle control and b,represents significant difference (P<0.05) compared to MPTP control. Results were analysed using analysis of variance Tukey’s post-hoc test. a =P<0.05 vs. VEH-VEH control; b =P<0.05 vs. MPTP control
Rota rod test
Effect of Ethanolic extract of Delphinium denudatum (Jadwar) on rota rod test
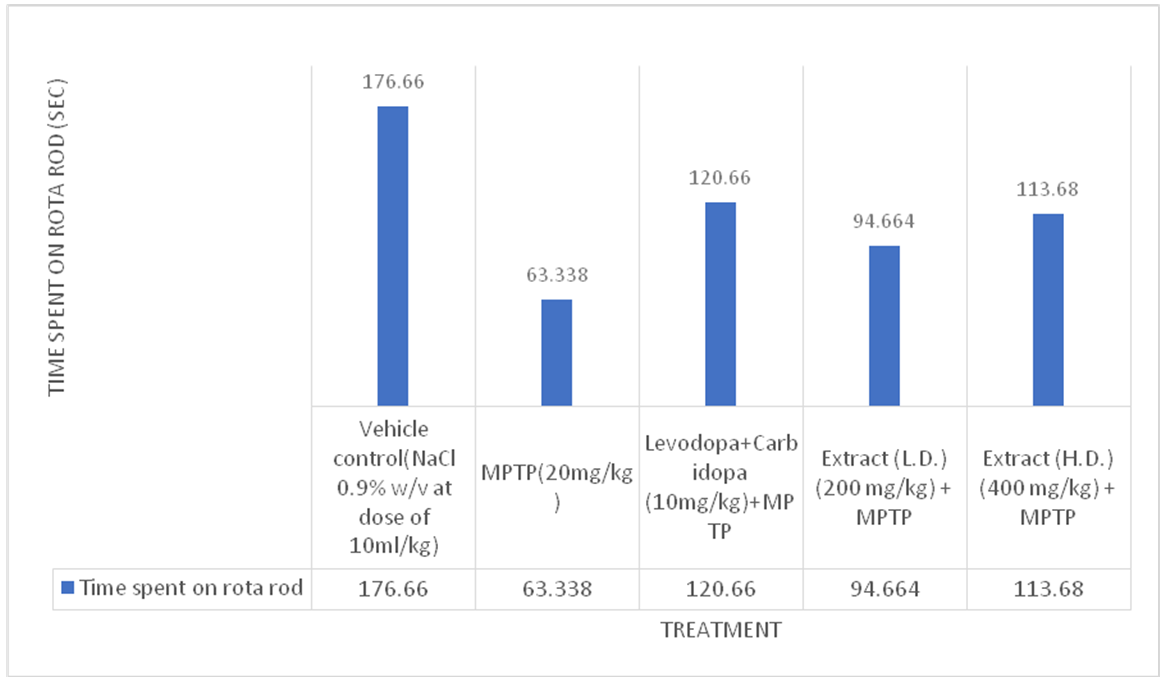
Fig. 4: Effect of ethanolic extracts of Delphinium denudatum on fall time in seconds in Accelerated Rotarod Test. All values are represented as mean±standard error of the mean; n = 6, a represents significant difference (P<0.05) compared to vehicle control and b,represents significant difference (P<0.05) compared to MPTP control. Results were analyzed using analysis of variance Tukey’s post-hoc test. a =P<0.05 vs. VEH-VEH control; b =P<0.05 vs. MPTP control
Table 1: Details of experimental protocols: for 7 d
| Group name | Number of animals required |
| Naïve animal received a standard pellet diet and water ad libitum daily. Control group, normal saline 0.9% NaCl w/v (10 ml/kg, or 1 ml of/100 g body weight orally) for 7 consecutive days | 1 X 6=6 |
| Group treated with MPTP 20 mg/kg for 7 consecutive days | 1 X 6=6 |
| Group treated with Syndopa(Levodopa (100 mg)+Carbidopa (10 mg)/kg) Followed by MPTP 20 mg/kg for 7 consecutive days | 1 X 6=6 |
Ethanolic extract of the Flower of Delphinium denudatum(Jadwar)Low dose (200 mg/kg b. wt) Followed by MPTP 20 mg/kg for 7 consecutive days |
1 X 6=6 |
Ethanolic extract of the Flower of Delphinium denudatum(Jadwar)Low dose (400 mg/kg b. wt) Followed by MPTP 20 mg/kg for 7 consecutive days |
1 X 6=6 |
| Total number of animals required: n=6; N=5; = 30 mice |
Note: All the parameters will perform with suitable time interval to prevent unwanted stress in animals.
Forced swim test
Effect of Ethanolic extract of Delphinium denudatum (Jadwar) on forced swim test
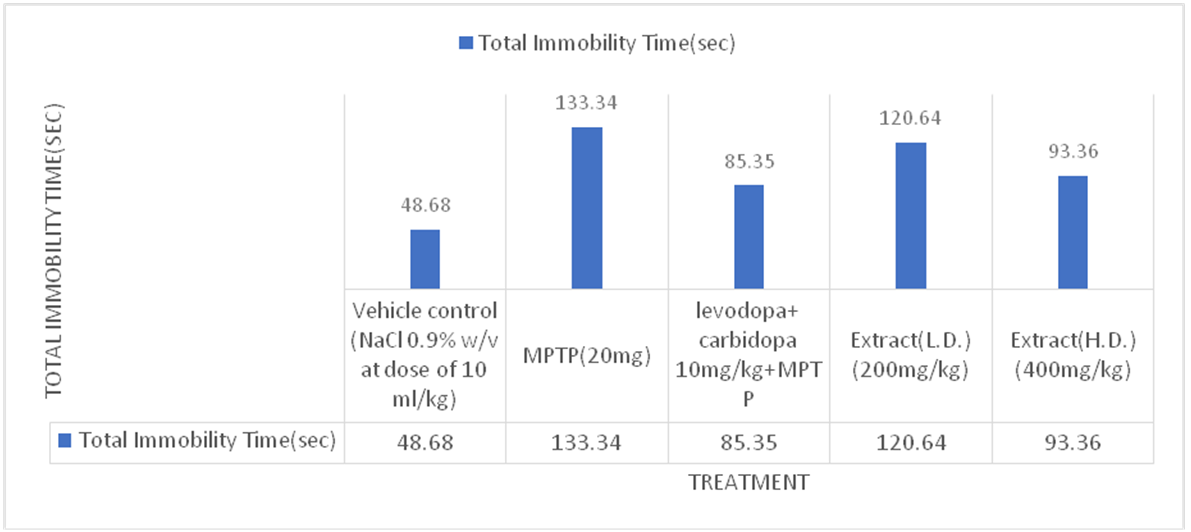
Fig. 5: Effect of ethanolic extracts of Delphinium denudatum on immobility time in seconds in Forced Swim Test. All values are represented as mean±standard error of the mean; n = 6, a represents significant difference (P<0.05) compared to vehicle control and b,represents significant difference (P<0.05) compared to MPTP control. Results were analysed using analysis of variance Tukey’s post-hoc test. a =P<0.05 vs. VEH-VEH control; b =P<0.05 vs. MPTP control
Accelerated rotarod test
Effects of ethanolic extract of delphinium denudatum (Jadwar) on accelerated rota rod test
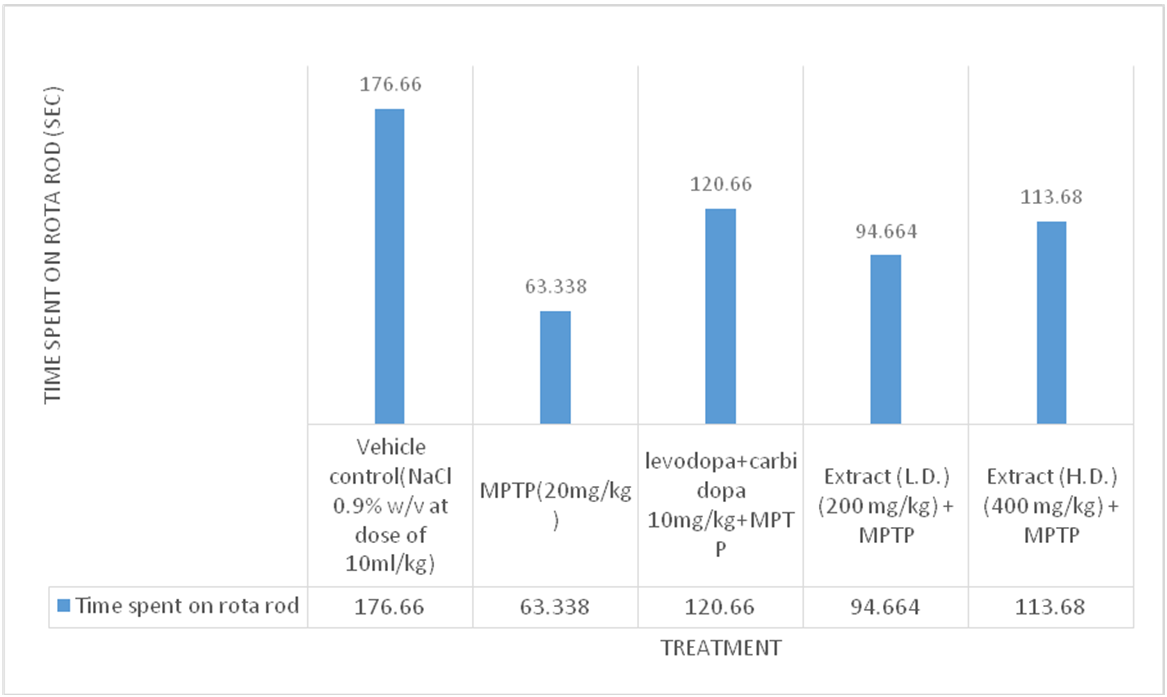
Fig. 6: Effect of ethanolic extracts of Delphinium denudatumon fall time in seconds in Accelerated Rotarod Test. All values are represented as mean±standard error of the mean; n = 6, arepresents significant difference (P<0.05) compared to vehicle control and b,represents significant difference (P<0.05) compared to MPTP control. Results were analysed using analysis of variance Tukey’s post-hoc test. a =P<0.05 vs. VEH-VEH control; b =P<0.05 vs. MPTP control
Actophotometer test
Effects of ethanolic extract of delphinium denudatum (Jadwar) on actophotometer test
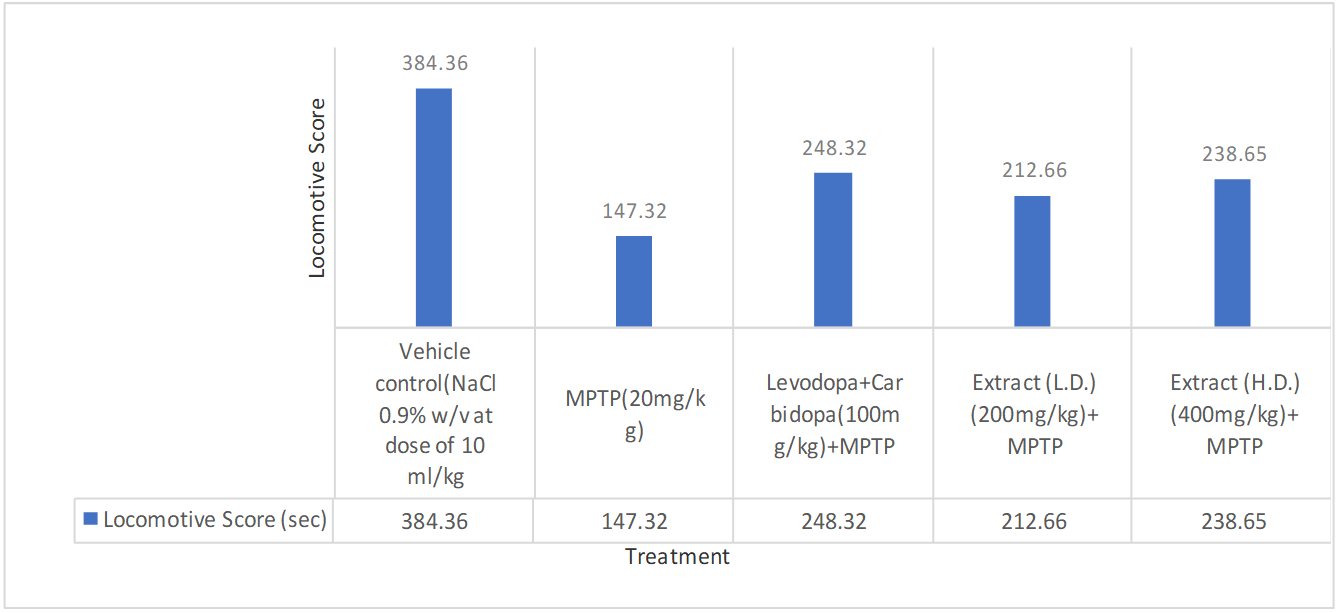
Fig. 7: Effect of ethanolic extracts of Delphinium denudatum on locomotor activity in actophotometer test. All values are represented as mean±standard error of the mean; n = 6, arepresents significant difference (P<0.05) compared to vehicle control and b,represents significant difference (P<0.05) compared to MPTP control. Results were analysed using analysis of variance Tukey’s post-hoc test. a =P<0.05 vs. VEH-VEH control; b =P<0.05 vs. MPTP control
Elevated plus maze test
Effects of ethanolic extract of delphinium denudatum (Jadwar) on elevated plus maze test
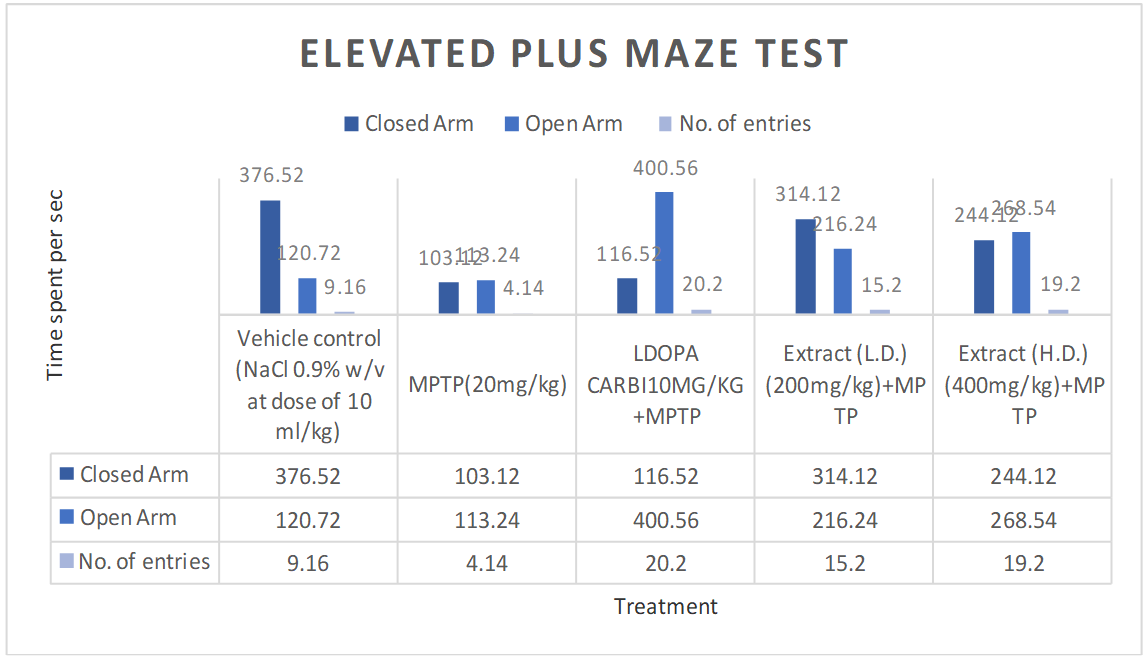
Fig. 8: Effect of ethanolic extracts of Delphinium denudatum on locomotor activity in Elevated Plus Maze test. All values are represented as mean±standard error of the mean; n = 6, arepresents significant difference (P<0.05) compared to vehicle control and b,represents significant difference (P<0.05) compared to MPTP control. Results were analysed using analysis of variance Tukey’s post-hoc test. a =P<0.05 vs. VEH-VEH control; b =P<0.05 vs. MPTP control
Open field test
Effects of ethanolic extract of delphinium denudatum (Jadwar) on open field test
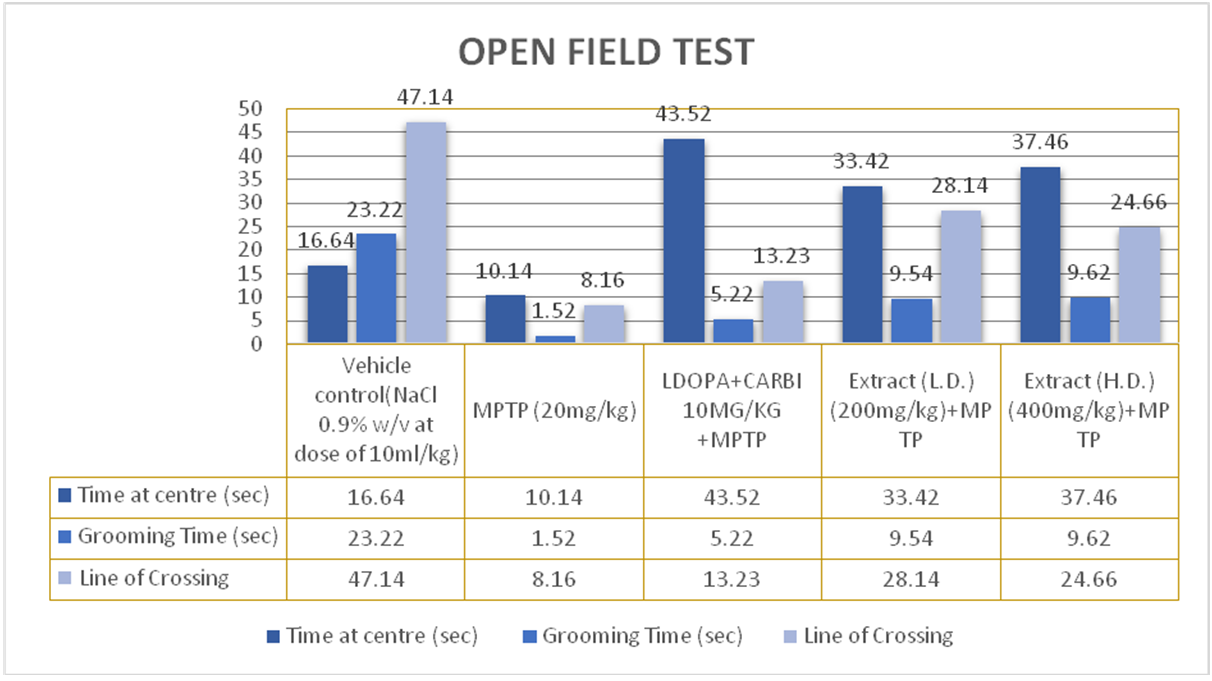
Fig. 9: Effect of ethanolic extracts of Delphiniumdenudatum on locomotor activity in the Open Field test. All values are represented as mean±standard error of the mean; n = 6, a represents significant difference (P<0.05) compared to vehicle control, and brepresents significant difference (P<0.05) compared to MPTP control. Results were analyzed using analysis of variance Tukey’s post-hoc test. a =P<0.05 vs. VEH-VEH control; b =P<0.05 vs. MPTP control
CONCLUSION
The studies showed that the anti-Parkinsonian actions of natural medicinal plants and active components. We identified a wide range of anti-Parkinsonian effects of different herbal extracts and natural compounds. Several PD neurotoxin models allow for the formulation of anti-Parkinsonian drugs. Even herbal treatment can be used for anti-Parkinsonian drugs. However, researchers need to utilize different types of plant extracts and active components to see their actions and efficacy in PD models. Additionally, there is insufficient discussion about the active components and the action of herbal extracts.
ACKNOWLEDGMENT
It’s our privilege to express the profound sense of gratitude and cordial thanks to our respected chairman Mr. Anil Chopra and Vice Chairperson Ms. Sangeeta Chopra, St. Soldier Educational Society, Jalandhar for providing the necessary facilities to complete this research work.
FUNDING
Nil
AUTHORS CONTRIBUTIONS
All authors have contributed equally
CONFLICT OF INTERESTS
Declared none
REFERENCES
Martinez Martin P, Rodriguez Blazquez C, Forjaz MJ, Frades Payo B, Aguera Ortiz L, Weintraub D. Neuropsychiatric symptoms and caregiver’s burden in Parkinson’s disease. Parkinsonism Relat Disord. 2015;21(6):629-34. doi: 10.1016/j.parkreldis.2015.03.024, PMID 25892660.
Surmeier DJ, Guzman JN, Sanchez Padilla J, Schumacker PT. The role of calcium and mitochondrial oxidant stress in the loss of substantia nigra pars compacta dopaminergic neurons in Parkinson’s disease. Neuroscience. 2011;198:221-31. doi: 10.1016/j.neuroscience.2011.08.045, PMID 21884755.
Rodriguez Pallares J, Parga JA, Joglar B, Guerra MJ, Labandeira Garcia JL. Mitochondrial ATP-sensitive potassium channels enhance angiotensin induced oxidative damage and dopaminergic neuron degeneration relevance for aging-associated susceptibility to Parkinson’s disease. Age (Dordr). 2012;34(4):863-80. doi: 10.1007/s11357-011-9284-7, PMID 21713375.
Cury RG, Galhardoni R, Fonoff ET, Perez Lloret S, Dos Santos Ghilardi MG, Barbosa ER. Sensory abnormalities and pain in Parkinson disease and its modulation by treatment of motor symptoms. Eur J Pain. 2016 Feb;20(2):151-65. doi: 10.1002/ejp.745, PMID 26147660.
Korczyn AD. Why have we failed to cure Alzheimer’s disease? J Alzheimers Dis. 2012;29(2):275-82. doi: 10.3233/JAD-2011-110359, PMID 22258512.
Meissner WG, Frasier M, Gasser T, Goetz CG, Lozano A, Piccini P. Priorities in Parkinson’s disease research. Nat Rev Drug Discov. 2011;10(5):377-93. doi: 10.1038/nrd3430, PMID 21532567.
Gazewood JD, Richards DR, Clebak K. Parkinson disease: an update. Am Fam Physician. 2013;87(4):267-73. PMID 23418798.
Aleem M, Ahmad E, Anis M. Botany phytochemistry pharmacology and unani traditional uses of jadwar (delphinium denudatum wall.): a review. J Phytopharmacol. 2020;9(5):378-83. doi: 10.31254/phyto.2020.9516.
Zafar S, Ahmad MA, Siddiqui TA. Protective role of Delphinium denudatum (Jadwar) against morphine induced tolerance and dependence in mice. J Ethnopharmacol. 2001 Nov 1;78(1):95-8. doi: 10.1016/s0378-8741(01)00317-8, PMID 11585695.
Kumar A, Sharma M, Richardson CD, Kelvin DJ. Potential of natural alkaloids from jadwar (delphinium denudatum) as inhibitors against main protease of COVID-19: a molecular modeling approach. Front Mol Biosci. 2022 May 10;9:898874. doi: 10.3389/fmolb.2022.898874, PMID 35620478.
Daneshfard B, Yekta NH, Khoshdel A, Heiran A, Cheraghi R, Yarmohammadi H. The effect of Delphinium denudatum (Jadwar) on fatigue: a randomized double blind placebo-controlled clinical trial. Complement Ther Med. 2019 Oct 1;46:29-35. doi: 10.1016/j.ctim.2019.05.027, PMID 31519284.